Abstract
Background
The benefit of adjuvant chemotherapy (ACT) in pathological T2N0M0 non-small-cell lung cancer (NSCLC) patients is not clear.
Methods
One thousand and fifty pathological T2N0M0 NSCLC patients were included and divided into two groups: with and without ACT. A propensity score matching analysis was carried out to minimize selection bias. The significance of ACT in high-risk patients was further analyzed. The Kaplan–Meier method and Cox proportional hazards model were used to assess the impact of ACT on the overall survival (OS), disease-free survival (DFS), and cancer-specific survival.
Results
For the entire cohort, 31.9% (335/1050) of patients received ACT. After propensity score matching, 325 pairs of patients were matched. OS and DFS were comparable between groups in the original or matched cohort, which was confirmed by the multivariate analysis (all P > 0.05). In high-risk patients, the data suggest that ACT could improve OS and DFS only in patients with tumours >4 cm (OS: P = 0.003; DFS: P = 0.013). ACT could significantly improve the 5-year OS in patients with wild-type epidermal growth factor receptor (EGFR) (P = 0.022). ACT, however, could not improve cancer-specific survival in any subgroup, including patients with tumours >4 cm or wild-type EGFR (all P > 0.05). For patients with other high-risk factors, ACT failed to benefit patients in long-term outcomes.
Conclusions
In resected pT2N0M0 NSCLC patients, those with tumours >4 cm and wild-type EGFR are real high-risk patients and could gain survival benefit from ACT. Further prospective study is needed to confirm the definition.
Key words: non-small cell lung cancer, T2N0M0, adjuvant chemotherapy, survival
Highlights
-
•
ACT could not improve long-term survival in pT2N0M0 NSCLC in general.
-
•
ACT could only improve OS and DFS in pT2N0M0 NSCLC >4 cm.
-
•
ACT could not improve CSS in any subgroup of pT2N0M0 NSCLC.
-
•
For patients with other high-risk factors, ACT failed to improve long-term survival.
Introduction
The role of adjuvant chemotherapy (ACT) in pathological stage T2N0M0 (pT2N0M0) non-small-cell lung cancer (NSCLC) remains controversial. The current National Comprehensive Cancer Network (NCCN) guideline recommends that patients with high-risk factors which include poorly differentiated tumours, vascular invasion, wedge resection, tumours >4 cm, visceral pleural involvement (VPI), and unknown lymph node status (Nx), should receive ACT.1 This recommendation, however, is only based on the post hoc subgroup analysis of CALGB-9633 and JBR.10,2,3 and thus lacks strong evidence. Data-driven evidence regarding the significance of ACT in these patients is rare and must be further elucidated.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15
Herein, we set a large cohort of pT2N0M0 NSCLC patients using the widely accepted definition of high-risk cases by NCCN guidelines1 to assess the significance of ACT in these patients, and verify the high-risk patients who can truly benefit from ACT for a tailed therapy.
Methods
Patient selection
We used a database of 5346 NSCLC patients who underwent consecutive pulmonary resections at Sun Yat-sen University Cancer Center between January 2001 and December 2014, as we previously described.16, 17, 18 The inclusion criteria of this study were as follows: (i) patients with primary NSCLC; (ii) patients received complete resection; (iii) pT2N0M0. Patients with the following characteristics were excluded: (i) adjuvant therapies other than ACT; (ii) other concurrent or previous primary cancers; (iii) neoadjuvant therapy; (iv) operative mortality; (v) incomplete data. Operative mortality was defined as death within 30 days of operation or at any time after the operation if the patient did not leave the hospital alive.19 Finally, 1050 patients were successfully included in this study and a flow chart of the inclusion process is shown in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100508. These patients were further divided into two groups: patients with ACT (ACT+ group) and patients without ACT (ACT− group). The 8th edition of the American Joint Committee on Cancer (AJCC) staging system for lung cancer was used to restage all of these cases.20 This study was approved by the institutional review board of Sun Yat-sen University Cancer Center, and the key raw data have been submitted to the Research Data Deposit public platform (www.researchdata.org.cn) with the approval RDD number RDDA2021001984.
Follow-up
Generally, post-operative follow-up is recommended every 3 months for the first 2 years, every 6 months for the next 3-5 years, and once a year thereafter.17,18 At each follow-up visit, a physical examination, serum tumour marker test, spiral contrast-enhanced chest computed tomography (CT) scan, and abdominal sonography were carried out. If the patient had specific symptoms, the examination was carried out as soon as possible for a more careful assessment. Abdomen CT scans, bone scans, and brain magnetic resonance imaging scans were carried out when clinically indicated.17,18 Follow-up information was last updated in April 2019 or on the date of death. The median time from the date of surgery to the last follow-up was 65 months (range, 1-210 months).
Definition of high-risk factors
There are six high-risk factors recommended by the NCCN guidelines: poorly differentiated tumours, vascular invasion, wedge resection, tumours >4 cm, VPI, and Nx.1 Based on previous studies, NSCLC patients with Nx or receiving wedge resection were considered incomplete resection cases,1,21, 22, 23, 24 and it has been widely accepted that post-operative therapy should be routinely carried out in these patients. Therefore, only poorly differentiated tumours, vascular invasion, tumours >4 cm, and VPI were identified as high-risk factors1 in this study.
Propensity score matching analysis
A propensity score matching (PSM) analysis was conducted to minimize the selection bias.25 Propensity scores were calculated by a logistic regression model including the sex, age, anatomical type, tumour location, tumour size, smoking history, comorbidity, surgical procedure, histology, cell differentiation, vascular invasion, and VPI. Patients from the two groups were matched in a 1 : 1 ratio based on their propensity scores. The R software (version 4.0.2, Bell Laboratories, Murray Hill, NJ; https://cran.r-project.org/bin/windows/base/R-4.0.2-win.exe) was used to carry out the PSM analysis.
Survival analysis
Both overall survival (OS) and disease-free survival (DFS) were set as the endpoints of the survival analysis; in addition, cancer-specific survival (CSS) was also analysed in this study. OS was defined as the time between the date of surgery and the date of death. DFS was calculated from the time of surgery to the time of recurrence or death from any cause. CSS was defined as the time between the date of surgery and the date of death caused by NSCLC. Patients without an event were censored at the last follow-up known to be alive. The long-term survival of the two groups was compared in the entire cohort and in the matched cohort. After PSM, a subgroup analysis based on high-risk factors (poorly differentiated tumours, vascular invasion, tumours >4 cm, and VPI) was conducted to verify whether pT2N0M0 NSCLC patients with these high-risk factors could benefit from ACT. In addition to these high-risk factors, other potential clinical characteristics were also explored if they could probably influence the benefit of ACT.
Statistical analysis
Continuous variables were compared using Mann–Whitney U test. Pearson’s χ2 test was used to determine significant differences between groups for categorical variables. OS, DFS, and CSS were evaluated using the Kaplan–Meier method, and the log-rank test was used to compare the differences between groups. The Cox proportional hazards regression model was conducted to identify independent prognostic factors that are associated with long-term survival. IBM SPSS Statistics (version 25.0, IBM Corp., Armonk, NY) was used to conduct the statistical analysis. Two-sided P < 0.05 was considered statistically significant.
Results
Patient characteristics
In total, 1050 pT2N0M0 NSCLC cases were included, and 325 pairs were matched after PSM. The clinicopathological characteristics of the entire cohort (N = 1050) and paired patients (N = 650) are summarized in Table 1. For the entire cohort, 31.9% (335/1050) of patients received ACT. Of the 335 patients with ACT, specific chemotherapy regimens of 318 (94.9%) patients were available. The commonly used regimens were pemetrexed plus platinum (32.1%, 102/318; including 52 with cisplatin and 50 with carboplatin), paclitaxel plus cisplatin (21.7%, 69/318), tegafur (14.8%, 47/318), docetaxel plus cisplatin (13.8%, 44/318), vinorelbine plus cisplatin (5.7%, 18/318), pemetrexed (5.3%, 17/318), and gemcitabine plus carboplatin (2.8%, 9/318). A total of 87.5% (293/335) of patients with ACT received at least four cycles of ACT. Patients with ACT were younger (P < 0.001) and had larger tumours (P = 0.019). Adenocarcinoma was the predominant pathological type, followed by squamous cell carcinoma (Table 1). After the PSM, all characteristics were well balanced between the ACT+ group and the ACT− group (Table 1).
Table 1.
General clinicopathological characteristics of patients with and without ACT before and after PSM
| Characteristics | Before PSM (N = 1050) |
After PSM (N = 650) |
||||
|---|---|---|---|---|---|---|
| Without ACT (N = 715) | With ACT (N = 335) | P | Without ACT (N = 325) | With ACT (N = 325) | P | |
| Sex, n (%) | ||||||
| Male | 491 (68.7) | 238 (71.0) | 0.437 | 227 (69.8) | 232 (71.4) | 0.667 |
| Female | 224 (31.3) | 97 (29.0) | 98 (30.2) | 93 (28.6) | ||
| Age (years) | ||||||
| Mean ± SD | 60.9 ± 9.7 | 57.4 ± 9.4 | <0.001 | 57.7 ± 10.0 | 57.9 ± 8.9 | 0.782 |
| Median (min, max) | 61.0 (29, 90) | 58.0 (24, 79) | 58.0 (29, 83) | 58.0 (25, 79) | ||
| ≤60 | 341 (47.7) | 204 (60.9) | <0.001 | 198 (60.9) | 195 (60.0) | 0.810 |
| >60 | 374 (52.3) | 131 (39.1) | 127 (39.1) | 130 (40.0) | ||
| Tumour size (cm) | ||||||
| Mean ± SD | 3.3 ± 1.1 | 3.5 ± 1.1 | 0.019 | 3.4 ± 1.2 | 3.5 ± 1.1 | 0.144 |
| Median (min, max) | 3.5 (0.5, 5.0) | 4.0 (0.6, 5.0) | 3.5 (1.0, 5.0) | 4.0 (0.6, 5.0) | ||
| ≤4 cm | 567 (79.3) | 256 (76.4) | 0.290 | 250 (76.9) | 249 (76.6) | 0.926 |
| >4 cm | 148 (20.7) | 79 (23.6) | 75 (23.1) | 76 (23.4) | ||
| Anatomical type, n (%) | ||||||
| Central | 82 (11.5) | 41 (12.2) | 0.718 | 39 (12.0) | 40 (12.3) | 0.904 |
| Peripheral | 633 (88.5) | 294 (87.8) | 286 (88.0) | 285 (87.7) | ||
| Tumour location, n (%) | ||||||
| RUL | 214 (29.9) | 102 (30.4) | 0.419 | 93 (28.6) | 100 (30.8) | 0.962 |
| RML | 63 (8.8) | 31 (9.3) | 31 (9.5) | 30 (9.2) | ||
| RLL | 142 (19.9) | 57 (17.0) | 54 (16.6) | 56 (17.2) | ||
| LUL | 191 (26.7) | 82 (24.5) | 87 (26.8) | 80 (24.6) | ||
| LLL | 105 (14.7) | 63 (18.8) | 60 (18.5) | 59 (18.2) | ||
| Comorbidity, n (%) | ||||||
| No | 543 (75.9) | 265 (79.1) | 0.257 | 248 (76.3) | 255 (78.5) | 0.512 |
| Yes | 172 (24.1) | 70 (20.9) | 77 (23.7) | 70 (21.5) | ||
| Smoking history, n (%) | ||||||
| Never | 319 (44.6) | 140 (41.8) | 0.592 | 144 (44.3) | 135 (41.5) | 0.586 |
| Current | 309 (43.2) | 156 (46.6) | 138 (42.5) | 151 (46.5) | ||
| Quit | 87 (12.2) | 39 (11.6) | 43 (13.2) | 39 (12.0) | ||
| Surgical resectiona, n (%) | ||||||
| Lobectomy | 621 (86.9) | 302 (90.1) | 0.263 | 290 (89.2) | 292 (89.8) | 0.890 |
| Bilobectomy | 43 (6.0) | 17 (5.1) | 19 (5.8) | 17 (5.2) | ||
| Pneumonectomy | 40 (5.6) | 10 (3.0) | 8 (2.5) | 10 (3.1) | ||
| Sublobar resection | 11 (1.5) | 6 (1.8) | 8 (2.5) | 6 (1.98) | ||
| Histology, n (%) | ||||||
| Adenocarcinoma | 453 (63.4) | 207 (61.8) | 0.048 | 208 (64.0) | 200 (61.5) | 0.809 |
| Squamous cell carcinoma | 226 (31.6) | 98 (29.3) | 92 (28.3) | 98 (30.2) | ||
| Adenosquamous carcinoma | 18 (2.5) | 15 (4.5) | 12 (3.7) | 14 (4.3) | ||
| Neuroendocrine carcinoma | 11 (1.5) | 10 (3.0) | 7 (2.2) | 9 (2.8) | ||
| Sarcomatoid carcinoma | 2 (0.3) | 3 (0.9) | 1 (0.3) | 3 (0.9) | ||
| Mucoepidermoid carcinoma | 4 (0.6) | 1 (0.3) | 4 (1.2) | 1 (0.3) | ||
| Pleomorphic carcinoma | 0 | 1 (0.3) | 0 | 0 | ||
| Basaloid carcinoma | 1 (0.1) | 0 | 1 (0.3) | 0 | ||
| Cell differentiation, n (%) | ||||||
| Well | 61 (8.5) | 24 (7.2) | 0.148 | 25 (7.7) | 24 (7.4) | 0.925 |
| Moderate | 337 (47.1) | 141 (42.1) | 142 (43.7) | 138 (42.5) | ||
| Poor | 317 (44.3) | 170 (50.7) | 158 (48.6) | 163 (50.2) | ||
| Vascular invasion, n (%) | ||||||
| No | 687 (96.1) | 317 (94.6) | 0.282 | 309 (95.1) | 310 (95.4) | 0.854 |
| Yes | 28 (3.9) | 18 (5.4) | 16 (4.9) | 15 (4.6) | ||
| Visceral pleural involvement, n (%) | ||||||
| No | 176 (24.6) | 82 (24.5) | 0.770 | 83 (25.5) | 79 (24.3) | 0.911 |
| Yes | 423 (59.2) | 195 (58.2) | 191 (58.8) | 192 (59.1) | ||
| NA | 116 (16.2) | 58 (17.3) | 51 (15.7) | 54 (16.6) | ||
| EGFR statusa, n (%) | ||||||
| Wild-type | 203 (61.9) | 126 (67.0) | 0.243 | 91 (61.5) | 123 (67.2) | 0.279 |
| Mutations | 125 (38.1) | 62 (33.0) | 57 (38.5) | 60 (32.8) | ||
| Treatment after recurrenceb, n (%) | ||||||
| No | 54 (48.6) | 29 (39.7) | 0.234 | 25 (48.1) | 26 (37.1) | 0.226 |
| Yes | 57 (51.4) | 44 (60.3) | 27 (51.9) | 44 (62.9) | ||
ACT, adjuvant chemotherapy; EGFR epidermal growth factor receptor; LLL, left lower lobe; LUL, left upper lobe; NA, not available; PSM, propensity score matching; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; SD, standard deviation.
Excluding patients without EGFR status record.
Excluding patients without recurrence.
Survival analysis
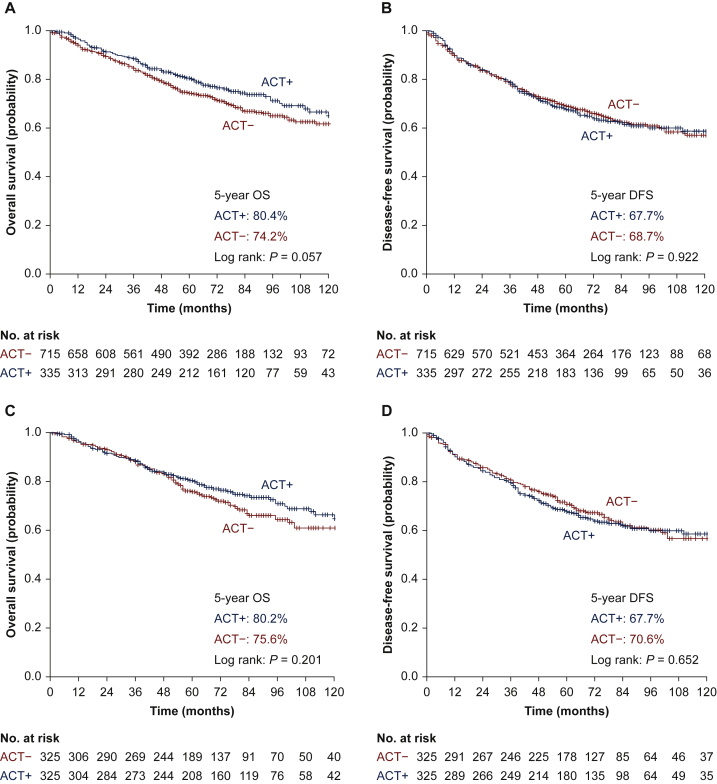
Before the PSM, the 5-year OS was mildly improved in patients receiving ACT without statistical significance (ACT− group versus ACT+ group: 74.2% versus 80.4%, P = 0.057, Figure 1A), but the 5-year DFS rate was comparable between the two groups (ACT− group versus ACT+ group: 68.7% versus 67.7%, P = 0.922, Figure 1B). The multivariate analysis demonstrated that sex, age, and tumour size were independent prognostic factors for OS (Table 2), whereas sex, age, and histology were independent prognostic factors for DFS (Table 2). ACT, however, had impact neither on OS [hazard ratio (HR) = 0.800, 95% confidence interval (CI) 0.621-1.030, P = 0.083, Table 2] nor DFS (HR = 1.023, 95% CI 0.822-1.273, P = 0.837, Table 2).
Figure 1.
Survival curves for patients with or without ACT before and after the PSM: (A) survival curves of OS before PSM; (B) survival curves of DFS before PSM; (C) survival curves of OS after PSM; (D) survival curves of DFS after PSM.
ACT, adjuvant chemotherapy; DFS, disease-free survival; OS, overall survival; PSM, propensity score matching.
Table 2.
Multivariate Cox regression analysis for prognostic factors before propensity score matching (N = 1050)
| Characteristics | OS |
DFS |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 0.620 (0.432-0.890) | 0.009 | 0.706 (0.515-0.968) | 0.031 |
| Age (years) | ||||
| ≤60 | Ref | Ref | ||
| >60 | 1.572 (1.235-2.002) | <0.001 | 1.336 (1.078-1.656) | 0.008 |
| Tumour size (cm) | ||||
| ≤4 cm | Ref | Ref | ||
| >4 cm | 1.345 (1.030-1.757) | 0.029 | 1.262 (0.986-1.615) | 0.064 |
| Anatomical type | ||||
| Central | Ref | Ref | ||
| Peripheral | 0.852 (0.587-1.235) | 0.397 | 0.832 (0.593-1.169) | 0.289 |
| Tumour location | ||||
| RUL | Ref | Ref | ||
| RML | 1.141 (0.725-1.796) | 0.570 | 1.116 (0.749-1.663) | 0.591 |
| RLL | 1.318 (0.952-1.824) | 0.096 | 1.144 (0.851-1.538) | 0.373 |
| LUL | 1.064 (0.778-1.455) | 0.700 | 1.031 (0.781-1.360) | 0.289 |
| LLL | 1.090 (0.763-1.558) | 0.635 | 1.055 (0.768-1.449) | 0.743 |
| Comorbidity | ||||
| No | Ref | Ref | ||
| Yes | 0.880 (0.661-1.173) | 0.384 | 0.864 (0.668-1.119) | 0.268 |
| Smoking history | ||||
| Never | Ref | Ref | ||
| Current/quit | 1.074 (0.788-1.463) | 0.652 | 1.075 (0.813-1.423) | 0.612 |
| Surgical resection | ||||
| Lobar resectiona | Ref | Ref | ||
| Sublobar resection | 1.094 (0.444-2.692) | 0.845 | 1.254 (0.586-2.686) | 0.559 |
| Histology | ||||
| Adenocarcinoma | Ref | Ref | ||
| Non-adenocarcinoma | 0.801 (0.609-1.054) | 0.113 | 0.726 (0.565-0.934) | 0.013 |
| Cell differentiation | ||||
| Well | Ref | Ref | ||
| Moderate | 0.745 (0.475-1.169) | 0.201 | 1.008 (0.660-1.538) | 0.972 |
| Poor | 1.169 (0.746-1.833) | 0.495 | 1.342 (0.875-2.057) | 0.177 |
| Vascular invasion | ||||
| No | Ref | Ref | ||
| Yes | 1.493 (0.886-2.514) | 0.132 | 1.280 (0.796-2.060) | 0.308 |
| Visceral pleural involvement | ||||
| No | Ref | Ref | ||
| Yes | 1.282 (0.991-1.658) | 0.058 | 1.232 (0.978-1.551) | 0.076 |
| NA | 0.956 (0.675-1.355) | 0.801 | 0.950 (0.697-1.295) | 0.746 |
| Adjuvant chemotherapy | ||||
| No | Ref | Ref | ||
| Yes | 0.800 (0.621-1.030) | 0.083 | 1.023 (0.822-1.273) | 0.837 |
CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; LLL, left lower lobe; LUL, left upper lobe; NA, not available; OS, overall survival; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Include lobectomy, bilobectomy, and pneumonectomy.
After the PSM, our data showed that both 5-year OS rate (ACT− group versus ACT+ group: 75.6% versus 80.2%, P = 0.201,Figure 1C) and 5-year DFS rate (ACT− group versus ACT+ group: 70.6% versus 67.7%, P = 0.652, Figure 1D) were comparable between the two groups. The multivariate analysis suggested that sex, age, tumour location, and histology were independent prognostic factors for OS, whereas sex and histology were independent prognostic factors for DFS. ACT again, however, failed to benefit the patients in either OS (HR = 0.824, 95% CI 0.616-1.104, P = 0.195, Table 3) or DFS (HR = 1.062, 95% CI, 0.820-1.376, P = 0.648, Table 3).
Table 3.
Multivariate Cox regression analysis for prognostic factors after propensity score matching (N = 650)
| Characteristics | OS |
DFS |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 0.390 (0.235-0.646) | <0.001 | 0.443 (0.289-0.681) | <0.001 |
| Age (years) | ||||
| ≤60 | Ref | Ref | ||
| >60 | 1.471 (1.081-2.003) | 0.014 | 1.234 (0.939-1.620) | 0.131 |
| Tumour size (cm) | ||||
| ≤4 cm | Ref | Ref | ||
| >4 cm | 1.220 (0.866-1.718) | 0.256 | 1.275 (0.937-1.734) | 0.122 |
| Anatomical type | ||||
| Central | Ref | Ref | ||
| Peripheral | 0.880 (0.548-1.413) | 0.596 | 0.870 (0.570-1.330) | 0.521 |
| Tumour location | ||||
| RUL | Ref | Ref | ||
| RML | 1.008 (0.550-1.848) | 0.980 | 1.030 (0.614-1.726) | 0.912 |
| RLL | 1.940 (1.276-2.948) | 0.002 | 1.409 (0.962-2.063) | 0.078 |
| LUL | 1.151 (0.766-1.730) | 0.497 | 1.076 (0.757-1.528) | 0.684 |
| LLL | 1.086 (0.688-1.716) | 0.722 | 1.006 (0.676-1.498) | 0.976 |
| Comorbidity | ||||
| No | Ref | Ref | ||
| Yes | 0.902 (0.619-1.317) | 0.594 | 0.913 (0.657-1.269) | 0.590 |
| Smoking history | ||||
| Never | Ref | Ref | ||
| Current/quit | 1.089 (0.737-1.608) | 0.669 | 1.067 (0.755-1.509) | 0.714 |
| Surgical resection | ||||
| Lobar resectiona | Ref | Ref | ||
| Sublobar resection | 0.954 (0.342-2.665) | 0.929 | 1.270 (0.548-2.942) | 0.578 |
| Histology | ||||
| Adenocarcinoma | Ref | Ref | ||
| Non-adenocarcinoma | 0.646 (0.456-0.915) | 0.014 | 0.616 (0.449-0.844) | 0.003 |
| Cell differentiation | ||||
| Well | Ref | Ref | ||
| Moderate | 0.829 (0.439-1.566) | 0.564 | 1.133 (0.625-2.053) | 0.681 |
| Poor | 1.489 (0.799-2.774) | 0.210 | 1.619 (0.896-2.925) | 0.111 |
| Vascular invasion | ||||
| No | Ref | Ref | ||
| Yes | 1.458 (0.773-2.752) | 0.245 | 1.421 (0.810-2.492) | 0.220 |
| Visceral pleural involvement | ||||
| No | Ref | Ref | ||
| Yes | 1.123 (0.807-1.562) | 0.491 | 1.082 (0.809-1.446) | 0.596 |
| NA | 0.955 (0.608-1.501) | 0.842 | 0.786 (0.523-1.180) | 0.245 |
| Adjuvant chemotherapy | ||||
| No | Ref | Ref | ||
| Yes | 0.824 (0.616-1.104) | 0.195 | 1.062 (0.820-1.376) | 0.648 |
CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; OS, overall survival; LLL, left lower lobe; LUL, left upper lobe; NA, not available; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Include lobectomy, bilobectomy, and pneumonectomy.
We further explored the impact of ACT on CSS. Before PSM, patients with ACT had a better CSS than those without ACT (5-year CSS: 79.1% versus 86.7%, P = 0.045), but ACT failed to improve CSS in matched cohort after PSM (5-year CSS: 83.2% versus 87.1%, P = 0.886). As predicted, multivariate analysis indicated that ACT was a prognostic factor for CSS neither before (P = 0.050) nor after (P = 0.750) PSM.
The significance of ACT in patients with high-risk factors
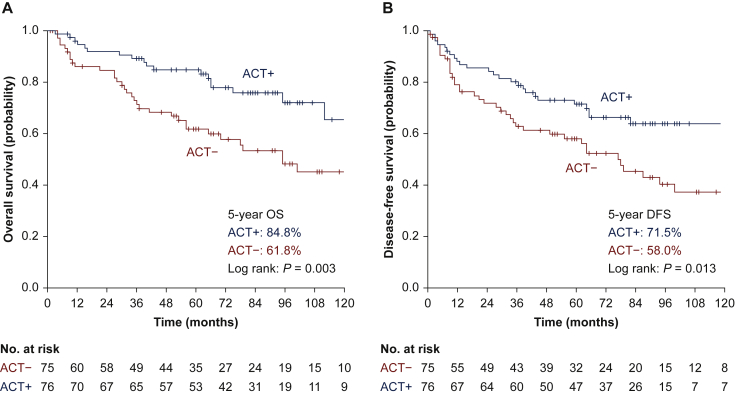
The current NCCN guideline of NSCLC proposes ACT for pT2N0M0 NSCLC patients with high-risk factors at grade 2A evidence.1 To investigate whether high-risk patients can truly benefit from ACT, we conducted further analyses for these patients. After the PSM, survival analyses were conducted for patients with high-risk factors, including poorly differentiated tumours, vascular invasion, tumours >4 cm, and VPI, based on the definition of the NCCN guidelines.1 The data suggested that ACT could significantly improve both OS (5-year OS rate: 61.8% versus 84.8%, P = 0.003, Figure 2A) and DFS (5-year DFS rate: 58.0% versus 71.5%, P = 0.013, Figure 2B) in patients whose tumour sizes exceeded 4 cm. ACT failed, however, to improve survival for patients with poorly differentiated tumour cell (OS, P = 0.699; DFS, P = 0.369), vascular invasion (OS, P = 0.340; DFS, P = 0.142), or VPI (OS, P = 0.207; DFS, P = 0.855) (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100508). The multivariate analysis confirmed that ACT was an independent factor that favoured both OS (HR = 0.378, 95% CI: 0.204-0.702, P = 0.002) and DFS (HR = 0.487, 95% CI: 0.282-0.839, P = 0.010) in patients with tumours >4 cm in diameter (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100508, online only).
Figure 2.
Subgroup analysis of the survival curves for patients with or without ACT after PSM with respect to tumours >4 cm: (A) survival curves of OS; (B) survival curves of DFS.
ACT, adjuvant chemotherapy; DFS, disease-free survival; OS, overall survival; PSM, propensity score matching.
Interestingly, we explored the association of ACT benefit with epidermal growth factor receptor (EGFR) mutation in 331 patients with available EGFR status after PSM. In 117 EGFR-mutated pT2N0M0 patients, ACT did not improve the long-term survival (OS, P = 0.965; DFS, P = 0.096), even in those with tumours >4 cm (OS: P = 0.620; DFS: P = 0.904). In patients with wild-type EGFR (N = 214), however, ACT significantly improved the 5-year OS (ACT− group versus ACT+ group: 72.2% versus 83.6%, P = 0.022, Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100508). ACT, however, could not improve CSS in any subgroup, even in patients with tumours >4 cm (P = 0.802) or wild-type EGFR tumours (P = 0.059) after PSM.
Discussion
The current NCCN guideline recommends ACT for stage II and III NSCLC patients, whereas the significance of ACT in pT2N0M0 (stages IB and IIA) has not been well elucidated.1 Although ACT is not routinely recommended for pT2N0M0 NSCLC patients,1,21,26 a postoperative multimodality evaluation is necessary to assess the risk of recurrence and potential benefits of ACT for individual patients. The NCCN guideline recommends ACT for selected pT2N0M0 NSCLC patients with high-risk factors at category 2A evidence. The definition of high-risk factors and their significance in guiding ACT, however, were not consistent. The NCCN guideline defines high-risk factors as VPI, poor cell differentiation, lymphovascular invasion, etc.1 The European Society of Medical Oncology (ESMO) guideline suggests that stage IB patients with primary tumours >4 cm can be considered to receive ACT.21 Except for the tumour size, the American Society of Clinical Oncology (ASCO) guideline recommends other factors be considered in decision making, such as histopathological features, presence of oncogenic drivers, and gene signatures.26 Nevertheless, there is no strong evidence to confirm the clinical significance of these high-risk factors and their guidance in ACT administration in pT2N0M0 NSCLC. The conclusions of previous studies were controversial regarding the role of ACT in high-risk pT2N0M0 NSCLC.12, 13, 14 Two possible reasons may contribute to the discrepancies; the definition of ‘high-risk’ factors was not consistent, and the sample sizes of pT2N0M0 NSCLC in some studies were too limited to draw a powerful evidence-based conclusion.
Here, we conducted a large cohort study to assess the significance of ACT in pT2N0M0 NSCLC patients with an emphasis on patients with high-risk factors (defined by NCCN guidelines), and PSM was carried out to minimize the selection bias. Our results suggested that ACT could not improve OS, DFS, or CSS in all pT2N0M0 NSCLC, but those with tumours >4 cm and wild-type EGFR could have better OS and DFS after ACT. For patients with other high-risk factors such as poorly differentiated tumours, vascular invasion, or VPI, ACT failed to benefit the patients in long-term survival. ACT, however, had no impact on CSS in any subset of pT2N0M0 NSCLC patients. Based on our findings, the real high-risk pT2N0M0 NSCLC patients who may benefit from ACT are those with tumours >4 cm and wild-type EGFR. These findings are important to help clinicians set a more tailored post-operative therapy for stage IB-IIA patients. This tailored strategy could avoid unnecessary ACT in some patients to reduce the adverse effects caused by overtreatment and economic burdens. To the best of our knowledge, this is the first study that focuses on high-risk pT2N0M0 NSCLC patients defined by the NCCN guidelines to study the significance of ACT. The large cohort size, the use of PSM to reduce selection bias, and the widely accepted definition of high-risk factors by NCCN lend much reliability and power to the results of this study.
Previous prospective randomized, controlled trials (RCTs) suggested that ACT could not benefit pT2N0M0 NSCLC in general,2,3,6,7 which is similar to ours. To the best of our knowledge, only CALGB-9633 was specifically designed for stage IB NSCLC patients to evaluate the significance of ACT. Hence, ACT had no impact on the long-term survival in these patients in general.2 Subgroup analyses suggested, however, that pT2N0M0 patients whose tumours were 4 cm or larger might benefit from ACT.2 Similar results were observed in the JBR.10 trial.3 Subgroup analyses of CALGB-9633 and JBR.10 indicated that the benefit of ACT in pT2N0M0 NSCLC patients was probably associated with the tumour size. In this study, our data demonstrated that ACT could improve long-term survival only in patients with tumours >4 cm, which was similar to these two RCTs.2,3 In the 8th edition of the AJCC TNM classification for lung cancer, tumour size >4 cm was defined as T2b, and stage T2bN0M0 escalated to stage IIA accordingly, which emphasizes the important threshold of the tumour diameter of 4 cm. Unfortunately, previous RCTs2,3 did not answer the significance of ACT in patients with other high-risk factors such as VPI, poor cell differentiation, and lymphovascular invasion. Considering previous studies and this study together, we believe that pT2N0M0 NSCLC patients with tumours >4 cm could benefit from ACT in long-term survival; ACT for patients with other high-risk factors defined by the NCCN guidelines1 should be cautious due to uncertain benefit.
Other retrospective studies also reported the value of ACT in pT2N0M0 NSCLC patients, but the results were diverse and inconsistent.9, 10, 11, 12, 13, 14 Some studies concluded that ACT could improve the long-term survival in pT2N0M0 NSCLC patients,9, 10, 11 whereas other studies concluded that ACT could not improve the long-term survival even in patients with high-risk factors.12, 13, 14 Different inclusion criteria and definitions of ‘high-risk’ factors may contribute to the discrepancies in these previous retrospective studies.9, 10, 11, 12, 13, 14 Li and colleagues14 reported in a retrospective PSM study that ACT could not improve survival in patients with stage IB (AJCC 7th edition27) NSCLC, even in patients with tumours ≥4 cm, different with our finding. We should note, however, that the patients’ number of tumours ≥4 cm (114) was smaller than that in our study (151 patients with tumours >4 cm), and median follow-up time (37 months) was shorter than ours (65 months), which may affect the results. Recently, Zhang’s retrospective PSM study found that ACT could not improve survival in the 4 < tumour ≤5 cm subgroup but patients with VPI in the 3 < tumour ≤ 4 cm subgroup may benefit more from ACT.28 VPI in that study seemed to be a more important factor to guide ACT in stage IB-IIA NSCLC patients. Based upon the previous and our study, we think that a prospective multicenter trial is required to address the role of ACT in pT2N0M0 NSCLC patients.
Our study explored the efficacy of ACT based on the EGFR mutation status and demonstrated that ACT improved the OS of EGFR wild-type pT2N0M0 patients but not EGFR-mutated patients. These results were consistent with Isaka et al.29 in stage II/III pulmonary adenocarcinoma, which confirms the insignificance of ACT on resected EGFR-mutated NSCLC. In the recently published ADAURA trial, adjuvant osimertinib reduced the risk of disease recurrence and improved DFS in resected EGFR-mutated stage IB-IIIA NSCLC patients, and the benefit existed regardless of the administration of ACT.30 Based on these findings, we propose that ACT should be cautiously administered to pT2N0M0 NSCLC patients who harbour sensitive EGFR mutations to avoid overtreatment. Besides, programmed death-ligand 1 (PD-L1) antibody atezolizumab after ACT was shown to extend DFS in resected stage II-IIIA NSCLC with PD-L1 ≥1%,31 and approved in the adjuvant setting. The role of ACT in high-risk pT2N0M0 NSCLC should be evaluated again in the era of adjuvant immunotherapy.
It was interesting that ACT could not improve CSS in any subset of pT2N0M0 NSCLC patients, even in patients with tumours >4 cm or EGFR wild-type tumours. On the one hand, this result indicated that the benefit of ACT in pT2N0M0 NSCLC patients was limited, which supported our main result that ACT might not be routinely recommended in pT2N0M0 NSCLC patients. On the other hand, for pT2N0M0 NSCLC patients who could benefit from ACT according to our study, this result indicated that only receiving ACT might not be enough to guarantee a satisfactory long-term survival. As mentioned previously, targeted therapy and immunotherapy showed an encouraging survival benefit as adjuvant therapy in some eligible patients with resected NSCLC. Therefore, some eligible pT2N0M0 NSCLC patients may have better CSS after receiving ACT and targeted therapy or immunotherapy. Moreover, on the basis of similar CSS, the differences in OS may be influenced by comorbidity and further treatment, and we believe that detailed information of subsequent treatment and use of EGFR tyrosine kinase inhibitors (TKIs) upon tumour relapse in both groups would help to clarify this result; unfortunately, these data are now unavailable for this study.
Limitations must be noted when interpreting our data. Firstly, this is a retrospective study from a single institution, which could inevitably cause selection bias. For example, the selection criteria for ACT and the chemotherapy regimen were not randomized but based upon physician preference. Secondly, although PSM was used to balance the comparison variables, other unknown confounding factors could not be controlled by PSM. Thirdly, some potential impact factors such as spread through air spaces, PD-L1 status, and status of other driver genes were not available in the current study due to the long-time span, which we think should be evaluated in the future with the advent of precision medicine. Additionally, we are unable to get the detailed information of subsequent treatment and use of EGFR TKIs upon tumour relapse in both groups, which confused the explanation of the similar CSS and different OS observed in the patients receiving ACT or not.
Conclusions
In conclusion, in resected pT2N0M0 NSCLC patients, those with tumours >4 cm and wild-type EGFR are real high-risk patients and could gain better OS and DFS from ACT, but ACT could not improve CSS in any subset of resected pT2N0M0 NSCLC patients. Further prospective study is needed to confirm the definition.
Acknowledgments
Funding
This work was supported by the Sun Yat-sen University Clinical Research 5010 Program [grant number 2019012, ChiCTR2000034737], the Natural Science Foundation of Guangdong Province [grant numbers 2018A030313410, 2020A151501311, 2022A1515012582], the National Natural Science Foundation of China [grant number 82072572], the Sun Yat-sen University Young Teacher Plan [grant number 19ykpy179], and the Guangzhou Science and Technology Program [grant numbers 202002020074, 202103000023].
Disclosure
None declared.
Supplementary data
References
- 1.National Comprehensive Cancer Network . NCCN; 2021. NCCN guideline for non-small cell lung cancer. Version 5. [Google Scholar]
- 2.Strauss G.M., Herndon J.E., II, Maddaus M.A., et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butts C.A., Ding K., Seymour L., et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waller D., Peake M.D., Stephens R.J., et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004;26:173–182. doi: 10.1016/j.ejcts.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 5.Arriagada R., Bergman B., Dunant A., et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 6.Winton T., Livingston R., Johnson D., et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 7.Douillard J.Y., Rosell R., De Lena M., et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 8.Pignon J.P., Tribodet H., Scagliotti G.V., et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 9.Park S.Y., Lee J.G., Kim J., et al. Efficacy of platinum-based adjuvant chemotherapy in T2aN0 stage IB non-small cell lung cancer. J Cardiothorac Surg. 2013;8:151. doi: 10.1186/1749-8090-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgensztern D., Du L., Waqar S.N., et al. Adjuvant chemotherapy for patients with T2N0M0 NSCLC. J Thorac Oncol. 2016;11:1729–1735. doi: 10.1016/j.jtho.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung J.J., Wu Y.C., Chou T.Y., et al. Adjuvant chemotherapy improves the probability of freedom from recurrence in patients with resected stage IB lung adenocarcinoma. Ann Thorac Surg. 2016;101:1346–1353. doi: 10.1016/j.athoracsur.2015.10.075. [DOI] [PubMed] [Google Scholar]
- 12.Park H.J., Park H.S., Cha Y.J., et al. Efficacy of adjuvant chemotherapy for completely resected stage IB non-small cell lung cancer: a retrospective study. J Thorac Dis. 2018;10:2279–2287. doi: 10.21037/jtd.2018.03.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon Y., Choi S.Y., Park J.K., et al. Prognostic factors in stage IB non-small cell lung cancer according to the 8(th) edition of the TNM staging system after curative resection. J Thorac Dis. 2019;11:5352–5361. doi: 10.21037/jtd.2019.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X., Zhang C., Sun Z., et al. Propensity-matched analysis of adjuvant chemotherapy for completely resected Stage IB non-small-cell lung cancer patients. Lung Cancer. 2019;133:75–82. doi: 10.1016/j.lungcan.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Pathak R., Goldberg S.B., Canavan M., et al. Association of survival with adjuvant chemotherapy among patients with early-stage non-small cell lung cancer with vs without high-risk clinicopathologic features. JAMA Oncol. 2020;6:1–10. doi: 10.1001/jamaoncol.2020.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang R.B., Yang J., Zeng T.S., et al. Incidence and distribution of lobe-specific mediastinal lymph node metastasis in non-small cell lung cancer: data from 4511 resected cases. Ann Surg Oncol. 2018;25:3300–3307. doi: 10.1245/s10434-018-6394-9. [DOI] [PubMed] [Google Scholar]
- 17.Yang M.Z., Hou X., Liang R.B., et al. The incidence and distribution of mediastinal lymph node metastasis and its impact on survival in patients with non-small-cell lung cancers 3 cm or less: data from 2292 cases. Eur J Cardiothorac Surg. 2019;56:159–166. doi: 10.1093/ejcts/ezy479. [DOI] [PubMed] [Google Scholar]
- 18.Yang M.Z., Hou X., Li J.B., et al. Impact of L4 lymph node dissection on long-term survival in left-side operable non-small-cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg. 2020;57:1181–1188. doi: 10.1093/ejcts/ezaa008. [DOI] [PubMed] [Google Scholar]
- 19.Yang H.X., Woo K.M., Sima C.S., et al. Long-term survival based on the surgical approach to lobectomy for clinical stage I non-small cell lung cancer: comparison of robotic, video-assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg. 2017;265:431–437. doi: 10.1097/SLA.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Detterbeck F.C., Boffa D.J., Kim A.W., et al. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Postmus P.E., Kerr K.M., Oudkerk M., et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 22.Howington J.A., Blum M.G., Chang A.C., et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 23.Osarogiagbon R.U., Allen J.W., Farooq A., et al. Outcome of surgical resection for pathologic N0 and Nx non-small cell lung cancer. J Thorac Oncol. 2010;5:191–196. doi: 10.1097/JTO.0b013e3181c8cc32. [DOI] [PubMed] [Google Scholar]
- 24.Osarogiagbon R.U., Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2013;96:1178–1189. doi: 10.1016/j.athoracsur.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 25.D’Agostino R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Kris M.G., Gaspar L.E., Chaft J.E., et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario clinical practice guideline update. J Clin Oncol. 2017;35:2960–2974. doi: 10.1200/JCO.2017.72.4401. [DOI] [PubMed] [Google Scholar]
- 27.Detterbeck F.C., Boffa D.J., Tanoue L.T. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 28.Zhang P., Duan J., Bai H., et al. Influence of adjuvant chemotherapy on survival for patients with stage IB and IIA non-small cell lung cancer. Thorac Cancer. 2021;12:30–39. doi: 10.1111/1759-7714.13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isaka T., Ito H., Nakayama H., et al. Efficacy of platinum-based adjuvant chemotherapy on prognosis of pathological stage II/III lung adenocarcinoma based on EGFR mutation status: a propensity score matching analysis. Mol Diagn Ther. 2019;23:657–665. doi: 10.1007/s40291-019-00419-9. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y.L., Tsuboi M., He J., et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383:1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 31.Felip E., Altorki N., Zhou C., et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398:1344–1357. doi: 10.1016/S0140-6736(21)02098-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.