Abstract
Background
The COVID‐19 pandemic has necessitated the rapid and widespread adoption of novel mechanisms of service delivery, including the use of telemedicine. The aim of this study was to examine the impact of COVID‐19 on cardiogenetics practices.
Methods
We retrospectively analyzed the clinical characteristics of patients who were seen for cardiogenetics visits pre‐pandemic (1 April–23 December 2019) and during the pandemic (1 April–23 December 2020) at Columbia University Irving Medical Center.
Results
Six percent (n = 6) of visits in 2019 were remote telemedicine encounters, whereas 80% (n = 106) of visits in 2020 were telemedicine encounters. In 2019, only 18% (n = 19) of the patients seen for genetic counseling were family members of probands; this percentage increased to 34% in 2020 (n = 45; p = .01). In 2020, the geographic reach of genetic counseling also extended far beyond New York State, reaching a total of 11 states as well as one patient in Puerto Rico. Genetic testing results were similar in 2019 and 2020.
Conclusion
Despite the health‐care delivery barriers created by the COVID‐19 pandemic, the use of telemedicine allowed us to expand the reach of cardiovascular genetic counseling and testing.
Keywords: cardiogenetics, telegenetics, telemedicine
The aim of this study was to examine the impact of COVID‐19 on cardiogenetics practices. We found that despite the health care delivery barriers created by the COVID‐19 pandemic, the use of telemedicine allowed us to not only continue seeing patients for cardiogenetics visits but also to expand the reach of cardiovascular genetic counseling and testing beyond the geographical boundaries of our previous catchment area.
1. BACKGROUND
Coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has now affected over 300 million people worldwide, with over 5 million fatalities (COVID‐19 Map ‐ Johns Hopkins Coronavirus Resource Center, n.d.). This unprecedented global pandemic has significantly impacted the access, coverage, quality, and safety of health‐care services (Blumenthal et al., 2020). Due to high rates of transmission, as well as high morbidity and mortality (Zhou et al., 2020), COVID‐19 has necessitated the rapid and widespread adoption of novel mechanisms of service delivery and workforce mobilization across all health‐care systems. As cases began to rise in the United States, there was a simultaneous prioritization of COVID‐related clinical care and research initiatives and a delay of non‐essential consultations and procedures (Keesara et al., 2020; Sayer et al., 2020). Additionally, to protect the health and well‐being of patients, hospitals recommended a delay of in‐person care for high‐risk groups (Santoli et al., 2020). These modifications in clinical prioritization impacted patients in all sectors of care but, in particular, those with cardiovascular disease who oftentimes require close clinical monitoring (Czeisler et al., 2020; DeFilippis et al., 2020).
In order to adjust to the restrictions of the pandemic, health‐care providers adopted telemedicine as a means to provide support and care to patients (Mann et al., 2020). In the field of genetic counseling specifically, telegenetics, or the use of technological platforms to deliver genetic services, became more commonplace during the pandemic (Gray et al., 2000; Wosik et al., 2020). Previously, telegenetics was predominantly used for patients in remote areas with a limited genetics health workforce (Bergstrom et al., 2020; Buchanan et al., 2016). However, COVID‐19 highlighted the unprecedented opportunities through which to expand the reach of genetic counseling services via telegenetics.
Within the context of a global pandemic, this platform for care delivery offers a safer option for patients and providers by reducing potential infectious exposures. Telegenetics also reduces geographical barriers, which are particularly relevant in the uptake of cascade screening in family members who may not live locally (Srinivasan et al., 2020). In addition, the ability to attend a consultation remotely allows patients the flexibility to reduce their time away from other responsibilities such as work or childcare and may increase participation for those who are medically or socially vulnerable or who do not have ready access to providers (Bashshur et al., 2020; Mahon, 2020). Conversely, there are also a number of barriers to implementing telegenetics in clinical practice, including interstate licensing restrictions for health‐care providers, reimbursement limitations, and technology‐related issues (Bhate et al., 2020; Gadzinski et al., 2020; Wilson et al., 2017).
While many clinics have re‐established in‐person clinical encounters, telegenetics is likely to continue to play an integral role in the future of health service delivery (Sayer et al., 2020; Wosik et al., 2020). Since there is limited information about the utility of telegenetics in clinical practice, this study aimed to characterize the impact of telegenetics during the 2020 pandemic within the cardiogenetics clinical practices at a large tertiary referral center in New York City.
2. METHODS
2.1. Study design and sample
We retrospectively collected demographic and clinical characteristics of adult patients (age ≥ 18 years) who were seen for cardiovascular genetic counseling visits between 1 April 2019 to 23 December 2019 (267 days) and 1 April 2020 to 23 December 2020 (267 days) at Columbia University Irving Medical Center (CUIMC; New York, NY). We chose 1 April as the starting date in 2020 given that cardiovascular genetic counseling visits were on hold at our institution in February and March due to the rapid spread of the COVID‐19 pandemic in New York City and only re‐initiated in April. We then chose 1 April as the starting date in 2019 to create the closest comparison group. The focus of the study was on patients who underwent genetic testing, as data were not uniformly available on patients who underwent genetic counseling but did not pursue genetic testing across all providers. The CUIMC Institutional Review Board approved the study protocol, with a waiver of consent for retrospective data review.
2.2. Cardiogenetics visits
The same providers saw patients for cardiogenetics visits in 2019 and 2020. M.W. saw patients in the electrophysiology clinic, F.L. and N.U. saw patients in the heart failure clinic, and M.R. saw patients in the preventative cardiology clinic. I.K. saw patients in all three cardiogenetics clinics as a cardiovascular genetic counselor. Notably, since New York State does not currently license genetic counselors, I.K. did not bill for services; this practice was the same in 2019 and 2020 (Roberts et al., 2017). M.W., F.L., N.U., and M.R. are physicians licensed in New York State. They did not obtain licensure in other states for telegenetics visits given the waiver of the requirement during the COVID‐19 pandemic (Slomski, 2020). In‐person visits were conducted over 60 min on‐site at CUIMC. In‐person visits with M.W., F.L., N.U., and M.R. included full physical examinations. Telegenetics visits were also allotted 60 min and were conducted via telephone from 1 April 2020 to 5 May 2020. After 5 May 2020, video visits became available via institutional patient portals and were the preferred method for telegenetics visits unless precluded by Internet instability or technical difficulties. In all cases, the decision to pursue genetic testing was based on patient–provider shared decision‐making. Genetic testing results were disclosed to patients via telephone, video, or in‐person consults. Patients were provided with their results and a consult letter through electronic correspondence. Additional information in the form of patient educational material and referrals was provided per individual patient's request. Standards for the Reporting of Genetic Counseling Interventions in Research and Other Studies checklists are included in Supplemental Tables S1 and S2 for in‐person and telegenetics visits, respectively (Hooker et al., 2017).
Both in‐person and remote genetic counseling appointments included content pertaining to the indication for the appointment as well as educational and psychotherapeutic content. Additionally, the providers (physician and genetic counselor) involved in both service delivery models were consistent. During in‐person encounters, the providers jointly delivered care to patients; however, during remote appointments, the physician and counselor provided care to patients on different days. The genetic counselor would conduct genetic counseling appointments prior to the physician appointment to collect medical history, construct a pedigree, and discuss the genetic testing process. This information would inform discussions during the remote appointment with the physician.
Remote and in‐person appointments also differed in that educational tools such as a flip‐book, which were made available during in‐person encounters but were not utilized in remote sessions. Additionally, while in‐person appointments routinely took place in a private office location, remote appointments were at the discretion of the patient. Therefore, appointments could occur within various settings such as the home, car, or office, and the element of privacy was in the control of the patient.
2.3. Genetic counseling outcome measures
Genetic testing results were recorded for each encounter when available. Variants categorized as “definitely pathogenic” or “likely pathogenic” were considered positive in the analysis. Variants classified as “likely benign” or “benign” were considered negative. “Variants of uncertain significance” (VUS) were separately coded. All patients in the study were offered genetic testing using commercial gene panels from Clinical Laboratory Improvement Amendments‐approved laboratories. The particular testing kit was chosen based on indication for testing, patient and provider preference, and insurance coverage. Genetic testing kits were mailed to patients seen via telemedicine visits. If patients were sent a testing kit but did not return their sample, this was noted as a separate outcome (“not returned”).
2.4. Indication for genetic testing
Indication for genetic testing was extracted from an electronic medical record chart review. Patients were organized into the following 10 categories: abnormal electrocardiogram, abnormal lipids, aortic pathology, connective tissue disease, familial pathogenic variant, family history of sudden cardiac arrest, muscular dystrophy, nonischemic cardiomyopathy, sudden cardiac arrest, and syncope. Abnormal electrocardiogram included patients with suspected Brugada syndrome (Juang & Horie, 2016) and long QT syndrome (Yunis & Bhonsale, 2020). Abnormal lipids included patients undergoing testing due to profound hyperlipidemia or hypertriglyceridemia (Naukkarinen et al., 2006). Aortic pathology included patients with a history of aortic aneurysm, aortic dilatation, or aortic dissection (Pinard et al., 2019). Connective tissue disease included patients with suspected Marfan syndrome and Vascular Ehlers‐Danlos syndrome (Murphy‐Ryan et al., 2010). Patients being tested due to a familial pathogenic variant were grouped together in one category, regardless of the underlying variant. Patients being tested for nonischemic cardiomyopathy were subdivided into hypertrophic cardiomyopathy (Maron et al., 2012), dilated cardiomyopathy (Kärkkäinen & Peuhkurinen, 2007; Tayal et al., 2017), and other nonischemic cardiomyopathies. Other nonischemic cardiomyopathies (Marcus et al., 2013) included patients with arrhythmogenic right ventricular cardiomyopathy as well as patients with cardiac amyloidosis (Maurer et al., 2016).
2.5. Data analysis
All statistical analyses were performed using R (version 4.0.2). Values for continuous characteristics were presented as mean ± standard deviation for normally distributed variables or median [interquartile range] for non‐normal distributions. Univariable comparison of baseline characteristics between patients seen in 2019 and those seen in 2020 was made using the chi‐squared test, Fisher exact test, Student's t‐test, or the Kruskal–Wallis test where appropriate. p value of < .05 was considered statistically significant. Adjustments were not made for multiple comparisons given the exploratory nature of the study.
The map of genetic testing was created using the usmap and maps packages in R by plotting patient zip codes to their corresponding latitude and longitude (Lorenzo & Maintainer, 2020; Package “maps” Title Draw Geographical Maps, 2018). The “distance” variable was calculated using the sp package in R to find the great circle distance (orthodromic/spherical distance) in kilometers between the coordinates of CUIMC and the coordinates of each patient's zip code (Package “sp” Title Classes and Methods for Spatial Data, 2020).
3. RESULTS
3.1. Patient demographics
Overall, 104 patients had a cardiovascular genetic counseling visit for genetic testing in 2019 compared to 132 patients in 2020 (Table 1). Only 6% (n = 6) of visits in 2019 were remote telegenetics encounters, whereas 80% (n = 106) of visits in 2020 were telegenetics encounters. Of the six telegenetics encounters in 2019, three were for family members of a patient with familial hypercholesterolemia and two were for family members of a patient with genotype‐positive hypertrophic cardiomyopathy. The mean age was not significantly different between 2019 and 2020 (p = .54), although qualitatively the distribution of ages differed (Figure 1), with the highest density of ages clustered around 60 years of age in 2019 compared to 40 years of age in 2020. There was no significant difference in race/ethnicity of patients seen in 2019 versus 2020 (p = .65).
TABLE 1.
Characteristics of patients who underwent genetic counseling by year
| Characteristics a | 2019 | 2020 | p value |
|---|---|---|---|
| n = 104 | n = 132 | ||
| Age (years) | 48 ± 19 | 49 ± 17 | .54 |
| Male (%) | 60 (58) | 72 (55) | .93 |
| Self‐reported race and ethnicity (%) | .65 | ||
| White, non‐Hispanic | 46 (44) | 54 (41) | – |
| Black, non‐Hispanic | 7 (7) | 12 (9) | – |
| Hispanic/Latino | 20 (19) | 37 (28) | – |
| Asian, non‐Hispanic | 4 (4) | 5 (4) | – |
| Other, non‐Hispanic | 4 (4) | 9 (7) | – |
| Telemedicine (%) | 6 (6) | 106 (80) | <.001 |
| Family member of proband (%) | 19 (18) | 45 (34) | .01 |
| Distance from CUIMC (km) b | 17 [5–42] | 19 [5–60] | .18 |
| State of residence (%) | .01 | ||
| New York | 82 (79) | 85 (64) | – |
| New Jersey | 18 (17) | 27 (20) | – |
| Connecticut | 4 (4) | 1 (1) | – |
| Florida | 0 | 7 (5) | – |
| Virginia | 0 | 3 (2) | – |
| Maryland | 0 | 2 (2) | – |
| California | 0 | 2 (2) | – |
| Massachusetts | 0 | 1 (1) | – |
| Michigan | 0 | 1 (1) | – |
| Pennsylvania | 0 | 1 (1) | – |
| Georgia | 0 | 1 (1) | – |
| Puerto Rico | 0 | 1 (1) | – |
Abbreviation: CUIMC, Columbia University Irving Medical Center.
Data were expressed as number (percentage), mean ± standard deviation, or median [interquartile range].
Distance calculated as great circle distance (orthodromic/spherical distance) in kilometers between the coordinates of CUIMC and the coordinates of patient zip codes.
FIGURE 1.
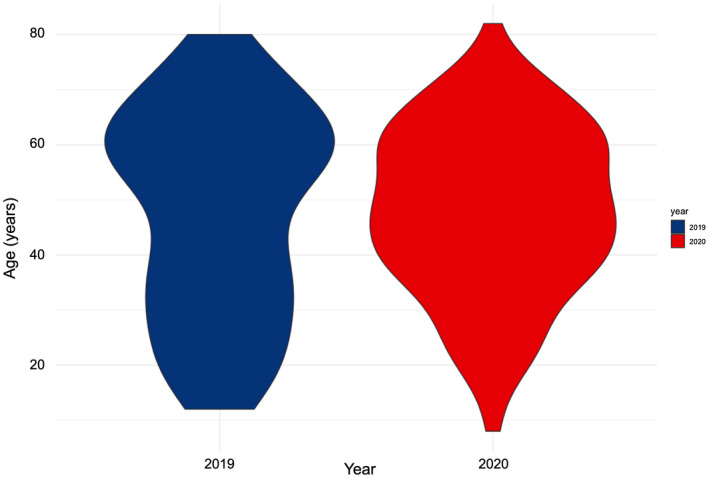
Distribution of age by year: Density plot of age in 2019 (blue) and 2020 (red)
Notably, there was a significant increase in the number of family members seen for genetic counseling in 2020 (Figure 2); in 2019, only 18% (n = 19) of the patients seen for genetic counseling were family members of probands, whereas this percentage increased to 34% in 2020 (n = 45; p = .01). In addition, in 2020, the geographic reach of genetic counseling extended far beyond New York State, reaching a total of 11 states as well as one patient in Puerto Rico (Figure 3). Although the distance between patient home address coordinates and CUIMC coordinates did not differ significantly between 2019 and 2020 (p = .18), the difference in states reached did achieve significance (p = .01).
FIGURE 2.
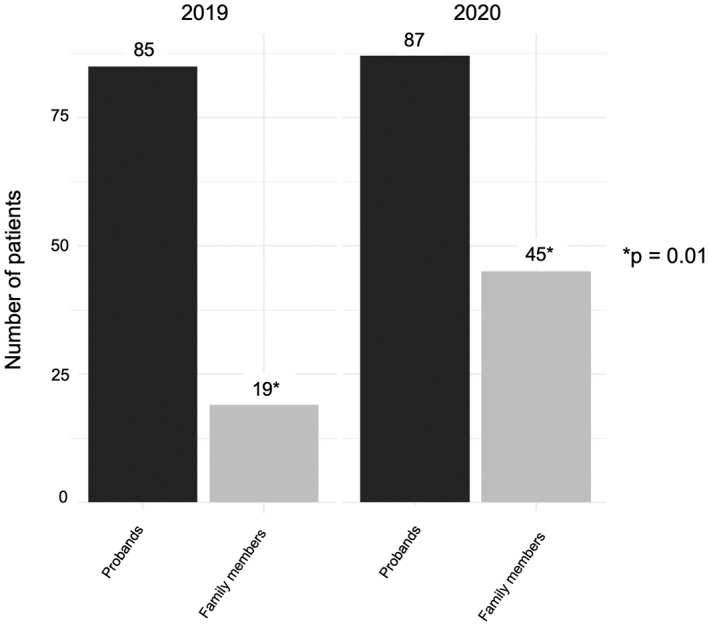
Proband versus family member testing by year: Number of patients who were probands (black) versus family members (gray) in 2019 and 2020
FIGURE 3.
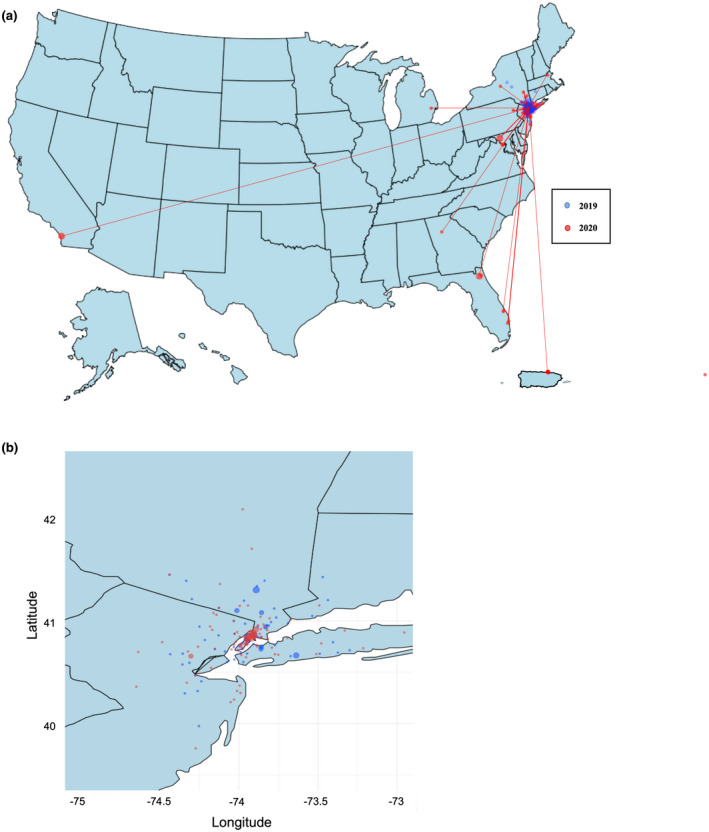
Geographic distribution of genetic counseling visits by year. (a) U.S. Map: Zip codes of patients seen for genetic counseling in 2019 (blue) and 2020 (red) plotted by latitude and longitude. (b) New York and surrounding states: Zip codes of patients seen for genetic counseling within the tri‐state area (longitude −75 to −73 and latitude 40–42) in 2019 (blue) and 2020 (red)
One provider (M.W.) did have data on all patients who underwent genetic counseling, including those who did not undergo genetic testing, and this information is reported in Table 2. Similar trends were seen in this clinic, with an increase in family members seen in 2020 (n = 8 [17%]) compared to 2019 (n = 3 [6%]), though this did not reach significance in this smaller cohort (p = .12). Genetic testing rates were comparable in 2019 (n = 40 [85%]) and 2020 (n = 40 [83%]) in this clinic (p = .81).
TABLE 2.
Characteristics of patients who underwent genetic counseling by year in the cardiogenetics electrophysiology clinic
| Characteristics | 2019 | 2020 | p value |
|---|---|---|---|
| n = 47 | n = 48 | ||
| Age (years) | 56 ± 18 | 54 ± 14 | .53 |
| Male (%) | 26 (55) | 31 (65) | .36 |
| Self‐reported race and ethnicity (%) | .69 | ||
| White, non‐Hispanic | 46 (44) | 32 (67) | – |
| Black, non‐Hispanic | 2 (4) | 3 (6) | – |
| Hispanic/Latino | 9 (19) | 11 (23) | – |
| Asian, non‐Hispanic | 5 (11) | 2 (4) | – |
| Other, non‐Hispanic | 0 (0) | 0 (0) | – |
| Telemedicine (%) | 0 (0) | 48 (100) | <.001 |
| Family member of proband (%) | 3 (6) | 8 (17) | .12 |
| State of residence (%) | .21 | ||
| New York | 38 (81) | 29 (60) | |
| New Jersey | 8 (17) | 14 (29) | |
| Connecticut | 1 (2) | 2 (4) | |
| Florida | 0 | 1 (2) | |
| Maryland | 0 | 1 (2) | |
| Massachusetts | 0 | 1 (2) | |
| Genetic testing (%) | 40 (85) | 40 (83) | .81 |
3.2. Genetic testing results
Genetic testing results were similar between the 2 years with 29% of patients testing positive for a pathogenic variant in 2019 and 28% of patients testing positive in 2020 (p = .91; Table 3). These findings were similar to what has been previously reported in the literature (Alfares et al., 2015; Bai et al., 2009; Seidelmann et al., 2017). For patients receiving genetic testing due to a family history of a genetic cardiovascular disease, detection of pathogenic variants was predictably higher, though the difference did not reach statistical significance (47% in 2019 and 40% in 2020, p = .31). Of those patients who underwent telegenetics visits and were sent genetic testing kits (n = 106), 14% (n = 15) did not return a sample. An additional three patients did return their samples, but genetic testing results were still pending at the end of the study period. Therefore, in 2020, only 114 patients were included in the analysis of genetic testing results as shown in Table 3. Indication for testing was significantly different between 2019 and 2020 (p < .001), with more patients being tested in the setting of familial pathogenic variants in 2020 compared to 2019.
TABLE 3.
Genetic testing results
| Characteristics a | 2019 | 2020 | p value |
|---|---|---|---|
| n = 104 | n = 114 | ||
| Testing Results (%) | .91 | ||
| Positive | 30 (29) | 32 (28) | – |
| Variant of uncertain significance | 34 (33) | 35 (31) | – |
| Negative | 40 (38) | 47 (41) | – |
| Indication for testing (%) | <.001 | ||
| Familial pathogenic variant | 19 (18) | 43 (38) | – |
| Nonischemic cardiomyopathy | |||
| Hypertrophic cardiomyopathy | 35 (34) | 15 (13) | – |
| Dilated cardiomyopathy | 4 (4) | 12 (11) | – |
| Other nonischemic cardiomyopathies | 8 (8) | 14 (12) | – |
| Abnormal lipids | 16 (15) | 7 (6) | – |
| Aortic pathology | 9 (11) | 9 (8) | – |
| Abnormal electrocardiogram | 4 (4) | 9 (8) | – |
| Sudden cardiac arrest | 4 (4) | 1 (1) | – |
| Connective tissue disease | 2 (2) | 0 (0) | – |
| Syncope | 1 (1) | 1 (1) | – |
| Family history of sudden cardiac arrest | 1 (1) | 2 (2) | – |
| Muscular dystrophy | 1 (1) | 1 (1) | – |
Data were expressed as number (percentage).
4. DISCUSSION
In this study, we found that despite the health‐care delivery barriers created by the COVID‐19 pandemic, the use of telemedicine allowed us to not only continue seeing patients for genetic counseling visits but also expand the reach of genetic counseling and testing far beyond the geographical boundaries of our previous catchment area. Removal of geographical constraints facilitated increased cascade screening of family members who may not otherwise have received genetic testing. In addition, this was also likely facilitated in large part by practice changes; telegenetics visits were not routinely offered to family members in 2019 but were routinely offered in 2020. This was the first study to quantitatively and qualitatively examine the impact of telemedicine on cardiovascular genetic counseling and testing during the COVID‐19 pandemic (Mahon, 2020; Pagliazzi et al., 2020). Our results support the ongoing use of telegenetics as a means to improve patient access to genetic counseling and testing services.
Although telemedicine was already established to some degree at our institution prior to the COVID‐19 pandemic, its use had been limited by lack of awareness and patient and provider unfamiliarity (Ohannessian et al., 2020). Many challenges still remain in the use of telegenetics consultations, including technology‐related issues, communication barriers, licensing, and reimbursement limitations (Terry et al., 2019). However, despite these drawbacks, prior studies have largely demonstrated high patient and provider satisfaction with the telemedicine format (Vrečar et al., 2017). Increasing familiarity with telegenetics and better integration within the health‐care system will allow for continued improvements to the patient–provider experience.
4.1. Cascade screening via telegenetics
One of the most notable findings of our study was the increased familial testing uptake in 2020. A recent systematic review of barriers and facilitators for cascade testing in genetic conditions identified a lack of accessibility to genetic testing as a major barrier to cascade testing of family members (Srinivasan et al., 2020). Our study results corroborate this finding, given the significant increase in cascade screening with the adoption of telegenetics. Other factors that were identified included attitudes, beliefs, and emotional responses relating to the individual and their relatives, which are less likely to be affected by the platform of care delivery.
4.2. Limitations of telegenetics
Our study also highlighted some of the limitations of utilizing telegenetics. During an in‐person patient encounter, obtaining a patient sample through either blood or saliva is fairly seamless. However, we experienced 14% of patients who received a saliva or buccal kit but failed to mail their kit to the genetic testing laboratory. The number of unreturned kits was the greatest for the month of December, as there was a limited amount of time for kits to be returned and included in the analysis of this study. In addition, there were major shipping service delays during this time period which may have further delayed the return of testing kits. Another difficulty of not having a health‐care professional at the time of obtaining a patient specimen is receiving a sample that does not meet the threshold for genetic testing, necessitating another kit to be sent to the patient. This could delay results that may impact clinical management. Moreover, we found that several older patients preferred to defer telegenetics visits in favor of obtaining in‐person visits, despite their higher risk for COVID‐19. This may have been due to a lower comfort level with telegenetics technology in older adults. Indeed, although the mean age was not significantly different between patients seen in 2019 and 2020 (p = .54), the distribution of ages did appear to differ, with the highest density of ages clustered around 60 years of age in 2019 compared to 40 years of age in 2020.
Physician providers were also unable to perform physical examinations on patients via telemedicine, which led to overall decreases in billing level and greater use of time‐based billing. In addition, 24 states including New York State do not currently license genetic counselors; this creates a lack of billing structure which can be a significant hurdle to expanding telegenetics in these states. For states that do offer licensure, requirements vary by state, and obtaining licensure from multiple states is costly in terms of both time and resources.
4.3. Study limitations
Additional limitations specific to our study include the retrospective nature of its design and its focus on only cardiogenetics. In addition, adjustments were not made for multiple comparisons in our statistical analyses. Notably, we also excluded some patients who were seen for genetic counseling but declined genetic testing or previously had genetic testing performed, which could have affected our analysis. For example, there may have been more patients seen in total for genetic counseling in 2019 than in 2020 who did not qualify for genetic testing, but this would not have been captured in our approach. However, based on our data from the electrophysiology clinic, for which we did have complete data on all patients who underwent genetic counseling, it seems that genetic testing percentages were similar between 2019 and 2020. Our study also did not address patient or provider satisfaction with the telegenetics format, which is an important consideration that should be addressed in future qualitative studies as telegenetics continues to expand. Additionally, we did not have information available regarding specific technical difficulties that may have been encountered during telegenetics visits. Finally, it was outside of the scope of this study to examine the downstream impacts of genetic testing results, including whether out‐of‐state patients with positive results were able to establish appropriate care with a local cardiology provider.
4.4. Conclusions
However, despite these limitations, our study demonstrated the potential of telegenetics to expand the reach of genetic counseling. Even beyond the context of the pandemic, telegenetics offers patients and clinicians convenience and flexibility and has likely earned its permanent place in clinical practice. Future efforts are warranted to study the longer term clinical impacts of telegenetics as well as to continue to improve telemedicine technology and standardize licensure of genetic counselors, with the ultimate aim of increasing patient access to personalized genomic medicine.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
LWL contributed to the study concept and design, extracted data, performed the statistical analysis, and wrote up the manuscript. IK contributed to the study concept and design, extracted data, helped interpret the results, and wrote up the manuscript. FL, MW, GS, YS, MR, and NU contributed to the study concept and design, helped interpret the results, and critically revised the manuscript. All authors approved the manuscript. Both LL and IK had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
HUMAN STUDIES AND INFORMED CONSENT
The CUIMC Institutional Review Board approved the study protocol, with a waiver of consent for retrospective data review.
Supporting information
Tables S1–S2
ACKNOWLEDGMENTS
None.
Liang, L. W. , Kalia, I. , Latif, F. , Waase, M. P. , Shimada, Y. J. , Sayer, G. , Reilly, M. P. & Uriel, N. (2022). The use of telemedicine in cardiogenetics clinical practice during the COVID‐19 pandemic. Molecular Genetics & Genomic Medicine, 10, e1946. 10.1002/mgg3.1946
Lusha W. Liang and Isha Kalia contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alfares, A. A. , Kelly, M. A. , McDermott, G. , Funke, B. H. , Lebo, M. S. , Baxter, S. B. , Shen, J. , McLaughlin, H. M. , Clark, E. H. , Babb, L. J. , Cox, S. W. , Depalma, S. R. , Ho, C. Y. , Seidman, J. G. , Seidman, C. E. , & Rehm, H. L. (2015). Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: Expanded panels offer limited additional sensitivity. Genetics in Medicine, 17(11), 880–888. 10.1038/gim.2014.205 [DOI] [PubMed] [Google Scholar]
- Bai, R. , Napolitano, C. , Bloise, R. , Monteforte, N. , & Priori, S. G. (2009). Yield of genetic screening in inherited cardiac channelopathies how to prioritize access to genetic testing. Circulation. Arrhythmia and Electrophysiology, 2(1), 6–15. 10.1161/CIRCEP.108.782888 [DOI] [PubMed] [Google Scholar]
- Bashshur, R. , Doarn, C. R. , Frenk, J. M. , Kvedar, J. C. , & Woolliscroft, J. O. (2020). Telemedicine and the COVID‐19 pandemic, lessons for the future. Telemedicine and E‐Health, 26(5), 571–573. 10.1089/tmj.2020.29040.rb [DOI] [PubMed] [Google Scholar]
- Bergstrom, K. L. , Brander, T. E. , Breen, K. E. , & Naik, H. (2020). Experiences from the epicenter: Professional impact of the COVID‐19 pandemic on genetic counselors in New York. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 187(1), 28–36. 10.1002/ajmg.c.31855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhate, C. , Ho, C. H. , & Brodell, R. T. (2020). Time to revisit the health insurance portability and accountability act (HIPAA)? Accelerated telehealth adoption during the COVID‐19 pandemic. Journal of the American Academy of Dermatology, 83(4), e313–e314. 10.1016/j.jaad.2020.06.989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal, D. , Fowler, E. J. , Abrams, M. , & Collins, S. R. (2020). Covid‐19 — Implications for the health care system. New England Journal of Medicine, 383(15), 1483–1488. 10.1056/nejmsb2021088 [DOI] [PubMed] [Google Scholar]
- Buchanan, A. H. , Rahm, A. K. , & Williams, J. L. (2016). Alternate service delivery models in cancer genetic counseling: A mini‐review. In Temkin S. M. (Ed.), Frontiers in oncology (Vol. 6, issue MAY, p. 120). Frontiers media S.a. 10.3389/fonc.2016.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID‐19 Map ‐ Johns Hopkins Coronavirus Resource Center . (n.d.). Retrieved December 28, 2020, from https://coronavirus.jhu.edu/map.html
- Czeisler, M. É. , Marynak, K. , Clarke, K. E. N. , Salah, Z. , Shakya, I. , Thierry, J. M. , Ali, N. , McMillan, H. , Wiley, J. F. , Weaver, M. D. , Czeisler, C. A. , Rajaratnam, S. M. W. , & Howard, M. E. (2020). Delay or avoidance of medical care because of COVID‐19–related concerns — United States, June 2020. MMWR. Morbidity and Mortality Weekly Report, 69(36), 1250–1257. 10.15585/mmwr.mm6936a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis, E. M. , Reza, N. , Donald, E. , Givertz, M. M. , Lindenfeld, J. A. , & Jessup, M. (2020). Considerations for heart failure care during the COVID‐19 pandemic. In Greenberg B. (Ed.), JACC: Heart failure (Vol. 8, issue 8, pp. 681–691). Elsevier Inc. 10.1016/j.jchf.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo, P. , & Maintainer . (2020). Title US maps including Alaska and Hawaii. CRAN R‐project. [Google Scholar]
- Gadzinski, A. J. , Gore, J. L. , Ellimoottil, C. , Odisho, A. Y. , & Watts, K. L. (2020). JU forum ‐ implementing telemedicine in response to the. The Journal of Urology, 204(July), 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, J. , Brain, K. , Iredale, R. , Alderman, J. , France, E. , & Hughes, H. (2000). A pilot study of telegenetics. Journal of Telemedicine and Telecare, 6(4), 245–247. 10.1258/1357633001935329 [DOI] [PubMed] [Google Scholar]
- Hooker, G. W. , Babu, D. , Myers, M. , Zierhut, H. , & McAllister, M. (2017). Standards for the reporting of genetic counseling interventions in research and other studies (GCIRS): An NSGC task force report. Journal of Genetic Counseling, 26(3), 355–360. 10.1007/S10897-017-0076-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang, J. M. J. , & Horie, M. (2016). Genetics of Brugada syndrome. In Matsumoto K. & Chen S.‐A. (Eds.), Journal of arrhythmia (Vol. 32, issue 5, pp. 418–425). Elsevier B.V. 10.1016/j.joa.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärkkäinen, S. , & Peuhkurinen, K. (2007). Genetics of dilated cardiomyopathy. Annals of Medicine, 39(2), 91–107. 10.1080/07853890601145821 [DOI] [PubMed] [Google Scholar]
- Keesara, S. , Jonas, A. , & Schulman, K. (2020). Covid‐19 and health Care's digital revolution. New England Journal of Medicine, 382(23), e82. 10.1056/nejmp2005835 [DOI] [PubMed] [Google Scholar]
- Mahon, S. M. (2020). Telegenetics remote counseling during the covid‐19 pandemic. Clinical Journal of Oncology Nursing, 24(3), 244–248. 10.1188/20.CJON.244-248 [DOI] [PubMed] [Google Scholar]
- Mann, D. M. , Chen, J. , Chunara, R. , Testa, P. A. , & Nov, O. (2020). COVID‐19 transforms health care through telemedicine: Evidence from the field. Journal of the American Medical Informatics Association, 27(7), 1132–1135. 10.1093/jamia/ocaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus, F. I. , Edson, S. , & Towbin, J. A. (2013). Genetics of arrhythmogenic right ventricular cardiomyopathy: A practical guide for physicians. Journal of the American College of Cardiology, 61(19), 1945–1948. 10.1016/j.jacc.2013.01.073 [DOI] [PubMed] [Google Scholar]
- Maron, B. J. , Maron, M. S. , & Semsarian, C. (2012). Genetics of hypertrophic cardiomyopathy after 20years: Clinical perspectives. In Fuster V. (Ed.), Journal of the American College of Cardiology (Vol. 60, issue 8, pp. 705–715). Elsevier. 10.1016/j.jacc.2012.02.068 [DOI] [PubMed] [Google Scholar]
- Maurer, M. S. , Hanna, M. , Grogan, M. , Dispenzieri, A. , Witteles, R. , Drachman, B. , Judge, D. P. , Lenihan, D. J. , Gottlieb, S. S. , Shah, S. J. , Steidley, D. E. , Ventura, H. , Murali, S. , Silver, M. A. , Jacoby, D. , Fedson, S. , Hummel, S. L. , Kristen, A. V. , Damy, T. , … Rapezzi, C. (2016). Genotype and phenotype of Transthyretin cardiac amyloidosis: THAOS (Transthyretin amyloid outcome survey). Journal of the American College of Cardiology, 68(2), 161–172. 10.1016/j.jacc.2016.03.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy‐Ryan, M. , Psychogios, A. , & Lindor, N. M. (2010). Hereditary disorders of connective tissue: A guide to the emerging differential diagnosis. Genetics in Medicine, 12(6), 344–354. 10.1097/GIM.0b013e3181e074f0 [DOI] [PubMed] [Google Scholar]
- Naukkarinen, J. , Ehnholm, C. , & Peltonen, L. (2006). Genetics of familial combined hyperlipidemia. Current Opinion in Lipidology, 17(3), 285–290. 10.1097/01.mol.0000226121.27931.3f [DOI] [PubMed] [Google Scholar]
- Ohannessian, R. , Duong, T. A. , & Odone, A. (2020). Global telemedicine implementation and integration within health systems to fight the COVID‐19 pandemic: A call to action. JMIR Public Health and Surveillance, 6(2), e18810. 10.2196/18810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Package “maps” Title Draw Geographical Maps . (2018).
- Package “sp” title classes and Methods for spatial data . (2020).
- Pagliazzi, A. , Mancano, G. , Forzano, G. , Giovanni, F. , Gori, G. , Traficante, G. , Iolascon, A. , & Giglio, S. (2020). Genetic counseling during COVID‐19 pandemic: Tuscany experience. Molecular Genetics & Genomic Medicine, 8(10), e1433. 10.1002/mgg3.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard, A. , Jones, G. T. , & Milewicz, D. M. (2019). Genetics of thoracic and abdominal aortic diseases: Aneurysms, dissections, and ruptures. In Freedman J. E. (Ed.), Circulation research (Vol. 124, issue 4, pp. 588–606). Lippincott Williams and Wilkins. 10.1161/CIRCRESAHA.118.312436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, M. C. , Wood, E. M. , Gaieski, J. B. , & Bradbury, A. R. (2017). Possible barriers for genetic counselors returning actionable genetic research results across state lines. Genetics in Medicine, 19(11), 1202–1204. 10.1038/gim.2017.34 [DOI] [PubMed] [Google Scholar]
- Santoli, J. M. , Lindley, M. C. , DeSilva, M. B. , Kharbanda, E. O. , Daley, M. F. , Galloway, L. , Gee, J. , Glover, M. , Herring, B. , Kang, Y. , Lucas, P. , Noblit, C. , Tropper, J. , Vogt, T. , & Weintraub, E. (2020). Effects of the COVID‐19 pandemic on routine pediatric vaccine ordering and administration — United States, 2020. MMWR. Morbidity and Mortality Weekly Report, 69(19), 591–593. 10.15585/mmwr.mm6919e2 [DOI] [PubMed] [Google Scholar]
- Sayer, G. , Horn, E. M. , Farr, M. A. , Axsom, K. , Kleet, A. , Gjerde, C. , Latif, F. , Sobol, I. , Kelley, N. , Lancet, E. , Halik, C. , Takeda, K. , Naka, Y. , Yuzefpolskaya, M. , Kumaraiah, D. , Colombo, P. C. , Maurer, M. S. , & Uriel, N. (2020). Transition of a large tertiary heart failure program in response to the COVID‐19 pandemic: Changes that will endure. Circulation: Heart Failure, 13(9), 425–432. 10.1161/CIRCHEARTFAILURE.120.007516 [DOI] [PubMed] [Google Scholar]
- Seidelmann, S. B. , Smith, E. , Subrahmanyan, L. , Dykas, D. , Ziki, M. D. A. , Azari, B. , Hannah‐Shmouni, F. , Jiang, Y. , Akar, J. G. , Marieb, M. , Jacoby, D. , Bale, A. E. , Lifton, R. P. , & Mani, A. (2017). Application of whole exome sequencing in the clinical diagnosis and Management of Inherited Cardiovascular Diseases in adults. Circulation: Cardiovascular Genetics, 10(1), e001573. 10.1161/CIRCGENETICS.116.001573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomski, A. (2020). Telehealth success spurs a call for greater post–COVID‐19 license portability. JAMA, 324(11), 1021–1022. 10.1001/JAMA.2020.9142 [DOI] [PubMed] [Google Scholar]
- Srinivasan, S. , Won, N. Y. , Dotson, W. D. , Wright, S. T. , & Roberts, M. C. (2020). Barriers and facilitators for cascade testing in genetic conditions: A systematic review. European Journal of Human Genetics, 28(12), 1631–1644. 10.1038/s41431-020-00725-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayal, U. , Prasad, S. , & Cook, S. A. (2017). Genetics and genomics of dilated cardiomyopathy and systolic heart failure. In Genome medicine (Vol. 9, Issue 1). BioMed Central Ltd. 10.1186/s13073-017-0410-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry, A. B. , Wylie, A. , Raspa, M. , Vogel, B. , Sanghavi, K. , Djurdjinovic, L. , Caggana, M. , & Bodurtha, J. (2019). Clinical models of telehealth in genetics: A regional telegenetics landscape. Journal of Genetic Counseling, 28(3), 673–691. 10.1002/jgc4.1088 [DOI] [PubMed] [Google Scholar]
- Vrečar, I. , Hristovski, D. , & Peterlin, B. (2017). Telegenetics: An update on availability and use of telemedicine in clinical genetics service. Journal of Medical Systems, 41(2), 21. 10.1007/s10916-016-0666-3 [DOI] [PubMed] [Google Scholar]
- Wilson, F. A. , Rampa, S. , Trout, K. E. , & Stimpson, J. P. (2017). Reimbursements for telehealth services are likely to be lower than non‐telehealth services in the United States. Journal of Telemedicine and Telecare, 23(4), 497–500. 10.1177/1357633X16652288 [DOI] [PubMed] [Google Scholar]
- Wosik, J. , Fudim, M. , Cameron, B. , Gellad, Z. F. , Cho, A. , Phinney, D. , Curtis, S. , Roman, M. , Poon, E. G. , Ferranti, J. , Katz, J. N. , & Tcheng, J. (2020). Telehealth transformation: COVID‐19 and the rise of virtual care. In Bakken S. (Ed.), Journal of the American Medical Informatics Association (Vol. 27, issue 6, pp. 957–962). Oxford University Press. 10.1093/jamia/ocaa067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis, A. , & Bhonsale, A. (2020). Long QT syndrome. In Natale A., Wang P. J., Al‐Ahmad A., & Mark Estes N. A. (Eds.), Cardiac electrophysiology: Clinical case review (pp. 215–218). Springer International Publishing. 10.1007/978-3-030-28533-3_52 [DOI] [Google Scholar]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , Xiang, J. , Wang, Y. , Song, B. , Gu, X. , Guan, L. , Wei, Y. , Li, H. , Wu, X. , Xu, J. , Tu, S. , Zhang, Y. , Chen, H. , & Cao, B. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. The Lancet, 395(10229), 1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


