Abstract
Diseases are major drivers of the deterioration of coral reefs and are linked to major declines in coral abundance, reef functionality, and reef-related ecosystems services. An outbreak of a new disease is currently rampaging through the populations of the remaining reef-building corals across the Caribbean region. The outbreak was first reported in Florida in 2014 and reached the northern Mesoamerican Reef by summer 2018, where it spread across the ~450-km reef system in only a few months. Rapid spread was generalized across all sites and mortality rates ranged from 94% to <10% among the 21 afflicted coral species. Most species of the family Meandrinadae (maze corals) and subfamily Faviinae (brain corals) sustained losses >50%. This single event further modified the coral communities across the region by increasing the relative dominance of weedy corals and reducing reef functionality, both in terms of functional diversity and calcium carbonate production. This emergent disease is likely to become the most lethal disturbance ever recorded in the Caribbean, and it will likely result in the onset of a new functional regime where key reef-building and complex branching acroporids, an apparently unaffected genus that underwent severe population declines decades ago and retained low population levels, will once again become conspicuous structural features in reef systems with yet even lower levels of physical functionality.
Subject terms: Biodiversity, Ecosystem ecology
A new deadly coral disease, known as stony coral tissue loss disease, has modified the coral communities across the Caribbean region by disproportionately affecting key reef-building corals and reducing reef functionality.
Introduction
Disease outbreaks are often associated with mass mortality events that can rapidly and drastically reduce populations over short periods of time1–3. When these events affect foundation species, population losses result in changes in the local environment, upon which a variety of other species depend, and altering the structure and functioning of the entire ecosystem4,5. In the marine realm, Caribbean coral reefs offer some of the best examples of such catastrophic events. This region is a well-known hot spot for diseases6–8 and many have decimated the populations of primary reef-builders, resulting in devastating changes to the spatial heterogeneity of the reefscape, its capacity to provide ecosystem services, and the ability to track sea-level rise6,9. In the late 1970s, a region-wide outbreak of white-band disease led to population losses of nearly 80% of the major reef-building corals Acropora palmata and A. cervicornis10, resulting in a notable reduction in reef functionality (e.g.,11–13). Since then, multiple disease outbreaks have nearly decimated the populations of many other key reef-building corals6,7. Moreover, there is an ever-increasing risk of diseases that may further impact the stability of reef ecosystems, as the frequency and intensity of disease outbreaks are often related to rapidly increasing human-induced pressures, such as rising sea temperatures, decreased water quality, and nutrient enrichment14–18.
In 2014, the south-eastern Florida sub-region saw the onset of a new deadly coral disease, known as stony coral tissue loss disease (SCTLD;19). The disease spread across the Caribbean with reports of SCTLD in the Western Caribbean, Bahamas, Puerto Rico, and the US Virgin Islands and recent reports in the Lesser and Greater Antilles20. The sources of transmission and specific causative agents are not yet fully understood, although the disease is clearly transmitted through seawater with bacteria being involved at some level in disease progression, and viruses of the algal symbionts being found in pathological studies, suggesting a disruption of host–symbiont physiology21–23. Most evidence of the devastating effects of SCTLD comes from Florida and Mexico24,25, yet similar outcomes have been reported elsewhere in the region. SCTLD affects nearly 30 different coral species and is highly virulent, spreading rapidly within reef systems26,27. Highly susceptible species (e.g., Meandrina meandrites and Dendrogyra cylindrus) are rapidly infected and can die within weeks19,28. Coral density and composition and environmental conditions, including nutrient concentrations and turbidity, likely influence disease prevalence and progression24,25,29,30. However, high water temperatures do not appear to directly influence SCTLD prevalence or virulence21,24,26,27.
SCTLD now threatens many coral species that serve as important reef-builders and habitat providers in most of today’s Caribbean reefs31, which raises the question of whether post-SCTLD coral assemblages will be able to maintain key geo-ecological functions, such as reef framework production, sediment generation, the maintenance of reef habitat complexity, and the capacity for coral reef growth that is needed to track future sea level increases9. These functions largely rely on the capacity of coral assemblages to create complex three-dimensional structures by means of calcium carbonate precipitation – defined hereinafter as reef physical functionality. This is particularly relevant in the Caribbean, as lower levels of functional diversity are present compared to those in other regions (e.g., the Great Barrier Reef). Moreover, many key functional attributes of reef-building corals are redundant, and only a few species determine the physical functionality of the coral community32,33. Here, we used extensive pre- and post-SCTLD data along a 450-km reef track in the Mexican Caribbean to examine the regional effects of this emerging threat and to identify the potential ecological and environmental covariates that influenced the occurrence and severity of SCTLD. We then sought to answer how the severe population declines of SCTLD-afflicted species would impact functional diversity and coral community calcification in Caribbean coral reefs. We characterize the functional diversity of Caribbean coral species using six different traits known to be important for reef physical functionality32,33 (i.e., skeletal density, growth rate, rugosity index, colony size, reproduction strategy, and corallite width) and show that coral mortality was widespread, with corals with intimate phylogenetic (i.e., Meandrinadae and Faviinae) and functional trait relationships being disproportionally affected by SCTLD, favoring the domination of coral species that poorly contribute to reef functionality.
Results and discussion
Impacts on coral communities
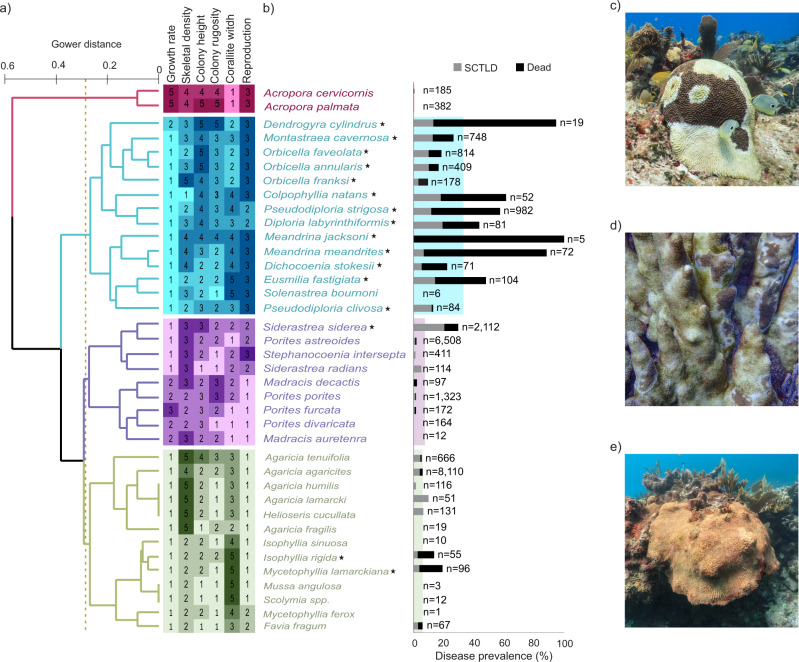
In only a few months, SCTLD spread across hundreds of kilometers and triggered an unprecedented loss of corals (Figs. 1, 2). Out of the 29,095 colonies surveyed between July 2018 and January 2020, 17% were already dead with signs of recent mortality (e.g., bare skeletons or the presence of a thin layer of filamentous algae covering the colony; Fig. 1e) and an additional 10% were afflicted by the disease (Fig. 1c, d). However, susceptibility and mortality varied greatly between species. Twenty-five of the 48 recorded species were affected by the disease, with disproportionate effects observed in a single morpho-functional group largely defined by massive species with mid-to-large sizes, dense skeletons, low growth rates, and broadcasting sexual reproduction (Fig. 1). Species from the family Meandrinadae and subfamily Faviinae were the most severely affected. In particular, Dendrogyra cylindrus and Meandrina spp. experienced disease prevalence and population losses greater than 80% (Fig. 1). A temporal comparison of community compositions with pre- and post-outbreak data revealed that less conspicuous species, such as Dichocoenia stokesii or those within the Mussinae subfamily, were noticeably less abundant after the outbreak (Fig. S1), indicating that these species were more severely affected than what our post-outbreak surveys suggested. This is likely because the skeletons of the small and encrusting species killed by the disease were rapidly covered by algae or sediment, rendering them inaccessible during post-outbreak surveys.
Fig. 1. Morpho-functional groups of Caribbean corals and their susceptibility to stony coral tissue loss disease (SCTLD).
a Hierarchical clustering dendrogram based on a Gower dissimilarity analysis and heat map representation of functional traits. Numerical values from 1 to 5 correspond to the categories listed in Table S6. The variation in color intensity within each group (light to deep) corresponds to the trait numerical value (given inside each square). b Prevalence of SCTLD for coral species across 101 reef sites in the Mexican Caribbean (n = number of colonies). We included coral colonies with total mortality whose deaths could be attributable to SCTLD. The shaded area corresponds to the prevalence of SCTLD for each morpho-functional group in (a). The asterisk (*) indicates species with >10% disease prevalence (these were considered highly susceptible species, see Methods for more details). c Pseudodiploria strigosa colony with the characteristic lesions produced by SCTLD in Puerto Morelos (July 2018). d Dendrogyra cylindrus colony afflicted with SCTLD showing extensive recent mortality in Sian Ka’an (September 2018). e A recently deceased colony of Meandrina sp. (covered by a homogenous, thin layer of filamentous algae) in Akumal (September 2018). Photo credits: Lorenzo Alvarez-Filip.
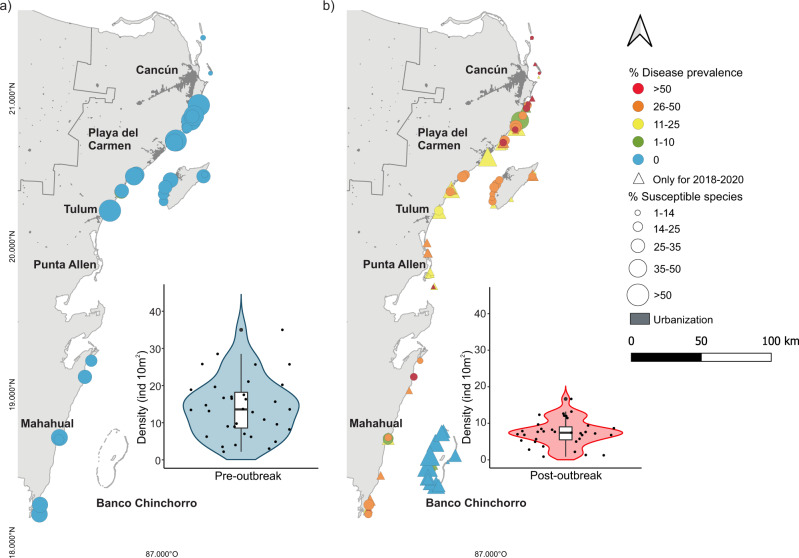
Fig. 2. Prevalence of stony coral tissue loss disease (SCTLD) in highly susceptible species in the Mexican Caribbean.
a White plague-type disease prevalence in species highly susceptible to SCTLD in 35 sites surveyed before the outbreak of the disease (2016 and 2017). b SCTLD prevalence (i.e., diseased and recently deceased colonies) in highly susceptible species during or after the SCTLD outbreak (2018 and 2020). In (a) and (b), the circles represent the locations of the reefs that were sampled before and after the outbreak. The triangles represent the sites that were only surveyed during or after the outbreak. The size of the figure indicates the percentage of healthy colonies of highly susceptible species based on the total number of surveyed colonies at each site and time period (including all coral species). The insets in (a) and (b) represent the distributions of the densities of live coral colonies of highly susceptible species across all surveyed sites for each period. The data points represent each surveyed site, and the box plots depict the median (horizontal line), the first and third quartiles (box height), and 95th percentiles (whiskers). The shaded area (violin plot) depicts the kernel density showing the probability of the data at different values. Highly susceptible species are those with more than 10% disease prevalence (see Methods and Fig. 1).
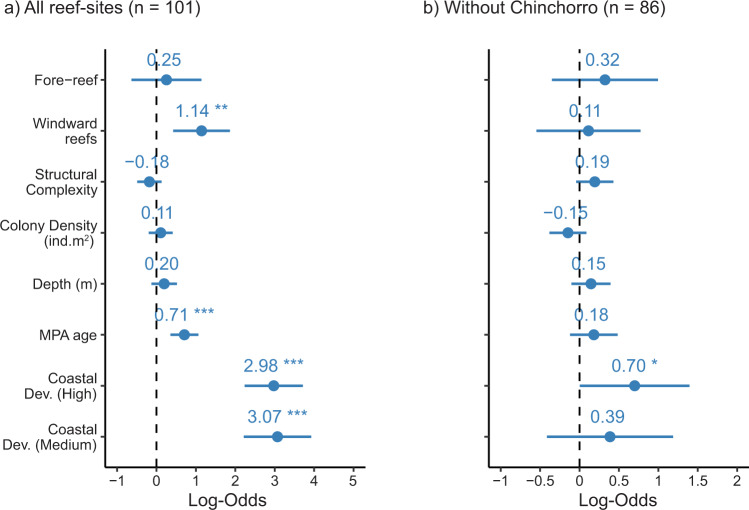
Across all surveyed sites, disease prevalence (considering both diseased and dead colonies) in highly susceptible species showed no statistical differences with regard to depth, reef zone, structural complexity, or coral density (Fig. 3a; Table S1; Fig. S2), suggesting that the spread of SCTLD is primarily controlled by the capacity of the pathogen(s) to be transported in the water column within and between reef sites. However, we found a strong effect of the threat of coastal development on disease prevalence, indicating that sites close to developed areas were considerably more affected than those in isolated regions. In addition, wind exposure (i.e., windward areas) and the age of marine protected areas were also related to higher disease prevalence (Fig. 3a; Table S1; Fig. S2). The observed significant effects were strongly driven by the lack of SCTLD in the reef sites of Banco Chinchorro (which remained unaffected until at least December 2021). Banco Chinchorro is an isolated offshore bank with restricted access and minimal human infrastructure (i.e., fisher campsites and research and military stations) that is separated from the mainland by a deep-water channel in which the strong northward Yucatan Current likely acts as a physical barrier to biological connectivity and land-based perturbations. Species susceptible to SCTLD are abundant in Banco Chinchorro (Fig. 2b), thus the absence of the disease is not due to the lack of potential host species.
Fig. 3. Disease prevalence predictors in coral reefs in the Mexican Caribbean.
a Disease prevalence predictors for 101 reef sites in the Mexican Caribbean and (b) without the Banco Chinchorro reefs (86 sites). Effect sizes are the logistic mixed models with the dots and lines representing the means and 95% confidence intervals, respectively. All continuous predictive variables were scaled to z-scores with the scale function in R. In the model, categorical variables, such as coastal development (low), leeward reefs, and back-reefs, were used as arbitrary references. Asterisks denote statistical differences (*p < 0.05, **p < 0.01, ***p < 0.001).
When the reefs of Banco Chinchorro were removed from the analyses, high-level coastal development remained significant, whereas the rest of the covariates ceased to have significant effects (Fig. 3b; Table S2; Fig. S3). The effects of coastal development and wind exposure might be linked and partly explained by the fact that the reefs adjacent to the mainland (all windward sites) were also closer to the main urban and tourist developments in the region. For example, most sites experiencing disease prevalence >50% are located in the northernmost region, where coastal development is high (Fig. 2;34). Coastal reefs in the Mexican Caribbean are strongly influenced by freshwater inflow from combined runoff and submarine groundwater discharges, which load the seawater with pathogens, sediments, nutrients, and pollutants35,36, many of which have been found to serve as vectors and influence disease progression while reducing coral resistance (e.g.,15,16,37–39). These negative effects may be exacerbated by low water recirculation typical of reef lagoons in fringing reefs, which increases the influx and retention of coastal waters within the system35.
Overall, the spatial pattern of afflicted reefs suggests that anthropogenic pressures in the form of coastal development modulate the vulnerability of coral communities to a certain degree, resulting in greater or lesser disease prevalence. Therefore isolation from anthropogenic pressures might provide some degree of protection. However, given the contagiousness and the high mortality rate after infection, coral mortality and the disappearance of key reef-building species will most likely occur regardless of local-scale conditions and geomorphological features after SCTLD reaches a site (Fig. 2;19,25,31,40).
Impacts on functional integrity
Abrupt coral die-off radically reduced the abundance of species and the traits that support the physical functionality of coral reefs. Most of the reefs shifted further away from the dominance of reef-building species that are key providers of three-dimensionality to depauperate assemblages dominated by taxa with simpler morphological attributes and slower growth rates. The results of the similarity percentage (SIMPER) analysis show that even before the outbreak, most coral reefs in the region were already largely dominated by encrusting and sub-massive agaricids and Porites astreoides, which are weedy coral species that accounted for 63.33% and 71.83% of the similarity between sites before the outbreak and after the coral die-off, respectively (Fig. S4). The relative increase in the abundance of these two groups accounted for 50.42% of the dissimilarity between periods (pre- and post-outbreak), while decreases of highly susceptible species accounted for only 13.06% of the dissimilarity, as many of these were either uncommon or rare species (Table S3).
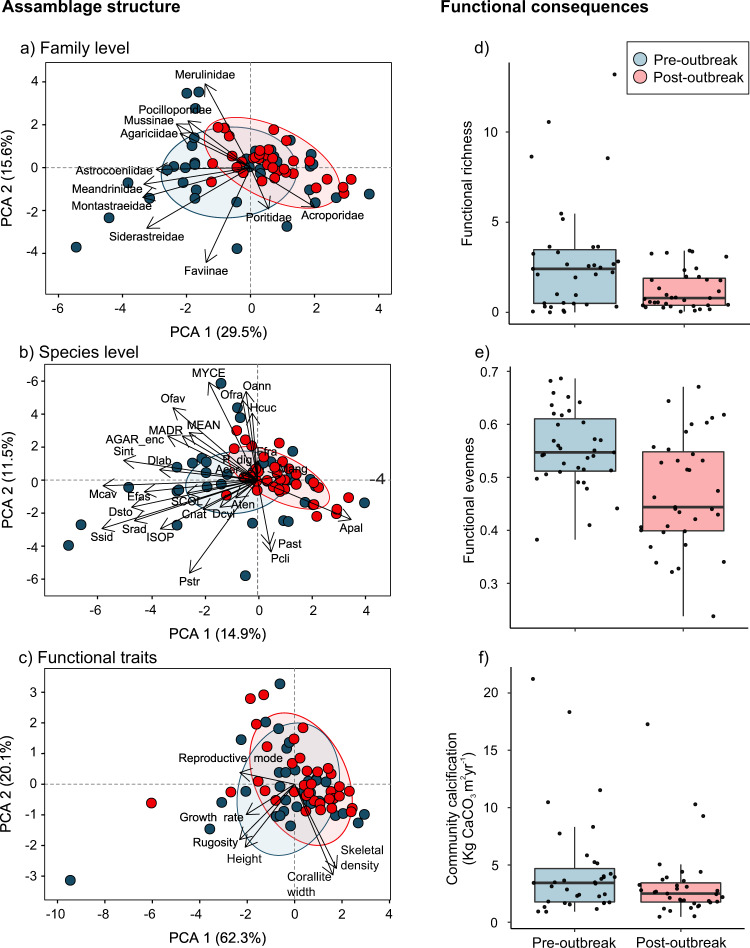
Given that the species that suffered the most severe losses share key life-history traits (Fig. 1), the functional space of the coral assemblages was considerably reduced at regional scales after the outbreak. A before-after analysis at species and family levels and the multi-dimensional trait space of the coral assemblages weighted by the absolute abundance of taxa contributing to each trait revealed a drastic transformation towards more homogenous assemblages, as determined by taxonomic and functional trait data, with a notorious lack of contributions from the most severely afflicted species during the post-outbreak period (Fig. 4a–c). These emerging novel assemblages were remarkably characterized by the presence of acroporid corals and their life-history traits (Fig. 4a–c) despite their low abundance across surveyed sites (Table S3). Not surprisingly, composition changes resulted in significant losses of functional richness (t = 2.67, df = 46.04, p = 0.01) and functional evenness (t = 3.81, df = 65.54, p > 0.01) in the coral assemblages (Fig. 4d, e) despite an apparent increase in species richness (t = −2.36, df = 65.19, p = 0.02) that resulted from the increased survey effort during the post-outbreak period (Fig. S5; see methods). Ultimately, increased mortality was reflected in a marked reduction in coral community calcification (regional mean ± SE; 4.60 ± 0.77 G = Kg CaCO3 m2 yr−1 before the outbreak to 3.27 ± 0.53 G after the outbreak; t = −3.0, df = 34, p = 0.004; Fig. 3f) that was largely driven by the loss of highly susceptible species (3.04 ± 0.62 G pre-outbreak to 1.91 ± 0.34 G post-outbreak).
Fig. 4. Shifts in coral community composition and functioning following the stony coral tissue loss disease (SCTLD) outbreak.
a–c Principal component biplots of the shifts in the coral assemblages in 35 reef sites along the Mexican Caribbean between the pre- (blue) and post-outbreak (red) periods in (a) coral assemblages, (b) family assemblages, and (c) functional traits based on community weighted means (CWM). The points in (a–c) represent the reef sites for each period. Vectors represent the absolute contributions of families, species, and traits. The colored ellipses represent the 95% confidence intervals around the weighted average of the site scores for each period. d–f Box plots of the functional shifts between the pre- (blue) and post-outbreak (red) periods in the same reef sites with regard to (d) functional richness, (e) functional evenness, and (f) coral community calcification (Kg CaCO3 m2 yr−1). The points in (d–f) represent each surveyed reef site and box plots show the median (horizontal line), first and third quartiles (box height), and the minimum and maximum values (whiskers; excluding outliers). Species key in (b): Acer: Acropora cervicornis; AGAR_enc: Agaricia encrusting Apal: Acropora palmata; Aten: Agaricia tenuifolia; Cnat: Colpophyllia natans; Dcyl: Dendrogyra cylindrus; Dlab: Diploria labyrinthiformis; Dsto: Dichocoenia stokesii; Efas: Eusmilia fastigiata; Ffra: Favia fragum; Hcuc: Helioseris cucullata; ISOP: Isophyllia spp; MADR: Madracis spp; Mang: Mussa angulosa; Mcav: Montastraea cavernosa; MEAN: Meandrina spp; MYCE: Mycetophyllia spp; Oann: Orbicella annularis; Ofav: Orbicella faveolata; Ofra: Orbicella franksi; P_dig: Branching Porites; Past: Porites astreoides; Pcli: Pseudodiploria clivosa: Pstr: Pseudodiploria strigosa: SCOL: Scolymia spp; Sint: Stephanocoenia intersepta; Srad: Siderastrea radians; Ssid: Siderastrea siderea.
The ecology and physical functionality of coral assemblages in the Caribbean were undergoing severe ecological changes prior to the SCTLD outbreak. Chronic and acute disturbances had progressively driven a decline in the abundance of the main reef-building corals that was accompanied by a concomitant increase in the relative or absolute abundance of opportunistic species characterized by small-sized colonies that do not notably contribute to reef structure and are known to be tolerant to environmental stress (Fig. 5;9,32). The pre-SCTLD communities were described as ‘shifted’ coral assemblages, and the contributions of formerly dominant acroporids were often negligible given their reduced abundance, whereas large massive species remained and contributed the most to ecosystem structure and functionality (Fig. 5;32,41–43). However, the resulting wide-spread coral mortality described here was dictated by the vulnerability to SCTLD of some species that share key morpho-functional traits (Fig. 1a), and thus caused non-random changes in community structure that further and radically affected the functional integrity of the coral communities.
Fig. 5. Conceptual diagram of the long-term trajectory of the physical functionality of Caribbean reefs based on published temporal trends (Table S4) and the recent impacts of stony coral tissue loss disease (SCTLD).
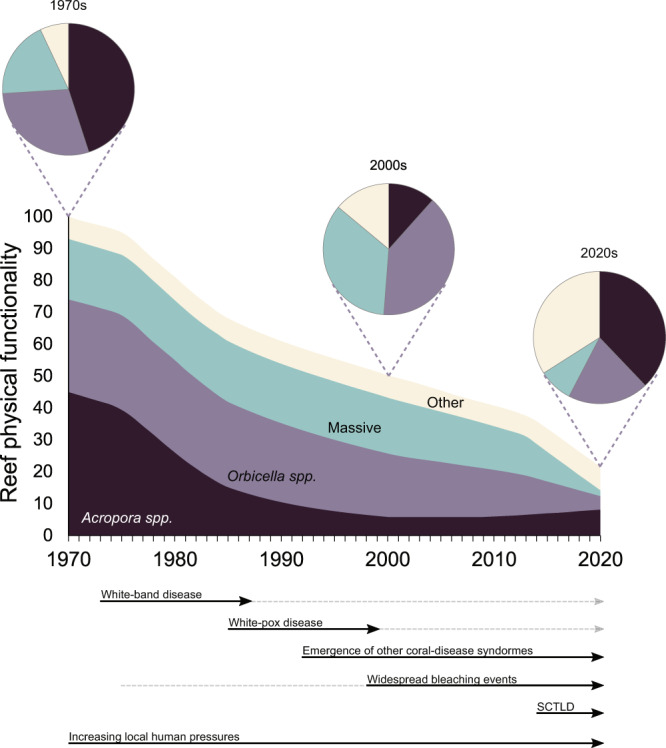
The physical functionality of reefs depends on the abundance (or cover), capacity to accumulate CaCO3, and structural complexity of each species present in the system32. The stacked plot represents the functional contributions of four coral groups over time. The pie charts illustrate the proportional contributions of each coral group during three different periods. Acropora spp. and Orbicella spp. contain all the species for each of these genera and are illustrated as a single group, as they are the main reef-building corals in the Caribbean and have dominated shallow-water coral-reef habitats throughout the region in geological times42. The group of massive corals includes important reef framework builders from the Diploria, Pseudodiploria, Colpophyllia, Montastraea, and Dendrogyra genera (many of which were severely affected by SCTLD and were included in the second morpho-functional group from the top in Fig. 1a). The other group includes all other coral species, which are largely classified as weedy, submassive, or foliose-digitate corals (included in the third and fourth morpho-functional groups from the top in Fig. 1a) for which little evidence of declines exists. The black arrows indicate major sources of coral decline widely recognized in the literature. White-band disease resulted in severe population declines of acroporids10. The white-pox epidemic has infected many of the remaining colonies of this genus since the 1990s87. Other coral-disease syndromes (e.g., white plague and Caribbean yellow band) that mainly affect Orbicella and other massive species have increased in frequency and virulence over the last three decades (e.g.,7,88). Coral mortality has also continued to increase in the Caribbean and is associated with warm-water bleaching events and other local-scale anthropogenic impacts13,34,89. The grey-dashed arrows indicate that the source of stress remains, although the effects on widespread coral mortality are unclear.
The morpho-functional groups comprised of large and massive species were the most afflicted by the SCTLD outbreak (Fig. 1), whereas the species mildly affected by the disease showed relative increases in abundance. The post-SCTLD coral communities are now represented by a hyper-domination of opportunistic corals, although this remarkably seems to be accompanied by an apparent resurgence of acroporids as key functional elements (Figs. 4a–c, 5). However, the increase of acroporids is primarily an artefact of the drastic reductions in the relative contributions of many other species due to SCTLD (Fig. 5). Only a minor proportion of the increase in the contributions of acroporids may be explained by population recovery or re-sheeting growth over relict reef structures44–46. In fact, the acroporid populations have remained low compared with their historical estimations12,13, as these species have low biological connectivity, low recruitment rates, reduced genetic diversity, impaired recovery abilities, and high vulnerability to regional and global stressors13,47. Although it is encouraging that acroporid populations have remained relatively unchanged after the SCTLD outbreak, it is unlikely that these species will considerably improve the structure and dynamics of rapidly changing Caribbean coral assemblages given current conditions.
The large-scale loss of the functionally important corals defined radical shifts in reef conditions and dynamics, exacerbating further losses of ecological integrity along the entire reef track. The outcomes of coral die-off from the SCTLD outbreak will compromise key functions that are supported by living reef-building corals such as reef framework production, the maintenance of reef habitat complexity, and the potential for growth9. These functions largely depend on the capacity of coral assemblages to accumulate calcium carbonate at higher rates than the rate of loss due to biological, chemical, or physical erosion. If erosive processes equal or exceed reef carbonate production, reef frameworks may be destroyed faster than they are produced, resulting in a net negative carbonate budget48,49. In this study, we observed a nearly 30% reduction in the capacity of coral communities to produce calcium carbonate. This is alarming because levels of community calcification prior to the impacts of SCTLD were substantially below the optimal rates that have been reported under high coral cover states in the Caribbean49,50. In the absence of recovery, the ultimate consequences of coral mortality will thus be modulated by destructive forces like bioerosion or the biogenic dissolution of reef structures51. This is particularly relevant as both bioerosion rates and skeletal dissolution are thought to become pervasive when the water chemistry changes or the temperatures increase48,51. Our understanding of how the increased availability of substrate for bioeroders will interact with rapid environmental changes remains limited. However, if the ultimate objective is preserving coral reef functioning and services, it may be necessary to focus on replenishing and favoring the recovery of coral communities while improving our understanding of how to control and modulate the destructive forces operating within coral reefs.
Implications for conservation
The widespread coral die-off associated with SCTLD has affected the populations of many important reef-building species (Fig. 1). Although the impacts of this highly virulent disease are consistent across affected regions19,24–27, the wide-spread consequences of this outbreak are yet to be known for the entire Caribbean. However, the rapid movement of the disease across the region20 and the overlapping distribution ranges of most species within the Greater Caribbean region33,52, suggest that the outbreak will affect the entire region, as has occurred with previous disease outbreaks6,8,10. Therefore, some species will rapidly be at a clear risk of extinction across their distribution ranges (e.g., D. cylindrus; Fig.1a;28), while other susceptible species that underwent comparatively lower declines (30–70%; e.g., brain corals) will also have compromised abilities to overcome future sources of stress. For example, the evident declines in the populations of species belonging to the family Meandrinidae and subfamily Faviinae could reduce their levels of genetic diversity (e.g.,53), putting these species at risk of bottleneck events that would limit their ability to cope with environmental change54. In addition, the levels of isolation of the remaining colonies will reduce or hinder the capacity for sexual reproduction (e.g., Allee effect) and genetic recombination, further diminishing the abilities of populations to adapt to rapidly changing conditions55. Moreover, many afflicted species are slow growing (Fig. 1), and the replacement of dead corals will undoubtedly take decades while many acute and chronic stressors operate on smaller temporal scales56. This is particularly relevant given that corals weakened by SCTLD are likely to be more susceptible to subsequent disease outbreaks and to other threats like bleaching.
One key question for the coming years is whether populations of highly afflicted species will be able to recover and sustain key geo-ecological functions. To date, we have little evidence in this regard. Empirical observations during our surveys have shown that small (<5 cm) Meandrina, Diploria, or Pseudodiploria corals have apparently remained unaffected by SCTLD, even in sites that underwent severe losses in adult populations (Fig. S6). Furthermore, recent evidence shows that coral colonies with evident lesions associated with SCTLD can spawn and produce viable gametes57. However, the replacement of dead corals through larval recruitment or the growth of small juveniles will take several years given low larval survival and the slow growth rates of most species58,59. In addition, coral recruitment (i.e., the successful settlement of coral larvae) and subsequent survival will largely depend on suitable ecological conditions, such as low densities of harmful fleshy macroalgae60. Unfortunately, in our study region and elsewhere in the Caribbean, there is extensive evidence indicating that macroalgae cover is progressively becoming a dominant component of benthic reef communities61,62. It is likely that macroalgae will rapidly overtake the free space left by recently deceased corals (e.g.,27), hindering coral recovery by impeding the settlement of new recruits and reducing the likelihood of colonies being able to either slow or halt disease progression by recolonizing their own structures or neighboring substrates (e.g.,32,63). Natural processes might therefore be insufficient to restore the severe population losses of many coral species due to the SCTLD outbreak. Rather, it is likely that human interventions in the form of rescuing colonies of vulnerable species, preserving their genetic material, and implementing restoration efforts will be needed to facilitate recovery and prevent the region-wide extinction of some species (e.g.,28). We believe, however, that these actions will only succeed if they are accompanied by stringent controls that take into consideration climate change, coastal development, and wastewater treatment to improve local conditions and ecosystem resilience.
Methods
Field surveys and SCTLD prevalence
To assess the spread and impacts of SCTLD, extensive surveys were conducted across the Mexican Caribbean between July 2018 and January 2020 (post-outbreak period). In total, 101 sites were surveyed (82 fore-reefs, 19 back-reefs, and four reef-crests) in depths ranging from 1 to 24 m (Fig. 2; Supplementary Data 1). Thirty-five of these sites were also surveyed in 2016 and 2017 (pre-outbreak period) as part of a separate effort24,34,64 and were used to investigate the ecological and functional consequences of the SCTLD outbreak. These 35 resampled sites are also distributed across the Mexican Caribbean (Fig. 2) and cover similar habitats and depth gradients (thirty reef sites are fore-reefs and five are back-reefs). For the pre-outbreak period, white plague-type disease prevalence is reported, as there were no reports of SCTLD and a notable overlap between host ranges and the susceptibility to both diseases was present.
All sites were surveyed using the Atlantic and Gulf Rapid Reef Assessment protocol65. At each site, coral assemblages were surveyed in 10 × 1 m transects that were haphazardly placed within the reef structure at the same depth and reef-zone. For the pre-outbreak period, 1–7 transects (mean = 2.8; SD = 1.4) were evaluated in each site, and for the post-outbreak period between 3–23 transects (mean = 7.1; SD = 3.3) were conducted in each site. The higher number of transects conducted in the post-outbreak period was not expected to artificially increase the relative abundance of coral colonies nor disease prevalence, as the pre-SCTLD effort was already robust enough to capture ecological patterns65. Rather, we increased the effort to increase the probability of recording rare species that we knew were highly vulnerable to disease. The following information was recorded for each living coral colony within each transect: species name, colony size (maximum diameter, diameter perpendicular to the maximum diameter, and height), bleaching percentage, partial mortality percentage (new, transition, and old), and the presence of SCTLD or other diseases65. For this study, we also recorded colonies with 100% mortality that could be attributed to SCTLD (i.e., recent or transient mortality was still evident; e.g., Fig. 1e). Mortality was deemed to be recent when the superficial structure of the colonies was bare (white coral skeleton) or covered by a thin layer of sediment or filamentous algae, indicating that the soft tissue had died within a time frame of hours to weeks. Only 241 (out of 29,095) post-outbreak colonies were recorded to have been affected by other diseases, none with evidence of rapid disease progression or severe coral mortality. During the surveys, we did not find evidence of the outbreak of other diseases, therefore, we assumed that coral mortality was produced by SCTLD because this disease has a high rate of lethality and progression19,26. Also, to differentiate SCTLD from bleaching, we carefully observed if the colony presented live tissue. When a colony presented signs of bleaching, the remaining tissue had a pale or transparent color that was still visible, which contrasts with what is present with SCTLD, as SCTLD kills the living tissue of the colony. We considered both diseased and recently deceased colonies to prevent underestimating the effects of the disease, as has been done in other similar studies24,25,27.
We focused on exploring the prevalence and geographical and temporal trends of the most ‘highly susceptible species’, which were species with more than 10% disease prevalence (considering diseased and recently deceased colonies). These species are: Colpophyllia natans, Dendrogyra cylindrus, Diploria labyrinthiformis, Dichocoenia stokesii, Eusmilia fastigiata, Isophyllia rigida, Montastraea cavernosa, Meandrina meandrites, M. jacksoni, Mycetophyllia lamarckiana, Orbicella annularis, O. faveolata, O. franksi, Pseudodiploria clivosa, P. strigosa and Siderastrea siderea (Fig. 1b). For this, we used the information from the 101 sites surveyed during the post-outbreak period to calculate SCTLD prevalence for each site and coral species. Although S. siderea showed different signs of infection (termed white-blotch syndrome in some studies;25), we considered this species to be affected by SCTLD due to the timing of the onset of signs and the disease progression being similar to what we observed with other species. In addition, to generate an overview of the outbreak status of the Mexican Caribbean, we calculated SCTLD prevalence for each site and each period using only highly susceptible species.
Effect of environmental and anthropogenic covariates on SCTLD prevalence
We modeled the percentage of afflicted colonies as a function of coral colony density (prior to the impacts of the disease), reef structural complexity, reef zonation, depth, and the degree of exposure to dominant winds. In addition, we evaluated the influence from land-based human activities using the Coastal Development level (World Resources Institute database,66) and protection status based on MPA age. These variables were selected based on their importance to coral reef health and the known susceptibility of coral assemblages to disturbance (34,64, see Table S5 for details), depending on the availability of information for the 101 sites (Supplementary Data 2). Water temperature or thermal stress were not included, as remote sensing data do not capture local variation at the necessary resolution (4 km;67). Furthermore, previous studies have shown that temperature is a poor predictor of the spatial variation of coral reef conditions in this ecoregion68 and that high water temperatures do not affect SCTLD prevalence or virulence21,24,26,27.
We used a binomial logistic generalized linear mixed model, setting the percentage of afflicted colonies as the response variable (i.e., total number of colonies/number of colonies afflicted) and the aforementioned factors as the predictive variables. All continuous predictive variables were scaled to z-scores with the scale function in R. In the model, categorical variables, such as coastal development (low), leeward reefs, and back-reefs, were used as arbitrary references. We included site and transect nested within site as random effects to account for spatial heterogeneity and site-level stochasticity due to the repeated sampling of sites. Moreover, we also included species as a random effect in the regression model to account for species identity and that individuals from the same species may respond to SCTLD more similarly when compared with individuals of other species. Furthermore, we included the sampling size area as a weighting factor to account for the potential effect of effort among sites into our analysis. Statistical analyses were carried out using a 95% confidence interval (α = 0.05), and model assumptions were validated with residual plots. Regression models were constructed using the glmer function within the lme4 package69 in R Version 3.6.170.
Coral morpho-functional groups and community shifts
The functional diversity of coral communities was estimated using six different traits: skeletal density, growth rate, rugosity index, colony size, reproduction strategy, and corallite width (Table S6). Some or all of these traits have been previously used in other studies to represent the functioning of reef-building corals in a multidimensional space33,71. The information on species-level traits was obtained from different sources that provide comprehensive details for each selected trait (32,72,73; Table S7). To better compare traits given the contribution disparities between species (see74,75), the traits were categorized into numerical groups (1–5). Hierarchical clustering was performed to identify groups in the data set and estimate trait similarity. We grouped the reef corals into morpho-functional groups using a Gower dissimilarity matrix (‘vegan’ package in R;76) and average-linkage hierarchical clustering, which calculates the average distance between clusters before merging (‘cluster’ package in R;77). Groups were defined using 65% dissimilarity because it was the most evident grouping and provided a concise number of groups (Fig. 1).
We measured the contribution of each species to compositional similarity within periods and dissimilarity between periods with a similarity percentage (SIMPER;78) analysis. SIMPER identifies the species that are most responsible for the observed patterns (e.g., the species that typify each factor level and those that contribute the most to dissimilarity between levels) by disaggregating the Bray-Curtis similarities between samples. The more abundant a species is within a group, the more it contributes to intra-group similarity; species with consistently high contributions to the dissimilarity between groups are good discriminating species79.
To explore temporal changes in coral composition and the traits of those assemblages, a principal component analysis (PCA) was performed using the density (ind/10 m2) of the colonies of each species in each of the 35 reef sites with pre- and post-outbreak information. We evaluated changes in coral-assemblage composition at species and family levels. Changes in functional trait assemblages were assessed through a community-weighted means (CWM) analysis that calculates the relative contribution of a given trait to the coral assemblage, which largely determines ecosystem processes80,81. Changes in the functional diversity of coral assemblages were evaluated with functional richness, which represents the volume of the convex hull covering all species in the functional space, and functional evenness, which measures the regularity of the abundance distribution in the functional space82. These indices have been widely used to account for functional diversity changes in coral communities83,84. Statistical differences between periods were tested with a paired Welch’s t-test. All statistical and functional diversity analyses were performed in R70 using the ‘FD’ package85.
Coral community calcification
To estimate the effects of SCTLD on the physical functionality of coral reefs, we calculated the potential calcification (i.e., kg CaCO3 m−2 yr−1) of the coral assemblages for each period using the sum of the calcification rate of each colony proportional to the sampled reef area (m2). We used a reef-level estimation to calculate mean coral community calcification for each period. For this, we estimated the calcification rate of each colony within each study site considering the size, mean annual growth rate (cm yr−1), mean skeletal density (g cm−3), and morphological growth of each species following the methodology described by Gonzalez-Barrios et al.86 (see also31). For each period, the calcification rate was estimated as the annual volume of calcium carbonate accumulated by the living tissue of the colony for each time period using the information from the 35 resampled sites.
Statistics and reproducibility
Raw data are accessible in Supplementary Data 1 and 2. The methods for statistical analysis and sizes of the samples (defined as n) are given in the respective sections of results and methods. Statistical analyses were conducted in R Version 3.6.173 using the cited packages, and reproducibility can be achieved using the parameters reported in the Methods.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This study was supported by the Mexican Council of Science and Technology (CONACyT, grant number: FORDECYT-PRONACES/425888/2020), the Comisión Nacional de Áreas Naturales Protegidas of Mexico (grant number: PROREST/CER/56/2019), the Universidad Nacional Autónoma de México (UNAM; UNAM-DGAPA-PAPIIT grant number: IN-205019), and a Royal Society Newton Advanced Fellowship (grant number: NA150360). We are grateful three anonymous referees for their comments and suggestions that greatly improved our analysis and the manuscript. We also thank Andrea Lievana for editing the manuscript.
Author contributions
Conceptualization, Funding acquisition, Project administration, and Writing original draft: L.A.-F.; Methodology and Investigation: L.A.-F., F.J.G.-B., E.P.-C., A.M.-H., N.E.-S.; Data curation: E.P.-C., N.E.-S.; Formal analysis: L.A.-F., F.J.G.-B., A.M.-H., N.E.-S.; Visualization: L.A.-F., F.J.G.-B., N.E.-S.; Writing – review & editing: F.J.G.-B., E.P.-C., A.M.-H., N.E.-S.
Peer review
Peer review information
Communications Biology thanks Colin Shea, Thierry Work and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Caitlin Karniski. Peer reviewer reports are available.
Data availability
All data are available in the main text or in Supplementary Data 1 and 2.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-022-03398-6.
References
- 1.Dungan ML, Miller TE, Thomson DA. Catastrophic decline of a top carnivore in the Gulf of California rocky intertidal zone. Science. 1982;216:989–991. doi: 10.1126/science.216.4549.989. [DOI] [PubMed] [Google Scholar]
- 2.Pounds JA, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls, H. Mysterious die-off sparks race to save saiga antelope. Nature 1–2. 10.1038/nature.2015.17675 (2015).
- 4.Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife - Threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 5.Peters, E. C. Diseases of Coral Reef Organisms. in Coral Reefs in the Anthropocene (ed. Birkeland, C.) 147–178 (Springer Netherlands, 2015). 10.1007/978-94-017-7249-5_8.
- 6.Weil, E. Coral Reef Diseases in the Wider Caribbean. in Coral Health and Disease (eds. Rosenberg, E. & Loya, Y.) 35–68 (Springer, 2004). 10.1007/978-3-662-06414-6_2.
- 7.Harvell CD, et al. Coral diseases, Environmental drivers and the balance between corals and microbial associates. Oceanography. 2007;20:172–195. doi: 10.5670/oceanog.2007.91. [DOI] [Google Scholar]
- 8.Lessios HA, Robertson DR, Cubit JD. Robertson, D. R. & Cubit, J. D. Spread of Diadema mass mortality through the Caribbean. Science. 1984;226:335–337. doi: 10.1126/science.226.4672.335. [DOI] [PubMed] [Google Scholar]
- 9.Perry CT, Alvarez-Filip L. Changing geo-ecological functions of coral reefs in the Anthropocene. Funct. Ecol. 2019;33:976–988. [Google Scholar]
- 10.Aronson RB, Precht WF. White-band disease and the changing face of Caribbean coral reefs. in. Hydrobiologia. 2001;460:25–38. doi: 10.1023/A:1013103928980. [DOI] [Google Scholar]
- 11.Alvarez-Filip, L., Dulvy, N. K., Gill, J. a, Côté, I. M. & Watkinson, A. R. Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proceedings. Biological sciences / The Royal Society276, 3019–3025 10.1098/rspb.2009.0339 (2009). [DOI] [PMC free article] [PubMed]
- 12.Estrada-Saldívar N, Jordán-Dahlgren E, Rodriguez-Martinez RE, Perry CT, Alvarez-Filip L. Functional consequences of the long-term decline of reef-building corals in the Caribbean: evidence of across-reef functional convergence. R. Soc. Open Sci. 2019;6:1–15. doi: 10.1098/rsos.190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cramer, K. L. et al. Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci. Adv.610.1126/sciadv.aax9395 (2020). [DOI] [PMC free article] [PubMed]
- 14.Bruno JF, et al. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 2007;5:1220–1227. doi: 10.1371/journal.pbio.0050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vega Thurber R, et al. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob. Change Biol. 2014;20:544–554. doi: 10.1111/gcb.12450. [DOI] [PubMed] [Google Scholar]
- 16.Wear SL, Thurber RV. Sewage pollution: Mitigation is key for coral reef stewardship. Ann. N. Y. Acad. Sci. 2015;1355:15–30. doi: 10.1111/nyas.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randall CJ, Van Woesik R. Some coral diseases track climate oscillations in the Caribbean. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-05763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lapointe, B. E., Brewton, R. A., Herren, L. W., Porter, J. W. & Hu, C. Nitrogen enrichment, altered stoichiometry, and coral reef decline at Looe Key, Florida Keys, USA: a 3 ‑ decade study. Marine Biology166, (Springer Berlin Heidelberg, 2019) 10.1007/s00227-019-3538-9.
- 19.Precht WF, Gintert BE, Robbart ML, Fura R, van Woesik R. Unprecedented disease-related coral mortality in Southeastern Florida. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer, P. R., Roth, L. & Lang, J. Map of Coral Cover of Susceptible Coral Species to SCTLD. (2020). Available at: www.agrra.org. ArcGIS Online.
- 21.Aeby GS, et al. Pathogenesis of a tissue loss disease affecting multiple species of corals along the Florida Reef Tract. Front. Mar. Sci. 2019;6:1–18. doi: 10.3389/fmars.2019.00678. [DOI] [Google Scholar]
- 22.Landsberg JH, et al. Stony coral tissue loss disease in Florida is associated with disruption of Host–Zooxanthellae Physiology. Front. Mar. Sci. 2020;7:1–24. doi: 10.3389/fmars.2020.576013. [DOI] [Google Scholar]
- 23.Work TM, et al. Viral-like particles are associated with endosymbiont pathology in Florida Corals affected by stony coral tissue loss disease. Front. Mar. Sci. 2021;8:1–18. doi: 10.3389/fmars.2021.750658. [DOI] [Google Scholar]
- 24.Alvarez-Filip L, Estrada-Saldívar N, Pérez-Cervantes E, Molina-Hernández A, González-Barrios FJ. A rapid spread of the Stony Coral Tissue Loss Disease outbreak in the Mexican Caribbean. PeerJ. 2019 doi: 10.7717/peerj.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gintert BE, et al. Regional coral disease outbreak overwhelms impacts from local dredge project. Environ. Monit. Assess. 2019;191:1–39. doi: 10.1007/s10661-019-7767-7. [DOI] [PubMed] [Google Scholar]
- 26.Muller EM, Sartor C, Alcaraz NI, van Woesik R. Spatial epidemiology of the stony-coral-tissue-loss disease in Florida. Front. Mar. Sci. 2020;7:11. doi: 10.3389/fmars.2020.00011. [DOI] [Google Scholar]
- 27.Estrada-Saldívar N, Quiroga-García BA, Pérez-Cervantes E, Rivera-Garibay OO, Alvarez-Filip L. Effects of the stony coral tissue loss disease outbreak on coral communities and the benthic composition of cozumel reefs. Front. Mar. Sci. 2021;8:1–13. doi: 10.3389/fmars.2021.632777. [DOI] [Google Scholar]
- 28.Neely, K. L., Lewis, C. L., Lunz, K. & Kabay, L. Rapid population decline of the Pillar coral Dendrogyra cylindrus along the Florida Reef Tract. Front. Mar. Sci.810.3389/fmars.2021.656515 (2021).
- 29.Rippe JP, Kriefall NG, Davies SW, Castillo KD. Differential disease incidence and mortality of inner and outer reef corals of the upper Florida Keys in association with a white syndrome outbreak. Bull. Mar. Sci. 2019;95:305–316. doi: 10.5343/bms.2018.0034. [DOI] [Google Scholar]
- 30.Sharp WC, Shea CP, Maxwell KE, Muller EM, Hunt JH. Evaluating the small-scale epidemiology of the stony-coral -tissue-loss-disease in the middle. PLOS ONE. 2020;15:1–25. doi: 10.1371/journal.pone.0241871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estrada-Saldívar N, et al. Reef-scale impacts of the stony coral tissue loss disease outbreak. Coral Reefs. 2020 doi: 10.1007/s00338-020-01949-z. [DOI] [Google Scholar]
- 32.González-Barrios FJ, Cabral-Tena RA, Alvarez-Filip L. Recovery disparity between coral cover and the physical functionality of reefs with impaired coral assemblages. Glob. Change Biol. 2021;27:640–651. doi: 10.1111/gcb.15431. [DOI] [PubMed] [Google Scholar]
- 33.McWilliam M, et al. Biogeographical disparity in the functional diversity and redundancy of corals. Proc. Natl Acad. Sci. 2018;115:3084–3089. doi: 10.1073/pnas.1716643115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suchley A, Alvarez-Filip L. Local human activities limit marine protection efficacy on Caribbean coral reefs. Conserv. Lett. 2018;11:1–9. doi: 10.1111/conl.12571. [DOI] [Google Scholar]
- 35.Hernández-Terrones L, et al. Groundwater Pollution in a Karstic Region (NE Yucatan): baseline nutrient content and flux to coastal ecosystems. Water Air Soil Pollut. 2011;218:517–528. doi: 10.1007/s11270-010-0664-x. [DOI] [Google Scholar]
- 36.Cejudo, E., Acosta-González, G., Ortega-Camacho, D. & Ventura-Sanchez, K. Water quality in natural protected areas in Cancun, Mexico: A historic perspective for decision makers. Reg Stud Mar Sci4810.1016/j.rsma.2021.102035 (2021).
- 37.Iwanowicz DD, et al. Explor. Stony Coral Tissue Loss Dis. Bact. Pathobiome. 2020 doi: 10.1017/CBO9781107415324.004. [DOI] [Google Scholar]
- 38.Studivan MS, et al. Reef sediments can act as a stony coral tissue loss disease vector. Front. Mar. Sci. 2022;8:1–15. doi: 10.3389/fmars.2021.815698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruno JF, Petes LE, Harvell CD, Hettinger A. Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 2003;6:1056–1061. doi: 10.1046/j.1461-0248.2003.00544.x. [DOI] [Google Scholar]
- 40.Aeby GS, et al. Changing stony coral tissue loss disease dynamics through time in Montastraea cavernosa. Front. Mar. Sci. 2021;8:1–13. doi: 10.3389/fmars.2021.699075. [DOI] [Google Scholar]
- 41.Perry CT, et al. Regional-scale dominance of non-framework building corals on Caribbean reefs affects carbonate production and future reef growth. Glob. Change Biol. 2015;21:1153–1164. doi: 10.1111/gcb.12792. [DOI] [PubMed] [Google Scholar]
- 42.Toth, L. T. et al. The unprecedented loss of Florida’s reef‐building corals and the emergence of a novel coral‐reef assemblage. Ecology e02781. 10.1002/ecy.2781 (2019). [DOI] [PMC free article] [PubMed]
- 43.Alves C, et al. Twenty years of change in benthic communities across the Belizean Barrier Reef. PLoS ONE. 2022;17:1–36. doi: 10.1371/journal.pone.0249155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruno JF. Implications for reef restoration efforts. Science. 2014;345:879–880. doi: 10.1126/science.1258556. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez-Martínez RE, Banaszak AT, McField MD, Beltrán-Torres A, Alvarez-Filip L. Assessment of Acropora palmata in the mesoamerican reef system. PLoS ONE. 2014;9:1–7. doi: 10.1371/journal.pone.0096140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mudge L, Alves C, Figueroa-Zavala B, Bruno JF. Assessment of Elkhorn coral populations and associated herbivores in Akumal, Mexico. Front. Mar. Sci. 2019;6:1–12. doi: 10.3389/fmars.2019.00683. [DOI] [Google Scholar]
- 47.Baums IB, Miller MW, Hellberg ME. Regionally isolated populations of an imperiled Caribbean coral, Acropora palmata. Mol. Ecol. 2005;14:1377–1390. doi: 10.1111/j.1365-294X.2005.02489.x. [DOI] [PubMed] [Google Scholar]
- 48.Perry CT, et al. Changing dynamics of Caribbean reef carbonate budgets: emergence of reef bioeroders as critical controls on present and future reef growth potential. Proc. R. Soc. B: Biol. Sci. 2014;281:20142018–20142018. doi: 10.1098/rspb.2014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molina-Hernández, A., González-Barrios, F. J., Perry, C. T. & Álvarez-Filip, L. Two decades of carbonate budget change on shifted coral reef assemblages: Are these reefs being locked into low net budget states?: Caribbean reefs carbonate budget trends. Proc. Royal Soc. B: Biol. Sci.287. 10.1098/rspb.2020.2305rspb20202305 (2020). [DOI] [PMC free article] [PubMed]
- 50.Perry CT, et al. Loss of coral reef growth capacity to track future increases in sea level. Nature. 2018;558:396–400. doi: 10.1038/s41586-018-0194-z. [DOI] [PubMed] [Google Scholar]
- 51.Enochs IC, et al. Ocean acidification enhances the bioerosion of a common coral reef sponge: Implications for the persistence of the Florida Reef Tract. Bull. Mar. Sci. 2015;91:271–290. doi: 10.5343/bms.2014.1045. [DOI] [Google Scholar]
- 52.Veron J, Stafford-Smith M, DeVantier L, Turak E. Overview of distribution patterns of zooxanthellate Scleractinia. Front. Mar. Sci. 2015;2:1–19. [Google Scholar]
- 53.Miller MW, Lohr KE, Cameron CM, Williams DE, Peters EC. Disease dynamics and potential mitigation among restored and wild staghorn coral, Acropora cervicornis. PeerJ. 2014;2014:1–30. doi: 10.7717/peerj.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes AR, Stachowicz JJ. Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc. Natl Acad. Sci. USA. 2004;101:8998–9002. doi: 10.1073/pnas.0402642101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sokolow SH. Effects of a changing climate on the dynamics of coral infectious disease: A review of the evidence. Dis. Aquat. Org. 2009;87:5–18. doi: 10.3354/dao02099. [DOI] [PubMed] [Google Scholar]
- 56.Bellwood DR, et al. Coral reef conservation in the Anthropocene: Confronting spatial mismatches and prioritizing functions. Biol. Conserv. 2019;236:604–615. doi: 10.1016/j.biocon.2019.05.056. [DOI] [Google Scholar]
- 57.Grosso-Becerra MV, Mendoza-Quiroz S, Maldonado E, Banaszak AT. Cryopreservation of sperm from the brain coral Diploria labyrinthiformis as a strategy to face the loss of corals in the Caribbean. Coral Reefs. 2021;40:937–950. doi: 10.1007/s00338-021-02098-7. [DOI] [Google Scholar]
- 58.Edmunds PJ. Long-term dynamics of coral reefs in St. John, US Virgin Islands. Coral Reefs. 2002;21:357–367. doi: 10.1007/s00338-002-0258-1. [DOI] [Google Scholar]
- 59.Vermeij MJA. Early life-history dynamics of Caribbean coral species on artificial substratum: The importance of competition, growth and variation in life-history strategy. Coral Reefs. 2006;25:59–71. doi: 10.1007/s00338-005-0056-7. [DOI] [Google Scholar]
- 60.Webster FJ, Babcock RC, Van Keulen M, Loneragan NR. Macroalgae inhibits larval settlement and increases recruit mortality at Ningaloo Reef, Western Australia. PLoS ONE. 2015;10:1–14. doi: 10.1371/journal.pone.0124162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suchley A, McField MD, Alvarez-Filip L. Rapidly increasing macroalgal cover not related to herbivorous fishes on Mesoamerican reefs. PeerJ. 2016;4:e2084. doi: 10.7717/peerj.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Contreras-Silva, A. et al. A meta-analysis to assess long-term spatiotemporal changes of benthic coral and macroalgae cover in the Mexican caribbean. 1–12. 10.1038/s41598-020-65801-8 (2020). [DOI] [PMC free article] [PubMed]
- 63.Meiling SS, Muller EM, Smith TB, Brandt ME. 3D photogrammetry reveals dynamics of stony coral tissue loss disease (SCTLD) Lesion progression across a thermal stress event. Front. Mar. Sci. 2020;7:1–12. doi: 10.3389/fmars.2020.597643. [DOI] [Google Scholar]
- 64.Espinosa-Andrade, N., Suchley, A., Reyes-Bonilla, H. & Alvarez-Filip, L. The no-take zone network of the Mexican Caribbean: assessing design and management for the protection of coral reef fish communities. Biodivers. Conserv.10.1007/s10531-020-01966-y (2020).
- 65.Lang, J. C., Marks, K. W., Kramer, P. R., Kramer, P. A. & Ginsburg, R. N. AGRRA Protocols. Version 5.5. The Atlantic and Gulf Rapid Reef Assessment (AGRRA) Program. (2012).
- 66.Burke L, Reytar K, Spalding M, Perry A. Reefs risk. Natl Geographic. 2011 doi: 10.1016/0022-0981(79)90136-9. [DOI] [Google Scholar]
- 67.Chollett I, Müller-Karger FE, Heron SF, Skirving W, Mumby PJ. Seasonal and spatial heterogeneity of recent sea surface temperature trends in the Caribbean Sea and southeast Gulf of Mexico. Mar. Pollut. Bull. 2012;64:956–965. doi: 10.1016/j.marpolbul.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 68.Cox C, Valdivia A, McField M, Castillo K, Bruno JF. Establishment of marine protected areas alone does not restore coral reef communities in Belize. Mar. Ecol. Prog. Ser. 2017;563:65–79. doi: 10.3354/meps11984. [DOI] [Google Scholar]
- 69.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 70.Core Team, R. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: URLhttps://www.R-project.org/ (2020).
- 71.Hughes TP, et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science. 2018;359:80–83. doi: 10.1126/science.aan8048. [DOI] [PubMed] [Google Scholar]
- 72.Riddle D. Coral reproduction, part one: A natural coral spawning in Hawaii, The cauliflower coral (Pocillopora meandrina) Adv. Aquarist’s Online Mag. 2008;7:10–16. [Google Scholar]
- 73.Madin JS, et al. The Coral Trait Database, a curated database of trait information for coral species from the global oceans. Sci. Data. 2016;3:160017. doi: 10.1038/sdata.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McWilliam, M., Pratchett, M. S., Hoogenboom, M. O. & Hughes, T. P. Deficits in functional trait diversity following recovery on coral reefs. Proc. Royal Soc. B: Biol. Sci.287. 10.1098/rspb.2019.2628 (2020). [DOI] [PMC free article] [PubMed]
- 75.Hughes TP, et al. Global warming transforms coral reef assemblages. Nature. 2018 doi: 10.1038/s41586-018-0041-2. [DOI] [PubMed] [Google Scholar]
- 76.Oksanen, J. Vegan: community ecology package version 1.8-6. http://cran.r-project.org (2007).
- 77.Maechler, M. et al. Cluster: Cluster Analysis Basics and Extensions. R package version 2.1.3. https://CRAN.R-project.org/package=cluster (2022).
- 78.CLARKE KR. Non‐parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993;18:117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x. [DOI] [Google Scholar]
- 79.Clarke, K. R. & Warwick, R. M. Change in marine communities: an approach to statistical analysis and interpretation. 2nd edition. Primer-E, Plymouth. Plymouth, United Kingsom: PRIMER-E 172 (2001).
- 80.Lavorel S, et al. Assessing functional diversity in the field - Methodology matters! Funct. Ecol. 2008;22:134–147. [Google Scholar]
- 81.Ricotta C, Moretti M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia. 2011;167:181–188. doi: 10.1007/s00442-011-1965-5. [DOI] [PubMed] [Google Scholar]
- 82.Villéger S, Mason NWH, Mouillot D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology. 2008;89:2290–2301. doi: 10.1890/07-1206.1. [DOI] [PubMed] [Google Scholar]
- 83.Denis V, Ribas-Deulofeu L, Sturaro N, Kuo CY, Chen CA. A functional approach to the structural complexity of coral assemblages based on colony morphological features. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-10334-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teixidó N, et al. Teixidó, N. et al. Functional biodiversity loss along natural CO2 gradients. Nat. Commun. 2018;9:1–9. doi: 10.1038/s41467-018-07592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laliberté, E. et al. FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1.0-12. (2014). [DOI] [PubMed]
- 86.González-Barrios FJ, Alvarez-Filip L. A framework for measuring coral species-specific contribution to reef functioning in the Caribbean. Ecol. Indic. 2018;95:877–886. doi: 10.1016/j.ecolind.2018.08.038. [DOI] [Google Scholar]
- 87.Patterson KL, et al. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc. Natl Acad. Sci. 2002;99:8725–8730. doi: 10.1073/pnas.092260099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edmunds PJ, Elahi R. The demographics of a 15-year decline in covers of the Caribbean reef coral Montastraea annularis. Ecol. Monogr. 2007;77:3–18. doi: 10.1890/05-1081. [DOI] [Google Scholar]
- 89.Eakin, C. M. et al. Caribbean corals in crisis: Record thermal stress, bleaching, and mortality in 2005. PLoS ONE5. 10.1371/journal.pone.0013969 (2010). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data are available in the main text or in Supplementary Data 1 and 2.