Abstract
The wide-ranging potencies of bioactive N-fused heterocycles inspire the development of synthetic transformations that simplify preparation of their complex, diverse structural motifs. Heteroaryl ketones are ubiquitous, readily available, and inexpensive molecular scaffolds, and are thus synthetically appealing as precursors in preparing N-fused heterocycles via intramolecular acyl-transfer. To best of our knowledge, acyl-transfer of unstrained heteroaryl ketones remains to be demonstrated. Here, we show an acyl transfer-annulation to convert heteroaryl ketones to N-fused heterocycles. Driven via aromatisation, the acyl of a heteroaryl ketone can be transferred from the carbon to the nitrogen of the corresponding heterocycle. The reaction commences with the spiroannulation of a heteroaryl ketone and an alkyl bromide, with the resulting spirocyclic intermediate undergoing aromatisation-driven intramolecular acyl transfer. The reaction conditions are optimised, with the reaction exhibiting a broad substrate scope in terms of the ketone and alkyl bromide. The utility of this protocol is further demonstrated via application to complex natural products and drug derivatives to yield heavily functionalised N-fused heterocycles.
Subject terms: Synthetic chemistry methodology, Homogeneous catalysis
Heteroaryl ketones are ubiquitous molecular scaffolds but seldom used as synthetic precusors. Here, the authors develop an acyl transfer-annulation to convert heteroaryl ketones to N-fused heterocycles, which are prevalent in bioactive molecules.
Introduction
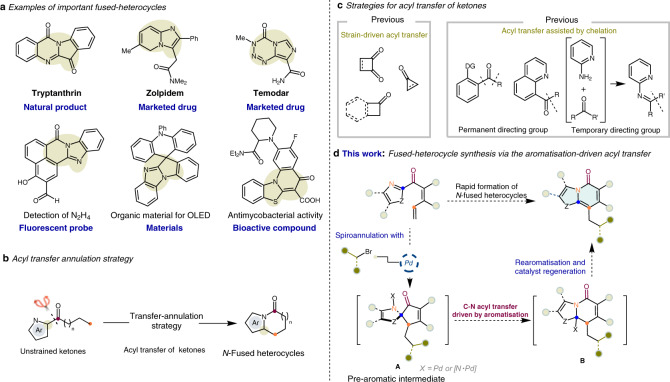
N-fused heterocyclic compounds, such as pharmaceuticals, agrochemicals, plastics, and dyes (Fig. 1a), are integrated into everyday life1–6. Big data analysis shows that heterocycle synthesis is one of the most common reactions in the field of medicinal chemistry7,8. Among the best-selling therapeutics, almost a third contain fused heterocyclic structures9. Due to the high value of N-fused heterocycles, their novel, effective, flexible, general syntheses require investigation10–12.
Fig. 1. Examples of critial fused-heterocycles and our reaction design.
a N-fused heterocycles are ubiquitous within critical molecules, including biologically active natural and synthetic compounds and fine chemicals for use in functional materials. b Transfer-annulation strategy for synthesis of N-fused heterocycles. c Different strategies used in acyl transfer of ketones. d Fused heterocycle synthesis in this study via aromatisation-driven acyl tranfer of heteroaryl ketones with alkyl bromides.
Acyl transfer is a critical process in various biological transformations13. In the field of organic synthesis, acyl transfer is frequently used in formation carbonyl compounds14–18. A typical acyl transfer employs a reactive carboxylic acid derivative (e.g. an acyl chloride or a thioester) as an acyl source. However, whether relatively inert ketones may serve as acyl transfer agents remains unclear?
Ketones are ubiquitous functional groups that not only occur widely in drug molecules and natural products but also act as bulk feedstocks in the syntheses of fine chemicals and materials. They are stable, non-toxic, and simple to prepare via various methods, rendering them ideal synthetic precursors19. If intramolecular acyl transfer of heteroaryl ketones can be realised, a transfer-annulation strategy may be employed in N-fused heterocycle preparation (Fig. 1b). However, owing to the kinetic inertness of C–C bonds, acyl transfer of ketones largely focuses on highly strained ketones20–26. For unstrained ketones27–32, the most common strategy involves using directing groups to form of a stable chelate (Fig. 1c)33–40. Although effective, the use of directing groups complicates the overall synthesis and limits the scope of the accessible products. Hence, a acyl transfer of unstrained ketones for use in N-fused heterocycle synthesis is warranted.
Aromatisation, which enables delocalisation of electron density, stabilising the molecule41, is a critical thermodynamic driving force in the field of organic chemistry42–45, e.g. aromatisation-driven deacylations of ketones are prominent bond-cleavage strategies46–48. Therefore, we conceived a approach for the acyl transfer of unstrained heteroaryl ketones driven by aromatisation of a pre-aromatic intermediate (Fig. 1d). This strategy may be suitable for use in the syntheses of N-fused heterocycles, and, critically, the directing group is no longer required. The next challenge in this strategy is the in situ formation of special, high-energy, pre-aromatic substrates. Transition metal-catalysed dearomatisation is a straightforward strategy to prepare spirocyclic scaffolds49–52. The spirocyclic intermediates, which are formed in situ from readily available heteroaryl ketones via dearomatisations53–56, should serve as pre-aromatic precursors to facilitate rearrangement (Fig. 1d). This likely involves a Pd-catalysed dearomative spirocyclisation of a heteroaryl ketone with an alkyl bromide to generate a pre-aromatic intermediate (A), which is then intramolecularly trapped by the heterocyclic nitrogen57–61. The resulting intermediate (B) may subsequently lose a hydrogen, restoring aromaticity to yield the fused heterocyclic product.
Here, we report an acyl transfer-annulation of heteroaryl ketones driven by aromatisation. This method is operationally simple, scalable, and applicable to late-stage modifications of natural products and drug derivatives, which make it a valuable method for the synthesis of organic N-fused heterocycles.
Results
Reaction optimisation
To explore this strategy, we initially used a heteroaryl ketone with a tethered olefin (1), which was prepared in one step using commercially available benzimidazole and 2-vinylbenzoyl chloride, as a model substrate. Because of the unique properties of difluoromethylene group (CF2) and its critical applications in medicinal chemistry62–64, ethyl bromodifluoroacetate (BrCF2COOEt) was employed as the coupling partner. After systematic screening, the desired rearrangement product (2) is obtained in a 90% yield using PdCl2 in combination with 1,1-bis(diphenylphosphino)pentane (dpppent, L1) as the ligand and Na2CO3 as the base in dioxane/tetrahydrofuran (THF) (Table 1, entry 1). The structure of 2 was unambiguously determined by X-ray crystallography. In addition, the Pd catalyst appears to be critical in this reaction. Using Pd(OAc)2 or Pd2(dba)3 (dba = dibenzylideneacetone) as the catalyst results in much lower yields (Table 1, entries 2–3), and other metals, such as NiCl2 and FeCl2, are completely ineffective (Table 1, entry 4). A study of the ligand effect further suggests that bidentate phosphine ligands are generally superior, with the yield increasing with the increasing bite angle of the phosphine employed, and L1 is the only ligand that generates full conversion with the optimal yield (Table 1, entry 5). The addition of a base improves the reaction outcome appreciably, likely by neutralising the in situ-generated HBr (Table 1, entry 6). A survey of different solvents reveals that dioxane and THF are individually good, albeit generating slightly lower yields than that obtained using the mixture (Table 1, entries 7–9).
Table 1.
Screening of reaction conditions.
Unless otherwise specified, all reactions were carried out using 1 (0.1 mmol) and ethyl bromodifluoroacetate (0.15 mmol, 1.5 equiv), with 10 mol% PdCl2, 12 mol% L1 and Na2CO3 (1.0 equiv) in dioxane/THF (1:2) at 130 °C for 24 h. The CCDC number of 2 is 2116750.
aIsolated yields after chromatography.
Substrate scope
With the conditions determined, the scope of alkyl bromides was examined first (Fig. 2). Ketone 1 is successfully coupled with various alkyl bromides, with 5-, 6-, 7-, or 12-membered cycloalkyls (3–6) generating good yields of the desired coupling products. Heterocyclic bromides, with moieties such as tetrahydropyrane (7) and THF (8), react smoothly, resulting in good yields. Remarkably, the polycyclic bromide derived from the natural steroid stanolone is also amenable to coupling under the reaction conditions (9). Linear alkyl bromides are also suitable for reaction (10–12). We then investigated substrates with a CF2 group. Bromofluoroacetate, bromodifluoromethyl ketone, perfluoroalkyl bromide, bromodifluoromethyl phosphonate, and bromodifluoromethyl sulfone effectively undergo the desired annulation (13–17).
Fig. 2. Substrate scope of alkyl bromides.
Unless otherwise specified, all the reactions were carried out using ketone 1 (0.1 mmol, 1.0 equiv) and alkyl bromide (0.15 mmol, 1.5 equiv.), PdCl2 (10 mol%), dpppent (12 mol%) and Na2CO3 (1.0 equiv) in dioxane/THF (1:2) at 130 °C. Isolated yields after chromatography are shown.
We further explored the rearrangements of various heteroaryl ketones with bromodifluoroacetate (Fig. 3). The rearrangement took place smoothly by using 2-acylimidazoles and 2-acylbenzimidazoles as substrates (18–41). Both electron-rich and deficient substrates are competent during the cyclization process. A range of functional groups are compatible, including aryl fluorides (28 and 40) and chlorides (20 and 39), trifluoromethyl (21 and 38), esters (23) and cyano (22), are all tolerated. Changing the nitrogen protecting group from methyl to isopropyl (30) and benzyl (31) did not significantly affect the reactivity.
Fig. 3. Substrate scope of heteroaryl ketones.
Isolated yields after chromatography are shown. The CCDC number of 43 is 2116753, 52 is 2116752. aThe reaction was performed under optimised condition A: ketone 1 (0.1 mmol, 1.0 equiv) and ethyl bromodifluoroacetate (0.15 mmol, 1.5 equiv), PdCl2 (10 mol%), dpppent (12 mol%) and Na2CO3 (1.0 equiv) in dioxane/THF (1:2) at 120 °C for 24 h. bThe reaction was conducted under optimised condition A with a slight modification: bis(2-diphenylphosphinophenyl)ether (DPEPhos) (12 mol%) was used as ligand during the reaction. cThe reaction was performed under optimised condition B: ketone 1 (0.1 mmol, 1.0 equiv) and ethyl bromodifluoroacetate (0.15 mmol, 1.5 equiv), PdCl2 (10 mol%), dppf (12 mol%) and K2CO3 (1.0 equiv) in dioxane/THF (1:1) at 130 °C for 24 h. dppf = 1,1′-bis(diphenylphosphino)ferrocene.
Compared to the substrate with 4,5-diphenylimidazole (32), the reactions of 4-phenylimidazole (33) and imidazole (34) yield lower conversions, indicating that aromatisation is essential to promote the reaction. Marketed drug-derived ketones, such as ketoconazole (41), also react smoothly despite the presence of several other functional groups. Significantly, numerous substrates are synthesised via direct acylation of commercially available imidazoles or benzimidazoles, with the resulting ketones directly undergoing rearrangement, which further highlights the efficiency of this process. Further, we examined other types of heterocycles, which should yield different heterocyclic cores via rearrangement. Heterocycles such as thiazole (42), benzothiazoles (43–51), benzoxazole (52), and oxazole (53) may also be incorporated, yielding pharmaceutically interesting fused-ring skeletons65,66.
Mechanistic considerations
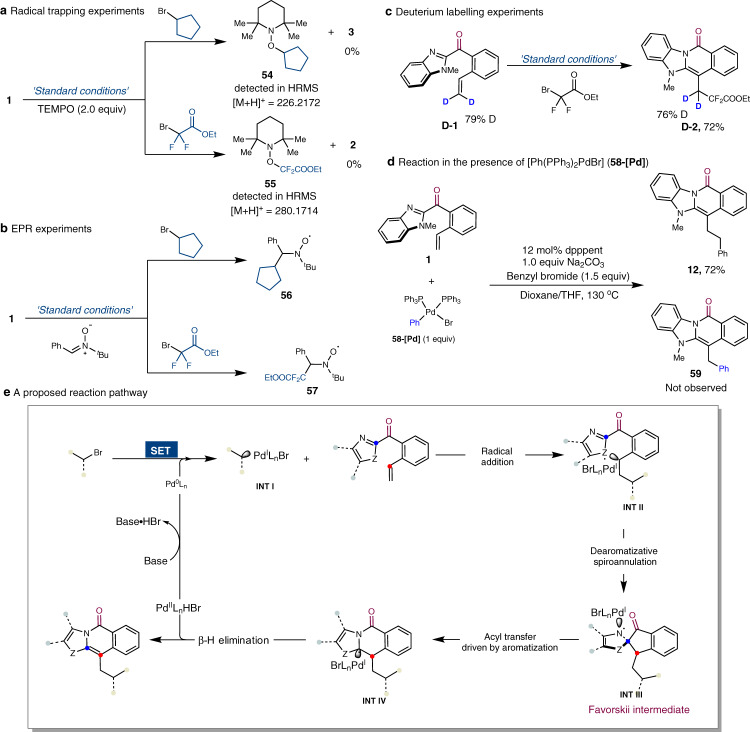
A study was performed to investigate the reaction pathway. To determine whether an alkyl radical exists during this Pd-catalysed process, a radical inhibition study was performed. When 2,2,6,6-tetramethylpiperidinooxy (TEMPO) is added to the reaction mixture, it traps alkyl radicals, indicating that the reaction involves radical species (Fig. 4a). An electron paramagnetic resonance (EPR) study of the reaction of bromocyclopentane with the spin-trapping agent phenyl-N-tert-butylnitrone reveals the presence of spin adducts of the trapped alkyl radicals 56 and 57 (Fig. 4b), as indicated by the EPR spectrum (see supporting information). Deuterium labelling studies were conducted using the heteroaryl ketone D-1 (79% deuterium content) as a substrate under the optimised conditions, with a significant level of the deuterated product D-2 (76% deuterium content) detected, suggesting that there were no reversible hydro-metallation in this process (Fig. 4c)67,68. Finally, we synthesised an aryl Pd complex (58-[Pd]), with 12 produced instead of 59 in the presence of 58-[Pd], benzyl bromide, and 1 (Fig. 4d). Therefore, the alkyl group of the fused heterocyclic product is not derived from the migratory insertion of the Pd(II) complex. The proposed reaction pathway is thus shown in Fig. 4e. The reaction may be initiated by a single electron transfer between Pd(0) and the alkyl bromide, producing hybrid alkyl Pd(I)-radical species INT I. Subsequently, radical addition to the alkene occurs, leading to the hybrid benzylic radical INT II, which then undergoes dearomatisative-spirocyclisation to form the spiro-N-radical INT III. Aromatisation-driven intramolecular acyl transfer may then occur to form the alkyl radical INT IV. Subsequent β-H elimination at the latter yields the product with concomitant regeneration of the Pd catalyst. This proposed mechanism is also supported by X-ray photoelectron spectroscopy, which revealed the presence of three distinct Pd oxidation states (Pd(0), Pd(I), and Pd(II)) during the process, suggesting that Pd(I) species may be involved.
Fig. 4. Mechanistic studies.
a Radical trapping study using TEMPO showing that alkyl radical species are involved in the reaction. b EPR studies also suggest that this reaction may involve alkyl radicals. c Deuterium labelling studies. d Reaction of 1 with benzyl bromide in the presence of [Ph(PPh3)2PdBr] (58-[Pd]). e A proposed reaction pathway.
Synthetic utility
Further studies were conducted to demonstrate the viability of this acyl transfer-annulation strategy. The protocol was applied in the late-stage modifications of natural products and drug derivatives (Fig. 5a). Various complex molecules with diverse structural features, such as steroids (62 and 69), N-heteroarenes (oxazole 63 and indole 68), alkaloids (66), and carbohydrates (72), are readily converted into the corresponding products in useful yields. This strategy provides a straightforward, versatile method of generating valuable N-fused heterocyclic moieties within complex molecules. Given the ubiquity of N-fused heterocycles in pharmaceuticals, this approach may be used in the field of medicinal chemistry.
Fig. 5. Synthetic applications.
a Using the tranfer-annulation strategy in the late-stage modifications of complex frameworks based on natural products and drug molecules. b Gram-scale synthesis and various useful transformations of 2. The CCDC number of 74 is 2131840.
To showcase the scalability of this process, a gram-scale reaction was carried out. Gratifyingly, a satisfactory 67% isolated yield (80% yield based on recovered 1) of product 2 could be obtained without modification of the optimised conditions (Fig. 5b). The N-fused heterocyclic scaffold can readily undergo various transformations to access a range of synthetically useful scaffolds. For example, the bromination of 2 proceeded to afford 74, excellent selectivity for the 9-position was observed, which allows follow-up fused heterocycle manipulations through cross-couplings. Treatment with mCPBA, deconstruction of N-fused heterocycle was observed, which afforded 75 in 53% yield. Diazidation product 76 was afforded in 48% yield via vicinal diazidation of olefin. Moreover, the ester moiety was smoothly reduced with NaBH4, affording the corresponding alcohol 77 in 68% yield.
In conclusion, a synthetically useful, mechanistically intriguing intramolecular acyl transfer of heteroaryl ketones was developed, which was suitable for use in fused-ring synthesis. The formation of a high-energy pre-aromatic spirocyclic intermediate was critical in the successful transformation, with aromatisation the driving force that facilitated C–C bond cleavage. Given the ready availability of the ketone moiety, this strategy could be used to simplify the syntheses of complex N-fused heterocyclic systems, which are privileged structures within numerous biologically active compounds. Moreover, the protocol enabled the late-stage modifications of intricate natural products and drug derivatives and may thus facilitate heterocyclic drug discovery.
Methods
General condition A for transfer-annulation of heteroaryl ketones derived from (benzo)imidazoles
In a nitrogen-filled glovebox, an oven-dried 10 mL sealed tube equipped with a Teflon-coated magnetic stir bar was charged successively with heteroaryl ketone 1 (0.1 mmol), alkyl bromide (0.15 mmol, 1.5 equiv), PdCl2 (0.01 mmol, 10 mol%), dpppent (0.012 mmol, 12 mol%), Na2CO3 (0.1 mmol, 1.0 equiv) and dioxane/THF (1.0 mL, 1:2). The tube then was sealed with a Teflon screw cap, moved out of the glovebox, and placed on a hotplate pre-heated to 130 °C for 24–36 h. After completion of the reaction, the mixture was filtered through a thin pad of silica gel. The filter cake was washed with ethyl acetate and the combined filtrate was concentrated under vacuum. The residue was purified via silica gel chromatography.
General condition B for transfer-annulation of heteroaryl ketones derived from (benzo)thiazoles and (benzo)oxazoles
In a nitrogen-filled glovebox, an oven-dried 10 mL sealed tube equipped with a Teflon-coated magnetic stir bar was charged successively with heteroaryl ketone 1 (0.1 mmol), difluorobromoethyl ester (0.15 mmol, 1.5 equiv), PdCl2 (0.01 mmol, 10 mol%), dppf (0.012 mmol, 12 mol%), K2CO3 (0.1 mmol, 1.0 equiv) and dioxane/THF (1.0 mL, 1:1). The tube then was sealed with a Teflon screw cap, moved out of the glovebox, and placed on a hotplate pre-heated to 120 °C for 24 h. After completion of the reaction, the mixture was filtered through a thin pad of silica gel. The filter cake was washed with ethyl acetate and the combined filtrate was concentrated under vacuum. The residue was purified via silica gel chromatography.
Supplementary information
Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (21971205), Key Research and Invention Program in Shaanxi Province of China (2021SF-299), Natural Science Basic Research Program of Shaanxi (2020JQ-574), Scientific Research Program of Shaanxi Education Department (No. 20JK0937) and Northwest University.
Author contributions
H.W. conceived and designed the project and composed the paper. D.Y., H.L., Y.H. and J.W. conducted the experiments and analysed the data. H.L. and Z.Z. discussed the experimental results and commented on the paper. H.W. conducted general guidance, project directing, and paper revisions.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Data relating to the optimisation studies, mechanistic studies, general methods, and the characterisation data of materials and products, are available in the Supplementary Information. Crystallographic parameters for compounds 2, 43, 52 and 74 are available free of charge from the Cambridge Crystallographic Data Centre under CCDC 2116750 (2), 2116753 (43), 2116752 (52) and 2131840 (74). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/getstructures.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dan Ye, Hong Lu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-31063-3.
References
- 1.Taylor RD, MacCoss M, Lawson AD. Rings in drugs. J. Med. Chem. 2014;57:5845–5859. doi: 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- 2.Ning J, et al. A highly sensitive and selective two-photon fluorescent probe for real-time sensing of cytochrome P450 1A1 in living systems. Mater. Chem. Front. 2018;2:2013–2020. doi: 10.1039/C8QM00372F. [DOI] [Google Scholar]
- 3.Kumar S, Bawal S, Gupta H. Biological activities of quinoline derivatives. Mini. Rev. Med. Chem. 2009;9:1648–1654. doi: 10.2174/138955709791012247. [DOI] [PubMed] [Google Scholar]
- 4.Bollini M, et al. New potent imidazoisoquinolinone derivatives as anti-trypanosoma cruzi agents: Biological evaluation and structure–activity relationships. Bioorg. Med. Chem. 2009;17:1437–1444. doi: 10.1016/j.bmc.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Cui J, et al. A highly sensitive and selective fluorescent probe for N2H4 in air and living cells. N. J. Chem. 2017;41:11891–11897. doi: 10.1039/C7NJ01943B. [DOI] [Google Scholar]
- 6.Hao Y, et al. Discovery of tryptanthrins as novel antiviral and anti-phytopathogenic-fungus agents. J. Agric. Food Chem. 2020;68:5586–5595. doi: 10.1021/acs.jafc.0c02101. [DOI] [PubMed] [Google Scholar]
- 7.Schneider N, Lowe DM, Sayle RA, Tarselli MA, Landrum GA. Big data from pharmaceutical patents: a computational analysis of medicinal chemists’ bread and butter. J. Med. Chem. 2016;59:4385–4402. doi: 10.1021/acs.jmedchem.6b00153. [DOI] [PubMed] [Google Scholar]
- 8.Brown DG, Boström J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 2016;59:4443–4458. doi: 10.1021/acs.jmedchem.5b01409. [DOI] [PubMed] [Google Scholar]
- 9.McGrath NA, Brichacek M, Njardarson JT. A graphical journey of innovative organic architectures that have improved our lives. Chem. Educ. 2010;87:1348–1349. doi: 10.1021/ed1003806. [DOI] [Google Scholar]
- 10.Royer J. Asymmetric Synthesis of Nitrogen Heterocycles (Wiley-VCH, 2009).
- 11.Eicher, T., Hauptmann, S. & Speicher, A. The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications (Wiley-VCH, 2012).
- 12.Wu, X.-F. Transition Metal-Catalyzed Heterocycle Synthesis via C–H Activation (Wiley-VCH, 2015).
- 13.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu. Rev. Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 14.Burke HM, McSweeney L, Scanlan EM. Exploring chemoselective S-to-N acyl transfer reactions in synthesis and chemical biology. Nat. Commun. 2017;8:15655. doi: 10.1038/ncomms15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramakers BEI, van Hesta JCM, Löwik DWPM. Molecular tools for the construction of peptide-based materials. Chem. Soc. Rev. 2014;43:2743–2756. doi: 10.1039/c3cs60362h. [DOI] [PubMed] [Google Scholar]
- 16.Penteado F, et al. α-Keto acids: acylating agents in organic synthesis. Chem. Rev. 2019;19:7113–7278. doi: 10.1021/acs.chemrev.8b00782. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Szostak M. Transition-metal-free activation of amides by N−C bond cleavage. Chem. Rec. 2020;20:649–659. doi: 10.1002/tcr.201900072. [DOI] [PubMed] [Google Scholar]
- 18.Shi S, Nolan SP, Szostak M. Well-defined palladium(II)–NHC precatalysts for cross-coupling reactions of amides and esters by selective N–C/O–C cleavage. Acc. Chem. Res. 2018;51:2589–2599. doi: 10.1021/acs.accounts.8b00410. [DOI] [PubMed] [Google Scholar]
- 19.March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (8th ed) pp 579–586, pp 891–899 (John Wiley & Sons, 2020).
- 20.Chen P-h, Billett BA, Tsukamoto T, Dong G. “Cut and sew” transformations via transition-metal-catalyzed carbon-carbon bond activation. ACS Catal. 2017;7:1340–1360. doi: 10.1021/acscatal.6b03210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami M, Ishida N. Cleavage of carbon-carbon σ-bonds of four-membered rings. Chem. Rev. 2021;121:264–299. doi: 10.1021/acs.chemrev.0c00569. [DOI] [PubMed] [Google Scholar]
- 22.Fumagalli G, Stanton S, Bower JF. Recent methodologies that exploit C−C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 2017;117:9404–9432. doi: 10.1021/acs.chemrev.6b00599. [DOI] [PubMed] [Google Scholar]
- 23.Seiser T, Saget T, Tran DN, Cramer N. Cyclobutanes in catalysis. Angew. Chem. Int. Ed. 2011;50:7740–7752. doi: 10.1002/anie.201101053. [DOI] [PubMed] [Google Scholar]
- 24.Murakami M, Amii H, Ito Y. Selective activation of carbon–carbon bonds next to a carbonyl. Nature. 1994;370:540–541. doi: 10.1038/370540a0. [DOI] [Google Scholar]
- 25.Murakami, M. & Chatani, N. Cleavage of carbon-carbon single bonds by transition metals (Wiley-VCH, 2015).
- 26.Bender M, Turnbull BWH, Ambler BR, Krische MJ. Ruthenium-catalyzed insertion of adjacent diol carbon atoms into C–C bonds: entry to type II polyketides. Science. 2017;357:779–781. doi: 10.1126/science.aao0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souillart L, Cramer N. Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 2015;115:9410–9464. doi: 10.1021/acs.chemrev.5b00138. [DOI] [PubMed] [Google Scholar]
- 28.Deng L, Dong G. Carbon-carbon bond activation of ketones. Trends Chem. 2020;2:183–198. doi: 10.1016/j.trechm.2019.12.002. [DOI] [Google Scholar]
- 29.Xia Y, Dong G. Temporary or removable directing groups enable activation of unstrained C–C bonds. Nat. Rev. Chem. 2020;4:600–614. doi: 10.1038/s41570-020-0218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen F, Wang T, Jiao N. Recent advances in transition-metal-catalyzed functionalization of unstrained carbon-carbon bonds. Chem. Rev. 2014;114:8613–8661. doi: 10.1021/cr400628s. [DOI] [PubMed] [Google Scholar]
- 31.Lu H, Yu T-Y, Xu P-F, Wei H. Selective decarbonylation via transition-metal-catalyzed carbon-carbon bond cleavage. Chem. Rev. 2021;121:365–411. doi: 10.1021/acs.chemrev.0c00153. [DOI] [PubMed] [Google Scholar]
- 32.Song F, Gao T, Wang B-Q, Shi Z-J. Catalytic activations of unstrained C–C bond involving organometallic intermediates. Chem. Soc. Rev. 2018;47:7078–7115. doi: 10.1039/C8CS00253C. [DOI] [PubMed] [Google Scholar]
- 33.Dreis AM, Douglas CJ. Catalytic carbon-carbon σ bond activation: an intramolecular carbo-acylation reaction with acylquinolines. J. Am. Chem. Soc. 2009;131:412–413. doi: 10.1021/ja8066308. [DOI] [PubMed] [Google Scholar]
- 34.Jun C-H, Lee H. Catalytic carbon-carbon bond activation of unstrained ketone by soluble transition-metal complex. J. Am. Chem. Soc. 1999;121:880–881. doi: 10.1021/ja983197s. [DOI] [Google Scholar]
- 35.Xia Y, et al. Catalytic activation of carbon-carbon bonds in cyclopentanones. Nature. 2016;539:546–550. doi: 10.1038/nature19849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rong ZQ, et al. Intramolecular acetyl transfer to olefins by catalytic C–C bond activation of unstrained ketones. Angew. Chem. Int. Ed. 2018;57:475–479. doi: 10.1002/anie.201711394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia Y, et al. Two-carbon ring expansion of 1-indanones via insertion of ethylene into carbon-carbon bonds. J. Am. Chem. Soc. 2019;141:13038–13042. doi: 10.1021/jacs.9b07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao P, Yu T, Lu H, Xu P-F, Wei H. Regiodivergent access to 2- or 3-substituted indanones: catalyst-controlled carboacylation via C–C bond activation. CCS Chem. 2020;2:1862–1871. [Google Scholar]
- 39.Zhang R, Xia Y, Dong G. Intermolecular [5+2] annulation between 1-indanones and internal alkynes by rhodium-catalyzed C–C activation. Angew. Chem. Int. Ed. 2021;60:20476–20482. doi: 10.1002/anie.202106007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J, Zhang R, Wu X, Dong G, Xia Y. Intramolecular one-carbon homologation of unstrained ketones via C–C activation-enabled 1,1-insertion of alkenes. Org. Lett. 2022;24:2436–2440. doi: 10.1021/acs.orglett.2c00716. [DOI] [PubMed] [Google Scholar]
- 41.Schleyer PVR, Pühlhofer F. Recommendations for the evaluation of aromatic stabilization energies. Org. Lett. 2002;4:2873–2876. doi: 10.1021/ol0261332. [DOI] [PubMed] [Google Scholar]
- 42.King RB, Efraty A. Pentamethylcyclopentadienyl derivatives of transition metals. II. Synthesis of pentamethylcyclopentadienyl metal carbonyls from 5-acetyl-1,2,3,4,5-pentamethylcyclopentadiene. J. Am. Chem. Soc. 1972;94:3773–3779. doi: 10.1021/ja00766a018. [DOI] [Google Scholar]
- 43.Crabtree RH, Dion RP, Gibboni DJ, Mcgrath DV, Holt EM. Carbon–carbon bond cleavage in hydrocarbons by iridium complexes. J. Am. Chem. Soc. 1986;108:7222–7227. doi: 10.1021/ja00283a015. [DOI] [Google Scholar]
- 44.Youn SW, Kim BS, Jagdale AR. Pd-catalyzed sequential C–C bond formation and cleavage: evidence for an unexpected generation of arylpalladium(II) species. J. Am. Chem. Soc. 2012;134:11308–11311. doi: 10.1021/ja304616q. [DOI] [PubMed] [Google Scholar]
- 45.Smits G, Audic B, Wodrich MD, Corminboeuf C, Cramer N. A β-carbon elimination strategy for convenient in situ access to cyclopentadienyl metal complexes. Chem. Sci. 2017;8:7174–7179. doi: 10.1039/C7SC02986A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Y, et al. Deacylative transformations of ketones via aromatization-promoted C–C bond activation. Nature. 2019;567:373–378. doi: 10.1038/s41586-019-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X, Xu Y, Dong G. Deacylation-aided C–H alkylative annulation through C–C cleavage of unstrained ketones. Nat. Catal. 2021;4:703–710. doi: 10.1038/s41929-021-00661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X, Xu Y, Dong G. Olefination via Cu-mediated dehydroacylation of unstrained ketones. J. Am. Chem. Soc. 2021;143:20042–20048. doi: 10.1021/jacs.1c09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roche SP, Porco JA., Jr. Dearomatization strategies in the synthesis of complex natural products. Angew. Chem. Int. Ed. 2011;50:4068–4093. doi: 10.1002/anie.201006017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuo C-X, Zhang W, You S-L. Catalytic asymmetric dearomatization reactions. Angew. Chem. Int. Ed. 2012;51:12662–12686. doi: 10.1002/anie.201204822. [DOI] [PubMed] [Google Scholar]
- 51.Wu W-T, Zhang L, You S-L. Catalytic asymmetric dearomatization (CADA) reactions of phenol and aniline derivatives. Chem. Soc. Rev. 2016;45:1570–1580. doi: 10.1039/C5CS00356C. [DOI] [PubMed] [Google Scholar]
- 52.Zheng C, You S-L. Catalytic asymmetric dearomatization (CADA) reaction-enabled total synthesis of indole-based natural products. Nat. Prod. Rep. 2019;36:1589–1605. doi: 10.1039/C8NP00098K. [DOI] [PubMed] [Google Scholar]
- 53.Flynn AR, McDaniel KA, Hughes ME, Vogt DB, Jui NT. Hydroarylation of arenes via reductive radical-polar crossover. J. Am. Chem. Soc. 2020;142:9163–9168. doi: 10.1021/jacs.0c03926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams K, et al. An iron-catalysed C–C bond-forming spirocyclization cascade providing sustainable access to new 3D heterocyclic frameworks. Nat. Chem. 2016;9:396–401. doi: 10.1038/nchem.2670. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Zheng C, You S-L. Iridium-catalyzed asymmetric allylic dearomatization by a desymmetrization strategy. Angew. Chem. Int. Ed. 2017;56:15093–15097. doi: 10.1002/anie.201708419. [DOI] [PubMed] [Google Scholar]
- 56.Zheng C, Xia Z-L, You S-L. Unified mechanistic understandings of pictet-spengler reactions. Chem. 2018;4:1952–1966. doi: 10.1016/j.chempr.2018.06.006. [DOI] [Google Scholar]
- 57.Roque JB, Kuroda Y, Göttemann LT, Sarpong R. Deconstructive fluorination of cyclic amines by carbon-carbon cleavage. Science. 2018;361:171–174. doi: 10.1126/science.aat6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ota E, Wang H, Frye NL, Knowles RR. A redox strategy for light-driven, out-of-equilibrium isomerizations and application to catalytic C–C bond cleavage reactions. J. Am. Chem. Soc. 2019;141:1457–1462. doi: 10.1021/jacs.8b12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smaligo AJ, et al. Hydrodealkenylative C(sp3)–C(sp2) bond fragmentation. Science. 2019;364:681–685. doi: 10.1126/science.aaw4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Du J, Zuo Z. Selective C-C bond scission of ketones via visible-light-mediated cerium catalysis. Chem. 2020;6:266–279. doi: 10.1016/j.chempr.2019.11.009. [DOI] [Google Scholar]
- 61.Du J, et al. Photocatalytic aerobic oxidative ring expansion of cyclic ketones to macrolactones by cerium and cyanoanthracene catalysis. Angew. Chem. Int. Ed. 2021;60:5370–5376. doi: 10.1002/anie.202012720. [DOI] [PubMed] [Google Scholar]
- 62.Muller CK, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 63.Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008;37:320–330. doi: 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- 64.O’Hagan D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008;37:308–319. doi: 10.1039/B711844A. [DOI] [PubMed] [Google Scholar]
- 65.Michel, S., Tillequin, F. & Koch, M. Strellidimine: the first natural bis-ellipticine alkaloid. J. Chem. Soc. Chem. Commun. 229–230 https://pubs.rsc.org/en/content/articlelanding/1987/c3/c39870000229#!divAbstract (1987).
- 66.Teich L, et al. Synthesis and biological evaluation of new derivatives of emodin. Bioorg. Med. Chem. 2004;12:5961–5971. doi: 10.1016/j.bmc.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 67.Liu W, et al. Synthesis of spirocycles via Ni-catalyzed intramolecular coupling of thioesters and olefins. Chem. Eur. J. 2021;127:7651–7656. doi: 10.1002/chem.202100390. [DOI] [PubMed] [Google Scholar]
- 68.Lv, L., Yu, L., Qiu, Z. & Li, C.-J. Switch in selectivity formal hydroalkylation of 1,3-dienes and enynes with simple hydrazones. Angew. Chem. Int. Ed. 59, 6466–6472 (2020). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data relating to the optimisation studies, mechanistic studies, general methods, and the characterisation data of materials and products, are available in the Supplementary Information. Crystallographic parameters for compounds 2, 43, 52 and 74 are available free of charge from the Cambridge Crystallographic Data Centre under CCDC 2116750 (2), 2116753 (43), 2116752 (52) and 2131840 (74). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/getstructures.