Abstract
Objective: To investigate the prognosis of patients with early gastric carcinoma (EGC) treated by endoscopic submucosal dissection (ESD) and the risk factors for additional postoperative surgery. Methods: A retrospective analysis was performed on 100 patients with EGC admitted to our hospital from January 2017 to May 2019. According to different surgical methods, patients were divided into the ESD (n=60) and endoscopic mucosal resection (EMR) groups (n=40). Clinical efficacy, perioperative indexes, incidence of complications and risk factors for additional postoperative surgery were compared. Results: The ESD group had evidently prolonged operation time (P<0.01) but similar intraoperative blood loss (P>0.05) as compared with the EMR group. In comparison to the EMR group, the gastrointestinal recovery time and length of stay in the ESD group were notably shorter (P<0.01), the rates of en bloc resection and complete resection of lesions were markedly higher (P<0.05), and the postoperative fever/infection rate was noticeably lower (P<0.05). The two surgical methods had no significant difference on the overall survival rate of patients (P=0.302). It was identified that the infiltration depth and the positive surgical margin were independent risk factors for postoperative additional surgery (all P<0.05). ROC analysis revealed that positive surgical margin was quite valuable in judging the need for additional postoperative surgery. Conclusion: ESD can accelerate the postoperative recovery of patients with EGC, and positive surgical margin is independently tied to additional postoperative surgery in patients after ESD.
Keywords: Early gastric carcinoma, endoscopic submucosal dissection, prognosis, additional postoperative surgery, risk factors
Introduction
Gastric carcinoma (GC) is one of the most familiar gastrointestinal malignancies worldwide [1]. Survey data show that GC afflicts 1 million people every year, causing an annual death rate of more than 780,000 [2]. Today, early diagnosis and treatment for gastric carcinoma (EGC) has huge challenges and has attracted worldwide attention [3]. Gastric mucosa or submucosal cancer is defined as EGC, regardless of tumor size or lymph node metastasis [4]. As the onset of EGC is relatively subtle, most patients have no obvious symptoms in the early stage; hence, it cannot be identified until the patient is admitted to hospital after further development of the disease and the emergence of clinical symptoms [5]. As a result, patients tend to miss the optimal timing for treatment.
As medicine develops, the diagnostic rate of EGC has increased significantly, and the effective treatment for patients with EGC can greatly improve their postoperative survival and quality of life (QoL) [6]. Research has shown that the survival of patients with EGC is substantially prolonged and the recurrence rate is reduced after surgical treatment [7]. Traditional treatment for EGC is radical gastrectomy, but it has some trauma and slow recovery [8]. The emergence of endoscopic submucosal dissection (ESD) has greatly addressed this problem [9]. ESD has been clinically considered as the preferred treatment for EGC without lymph node metastasis [10]. Before the popularization of ESD, conventional endoscopic mucosal resection (EMR) with snares was mainly used in clinical scenarios. However, there are technical limitations of EMR for EGC or submucosal fibrosis with diameter greater than 20 mm [11]. No research has been conducted to clarify whether the two modalities have an impact on prognosis after ESD treatment.
In addition, a survey has found that some patients with EGC face incurable resection after ESD treatment [12]. Non-curative resection refers to incomplete resection, involving non-block resection and/or positive margin, as well as the existence of related risk factors for lymph node metastasis [13]. Some studies found that the 5-year survival rate of the group given additional surgery was higher than that given no additional surgery in the early stage of gastric cancer that is incurable under ESD [14,15]. However, there is still controversy about the risk factors of additional surgery.
Accordingly, we retrospectively analyzed the impacts of EMR and ESD as two frequently-used surgical methods on the prognosis of patients with EGC and explored the risk factors of additional surgery after ESD treatment, so as to provide reference for clinical treatment.
Methods and materials
Clinical data
Altogether 100 patients with EGC admitted to the First People’s Hospital of Shangqiu from January 2017 to May 2019 were analyzed retrospectively and grouped based on different treatment modalities. Among them, 60 cases were treated with ESD and 40 with EMR. This research was approved by the Medical Ethics Committee of the First People’s Hospital of Shangqiu (ethical approval No.: LL2020 (review) 041A (check)).
Inclusion and exclusion criteria
Inclusion criteria
Patients diagnosed with EGC by imaging and pathological biopsy; Patients with clinical stage T1; and patients who met the surgical indications. Patients and their families provided written informed consent before surgery.
Exclusion criteria
Patients with other tumors; Patients with follow-up failure; Patients with severe cardiopulmonary dysfunction; Patients with recent use of anticoagulant drugs.
Surgical methods
ESD treatment scheme
Indigo carmine 0.2% was used for staining to display the type and boundary of lesions under the endoscope. The site 5 mm outside the tumor boundary was marked with a needle knife, and mixed solution (10% glycerin, 0.9% sodium chloride, 5% fructose, indigo carmine, and epinephrine) was injected. The site was pre-cut with a needle knife, and a circular incision on the outside was marked with an IT or Flex knife. During the operation, submucosal injection was increased as appropriate, and the lesion should be fully raised to ensure clear anatomy. Coagrasper hemostatic forceps was used to treat mucosal defect wounds, and preventive hemostasis was implemented for visible blood vessels. A hemostatic clip was adopted to clamp the larger exposed blood vessels, and sucralfate suspension was sprayed on the wound surface to ensure complete hemostasis as a wound protective agent to prevent bleeding. During the operation, a small amount of bleeding was coagulated by heat, and a large amount of bleeding was washed repeatedly with normal saline. After the bleeding point was determined, thermocoagulation or hemostatic forceps were used to stop bleeding.
EMR treatment scheme
An injection needle was inserted through the channel for endoscopic biopsy. The needle tip was pierced into the bottom of polyp base, and 2-10 ml epinephrine + saline (1:10000) was injected into the submucosa 2-3 mm from the outer edge of the lesion via multi-point submucosa, so that the whole lesion was obviously raised, that is, the lifting sign was positive. The lesion was separated from the muscular layer, and the raised lesion was snared by an electric snare, followed by partial excision. Finally, complete excision was performed: electrocoagulation current was used, and then mixed current was used to remove the wound. The wound was observed for 1-2 min. In the case of no active bleeding, the endoscope was withdrawn.
Patients were fasted for 8 hours and water-deprived for 6 hours before operation. Routine blood work, electrolytes, liver and kidney function, five items of hepatitis B, blood type and four items of coagulation were examined to evaluate the general situation, coagulation function and postoperative bleeding risk. After that, the wound was carefully checked, and electrocoagulation was applied to the exposed blood vessels to prevent bleeding. On the first day after operation, the patient was prohibited from drinking water, and given intravenous fluids and electrolytes. Attention was paid to the changes of blood pressure, respiration, pulse and electrocardiogram of patients. If there was no abnormality in related examinations and clinical manifestations, the patient was allowed to take a small amount of liquid food on the second day after operation.
The main surgical tools included GIF-H260 gastroscope (Olympus Corp, Japan), ICC-200 (ERBR, Germany) and Coagrasper hemostatic forceps (Olympus Corp, Japan).
Outcome measures
Primary outcome measures
En bloc and complete resection rates of lesions were observed. The overall survival rate was compared between the two cohorts. Patients were enrolled to additional (n=13) and non-additional surgery groups (n=47) based on their postoperative additional surgery. The clinical data were collected and analyzed by Logistic regression. The indexes with differences in univariate analysis were selected for multivariate Logistic regression, and the backward LR method was used for analysis.
Secondary outcome measures
Perioperative indexes and complication rate were compared.
Statistical methods
Categorical variables were expressed as (%) and analyzed using the Chi-square test, Continuous variables in normal distribution were expressed as mean ± standard deviation (mean ± SD), and the inter-group comparison was conducted using independent samples t-test while the intra-group comparison was done using paired t-test. Logistic regression analysis was adopted to analyze the risk factors for postoperative additional surgery in patients undergoing ESD. SPSS 20.0 (SPSS, Chicago, USA) software was used for data analyses and P<0.05 was regarded as statistical significance.
Results
Clinical data comparison
There were no marked differences in age, gender, lesion location, tumor diameter, invasion depth and differentiation degree between the ESD and EMR groups (all P>0.05, Table 1).
Table 1.
Clinical data
| Factor | ESD group (n=60) | EMR group (n=40) | P value |
|---|---|---|---|
| Age | 0.185 | ||
| ≥55 (n=58) | 38 | 20 | |
| <55 (n=42) | 22 | 20 | |
| Gender | 0.281 | ||
| Male (n=59) | 38 | 21 | |
| Female (n=41) | 22 | 19 | |
| Lesion site | 0.825 | ||
| Cardia (n=24) | 14 | 10 | |
| Gastric body (n=31) | 20 | 11 | |
| Gastric antrum (n=45) | 26 | 19 | |
| Tumor diameter | 0.242 | ||
| ≤20 mm (n=47) | 47 | 35 | |
| >20 mm (n=13) | 13 | 5 | |
| Infiltration depth | 0.736 | ||
| Mucosal layer (M) (n=79) | 46 | 33 | |
| Submucosa (SM1) (n=16) | 11 | 5 | |
| Submucosa (SM2) (n=5) | 3 | 2 | |
| Differentiation | 0.451 | ||
| Poorly differentiated (n=12) | 6 | 6 | |
| Moderate and high differentiated (n=88) | 54 | 34 |
Note: Chi-square test was used.
Comparison of perioperative indexes of patients
This research first compared the changes of perioperative indexes between the two cohorts after different surgical treatments. The ESD group experienced notably longer operation time (P<0.01, Figure 1A) than the EMR group, and showed similar intraoperative blood loss (P>0.05, Figure 1B). Whereas, the gastrointestinal recovery time and length of stay in the ESD group were remarkably shorter than the EMR group (P<0.01, Figure 1C, 1D), suggesting that ESD treatment can accelerate patients’ gastrointestinal recovery.
Figure 1.
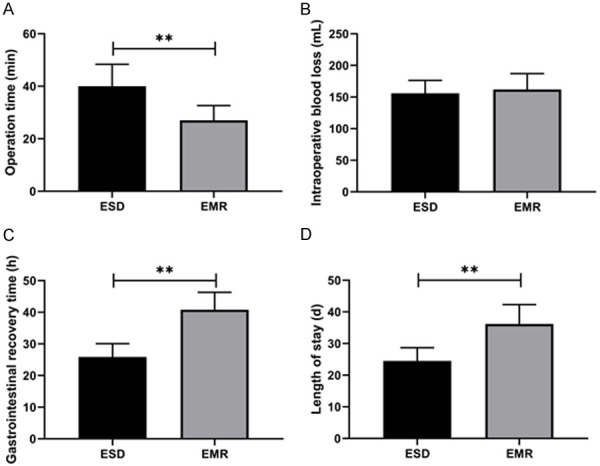
Comparison of perioperative indicators of patients. A. Comparison of operation time between two groups. B. Comparison of intraoperative blood loss between two groups. C. Comparison of gastrointestinal recovery time between two groups. D. Comparison of length of stay between two groups. Note: **P<0.01. The inter-group comparison was assessed by independent sample t-test.
Comparison of en bloc resection rate and complete resection rate of lesions
Furthermore, the en bloc and complete resection rates of lesions were compared, and markedly higher rates of en bloc resection and complete resection of lesions were found in the ESD group (P<0.05, Table 2).
Table 2.
En bloc resection rate and complete resection rate of lesions
| Groups | n | En bloc resection rate of lesions | Complete resection rate of lesions |
|---|---|---|---|
| ESD group | 60 | 58 (95.00%) | 58 (95.00%) |
| EMR group | 40 | 25 (62.50%) | 30 (75.00%) |
| χ2 value | 19.856 | 10.669 | |
| P value | <0.001 | 0.001 |
Note: Chi-square test was used.
Incidence of complications
The comparison of the incidence of postoperative complications between both groups revealed a notably lower incidence of postoperative fever/infection rate in the ESD group compared with the EMR group (P<0.05), but no statistical difference in intraoperative bleeding, postoperative delayed bleeding, perforation or subcutaneous emphysema (P>0.05, Table 3).
Table 3.
Incidence of complications
| Variables | ESD group (n=60) | EMR group (n=40) | χ2 value | P value |
|---|---|---|---|---|
| Postoperative fever/infection | 2 (3.33%) | 10 (25.00%) | 10.669 | 0.001 |
| Intraoperative bleeding | 6 (10.00%) | 5 (12.50%) | 0.153 | 0.696 |
| Postoperative delayed bleeding | 2 (3.33%) | 4 (10.00%) | 0.877 | 0.349 |
| Perforation | 1 (1.66%) | 1 (2.50%) | 0.085 | 0.771 |
| Subcutaneous emphysema | 5 (8.33%) | 4 (10.00%) | 0.081 | 0.775 |
Note: Chi-square test was used.
Comparison of overall survival of patients
The follow-up ended in May 2020, and patients were followed up successfully through outpatient review of electronic pathology records and telephone. We found that the two surgical methods had no marked difference on the overall survival rate of patients (P=0.302, Figure 2).
Figure 2.
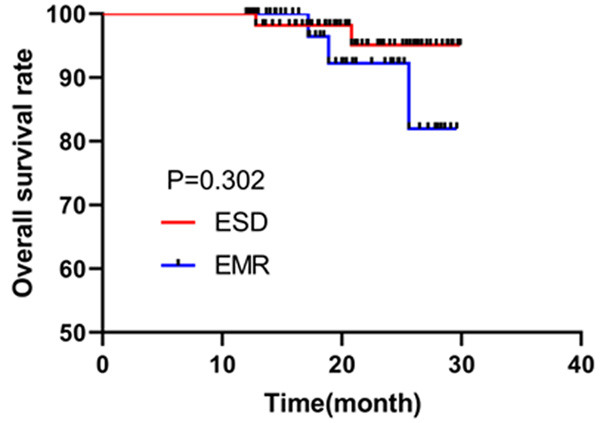
Comparison of overall survival rate between two groups. Kaplan-Meier survival curve was used to assess the survival of patients.
Analysis of risk factors of postoperative additional surgery after ESD
In this research, we also recorded the postoperative additional operations in the ESD group and analyzed the independent risk factors for additional operations using the Logistic regression test (Table 4). First of all, 60 patients were grouped based on the conditions of postoperative additional surgery, including 13 with additional surgery and 47 without. Univariate analysis was conducted to analyze the correlation of postoperative additional surgery with patients’ age, gender, lesion location, ulcer, tumor diameter, infiltration depth, differentiation degree, and positive surgical margin. This revealed that the independence of infiltration depth and the positive surgical margin were the risk factors for postoperative additional surgery in both groups (Table 5, P<0.05). Further, multivariate analysis confirmed the accuracy of the conclusion obtained from the Univariate analysis, that is, infiltration depth and positive surgical margin were the independent risk factors for postoperative additional surgery in patients who received ESD (Table 6, P<0.05). In view of the joint ROC curve-based analysis, the areas under the curves of infiltration depth and positive surgical margin for predicting the additional surgery after ESD was 0.659 and 0.733, respectively, and the area under the curve of infiltration depth was not statistically significant (Table 7, Figure 3, P>0.05).
Table 4.
Assignment
| Factors | Assignment |
|---|---|
| Infiltration depth | Mucosal layer (M) =0, Submucosal layer (SM1) =1, Submucosal layer (SM2) =2. |
| Surgical margin | Positive =0, Negative =1. |
| Additional surgery | Yes =0, No =1. |
Table 5.
Analysis of clinical data of patients
| Variables | Additional surgery group (n=13) | Non-additional surgery group (n=47) | P value |
|---|---|---|---|
| Age (years) | 0.423 | ||
| ≥55 (n=38) | 7 | 31 | |
| <55 (n=22) | 6 | 16 | |
| Gender | 0.879 | ||
| Male (n=38) | 8 | 30 | |
| Female (n=22) | 5 | 17 | |
| Lesion location | 0.975 | ||
| Cardia (n=14) | 3 | 11 | |
| Gastric body (n=20) | 4 | 13 | |
| Gastric antrum (n=26) | 6 | 23 | |
| Ulcer | 0.637 | ||
| With (n=7) | 2 | 5 | |
| Without (n=53) | 11 | 42 | |
| Tumor diameter (mm) | 0.368 | ||
| ≤20 (n=47) | 9 | 38 | |
| >20 (n=13) | 4 | 9 | |
| Infiltration depth | <0.001 | ||
| Mucous layer (M) (n=46) | 6 | 40 | |
| Submucosa (SM1) (n=11) | 4 | 7 | |
| Lamina propria (SM2) (n=3) | 3 | 0 | |
| Differentiation degree | 0.299 | ||
| Low differentiation (n=6) | 2 | 3 | |
| Medium + well differentiation (n=54) | 11 | 44 | |
| Character of surgical margin | <0.001 | ||
| Positive (n=15) | 8 | 7 | |
| Negative (n=46) | 5 | 40 |
Note: Chi-square test was used.
Table 6.
Multivariate analysis
| Variables | β | S.E | Wals | Sig. | Exp (β) | EXP (B) 95% CI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower bound | Upper bound | ||||||
| Infiltration depth | -1.618 | 0.65 | 6.19 | 0.013 | 0.198 | 0.055 | 0.709 |
| Surgical margin | 2.082 | 0.777 | 7.186 | 0.007 | 8.024 | 1.75 | 36.781 |
Table 7.
ROC curve parameters
| Index | AUC | 95% CI | P-value | Specificity | Sensitivity | Youden index |
|---|---|---|---|---|---|---|
| Infiltration depth | 0.658 | 0.473-0.844 | 0.081 | 82.97 | 53.84 | 29.13 |
| Surgical margin | 0.733 | 0.563-0.903 | 0.011 | 85.10 | 61.54 | 46.64 |
Note: AUC: area under curve.
Figure 3.
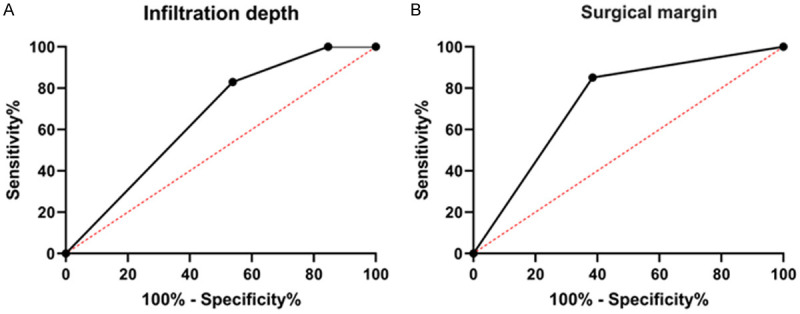
ROC curve of infiltration depth and surgical margin predicted by postoperative additional surgery. A. ROC curve of infiltration depth in predicting postoperative additional surgery. B. ROC curve of surgical margin in predicting postoperative additional surgery. ROC curve was used.
Discussion
Today, people’s eating habits have enormously changed as the life quality improves [16], which has led to a growing incidence of digestive system diseases [17]. GC, as one of the top three digestive system tumors, has seriously threatened the QoL and safety of human beings [18]. The existing investigation shows that the 5-year survival rate of EGC is as high as 90%, while that of patients with advanced GC do not even reach 30% [19]. Therefore, the detection, early diagnosis and early treatment of EGC are of utmost importance to the survival of patients [20]. Nowadays, as the medical level and people’s awareness of cancer prevention improve, the early diagnosis rate of GC has been continuously raised [21]. At this stage, EGC is mainly treated clinically by traditional surgical resection combined with lymph node dissection. However, such a treatment method can cause greater trauma, various postoperative complications and slow recovery of patients [22]. Some scholars believe that radical resection of EGC is an over-treatment.
Recent years have witnessed the increasing application of EMR and ESD in the treatment of EGC driven by the continuous advances in endoscopic techniques. The operation is carried out with different electric knives according to the condition of the lesion, and the tissue between the mucous layer and the muscularis propria is gradually separated through endoscopic observation. Then the diseased mucosa and submucosa are completely peeled off step by step, which greatly reduces the trauma to the patients [23]. However, it is not clear whether EMR and ESD have different impacts on the clinical efficacy on and prognosis of patients with EGC. Through this retrospective analysis, we found that despite a longer operation time, ESD contributed to notably shorter gastrointestinal recovery time and hospitalization time in patients receiving ESD treatment compared with EMR, which indicated that the postoperative recovery of patients with EGC after ESD treatment was significantly faster than that of patients undergoing EMR. In addition, notably higher rates of en bloc resection and complete resection were found in the ESD group, suggesting that the resection effect of ESD was superior to that of EMR. The research of Oka et al. also showed that ESD increased the overall and histological resection rates, which was consistent with our findings [24]. Furthermore, we compared the postoperative complications of patients, and found that the fever infection rate of patients after EMR treatment increased significantly, but there was no difference in overall survival rate between the two groups based on follow-up. Sun et al. [25] found that the 2-year survival rate of patients with ESD was higher than that of EMR, which was inconsistent with our results. We think this may be due to the small number of samples. All the above findings confirm that ESD treatment can substantially improve the postoperative recovery and complete resection rate.
As the preferred choice for the treatment of EGC, ESD can retain the original physiological and anatomical structure, thereby reducing the complications caused by gastrointestinal reconstruction and improving the postoperative QoL of patients [26]. However, for non-curative resection of vascular infiltration, a second surgical operation is required after the initial operation for radical excision [27]. Given that the risk factors of additional surgery are not clear, we conducted analysis in 60 patients who underwent ESD. It was found that postoperative additional surgery was correlated with infiltration depth and positive surgical margin, which was consistent with the research by Ding et al. [28]. However, through further ROC curve-based analysis, we found that the predictive value of infiltration depth for additional surgery was low, which was not statistically significant, while the area under the curve of positive surgical margin was greater than 0.7, which was statistically remarkable. These results indicate that positive surgical margin is expected to become a potential outcome measure for additional surgery after ESD.
In this study, the effectiveness of ESD for EGC was determined through analysis, and the risk factors of additional operation surgery post ESD were discussed. However, as a retrospective analysis, this study still has sample defects compared with random control analysis, which may lead to biased results. Second, the sample size was relatively small, and the follow-up time was short. Therefore, we will increase the sample size and extend the follow-up time in subsequent experiments to affirm our research conclusions.
To sum up, ESD can accelerate the postoperative recovery of patients with EGC, and the infiltration depth, positive surgical margin are independent risk factors for postoperative additional surgery in patients undergoing ESD.
Disclosure of conflict of interest
None.
References
- 1.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Venerito M, Link A, Rokkas T, Malfertheiner P. Gastric cancer-clinical and epidemiological aspects. Helicobacter. 2016;21(Suppl 1):39–44. doi: 10.1111/hel.12339. [DOI] [PubMed] [Google Scholar]
- 4.Choi YJ, Kim N. Gastric cancer and family history. Korean J Intern Med. 2016;31:1042–1053. doi: 10.3904/kjim.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. doi: 10.1177/1010428317714626. [DOI] [PubMed] [Google Scholar]
- 7.Hatta W, Gotoda T, Koike T, Masamune A. History and future perspectives in Japanese guidelines for endoscopic resection of early gastric cancer. Dig Endosc. 2020;32:180–190. doi: 10.1111/den.13531. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Choi CW. Common locations of gastric cancer: review of research from the endoscopic submucosal dissection era. J Korean Med Sci. 2019;34:e231. doi: 10.3346/jkms.2019.34.e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, Matsui T. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3–15. doi: 10.1111/den.12518. [DOI] [PubMed] [Google Scholar]
- 10.Nishizawa T, Yahagi N. Long-term outcomes of using endoscopic submucosal dissection to treat early gastric cancer. Gut Liver. 2018;12:119–124. doi: 10.5009/gnl17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakaya N, Nakamura T, Shimosegawa T. A scoring system to stratify curability after endoscopic submucosal dissection for early gastric cancer: “eCura system”. Am J Gastroenterol. 2017;112:874–881. doi: 10.1038/ajg.2017.95. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Hong SJ. Treatment strategies after non-curative endoscopic resection of early gastric cancer. Korean J Gastroenterol. 2017;70:128–133. doi: 10.4166/kjg.2017.70.3.128. [DOI] [PubMed] [Google Scholar]
- 13.Isomoto H. Differentiated-type predominant mixed-histology-type early gastric cancer is a significant risk factor for endoscopic non-curative resection regardless of tumor size. Dig Endosc. 2018;30:602–604. doi: 10.1111/den.13195. [DOI] [PubMed] [Google Scholar]
- 14.Jeon MY, Park JC, Hahn KY, Shin SK, Lee SK, Lee YC. Long-term outcomes after noncurative endoscopic resection of early gastric cancer: the optimal time for additional endoscopic treatment. Gastrointest Endosc. 2018;87:1003–1013. e1002. doi: 10.1016/j.gie.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Gotoda T, Hatta W, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Shimosegawa T. Survival benefit of additional surgery after non-curative endoscopic submucosal dissection for early gastric cancer: a propensity score matching analysis. Ann Surg Oncol. 2017;24:3353–3360. doi: 10.1245/s10434-017-6039-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Zhu C, Lu X. Advances in serum biomarkers for early diagnosis of gastric cancer. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019;48:326–333. doi: 10.3785/j.issn.1008-9292.2019.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21:4012. doi: 10.3390/ijms21114012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Yu C. Helicobacter pylori infection following endoscopic resection of early gastric cancer. Biomed Res Int. 2019;2019:9824964. doi: 10.1155/2019/9824964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engstrand L, Graham DY. Microbiome and gastric cancer. Dig Dis Sci. 2020;65:865–873. doi: 10.1007/s10620-020-06101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yusefi AR, Bagheri Lankarani K, Bastani P, Radinmanesh M, Kavosi Z. Risk factors for gastric cancer: a systematic review. Asian Pac J Cancer Prev. 2018;19:591–603. doi: 10.22034/APJCP.2018.19.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu D, Zhang P, Ma J, Xu J, Yang L, Xu W, Que H, Chen M, Xu H. Serum biomarker panels for the diagnosis of gastric cancer. Cancer Med. 2019;8:1576–1583. doi: 10.1002/cam4.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilson DH. Advances in the treatment of gastric cancer: 2019. Curr Opin Gastroenterol. 2019;35:551–554. doi: 10.1097/MOG.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 23.Petryszyn P, Chapelle N, Matysiak-Budnik T. Gastric cancer: where are we heading? Dig Dis. 2020;38:280–285. doi: 10.1159/000506509. [DOI] [PubMed] [Google Scholar]
- 24.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 25.Sun H, Qian YS. Clinical effects of ESD and EMR in the treatment of early gastric cancer and its influence on complications. Clinical Medical Research and Practice. 2021;6:63–65. [Google Scholar]
- 26.Makimoto S, Takami T, Hatano K, Kataoka N, Yamaguchi T, Tomita M, Shono Y. Additional surgery after endoscopic submucosal dissection for colorectal cancer: a review of 53 cases. Int J Colorectal Dis. 2019;34:1723–1729. doi: 10.1007/s00384-019-03370-7. [DOI] [PubMed] [Google Scholar]
- 27.Cheng P, Lu Z, Zhang M, Chen H, Guo Z, Zheng Z, Wang X. Is additional surgery necessary after non-curative endoscopic submucosal dissection for early colorectal cancer? J Invest Surg. 2021;34:889–894. doi: 10.1080/08941939.2019.1697770. [DOI] [PubMed] [Google Scholar]
- 28.Ding J, Yuan Y, Liu S, Feng M, Guan W. The risk factors analysis of additional surgery after endoscopic submucosal dissection (ESD) for early gastric cancer. Chin J Gen Surg (Electronic Edition) 2017;11:118–122. [Google Scholar]


