Abstract
Staphylokinase (SAK), a polypeptide secreted by Staphylococcus aureus, is a plasminogen activator with a therapeutic potential in thrombosis diseases. A Bacillus subtilis strain which is multiply deficient in exoproteases was transformed by an expression plasmid carrying a promoter and a signal sequence of subtilisin fused in frame with the sak open reading frame. However, the amount of SAK secretion was marginal (45 mg/liter). In contrast, disruption of the wprA gene, which encodes a subtilisin-type protease, strongly promoted the production of SAK in the stationary phase (181 mg/liter). In addition, the extracellular stability of mature SAK was dramatically enhanced. These data indicate a significant role of the wprA gene product in degrading foreign proteins, both during secretion and in the extracellular milieu.
Staphylokinase (SAK), a 136-amino-acid protein secreted by Staphylococcus aureus strains, acts as a plasminogen activator (11) by forming a complex with plasmin that activates other plasminogens (8). In contrast to streptokinase (SK), SAK exhibits fibrin-specific activation of plasminogen and low immunogenicity. Therefore, SAK has a potential for use in treatment of thrombosis (3, 19), and it has emerged as a thrombolytic agent for treatment of patients with acute myocardial infarction or peripheral arterial occlusion (5, 23).
Natural strains of S. aureus produce only a negligible amount of SAK (4). Thus, several efforts to produce SAK in large quantities have been made (2, 13, 21, 29). A Bacillus expression system exploiting the natural promoter and translation signals of SAK resulted in the secretion of 25 mg of recombinant SAK (rSAK) per liter in B. subtilis DB104, which is deficient in two major extracellular proteases (2, 7). Recently, rSAK was produced in B. subtilis WB700 (30), which is deficient in seven extracellular proteases, thereby preventing of the degradation of rSAK in the culture medium, by using the sucrose-inducible sacB promoter and the levansucrase signal sequence. Fermentation of WB700 resulted in the production of 337 mg of rSAK per liter (29).
An IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible system was designed and used for the investigation of the influence of the cell wall-associated protease WprA on the production of the Bacillus licheniformis alpha-amylase AmyL (22). The Bacillus protein, AmyL, was degraded by the wprA gene product during or shortly after the translocation across the membrane and in a cell-associated location (22). wprA codes for a 96-kDa protein that is processed to the CWBP23 propeptide and CWBP52 mature protease, forming a complex associated with the cell wall (14). Unexpectedly, the complex was also found in the culture supernatant of Bacillus subtilis WB600 (1, 28). Whether it is present in the cell wall or in the culture medium therefore seems to be a critical factor in the degradation of recombinant proteins. However, this hypothesis has not yet been tested with a wprA-deficient strain. Here we report that inactivation of the wprA gene in B. subtilis WB600 results in enhanced production of rSAK.
MATERIALS AND METHODS
Bacterial strains, culture medium, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Cultivation of recombinant B. subtilis was carried out in 250 ml of Luria-Bertani (LB) medium (1% [wt/vol] Bacto tryptone, 0.5% [wt/vol] yeast extract, 1% [wt/vol] sodium chloride) supplemented with kanamycin (5 mg/liter) at 37°C and 200 rpm. Cell growth was monitored by measurement of the optical density of the culture medium at 600 nm.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Genotype and genetic markers | Comments | Reference |
|---|---|---|---|
| Strains | |||
| B. subtilis DB431 | nprE aprA epr bpf | Expression host deficient in four exoproteases | 7 |
| B. subtilis WB600 | nprE aprA epr bpf mpr nprB | Expression host deficient in six exoproteases | 28 |
| B. subtilis LB700 | nprE aprA epr bpf mpr nprB wprA | Cell wall-associated protease, wprA-deficient WB600 | This study |
| E. coli MC1061 | F−araD139 Δ(ara-leu)7696 galE15 galK16 Δ(lac)X74 rpsL (Strr) hsdR2 (rK mK+) mcrA mcrB1 | Host for DNA manipulation | 24 |
| Plasmids | |||
| pKWZ | Neor; Bacillus ori | SPS | 17 |
| pSM704 | Neor Ampr; Bacillus ori, E. coli ori | Shuttle expression vector with SPS | This study |
| pSAK704 | Neor Ampr; Bacillus ori, E. coli ori | SAK secretion plasmid | This study |
| pUCTV2 | Ampr Tcr; Bacillus ts-ori, E. coli ori | Temperature-sensitive suicide vector | 25 |
| pDWPRA | Ampr Tcr; Bacillus ts-ori, E. coli ori | Gene replacement vector with inactivated copy of wprA | This study |
DNA manipulations and PCR.
Restriction enzymes, Vent DNA polymerase, and XhoI linker were purchased from New England Biolabs Inc. Competent cells of B. subtilis and Escherichia coli were transformed with plasmids by standard protocols (9, 18). Genomic DNA from B. subtilis was prepared by Doi's method (6). All DNA manipulations were carried out by standard protocols (20). PCR screening for disruption of the wprA gene in B. subtilis was performed with two primers, WPRP-F (5′-TGCCAATTGGTTTTCAATTGTTTTAATAGA) and TETP-R (5′-TAAAATTTGGTTGTGTCGTAAATTCGATTG). Southern blotting and hybridizations were performed using the nonradioactive ECL kit (Amersham Life Sciences). The hybridization probe was prepared with PCR amplification using WPRS-F (5′-ACTTAAATTAATCACCTTTGCTCCTTTGTC) and WPRS-R (5′-CTGCGCTGACAGCCTTCATGACATTCA).
Construction of shuttle expression plasmid pSAK704.
The subtilisin promoter and signal sequences (SPS) in plasmid pKWZ (17) were subcloned into the HindIII-EcoRI site of pUC18. SPS was amplified by PCR with the primer pair SPS-F (5′-AGCGGATAACAATTTCACACAGGA) (New England Biolabs, Inc.) and SPS-R (5′-TTGAGCTCGCCGGCCTGCGCAGACATGTTGCT), which contain additional SacI and NaeI restriction sites. The EcoRI- and SacI-digested SPS was subcloned into the EcoRI-SacI site of pUC19, digested with EcoRI and PstI, and finally inserted into EcoRI-PstI site of plasmid pKK223-3. The origin of replication and kanamycin resistance gene from pUB110 (15) were inserted into the PvuII-EcoRI site of SPS-containing pKK223-3. The resulting plasmid, a shuttle expression vector, was termed pSM704.
The SAK-encoding gene was amplified from chromosomal DNA of S. aureus NCTC10033 by PCR with the oligonucleotide pair SAK-F (5′-TCAAGTTCATTCGACAAAGGAAAAT) and SAK-R (5′-GGGAAGCTTATTTCTTTTCTATAACAACCTTTG). The PCR product was cleaved with HindIII and inserted into the NaeI-HindIII site of pSM704 to drive rSAK expression (Fig. 1). The correct in-frame fusion between SPS and rSAK was confirmed by sequencing.
FIG. 1.
SAK expression vector pSAK704 (7.2 kb) (A) and inactivation of the wprA gene by plasmid pDWPRA (B). D, H, and S, catalytic amino acids of the WprA protease.
Construction of gene replacement vector pDWPRA.
wprA (3.1 kb) was amplified from chromosomal DNA of B. subtilis WB600 with the oligonucleotide pair WPR-F (5′-CCCGAATTCTTGATAGAGCTGGTTTTTTTTATA) and WPR-R (5′-GCAGAATTCGCACAGCTTCGGCTTATCGGAATT). The PCR product was digested with EcoRI and subcloned into pUC19. The resulting plasmid, pUC19-wprA, was further digested with Bst1107I and EcoRV, generating a wprA-containing fragment which was subsequently fused to an XhoI linker. The tetracycline resistance gene, tet (1.7 kb) (16), was obtained by digestion of pUCTV2 (25) with XhoI and finally was inserted into the XhoI site of the wprA-containing plasmid fragment. The tet-disrupted wprA fragment was ligated into the EcoRI site of XhoI-digested pUCTV2, and the resulting plasmid was termed pDWPRA (Fig. 1).
SDS-PAGE and immunoblot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out using Laemmli's method (12). Purified rSAK from E. coli (13) was used to raise antibodies in rabbits. Western blot analysis was performed by standard protocols (20). Anti-rabbit immunoglobulin G–peroxidase conjugate (A-9169; Sigma) was used as the secondary antibody. The light-emitting nonradioactive ECL kit (Amersham Life Sciences) was used for signal detection.
Determination of SAK activity.
B. subtilis transformants carrying plasmid pSAK704 were plated on LB-kanamycin agar and incubated for 24 h at 37°C. Five milliliters of top agar solution containing 50 mM Tris-Cl (pH 8.0), 150 mM NaCl, 0.7% (wt/vol) agar, 3% (wt/vol) skim milk, and 50 μg of human plasminogen was then poured onto the agar surface, and the plate was incubated for a further 2 h at 37°C. SAK activity was determined by the clear zone around colony. Quantitative SAK activity was determined by the plasminogen-coupled chromogenic substrate assay (21) with following modifications. Forty microliters of SAK (final concentration, 0 to 10 nM) and 40 μl of human plasminogen (final concentration, 0.5 μM) in 20 mM sodium phosphate buffer (pH 7.5) were mixed and incubated for 25 min at 25°C. Twenty microliters of the chromogenic substrate S-2251 (final concentration, 1 mM) (V-0882; Sigma) was then added, and the absorbance at 450 nm was measured after a further 5 min (Bio-Rad microwell plate reader, model 550).
Purification of rSAK.
Two hundred milliliters of B. subtilis LB700 broth was centrifuged (6,000 × g, 10 min). After removal of the microbial cells, solid ammonium sulfate was added to the supernatant and mixed to achieve 80% saturation. After the mixture was allowed to stand at 4°C for 4 h, the precipitated protein was collected by centrifugation (8,000 × g, 30 min, 4°C). The precipitate was dissolved in 5 ml of 50 mM sodium phosphate (pH 7.5) containing 150 mM NaCl and then dialyzed against the same buffer. The dialyzed sample was then applied to a Superdex 75 column, previously equilibrated with the same buffer (Pharmacia Biotech), operated with fast protein liquid chromatography equipment (flow rate, 0.5 ml/min) (Pharmacia Biotech). Purified fractions of rSAK were analyzed by SDS-PAGE, electroblotted to polyvinylidene difluoride membranes (Schleicher & Schuell), and certified by peptide sequencing (Perkin-Elmer ABI 476).
Stability of SAK and SK in the spent medium.
A 24-h-old culture broth of B. subtilis was centrifuged (10,000 × g, 10 min) and passed through a 0.45-μm-pore-size filter (Millipore). Five milligrams of purified rSAK was then added to 1 ml of this filtrate and incubated at 37°C. The residual SAK activity was assayed by the chromogenic substrate method as described above. One milligram of purified recombinant SK (rSK) from E. coli (10) was added to 1 ml of the filtrate and incubated at 37°C. The residual SK activity was measured by the plasminogen color coupling assay (10).
RESULTS
Inactivation of wprA in the chromosome of B. subtilis WB600.
B. subtilis WB600 was transformed with plasmid pDWPRA, and tetracycline-resistant clones were selected at 30°C and subsequently were singly transferred to 5 ml of LB medium (42°C) to eliminate the unincorporated plasmid. Ten colonies were randomly selected, and clones with an inactivated copy of wprA in the chromosome were identified by PCR screening with primers WPRP-F and TETP-R. PvuII-digested genomic DNAs of WB600 and a candidate clone, LB700, were hybridized with a wprA PCR fragment amplified with WPRS-F and WPRS-R as a probe, which corresponds to the region between nucleotides 2050 and 2340, also encoding the catalytic serine residue. A hybridization band of 1.4 kb indicated that the wprA gene in B. subtilis LB700 was indeed disrupted by the tet gene (data not shown).
Enhanced secretion of rSAK by wprA inactivation.
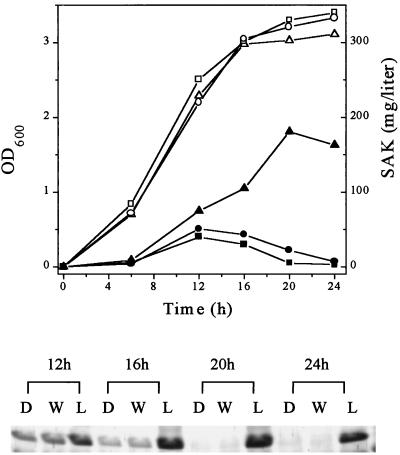
B. subtilis LB700, WB600, and DB431 were transformed with pSAK704, and several colonies were picked for further investigation. Preliminary tests on skim milk-plasminogen plates showed that LB700 displayed a much larger and clearer zone around the colony than WB600 or DB431 transformants (data not shown). Thus, the deletion of wprA seemed to enhance the production of rSAK. Consequently, selected transformants were tested in liquid culture. The secretion of rSAK by LB700 was dramatically enhanced during the stationary phase, whereas it was severely degraded in DB431 and WB600 after 20 h (Fig. 2). The final concentration of rSAK secreted by B. subtilis LB700 transformants was 181 mg/liter as determined by the chromogenic assay. This contrasts with only 45 mg/liter secreted by WB600 or DB431 transformants (Fig. 2). Moreover, secretion of rSAK by LB700 continued for more than 36 h (data not shown).
FIG. 2.
Time course of SAK production by B. subtilis and SDS-PAGE analysis. Open symbols, growth; closed symbols, concentration of secreted SAK; squares, B. subtilis DB431; circles, WB600; triangles, LB700. Lanes D, W, and L, B. subtilis DB431, WB600, and LB700, respectively. OD600, optical density at 600 nm.
SDS-PAGE and Western blot analysis indicated that secreted rSAK constituted a major band in a Coomassie brilliant blue-stained gel (data not shown). Amino acid sequencing analysis of the purified mature rSAK showed the wild-type SAK sequence SSSFD at the amino-terminal region.
Stability of SAK in the extracellular medium.
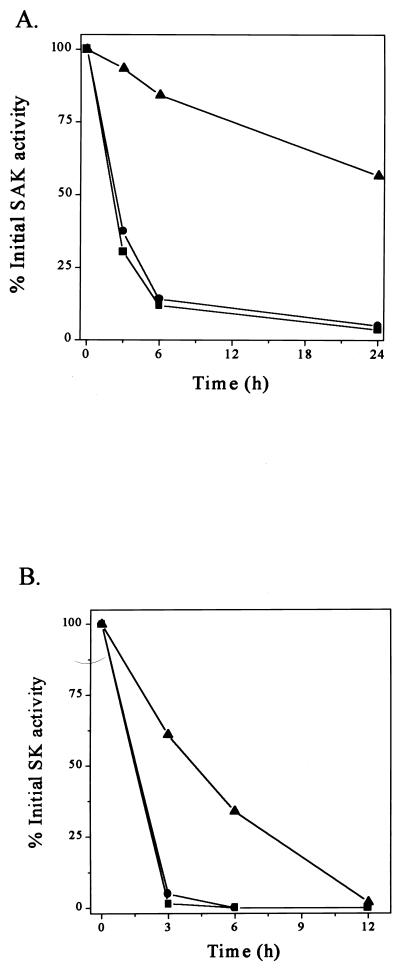
The WprA protease was reported to be present in the culture supernatant of B. subtilis WB600 (1). We have therefore investigated whether the disruption of wprA influences the stability of rSAK in the extracellular medium. Figure 3A shows that 11.9 and 14.1% of the original rSAK activity was recovered after 6 h of incubation of rSAK at 37°C with culture filtrates of B. subtilis DB431 and WB600, respectively. However, significantly less degradation of rSAK occurred in the culture filtrate of LB700, and more than 55% of the original rSAK activity was still recovered after 24 h (Fig. 3A). Hence, the extracellular WprA protease is significantly involved in the degradation of secreted rSAK in B. subtilis.
FIG. 3.
Enhanced stability of recombinant proteins in spent medium of B. subtilis LB700. Stability of SAK (A) and SK (B) in spent medium of B. subtilis DB431 (■), WB600 (●), and LB700 (▴) at 37°C is shown.
Stability of SK in the culture filtrate.
In order to find out whether wprA gene disruption also improves SK stability, a similar experiment was performed with rSK. Figure 3B shows that upon incubation with culture filtrate of strain WB600, most of the rSK activity disappeared within 3 h. In contrast, 61% of the original SK activity was recovered after the same time upon incubation with culture filtrate of LB700 (Fig. 3B). Therefore, wprA disruption also improves the stability of SK, although not as much as that of rSAK. In fact, the rate of degradation of rSK was about five times higher than that of rSAK. SK appears to be more susceptible to the residual extracellular proteases than SAK.
DISCUSSION
In B. subtilis LB700 carrying plasmid pSAK704, secretion of rSAK was dramatically increased in the stationary phase compared with that in the other B. subtilis strains (Fig. 2). This result indicated that secretion of rSAK was restricted by WprA protease associated with the cell wall. Because the mature Bacillus protein AmyL was reported to be stable in spent culture medium, while it was degraded in the membrane translocation (22), rSAK in the secretion process seemed to be susceptible to WprA protease in the cell wall.
Recently, the wprA gene products CWBP52 and CWBP23 were found as a complex in the culture medium (1). The influence of WprA on mature rSAK in the spent culture medium was investigated. Mature rSAK was significantly degraded in the spent media of WB600 and DB431, in contrast to the case for the spent medium of LB700 (Fig. 3A). It was deduced that the stability of rSAK was influenced not only by WprA in the cell wall but also by released WprA in the extracellular medium. The stability of SK was also significantly affected by WprA protease in the culture supernatant (Fig. 3B). SK was reportedly produced in B. subtilis WB600 (27). However, the secretion amount was not notable. It was thought that removal of WprA could enhance the secretory production level of SK in B. subtilis.
With the extracellular protease level from B. subtilis 168 set as 100%, Mpr, NprB, and Vpr have levels of 2.2, 0.48, and 0.18%, respectively (28, 29). Extracellular WprA constitutes less than 0.14% of the total extracellular protease activity. It was reported that the remaining 0.1% of extracellular protease activity is still sufficient to degrade foreign proteins that are highly sensitive to proteases (26). While WprA is less significant than Mrp and NprB in total extracellular protease activity, mature SAK and SK were more sensitive to the trivial WprA protease than to Mpr and NprB in the extracellular milieu.
Therefore, it is necessary to reconsider the role of WprA, which has been thought to be restricted to being an authenticator of misfolded secretory proteins in the cell wall (22). The role of the wprA gene product may be in proteolysis of nutrients in the proximal area. The colony growth of the wprA+ strain was qualitatively greater than that of the wprA mutant after 30 h (data not shown). This may be observed only in a multiple-protease-deficient strain. However, the growth in the broth was not distinguishable (Fig. 2).
In summary, it was shown that the cell wall-associated protease WprA was essential for the degradation of heterologous rSAK in B. subtilis. The severe degradation of SAK and SK in the extracellular milieu was prevented by inactivation of the minor WprA protease. These data indicate a significant role of the wprA gene product in degrading foreign proteins, both during secretion and in the extracellular milieu. It is important to identify and inactivate a heterologous protein digestion apparatus for the production of a recombinant protein.
ACKNOWLEDGMENTS
This work was supported in part by a research grant from the Korea Ministry of Science and Technology (1999) and in part by KOSEF for SRC (RCNBMA, SNU).
We are grateful to S. L. Wong for providing B. subtilis WB600. We thank K.-D. Wittchen for supplying plasmid pUCTV2 and W. J. Oh for technical support.
REFERENCES
- 1.Babe L M, Schmidt B. Purification and biochemical analysis of WprA, a 52-kDa serine protease secreted by B. subtilis as an active complex with its 23-kDa propeptide. Biochim Biophys Acta. 1998;1386:211–219. doi: 10.1016/s0167-4838(98)00110-1. [DOI] [PubMed] [Google Scholar]
- 2.Behnke D, Gerlach D. Cloning and expression in Escherichia coli, Bacillus subtilis, and Streptococcus sanguis of a gene for staphylokinase, a bacterial plasminogen activator. Mol Gen Genet. 1987;210:528–534. doi: 10.1007/BF00327208. [DOI] [PubMed] [Google Scholar]
- 3.Collen D, De Cock F, Stassen J M. Comparative immunogenicity and thrombolytic properties toward arterial and venous thrombi of streptokinase and recombinant staphylokinase in baboons. Circulation. 1993;87:996–1006. doi: 10.1161/01.cir.87.3.996. [DOI] [PubMed] [Google Scholar]
- 4.Collen D, Silence K, Demarsin E, De Mol M, Lijnen H R. Isolation and characterization of natural and recombinant staphylokinase. Fibrinolysis. 1992;6:203–213. [Google Scholar]
- 5.Collen D, Van de Werf F. Coronary thrombolysis with recombinant staphylokinase in patients with evolving myocardial infarction. Circulation. 1993;87:1850–1853. doi: 10.1161/01.cir.87.6.1850. [DOI] [PubMed] [Google Scholar]
- 6.Doi R H. Isolation of Bacillus subtilis chromosomal DNA. In: Rodriuez R L, Tait R C, editors. Recombinant DNA techniques. Reading, Mass: Addison-Wesley Publishing Co., Inc.; 1983. pp. 162–163. [Google Scholar]
- 7.Doi R H, He X S, McCready P, Bakheit N. Bacillus subtilis: a model system for heterologous gene expression. In: Kelly J W, Baldwin T O, editors. Applications of enzyme biotechnology. New York, N.Y: Plenum Press; 1991. pp. 261–272. [Google Scholar]
- 8.Grella D K, Castellino F J. Activation of human plasminogen by staphylokinase. Direct evidence that preformed plasmin is necessary for activation to occur. Blood. 1997;89:1585–1589. [PubMed] [Google Scholar]
- 9.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 10.Ko J H, Park D K, Kim I C, Lee S H, Byun S M. High-level expression and secretion of streptokinase in Escherichia coli. Biotechnol Lett. 1995;17:1019–1024. [Google Scholar]
- 11.Lack C H. Staphylokinase: an activator of plasma protease. Nature. 1948;161:559–560. doi: 10.1038/161559b0. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Lee S J, Kim I C, Kim D M, Bae K H, Byun S M. High level secretion of recombinant staphylokinase into periplasm of Escherichia coli. Biotechnol Lett. 1998;20:113–116. [Google Scholar]
- 14.Margot P, Karamata D. The wprA gene of Bacillus subtilis 168, expressed during exponential growth, encodes a cell-wall-associated protease. Microbiology. 1996;142:3437–3444. doi: 10.1099/13500872-142-12-3437. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie T, Hoshino T, Tanaka T, Sueoka N. The nucleotide sequence of pUB110: some salient features in relation to replication and its regulation. Plasmid. 1986;15:93–103. doi: 10.1016/0147-619x(86)90046-6. [DOI] [PubMed] [Google Scholar]
- 16.Palva A, Vigren G, Simonen M, Rintala H, Laamanen P. Nucleotide sequence of the tetracycline resistance gene of pBC16 from Bacillus cereus. Nucleic Acids Res. 1990;18:1635. doi: 10.1093/nar/18.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S S, Wong S L, Wang L F, Doi R H. Bacillus subtilis subtilisin gene (aprE) is expressed from a sigma A (sigma 43) promoter in vitro and in vivo. J Bacteriol. 1989;171:2657–2665. doi: 10.1128/jb.171.5.2657-2665.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadaie Y, Kada T. Formation of competent Bacillus subtilis cells. J Bacteriol. 1983;153:813–821. doi: 10.1128/jb.153.2.813-821.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai M, Watanuki M, Matsuo O. Mechanism of fibrin-specific fibrinolysis by staphylokinase: participation of a2-plasmin inhibitor. Biochem Biophys Res Commun. 1989;162:830–837. doi: 10.1016/0006-291x(89)92385-1. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 21.Schlott B, Hartmann M, Gührs K, Birch-Hirschfeid E, Pohl H, Vanderschueren S, Van der Werf F, Michoel A, Collen D, Behnke D. High yield production and purification of recombinant staphylokinase for thrombolytic therapy. Bio/Technology. 1994;12:185–189. doi: 10.1038/nbt0294-185. [DOI] [PubMed] [Google Scholar]
- 22.Stephenson K, Harwood C R. Influence of a cell-wall-associated protease on production of alpha-amylase by Bacillus subtilis. Appl Environ Microbiol. 1998;64:2875–2881. doi: 10.1128/aem.64.8.2875-2881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanderschueren S, Stockx L, Wilms G, Lacroix H, Verhaeghe R, Vermylen J, Collen D. Thrombolytic therapy of peripheral arterial occlusion with recombinant staphylokinase. Circulation. 1995;92:2050–2057. doi: 10.1161/01.cir.92.8.2050. [DOI] [PubMed] [Google Scholar]
- 24.Wertman K F, Wyman A R, Bostein D. Host/vector interactions which affect the viability of recombinant phage lambda clones. Gene. 1986;49:253–262. doi: 10.1016/0378-1119(86)90286-6. [DOI] [PubMed] [Google Scholar]
- 25.Wittchen K D, Meinhardt F. Inactivation of the major extracellular protease from Bacillus megaterium DSM319 by gene replacement. Appl Microbiol Biotechnol. 1995;42:871–877. doi: 10.1007/BF00191184. [DOI] [PubMed] [Google Scholar]
- 26.Wong S-L. Advances in the use of Bacillus subtilis for the expression and secretion of heterologous proteins. Curr Opin Biotechnol. 1995;6:517–522. doi: 10.1016/0958-1669(95)80085-9. [DOI] [PubMed] [Google Scholar]
- 27.Wong S-L, Ye R, Nathoo S. Engineering and production of streptokinase in a Bacillus subtilis expression-secretion system. Appl Environ Microbiol. 1994;60:517–523. doi: 10.1128/aem.60.2.517-523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X C, Lee W, Tran L, Wong S L. Engineering a Bacillus subtilis expression-secretion system with a strain deficient in six extracellular proteases. J Bacteriol. 1991;173:4952–4958. doi: 10.1128/jb.173.16.4952-4958.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye R, Kim J-H, Kim B-G, Szarka S, Sihota E, Wong S-L. High level production of intact, biologically active staphylokinase from Bacillus subtilis. Biotechnol Bioeng. 1999;62:87–96. [PubMed] [Google Scholar]
- 30.Ye R, Yang L P, Wong S-L. Proceedings of the International Symposium on Recent Advances in Bioindustry 1996. Seoul, Korea: The Korean Society for Applied Microbiology; 1996. Construction of protease-deficient Bacillus subtilis strains for expression studies: inactivation of seven extracellular proteases and the intracellular LonA protease; pp. 160–169. [Google Scholar]