Highlights
-
•
Intermediate/high risk elderly prostate cancer benefits less from received standards of care.
-
•
Age is an independent factor for disease control and tolerance.
-
•
Brachytherapy boost remains efficient and feasible in the elderly.
-
•
Careful discussion for super elderly patients (>80 y) is warranted.
-
•
Oncogeriatric assessment is necessary to identify best candidates.
Abbreviations: ADT, androgen deprivation therapy; BT, brachytherapy; bRFS, biochemical relapse free survival; CSS, cancer specific survival; CT, computerized tomography; CTCAE, common terminology criteria for adverse events; CTV, clinical target volume; D2cc, dose delivered to 2cc of the organ at risk; D90, dose delivered to 90% of the clinical target volume; D100, dose delivered to 100% of CTV; DFS, disease-free survival; DNR, dose non-homogeneity ratio; EBRT, external beam radiation therapy; EQD2, equivalent dose at 2 Gy per fraction; GI, gastro-intestinal; GU, genito-urinary; HDB, high-dose rate brachytherapy; HR, high risk; IMRT, intensity modulated radiation therapy; IR, intermediate risk; ISUP, International Society of Urological Pathology; LDR, low dose-rate; LR, low risk; lRFS, local relapse free survival; MFU, median follow up; mRFS, metastatic relapse-free survival; MRI, magnetic resonance imaging; NCCN, national comprehensive cancer network; OAR, organs at risks; OS, overall survival; PC, prostate cancer; PET, positron emission tomography; PSA, prostate specific antigen; pts, patients; QoL, quality of life; RCT, randomized clinical trial; rRFS, regional lymph-node relapse free survival; TD, total dose; V100, percentage of the clinical target volume receiving 100% of the prescribed dose; V150, percentage of the clinical target volume receiving 150% of the prescribed dose; V200, percentage of the clinical target volume receiving 200% of the prescribed dose; Vr90, percentage of the rectum volume receiving 90% of the prescribed dose; Vr100, percentage of the rectum volume receiving 100% of the prescribed dose; Vu115, percentage of the urethra volume receiving 115% of the prescribed dose; Vu125, percentage of the urethra volume receiving 125% of the prescribed dose; 3DRT, three-dimensional radiation therapy
Keywords: Prostate cancer, High-risk, Brachytherapy, Boost, Eldery, Comorbidity, Oncogeriatric assessment
Abstract
Purpose
To analyze the oncological outcome in elderly (>70 years) prostate cancer after high-dose rate brachytherapy (HDB) boost.
Materials/methods
In this retrospective study, patients with intermediate (IR) and high-risk (HR) prostate cancer underwent external beam radiation therapy (EBRT) followed by HDB boost with/without androgen deprivation therapy (ADT). The impact of age (≤70y vs. > 70y) was investigated. Oncological outcome focused on biochemical relapse-free survival (bRFS), cause-specific (CSS) and overall survival (OS). Late genito-urinary (GU) and gastro-intestinal (GI) toxicities were investigated.
Results
From 07/08 to 01/22, 518 pts received a HDB boost, and 380 were analyzed (≤70y:177pts [46.6%] vs. > 70y:203pts [53.4%]). Regarding NCCN classification, 98 pts (≤70y: 53pts; >70y: 45pts; p = 0.107) and 282 pts (≤70y: 124pts; >70y: 158pts; p = NS) were IR and HR pts respectively. Median EBRT dose was 46 Gy [37.5–46] in 23 fractions [14–25]. HDB boost delivered a single fraction of 14/15 Gy (79%). ADT was used in 302 pts (≤70y: 130pts; >70y: 172pts; p = 0.01). With MFU of 72.6 months [67–83] for the whole cohort, 5-y bRFS, 5-y CSS and 5-y OS were 88% [85–92], 99% [97–100] and 94% [92–97] respectively; there was no statistical difference between the two age groups except for 5-y CSS (p = 0.05). Late GU and GI toxicity rates were 32.4% (G ≥ 3 7.3%) and 10.1% (no G3) respectively.
Conclusions
For IR and HR prostate cancers, HDB boost leads to high rates of disease control with few late G ≥ 3 GU/GI toxicities. For elderly pts, HDB boost remains warranted mainly in HR pts, while competing comorbidity factors influence OS.
Introduction
Prostate cancer (PC) is the second most common cancer in the world, and the most frequent after the age of 70. According to estimates, its incidence in the next twenty years will double in men over 70, with 1.44 million new cases (compared to 709,000 today) [1], [2]. The management of localized PC in older adults is already a challenge for clinicians and will be a major public health issue in the future.
The current recommended standard of care for localized intermediate (IR) to high-risk (HR) PC is prostatectomy or a combination of androgen deprivation therapy (ADT) plus definitive radiotherapy, which may be external beam radiotherapy (EBRT) with or without brachytherapy (BT) boost allowing dose escalation [3]. In the 3 randomized clinical trials (RCT) which compared PC irradiation with or without BT boost, the latter demonstrated a significant biochemical relapse-free survival (bRFS) benefit, with no overall survival (OS) impact [4], [5], [6], [7]. In the ASCENDE-RT trial, brachytherapy boost significantly decreased urinary function [7]. However, these RCT included patients up to 80 years old, without specific sub-group analysis related to age which was only reported as a predictive factor of OS [7]. Elderly patients remain under-represented or under-evaluated in RCTs, making elderly PC management complex and controversial due to lack of robust and consensual data. Furthermore, the National Comprehensive Cancer Network (NCCN) guidelines are less applied to HR subjects over 75 y, which can lead to a risk of inappropriate or even under-treatment [8], [9], [10], [11], [12], [13], [14], [15]. However, chronological age is not the only factor to consider in the management of older adults, whose PC is often associated with poorer histological criteria combined with competing comorbidities [16].
Elderly PC irradiation has recently been investigated, showing both oncological outcomes and toxicity profiles comparable to younger populations [17], [18], [19], [20], [21]. The impact of EBRT plus BT boost was also investigated in IR and HR elderly PC populations. Although this treatment improved bRFS, cause-specific survival (CSS) and OS, results must be viewed with caution owing to the lack of data regarding patient comorbidities and treatment-related toxicities [21], [22], [23], [24].
We therefore conducted a study comparing EBRT with high-dose-rate (HDR) BT boost and ADT in men under, and over, 70 y with IR to HR-PC.
Material and methods
An observational, retrospective, single institution study was performed in the Antoine Lacassagne Cancer Center in Nice (France) for patients with IR to HR-PC receiving HDR-BT boost. Before data collection, the consent of all patients was obtained. In accordance with current legislation, data collection was registered at the National Health Data Hub under the number F20201008194116.
Patients
Patients with histologically proven localized PC, who received EBRT combined with HDR-BT boost, were eligible. Exclusion criteria were lymph node involvement, metastatic disease and other non-controlled neoplasia. Patients with IR or HR-PC with a follow-up of at least 18 months were selected. Patients underwent a clinical rectal examination, prostate-specific antigen (PSA) blood test, pelvic magnetic resonance imaging (MRI) and/or computed tomography scan (CT) and bone scan at the time of diagnosis. Tumors were classified according to NCCN guidelines and the International Society of Urological Pathology (ISUP) grading [3], [25].
Treatment modalities
External beam radiotherapy (EBRT)
The planning CT-scan was performed in the treatment position with 2.5 mm slices. The target volume (CTV) was defined using the planning CT-scan and covered the prostate gland up to the first third of the seminal vesicles with or without pelvic lymph node irradiation (obturator, bilateral internal/external iliac vessels up to L5/S1 or 1.5 cm above their bifurcation). A 10 mm margin (PTV) reduced to 5 mm at the rectal level was applied to the predefined CTV. The EBRT delivered dose was 46 Gy (ICRU point) in 23 fractions of 2 Gy, based on three-dimensional conformational (3DRT) radiotherapy or intensity modulated radiotherapy (IMRT), using 6 or 10 MV X-photons. Organs at risk (OARs) included rectum, bladder, and bowel. Delineation and dose constraints for OARs were based on the RECORAD 2.0 recommendations [26].
High-dose-rate brachytherapy boost (HDR-BT boost)
The brachytherapy technique has already been described [27]. Briefly, HDR-BT boost was performed during the month before or after EBRT. Under general or spinal anesthesia, a triple lumen urinary catheter was inserted into the bladder. Transperineal needles were then implanted under endorectal ultrasound guidance using a dedicated perineal template sutured to the skin. After leaving the recovery room, patients underwent a post-implant CT-scan for treatment planning purposes. The CTV included the entire prostate and OARs (rectum, urethra and bowel) were delineated. Dose-volume adaptation was performed manually by graphical optimization (OncentraBrachy, Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden; Sagiplan™ Eckert&Ziegler BEBIG, Berlin, Germany). Three different irradiation regimens were used: 3 fractions (total dose- TD: 18 Gy), 2 fractions (TD: 18 Gy) and 1 fraction (TD: 14 then 15 Gy). Prostate dose constraints were V100 > 95%, V150 < 35%, and V200 < 15%, D90 > 105%, and D100 > 80%. Because of dose escalation between multi- and mono-fractioned schemes, dose constraints used for OARs relied on the BT regimen groups. For 2 or 3 fraction BT regimens, Vu125 and Vr100 should be<1%, while for 1 fraction BT protocols, Vu115 and Vr90 should be<1%. The different BT regimens improved comparable efficacy and tolerance [28], [27]. After the last session, the needles were removed and the urinary catheter kept until hematuria resolved. The catheter was then removed and the patient was discharged after micturition resumed.
Androgen deprivation therapy (ADT)
According to the NCCN recommendations [3], short (6 months) ADT was prescribed to unfavorable IR patients while HR patients received prolonged ADT for 18 to 30 months depending on patient tolerance and background (especially cardiovascular or cognitive impairment comorbidities). ADT comprised a LHRH agonist (Leuprorelin, Triptorelin or Degarelix) combined with a non-steroid anti-androgen for the first month to prevent flare-up (Bicalutamide 50 mg daily).
Follow-up and evaluation
Oncological outcome was analyzed based on biochemical, local, regional (pelvic lymph-node), and metastatic recurrence. Biochemical relapse was defined according to the Phoenix definition: nadir + 2 ng/mL. However, in the event of PSA increase > nadir + 2, pts underwent prostate MRI and PET-scan in order to detect local and/or distant disease progression followed by prostate biopsies if local relapse was suspected. Local recurrence was defined as a relapse occurring in the prostate gland. Regional node relapse was defined as lymph-node failure confirmed by imaging encompassing the pelvic to para-aortic area. Metastatic recurrence was defined as distant failure confirmed by CT-scan, bone-scan or choline/PSMA PET-CT.
Toxicity analysis focused on genitourinary (GU) and gastrointestinal (GI) side effects using CTCAE, version 4.0 criteria (National Cancer Institute Common Toxicity Criteria). Toxicity assessment was performed weekly during the EBRT course, 1 month after HDR-BT boost, then every 6 months for the first 5 years of follow-up, then annually. The cut-off between acute and late toxicities was fixed at 6 months after the end of HDR-BT. Late toxicities were evaluated using their highest grade at any given consultation starting 6 months after treatment and the grade at last follow-up.
Statistical analysis
The primary endpoint was biochemical relapse-free survival (bRFS). Secondary endpoints were local- (lRFS), regional lymph node- (rRFS) and metastatic relapse-free survival (mRFS), disease-free (DFS), cancer-specific (CSS) and overall survival (OS). Late GU and GI toxicities were also investigated. Qualitative data were summarized using absolute and relative frequencies and quantitative data using median and range. Patients were censored at their last follow-up. BRFS, lRFS, mRFS, DFS, CSS and OS were estimated using the Kaplan-Meier method and compared using a log-rank test and Cox multivariate analysis when required. BRFS was defined between the date of diagnosis (biopsy) and date of the first biochemical event. LRFS, rRFS, mRFS were defined between the date of diagnosis and date of the first local, regional lymph-node and distant event respectively. DFS was defined between the date of diagnosis and date of the first event (biochemical, local, regional or distant recurrence as well as all causes of death). CSS was defined between the date of diagnosis and date of death due to PC. OS was defined as the period between diagnosis and date of death (all causes of death).
Statistical analysis was performed using R.3.6.1 Software for Windows. All tests were two-sided, and p-values < 0.05 were considered as significant results.
Results
Patient characteristics
Between 02/2008 to 01/2022, 518 pts treated with HDR-BT were eligible, of whom 380 IR or HR-PC pts with a minimal follow-up of 18 months were included in this study: 177 pts ≤ 70 y (46.6%) and 203 pts > 70 y (53.4%) with a median age of 64 y [47–70] and 75 y [70 – 85.3] respectively. Patient and treatment characteristics are reported in Table 1. Except for ADT, the two age groups were comparable. Ninety-eight pts (25.8%; ≤ 70 y (29.9%) vs. > 70 y (22.2%); p = 0.107) and 282 pts (74.2%; ≤ 70 y (70.1%) vs. > 70 y (77.8%); p = 0.107) were IR and HR respectively. EBRT was mainly performed through IMRT (61.9%) on a pelvic field (86.6%) delivering a median dose of 46 Gy [37.5–46] in 15 to 23 fractions. HDR-BT boost was mostly delivered in a single fraction of 14/15 Gy (79%), with median V100 and median D90 of 98 % [79–100] and 110% [78–140] respectively (Table 1 & e-Table Supplementary data). ADT was used in 302 pts (79.4%; ≤ 70 y (73.4%) vs. > 70 y (84.7%); p = 0.01) for a median time of 18 months [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41] (IR: 6 months [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33]; HR: 19 months [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]).
Table 1.
Patient and treatment features.
| Whole cohort |
≤ 70 y |
> 70 y |
p value |
||||
|---|---|---|---|---|---|---|---|
| data |
%/[min–max] |
data |
%/[min–max] |
data |
%/[min–max] |
||
| # pts | 380 | 177 | 46.6 | 203 | 53.4 | ||
| Median age (years) | 70.6 | [47 – 85] | 64.4 | [47 – 70] | 74.3 | [70 – 85.3] | |
| MFU (months) | 73.6 | [67 – 83] | 60 | [60 – 60] | 60 | [54 – 60] | |
| NCCN classification | 0.107 | ||||||
| Intermediate-risk | 98 | 25.8 | 53 | 29.9 | 45 | 22.2 | |
| High-risk | 282 | 74.2 | 124 | 70.1 | 158 | 77.8 | |
| T (TNM) | 0.936 | ||||||
| T1 | 40 | 10.5 | 20 | 11.3 | 20 | 9.9 | |
| T2 | 138 | 36.3 | 62 | 35.0 | 76 | 37.4 | |
| T3 | 196 | 51.6 | 92 | 52.0 | 104 | 51.2 | |
| T4 | 4 | 1.1 | 2 | 1.1 | 2 | 1.0 | |
| TX | 2 | 0.5 | 1 | 0.6 | 1 | 0.5 | |
| Median iPSA (ng/ml) | 9.5 | [1 – 369] | 9 | [6.6 – 369] | 10 | [1 – 16.1] | 0.154 |
| ISUP | 0.132 | ||||||
| 1 | 71 | 18.7 | 38 | 21.5 | 33 | 16.3 | |
| 2 | 101 | 26.6 | 56 | 31.6 | 45 | 22.2 | |
| 3 | 61 | 16.1 | 25 | 14.1 | 36 | 17.6 | |
| 4 | 73 | 19.2 | 28 | 15.8 | 45 | 22.2 | |
| 5 | 71 | 18.7 | 29 | 16.4 | 42 | 20.7 | |
| X | 3 | 0.8 | 1 | 0.6 | 2 | 1.0 | |
| EBRT | |||||||
| 3DRT | 144 | 38.1 | 70 | 39.5 | 74 | 3.4 | 0.256 |
| IMRT | 234 | 61.9 | 107 | 60.4 | 127 | 63.2 | 0.301 |
| Pelvic LN irradiation | 329 | 86.6 | 146 | 82.5 | 183 | 90.1 | 0.042 |
| Median duration (d) | 35.4 | [14 – 62] | 35 | [18 – 56] | 35.7 | [14 – 62] | 0.112 |
| Median dose (Gy) | 46 | [37.5 – 46] | 46 | [37.5 – 46] | 46 | [37.5 – 46] | 0.85 |
| Median # fraction | 23 | [14 – 25] | 23 | [14 – 25] | 23 | [15 – 23] | 0.253 |
| BT | |||||||
| Time interval EBRT/BT (d) | 14.1 | [0 – 43] | 13 | [0 – 39] | 13 | [0 – 43] | 0.951 |
| 1 fraction (14/15 Gy) | 300 | 79 | 133 | 75.1 | 167 | 82.3 | 0.116 |
| 2/3 fractions (18 Gy) | 80 | 21 | 44 | 24.9 | 36 | 17.7 | 0.201 |
| Median CTV (cc) | 39 | [11 – 140] | 38 | [11 – 120] | 41 | [15 – 140] | 0.286 |
| Median D90 (%) | 110 | [78 – 140] | 110 | [78 – 120] | 110 | [95 – 140] | 0.718 |
| Median V100 (%) | 98 | [79 – 100] | 98 | [79 – 100] | 98 | [87 – 100] | 0.05 |
| Median DNR | 0.28 | [0.13 – 0.38] | 0.29 | [0.17 – 0.34] | 0.27 | [0.13 – 0.38] | 0.07 |
| D2cc urethra (%) | 95 | [81 – 110] | 91 | [90 – 110] | 97 | [81 – 110] | 0.002 |
| D2cc rectum (%) | 66 | [60 – 93] | 67 | [60 – 93] | 66 | [64 – 92] | 0.09 |
| ADT | 302 | 79.4 | 130 | 73.4 | 172 | 84.7 | 0.01 |
| Median duration (months) | 18 | [3 – 41] | 18 | [6 – 26.9] | 18 | [8.9 – 23] | 0.495 |
| Median duration IR (months) | 6 | [5 – 33] | 6 | [5.8 – 33] | 6 | [5 – 17] | 0.552 |
| Median duration HR (months) | 19 | [3 – 41] | 23 | [5.3 – 41] | 18 | [3 –41] | 0.262 |
#pts: number of patients; MFU: median follow-up; NCCN: National Comprehensive Cancer Network; T: tumor; iPSA: initial prostate specific antigen; ISUP: International Society of Urological Pathology; EBRT: external beam radiation therapy; 3DRT: three-dimensional radiation therapy; IMRT: intensity modulated radiation therapy; LN: lymph-node; # fraction: number of fractions; BT: brachytherapy; CTV: clinical target volume; D90: dose delivered to 90% of the CTV, V100: percentage of the clinical target volume receiving 100% of the prescribed dose; DNR: dose non-homogeneity ratio D2cc: dose delivered to 2 cc of the organ at risk; ADT: androgen deprivation therapy; IR: intermediate risk; HR: high risk.
Oncological outcomes
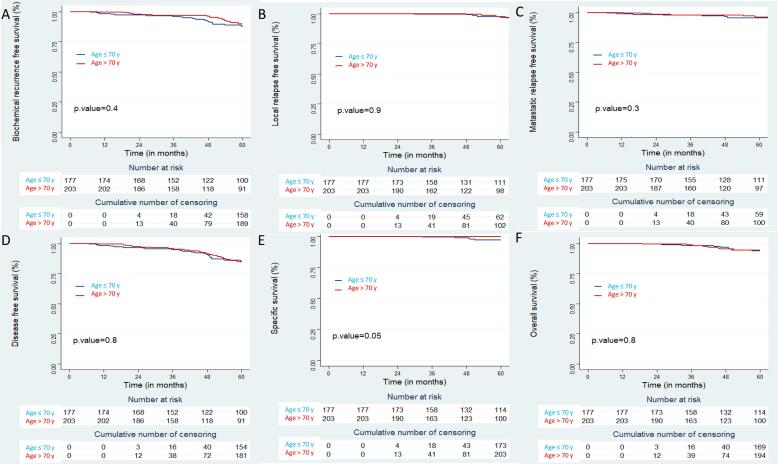
With a median follow-up (MFU) of 73.6 months [67.4 – 83.4] for the whole cohort, 5-year oncological outcomes were: 5-y bRFS: 88% [CI95%: 85–92], 5-y lRFS: 97% [CI95%: 95–99], 5-y rRFS: 99% [CI95%: 98 – 100] and 5-y mRFS: 96% [CI95%: 94–98]. Five-y DFS, 5-y CSS and 5-y OS were 85% [CI95%: 81–89], 99% [CI95%: 97–100] and 94% [CI95%: 92–97] respectively (e-Fig. 1 Supplementary data). With a comparable MFU, no statistical oncological outcome difference was reported in the two age-groups, except for 5-y CSS, with a better result in the oldest group (≤70 y: 97% [CI95%: 94–100] vs. > 70 y: 100%; p = 0.05) (Fig. 1, Table 2). In a subgroup analysis comparing elderly (70 y < pts ≤ 80 y [182 pts]) vs. super-elderly (>80 y pts [21 pts]), 5-y bRFS (p = 0.3), 5-y lRFS (p = 0.3) and 5-y CSS (p = NA) were not statistically different between the two age-groups; however, a significant difference was observed for 5-y OS (p = 0.003) (e-Fig. 2 Supplementary data).
Fig. 1.
Oncological outcome according to patient age: Biochemical recurrence free survival (A); Local recurrence free survival (B); Metastatic recurrence free survival (C); Disease free survival (D); Specific survival (E); Overall survival (F).
Table 2.
Oncological outcome.
| Whole cohort |
≤ 70 y |
> 70 y |
p value |
||||
|---|---|---|---|---|---|---|---|
| data |
%/CI95% |
data |
%/CI95% |
data |
%/CI95% |
||
| # pts | 380 | 177 | 46.6 | 203 | 53.3 | ||
| Oncological outcome | |||||||
| Biochemical relapse | 43 | 11.3 | 24 | 55.8 | 19 | 44.2 | 0.26 |
| Local relapse | Nov-43 | 25.6 | 6 | 54.5 | 5 | 45.5 | 0.76 |
| Pelvic LN relapse | Jun-43 | 14.0 | 4 | 66.6 | 2 | 33.4 | 0.42 |
| Distant M relapse | 19/43 | 44.2 | 12 | 63.1 | 7 | 36.9 | 0.16 |
| Unknown | Jul-43 | 16.3 | – | – | – | – | – |
| 5-y bRFS | 88 | [85 – 92] | 87 | [82 – 93] | 90 | [85 – 95] | 0.4 |
| 5-y lRFS | 97 | [95 – 99] | 97 | [94 – 100] | 98 | [95 – 100] | 0.9 |
| 5-y rRFS | 99 | [98 – 100] | 99 | [97 – 100] | 99 | [98 – 100] | 0.5 |
| 5-y mRFS | 96 | [94 – 98] | 96 | [93 – 99] | 96 | [93 – 99] | 0.3 |
| 5-y DFS | 85 | [81 – 89] | 84 | [79 – 91] | 85 | [79 – 91] | 0.8 |
| 5-y CSS | 99 | [97 – 100] | 97 | [94 – 100] | 100 | [100 – 100] | 0.05 |
| 5-y OS | 94 | [92 – 97] | 95 | [91 – 98] | 94 | [90 – 98] | 0.8 |
# pts: number of patients; LN: lymph-node; M: metastatic; bRFS: biochemical relapse free survival; lRFS: local relapse free survival; rRFS: regional lymph-node relapse free survival; mRFS: metastatic relapse free survival; DFS: disease-free survival; CSS: cancer specific survival; OS: overall survival.
Toxicity
Late GU toxicity was observed in 123 pts (32.4%), including 10.6% G2 and 7.3% G3 toxicities, with no grade 4. No statistical difference between the two age-groups was observed for late GU toxicity (p = 0.669) (Table 3). Late GI toxicity was observed in 39 pts (10.1%) with 12.8% G2 toxicity and no grade ≥ 3. No statistical difference between the two age-groups was observed for late GI toxicity (p = 1) (Table 3). However, in the subgroup analysis comparing elderly vs. super-elderly, late GU toxicity was observed in 55 pts ≤ 80 y (30.4%) versus 13 pts > 80 y (61.9%), a statistical difference (p = 0.008). Late GI toxicity profile was non-significantly different between elderly and super-elderly (e-Table 2 Supplementary data).
Table 3.
Toxicity.
| Whole cohort |
≤ 70 y |
> 70 y |
p value |
||||
|---|---|---|---|---|---|---|---|
| # pts |
% |
# pts |
% |
# pts |
% |
||
| # pts | 380 | 177 | 46.6 | 203 | 53.3 | ||
| Late GU toxicities | 123 | 32.4 | 55 | 31.1 | 68 | 33.7 | 0.669 |
| G1 | 101/123 | 82.1 | 44/55 | 80.0 | 57/68 | 83.8 | 0.389 |
| G2 | 13/123 | 10.6 | Aug-55 | 14.5 | May-68 | 7.4 | |
| G3 | 9/123 | 7.3 | Mar-55 | 5.5 | Jun-68 | 8.8 | |
| Late GI toxicities | 39 | 10.1 | 19 | 11.7 | 20 | 9.7 | 1 |
| G1 | 34/39 | 87.2 | 17/19 | 89.5 | 17/20 | 85 | 1 |
| G2 | May-39 | 12.8 | 19-Feb | 10.5 | 20-Mar | 15 | |
#pts: number pf patients; GU: genito-urinary; GI: gastro-intestinal.
Discussion
The combination of EBRT, BT boost and ADT is one of the standards of care for IR/HR localized PC. These recommendations are not as yet applied to elderly pts, who are often mis- or undertreated because only their age is taken into account. As a result, their treatment remains complex and controversial.
Oncological outcomes in our analysis of 380 IR to HR-PC pts with a MFU of 72.3 months 5-y results were: bRFS 87 vs. 90% (p = 0.4), CSS 97 vs. 100% (p = 0.05) and OS 95 vs. 94% (p = 0.8) for ≤ 70 y and > 70 y age groups respectively. The only significant difference was observed for 5-yCSS. However, this result should be viewed with caution in light of the insufficient number of clinical events (4 vs. 0 for ≤ 70 y and > 70 y respectively), which makes this result less relevant. Patient characteristics were comparable in the two age-groups, except for the use of ADT (≤70 y 130 pts vs. > 70 y 172 pts; p = 0.01); there was no statistical difference in ADT indication or duration. Geinitz et al. [18] analyzed the impact of age (>75 y) in pts treated with EBRT +/- ADT. The authors reported a better 4-y bRFS in the elderly group (76% vs. 61%, p = 0.042), which could possibly be explained by lower testosterone levels after ADT in elderly patients.
Our data were consistent with available retrospective studies reporting BT boost in older adults, with some heterogeneity in population, follow-up, and treatment modalities. Stromberg et al. [23] compared 3 different regimens (3DRT, EBRT + HDR-BT boost, IMRT) in 443 IR or HR-PC patients > 70 y and reported a 5-y bRFS and OS of 79.4% and 87.7% respectively for the BT regimen. Strouthos et al. [29] studied HDR-BT boost in 303 HR-PC pts where 63% were ≥ 70 y. With a MFU of 71.6 months, the authors reported an estimated bRFS and OS at 72 months of 85.7% and 88.3%. Yamazaki et al. [30], [31] in their two studies comparing younger and elderly pts (>75 y then 80 y) with all risk PC, reported comparable outcomes in the cohorts with no impact of age. However, in a retrospective study including 2701 pts > 80 y treated with BT (77% with IR or HR-PC), Valdivieso R et al. [32] analyzed the oncological outcome at 5 and 10 years. The authors observed that, in this super-elderly population, the risk of dying of PC is 10 times less compared to all other cause death risk (2% vs. 19% for 5-y cause specific mortality and 5-y other causes mortality respectively). Consequently, the advantage of BT may disappear due to the shorter life expectancy.
Regarding toxicity analysis, our data were consistent with results reported in literature [29], [30], [31], [33], [34]. HDR-BT was well tolerated with 31.1% and 33.7% of late GU toxicity for ≤ 70 y and > 70 y respectively (p = 0.669) and 11.7% and 9.7% of late GI toxicity for ≤ 70 y and > 70 y respectively (p = 1). In our cohort, late G ≥ 2 GU/GI toxicity was ≤ 7%, better than results reported in literature [21], which was mainly based on references using 3DRT. We may posit that our toxicity results were improved by the use of IMRT in 62%, as already reported by Löser at al. [34]. We do not report statistical differences in terms of toxicity between the two age-groups, while several retrospective studies confirmed comparable toxicity outcomes in younger and elderly patients [30], [31], [33]. However, Chen et al. [35], in their large cohort of 5621 pts > 65 y, highlighted age (comorbidities and addition of EBRT) as a factor behind post BT complications [35]. We also reported an increased late GU toxicity in pts > 80 y (p = 0.008), as described by Yamazaki et al. [31], who reported a higher incidence of GU toxicity than in other age-groups, especially with the use of BT. For > 80 y pts, the risk of BT toxicity (mainly GU) seems to increase and its oncological benefit remains open to debate.
This analysis contains a number of limitations. As in all observational studies and despite extensive corrections, our results may have been influenced by unknown residual confounding factors. It was a retrospective, monocentric analysis with data collection over a long period time (from 2008 to 2021); there may therefore be missing data or discrepancies between treatment modalities. Regarding irradiation technique, we mixed 3DRT and IMRT; also, the use of BT may lead to significant selection bias owing to patient BT feasibility status (anesthesia, comorbidity associated factors …). One of the major limitations of our study is the lack of comorbidity analysis in a heterogeneous, aged population. Comorbidity impact on life expectancy becomes ever greater with age, with competing factors of death. Cardiovascular and cognitive morbidity factors in particular can influence oncological outcomes and treatment tolerance especially with the addition of ADT [36], [37], [38], [39]. Indeed, retrospective studies discussed the benefit of the combination EBRT + ADT for older adults with moderate/severe comorbidities or the super-aged, highlighting the importance of oncogeriatric assessment [40], [41]. In a prospective study, Goineau et al. [42] analyzed quality of life (QoL) in 208 pts ≥ 75 y with localized PC treated after EBRT+/-ADT (without BT boost). The authors reported that, compared to baseline results, QoL was not significantly different in 75% of the pts. No predictive factor for QoL deterioration was observed (oncogeriatric assessment or tumor/treatment features).
Conclusion
For IR and HR prostate cancers in the elderly (>70 y), BT boost remains warranted in order to achieve optimal oncological outcome. In this population, the toxicity profile of BT boost appears acceptable. However, this treatment should be carefully discussed in super senior pts (>80 y) with competing comorbidity factors, which could significantly affect OS and the risk of side effects. The results of prospective trials to identify patients with the greatest benefit/risk balance are awaited.
Due to the increase in life expectancy, PC management in the elderly is a real challenge for patients, clinicians, health care providers and payers. Elderly patients remain candidates for optimal curative treatment (i.e. regardless of age) after oncogeriatric assessment to judiciously identify the patients who stand to benefit most.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2022.05.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cancer today [Internet]. [cited 2021 Jun 18]. Available from: http://gco.iarc.fr/today/home.
- 2.Cancer Tomorrow [Internet]. [cited 2021 Jun 18]. Available from: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=27&single_unit=50000&age_start=14.
- 3.Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology in: Journal of the National Comprehensive Cancer Network Volume 17 Issue 5 (2019) [Internet]. [cited 2021 Jun 27]. Available from: https://jnccn.org/view/journals/jnccn/17/5/article-p479.xml. [DOI] [PubMed]
- 4.Sathya J.R., Davis I.R., Julian J.A., Guo Q., Daya D., Dayes I.S., et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005;23(6):1192–1199. doi: 10.1200/JCO.2005.06.154. [DOI] [PubMed] [Google Scholar]
- 5.Hoskin P.J., Rojas A.M., Bownes P.J., Lowe G.J., Ostler P.J., Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103(2):217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Hoskin P.J., Rojas A.M., Ostler P.J., Bryant L., Lowe G.J. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: Mature 12-year results. Radiother Oncol. 2021;154:214–219. doi: 10.1016/j.radonc.2020.09.047. [DOI] [PubMed] [Google Scholar]
- 7.Morris W.J., Tyldesley S., Rodda S., Halperin R., Pai H., McKenzie M., et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiation Oncol Biol Phys. 2017;98(2):275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Chen R.C., Carpenter W.R., Hendrix L.H., Bainbridge J., Wang A.Z., Nielsen M.E., et al. Receipt of guideline-concordant treatment in elderly prostate cancer patients. Int J Radiat Oncol Biol Phys. 2014;88(2):332–338. doi: 10.1016/j.ijrobp.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz K.L., Alibhai S.M.H., Tomlinson G., Naglie G., Krahn M.D. Continued undertreatment of older men with localized prostate cancer. Urology. 2003;62(5):860–865. doi: 10.1016/s0090-4295(03)00690-3. [DOI] [PubMed] [Google Scholar]
- 10.Alibhai S.M.H., Naglie G., Nam R., Trachtenberg J., Krahn M.D. Do Older Men Benefit From Curative Therapy of Localized Prostate Cancer? JCO. 2003 Sep 1;21(17):3318–3327. doi: 10.1200/JCO.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Bechis S.K., Carroll P.R., Cooperberg M.R. Impact of Age at Diagnosis on Prostate Cancer Treatment and Survival. JCO. 2011;29(2):235–241. doi: 10.1200/JCO.2010.30.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alibhai S.M.H., Krahn M.D., Cohen M.M., Fleshner N.E., Tomlinson G.A., Naglie G. Is there age bias in the treatment of localized prostate carcinoma? Cancer. 2004;100(1):72–81. doi: 10.1002/cncr.11884. [DOI] [PubMed] [Google Scholar]
- 13.Yang D.D., Mahal B.A., Muralidhar V., Boldbaatar N., Labe S.A., Nezolosky M.D., et al. Receipt of definitive therapy in elderly patients with unfavorable-risk prostate cancer. Cancer. 2017;123(24):4832–4840. doi: 10.1002/cncr.30948. [DOI] [PubMed] [Google Scholar]
- 14.Bratt O., Folkvaljon Y., Hjälm Eriksson M., Akre O., Carlsson S., Drevin L., et al. Undertreatment of Men in Their Seventies with High-risk Nonmetastatic Prostate Cancer. Eur Urol. 2015;68(1):53–58. doi: 10.1016/j.eururo.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Aas K., Dorothea Fosså S., Åge Myklebust T., Møller B., Kvåle R., Vlatkovic L., et al. Increased curative treatment is associated with decreased prostate cancer-specific and overall mortality in senior adults with high-risk prostate cancer; results from a national registry-based cohort study. Cancer Med. 2020;9(18):6646–6657. doi: 10.1002/cam4.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brassell S.A., Rice K.R., Parker P.M., Chen Y., Farrell J.S., Cullen J., et al. Prostate Cancer in Men 70 Years Old or Older, Indolent or Aggressive: Clinicopathological Analysis and Outcomes. J Urol. 2011;185(1):132–137. doi: 10.1016/j.juro.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Villa S., Bedini N., Fallai C., Olmi P. External beam radiotherapy in elderly patients with clinically localized prostate adenocarcinoma: age is not a problem. Critical Reviews in Oncology/Hematology. 2003 Nov;48(2):215–225. doi: 10.1016/j.critrevonc.2003.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Geinitz H., Zimmermann F.B., Thamm R., Schumertl A., Busch R., Molls M. 3D conformal radiation therapy for prostate cancer in elderly patients. Radiother Oncol. 2005 Jul;76(1):27–34. doi: 10.1016/j.radonc.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen T.D., Azria D., Brochon D., Poortmans P., Miller R.C., Scandolaro L., et al. Curative external beam radiotherapy in patients over 80 years of age with localized prostate cancer: A retrospective rare cancer network study. Critical Reviews in Oncology/Hematology. 2010;74(1):66–71. doi: 10.1016/j.critrevonc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Okonogi N., Katoh H., Kawamura H., Tamaki T., Kaminuma T., Murata K., et al. Clinical outcomes of helical tomotherapy for super-elderly patients with localized and locally advanced prostate cancer: comparison with patients under 80 years of age. J Radiat Res. 2015;56(6):889–896. doi: 10.1093/jrr/rrv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marotte D., Chand-Fouche M.-E., Boulahssass R., Hannoun-Levi J.-M. Irradiation of localized prostate cancer in the elderly: A systematic literature review. Clinical and Translational Radiation Oncology. 2022;35:1–8. doi: 10.1016/j.ctro.2022.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman K.E., Chen M.-H., Moran B.J., Braccioforte M.H., Dosoretz D., Salenius S., et al. Prostate cancer-specific mortality and the extent of therapy in healthy elderly men with high-risk prostate cancer. Cancer. 2010;116(11):2590–2595. doi: 10.1002/cncr.24974. [DOI] [PubMed] [Google Scholar]
- 23.Stromberg J.S., Kestin L.L., Gustafson G.S., Vicini F.A., Ghilezan M.I., Martinez A.A. Improved Outcome with Combined Pelvic External Beam Radiation and HDR Boost Brachytherapy or IMART Compared with Conventional External Beam Prostate Radiation for Elderly Men with High-intermediate to High-risk Prostate Cancer. Int J Radiation Oncol Biol Phys. 2009;75(3):S337–S338. [Google Scholar]
- 24.Kent A.R., Matheson B., Millar J.L. Improved survival for patients with prostate cancer receiving high-dose-rate brachytherapy boost to EBRT compared with EBRT alone. Brachytherapy. 2019 May;18(3):313–321. doi: 10.1016/j.brachy.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Egevad L., Delahunt B., Srigley J.R., Samaratunga H. International Society of Urological Pathology (ISUP) grading of prostate cancer - An ISUP consensus on contemporary grading. APMIS. 2016 Jun;124(6):433–435. doi: 10.1111/apm.12533. [DOI] [PubMed] [Google Scholar]
- 26.de Crevoisier R., Supiot S., Créhange G., Pommier P., Latorzeff I., Chapet O., et al. External radiotherapy for prostatic cancers. Cancer Radiother. 2022;26(1-2):329–343. doi: 10.1016/j.canrad.2021.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Falk A.T., Demontoy S., Chamorey E., Chand M.-E., Gautier M., Azria D., et al. High-dose-rate brachytherapy boost for prostate cancer: Comparison of three different fractionation schemes. Brachytherapy. 2017;16(5):993–999. doi: 10.1016/j.brachy.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Hijazi H., Chevallier D., Gal J., Chand M.E., Gautier M., Hannoun-Levi J.M. Prostate cancer boost using high-dose-rate brachytherapy: early toxicity analysis of 3 different fractionation schemes. jcb. 2013;4:203–209. doi: 10.5114/jcb.2013.38657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strouthos I., Chatzikonstantinou G., Zamboglou N., Milickovic N., Papaioannou S., Bon D., et al. Combined high dose rate brachytherapy and external beam radiotherapy for clinically localised prostate cancer. Radiother Oncol. 2018;128(2):301–307. doi: 10.1016/j.radonc.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki H., Masui K., Suzuki G., Nakamura S., Aibe N., Shimizu D., et al. Radiothrerapy for Elderly Patients Aged ≥75 Years with Clinically Localized Prostate Cancer-Is There a Role of Brachytherapy? J Clin Med. 2018 doi: 10.3390/jcm7110424. Nov 8;7(11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamazaki H., Masui K., Suzuki G., Shimizu D., Aibe N., Yamada K., et al. Radiotherapy for elder patients aged ≥80 with clinically localized prostate cancer - Brachytherapy enhanced late GU toxicity especially in elderly. Clin Transl Radiat Oncol. 2020;25:67–74. doi: 10.1016/j.ctro.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valdivieso R., Boehm K., Meskawi M., Larcher A., Tian Z., Parent M.-E., et al. Patterns of use and patient characteristics: brachytherapy for localized prostate cancer in octo- and nonagenarians. World J Urol. 2015;33(12):1985–1991. doi: 10.1007/s00345-015-1553-0. [DOI] [PubMed] [Google Scholar]
- 33.Stromberg J., Kestin L., Gustafson G., Edmundson G., Gonzalez J., Spencer W., et al. Acute and late toxicity in men ≥70 years old compared with men < 70 years old treated with high dose rate prostate brachytherapy boost and pelvic external irradiation for prostate cancer. Int J Radiat Oncol Biol Phys. 2001 Nov 1;51(3):308. [Google Scholar]
- 34.Löser A., Beyer B., Carl C.O., Löser B., Nagaraj Y., Frenzel T., et al. Toxicity and risk factors after combined high-dose-rate brachytherapy and external beam radiation therapy in men ≥75 years with localized prostate cancerToxizität und Risikofaktoren nach kombinierter Hochdosis-Brachytherapie und perkutaner Bestrahlung bei Männern ≥75 Jahren mit lokalisiertem Prostatakarzinom. Strahlenther Onkol. 2019;195(5):374–382. doi: 10.1007/s00066-018-1380-5. [DOI] [PubMed] [Google Scholar]
- 35.Chen A.B., D’Amico A.V., Neville B.A., Earle C.C. Patient and Treatment Factors Associated With Complications After Prostate Brachytherapy. J Clin Oncol. 2006;24(33):5298–5304. doi: 10.1200/JCO.2006.07.9954. [DOI] [PubMed] [Google Scholar]
- 36.Merrick G.S., Wallner K.E., Galbreath R.W., Butler W.M., Brammer S.G., Allen Z.A., et al. Prostate brachytherapy in men > or =75 years of age. Int J Radiat Oncol Biol Phys. 2008 Oct 1;72(2):415–420. doi: 10.1016/j.ijrobp.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Levine G.N., D'Amico A.V., Berger P., Clark P.E., Eckel R.H., Keating N.L., et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010;60(3):194–201. doi: 10.3322/caac.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nanda A., Chen M.-H., Moran B.J., Braccioforte M.H., Dosoretz D., Salenius S., et al. Predictors of prostate cancer-specific mortality in elderly men with intermediate-risk prostate cancer treated with brachytherapy with or without external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2010;77(1):147–152. doi: 10.1016/j.ijrobp.2009.04.085. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen P.L., Alibhai S.M.H., Basaria S., D’Amico A.V., Kantoff P.W., Keating N.L., et al. Adverse Effects of Androgen Deprivation Therapy and Strategies to Mitigate Them. Eur Urol. 2015;67(5):825–836. doi: 10.1016/j.eururo.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen P.L., Chen M.-H., Renshaw A.A., Loffredo M., Kantoff P.W., D'Amico A.V. D’Amico AV. Survival Following Radiation and Androgen Suppression Therapy for Prostate Cancer in Healthy Older Men: Implications for Screening Recommendations. Int J Radiation Oncol Biol Phys. 2010;76(2):337–341. doi: 10.1016/j.ijrobp.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 41.Dell’Oglio P., Bandini M., Leyh-Bannurah S.-R., Tian Z., Trudeau V., Larcher A., et al. External beam radiotherapy with or without androgen deprivation therapy in elderly patients with high metastatic risk prostate cancer. Urologic Oncology: Seminars and Original Investigations. 2018;36(5):239.e9–239.e15. doi: 10.1016/j.urolonc.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Goineau A., Campion L., Commer J.-M., Vié B., Ghesquière A., Béra G., et al. Can Comprehensive Geriatric Assessment Predict Tolerance of Radiotherapy for Localized Prostate Cancer in Men Aged 75 Years or Older? Cancers (Basel) 2020;12(3):635. doi: 10.3390/cancers12030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.