Abstract
Campylobacter is the primary agent of human bacterial gastroenteritis worldwide. In contrast to temperate zones, weather effects on Campylobacter prevalence in broilers under tropical conditions are under-researched. We examined the association between weather and Campylobacter prevalence in slaughtered broilers in Sri Lanka, a tropical country with weather variations led by monsoons. Each month (October 2009–July 2011), 20–30 broiler batches referring to two semi-automated slaughterhouses from five Sri Lankan provinces were tested for Campylobacter contamination and analysed in relation to temperature, humidity and rainfall. Overall prevalence was 63.8% (95% CI 59.6–67.9%, n = 542), peaking in September–November. Each 1 °C increase in monthly mean temperature up to 26 °C increased Campylobacter-positive batches by 16.4% (95% CI 0.4–35.1%). For each 10 mm increase in monthly total rainfall up to 300 mm, Campylobacter-positive batches increased significantly by 0.8% (0.1–1.5%) at 1-month lag. For each 1% increase in relative humidity up to 80% at 1- and 2-month lags, Campylobacter-positive batches increased of respectively 4.2% (1.9–6.7%) and 4.0% (1.5–6.5), and decreased by 3.6% (2.6–4.6%) and 4.0% (2.6–5.4%) for unit increases above 80%. These results suggest that even in tropical countries without marked seasons, there are weather effects possibly reflecting Campylobacter potential to colonise its preferred host and/or survive in the environment.
Key words: Campylobacter, poultry, seasonality, tropical weather
Introduction
Campylobacter inhabits the intestinal tract of many animal species, but colonisation of the chicken's gut by Campylobacter is of particular interest due to its recognised role as the primary source of human campylobacteriosis [1, 2]. The main disease-causing zoonotic Campylobacter species of public health significance, i.e. Campylobacter jejuni and Campylobacter coli, are commensal in chickens and can contaminate chicken meat during slaughtering and carcass processing, thereby infecting humans via the foodborne route [3].
Campylobacteriosis is the most common cause of bacterial gastroenteritis in humans throughout the world. A distinctive feature of this disease is its seasonality, with a peak in human disease incidence observed during the summer in countries with a generally temperate climate. This is demonstrated by specific studies carried out in England [4], Scotland [5] and New Zealand [6], as well as by a multi-country study including nine European countries (Austria, Denmark, Finland, France, Germany, Norway, Scotland, Sweden and Wales) and New Zealand [7]. There is no such seasonality described in tropical countries. To date, only an Australian study has compared Campylobacter infection rates in humans in temperate and sub-tropical regions of the country where seasons are not so marked. The study reported differences in the association between campylobacteriosis notifications and some weather variables in the two cities under study (Brisbane and Adelaide), which had different climatic conditions [8].
Further research has been conducted to understand the basis of human campylobacteriosis seasonality, with climate being the primary factor investigated along with the link between human campylobacteriosis incidence and Campylobacter prevalence in broiler flocks. For instance, a Danish study [9], as well as an international study comprising Europe, Canada, Australia and New Zealand [10], revealed a significant correlation between ambient temperature and the number of human campylobacteriosis cases. A strong correlation between the same factors was also found in England and Wales, especially in children under 5 years of age [11]. Furthermore, by comparing longitudinal surveillance data in six North European countries, including Denmark, Finland, Iceland, the Netherlands, Norway and Sweden, a significant degree of co-seasonality in Campylobacter incidence in humans and broilers was identified [12]. Whether such seasonality is attributed to a common factor for both humans and chickens, or whether humans acquire Campylobacter from the colonised chickens, is debatable. Yet, chicken contributing to human Campylobacter infections is supported by the findings of a Danish study showing a direct effect of season on the occurrence of Campylobacter in Danish broiler meat at retail [13].
Seasonal trends in Campylobacter-positive broiler flocks have been explained with several hypotheses, including geographical location and local climate, flock management practices, especially biosecurity measures [14] and factors influencing the survival and spread of Campylobacter in the environment. As an example, a Danish study [15] has identified flies as an important mechanical vector for the introduction of Campylobacter into broiler chicken barns during the high prevalence season. Additionally, environmental parameters like daylight hours, minimum and maximum ambient temperatures have been shown to influence carriage rates of thermophilic Campylobacter in the chicken's gut [16]. Given the attention on global climate change and its consequences on microbial threats [17], exploring how weather relates to Campylobacter prevalence in broilers may provide valuable information for predictive studies and preventative measures. This may be particularly insightful when such information originates from areas under a tropical climate, having temperatures that remain relatively constant throughout the year and seasonal variations are essentially dominated by monsoonal precipitation.
Sri Lanka is a tropical island country with no marked seasons. The island is situated at the Southern tip of the Indian sub-continent, extending from 5°55′ to 9°51′N and from 79°42′ to 81°53′E. Ambient temperature varies only slightly during the year in most of the country but in the inland mountainous region, whereas the largest weather variations throughout the year concern the rainfall [18]. In Sri Lanka, Campylobacter prevalence rates in broilers and layers have been documented [19], with more than 65% of broiler flocks in some areas of the country being Campylobacter positive. Deep-litter open-house rearing systems, which have generally low biosecurity standards, are common practice for broiler farming in Sri Lanka given the tropical climate and level of economic development. As there is little evidence about how weather relates to Campylobacter colonisation levels in broilers reared under tropical conditions, the objective of this study was to determine the correlation between meteorological conditions and Campylobacter prevalence in broilers at slaughter in Sri Lanka.
Methods
Sample collection
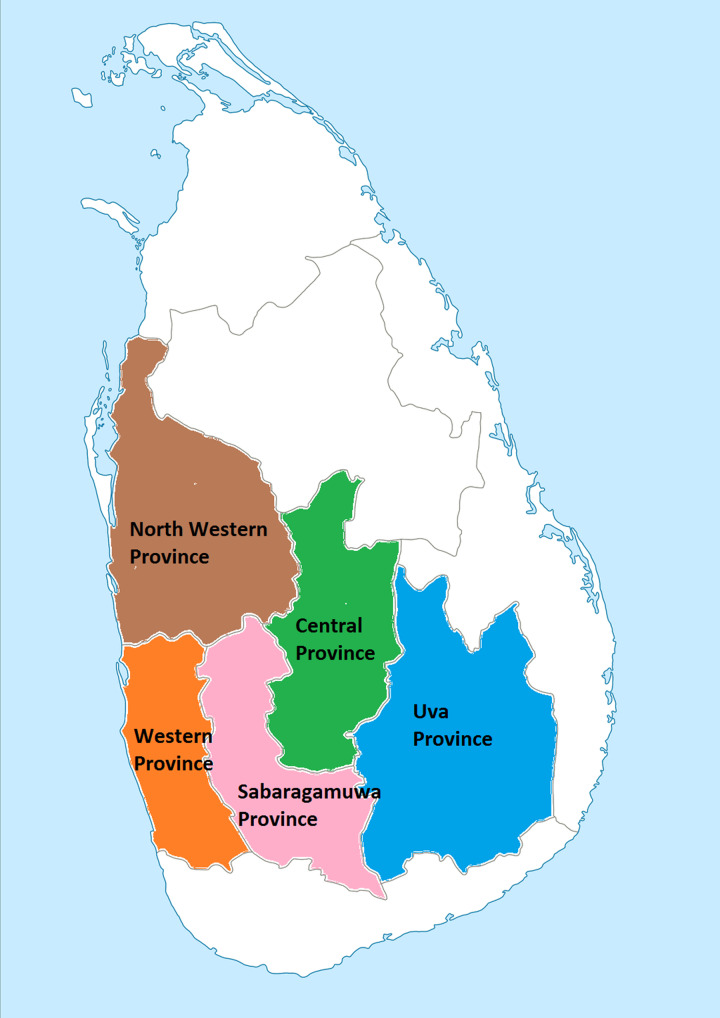
Sampling operations were performed from October 2009 to July 2011. Sampling locations consisted of two semi-automated broiler meat processing plants situated in the cities of Gampaha and Gampola in Sri Lanka. These broiler meat processing plants received broilers from different areas of the country representing five provinces (Fig. 1). Sampling was discontinued between March and May 2011. Each month, 20–30 slaughter batches of broilers were tested for Campylobacter spp. A minimum of 10 pairs of caecae was collected from randomly selected broilers at the point of evisceration in every batch studied and transported on ice to the laboratory with the accompanying information on the flock/farm of origin. Isolation and identification of Campylobacter spp. were carried out according to ISO 10272:2006 standards. Samples were not collected on a specific day of the month. Instead, samples were collected in three to four lots per month (with each lot containing samples from five to ten slaughter batches). The time between sample lot collections varied from 1 to 2 weeks so that samples were collected throughout each month under study.
Fig. 1.
Map of Sri Lanka showing the provinces where the sampled broilers were reared.
Isolation and identification of Campylobacter
Caecal materials were extracted from the 10 pair of caeca collected from each slaughter batch and pooled to make two samples each containing caecal extracts from five birds. Each pooled sample was mixed using a sterile cotton bud and streaked on modified Charcoal Cefoperazone Deoxycholate Agar (mCCDA) directly. As per ISO 10272:2006, plates were incubated under a micro-aerobic atmosphere at 42 °C for 48 h. Plates were then observed for specific colony morphology. Presumptive Campylobacter spp. colonies on mCCDA agar were then sub-cultured in blood agar plates and incubated micro-aerobically at 42 °C for 48 h. For the confirmation of thermotolerant Campylobacter spp. colony morphology both on mCCDA and blood agar, Gram staining, motility, catalase test, oxidase reaction, aerobic growth at 42 °C and microaerobic growth at 25 °C were utilised.
Climatological data
Monthly average temperature (°C), monthly total rainfall (mm) and monthly average relative humidity (%) measured at the provinces where the broilers were reared, were obtained from the Global Web Site (http://worldweather.wmo.int/en/home.html), which provides official weather observations, forecasts and other climatological information for selected cities supplied by the National Meteorological & Hydrological Services worldwide that make official weather observations in their respective countries.
Data analysis
Given the clustered and longitudinal nature of the dataset, generalised estimating equation (GEE) regression models with a log link function and negative binomial error distribution were used to examine the associations between the monthly occurrence of Campylobacter-positive poultry batches (dependent variable) and the meteorological variables (independent variables). The province of origin was thus set as the panel (cluster) variable and the month/year of sampling as the time scale. The unit of analysis consisted of 110 province-month observations (i.e. five provinces followed for 22 months, from October 2009 to July 2011). Generalised estimating equations accounted for the within-province correlation resulting from repeated measurements made over months in the same provinces using quasi-likelihood methods and cluster-correlated robust variance estimators [20]. While the dependent variable was the monthly number of Campylobacter-positive batches from each province, the corresponding monthly total number of analysed batches from each province (i.e. the underlying denominator of the positive batches) was included as an offset term in the models to obtain models of Campylobacter positivity rates. Associations were expressed as incidence rate ratios (IRR) and corresponding 95% confidence intervals (95% CIs); the percent increase in the monthly rate of Campylobacter-positive poultry batches for each unit increase in each weather variables was then calculated as (IRR-1) × 100%. The quasi-likelihood under the independence model information criterion (QIC) was used to guide the selection of the most parsimonious model and the best-fitting within-group working correlation structure [21]. To allow for delayed effects of weather variables on Campylobacter prevalence, lags of 1–3 months were also assessed. Collinearities between weather variables were checked prior to regression analyses by calculating Spearman's correlation coefficients and selection between collinear variables was selected based on improved model fit. Both univariate (each weather variable at a time) and multivariate (adjusted for all other weather variables) analyses were performed.
Similar to the analytical approach used elsewhere [22, 23], the linearity of associations between the outcome variable and each weather variable under study was examined graphically prior to GEE regression analysis using exposure-response curves based on restricted cubic splines [24], with three knots at Harrell's recommended percentiles (25%, 50% and 75%) [25]. The significance of the departure from linearity was assessed by testing the null hypothesis that the coefficients of the non-linear terms were equal to zero [26]. This allowed for the identification of non-linear relationships and inflection points, if present, where Campylobacter positivity increased or decreased as a function of the weather variables in question. Statistical analysis was performed using STATA 13 (StataCorp LP, College Station, TX, USA).
Results
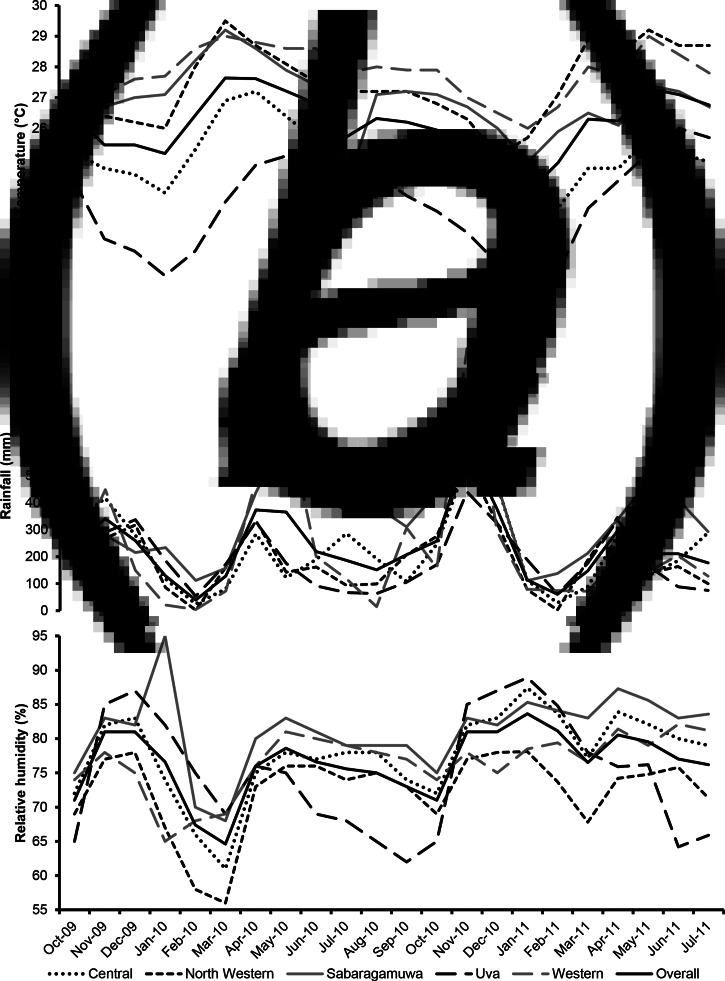
Overall, there were 346 Campylobacter-positive poultry batches (out of 542 batches tested, 63.8%, 95% CI 59.6–67.9%) over the five provinces during the entire study period. The Campylobacter prevalence and descriptive summary statistics of the weather variables in each province are shown in Table 1. Except for the province of Uva where only six batches were sampled, the highest positivity rate (70.8%) occurred in the Western province (Table 1). The overall monthly mean temperature, rainfall and relative humidity were 26.1 °C (±2.0 °C standard deviation (s.d.), range: 20.7–29.5 °C), 232.2 mm (±165.6 mm s.d., range: 1.5–971.5 mm) and 76.5% (± 6.9% s.d., range: 56–95%), respectively. The weather variables and Campylobacter prevalence in each of the five provinces and month of the study period are shown in Figures 2 and 3, respectively. The highest monthly mean temperatures were recorded in the months of March–May, especially in the Western and North Western provinces (Fig. 2). Temperatures varied less (coefficient of variation (CV): 7.7%) than rainfall (CV: 71.3%) and relative humidity (CV: 9.0%), whose highest values were recorded in the months of April and November (rainfall) and November-January (relative humidity), especially in the Western and Sabaragamuwa provinces (Fig. 2). A significant negative correlation was found between mean temperature and relative humidity (rho = −0.25, P = 0.008), whereas a significant positive correlation was found between rainfall and relative humidity (rho = 0.43, P < 0.0001).
Table 1.
Campylobacter positivities in poultry batches and summary statistics (monthly mean ± standard deviation and min–max values) of the weather variables in the five provinces under study, Sri Lanka, October 2009 to July 2011
| Province | No. of Campylobacter-positive batches/total no. of batches sampled | Positivity rate (95% CI) | Temperature (°C) | Rainfall (mm) | Relative humidity (%) |
|---|---|---|---|---|---|
| Central | 34/52 | 65.4% (50.9–78.0%) | 24.9 ± 1.1 (22.2–27.2) | 218.3 ± 139.2 (24.4–546.3) | 77.7 ± 6.2 (61.0–87.4) |
| Western | 51/72 | 70.8% (58.9–81.0%) | 27.8 ± 0.8 (26.0–29.0) | 230.2 ± 239.3 (4.5–971.5) | 76.6 ± 4.5 (65.0–82.1) |
| Sabaragamuwa | 142/231 | 61.5% (54.9–67.8%) | 26.9 ± 1.1 (24.1–29.2) | 334.9 ± 145.4 (111.1–658.5) | 81.2 ± 5.7 (68.0–95.0) |
| North Western | 113/181 | 62.4% (54.9–69.5%) | 27.5 ± 1.2 (25.1–29.5) | 189.3 ± 129.5 (1.5–550.5) | 72.4 ± 6.0 (56.0–78.1) |
| Uva | 6/6 | 100% (54.1–100%)a | 23.5 ± 1.6 (20.7–26.0) | 188.6 ± 112.4 (53.4–440.3) | 75.0 ± 8.8 (62.0–88.9) |
One-sided, 97.5% confidence interval.
Fig. 2.
Monthly variation of the average temperature (a), total rainfall (b) and average relative humidity (c) in each of the five provinces under study and overall, at each month of the study period. Sri Lanka, October 2009–July 2011.
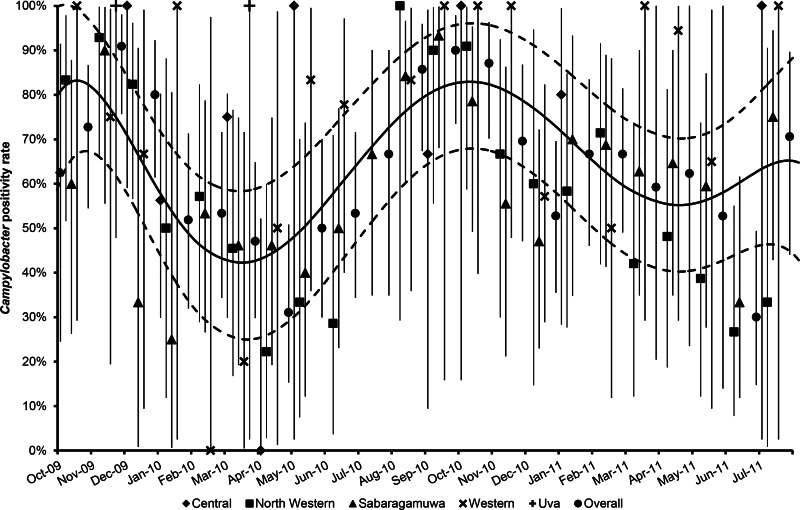
Fig. 3.
Monthly variation of Campylobacter positivity rates in poultry batches from each of the five provinces under study and overall, at each month of the study period. Sri Lanka, October 2009– July 2011. Error bars of the marker symbols indicate 95% confidence intervals of the monthly Campylobacter positivity rates in poultry batches. A locally weighted scatterplot smoothing (LOWESS) curve (solid back line) with corresponding 95% confidence intervals (dotted back line) is fitted to the observed data. The South-West monsoon usually lasts from May to September.
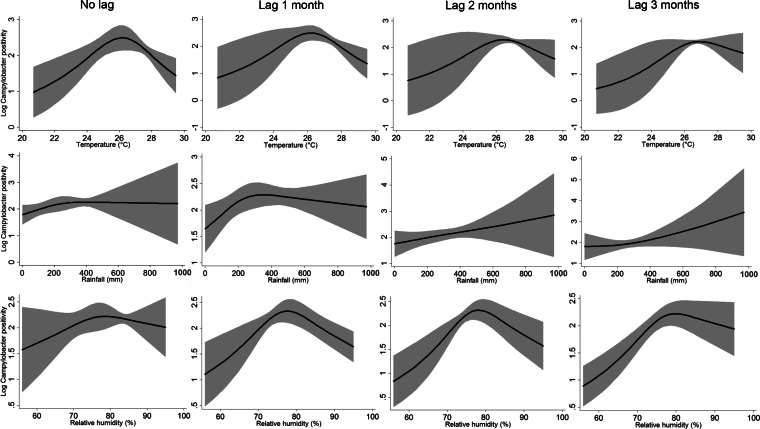
The major peak months of Campylobacter prevalence occurred between September and November, particularly evident in the North Western and Sabaragamuwa provinces (Fig. 3). The analysis of departure from linearity (Fig. 4) indicated a significant non-linear association between the logarithm of the monthly occurrence of Campylobacter-positive broiler batches with the average temperature of the current month (no lag: P = 0.0137), as well as a borderline significant departure from linearity for the temperature of the previous month (lag −1: P = 0.0655), whereas significant non-linearity was absent at lags of 2 (P = 0.157) and 3 (P = 0.1372) months. For rainfall, no significant departure from linearity was observed for the current month (no lag: P = 0.2977) and at lags of 2 (P = 0.6031) and 3 (P = 0.5818) months, whereas a borderline significant departure from linearity was detected for the rainfall of the previous month (lag −1: P = 0.0532). For relative humidity, significant departures for linearity were found at any lag (lag −1: P = 0.018; lag −2: P = 0.006; lag −3:P = 0.037), but not for the current month (P = 0.3162). Upon inspection of the exposure-response curves (Fig. 4), the effect of increasing temperature on Campylobacter positivity appeared to be (linearly) positive until a threshold of 26 °C and negative afterwards. Similarly, the inflection points for the (lagged) rainfall and relative humidity were located at 300 mm and at 80%, respectively (Fig. 4). These thresholds were therefore used to define knots of linear splines for the inclusion of the respective variables in the GEE regression models.
Fig. 4.
Smoothing plots of weather variables vs. Campylobacter positivity rates in poultry batches from the five provinces under study, Sri Lanka, October 2009– July 2011. The grey areas around the black curves indicate the 95% confidence intervals.
The associations between the weather variables and Campylobacter positivity rates are presented in Table 2. In the multivariate analysis (where the effects of the other weather variables are controlled for), significant positive associations were observed for temperature ⩽26 °C, rainfall ⩽300 mm and relative humidity ⩽80% and significant negative associations were found for relative humidity >80%, for some of the lag months (Table 2). Specifically, for each 1 °C increase in the mean monthly temperature up to 26 °C, the rate of Campylobacter-positive poultry batches was expected to increase significantly by 16.4% (95% CI 0.4–35.1%) in the current month. Moreover, for each 10 mm increase in the total monthly rainfall up to 300 mm, the Campylobacter positivity rate increased significantly by 0.8% (95% CI 0.1–1.5%) in the following month. Finally, for each 1% increase in relative humidity up to 80% at lags of 2 and 3 months, a significant increase of, respectively, 4.2% (95% CI 1.9–6.7%) and 4.0% (95% CI 1.5–6.5) in the rate of positive batches was expected, whereas significant decreases of 3.6% (95% CI 2.6–4.6%) and 4.0% (95% CI 2.6–5.4%) for each 1% increase above 80% relative humidity was observed at lags of 1 and 2 months.
Table 2.
Incidence rate ratios (IRR) and 95% confidence intervals (CI) for the associations between weather variables and the occurrence of Campylobacter-positive poultry batches
| Variable | Univariate IRR (95% CI) | Multivariate IRR (95% CI)a |
|---|---|---|
| Temperature (per 1 °C increment) | ||
| Current month (no lag); ⩽26 °C | 1.172 (1.012–1.358)* | 1.164 (1.004–1.351)* |
| Current month (no lag); >26 °C | 0.866 (0.759–0.987)* | 0.867 (0.727–1.035) |
| Previous month (lag −1); ⩽26 °C | 1.180 (0.917–1.518) | 1.188 (0.947–1.489) |
| Previous month (lag −1); >26 °C | 0.861 (0.754–0.982)* | 0.875 (0.755–1.014) |
| Two months before (lag −2) | 1.009 (0.878–1.158) | 1.043 (0.917–1.185) |
| Three months before (lag −3) | 1.064 (0.934–1.212) | 1.109 (0.973–1.263) |
| Rainfall (per 10 mm increment) | ||
| Current month (no lag) | 1.003 (0.998–1.007) | 0.999 (0.981–1.018) |
| Previous month (lag −1); ⩽300 mm | 1.011 (1.001–1.021)* | 1.008 (1.001–1.015)* |
| Previous month (lag −1); >300 mm | 0.998 (0.991–1.005) | 0.997 (0.991–1.002) |
| Two months before (lag −2) | 1.005 (0.999–1.012) | 1.000 (0.994–1.006) |
| Three months before (lag −3) | 1.006 (0.997–1.016) | 1.002 (0.994–1.010) |
| Relative humidity (per 1% increment) | ||
| Current month (no lag) | 1.007 (1.000–1.015)* | 1.002 (0.995–1.008) |
| Previous month (lag −1); ⩽80% | 1.027 (1.011–1.043)** | 1.017 (0.997–1.038) |
| Previous month (lag −1); >80% | 0.966 (0.951–0.982)** | 0.964 (0.954–0.974)*** |
| Two months before (lag −2); ⩽80% | 1.039 (1.019–1.059)*** | 1.042 (1.019–1.067)*** |
| Two months before (lag −2); >80% | 0.958 (0.932–0.984)** | 0.960 (0.946–0.974)*** |
| Three months before (lag −3); ⩽80% | 1.035 (1.021–1.050)*** | 1.040 (1.015–1.065)** |
| Three months before (lag −3); >80% | 0.979 (0.961–0.998)* | 0.986 (0.972–1.000) |
*P < 0.05; **P < 0.01; ***P < 0.001.
Adjusted for the other weather variables.
Discussion
Seasonal trends in Campylobacter prevalence in broilers have been widely explained in the literature as the result of factors ranging from broiler management practices to climate, but current knowledge is largely based on studies carried out in industrialised countries with a temperate climate. This study investigated the association between the weather and Campylobacter prevalence in broilers reared in a developing country with a tropical climate, using a multivariate modeling approach. The lack of similar studies from tropical areas hinders the direct comparison of our results to published research and comparison of our data with published reports from temperate countries may not be completely appropriate due to major differences in management practices, among others.
Horizontal transmission from the environment is believed to be the most important source of Campylobacter in poultry. Thus, the implementation of strict biosecurity is the best way to control flock colonisation [14, 27]. Other than that, seasonality in Campylobacter prevalence in poultry may be explained, at least in part, by flies as a potential source of infection [15, 28], as fly populations follow closely the temperature pattern [29]. Accordingly, fly screens proved to be effective in reducing the number of Campylobacter-positive flocks in Denmark [30]. However, in countries like Sri Lanka, broilers are reared under deep-litter open-house systems all year round, meaning that the birds are constantly exposed to the outside environment and flies are free to move in and out the farm premises. Even though the present study did not collect data on the specific biosecurity levels of the farms from which the sampled chickens originated, it is well known that these are minimal and that poultry reared in such circumstances is at increased risk of Campylobacter colonisation [31, 32]. Temporal variations in Campylobacter prevalence can, therefore, be mainly ascribed to weather.
Being in the equatorial tropical zone, Sri Lanka always has a generally warm weather, with only small variations in temperature throughout the year. On average, the difference between the coolest months of the year (November–February) and the warmest ones (April and May) is around 2 °C. Additionally, being an island, relative humidity is high and it is generally about 70% during the day and above 90% at night (http://www.statistics.gov.lk/Abstract2014/Pages/chap1.htm). The only factor leading to some seasonality is the Indian Ocean Monsoons. Indeed, Sri Lanka experiences two monsoons that give rise to four distinct (monsoonal) seasons, namely the South-West monsoon, the North-East monsoon and two inter-monsoonal periods, in a calendar year [18]. Rainfall during the South-West monsoon, which lasts from May to September, hits mainly the South-Western parts of the country. We found that Campylobacter prevalence in broiler batches varied to some extent throughout the year, revealing that the major peak months were those between September and November (Fig. 3). This may be related to the weather changes of the South-West monsoonal rains. Indeed, the onset of the prevalence peak coincided with the warmer weather and commencement of the South-West monsoon in May (Fig. 3). Furthermore, the decline of positivity rate seems to coincide with the dry season accompanied by comparatively lower temperatures (November–February). Moreover, most of the positive batches studied originated from those areas more heavily hit by the South-West monsoons.
All three weather parameters tested (i.e. temperature, rainfall and relative humidity) were significantly associated with Campylobacter prevalence at some lags in time. Our analyses also suggested that there were thresholds for (lagged) weather effects, with linear and positive effects for average monthly temperature up to 26 °C, for total monthly rainfall up to 300 mm and for average monthly relative humidity up to 80% (and some negative effects above 80% relative humidity). The positive correlation with temperature is in agreement with other studies. For example, strong correlations have been reported between the temperature 3–4 weeks before slaughter and the percentage of broiler flocks colonised with Campylobacter [9]. Additionally, Jore et al. [12] found an association between Campylobacter incidence in broilers in a given month and the mean temperature of the Northern hemisphere in the same and preceding month. Furthermore, the Australian study has found the temperature to be positively associated with human Campylobacter infections in the sub-tropical climate of Brisbane, but negatively correlated with the number of cases in the temperate climate city of Adelaide [8]. Also, the positive associations with rainfall and relative humidity are in agreement with the literature, as humidity and/or precipitation allowing for moist conditions are known to support the survival of Campylobacter in the environment [9]. Furthermore, because of high susceptibility to UV radiation and desiccation, Campylobacter spp. have been found more frequently in broiler flock surroundings in rainy days when compared with sunny days [33]. The identification of thresholds above which such positive correlations are no longer (linearly) significant and sometimes even become negative, deserves further investigation as to understand the possible underlying biological processes. Positive and negative correlations between Campylobacter prevalence and weather have also been explained by varying Campylobacter survival in the environment, including biofilm formation by Campylobacter in water systems of poultry farms [34], as well as differing sources of contamination, such as the increased number of wild birds and pests in the warm vs. cool months [9]. However, in open housing systems, broilers are always exposed to wild birds and pests due to poor biosecurity. Therefore, such a factor influencing the prevalence of Campylobacter does not appear to be justified despite the fact that wild birds and pests tend to enter these poultry houses more often seeking water and shelter in hot or rainy periods.
It is possible for weather conditions to influence not only the bacterium but also the host, i.e. broiler chickens in our case. Previous studies on Campylobacter seasonality in poultry, being carried out in temperate countries, focused primarily on pathogen-related factors, as in those countries broilers tend to be reared in controlled environments; thus, animals are not directly exposed to outdoor conditions. The situation is different when broilers are reared in open-houses, which are the most appropriate type of poultry houses in a developing country with a tropical climate like Sri Lanka because of the easier heat loss through natural ventilation, although they directly expose broilers to the outside environment. It is known that high ambient temperatures may have a major detrimental impact on poultry health and productivity, and when high temperatures are coupled with high humidity, the outcome may be worse. The situation may be further aggravated by the usually high poultry density in these houses [35]. The neutral temperature for broilers is 16–19 °C and temperatures above this range leads to heat stress, which affects the physiology and productive performance of the birds [36, 37]. Heat stress is a recognised problem in broiler production in the tropics [38], with breathing difficulties, decreased feed intake and growth being often observed. As the studied flocks are usually exposed to temperatures almost constantly above 25 °C with high humidity for at least 6 h a day, they are often in the heat stress state. Studies have also shown significant changes in the normal intestinal microbiota and intestinal morphology in heat stressed poultry [39]. Experimental exposure of broilers to high temperatures (30 °C) for 24 h has been shown to change significantly the intestinal microbial community structure, evoking susceptibility to Salmonella [40]. Additionally, broilers in heat stress have an impaired immune system [39, 41]. Altogether these factors may contribute to explain the observed Campylobacter prevalence associated with variations in temperature and humidity.
The biggest limitation of this study is due to its ecological nature, i.e. to the spatiotemporal resolution of the meteorological and prevalence data. Indeed, we used monthly average temperature, monthly total rainfall and monthly average relative humidity at the level of provinces where the broilers originated from, as it was not possible to obtain weather variables at smaller geographical units like the farm premises themselves. The main factors to consider when selecting the time scale to assess meteorological effects on disease occurrence have been summarised elsewhere [42]. Our analysis was based on a monthly time scale, which may be too large to appreciate potential short-term (or ‘acute’) weather effects, so we cannot rule out that these effects could be finer than those evidenced here. However, broilers were examined at slaughter and broilers’ production cycles do not usually last less than 6 weeks, so our results are meant to reflect the potential (average) effects of weather during most of broilers’ production cycle. Another limitation is related to the lack of data on transport conditions from farms to the processing plant that may also have had an effect, although all broilers were transported within 2–3 h.
Conclusions
Even in a tropical country like Sri Lanka without marked differences among seasons and with relatively stable (warm and humid) weather conditions throughout the year, the levels of Campylobacter contamination of broilers at slaughter show significant correlations with meteorological variables like temperature, rainfall and relative humidity, with some thresholds for differential (linear and non-linear) effects of these weather variables, suggesting that they may influence the potential of Campylobacter to colonise its preferred host and/or to survive in the environment.
Acknowledgements
We thank Dr G.C. Hettiarchchi for helping us with sampling for this study. WHO-Global Foodborne Infections Network (WHO-GFN) is acknowledged for facilitating the exchange of researchers and knowledge between research groups.
References
- 1.Boysen L, et al. (2014) Source attribution of human campylobacteriosis in Denmark. Epidemiology and Infection 142(8), 1599–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagenaar JA, et al. (2015) Campylobacter: animal reservoirs, human infections and options for control. In Sing A (ed). Zoonoses-Infections Affecting Humans and Animals. Dordrecht: Springer, pp. 159–177. [Google Scholar]
- 3.Newell D, et al. (2017) Campylobacter epidemiology-sources and routes of transmission for human infection. In Klein G (ed). Campylobacter Features, Detection, and Prevention of Foodborne Disease. Germany: Elsevier, pp. 85–110. [Google Scholar]
- 4.Sopwith W, et al. (2003) Enhanced surveillance of campylobacter infection in the North West of England 1997–1999. Journal of Infection 46(1), 35–45. [DOI] [PubMed] [Google Scholar]
- 5.Miller G, et al. (2004) Human campylobacteriosis in Scotland: seasonality, regional trends and bursts of infection. Epidemiology and Infection 132(4), 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hearnden M, et al. (2003) The regionality of campylobacteriosis seasonality in New Zealand. International Journal of Environmental Health Research 13(4), 337–348. [DOI] [PubMed] [Google Scholar]
- 7.Nylen G, et al. (2002) The seasonal distribution of campylobacter infection in nine European countries and New Zealand. Epidemiology and Infection 128(3), 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi P, et al. (2008) Weather and notified Campylobacter infections in temperate and sub-tropical regions of Australia: an ecological study. Journal of Infection 57(4), 317–323. [DOI] [PubMed] [Google Scholar]
- 9.Patrick ME, et al. (2004) Effects of climate on incidence of Campylobacter spp. in humans and prevalence in broiler flocks in Denmark. Applied and Environmental Microbiology 70(12), 7474–7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovats RS, et al. (2005) Climate variability and campylobacter infection: an international study. International Journal of Biometeorology 49(4), 207–214. [DOI] [PubMed] [Google Scholar]
- 11.Louis VR, et al. (2005) Temperature-driven Campylobacter seasonality in England and Wales. A Applied and Environmental Microbiology 71(1), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jore S, et al. (2010) Trends in Campylobacter incidence in broilers and humans in six European countries, 1997–2007. Preventive Veterinary Medicine 93(1), 33–41. [DOI] [PubMed] [Google Scholar]
- 13.Boysen L, Vigre H and Rosenquist H (2011) Seasonal influence on the prevalence of thermotolerant Campylobacter in retail broiler meat in Denmark. Food Microbiology 28(5), 1028–1032. [DOI] [PubMed] [Google Scholar]
- 14.Newell DG, et al. (2011) Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Applied and Environmental Microbiology 77(24), 8605–8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hald B, et al. (2004) Flies and Campylobacter infection of broiler flocks. Emerging Infectious Diseases 10(8), 1490–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace JS, et al. (1997) Seasonality of thermophilic Campylobacter populations in chickens. Journal of Applied Microbiology 82(2), 219–224. [PubMed] [Google Scholar]
- 17.Stewart LD and Elliott CT (2015) The impact of climate change on existing and emerging microbial threats across the food chain: an island of Ireland perspective. Trends in Food Science & Technology 44(1), 11–20. [Google Scholar]
- 18.Burt T and Weerasinghe K (2014) Rainfall distributions in Sri Lanka in time and space: an analysis based on daily rainfall data. Climate 2(4), 242–263. [Google Scholar]
- 19.Kalupahana RS, et al. (2013) Colonization of Campylobacter spp. in broiler chickens and laying hens reared in tropical climates with low-biosecurity housing. Applied and Environmental Microbiology 79(1), 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James W and Hardin JMH (2012) Generalized Estimating Equations. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- 21.Cui J (2007) QIC program and model selection in GEE analyses. Stata Journal 7(2), 209. [Google Scholar]
- 22.Zhang Z, et al. (2016) Short-term effects of meteorological factors on hand, foot and mouth disease among children in Shenzhen, China: non-linearity, threshold and interaction. Science of the Total Environment 539, 576–582. [DOI] [PubMed] [Google Scholar]
- 23.Li ZJ, et al. (2015) Nonlinear and threshold of the association between meteorological factors and bacillary dysentery in Beijing, China. Epidemiology and Infection 143(16), 3510–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desquilbet L and Mariotti F (2010) Dose-response analyses using restricted cubic spline functions in public health research. Statistics in Medicine 29(9), 1037–1057. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE (2001) Regression Modeling Strategies, with Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag. [Google Scholar]
- 26.Marrie RA, Dawson NV and Garland A (2009) Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. Journal of Clinical Epidemiology 62(5): 511–517.e511. [DOI] [PubMed] [Google Scholar]
- 27.Guerin MT, et al. (2008) Temperature-related risk factors associated with the colonization of broiler-chicken flocks with Campylobacter spp. in Iceland, 2001–2004. Preventive Veterinary Medicine 86(1–2), 14–29. [DOI] [PubMed] [Google Scholar]
- 28.Nichols GL (2005) Fly transmission of Campylobacter. Emerging Infectious Diseases 11(3), 361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goulson D, et al. (2005) Predicting calyptrate fly populations from the weather, and probable consequences of climate change. Journal of Applied Ecology 42(5), 795–804. [Google Scholar]
- 30.Hald B, Sommer HM and Skovgard H (2007) Use of fly screens to reduce Campylobacter spp. introduction in broiler houses. Emerging Infectious Diseases 13(12), 1951–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conan A, et al. (2012) Biosecurity measures for backyard poultry in developing countries: a systematic review. BMC Veterinary Research 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen VM, et al. (2011) Influence of production system on the rate of onset of Campylobacter colonization in chicken flocks reared extensively in the United Kingdom. British Poultry Science 52(1), 30–39. [DOI] [PubMed] [Google Scholar]
- 33.Hansson I, et al. (2007) Correlations between Campylobacter spp. prevalence in the environment and broiler flocks. Journal of Applied Microbiology 103(3), 640–649. [DOI] [PubMed] [Google Scholar]
- 34.Reeser RJ, et al. (2007) Characterization of Campylobacter jejuni biofilms under defined growth conditions. Applied and Environmental Microbiology 73(6), 1908–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bandara R and Dassanayake D (2006) A quantitative analysis on factors affecting profitability of small scale broiler production. Journal of Agricultural Sciences 2(3), 45–50. [Google Scholar]
- 36.Sohail M, et al. (2010) Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and Lactobacillus-based probiotic: dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poultry Science 89(9), 1934–1938. [DOI] [PubMed] [Google Scholar]
- 37.Lara LJ and Rostagno MH (2013) Impact of heat stress on poultry production. Animals 3(2), 356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borges S, et al. (2003) Dietary electrolyte balance for broiler chickens exposed to thermoneutral or heat-stress environments. Poultry Science 82(3), 428–435. [DOI] [PubMed] [Google Scholar]
- 39.Quinteiro-Filho WM, et al. (2012) Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute hypothalamic-pituitary-adrenal axis activation. Journal of Animal Science 90(6), 1986–1994. [DOI] [PubMed] [Google Scholar]
- 40.Burkholder K, et al. (2008) Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poultry Science 87(9), 1734–1741. [DOI] [PubMed] [Google Scholar]
- 41.Mashaly M, et al. (2004) Effect of heat stress on production parameters and immune responses of commercial laying hens. Poultry Science 83(6), 889–894. [DOI] [PubMed] [Google Scholar]
- 42.Wu H, et al. (2014) The effect of meteorological factors on adolescent hand, foot, and mouth disease and associated effect modifiers. Global Health Action 7, 24664. [DOI] [PMC free article] [PubMed] [Google Scholar]