Abstract
Objective: To investigate the predictive value of N-terminal pro brain natriuretic peptide (NT-proBNP), procalcitonin and central venous pressure (CVP) for new atrial fibrillation after cardiac surgery. Methods: Patients underwent cardiac surgery in Huai’an First People’s Hospital from June 2019 to December 2020 were enrolled in this study. Patients without POAF after surgery were included in the control group, and patients with POAF after surgery were included in the POAF group. Clinical data of patients were collected and retrospectively analyzed. Results: The incidence of in-hospital complications, length of stay and cost of hospitalization in the POAF group were significantly higher than those in the control group; Moreover, there were significant differences in central venous pressure, peak cTnI, NT-proBNP, procalcitonin, and white blood cell counts between the two groups. NT-proBNP, procalcitonin and elevated central venous pressure are independent risk factors for POAF in patients after cardiac surgery. Conclusion: NT-proBNP, procalcitonin and CVP were closely related with atrial fibrillation after cardiac surgery. The combination of NT-proBNP, procalcitonin and CVP had a better discriminative ability for new atrial fibrillation after cardiac surgery compared to either of them alone.
Keywords: Cardiac surgery, atrial fibrillation, serum markers
Introduction
Postoperative atrial fibrillation (POAF) is considered as the most commonly faced supraventricular arrhythmia after cardiovascular surgery. The incidence of POAF after cardiac surgery is about 20%-50%, especially for valve and bypass surgery [1,2]. Studies have shown that POAF is related to many factors, including obesity [3], gender, chronic obstructive disease, valvular heart disease [4,5], epicardial adipose tissue [6], and obstructive sleep apnea [7]. But the exact mechanism is still unclear. The onset and development of POAF was associated with inflammation, cardiac ischemia and sympathetic activation. POAF not only increases the length of hospitalization and hospitalization costs, but also increases the incidence of perioperative complications and mortality, leading to a poor prognosis.
Therefore, preventing the occurrence of POAF after cardiac surgery and finding the specific biomarkers for the prediction are particularly important.
It has been reported that procalcitonin could predict the incidence of POAF after cardiac surgery, including coronary artery bypass grafting [8]. There are increased evidences which revealed that elevated level of N-terminal pro brain natriuretic peptide (NT-proBNP) was correlated with postoperative major adverse cardiovascular events in general and POAF in particular [9]. Central venous pressure (CVP), one of the most common hemodynamic indexes, is increased in response to various stimuli including elevated wall stress and fulfilling pressure, and cardiac ischemia [10]. Nevertheless, there is still a lack of ideal biomarkers for early prediction of POAF. The aim of this study was to explore the independent risk factors for new atrial fibrillation after cardiac surgery, and at the same time to construct a joint predictive model with multiple risk factors and to evaluate its predictive value, so as to guide prevention and early treatment of the new atrial fibrillation after cardiac surgery.
Materials and methods
Research objects
In this retrospective study, patients who underwent cardiac surgery in Huai’an First People’s Hospital from June 2019 to December 2020 were consecutively included. POAF is defined as any documented atrial fibrillation episode lasting >30 seconds captured on a standard 12-lead electrocardiogram during the period from immediately after cardiac surgery to discharge. This study was approved by the ethics committee of Huai’an First People’s Hospital (Approval number: 2019214) and complied with “Declaration of Helsinki” revised in 2013. The research subjects were exempted from signing informed consent for the retrospective nature.
The inclusion criteria were as follows: patients who underwent the elective first cardiac surgery; patients with confirmed sinus rhythm before surgery; patients with an age over 18 years old; and patients with completed data. The exclusion criteria were as follows: patients were with previous paroxysmal atrial fibrillation; patients were diagnosed as primary myocardiopathy or congenital heart disease; or patients with implanted cardiac devices and electrophysiologic ablation history.
Research groups
According to the occurrence of POAF, patients without POAF were included in the control group, and patients with POAF were included in the POAF group. The POAF group was treated with amiodarone (Sanofi Company, France) according to the conventional regimen to treat new postoperative POAF. According to the recommendations, a loading dose of amiodarone 150 mg was given intravenously, then the maintenance amiodarone was intravenously given with 0.5-2 mg per minute. This intravenous medication was gradually switched to oral dosing. The oral amiodarone (200 mg three times daily starting 5 days and 200 mg once daily continuing for one month) was performed in patients after surgery.
Collection of clinical data
Clinical data of selected patients were collected from the electronic medical record system, including: (1) Basic information: gender, age, New York heart function classification at admission, length of stay (days) and cost; (2) Past medical history: Diabetes, hypertension, coronary heart disease, mean arterial pressure and heart rate; (3) Clinical prognosis: perioperative complications include stroke, hemorrhage, infection, renal function or respiratory failure, cardiac arrest, and cerebral complications; (4) Laboratory Data and auxiliary examination: preoperative and postoperative white blood cell count, procalcitonin, NT-proBNP and cardiac troponin I (cTnI) which were detected by C6000 type automatic biochemistry analyser (Roche, USA). Central venous pressure was detected through the insertion of a catheter into a jugular vein.
The primary outcomes included new onset of postoperative atrial fibrillation, white blood cell count, procalcitonin, NT-proBNP, cTnI and central venous pressure. The secondary outcomes consisted of in-hospital complication rate, mortality, hospital stay and hospital costs.
Statistical analysis
SPSS 20.0 software and MedCalc 19.3 software were used for data analysis. Normally distributed measurement data were expressed as Mean ± standard deviation (SD), and the independent sample t test was used for comparison between groups. Enumeration data were expressed as a percentage (%), and the chi-square test was used for comparison between groups. The control group and the POAF group were matched at 1:1 by the nearest neighbor matching method. The variables with P<0.05 in the univariate analysis were incorporated into the multivariate logistic regression model to analyze the independent risk factors for POAF in patients after cardiac surgery, and the logistic model equation was used to transform to construct the combined predictive factor, according to the work of the subjects. The receiver operating characteristic (ROC) curve was used to determine the predictive performance of the screened factors. The difference was statistically significant with P<0.05.
Results
Comparison of hospitalization outcomes
A total of 137 patients who underwent cardiac surgery were included in this study, including 66 in the normal control group and 71 in the POAF group. The incidence of in-hospital complications, length of stay, and cost of hospitalization in the POAF group were significantly higher than those in the normal control group (all P<0.05, Table 1).
Table 1.
Comparison of hospitalization outcomes between POAF group and normal control group
| Group | In-hospital complication rate N (%) | Mortality N (%) | Hospital stay (days) | Hospital costs [Ten thousand, M (Q1, Q3)] |
|---|---|---|---|---|
| POAF group (n=71) | 11 (15.5) | 2 (2.8) | 15.1±3.3 | 11.2 (8.9, 13.1) |
| Control group (n=66) | 4 (6.1) | 1 (1.5) | 11.6±2.1 | 7.4 (6.5, 8.5) |
| χ2/t/Z value | 4.468 | 0.271 | 7.343 | 4.574 |
| P value | 0.035 | 0.603 | <0.001 | <0.001 |
Comparison of general information
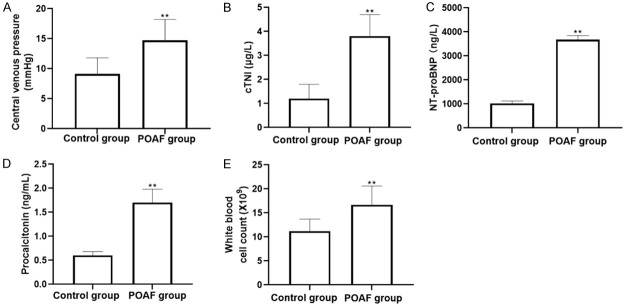
Taking into account the large difference in sample size between the two groups and the large number of observation indicators, in order to ensure the comparability between the two groups, the propensity score matching was carried out on the two sets of samples. After matching the patients in the normal control group and the POAF group with 1:1, the patients who finally matched successfully constitute a new data set for further statistical analysis. Comparison of baseline clinical data between two groups of patients after matching age, gender, preoperative heart rate, preoperative mean arterial pressure, percentage of patients with a history of diabetes, percentage of patients with a history of hypertension, and a history of coronary heart disease, showed that there were no statistically significant differences in baseline data (all P>0.05, Table 2). The differences in central venous pressure, peak cTnI, NT-proBNP, procalcitonin, and white blood cell counts between the two groups were statistically significant (P<0.01) (Table 3 and Figure 1).
Table 2.
Comparison of baseline clinical data between two groups of patients after propensity matching
| Project | Control group (n=49) | POAF group (n=49) | χ2/t Value | P Value |
|---|---|---|---|---|
| Age (years) | 61.3±3.1 | 62.5±2.6 | 1.730 | 0.087 |
| Male [N (%)] | 28 (57.1) | 26 (53.1) | 0.165 | 0.685 |
| Heart rate (bpm/min) | 78.1±11.3 | 76.9±14.2 | 0.463 | 0.645 |
| Mean arterial pressure (mmHg) | 81.9±15.7 | 83.4±16.1 | 0.467 | 0.642 |
| Diabetes [N (%)] | 15 (30.6) | 16 (32.6) | 0.047 | 0.828 |
| Hypertension [N (%)] | 14 (28.6) | 15 (30.6) | 0.049 | 0.825 |
| Coronary heart disease [N (%)] | 13 (26.5) | 13 (26.5) | 0.000 | >1.000 |
Table 3.
Comparison of central venous pressure combined serum markers between two groups
| Parameters | Control group (n=49) | POAF group (n=49) | t/Z Value | P Value |
|---|---|---|---|---|
| Central venous pressure (mmHg) | 9.1±2.7 | 14.7±3.5 | 5.237 | 0.005 |
| cTnI peak [μg/L, M (Q1, Q3)] | 1.2 (0.7, 1.3) | 3.8 (3.1, 4.2) | 8.047 | 0.003 |
| NTproBNP [ng/L, M (Q1, Q3)] | 1024 (935, 1176) | 3679 (3082, 4249) | 6.556 | 0.006 |
| Procalcitonin [ng/ml, M (Q1, Q3)] | 0.6 (0.4, 0.9) | 1.7 (1.5, 2.1) | 3.932 | 0.008 |
| White blood cell count (×109/L) | 11.2±2.5 | 16.7±3.9 | 8.553 | 0.002 |
Figure 1.
Comparison of central venous pressure and serum markers between two groups. A. Central venous pressure; B. cTNI level; C. NT-proBNP level; D. Procalcitonin level; E. White blood cell count.
Univariate and multivariate analysis of the factors for POAF after cardiac surgery
The factors with statistical significance in the univariate analysis were brought into the multivariate logistic regression model, and the analysis results showed that NT proBNP, procalcitonin and central venous pressure were independent risk factors for POAF in patients after cardiac surgery (both P<0.05, Tables 4 and 5).
Table 4.
Univariate analysis of the risk factors for POAF after cardiac surgery
| Factors | β value | OR value | 95% CI | P value |
|---|---|---|---|---|
| Central venous pressure | 0.036 | 1.078 | 0.842-1.312 | 0.009 |
| cTnI peak | -0.015 | 0.862 | 0.764-1.239 | 0.105 |
| NT-proBNP | 0.031 | 1.062 | 0.911-1.352 | 0.032 |
| Procalcitonin | 0.312 | 1.289 | 1.042-1.624 | <0.001 |
| White blood cell count | -0.009 | 0.939 | 0.887-1.025 | 0.305 |
Table 5.
Multivariate logistic regression analysis results of POAF after cardiac surgery
| Factors | β value | OR value | 95% CI | P value |
|---|---|---|---|---|
| Central venous pressure | 0.028 | 1.092 | 0.911-1.239 | 0.011 |
| cTnI peak | -0.010 | 0.901 | 0.811-1.097 | 0.093 |
| NT-proBNP | 0.026 | 1.011 | 0.935-1.212 | 0.021 |
| Procalcitonin | 0.249 | 1.179 | 1.036-1.412 | <0.001 |
| White blood cell count | -0.011 | 0.946 | 0.936-1.011 | 0.288 |
ROC curves analysis
As shown in Figure 2 and Table 6, Analysis of ROC curve showed that AUC of NT-proBNP for predicting POAF after cardiac surgery was 0.714, AUC of procalcitonin for predicting POAF after cardiac surgery was 0.824, and AUC of central venous pressure for predicting POAF after cardiac surgery was 0.682, all of which had good predictive value. In additional, the cut off value for NT-proBNP, procalcitonin and central venous pressure were 3886.0 ng/L, 1.8 μg/L and 15.2 mmHg, respectively. Compared with either NT-proBNP, procalcitonin or central venous pressure, the combination of these three indicators had higher AUC value.
Figure 2.
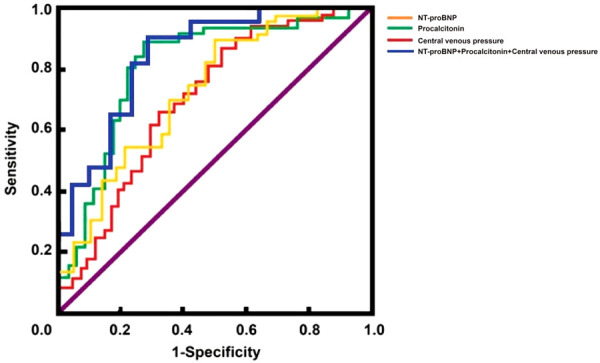
ROC curves of NT-proBNP, Procalcitonin, central venous pressure predicting the POAF after cardiac surgery.
Table 6.
ROC parameters of NT-proBNP, Procalcitonin, central venous pressure for predicting the POAF after cardiac surgery
| Indicators | AUC | 95% CI | S.E | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| NT-proBNP | 0.714 | 0.682-0.923 | 0.041 | 72.89 | 87.92 |
| Procalcitonin | 0.824 | 0.703-0.874 | 0.047 | 92.11 | 71.30 |
| CVP | 0.682 | 0.718-0.920 | 0.052 | 70.36 | 84.82 |
| Combination of the above three indicators | 0.914 | 0.642-0.911 | 0.049 | 94.82 | 80.75 |
Discussion
The occurrence of POAF may involve a variety of factors, including cardiac disordered structural foundation, inflammation and oxidative stress, electrolyte and internal environment and so on [11,12]. About 35% to 50% of POAF usually occurs in 2nd to 5th day after cardiac surgery [13-15]. Clinical efforts to prevent and control POAF after cardiac surgery are facing significant challenges, and the results are not ideal.
Cardiac surgery leads to local inflammation around the heart and increased pericardial effusion. Postoperative pericardial fluid including blood, hemolysis of blood cells, hemoglobin, white blood cells and platelet activation markers are highly oxidized [16,17]. Myocardia itself produces proinflammatory cytokines, resulting in local inflammation and directly alters cardiac function. Inflammation within the pericardial space may lead to myocardial electrical activity and apoptosis changes, cause elevated white blood cell count, and thus result in the formation of action potential heterogeneity and arrhythmia and dissemination [18,19]. There are statistically significant differences between control group and POAF group in terms of procalcitonin and white blood cell count. Procalcitonin could reflect the activity of systemic inflammatory response. It was an independent risk factor for POAF in postoperative cardiac patients, indicating that the degree of inflammation caused by surgery was directly correlated with the incidence of POAF. Prevention of inflammation and pericardial effusion may be able to reduce the incidence of POAF, which was in consistent with previous results [20].
CVP is one of the hallmarks of volume load after cardiac surgery. NT proBNP is a polypeptide secreted by cardiac myocytes after increased load capacity. The load capacity was determined by the biological markers [21,22]. It was reported that the cardiac function and prognosis in patients with heart failure could be evaluated through central venous pressure combined with NT proBNP [23-25]. Recent studies showed that high volume could lead to increased intravascular volume and stimulate the right atrium diastole and left atrial enlargement. The elevated level of NT proBNP was closely associated with the degree of load capacity [26,27]. Christians et al. [28] found that 88% of patients after a new atrial fibrillation had positive fluid balance. The study also found that patients with new-onset atrial fibrillation after cardiac surgery had liquid positive balance, elevated central venous pressure and increased NT proBNP, compared with those in control group, indicating that increased NT-proBNP and elevated central venous pressure after surgery were the risk factors for POAF. These results were in accordance with the report by Bjerrum et al. [29].
There are some limitations in this study. First, this is a retrospective study, and there were no follow-up results. Second, the development of atrial fibrillation is involved in many factors. The parameters of this study was limited to age, gender, preoperative heart rate, mean arterial pressure, history of diabetes, history of hypertension, and history of coronary artery disease. Third, all patients’ data were collected from this single center, which may lead to biased results.
In conclusion, the results of this study showed that NT-proBNP, procalcitonin and central venous pressure are closely related to atrial fibrillation after cardiac surgery. Joint detection of the three biomarkers has a better performance for predicting new-onset atrial fibrillation after cardiac surgery compared with each of the single factors, which may help clinicians make a preliminary judgment on such patients and offer a possibility of early intervention.
Acknowledgements
This work was supported by Huai’an Health Research Project (HAWJ 202009), Nanjing Medical University Research Development Project (NMUB 2019352) and Huai’an First Hospital Affiliated to Nanjing Medical University High-level Talent Research Fund (YGRX 201914).
Disclosure of conflict of interest
None.
References
- 1.Qureshi M, Ahmed A, Massie V, Marshall E, Harky A. Determinants of atrial fibrillation after cardiac surgery. Rev Cardiovasc Med. 2021;22:329–341. doi: 10.31083/j.rcm2202040. [DOI] [PubMed] [Google Scholar]
- 2.Baeza-Herrera LA, Rojas-Velasco G, Marquez-Murillo MF, Portillo-Romero ADR, Medina-Paz L, Alvarez-Alvarez R, Ramos-Enriquez A, Baranda-Tovar FM. Atrial fibrillation in cardiac surgery. Arch Cardiol Mex. 2019;89:348–359. doi: 10.24875/ACM.19000134. [DOI] [PubMed] [Google Scholar]
- 3.Girerd N, Pibarot P, Fournier D, Daleau P, Voisine P, O’Hara G, Despres JP, Mathieu P. Middle-aged men with increased waist circumference and elevated C-reactive protein level are at higher risk for postoperative atrial fibrillation following coronary artery bypass grafting surgery. Eur Heart J. 2009;30:1270–1278. doi: 10.1093/eurheartj/ehp091. [DOI] [PubMed] [Google Scholar]
- 4.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ Jr, Cohn LH, Burstin HR. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 5.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT Investigators of the Ischemia Research and Education Foundation; Multicenter Study of Perioperative Ischemia Research Group. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 6.Opolski MP, Staruch AD, Kusmierczyk M, Witkowski A, Kwiecinska S, Kosek M, Jastrzebski J, Pregowski J, Kruk M, Rozanski J, Demkow M, Ruzyllo W, Kepka C. Computed tomography angiography for prediction of atrial fibrillation after coronary artery bypass grafting: proof of concept. J Cardiol. 2015;65:285–292. doi: 10.1016/j.jjcc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Qaddoura A, Baranchuk A. Risk factors for post coronary artery bypass graft atrial fibrillation: role of obstructive sleep apnea. Medwave. 2016;16:e6810. doi: 10.5867/medwave.2016.6810. [DOI] [PubMed] [Google Scholar]
- 8.Selcuk M, Cinar T, Saylik F, Dogan S, Selcuk I, Orhan AL. Predictive value of systemic immune inflammation index for postoperative atrial fibrillation in patients undergoing isolated coronary artery bypass grafting. Medeni Med J. 2021;36:318–324. doi: 10.4274/MMJ.galenos.2021.37998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnaubelt S, Pilz A, Koller L, Kazem N, Hofer F, Fleck T, Laufer G, Steinlechner B, Niessner A, Sulzgruber P. The impact of volume substitution on post-operative atrial fibrillation. Eur J Clin Invest. 2021;51:e13456. doi: 10.1111/eci.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kogo H, Sezai A, Osaka S, Shiono M, Tanaka M. Does epicardial adipose tissue influence postoperative atrial fibrillation? Ann Thorac Cardiovasc Surg. 2019;25:149–157. doi: 10.5761/atcs.oa.18-00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng S, Wang J, Yu H, Cao G, Liu P. Influence of dexmedetomidine on post-operative atrial fibrillation after cardiac surgery: a meta-analysis of randomized controlled trials. Front Cardiovasc Med. 2021;8:721264. doi: 10.3389/fcvm.2021.721264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Z, Zhou H, Ni Y, Wu C, Zhang C, Ling X. Can dexmedetomidine reduce atrial fibrillation after cardiac surgery? A systematic review and meta-analysis. Drug Des Devel Ther. 2018;12:521–531. doi: 10.2147/DDDT.S153834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malhotra P, Pande S, Mahindru S, Thukral A, Kotwal AS, Gupta RP, Tewari P, Agarwal SK. Postoperative atrial fibrillation in coronary artery bypass grafting herald poor outcome. Ann Card Anaesth. 2021;24:464–469. doi: 10.4103/aca.ACA_30_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngaage DL, Schaff HV, Mullany CJ, Sundt TM 3rd, Dearani JA, Barnes S, Daly RC, Orszulak TA. Does preoperative atrial fibrillation influence early and late outcomes of coronary artery bypass grafting? J Thorac Cardiovasc Surg. 2007;133:182–189. doi: 10.1016/j.jtcvs.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Kunt A, Ozcan S, Kucuker A, Odabasi D, Sami Kunt A. Effects of perioperative statin treatment on postoperative atrial fibrillation and cardiac mortality in patients undergoing coronary artery bypass grafting: a propensity score analysis. Med Glas (Zenica) 2015;12:190–195. doi: 10.17392/796-15. [DOI] [PubMed] [Google Scholar]
- 16.Lehto J, Kiviniemi T. Postpericardiotomy syndrome after cardiac surgery. Ann Med. 2020;52:243–264. doi: 10.1080/07853890.2020.1758339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehto J, Virolainen J. Postpericardiotomy syndrome. Duodecim. 2017;133:411–416. [PubMed] [Google Scholar]
- 18.Imazio M, Brucato A, Ferrazzi P, Pullara A, Adler Y, Barosi A, Caforio AL, Cemin R, Chirillo F, Comoglio C, Cugola D, Cumetti D, Dyrda O, Ferrua S, Finkelstein Y, Flocco R, Gandino A, Hoit B, Innocente F, Maestroni S, Musumeci F, Oh J, Pergolini A, Polizzi V, Ristic A, Simon C, Spodick DH, Tarzia V, Trimboli S, Valenti A, Belli R, Gaita F COPPS-2 Investigators. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. JAMA. 2014;312:1016–1023. doi: 10.1001/jama.2014.11026. [DOI] [PubMed] [Google Scholar]
- 19.Imazio M, Belli R, Brucato A, Ferrazzi P, Patrini D, Martinelli L, Polizzi V, Cemin R, Leggieri A, Caforio AL, Finkelstein Y, Hoit B, Maisch B, Mayosi BM, Oh JK, Ristic AD, Seferovic P, Spodick DH, Adler Y. Rationale and design of the COlchicine for prevention of the post-pericardiotomy syndrome and post-operative atrial fibrillation (COPPS-2 trial): a randomized, placebo-controlled, multicenter study on the use of colchicine for the primary prevention of the postpericardiotomy syndrome, postoperative effusions, and postoperative atrial fibrillation. Am Heart J. 2013;166:13–19. doi: 10.1016/j.ahj.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Racca V, Torri A, Grati P, Panzarino C, Marventano I, Saresella M, Castiglioni P. Inflammatory cytokines during cardiac rehabilitation after heart surgery and their association to postoperative atrial fibrillation. Sci Rep. 2020;10:8618. doi: 10.1038/s41598-020-65581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pudjiadi AH, Rahayu T, Wijaya S, Alatas FS. Serum NT-Pro-BNP versus noninvasive bedside inotropic index in paediatric shock: a contest of myocardial performance in response to fluid loading. Crit Care Res Pract. 2021;2021:7458186. doi: 10.1155/2021/7458186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knobloch K, Tepe J, Rossner D, Lichtinghagen R, Luck HJ, Busch KH, Vogt PM. Combined NT-pro-BNP and CW-Doppler ultrasound cardiac output monitoring (USCOM) in epirubicin and liposomal doxorubicin therapy. Int J Cardiol. 2008;128:316–325. doi: 10.1016/j.ijcard.2007.04.182. [DOI] [PubMed] [Google Scholar]
- 23.Lieppman K, Kramer-Clark L, Tobias JD. Plasma B-type natriuretic peptide monitoring to evaluate cardiovascular function prior to organ procurement in patients with brain death. Paediatr Anaesth. 2008;18:852–856. doi: 10.1111/j.1460-9592.2008.02652.x. [DOI] [PubMed] [Google Scholar]
- 24.Christenson RH. What is the value of B-type natriuretic peptide testing for diagnosis, prognosis or monitoring of critically ill adult patients in intensive care? Clin Chem Lab Med. 2008;46:1524–1532. doi: 10.1515/CCLM.2008.294. [DOI] [PubMed] [Google Scholar]
- 25.Tobin G, Chacko AG, Simon R. Evaluation of NT-ProBNP as a marker of the volume status of neurosurgical patients developing hyponatremia and natriuresis: a pilot study. Neurol India. 2018;66:1383–1388. doi: 10.4103/0028-3886.241401. [DOI] [PubMed] [Google Scholar]
- 26.Whitman IR, Vittinghoff E, DeFilippi CR, Gottdiener JS, Alonso A, Psaty BM, Heckbert SR, Hoogeveen RC, Arking DE, Selvin E, Chen LY, Dewland TA, Marcus GM. NT-proBNP as a mediator of the racial difference in incident atrial fibrillation and heart failure. J Am Heart Assoc. 2019;8:e010868. doi: 10.1161/JAHA.118.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almuwaqqat Z, O’Neal WT, Norby FL, Lutsey PL, Selvin E, Soliman EZ, Chen LY, Alonso A. Joint associations of obesity and NT-proBNP with the incidence of atrial fibrillation in the ARIC study. J Am Heart Assoc. 2019;8:e013294. doi: 10.1161/JAHA.119.013294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christians KK, Wu B, Quebbeman EJ, Brasel KJ. Postoperative atrial fibrillation in noncardiothoracic surgical patients. Am J Surg. 2001;182:713–715. doi: 10.1016/s0002-9610(01)00799-1. [DOI] [PubMed] [Google Scholar]
- 29.Bjerrum E, Wahlstroem KL, Gogenur I, Burcharth J, Ekeloef S. Postoperative atrial fibrillation following emergency noncardiothoracic surgery: a systematic review. Eur J Anaesthesiol. 2020;37:671–679. doi: 10.1097/EJA.0000000000001265. [DOI] [PubMed] [Google Scholar]