Abstract
Introduction
Although common in individuals with cancer as well as nerve root compression, neuropathic pain can be difficult to prevent and manage due to the complex pain mechanisms involved in its pathophysiology. Although contrast baths have a long history of use for pain management, there is little known about their efficacy in the acute rehabilitation setting or in patients with cancer related neuropathic pain.
Case presentation
A 54-year-old man with multiple myeloma presented with progressive lower extremity weakness and 8/10 neuropathic pain intensity in both feet due to extensive myelomatous involvement of his lumbar spine. The patient’s pain limited his participation in physical therapy despite an extensive pain medication regimen. At the discretion of the patient’s physical therapist, contrast bath therapy for this patient was initiated with a reduction in pain intensity to 3/10. This analgesic effect lasted for a period of up to 10 h, which allowed the patient to participate in 3 h of therapy daily and to sleep comfortably.
Discussion
Contrast baths are a simple and cost-effective therapy that can be performed independently. As demonstrated in this case, they can potentially provide marked improvement in neuropathic pain and, in the acute rehabilitation setting, may enable patients to better participate in intensive physical therapy.
Subject terms: Quality of life, Chronic pain, Neuropathic pain
Introduction
Neuropathic pain is common in patients with cancer, with a prevalence as high as 40% [1]. It is also a defining feature of those with spinal nerve root compression [2]. Unfortunately, it can be difficult to prevent and manage due to the complex interweaving pain mechanisms involved in the pathophysiology of neuropathic pain. Although many interventions appear to show benefit in the clinical setting, they often have limited supportive evidence in the literature [3].
Contrast baths are a classic therapeutic modality that has been used primarily in sports, orthopedic, and diabetic populations for swelling, pain, and healing [4, 5]. There is currently no data describing the effects of contrast bath therapy on neuropathic cancer-related pain. This case describes a 54-year-old man with spinal nerve root compression secondary to multiple myeloma who presented to acute rehabilitation with unrelenting neuropathic pain treated successfully with contrast bath therapy.
Case presentation
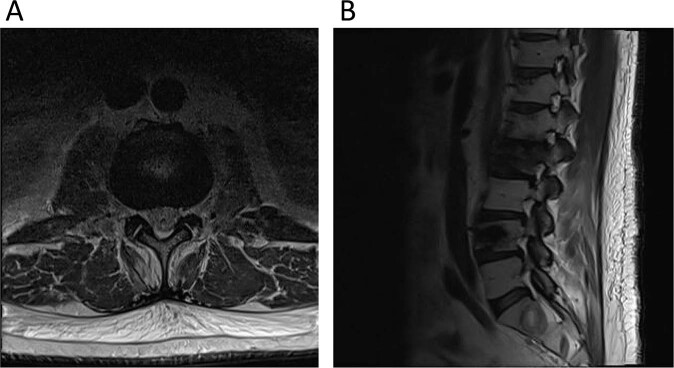
A 54-year-old man with lambda light chain myeloma and known metastatic disease to the sternum and thoracolumbar spine (status post previous radiation and chemotherapy) presented to acute care with lower extremity weakness. An MRI of the lumbar spine demonstrated extensive myelomatous involvement of the lumbar spine with multilevel compression fractures and multilevel neuroforaminal stenosis (right L1-L3 and left L2-L4) due to a combination of collapsed osseous structures and foraminal neoplasms (Fig. 1). There was no severe central canal stenosis.
Fig. 1. Non-contrast MRI of the lumbar spine.
A Axial image at the L2-L3 level demonstrating bilateral neuroforaminal stenosis. B T2-Weighted Images MRI Images of the lumbar spine demonstrating pathologic compression fractures of the L2 and L4 vertebral bodies along with multilevel stenosis of the right neuroforamen.
The patient was treated with a modified VD-PACE regimen and 8 fractions of palliative radiation therapy. He was subsequently treated with a steroid taper regimen.
Following his acute hospital course, the patient presented to acute inpatient rehabilitation. Upon presentation, the patient reported 3 months of constant pain, initially 6/10 with worsening over the past month to 8/10 pain. He attributed this to a pain medication regimen change (fentanyl to methadone). The pain was located on dorsal and ventral surfaces of his feet, sparing the toes and ankle. It was always bilateral and did not radiate. His pain was most severe in the morning and evening, and he reported spikes of discomfort every 2–4 h without clear triggers. He did not experience any moments of complete pain relief. The pain was characterized as a numb pressure with a severe burning sensation. The patient also described “jello pads on the soles of [his] feet” and feeling “like [his] feet were in boiling water”. He also began developing similar numbness and burning in his upper thighs.
The patient had been seen by a palliative medicine physician prior to his rehabilitation admission and was placed on a regimen of methadone (10 mg every 8 h), gabapentin (900 mg every 8 h), duloxetine (60 mg daily), hydromorphone (16 mg every 3 h as needed), and lidocaine cream (twice a day as needed). He reported that despite this pain regimen, his pain remained 8/10. He felt gabapentin intermittently relieved his pain and hydromorphone provided breakthrough pain relief for about 30 min at a time. Lidocaine cream would “take the edge off” his pain. Other attempts at pain control over the previous 3 months included acupuncture, which had relieved his pain transiently. Massages, foot rubs, compression stockings, and ACE wraps provided no significant relief.
On exam, the patient had 1/5 movement bilaterally in extensor hallucis longus, ankle dorsiflexion, and ankle plantarflexion, as well as 1/5 movement in left hip flexion on the Medical Research Council Scale. He reported impaired sensation below T12 and was areflexic bilaterally in the lower extremities with mute Babinski reflexes. He was unable to ambulate independently.
At the discretion of the physical therapist treating the patient, contrast bath therapy was initiated following failed attempts at other pain management techniques as previously noted. A trial contrast bath was performed using 49 °F (9.4 °C) water and 104 °F (40 °C) water. The patient submerged his feet to ankle depth in shallow water basins, alternating 2 min in warm water and 1 min in cold water for a total of three cycles. Water temperature was monitored by a thermometer to ensure that it remained in a safe range due to the patient’s impaired sensation.
The patient reported immediate significant pain relief to a level of 3/10 in the submerged areas of his feet. The pain was alleviated for 10 h after 2 rounds of contrast baths and allowed for comfortable sleep. After 10 h, the burning sensation would begin to mount again. Repeated contrast bath immediately suppressed the pain back to a level of 3/10. He also noted decreased edema in his feet following initiation of contrast bath therapy. Prior to contrast bath therapy, the patient was unable to tolerate full physical therapy sessions. With the addition of contrast bath therapy, he could tolerate his 3 h of physical and occupational therapy a day. Notably, the patient began performing contrast bath therapy in his own free time, which allowed him to maximize time for functional rehabilitation.
When asked about his experience with contrast bath therapy, the patient stated that he was “cautiously optimistic” and was happy that he “could function now, because [he] couldn’t function before”. He noted, “When you’re in pain you can’t think straight or talk straight. I feel more in control now”.
Discussion
Although many articles cite ancient origins of contrast baths [3], the exact history of contrast baths are unknown. They have been used in the medical setting since at least 70 years ago with the earliest reports of its use in hand therapy [4]; however, there is little known about the efficacy of contrast bath therapy in the acute rehabilitation settings or in patients with cancer related neuropathic pain. It is thought that altering peripheral vasoconstriction and vasodilation can decrease edema and increase circulation [4]. However, there is a need for evidence of a relationship to functional outcomes such as the ability to undertake activities of daily living [3].
Contrast bath therapy has previously been shown to be therapeutic in treatment of muscle injuries. A study using near-infrared spectroscopy to study the effects of contrast bath therapy found that it induced a transient increase in intramuscular hemodynamics and oxygenation of the lower limb [5]. This increase in hemodynamics and oxygenation is thought to facilitate muscle healing. In addition, a systematic review of contrast bath therapy effectiveness found evidence that it increases blood flow in the superficial skin [4]. The alternation of cold and warm water immersion used in contrast bath therapy is felt to increase vasodilation as compared to warm water immersion alone [3]. Contrast bath therapy has also demonstrated effectiveness in patients with complex regional pain syndrome when used as desensitization therapy [6]. There are no known case studies of contrast bath therapy used for neuropathic pain due to spinal root compression.
Conventional pain management techniques to manage neuropathic pain include several pharmacological modalities. The World Health Organization recommends using an analgesic ladder as a framework for a stepwise approach to cancer-related pain, with close monitoring of efficacy and safety [7, 8]. Mild pain is typically managed using nonopioid analgesics such as acetaminophen or non-steroidal anti-inflammatory drugs. Opioid medications can be considered for moderate and severe pain. Other common adjuvant agents include gabapentinoids, antidepressants including serotonin-norepinephrine reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs), and other anticonvulsants [9–12]. Other medications with less evidence although sometimes used in refractory cases include sodium channel blockers, ketamine, benzodiazepines, muscle relaxants, and cannabinoids [13–16]. Studies regarding the effectiveness of these adjuvant medications for treatment of cancer pain are limited, with many suggesting only modest benefit [17].
Unfortunately, there are many adverse reactions associated with the use of oral pain medications that may not be acceptable to patients with cancer related neuropathic pain. As an alternative, non-pharmacological pain modalities have been trialed. Non-pharmacological therapies for neuropathic cancer related pain include hydrotherapy, orthoses, heat/cold, transcutaneous electrical nerve stimulation, scrambler therapy, hypnotic analgesia, meditation, and acupuncture [18–21]. There is little data evaluating these therapies in terms of efficacy and safety in this population.
Contrast bath therapy can be an effective non-pharmacological modality to address refractory neuropathic pain. Administration of the therapy is simple and can be done without trained expertise. Since contrast bath therapy can be done independently by the patient, less therapy time is lost. Contrast bath therapy does not require the use of medications or invasive procedures, thus posing minimized risk to the patient relative to other pain management modalities. However, the water temperature must be monitored carefully as risk of burns is high in insensate patients. Contrast bath therapy has a minimal cost, requiring only a basin, warm water, and a thermometer. As demonstrated in this case, contrast bath therapy may also provide significant benefit to patients with debilitating neuropathic pain and allow better participation in intensive rehabilitative therapy. Larger and longer-term studies are needed to verify this finding and measure continued pain relief over time. Future studies are also need to determine whether contrast bath therapy can be effective independently or are best used as adjunctive therapy.
Supplementary information
Author contributions
All authors participated in conception, evaluation, and writing of this case report.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elaine Hong Hatch, Email: elainehonghatch@gmail.com.
Christopher Gorrell, Email: Christopher.Gorrell@pennmedicine.upenn.edu.
Benjamin A. Abramoff, Email: Benjamin.Abramoff@pennmedicine.upenn.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41394-022-00526-6.
References
- 1.Oosterling A, te Boveldt N, Verhagen C, van der Graaf WT, Van Ham M, Van der Drift M, et al. Neuropathic pain components in patients with cancer: prevalence, treatment, and interference with daily activities. Pain Pr. 2016;16:413–21. doi: 10.1111/papr.12291. [DOI] [PubMed] [Google Scholar]
- 2.Freynhagen R, Baron R, Tölle T, Stemmler E, Gockel U, Stevens M, et al. Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: A prospective observational pilot study (MIPORT) Curr Med Res Opin. 2006;22:529–37. doi: 10.1185/030079906X89874. [DOI] [PubMed] [Google Scholar]
- 3.Petrofsky J, Lohman E, 3rd, Lee S, de la Cuesta Z, Labial L, Iouciulescu R, et al. Effects of contrast baths on skin blood flow on the dorsal and plantar foot in people with type 2 diabetes and age-matched controls. Physiother Theory Pr. 2007;23:18997. doi: 10.1080/09593980701209295. [DOI] [PubMed] [Google Scholar]
- 4.Breger Stanton DE, Lazaro R, Macdermid JC. A systematic review of the effectiveness of contrast baths. J Hand Ther. 2009;22:57–69. doi: 10.1016/j.jht.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Shadgan B, Pakravan AH, Hoens A, Reid WD. Contrast baths, intramuscular hemodynamics, and oxygenation as monitored by near-infrared spectroscopy. J Athl Train. 2018;53:782–7. doi: 10.4085/1062-6050-127-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soylev GO, Boya H. A rare complication of total knee arthroplasty: Type 1 complex regional pain syndrome of the foot and ankle. Acta Orthopaedica et Traumatologica Turc. 2016;50:592–5. doi: 10.1016/j.aott.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. https://www.who.int/cancer/palliative/painladder/en/.
- 8.Esin E, Yalcin S. Neuropathic cancer pain: What we are dealing with? How to manage it? Onco Targets Ther. 2014;7:599–618. doi: 10.2147/OTT.S60995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caraceni A, Zecca E, Bonezzi C, Arcuri E, Yaya Tur R, Maltoni M, et al. Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. J Clin Oncol. 2004;22:2909. doi: 10.1200/JCO.2004.08.141. [DOI] [PubMed] [Google Scholar]
- 10.Jongen JL, Huijsman ML, Jessurun J, Ogenio K, Schipper D, Verkouteren DR, et al. The evidence for pharmacologic treatment of neuropathic cancer pain: beneficial and adverse effects. J Pain Symptom Manag. 2013;46:581. doi: 10.1016/j.jpainsymman.2012.10.230. [DOI] [PubMed] [Google Scholar]
- 11.Hepner S, Claxton R. Anti-epileptic drugs for pain #271. J Palliat Med. 2013;16:799. doi: 10.1089/jpm.2013.9497. [DOI] [PubMed] [Google Scholar]
- 12.Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Garzón-Rodríguez C, Casals Merchan M, Calsina-Berna A, López-Rómboli E, Porta-Sales J. Lidocaine 5% patches as an effective short-term co-analgesic in cancer pain. Preliminary results. Support Care Cancer. 2013;21:3153. doi: 10.1007/s00520-013-1948-7. [DOI] [PubMed] [Google Scholar]
- 14.Challapalli V, Tremont-Lukats IW, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev. 2005;2015:CD003345. [DOI] [PMC free article] [PubMed]
- 15.Ben-Ari A, Lewis MC, Davidson E. Chronic administration of ketamine for analgesia. J Pain Palliat Care Pharmacother. 2007;21:7. doi: 10.1080/J354v21n01_04. [DOI] [PubMed] [Google Scholar]
- 16.Yomiya K, Matsuo N, Tomiyasu S, Yoshimoto T, Tamaki T, Suzuki T, et al. Baclofen as an adjuvant analgesic for cancer pain. Am J Hosp Palliat Care. 2009;26:112. doi: 10.1177/1049909108327968. [DOI] [PubMed] [Google Scholar]
- 17.Bennett MI. Effectiveness of antiepileptic or antidepressant drugs when added to opioids for cancer pain: systematic review. Palliat Med. 2011;25:553. doi: 10.1177/0269216310378546. [DOI] [PubMed] [Google Scholar]
- 18.Rhiner M, Ferrell BR, Ferrell BA, Grant MM. A structured nondrug intervention program for cancer pain. Cancer Pr. 1993;1:137. [PubMed] [Google Scholar]
- 19.Hurlow A, Bennett MI, Robb KA, Johnson MI, Simpson KH, Oxberry SG. Transcutaneous electric nerve stimulation (TENS) for cancer pain in adults. Cochrane Database Syst Rev. 2012;2012:CD006276. [DOI] [PMC free article] [PubMed]
- 20.Park HS, Sin WK, Kim HY, Moon JY, Park SY, Kim YC, et al. Scrambler therapy for patients with cancer pain - case series. Korean J Pain. 2013;26:65. doi: 10.3344/kjp.2013.26.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Center for Complementary and Integrative Health (https://nccih.nih.gov/).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.