Abstract
Reproductive function is suppressed during lactation owing to the suckling-induced suppression of the kisspeptin gene (Kiss1) expression in the arcuate nucleus (ARC) and subsequent suppression of luteinizing hormone (LH) release. Our previous study revealed that somatostatin (SST) neurons mediate suckling-induced suppression of LH release via SST receptor 2 (SSTR2) in ovariectomized lactating rats during early lactation. This study examined whether central SST-SSTR2 signaling mediates the inhibition of ARC Kiss1 expression and LH release in lactating rats during late lactation and whether the inhibition of glutamatergic neurons, stimulators of LH release, is involved in the suppression of LH release mediated by central SST-SSTR2 signaling in lactating rats. A central injection of the SSTR2 antagonist CYN154806 (CYN) significantly increased ARC Kiss1 expression in lactating rats on day 16 of lactation. Dual in situ hybridization revealed that few ARC Kiss1-positive cells co-expressed Sstr2, and some of the ARC Slc17a6 (a glutamatergic neuronal marker)-positive cells co-expressed Sstr2. Furthermore, almost all ARC Kiss1-positive cells co-expressed Grin1, a subunit of N-methyl-D-aspartate (NMDA) receptors. The numbers of Slc17a6/Sstr2 double-labeled and Slc17a6 single-labeled cells were significantly lower in lactating dams than in non-lactating rats whose pups had been removed after parturition. A central injection of an NMDA antagonist reversed the CYN-induced increase in LH release in lactating rats. Overall, these results suggest that central SST-SSTR2 signaling, at least partly, mediates the suppression of ARC Kiss1 expression and LH release by inhibiting ARC glutamatergic interneurons in lactating rats.
Keywords: Glutamate, Kisspeptin, Lactation, Luteinizing hormone, Somatostatin
Ovarian functions, such as follicular development and ovulation, are severely suppressed during lactation in species such as rodents, ruminants, and primates, including humans, resulting in lactational anestrus in these species [1,2,3]. This suppression is assumed to be primarily due to the suckling-induced inhibition of pulsatile release of gonadotropin-releasing hormone (GnRH) and the subsequent inhibition of gonadotropin release. Luteinizing hormone (LH) pulses were strongly suppressed in lactating rats, and LH pulses were restored several hours after the removal of pups from lactating rats [4,5,6,7].
Previous studies have revealed that the suppression of pulsatile GnRH/LH release in lactating rats is largely due to a profound inhibition of kisspeptin and kisspeptin gene (Kiss1) expression in the hypothalamic arcuate nucleus (ARC) [8, 9]. The ARC kisspeptin neurons co-express neurokinin B (Tac3) and dynorphin A (Pdyn) genes, which are referred to as KNDy neurons [10,11,12]. The KNDy neurons are considered to be the GnRH/gonadotropin pulse generator in mammals, including rodents and ruminants [12,13,14,15]. Our previous study revealed that among the KNDy genes, Kiss1 gene expression, but not Tac3 or Pdyn gene expression, was strongly suppressed in the ARC of ovariectomized (OVX) lactating rats during early lactation [16]. Furthermore, the study suggested that somatostatin (SST) and SST receptor 2 (SSTR2, an SST receptor) signaling at least partially mediates the suppression of pulsatile LH secretion during early lactation as the central administration of SSTR2 antagonist increased LH release in OVX lactating rats. Additionally, the expression of the SST gene (Sst) in the thalamus and SSTR2 gene (Sstr2) in the ARC was significantly increased in lactating rats compared to that in non-lactating controls [16]. In addition, we suggested that SST might indirectly act on ARC kisspeptin neurons via interneuron(s) in the hypothalamus, as histological examination revealed low Sstr2 expression in the ARC kisspeptin neurons (< 1%) in lactating rats [16]. Glutamatergic neurons are potential interneurons as glutamate is a major stimulatory neurotransmitter for GnRH/LH secretion and acts by stimulating kisspeptin neurons. Glutamatergic neurons are abundantly located in the ARC in female rats [17,18,19,20]. N-methyl-D-aspartate (NMDA), an ionotropic glutamatergic receptor agonist, immediately stimulates LH secretion in wild-type female rats but not in Kiss1 knockout rats [21]. Furthermore, our previous studies have revealed that the expression of NR1 gene (Grin1), a subunit of the NMDA receptor, can be observed in most ARC kisspeptin neurons in female rats [22], and glutamate treatment largely enhanced in vitro GnRH release from the ARC-median eminence tissue taken from female rats [23]. Notably, previous studies have indicated the suppressive effect of SST-SSTR2 signaling on glutamate release from glutamatergic neurons in the cerebral cortex and retina of mice [24, 25] and in the hippocampus and basal forebrain of rats [26, 27]. These results indicate that glutamatergic interneurons may mediate suckling-induced suppression of LH release by serving as an action site of SST-SSTR2 signaling during lactation.
The present study examined whether SST-SSTR2 signaling is involved in the suckling-induced suppression of ARC Kiss1 expression and LH secretion and whether the ARC glutamatergic neurons act as interneurons in mediating the suckling-induced suppression of LH release by SST-SSTR2 signaling in rats during late lactation. To address these issues, we examined the effect of central administration of an SSTR2 antagonist on ARC Kiss1 expression in ovary-intact lactating rats on day 16 of lactation. Furthermore, we investigated the expression of Sstr2 or Grin1 in ARC Kiss1-expressing neurons using double in situ hybridization (ISH) in estradiol-17β (E2)-treated OVX non-lactating rats. We also investigated whether the suckling stimulus reduces the expression of Slc17a6, a glutamatergic neuronal marker gene, by comparing the expression of Slc17a6 between lactating and non-lactating rats. We also investigated the co-expression of Sstr2 and Slc17a6 by double ISH in the ARC of lactating and non-lactating rats. Finally, we investigated whether a central injection of an NMDA antagonist reverses the SSTR2 antagonist-induced increase in LH release in lactating rats.
Materials and Methods
Animals and experimental design
Wistar-Imamichi female rats (Institute for Animal Reproduction, Kasumigaura, Japan) were housed in a controlled environment (14 h light/10 h dark cycle; temperature at 22 ± 3°C) with free access to food and water. Female rats with at least two consecutive estrous cycles were mated with male rats. Pregnant female rats were housed individually until the day of parturition, and the day of parturition was designated as day 0 of lactation. The litter size in the lactating group was adjusted to eight on day 1. Some female dams were deprived of their litters on day 1, were OVX, and then subcutaneously implanted with a Silastic tubing (inner diameter 1.5 mm; outer diameter 3.0 mm; length 25 mm; Dow Corning, Midland, MI, USA) containing E2 (Sigma Aldrich, St Louis, MO, USA) dissolved in peanut oil (Sigma Aldrich) at 20 µg/ml to mimic the plasma E2 levels in intact lactating rats [9, 28, 29] to act as OVX + low E2 non-lactating rats. OVX and low E2 treatments were administered to avoid steroidal fluctuations associated with estrous cycles after pup removal. This treatment has been confirmed to suppress LH release during late lactation, as shown in ovary-intact lactating rats [9]. The low E2 treatment did not exhibit an inhibitory effect on ARC Kiss1 expression in virgin OVX female rats and non-lactating OVX rats 16 days after parturition [9, 30]. The ovary-intact lactating dams were used to examine the effect of central SSTR2 antagonism on the expression of Kiss1 in the ARC during the late lactation period (day 16 of lactation). Some lactating and non-lactating rats were used to compare Sstr2 and Slc17a6 expression in the ARC between lactating and non-lactating conditions. Furthermore, some lactating rats were used to investigate the effect of central glutamatergic antagonism on LH release recovered by the SSTR2 antagonist. Expression of Sstr2 or Grin1 in ARC Kiss1-expressing cells was determined in non-lactating rats. The non-lactating rats were used as ARC Kiss1 expression is largely inhibited in lactating dams [9]. Thus, some ARC Kiss1-expressing cells could not be identified in the lactating rats. If not otherwise specified, the surgical procedures were performed under anesthesia with an intraperitoneal injection of ketamine (27 mg/kg; Fujita, Tokyo, Japan)/xylazine (5.3 mg/kg; Bayer AG, Leverkusen, Germany) mixture followed by inhalation of 1% to 2% isoflurane (Pfizer Japan, Tokyo, Japan). The present study was approved by the Committee on Animal Experiments of the Graduate School of Bioagricultural Sciences, Nagoya University.
Administration of central SSTR2 antagonist with/without NMDA antagonist and blood sampling
Lactating or non-lactating rats were stereotaxically implanted with a stainless-steel guide cannula (22 gauge; P1 Technologies, Roanoke, VA, USA) for intracerebroventricular (icv) injection into the third ventricle (3V) on day 7 of lactation to investigate the effects of SSTR2 antagonist on ARC Kiss1 expression or LH pulses. The brain coordinates for the 3V injection were 0.8 mm posterior and 7.5 mm ventral to the bregma according to the rat brain atlas [31].
CYN154806 (CYN, Sigma Aldrich), an SSTR2-specific antagonist that binds to SSTR2 with high affinity (pIC50 8.6) and to other SST receptors with low affinity (pIC50 5.4–6.5) [32], was dissolved in ultrapure water (10 nmol/2 µl) and MK801 (Sigma Aldrich), an NMDA receptor antagonist, was dissolved in artificial cerebrospinal fluid (CSF) (100 nmol/3 µl). Free-moving conscious lactating rats were administered CYN (n = 4) to the 3V at a flow rate of 1 µl/min for 2 min on day 16 of lactation, using a microsyringe pump (EICOM, Kyoto, Japan) immediately after the first blood collection. Some lactating rats were administered MK801 (n = 4) to the 3V at a flow rate of 1 µl/min for 3 min followed by the 3V CYN administration. Control lactating rats (n = 4) were administered vehicle at the 3V. The CYN dose was selected according to our previous study, which revealed that the same dose of CYN significantly increased LH release in OVX lactating mother rats during early lactation (day 8 of lactation) [16]. The dose of MK801 was chosen according to a previous study, which showed that an icv injection of the same dose of MK801 blocked fos expression induced by dehydration in the median preoptic nucleus, supraoptic nucleus, and paraventricular nucleus in male rats [33]. Blood samples (150 µl) were collected every 6 min for 3 h through a silicone cannula (Shin-Etsu Polymer, Tokyo, Japan) that had been inserted into the right atrium via the jugular vein on the day before blood sampling. An equivalent volume of rat red blood cells, taken from donor rats and diluted with heparinized saline, was replaced through the atrial cannula after each blood collection. Plasma samples (50 µl) were obtained by immediate centrifugation and stored at −20°C until assayed for LH. At the end of blood sampling, the placement of the icv cannula was verified by visual inspection after the injection of the same amount of 3% brilliant blue solution using a microsyringe pump.
LH assays
Plasma LH concentrations were measured by a double-antibody radioimmunoassay (RIA) using a rat LH RIA kit provided by the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA, USA) and were expressed in terms of the NIDDK rat LH-RP-3. The lowest detectable level was 7.8 pg/tube for 50 µl plasma samples. Intra- and inter-assay coefficients of variation were 6.7% and 10.3%, respectively, at the level of 0.78 ng/ml.
Preparation of complementary RNA (cRNA) probes for ISH
Digoxigenin (DIG)-labeled antisense cRNA probes were designed for Kiss1 (GenBank accession number AY196983, nucleotide 33-348), Sstr2 (NM_019348, nucleotide 176-1092), Grin1 (NM_001270602, nucleotide 768-1678 and 1678-2609), and fluorescein isothiocyanate (FITC)-labeled antisense cRNA probes were designed for Slc17a6 (NM_053427.1, nucleotide 935-2053) and Kiss1. These probes were synthesized via in vitro transcription using complementary DNA (cDNA) obtained from the rat whole hypothalamus using DIG- or FITC-labeling mix (Roche Diagnostics, Basel, Switzerland), with the appropriate polymerase (T7 or T3) according to the direction of the cDNA insertion. The identification of cDNA insertions was confirmed using Sanger sequencing.
Brain sampling and single- and double-ISH for analysis of Kiss1, Sstr2, Grin1, and Slc17a6 expression in the ARC of lactating and non-lactating rats
Brain samples were collected from lactating and non-lactating rats 16 days after parturition. Some free-moving conscious lactating rats on day 16 of lactation were administered CYN or vehicle (n = 3–5) into the 3V as described above. Some non-lactating rats (n = 3) on postpartum day 16 were administered the vehicle at the 3V. One hour after injection, the animals were deeply anesthetized with sodium pentobarbital (40 mg/kg; Kyoritsu Seiyaku, Tokyo, Japan) and immediately perfused with 0.05 M phosphate-buffered saline (PBS) and subsequently with 4% paraformaldehyde (PFA) in 0.05 M phosphate buffer (PB). The brains were removed from the skulls and post-fixed with 4% PFA overnight at 4°C and retained in 30% sucrose in 0.05 M PB at 4°C until they sank under RNase-free conditions for 4 days. Serial hypothalamic coronal sections (50 µm in thickness) through the ARC, prepared using a cryostat (CM1800, Leica Biosystems, Wetzlar, Germany), were subjected to single ISH for Kiss1 (n = 3–5) or double ISH for Sstr2 and Slc17a6 (n = 3). Brain sections obtained from non-lactating rats (n = 3) were subjected to double ISH for Kiss1 and Sstr2, or Kiss1 and Grin1.
Free-floating single ISH for Kiss1 was conducted as previously described [34, 35]. Briefly, every fourth rat brain section was used for analyzing the ARC (13 sections, 1.72 to 4.36 mm posterior to the bregma) according to the rat brain atlas [31]. The sections were washed with 0.05 M PBS, treated with 1 µg/ml protease K for 15 min at 37°C, and incubated with 0.25% acetic anhydride in 0.1 M triethanolamine for 10 min. The sections were hybridized with 1.0 µg/µl DIG-labeled antisense cRNA probes overnight at 60°C. After hybridization, the sections were washed twice with 2 × saline-sodium citrate (SSC)-50% formamide for 15 min at 60°C, treated with 20 µg/ml RNase A for 30 min at 37°C, and steeped in 2 × SSC, 0.5 × SSC, and DIG-1 buffer (100 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.01% Tween-20) for 15 min twice. The sections were soaked in 1.5% blocking reagent (Boehringer Mannheim GmbH, Mannheim, Germany) in DIG-1 buffer for 1 h at 37°C and incubated with alkaline phosphatase-conjugated anti-DIG Fab fragment (1:1000; #11093274910, Roche Diagnostics; RRID: AB_2734716) for 2 h at 37°C. Subsequently, the sections were washed twice with DIG-1 buffer and then with DIG-3 buffer (100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 50 mM MgCl2). The hybridized and immuno-labeled probes were visualized with 4-nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indoyl-phosphate in DIG-3 buffer. The visualization reaction was terminated by a reaction stop solution (10 mM Tris-HCl, pH 7.5, 1 mM ethylenediaminetetraacetic acid, pH 8.0). The sections were observed under an Olympus BX53 light microscope, and the number of Kiss1-expressing cells throughout the ARC was bilaterally counted thrice in each section and then averaged.
Free-floating double labeling ISH was conducted, as described previously [16, 36], for the following combinations: Kiss1 and Sstr2, Kiss1 and Grin1, and Slc17a6 and Sstr2. Briefly, every fourth rat brain section containing the ARC was hybridized with 1.0 µg/µl FITC- and DIG-labeled antisense cRNA probes overnight at 60°C. The hybridized FITC-labeled probe was detected using peroxidase-conjugated anti-FITC Fab fragment (1:1000, #11426346910, Roche Diagnostics; RRID: AB_840257) and the TSA Plus FITC Kit (Perkin Elmer, Waltham, MA, USA), according to the manufacturer’s instructions. After inactivation of the peroxidase by incubating the brain sections in 0.1 N hydrochloric acid for 30 min, the DIG-labeled probe was detected using peroxidase-conjugated anti-DIG Fab fragment (1:500, #11207733910, Roche Diagnostics; RRID: AB_514500), TSA Plus Biotin Kit (Perkin Elmer), and DyLight594-conjugated streptavidin (Thermo Fisher Scientific, Waltham, MA, USA). Fluorescent images were captured and investigated under a fluorescence microscope with ApoTome optical sectioning (Carl Zeiss, Oberkochen, Germany). The numbers of Kiss1-,Sstr2-, Grin1-, and Slc17a6-expressing cells throughout the ARC were unilaterally counted thrice in each section and then averaged.
Statistical analysis
LH pulses were identified by the PULSAR computer program [37,38,39], and the mean LH concentrations and frequency and amplitude of LH pulses for a 3 h sampling period were calculated for each individual and then for the group. Statistical differences in the LH pulse parameters (mean LH concentrations and frequency of LH pulses) between groups, and the number of Kiss1-, Slc17a6-, Slc17a6/Sstr2-, and Sstr2-expressing cells in the ARC between groups were determined by one-way ANOVA followed by the Bonferroni test. Statistical differences in the amplitude of LH pulses between vehicle- and CYN-treated groups were determined using the Student’s t-test. All tests were conducted using R statistical software (release 3.5.1).
Results
Central SSTR2 antagonism increased the ARC Kiss1 expression in lactating rats
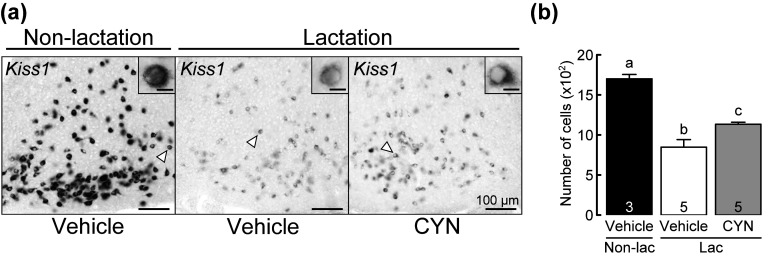
Figure 1a shows the photomicrographs of Kiss1-expressing cells in the ARC of representative non-lactating rats treated with vehicle at the 3V, and those of lactating rats treated with CYN or vehicle at the 3V. Kiss1 signals were strongly and abundantly found in the ARC of non-lactating rats. Kiss1 signals were also found in the ARC of both vehicle- and CYN-treated lactating dams, and were weaker than those in non-lactating rats. The number of Kiss1-expressing cells in the ARC is shown in Fig. 1b. One-way ANOVA revealed a significant difference in the number of ARC Kiss1-expressing cells between the groups [F (2,10) = 32.797, P < 0.001]. In particular, the Bonferroni test revealed that the number of ARC Kiss1-expressing cells in vehicle-treated (P < 0.001) or CYN-treated (P < 0.001) lactating rats was significantly lower than that of vehicle-treated non-lactating rats (n = 3–5, Fig. 1b). Importantly, the number of ARC Kiss1-expressing cells in CYN-treated lactating rats was significantly higher than that in vehicle-treated lactating rats (P = 0.032, Fig. 1b).
Fig. 1.
Effects of central administration of somatostatin receptor 2 (SSTR2) antagonist (CYN) on Kiss1 mRNA expression in the arcuate nucleus (ARC) of lactating rats. (a) Representative photomicrographs showing Kiss1 mRNA expression in the ARC of non-lactating rats injected with vehicle in the third ventricle (3V) and lactating rats injected with vehicle or CYN in the 3V 1 h before brain sampling. Insets indicate Kiss1-expressing cells at higher magnification. Scale bars in the insets represent 10 µm. (b) Number of Kiss1-expressing cells in the ARC of vehicle-treated non-lactating rats (closed column), vehicle-treated lactating rats (open column), and CYN-treated lactating rats (shaded column). Values are the means ± SEM. Numbers in each column indicate the number of animals used. Values marked with different letters are significantly different (P < 0.05, one-way ANOVA followed by Bonferroni test).
Determination of Sstr2 or Grin1 expression in the ARC Kiss1-positive cells in female rats
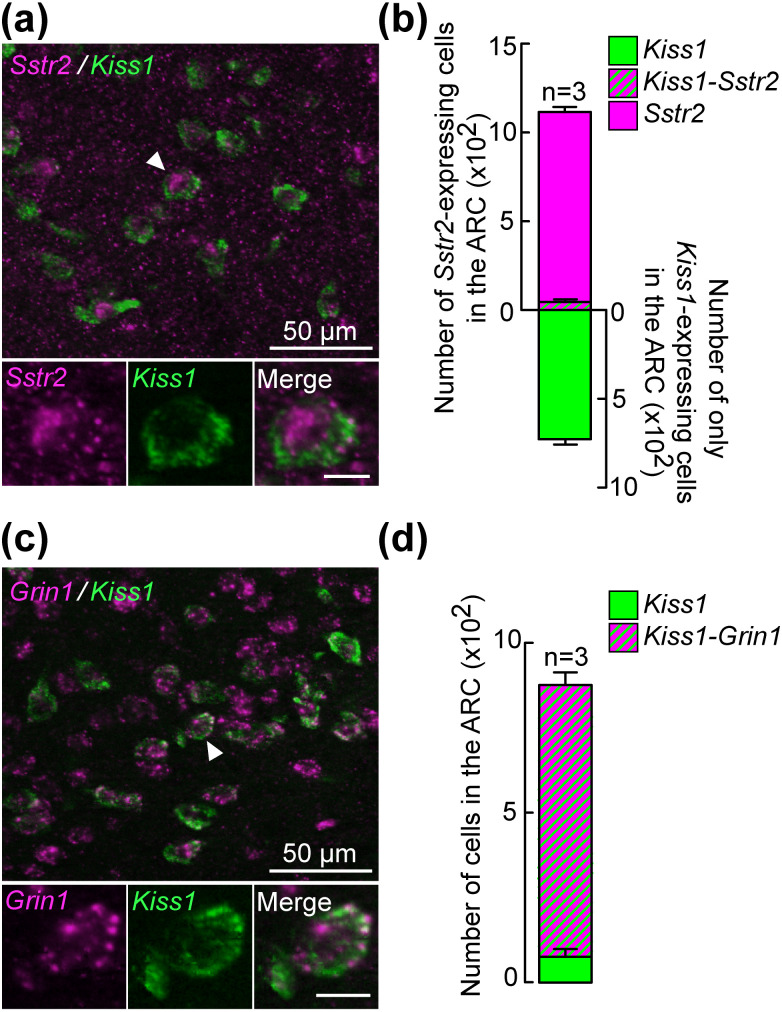
Figure 2a shows the representative photomicrographs of Sstr2 and Kiss1 signals determined by a double ISH in the ARC of non-lactating rats 16 days after parturition. Several Sstr2- or Kiss1-positive cells were found in the ARC of the animals. Quantitative analysis revealed that 5.6% of Kiss1-expressing cells (44.4 ± 15.6 out of 772.8 ± 44.3 cells, n = 3) were dual-labeled with Sstr2 signals in the ARC (Fig. 2b). Figure 2c shows the representative photomicrographs of Grin1- and Kiss1-positive cells in the ARC of a non-lactating animal. Quantitative analysis revealed that a majority (91.2%, 799.7 ± 37.4 out of 876.0 ± 17.3 cells, n = 3) of Kiss1-expressing cells showed Grin1 signals in the ARC of the animals (Fig. 2d).
Fig. 2.
Co-expression of Sstr2 (SSTR2 gene) or Grin1, an N-methyl-D-aspartate (NMDA) receptor marker gene, in the ARC Kiss1-expressing cells of non-lactating rats. (a) Representative photomicrographs showing Sstr2- (magenta) and Kiss1- (green) expressing cells in the ARC of non-lactating rats. A dual-labeled cell marked with an arrowhead is shown as magnified images showing Sstr2- and Kiss1-positive and merged cells. Scale bars in the magnified images represent 10 µm. (b) Number of cells expressing Sstr2 alone (magenta), both Sstr2 and Kiss1 (magenta/green), or Kiss1 alone (green) in the ARC were quantified and shown in a stacked bar graph. Values are the means ± SEM. (c) Representative photomicrographs showing Grin1- (magenta) and Kiss1- (green) expressing cells in the ARC of non-lactating rats. A dual-labeled cell marked with an arrowhead is shown as a magnified image showing Grin1- and Kiss1-positive and merged cells. (d) Number of cells expressing both Grin1 and Kiss1 (magenta/green) or Kiss1 alone (green) in the ARC were quantified and are shown in a stacked bar graph. Values are the means ± SEM.
Decrease in the number of Slc17a6-expressing cells and of Slc17a6- and Sstr2-co-expressing cells in the ARC of lactating rats
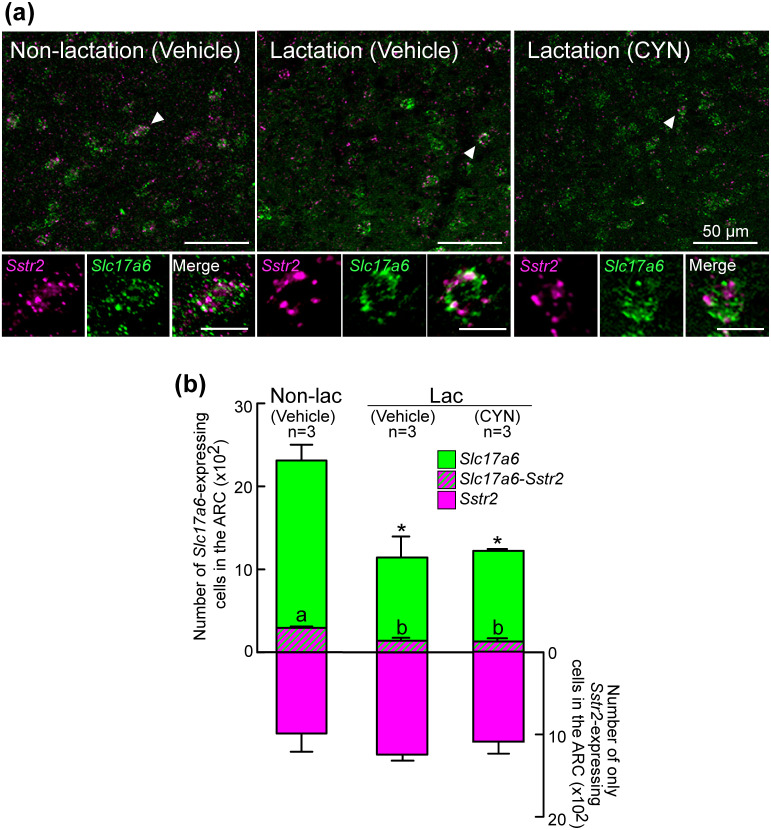
Figure 3a shows the representative photomicrographs of Sstr2 and Slc17a6 signals in the ARC of lactating rats treated with 3V injection of CYN or vehicle and non-lactating rats treated with 3V injection of vehicle. Many Slc17a6-expressing cells were found in the ARC of non-lactating rats, whereas fewer Slc17a6-expressing cells were found in the ARC of lactating groups. Sstr2-positive cells were also found in the ARC in all the groups. One-way ANOVA revealed a significant difference in the number of Slc17a6-positive cells between the groups [F (2,6) = 12.33, P = 0.007]. In particular, the Bonferroni test revealed that the number of Slc17a6-positive cells in vehicle-treated (P = 0.013) or CYN-treated (P = 0.018) lactating rats was significantly lower than that of non-lactating rats (Fig. 3b). No significant difference was found in the number of Slc17a6-positive cells between the lactating groups with and without CYN treatment.
Fig. 3.
Sstr2 (SSTR2 gene) and Slc17a6 (a glutamatergic neuronal marker gene) expression in the ARC of non-lactating and lactating rats. (a) Representative photomicrographs showing Sstr2- (magenta) and Slc17a6- (green) expressing cells in the ARC of vehicle-treated non-lactating rats, and vehicle- or CYN-treated lactating rats. A dual-labeled cell marked with arrowheads was shown as magnified images showing Sstr2- and Slc17a6-positive and merged cells. Scale bars in the magnified images represent 10 µm. (b) Number of cells expressing Sstr2 alone (magenta), both Sstr2 and Slc17a6 (magenta/green), or Slc17a6 alone (green) in the ARC of each group were quantified and are shown in a stacked bar graph. Values are shown as the means ± SEM. Values marked with an asterisk are significantly different (P < 0.05, one-way ANOVA followed by Bonferroni test) in the number of Slc17a6-expressing cells in the ARC from that in the non-lactating group. Values marked with different letters are significantly different (P < 0.05, one-way ANOVA followed by Bonferroni test) from each other in the number of the ARC cells co-expressing Sstr2 and Slc17a6.
Sstr2 signals were found in a part of Slc17a6-positive cells in the ARC of non-lactating rats (12.9%, 294.8 ± 18.5 out of 2312.2 ± 201.0 Slc17a6-expressing cells, n = 3), vehicle-treated lactating rats (11.9%, 137.7 ± 35.5 out of 1137.8 ± 253.1 Slc17a6-expressing cells, n = 3), and CYN-treated lactating rats (10.5%, 128.6 ± 20.9 out of 1221.2 ± 4.8 Slc17a6-expressing cells, n = 3). One-way ANOVA revealed a significant difference in the number of Sstr2-expressing Slc17a6-positive cells [F (2,6) = 12.858, P = 0.007] between the groups. In particular, the Bonferroni test revealed that the number of Sstr2-expressing Slc17a6-positive cells in vehicle-treated (P = 0.016) or CYN-treated (P = 0.012) lactating rats was significantly lower than that of non-lactating rats (n = 3, Fig. 3b). No significant difference was found in the number of Sstr2-expressing cells between the groups.
Central glutamatergic antagonism blocked the increase in LH release induced by SSTR2 antagonist in lactating rats
Figure 4a shows the LH release profile in lactating rats bearing a 3V injection of vehicle or CYN with or without co-administration of glutamatergic NMDA receptor antagonist (MK801) on day 16 of lactation. LH release was inhibited in vehicle-injected control lactating dams and could be detected in lactating dams treated with 3V CYN. In contrast, LH release was inhibited in CYN-treated lactating rats when MK801 was co-administered into the 3V. One-way ANOVA revealed a significant difference in mean LH concentrations [F (2, 9) = 88.672, P < 0.001] between the groups. In particular, the Bonferroni test revealed that the mean LH concentrations in lactating rats treated with CYN were significantly higher than those in vehicle-treated lactating rats (P < 0.001), and that the mean LH concentrations in lactating rats treated with both CYN and MK801 were significantly lower than those in the vehicle (P < 0.001) or CYN-treated (P = 0.021) lactating rats (Fig. 4b). LH pulse frequency tended to increase in CYN-treated animals and decrease in CYN/MK801-treated lactating dams compared to that in vehicle-treated controls. However, significant differences were not observed between groups (Fig. 4c). LH pulse amplitude in lactating animals treated with CYN was significantly higher than that in vehicle-treated controls (P = 0.005, Student's t-test). Additionally, statistical analysis between the three groups was not applicable for the amplitude of LH pulses as LH pulses were found in two out of four CYN- and MK801-treated lactating dams (Fig. 4d).
Fig. 4.
Effects of the 3V injection of SSTR2 antagonist (CYN) with or without co-administration of glutamatergic NMDA receptor antagonist (MK801) on LH secretion in lactating rats on day 16 of lactation. (a) Profile of plasma LH levels in lactating rats treated with vehicle or CYN in combination with vehicle (CSF) or MK801 immediately after the onset of blood sampling (arrows). Arrowheads indicate the peaks of LH pulses detected by the PULSAR computer program. Mean LH concentrations (b) and the frequency (c) and amplitude of LH pulses (d) of lactating rats in each group. Open, shaded, and hatched bars show values (means ± SEM) in vehicle-, CYN-, and CYN/MK801-treated lactating rats, respectively. Numbers in each column indicate the number of animals used. Values marked with different letters are significantly different (P < 0.05, one-way ANOVA followed by Bonferroni test) from each other. The amplitude of LH pulses between vehicle- and CYN-treated lactating rats is significantly different (* P = 0.005, Student’s t-test).
Discussion
The present study demonstrates that SST-SSTR2 signaling, at least partly, mediates the suckling-induced suppression of LH release by inhibiting kisspeptin neurons in lactating rats as a central administration of SSTR2 antagonist significantly increased plasma LH levels and Kiss1 expression in the ARC of rats during late lactation. The present study also suggests that the ARC glutamatergic neurons mediate the suckling-induced suppression of LH release by SST-SSTR2 signaling in lactating rats as central administration of an NMDA antagonist reversed the SSTR2 antagonist-induced increase in LH release in lactating dams. Furthermore, the present study revealed that a part of the ARC Slc17a6-positive glutamatergic neurons co-expressed Sstr2, most of the ARC Kiss1-positive cells co-expressed Grin1 (NMDA receptor marker gene), and the number of Slc17a6-expressing cells decreased in the ARC of lactating rats as compared to that in non-lactating rats. Taken together, these results suggest that the suckling stimulus suppresses ARC Kiss1 expression and consequent LH release through, at least partly, inhibitory SST-SSTR2 signaling and suppression of the ARC glutamatergic neurons in lactating rats.
Our previous study revealed that in early lactating OVX rats, central administration of an SSTR2 antagonist significantly restored LH pulses [16]. This study revealed the involvement of SST-SSTR2 signaling in lactational LH suppression during the late lactation period. These findings suggest that SST-SSTR2 signaling is involved in LH suppression throughout the lactation period in rats. The involvement of SST-SSTR2 signaling in suppressing LH release during late lactation in rats is consistent with a previous study, which showed that central SST signaling could be involved in the estradiol negative feedback effects on LH release in ewes [40]. Taken together, these findings suggest that SST-SSTR2 signaling mediates suckling stimulus-induced inhibition of ARC Kiss1 expression, resulting in the suppression of GnRH/LH release during the entire lactation period in rats.
Glutamatergic neurons stimulate GnRH/LH secretion by activating kisspeptin neurons [21]. The present study revealed that SSTR2 expression in ARC kisspeptin neurons was extremely limited in female rats, suggesting that SST might indirectly inhibit ARC Kiss1 expression and consequent GnRH/LH release by affecting stimulatory interneurons for kisspeptin neuronal activity. As expected, the present study suggests that the inhibitory SST-SSTR2 signal, at least partly, affects ARC glutamatergic neurons. This is because the current central NMDA receptor antagonism reversed the increase in LH release induced by the SSTR2 antagonist in lactating dams. Furthermore, Sstr2 was co-expressed in some ARC glutamatergic neurons, and Grin1, a subunit of the NMDA receptor, was evident in most ARC kisspeptin neurons in female rats. Notably, the number of ARC Slc17a6-positive glutamatergic cells in lactating dams was significantly lower than that in non-lactating controls, regardless of the SSTR2 antagonist treatment. This suggests that the suckling stimulus reduced the ARC glutamatergic neurons via the SSTR2-independent pathway. Furthermore, this study revealed that numerous non-glutamatergic Sstr2-expressing cells were abundantly located in the ARC of lactating dams. These findings imply that interneurons other than glutamatergic neurons may also be involved in the SST-induced inhibition of LH release in late lactating rats. Further studies are required to address these issues.
Notably, the current central administration of the SSTR2 antagonist significantly increased the mean LH levels but failed to affect the LH pulse frequency. These results suggest that SST-SSTR2 signaling may not be involved in the direct inhibition of GnRH pulse generator activity. This notion is supported by the current result that Sstr2 expression is limited in ARC kisspeptin neurons, namely KNDy neurons, which are responsible for GnRH pulse generation [13]. Furthermore, it is also possible that the current SSTR2 antagonist may directly affect GnRH neurons, as SSTR2 is highly expressed in GnRH neurons and that SST-immunoreactive fibers are closely localized in GnRH neuronal fibers in the organum vasculosum of the lamina terminalis in female rats [41]. Additionally, SST-immunoreactive fibers are appositions with 50–60% of GnRH neurons in male and female mice [42].
In this study, the number of ARC Sstr2-expressing cells was comparable between lactating and non-lactating rats during the late lactation period. Our previous study revealed that the number of ARC Sstr2-expressing cells in early lactating rats was comparable to that in non-lactating control rats; while Sstr2 mRNA levels in the ARC-ME region in lactating rats were significantly higher than those in non-lactating rats [16]. These results imply that Sstr2 mRNA levels per cell would be increased by suckling stimulus in both early and late lactation periods.
Our previous study revealed that the suckling stimulus increased Sst expression in the posterior intralaminar complex of the thalamus (PIL) and Sstr2 expression in the ARC, in which the Sstr2 expression was higher than other SST receptor mRNAs in rats during early lactation [16]. Thus, as shown in Fig. 5, we envisioned that the suckling stimulus may also activate the PIL SST neurons during late lactation. The resultant direct (on kisspeptin neurons) and indirect (on glutamatergic neurons) SST-SSTR2 signaling may inhibit Kiss1 expression and subsequent GnRH/LH release during late lactation. Interestingly, Dufourny et al. demonstrated that most ARC kisspeptin neurons showed SST appositions in both male and female rats and that one-third of kisspeptin neurons expressed SST receptor 1 (SSTR1)-immunoreactivity in male rats [43]. Thus, SST may directly inhibit ARC kisspeptin neurons via SSTR1 in female rats during lactation. Further studies are needed to understand the roles of SST-SSTR1 signaling in lactational anestrus.
Fig. 5.
Schematic showing the possible brain mechanism underlying suckling stimulus-induced inhibition of ARC Kiss1 expression and consequent GnRH/LH pulses in lactating rats based on the present and previous studies [16]. The suckling stimulus seems to activate the PIL SST neurons, and the resultant inhibitory SST-SSTR2 signaling may result in the inhibition of Kiss1 expression in KNDy neurons and subsequent GnRH/LH pulses via direct (on kisspeptin neurons) and indirect (on stimulatory glutamatergic neurons) pathways. Glu, Glutamatergic; GluR, Glutamatergic receptor.
In conclusion, the present study demonstrates that SST-SSTR2 signaling, at least partly, mediates suckling-induced suppression of GnRH/LH release and ARC Kiss1 expression via inhibition of hypothalamic glutamatergic neurons during the late lactating period in rats.
Conflict of interests
The authors declare no conflicts of interest.
Acknowledgments
We acknowledge the great contributions of the late Dr. Kei-ichiro Maeda for his expert advice and supervision of the study on lactational anestrus. The authors are grateful to the National Hormone and Peptide Program for the rat LH assay kit. The radioimmunoassay was conducted at the Nagoya University Radioisotope Research Center. We also thank Dr. Kana Ikegami, Dr. Youki Watanabe, and Dr. Nahoko Ieda for their technical advice. This work was partially supported by a Grant-in-Aid for a Research Fellow of the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 16J05404 (to AS), JSPS KAKENHI Grant Numbers 23380163, 18H03973, 21H05031 (to H Tsuk), 19H03103 (to NI), and 20H03127 (to YU). This research was also partially supported by JSPS KAKENHI Grant Number 24380157 to the late Dr. Kei-ichiro Maeda (The University of Tokyo).
References
- 1.Tsukamura H. Kobayashi Award 2019: The neuroendocrine regulation of the mammalian reproduction. Gen Comp Endocrinol 2022; 315: 113755. [DOI] [PubMed] [Google Scholar]
- 2.Van Ginneken JK. Prolonged breastfeeding as a birth spacing method. Stud Fam Plann 1974; 5: 201–206. [PubMed] [Google Scholar]
- 3.Walters DL, Short RE, Convey EM, Staigmiller RB, Dunn TG, Kaltenbach CC. Pituitary and ovarian function in postpartum beef cows. III. Induction of estrus, ovulation and luteal function with intermittent small-dose injections of GnRH. Biol Reprod 1982; 26: 655–662. [DOI] [PubMed] [Google Scholar]
- 4.Ford JJ, Melampy RM. Gonadotropin levels in lactating rats: effect of ovariectomy. Endocrinology 1973; 93: 540–547. [DOI] [PubMed] [Google Scholar]
- 5.Hammons JA, Velasco M, Rothchild I. Effect of the sudden withdrawal or increase of suckling on serum LH levels in ovariectomized postparturient rats. Endocrinology 1973; 92: 206–211. [DOI] [PubMed] [Google Scholar]
- 6.Smith MS, Neill JD. Inhibition of gonadotropin secretion during lactation in the rat: relative contribution of suckling and ovarian steroids. Biol Reprod 1977; 17: 255–261. [DOI] [PubMed] [Google Scholar]
- 7.Maeda KI, Tsukamura H, Yokoyama A. Suppression of luteinizing hormone secretion is removed at late lactation in ovariectomized lactating rats. Endocrinol Jpn 1987; 34: 709–716. [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, Adachi S, Inoue K, Maeda KI, Tsukamura H. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology 2007; 148: 2226–2232. [DOI] [PubMed] [Google Scholar]
- 9.Yamada S, Uenoyama Y, Deura C, Minabe S, Naniwa Y, Iwata K, Kawata M, Maeda KI, Tsukamura H. Oestrogen-dependent suppression of pulsatile luteinising hormone secretion and Kiss1 mRNA expression in the arcuate nucleus during late lactation in rats. J Neuroendocrinol 2012; 24: 1234–1242. [DOI] [PubMed] [Google Scholar]
- 10.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 2007; 148: 5752–5760. [DOI] [PubMed] [Google Scholar]
- 11.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009; 29: 11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda KI, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagae M, Uenoyama Y, Okamoto S, Tsuchida H, Ikegami K, Goto T, Majarune S, Nakamura S, Sanbo M, Hirabayashi M, Kobayashi K, Inoue N, Tsukamura H. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci USA 2021; 118: e2009156118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA 2017; 114: E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology 2013; 154: 4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugimoto A, Tsuchida H, Ieda N, Ikegami K, Inoue N, Uenoyama Y, Tsukamura H. Somatostatin-somatostatin receptor 2 signaling mediates LH pulse suppression in lactating rats. Endocrinology 2019; 160: 473–483. [DOI] [PubMed] [Google Scholar]
- 17.Brann DW, Mahesh VB. Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation. Front Neuroendocrinol 1994; 15: 3–49. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami S, Ichikawa M, Murahashi K, Hirunagi K, Tsukamura H, Maeda KI. Excitatory amino acids act on the median eminence nerve terminals to induce gonadotropin-releasing hormone release in female rats. Gen Comp Endocrinol 1998; 112: 372–382. [DOI] [PubMed] [Google Scholar]
- 19.Smith MJ, Jennes L. Neural signals that regulate GnRH neurones directly during the oestrous cycle. Reproduction 2001; 122: 1–10. [DOI] [PubMed] [Google Scholar]
- 20.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 2011; 173: 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uenoyama Y, Nakamura S, Hayakawa Y, Ikegami K, Watanabe Y, Deura C, Minabe S, Tomikawa J, Goto T, Ieda N, Inoue N, Sanbo M, Tamura C, Hirabayashi M, Maeda KI, Tsukamura H. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol 2015; 27: 187–197. [DOI] [PubMed] [Google Scholar]
- 22.Ieda N, Assadullah, Minabe S, Ikegami K, Watanabe Y, Sugimoto Y, Sugimoto A, Kawai N, Ishii H, Inoue N, Uenoyama Y, Tsukamura H. GnRH(1-5), a metabolite of gonadotropin-releasing hormone, enhances luteinizing hormone release via activation of kisspeptin neurons in female rats. Endocr J 2020; 67: 409–418. [DOI] [PubMed] [Google Scholar]
- 23.Murahashi K, Tsukahara S, Tsukamura H. The arcuate nucleus mediates facilitating effect of estrogen on glutamate-induced in vitro GnRH release from nerve terminals of female rats. J Reprod Dev 2002; 48: 183–188. [Google Scholar]
- 24.Dal Monte M, Petrucci C, Cozzi A, Allen JP, Bagnoli P. Somatostatin inhibits potassium-evoked glutamate release by activation of the sst(2) somatostatin receptor in the mouse retina. Naunyn Schmiedebergs Arch Pharmacol 2003; 367: 188–192. [DOI] [PubMed] [Google Scholar]
- 25.Grilli M, Raiteri L, Pittaluga A. Somatostatin inhibits glutamate release from mouse cerebrocortical nerve endings through presynaptic sst2 receptors linked to the adenylyl cyclase-protein kinase A pathway. Neuropharmacology 2004; 46: 388–396. [DOI] [PubMed] [Google Scholar]
- 26.Kozhemyakin M, Rajasekaran K, Todorovic MS, Kowalski SL, Balint C, Kapur J. Somatostatin type-2 receptor activation inhibits glutamate release and prevents status epilepticus. Neurobiol Dis 2013; 54: 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Momiyama T, Zaborszky L. Somatostatin presynaptically inhibits both GABA and glutamate release onto rat basal forebrain cholinergic neurons. J Neurophysiol 2006; 96: 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cagampang FRA, Maeda KI, Tsukamura H, Ohkura S, Ota K. Involvement of ovarian steroids and endogenous opioids in the fasting-induced suppression of pulsatile LH release in ovariectomized rats. J Endocrinol 1991; 129: 321–328. [DOI] [PubMed] [Google Scholar]
- 29.Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol 2009; 21: 527–537. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda KI. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005; 146: 4431–4436. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The rat brain in sterotaxic coordinates. 6th ed. San Diego: Academic press; 2008. [Google Scholar]
- 32.Feniuk W, Jarvie E, Luo J, Humphrey PPA. Selective somatostatin sst(2) receptor blockade with the novel cyclic octapeptide, CYN-154806. Neuropharmacology 2000; 39: 1443–1450. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z, Herbert J. Effects of intracerebroventricular dizocilpine (MK801) on dehydration-induced dipsogenic responses, plasma vasopressin and c-fos expression in the rat forebrain. Brain Res 1998; 784: 91–99. [DOI] [PubMed] [Google Scholar]
- 34.Minabe S, Nakamura S, Fukushima E, Sato M, Ikegami K, Goto T, Sanbo M, Hirabayashi M, Tomikawa J, Imamura T, Inoue N, Uenoyama Y, Tsukamura H, Maeda KI, Matsuda F. Inducible Kiss1 knockdown in the hypothalamic arcuate nucleus suppressed pulsatile secretion of luteinizing hormone in male mice. J Reprod Dev 2020; 66: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikegami K, Goto T, Nakamura S, Watanabe Y, Sugimoto A, Majarune S, Horihata K, Nagae M, Tomikawa J, Imamura T, Sanbo M, Hirabayashi M, Inoue N, Maeda KI, Tsukamura H, Uenoyama Y. Conditional kisspeptin neuron-specific Kiss1 knockout with newly generated Kiss1-floxed and Kiss1-Cre mice replicates a hypogonadal phenotype of global Kiss1 knockout mice. J Reprod Dev 2020; 66: 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Assadullah, Ieda N, Kawai N, Ishii H, Ihara K, Inoue N, Uenoyama Y, Tsukamura H. Co-expression of the calcitonin receptor gene in the hypothalamic kisspeptin neurons in female rats. Reprod Med Biol 2018; 17: 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda KI, Tsukamura H, Uchida E, Ohkura N, Ohkura S, Yokoyama A. Changes in the pulsatile secretion of LH after the removal of and subsequent resuckling by pups in ovariectomized lactating rats. J Endocrinol 1989; 121: 277–283. [DOI] [PubMed] [Google Scholar]
- 38.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol 1982; 243: E310–E318. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchida H, Kawai N, Yamada K, Takizawa M, Inoue N, Uenoyama Y, Tsukamura H. Central µ-opioid receptor antagonism blocks glucoprivic LH pulse suppression and gluconeogenesis/feeding in female rats. Endocrinology 2021; 162: 1–12. [DOI] [PubMed] [Google Scholar]
- 40.Pillon D, Caraty A, Fabre-Nys C, Lomet D, Cateau M, Bruneau G. Regulation by estradiol of hypothalamic somatostatin gene expression: possible involvement of somatostatin in the control of luteinizing hormone secretion in the ewe. Biol Reprod 2004; 71: 38–44. [DOI] [PubMed] [Google Scholar]
- 41.Koyama M, Yin C, Ishii H, Sakuma Y, Kato M. Somatostatin inhibition of GnRH neuronal activity and the morphological relationship between GnRH and somatostatin neurons in rats. Endocrinology 2012; 153: 806–814. [DOI] [PubMed] [Google Scholar]
- 42.Bhattarai JP, Kaszás A, Park SA, Yin H, Park SJ, Herbison AE, Han SK, Abrahám IM. Somatostatin inhibition of gonadotropin-releasing hormone neurons in female and male mice. Endocrinology 2010; 151: 3258–3266. [DOI] [PubMed] [Google Scholar]
- 43.Dufourny L, Delmas O, Teixeira-Gomes AP, Decourt C, Sliwowska JH. Neuroanatomical connections between kisspeptin neurones and somatostatin neurones in female and male rat hypothalamus: A possible involvement of SSTR1 in kisspeptin release. J Neuroendocrinol 2018; 30: 0–2. [DOI] [PubMed] [Google Scholar]