Abstract
A method was developed to deposit a visible layer of water-insoluble compounds via sublimation onto the surface of solid media. The compound is sublimed from a heated aluminum dish containing the compound onto the surface of an inverted, ice-cooled, inoculated agar petri dish. The method results in the deposition of a thin, even layer on the agar surface without the use of solvent. After incubation, clearing zones around colonies indicate the presence of compound-degrading microorganisms.
In 1982, Kiyohara et al. described a method for the screening of hydrocarbon-degrading bacteria (9). This method involved spraying polycyclic aromatic hydrocarbons (PAHs) with a thin-layer chromatography sprayer onto mineral salts agar plates. The spray-plate method was developed because water-insoluble compounds cannot be incorporated into agar in the usual way and must be dissolved in organic solvents. Colonies showing clearing zones around them after incubation were picked as degrading species. Because many of these compounds are carcinogenic, spraying had to be carefully performed in a hood with the appropriate personal safety equipment.
The spray-plate method was reviewed in Manual of Environmental Microbiology (13), and many publications cite this method as the technique used for initial isolation of PAH-degrading bacteria (3–6, 8, 10). However, several problems exist with the spray-plate method. The compound must be solubilized with acetone or ether, which may have toxic effects on the bacteria or may be used as an alternate carbon source. If the spray-plate method is implemented before streaking, the compound layer may be disrupted. When plates are sprayed after inoculation, colonies may spread or be disrupted by impacting solvent droplets. The quantity of PAH sprayed onto the agar cannot be easily controlled, compound may be lost by overspraying, and overspraying may result in contamination of large areas of the hood. Because it is difficult to spray plates in an aseptic manner, this method cannot confidently be used for final isolation or enumeration of PAH-degrading bacteria. Other methods, such as spread-plating or the agar-overlay technique (1), involve either direct contact of solvent with the agar or incorporation of solvent directly into the agar. A sublimation method was developed to avoid the problems described above. This method safely deposits an even, thin, visible layer of compound onto the agar surface without the use of solvents.
(This article was presented as poster Q-201 at the 99th General Meeting of the American Society for Microbiology in Chicago, Ill., on 1 June 1999.)
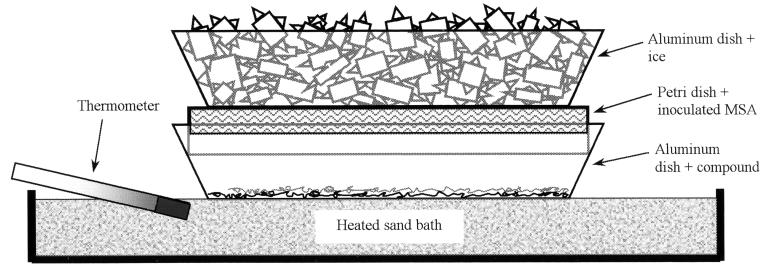
The sublimation system consists of two aluminum dishes, an inoculated mineral salts agar (MSA) standard plastic petri plate (100 by 15 mm), a thermometer, and a heated Pyrex petri dish bottom (diameter, 140 mm) filled to a depth of ∼16 mm with sand (Fig. 1). A thermostatically controlled, aluminum-top hot plate (type 2200; Thermolyne) with a sand bath was placed in a fume hood. The sand was packed and smoothed to a flat surface. An empty aluminum dish was left on the sand bath when the system was not in use to stabilize the bath temperature. A thermometer (FisherBrand 14-985-G) was placed at the smallest possible angle on one side and just below the surface of the sand bath so that the temperature near the surface of the sand could be estimated. The temperature on the aluminum dish surface was determined by melting several compounds with known melting points as the sand was heated and correlating this temperature to the temperature recorded by the thermometer. The highest temperature at which a plastic petri dish would no longer deform and/or stick to the aluminum dish defined the upper temperature limit of the system (135°C). Urea (melting point, 133 to 135°C) was used to verify this temperature. Pyrex petri plates were not used because they adversely affected the evenness of compound deposition. Finely divided crystals of the compound to be sublimed (200 mg) were evenly spread in an aluminum dish (FisherBrand 08-732-10D; top inside diameter, 110 mm). The compound was spread with a spatula, followed by a slight raking of the spatula across the top ridges of the aluminum dish to create vibration. The dish and compound were then held on the heated sand bath for several seconds so that even thermal expansion of the dish bottom occurred. Disrupting the sand surface by pressing the aluminum dish into the sand bath can result in uneven deposition of the compound. Plastic petri plates containing sterile MSA were inoculated at an ambient temperature and subsequently cooled to about 10°C by placing another aluminum dish containing crushed ice onto the inverted plates for about 5 min or refrigerating the plates for about 45 min. MSA contained 1.0 g of KNO3, 0.38 g of K2HPO4, 0.2 g of MgSO4 · 7H2O, 0.05 g of FeCl3 · 6H2O, and 17.0 g of Difco granulated agar per liter of water, and the pH was adjusted to 7.0 with 10% HCl (vol/vol). A cooled, inoculated MSA plate was placed upside-down into the heated aluminum dish containing the compound to be sublimed. A second aluminum dish filled with crushed ice was then placed onto the inverted MSA petri plate. Because of the high temperature of the hot plate, the ice must be maintained throughout the sublimation process. Sublimation of the compound was allowed to occur for a period of time sufficient to deposit a visible layer on the inoculated agar (Table 1). Latex gloves were worn throughout the process. Evenness of PAH deposition was evaluated by observing variations in the fluorescence of the sublimed PAH layer under UV light.
FIG. 1.
Diagram of the sublimation system. The compound to be sublimed and an inverted plastic petri plate containing inoculated MSA rest in a heated aluminum dish. While resting on the petri plate, the second aluminum dish containing ice serves to cool the agar during sublimation. The sand bath was placed on a thermostatically controlled hot plate, and the temperature was monitored with a thermometer placed below the surface of the sand.
TABLE 1.
Compound properties, aluminum dish temperature, and approximate time required to sublime a visible layer of compound onto the agar surface
| Compounda | Melting point (°C) | Vapor pressure (mm Hg)b | Aluminum dish temp (°C) | Approx time (min) | Reference |
|---|---|---|---|---|---|
| Naphthalene | 80 | 3.1 × 10−1 | 75 | 0.5 | 7 |
| p,p′-DDE | 88 | 1.9 × 10−5 | 75 | 35 | 7 |
| Phenanthrene | 100 | 1.0 × 10−3 | 95 | 5 | 7 |
| Fluoranthene | 107 | 6.4 × 10−5 | 95 | 10 | 7 |
| p,p′-DDT | 109 | 2.4 × 10−6 | 95 | 30 | 7 |
| Anthracene | 218 | 6.5 × 10−4 | 135 | 5 | 7 |
| Dieldrin | 175 | 1.6 × 10−4 | 135 | 5 | 7 |
| Mirex | 485 | 2.1 × 10−6 | 135 | 25 | 7 |
| Captan | 178 | 9.8 × 10−6 | 135 | 25 | EXTOXNET website (http://ace.ace.orst.edu/info/extoxnet/) |
| Triphenylene | 197 | 1.7 × 10−8 | 135 | 30 | 2 |
| Octacosane | 61 | 1.1 × 10−9 | 135 | 105 | 12 |
| 9,10-DPA | 245 | 1.4 × 10−10 | 135 | 175 | 11 |
DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; DPA, 9,10-diphenylanthracene.
Vapor pressures at 25°C.
Aluminum dish temperatures used in this study were 75, 95, and 135°C. With the exception of octacosane, a temperature of at least 5°C below the melting point of the compound was chosen so that the compound would remain in the solid state. There was a concern that the agar temperature would rise to the point where thermal death of the bacteria would occur. Positioning the thermocouple of a digital thermometer at the surface of the agar and at one edge of the plate so that the temperature of the agar surface could be measured during the sublimation procedure tested this question. The plate was cooled at 10°C and placed in the sublimation system, and the temperature of the agar surface was recorded every 2 min for 30 min. The aluminum dish containing ice effectively maintained the agar temperature below the ambient temperature (23°C) at a heated-aluminum-dish temperature of 135°C. The crystals of compounds with higher vapor pressures, such as naphthalene, tend to clump and occasionally must be broken apart to prevent uneven deposition of the compound. Although lower-vapor-pressure compounds require longer sublimation times and/or higher temperatures of the sand bath, they usually do not clump. Compounds that were in the liquid state during the sublimation process (octacosane) were mixed with 1 to 2 g of alumina during the heating process. The alumina served to spread the liquid evenly in the aluminum dish and prevent puddling of the compound. Compounds in the liquid form were deposited less evenly because of an increased sensitivity to uneven heating on the sand bath.
The sublimation method was much less prone to contamination than the spray-plate method. Forty sterile Difco nutrient agar plates sprayed with acetone for several seconds with a thin-layer chromatography sprayer showed 85% contamination. Thirty sterile Difco nutrient agar plates placed in the sublimation system for 1 min on an aluminum dish heated to 75°C resulted in 6% contamination. Both sets of plates were incubated for 3 days at 30°C and then examined for the presence of microbial colonies.
The amount of compound deposited on the agar surface with respect to time was evaluated qualitatively by a paper disk assay and quantitatively by scanning UV spectrophotometry. Paper disks (∼1 cm in diameter) from a hole punch were randomly placed on the agar surface of a petri plate. The plate was placed in the sublimation system, and a paper disk was removed at appropriate intervals until a difference between the cleared zone created by the removed disk and the deposited compound layer could be seen. A relationship between vapor pressure and the approximate amount of time required to sublime a visible layer of compound onto the agar surface was found (Table 1). This sublimation method effectively deposited a consistent amount of compound onto the agar surface. A visible layer of anthracene was obtained by subliming the compound for 5 min at 135°C onto 40 ml of solidified agar in a petri dish. The anthracene was extracted with methylene chloride and quantified on an HP 8453 scanning UV spectrophotometer. The amount of anthracene on the agar surface averaged 0.46 mg with a standard deviation of 0.032 (n = 8). The average depth of the anthracene layer (density = 1.25) was calculated to be 60.5 nm.
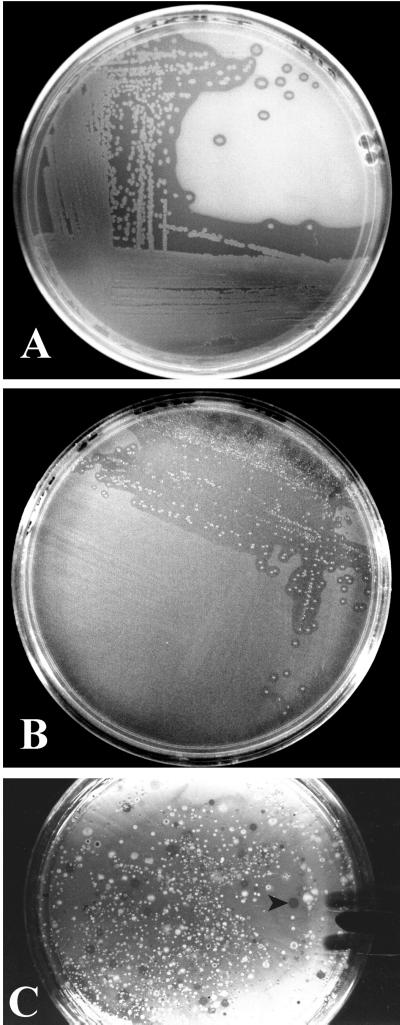
Bacteria capable of degrading naphthalene, phenanthrene, and anthracene were obtained from soil contaminated with JP-4 jet fuel. Enumeration of phenanthrene-degrading bacteria was accomplished by spreading a serial dilution of the soil sample onto the surface of MSA followed by sublimation of phenanthrene onto the agar. After incubation, the ability to metabolize phenanthrene was indicated by the presence of clearing zones (Fig. 2C). Colonies with clearing zones in the PAH layer were picked and streaked for isolation (Fig. 2A and B). Naphthalene-degrading bacteria were incubated at 30°C for 2 days, phenanthrene-degrading bacteria were incubated for 5 days, and anthracene-degrading bacteria were incubated for 10 days. Because many bacterial species will grow on agar without added substrate, the presence of clearing zones in a sublimed compound layer is more definitive for the isolation of degrading species than simply observing growth of bacteria in a plate with naphthalene crystals placed on the lid. The relatively high vapor pressure of naphthalene required that a filter paper disk (FisherBrand 09-795C, 9 cm in diameter) dipped in liquid naphthalene be placed on the lid of the MSA plate during incubation to prevent the compound from leaving the agar surface. Airflow was reduced by placing a weight (250-ml Erlenmeyer flask containing water) on top of the naphthalene-coated agar plate(s) during incubation. The naphthalene-soaked filter paper placed on the petri plate lid maintained a thin layer of naphthalene on the agar for more than 10 days.
FIG. 2.
After incubation, clearing zones can be seen around colonies that degrade naphthalene (A) and phenanthrene (B). Enumeration of phenanthrene-degrading species was carried out by dilution of a soil sample contaminated with JP-4 jet fuel. Colonies with clearing zones (arrow) are phenanthrene-degrading species (C).
This sublimation method will likely prove useful for biodegradation studies of many water-insoluble compounds. Naphthalene served as a model for volatile, high-vapor-pressure compounds, while octacosane and 9,10-diphenylanthracene were investigated as models for the very low-vapor-pressure compounds. Compounds such as the highly carcinogenic benzopyrenes have vapor pressures that range between 5.9 × 10−9 and 3.9 × 10−10 mm Hg (11), comparable to those of octacosane and 9,10-diphenylanthracene (Table 1).
Acknowledgments
We thank Diagnostic Instrument/Analytical Laboratory for use of a hand-held infrared thermometer and help with thermal control for this method, E. Alley and Mississippi State Chemical Laboratory for use of analytical instruments, and T. Stewart of Columbus Airforce Base for providing contaminated soil samples.
REFERENCES
- 1.Bogardt A H, Hemmingsen B B. Enumeration of phenanthrene-degrading bacteria by an overlayer technique and its use in evaluation of petroleum-contaminated sites. Appl Environ Microbiol. 1992;58:2579–2582. doi: 10.1128/aem.58.8.2579-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Kruif C G. Enthalpies of sublimation and vapour pressures of 11 polycyclic hydrocarbons. J Chem Thermodynamics. 1980;12:243–248. [Google Scholar]
- 3.Foght J M, Westlake D W S. Transposon and spontaneous deletion mutants of plasmid-borne genes encoding polycyclic aromatic hydrocarbon degradation by a strain of Pseudomonas fluorescens. Biodegradation. 1996;7:353–366. doi: 10.1007/BF00115749. [DOI] [PubMed] [Google Scholar]
- 4.Goyal A K, Zylstra G J. Molecular cloning of novel genes for polycyclic aromatic hydrocarbon degradation from Comamonas testosteroni GZ39. Appl Environ Microbiol. 1996;62:230–236. doi: 10.1128/aem.62.1.230-236.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerin W F, Jones G E. Two-stage mineralization of phenanthrene by estuarine enrichment cultures. Appl Environ Microbiol. 1988;54:929–936. doi: 10.1128/aem.54.4.929-936.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heitkamp M A, Franklin W, Cerniglia C E. Microbial metabolism of polycyclic aromatic hydrocarbons: isolation and characterization of a pyrene-degrading bacterium. Appl Environ Microbiol. 1988;54:2549–2555. doi: 10.1128/aem.54.10.2549-2555.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinckley D A, Bidleman T F, Foreman W T, Tuschall J R. Determination of vapor pressures for nonpolar and semipolar organic compounds from gas chromatographic retention data. J Chem Eng Data. 1990;35:232–237. [Google Scholar]
- 8.Kästner M, Breuer-Jammali M, Mahro B. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons (PAH) Appl Microbiol Biotechnol. 1994;41:267–273. [Google Scholar]
- 9.Kiyohara H, Nagao K, Yana K. Rapid screen for bacteria degrading water-insoluble, solid hydrocarbons on agar plates. Appl Environ Microbiol. 1982;43:454–457. doi: 10.1128/aem.43.2.454-457.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen T, Kristensen P. Effects of bacterial inoculation and nonionic surfactants on degradation of polycyclic aromatic hydrocarbons in soil. Environ Toxicol Chem. 1997;16:631–637. [Google Scholar]
- 11.Niederfellner J, Lenoir D, Matuschek G, Rehfeldt F, Utschick H, Brüggemann R. Description of vapour pressures of polycyclic aromatic compounds by graph theoretical indices. Quant Struct-Act Relat. 1997;16:38–48. [Google Scholar]
- 12.Piacente V, Fontana D, Scardala P. Enthalpies of vaporization of a homologous series of n-alkanes determined from vapor pressure measurements. J Chem Eng Data. 1994;39:231–237. [Google Scholar]
- 13.Shuttleworth K L, Cerniglia C E. Practical methods for the isolation of polycyclic aromatic hydrocarbon (PAH)-degrading microorganisms and the determination of PAH mineralization and biodegradation intermediates. In: Hurst C J, Knudsen G R, McInerney M J, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C.: American Society for Microbiology; 1997. pp. 766–775. [Google Scholar]