Abstract
Objective
To identify the demographic and clinical characteristics associated with adverse COVID-19 outcomes across a 12-month period in 2020 and 2021.
Research design and methods
We conducted a retrospective cohort study using electronic health records from five academic health systems in Pennsylvania and Maryland, including patients with COVID-19 with type 2 diabetes or at risk of type 2 diabetes. Patients were classified based on 30-day outcomes: (1) no hospitalization; (2) hospitalization only; or (3) a composite measure including admission to the intensive care unit (ICU), intubation, or death. Analyses were conducted in patients with type 2 diabetes and patients at risk of type 2 diabetes separately.
Results
We included 15 725 patients with COVID-19 diagnoses between March 2020 and February 2021. Older age and higher Charlson Comorbidity Index scores were associated with higher odds of adverse outcomes, while COVID-19 diagnoses later in the study period were associated with lower odds of severe outcomes. In patients with type 2 diabetes, individuals on insulin treatment had higher odds for ICU/intubation/death (OR=1.59, 95% CI 1.27 to 1.99), whereas those on metformin had lower odds (OR=0.56, 95% CI 0.45 to 0.71). Compared with non-Hispanic White patients, Hispanic patients had higher odds of hospitalization in patients with type 2 diabetes (OR=1.73, 95% CI 1.36 to 2.19) or at risk of type 2 diabetes (OR=1.77, 95% CI 1.43 to 2.18.)
Conclusions
Adults who were older, in racial minority groups, had multiple chronic conditions or were on insulin treatment had higher risks for severe COVID-19 outcomes. This study reinforced the urgency of preventing COVID-19 and its complications in vulnerable populations.
Trial registration number
Keywords: Hospitalizations; Diabetes Mellitus, Type 2; COVID-19; Mortality
WHAT IS ALREADY KNOWN ON THIS TOPIC
Certain individuals, including those with diabetes, have an increased risk for COVID-19-related hospitalization and mortality.
WHAT THIS STUDY ADDS
This study included both ambulatory and inpatient populations in five health systems in Pennsylvania and Maryland.
In patients with type 2 diabetes, individuals on insulin treatment had higher odds for intensive care unit/intubation/death, whereas those on metformin had lower odds. Patients on metformin, glucagon-like peptide-1 receptor agonists, or dipeptidyl peptidase-4 inhibitors had lower odds for hospitalization.
Non-Hispanic Black and Hispanic patients who were at risk of diabetes had 54%–77% increased odds of hospitalization than White patients, and the associations were consistent across age, sex, and COVID-19 diagnosis periods.
COVID-19 diagnoses later in the study period were associated with lower odds of severe outcomes.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
This study reinforced the urgency of preventing COVID-19 and its complications in vulnerable populations.
Introduction
Systematic reviews and meta-analyses have reported a twofold to threefold increased risk of mortality due to COVID-19 for individuals with type 1 or type 2 diabetes in comparison to individuals without diabetes.1 Increased susceptibility in patients with diabetes may result from an impaired immune response to SARS-CoV-2 pathogens, particularly in a high glucose environment.2 3 In addition, phenotypes related to more severe forms of diabetes, such as uncontrolled blood glucose, a higher body mass index (BMI), and additional chronic conditions, are linked to admission to an intensive care unit (ICU), intubation, or early death in patients positive for COVID-19.4 However, most studies that included patients with diabetes were conducted primarily in COVID-19 hotspots,5 6 based on a single health system,7 or limited to the early period of the COVID-19 pandemic.5–9 Moreover, several studies in individuals with diabetes only included patients who were admitted to the hospital, which may result in biased conclusions.7–9 As diabetes itself or the severity of COVID-19 disease could prompt admission to the hospital, the associations observed between the risk factors and outcomes in the inpatient setting might be spurious without a non-hospitalized comparison group.10
The objective of this study was to identify demographic and clinical characteristics associated with COVID-19 hospitalization and mortality in the PaTH to Health: Diabetes study, including hospitalized and non-hospitalized patients, conducted in Pennsylvania and Maryland. Since minority populations, particularly Hispanics, have grown significantly in these two states in the past decade, we devoted additional attention to racial disparities in COVID-19 outcomes.
Methods
Study population
This retrospective study included adult patients with type 2 diabetes or at risk of type 2 diabetes using the PaTH Toward a Learning Health System (PaTH) clinical data research network. PaTH is one of eight clinical data research networks that comprise PCORnet, a national network for patient-centered outcomes research funded by the Patient-Centered Outcomes Research Institute. For this analysis, we included PaTH electronic health record (EHR) data from five academic health systems in Pennsylvania and Maryland: Penn State Health Milton S Hershey Medical Center, UPMC, Geisinger Health System, Temple Health System, and the Johns Hopkins Health System. EHR data from individual health systems were deidentified per data sharing policies. EHR data were encoded using standard healthcare terminologies and then combined using the PCORnet-specified Common Data Model (CDM).11 The PCORnet CDM transforms each healthcare system’s dialect into a common language standardized on the meaningful use-recommended vocabularies (SNOMED, RxNORM, and LOINC).
Type 2 diabetes was defined based on the SUrveillance, PREvention, and ManagEment of Diabetes Mellitus (SUPREME-DM) criteria adapted to our data source.12 Included patients with type 2 diabetes since 1 January 2012 met the following criteria: had one or more inpatient diagnosis codes for diabetes mellitus or had two or more of any of the following that occurred on separate days, no more than 2 years apart: (1) received a diagnosis code for type 2 diabetes mellitus in an ambulatory office visit (International Classification of Diseases 10th Revision codes E10.x and E11.x); (2) were dispensed diabetes medication (unless the medication was metformin, a thiazolidinedione, or exenatide and no other criteria were met); (3) had a hemoglobin A1c (HbA1c) level ≥6.5%; and (4) had a random plasma glucose level ≥200 mg/dL. We did not use the SUPREME-DM criteria for fasting plasma glucose levels and the 2-hour oral glucose tolerance test (OGTT), due to unreliable ascertainment of fasting status and OGTT data from the EHR.
Patients at risk of type 2 diabetes were defined based on having any BMI ≥25 kg/m2 in the PaTH database, at least one inpatient pre-diabetes diagnosis, at least two outpatient pre-diabetes diagnoses, or HbA1c levels between 5.7% and 6.4%. Approximately 86% of the individuals were included in the at-risk cohort based on elevated BMI criteria alone.
In this study, our analysis included adult patients (18 years or older) with their first COVID-19 diagnosis between 1 March 2020 and 28 February 2021. All patients either had type 2 diabetes or were at risk of type 2 diabetes, and all were continuing patients with at least one ambulatory visit (primary care or endocrinology) in the PaTH health system during 2019 (see online supplemental figure S1 for the study flow diagram). Details about the PaTH to Health: Diabetes study were previously described elsewhere.12
bmjdrc-2022-002774supp001.pdf (213.3KB, pdf)
Study outcomes
The outcomes of interest were proxy measures of COVID-19 severity. Patients were classified into three categories based on 30-day outcomes as indicated by site of care: (1) no hospitalization; (2) hospitalization only; or (3) admission to the ICU, intubation, or death.
Patient characteristics
Demographic data including age, sex, race/ethnicity, smoking status, and rurality were extracted from EHRs. Rural or urban designation was defined using rural-urban commuting area codes, a scheme for delineating subcounty components of rural and urban areas using zip code. Chronic conditions were defined by diagnosis codes based on validated algorithms used in prior studies.9 10 Because chronic conditions were not consistently recorded at each encounter, particularly if they were not related to the reason(s) for the patient’s visit, they were instead assessed based on patient records for 3 years prior to COVID-19 diagnosis. Comorbid conditions were further summarized using the Charlson Comorbidity Index (CCI), which examines 19 medical conditions that are each assigned an integer weight between 1 and 6, with a weight of 6 representing the most severe morbidity. The summation of the weighted comorbidity scores results in the final CCI score.13 14 Clinical variables, including BMI, systolic blood pressure, diastolic blood pressure, high-density lipoprotein cholesterol level, low-density lipoprotein cholesterol level, and HbA1c level, were based on the most recent values prior to the COVID-19 diagnosis. The 12-month study period (from March 2020 to February 2021) was divided into three 4-month intervals, which also coincided with advances in treatment, changes in viral variant, or other factors that might affect care and patient outcomes (March 2020 to June 2020; July 2020 to October 2020; and November 2020 to February 2021).
Statistical analysis
Analyses were conducted in patients with type 2 diabetes and patients at risk of type 2 diabetes separately. The associations between the patient characteristics and outcomes of interest were first assessed using χ2 test, analysis of variance, or Kruskal-Wallis test. Next, multivariable multinomial logistic regressions were conducted, including all covariates that were significant in the bivariable analyses. Prior to modeling, the predictors were assessed for multicollinearity using variance inflation factor statistics, but none were found. ORs were used to quantify the magnitude and direction of any significant associations while adjusting for the other variables included in the model. Subgroup analyses by age, sex, race, and time of COVID-19 diagnosis were performed. Additional analyses were conducted to compare the demographic and clinical variables in non-Hispanic White, non-Hispanic Black, and Hispanic patients with COVID-19 and to evaluate racial differences in COVID-19 outcomes. All analyses used SAS software V.9.4 (SAS Institute), and two-tailed p values <0.05 were considered statistically significant.
Results
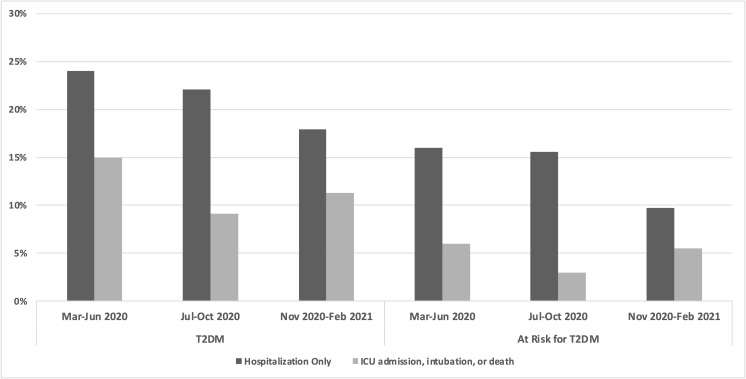
There were 15 725 patients identified (4944 with type 2 diabetes, 10 781 at risk of type 2 diabetes) with a COVID-19 diagnosis between March 2020 and February 2021. A total of 3490 patients (22.2%) had at least one adverse event, including 2404 patients with hospitalizations only and 1086 patients with ICU admission, intubation, or death within 30 days of diagnosis (777 (4.9%) ICU admissions, 291 (1.9%) intubations, and 502 (3.2%) deaths; not mutually exclusive). The prevalence of these outcomes varied over time: the rate of hospitalization was lowest in patients diagnosed between November 2020 and February 2021, while the rate of ICU admission, intubation, or death was the lowest from July 2020 to October 2020. The increase in ICU admission, intubation, or death between November 2020 and February 2021 was consistent with the COVID-19 infection uptick in late 2020. In all time periods, patients with type 2 diabetes had a higher prevalence of adverse outcomes than those at risk of type 2 diabetes (figure 1).
Figure 1.
Prevalence of 30-day outcomes by COVID-19 pandemic period (March 2020 to February 2021) in patients positive for COVID-19 with type 2 diabetes (T2DM) or at risk of type 2 diabetes. ICU, intensive care unit.
As shown in table 1, in patients with type 2 diabetes, the mean age was 62.3 years, 46.2% were men, 28.8% were non-Hispanic Black, 13.4% were Hispanic, 3.9% were other non-Hispanic (including Asians), and 94.5% resided in urban area. The mean BMI was 34.2 kg/m2, and the mean CCI score was 5.4. Based on EHRs, 33.5% of the patients had chronic pulmonary disease, and 28.8% had renal disease. Furthermore, the mean HbA1c level was 7.6%; 17.2% of the patients were taking sulfonylureas, 37.3% were on insulin treatment; 42.3% received metformin; 10.7% were on dipeptidyl peptidase-4 (DPP-4) inhibitors; 4.4% were on glucagon-like peptide-1 (GLP-1) agonists; and 9.6% were on sodium-glucose co-transporter 2 (SGLT2) inhibitors. In patients at risk of type 2 diabetes, the mean age was 52.4 years, 37.7% were men, 21.0% were non-Hispanic Black, 10.1% were Hispanic, 3.1% were other non-Hispanic (including Asians), and 95.7% resided in urban area. The mean BMI was 32.3 kg/m2, and the mean CCI score was 2.2. Chronic pulmonary disease was identified in 24.3% of the patients (table 1).
Table 1.
Patient characteristics by 30-day outcome in patients with COVID-19 with type 2 diabetes or at risk of type 2 diabetes
| Type 2 diabetes* | At risk of type 2 diabetes†‡ | |||||||
| Total (n=4944) |
No hospitalization (n=3333) |
Hospitalization only (n=1025) |
ICU, intubation, death (n=586) | Total (n=10 781) |
No hospitalization (n=8902) |
Hospitalization only (n=1379) |
ICU, intubation, death (n=500) | |
| Age (year) | 62.3 (14.0) | 60.1 (13.6) | 65.8 (13.8) | 68.8 (13.5) | 52.4 (17.3) | 50.4 (16.4) | 59.8 (17.4) | 69.2 (17.2) |
| Age group (%) | ||||||||
| <65 | 56.5 | 63.5 | 45.2 | 36.2 | 75.8 | 80.8 | 57.7 | 35.8 |
| ≥65 | 43.5 | 36.5 | 54.8 | 63.8 | 24.2 | 19.2 | 42.3 | 64.2 |
| Sex (%) | ||||||||
| Men | 46.2 | 44.8 | 47.6 | 51.9 | 37.7 | 36.4 | 40.8 | 53.0 |
| Women | 53.8 | 55.2 | 52.4 | 48.1 | 62.3 | 63.6 | 59.3 | 47.0 |
| Race/ethnicity (%) | ||||||||
| White, non-Hispanic | 52.0 | 49.7 | 49.8 | 69.3 | 63.7 | 63.2 | 61.0 | 80.2 |
| Black, non-Hispanic | 28.8 | 30.7 | 28.4 | 18.9 | 21.0 | 21.0 | 24.3 | 11.6 |
| Other, non-Hispanic | 3.9 | 4.5 | 3.3 | 1.4 | 3.1 | 3.2 | 2.7 | 2.4 |
| Hispanic | 13.4 | 12.8 | 17.7 | 9.9 | 10.1 | 10.3 | 10.9 | 5.8 |
| Unknown | 1.8 | 2.3 | 0.9 | 0.5 | 2.1 | 2.3 | 1.2 | 0.0 |
| Smoking (%) | ||||||||
| Current | 4.8 | 4.2 | 5.0 | 7.7 | 5.6 | 5.4 | 6.1 | 7.2 |
| Former | 19.5 | 15.7 | 23.0 | 35.0 | 16.7 | 14.6 | 23.4 | 35.0 |
| Never | 32.6 | 32.3 | 31.5 | 35.8 | 40.1 | 39.8 | 45.0 | 48.8 |
| Unknown | 43.1 | 47.8 | 40.5 | 21.5 | 36.9 | 40.2 | 25.5 | 9.0 |
| Location (%) | ||||||||
| Urban | 94.5 | 97.6 | 97.8 | 71.3 | 95.7 | 97.1 | 97.5 | 67.6 |
| Rural Unknown |
1.9 3.6 |
2.1 0.3 |
1.6 0.7 |
1.2 27.5 |
2.5 1.8 |
2.7 0.3 |
1.9 0.7 |
0.8 31.6 |
| Comorbidity (%) | ||||||||
| MI | 12.6 | 9.1 | 18.9 | 21.5 | 3.2 | 2.5 | 5.9 | 8.4 |
| Heart failure | 24.3 | 17.0 | 36.7 | 44.4 | 7.4 | 5.0 | 14.7 | 29.2 |
| Peripheral vascular disease | 19.0 | 14.2 | 28.5 | 30.0 | 6.3 | 4.6 | 11.5 | 22.8 |
| Cerebrovascular disease | 17.7 | 13.4 | 25.8 | 28.0 | 7.3 | 5.7 | 13.4 | 20.0 |
| Chronic pulmonary disease | 33.5 | 31.4 | 36.5 | 39.9 | 24.3 | 23.2 | 27.4 | 35.2 |
| Liver disease, mild | 15.1 | 15.1 | 15.6 | 14.5 | 7.6 | 7.3 | 9.84 | 8.0 |
| Renal disease, mild-moderate | 28.8 | 20.5 | 41.8 | 53.2 | 8.1 | 5.5 | 17.6 | 28.2 |
| Any malignancy | 10.9 | 9.3 | 12.9 | 16.2 | 8.7 | 6.7 | 18.5 | 17.8 |
| CCI score | 5.4 (3.1) | 4.7 (2.8) | 6.6 (3.2) | 7.3 (3.2) | 2.2 (2.4) | 1.8 (2.0) | 3.6 (2.9) | 5.0 (3.1) |
| BMI (kg/m2) | 34.2 (8.2) | 33.9 (8.2) | 32.4 (8.2) | 32.8 (9.3) | 32.3 (7.1) | 32.4 (7.1) | 31.8 (7.5) | 31.0 (6.4) |
| BMI category (%) | ||||||||
| <18.5 | 0.4 | 0.3 | 0.6 | 0.9 | 0.4 | 0.2 | 0.6 | 0.2 |
| 18.5 to <25 | 9.0 | 8.3 | 11.0 | 9.7 | 8.7 | 7.8 | 12.3 | 14.8 |
| 25–29.9 | 22.2 | 20.9 | 26.8 | 21.7 | 33.3 | 33.5 | 32.3 | 32.4 |
| 30–39.9 | 45.6 | 47.1 | 41.3 | 44.5 | 42.1 | 42.6 | 38.9 | 42.4 |
| 40+ | 21.0 | 21.7 | 18.4 | 21.3 | 13.1 | 13.4 | 12.6 | 8.4 |
| Unknown | 1.8 | 1.8 | 1.9 | 1.9 | 2.5 | 2.5 | 3.3 | 1.8 |
| SBP (mm Hg)§ | 132.3 (18.1) | 131.6 (17.2) | 134.3 (19.2) | 132.8 (19.6) | 126.7 (16.3) | 126.2 (15.9) | 129.1 (17.8) | 129.3 (18.2) |
| DBP (mm Hg)¶ | 76.1 (11.2) | 77.1 (10.8) | 75.0 (11.7) | 72.9 (11.7) | 76.9 (10.5) | 77.2 (10.4) | 75.6 (10.8) | 73.6 (11.0) |
| HDL cholesterol (mg/dL)** | 46.2 (14.2) | 46.5 (13.9) | 45.3 (14.8) | 44.8 (14.6) | 52.9 (15.4) | 53.0 (15.1) | 52.9 (16.7) | 50.1 (17.1) |
| LDL cholesterol (mg/dL)†† | 136.2 (47.5) | 139.3 (47.6) | 129.5 (45.5) | 126.4 (48.4) | 152.3 (41.7) | 143.7 (40.6) | 146.9 (45.6) | 138.0 (47.9) |
| Time of COVID-19 diagnosis (%) | ||||||||
| March to June 2020 | 29.1 | 26.3 | 33.9 | 36.9 | 25.1 | 24.0 | 30.8 | 30.0 |
| July to October 2020 | 24.6 | 25.1 | 26.2 | 18.9 | 26.6 | 26.2 | 32.4 | 17.4 |
| November to January 2021 | 46.3 | 48.6 | 40.0 | 44.2 | 48.3 | 49.9 | 36.8 | 52.6 |
| HbA1c category (%) | ||||||||
| <6.0% | 10.8 | 10.6 | 10.2 | 13.1 | ||||
| 6.0%–6.9% | 23.9 | 24.9 | 22.2 | 21.2 | ||||
| 7.0%–7.9% | 16.5 | 16.5 | 17.1 | 15.2 | ||||
| 8.0%–8.9% | 8.8 | 9.0 | 8.0 | 9.2 | ||||
| ≥9.0% | 13.6 | 13.5 | 14.3 | 12.8 | ||||
| Unknown | 26.4 | 25.5 | 28.3 | 28.5 | ||||
| HbA1c level (%)‡‡ | 7.6 (1.90) | 7.6 (1.87) | 7.6 (1.99) | 7.6 (2.07) | ||||
| Any sulfonylureas (%) | 17.2 | 19.8 | 14.5 | 13.5 | ||||
| Any metformin (%) | 42.3 | 49.1 | 30.9 | 23.9 | ||||
| Any insulin (%) | 37.3 | 31.3 | 49.5 | 50.0 | ||||
| Any DPP-4 inhibitor (%) | 10.7 | 11.1 | 9.0 | 10.9 | ||||
| Any GLP-1R agonist (%) | 4.4 | 5.0 | 2.8 | 4.3 | ||||
| Any SGLT2 inhibitor (%) | 9.6 | 10.5 | 8.0 | 7.0 | ||||
Data presented as mean (SD) or per cent.
*All p values <0.05 except location, liver disease, BMI category, and HbA1c category.
†All p values <0.05 except location.
‡At risk defined based on elevated BMI, pre-diabetes diagnosis, or elevated HbA1c.
§SBP n=15 439.
¶DBP n=15 438.
**HDL n=8369.
††LDL n=8376.
‡‡HbA1c n=6720.
BMI, body mass index; CCI, Charlson Comorbidity Index; DBP, diastolic blood pressure; DPP-4, dipeptidyl peptidase-4; GLP-1R, glucagon-like peptide-1 receptor; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; ICU, intensive care unit; LDL, low-density lipoprotein; MI, myocardial infarction; SBP, systolic blood pressure; SGLT2, Sodium-glucose cotransporter-2.
The bivariate analyses showed that in patients with type 2 diabetes, being older, male, non-Hispanic White, a former smoker, or having a COVID-19 diagnosis earlier in the pandemic were more prevalent in hospitalization-only patients and patients with ICU admission, intubation, or death. The mean CCI scores were higher in patients with adverse outcomes. Insulin use was more prevalent in those with hospitalization or with ICU admission, intubation, or death, while the uses of sulfonylureas, metformin, DPP-4 inhibitor, glucagon-like peptide-1 receptor (GLP-1R) agonist, and SGLT2 inhibitor were more prevalent in those who had no hospitalization. HbA1c levels were not associated with adverse outcomes (table 1). In patients at risk of type 2 diabetes, being older, male, non-Hispanic White, a former smoker, and having a COVID-19 diagnosis earlier in the pandemic were more prevalent in hospitalization-only patients, as well as in patients with ICU admission, intubation, or death. The mean CCI scores were higher in patients with adverse outcomes. Underweight or normal weight was more prevalent in patients who were hospitalized or with ICU admission, intubation, or death (table 1).
Table 2 shows the incidence of COVID-19 outcomes by demographic and clinical variables. As expected, patients with type 2 diabetes had a higher incidence of adverse outcomes due to COVID-19 than those at risk of diabetes across age, sex, and race/ethnicity groups. Adjusted ORs of hospitalization only and more severe outcome (ICU admission, intubation, or death) were estimated respectively from the multinomial logistic models with no hospitalization being the reference category (table 2). In patients with type 2 diabetes, those who were older, Hispanic (vs White, OR=1.73, 95% CI 1.36 to 2.19), had high CCI scores (OR=2.06 (95% CI 1.76 to 2.42) per 5-unit increase), or were on insulin treatment (OR=1.63, 95% CI 1.37 to 1.94) had significantly higher odds of hospitalization. On the other hand, patients diagnosed later in the COVID-19 pandemic or those on metformin, DPP-4 inhibitor, or GLP-1 agonist had lower odds of hospitalization. BMI and the uses of sulfonylureas or SGLT2 inhibitor were not significantly associated with hospitalization (table 2). For ICU admission, intubation, or death, being aged 65 or older, having BMI ≥40 kg/m2, having a higher CCI score, and being on insulin treatment were associated with higher odds, while being non-Hispanic Black, diagnosed in later periods, and on metformin treatment were associated with lower odds of the most severe outcomes. In contrast to hospitalization only, medications such as DPP-4 inhibitor, GLP-1 agonist, or SGLT2 inhibitor were not significantly associated with the most severe outcomes (table 2).
Table 2.
Incidence and adjusted ORs* for hospitalization only and severe outcomes in patients with type 2 diabetes or at risk of type 2 diabetes
| Type 2 diabetes | At risk of type 2 diabetes† | |||||||
| Hospitalization only (%) | ICU, intubation, death (%) | Hospitalization OR (95% CI) |
ICU, intubation, death OR (95% CI) |
Hospitalization only (%) | ICU, intubation, death (%) | Hospitalization OR (95% CI) |
ICU, intubation, death OR (95% CI) |
|
| Age <65 | 16.6 | 7.6 | Ref | Ref | 9.7 | 2.2 | Ref | Ref |
| Age ≥65 | 26.1 | 17.4 | 1.24 (1.02 to 1.49)‡ |
1.45 (1.13 to 1.85)‡ |
22.4 | 12.3 | 1.20 (1.01 to 1.43)‡ |
2.05 (1.57 to 2.67)‡ |
| Women | 20.2 | 10.6 | Ref | Ref | 12.2 | 3.5 | Ref | Ref |
| Men | 21.4 | 13.3 | 1.03 (0.88 to 1.21) |
1.21 (0.98 to 1.49) |
13.8 | 6.5 | 1.14 (1.00 to 1.30) |
1.62 (1.32 to 1.99)‡ |
| White | 19.8 | 15.8 | Ref | Ref | 12.3 | 5.8 | Ref | Ref |
| Black | 20.4 | 7.8 | 1.08 (0.89 to 1.32) |
0.68 (0.52 to 0.89)‡ |
14.8 | 2.6 | 1.54 (1.31 to 1.80)‡ |
0.81 (0.43 to 1.52) |
| Hispanic | 27.3 | 8.7 | 1.73 (1.36 to 2.19)‡ |
1.10 (0.78 to 1.55) |
13.7 | 2.7 | 1.77 (1.43 to 2.18)‡ |
1.23 (0.81 to 1.87) |
| March to June 2020 | 24.1 | 15.0 | Ref | Ref | 15.7 | 5.5 | Ref | Ref |
| July to October 2020 | 22.1 | 9.1 | 0.72 (0.59 to 0.89)‡ |
0.35 (0.27 to 0.47)‡ |
15.6 | 3.0 | 0.89 (0.76 to 1.05) |
0.39 (0.29 to 0.53)‡ |
| November 2020 to February 2021 | 17.9 | 11.3 | 0.55 (0.45 to 0.66)‡ |
0.36 (0.29 to 0.46)‡ |
9.8 | 5.1 | 0.52 (0.44 to 0.60)‡ |
0.53 (0.42 to 0.67)‡ |
| BMI <18.5 | 28.6 | 23.8 | 1.49 (0.49 to 4.55) |
2.17 (0.60 to 7.79) |
34.8 | 4.4 | 1.80 (0.67 to 4.84) |
0.63 (0.07 to 5.28) |
| BMI 18.5–24.9 | 25.4 | 12.8 | Ref | Ref | 18.0 | 7.9 | Ref | Ref |
| BMI 25–29.9 | 25.1 | 11.6 | 1.18 (0.89 to 1.56) |
1.10 (0.75 to 1.61) |
12.4 | 4.5 | 0.73 (0.59 to 0.91)‡ |
0.66 (0.48 to 0.92)‡ |
| BMI 30–39.9 | 18.8 | 11.6 | 0.92 (0.70 to 1.20) |
1.17 (0.81 to 1.67) |
11.8 | 4.7 | 0.77 (0.62 to 0.96)‡ |
0.88 (0.64 to 1.22) |
| BMI ≥40 | 18.2 | 12.1 | 1.05 (0.77 to 1.43) |
1.52 (1.02 to 2.26)‡ |
12.3 | 3.0 | 0.93 (0.71 to 1.21) |
0.81 (0.52 to 1.27) |
| CCI score (every 5 units) | 2.06 (1.76 to 2.42)‡ |
2.75 (2.26 to 3.35)‡ |
4.24 (3.64 to 4.93)‡ |
6.12 (4.94 to 7.60)‡ |
||||
| No insulin | 16.7 | 9.5 | Ref | Ref | ||||
| Any insulin | 27.5 | 15.9 | 1.63 (1.37 to 1.94)‡ |
1.59 (1.27 to 1.99)‡ |
||||
| No metformin | 24.8 | 15.6 | Ref | Ref | ||||
| Any metformin | 15.2 | 6.7 | 0.63 (0.53 to 0.75)‡ |
0.56 (0.45 to 0.71)‡ |
||||
| No DPP-4 inhibitor | 21.1 | 11.8 | Ref | Ref | ||||
| Any DPP-4 inhibitor | 17.5 | 12.1 | 0.75 (0.58 to 0.98)‡ |
1.03 (0.75 to 1.42) |
||||
| No GLP-1R agonist | 21.1 | 11.9 | Ref | Ref | ||||
| Any GLP-1R agonist | 13.4 | 11.5 | 0.61 (0.40 to 0.93)‡ |
1.07 (0.66 to 1.72) |
||||
| No SGLT2 inhibitor | 21.1 | 12.2 | Ref | Ref | ||||
| Any SGLT2 inhibitor | 17.4 | 8.7 | 1.08 (0.82 to 1.42) | 1.06 (0.73 to 1.55) |
||||
*Adjusted for age, sex, race/ethnicity, smoking status, CCI score, time of COVID-19 diagnosis, BMI category, SBP (quartile), DBP (quartile), HDL level (quartile), and LDL level (quartile). Additional adjustments for HbA1c level, insulin use, metformin use, DPP-4 inhibitor use, GLP-1R agonist use, and SGLT2 inhibitor use in analyses of patients with type 2 diabetes.
†At risk defined based on elevated BMI, pre-diabetes diagnosis, or elevated HbA1c.
‡P<0.05.
BMI, body mass index; CCI, Charlson Comorbidity Index; DBP, diastolic blood pressure; DPP-4, dipeptidyl peptidase-4; GLP-1R, glucagon-like peptide-1 receptor; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; ICU, intensive care unit; LDL, low-density lipoprotein; SBP, systolic blood pressure; SGLT2, Sodium-glucose cotransporter-2.
In patients at risk of type 2 diabetes, non-Hispanic Black (OR=1.54, 95% CI 1.31 to 1.80), Hispanic patients (OR=1.77, 95% CI 1.43 to 2.18) and those with higher CCI scores (OR=4.24 (95% CI 3.64 to 4.93) per 5-unit increase) had significantly higher odds of hospitalization. Patients with overweight or obesity had lower odds of hospitalization than those with normal weight. Patients diagnosed between November 2020 and February 2021 had significantly lower odds of hospitalization than those diagnosed during the early period of the pandemic (table 2). Patients who were aged 65 or older, were male, or who had higher CCI scores (OR=6.12 (95% CI 4.94 to 7.60) for every 5-unit increase) were more likely to experience ICU admission, intubation, or death. Compared with those with normal weight, patients who were overweight had lower odds of the most severe outcomes. Patients with later COVID-19 diagnoses had lower odds than those diagnosed in March to June 2020.
We conducted a series of subgroup analyses by age, sex, race/ethnicity, and COVID-19 diagnosis period for hospitalization only and ICU, intubation, or death in patients with type 2 diabetes or at risk of type 2 diabetes. The associations between the risk factors and outcomes were generally similar across the subgroups (data not shown).
Additional analyses on racial disparity
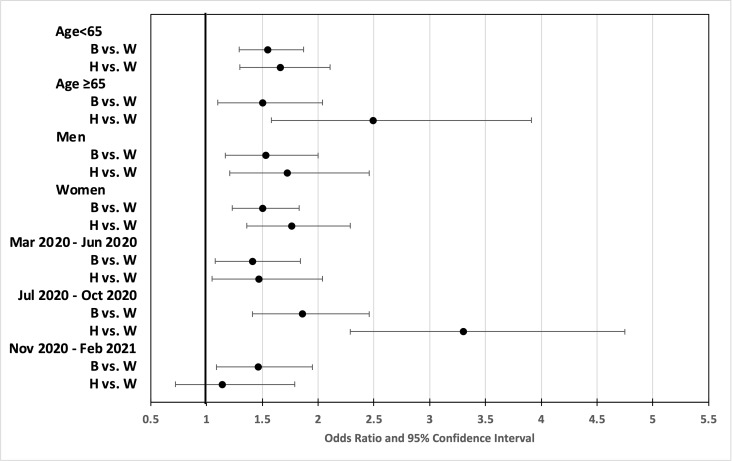
We conducted additional analyses focusing on racial differences. Compared with White patients, non-Hispanic Black and Hispanic patients were younger, more likely to be women, had lower CCI scores, and were more likely to be diagnosed with COVID-19 earlier in the pandemic. In addition, among patients with type 2 diabetes, compared with White patients, Black and Hispanic patients had higher HbA1c levels and were more likely to be on metformin and insulin (online supplemental table S1). In the multivariable analyses adjusted for all covariates, in patients with type 2 diabetes, compared with White patients, Hispanic patients had 33%–143% increased odds of hospitalizations across age groups, sex, and COVID-19 diagnosis periods (online supplemental table S2). In the patients at risk of type 2 diabetes, compared with White patients, Black patients had 41%–86% increased odds of hospitalization, and Hispanic patients had 14%–230% increased odds of hospitalization across the subgroups (figure 2 and online supplemental table S2). For the composite variable of ICU admission, intubation, or death, Black patients and non-Hispanic patients did not have increased odds, except in Hispanic patients during July to October 2020 (online supplemental table S2).
Figure 2.
ORs (and 95% CIs) for hospitalization in patients positive for COVID-19 at risk of type 2 diabetes. B, non-Hispanic Black; H, Hispanic; W, non-Hispanic White.
bmjdrc-2022-002774supp002.pdf (141KB, pdf)
Discussion
In our sample of patients diagnosed with COVID-19 and type 2 diabetes or at risk of type 2 diabetes, 22% were hospitalized, 5% were admitted to ICUs, 2% were intubated, and 3% died within 30 days of receiving a COVID-19 diagnosis from March 2020 to February 2021.
Vulnerable populations experienced worse outcomes with COVID-19 infections throughout the pandemic in 2020, including the elderly, people of color, and those with pre-existing or comorbid conditions, such as diabetes.1 15–18 Our findings were consistent with prior studies. As our analysis included data until early 2021, we were also able to show that the prevalence of severe outcomes decreased over time. Compared with the initial period (March to June 2020), patients diagnosed with COVID-19 in later periods were ~40%–65% less likely to experience the most severe COVID-19 outcomes. Although this shift in outcomes may reflect changing demographics in that healthier patients were infected later in the pandemic, the shift also suggests advances in the science and understanding of COVID-19 and the implementation of medical protocols to better respond to and manage COVID-19.
In the analyses of racial differences, we found non-Hispanic Black and Hispanic patients with type 2 diabetes were younger and had higher HbA1c levels (online supplemental table S1). These observations were consistent with population-level data reported in prior studies. In an analysis of the National Health and Nutrition Examination Survey (NHANES) data, Wang et al reported that Mexican American and non-Hispanic Black adults had a significantly younger mean age at diabetes diagnosis (mean age 47.2 and 44.9 years, respectively) relative to non-Hispanic White adults (mean age 51.8 years).19 Another NHANES study found non-Hispanic Black and Mexican American adults were significantly less likely to achieve HbA1c targets (60.4% and 55.7%, respectively) as compared with non-Hispanic White adults (68.3%).20 After controlling for covariates, our data indicated that patients who were non-Hispanic Black or Hispanic had significantly higher odds of hospitalization than White patients, and the associations were consistent across age, sex, and COVID-19 diagnosis periods. Although disparities were not apparent in the most severe outcome category (ie, ICU admission, intubation, or death), individuals in minority groups may have higher COVID-19 incidence or be diagnosed later, leading to increased odds of hospitalization. Once admitted to a hospital, the disease prognosis of these patients was comparable to that of their White counterparts. These findings were consistent with studies conducted in large health systems in Houston and Milwaukee,21 22 in which non-Hispanic Black and Hispanic patients had a higher likelihood of hospitalization, but there were no differences in ICU utilization, in-hospital mortality, ventilator use, or treatment parameters.22
Patients with type 2 diabetes using insulin had significantly higher odds of adverse events, which was expected, as insulin use is an indicator of an advanced stage of diabetes. This finding was consistent with other large studies.23–25 For instance, among 64 892 veterans with diabetes and COVID-19, insulin use was associated with higher odds of hospitalization and risk of death.25 A meta-analysis of 33 studies reported an OR of 1.70 (95% CI 1.33 to 2.19) as comparing patients on insulin to non-users.26 Because the coronavirus replicates faster in a high glucose environment and glucose fluctuations make it more challenging to treat COVID-19,2 patients with uncontrolled diabetes may experience more severe COVID-19 outcomes. This suggests that providers treating patients with COVID-19 with diabetes should remain vigilant, and patients with diabetes on insulin may require further attention regarding COVID-19 prevention and management.
Our study also suggested that metformin use was associated with a protective effect; patients with diabetes on metformin had ~40% lower odds of severe COVID-19 outcomes than those not taking metformin, after adjusting for HbA1c levels. Some previous studies noted similar reduced risks in patients on metformin.24 25 In a nationwide observational cohort study in England, metformin use was associated with 23% reduced mortality in people with type 2 diabetes.25 A meta-analysis reported a pooled OR of 0.54 (95% CI 0.47 to 0.62) for metformin use and mortality.26 Several potential mechanisms might explain the protective effect of metformin, either by reducing the likelihood of SARS-CoV-2 infection or by decreasing COVID-19 severity. Metformin reduces blood glucose levels; worse glucose control has been associated with higher mortality and end-organ complications in patients with COVID-19.18 Metformin could decrease endothelial injury, an important factor and therapeutic target in mitigating COVID-19 complications.27 28 Metformin also inhibits neutrophil extracellular trap release, alleviating the development of downstream lung injury.29–31 Metformin could also decrease the viral cycle, with efficacy against Middle East respiratory syndrome and COVID-19.32 33 While we do not have data regarding medication dosage or combination therapies to support the causal relationship between metformin and less severe COVID-19 outcomes, further research on these mechanisms may contribute to the prevention of COVID-19 and other viral infections.
GLP-1R agonists and DPP-4 inhibitors are glucose-regulating medications known to have anti-inflammatory effects that may improve outcomes in patients with SARS-CoV-2 infection. In a multination study of TriNetX COVID-19 Research Network, the use of GLP-1R agonists was associated with significant reductions in hospital admission, respiratory complications, and mortality; the use of DPP-4 inhibitors was associated with a reduction in respiratory complications and subsequent hospitalizations.34 Moreover, in a study among veterans with diabetes and COVID-19, SGLT2 inhibitors and GLP-1R agonist were associated with lower odds of hospitalization; SGLT2 inhibitor use was also associated with lower odds of death.35 In our study, we found patients with type 2 diabetes on DPP-4 inhibitor or GLP-1R agonist had significantly lower odds of being hospitalized, whereas there were no associations with ICU admission, intubation, or death. There were no significant associations between SGLT2 use and COVID-19 adverse outcomes. The inconsistency across observational studies may be attributed to differences in study populations, strategies of adjusting for confounding, or study power because of smaller proportions of patients with diabetes on those newer medications.
Unlike some previous studies,36–38 our study did not find higher HbA1c levels were significantly associated with more severe COVID-19 outcomes. As shown in table 1, HbA1c data were missing in 26.4% of the patients with type 2 diabetes, with slightly higher percentages in those who had hospitalization (28.3%) or ICU/intubation/death (28.5%) than those without hospitalizations (25.5%). We cannot rule out the possibility that those with hospitalization or ICU/intubation/death had higher HbA1c. Nonetheless, in the multivariable regressions, we adjusted for the HbA1c levels as a categorical variable, including the ‘unknown’ category.
In patients at risk of type 2 diabetes, we found that being underweight was associated with higher odds of hospitalization, while having overweight and obesity was associated with lower odds, compared with patients of normal weight. These findings were not consistent with some prior studies.6 39 However, the at-risk patients in our study entered the cohort largely based on elevated BMI, a small proportion of the study cohort had normal weight or underweight. This group may represent a more susceptible population, that is, pre-diabetes, with different phenotypes. Likewise, the lack of significant associations between higher BMIs and adverse COVID-19 outcomes in patients with type 2 diabetes requires further study. It is possible that more aggressive care was provided to patients who were perceived as high risk, including those with both type 2 diabetes and obesity. Additional care may mitigate the negative impact of obesity.
Our study had several strengths. First, the study population consisted of patients from two states, including both hotspot and non-hotspot areas, suggesting broader generalizability. Second, our data included patients with COVID-19 diagnosed over a 12-month period, reflecting the different stages of prevention and treatment protocols before vaccines became available. Third, our data included patients from five health systems, with a substantial number of people in under-represented minority groups, from different clinical practice settings, and urban and rural areas.
There are limitations to our study. First, our patients were seen in tertiary academic health systems, and therefore, our results may not be applicable to patients seen in community settings. Second, missing information is not uncommon in EHR data, and the missingness on smoking status and HbA1c levels cannot be ignored. We cannot rule out the possibility of misclassification due to missing data. Third, EHR data may not capture COVID-19 outcomes outside the five health systems we examined, potentially underestimating the proportion of adverse events. Fourth, the association between covariates and outcomes could be overestimated due to residual confounding factors, such as insurance type and stage of COVID-19 illness at the time of diagnosis. Moreover, additional socioeconomic factors, such as education, income, and census track-level deprivation data, were not consistently coded across institutions, which limited the feasibility to study additional factors of COVID-19 hospitalizations. Finally, the original PaTH to Health: Diabetes study was a natural experiment to evaluate the effects of intensive behavioral therapy (IBT), implemented by the Centers for Medicare and Medicaid Services and subsequently by most private plans, on diabetes-related outcomes. As the BMI cut-off for the IBT services does not account for the ethnicity of the individual, Asians who reach overweight at the BMI of 23 kg/m2 and are at a greater risk for developing diabetes at a lower BMI were not included in the study population.
In conclusion, our study further emphasized that patients with type 2 diabetes or at risk of diabetes who were older, in racial minority groups, who had multiple chronic conditions, and were on insulin treatment had higher risks for severe COVID-19 outcomes. This reinforces the urgency to prevent COVID-19 and its complications, which subsequently can overburden medical resources. Given that racial disparities for COVID-19 vaccinations remain in Pennsylvania and Maryland, as well as across the USA, increased community outreach is needed to prevent COVID-19 infections and increase the public’s knowledge of and confidence in COVID-19 vaccines.
Acknowledgments
The authors acknowledge the participation of their partnering health systems within the PaTH Network and thank all the investigators and staff for their efforts.
Footnotes
Contributors: All authors contributed to the concept and design of the paper. H-CY, JLK, LK, EBL, and CLB contributed to acquisition, analysis, or interpretation of data. H-CY drafted the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. LK and EBL conducted the statistical analysis. JLK obtained the funding. JMP and EF provided administrative, technical, or material support. H-CY is the guarantor and takes responsibility for the conduct and the contents of the article.
Funding: Research reported in this publication was funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (PCORI NEN-1509-32304).
Disclaimer: PCORI had no role in the study design, data collection, interpretation, or writing or approving the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. The data sets generated and/or analyzed during the current study are not publicly available due to data sharing agreements with electronic health record data. However, opportunities exist for collaborations with the PaTH Network.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was reviewed and approved by the Institutional Review Board (IRB) of Johns Hopkins School of Medicine, the central IRB for all participating institutions.
References
- 1.Apicella M, Campopiano MC, Mantuano M, et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol 2020;8:782–92. 10.1016/S2213-8587(20)30238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract 2020;162:108142. 10.1016/j.diabres.2020.108142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin Y, Rohli KE, Shen P, et al. The epidemiology, pathophysiological mechanisms, and management toward COVID-19 patients with type 2 diabetes: a systematic review. Prim Care Diabetes 2021;15:899–909. 10.1016/j.pcd.2021.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlesinger S, Neuenschwander M, Lang A, et al. Risk phenotypes of diabetes and association with COVID-19 severity and death: a living systematic review and meta-analysis. Diabetologia 2021;64:1480–91. 10.1007/s00125-021-05458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA 2020;323:2052–9. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med 2020;173:773–81. 10.7326/M20-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla AP, Tchang BG, Lam T, et al. Preadmission predictors of severe COVID-19 in patients with diabetes mellitus. J Diabetes Complications 2021;35:107967. 10.1016/j.jdiacomp.2021.107967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia 2020;63:1500–15. 10.1007/s00125-020-05180-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wargny M, Potier L, Gourdy P, et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia 2021;64:778–94. 10.1007/s00125-020-05351-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith GJ, Morris TT, Tudball MJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun 2020;11:5749. 10.1038/s41467-020-19478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Common Data Model. Patient Centered Outcomes Research Institute . The National patient-centered clinical research network. accessed January 7, 2019. Available: http://www.pcornet.org/resource-center/pcornet-common-data-model/
- 12.Kraschnewski JL, Kong L, Francis E, et al. A patient-centered path to address diabetes: protocol for a study on the impact of obesity counseling. JMIR Res Protoc 2019;8:e12054. 10.2196/12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 14.Glasheen WP, Cordier T, Gumpina R, et al. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am Health Drug Benefits 2019;12:188–97. [PMC free article] [PubMed] [Google Scholar]
- 15.Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is Tripled in the diabetes community: a prospective analysis of the pandemic's impact in type 1 and type 2 diabetes. Diabetes Care 2021;44:526–32. 10.2337/dc20-2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SM, Boppana A, Traupman JA, et al. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J Med Virol 2021;93:409–15. 10.1002/jmv.26227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vahidy FS, Drews AL, Masud FN, et al. Characteristics and outcomes of COVID-19 patients during initial peak and resurgence in the Houston metropolitan area. JAMA 2020;324:998–1000. 10.1001/jama.2020.15301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L, She Z-G, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020;31:1068–77. 10.1016/j.cmet.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang MC, Shah NS, Carnethon MR, et al. Age at diagnosis of diabetes by race and ethnicity in the United States from 2011 to 2018. JAMA Intern Med 2021;181:1537–9. 10.1001/jamainternmed.2021.4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999-2018. JAMA 2021;326:704–13. 10.1001/jama.2021.9883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz-Price LS, Nattinger AB, Rivera F, et al. Racial disparities in incidence and outcomes among patients with COVID-19. JAMA Netw Open 2020;3:e2021892. 10.1001/jamanetworkopen.2020.21892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health 2021;21:1330. 10.1186/s12889-021-11431-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boye KS, Tokar Erdemir E, Zimmerman N, et al. Risk factors associated with COVID-19 hospitalization and mortality: a large Claims-Based analysis among people with type 2 diabetes mellitus in the United States. Diabetes Ther 2021;12:2223–39. 10.1007/s13300-021-01110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khunti K, Knighton P, Zaccardi F, et al. Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: a nationwide observational study in England. Lancet Diabetes Endocrinol 2021;9:293–303. 10.1016/S2213-8587(21)00050-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wander PL, Lowy E, Beste LA. Prior glucose-lowering medication use and 30-day outcomes among 64,892 veterans with diabetes and COVID-19. Diabetes Care 2021;44:2708–13. 10.2337/dc21-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen NN, Ho DS, Nguyen HS, et al. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: a meta-analysis. Metabolism 2022;131:155196. 10.1016/j.metabol.2022.155196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120–8. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xin G, Wei Z, Ji C, et al. Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtDNA release. Sci Rep 2016;6:36222. 10.1038/srep36222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron AR, Morrison VL, Levin D, et al. Anti-Inflammatory effects of metformin irrespective of diabetes status. Circ Res 2016;119:652–65. 10.1161/CIRCRESAHA.116.308445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bendib I, de Chaisemartin L, Granger V, et al. Neutrophil extracellular traps are elevated in patients with pneumonia-related acute respiratory distress syndrome. Anesthesiology 2019;130:581–91. 10.1097/ALN.0000000000002619 [DOI] [PubMed] [Google Scholar]
- 31.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020;5:e138999. 10.1172/jci.insight.138999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kindrachuk J, Ork B, Hart BJ, et al. Antiviral potential of ERK/MAPK and PI3K/Akt/mTOR signaling modulation for middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother 2015;59:1088–99. 10.1128/AAC.03659-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia G, Sharma A, Ramaiah A. Antiviral drug screen of kinase inhibitors identifies cellular signaling pathways critical for SARS-CoV-2 replication. bioRxiv 2020. [Google Scholar]
- 34.Nyland JE, Raja-Khan NT, Bettermann K. Diabetes, drug treatment, and mortality in COVID-19: a multinational retrospective cohort study. Diabetes 2021;70:2916. 10.2337/db21-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wander PL, Lowy E, Beste LA. Prior glucose-lowering medication use and 30-day outcomes among 64,892 veterans with diabetes and COVID-19. Diabetes Care 2021;44:2708–13. 10.2337/dc21-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong R, Hall M, Vaddavalli R. Glycemic control and clinical outcomes in US. patients with COVID-19: data from the National COVID cohort collaborative (N3C) database. Diabetes Care 2022;24:dc212186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merzon E, Green I, Shpigelman M, et al. Haemoglobin A1c is a predictor of COVID-19 severity in patients with diabetes. Diabetes Metab Res Rev 2021;37:e3398. 10.1002/dmrr.3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol 2020;8:823–33. 10.1016/S2213-8587(20)30271-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahamat-Saleh Y, Fiolet T, Rebeaud ME, et al. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: a systematic review and meta-analysis of observational studies. BMJ Open 2021;11:e052777. 10.1136/bmjopen-2021-052777 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2022-002774supp001.pdf (213.3KB, pdf)
bmjdrc-2022-002774supp002.pdf (141KB, pdf)
Data Availability Statement
No data are available. The data sets generated and/or analyzed during the current study are not publicly available due to data sharing agreements with electronic health record data. However, opportunities exist for collaborations with the PaTH Network.