Abstract
Objective: To investigate the effect of liraglutide combined with metformin or acarbose on glucose control in patients with type 2 diabetes mellitus (T2DM) and to analyze the risk factors of gastrointestinal adverse reactions. Methods: This retrospective study was conducted on 88 T2DM patients who were treated in our hospital from February 2019 to August 2021. The patients were divided into Group A (n=40) and Group B (n=48) according to different treatment methods. Group A was treated with liraglutide and metformin, while Group B was given liraglutide and acarbose. The effects of glucose control (FPG, 2hPG, HbA1c), inflammatory indexes (IL-6, CRP, SAA), fasting C-peptide, 2-h postprandial C-peptide levels and adverse reactions were compared. Afterwards, The risk factors of gastrointestinal adverse reactions were assessed via logistics regression. Results: It was found that the FPG, 2hPG and HbA1c levels after treatment were lower than those before treatment (P<0.05), and the levels in group A were lower than those in group B (P<0.05). The serum IL-6, CRP and SAA levels after treatment were lower than those before treatment (P<0.05), but there was no marked difference between the two groups after treatment (P>0.05). The fasting C-peptide and 2-h postprandial C-peptide levels in group A after treatment were higher than those in group B (P<0.05). Logistics regression analysis revealed that complicated digestive system diseases and combined use of acarbose were independent risk factors. Conclusion: Compared with liraglutide and acarbose, liraglutide and metformin has better glucose control effect in T2DM. Although there is no obvious difference in eliminating inflammation, liraglutide combined with acarbose will increase the incidence of gastrointestinal adverse reactions in patients. So, liraglutide combined with metformin is recommended for T2DM treatment.
Keywords: Liraglutide, metformin, acarbose, type 2 diabetes mellitus, gastrointestinal adverse reactions, risk factors analysis
Introduction
Type 2 diabetes mellitus (T2DM) is a kind of chronic metabolic disorder, which has increased exponentially in many third world countries [1]. It will not only cause pathological hyperglycemia in patients, but also reduce the secretion of insulin, leading to multiple complications, affecting the functions of various organs, and ultimately causing great harm to their quality of life and prognosis [2,3]. Overweight, obesity, and unbalanced life and rest can easily lead to type 2 diabetes and aggravate the illness [4]. The aggravation of T2DM usually brings a heavy burden to limited medical resources, and it is still a challenge to the treatment and prognosis of diabetes [5,6].
Drug therapy is the first choice for clinical treatment of T2DM, in which liraglutide is widely used in various trials on diabetes [7-9]. Liraglutide is one of the long-acting analogues of glucagon-like peptide-1 and can cross the blood-brain barrier and play an essential role in diabetes and its oxidative stress and apoptosis [10]. However, liraglutide can cause a variety of cardiovascular-related adverse reactions, while reducing patients’ appetite and causing gastrointestinal adverse reactions [11,12]. Metformin, which can effectively control the glycosylated blood glucose and blood glucose level of patients, so as to avoid complications, is widely used in treating diabetes [13]. Metformin has been used clinically for 60 years. It can effectively improve the cardiovascular function caused by diabetes [14]. Acarbose is a kind of α-glycosidase inhibitor, which can effectively inhibit the synthesis of sucrase, pancreatic amylase and small intestinal epithelial glucoamylase, hinder the degradation of intestinal carbohydrates and reduce plasma insulin secretion [15].
Liraglutide in combination with metformin or acarbose has been proved to be effective in T2DM treatment. But it is vague whether there is a difference in the efficacy of the two schemes. Early studies have shown that combination of drugs can reduce the incidence of adverse reactions in patients. Whether the two regimen can reduce the incidence of gastrointestinal adverse reactions needs further analysis.
The purpose of this research was to analyze the efficacy of liraglutide combined with metformin or acarbose in T2DM patients and to explore the risk factors of gastrointestinal adverse reactions.
Methods
General data
A retrospective analysis was conducted on 88 T2DM patients who were treated in our hospital from February 2019 to August 2021. The patients were divided into group A (n=40) and group B (n=48) according to different treatment methods. The research was approved by the Ethics Committee of our hospital, and the subjects and their guardians were informed and signed a fully informed consent form. Ethics code: 2019A041LL (trial).
Inclusion criteria: ① Patients met the World Health Organization diagnostic criteria for diabetes [16] and were diagnosed as T2DM; ② Patients with complete clinical data; ③ Patients who could cooperate in the study. Exclusion criteria: ① Patients who were allergic to insulin; ② Patients with severe hypoglycemia; ③ Patients with acute complications of diabetes in the last 6 months; ④ Patients with hypertension nephropathy and nephritis hematuria; ⑤ Patients with irregular life pattern; ⑥ Patients who were transferred to the hospital midway, switched to other treatment schemes, lost follow-up or dropped out of the experiment; ⑦ Patients with poor compliance and could not complete the test as required.
Treatment methods
Group A was treated with liraglutide and metformin. The patients were given subcutaneous injection of liraglutide [Novo Nordisk (China) Pharmaceutical Co., Ltd. SFDA Approval No. J20160037] 0.6 mg each time, once a day for 15 weeks. On this basis, 0.85 g metformin hydrochloride tablets (Sino-American Shanghai Squibb Pharmaceuticals Co., Ltd., SFDA Approval No. H20023370) were given orally once a day.
Group B: Patients were given subcutaneous injection of liraglutide [Novo Nordisk (China) Pharmaceutical Co., Ltd. SFDA Approval No. J20160037] 0.6 mg each time, once a day for 15 weeks. Additionally, acarbose tablets (Bayer Healthcare Co., Ltd., SFDA Approval No. H20023370) were given 50 mg each time, three times a day.
During this period, the diet of both groups was controlled. The patients were required to reduce the intake of sugar and have more exercies.
Serum collection
The venous blood 5 mL of patients was collected and placed at room temperature for 30 min, then centrifuged at 4°C, 1500× g for 10 min, and stored in a refrigerator at -70°C.
Outcome measures
Main outcome measures
Glucose control effect
The levels of blood glucose and insulin in both groups were evaluated before and after treatment (1 day after treatment). Fasting blood glucose (FBG) and 2-hour postprandial blood glucose (2hPBG) were measured by blood glucose analyzer. Patients’ glycated hemoglobin (HbAlc) was measured by an automatic biochemical analyzer.
Determination of C-peptide
C-peptide was detected before and 1 day after treatment in two groups of patients. It was then determined by radioimmunoassay kit of Depp (Tianjin). The inter- and intra-assay coefficients of variation (CVs) were 4.4% and 9.8%, respectively. The levels of fasting C-peptide and postprandial 2h C-peptide in venous blood of patients were measured.
Inflammation index
Inflammation indexes of patients were detected before and 1 day after treatment. Enzyme-linked immunosorbent assay (ELISA) was applied to detect C-reactive protein (CRP, Shanghai, China, mlbio, ml057570), interleukin-6 (IL-6, Shanghai, China, mlbio, ml058097) and serum amyloid A (SAA, Shanghai, China, mlbio, ml060332).
Efficacy evaluation
The efficacy evaluation of two groups of patients was tested. Markedly effective: After treatment, the symptoms of patients basically disappeared (FBG<7.2 mmol/L, 2hPBG<8.3 mmol/L). Effective: After treatment, the symptoms of patients were obviously improved (FGB<8.3 mmol/L and 2hPBG<10.0 mmol/L). Ineffective: After treatment, the symptoms of patients was not improved, and the blood glucose did not meet the above standards. Total effective rate = (markedly effective + effective)/total cases ×100%.
Secondary outcome measures
Adverse reactions
A series of adverse reactions during the treatment was observed, including edema, nausea, gastrointestinal discomfort and hypoglycemia. Logistic regression was used to assess the risk factors of gastrointestinal adverse reactions.
Statistical methods
SPSS22.0 statistical software (Easybio, China) was used for data analysis. The counting data were tested by χ2 test. The measured data, expressed as (mean ± standard deviation), were assessed via independent sample t-test. While those within groups were evaluated via paired t-test. Logistic regression method was used to evaluate the risk factors for gastrointestinal adverse reactions in T2DM patients. GraphPadPrism8 software was used to for figure rendering. P<0.05 was regarded as statistically significant.
Results
General data
According to the baseline data of both groups, there was no obvious difference in average age, gender, body mass index (BMI), and drinking or smoking history (P>0.05), indicating the two groups were comparable (Table 1).
Table 1.
General data of two groups of patients [n (%) (Means ± SD)]
| Factor | Group A (n=40) | Group B (n=48) | t/χ2 value | P value |
|---|---|---|---|---|
| Average age (years) | 50.53±6.96 | 51.21±6.53 | 0.47 | 0.638 |
| BMI (kg/m2) | 23.26±2.12 | 22.98±2.57 | 0.55 | 0.584 |
| Average course of disease (years) | 3.54±0.32 | 3.61±0.29 | 1.08 | 0.285 |
| Gender | 0.01 | 0.907 | ||
| Man | 18 (45.00) | 21 (43.75) | ||
| Woman | 22 (55.00) | 27 (56.25) | ||
| Residence | 0.04 | 0.851 | ||
| Villages | 14 (35.00) | 17 (35.42) | ||
| Cities and towns | 26 (65.00) | 29 (64.58) | ||
| Working condition | 0.23 | 0.633 | ||
| Lay-off/retirement | 23 (57.50) | 30 (62.50) | ||
| Incumbent | 17 (42.50) | 18 (37.50) | ||
| Drinking | 0.05 | 0.815 | ||
| Yes | 21 (52.50) | 24 (50.00) | ||
| No | 19 (47.50) | 24 (50.00) | ||
| Smoking | 1.52 | 0.218 | ||
| Yes | 28 (70.00) | 39 (81.25) | ||
| No | 13 (30.00) | 9 (18.75) | ||
| Past medical history | ||||
| Hypertension | 18 | 27 | 1.105 | 0.293 |
| Hyperlipidemia | 10 | 11 | 0.052 | 0.819 |
| Chronic obstructive pulmonary disease | 6 | 8 | 0.045 | 0.831 |
| Complicated by digestive system diseases | 12 | 15 | 0.016 | 0.899 |
Effect on Glucose control (FPG, 2hPG, HbA1c)
It was found that before treatment, there was no difference in the FPG, 2hPG and HbA1c levels between group A and B (P>0.05); But after one day of treatment, those levels in both groups reduced, and the levels in group A were even lower than those in group B (P<0.05) (Figure 1).
Figure 1.
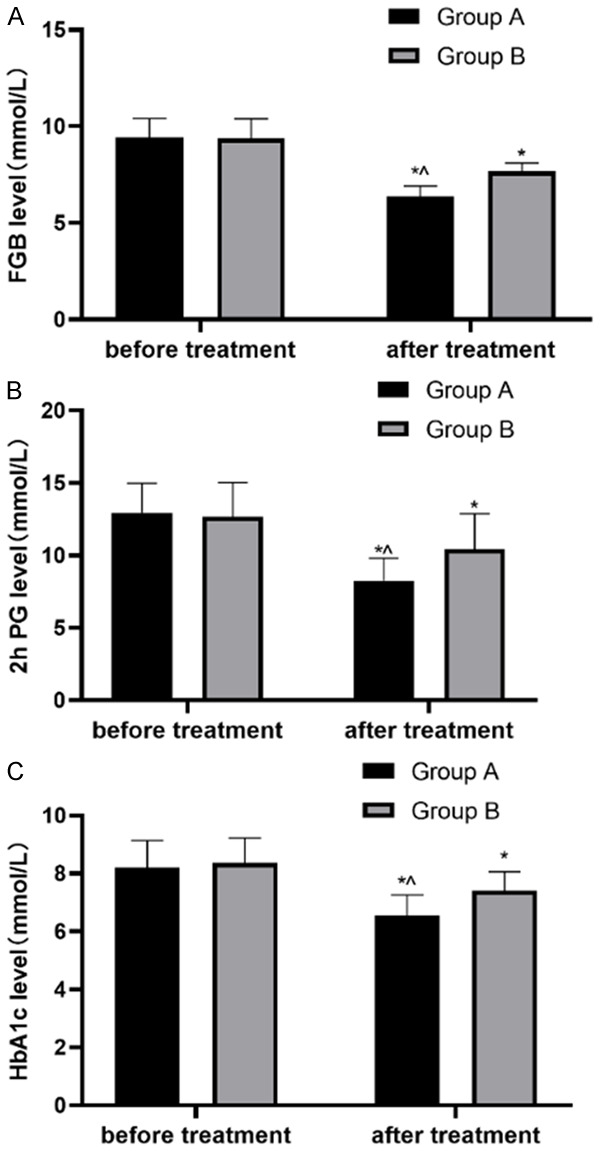
Changes of blood glucose in both groups. A. FGB level in patients before and after treatment; B. 2hPG level before and after treatment in both groups; C. HbA1c level before and after treatment. Note: *P<0.05, compared with before treatment; ^P<0.05, compared with group B after treatment.
C-peptide determination
It was found that there was no difference in fasting C-peptide and postprandial 2h C-peptide levels between two groups before treatment (P>0.05). After treatment, the levels in both groups were higher than those before treatment (all P<0.05), and the levels in group A were significantly higher than those in group B (both P<0.05) (Figure 2).
Figure 2.
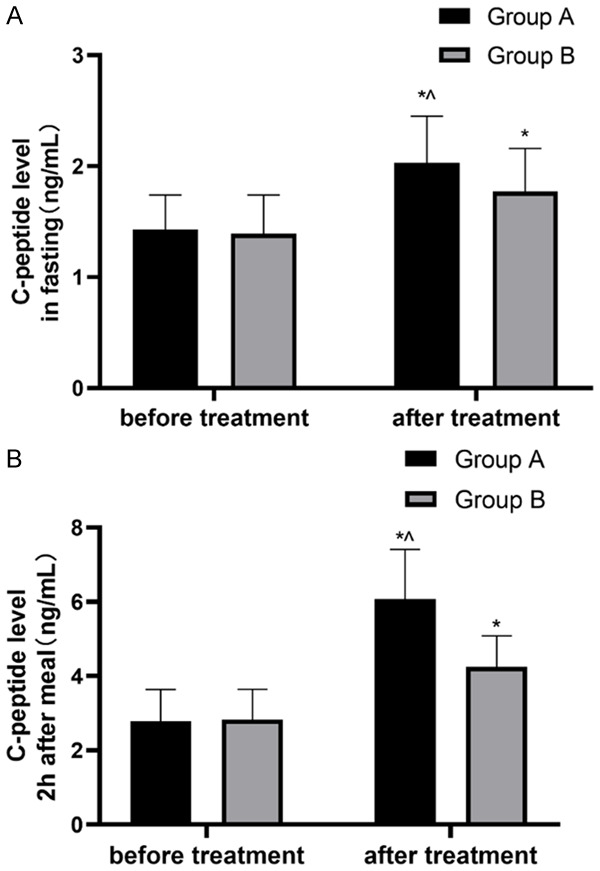
Changes of C-peptide levels in both groups of patients. A. C-peptide level in patients before and after treatment; B. 2h C-peptide level in patients before and after treatment. Note: *P <0.05, compared with before treatment; ^P<0.05, compared with group B after treatment.
Inflammatory indicators
It was found that the IL-6, CRP and SAA levels in both groups were not different before treatment (P>0.05), but after treatment, the levels were lower than those before treatment (P<0.05). However, there was no difference in inflammatory indexes between groups (P>0.05) (Figure 3).
Figure 3.
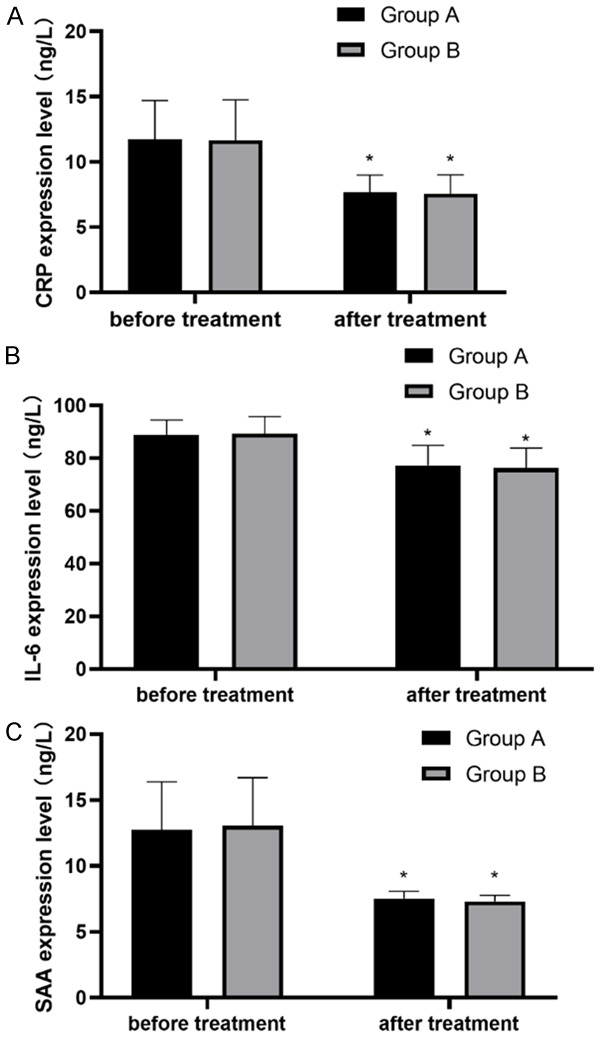
Changes of inflammatory indexes in both groups before and after treatment. A. IL-6 level in patients before and after treatment; B. CRP level in patients before and after treatment; C. SAA level in patients before and after treatment. Note: *P<0.05, compared with before treatment.
Efficacy
It was found that the total effective rate in patients of Group A was higher than that in group B (P<0.05) (Table 2).
Table 2.
Comparison of efficacy between two groups after treatment [n (%)]
| Group A (n=40) | Group B (n=48) | χ2 | P | |
|---|---|---|---|---|
| Markedly effective | 26 (65.00) | 21 (43.75) | - | - |
| Effective | 12 (30.00) | 15 (31.25) | - | - |
| Ineffective | 2 (5.00) | 12 (25.00) | - | - |
| Total effective rate | 38 (95.00) | 36 (75.00) | 6.52 | 0.011 |
Adverse reactions
There was no marked difference in the incidence of adverse reactions between Groups A and B (P<0.05) (Table 3).
Table 3.
Comparison of efficacy between two groups after treatment [n (%)]
| Group A (n=40) | Group B (n=48) | χ2 | P | |
|---|---|---|---|---|
| Edema | 0 (0.00) | 1 (2.08) | - | - |
| Nausea | 1 (2.50) | 1 (2.08) | - | - |
| Hypoglycemia | 0 (0.00) | 1 (2.08) | - | - |
| Incidence of adverse reactions | 2 (5.00) | 3 (20.83) | 0.064 | 0.801 |
Analysis of risk factors of gastrointestinal adverse reactions
The adverse reactions of two groups of patients were counted and analyzed. The results manifested that there were 16 cases of gastrointestinal reaction in group A and 35 in group B. There was marked difference in gastrointestinal adverse reactions between two groups through chi-square test (P<0.05) (Table 4). Then patients were divided into groups based on the occurrence of gastrointestinal tract, and the clinical data were collected (Table 5). Through univariate and multivariate analysis, it was found that digestive system diseases and treatment were independent factors of gastrointestinal reaction (Table 6).
Table 4.
Comparison of incidence of gastrointestinal reactions
| Groups | Occurred | Not occurred | χ2 value | P value |
|---|---|---|---|---|
| Group A (n=40) | 16 | 24 | 9.702 | 0.002 |
| Group B (n=48) | 35 | 13 |
Table 5.
Assignment table
| Factor | Assignment |
|---|---|
| Age (X) | <50=0; ≥50=1 |
| BMI (X) | <23 kg/m2 =0; ≥23 kg/m2 =1 |
| Course of disease (X) | <3.5 years =0; ≥3.5 years =1 |
| Gender (X) | Male =0; Female =1 |
| History of alcoholism (X) | Yes =0; No =1 |
| History of smoking (X) | Yes =0; No =1 |
| Hypertension (X) | Yes =0; No =1 |
| Diabetes (X) | Yes =0; No =1 |
| Obstructive pulmonary emphysema (X) | Yes =0; No =1 |
| Complicated with digestive system diseases (X) | Yes =0; No =1 |
| Treatment plans (X) | Liraglutide combined with metformin =0; Liraglutide combined with acarbose =1 |
| Gastrointestinal adverse reactions (Y) | Yes =1; No =0 |
Table 6.
Logistics regression analysis
| Factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| P value | OR value | 95 CI% | P value | OR value | 95 CI% | |
| Age | 0.871 | 0.924 | 0.353-2.414 | |||
| BMI | 0.259 | 0.611 | 0.260-1.438 | |||
| Course of disease | 0.956 | 0.975 | 0.407-2.337 | |||
| Gender | 0.259 | 1.636 | 0.696-3.848 | |||
| History of alcoholism | 0.370 | 1.477 | 0.630-3.460 | |||
| History of smoking | 0.554 | 0.743 | 0.277-1.990 | |||
| Hypertension | 0.209 | 0.578 | 0.246-1.358 | 0.021 | 3.217 | 1.196-8.652 |
| Diabetes | 0.274 | 1.735 | 0.646-4.656 | |||
| Obstructive pulmonary emphysema | 0.218 | 2.069 | 0.651-6.579 | |||
| Complicated with digestive system diseases | 0.002 | 4.038 | 1.646-9.908 | 0.003 | 4.536 | 1.698-12.122 |
| Treatment plans | 0.015 | 0.276 | 0.098-0.780 | 0.013 | 0.236 | 0.075-0.740 |
Discussion
As the fifth leading cause of death worldwide, the number of people with diabetes is rising sharply [17,18]. T2DM is the most common type of diabetes, accounting for almost 90% of the patients. It causes many complications, thus bringing about great damage to the quality of life and organ function of patients [19]. Hence, the treatment plan, the inhibition of inflammation caused by various complications of diabetes, and the prognosis have become research hotspots [20].
2hPG, FPG and HbA1c play an essential role in the detection and prognosis of diabetes, and these three indexes are independent of each other [21]. HbA1c is widely used as a diagnostic marker for T2DM, which reflects the average plasma glucose level in the first 2-3 months [22]. Although the measurements of FPG and 2hPG can reflect patients’ blood glucose levels to some extent, additional detection of HbA1c can provides more accurate result and can predict the risk of future diabetic complications such as cardiovascular disease. Meanwhile higher HbA1c is associated with higher CVD risk and total mortality [23]. As shown in this study, the 2hPG, FPG and HbA1c levels in group A after treatment were markedly lower than those in group B. After all, acarbose is still a kind of oligosaccharide containing pseudosaccharides, and its inhibitory effect on various intestinal maltase, sucrase, dextrinase and glucosamylase is very limited. It can only inhibit or postpone the absorption of various carbohydrates [24]. As a natural product used in herbs, metformin can directly or indirectly act on the liver to reduce glucose production and play a role in the intestinal part to increase glucose utilization, and most importantly, it can enhance insulin sensitivity [25]. Thus, in group A, combined use of liraglutide and metformin has a better effect on glucose control in patients with diabetes.
From the level of C-peptide determination, the two indexes of C-peptide in group A were higher than those in group B after treatment. C-peptide and insulin are secreted from islet cells in an equal molecular way. Compared with insulin, C-peptide is higher and more stable, so it is generally used to evaluate islet cell function [26]. In view of the results of this study, the efficacy of liraglutide combined with metformin in group A was better.
As for inflammatory factors, the postoperative inflammatory factors of both groups decreased, but there was no obvious difference between two groups. Metformin can not only improve blood glucose control, but also increase the autophagy of CD4+ T cells, and the enhanced function of mitochondrial bioenergy can effectively reduce oxidation and inflammation [27]. However, acarbose also reduced the levels of inflammatory factors in T2DM patients [28]. Although both the two regimens reduced inflammatory factors, it is still vague which one was more effective. As to the adverse reactions, it was found that the incidence of inflammatory reactions, especially gastrointestinal adverse reactions, in group A was less than that in group B, indicating that liraglutide combined with metformin is safer and more friendly to patients’ gastrointestinal function.
To better determine the occurrence of gastrointestinal adverse reactions in patients, we carried out regression analysis. We found that the complicated with digestive system diseases and the treatment regimen were independently tied to gastrointestinal adverse reactions. As the most representative drug of α-glycosidase inhibitor, acarbose can delay the absorption of carbohydrates in the small intestine, and a large quantity of undigested carbohydrates reaches the large intestine. It is further decomposed under the action of intestinal bacteria, resulting in excessive gas production in the intestine [29]. The use of acarbose should start from a low dose, and the dosage can be gradually increased without gastrointestinal adverse reactions. If patients have gastrointestinal adverse reactions, it is necessary to change the drug treatment. The doctors should comprehensively evaluate patients’ gastrointestinal system and formulate a gradually increased drug administration and slow feeding program, so as to avoid poor tolerance and compliance. Digestive tract disease is not a contraindication of liraglutide, but GLP-1 receptor agonists can delay gastric emptying and may aggravate the disease. It is suggested that the patients with digestive tract disease should switch to other hypoglycemic drugs with less gastrointestinal stimulation, such as insulin, sulfonylurea or glinide, etc. If it is necessary to use liraglutide, a low initial dose with gradual increase is recommended.
This research still has some shortcomings. First of all, there is no long-term follow-up of patients. Diabetes is a lifelong disease; The long-term use of the two drugs may cause drug resistance, whether the efficacy would be impaired needs further study. Secondly, the regression study has sample bias. Although we have compared the baseline data, the bias of the results cannot be avoided. Thus, we hope to conduct long-term follow-up and randomized controlled trials to improve the conclusions.
To sum up, compared with liraglutide and acarbose, liraglutide combined with metformin is more effective in controlling glucose during T2DM treatment. Although there is no remarkable difference in eliminating inflammation, liraglutide combined with acarbose can increase the incidence of gastrointestinal adverse reactions in T2DM patients. It is recommended to use liraglutide combined with metformin.
Acknowledgements
This study was supported by project of Henan Province Mdical Science and Technology Tackling Program (Grant No: LHGJ20190263 and LHGJ20190218), and Key scientific research projects of universities in Henan Province (Grant No: 20A320034).
Disclosure of conflict of interest
None.
References
- 1.Tong HV, Luu NK, Son HA, Hoan NV, Hung TT, Velavan TP, Toan NL. Adiponectin and pro-inflammatory cytokines are modulated in Vietnamese patients with type 2 diabetes mellitus. J Diabetes Investig. 2017;8:295–305. doi: 10.1111/jdi.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu L, Li Y, Du C, Zhao W, Zhang H, Yang Y, Sun A, Song X, Feng Z. Pattern recognition receptor-mediated chronic inflammation in the development and progression of obesity-related metabolic diseases. Mediators Inflamm. 2019;2019:5271295. doi: 10.1155/2019/5271295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang K, Liang Y, Su Y, Wang L. DhHP-6 ameliorates hepatic oxidative stress and insulin resistance in type 2 diabetes mellitus through the PI3K/AKT and AMPK pathway. Biochem J. 2020;477:2363–2381. doi: 10.1042/BCJ20200402. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Cao YF, Sun XY, Han L, Li SN, Gu WQ, Song M, Jiang CT, Yang X, Fang ZZ. Plasma tyrosine and its interaction with low high-density lipoprotein cholesterol and the risk of type 2 diabetes mellitus in Chinese. J Diabetes Investig. 2019;10:491–498. doi: 10.1111/jdi.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonio-Villa NE, Bello-Chavolla OY, Vargas-Vazquez A, Mehta R, Aguilar-Salinas CA Metabolic Syndrome Study Group. The combination of insulin resistance and visceral adipose tissue estimation improves the performance of metabolic syndrome as a predictor of type 2 diabetes. Diabet Med. 2020;37:1192–1201. doi: 10.1111/dme.14274. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G 2010 China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen LV, Flint A, Olsen AK, Ingwersen SH. Liraglutide in type 2 diabetes mellitus: clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2016;55:657–672. doi: 10.1007/s40262-015-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seppa K, Toots M, Reimets R, Jagomae T, Koppel T, Pallase M, Hasselholt S, Krogsbaek Mikkelsen M, Randel Nyengaard J, Vasar E, Terasmaa A, Plaas M. GLP-1 receptor agonist liraglutide has a neuroprotective effect on an aged rat model of Wolfram syndrome. Sci Rep. 2019;9:15742. doi: 10.1038/s41598-019-52295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris S, Abrahamson MJ, Ceriello A, Charpentier G, Evans M, Lehmann R, Liebl A, Linjawi S, Holt RIG, Hosszúfalusi N, Rutten G, Vilsbøll T. Clinical considerations when initiating and titrating insulin degludec/liraglutide (IDegLira) in people with type 2 diabetes. Drugs. 2020;80:147–165. doi: 10.1007/s40265-019-01245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu H, Zhang Y, Shi Z, Lu D, Li T, Ding Y, Ruan Y, Xu A. The neuroprotection of liraglutide against ischaemia-induced apoptosis through the activation of the PI3K/AKT and MAPK pathways. Sci Rep. 2016;6:26859. doi: 10.1038/srep26859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Ambrosy AP, DeVore AD, Margulies KB, McNulty SE, Mentz RJ, Hernandez AF, Michael Felker G, Cooper LB, Lala A, Vader J, Groake JD, Borlaug BA, Velazquez EJ. Liraglutide and weight loss among patients with advanced heart failure and a reduced ejection fraction: insights from the FIGHT trial. ESC Heart Fail. 2018;5:1035–1043. doi: 10.1002/ehf2.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Madsen KS, Kahler P, Kahler LKA, Madsbad S, Gnesin F, Metzendorf MI, Richter B, Hemmingsen B. Metformin and second- or third-generation sulphonylurea combination therapy for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2019;4:CD012368. doi: 10.1002/14651858.CD012368.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zilov AV, Abdelaziz SI, AlShammary A, Al Zahrani A, Amir A, Assaad Khalil SH, Brand K, Elkafrawy N, Hassoun AAK, Jahed A, Jarrah N, Mrabeti S, Paruk I. Mechanisms of action of metformin with special reference to cardiovascular protection. Diabetes Metab Res Rev. 2019;35:e3173. doi: 10.1002/dmrr.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang F, Xu S, Tang L, Pan X, Tong N. Acarbose with comparable glucose-lowering but superior weight-loss efficacy to dipeptidyl peptidase-4 inhibitors: a systematic review and network meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) 2020;11:288. doi: 10.3389/fendo.2020.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulgar Suarez M, Gomez Guedes P, Aguado Diaz M, Menendez Alvarez S, Garcia Garaboa A, Rodriguez Gonzalez I, Gonzalez Pelaez C. Validity of new diagnostic criteria for type 2 diabetes mellitus. Impact of its application in a health care area. Aten Primaria. 2001;27:111–115. doi: 10.1016/S0212-6567(01)78783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) Edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 19.Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016;4:537–547. doi: 10.1016/S2213-8587(16)30010-9. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Fu R, Lei C, Deng Y, Lou W, Wang L, Zheng Y, Deng X, Yang S, Wang M, Zhai Z, Zhu Y, Xiang D, Hu J, Dai Z, Gao J. Estimates of type 2 diabetes mellitus burden attributable to particulate matter pollution and its 30-year change patterns: a systematic analysis of data from the Global Burden of Disease Study 2019. Front Endocrinol (Lausanne) 2021;12:689079. doi: 10.3389/fendo.2021.689079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Yang Z, Zhang M, Zhu Y, Zhao Z, Huang Z, Li C, Zhou M, Farmer AJ, Tang J, Wang L. Independent effects of 2hPG, FPG and HbA1c on cardiovascular risk: analysis of a nationally representative sample from China. Diabetes Res Clin Pract. 2021;173:108672. doi: 10.1016/j.diabres.2021.108672. [DOI] [PubMed] [Google Scholar]
- 22.Petersmann A, Muller-Wieland D, Muller UA, Landgraf R, Nauck M, Freckmann G, Heinemann L, Schleicher E. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2019;127(Suppl 1):S1–S7. doi: 10.1055/a-1018-9078. [DOI] [PubMed] [Google Scholar]
- 23.Wisgerhof W, Ruijgrok C, den Braver NR, Borgonjen-van den Berg KJ, van der Heijden A, Elders PJM, Beulens JWJ, Alssema M. Phenotypic and lifestyle determinants of HbA1c in the general population-The Hoorn Study. PLoS One. 2020;15:e0233769. doi: 10.1371/journal.pone.0233769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q, Luo Y, Zhang X, Kang Q, Zhang D, Zhang L, Bai L, Deng Z. A severe leakage of intermediates to shunt products in acarbose biosynthesis. Nat Commun. 2020;11:1468. doi: 10.1038/s41467-020-15234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ismail HM, Evans-Molina C, DiMeglio LA, Becker DJ, Libman I, Sims EK, Boulware D, Herold KC, Rafkin L, Skyler J, Cleves MA, Palmer J, Sosenko JM Type 1 Diabetes Trial Net and Diabetes Prevention Trial-Type-1 Study Group. Associations of HbA1c with the timing of C-peptide responses during the oral glucose tolerance test at the diagnosis of type 1 diabetes. Pediatr Diabetes. 2019;20:408–413. doi: 10.1111/pedi.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, Jiang K, Liu R, Guo Z, Deeney J, Apovian CM, Snyder-Cappione J, Hawk GS, Fleeman RM, Pihl RMF, Thompson K, Belkina AC, Cui L, Proctor EA, Kern PA, Nikolajczyk BS. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 2020;32:44–55. e6. doi: 10.1016/j.cmet.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mo D, Liu S, Ma H, Tian H, Yu H, Zhang X, Tong N, Liao J, Ren Y. Effects of acarbose and metformin on the inflammatory state in newly diagnosed type 2 diabetes patients: a one-year randomized clinical study. Drug Des Devel Ther. 2019;13:2769–2776. doi: 10.2147/DDDT.S208327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LiverTox: clinical and research information on drug-induced liver injury. Bethesda (MD) 2012 [PubMed] [Google Scholar]


