Abstract
To observe the effects and safety of Sacubitril/Valsartan (SV) on heart function and blood pressure in maintenance hemodialysis (MHD) patients with chronic heart failure (CHF). The clinical data and biochemical parameters of MHD patients were retrospectively analyzed. These MHD patients, who were collected from January 2020 to June 2021 in the Blood Purification Center of the First Affiliated Hospital of Chongqing Medical University, received SV treatment to control heart failure (HF). Altogether 54 MHD patients complicated with CHF who received SV treatment were selected for this self-controlled study. The changes of serum biochemical indexes, left anteroposterior atrial diameter (LAD), left ventricular end diastolic diameter (LVID), left ventricular ejection fraction (LVEF), right atrial transverse diameter (RAD), right anteroposterior ventricular diameter (RVD), blood pressure and antihypertensive drug dosage before and after treatment were assessed. The adverse reactions such as hyperkalemia, hypotension before dialysis, angina pectoris, myocardial infarction, cerebral infarction, cerebral hemorrhage and hospitalization due to HF were recorded before and after treatment. After treatment, LAD and LVID, incidence of angina pectoris, duration of hospitalization for HF, systolic blood pressure and diastolic blood pressure before dialysis, and the calibration value of antihypertensive drugs were all reduced, while LVEF was increased. The incidence of hyperkalemia (serum potassium >5.5 mmol/L) also increased after treatment compared with before treatment (P<0.05). The incidence of hypotension, angina pectoris, myocardial infarction, cerebral infarction and cerebral hemorrhage during treatment was similar to that before treatment (P>0.05). SV can effectively improve left atrial and left ventricular remodeling in MHD patients with CHF, improve LVEF, reduce the incidence of angina pectoris and duration of hospitalization due to HF in MHD patients, which is conducive to the control of blood pressure in MHD patients with hypertension. The incidence of hyperkalemia increased during SV treatment. SV did not increase the incidence of hypotension, myocardial infarction, cerebral infarction, cerebral hemorrhage and other events in MHD patients.
Keywords: Sacubitril/Valsartan (SV), maintenance hemodialysis (MHD), adverse reaction (ADR), safety
Introduction
With the improvement of economy, there are more and more patients who suffer from chronic kidney disease (CKD) and maintenance hemodialysis (MHD) in China. According to the data of China Blood Purification Case Registration System, the number of MHD patients reached 633,000 in 2019 [1].
There are many factors affecting the prognosis of MHD patients, among which cardiovascular diseases and events are the biggest threats [2,3]. Among MHD patients with known causes of death, more than 50% cases die from cardiovascular disease [4,5], which is the leading cause of death in dialysis patients. Heart failure (HF) is one of the most common complications in dialysis patients. Studies have shown that the proportion of MHD patients with HF in China is as high as 45.45% [6,7], and its 5-year survival rate is only 12.5%. How to improve the prognosis and survival rate of these patients is a challenging task.
Recently, research has confirmed that Sacubitril/Valsartan (SV) can effectively reduce the mortality and hospitalization rate of patients with chronic heart failure (CHF) combined with reduced ejection fraction, and its effect is superior to angiotensin converting enzyme inhibitor (ACEI) [8]. It is one of the most promising drugs in CHF treatment [9,10]. However, there are insufficient clinical data on the efficacy and safety of this drug in MHD patients complicated with CHF. The purpose of this research is to observe SV’s effect on the cardiac structure and function of MHD patients complicated with CHF, and to investigate its safety in MHD patients, hoping to provide reference for improving their prognosis and quality of life.
Data and methods
Objects of study
This research is a self-controlled study. Altogether 54 MHD patients complicated with chronic heart failure who had received SV treatment from January 2020 to June 2021 were selected in the Blood Purification Center of the First Affiliated Hospital of Chongqing Medical University and analyzed retrospectively. Ethical batch number: LL2020 (Review) A54 (Nuclear) 012.
Inclusion criteria
1) Patients with the end-stage renal disease who received maintenance hemodialysis for at least 3 months; 2) Those who met the CHF diagnostic criteria in Chinese Guidelines for the diagnosis and treatment of heart Failure 2018 [11]; 3) LVEF<55% before treatment; 4) Before dialysis, systolic blood pressure >100 mmHg and diastolic blood pressure >60 mmHg.
Exclusion criteria
1) Patients with insufficient data that affected the results; 2) Patients combined with malignancy, congenital heart disease, tuberculosis, HIV and cardiac resynchronization treatment; 3) Serum potassium ≥6.5 mmol/L within 3 months before treatment.
All patients received regular hemodialysis (3 times/week, 4 h/time) with control of blood pressure. All patients were treated with one or more of the antihypertensive drugs such as CCB/ACEI/ARB/β-blocker/α-blocker to control their blood pressure before treatment. At the beginning of SV treatment, the original antihypertensive drugs were directly switched to SV, or the ACEI/ARB drugs in the original antihypertensive regimens were switched to SV, or SV was added to the original antihypertensive regimens to control blood pressure. If the early morning systolic blood pressure ≤100 mmHg or diastolic blood pressure ≤60 mmHg lasted for more than 2 days, the antihypertensive drugs except SV should be reduced until the drug was stopped, and then the amount of SV should be reduced. Moreover, improvement of renal anemia (iron, recombinant human erythropoietin), regulation of calcium and phosphorus metabolism and other treatments should be conducted. The used SV tablets were from Beijing Novartis Pharmaceutical Co., LTD., national medicine standard J20190002, titrated dose 50-200 mg, orally, twice a day. All patients were treated for 6 months.
Research methods
Follow-up visit
All patients were followed up by outpatient and telephone once every month for an average of 6 months.
General clinical data, biochemical parameters and cardiac ultrasound data collection before and after SV treatment
General clinical data
The following data were collected: Age, gender, BMI, dialysis age, dry weight, urea clearance rate, primary disease composition, NYHA cardiac function grading, pre-dialysis systolic blood pressure (SBP), pre-dialysis diastolic blood pressure (DBP), and medication (The dosage of antihypertensive drugs was measured by the ratio of the actual dosage to the defined daily dose (DDD) of patients. When the same patient received multiple antihypertensive drugs, the calibrated dose is the sum of the ratio of the actual dose of each drug and DDD of the drug).
Biochemical criteria
Hemoglobin, blood calcium, blood phosphorus, iPTH, blood creatinine, urea nitrogen and blood potassium were examined monthly before and during SV treatment, and blood samples were taken from hemodialysis pipeline before dialysis. The biochemical indexes of hemoglobin were tested by automatic blood routine tester (China, Shenzhen, Mindray, BC-5600) and automatic biochemical analyzer (Japan, Tokyo, Hitachi, 5800). All steps are strictly operated in accordance with the kits and instrument instructions.
Cardiac ultrasound data
Echocardiographic measurements of patients before and after SV treatment were collected, including left anteroposterior atrial diameter (LAD), left ventricular end diastolic diameter (LVID), left ventricular ejection fraction (LVEF), right atrial transverse diameter (RAD), and right anteroposterior ventricular diameter (RVD). It was determined by color Doppler echocardiography (Amsterdam, Netherlands, EPIQ-5).
Adverse reactions record during SV treatment
Adverse reactions during treatment were recorded, including hyperkalemia, hypotension before dialysis, angina pectoris, myocardial infarction, cerebral infarction, cerebral hemorrhage, and hospitalization due to heart failure.
Hyperkalemia was defined as blood potassium exceeding 5.5 mmol/L and the hyperkalemia of the same patient before and after a single dialysis was limited to one person time; Predialysis hypotension was defined as a mean SBP<90 mmHg or a mean DBP<60 mmHg over two sessions before dialysis; Angina pectoris was limited to symptoms such as chest area tightness, crushing feeling, and chest pain, which could be relieved within 10 min or relieved within 10 min after nitroglycerin and Suxiaojiuxin pills were taken under the tongue; Myocardial infarction was defined as acute myocardial infarction with obvious abnormality of electrocardiogram and myocardial enzyme spectrum; Cerebral infarction and cerebral hemorrhage were defined as newly emerging limb movement disorders and the new onset was clearly diagnosed by cranial imaging examination; The occurrence of the same adverse reaction in the same patient and day was limited to one person at a time.
Statistical methods
SPSS 22.0 [12] statistical software was conducted to analyze the data, and Graphpad [13] PRISM 6.0 was employed to plot the data. The measurement data were expressed as (x̅±s). For the comparison of mean values before and after treatment, paired sample T-test was used if the difference followed normal distribution; Wilcoxon signed rank test of paired sample was used if the difference did not follow normal distribution. P<0.05 was considered statistically remarkable.
Results
Baseline clinical data
A total of 54 patients were enrolled in this research, including 29 males and 25 females, with an average age of 50.9±14.6 years old, dialysis age of 35.81±24.42 months. KT/V was 1.28±0.14, SV titrated dose was 50-200 mg, twice a day, and SV dose was adjusted up and down according to patients’ blood pressure and blood potassium (Table 1).
Table 1.
General clinical data of patients before treatment (n=54)
| Age | 50.9±14.6 |
| Gender (male/female) | 29/25 |
| Dialysis age (months) | 32.81±20.42 |
| BMI (Kg/m2) | 22.81±2.89 |
| Dry Weight(DW) (Kg) | 61. 35±14.38 |
| KT/V | 1.28±0.14 |
| Primary Component n (%) | |
| Chronic Glomerulonephritis (CG) | 32 (59.2) |
| Diabetic Nephropathy (DN) | 6 (11.1) |
| Hypertensive Nephropathy | 9 (16.7) |
| Other | 7 (13.0) |
| NYHA Heart Function Classification (%) | |
| I | 0 (0) |
| II | 13 (24.0) |
| III | 34 (63.0) |
| IV | 7 (13.0) |
| Systolic pressure before dialysis (mmHg) | 151±19 |
| Diastolic pressure before dialysis (mmHg) | 90±21 |
| Drug Usage | |
| Metoprolol, n (%) | 51 (94.4) |
| Ivabradine, n (%) | 0 (0) |
| Digitalis, n (%) | 13 (24.1) |
| Nitrates, n (%) | 28 (51.9) |
| SV maintenance dose, n (%) | |
| 50 mg, twice/d | 8 (14.8) |
| 100 mg, twice/d | 41 (75.9) |
| 200 mg, twice/d | 5 (9.3) |
Serum biochemical indices before and after SV treatment
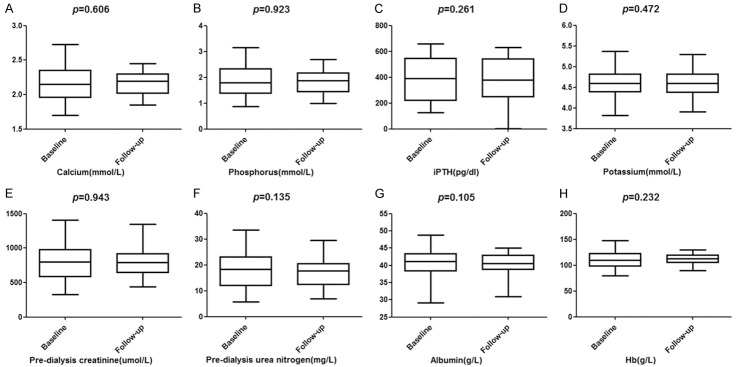
All patients were followed up once a month on average for hemoglobin, blood calcium, blood phosphorus, iPTH, pre-dialysis serum creatinine, pre-dialysis urea nitrogen, albumin and blood potassium, blood samples were taken through hemodialysis pipeline before dialysis. It manifested that there was no marked difference in total serum potassium level between before and after SV treatment (P>0.05; Figure 1), but the incidence of hyperkalemia decreased after SV treatment (P<0.05; Table 2). There were no significant differences in hemoglobin, serum calcium, serum phosphorus, iPTH, pre-dialysis serum creatinine, pre-dialysis urea nitrogen and albumin before and after treatment (P>0.05; Figure 1).
Figure 1.
Comparison of laboratory biochemical indices before and after SV treatment. A. Changes of Calcium (mmol/L) before and after treatment; B. Changes of Phosphorus (mmol/L) before and after treatment; C. Changes of iPTH (pg/dl) before and after treatment; D. Changes of potassium (mmol/L) before and after treatment; E. Changes of Pre-dialysis creatinine (μmol/L) before and after treatment; F. Changes of Pre-dialysis urea nitrogen (mg/L) before and after treatment; G. Changes of Albumin (g/L) before and after treatment; H. Changes of Hb (g/L) before and after treatment.
Table 2.
Comparison of adverse reactions
| Group | Hyperkalemia | Hypotension | Angina | Miocardial infarction | Cerebral infarction | Cerebral hemorrhage | Hospitalization for HF |
|---|---|---|---|---|---|---|---|
| Before treatment | 6 | 5 | 12 | 5 | 4 | 2 | 2 |
| After treatment | 15 | 6 | 4 | 3 | 2 | 1 | 10 |
| χ2 value | 4.788 | 0.101 | 4.696 | 0.540 | 0.706 | 0.343 | 6.000 |
| P value | 0.029 | 0.750 | 0.030 | 0.462 | 0.401 | 0.558 | 0.014 |
Comparison of cardiac color doppler ultrasonography before and after SV treatment
By cardiac ultrasound examination, the measured values of LAD and LVID after treatment were lower than those before treatment, and LVEF was higher than those before treatment (P<0.001). The difference was statistically marked, but RAD and RVD had no statistical difference before and after treatment (P>0.05; Figure 2).
Figure 2.
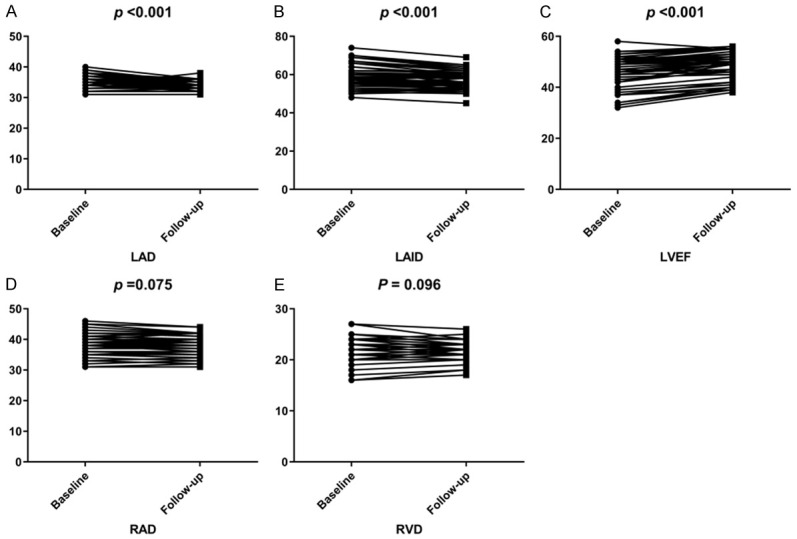
LAD, LVID, LVEF, RAD, RVD changes before and after treatment. A. Changes of LAD before and after treatment; B. Changes of LVID before and after treatment; C. Changes of LVEF before and after treatment; D. Changes of RAD before and after treatment; E. Changes of RVD before and after treatment.
Comparison of systolic and diastolic blood pressure before and after SV treatment
After 6 months of SV treatment, patients’ SBP and DBP before dialysis decreased compared with that before treatment (P<0.001; Figure 3), and the dosage or type of antihypertensive drugs decreased compared with that before treatment (P<0.05; Figure 4).
Figure 3.
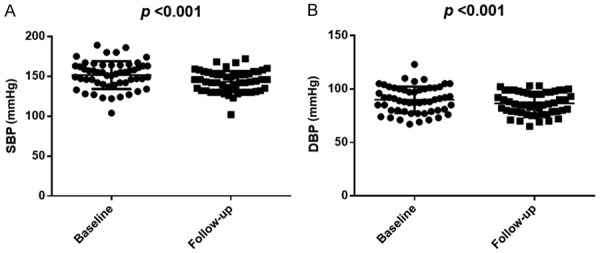
SBP, DBP changes before and aftertreatment. A. Changes of SBP before and after treatment; B. Changes of DBP before and after treatment.
Figure 4.
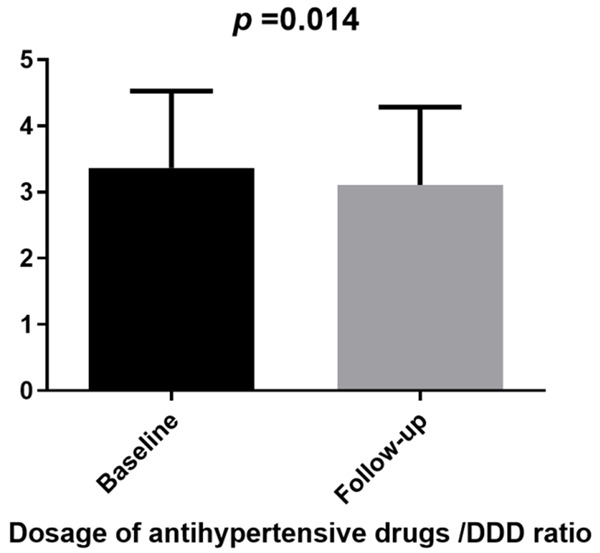
Change of antihypertensive drug dose before and after treatment.
Adverse reactions during SV treatment
In this research, 54 patients with hyperkalemia, hypotension before dialysis, angina pectoris, myocardial infarction, cerebral infarction, cerebral hemorrhage and HF were followed up within 6 months before SV treatment. Meanwhile, the incidence of these same adverse reactions was recorded during the 6-month period of SV treatment for all patients. After treatment, the incidence of hyperkalemia (serum potassium >5.5 mmol/L), angina pectoris and hospitalization due to HF were all lower than those before treatment. There was no marked difference in the incidence of hypotension, myocardial infarction, cerebral infarction and cerebral hemorrhage before and after dialysis ( P>0.05; Table 2).
Discussion
Maintenance hemodialysis patients are prone to ventricular remodeling and reduced myocardial contractility due to uremic toxin, renal hypertension, activation of RAAS system, nutrient intake, synthesis deficiency, etc [14]. As one of the most common complications in patients with end-stage renal disease, HF is a serious threat to patients’ life safety and affects their quality of life [15]. Cardiovascular and cerebrovascular events are the main causes of death in MHD patients [16]. How to improve patients’ prognosis is a persistent concern of hemodialysis doctors.
SV is a eutectic of angiotensin receptor antagonist and enkephalin inhibitor [17]. By inhibiting enkephalinase, it increases the levels of natriuretic peptide hormones such as ANP, BNP and CNP, and inhibits the activation of RAAS system, which plays a dual role in inhibiting sympathetic activity, reducing aldosterone secretion, relaxing blood vessels Anti myocardial hypertrophy and anti myocardial fibrosis [18]. Many clinical studies have confirmed that SV could effectively reduce cardiovascular mortality and hospitalization rate due to HF in CHF patients [19], and could improve ventricular remodeling and enhance LVEF. It has recently been reported that SV has a good effect on lowering blood pressure and has a significant effect on refractory hypertension such as renal hypertension [20]. However, there are few clinical data of SV for MHD complicated with CHF and hypertension. It’s therefore urgent to verify the efficacy and safety of SV in dialysis patients.
This study retrospectively analyzed the clinical data of MHD patients complicated with CHF who received SV treatment in the blood purification center of our hospital. The results showed that there were no significant differences in serum calcium, phosphorus, iPTH, hemoglobin, pre-dialysis creatinine, pre-dialysis urea nitrogen, albumin and other indexes in all patients before and after treatment. In 6 patients, urine volume increased after treatment, ultrafiltration volume decreased, pre-dialysis urea nitrogen and creatinine levels decreased before dialysis. It might be related to the fact that SV increases the levels of ANP and BNP, inhibits RAAS, increases renal blood flow and glomerular filtration rate, and thus plays a diuretic role.
Compared with the values of 54 patients before and after SV treatment, we found that LAD and LVID of patients decreased after treatment, while LVEF increased after treatment, the differences were statistically significant. After treatment, the symptoms of HF and activity tolerance of patients were improved, suggesting that SV could improve left atrial and left ventricular remodeling and enhance LVEF in MHD patients with CHF. This result is consistent with the conclusion of Lee et al. [21], and is the few clinical data confirming SV efficacy. Among patients selected in this research, 18 patients had LVEF above 50% before treatment, but the LVEF of these patients was still improved after treatment. LVEF of 15 patients gradually increased after conversion from ACEI/ARB to SV treatment three months later. These results suggested that SV could still improve ventricular remodeling and enhance LVEF in MHD patients with retained ejection fraction and CHF, and is superior to ACEI/ARB in improving ventricular remodeling and enhancing LVEF. We consider that this is relevant to the inhibition of enkephalin and the increase of natriuretic peptide levels by sacurbutri in drug structure. The increased levels of ANP, BNP and CNP play a great role in antimyocardial hypertrophy, anti-fibrosis, sympathetic nerve inhibition, vasodilation, etc. In addition, SV combined with equal molar quantities of valsartan further inhibits RAAS activation. Both mechanisms are related to the reversal of ventricular remodeling.
Hypertension is very common in MHD patients [22], and many patients have “refractory hypertension” whose blood pressure still cannot be controlled at the target level after combined application of 3 or more antihypertensive drugs [23]. British HARP-III study manifested that SV could reduce blood pressure in CKD patients by an average of 5.4 mmHg (95% CI-7.4~-3.4) in SBP and 2.1 mmHg (95% CI-3.3~-1.0) in DBP [24]. Our study also observed that the SBP and DBP of patients before dialysis decreased after SV treatment compared with those before treatment, and the dose or type of oral antihypertensive drugs decreased, with statistically marked differences. It is suggested that SV can effectively reduce the blood pressure of MHD patients with hypertension, which is considered to be related to the dual antihypertensive effect of SV. Some hypertensive patients who received SV therapy also received other antihypertensive drugs. After SV treatment, the calibration value of other oral antihypertensive drugs decreased, indicating the antihypertensive effect of SV, which may add new options for the treatment of “refractory hypertension”.
Our study also recorded the occurrence of related adverse reactions in 54 patients within 6 months before and during SV treatment. It was found that the number of patients with hyperkalemia increased after treatment, while the number of those with angina pectoris and hospitalization for HF decreased after treatment, with statistically significant differences. Although the serum potassium of MHD patients is affected by dialysis, diet, drugs, etc, there is a large fluctuation. However, the difference in the incidence of hyperkalemia before and after treatment should be noted. We considered that the increase in hyperkalemia was mainly related to the dual antagonistic effect of natriuretic peptide and RAAS inhibitor against aldosterone. This was also confirmed by the observation that hyperkalemia after treatment mainly occurred in MHD patients with urine. After treatment, the number of angina pectoris and hospitalization for HF decreased, which was mainly related to the effects of SV on atherosclerosis, cardiac remodeling and LVEF enhancement. Symptomatic hypotension, myocardial infarction, cerebral infarction, cerebral hemorrhage and other events associated with SV were not observed, and there was no statistical difference before and after treatment.
Nevertheless, the present study still has some limitations. First, this was a retrospective study, but our sample size was inadequate. Second, our patients with MHD and CHF were treated only with the SV regimen, which prevented us from collecting additional samples. Third, our follow-up time is relatively short, whether there is a long-term effect of SV regimen on patients needs further study. Hence, we hope to conduct a randomized controlled study to continuously improve our conclusions.
Conclusion
In conclusion, SV could improve left atrial and left ventricular remodeling in MHD patients with CHF, enhance LVEF, improve cardiac function, and reduce the incidence of angina pectoris and hospitalization for HF. In MHD patients combined with hypertension, it could effectively control blood pressure, and does not increase the occurrence of myocardial infarction, cerebral infarction, cerebral hemorrhage and other events in patients. Because SV has the dual antagonistic effect on aldosterone, attention should be paid to the prevention of hyperkalemia in MHD patients with urine. This is an observational study in the real world with a small sample size, and its results need to be further confirmed by more rigorous studies. In the near future, RCT studies should be designed to further explore the SV effects on the heart, blood pressure and blood vessels in MHD patients.
Disclosure of conflict of interest
None.
References
- 1.Chen MX. Current situation and future of nephrology in China. Chin Med News. 2021;36:19–19. [Google Scholar]
- 2.Wakasugi M, Narita I, Iseki K, Asahi K, Yamagata K, Fujimoto S, Moriyama T, Konta T, Tsuruya K, Kasahara M, Shibagaki Y, Kondo M, Watanabe T. The effect of CKD on associations between lifestyle factors and all-cause, cancer, and cardiovascular mortality: a population-based cohort study. Intern Med. 2021;60:2189–2200. doi: 10.2169/internalmedicine.6531-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankowski J, Floege J, Fliser D, Bohm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16:573–585. doi: 10.1038/s41581-020-0315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M, Li Y, Lv J, Xu H, Wu X, Wen W, Wang W, Yang H. The effects of glucose-free and glucose-containing dialysate during dialysis in MHD patients: a prospective cross-over study. Perfusion. 2021:2676591211042726. doi: 10.1177/02676591211042726. [DOI] [PubMed] [Google Scholar]
- 6.Bozkurt B, Khalaf S. Heart failure in women. Methodist Debakey Cardiovasc J. 2017;13:216–223. doi: 10.14797/mdcj-13-4-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen XN, Pan XX, Yu HJ, Shen PY, Zhang QY, Zhang W, Ren H, Qian Y, Zhu P, Chen N. Analysis of cardiovascular disease in Chinese in-patients with chronic kidney disease. Intern Med. 2011;50:1797–1801. doi: 10.2169/internalmedicine.50.5158. [DOI] [PubMed] [Google Scholar]
- 8.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Committees Investigators. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF) Eur J Heart Fail. 2014;16:817–825. doi: 10.1002/ejhf.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayard G, Da Costa A, Pierrard R, Romeyer-Bouchard C, Guichard JB, Isaaz K. Impact of sacubitril/valsartan on echo parameters in heart failure patients with reduced ejection fraction a prospective evaluation. Int J Cardiol Heart Vasc. 2019;25:100418. doi: 10.1016/j.ijcha.2019.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vecchis R, Ariano C, Soreca S. Antihypertensive effect of sacubitril/valsartan: a meta-analysis. Minerva Cardioangiol. 2019;67:214–222. doi: 10.23736/S0026-4725.19.04869-2. [DOI] [PubMed] [Google Scholar]
- 11.Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association; Chinese Heart Failure Association of Chinese Medical Doctor Association; Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46:760–789. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Dudley WN, Benuzillo JG, Carrico MS. SPSS and SAS programming for the testing of mediation models. Nurs Res. 2004;53:59–62. doi: 10.1097/00006199-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Le Berre M, Gerlach JQ, Dziembala I, Kilcoyne M. Calculating half maximal inhibitory concentration (IC50) values from glycomics microarray data using graphpad prism. Methods Mol Biol. 2022;2460:89–111. doi: 10.1007/978-1-0716-2148-6_6. [DOI] [PubMed] [Google Scholar]
- 14.Lu W, Ren C, Han X, Yang X, Cao Y, Huang B. The protective effect of different dialysis types on residual renal function in patients with maintenance hemodialysis: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e12325. doi: 10.1097/MD.0000000000012325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wouk N. End-stage renal disease: medical management. Am Fam Physician. 2021;104:493–499. [PubMed] [Google Scholar]
- 16.Tangren J, Nadel M, Hladunewich MA. Pregnancy and end-stage renal disease. Blood Purif. 2018;45:194–200. doi: 10.1159/000485157. [DOI] [PubMed] [Google Scholar]
- 17.Docherty KF, Vaduganathan M, Solomon SD, McMurray JJV. Sacubitril/Valsartan: neprilysin inhibition 5 years after PARADIGM-HF. JACC Heart Fail. 2020;8:800–810. doi: 10.1016/j.jchf.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heyse A, Manhaeghe L, Mahieu E, Vanfraechem C, Van Durme F. Sacubitril/valsartan in heart failure and end-stage renal insufficiency. ESC Heart Fail. 2019;6:1331–1333. doi: 10.1002/ehf2.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CH. Critical questions about PARADIGM-HF and the future. Acta Cardiol Sin. 2016;32:387–396. doi: 10.6515/ACS20151120A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croteau D, Qin F, Chambers JM, Kallick E, Luptak I, Panagia M, Pimentel DR, Siwik DA, Colucci WS. Differential effects of Sacubitril/Valsartan on diastolic function in mice with obesity-related metabolic heart disease. JACC Basic Transl Sci. 2020;5:916–927. doi: 10.1016/j.jacbts.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Oh J, Kim H, Ha J, Chun KH, Lee CJ, Park S, Lee SH, Kang SM. Sacubitril/valsartan in patients with heart failure with reduced ejection fraction with end-stage of renal disease. ESC Heart Fail. 2020;7:1125–1129. doi: 10.1002/ehf2.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song L, Quan ZL, Zhao LY, Cui DM, Zhong M, Zhou LF, Sun CY, Chen YG, Mo YW, Feng Z, Tao Y, Ye Z, Chen Y, Liang H, Lin T, Liu S, Liang XL, Fu X. Impact of pulmonary hypertension on arteriovenous fistula failure of hemodialysis patients: a 10 years follow-up cohort study. J Vasc Access. 2021:11297298211027408. doi: 10.1177/11297298211027408. [DOI] [PubMed] [Google Scholar]
- 23.Mahfoud F, Himmel F, Ukena C, Schunkert H, Bohm M, Weil J. Treatment strategies for resistant arterial hypertension. Dtsch Arztebl Int. 2011;108:725–731. doi: 10.3238/arztebl.2011.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UK HARP-III Collaborative Group. Randomized multicentre pilot study of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease: United Kingdom Heart and Renal Protection (HARP)-III-rationale, trial design and baseline data. Nephrol Dial Transplant. 2017;32:2043–2051. doi: 10.1093/ndt/gfw321. [DOI] [PMC free article] [PubMed] [Google Scholar]