Abstract
The importance of resource limitation in controlling bacterial growth in the high-nutrient, low-chlorophyll (HNLC) region of the Southern Ocean was experimentally determined during February and March 1998. Organic- and inorganic-nutrient enrichment experiments were performed between 42°S and 55°S along 141°E. Bacterial abundance, mean cell volume, and [3H]thymidine and [3H]leucine incorporation were measured during 4- to 5-day incubations. Bacterial biomass, production, and rates of growth all responded to organic enrichments in three of the four experiments. These results indicate that bacterial growth was constrained primarily by the availability of dissolved organic matter. Bacterial growth in the subtropical front, subantarctic zone, and subantarctic front responded most favorably to additions of dissolved free amino acids or glucose plus ammonium. Bacterial growth in these regions may be limited by input of both organic matter and reduced nitrogen. Unlike similar experimental results in other HNLC regions (subarctic and equatorial Pacific), growth stimulation of bacteria in the Southern Ocean resulted in significant biomass accumulation, apparently by stimulating bacterial growth in excess of removal processes. Bacterial growth was relatively unchanged by additions of iron alone; however, additions of glucose plus iron resulted in substantial increases in rates of bacterial growth and biomass accumulation. These results imply that bacterial growth efficiency and nitrogen utilization may be partly constrained by iron availability in the HNLC Southern Ocean.
The factors that regulate the growth of marine heterotrophic bacteria are ecologically and biogeochemically important to the cycling of energy and materials in the ocean. Bacterioplankton often dominate the biomass of planktonic food webs, making their role in nutrient and energy fluxes crucial for the organization of marine ecosystems (1, 12, 18, 21, 22, 23, 28). Constraints on the growth rates of bacterioplankton may not be consistent in different marine environments. Bacterial growth rates may be limited by dissolved organic matter (DOM) quality (8, 11, 34), inorganic nutrients (51, 57), temperature (36, 45, 54, 61), viral infection (48), or micronutrients such as iron (29, 30, 43). Bacterial stocks are the result of growth and removal processes that include grazing (19, 63), viral infection (48), and physical mixing (17). Each of these factors may limit bacterial growth over different temporal and spatial scales (17).
The motivation behind this study was to identify the factors limiting bacterial growth in the pelagic Southern Ocean. The Southern Ocean (3, 42), the equatorial Pacific (7, 20, 33), and the subarctic Pacific (34, 37) have all been characterized as high-nutrient, low-chlorophyll (HNLC) oceans. Many hypotheses have been proposed to explain the existence of HNLC regions, but most attention has focused on the importance of iron in limiting phytoplankton growth in HNLC systems (15, 38, 41, 42). HNLC oceans typically have subnanomolar concentrations of dissolved iron (41, 42, 52, 53). Such low concentrations of dissolved iron are known to limit phytoplankton growth, but because bacteria are widely regarded as more efficient competitors for limiting nutrients, limitation of bacterial growth by dissolved iron has been relatively neglected. Iron is an essential nutrient for bacteria because it is part of cytochrome c, a component of the electron transport chain in the respiratory system. Thus, iron deficiency might restrict the growth efficiency of heterotrophic bacteria (58). The few investigations into the dependence of bacteria on iron indicate that iron may restrict bacterial conversion efficiency and growth rates in oceanic and coastal regions of HNLC oceans (29, 43, 58).
Studies in the equatorial and subarctic Pacific indicated that the microbial loop might serve an important role in regulating fluxes of material in HNLC systems (7, 20, 33, 37). DOM constitutes a potentially large, exportable pool of reduced carbon, and quantification of DOM fluxes is essential for understanding the ocean carbon cycle (7, 9, 62). The quality of available resources may control bacterial growth rates and standing stocks in some systems. The persistence of DOM in many marine systems suggests that DOM production and subsequent bacterial consumption may be uncoupled in either space or time. Such persistence indicates that some factor limits bacterial utilization of the available DOM substrates (8, 9, 11, 57, 62, 64). Determination of whether bacterial growth limitation results from poor-quality DOM requires experiments that monitor the bacterial response to representative DOM substrates added to seawater batch cultures (34, 36, 63).
Several investigations have evaluated how the quality of organic material affects bacterial growth in marine systems. Bacterial growth efficiency and growth rates may depend on the stoichiometric C/N ratio of the organic substrate rather than on the specific type of organic substrates utilized for growth (24, 25). In the Sargasso Sea, bacterial growth may be limited by input of high-quality (labile) organic carbon (8). In contrast, Kirchman (34) found that bacterial growth rates in the subarctic Pacific depended more on specific organic substrates.
Bacterial growth can also depend on the supply and quality of inorganic nutrients (51, 64). Bacteria have been shown to be effective competitors with phytoplankton for ammonium and phosphate (14, 35, 56), and in some systems bacterial uptake of mineral nutrients dominates nutrient fluxes (13, 60). The nitrogen and phosphorus requirements of bacteria are large because of their high cellular nucleic acid and protein contents, which require them to sustain low intracellular C/N/P ratios (24, 25). Studies from both open ocean and coastal environments suggest that most marine bacterial nitrogen requirements can be met by dissolved free amino acids (DFAA) or ammonium (NH4+) (31, 32, 35). Specific carbon sources used to fulfill oceanic bacterial demands are not as well defined, but monosaccharides like glucose appear to support a large fraction of the bacterial carbon requirement (49). The complexity of microbial communities requires investigations that focus on the possibility that multiple factors may interact to control bacterial growth. Resource limitation may construct synergistic or antagonistic relationships within marine food webs, potentially controlling the growth of marine bacteria. For example, Hutchins et al. (29) hypothesized that iron-limited phytoplankton growth could restrict carbon flow to heterotrophic bacteria, resulting in an interaction between iron and carbon as possible limiting resources.
The present study sought to determine whether resources limited bacterial growth in the pelagic Southern Ocean. Specifically, we tested whether additions of labile organic material (glucose and amino acids) and/or inorganic nutrients (ammonium, phosphate, and dissolved iron) stimulated rates of bacterial growth. By assessing how rates of bacterial production change relative to changes in standing biomass, we address the relative importance of DOM and iron in controlling rates of bacterial growth in the Southern Ocean.
MATERIALS AND METHODS
Study site.
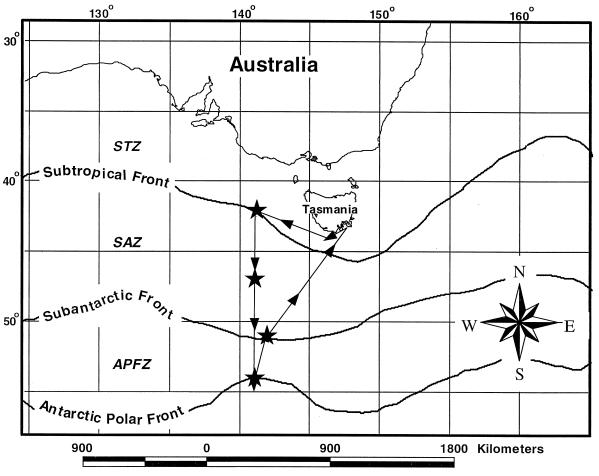
Sampling for these experiments took place aboard the Australian vessel R/V Aurora Australis between 2 and 26 March 1998. The cruise followed a southerly transect along 141°E between 42°S and 55°S (Fig. 1). The cruise track intersected several frontal systems, including the subtropical front (STF) (42°S), the subantarctic front (SAF) (51°S), and the Antarctic polar front (APF) (54°S). Experiments were performed in each of these fronts and at one location inside the subantarctic zone (SAZ) at 47°S. Although they are variable in their locations, each frontal system has a characteristic chemical and physical hydrographic signature (5, 50). Generally, concentrations of major nutrients (NO3− and PO4+) increased along a southerly gradient, while the trace nutrient iron was found in subnanomolar concentrations throughout the entire study region (40, 52, 53; P. W. Boyd, A. C. Crossley, G. R. DiTullio, F. B. Griffiths, D. A. Hutchins, B. Queguiner, P. N. Sedwick, and T. W. Trull, submitted for publication).
FIG. 1.
Study site and cruise track of R/V Aurora Australis voyage 6, 28 February to 3 April 1998. Stars represent stations where experiments were conducted (42°S, 47°S, 51°S, and 54°S at 141°E). Approximate locations of permanent frontal and water-mass boundaries are indicated.
Experimental design.
The overall design of these experiments was to add amendments (organic and/or inorganic) to unmanipulated (whole) seawater, incubate at in situ temperatures, and monitor changes in bacterial growth, abundance, and biomass over a 4- to 5-day incubation period. The decision to use unfiltered rather than size-fractionated seawater (i.e., grazer-reduced treatments) was made to minimize risks of potential contamination of samples with metals or DOM as an artifact of filtration (8).
Water was collected, supplemented with amendments, and then placed in darkened incubators for the duration of the experiment. Incubation in the dark obviated stimulation of phytoplankton growth and subsequent organic enrichment artifacts. Seawater was collected from a depth of approximately 15 m with an all-Teflon, trace-metal-clean pump system (29). The seawater was pumped directly into a trace-metal clean incubation laboratory van, where water was dispensed into 2-liter polycarbonate bottles. All bottles used in these experiments were soaked for 48 h in 10% HCl and then rinsed three times with sample water. The 2-liter polycarbonate bottles were filled, capped, and carried to a positive-pressure hood, where 175-ml polyethylene bottles were filled for each treatment. Duplicate treatments were prepared and sampled for all experiments. The sample handling and setup were designed to reduce potential metal contamination, although contamination was not directly measured. Plastic gloves were worn during all sample transfers.
All substrates were prepared from commercially available reagents. Glucose, ammonium, and phosphate additions were made from dry stocks dissolved in MilliQ-water. Stocks were sterilized through 0.2-μm-pore-size Acrodisc filters (HT Tuffryn membrane) which had been flushed several times with MilliQ-water prior to sample filtration. No efforts were made to remove possible metal contaminates in the glucose stocks; however, the effect of inadvertent contamination may have been minimized, because differential responses to treatments containing glucose plus Fe relative to treatments containing glucose alone were observed (see Results). Initial iron stocks (17.9 μM FeCl2) were made in 0.01% N HCl. Controls consisted of untreated, whole seawater without amendments. Combined nutrient and substrate additions contained the same concentrations of glucose, ammonium, phosphate, and iron as for treatments where these amendments were added individually (Table 1). The concentrations of glucose differed in each experiment, while all other treatment concentrations were constant for all experiments. Amino acid additions were from a commercially available mixture of 20 amino acids (Pierce Chemical), from which metals had been removed using a Chelex ion-exchange resin column (47).
TABLE 1.
Net accumulation rates,a specific growth rates, and biomass yield
| Location, temp, and date | Treatment (concn) | Abundance (day−1) | Biovolume (day−1) | Maximum P/Bb (day−1) | Biomass yieldc (μg of C liter−1) |
|---|---|---|---|---|---|
| Station 1 (42°S, 141°E), 13°C, 2 March | Control | 0.13 | NSd | 0.27 | 0.38 |
| Glucose (6.7 μM C) | 0.28 | NS | 0.43 | 32.44 | |
| Fe (2.5 nM) | 0.21 | 0.22 | 0.23 | NS | |
| Glucose (6.7 μM) + Fe (2.5 nM) | 0.31 | NS | 0.44 | 31.52 | |
| NH4 (10 μM) + PO4 (1.6 μM) | 0.22 | NS | 0.28 | NS | |
| Glucose (6.7 μM) + NH4 (10 μM) + PO4 (1.6 μM) | 0.44 | NS | 1.10 | 46.99 | |
| Glucose (6.7 μM) + NH4 (10 μM) + PO4 (1.6 μM) + Fe (2.5 nM) | 0.42 | NS | 0.78 | 38.87 | |
| Station 2 (47°S, 141°E), 11°C, 9 March | Control | 0.04 | NS | 0.19 | 0.57 |
| Glucose (1 μM C) | 0.13 | 0.17 | 0.14 | NS | |
| Fe (2.5 nM) | NS | NS | 0.13 | NS | |
| Glucose (1 μM) + Fe (2.5 nM) | 0.13 | 0.16 | 0.33 | NS | |
| DFAA (1 μM) | 0.36 | 0.71 | 1.87 | 68.14 | |
| DFAA (1 μM) + Fe (2.5 nM) | 0.33 | 0.79 | 1.95 | 46.61 | |
| Station 3 (51°S, 142°E), 8.5°C, 26 March | Control | NS | NS | 0.06 | 1.08 |
| Glucose (10 μM C) | NS | 0.35 | 0.51 | NS | |
| Fe (2.5 nM) | NS | NS | 0.06 | NS | |
| Glucose (10 μM) + Fe (2.5 nM) | 0.16 | 0.53 | 0.70 | 21.36 | |
| DFAA (1 μM) | 0.32 | NS | 0.79 | 40.96 | |
| DFAA (1 μM) + Fe (2.5 nM) | 0.33 | 0.69 | 1.13 | 38.41 | |
| Station 4 (54°S, 141°E), 4.5°C, 18 March | Control | 0.11 | 0.23 | 0.05 | 3.24 |
| Glucose (5 μM C) | NS | NS | 0.05 | NS | |
| Fe (2.5 nM) | NS | NS | 0.05 | NS | |
| Glucose (5 μM) + Fe (2.5 nM) | 0.12 | 0.15 | 0.03 | NS | |
| DFAA (1 μM) | 0.11 | NS | 0.03 | NS | |
| DFAA (1 μM) + Fe (2.5 nM) | 0.07 | NS | 0.04 | NS |
Net accumulation rates derived from linear regression of natural log of cell abundance or biovolume through day 4 in all treatments.
Maximum specific growth rate estimated as P/B at each time point.
Biomass yield calculated as biomass at final time point − biomass at initial time point (t = 0).
NS, regression coefficient (slope) not significantly different from zero (P > 0.05) or treatment effect not significantly different from in situ control.
All sampling was done in a positive-pressure, trace-metal-clean incubation van. Daily samples of approximately 30 ml were poured from polyethylene incubation bottles into acid-cleaned, MilliQ-water-rinsed 50-ml polycarbonate tubes. The tubes were then transferred to a radiation van for preparation of incubation mixtures for [3H]thymidine (TdR) and [3H]leucine (Leu) incorporation assays. TdR incorporation may be used as a proxy for cell division and DNA synthesis, while Leu incorporation estimated prokaryotic protein production rates.
TdR and Leu incorporation assays.
Incubations for measurements of TdR and Leu incorporation were carried out in on-deck, flowthrough incubators or in shipboard refrigerated incubators. On-deck incubators were maintained at surface water temperatures with circulating surface seawater. Incorporation of TdR and Leu was measured by the microcentrifugation procedure described by Smith and Azam (55). High-specific-activity TdR and Leu (TdR, 79 Ci mmol−1; Leu, 179 Ci mmol−1) (New England Nuclear) were added to 2-ml microcentrifuge tubes, followed by the addition of 1.5 ml of sample water to start the incubations. The final concentration of both TdR and Leu was 20 nM. Triplicate samples of both TdR and Leu from each treatment bottle and each time point were incubated. Time zero blanks for both TdR and Leu were killed with 5% (final concentration) trichloroacetic acid. Microcentrifuge tubes for TdR and Leu incorporation were placed in darkened floating racks in shaded incubators and incubated for 4 to 16 h, depending on the expected activity of the samples and the temperature of the water.
Incubations were terminated by the addition of 100 μl of 100% trichloroacetic acid (final concentration, 5%). Samples were immediately frozen for subsequent radioassay following the cruise. Samples were processed as described by Smith and Azam (55) and Carlson et al. (10). Packard Ultima Gold scintillation cocktail was added to the pellet, and the radioactivity of each sample was counted with a Wallac 1409 liquid scintillation counter. Rates of isotope incorporation for each sample were calculated as the average from three replicates minus the value of the blank. Disintegrations per minute were calculated based on counts per minute standardized to external quench parameters and the counting efficiency. Counting errors were less than 20%.
Cellular abundance and volumes.
Samples for determination of bacterial abundance and cell volume were collected in 50-ml polyethylene tubes and preserved in filtered glutaraldehyde (0.2-μm-pore-size filter; 2% final concentration). Samples were immediately filtered onto blackened 0.2-μm-pore-size polycarbonate membrane filters (Poretics Corp.) and stained with a 0.05% solution of acridine orange. The volume filtered varied depending on cell density, with the objective of evenly distributing 100 to 300 cells per microscope field for image analysis. Filters were affixed to microscope slides with a small drop of Resolve immersion oil and mounted with a cover slide (28). Filters were frozen until processing.
Cell volumes were determined using a video image analysis system. Cells were enumerated on a Zeiss Axiophot epifluorescence microscope at a magnification of ×1,000. Video images of the 24- by 24-μm microscope field were captured and stored using Zeiss VIDAS VIDEOPLAN image analysis software. Fluorescence was achieved using blue excitation (450 to 490 nm) and a 520-nm emission filter from a 200-W mercury lamp. Sufficient video images were captured from each filter to yield between 300 and 1,000 measurements of individual cells. The length, width, area, and perimeter of each cell were measured, and cell volumes were calculated using algorithms calibrated with fluorescent beads (2). Algorithms included edge detection to minimize halo effects (4). Cell abundance was determined by visual cell counts where at least 300 cells per filter were counted.
Conversion factors.
Conversion factors from the literature were employed to translate incorporation and biovolume (abundance × mean cell volume) into carbon-based estimates of production and biomass. TdR incorporation rates were converted using 2 × 1018 cells mol of TdR−1 and 120 μg of C μm−3 (18, 22, 39). Leu incorporation could not be measured for the DFAA and DFAA plus Fe additions because the amino acid mixture contained unlabeled leucine and extracellular isotope dilution prevented signal detection. For this reason, estimates of bacterial production (micrograms of C liter−1 day−1) were derived exclusively from TdR incorporation rates. Leu incorporation was employed as an extra index of growth limitation for all other treatments. Bacterial production estimates were derived from TdR incorporation rates and estimates of mean cell volumes. Biomass was determined as cell abundance × mean cell volume × 120 fg of C μm−3) (39).
Data analysis.
Duplicate incubation samples were analyzed for each treatment. Data were analyzed statistically using multivariate analysis of variance. Statistically significant results were analyzed using Student-Newman-Kuels (SNK) multiple comparison tests, with statistical significance determined at a P value of <0.05 (59). For these experiments, SNK tests were used to distinguish differences between treatments and significant differences over time for the variables cell abundance, cell volume, rate of bacterial production, and specific growth rate (production rate divided by standing biomass [P/B]).
The rates of change of various properties were estimated using regression coefficients from model I least-squares fits on the natural logarithms of the individual data versus incubation time. Growth rates were derived by several methods. Calculation of P/B for given time points yielded estimates of the intrinsic specific growth rates (day−1), taking into account changes in both abundance and biovolume. Net accumulation rates were determined from the rate of increase of cell abundance over time, providing an indication of population growth based on cell division. Growth rates computed from changes in total biovolume used the rate of increase in the natural logarithm of (cell abundance × mean cell volume), which accounts for increases in cell size and cell division. Regressions were performed over appropriate intervals following inspection of the experimental time course plots. These net rates reflect the intrinsic growth rate as reduced by removal processes (e.g., grazing and viral lysis).
RESULTS
Bacterial production and distribution.
A strong zonal gradient in surface water temperatures was observed along the cruise transect. The most northern station had warm surface waters (14°C), while the surface water temperature at the southern station fell to ∼4°C. Major nutrient concentrations increased from north to south. The surface nitrate and phosphate concentrations in the subtropical zone (STZ) were 7 and 0.6 μM, respectively, while concentrations in the APF were 26 and 1.2 μM, respectively. Surface silicic acid concentrations showed an opposite trend, ranging between 0.1 and 2.5 μM, with substantial depletion in the STZ and increasing in abundance in the APF (53; Boyd et al., submitted). Dissolved iron concentrations were subnanomolar (0.05 to 0.70 nM) within the entire study region and generally decreased along a southerly gradient (53; Boyd et al., submitted). The phytoplankton community structure changed dramatically along the meridional transect; waters in the STZ were dominated by cyanobacteria, with a shift to eukaryotic dominance in the SAZ and APF (Boyd et al., submitted).
The rates of bacterial production in the upper mixed layer showed a north-south gradient, declining nearly an order of magnitude between the STF and the APF (0.3 to 0.03 μg of C liter−1 day−1). The bacterial abundance in the upper mixed layer displayed a similar zonal gradient, with a greater cell abundance at the northern end of the transect and a decrease towards the APF.
Responses to potential growth-limiting substances.
The major result of the four addition experiments performed in this study was that labile DOM stimulated rates of bacterial growth and enhanced biomass production (Table 1). Bacteria responded favorably to both glucose and amino acids, indicating that bacterial growth may be constrained by both organic carbon and nitrogen. Additions of dissolved iron or ammonium and phosphate alone had no significant impact on rates of bacterial growth or biomass production in any experiment. The two experiments in the SAZ and SAF indicated that bacterial growth responded to combined additions of glucose plus Fe. In two of three cases, bacterial growth showed dependence on the type of DOM substrate (glucose versus DFAA) used in the growth media (see below). Results were time dependent, with relative responses to each substrate differing during some time courses.
(i) Experiment in the STF (42°S, 141°E).
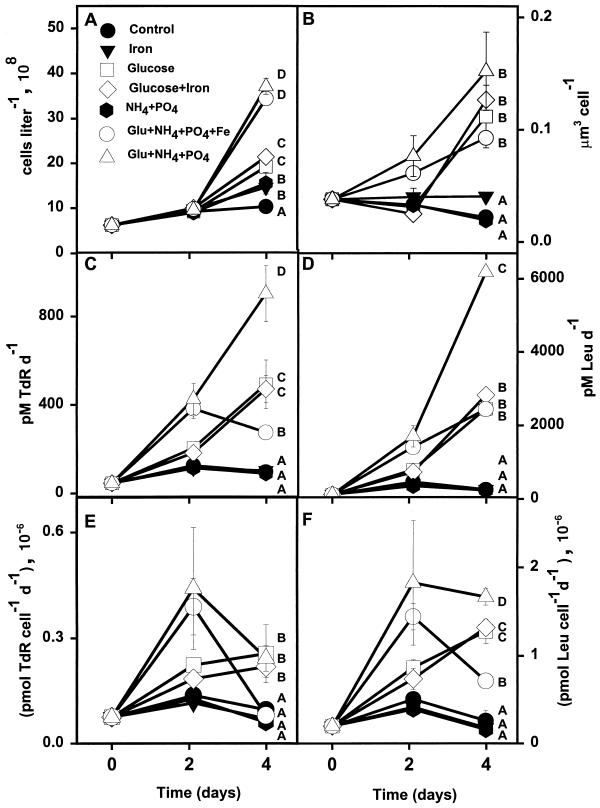
The addition of glucose and combinations of glucose with ammonium, phosphate, and/or iron all significantly stimulated bacterial growth rates and the production of bacterial biomass in seawater collected from the STF (Fig. 2; Table 2). By day 4, all samples receiving glucose treatments displayed elevated cell abundance relative to the controls (Fig. 2A). All samples receiving glucose showed significant increases in bacterial cell volume (micrometers3 cell−1) (Fig. 2B) and rates of bacterial production (Table 2). Rates of Leu incorporation in the cells given glucose plus NH4+ plus PO43− treatment were ∼25 times greater than those in cells given control treatments (Fig. 2D). Additions of glucose plus NH4+ plus PO43− and glucose plus NH4+ plus PO43− plus Fe dramatically increased rates of TdR incorporation per cell after 2 days (Fig. 2E). Overall, additions of NH4+ plus PO43− with glucose resulted in greater enhancement of cell growth rates than did glucose treatment without NH4+ plus PO43−. Specific growth rates (P/B) for the control, Fe, and NH4+ plus PO43− treatments were statistically indistinguishable throughout the experiment.
FIG. 2.
Responses of various bacterial properties to enrichment treatments in the STF (42°S, 141°E). (A) Cell abundance; (B) mean cell volume; (C) TdR incorporation; (D) Leu incorporation; (E) TdR incorporation per cell; (F) Leu incorporation per cell. Open symbols, organic amendments; closed symbols, inorganic amendments and unamended controls. Error bars are standard errors for duplicate samples. Letters represent results of SNK multiple-comparison tests at the final time point. Results for treatments with the same letter are statistically indistinguishable (P > 0.05).
TABLE 2.
Bacterial cell properties from experiment at 42°S
| Time (days) | Treatment | Bacterial biomass (μg of C liter−1)a | Bacterial production (μg of C liter−1 day−1)a |
|---|---|---|---|
| 0 | 3.56 | 0.54 | |
| 2 | Control | 4.71 (0.31) A | 1.29 (0.04) A |
| Glucose | 4.64 (0.58) A | 2.01 (0.04) B | |
| Fe | 5.91 (1.52) A | 1.34 (0.26) A | |
| Glucose + Fe | 4.51 (0.18) A | 1.52 (0.06) B | |
| NH4 + PO4 | 4.17 (0.21) A | 1.18 (0.20) A | |
| Glucose + NH4 + PO4 | 8.39 (0.40) A | 9.26 (4.10) C | |
| Glucose + NH4 + PO4 + Fe | 9.67 (1.91) A | 6.52 (0.64) C | |
| 4 | Control | 3.94 (0.44) A | 0.75 (0.01) A |
| Glucose | 36.00 (0.99) B | 15.42 (7.90) B | |
| Fe | 8.85 (0.22) A | 1.24 (0.05) C | |
| Glucose + Fe | 35.08 (0.02) B | 15.37 (1.49) B | |
| NH4 + PO4 | 5.45 (1.06) A | 0.63 (0.00) A | |
| Glucose + NH4 + PO4 | 50.55 (2.08) C | 24.61 (3.00) B | |
| Glucose + NH4 + PO4 + Fe | 42.43 (1.50) D | 6.78 (0.13) D |
Results with the same letter designations within a time point are statistically indistinguishable at a P value of >0.05. Numbers in parentheses are standard errors for duplicate samples.
(ii) Experiment in the SAZ (47°S, 141°E).
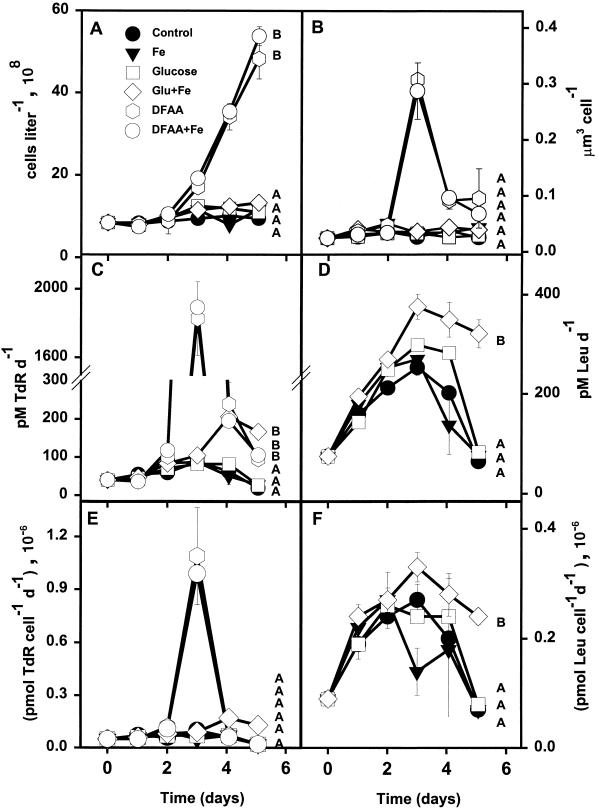
DFAA additions significantly stimulated rates of bacterial growth and enhanced bacterial biomass in amendment experiments performed in the SAZ (Fig. 3). Additions of DFAA and DFAA plus Fe produced roughly ten times more biomass than all other treatments, including glucose (Table 3). The addition of DFAA and DFAA plus Fe resulted in cell volumes (micrometers3 cell−1) approximately eight times greater than those with all other treatments by day 3 (Fig. 3B). DFAA and DFAA plus Fe additions resulted in rates of TdR incorporation ∼45 times higher than in situ rates measured at day 0. Rates of isotope incorporation (picomoles liter−1 day−1) with the glucose and glucose plus Fe additions increased slightly by days 4 and 5 but never attained the levels observed with the DFAA additions. The addition of glucose alone did not affect cell growth rates throughout the experiment, but the addition of glucose plus Fe increased growth rates more than five times above the control value by day 5. Samples receiving glucose plus Fe also showed significantly greater rates of TdR and Leu incorporation than those receiving glucose alone (Fig. 3C and D). Combined additions of Fe and DFAA had no measurable effect on growth rates relative to additions of DFAA alone.
FIG. 3.
Responses of various bacterial properties to enrichment treatments in the SAZ (47°S, 141°E). (A) Cell abundance; (B) mean cell volume; (C) TdR incorporation; (D) Leu incorporation; (E) TdR incorporation per cell; (F) Leu incorporation per cell. Open symbols, organic amendments; closed symbols, iron-only amendments or unamended controls. Error bars are standard errors for duplicate samples. Letter designations are the same as for Fig. 2.
TABLE 3.
Bacterial cell properties from experiment at 47°S
| Time (days) | Treatment | Bacterial biomass (μg of C liter−1)a | Bacterial production (μg of C liter−1 day−1)a |
|---|---|---|---|
| 0 | 3.50 | 0.34 | |
| 3 | Control | 4.13 (0.16) A | 0.78 (0.10) A |
| Glucose | 6.47 (1.95) A | 0.87 (0.30) A | |
| Fe | 9.01 (0.16) A | 0.93 (0.03) A | |
| Glucose + Fe | 6.35 (1.00) A | 1.15 (0.16) A | |
| DFAA | 74.55 (15.98) B | 139.34 (19.62) B | |
| DFAA + Fe | 69.35 (19.92) B | 135.35 (34.22) B | |
| 4 | Control | 5.73 (0.80) A | 0.72 (0.10) A |
| Glucose | 5.14 (0.58) A | 0.71 (0.11) A | |
| Fe | 4.22 (0.61) A | 0.53 (0.20) A | |
| Glucose + Fe | 6.43 (0.60) A | 2.14 (0.14) B | |
| DFAA | 40.45 (3.17) B | 5.54 (0.18) B | |
| DFAA + Fe | 45.32 (4.21) B | 4.98 (0.23) B | |
| 5 | Control | 4.07 (0.55) A | 0.17 (0.06) A |
| Glucose | 5.19 (0.41) A | 0.25 (0.13) B | |
| Fe | 7.36 (0.47) A | 0.37 (0.03) B | |
| Glucose + Fe | 7.90 (1.48) A | 1.97 (0.32) C | |
| DFAA | 71.64 (17.69) B | 2.34 (1.61) C | |
| DFAA + Fe | 50.11 (0.83) B | 1.98 (1.19) C |
Letter designations are the same as in Table 2. Numbers in parentheses are standard errors for duplicate samples.
(iii) Experiment in the SAF (51°S, 142°E).
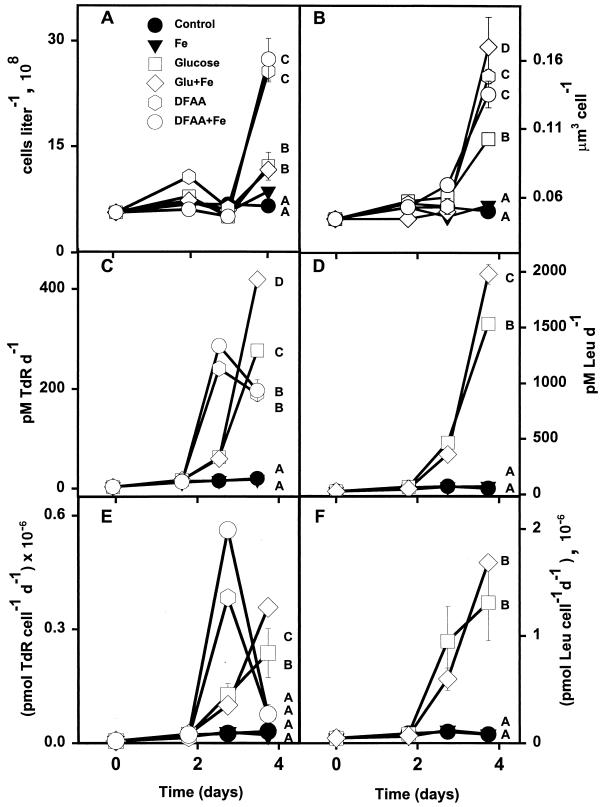
Bacterial growth in the SAF increased in response to treatments with both amino acids and glucose (Fig. 4; Table 4). Bacterial cell abundance and volume increased with both glucose treatments and amino acid treatments; however, the amino acid treatments produced larger cells than the glucose treatments (Fig. 4A and B). There were no significant differences in abundance, volume, biomass, production, or growth rates between Fe and control treatments (Table 4). DFAA and DFAA plus Fe treatments produced about three times more biomass than the glucose treatment and about twice the biomass of the glucose plus Fe treatment (Table 4). The glucose plus Fe addition resulted in nearly twice as much as biomass as the glucose-alone addition (Table 4). Additions of both glucose and amino acids stimulated rates of bacterial production in the SAF (Table 4). The glucose plus Fe treatment increased rates of TdR and Leu incorporation significantly above those with the glucose treatment (Fig. 4C and D). DFAA and glucose treatments resulted in more than a 100-fold increase in TdR uptake per cell relative to control and Fe treatments (Fig. 4E). DFAA additions provoked large increases in rates of TdR incorporation per cell through the first 3 days of the incubation (Fig. 4E), while glucose treatments significantly increased thymidine incorporation through all 4 days of the incubation. Bacterial growth was stimulated most dramatically by additions of amino acids and by the treatments containing glucose plus Fe (Fig. 4; Table 4).
FIG. 4.
Responses of various bacterial properties to enrichment treatments in the SAF (51°S, 142°E). (A) Cell abundance; (B) mean cell volume; (C) TdR incorporation; (D) Leu incorporation; (E) TdR incorporation per cell; (F) Leu incorporation per cell. Symbols are same as in Fig. 3. Error bars are standard errors for duplicate samples. Letter designations are the same as for Fig. 2.
TABLE 4.
Bacterial cell properties from experiment at 51°S
| Time (days) | Treatment | Bacterial biomass (μg of C liter−1)a | Bacterial production (μg of C liter−1 day−1)a |
|---|---|---|---|
| 0 | 3.01 | 0.03 | |
| 3 | Control | 4.32 (0.07) A | 0.19 (0.01) A |
| Glucose | 3.93 (0.49) A | 0.90 (0.03) B | |
| Fe | 2.95 (0.05) A | 0.18 (0.00) A | |
| Glucose + Fe | 3.56 (0.39) A | 0.72 (0.18) B | |
| DFAA | 3.93 (0.74) A | 3.11 (0.59) C | |
| DFAA + Fe | 4.18 (0.14) A | 4.74 (0.12) D | |
| 4 | Control | 4.09 (0.14) A | 0.23 (0.01) A |
| Glucose | 13.29 (1.33) B | 6.81 (0.06) B | |
| Fe | 5.40 (0.69) A | 0.24 (0.01) A | |
| Glucose + Fe | 24.37 (4.77) C | 17.11 (3.09) C | |
| DFAA | 43.97 (4.09) D | 6.75 (0.10) B | |
| DFAA + Fe | 41.42 (7.64) D | 6.33 (0.05) B |
Letter designations are the same as in Table 2. Numbers in parentheses are standard errors for duplicate samples.
(iv) Experiment in the APF (54°S, 141°E).
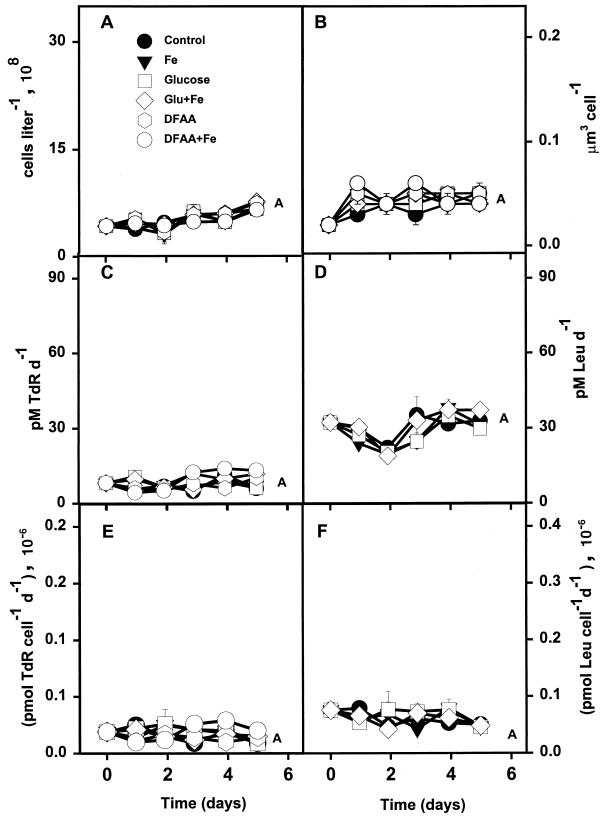
The most striking feature of the experiment conducted in the APF (54°S) was the lack of bacterial response to any of the treatments (Fig. 5). Bacterial abundance, cell volume, and rates of isotope incorporation all increased slightly with every treatment over time, but none of the measured cell properties changed significantly relative to one another. The initial biomass at day 0 was lower than at any other station by a factor of three, while in situ rates of TdR and Leu incorporation were nearly the same as those observed at the SAF (Fig. 4C and D and 5C and D). TdR incorporation ranged between 0.01 × 106 and 0.03 × 106 pmol cell−1 day−1 for all treatments over all time points (Fig. 5E).
FIG. 5.
Responses of various bacterial properties to enrichment treatments in the APF (54°S, 141°E). (A) Cell abundance; (B) mean cell volume; (C) TdR incorporation; (D) Leu incorporation; (E) TdR incorporation per cell; (F) Leu incorporation per cell. Symbols are the same as in Fig. 3. Error bars are standard errors for duplicate samples. Letter designations are the same as for Fig. 2.
DISCUSSION
Predominance of DOM stimulation of bacterial growth.
Based on these experiments, rates of bacterial growth in the Southern Ocean appeared to be controlled primarily by glucose or DFAA availability. Dissolved iron may have been important in regulating the complete response to labile DOM, but the primary limitation to bacterial growth was availability of labile organic substrates. These experiments also indicate that bacterial growth rates were most severely constrained by supplies of substrates that contain reduced nitrogen.
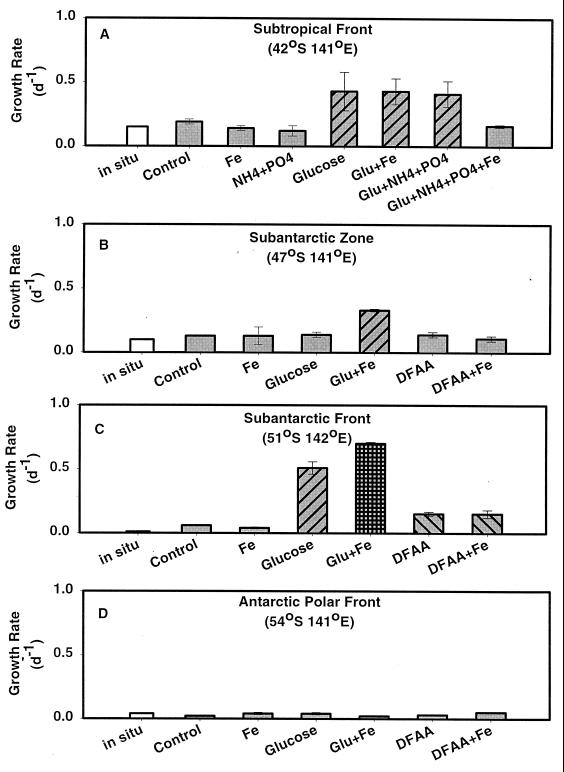
The most pronounced trend in three of the four studies was that the addition of DOM, as either glucose or amino acids, enhanced rates of bacterial growth and resulted in significant increases in bacterial abundance, production, and biomass. Additions of either glucose or amino acids increased production rates and resulted in the accumulation of larger bacterial cells than control treatments. Nine of the 16 treatments with DOM additions showed significant increases in bacterial abundance and biomass yields (Table 1). Fourteen out of the 16 treatments with either glucose or DFAA showed significantly increased net accumulation rates (abundance) relative to the control and Fe treatments (Table 1). Mean rates of TdR incorporation per cell for the glucose and DFAA treatments were ∼300% higher than those for control treatments in three of the four experiments. Finally, large increases in specific growth rates (P/B) were seen with nearly all of the DOM additions (Fig. 6).
FIG. 6.
Specific growth rates (P/B) at day 4 for experimental treatments. In situ rates are estimated at day 0 from control treatments. Error bars represent standard errors for duplicate treatments. Results with similar bar fill patterns are statistically indistinguishable (P > 0.05) for each experiment.
Patterns of response to DOM.
Patterns of bacterial growth changed in response to the types of organic substrates provided in these experimental treatments. Treatments with dissolved organic nitrogen (DFAA) or glucose plus NH4+ plus PO43− stimulated rates of bacterial growth and production of biomass to a greater extent than additions of glucose alone. Six of the eight treatments with either DFAA or glucose plus NH4+ plus PO43− resulted in larger maximal specific growth rates (P/B) and produced between 2 and 25 times more biomass than glucose and glucose plus Fe treatments. Mean bacterial biomass, production, and growth rates were greater for treatments with DFAA or glucose plus NH4+ plus PO43− than for treatments with glucose alone (Tables 2 to 4). In two of the four experiments, maximal rates of TdR incorporation occurred with either DFAA or glucose plus NH4+ plus PO43− (Fig. 2C and 3C).
One explanation for the observed bacterial growth response may be that bacterial growth in the Southern Ocean is limited by the availability of reduced nitrogen. Increased bacterial growth efficiency (BGE) on DFAA or glucose plus NH4+ plus PO43− substrates may have spurred the increases in growth rates with these treatments relative to treatments with organic carbon alone. Amino acids can be assimilated into cellular constituents (proteins and nucleic acids), providing cellular energy conservation by circumventing the cell's need to expend energy in construction of amino acids from simple carbon, nitrogen, and phosphorus substrates (11, 34, 44).
Reduced nitrogen substrates may have triggered substantial increases in BGE, resulting in large increases in growth rates. Recent efforts to measure BGE with bacteria growing on naturally occurring substrates indicate a range of 4 to 40% (6, 16). BGE is dependent in part on the quality of the substrate used to support bacterial growth. Goldman et al. (25) and Goldman and Dennett (24) observed no differences in BGE in laboratory enrichment cultures grown on several substrates, including DFAA or glucose plus NH4+. They found that the BGE ranged between ∼40 and 95%, with lower conversion efficiency on substrates with higher C/N ratios. Carlson and Ducklow (8) estimated BGE in amendment experiments in the Sargasso Sea and found a range of 4 to 30% for DFAA and glucose treatments. Kirchman (34) estimated a BGE of 34% for bacteria grown on DFAA in the subarctic Pacific.
In the present study, four of the eight treatments enriched in reduced nitrogen resulted in higher rates of bacterial production than glucose additions. Mean biomass yields and growth rates with the DFAA and glucose + NH4+ additions were consistently larger than those with glucose additions alone (Table 1). These results hint that bacterial growth on glucose alone may have been less efficient than growth on organic amendments containing reduced nitrogen. Carbon-rich substrates such as glucose provide energy for cellular maintenance but may not provide essential nutrients needed to facilitate growth. Additions of reduced nitrogen (as either DFAA or NH4+) may have alleviated possible nitrogen limitation and increased BGE.
Similar to Cherrier et al. (11) in the eastern north Pacific and Kirchman (34) in the subarctic Pacific, we observed that combined additions of organic carbon with reduced nitrogen stimulated bacterial growth rates. However, we also observed that additions of glucose and glucose plus Fe frequently resulted in large increases in biomass. Clearly, bacterial growth in the Southern Ocean is not limited simply by organic carbon or by nitrogen. Labile organic matter appeared to be the primary control over bacterial growth rates in the Southern Ocean, with three of the four experiments showing specific limitation by reduced nitrogen containing DOM.
The major difference between this study in the HNLC region of the Southern Ocean and similar studies in the HNLC subarctic Pacific (34, 37) and equatorial Pacific (33, 36) was that we observed large increases in bacterial biomass in response to treatments with DFAA and glucose plus NH4+. In the north Pacific, where water temperatures are comparable to temperatures in this study, additions of DFAA and glucose plus NH4+ resulted in large increases in bacterial production but only small increases in bacterial abundance (34). Similarly, amendment experiments in the equatorial Pacific indicated that DFAA and glucose plus NH4+ treatments stimulated rates of production but had no effect on bacterial abundance (36). Removal processes (predation and viral lysis) apparently had sufficient capability to respond to increases in bacterial production in these two systems, whereas in the Southern Ocean, removal processes appear to be temporally uncoupled from bacterial growth.
The most dramatic increases in bacterial biomass observed in this experiment were in response to organic additions containing reduced nitrogen. In two of the four experiments, glucose and glucose plus Fe treatments also significantly increased cell abundance over that with the control treatment, but the response was lower than that with treatments containing reduced nitrogen. Furthermore, DFAA and glucose plus NH4+ plus PO43− treatments frequently led to production of larger cells. Studies in other HNLC regions found that bacterial growth rates and rates of production are a function of DOM, while biomass is tightly constrained by removal processes (11, 34, 36). However, in the Southern Ocean, both biomass production and growth rates appear to be functions of the specific types of organic substrates that support bacterial growth.
Differences between the apparent coupling between DOM and bacterial biomass in the Southern Ocean and other HNLC oceans may be a reflection of fundamental differences in the structures of the microbial food webs among the different HNLC systems. Kirchman (34) and Kirchman and Rich (36) observed a close coupling between bacterial growth and removal in the subarctic and equatorial Pacific despite additions of labile DOM. Although our experiments were manipulative and may not accurately model in situ processes, they provide evidence that increased fluxes of labile DOM in the Southern Ocean might result in a temporal uncoupling between bacterial growth and removal processes.
Colimitations: DOM and iron.
Another important difference between this study and other studies in the subarctic and equatorial Pacific is that the studies conducted in the subarctic and equatorial Pacific did not investigate the potential role of dissolved iron in regulating bacterial growth, and the resulting experimental treatments should be considered iron replete. The work described in this study was done under trace-metal-clean conditions, providing insight into possible limitation by both iron and organic material.
Additions of iron alone did not significantly increase bacterial growth rates above those with the control treatments in any of these experiments, unlike the results of Pakulski et al. (43) from another part of the Southern Ocean. However, a combined addition of glucose and iron frequently resulted in higher growth rates, rates of production, and accumulation of biomass than control treatments. In three of the four experiments, glucose alleviated growth limitation, while in two of the four experiments, the combined additions of dissolved iron and glucose resulted in larger increases in bacterial biomass than additions of glucose alone. Iron appeared to have a less important effect on bacterial growth with DFAA treatments. The bacterial growth responses to DFAA plus Fe and glucose plus Fe treatments suggest that BGEs in the DFAA treatments were already near maximal levels, and the addition of iron had no measurable impact on bacterial growth. In laboratory cultures, Tortell et al. (58) showed that BGE could be limited by the availability of iron. In iron-limited systems such as the HNLC Southern Ocean, bacterial cells may lack iron necessary for optimal functioning of the electron transport chain, reducing the total energy yield produced by organic matter oxidation. Additions of iron to glucose treatments may have increased the conversion efficiency of glucose, resulting in increased biomass production rates.
Our experiments indicate that bacterial growth in the iron-limited HNLC Southern Ocean is partly constrained by the availability of reduced nitrogen. One possible pathway through which iron and reduced nitrogen interact is assimilatory nitrate reduction. The cytoplasmic enzymes catalyzing the reduction of nitrate to ammonium require iron. Bacterial utilization of nitrate is generally low due to the high energetic costs associated with the reduction of nitrate to ammonium (36). We did not observe any response to experimental treatments amended with only iron, indicating that direct iron inhibition of bacterially mediated nitrate reduction was not the primary factor controlling rates of bacterial growth. However, our results indicate that bacterial growth is at least partly constrained by the availability of labile organic matter. This suggests that if nitrate reductase is inhibited by low in situ iron concentrations, its expression may be linked to the availability of an energy source such as labile DOM.
The possible inhibition of nitrate reductase by low iron availability could also affect the phytoplankton community and thus indirectly control bacterial growth. Phytoplankton growth on nitrate as a nitrogen source requires nitrate reductase. One possible implication of iron-inhibited nitrate reductase activity might be a decrease in inputs of reduced-nitrogen-containing DOM from phytoplankton, thus indirectly limiting bacterial growth. Various investigations into the factors limiting bacterial growth in other HNLC oceans support our observations that bacterial growth may be largely constrained by reduced-nitrogen-containing DOM (34, 35, 37).
Bacterial growth in the APF showed no stimulation by organic enrichments or dissolved iron, indicating that some factor other than DOM exerted specific control over rates of bacterial biomass production. It is possible that treatments in this experiment were contaminated with dissolved iron. Boyd et al. (submitted) noted significant iron contamination of water obtained at the same time as water for our experiment in the APF. As a result of this potential contamination, no firm conclusions about the role of Fe alone as a limiting factor may be drawn from this experiment; however, potential iron contamination does not detract from the striking lack of bacterial response to organic amendments observed in this experiment. In situ cell abundance and incorporation rates were lower at the APF than at any other station (Fig. 5). Furthermore, temperatures in the APF were ∼4°C cooler than those in the SAF. Kirchman and Rich (36) noted that the time scale of the bacterial response to increased DOM concentrations was longer at lower temperatures in the equatorial Pacific during normal conditions relative to El Niño conditions, when the temperature was nearly 5°C higher. It is possible that the 5-day incubation period used in this study was insufficient to observe a significant bacterial response to the amendments.
Pomeroy and Deibel (45) observed significant suppression of microbial metabolism at cold temperatures (−1.8 to +1°C). Pomeroy et al. (46) and Wiebe et al. (61) concluded that an interaction between temperature and organic substrate concentrations might partly constrain bacterial growth rates. Both investigations found that bacterial growth rates were sensitive to manipulations of both temperature and substrate, providing evidence that when bacteria are grown at the low end of their temperature range, they may require higher substrate concentrations to maintain cellular activity (45, 61). The APF marks the confluence of warm subantarctic water and cold polar water. Bacterioplankton sampled in the APF may be adapted to the higher water temperatures characteristic of the subantarctic waters (∼8°C). Those organisms adapted to warmer water would show optimal rates of growth at temperatures greater than in situ temperatures found in the APF (∼4°C). Bacterial growth in the APF may have been partly constrained by the low water temperatures observed in the APF. Past investigations into bacterial dynamics in the pelagic Southern Ocean indicated that metabolic activity and biomass tended to decrease south of the STF, increasing again south of the APF (26, 27).
We hypothesize that no single factor limited rates of bacterial growth in the Southern Ocean. Rather, a combination of factors, including carbon, nitrogen, temperature, and iron, combined to restrict the growth of heterotrophic bacteria. Growth-limiting factors may stem from an array of resource limitations across several trophic levels whose combined effect is to reduce in situ bacterial growth rates. Overall, bacterial growth in the HNLC Southern Ocean appeared to be limited by inputs of dissolved organic nitrogen and carbon. However, bacterial growth in the APF may be more tightly constrained by low temperatures than by resources. In the SAF and SAZ, additions of carbon, nitrogen, and iron may all interact to control bacterial stocks and rates of bacterial growth.
ACKNOWLEDGMENTS
We gratefully acknowledge Peter Sedwick and Tom Trull (University of Tasmania) for providing the opportunity to conduct this work. We are grateful to the officers and crew of the R.V. Aurora Australis and the Australian Antarctic Division of CSIRO. Philip Boyd (University of Otago) provided equipment and provocative insights into the ecology of HNLC oceans. Flynn Cunningham provided technical assistance in the image analysis part of this work. Scott Polk provided the study site map, and Jessica Morgan aided with statistical analyses.
This work was supported by NSF grants OPP 95-30734 to H.W.D. and INT 9802132 and OCE 9730334 to D.A.H.
Footnotes
U.S. J.G.O.F.S. contribution number 545. V.I.M.S. contribution number 2277.
REFERENCES
- 1.Azam F, Hodson R E. Size distribution and activity of marine microheterotrophs. Limnol Oceanogr. 1977;22:492–501. [Google Scholar]
- 2.Baldwin W W, Bankston P W. Measurement of live bacteria by Nomarski interference microscopy and steriologic methods as tested with macroscopic rod-shaped models. Appl Environ Microbiol. 1988;54:105–109. doi: 10.1128/aem.54.1.105-109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banse K. Low seasonality of low concentrations of surface chlorophyll in the Subantarctic water ring: underwater irradiance, iron, or grazing? Prog Oceanogr. 1996;37:241–291. [Google Scholar]
- 4.Bjornsen P K, Kuparinen J. Determination of bacterioplankton biomass, net production and growth efficiency in the Southern Ocean. Mar Ecol Proc Ser. 1991;71:185–194. [Google Scholar]
- 5.Butler E C V, Butt J A, Lindstrom E J, Tildesley P C, Pickmere S, Vincent W F. Oceanography of the Subtropical Convergence Zone around southern New Zealand. New Zealand J Mar Fresh Res. 1992;26:131–154. [Google Scholar]
- 6.Carlson, C. A., N. R. Bates, H. W. Ducklow, and D. A. Hansell. Estimation of bacterial respiration and growth efficiency in the Ross Sea, Antarctica. Aquat. Microb. Ecol., in press.
- 7.Carlson C A, Ducklow H W. Dissolved organic carbon in the upper ocean of the central equatorial Pacific ocean: daily and finescale vertical variations. Deep-Sea Res II. 1996;42:639–656. [Google Scholar]
- 8.Carlson C A, Ducklow H W. Growth of bacterioplankton and consumption of dissolved organic carbon in the Sargasso Sea. Aquat Microb Ecol. 1996;10:69–85. [Google Scholar]
- 9.Carlson C A, Ducklow H W, Michaels A F. Annual flux of dissolved organic carbon from the euphotic zone in the northwest Sargasso Sea. Nature. 1994;371:405–408. [Google Scholar]
- 10.Carlson C A, Ducklow H W, Hansell D A, Smith W O., Jr Organic carbon partitioning during spring phytoplankton blooms in the Ross Sea polyna and the Sargasso Sea. Limnol Oceanogr. 1998;43:375–386. [Google Scholar]
- 11.Cherrier J, Bauer J E, Druffel E R M. Utilization and turnover of labile dissolved organic matter by bacterial heterotrophs in eastern North Pacific surface waters. Mar Ecol Prog Ser. 1996;139:267–279. [Google Scholar]
- 12.Cho B C, Azam F. Major role of bacteria in biogeochemical fluxes in the ocean's interior. Nature. 1988;332:441–443. [Google Scholar]
- 13.Cotner J J B, Wetzel R G. Uptake of dissolved inorganic and organic phosphorous compounds by phytoplankton and bacterioplankton. Limnol Oceanogr. 1992;37:232–243. [Google Scholar]
- 14.Currie D J, Kalff J. Can bacteria outcompete phytoplankton for phosphorus? A chemostat test. Microb Ecol. 1984;10:205–216. doi: 10.1007/BF02010935. [DOI] [PubMed] [Google Scholar]
- 15.deBaar H J W, de Jong J T M, Bakker D C E, Loscher B M, Veth C, Bathmann U, Smetacek V. Importance of iron for plankton blooms and carbon dioxide drawdown in the Southern Ocean. Nature. 1995;373:412–415. [Google Scholar]
- 16.del Giorgio P, Cole J J. Bacterial growth efficiency in natural aquatic systems. Annu Rev Ecol Syst. 1998;29:503–541. [Google Scholar]
- 17.Ducklow H W. Factors regulating bottom-up control of bacteria biomass in open ocean plankton communities. Arch Hydrobiol Beih. 1992;37:207–217. [Google Scholar]
- 18.Ducklow H W, Carlson C A. Oceanic bacterial production. Adv Microb Ecol. 1992;12:113–181. [Google Scholar]
- 19.Ducklow H W, Hill S M. The growth of heterotrophic bacteria in the surface waters of warm core rings. Limnol Oceanogr. 1985;30:239–259. [Google Scholar]
- 20.Ducklow H W, Quinby H L, Carlson C A. Bacterioplankton dynamics in the equatorial Pacific during the 1992 El-Nino. Deep-Sea Res II. 1995;42:621–638. [Google Scholar]
- 21.Fuhrman J A, Sleeter T D, Carlson C A, Proctor L M. Dominance of bacterial biomass in the Sargasso Sea and its ecological implications. Mar Ecol Prog Ser. 1989;57:207–217. [Google Scholar]
- 22.Fuhrman J A, Azam F. Bacterioplankton secondary production estimates for coastal waters of British Columbia, Antarctica, and California. Appl Environ Microbiol. 1980;39:1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuhrman J, Azam F. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Mar Bio. 1982;66:109–120. [Google Scholar]
- 24.Goldman J C, Dennett M R. Ammonium regeneration and carbon utilization by marine bacteria grown on mixed substrates. Mar Biol. 1991;109:369–378. [Google Scholar]
- 25.Goldman J C, Caron D A, Dennett M R. Regulation of gross growth efficiency and ammonium regeneration in bacteria by substrate C:N ratio. Limnol Oceanogr. 1987;32:1239–1252. [Google Scholar]
- 26.Hanson R B, Lowery K. Spatial distribution, structure, biomass, and physiology of microbial assemblages across the Southern Ocean frontal zones during late austral winter. Appl Environ Microbiol. 1985;49:1029–1039. doi: 10.1128/aem.49.5.1029-1039.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanson R B, Shafer D, Ryan T, Pope D H, Lowery K. Bacterioplankton in Antarctic ocean waters during late austral winter: abundance, frequency of dividing cells, and estimates of production. Appl Environ Microbiol. 1983;45:1622–1632. doi: 10.1128/aem.45.5.1622-1632.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hobbie J E, Daley R J, Jasper S. Use of Nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchins D A, DiTullio G R, Zhang Y, Bruland K W. An iron limitation mosaic in the California upwelling regime. Limnol Oceanogr. 1998;43:1037–1054. [Google Scholar]
- 30.Hutchins D A, Franck V M, Brezinski M A, Bruland K W. Inducing phytoplankton iron limitation in iron-replete coastal waters with a strong chelating ligand. Limnol Oceanogr. 1999;44:1009–1018. [Google Scholar]
- 31.Keil R G, Kirchman D L. Contribution of dissolved free amino acids and ammonium to the nitrogen requirements of heterotrophic bacterioplankton. Mar Ecol Prog Ser. 1991;73:1–10. [Google Scholar]
- 32.Keil R G, Kirchman D L. Dissolved combined amino acids: chemical form and utilization by marine bacteria. Limnol Oceanogr. 1993;38:1256–1270. [Google Scholar]
- 33.Kirchman D L, Rich J H, Barber R T. Biomass and biomass production of heterotrophic bacteria along 140°W in the Equatorial Pacific: effect of temperature on the microbial loop. Deep-Sea Res II. 1995;42:603–619. [Google Scholar]
- 34.Kirchman D L. Limitation of bacterial growth by dissolved organic matter in the subarctic Pacific. Mar Ecol Prog Ser. 1990;62:47–54. [Google Scholar]
- 35.Kirchman D L. The uptake of inorganic nutrients by heterotrophic bacteria. Microb Ecol. 1994;28:255–271. doi: 10.1007/BF00166816. [DOI] [PubMed] [Google Scholar]
- 36.Kirchman D L, Rich J H. Regulation of bacterial growth by dissolved organic carbon and temperature in the Equatorial Pacific Ocean. Microb Ecol. 1997;33:11–20. doi: 10.1007/s002489900003. [DOI] [PubMed] [Google Scholar]
- 37.Kirchman D L, Keil R G, Simon M, Welschmeyer N A. Biomass and production of heterotrophic bacterioplankton in the oceanic subarctic Pacific. Deep-Sea Res II. 1993;40:967–988. [Google Scholar]
- 38.Landry M R, Barber R T, Bidigare R, Chai F, Coale K H, Dam H G, Lewis M R, Lindley S T, McCarthy J J, Roman M R, Stoecker D K, Verity P G, White J R. Iron and grazing constraints on primary production in the central equatorial Pacific: an EqPac synthesis. Limnol Oceanogr. 1997;42:405–418. [Google Scholar]
- 39.Lee S, Fuhrman J A. Relationships between biovolume and biomass of naturally-derived marine bacterioplankton. Appl Environ Microbiol. 1987;52:1298–1303. doi: 10.1128/aem.53.6.1298-1303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longhurst A R. Ecological geography of the sea. San Diego, Calif: Academic Press; 1998. [Google Scholar]
- 41.Martin J H, Fitzwater S E. Iron deficiency limits phytoplankton growth in the N.E. Pacific Subarctic Nature. 1988;331:341–343. [Google Scholar]
- 42.Martin J H, Gordon R M, Fitzwater S E. Iron in Antarctic water. Nature. 1990;345:156–158. [Google Scholar]
- 43.Pakulski J D, Coffin R B, Kelley C A, Holder S L, Downer R, Aas P, Lyons M M, Jeffrey W H. Iron stimulation of Antarctic bacteria. Nature. 1996;383:133–134. [Google Scholar]
- 44.Payne W J, Weibe W J. Growth yield and efficiency in chemosynthetic organisms. Annu Rev Microbiol. 1978;32:155–183. doi: 10.1146/annurev.mi.32.100178.001103. [DOI] [PubMed] [Google Scholar]
- 45.Pomeroy L R, Deibel D. Temperature regulation of bacterial activity during the spring bloom in Newfoundland coastal waters. Science. 1986;233:359–361. doi: 10.1126/science.233.4761.359. [DOI] [PubMed] [Google Scholar]
- 46.Pomeroy L R, Wiebe W J, Deibel D, Thompson R J, Rowe G T, Pakulski J D. Bacterial response to temperature and substrate concentration during the Newfoundland spring bloom. Mar Ecol Prog Ser. 1991;75:143–159. [Google Scholar]
- 47.Price N M, Harrison G I, Hering J G, Hudson R J, Nirel P M V, Palenik B, Morel F M M. Preparation and chemistry of the artificial algal culture medium. Aquil Biol Oceanogr. 1989;6:443–461. [Google Scholar]
- 48.Proctor L M, Fuhrman J A. Viral mortality of marine bacteria and cyanobacteria. Nature. 1990;343:60–62. [Google Scholar]
- 49.Rich J H, Ducklow H W, Kirchman D L. Concentrations and uptake of neutral monosaccharides along 140°W in the equatorial Pacific: contribution of glucose to heterotrophic bacterial activity and the DOM flux. Limnol Oceanogr. 1997;41:595–604. [Google Scholar]
- 50.Rintoul S R, Donguy J R, Roemmich D H. Seasonal evolution of the upper ocean thermal structure between Tasmania and Antarctica. Deep-Sea Res I. 1997;44:1185–1202. [Google Scholar]
- 51.Rivkin R B, Anderson M R. Inorganic nutrient limitation of oceanic bacterioplankton. Limnol Oceanogr. 1997;42:730–740. [Google Scholar]
- 52.Sedwick P N, Edwards P R, Mackey D J, Griffiths F B, Parslow J S. Iron and manganese in the surface waters of the Australian subantarctic region. Deep-Sea Res I. 1997;44:1239–1253. [Google Scholar]
- 53.Sedwick P N, DiTullio G R, Hutchins D A, Boyd P W, Griffiths F B, Crossley A C, Trull T W, Queguiner B. Limitation of algal growth by iron deficiency in the Australian Subantarctic region. Geophys Res Lett. 1998;26:2865–2868. [Google Scholar]
- 54.Shiah F-K, Ducklow H W. Temperature regulation of heterotrophic bacterioplankton abundance, production, and specific growth rate in Chesapeake Bay. Limnol Oceanogr. 1994;39:1243–1258. [Google Scholar]
- 55.Smith D C, Azam F. A simple, economical method for measuring bacterial protein synthesis in seawater using 3H-leucine. Mar Microb Food Webs. 1992;6:107–114. [Google Scholar]
- 56.Suttle C A, Fuhrman J A, Capone D G. Rapid ammonium cycling and concentration-dependent partitioning of ammonium and phosphate: implications for carbon transfer in planktonic communities. Limnol Oceanogr. 1990;35:424–433. [Google Scholar]
- 57.Thingstad T F, Hagstrom A, Rassoulzadegan F. Accumulation of degradable DOC in surface waters: is it caused by a malfunctioning microbial loop? Limnol Oceanogr. 1997;42:398–404. [Google Scholar]
- 58.Tortell P D, Maldonado M T, Price N M. The role of heterotrophic bacteria in iron-limited ocean ecosystems. Nature. 1996;383:330–332. [Google Scholar]
- 59.Underwood A J. Experiments in ecology: their logical design and interpretation using analysis of variance. New York, N.Y: Cambridge University Press; 1997. [Google Scholar]
- 60.Wheeler P A, Kirchman D L. Utilization of inorganic and organic nitrogen by bacteria in marine systems. Limnol Oceanogr. 1986;31:998–1009. [Google Scholar]
- 61.Wiebe W J, Sheldon W M, Pomeroy L R. Evidence of an enhanced substrate requirement by marine mesophilic bacterial isolates at minimal growth temperatures. Microb Ecol. 1993;25:151–160. doi: 10.1007/BF00177192. [DOI] [PubMed] [Google Scholar]
- 62.Williams P J L B. Evidence for the seasonal accumulation of carbon-rich dissolved organic material, its scale in comparison with changes in particulate material and consequential effect on net C/N assimilation rates. Mar Chem. 1995;51:17–29. [Google Scholar]
- 63.Wright R T, Coffin R B. Factors affecting bacterioplankton density and productivity in salt marsh estuaries. In: Klug M J, Reddy C A, editors. Current perspectives in microbial ecology. Washington, D.C.: American Society for Microbiology; 1984. pp. 485–494. [Google Scholar]
- 64.Zweifel U L. Factors controlling accumulation of labile dissolved organic carbon in the Gulf of Riga. Estuar Coast Shelf Sci. 1999;48:357–370. [Google Scholar]