Abstract
We report our retrospective analysis on 34 relapsed/refractory multiple myeloma (RRMM) patients treated with daratumumab based triplets. Twenty patients were females and 14 males. Median age was 73.2. Daratumumab was associated to lenalidomide in and dexamethasone (DRd) in 30 (88,3%) and to bortezomib and dexamethasone (DVd) in 4 cases (11,7%). The ORR was 88%. CR occurred in 12% of cases, VGPR in 44% and PR in 32%. The 12 months PFS and OS rates were 78% and 86,5%, respectively. Present data confirm those recently reported in the literature and further reinforce the early use of daratumumab-based triplets for RRMM patients.
Keywords: Multiple myeloma, Relapsed refractory, Daratumumab, Real-word experience
1. Introduction
Daratumumab (DARA), a monoclonal antibody targeting the CD38 cell surface receptor that is highly expressed on malignant plasma cells, is one of the most relevant new drugs recently introduced in the treatment strategy of multiple myeloma (MM), producing a direct anti-tumor activity and immunomodulatory effects [1]. The efficacy and safety of this drug became evident from the initial studies in relapsed/refractory MM (RRMM) patients in which DARA was used as a single agent [2]. Subsequently, the prospective randomized phase III POLLUX trial, in which the combination of daratumumab with lenalidomide and dexamethasone (DRd) was tested, reported impressive response rates and clinical outcomes [3]. Therefore, daratumumab was rapidly moved to the first line of treatment for both older patients, in combination with lenalidomide [4] or bortezomib + melphalan [5], and younger patients eligible for transplant in combination with the VTD scheme [6].
Still, there is limited data regarding the daily use of daratumumab, whether administered alone or in combination with other drugs [7], [8], [9], [10], [11], [12]. To date, only one recent study conducted by a group from Florence, reported a “real life” experience that focused on the use of DRd [13], confirming the efficacy and safety of this triple combination even in an advanced disease subset.
“Real-life” experiences are believed to be of utmost relevance to verify the efficacy and safety reported in the various registration studies, throughout particular contexts regarding elderly, comorbid, or frail patients, and to identify new prognostic factors. Therefore, herein we report the data of our single-center retrospective analysis on 34 RRMM patients treated at our institution (Hematology unit of the S.M. Goretti Hospital of Latina, Italy) from August 2018 to August 2021 with Dara-based triplets that, in the great majority of cases, consisted of the DRd scheme.
2. Case series
The characteristics of patients are outlined in Table 1. Twenty of the 34 MM patients were females and 14 were males, the median age was 73,2 years (range 64.6–80.3) and the median time from diagnosis of MM to the first treatment with daratumumab was 37 months (range 235–3). Cytogenetic characteristics were obtained from only 7 patients, of these only one was unfavorable. Each patient received one line of therapy and 6 (14,7%) patients received two lines or more. All 34 RRMM patients received a previous bortezomib-based therapy in which bortezomib was given with dexamethasone alone (3 cases) or combined with melphalan (16 cases) or with thalidomide (15 cases). In addition, 4 patients received lenalidomide, 3 patients received pomalidomide and 1 patient received Carfilzomib. Before DRd, seven patients (20,6%) underwent to a prior autologous stem cell transplantation (ASCT), that it is consisted in a single ASCT in 5 patients and in a double ASCT in the other 2 cases.
Table 1.
Summary of patients’ characteristics.
| Characteristics | Patients (n = 34)(%) |
|---|---|
| Sex (M/F) | 14/20 (41,2%−58,8%) |
| Median age (years) | 73,2 (64,6–84,3) |
| Median time from diagnosis (months) | 37 (3–235) |
| Number of previous lines of therapy: | % |
| One | 85.3 |
| Two or more | 14,7 |
| Type of previous lines of therapy: | n (%) |
| Bortezomib | 34 (100%) |
| Lenalidomide | 4 (11,8%) |
| Thalidomide | 15 (44,1%) |
| Pomalidomide | 3 (8,8%) |
| Carfilzomib | 1 (2,9%) |
| ASCT | 7 (20,6%) |
| CRAB evaluation at relapse: | n (%) |
| Hemoglobin < 10 g/dl | 8 (23,5%) |
| Creatinine > 2 mg/dl | 3 (8,8%) |
| Serum Calcium > 11 mg/dl | 0 (0%) |
| Progression in bone involvement | 19 (55,9%) |
| MC (serum) > 2 g/dl | 18 (53%) |
| Positive urinary immunofixation | 18 (53%) |
| Main comorbidities: | n (%) |
| CV diseases | 11 (32,4%) |
| Solid Tumors | 4 (11,8%) |
| Metabolic disorders | 3 (8,8%) |
| Other hematological disorders | 2 (5,9%) |
Sixteen patients (47%) presented one or more comorbidities: cardiovascular diseases (11), solid tumors (4), metabolic disorders (3), other hematological disorders (2), rheumatological diseases (1), pulmonary diseases (1), neurological diseases (1) and gastrointestinal diseases (1).
Twelve patients (36%) were refractory to the previous therapy. Eight patients (23,5%) presented with anemia (Hb < 10 g/dl), 3 (8,8%) with chronic kidney disease (creatinine > 2 mg/dl), 19 (55,9%) with bone lesions, while none of the patients presented with hypercalcemia (Ca2+ > 11 mg/dl). Among the 34 patients, 18 (53%) had a monoclonal component (CM) > 2 g/dl and 18 patients (53%) had a Bence-Jones proteinuria.
All RRMM patients received daratumumab IV at the standard dose of 16 mg/kg whereas in 30 (88,3%) patients it was combined with lenalidomide and dexamethasone (DRd) and in the remaining 4 cases (11,7%) with bortezomib and dexamethasone (DVd). A median number of 8 cycles (range 1–32) was administered within a median follow-up of 16 months (range 1–35). Among DRd treated patients, the lenalidomide starting dose was reduced in 12 patients (35%) according to the renal function or due to neutropenia or thrombocytopenia. At the time of analysis, 25 (73,5%) patients were currently receiving treatment. Nine patients (26,5%) discontinued treatment for progressive disease (8 patients) and intolerance (1 case).
Patients who received at least two doses of daratumumab-based triplets were included in the statistical analysis. Progression-free survival (PFS) and overall survival (OS) were calculated using the Kaplan-Meier product-limit estimator.
The overall response rate (ORR) was 88%. A Complete Response (CR) occurred in 12% of cases, a Very Good Partial Response (VGPR) in 44%, and a Partial Response (PR) in 32%. The Median time to achieve at least a PR was 40 days (range 30–110 days). Progressive disease was observed in 8 patients (23,5%), in which 5 cases (14,7%) led to death. Twenty-six patients (76,4%) were responding to treatment at the time of analysis.
Moreover, 4 transplant-eligible patients, however refractory to the VTD scheme after a median of 6 cycles of daratumumab, deepened their response, allowed themselves to receive an ASCT, and then continued DRD therapy. One of these four patients progressed and died within 100 days after the transplant.
Adverse events are summarized in Table 2. The most common hematological toxicities of any grade were neutropenia (15 patients; 44%), managed with lenalidomide dose reduction, and thrombocytopenia (10 patients; 29%). Other common non-hematological toxicities of grade 1 involved 10 patients (29%), including asthenia, light-headedness and breathlessness, edema of lower extremities, intestinal intolerance with diarrheal episodes, and epigastralgia. Two patients (5,4%) presented pulmonary AEs, one case with pneumonia and the other case with a chronic obstructive pulmonary diseases (COPD) exacerbation. The only grade 4 AE case was observed at the beginning of the third cycle and consisted of intolerance with persistent gastrointestinal disorders that required patient hospitalization leading to a discontinuation of the therapy.
Table 2.
Summary of main adverse events.
| Adverse Events (AEs) | Patients (%) |
|---|---|
| Hematological | |
| Neutropenia | 44,0% |
| Thrombocytopenia | 29,0% |
| Non-hematological | |
| Asthenia | 8,2% |
| Lightheadedness/breathlessness | 5,9% |
| Edema of lower extremities | 5,9% |
| Diarrhea | 8,8% |
| Epigastralgia | 2,9% |
| Pneumonia | 2,9% |
| COPD exacerbation | 2,9% |
After an extensive oral premedication of our patients consisting of cetirizine dihydrochloride 10 mg, montelukast 10 mg, paracetamol 1000 mg and dexamethasone 20 mg that was administered once/daily from the day before to the day after the infusion [14], we observed a DARA infusion-related reactions (IRRs) in only 3 patients (8,8%). All IRRs were a grade 1 – 2 and occurred during the first infusion, with urticaria, tachycardia, and respiratory symptoms, such as bronchospasm and dyspnea.
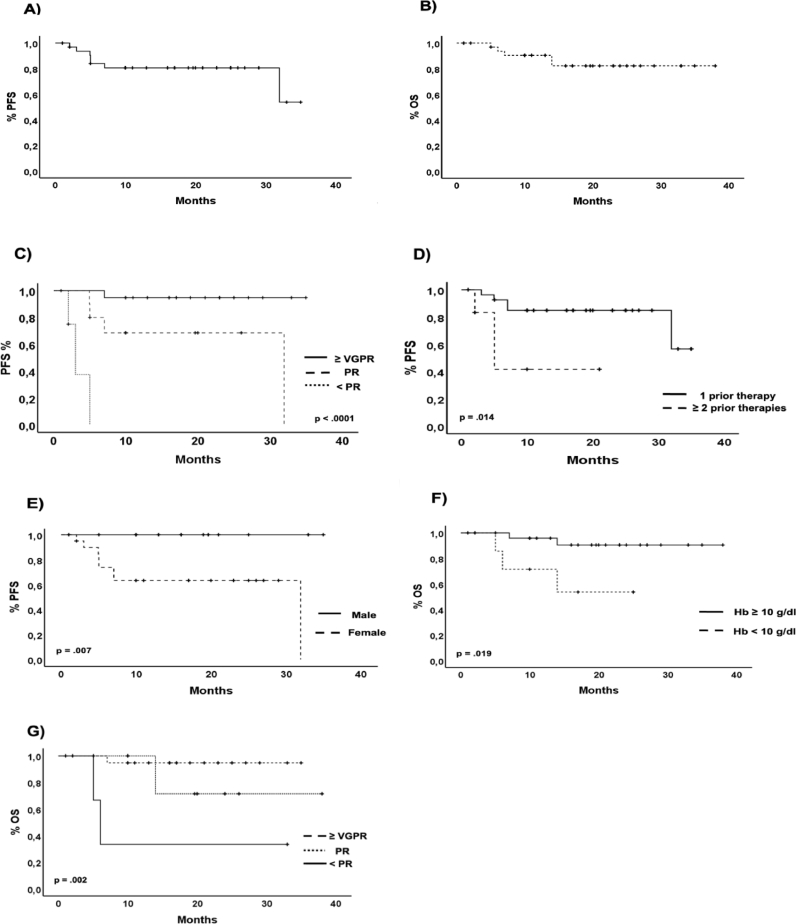
At a median follow-up of 16 months (range 35 – 1), the median PFS and OS have not been reached (NR); the 12-month PFS and OS rates were 78% and 86,5%, respectively (Fig. 1. A and B). In the univariate analysis, the achievement of at least a VGPR, receiving just one line of previous therapy and the male gender resulted in a statistically significant better PFS (Fig. 1. C, D, and E), whereas the absence of anemia at the start of the daratumumab-based triplets and the achievement of at least a VGPR predicted a better OS (Fig. 1. F and G).
Fig. 1.
Kaplan–Meyer survival curves. (A) PFS in all patients, (B) OS in all patients, (C) PFS according to the best response, (D) PFS according to the previous lines of therapy, (E) PFS according to the sex, (F) OS according to the presence of anemia at relapse, G) OS according to the best response.
In the multivariate analysis, only the achievement of at least a VGPR resulted as an independent predictor of prolonged PFS, showing an HR of 0.037 (95% CI of 0.0001–0.988; p= .049). Interestingly, other potential predictors of unfavorable prognoses, such as the condition of early or late relapse, did not impact patients' clinical outcomes after DRd.
In the present series, 4 patients received DVd because they were previously exposed to lenalidomide. Compared to the DRd treated patients, they showed a worse PFS and a similar OS. Once again, the small number of cases did not allow conclusions to be drawn concerning this issue. Apart from the daratumumab triplet used, many other factors may have contributed to the worse outcome of these four patients, as for example, the fact that they all had received more than one line of previous therapies.
3. Discussion
Despite the small number of our sample and the relatively short follow-up time, our results seem to confirm both the registration studies [3,15] and the Florence real-life experience [13].
In the POLLUX study, the DRd triplet showed an ORR of 92%, which is similar to the 88% reported in our series [3]. By contrast, the 43,1% CR rate reported in the POLLUX study was higher than the 12% observed in the present series. This fact may likely derive from the difficulties in assessing the remission status of the DARA exposed MM patients, for the known interferences of the therapeutic monoclonal antibody in serum electrophoresis and immunoprecipitation electrophoresis. This suggestion is further supported by the finding that our patients who obtained at least a VGPR had similar PFS and OS to that of the registration and real-life studies. In addition, we confirm the benefit of daratumumab-based triplets, even in pretreated and frail patients with several comorbidities. Finally, we confirm the daratumumab-based triplets’ safety in a real-life context, with only one grade 4 serious AE. This finding appears to be more relevant especially if we consider that our patients were clinically challenging, as 47% of them suffered from several comorbidities such as cardiovascular disease, diabetes, diverticulitis, concomitant or prior solid tumors (breast cancer, kidney cancer, prostate cancer, and thyroid cancer).
In conclusion, although the size of our cohort may be limited in addressing the robustness of our results, they nonetheless compared favorably to those recently reported in the randomized clinical trial POLLUX study and the Florence real-life experience, further reinforcing the early use of daratumumab-based triplets for the treatment of RRMM patients.
Declaration of Competing Interest
No potential conflict of interest was reported by the authors.
Informed consent
All patients were treated according to the national authorization indications. Therefore, it was not considered necessary to obtain a formal release of informed consent
References
- 1.Sanchez L., Wang Y., Siegel D.S., Wang M.L. Daratumumab: a first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J. Hematol. Oncol. 2016;9:51. doi: 10.1186/s13045-016-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonioli E., Staderini M., Pilerci S., Perfetto F., Cappelli F., Allinovi M., Nozzoli C., Attucci I., Buzzichelli A., Messeri M., Bosi A. Daratumumab, lenalidomide, and dexamethasone combination in relapsed/refractory myeloma patients: a real-life single-center experience. Leuk. Lymphoma. 2020;61:3255–3258. doi: 10.1080/10428194.2020.1802452. [DOI] [PubMed] [Google Scholar]
- 3.Dimopoulos M.A., Oriol A., Nahi H., San-Miguel J., Bahlis N.J., Usmani S.Z., Rabin N., Orlowski R.Z., Komarnicki M., Suzuki K., Plesner T., Yoon S.-.S., ben Yehuda D., Richardson P.G., Goldschmidt H., Reece D., Lisby S., Khokhar N.Z., O'Rourke L., Chiu C., Qin X., Guckert M., Ahmadi T., Moreau P. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016;375:1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 4.Durie B.G.M., Kumar S.K., Usmani S.Z., Nonyane B.A.S., Ammann E.M., Lam A., Kobos R., Maiese E.M., Facon T. Daratumumab-lenalidomide-dexamethasone vs standard-of-care regimes: efficacy in transplant-ineligible untreated myeloma. Am. J. Hematol. 2020;95:1486–1494. doi: 10.1002/ajh.25963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mateos M.V., Cavo M., Blade J., Dimopoulos M.A., Suzuki K., Jakubowiak A., Knop S., Doyen C., Lucio P., Nagy Z., Pour L., Cook M., Grosicki S., Crepaldi A., Liberati A.M., Campbell P., Shelekhova T., Yoon S.S., Iosava G., Fujisaki T., Garg M., Krevvata M., Chen Y., Wang J., Kudva A., Ukropec J., Wroblewski S., Qi M., Kobos R., San-Miguel J. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomized, open-label, phase 3 trial. Lancet. 2020;395:132–141. doi: 10.1016/S0140-6736(19)32956-3. [DOI] [PubMed] [Google Scholar]
- 6.Moreau P., Attal M., Hulin C., Arnulf B., Belhadj K., Benboubker L., Béné M.C., Broijl A., Caillon H., Caillot D., Corre J., Delforge M., Dejoie T., Doyen C., Facon T., Sonntag C., Fontan J., Garderet L., Jie K.S., Karlin L., Kuhnowski F., Lambert J., Leleu X., Lenain P., Macro M., Mathiot C., Orsini-Piocelle F., Perrot A., Stoppa A.M., van de Donk N.W., Wuilleme S., Zweegman S., Kolb B., Touzeau C., Roussel M., Tiab M., Marolleau J.P., Meuleman N., Vekemans M.C., Westerman M., Klein S.K., Levin M.D., Fermand J.P., Escoffre-Barbe M., Eveillard J.R., Garidi R., Ahmadi T., Zhuang S., Chiu C., Pei L., de Boer C., Smith E., Deraedt W., Kampfenkel T., Schecter J., Vermeulen J., Avet-Loiseau H., Sonneveld P. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394:29–38. doi: 10.1016/S0140-6736(19)31240-1. [DOI] [PubMed] [Google Scholar]
- 7.Afram G., Gran C., Borg Bruchfeld J., Wagner A.K., Hussain A., Alici E., Nahi H. Impact of performance status on overall survival in patients with relapsed and/or refractory multiple myeloma: real-life outcomes of daratumumab treatment. Eur. J. Haematol. 2020;105:196–202. doi: 10.1111/ejh.13426. [DOI] [PubMed] [Google Scholar]
- 8.Vozella F., Siniscalchi A., Rizzo M., Za T., Antolino G., Coppetelli U., Piciocchi A., Andriani A., Annibali O., de Rosa L., Cimino G., la Verde G., de Stefano V., Cantonetti M., di Toritto T.C., Petrucci M.T. Daratumumab in multiple myeloma: experience of the multiple myeloma GIMEMA Lazio group. Ann. Hematol. 2021;100:1059–1063. doi: 10.1007/s00277-020-04374-y. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi H., Tsushima T., Terao T., Abe Y., Miura D., Narita K., Kitadate A., Takeuchi M., Matsue K. Evaluation of the safety and efficacy of daratumumab outside of clinical trials. Int. J. Hematol. 2019;109:665–672. doi: 10.1007/s12185-019-02648-4. [DOI] [PubMed] [Google Scholar]
- 10.Szabo A.G., Klausen T.W., Levring M.B., Preiss B., Helleberg C., Breinholt M.F., Hermansen E., Gjerdrum L.M.R., Bønløkke S.T., Nielsen K., Kjeldsen E., Iversen K.F., Teodorescu E.M., Dokhi M., Kurt E., Strandholdt C., Andersen M.K., Vangsted A.J. The real-world outcomes of multiple myeloma patients treated with daratumumab. PLoS One. 2021;16 doi: 10.1371/journal.pone.0258487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvanová Ľ., Štulajterová V., Guman T., Ladická M., Hlebašková M., Chudej J., Šimek M., Štecová N., Flochová E., Kubala J., Simančíková I., Drgoňa Ľ., Vranovský A., Wild A., Mistrík M., Bátorová A. Real-world effectiveness and safety of daratumumab, bortezomib and dexamethasone in relapsed/refractory multiple myeloma in Slovakia. Neoplasma. 2021;68:626–630. doi: 10.4149/neo_2021_201113N1223. [DOI] [PubMed] [Google Scholar]
- 12.Atrash S., Thompson-Leduc P., Tai M.-.H., Kaila S., Gray K., Ghelerter I., Lafeuille M.-.H., Lefebvre P., Rossi A. Correction to: treatment patterns and effectiveness of patients with multiple myeloma initiating Daratumumab across different lines of therapy: a real-world chart review study. BMC Cancer. 2021;21:1291. doi: 10.1186/s12885-021-09015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonioli E., Staderini M., Pilerci S., Perfetto F., Cappelli F., Allinovi M., Nozzoli C., Attucci I., Buzzichelli A., Messeri M., Bosi A. Daratumumab, lenalidomide, and dexamethasone combination in relapsed/refractory myeloma patients: a real-life single-center experience. Leuk. Lymphoma. 2020;61:3255–3258. doi: 10.1080/10428194.2020.1802452. [DOI] [PubMed] [Google Scholar]
- 14.Moore D.C., Arnall J.R., Thompson D.L., Martin A.L., Robinson J., Ndiaye A., Paul B., Atrash S., Bhutani M., Voorhees P.M., Usmani S.Z. Evaluation of montelukast for the prevention of infusion-related reactions with daratumumab. Clin. Lymphoma Myeloma Leuk. 2020;20:e777–e781. doi: 10.1016/j.clml.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo A., Chanan-Khan A., Weisel K., Nooka A.K., Masszi T., Beksac M., Spicka I., Hungria V., Munder M., v Mateos M., Mark T.M., Qi M., Schecter J., Amin H., Qin X., Deraedt W., Ahmadi T., Spencer A., Sonneveld P. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016;375:754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]