Abstract
Introduction
The Flow Redirection Endoluminal Device (FRED; MicroVention) is a dual-layered flow diverter used for the treatment of intracranial aneurysms. The objective of this systematic review was to compile device-related safety and effectiveness data.
Methods
The literature from January 1, 2013 to April 30, 2021 was searched for studies describing use of the FRED for intracranial aneurysm treatment irrespective of aneurysm location and morphology. The review included anterior and posterior circulation ruptured and unruptured saccular, fusiform or dissection, and blister aneurysms. MeSH terms related to “flow re-direction endoluminal device” and “FRED for aneurysms” were used. Data related to indication, complications, and rates of aneurysm occlusion were retrieved and analyzed.
Results
Twenty-two studies with 1729 intracranial aneurysms were included in this review. Overall reported morbidity was 3.9% (range 0–20%). Overall procedure-related mortality was 1.4% (range 0–6%). Complication rates fell into 5 categories: technical (3.6%), ischemic (3.8%), thrombotic or stenotic (6%), hemorrhagic (1.5%), and non-neurological (0.8%). The aneurysm occlusion rate between 0 and 3 months (reported in 11 studies) was 47.8%. The occlusion rate between 4 and 6 months (reported in 14 studies) was 73.8%. Occlusion rates continued to increase to 75.1% at 7–12 months (reported in 10 studies) and 86.6% for follow-up beyond 1 year (reported in 10 studies).
Conclusion
This review indicated that the FRED is a safe and effective for the treatment of intracranial aneurysms. Future studies should directly compare the FRED with other flow diverters for a better understanding of comparative safety and effectiveness among the different devices.
Keywords: Flow diversion, Flow Redirection Endoluminal Device (FRED), Intracranial aneurysms
Introduction
Flow diversion has revolutionized the treatment of intracranial aneurysms. Over recent years, several flow-diverting devices have been introduced. These differ in their design, ease of deployment, and intended metal coverage across the aneurysm neck. The Flow Re-direction Endoluminal Device (FRED; MicroVention, Aliso Viejo, California, USA) is a dual-layered flow diverter used in the treatment of intracranial aneurysms. The outer stent portion consists of 16 nitinol wires and allows for proper positioning of the device, whereas the inner flow-diverting portion consists of 48 nitinol wires.1,2 The device is claimed to allow better navigation through the parent vessels, especially in vessels that tend to be convoluted. 3 The FRED was approved in the United States by the Food and Drug Administration in 2019 and is available in 2 forms, i.e., FRED 27 and FRED 21 (FRED Jr.). The 2 devices come in various diameters and lengths. Mechanical and technical advantages of using the FRED as opposed to other flow-diverter devices include the self-expanding ability of the device allowing for improved accuracy of placement, multiple radiopaque markers to allow for greater visibility, and the ability to retrieve the device for repositioning before it is fully deployed. 2 The use of the FRED for intracranial aneurysm treatment is still relatively recent, and there is no known literature compiling data from existing studies. The aim of this review was to analyze the safety and effectiveness data of this device.
Methods
Search criteria
To identify studies on the treatment of intracranial aneurysms with the FRED, searches of the PubMed, Embase, and ClinicalKey databases were performed. Search terms included “FRED for aneurysm” and “flow re-direction endoluminal device.” Search results were screened for studies specifically related to FRED devices in the neurointerventional field. FRED received the CE mark in 2013; therefore, the literature was reviewed from that year until April 2021.
Selection criteria
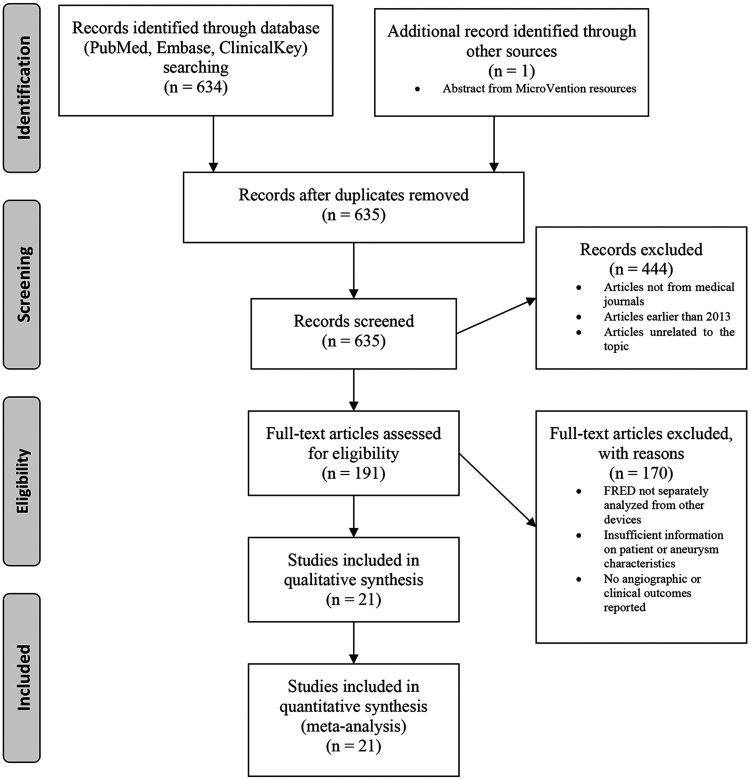
Studies reporting the use of the FRED device for intracranial aneurysms and its related procedural complications, aneurysm occlusion rates, and mortality were included in this review. Figure 1 illustrates the progression from the initial search results to the final studies included in this review using the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) 4-phase flow diagram. 4
Figure 1.
Flowchart for study selection using the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) 4-phase flow diagram. 4
Data analyzed
Data from all selected studies were pooled to calculate number of patients, proportion of male and female patients, mean age, proportion of anterior and posterior circulation location, proportion of each aneurysm’s morphology (saccular, blister, fusiform, or dissecting), proportion of ruptured aneurysms at presentation, rate of technical complications, ischemic and hemorrhagic adverse events, overall morbidity and mortality, and occlusion rates at each follow-up interval.
Results
Study characteristics
Twenty-two studies published between January 1, 2013 and April 30, 2021 are included in this review.1–3,5–24 One study published results in 2 separate papers.2,3 Table 1 outlines the characteristics of each study. Twenty-one studies were full-text articles and 1 was an abstract. 16 Sixteen of the studies were retrospective and 6 were prospective. Fifteen used the FRED 27 exclusively, 3 used the FRED 21 exclusively, and 4 used a combination of the 2 devices. The 22 studies included a total of 1831 patients with 1994 intracranial aneurysms.
Table 1.
Characteristics of the included studies.
| References | Country | Study Design | Number of Patients | Number of Aneurysms | Device Used | Location of Aneurysms | Size of Aneurysms | Occlusion Rates | Morbidity | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| Diaz et al. 5 | Columbia andUnited States | Retrospective | 13 | 14 | FRED 27 | 5 PComA, 1 ophthalmic ICA, 4 paraophthalmic ICA, 2 superior hypophyseal artery, 1 cavernous ICA, 1 vertebrobasilar system | Size range: 2.0–10.0 mm | Immediate flow reduction 14/14 (100%) | 0% | 0% |
| Poncyljusz et al. 6 | Poland | Retrospective | 6 | 8 | FRED 27 | 6 ophthalmic segment of ICA, 1 clinoidal segment of ICA, 1 ICA-PICA junction | Dome size range: 6.0–35.0 mmNeck size range: 5.0–14.0 mm | 8/8 (100%) complete occlusion 3 months post-procedure | 0% | 0% |
| Kocer et al. 7 | Turkey | Retrospective | 33 | 37 | FRED 27 | 31 paraophthalmic segment of ICA, 2 petrous segment of ICA, 1 ACA, 1 C1-C2 segment of ICA, 2 VA | Size range: 2.7–15.0 mmNeck size range: 2.0–7.1 mm | Complete occlusion 6/19 (32%) 0–1 month post-procedureComplete occlusion 8/12 (67%) 2–3 months post-procedureComplete occlusion 4/5 (80%) 4–6 months post-procedureComplete occlusion 8/8 (100%) 7–12 months post-procedure | 0% | 0% |
| Möhlenbruch et al. 1 | Germany | Prospective | 29 | 34 | FRED 27 | 23 ICA/posterior PComA, 3 ACA, 8 posterior circulation | Diameter range: 0.6–39.0 mmNeck size range: 1.0–16.3 mmParent Vessel Diameter Range: 2.0–5.3 mm proximally, 1.9–5.7 mm distally | 9/34 (26%) complete early occlusion 30 minutes after insertion19/34 (56%) complete occlusion 3 months post-procedure22/30 (73%) complete occlusion 6 months post-procedure | 3.4% | 0% |
| Briganti et al. 8 | Italy | Retrospective | 20 | 24 | FRED 27 | 10 paraophthalmic ICA, 5 cavernous ICA, 8 PComA, 1 AChA | Diameter range: 2.0–25.0 mmNeck size range: 2.0–12.0 mm | 10/24 (42%) complete occlusion 3 months post procedure8/24 (33%) complete occlusion 6 months post procedure2/24 (8%) complete occlusion 12 months post procedureOverall complete occlusion rate 20/24 (83%) | 0% | 0% |
| Drescher et al. 9 | Germany | Retrospective | 50 | 52 | FRED 27 | 8 cervical ICA, 3 cavernous ICA, 21 paraophthalmic ICA, 3 PComA, 2 ACA, 2 MCA, 2 BA, 9 VA, 2 PCA | Diameter range: 2.0–35.0 mmNeck width range: 2.0–20.0 mm | 25/43 (58.1%) complete occlusion 3 months post-procedure27/36 (75%) complete occlusion 12 months post-procedureOverall complete occlusion and near-complete occlusion rate 97.2% | 4% | 2% |
| Luecking et al. 10 | Germany | Prospective | 50 | 52 | FRED 27 | 46 ICA and carotid bifurcation including PComA origin, 1 MCA, 3 intracranial part of VA, 1 PICA origin, 1 basilar tip | Diameter range: 2.0–18.5 mm | 9 immediate complete occlusion34/47 (72.3%) complete occlusion 3 months post-procedure36/47 (76.6%) complete occlusion 6 months post-procedureOverall occlusion rate 87.2% | 0% | 0% |
| Mahboobani et al. 11 | Hong Kong | Retrospective | 11 | 11 | FRED 27 | 8 distal ICA, 3 VA | Diameter range: 3.0–26.0 mm Neck size range: 2.0–16.0 mmParent artery diameter range: 3.3–4.8 mm | Complete occlusion 6/8 (75%) 6 months post-procedure | 0% | 0% |
| Möhlenbruch et al. 12 | Germany, Turkey, Austria | Retrospective | 42 | 47 | FRED 21 | 18 MCA,13 ACA,2 PCA,1 SCA,1 VA, 11 anterior communicating artery,1 ICA | Diameter range: 1.3–25.2 mmNeck size range: 1.3–14.5 mmParent Vessel Diameter Range: 1.4–3.6 mm proximally, 1.5–3.4 mm distally | 6/41 (15%) complete occlusion 1 month post-procedure19/27 (70%) complete occlusion 6 months post-procedure8/11 (73%) complete occlusion 12 months post-procedure | 0% | 0% |
| Pierot et al.a2,3 | France | Prospective | 103 | 103 | FRED 21 & FRED 27 | 71 supraclinoid ICA, 15 cavernous ICA, 9 ACA/AComA, 8 MCA | Diameter: 71 < 10mm, 29 10–24 mm, 3 > 25 mmNeck size: 34 < 4 mm, 69 > 5 mm | Complete occlusion 58/95 (61.1%) 6 months post-procedureComplete occlusion 66/90 (73.3%) 1 year post-procedure | 2.9% | 1.9% |
| Killer et al. 13 | Austria, Turkey, United States, Germany, Denmark | Retrospective | 531 | 579 | FRED 27 | 50 PComA, 270 paraophthalmic ICA, 45 cavernous ICA segment, 67 other segments of ICA, 37 MCA, 16 ACA, 4 AComA, 13 pericallosal artery, 30 BA, 9 PCA, 2 SCA, 36 other posterior circulation locations | Diameter range: 1.0–36.6 mmNeck diameter range: 1.0–30.0 mm | Overall complete occlusion rate 69.2%Complete occlusion 20% at 3 months post-procedureComplete occlusion 82.5% 6 months post-procedureComplete occlusion 91.3% 1 year post-procedureComplete occlusion 95.3% over 1 year post-procedure | 4.0% | 1.5% |
| Manzato et al. 14 | Brazil | Retrospective | 28 | 38 | FRED 27 | 34 ICA, 1 MCA, 1 ACA, 1 PICA, 1 BA | Diameter range: 3.0–16.0 mm | 19/24 (79%) complete occlusion 6 months post-procedure | 10.3% | 0% |
| Piano et al. 15 | Italy | Prospective | 162 | 165 | FRED 27 | 80 paraophthalmic ICA, 26 PComA/AChA, 19 cavernous segment of ICA, 6 terminal segment of ICA, 5 MCA, 5 BA, 4 VBJ, 7 PICA, 13 VA | 65 < 10mm90 10–25mm 10 > 25mm | 72/113 (64%) complete occlusion 3 months post procedure110/143 (77%) complete occlusion137/143 (96%) curative at 12–24 months post procedure | 6.2% | 2.5% |
| Pabon et al. 16 | Columbia | Retrospective | 252 | 268 | FRED 27 | Not discussed | Mean aneurysm size: 12.4 mm | 92/105 (87.6%) complete occlusion at last follow up post-procedure (median follow up 16 months) | 7.5% | 2.3% |
| Rautio et al. 17 | Finland | Retrospective | 15 | 15 | FRED 21 | 6 AComA, 1 PComA, 2 pericallosal artery, 2 MCA, 2 PCA, 2 ACA | Diameter range: 1.0–15.0 mmParent vessel diameter range: 1.3–2.3 mm proximally, 1.3–2.3 mm distally | 9/15 (60%) complete occlusion 3–6 months post-procedure13/15 (87%) complete occlusion 6–24 months post-procedure | 0% | 0% |
| Laukka et al. 18 | Finland | Retrospective | 5 | 5 | FRED 27 & FRED 21 | 2 PCA, 2 PICA, 1 SCA | Size range: 4.0–4.4 mmParent artery diameter range: 1.2–2.3 mm | 5/5 (100%) complete occlusion 3–11 months post-procedure2/2 (100%) complete occlusion 18–22 months post-procedure | 20% | 0% |
| Sivasankar et al. 19 | India | Retrospective | 12 | 15 | FRED 21 | 6 distal ACA, 4 AComA, 3 MCA, 1 VA, 1 PCA | Size range: 1.4–11.0 mmNeck size range: 2.5–5.0 mmParent artery size range: 2.2–3.1 mm proximally, 1.9–2.6 mm distally | 6/10 (60%) complete occlusion 6–12 months post-procedure | 0% | 0% |
| Guimaraens et al. 20 | Spain | Prospective | 150 | 184 | FRED 27 & FRED 21 | 3 cervical carotid artery, 85 carotid siphon, 25 MCA, 21 PComA, 18 AComA, 12 BA, 5 AChA, 5 PICA, 3 ACA, 3 PCA, 5 VA | 86 small (<5 mm)27 medium (5–10 mm)11 large (>11 mm) | 132/156 (84.6%) complete occlusion at end of follow up31/47 (66%) complete occlusion within first year post procedure101/109 (92.7%) complete occlusion after 1 year post procedure | 0.5% | 0.5% |
| Griessenauer et al. 21 | Austria, United States, Germany, Turkey, | Retrospective | 84 | 84 | FRED 27 | 42 VA, 22 BA, 7 PCA, 4 SCA, 4 extradural VA, 3 PICA, 2 VBJ | Size range: 2.0–67.0 mm | 78.2% complete occlusion (median follow up 24 months post procedure) | 6% | 6% |
| Luecking et al. 22 | Germany | Retrospective | 78 | 78 | FRED 27 | 71 ICA including PComA and AChA origins, 7 posterior circulation (VA and BA) | Size range: 2.0–18.5 mm | 58/74 (77.3%) complete occlusion 6 months post procedure67/73 (90.5%) complete occlusion at long term follow up (median follow up 36.9 months post procedure) | 2.6% | 0% |
| Gan et al. 23 | United Kingdom | Retrospective | 21 | 25 | FRED 27 | 25 ICA | Size range 2–27mm | 22/25 (88%) at 6 months | 14.3% | 0% |
| Dinc et al. 24 | Turkey | Prospective | 136 | 155 | FRED 27 & FRED 21 | 123 ICA, 21 MCA, 1 ACA, 7 VA, 2 BA, 1 AICA | 104 small (≤ 10 mm)45 large (10–25 mm)6 giant (≥25 mm) | 103/143 (72%) complete occlusion 3–9 months104/123 (84.6%) complete occlusion 12–24 months84/102 (82.4%) complete occlusion >24 months | 1.5% | 0.7% |
ACA: anterior cerebral artery; AChA: anterior choroidal artery; AComA: anterior communicating artery; BA: basilar artery; ICA: internal carotid artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; PComA: posterior communicating artery; PICA: posterior inferior cerebellar artery; SCA: superior cerebellar artery; VA: vertebral artery; VBJ: vertebrobasilar junction.
aStudy sponsored by industry.
Patient characteristics
The 22 studies in this review included 1831 patients. Table 2 outlines patient demographics. Patient sex was specified in 21 studies for 1562 patients. Women comprised most of the patient population (72.9%). The age of the patient population was specified in 20 studies (N = 1572). The mean age was 58.5 years with a wide age range of 13 to 86 years.
Table 2.
Patient and aneurysm characteristics.a
| Patient characteristics | N (%) | |
|---|---|---|
| Sex (21 studies, 1562 patients) | Female | 1139 (72.9) |
| Male | 423 (27.1) | |
| Age, years (20 studies, 1572 patients) | 58.5 (mean) | |
| Aneurysm characteristics | ||
| Location (21 studies, 1729 aneurysms) | Anterior circulation | 1455 (84.2) |
| Posterior circulation | 274 (15.8) | |
| Morphology (17 studies, 1552 aneurysms) | Saccular | 1287 (82.9) |
| Fusiform or dissecting | 200 (12.9) | |
| Blister | 65 (44.2) | |
| Rupture status (20 studies, 1700 aneurysms) | Ruptured | 292 (17.2) |
| Unruptured | 1408 (82.8) |
aAs reported in the studies.
Aneurysm characteristics
The 22 studies include a total of 1729 aneurysms treated with the FRED. Table 2 outlines aneurysm characteristics. The location was specified in 21 studies for 1729 aneurysms. Most aneurysms were located in the anterior circulation as opposed to the posterior circulation ((84.2% vs 15.8%) The exact location of the aneurysms reported in each study is provided in Table 1.
Aneurysm morphology was specified in 17 studies for 1552 aneurysms. Most aneurysms were saccular (82.9%). Fusiform or dissecting was the next most common morphology (12.9%). Blister-like was the least common morphology (4.2%). Size or diameter ranges, neck size ranges, and parent artery size ranges are summarized in Table 1.
Rupture status was specified in 20 studies for 1700 aneurysms. Most aneurysms were unruptured at the time of treatment (82.8%).
Complications
Complications rates were reported in all 22 studies (Table 1) and are summarized in Table 3. These included complications that occurred intraprocedurally and/or during the follow-up period (mean 14.3 months) of the respective study.
Table 3.
Procedure-related complications and angiographic occlusion results.
| Complications | No. of events/procedures (%)(22 studies) |
|---|---|
| Technical complications | 68/1895 (3.6) |
| Ischemic complications | 72/1895 (3.8) |
| Thrombotic or stenotic complications | 113/1895 (6) |
| Hemorrhagic complications | 28/1895 (1.5) |
| Non-neurological complications | 15/1895 (0.8) |
| Overall morbidity | 3.9% (range 0–20) |
| Overall mortality | 1.4% (range 0–6) |
| Occlusion results | Mean follow up: 14.3 months |
| Immediate (2 studies) | 18/86 (20.9) |
| 0–3 months (9 studies) | 206/431 (47.8) |
| 4–6 months (12 studies) | 402/545 (73.8) |
| 7–12 months (9 studies) | 269/358 (75.1) |
| >12 months (9 studies) | 672/776 (86.6) |
| Overall occlusion rates at last follow-up (20 studies) | 82.8% (range 60–100) |
Technical complications were encountered in 3.6% of cases. They included misdeployment of the FRED during the procedure, migration of the device, or two phenomena known as “fish-mouthing” and “foreshortening.” “Fish-mouthing” is a change in the device’s morphology where there is an inward crimping either proximally, distally, or both. 7 “Foreshortening” is a change in the device’s morphology where it is no longer long enough to cover the neck of the aneurysm. With either, the stent must be repositioned or a longer stent must be used.
Ischemic complications occurred at a rate of 3.8% among the 22 studies reviewed. These complications included major and minor strokes and transient ischemic attacks.
Thrombotic or stenotic complications were seen in 6% of cases. These complications included in-stent stenosis and thrombosis and parent artery stenosis and thrombosis.
Hemorrhagic complications occurred at an incidence of 1.5%. These complications included aneurysm rupture (both delayed and intraprocedural), intraparenchymal hemorrhage, and subarachnoid hemorrhage. There was 1 case of aneurysm rupture due to wire-related perforation. 22
Several non-neurological complications were reported. These complications were relatively rare, seen in 0.8% of cases. Non-neurological complications include hemoptysis post extubation, intragastric bleeding, and femoral pseudoaneurysms at the puncture site.
Morbidity and mortality data were collected for all 22 studies. Average morbidity was 3.9%, ranging from 0% to 20%. There were 9 studies that reported 0% morbidity rates. Average mortality was 1.4%, ranging from 0% to 6%. Fourteen studies reported no deaths due to the procedure.
Occlusion results
The overall mean occlusion rate at the final follow up (mean follow up 14.3 months) was 82.8% with a range of 60%–100%. This rate included 20 of the 22 studies. Only immediate results and no follow-up results were reported in 1 study. Those investigators reported immediate flow reduction in 100% of cases. 5 Immediate aneurysm occlusion was reported in 2 studies with an overall rate of 20.9%.1,10
The earliest post-procedural follow up occurred between 0 and 3 months and was reported in 9 studies, with a mean occlusion rate of 47.8%. One study reported a 100% occlusion rate at this point in time. 6
Twelve studies reported occlusion results between 4 and 6 months. The mean occlusion rate increased to 73.8%. Occlusion rates continued to increase to 75.1% at 7–12 months (reported in 9 studies) and 86.6% for follow up beyond 1 year (reported in 9 studies). Occlusion rates for individual studies are outlined in Table 1 and summarized in Table 3.
Discussion
Flow-diverter devices are a new generation of endovascular devices developed to treat intracranial aneurysms. They are placed in the parent artery across the aneurysm neck to disrupt the flow of blood into the aneurysm. Several flow diverters have been approved in addition to the FRED, including the Pipeline Embolization Device (PED; Medtronic, Dublin, Ireland), the Silk (Balt Extrusion, Montmorency, France), the p64 (phenox, Bochum, Germany), and the Surpass Flow Diverter (Stryker Neurovascular, Fremont, California). Flow diverters are a suitable alternative treatment for aneurysms that have been difficult to treat with other procedures (giant, wide-necked, bifurcating aneurysms). 15 There are several unique complications encountered with the use of these devices, such as occlusion of collateral branches, in-stent thrombosis/stenosis, and delayed aneurysm rupture. 3
Briganti et al. performed a systematic review to determine the safety and efficacy of several flow diverters, including the FRED. Occlusion rates seen in 8 studies using the PED were 88.2%, 83% in 7 studies using the Silk (Balt Extrusion, Montmorency, France), and 75% in 1 study using the Surpass (Stryker Neurovascular, Fremont, CA, USA). Overall morbidity and mortality for the review were 3.5% and 3.4%, respectively. 25 This review specifically looked at studies using exclusively the FRED, and the overall occlusion rate was 82.8% which is comparable to other flow diverters. Additionally, the overall morbidity and mortality rates for the studies included in this review were 3.9% and 1.4%, respectively. The morbidity is comparable to that for other flow diverters, whereas the mortality rate seems to be much lower in the FRED studies.
Flow diverters have been indicated mainly for the treatment of internal carotid artery aneurysms with success seen in the posterior circulation (although posterior circulation use is off-label in the US). Treatment for posterior circulation aneurysms has a higher risk of ischemic complications due to the presence of perforators and branches to the brainstem. 25 Few studies have explored the use of flow diversion in the posterior circulation. Grissenauer et al. 21 performed a subgroup analysis of the European FRED (EuFRED) study 13 of patients treated with the FRED for posterior circulation aneurysms. Complete occlusion at last follow up was 78.2%, which is comparable to occlusion rates seen in the studies presented in this review. Posterior circulation aneurysms are complex in nature due to the presence of perforating vessels that can become occluded with the introduction of a flow diverter. 21 Although, overall complication rates and outcomes of posterior circulation aneurysms as presented in the review are encouraging, more studies are need on flow diversion for the posterior circulation.
The rate of technical complications in these studies is relatively low (3.8%), which may be in part due to the “stent within a stent” structure of the FRED. The outer stent portion has only 16 wires, which allows for better anchoring of the device in the parent vessel and a smoother deployment during the procedure. A larger number of wires, as seen in other flow diverters, can increase the friction between the flow diverter and parent vessel. 19 However, the lower rate of complications may also be related to the fact that operators have now been through a longer learning curve owing to their experience with previously marketed flow diverters.
There are 2 FRED systems that can be used to treat intracranial aneurysms: FRED and FRED Jr. (FRED 27 and FRED 21, respectively). The 2 devices have a similar design, although the FRED Jr. was specifically designed for smaller vessels (<3 mm). Flow diversion has not usually been used for the treatment of aneurysms in small vessels. Three of the 20 studies used the FRED Jr. exclusively with promising results. All 3 studies reported morbidity and mortality rates of 0% with high occlusion rates (range 60%–87%).12,17,19 These studies provide promising results for a safe and effective alternative for previously difficult-to-treat aneurysms. One study that used both the FRED and FRED Jr. compared the 2 treatment groups. The results showed similar effectiveness between the 2 groups but a higher rate of complications in the FRED Jr. group (13.9% vs. 6.5%). 20 More studies will need to be completed to compare the safety and effectiveness of the FRED Jr.
Most (72.7%) of the studies included in this review were performed retrospectively. This was a major limitation discussed in these studies. Another major limitation was the small patient populations. Only 6 studies had more than 100 patients. Most authors discussed the need for larger-scale, multicenter, prospective studies. Additionally, only 4 of the 22 studies reviewed reported statistical data for variables such as aneurysm size versus occlusion and aneurysm size or location versus complications.12,13,20,22 These data could be important in the decision to use the FRED over another flow diverter or a treatment modality other than flow diversion. Further studies using the FRED should include these kinds of statistical data. Compared to the systematic review by Briganti et al., 25 the follow-up data for this review was longer (14.3 months vs. 9 months). However, it is still important to collect long-term follow up data to better determine FRED safety and occlusion rates.
Our systematic review has several limitations. These include heterogeneity of the methods and data points among the studies included. Therefore, a meta-analysis including all published studies was not possible. The review also does not compare complications and occlusion rates of the FRED with the results for other flow diverters. Relatively fewer cases of the FRED Jr are described by the studies. Thus, the safety and efficacy profile of FRED may not be applicable to that of the FRED Jr.
Conclusion
The FRED is a safe and efficacious device for treating intracranial aneurysms. Its dual-layer design allows for improved deployment and is more easily maneuvered through tortuous vessels. Occlusion results are promising for the FRED to be used as an alternative to other flow diverters for aneurysms that tend to be difficult to treat. Large-scale prospective studies using the FRED are lacking. These studies need to be performed as well as include an adequate follow-up period to better determine long-term occlusion rates.
Highlights
There are little data available on the safety and effectiveness (angiographic occlusion rates) of the Flow Redirection Endoluminal Device (FRED; MicroVention) for the treatment of intracranial aneurysms.
To our knowledge, this is the first systematic review that describes these data.
The findings of the review suggest that safety and occlusion rates after FRED deployment are comparable to those described for the Pipeline embolization device (Medtronic).
Acknowledgments
The authors thank Paul H Dressel BFA for formatting the illustration and Debra J Zimmer for editorial assistance.
Footnotes
Contributorship: Conception and design: MW, EIL Acquisition of the data: all authors. Analysis and interpretation of the data: all authors. Drafting the manuscript: MW, JN, MK. Critically revising the manuscript: all authors. Reviewed submitted version of manuscript: all authors.
Ethical considerations: Because the study did not involve human interaction or patient data, an institution board ethical review was deemed unnecessary.
Data availability: Data that support the findings of this study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: MW, RHD, MA, JN, JMC, VL, JL, AM: None
AK: Research grant from the Scoliosis Research Society to study scoliosis in Chiari patients.
JMD: Research grant: National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR001413 to the University at Buffalo. Consulting: Medtronic; Honoraria: Neurotrauma Science, LLC; shareholder/ownership interests: Cerebrotech, RIST Neurovascular.
AHS: Financial interest/investor/stock options/ownership: Adona Medical, Inc, Amnis Therapeutics, (Purchased by Boston Scientific October 2017), Blink TBI Inc., Buffalo Technology Partners Inc., Cerebrotech Medical Systems, Inc., Cognition Medical, Endostream Medical Ltd., Imperative Care, International Medical Distribution Partners, Neurovascular Diagnostics Inc., Q’Apel Medical Inc, Rebound Therapeutics Corp. (Purchased 2019 by Integra Lifesciences, Corp), Rist Neurovascular Inc., Sense Diagnostics, Inc., Serenity Medical Inc., Silk Road Medical, Spinnaker Medical, Inc., StimMed, Synchron, Three Rivers Medical Inc., Vastrax, LLC, VICIS, Inc., Viseon Inc; Consultant/advisory board: Amnis Therapeutics, Boston Scientific, Canon Medical Systems USA Inc., Cerebrotech Medical Systems Inc., Cerenovus, Corindus Inc., Endostream Medical Ltd., Imperative Care, Inc. Integra LifeSciences Corp., Medtronic, MicroVention, Minnetronix Neuro, Inc., Northwest University–DSMB Chair for HEAT Trial, Penumbra, Q’Apel Medical Inc., Rapid Medical, Rebound Therapeutics Corp.(Purchased by Integra LifeSciences Corp.), Serenity Medical Inc., Silk Road Medical, StimMed, Stryker, Three Rivers Medical, Inc., VasSol, W.L. Gore & Associates; Principal investigator/steering committee for the following trials: Cerenovus NAPA and ARISE II; Medtronic SWIFT PRIME and SWIFT DIRECT; MicroVention FRED & CONFIDENCE; MUSC POSITIVE; and Penumbra 3 D Separator, COMPASS, INVEST, TIGER.
EIL: Shareholder/Ownership interests: NeXtGen Biologics, RAPID Medical, Claret Medical, Cognition Medical, Imperative Care (formerly the Stroke Project), Rebound Therapeutics, StimMed, Three Rivers Medical; National Principal Investigator/Steering Committees: Medtronic (merged with Covidien Neurovascular) SWIFT Prime and SWIFT Direct Trials; Honoraria: Medtronic (training and lectures); Consultant: Claret Medical, GLG Consulting, Guidepoint Global, Imperative Care, Medtronic, Rebound, StimMed; Advisory Board: Stryker (AIS Clinical Advisory Board), NeXtGen Biologics, MEDX, Cognition Medical, Endostream Medical; Site Principal Investigator: CONFIDENCE study (MicroVention), STRATIS Study—Sub I (Medtronic).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Muhammad Waqas https://orcid.org/0000-0003-4500-7954
Jason M Davies https://orcid.org/0000-0002-5225-3072
Elad I Levy https://orcid.org/0000-0002-6208-3724
References
- 1.Möhlenbruch MA, Herweh C, Jestaedt L, et al. The FRED flow-diverter stent for intracranial aneurysms: clinical study to assess safety and efficacy. AJNR Am J Neuroradiol 2015; 36: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierot L, Spelle L, Berge J, et al. Feasibility, complications, morbidity, and mortality results at 6 months for aneurysm treatment with the flow re-direction endoluminal device: report of SAFE study. J NeuroIntervent Surg 2018; 10: 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierot L, Spelle L, Berge J, et al. SAFE study (safety and efficacy analysis of FRED embolic device in aneurysm treatment): 1-year clinical and anatomical results. J NeuroIntervent Surg 2019; 11: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, et al.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz O, Gist TL, Manjarez G, et al. Treatment of 14 intracranial aneurysms with the FRED system. J Neurointerv Surg 2014; 6: 614–617. [DOI] [PubMed] [Google Scholar]
- 6.Poncyljusz W, Sagan L, Safranow K, et al. Initial experience with implantation of novel dual layer flow-diverter device FRED. Wideochir Inne Tech Maloinwazyjne 2013; 8: 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kocer N, Islak C, Kizilkilic O, et al. Flow re-direction endoluminal device in treatment of cerebral aneurysms: initial experience with short-term follow-up results. J Neurosurg 2014; 120: 1158–1171. [DOI] [PubMed] [Google Scholar]
- 8.Briganti F, Leone G, Ugga L, et al. Safety and efficacy of flow re-direction endoluminal device (FRED) in the treatment of cerebral aneurysms: a single center experience. Acta Neurochir (Wien) 2016; 158: 1745–1755. [DOI] [PubMed] [Google Scholar]
- 9.Drescher F, Weber W, Berlis A, et al. Treatment of intra- and extracranial aneurysms using the flow-redirection endoluminal device: multicenter experience and follow-up results. AJNR Am J Neuroradiol 2017; 38: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luecking H, Engelhorn T, Lang S, et al. FRED flow diverter: a study on safety and efficacy in a consecutive group of 50 patients. AJNR Am J Neuroradiol 2017; 38: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahboobani NR, Chong WH, Lam SS, Siu JC, et al. Treatment of intracranial aneurysms with flow re-direction endoluminal device – a single centre experience with short-term follow-up results. Neurointervention 2017; 12: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Möhlenbruch MA, Kizilkilic O, Killer-Oberpfalzer M, et al. Multicenter experience with FRED Jr flow re-direction endoluminal device for intracranial aneurysms in small arteries. AJNR Am J Neuroradiol 2017; 38: 1959–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killer-Oberpfalzer M, Kocer N, Griessenauer CJ, et al. European multicenter study for the evaluation of a dual-layer flow-diverting stent for treatment of wide-neck intracranial aneurysms: the European flow-redirection intraluminal device study. AJNR Am J Neuroradiol 2018; 39: 841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzato LB, Santos RB, Teixeira DO, et al. Initial experience with a flow redirection endoluminal device stent – a Brazilian multicenter study. J Stroke Cerebrovasc Dis 2018; 27: e158–e64. [DOI] [PubMed] [Google Scholar]
- 15.Piano M, Valvassori L, Lozupone E, et al. FRED Italian registry: a multicenter experience with the flow re-direction endoluminal device for intracranial aneurysms. J Neurosurg 10; 133(1): 174–81. [DOI] [PubMed] [Google Scholar]
- 16.Pabon B, Diaz C, Fonseca M, et al. P-021 COMFORT- Colombian multicenter flow-diverter observational reconstruction trial. local experience in the endovascular treatment of intracranial aneurysms with FRED stent. J Neurointerv Surg 2019; 11: A36. [Google Scholar]
- 17.Rautio R, Rahi M, Katila A, et al. Single-center experience with six-month follow-up of FRED Jr® flow diverters for intracranial aneurysms in small arteries. Acta Radiol 2019; 60: 917–924. [DOI] [PubMed] [Google Scholar]
- 18.Laukka D, Rautio R, Rahi M, et al. Acute treatment of ruptured fusiform posterior circulation posterior cerebral, superior cerebellar, and posterior inferior cerebellar artery aneurysms with FRED flow diverter: report of 5 cases. Oper Neurosurg (Hagerstown) 2019; 16: 549–556. [DOI] [PubMed] [Google Scholar]
- 19.Sivasankar R, Shrivastava M, Limaye US. Experience with FRED junior flow diverter in treatment of cerebral aneurysms at or distal to the circle of willis. J Clin Neurosci 2019; 69: 166–169. [DOI] [PubMed] [Google Scholar]
- 20.Guimaraens L, Vivas E, Saldaña J, et al. Efficacy and safety of the dual-layer flow-diverting stent (FRED) for the treatment of intracranial aneurysms. J Neurointerv Surg 2020; 12: 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griessenauer CJ, Möhlenbruch MA, Hendrix P, et al. The FRED for cerebral aneurysms of the posterior circulation: a subgroup analysis of the EuFRED registry. AJNR Am J Neuroradiol 2020; 41: 658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lücking H, Doerfler A, Goelitz P, et al. Two- to five-year follow-up of 78 patients after treatment with the flow redirection endoluminal device. Interv Neuroradiol 2019; 26: 1591019919878551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan CL, Yang Z, Salahia G, et al. A single-Centre experience and literature review of flow Re-Directional endoluminal device (FRED) in endovascular treatment of intracranial aneurysms. Clin Radiol 2021; 76: 238. e1–e8. [DOI] [PubMed] [Google Scholar]
- 24.Dinc H, Saatci I, Oguz S, et al. Long-term clinical and angiographic follow-up results of the dual-layer flow diverter device (FRED) for the treatment of intracranial aneurysms in a multicenter study. Neuroradiology 2021; 63(6): 943–52. [DOI] [PubMed]
- 25.Briganti F, Leone G, Marseglia M, et al. Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J 2015; 28: 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]