Abstract
French Guiana, a tropical country, is characterised by a young and multi-ethnic population. Difficulties in accessing safe water sources lead to outbreaks of gastroenteritis. The objectives of this study were (1) to describe the microbiological profile of shigella strains isolated in western French Guiana, including antimicrobial susceptibility and the distribution of strains in terms of species and serotypes and (2) to estimate the incidence of shigellosis in children under 5 years old. A retrospective observational study was conducted of 213 cases of shigellosis diagnosed in the biology department of the hospital centre for western French Guiana between 2000 and 2012 in children under 5 years old. The serogroups (formerly known as species) that predominates in French Guiana was Shigella flexneri. No resistance was observed to fluoroquinolones or to third-generation cephalosporins. The average incidence of shigellosis in children under 5 years old in western French Guiana was estimated at 189.6 cases per 100 000 inhabitants per year. Shigellosis is a public health problem in western French Guiana. These infections suggest the difficulties in accessing safe water sources and the lack of public sanitation. A quadrivalent vaccine containing Shigella sonnei and three serotypes of S. flexneri (S. flexneri 2a, 3a and 6) could provide broad coverage against shigella infections.
Key words: Antibiotic resistance, diarrhoea, dysentery (bacillary), epidemiology, shigella
Introduction
One hundred sixty-five million episodes of shigellosis are estimated to occur annually worldwide, and the vast majority of cases and deaths occur in low- to middle-income countries in children under 5 years of age [1]. The Global Enteric Multicentre Study, a matched case–control study of moderate-to-severe diarrhoea in children aged 0–24 months, showed that shigella is among the top ranking pathogens in the South American countries (Peru and Brazil) [2]. The shigella genus is composed of four serogroups (formerly known as species), i.e. Shigella flexneri, Shigella dysenteriae, Shigella boydii and Shigella sonnei, which are the aetiological agents of bacillary dysentery and shigellosis. A uniquely small inoculum of shigella (as few as 10 organisms) is required to cause dysentery, which facilitates person-to-person transmission [3].
High rates of resistance to conventional antibiotics such as ampicillin, chloramphenicol and cotrimoxazole have been described in many studies [4]. Thus, extended-spectrum cephalosporins, fluoroquinolones and azithromycin have come into use as front-line antibiotics. The clinical severity of shigellosis and the emergence of resistance against first-line therapies highlight the growing need to develop a vaccine against shigella infections. However, the existence of four different serogroups and at least 50 antigenically distinct serotypes is a barrier. Indeed, the larger the number of serotypes to be included, the more complex and expensive the vaccine becomes. As the serotype distribution differs by geographical region, a better understanding of the serotype distribution of shigella species at a national level is important in order to inform vaccine development [5].
French Guiana is a French overseas department located in South America. The subequatorial climate is characterised by a dry season (from the end of July to mid-November) alternating with a rainy season (from mid-November to the end of July), which is sometimes interrupted by a small dry season in March. The majority of the population lives on the coast (90%), and 10% of the population lives along the rivers – especially the Maroni River and the Oyapock River – or far inland. Some people have difficulty accessing drinking water. In 2010, almost 15% of the population did not have drinking water at home, including 70% of people living in rural areas. These drinking-water access problems are responsible for the spread of waterborne pathologies – such as typhoid fever – which cause epidemics, and can even become endemic [6].
There are few data available on shigellosis in French Guiana. A retrospective study was conducted in Saint-Laurent du Maroni's Hospital between 2000 and 2014 among pregnant women (37 cases of shigellosis diagnosed). S. flexneri and S. sonnei were the two species isolated from maternal stools [7].
This article aims to describe the strains of shigella isolated in children under 5 years of age (prevalence, distribution of species and serotypes, antimicrobial resistance), to identify seasonality in case distribution and to estimate the incidence of disease.
Materials and methods
Study population
A retrospective study was carried out of all shigella strains isolated between 2000 and 2012 from stools, urines, gastric samples and blood in children under 5 years of age at the bacteriological laboratory of Saint-Laurent du Maroni Hospital. This hospital is the second hospital in French Guiana and the only secondary health care facility in western French Guiana. The catchment area of this hospital included 79 500 inhabitants of which 51% lived in Saint-Laurent du Maroni the main town and 27% in rural areas in inland. The population is young and multi-ethnic. Children under 5 years of age accounted for 18% in 2010 [8].
Data collection
We collected from the laboratory dataset: demographics data (age, gender).
Bacterial strains, serotyping and antibiotics susceptibility of shigella strains
Shigella was identified by conventional methods and serotyping was performed by slide agglutination assays with a complete set of antisera recognizing all the described shigella serotypes [9]. The susceptibility of the shigella isolates to antibiotics was assessed using the VITEK 2 Compact system in accordance with the recommendations of the European Committee on Antimicrobial Susceptibility Testing [10]. The antibiotics tested were: cotrimoxazole, amoxicillin/clavulanic acid, amoxicillin, ampicillin, ticarcillin, piperacillin/tazobactam, cefalotin, cefotaxim, ceftazidim, cefoxitin, imipenem, tobramycin, gentamycin, amikacin, netilmycin, nalidixic acid, ofloxacin and ciprofloxacin. If more than one isolate with the same serotype and antimicrobial resistance phenotype was recovered from the same patient, only the first was included.
Statistical analysis
The number of cases every month was compared with the average monthly precipitation between 2002 and 2012. The data were obtained from the Météo France website [11].
The incidence rate between 2006 and 2012 was estimated from a census of children under 5 years of age in western French Guiana during the same period.
A series of inferential statistics was conducted to compare antimicrobial resistance across shigella serogroups or time periods, using χ2 test, Yates corrections or Fisher's exact test applied as needed. Spearman's coefficient correlation was used to demonstrate a correlation between the number of cases each month and average monthly precipitation. The α level was set at 5%.
The study was carried out with the approval of the Saint-Laurent du Maroni hospital ethics committee.
Results
Prevalence, serogroups and serotypes
Shigella was the second bacterial agent most frequently isolated, identified in 3366 stool samples (6.2%), after the enteropathogen Escherichia coli (8.3%). The other genera of bacteria isolated were Salmonella sp (3.6%), Campylobacter sp (3.1%) and Yersinia sp (0.01%).
In total, 213 strains of shigella were isolated between 2000 and 2012 from 213 infants and children under the age of 5 years with diarrhoea: 210 strains were isolated from stool samples, two from urine and one from a gastric sample. No strains were isolated from blood.
Male accounted for 51% of the patients (n = 109). The median age was 1.1 years, the 25th percentile was 0.7 years and the 75th percentile was 2.1 years. Children under 1 year of age accounted for 45% of the cases, including 15% of children under 6 months. Three neonatal cases defined by the presence of shigella in stool in children under the age of 1 month were observed.
The serogroup was determined for 210 strains of shigella, including 161 (77%) S. flexneri strains, 48 S. sonnei strains (23%) and one S. boydii strain. The indeterminate strains are referred to as Shigella spp hereafter. No S. dysenteriae strains were isolated.
Eight serotypes of S. flexneri were identified. The four major serotypes were serotype 2a (n = 100, 62%), serotype 3a (n = 26, 16%), serotype 1b (n = 12, 7%) and serotype 6 Boyd 88 (n = 9, 6%). The other serotypes were serotype 3b (n = 2, 2%), X, Y and 1a (n = 1, 1% each one). The serotype for nine S. flexneri strains was indeterminate. Serotype 2a was predominant throughout the study period. All S. sonnei strains belonged to biotype g. The biotype for two S. sonnei strains was indeterminate. The S. boydii strain belonged to serotype 20.
Antimicrobial resistance
The antimicrobial resistance is summarised in Table 1. The resistance profiles of S. flexneri and S. sonnei differed. Significantly more S. sonnei strains than S. flexneri strains were resistant to cotrimoxazole (96% vs. 69%, P < 0.001). A higher proportion of S. flexneri strains than S. sonnei strains were resistant to ampicillin (73% vs. 10%, P < 0.001), amoxicillin (37% vs. 0%, P < 0.001), ticarcillin (74% vs. 6%, P < 0.001) and cefalotin (17% vs. 4%, P = 0.03). The S. boydii strain was resistant to amoxicillin and ticarcillin, but was sensitive to cotrimoxazole. All of the strains were sensitive to aminoglycoside, fluoroquinolone and third-generation cephalosporins. One S. sonnei strain was resistant to nalidixic acid.
Table 1.
Antimicrobial resistance in Shigella flexneri, Shigella sonnei and Shigella spp strains
| Antibiotic | S. flexneri (n = 161) | % | S. sonnei (n = 48) | % | Shigella spp (n = 3) | % | Total of Shigella (n = 213) | % | P-value* |
|---|---|---|---|---|---|---|---|---|---|
| Cotrimoxazole | 108/156 | 69 | 46/48 | 96 | 2/3 | 67 | 156/207 | 75 | <0.001 |
| Amoxicillin – clavulanic acid | 25/156 | 16 | 0/48 | 0 | 0/3 | 0 | 25/207 | 12 | 0.003 |
| Ampicillin | 65/89 | 73 | 3/29 | 10 | _ | _ | 68/118 | 58 | <0.001 |
| Amoxicillin | 51/67 | 67 | 0/19 | 0 | 3/3 | 100 | 54/89 | 61 | <0.001 |
| Ticarcillin | 115/156 | 74 | 3/48 | 6 | 3/3 | 100 | 121/207 | 58 | <0.001 |
The results are expressed in number of resistant strains/number of strains tested and then in per cent.
*The P-value referenced the comparison between S. flexneri and S. sonnei strains.
The changes in resistant strains of S. flexneri and S. sonnei during 2000–2004, 2005–2008 and 2009–2012 are summarised in Tables 2 and 3. Among the S. flexneri strains, the resistance to clavulanic acid/amoxicillin increased, reaching 30% of strains between 2009 and 2012 (P < 0.001). The resistance to cotrimoxazole dropped between 2005–2008 and 2009–2012 (from 93% to 54%, respectively, P < 0.001). The resistance to ampicillin and ticarcillin decreased, but the difference was not significant. Among the S. sonnei strains, the resistance to cotrimoxazole increased but the difference was not significant. All the S. sonnei strains tested from the 2009–2012 period were resistant to cotrimoxazole. Few strains were resistant to β-lactam antibiotics, and only 8% of strains tested from 2009 to 2012 were resistant to ampicillin and ticarcillin.
Table 2.
Evolution of the antimicrobial resistance in Shigella flexneri strains between 2000 and 2012
| S. flexneri | |||||||
|---|---|---|---|---|---|---|---|
| Antibiotic | 2000/2004 | % | 2005/2008 | % | 2009/2012 | % | P-value |
| Cotrimoxazole | 25/27 | 93 | 41/51 | 80 | 42/78 | 54 | <0.001 |
| Amoxicillin – clavulanic acid | 0/27 | 0 | 2/51 | 4 | 23/78 | 30 | <0.001 |
| Ampicillin | – | – | 14/15 | 93 | 51/74 | 69 | 0.06 |
| Amoxicillin | 23/27 | 85 | 25/36 | 69 | 3/4 | 75 | 0.3 |
| Ticarcillin | 23/27 | 85 | 39/51 | 77 | 53/78 | 68 | 0.2 |
Table 3.
Evolution of the antimicrobial resistance in Shigella sonnei strains between 2000 and 2012
| S. sonnei | |||||||
|---|---|---|---|---|---|---|---|
| Antibiotic | 2000/2004 | % | 2005/2008 | % | 2009/2012 | % | P-value |
| Cotrimoxazole | 6/7 | 86 | 15/16 | 94 | 25/25 | 100 | 0.1 |
| Amoxicillin – clavulanic acid | 0/7 | 0 | 0/16 | 0 | 0/25 | 0 | NA |
| Ampicillin | – | – | 1/4 | 25 | 2/25 | 8 | NA |
| Amoxicillin | 0/7 | 0 | 0/12 | 0 | – | – | NA |
| Ticarcillin | 0/7 | 0 | 1/16 | 6 | 2/25 | 8 | NA |
Seasonality
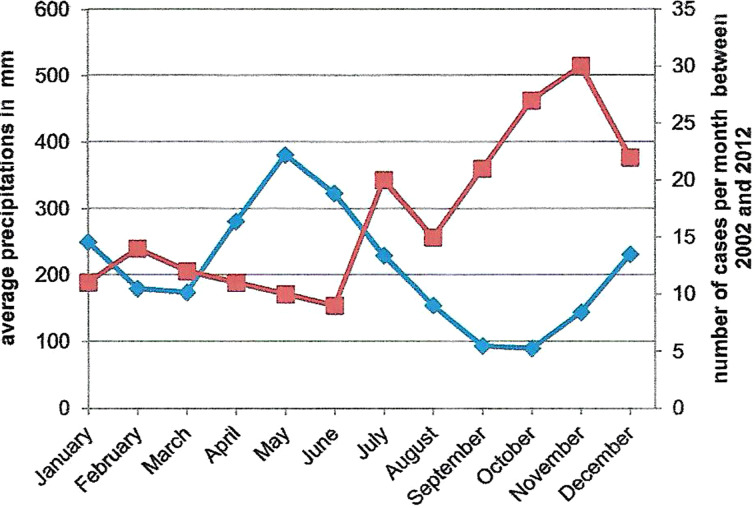
The relationship between average monthly precipitation and the number of cases per month is shown in Figure 1. An inverse relation was observed. More strains were isolated during the warmer and dryer months of the year (July to December). The correlation was high and significant (Spearman's coefficient correlation = −0.8, P < 0.001).
Fig. 1.
Comparison between average monthly precipitations (bleu curve) and the number of cases per month from 2002 to 2012 (red curve) in Saint-Laurent du Maroni.
Incidence
The incidence in western French Guiana in children under 5 years of age varied between 112.6 and 277.3 cases per 100 000 inhabitants between 2006 and 2012. The average incidence was 189.6 cases per 100 000 inhabitants.
Discussion
This is the first study of shigellosis in children under 5 years of age in western French Guiana. This is the population in which the morbidity is the most significant [1]. The incidence is underestimated for at least two reasons. First, shigella is fragile, which makes it difficult to isolate this bacterium. Stool samples need to be inoculated within half an hour following their collection, and specific storage conditions are needed. The bacteriological examination can be negative even when the sample contains bacteria [12]. Second, cases that occur in the isolated areas along the Maroni River are not accounted for. Because these areas are only accessible by boat or plane, transporting samples under the proper conditions is difficult.
The predominance of S. sonnei over other serogroups is observed when economic conditions improve and the level of sanitation increases. The frequency of isolation is directly correlated with the gross domestic product per capita [13]. The clear predominance of S. flexneri in western French Guiana reveals the presence of unsanitary conditions. This finding is consistent with the known distribution of shigella serogroups in countries with a low socio-economic level [1].
To the contrary of serotypes in S. dysenteriae (15 serotypes) and S. boydii (20 serotypes), major cross-reactions were observed in animal experiments for 14 of the 19 serotypes of S. flexneri, due to a degree of antigenic relatedness attributable to a common repeating tetrasaccharide unit [14]. Thus, a multivalent vaccine including O antigens from S. flexneri 2a and 3a, in addition to direct protection against S. flexneri 2b and 3b, would provide cross-protection against S. flexneri 1a, 1b, 4a, 4b, 5a, 5b, 7b, X and Y [15, 16].
Extrapolating these data to humans, a quadrivalent vaccine including S. sonnei (only one serotype described), S. flexneri 2a, 3a and 6 would provide broad coverage against shigella, through direct (87%) and cross-protection (94%). This level is in agreement with the rate observed in two multicentre studies at four sites in Africa and nine sites at Asia, in which a quadrivalent vaccine including the serotypes listed above would have provided protection against at least 85% of the serotyped strains [5, 17]. Although these cross-reactions remain theoretical and discrepancies exist between data for humans and animals [18], a quadrivalent vaccine containing a limited number of serogroups and serotypes could provide broad coverage against shigella.
In Thailand, the incidence in the overall population and among children under 5 years of age is estimated at 600 and 4000 cases per 100 000 inhabitants per year, respectively [19]. In Kenya, the incidence in the overall population is estimated at 408 cases per 100 000 inhabitants per year, and ranges from 136 to 369 cases per 100 000 inhabitants per year among children under 5 years of age [20]. In mainland France, the infection spreads in an epidemic pattern. Epidemics often occur in communities and involve young children. The main serogroup isolated is S. sonnei, and the incidence is low, on the order of 1.8 cases per 100 000 inhabitants per year [21]. In Canada and Belgium, the incidences are estimated at 1.9 and 3.7 cases per 100 000 inhabitants per year, respectively, in the overall population [22, 23]. Our data suggest that western French Guiana's incidence in children under 5 years of age is closer to low- than high-resource settings.
The shigella strains were resistant to antibiotics that are no longer recommended for treating shigellosis. All of the strains were sensitive to fluoroquinolones and third-generation cephalosporin. The sensitivity to azithromycin was not determined. The same resistance profiles have been observed in strains isolated in eastern and northern Brazil [24].
Individuals carrying enterobacteria resistant to third-generation cephalosporin have been reported in French Guiana: a study carried out in Trois Sauts – an Amerindian village in a protected area – estimated that 8% of healthy adults carried resistant enterobacteria. Resistance to third-generation cephalosporin was only observed in E. coli [25]. These strains carried heterogenic CTX-M type β-lactamases, of which 4.3% were CTX-M2 type. These data indicate that resistant strains circulate even in remote corners of French Guiana. The emergence of shigella or salmonella strains that are resistant to third-generation cephalosporin could occur due to the acquisition of plasmids that encode β-lactamase from the intestinal flora.
Breastfeeding decreases the severity of the disease and protects the host against shigella invasion. This protective effect seems to be related to the transmission of immunoglobulin A (IgA) antibodies and the action of lactoferrin – an antimicrobial glycoprotein that is present in breast milk and in most human mucosal secretions [26, 27]. Both IgA and lactoferrin seem to be bind to superficial proteins involved in the mechanism of the cell invasion. The significant prevalence of shigellosis in children under 1-year old in this study (45%) could be due to insufficient breastfeeding in this population, and could highlight a lack of hygiene in preparing baby bottles or the use of contaminated water. This hypothesis is supported by data from a survey on breastfeeding conducted between 2010 and 2012 among 96 women from the pregnancy network ‘Périnat Guyane’ (data not published). In the maternity ward, the rate of breastfeeding was estimated at 94%. However, the rate decreased rapidly after the first month, reaching 43% at 3 months and 32% at 6 months. The partial breastfeeding rate increased over time, reaching 46% at 6 months.
The seasonal peak of infection in the intertropical zone coincides with the warmer months. In Asia and Brazil, it corresponds with the rainy season [19, 28–30]. In Turkey, most cases are observed during the dry season [31]. Several factors could explain the seasonality in western French Guiana. Nearly half of the population that lives near the Maroni River does not have access to safe water, and uses rain water or surface water as a source of drinking water. During the warmer and dryer months, the storage tanks are often empty, and the population has little choice but to obtain water from the river. Between 1995 and 2007, 13 outbreaks of typhoid fever were identified. In most cases, people did not have access to the public supply system and used unsafe water (water from the river in rural areas and rain water in urban areas) [32].
Conclusion
Shigellosis is a frequent cause of diarrhoea among children under 5 years of age, especially among children under 1 year of age. Similar to other developing countries, the main species isolated was S. flexneri, and serotype 2a was highly represented.
The seasonal peak of shigellosis coincided with the warm and dry season. These infections suggest safe water source and proper sanitation may be difficult for this population. The high incidence rate should alert public authorities to the need for immediate action to improve water safety.
This study confirms the usefulness of fluoroquinolones and third-generation cephalosporin antibiotics in the treatment of shigellosis. However, continuous surveillance of circulating strains is important for adapting empiric treatment and preventing the emergence of antimicrobial resistance.
Acknowledgements
The study was carried out with the approval of the local ethics committee of Saint-Laurent du Maroni Hospital.
Declaration of interest
None.
References
- 1.Kotloff KL, et al. (1999) Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bulletin of the World Health Organization 77, 651–666. [PMC free article] [PubMed] [Google Scholar]
- 2.Platts-Mills JA, et al. (2015) Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). The Lancet Global Health 3, 564–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson M, Sansonetti PJ and Marteyn BS (2016) Shigella diversity and changing landscape: insights for the twenty-first century. Frontiers in Cellular and Infection Microbiology 6, Published online: 19 April 2016. doi: 10.3389/fcimb.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niyogi SK (2007) Increasing antimicrobial resistance – an emerging problem in the treatment of shigellosis. Clinical Microbiology and Infection 13, 1141–1143. [DOI] [PubMed] [Google Scholar]
- 5.Livio S, et al. (2014) Shigella isolates from the global enteric multicenter study inform vaccine development. Clinical Infectious Diseases 59, 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansotte F, Margueron T and Maison D (2010) L'alimentation en eau potable en Guyane: problématique et solutions appropriées. Santé Publique 22, 181–192. [PubMed] [Google Scholar]
- 7.Parisot M, et al. (2016) Shigellosis and pregnancy in French Guiana: obstetric and neonatal complications. American Journal of Tropical Medicine and Hygiene 95, 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute of Statistics and Economic Studies (INSEE) Database. Available at https://insee.fr (Accessed 4 March 2018).
- 9.Langendorf C, et al. (2015) Enteric bacterial pathogens in children with diarrhea in Niger: diversity and antimicrobial resistance. PLoS ONE 10(3). Published online: 23 March 2015. doi: 10.1371/journal.pone.0120275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Committee on Antimicrobial Susceptibility Testing (ECAST) Database. Available at http://www.sfm-microbiologie.org/UserFiles/files/casfm/CASFMV1_0_MARS_2017.pdf (Accessed 7 January 2018).
- 11.Meteo France Database. Available at http://www.meteofrance.gp/climat/antilles-guyane/saint-laurent/97311001/normales (Accessed 7 January 2018).
- 12.Breurec S, et al. (2016) Etiology and epidemiology of diarrhea in hospitalized children from low income country: a matched case-control study in Central African Republic. PLoS Neglected Tropical Diseases 10(1), Published online: 15 January 2016. doi: 10.1371/journal.pntd.0004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ram PK, Part II, et al. (2008) Analysis of data gaps pertaining to Shigella infections in low and medium human development index countries, 1984–2005. Epidemiology and Infection 136, 577–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine MM (2006) Enteric infections and the vaccines to counter them: future directions. Vaccine 24, 3865–3873. [DOI] [PubMed] [Google Scholar]
- 15.Levine MM, et al. (2007) Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nature Reviews Microbiology 5, 540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noriega FR, et al. (1999) Strategy for cross-protection among Shigella flexneri serotypes. Infection and Immunity 67, 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Von Seidlein L, et al. (2006) A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Medicine 3, 1556–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farzam N, et al. (2017) Vaccination with Shigella flexneri 2a conjugate induces type 2a and cross-reactive type 6 antibodies in humans but not in mice. Vaccine 35, 4990–4996. [DOI] [PubMed] [Google Scholar]
- 19.Chompook P, et al. (2005) Estimating the burden of shigellosis in Thailand: 36-month population-based surveillance study. Bulletin of the World Health Organization 83, 739–746. [PMC free article] [PubMed] [Google Scholar]
- 20.Njuguna HN, et al. (2013) Use of population-based surveillance to define the high incidence of shigellosis in an urban slum in Nairobi, Kenya. PLoS ONE 8(3), Published online: 7 March 2013. doi: 10.1371/journal.pone.0058437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agence Nationale de Sécurité du Médicament et des Produits de Santé (2018) Available at ansm.sante.fr/content/download/10302/120849/version/6/file/mp040601.pdf (Accessed 7 January 2018).
- 22.Vrbova L, et al. (2012) A descriptive study of reportable gastrointestinal illnesses in Ontario, Canada, from 2007 to 2009. BMC Public Health 12(1), Published online: 12 November 2012. doi: 10.1186/1471-2458-12-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertrand S. Données de surveillance du Centre National de Référence des Salmonella et Shigella, Belgique. Available at http://bacterio.wiv-isp.be/ (Accessed 7 January 2018).
- 24.Bastos FC and Loureiro ECB (2011) Antimicrobial resistance of shigella spp isolated in the state of pará, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 44, 607–610. [DOI] [PubMed] [Google Scholar]
- 25.Woerther PL (2012) Emergence, circulation et déterminants moléculaires des souches d'entérobactéries productrices de ß-lactamases à spectre étendu (BLSE) chez des populations soumises à des pressions de sélection variables (thesis). Paris, France: University Paris, 7, pp. 89. [Google Scholar]
- 26.Gomez HF, et al. (2001) Protective role of human lactoferrin against invasion of Shigella flexneri M90T. Advances in Experimental Medicine and Biology 501, 457–467. [DOI] [PubMed] [Google Scholar]
- 27.Willer EM, Lima RL and Giugliano LG (2004) In Vitro adhesion and invasion inhibition of Shigella dysenteriae, Shigella flexneri and Shigella sonnei clinical strains by human milk proteins. BMC Microbiology 4, Published online: 28 April 2004. doi: 10.1186/1471-2180-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ângela Bernardes Sousa M, et al. (2013) Shigella in Brazilian children with acute diarrhoea: prevalence, antimicrobial resistance and virulence genes. Memorias do Instituto Oswaldo Cruz 108, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinh H, et al. (2009) A changing picture of shigellosis in southern Vietnam: shifting species dominance, antimicrobial susceptibility and clinical presentation. BMC Infectious Diseases 9, Published: 15 December 2009. doi: 10.1186/1471-2334-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang D, et al. (2008) Investigating the effects of climate variations on bacillary dysentery incidence in northeast China using ridge regression and hierarchical cluster analysis. BMC Infectious Diseases 8, Published online: 25 September 2008. doi: 10.1186/1471-2334-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Özmert EN, et al. (2011) Clinical characteristics and antibiotic resistance of Shigella gastroenteritis in Ankara, Turkey between 2003 and 2009, and comparison with previous reports. International Journal of Infectious Diseases 15, 849–853. [DOI] [PubMed] [Google Scholar]
- 32.Mansotte F, Ravachol F and Ardillon V (2009) Les épidémies de typhoïde en Guyane française, 13 ans de veille et de gestion sanitaires. Bull d'Information en Santé Environnementale 20, 1–5. [Google Scholar]