Abstract
There is an urgent need for accurate and sensitive diagnostic tools that can overcome the current challenge to distinguish individuals with latent tuberculosis infection (LTBI) from individuals with active tuberculosis (TB). Recent literature has suggested that a group of cytokines may serve as biomarkers of TB disease progression. Using a multiplex ELISA, we quantified 27 circulatory markers present within the unstimulated plasma of individuals in Durban, South Africa who were healthy (n=20), LTBI (n=13), or had active TB (n=30). RT-qPCR was performed to measure gene expression of the cytokines of interest, using RNA isolated from healthy (n=20), LTBI (n=20), or active TB (n=30). We found that at the protein level, IL-1RA, IL-6, and IP-10 were significantly more abundant in participants with active TB (p< 0.05) compared to those with LTBI individuals. IP-10 also showed the strongest association with active TB compared to healthy and LTBI at mRNA level. Our data shows that these proteins may serve as biomarkers of TB at both the protein and gene level.
Keywords: latent TB, tuberculosis, biomarkers, IP-10, TB diagnosis, IL-1RA, disease progression
Introduction
Mycobacterium tuberculosis (Mtb) remains the leading cause of death by a single infectious agent (WHO, 2021). According to the World Health Organization (WHO) ~ 1.5 million people died from tuberculosis (TB) in 2021 (WHO, 2021). TB ranges from latent, to subclinical disease, and active TB disease (Pai et al., 2016). However, diagnosing and differentiating between disease states is difficult, and diagnostic tools lack sensitivity (Dorman, 2015) and can result in improper treatment or misdiagnosis.
Current methods of identifying those with TB infection include smear microscopy by staining for acid fast bacilli and by culturing Mtb, which is a tedious and a time-consuming process [as reviewed by (Sudbury et al., 2020)]. In addition, recent diagnostic tools such as GeneXpert are expensive and are mainly located at provincial hospitals and not in rural clinics, emphasizing the need for affordable and accessible means of diagnosing TB (Bassett et al., 2019). Current gold standards of testing for LTBI is the tuberculin skin test (TST) and the interferon gamma release assay (IGRA), which confirms the presence of interferon (IFN)-γ production as a surrogate marker of T cell responses (Diel et al., 2011). However, TST and IGRA cannot distinguish between those with LTBI and TB, which is an important distinction to make when determining correct courses of treatment. Therefore, identifying potential biomarkers of protective immunity or surrogates of disease progression remains an important need in the fight against TB (Leem et al., 2018).
Several studies have investigated the validity of using Mtb-specific cytokines to discriminate between LTBI and TB (Sudbury et al., 2020). Various cytokines have been implicated in protection during TB disease, including IFN-γ and interleukin (IL)-1RA (Cooper, 2009; O'Garra et al., 2013; Moreira-Teixeira et al., 2018; Sia and Rengarajan, 2019). IFN-γ is a well-known immune correlate in TB (Reichler et al., 2020). Recently, a type 1 IFN inflammatory gene signature in the blood of mice was shown to exacerbate TB pathogenesis and drive lung pathological damage in mice, and also served to differentiate those with active TB (Moreira-Teixeira et al., 2020). However, studies have also suggested that it is difficult to identify a single cytokine as a biomarker of disease, suggesting that a group or several cytokines may be key in identifying potential bio-signatures of disease as well as immune correlates of TB (Weiner et al., 2013). Another study showed that IFN-γ, IL-2, IL-5 and IL-10, IL-1RA, and MCP-1 distinguished between individuals with LTBI and TB (Suzukawa et al., 2016). In addition, stimulating plasma with Mtb specific antigens is limited by its ability to only distinguish those who are infected with Mtb and those who have been BCG vaccinated (Richeldi, 2006; Parida and Kaufmann, 2010). Stimulating plasma does not allow for the discrimination of individuals who are LTBI and those with active TB, which poses a considerable challenge to accurately diagnosing TB (Yao et al., 2017). Identifying circulatory markers that can accurately and sensitively differentiate between LTBI and TB is important in narrowing the search for an appropriate diagnostic marker that can overcome the challenges associated with current diagnostic tools.
In this study, we assessed the validity of using plasma isolated from individuals diagnosed with TB using GeneXpert and those with LTBI, diagnosed using the IGRA. We report that in unstimulated plasma, the cytokines IL-1RA, IL-6, and the chemokine IP-10 discriminate between healthy, LTBI, and TB patients.
Methods
Study Design and Setting
This study included participants from healthcare facilities that service the eThekwini district in KwaZulu-Natal (KZN), South Africa. Participants were recruited from Kwadabeka clinic and
Prince Cyril Zulu Communicable Disease Centre. South Africa had a TB prevalence rate of 737 in 100,000 in 2017 (Richeldi, 2006) with eThekwini described as having the highest TB prevalence rate in KZN. This study was approved by the Biomedical Research Ethics Committee (BREC) at the University of KwaZulu-Natal (BE022/13). We confirm that all research was performed in accordance with relevant guidelines/regulations. Informed consent was obtained from all participants and/or their legal guardians. We recruited TB patients who were newly diagnosed as GeneXpert positive (n=30), and LTBI (n=20) who were quantiferon (QFT) positive and healthy individuals (n=20) (QFT negative). These participants were treatment naïve. Clinical characteristics of all 70 participants used in this study are listed in Table 1 .
Table 1.
Clinical characteristics of n=70 participants used in this study.
| Characteristics | Healthy (IGRA-) | LTBI (IGRA+) | TB (GeneXpert+) |
|---|---|---|---|
| n | 20 | 20 | 30 |
| Age (mean and SD) | 30.90± 12.59 | 32.00 ± 11.95 | 35.37 ± 10.93 |
| Sex | |||
| Male | 35.% (7) | 45% (9) | 73.33% (22) |
| Female | 65% (13) | 55% (11) | 23.33% (7) |
| Not disclosed | 3.33% (1) | ||
| Assays | |||
| Plasma- Bio-Plex | 20 | 13 | 30 |
| qPCR on whole blood RNA | 20 | 20 | 30 |
Sample Collection
Whole blood was collected in heparin tubes. There was 20 ml of blood layered onto Ficoll density gradient medium and centrifuged at 800×g for 30 min with breaks off. PBMCs were collected and stored in liquid nitrogen storage facilities based at the AHRI, Durban, South Africa. The plasma was collected by centrifuging whole blood at 1000 ×g for 10 min with acceleration at maximum and deceleration off. The plasma was collected and stored at -80°C until use. GeneXpert diagnostic experiments were conducted at the National Health Laboratory Services, Durban, South Africa, according to the manufacturer’s instructions (Cepheid, CA). QFT-gold plus (Qiagen) experiments were repeated by the AHRI diagnostic department according to the manufacturer’s instructions.
Measurement of Plasma Cytokines by Multiplex ELISA
A multiplex Luminex assay was performed using the Bio-Plex 27 human cytokine screening panel, a 27-plex kit from Bio-Rad (Hercules, CA) to measure interleukin (IL)-1β, IL-1 receptor antagonist (IL-1RA), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, Eotaxin, fibroblast growth factor (FGF) basic, granulocyte colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF), interferon-γ (IFN- γ), interferon gamma inducible protein (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein alpha (MIP-1α), platelet derived growth factor (PDGF-bb), MIP-1β, regulated on activation, normal T cell expressed and secreted (RANTES), tumour necrosis factor (TNF- α), and vascular endothelial growth factor (VEGF). Assays were performed as per the manufacturer’s instructions and were obtained with the Bio-Plex 200 plate reader (CA). The sensitivity of the kit was 0.2-45.6 pg/ml for each of the 27-cytokine concentrations measured. The Bio-Plex-manager software version 6 was used to collect the data and a 5PL regression formula was used to generate the standard curves for each cytokine to interpolate the concentration of cytokines in the samples. Cytokines that were expressed lower than the lower limit of detection were reported as zero. The samples were diluted according to the manufacturer’s instruction for plasma samples.
RNA Isolation and cDNA Synthesis
There was 10 ml of whole blood collected in PAXgene™ tubes. RNA was isolated from whole blood according to the manufacturer’s instructions using the Paxgene™ kit (PreAnalytix, Switzerland). Complimentary DNA (cDNA) was synthesized from isolated RNA using the protocol described by Bio-Rad reverse transcription kit (Bio-Rad, Hercules, CA). Briefly, RNA was adjusted to a concentration of 500 ng for cDNA synthesis. Appropriate 5-20 µl of nuclease free water, 1 µl of RNA, and 4 µl of iScript 5× reaction mix were added for each sample to a total reaction volume of 20 µl. The T100 thermocycler from Bio-Rad (Hercules, CA) was set to 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C.
Real Time Quantitative Polymerase Chain Reaction for Gene Expression Analysis
Real time quantitative polymerase chain reaction (RT-qPCR) was done on the candidate genes as per the manufacturer’s instruction. The total reaction volume was 10 µl. There was 1 µl of cDNA added to each well with 9 µl of mastermix consisting of the appropriate forward and reverse primer at 0.5 µl each, 5 µl of iTaq™ Universal SYBR green supermix (Bio-Rad, Hercules, CA), and 3 µl of nuclease free water. The CFX 96 thermocycler (Bio-Rad, Hercules, CA) was set to the following protocol: 30 sec at 95°C, 5 sec at 95°C, and 30 sec at 56°C for 39 cycles. The melt curve analysis was done at 65-95°C at 0.5°C increments.
Primer Design
Primers were designed using the IDT primer design tool, PrimerQuest Tool, and sequences were blasted using the BlastN tool on NCBI. Primers were designed for IL-1RA, IL-6, and IP-10 (CXCL10) (see Table 2 ).
Table 2.
Primers used for gene expression studies.
| Target | Accession number | Forward primer | Reverse primer |
|---|---|---|---|
| IL-1RA | NM_001318914.2 | 5’-GCC TTC AGA ATC TGG GAT GTT-3’ | 5’-CGC CTT CGT CAG GCA TAT T-3’ |
| IL-6 | NM_000600 | 5’-TCT GGA TTC AAT GAG GAG ACT TG-3’ | 5’-GGA CTG CAG GAA CTC CTT AAA-3’ |
| IP-10 (CXCL10) | NM_001565.4 | 5’- CTC TAA GTG GCA TTC AAG GAG TA-3’ | 5’-ACC CTT GGA AGA TGG GAA AG-3’ |
Statistical Analysis
To identify any potential cytokine profiles that were associated with disease state, the data were log transformed and normalized and a principal component analysis (PCA) of the data was done. One-way ANOVA was done to compare disease groups, followed by a multiple comparison post-test using Tukey’s test. Values of p<0.05 were considered statistically significant. Data was analyzed using the GraphPad Prism version 9.4 software.
Results
Circulatory Cytokines May Distinguish Between Healthy, LTBI and Active TB Patients
To determine if circulatory cytokines are associated with active TB and LTBI, we performed a multivariate principal component analysis using unsupervised clustering. Cytokine protein concentrations that segregated according to disease, along PC2 (16.12%), were associated with higher protein abundance of cytokines such as IL-1RA, IL-6, IL-8, and IP-10 ( Figures 1A, C ). The remainder of the cytokines were loaded on PC1 (38.38%, Figures 1A, B ). Given the current understanding that a group of cytokines may serve as biomarkers of disease progression, these data suggest that certain cytokines may be differentially expressed in specific stages of disease.
Figure 1.
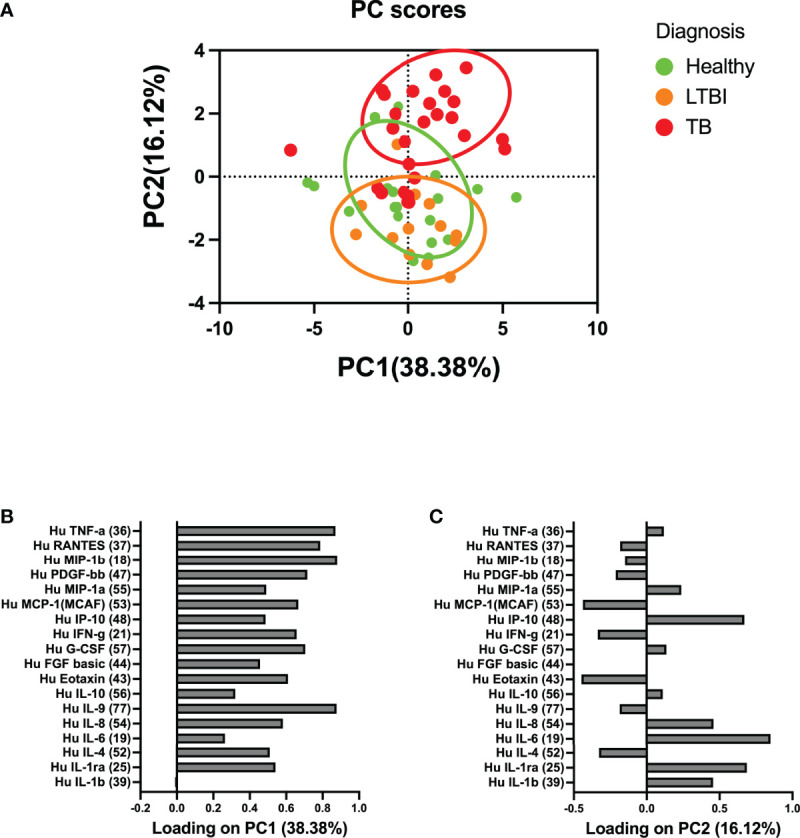
Principal component analysis (PCA) of the association between circulatory cytokines and TB disease states. (A) Circulatory cytokines separate according to whether individuals are healthy, latent TB infected, or with active TB disease. (B) PC1 accounted for 38.38% of the variance observed in the data set. (C) PC2 accounted for 16.12% of the variance observed in the data set. PC2 was used to define the association between circulatory cytokine and whether individuals were healthy, LTBI, or those who had active TB. Each dot represents a participant score on the loading components.
IL-1RA, IL-6, and IP-10 Are Abundant in TB Patients Compared to Healthy and LTBI
Following the findings that some circulatory markers were associated with disease and that participant scores loaded more highly on PC2, we sought to investigate whether there were any significant inflammatory circulatory biomarkers that could distinguish healthy individuals, LTBI, and active TB patients. We found that there was a difference between at least one of the groups for IL-1RA (p = 0.009), IL-6 (p = 0.0001), and IP-10 (p < 0.0001). Tukey’s test found that IL-1RA (p < 0.017, 91% CI (-605.9 to -50.41), Figure 2A ), IL-6 (p = 0.001, 91% CI (-10.91 to -2.357), Figure 2B ), and IP-10 (p < 0.0001, 91% CI (-1667 to -511.3), Figure 2C ) were abundant in the TB group, compared to the healthy group. IL-1RA (p = 0.051, 91% CI (-638.2 to 0.745), Figure 2A ), IL-6 (p = 0.001, 91% CI (-12.59 to -2.755), Figure 2B ), and IP-10 (p = 0.002, 91% CI (-1674 to -344.5), Figure 2C ) were also more abundant in the TB group compared to the LTBI group. Protein abundance of all 27 cytokines analyzed are listed in Table 3 .
Figure 2.
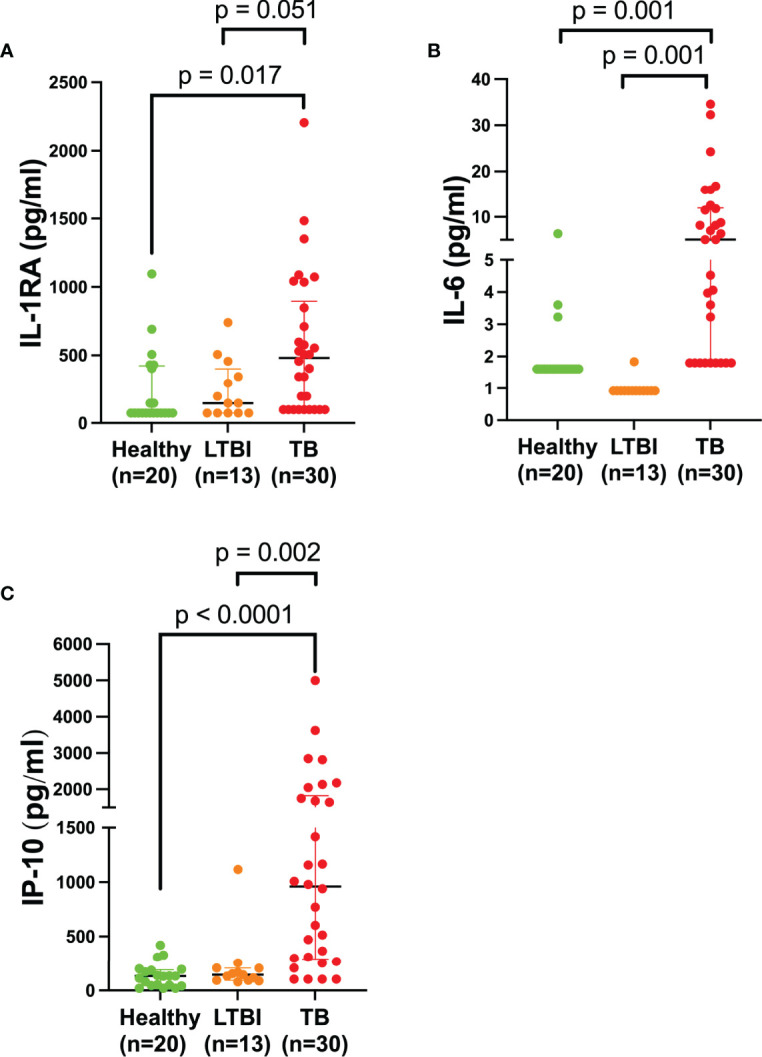
Plasma IL-RA, IL-6, and IP-10 are elevated in TB patients. Quantification of various cytokines in the plasma of healthy (n=20) individuals compared to LTBI (n=13) and TB (n=30) patients. Each dot represents a participant sample. (A) IL-1RA, (B) IL-6, and (C) IP-10 were quantified and measured in pg/ml. Significant differences were identified as those values that were less than p<0.05. Data was analyzed using the one-way ANOVA and followed by a multiple comparisons test using Tukey’s test. Tukey’s p-values have been reported in the above figure and illustrate the differences observed between groups.
Table 3.
Absolute protein abundance of all cytokines assessed in this study.
| Cytokine | Healthy | LTBI | TB | |||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| IL-1β | 1.165 | 1.165-1.175 | 0.205 | 0.205-0.205 | 1.08 | 1.08-1.08 |
| IL-1RA | 73.02 | 73.02-423.4 | 146 | 73.02-400.1 | 481.8 | 97.98-895.2 |
| IL-2 | N/A | N/A | N/A | N/A | N/A | N/A |
| IL-4 | 0.48 | 0.48-0.84 | 0.41 | 0.41-0.82 | 0.41 | 0.41-0.41 |
| IL-5 | N/A | N/A | N/A | N/A | N/A | N/A |
| IL-6 | 1.615 | 1.615-1.615 | 0.92 | 0.92-0.92 | 5.08 | 1.80-11.98 |
| IL-7 | N/A | N/A | N/A | N/A | N/A | N/A |
| IL-8 | 2.598 | 1.925-12.65 | 7.42 | 2.818-11.66 | 6.225 | 1.635-14.94 |
| IL-9 | 207 | 124.9-328.9 | 228.2 | 168.5-311.4 | 181.9 | 110.3-274.9- |
| IL-10 | 1.295 | 1.295-2.266 | 2.590 | 1.295-3.510 | 1.295 | 1.295-2.59 |
| IL-12 (p70) | N/A | N/A | N/A | N/A | N/A | N/A |
| IL-13 | N/A | N/A | N/A | N/A | N/A | N/A |
| IL-15 | N/A | N/A | N/A | N/A | N/A | N/A |
| IL-17 | N/A | N/A | N/A | N/A | N/A | N/A |
| Eotaxin | 8.035 | 2.533-29.30 | 6.870 | 4.425-30.06 | 5.370 | 3.990-17.66 |
| FGF basic | 8.245 | 8.245-8.245 | 8.245 | 8.245-12.37 | 9.885 | 9.885-9.885 |
| G-CSF | 62.40 | 33.20-116.1 | 94.04 | 71.27-149.7.0 | 113.6 | 45.10-156.5 |
| GM-CSF | N/A | N/A | N/A | N/A | N/A | N/A |
| IFN- γ | 1.855 | 1.350-7.620 | 3.710 | 2.360-5.07- | 1.180 | 1.180-4.390 |
| IP-10 | 131.9 | 46.84-192.4 | 145.8 | 93.98-207 | 958.7 | 291.3-1823 |
| MCP-1 | 14.61 | 7.560-36.30 | 17.90 | 10.51-37.60 | 11.56 | 5.918-20.57 |
| MIP-1 α | 1.990 | 1.990-1.990 | 1.105 | 1.105-2.7 | 1.660 | 0.83-4.123 |
| PDGF-bb | 139.9 | 38.80-374.20 | 213.9 | 98.61-509.6 | 143.4 | 31.65-283.1 |
| MIP-1 β | 132.5 | 75.38-165.9 | 157.8 | 120.8-208.5 | 119.7 | 76.33-142.4 |
| RANTES | 3736 | 1441-6231 | 34.74 | 17.14-45.95 | 3034 | 1496-4326 |
| TNF- α | 36.12 | 18.42-42.24 | 34.74 | 17.14-45.95 | 40.89 | 24.30-53.97 |
| VEGF | N/A | N/A | N/A | N/A | N/A | N/A |
N/A, these values were lower than the limit of detection.
Gene Expression of IP-10 Distinguishes Between Healthy, LTBI, and TB individuals
To determine if the circulatory signatures observed in the plasma were also expressed at the gene level, we measured the expression of IL-1RA, IL-6, and IP-10 in whole blood by qPCR. We found that there was a difference between one of the groups for IP-10 (p = 0.001). Tukey’s test found that IP-10 was upregulated in the TB group compared to the LTBI group (p = 0.004, 91% CI (-61.37 to -10.44), Figure 3C ). IP-10 gene was also upregulated in the TB group compared to healthy (p = 0.004, 91% CI (-62.33 to -10.55), Figure 3C ). Our data shows that IP-10 is more abundant at both the protein and gene level, and that it can be explored as a potential biomarker of TB disease. No significant differences were observed for IL-1RA and IL-6 at the mRNA level ( Figures 3A, B , respectively).
Figure 3.
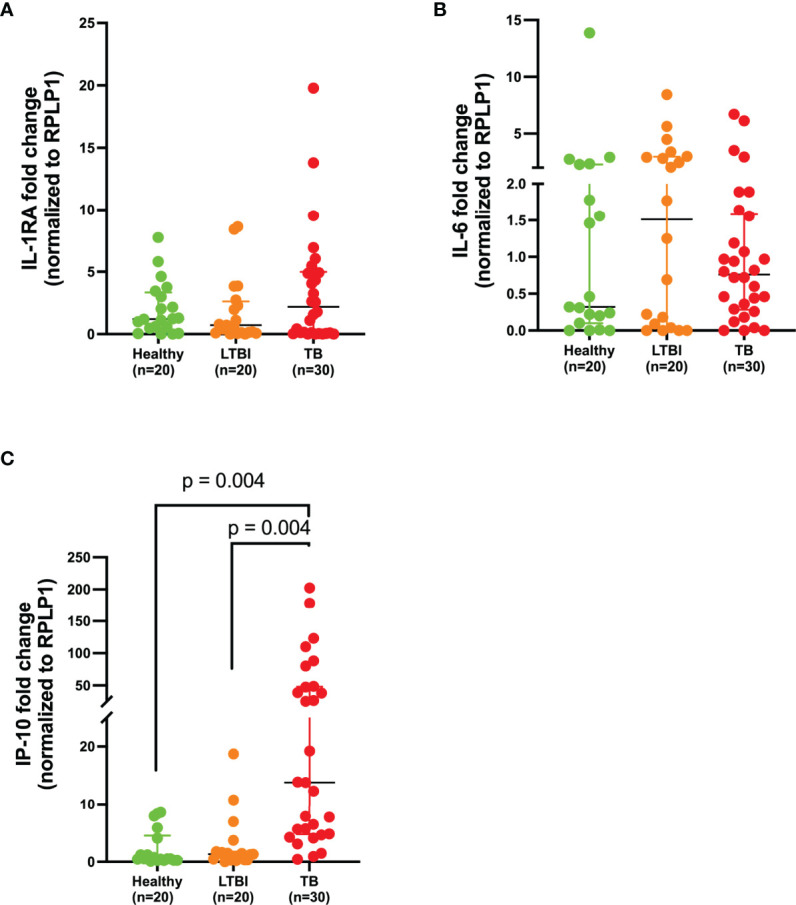
IP-10 is upregulated in whole blood of TB patients. Gene expression of (A) IL-1RA, (B) IL-6, and (C) IP-10 in the whole blood of healthy (n=20), LTBI (n=20), and TB (n=30) participants normalized to a house-keeping gene, RPLP1. P< 0.05 was considered statistically significant. Data were analyzed using the one-way ANOVA and followed by a multiple comparisons test using Tukey’s test. Tukey’s p-values have been reported in the above figure and illustrate the differences observed between groups.
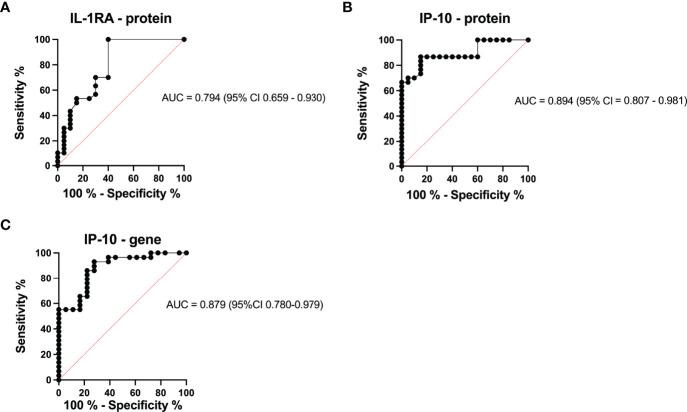
ROC Analysis Shows That IP-10 and IL-1RA Have Good Discriminatory Power Between Healthy Individuals and TB Patients
To assess the power of IL-1RA and IP-10 to discriminate between healthy individuals and those with TB, we performed ROC analysis. IL-1RA and IP-10 both showed a good area under the curve (AUC=0.794 and AUC=0.894, Figures 4A, B , respectively) with high specificity and sensitivity. IL-1RA had a positive predictive value (PPV) of 86.47% and a negative predictive value (NPV) of 44.95%. IP-10 showed more potential with PPV=92.39% and NPV= 68.15% ( Figure 4B ). To determine if IP-10 could distinguish between healthy and TB patients at the mRNA level, we performed ROC analysis based on the qPCR data. Even at the mRNA level, IP-10 had good positive and negative predictive values (AUC=0.879, with PPV=91, 58% and NPV=66, 79%) with high specificity (86, 20%) and sensitivity (77, 78%) ( Figure 4C ).
Figure 4.
ROC curve characteristics for IP-10 protein and IL-1RA protein as well as IP-10 mRNA, as discriminatory markers for TB. AUC, specificity, sensitivity, PPV, and NPV indicate the power of (A) IL-1RA protein abundance, (B) IP-10 protein abundance, and (C) IP-10 mRNA expression which are reported and indicate the power of these biomarkers for discriminating between those who are healthy and those with TB.
Discussion
This study investigated the potential of using plasma samples to identify circulatory markers that may distinguish between healthy, LTBI, and active TB disease. Of the 27 cytokines/chemokines assessed, IL-1RA, IL-6, and IP-10 were significantly different between individuals with LTBI and TB patients at the plasma protein levels. Gene expression profiles also validated IP-10 as a potential biomarker that can distinguish active TB disease from LTBI at the mRNA level.
A previous study identified the prognostic value of host soluble mediators as biomarkers for TB-associated mortality (Schutz et al., 2019). Common markers reported in our study and that of Schutz et al., identified cytokines IL-1RA, IL-6, and the chemokine IP-10 to be a marker of disease severity (Schutz et al., 2019). Furthermore, other studies have reported that IFN-γ, IL-2, IL-5, IL-10, IL-1RA, and MCP-1 distinguished between individuals with LTBI and TB individuals in QFT supernatants stimulated with TB antigen (Suzukawa et al., 2016). Our study differs from the approach taken by others (Suzukawa et al., 2016) in that we did not stimulate the plasma with any TB antigen. We also determined gene expression levels of the cytokines to validate their capacity to distinguish disease stages at the mRNA level. IP-10 discriminated TB from LTBI participants at both the protein and gene level.
IL-1RA is secreted by monocytes, eosinophils, and neutrophils and it antagonistically binds to IL-1α and IL-1β. Our findings of elevated IL-1RA in the TB group compared to the LTBI group corroborates a previous study which reported increased levels of IL-1RA in the serum of individuals with active TB (Juffermans et al., 1998). In addition, previous studies have also shown elevated levels of IL-1RA in QFT supernatants (Ruhwald et al., 2009) stimulated with TB antigens such as ESAT-6, CFP-10, and TB7.7 (Suzukawa et al., 2016). Given the highly inflammatory nature of TB, the repeated presence of IL-1RA in various settings such as in QFT TB antigen stimulation (Suzukawa et al., 2016), bronchoalveolar lavage (BAL) fluid (Tsao et al., 2000), and its presence in our study, albeit in unstimulated plasma, emphasizes the importance of IL-1RA as a potential biomarker of disease progression. IL-6 is well established as a marker of inflammation and plays a vital role in the cytokine storm associated with TB disease [as reviewed by (Boni et al., 2022)]. The abundant protein levels of IL-6 in the active TB group corroborates with previous findings that reported an association of IL-6 at the plasma level with pulmonary TB disease severity (Zambuzi et al., 2016). IL-6 has also been suggested as a biomarker to monitor TB disease in patients undergoing anti-TB treatment (Singh and Goyal, 2013; Chowdhury et al., 2014).
IP-10 is a chemokine that recruits T-lymphocyte cells to areas of inflammation and regulates the recruitment of Th1 cells (Azzurri et al., 2005; Blauenfeldt et al., 2018). As an interferon gamma inducible protein, the association of IP-10 with active disease supports the known relationship between IFN- γ and TB disease (Kannabus, 2021). Various studies have reported on the potential of IP-10 as a diagnostic marker to discriminate between individuals with LTBI and TB (Jacobs et al., 2016; Kumar et al., 2021) and in patients’ whole blood, stimulated with ESAT-6 and CFP-10 (Borgström et al., 2012; Jeong et al., 2015; Comella-Del-Barrio et al., 2019). IP-10 is highly detectable in the plasma of patients with active TB (Borgström et al., 2012; Jeong et al., 2015), measurably reduced in those on treatment, present in granulomas (Ferrero et al., 2003), and detectable in individuals cohabiting with a TB positive person, including children (Azzurri et al., 2005; Whittaker et al., 2008). Finally, IP-10 secretion is not influenced by the age of the person infected and is therefore useful in diagnosing both adults and children (Whittaker et al., 2008).
We hypothesize that these markers may serve as a good indicator to track and distinguish states in individuals diagnosed with LTBI. Accurate diagnosis may aid in preventative treatment which may reduce the likelihood of individuals progressing to TB disease if diagnosed early.
Our study shows that differences in circulatory cytokines/chemokines exist between individuals with LTBI and TB. These proteins may be exploited as biomarkers of disease progression. We were not able to correlate our circulatory markers with sputum bacterial loads, which would further inform on the relationship between diagnostic markers and bacterial load. This would provide more information on the strength of the immune response produced as a consequence of bacterial burden. However, a strength of our study is the association of the biomarkers with the disease both in the plasma and validation at the mRNA level. In addition, two of the markers we report here, namely IP-10 and IL-6, have also been reported in unstimulated plasma (Yao et al., 2017), as biomarkers for disease progression and validated in larger cohorts (Kumar et al., 2021). In addition, the high specificity and sensitivity observed in our ROC analysis for IP-10 indicate its potential in serving as a good diagnostic marker in conjunction with other inflammatory cytokine signatures. This data needs to be validated in a larger cohort in order to corroborate with previously reported discovery and validation cohorts (Yao et al., 2017; Kumar et al., 2021). The use of several cytokines as diagnostic markers has been suggested, and validating the identified cytokines is crucial for overcoming diagnostic challenges to improve health care guidelines currently in place for treatment of TB.
Our study supports the growing body of evidence which advocates for the use of plasma diagnostic biomarkers such as those detected in the circulation of individuals with LTBI and TB as a potential diagnostic tool. Further studies are needed to investigate the validity and viability of using specific cytokines and chemokines such as IL-1RA, IL-6, and IP-10 as potential biomarkers of disease progression in a high-disease burden setting.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Biomedical Research Ethics Committee (BREC) at the University of KwaZulu-Natal (BE022/13). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KF performed experimental work and wrote the manuscript. KF and MJM performed data analysis. OB, DM, and KR-B supported with experimental work and sample preparation. FK provided clinical samples. MJM, HN, and TN conceptualized the project and obtained funding for this study. All authors contributed to the article and approved the submitted version.
Funding
The work reported herein was made possible through funding by the South African Medical Research Council (SAMRC) through its Division of Research Capacity Development under the Internship Scholarship Programme (KF) and SAMRC Mid-Career Scientist Programme (MM) with funding received from the South African National Treasury. The content hereof is the sole responsibility of the authors and does not necessarily represent the official views of the SAMRC or the funders. MM was funded by the Wellcome Trust (grant#206751/A/17/Z) and Grand Challenges, an initiative of the Bill & Melinda Gates Foundation (grant #OPP1210776, grant# INV-016239), and SAMRC with funding from the SA Department of Health. HN was funded by NRF CSUR (grant # 116260).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all the study participants for their time and commitment to this study. We also thank the nurses and staff members and other personnel at clinical sites and all staff members at the clinical core at Africa Health Research Institute who helped with patient recruitment and sample processing.
References
- Azzurri A., Sow O. Y., Amedei A., Bah B., Diallo S., Peri G., et al. (2005). IFN-Gamma-Inducible Protein 10 and Pentraxin 3 Plasma Levels Are Tools for Monitoring Inflammation and Disease Activity in Mycobacterium Tuberculosis Infection. Microbes Infect. 7 (1), 1–8. doi: 10.1016/j.micinf.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Bassett I. V., Forman L. S., Govere S., Thulare H., Frank S. C., Mhlongo B., et al. (2019). Test and Treat TB: A Pilot Trial of GeneXpert MTB/RIF Screening on a Mobile HIV Testing Unit in South Africa. BMC Infect. Dis. 19 (1), 110. doi: 10.1186/s12879-019-3738-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauenfeldt T., Petrone L., Del Nonno F., Baiocchini A., Falasca L., Chiacchio T., et al. (2018). Interplay of DDP4 and IP-10 as a Potential Mechanism for Cell Recruitment to Tuberculosis Lesions. Front. Immunol. 9, 1456. doi: 10.3389/fimmu.2018.01456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni F. G., Hamdi I., Koundi L. M., Shrestha K., Xie J. (2022). Cytokine Storm in Tuberculosis and IL-6 Involvement. Infect. Genet. Evol. 97, 105166. doi: 10.1016/j.meegid.2021.105166 [DOI] [PubMed] [Google Scholar]
- Borgström E., Andersen P., Atterfelt F., Julander I., Källenius G., Maeurer M., et al. (2012). Immune Responses to ESAT-6 and CFP-10 by FASCIA and Multiplex Technology for Diagnosis of M. Tuberculosis Infection; IP-10 Is a Promising Marker. PloS One 7 (11), e43438. doi: 10.1371/journal.pone.0043438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I. H., Ahmed A. M., Choudhuri S., Sen A., Hazra A., Pal N. K., et al. (2014). Alteration of Serum Inflammatory Cytokines in Active Pulmonary Tuberculosis Following Anti-Tuberculosis Drug Therapy. Mol. Immunol. 62 (1), 159–168. doi: 10.1016/j.molimm.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Comella-Del-Barrio P., Abellana R., Villar-Hernández R., Jean Coute M. D., Sallés Mingels B., Canales Aliaga L., et al. (2019). A Model Based on the Combination of IFN-γ, IP-10, Ferritin and 25-Hydroxyvitamin D for Discriminating Latent From Active Tuberculosis in Children. Front. Microbiol. 10, 1855. doi: 10.3389/fmicb.2019.01855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. M. (2009). Cell-Mediated Immune Responses in Tuberculosis. Annu. Rev. Immunol. 27, 393–422. doi: 10.1146/annurev.immunol.021908.132703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel R., Goletti D., Ferrara G., Bothamley G., Cirillo D., Kampmann B., et al. (2011). Interferon-γ Release Assays for the Diagnosis of Latent Mycobacterium Tuberculosis Infection: A Systematic Review and Meta-Analysis. Eur. Respir. J. 37 (1), 88–99. doi: 10.1183/09031936.00115110 [DOI] [PubMed] [Google Scholar]
- Dorman S. (2015). Advances in the Diagnosis of Tuberculosis: Current Status and Future Prospects. Int. J. Tuberc. Lung Dis. 19 (5), 504–516. doi: 10.5588/ijtld.15.0048 [DOI] [PubMed] [Google Scholar]
- Ferrero E., Biswas P., Vettoretto K., Ferrarini M., Uguccioni M., Piali L., et al. (2003). Macrophages Exposed to Mycobacterium Tuberculosis Release Chemokines Able to Recruit Selected Leucocyte Subpopulations: Focus on Gammadelta Cells. Immunology 108 (3), 365–374. doi: 10.1046/j.1365-2567.2003.01600.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R., Malherbe S., Loxton A. G., Stanley K., van der Spuy G., Walzl G., et al. (2016). Identification of Novel Host Biomarkers in Plasma as Candidates for the Immunodiagnosis of Tuberculosis Disease and Monitoring of Tuberculosis Treatment Response. Oncotarget 7 (36), 57581–57592. doi: 10.18632/oncotarget.11420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y. H., Hur Y. G., Lee H., Kim S., Cho J. E., Chang J., et al. (2015). Discrimination Between Active and Latent Tuberculosis Based on Ratio of Antigen-Specific to Mitogen-Induced IP-10 Production. J. Clin. Microbiol. 53 (2), 504–510. doi: 10.1128/JCM.02758-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juffermans N. P., Verbon A., van Deventer S. J., van Deutekom H., Speelman P., van der Poll T. (1998). Tumor Necrosis Factor and Interleukin-1 Inhibitors as Markers of Disease Activity of Tuberculosis. Am. J. Respir. Crit. Care Med. 157 (4 Pt 1), 1328–1331. doi: 10.1164/ajrccm.157.4.9709126 [DOI] [PubMed] [Google Scholar]
- Kannabus A. (2021) TB Statistics South Africa TBFACTS.org: 2021. Available at: https://tbfacts.org/tb-statistics-south-africa/.
- Kumar N. P., Hissar S., Thiruvengadam K., Banurekha V. V., Suresh N., Shankar J., et al. (2021). Discovery and Validation of a Three-Cytokine Plasma Signature as a Biomarker for Diagnosis of Pediatric Tuberculosis. Front. Immunol. 12, 653898. doi: 10.3389/fimmu.2021.653898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem A. Y., Song J. H., Lee E. H., Lee H., Sim B., Kim S. Y., et al. (2018). Changes in Cytokine Responses to TB Antigens ESAT-6, CFP-10 and TB 7.7 and Inflammatory Markers in Peripheral Blood During Therapy. Sci. Rep. 8 (1), 1159. doi: 10.1038/s41598-018-19523-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira-Teixeira L., Mayer-Barber K., Sher A., O'Garra A. (2018). Type I Interferons in Tuberculosis: Foe and Occasionally Friend. J. Exp. Med. 215 (5), 1273–1285. doi: 10.1084/jem.20180325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira-Teixeira L., Stimpson P. J., Stavropoulos E., Hadebe S., Chakravarty P., Ioannou M., et al. (2020). Type I IFN Exacerbates Disease in Tuberculosis-Susceptible Mice by Inducing Neutrophil-Mediated Lung Inflammation and NETosis. Nat. Commun. 11 (1), 5566. doi: 10.1038/s41467-020-19412-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A., Redford P. S., McNab F. W., Bloom C. I., Wilkinson R. J., Berry M. P. (2013). The Immune Response in Tuberculosis. Annu. Rev. Immunol. 31, 475–527. doi: 10.1146/annurev-immunol-032712-095939 [DOI] [PubMed] [Google Scholar]
- Pai M., Behr M. A., Dowdy D., Dheda K., Divangahi M., Boehme C. C., et al. (2016). Tuberculosis. Nat. Rev. Dis. Primers 2, 16076. doi: 10.1038/nrdp.2016.76 [DOI] [PubMed] [Google Scholar]
- Parida S. K., Kaufmann S. H. (2010). The Quest for Biomarkers in Tuberculosis. Drug Discov 15 (3-4), 148–157. doi: 10.1016/j.drudis.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Reichler M. R., Hirsch C., Yuan Y., Khan A., Dorman S. E., Schluger N., et al. (2020). Predictive Value of TNF-α, IFN-γ, and IL-10 for Tuberculosis Among Recently Exposed Contacts in the United States and Canada. BMC Infect. Dis. 20 (1), 553. doi: 10.1186/s12879-020-05185-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L. (2006). An Update on the Diagnosis of Tuberculosis Infection. Am. J. Respir. Crit. Care Med. 174 (7), 736–742. doi: 10.1164/rccm.200509-1516PP [DOI] [PubMed] [Google Scholar]
- Ruhwald M., Bjerregaard-Andersen M., Rabna P., Eugen-Olsen J., Ravn P. (2009). IP-10, MCP-1, MCP-2, MCP-3, and IL-1RA Hold Promise as Biomarkers for Infection With M. Tuberculosis in a Whole Blood Based T-Cell Assay. BMC Res. Notes 2, 19. doi: 10.1186/1756-0500-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz C., Barr D., Andrade B. B., Shey M., Ward A., Janssen S., et al. (2019). Clinical, Microbiologic, and Immunologic Determinants of Mortality in Hospitalized Patients With HIV-Associated Tuberculosis: A Prospective Cohort Study. PloS Med. 16 (7), e1002840. doi: 10.1371/journal.pmed.1002840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia J. K., Rengarajan J. (2019). Immunology of Mycobacterium Tuberculosis Infections. Microbiolol. Spectrum 7 (4), 10. doi: 10.1128/microbiolspec.GPP3-0022-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P. P., Goyal A. (2013). Interleukin-6: A Potent Biomarker of Mycobacterial Infection. Springerplus 2, 686. doi: 10.1186/2193-1801-2-686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbury E. L., Clifford V., Messina N. L., Song R., Curtis N. (2020). Mycobacterium Tuberculosis-Specific Cytokine Biomarkers to Differentiate Active TB and LTBI: A Systematic Review. J. Infect. 81 (6), 873–881. doi: 10.1016/j.jinf.2020.09.032 [DOI] [PubMed] [Google Scholar]
- Suzukawa M., Akashi S., Nagai H., Nagase H., Nakamura H., Matsui H., et al. (2016). Combined Analysis of IFN-γ, IL-2, IL-5, IL-10, IL-1RA and MCP-1 in QFT Supernatant Is Useful for Distinguishing Active Tuberculosis From Latent Infection. PloS One 11 (4), e0152483. doi: 10.1371/journal.pone.0152483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao T. C., Hong J., Li L. F., Hsieh M. J., Liao S. K., Chang K. S. (2000). Imbalances Between Tumor Necrosis Factor-Alpha and Its Soluble Receptor Forms, and Interleukin-1beta and Interleukin-1 Receptor Antagonist in BAL Fluid of Cavitary Pulmonary Tuberculosis. Chest 117 (1), 103–109. doi: 10.1378/chest.117.1.103 [DOI] [PubMed] [Google Scholar]
- Weiner J., Maertzdorf J., Kaufmann S. H. (2013). The Dual Role of Biomarkers for Understanding Basic Principles and Devising Novel Intervention Strategies in Tuberculosis. Ann. New York Acad. Sci. 1283, 22–29. doi: 10.1111/j.1749-6632.2012.06802.x [DOI] [PubMed] [Google Scholar]
- Whittaker E., Gordon A., Kampmann B. (2008). Is IP-10 a Better Biomarker for Active and Latent Tuberculosis in Children Than IFNgamma? PloS One 3 (12), e3901. doi: 10.1371/journal.pone.0003901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2021) World Health Organization: Global Tuberculosis Report 2021. Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021.
- Yao X., Liu Y., Liu Y., Liu W., Ye Z., Zheng C., et al. (2017). Multiplex Analysis of Plasma Cytokines/Chemokines Showing Different Immune Responses in Active TB Patients, Latent TB Infection and Healthy Participants. Tuberculosis 107, 88–94. doi: 10.1016/j.tube.2017.07.013 [DOI] [PubMed] [Google Scholar]
- Zambuzi F. A., Cardoso-Silva P. M., Espindola M. S., Soares L. S., Galvão-Lima L. J., Brauer V. S., et al. (2016). Identification of Promising Plasma Immune Biomarkers to Differentiate Active Pulmonary Tuberculosis. Cytokine 88, 99–107. doi: 10.1016/j.cyto.2016.08.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.