Abstract
Objective: To explore the effects of ipratropium bromide combined with non-invasive ventilation for patients with both chronic obstructive pulmonary disease (COPD) and respiratory failure. Methods: A total of 110 patients with both COPD and respiratory failure who were admitted to our hospital from April 2018 to August 2019 were enrolled in this study; of which 52 patients were treated with a noninvasive ventilator as Group A, and the rest were treated with ipratropium bromide combined with noninvasive ventilation as Group B. The two groups were compared for blood gas indexes, pulmonary function, and treatment efficacy, and adverse reactions. Results: After treatment, Group B showed better blood gas indexes and pulmonary function than Group A (both P < 0.05), and Group B also showed significantly lower levels of inflammatory factors than Group A (P < 0.05). In addition, the efficacy and life quality of Group B were better than those of Group A, and adverse reactions of Group B were less than those of Group A (all P < 0.05). Conclusion: Ipratropium bromide combined with noninvasive ventilation is effective in the treatment of patients with both COPD and respiratory failure.
Keywords: Ipratropium bromide, noninvasive ventilator, COPD
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease caused by damage due to harmful chemicals [1]. These chemicals give rise to a series of inflammatory reactions, which not only destroys the lung structure, but also increases mucus production in the respiratory tract [2]. COPD is an incomplete reversible syndrome with limited air flow [3], which causes permanent damage to the respiratory tract and lungs, polypnea, and gradual deterioration with cough, and even further results in irreversible loss of the lung function, life quality decline and even death in serious cases [4]. Acute respiratory failure caused by hypercapnia is a common complication of COPD, which is associated with higher demand for respiratory support and a greater risk of death, and non-invasive ventilation is recommended as the gold standard for the treatment for it [5].
Non-invasive ventilation may temporarily reverse or slow down disease progression by providing ventilation support and avoiding tracheal intubation. Individuals can apply it to increase airflow and gas exchange and reduce the work of breathing under appropriate circumstances to improve lung mechanics. For patients, it contributes to clearing airway mucus and improving breathing during sleep, and it may also relieve fatigue of respiratory muscles [6]. Non-invasive ventilation has been widely applied in nursing of acute respiratory failure due to different causes [7], but its tolerance may be unfavorable. Ipratropium bromide is an anticholinergic drug [8], which can induce bronchiectasia by inhibiting cholinergic bronchodilation function [9]. One study has pointed out that ipratropium bromide therapy can improve the blood oxygen saturation and sleep quality of patients with moderate to severe COPD [10]. This study explored the effects of ipratropium bromide combined with non-invasive ventilation in patients with both COPD and respiratory failure.
Materials and methods
General data
A total of 110 patients with COPD with respiratory failure admitted to our hospital from April 2018 to August 2019 were enrolled, of which 52 patients were treated with a noninvasive ventilator as Group A and the rest were treated with ipratropium bromide combined with a noninvasive ventilator as Group B. Group A consisted of 32 males and 20 females, with an average age of (68.38 ± 5.29) years, and Group B consisted of 36 males and 22 females, with an average age of (69.32 ± 5.11) years. All patients were diagnosed with both COPD and respiratory failure by spirometry [11].
All enrolled patients and their family members signed an informed consent form after understanding this study, and the study was approved by the ethics committee of our hospital. The following patients were excluded: Patients with communication obstacles or mental health disorders, patients with hepatic or renal insufficiency, patients who were allergic to drugs used in this study, and those with other comorbid lung diseases.
Methods
Before treatment, both groups were treated with conventional anti-asthmatic, expectorant, and anti-inflammatory treatments and oxygen inhalation. Those infected with bacteria were given antibiotics according to their drug sensitivity test.
Each patient in Group A was treated with a noninvasive ventilator (Unique Biotechnology Co., Ltd., Beijing, Shanghai) under the S/T mode and the following parameter settings: Oxygen flow rate: 3-5 L/min; respiratory frequency: 12-18 times/min; inspiratory pressure: 10-18 cm Hg and expiratory pressure: 4-8 cm Hg. The patients were maintained with PaO2 > 60 mm Hg and SaO2 > 90%, and they were treated at 2-3 times/d and 2-4 h/time under parameters adjusted according to their tolerance during the process.
Each patient in Group B was treated with 500 μg ipratropium bromide (registration number: H20150159, Laboratoire Unither) in addition to the treatment in Group A as follows: The patient was given 500 μg ipratropium bromide and 1 ml normal saline through aerosol inhalation for 3 days. Patients in the two groups all insisted on receiving treatment, and the vital signs of each patient in the two groups were monitored throughout the treatment. The course of treatment for both groups was one week.
Outcome measures
(1) The blood gas indexes of the two groups before and after treatment were compared: Arterial carbon dioxide partial pressure (PaCO2) and arterial partial pressure of oxygen (PaO2). (2) The pulmonary function indexes of the two groups were evaluated before and 2 days after treatment: Total lung volume (TLC), residual volume (RV), vital capacity (VR), and forced expiratory volume in 1 second (FEV1). (3) Venous blood (5 ml) was sampled from each patient in the two groups after surgery, let to stand for 20 min, and then centrifuged at 10×g and 4°C for 15 min with a centrifuge (BMH Instruments Co., Ltd., Beijing, China) to separate the serum, and the serum was quickly frozen with liquid nitrogen and stored at -80°C for later analysis. The levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in the serum were determined using an enzyme-linked immuno-sorbent assay (ELISA) (Elisa Biotechnology Co., Ltd., Suzhou, China). (4) With a full score of 10 points, the Borg scale was adopted to evaluate the dyspnea symptoms of patients [12]. A higher Borg scale score indicates more severe dyspnea. (5) The efficacy on the two groups was evaluated according to the symptoms and characteristics of COPD complicated with respiratory failure [13]: Effective: The patients’ dyspnea symptoms and pulmonary function were improved obviously; Relief, the patient’s dyspnea symptoms and pulmonary function were improved; Ineffective: the patient’s clinical symptoms were not improved at all. (6) The MOS 36-Item Short-Form Health Survey (SF-36) was adopted to score the life quality of patients in four dimensions, body function, psychological function, social function, and material life, with a full score of 100 points for each dimension. A higher score indicated better life quality.
Statistical analyses
In this study, the data were statistically analyzed using SPSS 21.0 (SPSS, Inc., Chicago, Illinois, United States). Quantitative data were expressed as the mean ± standard deviation (x ± sd), compared between groups using the t test, and compared within groups before and after treatment using the paired t test. Enumeration data were expressed as [n (%)] and compared between groups using the chi-square test. P < 0.05 indicates a significant difference.
Results
There is no difference in general data between the two groups
There was no significant difference in general data, such as individual data about patient, living habits, and diseases between the two groups (all P > 0.05) Table 1.
Table 1.
General data of patients of the two groups (x̅ ± sd) [n (%)]
| Item | Group A (n=52) | Group B (n=58) | t/χ2 value | P-value |
|---|---|---|---|---|
| Sex | 0.003 | 0.954 | ||
| Male | 32 (61.54) | 36 (62.07) | ||
| Female | 20 (38.46) | 22 (37.93) | ||
| Height (cm) | 173.24 ± 6.54 | 174.11 ± 6.12 | 0.720 | 0.472 |
| Age (Y) | 68.38 ± 5.29 | 69.32 ± 5.11 | 0.947 | 0.345 |
| Weight (kg) | 66.31 ± 6.29 | 67.23 ± 6.23 | 0.769 | 0.443 |
| Education background | 0.022 | 0.880 | ||
| ≥ senior high school | 33 (63.46) | 36 (62.06) | ||
| < senior high school | 19 (36.54) | 22 (37.93) | ||
| Diabetes mellitus history | 0.025 | 0.872 | ||
| Yes | 27 (51.92) | 31 (53.45) | ||
| No | 25 (48.08) | 27 (46.55) | ||
| Hypertension history | 0.006 | 0.937 | ||
| Yes | 31 (59.62) | 35 (60.34) | ||
| No | 21 (40.38) | 23 (39.65) | ||
| Smoking frequency in a week | 0.387 | 0.533 | ||
| ≥ 5 | 42 (80.77) | 44 (75.86) | ||
| < 5 | 10 (19.23) | 14 (24.13) | ||
| Drinking frequency in a week | 0.003 | 0.954 | ||
| ≥ 3 | 32 (61.54) | 36 (62.07) | ||
| < 3 | 20 (38.46) | 22 (37.93) | ||
| Frequency of staying up late in a week | 1.122 | 0.289 | ||
| ≥ 5 | 24 (46.15) | 21 (36.21) | ||
| < 5 | 28 (53.85) | 37 (63.79) | ||
| Exercise frequency in a week | 0.199 | 0.654 | ||
| ≥ 3 | 30 (57.69) | 31 (53.45) | ||
| < 3 | 22 (42.31) | 27 (46.55) | ||
| Antibiotic use frequency | 3.501 | 0.061 | ||
| ≥ 2 | 16 (30.77) | 28 (48.28) | ||
| < 2 | 36 (69.23) | 30 (51.72) | ||
| Severity of illness | 2.774 | 0.249 | ||
| Mild | 18 (34.62) | 26 (44.83) | ||
| Moderate | 27 (51.92) | 21 (36.21) | ||
| Severe | 7 (13.46) | 11 (18.96) |
The blood gas indexes of patients treated through combined therapy are better
The PaCO2 of Group A before and after treatment was (65.28 ± 5.28) mmHg and (52.19 ± 4.43) mmHg, respectively, and the PaCO2 of Group B before and after treatment was (66.12 ± 5.37) mmHg and (42.47 ± 4.21) mmHg, respectively. In addition, the PaO2 of Group A before and after treatment was (52.48 ± 5.33) mmHg and (63.35 ± 6.42) mmHg, respectively, and the PaO2 of Group B before and after treatment was (53.14 ± 4.98) mmHg and (71.48 ± 5.92) mmHg, respectively. It can be seen that before treatment, there was no difference in related blood gas indexes between the two groups (P > 0.05), while after treatment, related blood gas indexes of Group B were better than those of Group A (P < 0.05) Figure 1.
Figure 1.
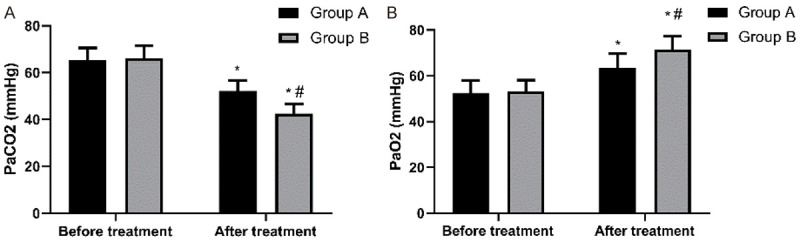
Comparison of blood gas indexes between the two groups before and after treatment. A. Comparison of PaCO2 between the two groups before and after treatment. Before treatment, there was no difference in PaCO2 between the two groups (P > 0.05), while after treatment, PaCO2 of both groups decreased (P < 0.05), and PaCO2 of Group B was lower than that of Group A (P < 0.05). Note: * it indicates that in comparison with the same group before treatment, P < 0.05. # indicates that in comparison with Group A, P < 0.05. B. Comparison of PaO2 between the two groups before and after treatment. Before treatment, there was no difference in PaO2 between the two groups (P > 0.05), while after treatment, PaO2 of both groups increased (P < 0.05), and PaO2 of Group B was higher than that of Group A (P < 0.05). Note: * indicates that in comparison with the same group before treatment, P < 0.05. # indicates that in comparison with Group A, P < 0.05.
The pulmonary function of patients treated through combined therapy is better
The TLC of Group A before and after treatment was (7.15 ± 0.89) L and (5.11 ± 0.23) L, respectively, and that of Group B before and after treatment was (7.14 ± 0.88) L and (5.78 ± 0.56) L, respectively. The RV of Group A before and after treatment was (4.78 ± 0.62) L and (1.89 ± 0.34) L, respectively, and that of Group B before and after treatment was (4.79 ± 0.64) L and (1.25 ± 0.26) L, respectively. The VC of Group A before and after treatment was (2.31 ± 0.43) L and (1.27 ± 0.21) L, respectively, and that of Group B was (2.32 ± 0.44) L and (1.78 ± 0.32) L, respectively. The FEV1 of Group A before and after treatment was (2.15 ± 0.79) L and (2.95 ± 0.54) L, respectively, and that of Group B before and after treatment was (2.14 ± 0.78) L and (3.68 ± 0.42) L, respectively. It can be seen that related lung function indexes of Group B were all better than those of Group A (all P < 0.05) Figure 2.
Figure 2.
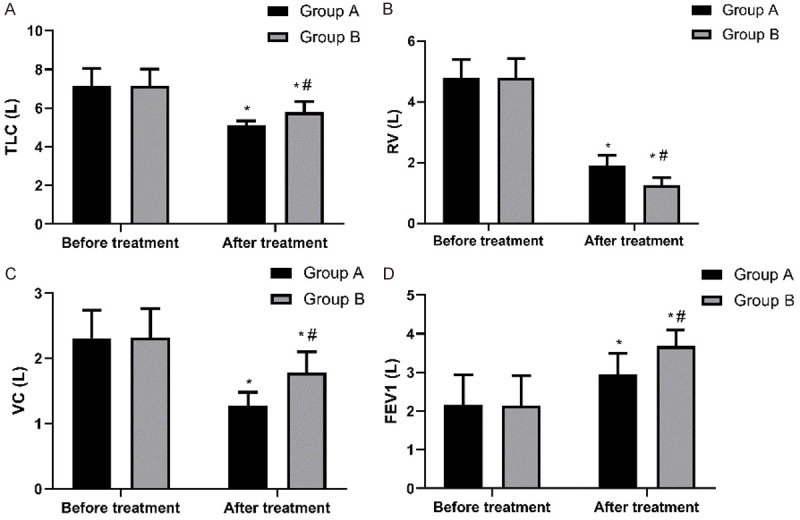
Comparison of pulmonary function indexes between the two groups before and after treatment. A. Comparison of TLC between the two groups before and after treatment. Before treatment, there was no difference in TLC between the two groups (P > 0.05), while after treatment, TLC of Group B was higher than that of Group A (P < 0.05). Note: * indicates that in comparison with the same group before treatment, P < 0.05. # indicates that in comparison with Group A, P < 0.05. B. Comparison of RV between the two groups before and after treatment. Before treatment, there was no difference in RV between the two groups (P > 0.05), while after treatment, RV of Group B was lower than that of Group A (P < 0.05). Note: * indicates that in comparison with the same group before treatment, P < 0.05. # indicates that in comparison with Group A, P < 0.05. C. Comparison of VC between the two groups before and after treatment. Before treatment, there was no difference in VC between the two groups (P > 0.05), while after treatment, VC of Group B was higher than that of Group A (P < 0.05). Note: * indicates that in comparison with the same group before treatment, P < 0.05. # indicates that in comparison with Group A, P < 0.05. D. Comparison of FEV1 between the two groups before and after treatment. Before treatment, there was no difference in FEV1 between the two groups (P > 0.05), while after treatment, FEV1 of Group B was higher than that of Group A (P < 0.05). Note: * indicates that in comparison with the same group before treatment, P < 0.05. # indicates that in comparison with Group A, P < 0.05.
The levels of inflammatory cytokines in patients treated through combined therapy are lower
The postoperative IL-6 level in Group A and Group B was (73.23 ± 8.24) ng/L and (56.79 ± 6.39) ng/L, respectively, and the postoperative TNF-α level in Group A and Group B was (63.72 ± 5.29) ng/L and (52.28 ± 4.54) ng/L, respectively, so the levels of IL-6 and TNF-α in Group B were lower than those in Group A (P < 0.05) Figure 3.
Figure 3.
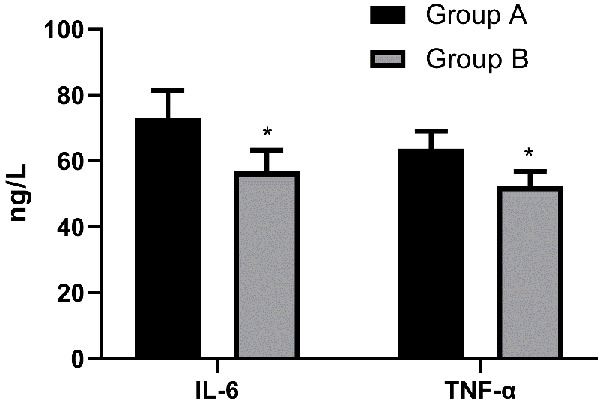
Comparison of inflammatory cytokines between the two groups after surgery. The levels of IL-6 and TNF-α in Group B were lower than those in Group A (P < 0.05). Note: * indicates that in comparison with Group A, P < 0.05.
The amelioration of dyspnea symptoms in patients treated with combination therapy is better
The Borg score of Group A before and after treatment was (7.38 ± 1.22) points and (5.82 ± 1.13) points, respectively, and that of Group B before and after treatment was (7.36 ± 1.21) points and (4.28 ± 1.04) points, respectively. It can be seen that before treatment, there was no difference between the two groups in Borg score (P > 0.05), while after treatment the Borg score of Group B was lower than that of Group A (P < 0.05) Figure 4.
Figure 4.
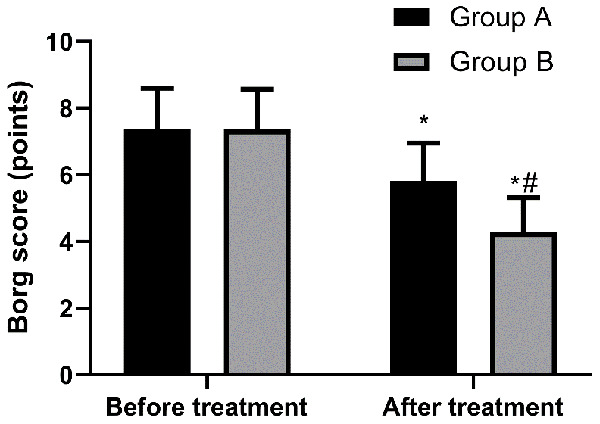
Comparison of Borg score of the two groups before and after treatment. Before treatment, there was no difference in Borg score between the two groups (P > 0.05), while after treatment, Borg score of Group B was lower than that of Group A (P < 0.05). Note: * indicates that in comparison with the same group before treatment, P < 0.05. # indicates that in comparison with Group A, P < 0.05.
The curative effect of patients treated through combined therapy is better
The effective rate in Group B was higher than that in Group A (P < 0.05) Table 2.
Table 2.
Comparison of efficacy between the two groups [n (%)]
| Efficacy | Group A (n=52) | Group B (n=58) | χ2 value | P-value |
|---|---|---|---|---|
| Effective | 14 (26.92) | 31 (43.45) | - | - |
| Relieved | 26 (50.00) | 23 (39.65) | - | - |
| Patients without effective treatment | 12 (23.08) | 4 (6.90) | - | - |
| Effective rate | 40 (76.92) | 54 (93.10) | 5.775 | 0.016 |
The incidence of adverse reactions in patients treated through combined therapy is lower
The incidence of adverse reactions in Group B was lower than that in Group A Table 3.
Table 3.
Comparison of adverse reactions between the two groups [n (%)]
| Evaluation of adverse reactions | Group A (n=52) | Group B (n=58) | χ2 value | P-value |
|---|---|---|---|---|
| Fever | 4 (7.69) | 1 (1.72) | - | - |
| Nausea | 3 (5.77) | 1 (1.72) | - | - |
| Vomiting | 2 (3.84) | 0 (0.00) | - | - |
| Diarrhea | 2 (3.84) | 0 (0.00) | - | - |
| Myasthenia of limbs | 1 (1.92) | 0 (0.00) | - | - |
| Dry mouth | 2 (3.84) | 2 (3.45) | - | - |
| The total incidence | 14 (26.92) | 6 (10.34) | 5.066 | 0.024 |
The life quality of patients treated through combined therapy is higher
Related scores of life quality (body function, life function, social function, and life quality) of Group B were all higher than those of Group A (all P < 0.05) Table 4.
Table 4.
Comparison of life quality between the two groups (x̅ ± sd)
| Group | n | Body function | Life function | Social function | Life quality |
|---|---|---|---|---|---|
| Group A | 52 | 75.29 ± 3.29 | 83.95 ± 3.52 | 77.49 ± 4.37 | 83.58 ± 4.21 |
| Group B | 58 | 85.49 ± 4.62 | 89.48 ± 2.49 | 86.55 ± 3.85 | 94.29 ± 3.22 |
| t | 13.200 | 9.587 | 11.560 | 15.070 | |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Discussion
COPD compromises life quality and increases morbidity and mortality, which may be related to disease deterioration. During the deterioration period, inflammation in the airway will increase [15]. One study has revealed that COPD is associated with chronic inflammation, and causes irreversible airflow restriction [16]. Chronic inflammation affects people both socially and behaviorally, with multiple variables that exert long-term effects on health, and IL-6 is one of the most commonly used biomarkers to measure these effects [17]. IL-6 plays a core role in the comprehensive immune defense network against infection. It is able to act through both classical and non-signaling pathways, posing different effects on immune ability [18]. In addition, IL-6 is also a key cytokine involved in malignant transformation and progression [19]. One study has pointed out that airway epithelial cells can strongly affect the pathogenesis of COPD through mechanisms such as the production of inflammatory mediators [20]. TNF-α not only has protective effects on diminishing inflammation, tissue regeneration and immune regulation, but it also has the pathogenicity of promoting inflammation and organ damage [21]. In our study, the inflammatory factor levels, pulmonary function, and blood gas indexes of Group B were better than those of group A, and we suspected that the reason for better pulmonary function of Group B may be due to the fact that ipratropium bromide suppressed inflammatory factor levels, and thus relaxed the trachea and helped reduce mucus secretion. In addition, the Borg score of Group B was lower than that of group A after treatment, indicating that patients treated with ipratropium bromide experienced a more mild dyspnea and enjoyed better efficacy. One study has revealed that anticholinergic drugs for COPD can alleviate dyspnea, lower COPD deterioration, and improve exercise tolerance, sleep quality and life quality [22]. Another other study supports the effectiveness and safety of inhaled ipratropium bromide in the long-term treatment of COPD [23]. In addition, one study has pointed out that adding ipratropium to the standard treatment for COPD can shorten the treatment time required in the emergency room [24]. These studies all indicate that ipratropium bromide is effective for COPD. Nebulization treatment of drugs is a non-invasive method, which can effectively treat respiratory diseases [25]. We also observed fewer adverse reactions in patients additionally treated with ipratropium bromide, One similar study has also pointed out that the adverse reactions of ipratropium bromide are rare and generally mild [26]. It may be due to the fact that nebulized drugs can reach the alveolar parts of the lung easily, improve the gas exchange in the lung and the tissue function with less invasiveness, and also brings about less adverse reactions. What’s more, the life quality of Group B was higher. One other study has shown that patients receiving ipratropium bromide scored higher in the quality of life questionnaire evaluating dyspnea, fatigue, emotional function, and proficiency [27]. In summary, ipratropium bromide combined with a noninvasive ventilator can significantly ameliorate circulatory inflammation and improve the pulmonary function and blood gas levels of patients, and thus provide higher treatment efficacy. Some studies also point out that the beneficial effects of bronchodilators on patients with COPD during ventilation with non-invasive devices may be due to the following reasons: After inhalation, the particle distribution of drugs will be affected by the equipment design and structural formation, which will reduce the particle diameter of drugs entering respiratory tract, reduce respiratory frequency, and increase tidal volume, thus enhancing drug delivery and improving drug bioavailability [28,29].
However, there are still some deficiencies in this study. We have not compared the therapeutic effects of ipratropium bromide with other anticholinergic drugs, nor have we conducted trapezoidal dose comparison, and we have also not evaluated the prognosis of patients. We will continue to conduct research from these points and update our results.
To sum up, ipratropium bromide is effective in treating patients with COPD with respiratory failure under non-invasive ventilation treatment, and if is beneficial to improving their pulmonary function.
Disclosure of conflict of interest
None.
References
- 1.Hansel NN, McCormack MC, Kim V. The effects of air pollution and temperature on COPD. COPD. 2016;13:372–379. doi: 10.3109/15412555.2015.1089846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;9:CD002309. doi: 10.1002/14651858.CD002309.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett NA, Kostakou E, Hart N, Douiri A, Camporota L. Extracorporeal carbon dioxide removal for acute hypercapnic exacerbations of chronic obstructive pulmonary disease: study protocol for a randomised controlled trial. Trials. 2019;20:465. doi: 10.1186/s13063-019-3548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herath SC, Normansell R, Maisey S, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD) Cochrane Database Syst Rev. 2018;10:CD009764. doi: 10.1002/14651858.CD009764.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun J, Li Y, Ling B, Zhu Q, Hu Y, Tan D, Geng P, Xu J. High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: an observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:1229–1237. doi: 10.2147/COPD.S206567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran F, Bradley JM, Piper AJ. Non-invasive ventilation for cystic fibrosis. Cochrane Database Syst Rev. 2017;2:CD002769. doi: 10.1002/14651858.CD002769.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S Paolo Navalesi Members Of The Steering Committee; Antonelli M, Brozek J, Conti G, Ferrer M, Guntupalli K, Jaber S, Keenan S, Mancebo J, Mehta S Suhail Raoof Members Of The Task Force. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50:1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 8.Nomura O, Morikawa Y, Hagiwara Y, Ihara T, Inoue N, Sakakibara H, Akasawa A. Ipratropium bromide for acute asthma in children: a retrospective trial. Arerugi. 2017;66:945–952. doi: 10.15036/arerugi.66.945. [DOI] [PubMed] [Google Scholar]
- 9.MacGregor TR, ZuWallack R, Rubano V, Castles MA, Dewberry H, Ghafouri M, Wood CC. Efficiency of ipratropium bromide and albuterol deposition in the lung delivered via a soft mist inhaler or chlorofluorocarbon metered-dose inhaler. Clin Transl Sci. 2016;9:105–113. doi: 10.1111/cts.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin RJ, Bartelson BL, Smith P, Hudgel DW, Lewis D, Pohl G, Koker P, Souhrada JF. Effect of ipratropium bromide treatment on oxygen saturation and sleep quality in COPD. Chest. 1999;115:1338–1345. doi: 10.1378/chest.115.5.1338. [DOI] [PubMed] [Google Scholar]
- 11.Riley CM, Sciurba FC. Diagnosis and outpatient management of chronic obstructive pulmonary disease: a review. JAMA. 2019;321:786–797. doi: 10.1001/jama.2019.0131. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MJ, Close L, Gillon SC, Molassiotis A, Lee PH, Farquhar MC Breathlessness Research Interest Group (BRIG) Use of the modified Borg scale and numerical rating scale to measure chronic breathlessness: a pooled data analysis. Eur Respir J. 2016;47:1861–1864. doi: 10.1183/13993003.02089-2015. [DOI] [PubMed] [Google Scholar]
- 13.Acarturk Tuncay E, Karakurt Z, Aksoy E, Salturk C, Gungor S, Ciftaslan N, Irmak I, Yavuz D, Ocakli B, Adiguzel N. Eosinophilic and non-eosinophilic COPD patients with chronic respiratory failure: neutrophil-to-lymphocyte ratio as an exacerbation marker. Int J Chron Obstruct Pulmon Dis. 2017;12:3361–3370. doi: 10.2147/COPD.S147261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zare R, Jafari P, Ghanizadeh A. Do Adult Attention Deficit Hyperactivity Disorder Quality-Of-Life (AAQoL) scale and the SF-36 scale measure the same construct of health-related quality of life? Atten Defic Hyperact Disord. 2017;9:39–45. doi: 10.1007/s12402-016-0206-5. [DOI] [PubMed] [Google Scholar]
- 15.Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193:965–74. doi: 10.1164/rccm.201509-1869OC. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61–75. doi: 10.1016/j.bbi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13:399–409. doi: 10.1038/nrrheum.2017.83. [DOI] [PubMed] [Google Scholar]
- 19.Bharti R, Dey G, Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: a snapshot of IL-6 mediated involvement. Cancer Lett. 2016;375:51–61. doi: 10.1016/j.canlet.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 20.Stolarczyk M, Amatngalim GD, Yu X, Veltman M, Hiemstra PS, Scholte BJ. ADAM17 and EGFR regulate IL-6 receptor and amphiregulin mRNA expression and release in cigarette smoke-exposed primary bronchial epithelial cells from patients with chronic obstructive pulmonary disease (COPD) Physiol Rep. 2016;4:e12878. doi: 10.14814/phy2.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemanetzoglou E, Andreadou E. CNS demyelination with TNF-alpha blockers. Curr Neurol Neurosci Rep. 2017;17:36. doi: 10.1007/s11910-017-0742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharya A, Bhargava S, Singh V, Talwar D, Whig J, Rebello J, Purandare S, Gogtay J. Efficacy and safety of ipratropium bromide/salbutamol sulphate administered in a hydrofluoroalkane metered-dose inhaler for the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1469–1476. doi: 10.2147/COPD.S89923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tashkin DP, Ashutosh K, Bleecker ER, Britt EJ, Cugell DW, Cummiskey JM, DeLorenzo L, Gilman MJ, Gross GN, Gross NJ, et al. Comparison of the anticholinergic bronchodilator ipratropium bromide with metaproterenol in chronic obstructive pulmonary disease. A 90-day multi-center study. Am J Med. 1986;81:81–90. doi: 10.1016/0002-9343(86)90468-7. [DOI] [PubMed] [Google Scholar]
- 24.Shrestha M, O’Brien T, Haddox R, Gourlay HS, Reed G. Decreased duration of emergency department treatment of chronic obstructive pulmonary disease exacerbations with the addition of ipratropium bromide to beta-agonist therapy. Ann Emerg Med. 1991;20:1206–1209. doi: 10.1016/s0196-0644(05)81472-6. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Wu L, Sun X. Intratracheal delivery of Nano- and microparticles and hyperpolarized gases: a promising strategy for the imaging and treatment of respiratory disease. Chest. 2020;157:1579–1590. doi: 10.1016/j.chest.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 26.Friedman M. A multicenter study of nebulized bronchodilator solutions in chronic obstructive pulmonary disease. Am J Med. 1996;100:30S–39S. doi: 10.1016/s0002-9343(96)80061-1. [DOI] [PubMed] [Google Scholar]
- 27.Colice GL. Nebulized bronchodilators for outpatient management of stable chronic obstructive pulmonary disease. Am J Med. 1996;100:11S–18S. doi: 10.1016/s0002-9343(96)80037-4. [DOI] [PubMed] [Google Scholar]
- 28.Jiang DH, Wang X, Liu LS, Ji DD, Zhang N. The effect of ventilator mask atomization inhalation of ipratropium bromide and budesonide suspension liquid in the treatment of COPD in acute exacerbation period on circulating levels of inflammation and prognosis. Eur Rev Med Pharmacol Sci. 2017;21:5211–5216. doi: 10.26355/eurrev_201711_13843. [DOI] [PubMed] [Google Scholar]
- 29.Rzepka-Wrona P, Skoczynski S, Wrona D, Barczyk A. Inhalation techniques used in patients with respiratory failure treated with noninvasive mechanical ventilation. Can Respir J. 2018;2018:8959370. doi: 10.1155/2018/8959370. [DOI] [PMC free article] [PubMed] [Google Scholar]


