Abstract
Glial fibrillary acidic protein (GFAP) is an intermediate filament protein that is characteristic for astrocytes and neural stem cells, and their malignant analogues in glioma. Since the discovery of the protein 50 years ago, multiple alternative splice variants of the GFAP gene have been discovered, leading to different GFAP isoforms. In this review, we will describe GFAP isoform expression from gene to protein to network, taking the canonical isoforms GFAPα and the main alternative variant GFAPδ as the starting point. We will discuss the relevance of studying GFAP and its isoforms in disease, with a specific focus on diffuse gliomas.
Keywords: GFAP, glia, astrocyte, glioma, cytoskeleton, splicing
Introduction
Glial fibrillary acidic protein (GFAP) is celebrating its 50th birthday after being first described by Lawrence Eng in a publication called ‘An acidic protein isolated from fibrous astrocytes’ published on May 7th 1971 (Eng et al., 1971; Helman et al., 2020). After its discovery in 1971 and official naming in 1972 (Uyeda et al., 1972), GFAP was recognized as a marker with high specificity for central nervous system (CNS) astrocytes (Bignami et al., 1972; Bignami and Dahl, 1974; Uyeda et al., 1972). GFAP was later classified as a type III intermediate filament (IF) protein along with vimentin, desmin, and peripherin based on sequence homology (Geisler and Weber, 1983). Unique to GFAP, in comparison to the other type III IF proteins, is the regulation of the GFAP pre-RNA transcript by alternative splicing and alternative polyadenylation. Since the discovery of the canonical isoform GFAPα, seven murine and twelve human splice-isoforms have been described (Middeldorp and Hol, 2011), of which the isoforms GFAPλ and GFAPμ were only discovered recently (Helman et al., 2020; van Bodegraven et al., 2021). Although the difference in functionality between the isoforms is not entirely known, neurological disorders are associated with alterations in isoform expression levels (Bugiani et al., 2011; Clairembault et al., 2014; Flint et al., 2012; Helman et al., 2020; Kamphuis et al., 2014; Stassen et al., 2017).
In this review, we summarize the history and current state of knowledge on GFAP alternative splicing, with a specific focus on the relevance for glioma. We start by describing the classification and assembly properties of IF proteins and GFAP, followed by an overview of the discovered GFAP isoforms. Next, we will focus on the expression regulation and protein characteristics of the two best-studied isoforms, GFAPα and GFAPδ. In the second part of the review, we will discuss the current state of knowledge on the clinical relevance of GFAP and the GFAPα/GFAPδ ratio as a glioma biomarker, and the known functions of the protein in relation to glioma.
GFAP and the Intermediate Filament (IF) Network
GFAP IF Network Assembly
The IF protein family consist of a large group of proteins that give rise to one of the three cytoskeletal networks in the cell. With a diameter of 10 nm, the filaments were originally distinguished by their intermediate size in comparison to microtubules (24nm) and actin filaments (7 nm), the other two cytoskeletal components in the cell (Herrmann and Aebi, 2016). With over 65 genes encoding for different IF proteins, the expression pattern of IFs is very cell type- and differentiation state-specific, a characteristic that sets it apart from the more ubiquitously expressed isoforms of actin and tubulin (Hesse et al., 2001). All IF proteins share a secondary structure consisting of an α-helical ‘rod’ domain flanked by a flexible N-terminal ‘head’ and a C-terminal ‘tail’ domain. Based on homology in sequence and assembly properties, the IF proteins are further classified into six subtypes. Although most subtypes contain proteins that form a network in the cytoplasm of the cell, the lamins of subtype V form a separate network on the inside of the nuclear envelope, also termed the nuclear lamina (Etienne-Manneville, 2018). GFAP is a type III IF protein that is most abundantly expressed in the brain, where it is classically used as a marker for astrocytes. However, its expression can also be found in several cell types outside the CNS, including liver stellate cells, fibroblasts, myoepithelial cells, chondrocytes, and lymphocytes (Messing and Brenner, 2020).
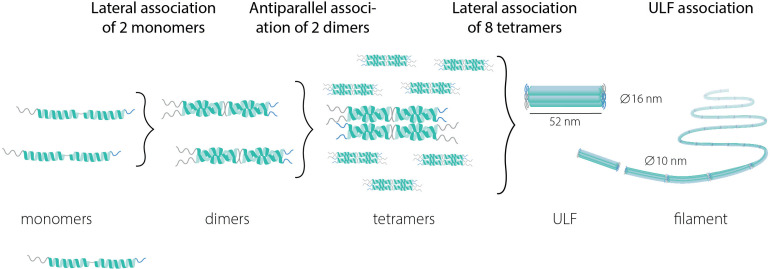
Assembly of IF proteins into the IF network is a multistep process (Figure 1) that occurs in the absence of cofactors and nucleotides (Etienne-Manneville, 2018). The first step in this process is the lateral association of monomers or heteromers to form a dimer. GFAP can form homodimers, but also heterodimers with vimentin. In the next step of IF network assembly, dimers bind in an antiparallel fashion to form tetramers, which then laterally associate into octamers forming structures called unit-length fragments (ULFs). Subsequent association of ULFs in a non-polar fashion leads to the formation of a filament, which then undergoes radial compaction leading to the final diameter of 10 nm. Because of the extreme stability of IFs, IF networks were long thought to be stagnant structures, but live-cell imaging has revealed that the IF network is, in fact, very dynamic and has a continuous turnover (Etienne-Manneville, 2018).
Figure 1.
The multi-assembly steps from intermediate protein monomers to filaments. ULF = unit length fragment.
GFAP Isoforms
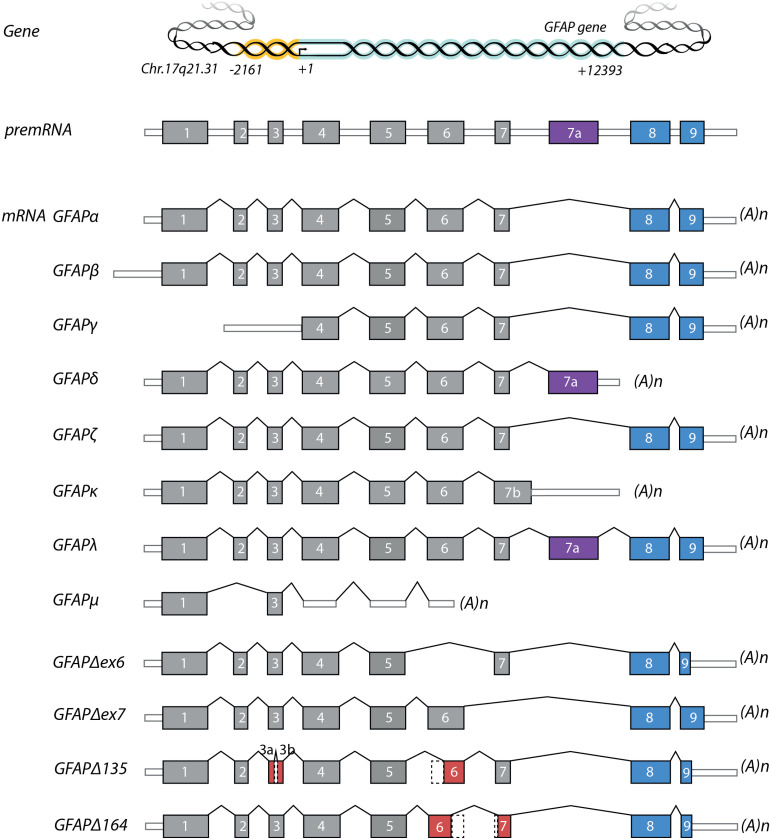
A GFAP IF network is likely to consist of several GFAP isoforms, in combination with other IF proteins, such as vimentin. Up till now, 12 GFAP isoforms (Table 1 and Figure 2) have been described in mice and humans that are either due to alternative starts sites or alternative splicing (Hol and Capetanaki, 2017; Middeldorp and Hol, 2011; van Bodegraven et al., 2019a). The first cDNA clone of mouse GFAP was sequenced in 1984 (Lewis et al., 1984) and of human GFAP in 1990 (Brenner et al., 1990). The cDNA of the canonical GFAP isoform, GFAPα, consists of 9 exons. In 1992 the first GFAP mRNA variant was described by Feinstein et al. in rat Schwann cells (Feinstein et al., 1992). This variant, GFAPβ, has an alternative start site 169 nt upstream of GFAPα. Later it was shown that this variant is also expressed in the central nervous system of rats (Condorelli et al., 1999b; Galea et al., 1995). GFAPγ is another variant with an alternative start, as it lacks exon1 and starts in intron 1, 130 nt upstream the start of exon 2 (Zelenika et al., 1995), and is expressed in mouse and human brain, and in mouse spleen. There is no definitive proof of protein expression of the GFAPβ and GFAPγ transcripts (Messing and Brenner, 2020). Several variants have been identified that are the result of alternative splicing (Table 1). In the paper of Zelenika et al. in 1995, besides GFAPγ another alternative splice variant was described with arretention of the last 284 nt of intron 8 (Zelenika et al., 1995). We confirmed the presence of the mRNA of this variant in mouse brains and termed it GFAPζ, however, the protein has not been identified yet (Kamphuis et al., 2012). GFAPδ was discovered in the rat brain and was first described to be the result of an extra exon; exon 7 + (Condorelli et al., 1999a). A few years later, GFAPε was found in a library of human fetal brain RNA. The cDNA of this isoform includes exon 1–7, plus exon 7a (which is highly similar to exon 7 + ) (Nielsen et al., 2002), but misses exon 8 and 9. GFAPδ protein is expressed in a subpopulation of astrocytes in the human brain (Roelofs et al., 2005) and is more widely expressed in the mouse brain (Kamphuis et al., 2012). The consensus now is that GFAPδ and GFAPε are the same variant (Middeldorp and Hol, 2011; Roelofs et al., 2005). In following years, more GFAP splice variants were discovered: GFAPΔ135, GFAPΔexon 6, and GFAPΔ164 in human and mouse brain (Hol et al., 2003; Kamphuis et al., 2012), GFAPΔexon 7 in mouse brain (Kamphuis et al., 2012), GFAPκ in pig brain (Blechingberg et al., 2007a), GFAPλ in human brain (Helman et al., 2020), and GFAPμ in human brain and glioma (van Bodegraven et al., 2021). Note that in the original GFAPδ paper the authors described 2 transcripts: (i) with an intron 7 retention (exon 7a) and alternative polyadenylation site ( = GFAPδ/ε) and (ii) with an intron 7 retention and including exon 8 and 9 ( = GFAPλ) (Condorelli et al., 1999a).
Table 1.
GFAP Isoforms and Proof of mRNA and Protein Expression.
| GFAP variant | mRNA | Protein | References (PMIDS) |
|---|---|---|---|
| α | Human, mouse, rat | Human, mouse, rat | (1, 2, 3) + many other papers |
| β | Human, mouse, rat | No proof (no specific antibody available) | (3, 4, 5, 6, 7) |
| γ | Human, mouse | No proof (no specific antibody available) | (3, 7) |
| δ = ε | Human, mouse, pig | Human, mouse | (3, 8, 9, 10, 11, 12, 13) |
| ζ | Human, mouse | No proof (no specific antibody available) | (3, 7, 14) |
| Δ135 | Human Not in: mouse |
No proof (because GFAP-pan antibodies cannot discriminate between α and Δ135) | (3, 15) |
| Δ164 | Human Not in: mouse |
Human: note-hGFAP+1 antibody cannot discriminate between Δ164, Δexon6, Δexon7 | (11, 15, 16) |
| Δexon6 | Human Not in: mouse |
See Δ164 | (11, 15, 16) |
| Δexon7 | Human, mouse | Human: note-hGFAP+1 antibody cannot discriminate between Δ164,
Δexon6, Δexon7. Not in: mouse (mGFAP+1 antibody recognizes Δ164, Δexon6, Δexon7 recombinant mouse proteins but does not stain mouse brain tissue) |
(3, 7) |
| κ | Human, mouse, pig | No evidence for protein expression in mice and humans: Three mouse-specific GFAPκ antibodies and 1 human-specific recognize recombinant protein but do not stain brain tissue. | (3, 7, 13) |
| λ | Human | Human (can be identified with anti-GFAPδ) | (17) |
| μ | Human | Human spinal cord (band of right size on Western blot) | (18) |
References: (1) (Lewis et al., 1984), (2) (Brenner et al., 1990), (3) (Kamphuis et al., 2012), (4) (Feinstein et al., 1992), (5) (Galea et al., 1995), (6) (Condorelli et al., 1999b), (7) (Kamphuis et al., 2014), (8) (Roelofs et al., 2005), (9) (Mamber et al., 2012), (10) (Middeldorp et al., 2010), (11) (Middeldorp et al., 2009), (12) (Stassen et al., 2017), (13) (Blechingberg et al., 2007a), (14) (Zelenika et al., 1995), (15) (Boer et al., 2010), (16) (Hol et al., 2003), (17) (Helman et al., 2020), (18) (van Bodegraven et al., 2021).
Figure 2.
The GFAP gene, premRNA and alternative splice variants. White rectangles depict 5′ and 3′ untranslated regions, grey rectangles depict translated regions. The alternative exons for the GFAP tail-region are represented in blue and purple. Exons with partial deletions are represented in red. Abbreviations: (A)n = poly-A-tail.
Taken together, it is clear that GFAP is alternatively spliced but the full extent of the splicing is not known yet. In ENSEMBL 38 different human transcripts are listed (GFAP ENSG00000131095), but these need further validation. In addition, novel antibodies are necessary to prove that the protein isoforms are also expressed. In the next part of this review, the focus will be on two of the most highly expressed isoforms: the canonical GFAPα and the alternative GFAPδ isoform, which differ in their last 41/42 amino-acid tail-region (Figure 3). We will describe how the two transcripts are regulated by posttranscriptional regulation and how post-translationally the difference in the tail-region lead to altered protein dynamics.
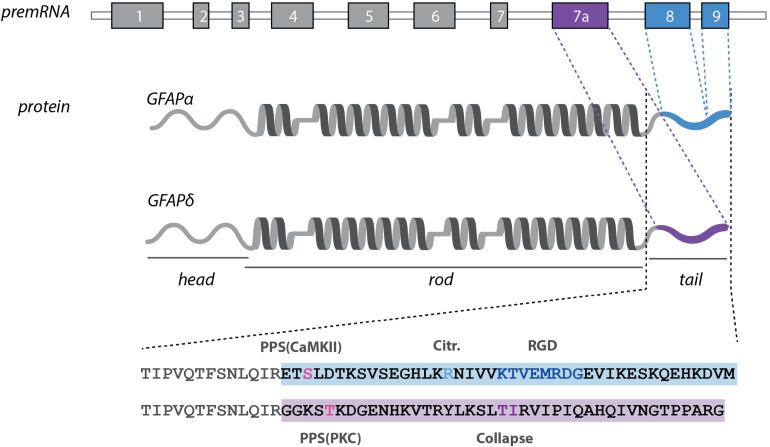
Figure 3.
The distinct features of the GFAPα and GFAPδ proteins. The GFAP protein consists of α-helical rod-domain (dark grey), flanked by a head- and tail-domain. The distinct tail-regions of the GFAPα and δ isoforms and the corresponding exons are marked in blue (GFAPα) and purple (GFAPδ). GFAPα contains an additional citrullination site (Jin et al., 2013) and a conserved RDG sequence (Chen and Liem, 1994). Both tail-regions have a different predicted phosphorylation site (Boyd et al., 2012). Collapse of GFAPδ has been assigned to T411 and T412 (Nielsen and Jørgensen, 2004). Abbreviations: aa = amino acids, PCS = predicted citrullination site, PPS = predicted phosphorylation site.
Regulation of GFAPα and GFAPδ Isoform Expression and Localisation
The human GFAP gene is located on chromosome 17q21 and spans over 10 kb of DNA (Huebner et al., 1991). The promoter region of the human GFAP gene extends from −2162 to +47 and contains consensus sequences and binding sites for a dozen transcription factors, as extensively discussed in Brenner and Messing (2021). Of the different transcription factors, AP-1, NF1, and STAT3 are considered most evidently involved in GFAP expression (Brenner and Messing, 2021). Both the GFAPα and GFAPδ splice variants share their RNA start site and their pre-mRNA consists out of nine exons, eight introns, four alternative exons, and two alternative introns (Middeldorp and Hol, 2011). Canonical splicing of the pre-mRNA leads to the inclusion of nine exons and results in the mRNA transcript of GFAPα. Upon an alternative splice event, the last two exons are replaced by an alternative exon 7a, which encodes for the tail-region of the GFAPδ protein. In addition to a difference in the last 123/126 nucleotides of the coding region of the mRNA, the transcript of GFAPδ has an alternative 3 prime untranslated region (3′ UTR) and polyadenylation site. A study by Blechingberg and colleagues showed that the activity of the polyadenylation signal rather than the 3′-splice site usage was the primary determent for processing into the GFAPα or GFAPδ/κ mRNA (Blechingberg et al., 2007b). Nevertheless, manipulation of splice enhancers and the polyadenylation signal in exon 7a within the minigene system used in this study also hinted towards an interplay between splicing and polyadenylation regulation, indicating a role for both mechanisms in the modulation of levels of GFAPα and GFAPδ isoforms (Blechingberg et al., 2007b). The effect of the GFAP gene transcription rate on isoforms balance has also been studied, although findings are inconclusive. Studies with the GFAP minigene with different promotors did not indicate an effect of transcriptional rate on polyadenylation site selection (Blechingberg et al., 2007b). On the other hand, inhibition of histone deacetylases activity in glioma cells and primary astrocytes was linked to decreased expression GFAP and a shift in the balance between the GFAPα to the GFAPδ isoform, a process modulated by splicing factor SR6 (Kanski et al., 2014). Although this suggests that lower transcriptional rates lead to increased alternative splicing, an increased transcriptional rate has also been linked to higher levels of GFAPδ and GFAPκ. In a transgenic mouse model carrying several copies of the human GFAP transgene, disproportionally increased levels of GFAPδ and GFAPλ were found (Lin et al., 2021).
Another level of post-transcriptional regulation of GFAP isoforms can come from microRNAs (miRNA). miRNAs are small non-coding RNAs that affect mRNA translation and stability. Upon association with the Argonaute protein, the seed sequence of the miRNA can bind to a complementary sequence of the mRNA, leading to mRNA cleavage or silencing (Matsuyama and Suzuki, 2020). Since miRNAs usually target the 3′ UTR of a transcript, and GFAPα and GFAPδ have different 3′ UTRs, their transcripts can be targeted by different miRNAs. Several studies have investigated the effect of miRNAs on GFAP expression in mouse models and human cell lines. However, direct regulation of miRNAs of specific human GFAP transcripts has so far not been proven, as aptly discussed by Brenner and Messing (2021).
Besides the regulation of GFAP mRNA isoform expression, also the localization of the different GFAP mRNAs within the cell has been investigated. Two independent studies made similar observations regarding distinct subcellular localisation patterns of the GFAPα and GFAPδ transcripts within astrocytes in vitro (Thomsen et al., 2013), and in mouse brain tissue (Oudart, 2012). Whereas GFAPδ localisation is mainly restricted to the soma of the cell, GFAPα mRNA is particularly enriched within the fine processes of the astrocyte (Oudart, 2012; Thomsen et al., 2013).
GFAPα and δ Protein Characteristics and Dynamics
The human canonical GFAPα protein consists of 432 amino acids with a molecular weight of 55 kDa. As a result of the alternative exon usage, the last 42 amino acids of the tail-region of the GFAPα isoform are replaced by 41 alternative amino acids in the GFAPδ isoform (Figure 3). Despite the similarity in length, the tail sequences of GFAPδ and α share no homology and have different isoelectric points (Boyd et al., 2012). Also on a phylogenetic level, the two isoforms are different. Most of the GFAP protein, including the tail-region of the GFAPα isoform, is highly conserved between species and shows more than 90% homology to mouse, rat, and pig GFAP and even 67% to the protein in zebrafish (Nielsen and Jørgensen, 2004). In contrast, the C-terminus of GFAPδ varies considerably between species, and is only conserved among higher primates, but not among other mammals (Singh et al., 2003). Since many of the non-conserved sequence changes in primates also alter the amino acid sequence of the C-terminus, it has been suggested that this change may have led to a positive selection of a novel function of the protein in higher primates. What this potential novel function is, remains to be investigated (Messing and Brenner, 2020; Singh et al., 2003).
On a functional level, the difference between the GFAPα and δ isoforms is best illustrated by the assembly properties of the proteins. Whereas GFAPα can self-assemble into a filamentous network, expression of solely GFAPδ leads to the formation of perinuclear aggregates (Nielsen and Jørgensen, 2004; Perng et al., 2008; Roelofs et al., 2005). Despite the incapacity of GFAPδ to form filaments by itself, the isoform can be incorporated into the IF network by forming heterodimers with GFAPα or vimentin. Nevertheless, only low amounts of GFAPδ are tolerated within the GFAPα/vimentin network and abundance of GFAPδ of more than 10% of all GFAPs leads to a collapse of the entire IF network (Moeton et al., 2016; Nielsen and Jørgensen, 2004). The lack of the highly conserved RGD-motif (KTXEMRDG) in the amino acid sequence of the C-terminus of GFAPδ was hypothesized to play a role in this incapacity to self-assemble into networks, as mutations in this motif in vimentin were associated with aberrant filament assembly (McCormick et al., 1993). Nevertheless, it is unlikely that the lack of filament-forming efficiency can be solely explained by the absence of this region, as the RGD-motif is not essential for homodimer formation of GFAPα (Chen and Liem, 1994). In addition, GFAP lacking the entire tail-domain can still dimerize and does not fully aggregate like GFAPδ. The inhibitory effect of the C-terminus of GFAPδ can likely be explained by its capacity to bind the coiled-coil 2B domain of the GFAP protein, pinpointed to the residues 411 and 412 of the sequence (Nielsen and Jørgensen, 2004). The presence of GFAPδ within the network can also lead to altered exchange dynamics. Fluorescence after photobleaching experiments showed that GFAPδ incorporates and dissociates slower from an IF network than GFAPα, and that collapse of the network slows down both isoforms even more (Moeton et al., 2016).
In addition to distinct assembly properties and dynamics, the difference in the tail-region between the GFAPα and δ isoforms can also lead to altered protein interactions. In a yeast two-hybrid screen it was shown that GFAPδ has decreased affinity for other IFs, like neurofilament light chain (NFL), peripherin, and internexin, but also non-IF proteins, like periplakin and envoplakin both focal adhesion proteins (Nielsen and Jørgensen, 2004). Presenilin, on the other hand, a protein that is part of the gamma-secretase complex involved in the cleavage of amyloid precursor protein and notch, was identified as a protein that specifically binds to GFAPδ, but not GFAPα (Nielsen et al., 2002). In addition to protein-protein interactions, GFAPα and δ also have different predicted kinase binding sites and phosphorylation residues (Boyd et al., 2012). Whether the predicted phosphorylation sites can indeed be phosphorylated and what the functional consequences are for the protein, remains to be investigated.
Clinical Relevance of GFAP and GFAPδ/α and in Diffuse Glioma
Shortly after the discovery of GFAP, the protein was discovered in primary brain tumours, where GFAP was found in gliomas with astrocyte-like characteristics (Uyeda et al., 1972). Since then, GFAP has been widely studied in the context of brain tumours, both to study the relevance of GFAP as a biomarker and to understand its function as a potential therapeutical target. After the discovery of GFAPδ in adult neural stem cells (Roelofs et al., 2005), also GFAP isoform research extended to the field of glioma biology, where neural stem cells are hypothesised to play a major role in the pathogenesis of glioma (Bradshaw et al., 2016). In the next part of the review, we give a short overview of the classification and clinical challenges of diffuse glioma, and if and how GFAP is relevant in the disease.
Classification and Clinicopathology of Diffuse Gliomas
Tumours in the CNS that arise from glial cells in the brain are classified as gliomas. Within this group, diffuse glioma is the most prevalent form in adults. This is characterized by infiltrative growth into the CNS parenchyma (Louis et al., 2016). Classically, diffuse gliomas are subdivided into different subgroups based on histopathological features, namely astrocytomas, oligodendrogliomas, and tumours with mixed astrocytic and oligodendroglial phenotypes. This sub-distinction is made based on the nuclear shape, with uniformly rounded nuclei generally considered to be oligodendrogliomas whereas nuclear irregularities and hyperchromasia point towards astrocytomas (Perry and Wesseling, 2016). In addition, the presence or absence of atypic nuclei, mitotic activity, microvascular proliferation, and necrosis are used to assign the tumour to a malignancy grade II, III, or IV, of which the latter is also referred to as glioblastoma multiforme (GBM) (Louis et al., 2016; Wesseling and Capper, 2018). This histological classification: i.e. astrocytoma, oligodendroglioma (grade II/III), and GBM is still used. However, in the most recent update of the World Health Organization (WHO) classification of CNS tumours, the molecular genetic features of the tumour play a more prominent role (Louis et al., 2021). The main driver mutations used for diffuse glioma classification are point mutations in the isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) genes, and mutations in these genes are associated with a more favourable prognosis (Hartmann et al., 2011; Sanson et al., 2009). In accordance, the majority of diffuse gliomas that present as grade IV at first diagnosis (primary GBM) are IDH wild-type (WT), whereas IDH1/IDH2 mutated grade IV gliomas more frequently develop from lower-grade tumours (secondary GBM) (Louis et al., 2016). In lower grade IDH1/IDH2 mutated gliomas, two additional molecular subgroups can be identified. IDH1/IDH2 mutations in combination with 1p/19q codeletion and TERT promoter mutation coincide with histological features of oligodendrogliomas, whereas IDH1/IDH2 mutations in combination with ATRX mutations and frequent TP53 mutations correspond to astrocytomas.
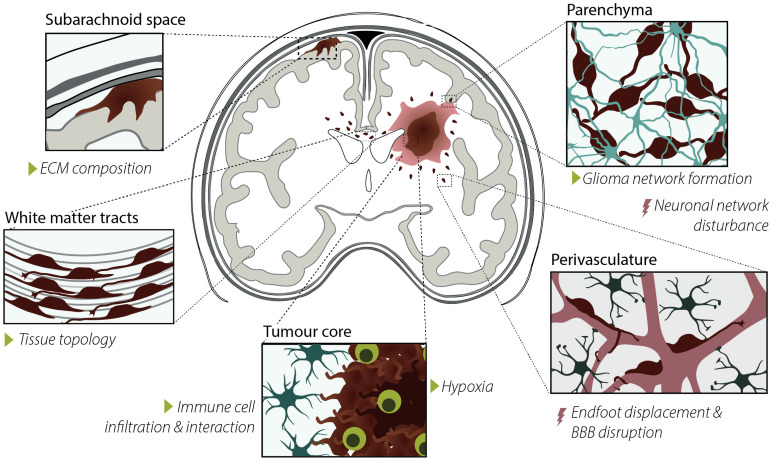
With an average of about 6.0 patients per 100,000 persons, glioma is a relatively rare disease (Ho et al., 2014; Ostrom et al., 2018). Nevertheless, the aggressiveness of the disease, particularly GBM, and the absence of a curative treatment makes glioma a serious health burden. Besides being the most severe form, GBMs are also the most prevalent form of diffuse gliomas, representing 52.9% of the cases (Ostrom et al., 2018). Even after therapy, the median survival of patients is only 14.6 months after initial diagnosis. Although lower-grade gliomas (LGGs) present with slower tumour growth, over time LGGs frequently develop into GBMs also making this tumour type not fully curable (Louis et al., 2016). The incurability of both low- and high-grade gliomas can be largely attributed to two clinical challenges in the treatment of the disease. First, similar to many other cancers, gliomas consist of a heterogeneous population of cells with many different subsets (Patel et al., 2014). Heterogeneity is one of the major challenges in developing novel curative treatments for the disease as heterogeneity can lead to an adaptive response of specific subsets of cells to existing treatment regimes, leading to treatment evasion (Wang et al., 2016). Reinitiation of developmental programs and neural stem cell hierarchies are thought to play an important role in glioma heterogeneity (Couturier et al., 2020; Neftel et al., 2019; Venteicher et al., 2017). A second major challenge in the treatment of gliomas is their infiltrative growth. Glioma cells typically migrate along pre-existing structures in the brain (Figure 4), which leads to therapy evasion and tumour recurrence (Claes et al., 2007). There is a great need for both novel biomarkers to follow tumour progression and recurrence, and novel therapeutical targets to treat the disease. In the next section, we give an overview of the GFAP IF network composition changes during glioma development and progression, and the functional consequences these alterations may have.
Figure 4.
Characteristics, drivers, and consequences of glioma cell invasion. Glioma cell invasion typically occurs along pre-existing structures in the brain, i.e. in the subarachnoid space and along the perivasculature and white-matter tracks. Glioma invasion is influenced by extracellular matrix (ECM) composition, tissue topology, immune cell interaction and infiltration, hypoxia, and glioma network formation, indicated with the green triangles. Consequences of invasion are neuronal network disturbances, astrocyte endfoot displacement, and blood-brain barrier disruption, indicated with the red lightning bolts.
The GFAP IF Network Composition in Diffuse Gliomas
The IF network in glioma cells is similar to the network in non-malignant glia. Gliomas with astrocytic characteristics, like astrocytomas and also GBMs, can express a combination of cytosolic IF proteins that are also found in immature, mature, and reactive astrocytes and neural stem cells. These IF proteins include GFAP, vimentin, nestin, and synemin (Hol and Pekny, 2015). Like the earlier described GFAP, vimentin is a class III IF that is similar in amino acid length and also has the property to self-assemble into networks. Nestin and synemin on the other hand are class IV IF proteins that cannot homodimerize and are characterized by an 8 to 10 times larger C-terminus. Heteropolymerisation of GFAP and vimentin was already discovered with immunoelectron microscopy in glioma cell lines and surgical biopsies over 30 years ago and more recently also nestin was observed to be part of the GFAP/vimentin-positive filaments in astrocytes (Leduc and Manneville, 2017; Paulus and Roggendorf, 1988; Wang et al., 1984). Depletion of either vimentin or GFAP leads to different IF network structures in astrocytes, with GFAP dominant networks being more compact and dense, and vimentin dominant networks more dispersed (Lepekhin et al., 2001; Menet et al., 2001). Although the effects of nestin and synemin and different isoforms of GFAP on the ultrastructure of IF networks have not yet been described in glia- and glioma cells, these findings show that different IF components within the same filament can have distinct contributions to the filament characteristics. In addition to cytosolic IF proteins, gliomas also express class IV IF proteins that make up the nuclear lamina. Of the four different lamins / lamin isoforms, laminB1 is the dominant protein expressed in glioma cells, and lamin A levels are relatively low in comparison to other cell types (Harada et al., 2014). In addition, laminB1 but not laminA depletion affects the organization of the cytosolic IF network in astrocytes (Dupin et al., 2011). Although this review will mainly focus on the cytosolic IF network, this and other findings suggest that the nuclear- and cytosolic IF networks interact and should not be regarded as two entirely separate entities.
The Heterogeneous Nature of GFAP Expression in Glioma
Although GFAP is commonly found in diffuse gliomas, the expression is highly heterogeneous both within and between tumours, as reviewed in van Bodegraven et al. (2019a). Within the tumour there is both regional diversity in GFAP expression, as well as variation in the cellular morphology of the GFAP expressing cells (van Bodegraven et al., 2019a). The last decade, (single cell) transcriptomics have better mapped the molecular diversity of cells within diffuse gliomas and have identified three molecular subtypes of grade IV gliomas: the classical, mesenchymal, and proneural subtypes (Verhaak et al., 2010; Wang et al., 2017), all of which can exist within the same tumour (Patel et al., 2014). Of these catogories, the mesenchymal subtype is associated with a less favorable patient prognosis (Verhaak et al., 2010; Wang et al., 2017), and mesenchymal-like cells are linked to treatment-resistence and invasiveness (Kim et al. 2021). Single-cell transcriptomics reveal that GFAP is mostly enriched in the classical subtype and is least abundantly expressed in the mesenchymal subtype, the latter being more associated with vimentin expression (Neftel et al., 2019; Celiku et al., 2014). The molecular subtypes are, however, highly plastic and transitions between mesenchymal and classical-like states are common (Neftel et al., 2019). The tumour microenvironment is described as an important driver of state transitions, and particularly the presence of immune cells like microglia and infiltrated monocyte-derived macrophages are closely associated with the mesenchymal signature (Kim et al., 2021). Interestingly, it has been reported that GFAP-positive neoplastic cells can express immune cell markers as well (Deininger et al., 2000; Leenstra et al., 1995; Takeuchi et al., 2008), leading to the hypothesis that cell fusions between neoplastic cells and macrophages/microglia can give rise to an aggressive and invasive population of glioma cells (Huysentruyt et al., 2011). These studies highlight the heterogeneous and dynamic nature of GFAP-expression in glioma and emphasize the need to better understand the regulation of the gene in the context of the disease.
Expression of GFAP in Relation to Diffuse Glioma Clinical Outcome
The IF network organization and general expression patterns of GFAP and other IF proteins have been studied in relation to glioma malignancy and overall survival, as reviewed in (van Bodegraven et al., 2019a). Based on semiquantitative immunohistochemical evaluation of vimentin, nestin, GFAP, and synemin expression in glioma tissue, Skalli and colleagues identified three subtypes of glioma: one group with high expression levels of all four IFs, one with low expression levels of all four IFs, and one group with strong nestin expression but low vimentin, GFAP, and synemin (Skalli et al., 2013). No correlation between staining patterns and survival was found in this study, and patient numbers were limited. The expression of GFAP is often regarded as a differentiation marker and is therefore associated with a less malignant tumour. Nevertheless, in a recent systematic review on GFAP expression levels in different malignancy grades, we show that GFAP is not consistently associated with more differentiated tumours (van Bodegraven et al., 2019a). Also, two recent studies that investigated the prognostic impact of GFAP immunopositivity in relation to GBM patient survival reported contradictory results. Ahmadipour and collegues found a significant correlation between high levels of GFAP and decreased long-term survival, independent of IDH1 mutation and MGMT methylation status (Ahmadipour et al., 2020). This is in contrast with the findings of Sommerlath and collegues, where GFAP-positivity was a positive predictor of long-term survival, particularly in patients with MGMT promotor hypermethylation (Sommerlath et al., 2022). The presence of GFAP in serum is, however, more consistently associated with malignancy grade, as serum GFAP is significantly elevated in grade IV patients, but not in lower grade gliomas or controls (van Asperen et al., 2022; van Bodegraven et al., 2019a). Together these studies highlight that GFAP protein levels are frequently altered in gliomas, but heterogeneity between patients and inconsistent findings between studies makes the use of pan GFAP levels as a biomarker uncertain.
GFAP Isoform Expression in Diffuse Glioma
In addition to general GFAP levels, multiple studies, including research performed in our group, have looked into the expression patterns of GFAP isoforms in tissue sections of gliomas (van Bodegraven et al., 2019b). In healthy adults, GFAPδ expression is limited to a subgroup of astrocytes located in the subpial zone of the cerebral cortex, the subgranular zone of the hippocampus, the subventricular zone, the rostral migratory stream, and the olfactory bulb (Roelofs et al., 2005; van den Berge et al., 2010). In accordance, whereas GFAPδ positive cells are absent in control autopsy brains in sections of cortical areas, GFAPδ positive cells have been reported in glioma tissue sections of different grades in the brain (Andreiuolo et al., 2009; Choi et al., 2009), and in the spinal cord gliomas (Heo et al., 2012). In contrast to general GFAP levels, multiple studies report an increase in GFAPδ levels in high- versus low-grade tumours (Andreiuolo et al., 2009; Brehar et al., 2015; Choi et al., 2009; van Bodegraven et al., 2019a). Brehar and colleagues compared the GFAPδ and nestin immunopositive staining of tissue biopsies to the macroscopic invasive properties of the tumour based on neuroimaging. They reported that neuroimaging features of highly invasive tumours correlate to a higher percentage of GFAPδ and nestin-positive cells in the corresponding tumour biopsy (Brehar et al., 2015). Since the biopsies were taken from the tumour core and areas of the tumour with the highest IF staining were selected, it remains to be investigated whether different regions of the tumour, like the invasive front, show the same increase in GFAPδ- and nestin-expressing cells. In addition to immunohistochemical analysis of gliomas, GFAP isoform expression patterns have also been investigated at the RNA level. Analysis of GFAP isoform expression in bulk RNA sequencing data of 528 high grade- and 516 low-grade gliomas, derived from the Cancer Genome Atlas (TCGA), showed that canonical isoform GFAPα is significantly lower in grade IV versus grade II and III tumours. GFAPδ on the other hand remains constant over the different malignancy grades. This results in a shift in ratios, with high GFAPδ/α ratios in high-grade tumours and a lower GFAPδ/α ratio in lower grade tumours (Stassen et al., 2017).
Although multiple research groups report changes in GFAPδ/α ratios in the different malignancy grades of glioma, it is important to note the high heterogeneity when it comes to GFAPδ expression. Andreiuoli and colleagues reported a large variation in GFAPδ staining levels, with strongly positive and negative foci in tumour tissue of GBM patients (Andreiuolo et al., 2009). Despite the high heterogeneity, some basal observations on the characteristics of the GFAPδ and GFAPα/pan positive cells were reported. First of all, Andreiuoli and colleagues described a high extend of overlap between GFAPδ and vimentin immunostaining in grade II and grade IV, although this observation has not been systematically quantified (Andreiuolo et al., 2009). Also, observations on morphological differences have been described. Choi and colleagues found a correlation between GFAPδ levels and the number of primary processes of the cell. In this study, increased levels of GFAPδ was associated with fewer primary processes and rounder cell morphology (Choi et al., 2009).The morphological analysis of GFAPα and GFAPδ expressing cells is performed in tissue biopsies that are frequently taken from areas of the tumour with high malignant cell densities. Whether similar morphological characteristics are found in cells that have invaded the brain parenchyma remains to be investigated. At last, although this review mainly focuses on adult diffuse gliomas, also in pediatric diffuse intrinsic pontine glioma (DIPG) GFAPδ-positive cells with distinctive morphologies are found, although no morphological characterisation has been performed in this context (Caretti et al., 2014). Based on these observations it can be hypothesised that the GFAPδ-positive cells in adult and diffuse gliomas form a distinct population of cells in the tumour. Given the restriction of GFAPδ-expression to adult neural stem cells in the human brain, GFAPδ-positive cells may represent a glioma stem cells population, although with current knowledge this is highly speculative and this hypothesis needs to be further investigated with additional characterization of the GFAPδ-positive population. Also how this population arises is currently not known. Whether alternative splicing in glioma is triggered by cell-intrinsic factors (e.g. as a downstream effect of mutated genes, or by reinitiation of developmental programs) or by extrinsic triggers (e.g. immune cell infiltration, hypoxia, or changes in tissue mechanics) remains to be experimentally tested in future research.
The Functional Role GFAP and GFAP Isoforms in Glioma Cell Behaviour
GFAP and GFAP isoforms have not only been studied as potential (bio)markers but their function has also been investigated. GFAP plays a role in a broad range of cellular processes, including but not limited to vesicle trafficking, localisation of organelles, and autophagy (Middeldorp and Hol, 2011). In the next paragraph, we will discuss some of the functions that have been discovered for GFAP in cell biological domains that are relevant to glioma malignancy, with a focus on GFAP isoforms where possible (Figure 5).
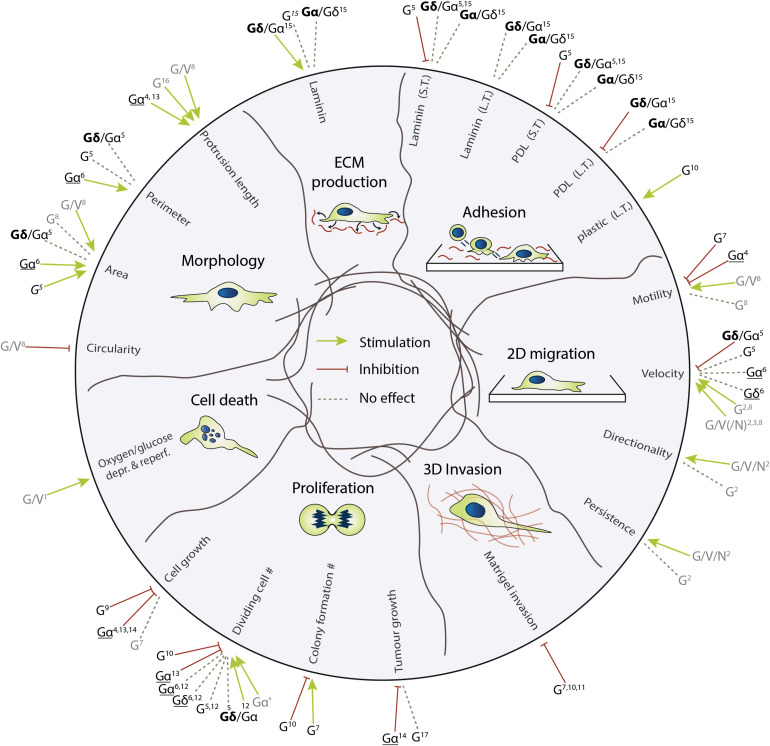
Figure 5.
The effect of modifications of the GFAP IF network on different biological domains relevant for glioma biology. The studies based on overexpression-expression are underlined, the other studies are based on endogenous expression levels, knock-down, or knock-out experiments. All studies in grey are based on experiments with (primary) astrocytes, the studies in black on studies with glioma cell lines. G = GFAP, Gδ/α = High GFAPδ/α-ratio cells, Gα/δ = Low GFAPδ/α-ratio cells, G/V/N = GFAP, vimentin, nestin, L.T. = long-term, S.T. = short-term (1) de Pablo et al. (2013); (2) de Pascalis et al. (2018); (3) Dupin et al. (2011); (4) Elobeid et al. (2000); (5) Moeton et al. (2014); (6) Moeton et al. (2016); (7) Murphy et al. (1998); (8) Lepekhin et al. (2001); (9) Pekny et al. (1998); (10) Rutka et al. (1994); (11) Rutka et al. (1998); (12) Stassen et al. (2017); (13) Toda et al. (1994); (14) Toda et al. (1999); (15) van Bodegraven et al. (2019b); (16) Weinstein et al. (1991); (17) Wilhelmsson et al. (2003).
Glioma Tumorigenesis, Cell Proliferation and Growth
Uncontrolled cell division is a hallmark of cancer. Several studies have investigated the link between manipulation of GFAP expression and tumorigenesis, cell proliferation, and cell cycle progression, however, the findings are contradictory. An early study investigated the effect of the presence of GFAP on spontaneous astrocytoma development in p53-negative mice exposed to carcinogen ethyl nitrosourea. When comparing GFAP wild-type mice to GFAP-null mice, tumour incidence, histological characteristics, and tumour progression were similar (Wilhelmsson et al., 2003). This is in contrast to a study performed by Toda and colleagues, where C6 rat glioma cells transfected with murine GFAP formed smaller tumours in athymic mice (Toda et al., 1999). Also, studies using in vitro models to investigate the effect of GFAP on cell proliferation are inconclusive. Most studies associate high levels of GFAP with decreased cell proliferation (Elobeid et al., 2000; Rutka et al., 1994; Toda et al., 1999) or find no effect (Murphy et al., 1998; Weinstein et al., 1991). However, one study described that a subpopulation of glioma cells with high GFAP expression generated more and larger colonies in a soft-agar colony formation assay in comparison to the GFAP low population (Murphy et al., 1998). Previous studies from our lab have investigated the effect of the GFAP isoforms on proliferation, but also here findings are somewhat contradictory. Whereas overexpression of GFAPα and GFAPδ does not affect proliferation based on BrdU incorporation, MTT assays, and PHP3 positivity (Moeton et al., 2016; Stassen et al., 2017), a higher fraction of BrdU positive cells was described in GFAPα versus GFAPpan depleted cells (Stassen et al., 2017). However, in these knockdown (KD) cells, proliferation was not affected based on Ki67 positivity or the number of cells in the G2/M/S phase of the cell cycle (Moeton et al., 2014).
GFAP has also been studied in the context of tumour suppressor gene regulation. The head domain of GFAP, and also vimentin, can bind to tumour suppressor protein menin, and colocalizes with menin during the S and early G2 phase of the cell cycle (Lopez-Egido et al., 2002). The authors suggest that the GFAP IF network is involved in sequestering menin during the S-G2 phase of the cell cycle, thereby regulating the activity of menin (Lopez-Egido et al., 2002). Whether these are interactions are involved in glioma malignancy, remains to be investigated.
Cellular Stress and Survival
GFAP and other IF proteins are frequently linked to the cellular response to stress, and regulators of apoptosis and cell death. GFAP interacts with Heat Shock Protein 27 (HSP27) and protein chaperone αB-crystallin (CRYAB), and the interaction of these proteins with GFAP prevents aggregation (Perng et al., 2008). GFAP is also identified as an interactor of c-Jun N-terminal Kinases (JNK) JNK1α1 and JNK3α1 in a yeast two-hybrid screen, linking GFAP to a major signalling pathway of apoptosis (Chen et al., 2014). In addition to regulatory pathways of apoptosis, another yeast two-hybrid screen linked GFAP to DNA Fragmentation Factor 45 (DFF45), a protein involved in the execution of apoptosis. The binding of GFAP to DFF45 prevents cleavage of the DFF45 protein by caspase-3, therewith preventing nuclease activity of the catalytic subunit DFF40 (Hanus et al., 2010). Many IF proteins, including GFAP and vimentin, also contain caspase 3 and 6 cleavage sites, showing that IFs are also targets of the apoptotic pathway (Byun et al., 2001; Messing and Brenner, 2020). The role of these interactions between IF proteins and stress- and cell survival-related pathways remain to be further investigated.
Although the effect of GFAP on cell survival has not been investigated in detail in glioma, in astrocytes GFAP was found to protect against cell death in response to cellular stress. In astrocytes challenged with oxygen-glucose deprivation, cell death was observed more frequently in GFAP/vimentin-null mice in comparison to astrocytes where the IF network remained intact (de Pablo et al., 2013). High-grade gliomas are frequently associated with hypoxia (Gérard et al., 2019; Jensen et al., 2014), therefore whether GFAP has a protective role in hypoxia-induced cell death in glioma is an interesting topic for further investigation.
Cell Motility, Migration, and Invasion
Several studies have investigated the role of GFAP in glial and glioma cell motility and migration. However, the effect of GFAP on motility and migratory behaviour is not straightforward. Early studies linked downregulation of GFAP with antisense oligonucleotides to increased velocity and motility of cell migration, suggesting an inhibitory effect of GFAP on migration (Murphy et al., 1998; Rutka et al., 1994, 1998). Also in Matrigel invasion studies, low levels of GFAP or depletion of GFAP in glioma cells led to higher invasiveness into Matrigel (Murphy et al., 1998; Rutka et al., 1994, 1998, 1999). However, in astrocytes isolated from mice lacking GFAP and vimentin, depletion of GFAP or vimentin alone led to a small defect in cell migration, but when both IFs were targeted a larger reduction in cell migration was observed (Lepekhin et al., 2001). Similar observations were made in primary astrocytes treated with siRNAs targeting GFAP, nestin, and/or vimentin, where the lack of all three IFs together leads to a stronger decrease in velocity, directionality, and persistence in comparison to depletion of individual IFs (de Pascalis et al., 2018; Dupin et al., 2011). When only GFAP was targeted in these cells, a reduction in velocity was observed, although no changes were seen in the directionality and persistence of the migration (de Pascalis et al., 2018). The role of GFAP isoforms has also been studied in the context of migration and motility. In glioma cells, shRNAs specifically targeting GFAPα reduced motility of glioma cells, whereas targeting all GFAP isoforms did not affect this (Moeton et al., 2014). Overexpression of GFAPα or GFAPδ in these cells on the other hand does not affect cell motility and migration (Moeton et al., 2016).
The cell migration machinery is a complex system that depends on many dynamic processes in the cell. In several of these processes, the IF network has been proven the play a role. One of the first steps in directional migration is cell polarization (Petrie et al., 2009). In migrating astrocytes, cell polarization is characterised by translocation of the nucleus to the rear of the cell, termed nucleus off-centring. Loss of GFAP, nestin, and vimentin in primary astrocytes leads to aberrant nucleus off-centring during cell polarization (Dupin et al., 2011). In contrast to nucleus off-centring, centrosome positioning during cell polarization is not dependent on the presence of GFAP but is regulated by nestin (Dupin et al., 2011). After the polarization of the cells, the next step in the process of glioma cell migration is the formation of focal adhesions through integrin-extracellular matrix (ECM) binding. The role of vimentin in the regulation of integrin- and focal adhesion dynamics is well established (Ivaska et al., 2007; Leube et al., 2015), and over the years the role of GFAP is becoming more apparent as well. The size of focal adhesions is affected by GFAP isoform expression, as both overexpression of GFAPα and GFAPδ leads to a smaller focal adhesion size (Moeton et al., 2016). GFAP can interact with focal adhesions through binding to vinculin and talin (de Pascalis et al., 2018), but whether this interaction directly affects focal adhesion size remains to be investigated. After the formation of adhesions, forces can be loaded on the integrin-ECM complexes by actomyosin contractility, facilitating cellular movement (Case and Waterman, 2015). Knockdown of vimentin, nestin, and GFAP in migrating astrocytes leads to stronger traction forces and a loss of force distribution in leader versus follower cells in collectively migrating astrocytes (de Pascalis et al., 2018). Also, cell-cell interactions are altered under these conditions, as the flow of N-cadherins at cell-cell adherent junctions is diminished. Interestingly, the morphology of the adherent junctions is also affected when GFAP is targeted alone, indicating a direct involvement of GFAP in the regulation of adherent junctions (de Pascalis et al., 2018).
In addition to the intracellular dynamics, glioma cell invasion into the brain parenchyma is also dependent on dynamic interactions with the surrounding cells and extracellular matrix. During tissue invasion, cells encounter ECM matrices with different topologies, ligand densities and distributions, all of which can affect the migration mode and strategy of a cell (Charras and Sahai, 2014). The function of GFAP in glioma appears to be strongly ECM-context dependent, as the outcome regarding adhesive- and migratory properties in response to depletion of the GFAP isoforms is dependent on the substrate (Moeton et al., 2014; van Bodegraven et al., 2019b). GFAP-isoforms also differentially regulate ECM production and remodelling. An increase in the GFAPδ/α-ratio leads to increased laminin-deposition of cells, and to altered gene expression of metalloproteinase 2 and A2M, genes that are involved in ECM remodelling (van Bodegraven et al., 2019b). The altered cell-ECM interaction in cells with a high GFAPδ/α-ratio is regulated by dual-specificity phosphatase 4, a gene that negatively correlates to glioma patient survival (Stassen et al., 2017; van Bodegraven et al., 2019b). In GFAPδ/α-high cells, DUSP4 is specifically localised to the focal adhesions of the cells, where it regulates laminin signalling (van Bodegraven et al., 2019b). These findings suggest that the GFAPδ/α-ratio particularly affects cellular processes that are relevant for cell migration within a three-dimensional context, potentially explaining the discrepancies in effect on cell migration on flat surfaces. Indeed, we recently have shown that GFAPδ/α-ratio high and low cells show different migratory behaviours when implanted in ex vivo organotypic brain slice cultures or in mice with a cranial imaging window (Uceda-Castro et al., 2022). GFAPδ/α-high cells show diffuse, directionally persistent invasion into the brain parenchyma, whereas GFAPδ/α-low cells migrate in a random-fashion. This shows that to fully understand the role of GFAP and GFAP isoforms in glioma cell invasion, it is important to investigate the protein within a cellular context that recapitulates the environmental conditions that glioma cells normally encounter.
Future Directions
In this review, we described the role of GFAP and its isoforms in glioma cell behaviour. The fully understand the function of GFAP and its isoforms on glial cell function we provide some directions for further research.
GFAP is a highly spliced gene and multiple splice variants have been identified in the mouse and human brain. Several subtypes of astrocytes express different isoforms, but the full spectrum of GFAP splice isoforms is still unknown. In the near future, targeted long-read RNAseq and single-cell RNA seq approaches will reveal which cell types express which GFAP variants. Importantly, the expression of the isoforms also needs to be confirmed at the protein level. This requires the development of a range of isoform-specific antibodies and analysis of the isoforms with proteomics.
The research on GFAP isoforms in glioma has been focused on GFAPα and GFAPδ. We have shown that a change in the ratio between GFAPα and GFAPδ changes cell-environment interactions. Thus, it is important to better understand the function of GFAP and its splice isoforms. This requires (1) novel mouse and cell models with targeted and timed deletion of specific GFAP isoforms and (2) tools and drugs to interfere with the GFAP IF network.
GFAP is a highly regulated gene and its expression is upregulated in several neurological diseases, mutations in GFAP are the cause of Alexander disease, a white matter disorder, and GFAP isoforms play a role in glioma malignancy. Thus understanding the regulation of GFAP gene expression and GFAP alternative splicing is important to understand how this protein is involved in these disorders. Targeting GFAP can be attractive to selectively and specifically interfere with the glial cell function in disease.
GFAP is part of the cytosolic IF network in cells, that consists of different IF proteins. Understanding the interactions of GFAP with other proteins in the IF network and the interaction of the IF network with other cytoskeletal systems (actin and microtubules) is essential to fully understand the cell biological and biomechanical function of GFAP and its isoforms.
All in all, fifty years after the first description of GFAP and due to the discoveries concerning isoforms and protein function, GFAP is still an exciting protein to study for a multidisciplinary group of researchers. Novel technologies in the field of genomics, biophysics, chemistry, molecular biology, cell biology, and neuroscience now make it possible to unravel the full function of this intriguing protein.
Abbreviations
- CRYAB
αB-crystallin
- DIPG
diffuse intrinsic pontine glioma
- DFF40/45
DNA Fragmentation Factor 40/45
- ECM
extracellular matrix
- GBM
glioblastoma multiforme
- GFAP
glial fibrillary acidic protein
- HSP27
Heat Shock Protein 27
- IDH1/2
isocitrate dehydrogenase 1 and 2
- IF
intermediate filament
- JNK
c-Jun N-terminal Kinases
- LGG
lower-grade glioma
- miRNA
microRNAs
- NFL
neurofilament light chain
- TCGA
the cancer genome atlas
- ULF
unit-length fragment
- WHO
World health organization
- WT
wild-type
Footnotes
Author Contributions: J.v.A. and E.H. wrote the manuscript. J.v.A., E.H., and P.R. edited and reviewed the manuscript. E.H. and P.R provided funding.
J.v.A and E.H were supported by the Dutch Cancer Society [KWF 101123] and P.R. by the T and P Bohnenn Foundation
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the KWF Kankerbestrijding, T and P Bohnenn Foundation, (grant number KWF 101123).
ORCID iD: Elly M. Hol https://orcid.org/0000-0001-5604-2603
References
- Ahmadipour Y., Gembruch O., Pierscianek D., Sure U., Jabbarli R. (2020). Does the expression of glial fibrillary acid protein (GFAP) stain in glioblastoma tissue have a prognostic impact on survival? Neurochirurgie, 66(3), 150–154. 10.1016/j.neuchi.2019.12.012 [DOI] [PubMed] [Google Scholar]
- Andreiuolo F., Junier M. P., Hol E. M., Miquel C., Chimelli L., Leonard N., Chneiweiss H., Daumas-Duport C., Varlet P. (2009). GFAPδ immunostaining improves visualization of normal and pathologic astrocytic heterogeneity. Neuropathology, 29(1), 31–39. 10.1111/j.1440-1789.2008.00936.x [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. (1974). Astrocyte-specific protein and neuroglial differentiation. An immunofluorescence study with antibodies to the glial fibrillary acidic protein. The Journal of Comparative Neurology, 153(1), 27–38. 10.1002/cne.901530104 [DOI] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. (1972). Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain research, 43(2), 429–435. [DOI] [PubMed] [Google Scholar]
- Blechingberg J., Holm I. E., Nielsen K. B., Jensen T. H., Jørgensen A. L., Nielsen A. L. (2007). Identification and characterization of GFAPκ, a novel glial fibrillary acidic protein isoform. Glia, 55(5), 497–507. 10.1002/glia.20475 [DOI] [PubMed] [Google Scholar]
- Blechingberg J., Lykke-andersen S., Jensen T. H., Jørgensen A. L., Nielsen A. L. (2007). Regulatory mechanisms for 3′-end alternative splicing and polyadenylation of the Glial Fibrillary Acidic Protein, GFAP, transcript. Nucleic Acids Research, 35(22), 7636–7650. 10.1093/nar/gkm931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer K., Middeldorp J., Spliet W. G. M., Razavi F., van Rijen P. C., Baayen J. C., Hol E. M., Aronica E. (2010). Immunohistochemical characterization of the out-of frame splice variants GFAP Delta164/Deltaexon 6 in focal lesions associated with chronic epilepsy. Epilepsy research, 90(1–2), 99–109. 10.1016/j.eplepsyres.2010.03.014 [DOI] [PubMed] [Google Scholar]
- Boyd S. E., Nair B., Ng S. W., Keith J. M., Orian J. M. (2012). Computational characterization of 3′ splice variants in the GFAP isoform family. PLoS ONE, 7(3), 36–41. 10.1371/journal.pone.0033565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw A., Wickremsekera A., Tan S. T., Peng L., Davis P. F., Itinteang T. (2016). Cancer Stem Cell Hierarchy in Glioblastoma Multiforme. Frontiers in Surgery, 3(April), 1–15. 10.3389/fsurg.2016.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehar F. M., Arsene D., Brinduse L. A., Gorgan M. R. (2015). Immunohistochemical analysis of GFAP-?? And nestin in cerebral astrocytomas. Brain Tumor Pathology, 32(2), 90–98. 10.1007/s10014-014-0199-8 [DOI] [PubMed] [Google Scholar]
- Brenner M., Lampel K., Nakatani Y., Mill J., Banner C., Mearow K., Dohadwala M., Lipsky R., Freese E. (1990). Characterization of human cDNA and genomic clones for glial fibrillary acidic protein. Brain Research. Molecular Brain Research, 7(4), 277–286. [DOI] [PubMed] [Google Scholar]
- Brenner M., Messing A. (2021). Regulation of GFAP Expression. In ASN Neuro (Vol. 13). https://doi.org/10.1177/1759091420981206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugiani M., Boor I., van Kollenburg B., Postma N., Polder E., van Berkel C., van Kesteren R. E., Windrem M. S., Hol E. M., Scheper G. C., Goldman S. A., van der Knaap M. S. (2011). Defective glial maturation in vanishing white matter disease. Journal of neuropathology and experimental neurology, 70(1), 69–82. 10.1097/NEN.0b013e318203ae74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun Y., Chen F., Chang R., Trivedi M., Green K. J., Cryns V. L. (2001). Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death and Differentiation, 8(5), 443–450. 10.1038/sj.cdd.4400840 [DOI] [PubMed] [Google Scholar]
- Caretti V., Bugiani M., Freret M., Schellen P., Jansen M., van Vuurden D., Kaspers G., Fisher P. G., Hulleman E., Wesseling P., Vogel H., Monje M. (2014). Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathologica, 128(4), 605–607. 10.1007/s00401-014-1307-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case L. B., Waterman C. M. (2015). Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nature Cell Biology, 17(8), 955–963. 10.1038/ncb3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celiku O., Johnson S., Zhao S., Camphausen K., Shankavaram U. (2014). Visualizing molecular profiles of glioblastoma with GBM-BioDP. PloS One, 9(7), e101239. 10.1371/journal.pone.0101239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras G., Sahai E. (2014). Physical influences of the extracellular environment on cell migration. Nature Reviews Molecular Cell Biology, 15(12), 813–824. 10.1038/nrm3897 [DOI] [PubMed] [Google Scholar]
- Chen W. J., Liem R. K. H. (1994). The endless story of the glial fibrillary acidic protein. Journal of Cell Science, 107(8), 2299–2311. 10.1242/jcs.107.8.2299 [DOI] [PubMed] [Google Scholar]
- Chen W. K., Yeap Y. Y. C., Bogoyevitch M. A. (2014). The JNK1/JNK3 interactome—Contributions by the JNK3 unique N-terminus and JNK common docking site residues. Biochemical and Biophysical Research Communications, 453(3), 576–581. 10.1016/j.bbrc.2014.09.122 [DOI] [PubMed] [Google Scholar]
- Choi K.-C., Kwak S.-E., Kim J.-E., Sheen S. H., Kang T.-C. (2009). Enhanced glial fibrillary acidic protein-δ expression in human astrocytic tumor. Neuroscience Letters, 463(3), 182–187. 10.1016/j.neulet.2009.07.076 [DOI] [PubMed] [Google Scholar]
- Claes A., Idema A. J., Wesseling P. (2007). Diffuse glioma growth: A guerilla war. Acta Neuropathologica, 114(5), 443–458. 10.1007/s00401-007-0293-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairembault T., Kamphuis W., Leclair-Visonneau L., Rolli-Derkinderen M., Coron E., Neunlist M., Hol E. M., Derkinderen P. (2014). Enteric GFAP expression and phosphorylation in Parkinson’s disease. Journal of Neurochemistry, 130(6), 805–815. 10.1111/jnc.12742 [DOI] [PubMed] [Google Scholar]
- Condorelli D. F., Nicoletti V. G., Dell'Barresi V., Conticello S. G., Caruso A., Tendi E. A., Giuffrida Stella A. M. (1999). Structural features of the rat GFAP gene and identification of a novel alternative transcript. Journal of Neuroscience Research, 56(3), 219–228. [DOI] [PubMed] [Google Scholar]
- Condorelli D. F., Nicoletti V. G., Dell'Albani P., Barresi V., Caruso A., Conticello S. G., Belluardo N., Stella A. M. G. (1999). GFAPbeta mRNA expression in the normal rat brain and after neuronal injury. Neurochemical research, 24(5), 709–714. [DOI] [PubMed] [Google Scholar]
- Couturier C. P., Ayyadhury S., Le P. U., Nadaf J., Monlong J., Riva G., Allache R., Baig S., Yan X., Bourgey M., Lee C., Wang Y. C. D., Wee Yong V., Guiot M. C., Najafabadi H., Misic B., Antel J., Bourque G., Ragoussis J., Petrecca K. (2020). Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nature Communications, 11(1), 1–19. 10.1038/s41467-020-17186-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pablo Y., Nilsson M., Pekna M., Pekny M. (2013). Intermediate filaments are important for astrocyte response to oxidative stress induced by oxygen-glucose deprivation and reperfusion. Histochemistry and Cell Biology, 140(1), 81–91. 10.1007/s00418-013-1110-0 [DOI] [PubMed] [Google Scholar]
- De Pascalis C., Pérez-González C., Seetharaman S., Boëda B., Vianay B., Burute M., Leduc C., Borghi N., Trepat X., Etienne-Manneville S. (2018). Intermediate filaments control collective migration by restricting traction forces and sustaining cell–cell contacts. The Journal of Cell Biology, 217(9), 3031–3044. 10.1083/jcb.201801162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger M. H., Seid K., Engel S., Meyermann R., Schluesener H. J. (2000). Allograft inflammatory factor-1 defines a distinct subset of infiltrating macrophages/microglial cells in rat and human gliomas. Acta Neuropathologica, 100(6), 673–680. 10.1007/s004010000233 [DOI] [PubMed] [Google Scholar]
- Dupin I., Sakamoto Y., Etienne-Manneville S. (2011). Cytoplasmic intermediate filaments mediate actin-driven positioning of the nucleus. Journal of cell science, 124(Pt 6), 865–872. 10.1242/jcs.076356 [DOI] [PubMed] [Google Scholar]
- Elobeid A., Bongcam-Rudloff E., Westermark B., Nistér M. (2000). Effects of inducible glial fibrillary acidic protein on glioma cell motility and proliferation. Journal of Neuroscience Research, 60(2), 245–256. 10.1002/(SICI)1097-4547(20000415)60:2<245::AID-JNR14>3.0.CO;2-1 [DOI] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. (1971). An acidic protein isolated from fibrous astrocytes. Brain Research, 28(2), 351–354. 10.1016/0006-8993(71)90668-8 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. (2018). Cytoplasmic Intermediate Filaments in Cell Biology. Annual review of cell and developmental biology, 34, 1–28. 10.1146/annurev-cellbio-100617-062534 [DOI] [PubMed] [Google Scholar]
- Feinstein D. L., Weinmaster G. A., Milner R. J. (1992). Isolation of cDNA clones encoding rat glial fibrillary acidic protein: Expression in astrocytes and in Schwann cells. Journal of Neuroscience Research, 32(1), 1–14. 10.1002/jnr.490320102 [DOI] [PubMed] [Google Scholar]
- Flint D., Li R., Webster L. S., Naidu S., Kolodny E., Percy A., van der Knaap M., Powers J. M., Mantovani J. F., Ekstein J., Goldman J. E., Messing A., Brenner M. (2012). Splice site, frameshift, and chimeric GFAP mutations in Alexander disease. Human Mutation, 33(7), 1141–1148. 10.1002/humu.22094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea E., Dupouey P., Feinstein D. L. (1995). Glial fibrillary acidic protein mRNA isotypes: Expression in vitro and in vivo. Journal of Neuroscience Research, 41(4), 452–461. 10.1002/jnr.490410404 [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. (1983). Amino acid sequence data on glial fibrillary acidic protein (GFA); implications for the subdivision of intermediate filaments into epithelial and non-epithelial members. The EMBO journal, 2(11), 2059–2063. 10.1002/j.1460-2075.1983.tb01700.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard M., Corroyer-Dulmont A., Lesueur P., Collet S., Chérel M., Bourgeois M., Stefan D., Limkin E. J., Perrio C., Guillamo J.-S., Dubray B., Bernaudin M., Thariat J., Valable S. (2019). Hypoxia Imaging and Adaptive Radiotherapy: A State-of-the-Art Approach in the Management of Glioma. Frontiers in Medicine, 6, 117 10.3389/fmed.2019.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzenko D., Chernyatina A. A., Strelkov S. V. (2017). Crystallographic studies of intermediate filament proteins. Sub-Cellular Biochemistry, 82(January), 151–170. 10.1007/978-3-319-49674-0_6 [DOI] [PubMed] [Google Scholar]
- Hanus J., Kalinowska-Herok M., Widlak P. (2010). Identification of novel putative regulators of the major apoptotic nuclease DNA fragmentation factor. Acta Biochimica Polonica, 57(4), 521–527. 10.18388/abp.2010_2438 [DOI] [PubMed] [Google Scholar]
- Harada T., Swift J., Irianto J., Shin J., Spinler K. R., Athirasala A., Diegmiller R., Dingal P. C. D. P., Ivanovska I. L., Discher D. E. (2014). Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. Journal of Cell Biology, 204(5), 669–682. 10.1083/jcb.201308029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C., Hentschel B., Tatagiba M., Schramm J., Schnell O., Seidel C., Stein R., Reifenberger G., Pietsch T., von Deimling A., Loeffler M., Weller M., & German Glioma Network. (2011). Molecular markers in low-grade gliomas: Predictive or prognostic? Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 17(13), 4588–4599. 10.1158/1078-0432.CCR-10-3194 [DOI] [PubMed] [Google Scholar]
- Helman G., Takanohashi A., Hagemann T. L., Perng M. D., Walkiewicz M., Woidill S., Sase S., Cross Z., Du Y., Zhao L., Waldman A., Haake B. C., Fatemi A., Brenner M., Sherbini O., Messing A., Vanderver A., Simons C. (2020). Type II Alexander disease caused by splicing errors and aberrant overexpression of an uncharacterized GFAP isoform. Human Mutation, 41(6), 1131–1137. 10.1002/humu.24008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo D. H., Kim S. H., Yang K.-M., Cho Y. J., Kim K. N., Yoon D. H., Kang T.-C. (2012). A histopathological diagnostic marker for human spinal astrocytoma: Expression of glial fibrillary acidic protein-δ. Journal of Neuro-Oncology, 108(1), 45–52. 10.1007/s11060-012-0801-z [DOI] [PubMed] [Google Scholar]
- Herrmann H., Aebi U. (2016). Intermediate filaments: Structure and assembly. Cold Spring Harbor Perspectives in Biology, 8(11), a018242. 10.1101/cshperspect.a018242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M., Magin T. M., Weber K. (2001). Genes for intermediate filament proteins and the draft sequence of the human genome: Novel keratin genes and a suprisingly high number of pseudogenes related to keratin genes 8 and 18. Journal of Cell Science, 114(14), 2569–2575. [DOI] [PubMed] [Google Scholar]
- Ho V. K. Y., Reijneveld J. C., Enting R. H., Bienfait H. P., Robe P., Baumert B. G., Visser O. (2014). Changing incidence and improved survival of gliomas. European Journal of Cancer, 50(13), 2309–2318. 10.1016/j.ejca.2014.05.019 [DOI] [PubMed] [Google Scholar]
- Hol E. M., Capetanaki Y. (2017). Type III Intermediate Filaments Desmin, Glial Fibrillary Acidic Protein (GFAP), Vimentin, and Peripherin. Cold Spring Harbor Perspectives in Biology, 9(12), a021642. 10.1101/cshperspect.a021642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol E. M., Pekny M. (2015). Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Current Opinion in Cell Biology, 32, 121–130. 10.1016/j.ceb.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Huebner K., Croce C. M., Betsholtz C., Wang J. L., Westermark B. (1991). Human Glial Fibrillary Acidic Protein: Complementary DNA Cloning, Chromosome Localization, and Messenger RNA Expression in Human Glioma Cell Lines of Various Phenotypes. Cancer Research, 51(5), 1553–1560. [PubMed] [Google Scholar]
- Huysentruyt L. C., Akgoc Z., Seyfried T. N. (2011). Hypothesis: Are neoplastic macrophages/microglia present in glioblastoma multiforme? ASN Neuro, 3(4), e00064. 10.1042/AN20110011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J., Pallari H. M., Nevo J., Eriksson J. E. (2007). Novel functions of vimentin in cell adhesion, migration, and signaling. Experimental Cell Research, 313(10), 2050–2062. 10.1016/j.yexcr.2007.03.040 [DOI] [PubMed] [Google Scholar]
- Jensen R. L., Mumert M. L., Gillespie D. L., Kinney A. Y., Schabel M. C., Salzman K. L. (2014). Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro-Oncology, 16(2), 280–291. 10.1093/neuonc/not148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Fu Z., Yang J., Troncosco J., Everett A. D., Van Eyk J. E. (2013). Identification and characterization of citrulline-modified brain proteins by combining HCD and CID fragmentation. Proteomics, 13(17), 2682–2691. 10.1002/pmic.201300064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis W., Mamber C., Moeton M., Kooijman L., Sluijs J. A., Jansen A. H. P., Verveer M., de Groot L. R., Smith V. D., Rangarajan S., Rodríguez J. J., Orre M., Hol E. M. (2012). GFAP Isoforms in Adult Mouse Brain with a Focus on Neurogenic Astrocytes and Reactive Astrogliosis in Mouse Models of Alzheimer Disease. PloS one, 7(8), e42823. 10.1371/journal.pone.0042823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis W., Middeldorp J., Kooijman L., Sluijs J. A., Kooi E. J., Moeton M., Freriks M., Mizee M. R., Hol E. M. (2014). Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiology of Aging, 35(3), 492–510. 10.1016/j.neurobiolaging.2013.09.035 [DOI] [PubMed] [Google Scholar]
- Kanski R., Sneeboer M. A. M., van Bodegraven E. J., Sluijs J. A., Kropff W., Vermunt M. W., Creyghton M. P., De Filippis L., Vescovi A., Aronica E., van Tijn P., van Strien M. E., Hol E. M. (2014). Histone acetylation in astrocytes suppresses GFAP and stimulates a reorganization of the intermediate filament network. Journal of cell science, 127(Pt 20), 4368–4380. 10.1242/jcs.145912 [DOI] [PubMed] [Google Scholar]
- Kim Y., Varn F. S., Park S.-H., Yoon B. W., Park H. R., Lee C., Verhaak R. G. W., Paek S. H. (2021). Perspective of mesenchymal transformation in glioblastoma. Acta Neuropathologica Communications, 9(1), 50. 10.1186/s40478-021-01151-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc C., Etienne-Manneville S. (2017). Intermediate filaments join the action. Cell Cycle, 16(15), 1389–1390. 10.1080/15384101.2017.1345230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc C., Manneville S. E. (2017). Regulation of microtubule-associated motors drives intermediate filament network polarization. Journal of Cell Biology 216(6), 1689–1704. 10.1083/jcb.201607045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenstra S., Das P. K., Troost D., de Boer O. J., Bosch D. A. (1995). Human malignant astrocytes express macrophage phenotype. Journal of Neuroimmunology, 56(1), 17–25. 10.1016/0165-5728(94)00128-b [DOI] [PubMed] [Google Scholar]
- Lepekhin E. A., Eliasson C., Berthold C. H., Berezin V., Bock E., Pekny M. (2001). Intermediate filaments regulate astrocyte motility. Journal of Neurochemistry, 79(3), 617–625. 10.1046/j.1471-4159.2001.00595.x [DOI] [PubMed] [Google Scholar]
- Leube R. E., Moch M., Windoffer R. (2015). Intermediate filaments and the regulation of focal adhesion. Current Opinion in Cell Biology, 32, 13–20. 10.1016/j.ceb.2014.09.011 [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Balcarek J. M., Krek V., Shelanski M., Cowan N. J. (1984). Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: Structural conservation of intermediate filaments. Proceedings of the National Academy of Sciences, 81(9), 2743–2746. 10.1073/pnas.81.9.2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N. H., Yang A. W., Chang C. H., & Perng, M. Der. (2021). Elevated GFAP isoform expression promotes protein aggregation and compromises astrocyte function. FASEB Journal, 35(5), e21614. 10.1096/fj.202100087R [DOI] [PubMed] [Google Scholar]
- Lopez-Egido J. R., Cunningham J., Berg M., Oberg K., Bongcam-Rudloff E., Gobl A. E. (2002). Menin’s interaction with glial fibrillary acidic protein and vimentin suggests a role for the intermediate filament network in regulating menin activity. Experimental Cell Research, 278(2), 175–183. 10.1006/excr.2002.5575 [DOI] [PubMed] [Google Scholar]
- Louis D. N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W. K., Ohgaki H., Wiestler O. D., Kleihues P., Ellison D. W. (2016). The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathologica, 131(6), 803–820. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- Louis D. N., Perry A., Wesseling P., Brat D. J., Cree I. A., Figarella-Branger D., Hawkins C., Ng H. K., Pfister S. M., Reifenberger G., Soffietti R., von Deimling A., Ellison D. W. (2021). The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology, 23(8), 1231–1251. 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamber C., Kamphuis W., Haring N. L., Peprah N., Middeldorp J., Hol E. M. (2012). GFAPδ expression in glia of the developmental and adolescent mouse brain. PloS One, 7(12), e52659. 10.1371/journal.pone.0052659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama H., Suzuki H. I. (2020). Systems and synthetic microRNA biology: From biogenesis to disease pathogenesis. International Journal of Molecular Sciences, 21(1), 1–23. 10.3390/ijms21010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick M. B., Kouklis P., Syder A., Fuchs E. (1993). The roles of the rod end and the tail in vimentin IF assembly and IF network formation. Journal of Cell Biology, 122(2), 395–407. 10.1083/jcb.122.2.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menet V., Giménez Y, Ribotta M., Chauvet N., Drian M. J., Lannoy J., Colucci-Guyon E., Privat A. (2001). Inactivation of the glial fibrillary acidic protein gene, but not that of vimentin, improves neuronal survival and neurite growth by modifying adhesion molecule expression. Journal of Neuroscience, 21(16), 6147–6158. 10.1523/jneurosci.21-16-06147.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing A., Brenner M. (2020). GFAP at 50. ASN Neuro, 12, 1759091420949680. 10.1177/1759091420949680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp J., Boer K., Sluijs J. A., De Filippis L., Encha-Razavi F., Vescovi A. L., Swaab D. F., Aronica E., Hol E. M. (2010). GFAPdelta in radial glia and subventricular zone progenitors in the developing human cortex. Development (Cambridge , England ), 137(2), 313–321. 10.1242/dev.041632 [DOI] [PubMed] [Google Scholar]
- Middeldorp J., Hol E. M. (2011). GFAP in health and disease. Progress in Neurobiology, 93(3), 421–443. 10.1016/j.pneurobio.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Middeldorp J., van den Berge S. A., Aronica E., Speijer D., Hol E. M. (2009). Specific human astrocyte subtype revealed by affinity purified GFAP antibody; unpurified serum cross-reacts with neurofilament-L in Alzheimer. PloS one, 4(11), e7663. 10.1371/journal.pone.0007663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeton M., Kanski R., Stassen O. M. J. A., Sluijs J. A., Geerts D., Van Tijn P., Wiche G., Van Strien M. E., Hol E. M. (2014). Silencing GFAP isoforms in astrocytoma cells disturbs laminin-dependent motility and cell adhesion. FASEB Journal, 28(7), 2942–2954. 10.1096/fj.13-245837 [DOI] [PubMed] [Google Scholar]
- Moeton M., Stassen O. M. J. A., Sluijs J. A., van der Meer V. W. N., Kluivers L. J., van Hoorn H., Schmidt T., Reits E. A. J., van Strien M. E., Hol E. M. (2016). GFAP isoforms control intermediate filament network dynamics, cell morphology, and focal adhesions. Cellular and Molecular Life Sciences, 73(21), 4101–4120. 10.1007/s00018-016-2239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K. G., Hatton J. D., Hoi S. H. (1998). Role of glial fibrillary acidic protein expression in the biology of human glioblastoma U-373MG cells. Journal of Neurosurgery, 89(6), 997–1006. 10.3171/jns.1998.89.6.0997 [DOI] [PubMed] [Google Scholar]
- Neftel C., Laffy J., Filbin M. G., Hara T., Shore M. E., Rahme G. J., Richman A. R., Silverbush D., Shaw M. L., Hebert C. M., Dewitt J., Gritsch S., Perez E. M., Gonzalez Castro L. N., Lan X., Druck N., Rodman C., Dionne D., Kaplan A., Suvà M. L. (2019). An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell, 178(4), 835–849.e21. 10.1016/j.cell.2019.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A. L., Holm I. E., Johansen M., Bonven B., Jørgensen P., Jørgensen A. L. (2002). A new splice variant of glial fibrillary acidic protein, GFAP epsilon, interacts with the presenilin proteins. Journal of Biological Chemistry, 277(33), 29983–29991. 10.1074/jbc.M112121200 [DOI] [PubMed] [Google Scholar]
- Nielsen A. L., Jørgensen A. L. (2004). Self-assembly of the cytoskeletal glial fibrillary acidic protein is inhibited by an isoform-specific C terminus. Journal of Biological Chemistry, 279(40), 41537–41545. 10.1074/jbc.M406601200 [DOI] [PubMed] [Google Scholar]
- Ostrom Q. T., Cote D. J., Ascha M., Kruchko C., Barnholtz-Sloan J. S. (2018). Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncology, 4(9), 1254–1262. 10.1001/jamaoncol.2018.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudart M. (2012). AstroDot – a new method for studying the spatial distribution of mRNA in astrocytes. Journal of cell science, 133(7), jcs239756. 10.1242/jcs.239756 [DOI] [PubMed] [Google Scholar]
- Patel A. P., Tirosh I., Trombetta J. J., Shalek A. K., Gillespie S. M., Wakimoto H., Cahill D. P., Nahed B. V., Curry W. T., Martuza R. L., Louis D. N., Rozenblatt-Rosen O., Suvà, M. L., Regev, A., & Bernstein, B. E. (2014). Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science, 344(6190), 1396–1401. 10.1126/science.1254257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus W., Roggendorf W. (1988). Vimentin and glial fibrillary acidic protein are codistributed in the same intermediate filament system of malignant glioma cells in vivo—A double-labeling immunoelectron-microscopical study. Virchows Archiv B Cell Pathology Including Molecular Pathology, 56(1), 67–70. 10.1007/BF02890003 [DOI] [PubMed] [Google Scholar]
- Pekny M., Eliasson C., Chien C. L., Kindblom L. G., Liem R., Hamberger A., Betsholtz C. (1998). GFAP-deficient astrocytes are capable of stellation in vitro when cocultured with neurons and exhibit a reduced amount of intermediate filaments and an increased cell saturation density. Experimental Cell Research, 239(2), 332–343. 10.1006/excr.1997.3922 [DOI] [PubMed] [Google Scholar]
- Perng M.-D., Wen S.-F., Gibbon T., Middeldorp J., Sluijs J., Hol E. M., Quinlan R. A. (2008). Glial fibrillary acidic protein filaments can tolerate the incorporation of assembly-compromised GFAP-delta, but with consequences for filament organization and alphaB-crystallin association. Molecular Biology of the Cell, 19(10), 4521–4533. 10.1091/mbc.E08-03-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A., Wesseling P. (2016). Histologic classification of gliomas. In Handbook of Clinical Neurology (1ste dr., Vol. 134). Elsevier B.V. 10.1016/B978-0-12-802997-8.00005-0 [DOI] [PubMed] [Google Scholar]
- Petrie R. J., Doyle A. D., Yamada K. M. (2009). Random versus directionally persistent cell migration. Nature Reviews Molecular Cell Biology, 10(8), 538–549. 10.1038/nrm2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs R. F., Fischer D. F., Houtman S. H., Sluijs J. A., Van Haren W., Van Leeuwen F. W., Hol E. M. (2005). Adult human subventricular, subgranular, and subpial zones contain astrocytes with a specialized intermediate filament cytoskeleton. Glia, 52(4), 289–300. 10.1002/glia.20243 [DOI] [PubMed] [Google Scholar]
- Rutka J. T., Ackerley C., Hubbard S. L., Tilup A., Dirks P. B., Jung S., Ivanchuk S., Kurimoto M., Tsugu A., Becker L. E. (1998). Characterization of glial filament-cytoskeletal interactions in human astrocytomas: An immuno-ultrastructural analysis. European Journal of Cell Biology, 76(4), 279–287. 10.1016/S0171-9335(98)80006-X [DOI] [PubMed] [Google Scholar]
- Rutka J. T., Hubbard S. L., Fukuyama K., Matsuzawa K., Dirks P. B., Becker L. E. (1994). Effects of antisense glial fibrillary acidic protein complementary DNA on the growth, invasion, and adhesion of human astrocytoma cells. Cancer Res ., 54(0008-5472 (Print)), 3267–3272. [PubMed] [Google Scholar]
- Rutka J. T., Ivanchuk S., Mondal S., Taylor M., Sakai K., Dirks P., Jun P., Jung S., Becker L. E., Ackerley C. (1999). Co-expression of nestin and vimentin intermediate filaments in invasive human astrocytoma cells. International Journal of Developmental Neuroscience, 17(5–6), 503–515. 10.1016/S0736-5748(99)00049-0 [DOI] [PubMed] [Google Scholar]
- Sanson M., Marie Y., Paris S., Idbaih A., Laffaire J., Ducray F., El Hallani S., Boisselier B., Mokhtari K., Hoang-Xuan K., Delattre J.-Y. (2009). Isocitrate Dehydrogenase 1 Codon 132 Mutation Is an Important Prognostic Biomarker in Gliomas. Journal of Clinical Oncology, 27(25), 4150–4154. 10.1200/JCO.2009.21.9832 [DOI] [PubMed] [Google Scholar]
- Singh R., Nielsen A. L., Johansen M. G., Jørgensen A. L. (2003). Genetic polymorphism and sequence evolution of an alternatively spliced exon of the glial fibrillary acidic protein gene, GFAP. Genomics, 82(2), 185–193. 10.1016/S0888-7543(03)00106-X [DOI] [PubMed] [Google Scholar]
- Skalli O., Wilhelmsson U., Örndahl C., Fekete B., Malmgren K., Rydenhag B., Pekny M. (2013). Astrocytoma grade IV (glioblastoma multiforme) displays 3 subtypes with unique expression profiles of intermediate filament proteins. Human Pathology, 44(10), 2081–2088. 10.1016/j.humpath.2013.03.013 [DOI] [PubMed] [Google Scholar]
- Sommerlath V. N., Buergy D., Etminan N., Brehmer S., Reuss D., Sarria G. R., Guiot M.-C., Hänggi D., Wenz F., Petrecca K., Giordano F. A. (2022). Molecular features of glioblastomas in long-term survivors compared to short-term survivors-a matched-pair analysis. Radiation Oncology (London , England ), 17(1), 15. 10.1186/s13014-022-01984-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen O. M. J. A., van Bodegraven E. J., Giuliani F., Moeton M., Kanski R., Sluijs J. A., van Strien M. E., Kamphuis W., Robe P. A. J., Hol E. M. (2017). GFAPδ/GFAPα ratio directs astrocytoma gene expression towards a more malignant profile. Oncotarget, 8(50), 88104–88121. 10.18632/oncotarget.21540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Kubota T., Kitai R., Nakagawa T., Hashimoto N. (2008). CD98 immunoreactivity in multinucleated giant cells of glioblastomas: An immunohistochemical double labeling study. Neuropathology: Official Journal of the Japanese Society of Neuropathology, 28(2), 127–131. 10.1111/j.1440-1789.2007.00859.x [DOI] [PubMed] [Google Scholar]
- Thomsen R., Daugaard T. F., Holm I. E., Nielsen A. L. (2013). Alternative mRNA Splicing from the Glial Fibrillary Acidic Protein (GFAP) Gene Generates Isoforms with Distinct Subcellular mRNA Localization Patterns in Astrocytes. PLoS ONE, 8(8), e72110. 10.1371/journal.pone.0072110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda M., Miura M., Asou H., Toya S., Uyemura K. (1999). Cell growth suppression of astrocytoma C6 cells by glial fibrillary acidic protein cDNA transfection. Jounal of neurochemistry , 63(5), 1975–1978. 10.1046/j.1471-4159.1994.63051975.x. [DOI] [PubMed] [Google Scholar]
- Toda M., Miura M., Asou H., Sugiyama I., Kawase T., Uyemura K. (1999). Suppression of glial tumor growth by expression of glial fibrillary acidic protein. Neurochemical Research, 24(2), 339–343. 10.1023/A:1022538810581 [DOI] [PubMed] [Google Scholar]
- Uceda-Castro R., van Asperen J. V., Vennin C., Sluijs J. A., van Bodegraven E. J., Margarido A. S., Robe P. A. J., van Rheenen J., Hol E. M. (2022). GFAP splice variants fine-tune glioma cell invasion and tumour dynamics by modulating migration persistence. Scientific Reports, 12(1), 424. 10.1038/s41598-021-04127-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda C. T., Eng L. F., Bignami A. (1972). Immunological study of the glial fibrillary acidic protein. Brain Research, 37(1), 81–89. 10.1016/0006-8993(72)90347-2 [DOI] [PubMed] [Google Scholar]
- van Asperen J. V., Fedorushkova D. M., Robe P. A. J. T., Hol E. M. (2022). Investigation of glial fibrillary acidic protein (GFAP) in body fluids as a potential biomarker for glioma: A systematic review and meta-analysis. Biomarkers: Biochemical Indicators of Exposure , Response, and Susceptibility to Chemicals, 27(1), 1–12. 10.1080/1354750X.2021.2006313 [DOI] [PubMed] [Google Scholar]
- van Bodegraven E. J., Sluijs J. A., Tan A. K., Robe P. A. J. T., Hol E. M. (2021). New GFAP splice isoform (GFAPµ) differentially expressed in glioma translates into 21 kDa N-terminal GFAP protein. FASEB Journal, 35(3), e21389. 10.1096/fj.202001767R [DOI] [PubMed] [Google Scholar]
- van Bodegraven E. J., van Asperen J. V., Robe P. A. J., Hol E. M. (2019). Importance of GFAP isoform-specific analyses in astrocytoma. Glia, 67(8), 1417–1433. 10.1002/glia.23594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bodegraven E. J., van Asperen J. V., Sluijs J. A., van Deursen C. B. J., van Strien M. E., Stassen O. M. J. A., Robe P. A. J., Hol E. M. (2019). GFAP alternative splicing regulates glioma cell-ECM interaction in a DUSP4-dependent manner. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 33(11), 12941–12959. 10.1096/fj.201900916R [DOI] [PubMed] [Google Scholar]
- van den Berge S. A., Middeldorp J., Eleana Zhang C., Curtis M. A., Leonard B. W., Mastroeni D., Voorn P., van de Berg W. D. J., Huitinga I., Hol E. M. (2010). Longterm quiescent cells in the aged human subventricular neurogenic system specifically express GFAP-δ. Aging Cell, 9(3), 313–326. 10.1111/j.1474-9726.2010.00556.x [DOI] [PubMed] [Google Scholar]
- Venteicher A. S., Tirosh I., Hebert C., Yizhak K., Neftel C., Filbin M. G., Hovestadt V., Escalante L. E., Shaw M. L., Rodman C., Gillespie S. M., Dionne D., Luo C. C., Ravichandran H., Mylvaganam R., Mount C., Onozato M. L., Nahed B. V., Wakimoto H., … M. L Suvà. (2017). Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science, 355(6332), eaai8478. 10.1126/science.aai8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaak R. G. W., Hoadley K. A., Purdom E., Wang V., Qi Y., Wilkerson M. D., Miller C. R., Ding L., Golub T., Mesirov J. P., Alexe G., Lawrence M., O’Kelly M., Tamayo P., Weir B. A., Gabriel S., Winckler W., Gupta S., Jakkula L., … D. N Hayes. (2010). Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH 1, EGFR, and NF1. Cancer Cell, 17(1), 98–110. 10.1016/j.ccr.2009.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Cairncross J. G., Liem R. K. H. (1984). Identification of glial filament protein and vimentin in the same intermediate filament system in human glioma cells. Proceedings of the National Academy of Sciences of the United States of America, 81(7 I), 2102–2106. https://doi.org/10.1073/pnas.81.7.2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Cazzato E., Ladewig E., Frattini V., Rosenbloom D. I. S., Zairis S., Abate F., Liu Z., Elliott O., Shin Y. J., Lee J. K., Lee I. H., Park W. Y., Eoli M., Blumberg A. J., Lasorella A., Nam D. H., Finocchiaro G., Iavarone A., Rabadan R. (2016). Clonal evolution of glioblastoma under therapy. Nature Genetics, 48(7), 768–776. 10.1038/ng.3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Hu B., Hu H., H K., S M., S L., Ac, deCarvalho, S, L., P, L., Y, L., F, B., Hj, C., Yh, L., N, S., E, M.-L., S, Z., E, C., Cg, S., A, O., … Rgw, V. (2017). Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell, 32(1). 10.1016/j.ccell.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D. E., Shelanski M. L., Liem R. K. H. (1991). Suppression by antisense mRNA demonstrates a requirement for the glial fibrillary acidic protein in the formation of stable astrocytic processes in response to neurons. Journal of Cell Biology, 112(6), 1205–1213. 10.1083/jcb.112.6.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling P., Capper D. (2018). WHO 2016 Classification of gliomas. Neuropathology and Applied Neurobiology, 44(2), 139–150. 10.1111/nan.12432 [DOI] [PubMed] [Google Scholar]
- Wilhelmsson U., Eliasson C., Bjerkvig R., Pekny M. (2003). Loss of GFAP expression in high-grade astrocytomas does not contribute to tumor development or progression. Oncogene, 22(22), 3407–3411. 10.1038/sj.onc.1206372 [DOI] [PubMed] [Google Scholar]
- Zelenika D., Grima B., Brenner M., Pessac B. (1995). A novel glial fibrillary acidic protein mRNA lacking exon 1. Molecular brain research, 30(2), 251–258. [DOI] [PubMed] [Google Scholar]