Abstract
Studies have shown that LIM domain kinase 1 (LIMK1) is upregulated in a variety of tumors and may be a potential detection target. The present study analyzed the expression difference of LIMK1 and its relationship with tumor clinicopathological characteristics and tumor microenvironment in colorectal cancer (CRC). The transcriptomic data of LIMK1 with CRC were downloaded from The Cancer Genome Atlas (TCGA) database and GEO databases for analyzing the expression of LIMK1 mRNA and the correlation with the prognosis of patients. The protein expression of LIMK1 was obtained from the Human Protein Atlas. The receiver operating characteristic (ROC) curve and Kaplan-Meier was used to evaluate the expression characteristics and prognostic differences of LIMK1 in CRC. STRING was used to analyze co-expression genes of LIMK1. The tumor immune estimation resource was applied to the correlation between LIMK1 expression and immune infiltrates. The present study verified LIMK1 expression at the level of clinical samples collected from the Tianjin Medical University General Hospital and cell lines using reverse transcription-quantitative PCR. The mRNA and protein expression of LIMK1 were both upregulated in tumor tissues compared with adjacent tissues in CRC. The expression levels of LIMK1 were positively associated with clinical-pathological features of CRC including lymphatic invasion (P=4.00×10−2) and high pathologic stages (P=4.20×10−2). The AUC value of LIMK1 in CRC was 0.937 (95% CI: 0.918-0.957) through ROC analysis. Under the best cut-off value (4.009), the sensitivity and specificity were 98 and 81.9%. LIMK1 expression was mainly related to CD4+ T cells, macrophages and dendritic cells in the immune microenvironment of CRC. In conclusion, the high expression of LIMK1 in CRC was closely related to the clinical features and prognosis of patients. Therefore, LIMK1 was a promising prognostic indicator and a potential target for immunotherapy in CRC.
Keywords: LIM domain kinase 1, colorectal cancer, immune infiltration, prognosis, biomarker
Introduction
Colorectal cancer (CRC) is one of the commonest and lethal malignancies worldwide. More than 1.2 million new CRC cases were reported globally in 2020 according to cancer prevalence statistics (1). Due to the effect of COVID-19 in 2020, poor medical conditions will certainly lead to a higher fatality rate in the future. With the growth health awareness among individuals, the detection rate of CRC is increasing. However, the metastatic rate of colorectal cancer remains high, especially liver metastasis of colorectal cancer, which leads to a low 5-year survival rate of colorectal cancer (2). Currently, CRC screening trials mainly rely on colonoscopy and some blood tests such as carcinoembryonic antigen (CEA), carbohydrate antigen 19–9 (CA19-9) and fecal occult blood test (FOBT) (3). Although a number of new molecular targets continue to be discovered as diagnostic and predictive biomarkers for CRC, such as microRNAs and circular RNAs (4,5), there remains a number of challenges that hinder the clinical practice for real applications. Thus, it is an urgent need to identify new biomarkers that can accurately predict CRC, especially metastatic CRC, to improve the prognosis and curative effect of adenocarcinoma.
LIM domain kinase 1 (LIMK1) is a kinase of the LIMK family and consists of two related proteins, LIMK1 and LIMK2 (6,7). LIMK1 is mainly expressed in the cytoplasm and small amounts in the nucleus (8). LIMK1 promotes actin polymerization and phosphorylates its downstream target cofilin, which further influences cell growth and functions, including cell proliferation, angiogenesis and cell cycle progression (9,10). In the initiation and progression of tumors, studies have verified that the abnormal expression of LIMK1 is closely related to the change of biological behavior of several human tumors, especially prostate cancer, breast cancer and gastric cancer (11–14). A study by Zhang et al (11) indicate that lung carcinoma cell proliferation and tumor metastasis are suppressed by inhibiting LIMK1 activity in vivo and in vitro. In a subsequent bioinformatics analysis, it is reported that the expression of LIMK1 is significantly correlated with tumor-infiltrating immune cells and poor prognosis of lung cancer (15). In CRC, a few studies have demonstrated that upregulation of LIMK1 through direct or indirect pathways can promote colorectal cancer cell proliferation, invasion and migration in vitro (16,17). Therefore, LIMK1 may be a suitable biomarker for the diagnosis and treatment of CRC.
Although the fact that regulation of LIMK1 expression could change malignant biological behaviors such as proliferation and migration of CRC (16), the association between LIMK1 and the immune microenvironment has not been reported in CRC. The present study hypothesized that LIMK1 might influence immune cells or other immune markers in CRC. To test this, the differential and prognostic value of LIMK1 was first explored in CRC based on data from The Cancer Genome Atlas (TCGA) database, GEPIA and TIMER 2.0 database. It was found that LIMK1 was indeed upregulated in CRC and this conclusion was verified using the Gene Expression Omnibus database (GEO) and clinical samples from Tianjin Medical University General Hospital. Moreover, the expression of LIMK1 was associated with multiple clinicopathological features of CRC patients. The association between LIMK1 and immune-related indicators in CRC was further evaluated. In brief, the association between the high expression of LIMK1 and CRC was analyzed from different perspectives.
Materials and methods
TCGA and GEO
The raw gene transcriptome data of LIMK1 and the clinical data of participants were downloaded from the TCGA (https://portal.gdc.cancer.gov/) and GEO websites (https://www.ncbi.nlm.nih.gov/geo/). The groups without a normal control group were excluded and the rest were included in the statistical analysis. For follow-up studies, the downloaded gene expression data was converted into TPM format and ID conversion performed. The processed data were then analyzed using ‘limma’ (version 3.50.3) and ‘ggplot2’ (version 3.3.5) (18,19) in R software (RStudio, Inc.; version 4.1.2; 64-bit; http://www.r-project.org/).
Survival analysis
The mRNA expression data of LIMK1 and survival data of CRC patients were downloaded from the TCGA websites. The median value of LIMK1 expression was set as the cut-off value used to separate patients into high and low expression groups. The overall survival (OS) rates, disease-specific survival (DSS) rates and progression-free interval (PFI) analyses were performed by drawing Kaplan-Meier curves to compare the survival differences. The data were analyzed and visualized using ‘survival’ (https://CRAN.R-project.org/package=survival; version 3.3-1), ‘survminer’ (https://CRAN.R-project.org/package=survminer; version 0.4.9) and ‘ggplot2’ in R software.
Tumor immune estimation resource 2.0 (TIMER 2.0) database
TIMER 2.0 (http://timer.cistrome.org/) is an online resource that provides comprehensive analysis and visualization functions of tumor-infiltrating immune cells (20). The efficacy of tumors and immunotherapy is largely influenced by the composition and abundance of immune cells in the tumor microenvironment (20). TIMER 2.0 allows users to select any gene of interest and visualize the correlation of its expression with immune infiltration levels in diverse cancer types. The present study analyzed the correction of LIMK1 expression and various immune cells in CRC. The correlation analysis was analyzed using Spearman's method.
The human protein atlas (HPA)
The expression of proteins in cells, normal tissues and cancerous tissues is shown in HPA (https://www.proteinatlas.org/; version 21.0) (21). The present study compared the protein expression of LIMK1 between CRC tumorous tissue and normal adjacent tissue by HPA.
Protein-protein interaction (PPI) networks and functional enrichment analysis
STRING (version 11.5) is available online and is user-friendly (22). It was used to search for co-expressed genes of LIMK1 by STRING and PPI networks were constructed using the top 10 genes by interaction scores. The correlations between LIMK1 expression and the top 10 genes were analyzed in CRC tumor samples. The correlation coefficient was analyzed using Spearman's method. Gene Ontology (GO) term enrichment (23) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (24) pathway enrichment of the genes obtained by screening were analyzed by the ‘ClusterProfiler’ package (version 4.2.2) (25) and ‘ggplot2’ package in R software.
Correlation analysis of immune cell markers in GEPIA
The online database Gene Expression Profiling Interactive Analysis (GEPIA) (http://gepia.cancer-pku.cn/index.html; version 1.0) has multiple functions, such as genetic difference analysis, survival prognosis and correlation analysis in multiple cancer types. A few gene markers that are currently widely recognized in immune cells were found. The correlations between LIMK1 and immune cell markers were analyzed by comparing their expression in the tumor tissues. The correlation coefficient was analyzed using Spearman's method.
Cell lines culture
The CRC cell lines SW480, LOVO, HCT116, DLD-1, SW620 and CRC normal epithelial cell line NCM460 were obtained from the Laboratory of General Surgery, Tianjin Medical University General Hospital (Tianjin, China). Mycoplasma testing (Beijing Solarbio Science & Technology Co., Ltd.) was performed for all cell lines used. These cell lines were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Hyclone; Cytiva) and 1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and incubated at 37°C with 5% CO2. Cell lines were tested for mycoplasma (PCR Mycoplasma; Venor GeM Mycoplasma Detection kit; MilliporeSigma and negative results were obtained.
RNA extraction and reverse transcription-quantitative (RT-q)PCR
The present study was approved by the Ethics Committee of the Tianjin Medical University General Hospital Ethical Committee (approval no. 2021-WZ-203). Between February 2020 and December 2020, 46 tumor tissue samples and normal adjacent tissue samples were collected from patients with CRC. All participants (Fig. 4F) signed an informed consent form. Tissue samples were stored in a refrigerator at −80°C. Total RNA from CRC cell lines and tissue samples was extracted with TRIzol® reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The concentration of RNA was measured using a NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific, Inc.). The 260/280 ratios of RNA from 1.7-2.0 were reversely transcribed using the FastQuant RT Supermix kit (Tiangen Biotech, Co., Ltd.). Subsequently, RT-qPCR was performed using SYBR Green qPCR Master Mix (Bimake Biotechnology). The thermocycling conditions were as follows: 95°C for 30 sec, followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec, finally denaturation at 95°C for 15 sec, 60°C for 60 sec and 95°C for 15 sec. mRNA expression was quantified using the 2−ΔΔCq method (26). The experiments were performed as three replicates. GAPDH was used to normalize LIMK1 expression. The primer sequences were as follows: LIMK1 forward, 5′-TTGCCAAGGACATCGCATCAGG-3′ and reverse, 5′-CGAAGTCAGCCACCACCACATT-3′; GAPDH forward, 5′-TGGCACCGTCAAGGCTGAGAA-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGACTC-3′.
Figure 4.
Verification of LIMK1 expression in GEO databases and tumor samples. (A-D) LIMK1 expression was verified higher than normal tissues in GSE 10715, GSE 18105, GSE 22598 and GSE 32323. (E) The expression in CRC cell lines (SW480, LOVO, HCT116, DLD-1 and SW620) and normal epithelial cell line (NCM460). (F) The LIMK1 expression level was upregulated in CRC tissues (n=46). ns, no significance; *P<0.05, ***P<0.001. LIMK1, LIM domain kinase 1; GEO, Gene Expression Omnibus; CRC, colorectal cancer.
Statistical analyses
All statistical analyses were performed and visualized using R software (4.1.0) and the R packages mentioned above. Comparisons between tumor tissues and normal tissues were performed using the paired t-test or Mann-Whitney U-test depending on data distribution. Comparisons between multiple groups were made using one-way analysis of variance (ANOVA). Bonferroni post-hoc tests were performed when appropriate. ROC curves were plotted and the ROC curve calculated using the R package ‘pROC’ (version 1.18.0) (27).
Results
mRNA expression differences and prognosis analysis of LIMK1 in pan-cancer
As mentioned in previous study (28), LIMK1 was differentially expressed in a variety of types of cancer. To determine the intracellular localization of LIMK1, the distribution of LIMK1 was studied in three tumor cell lines according to the HPA database. LIMK1 was expressed in varying degrees in the nucleus and cytoplasm (Fig. 1A and B). LIMK1 also had different levels of RNA expression in cell lines of different tissues (Fig. 1C). The expression of LIMK1 was analyzed in 33 types of cancer and the corresponding para cancer types. As shown in Fig. 1D, the expression of LIMK1 was significantly upregulated in 12 types of tumors, including colon cancer, rectal cancer, lung cancer and stomach cancer. However, LIMK1 was downregulated in brain lower grade glioma (LGG; P<0.01). Therefore, it was confirmed that LIMK1 was differentially expressed in different types of cancer. Subsequently, to verify whether the differential expression of LIMK1 was related to the patient's survival prognosis, the prognostic indicators of patients with differential expression of LIMK1 were analyzed. As shown in Fig. 2, the results showed that the overall survival of colon adenocarcinoma (COAD; HR=1.55, P=2.60×10−2), kidney renal papillary cell carcinoma (KIRP; HR=2.57, P=2.00×10−3), LGG (HR=2.39, P=1.00×10−3), lung adenocarcinoma (LUAD; HR=1.38, P=2.90×10−2) and rectum adenocarcinoma (READ; HR=1.22, P=2.20×10−2) were significantly different.
Figure 1.
LIMK1 location and expression at a different level. (A) The subcellular distribution of LIMK1, nucleus and microtubules of A-431, U-2 OS and U-251 MG cells as obtained from the HPA database (magnification, ×200). (B) LIMK1 expression pattern diagram. (C) The expression of LIMK1 in cell lines. (D) mRNA expression differences of LIMK1 in pan-cancer. Green dots represent normal adjacent tissue and red dots represented tumor tissue. The tumor abbreviation in red meant that LIMK1 was upregulated in tumor tissue. The tumor abbreviation in green meant the opposite. HPA, human protein atlas; LIMK1, LIM domain kinase 1; ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; COADREAD, colon adenocarcinoma/rectum adenocarcinoma esophageal carcinoma; DLBC, lymphoid neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIPAN, pan-kidney cohort (KICH + KIRC + KIRP); KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute myeloid leukemia; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; STES, stomach and esophageal carcinoma; TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma.
Figure 2.
Association between LIMK1 expression level and overall survival across various tumor types. The OS of COAD, KIRP, LGG, LUAD and READ were associated with LIMK1 expression. LIMK1, LIM domain kinase 1; OS, overall survival; COAD, colon adenocarcinoma; KIRP, kidney renal papillary cell carcinoma; LGG, brain lower grade glioma; LUAD, lung adenocarcinoma; READ, rectum adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma; HNSC, head and neck squamous cell carcinoma; KIRP, kidney renal papillary cell carcinoma; PAAD, pancreatic adenocarcinoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; THYM, thymoma; UCS, uterine carcinosarcoma.
Expression of LIMK1 mRNA and protein in CRC
To further examine the differential expression of LIMK1, the expression of LIMK1 mRNA and protein in CRC tissues was analyzed using data from the TCGA database and HPA. Paired and unpaired samples from the TCGA database were analyzed. As shown in Fig. 3A and B, analysis of paired data showed that the expression level of LIMK1 mRNA in CRC tissues (50) was significantly higher compared with that in normal tissues (50). The mean level of the normal group was 3.43±0.353 and that of the tumor group was 4.662±0.535 (paired t-test, P=1.12×10−3). Analysis of unpaired data also showed that the expression level of LIMK1 mRNA in CRC tissues (647) was significantly higher compared with that in normal tissues (51). The average level of the normal group was 3.434±0.351 and that of the tumor group was 4.501±0.620 (Mann-Whitney U test, P=1.08×10−3). As shown in Fig. 3C and D, LIMK1 protein expression was also upregulated in CRC tissues based on the immunohistochemical staining results from TPA.
Figure 3.
Expression of LIMK1 mRNA and protein in CRC. (A) In unpaired samples, mRNA expression levels of LIMK1 were significantly increased in tumor tissues compared with normal tissues (P=1.08×10−3). (B) In paired samples, mRNA expression levels of LIMK1 were significantly increased in tumor tissues compared with normal tissues (P=1.12×10−3). (C and D) The protein expression level of LIMK1 in (C) normal and (D) tumor tissues based on HPA (magnification of the lower left image, ×40; magnification of main image, ×400; scale bar, 100 µm). ***P<0.001. LIMK1, LIM domain kinase 1; CRC, colorectal cancer; HPA, human protein atlas.
Validation of LIMK1 mRNA differentially expressed level in CRC
To further prove the difference in LIMK1 expression in the TCGA database, the expression level of LIMK1 was analyzed in four GEO datasets (GSE 10715, GSE 18105, GSE 22598 and GSE 32323) and LIMK1 expression levels detected in CRC samples and peritumoral non-cancerous tissues (a distance of 5 cm to the tumour tissue) obtained from Tianjin Medical University General Hospital by RT-qPCR. As shown in Fig. 4A-D, LIMK1 was significantly upregulated in CRC tissues compared with that in normal tissues. As shown in Fig. 4E and F, in cell-level verification, the expression levels of LIMK1 were significantly increased in several CRC cell lines compared with the normal colonic epithelium cell line. The relative mRNA expression of LIMK1 was upregulated in CRC tissues compared with that in normal tissues (P=3.20×10−4). The above results confirmed that LIMK1 had obvious differences in expression in CRC.
Associations between the expression of LIMK1 mRNA and clinicopathological features of CRC patients
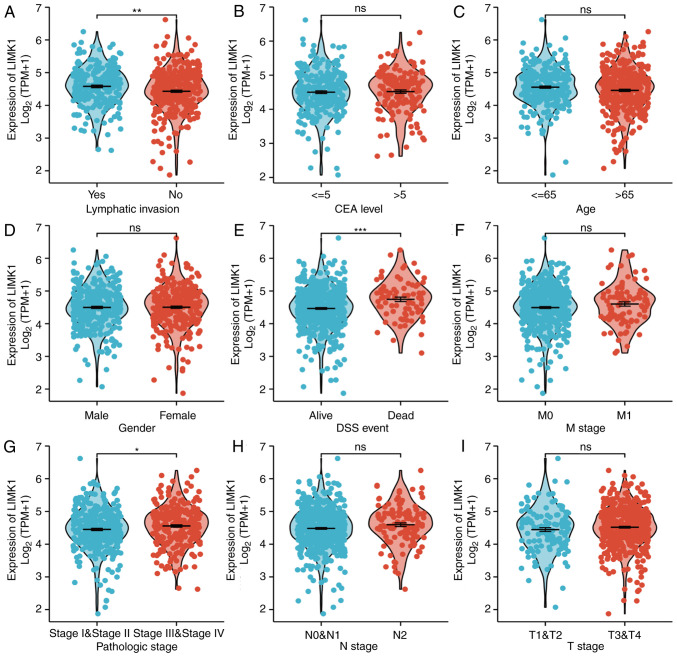
To verify the associations between LIMK1 expression and clinical indicators in patients, we explored the associations between LIMK1 mRNA levels and the clinicopathological features of CRC patients. Baseline characteristics were listed in Table I. As shown in Fig. 5, the expression levels of LIMK1 were positively associated with some clinical-pathological features of CRC, including DSS events (P<1.00×10−3) and lymphatic invasion (P=2.00×10−3). Although it did not make sense to analyze pathologic stage I–IV alone, patients who belonged to stage III–IV more highly expressed LIMK1 than those in stage I–II (P=4.20×10−2). This may be due to the small sample size in each subgroup. It was obvious that a higher tumor stage was associated with a higher expression level of LIMK1. There were no statistical differences in the rest of the clinicopathological characteristics. Overall, the above results suggested that LIMK1 could indicate the prognosis of CRC patients in some respects.
Table I.
Correlation between LIMK1 expression and pathological parameters of patients with colorectal cancer.
| Characteristic | Low expression of LIMK1 (n=322) | High expression of LIMK1 (n=322) | P-value |
|---|---|---|---|
| Sex | 1.000 | ||
| Female | 148 (23.0) | 153 (23.8) | |
| Male | 174 (27.0) | 169 (26.2) | |
| Age, years | 1.000 | ||
| ≤65 | 138 (21.4) | 138 (21.4) | |
| >65 | 184 (28.6) | 184 (28.6) | |
| T stage | 0.341 | ||
| T1 | 9 (1.4) | 11 (1.7) | |
| T2 | 63 (9.8) | 48 (7.5) | |
| T3 | 208 (32.4) | 228 (35.6) | |
| T4 | 39 (6.1) | 35 (5.5) | |
| N stage | 0.076 | ||
| N0 | 195 (30.5) | 173 (27.0) | |
| N1 | 74 (11.6) | 79 (12.3) | |
| N2 | 49 (7.7) | 70 (10.9) | |
| M stage | 0.408 | ||
| M0 | 234 (41.5) | 241 (42.7) | |
| M1 | 39 (6.9) | 50 (8.9) | |
| Lymphatic invasion | 0.004 | ||
| No | 196 (33.7) | 154 (26.5) | |
| Yes | 101 (17.4) | 131 (22.5) | |
| Pathologic stage | 0.306 | ||
| I | 59 (9.5) | 52 (8.3) | |
| II | 127 (20.4) | 111 (17.8) | |
| III | 84 (13.5) | 100 (16.1) | |
| IV | 41 (6.6) | 49 (7.9) | |
| CEA, ng/ml | 0.069 | ||
| ≤5 | 139 (33.5) | 122 (29.4) | |
| >5 | 67 (16.1) | 87 (21.0) | |
| DSS status | 0.002 | ||
| Alive | 289 (46.5) | 255 (41.0) | |
| Succumbed | 26 (4.2) | 52 (8.4) |
Values are expressed as n (%). CEA, carcinoembryonic antigen; DSS, disease-specific survival; LIMK1, LIM domain kinase 1.
Figure 5.
Relationship between LIMK1 mRNA and clinicopathological features of CRC including (A) lymphatic invasion, (B) CEA level, (C) age, (D) gender, (E) DSS event, (F) M stage, (G) pathologic stage, (H) N stage, and (I) T stage. Increased LIMK1 expression was highly associated with lymphatic invasion (A), DSS (E), and pathologic stage (G). No statistical differences were found in other features. ns, no significance; *P<0.05, **P<0.01, ***P<0.001. LIMK1, LIM domain kinase 1; CRC, colorectal cancer; DSS, disease-specific survival rate; CEA, carcinoembryonic antigen.
The association between LIMK1 and prognosis in patients with CRC
To investigate the association between LIMK1 expression and survival in patients with CRC, Kaplan-Meier curves were performed. As shown in Fig. 6A-C, the OS rates were significantly higher among CRC patients with low LIMK1 expression compared with those with high expression (HR=2.01; 95% CI: 1.24-3.23; P=5.00×10−3). Similarly, patients with high expression levels of LIMK1 had lower DSS rate (HR=2.48; 95% CI: 1.37-4.49; P=4.00×10−3). The same was true for PFI (HR=1.65; 95% CI: 1.08-2.54; P=2.10×10−2). Subsequently, a ROC curve analysis was performed to determine whether LIMK could distinguish between CRC samples and normal samples. As shown in Fig. 6D, the AUC of LIMK1 was 0.937 (95% CI: 0.918-0.957), according to the ROC curve. When each predictive variable was at its optimum cut-off value (cut-off=4.009), the sensitivity and specificity were 98 and 81.9%, respectively. These results suggested that LIMK1 might be a promising biomarker for CRC diagnosis.
Figure 6.
Kaplan-Meier curves and ROC curves of LIMK1 in CRC. (A-C) Kaplan-Meier curves indicated that patients with CRC with lower LIMK1 expression levels had longer (A) overall survival (P=5.00×10−3), (B) disease-specific survival (P=4.00×10−3) and (C) progression-free interval (P=2.10×10−2) than patients with higher expression. (D) The AUC value of LIMK1 in CRC was 0.937 (95% CI: 0.918-0.957). Under the best cut-off value (4.009), the sensitivity and specificity were 98 and 81.9%, respectively. ROC, receiver operating characteristic; AUC, area under the ROC curve; LIMK1, LIM domain kinase 1; CRC, colorectal cancer; OS, rate; TPR, true positive rate; FPR, false positive rate; HR, hazard ratio.
The PPI networks and functional annotations of LIMK1
The present study then attempted to determine the PPI networks and functional annotations of LIMK1 using GO, KEGG and STRING databases. The top 10 co-expressed genes of LIMK1 were analyzed and a network constructed according to the distance of the relationship (Fig. 7A). Subsequently, correlation analysis was performed to explore the correlations between LIMK1 expression and 10 co-expressed genes in CRC (Fig. 7B-K). Among them. CFL1, RHOB and RHOC had the most significant correlations with LIMK1. The correlations between LIMK1 and remaining genes had non-significant P-values or small r values in CRC. As shown in Fig. 7L, the cellular component (CC) of LIMK1 and its co-expressed genes in CRC mainly focused on lamellipodium, ruffle and cell leading edge. Molecular function (MF) was correlated with rho GTPase binding, ras GTPase binding and protein serine/threonine kinase activity. The biological process (BP) was correlated with the regulation of the actin filament-based process, regulation of actin cytoskeleton organization and actin filament organization. KEGG analyses indicated that these interrelated genes mainly concentrated on axon guidance, regulation of actin cytoskeleton and pathogenic E. coli infection pathways.
Figure 7.
PPI networks and functional annotations of LIMK1. (A) Network of LIMK1 and its co-expressed genes. (B-K) Correlations between LIMK1 and (B) PAK1, (C) PAK2, (D) ROCK1, (E) ROCK2, (F) CFL1, (G) CFL2, (H) RHOA, (I) RHOB, (J) RHOC and (K) RAC1 in CRC. Among them, CFL1 (r=0.428), RHOB (r=0.319), RHOC (r=0.534) had the most significant correlation with LIMK1. Other genes had non-significant P-values or small r values. (L) Functional enrichment analyses of LIMK1 and its co-expressed genes. The first three indicators, including pathogenic escherichia coli infection, regulation of actin cytoskeleton, and axon guidance, were obtained through KEGG analyses. Rho GTPase binding, ras GTPase binding and protein serine/threonine kinase were enriched terms in the GO category molecular function. Lamellipodium, ruffle and cell leading edge represented were significant terms in the GO category cellular component. Actin filament-based process, regulation of actin cytoskeleton organization and actin filament organization were terms accumulated in the GO category biological process. PPI, protein-protein interaction; LIMK1, LIM domain kinase 1; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; CRC, colorectal cancer; BP, biological process; CC, cellular component; MF, molecular function.
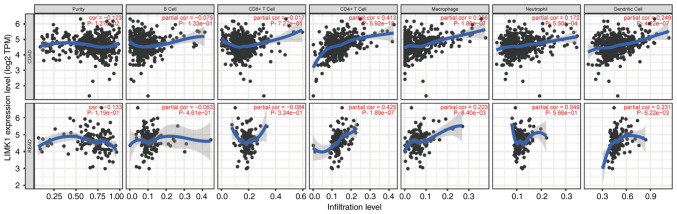
The correlations between LIMK1 expression and immune cell infiltration in CRC
To analyze the correlations between LIMK1 expression and immune cell infiltration in CRC, the correlations between LIMK1 and six immune cells was calculated using the TIMER database. As the TIMER database did not have CRC data, the correlation between LIMK1 and the six immune cells in COAD and READ, respectively, were calculated. The results revealed that the correlations between LIMK1 expression and immune cell infiltration in COAD were almost equivalent to READ. LIMK1 expression was mainly related to CD4+ T cells, macrophages and dendritic cells (Fig. 8). Furthermore, the correlations between LIMK1 expression and myeloid-derived suppressor cells (MDSCs) were also explored. The correlations between MDSCs and LIMK1 expression were relatively low in CRC (Fig. S1). The correlation coefficient was 0.104 (P=2.58×10−2) in COAD and 0.167 (P=3.1×10−2) in READ.
Figure 8.
The correlation of LIMK1 with immune cell infiltration in CRC. LIMK1 was correlated with the expression of multiple immune cells in the tumor microenvironment, among which CD4+ T cells macrophages and dendritic cells have the highest correlation. LIMK1, LIM domain kinase 1; CRC, colorectal cancer.
Correlation analysis between LIMK1 expression and immune cell markers
To explore the correlations between LIMK1 and multiple immune infiltrating cells, the changes in different immune cell subgroups were further analyzed. The correlations between LIMK1 and the immune markers of diverse cells were analyzed in the GEPIA database. It was observed that LIMK1 was indeed related to these immune cells (Table II). In addition, LIMK1 seemed to be more related to M2 macrophages according to the correlation results. Diverse T cell subsets in COAD and READ, such as Th1, Th2, Tfh, Th17, Tregs and T cell exhaustion were also analyzed. In the analysis of T cell subpopulations, the correlation of Tregs and T cell exhaustion marker genes appeared to be higher than others.
Table II.
Correlation analysis of LIMK1 and immune cell gene markers in GEPIA.
| COAD | READ | |||
|---|---|---|---|---|
|
|
|
|||
| Cell type or feature/gene markers | r | P-value | r | P-value |
| Th1 | ||||
| T-bet (TBX21) | 0.31 | 1.90×10−7 | 0.45 | 5.70×10−6 |
| STAT4 | 0.3 | 2.70×10−7 | 0.37 | 3.20×10−4 |
| STAT1 | 0.35 | 2.10×10−9 | 0.6 | 2.50×10−10 |
| IFN-γ (IFNG) | 0.21 | 4.40×10−4 | 0.45 | 5.50×10−6 |
| TNF-α (TNF) | 0.25 | 2.50×10−5 | 0.44 | 9.00×10−6 |
| Th2 | ||||
| STAT6 | 0.2 | 7.00×10−4 | 0.26 | 1.20×10−2 |
| STAT5A | 0.4 | 9.50×10−12 | 0.28 | 6.50×10−3 |
| IL13 | 0.22 | 3.10×10−4 | 0.093 | 3.80×10−1 |
| Tfh | ||||
| BCL6 | 0.42 | 6.60×10−13 | 0.44 | 1.00×10−5 |
| IL21 | 0.18 | 3.00×10−3 | 0.15 | 1.60×10−1 |
| Th17 | ||||
| STAT3 | 0.32 | 4.70×10−8 | 0.3 | 3.30×10−3 |
| IL17A | −0.09 | 1.30×10−1 | 0.004 | 9.70×10−1 |
| Treg | ||||
| FOXP3 | 0.44 | 1.50×10−14 | 0.46 | 4.10×10−6 |
| CCR8 | 0.43 | 7.20×10−14 | 0.41 | 5.80×10−5 |
| STAT5B | 0.36 | 4.60×10−10 | 0.36 | 9.30×10−4 |
| TGFβ | 0.42 | 3.80×10−13 | 0.52 | 1.40×10−7 |
| T-cell exhaustion | ||||
| PD-1 (PDCD1) | 0.28 | 3.10×10−6 | 0.41 | 5.90×10−5 |
| CTLA4 | 0.34 | 8.30×10−9 | 0.18 | 9.00×10−2 |
| LAG3 | 0.13 | 3.50×10−2 | 0.35 | 5.50×10−4 |
| TIM-3 | 0.44 | 1.90×10−14 | 0.55 | 1.90×10−8 |
| GZMB | 0.02 | 6.90×10−1 | 0.14 | 1.70×10−1 |
| M1 | ||||
| INOS (NOS2) | 0.13 | 3.50×10−2 | 0.35 | 5.50×10−3 |
| IRF5 | 0.35 | 2.10×10−9 | 0.42 | 2.90×10−5 |
| COX2 | 0.23 | 1.30×10−4 | 0.27 | 9.10×10−3 |
| M2 | ||||
| CD163 | 0.34 | 5.80×10−9 | 0.48 | 1.10×10−6 |
| VSIG4 | 0.41 | 1.20×10−12 | 0.37 | 2.70×10−4 |
| MS4A4A | 0.4 | 5.00×10−12 | 0.46 | 4.10×10−6 |
| Dendritic cells | ||||
| HLA-DPB1 | 0.36 | 4.50×10−10 | 0.35 | 6.10×10−4 |
| HLA-DQB1 | 0.2 | 7.60×10−4 | 0.067 | 5.30×10−1 |
| HLA-DRA | 0.31 | 1.80×10−7 | 0.38 | 2.20×10−4 |
| HLA-DPA1 | 0.33 | 2.90×10−8 | 0.31 | 2.30×10−3 |
| BDCA-1 | 0.33 | 1.30×10−8 | 0.058 | 5.80×10−1 |
| BDCA-4 | 0.46 | 8.90×10−16 | 0.48 | 1.10×10−6 |
| CD11c | 0.42 | 3.60×10−13 | 0.38 | 1.90×10−4 |
GEPIA, gene expression profiling interactive analysis; COAD, colon adenocarcinoma; READ, rectum adenocarcinoma; Th, helper T cell; Tfh, follicular helper T cell; Treg, regulatory T cell; M1, macrophage 1; M2, macrophage 2; STAT, signal transducer and activator of transcription; BCL, B cell lymphoma 6; FOXP3, forkhead box P3; PD-1, programmed cell death protein 1; CTLA4, cytotoxic T lymphocyte-associated antigen-4; LAG3, lymphocyte activation gene-3; TIM-3, T cell immunoglobulin-3; GZMB, granzyme B; INOS, inducible nitric oxide synthase; IRF5, interferon regulatory factor 5; COX2, cyclooxygenase 2; VSIG4, v-set and immunoglobulin domain-containing 4; MS4A4A, membrane spanning 4-domains A4A; HLA, human leukocyte antigen; BDCA, blood dendritic cells antigen.
LIMK1 expression was correlated with immune checkpoint genes in CRC
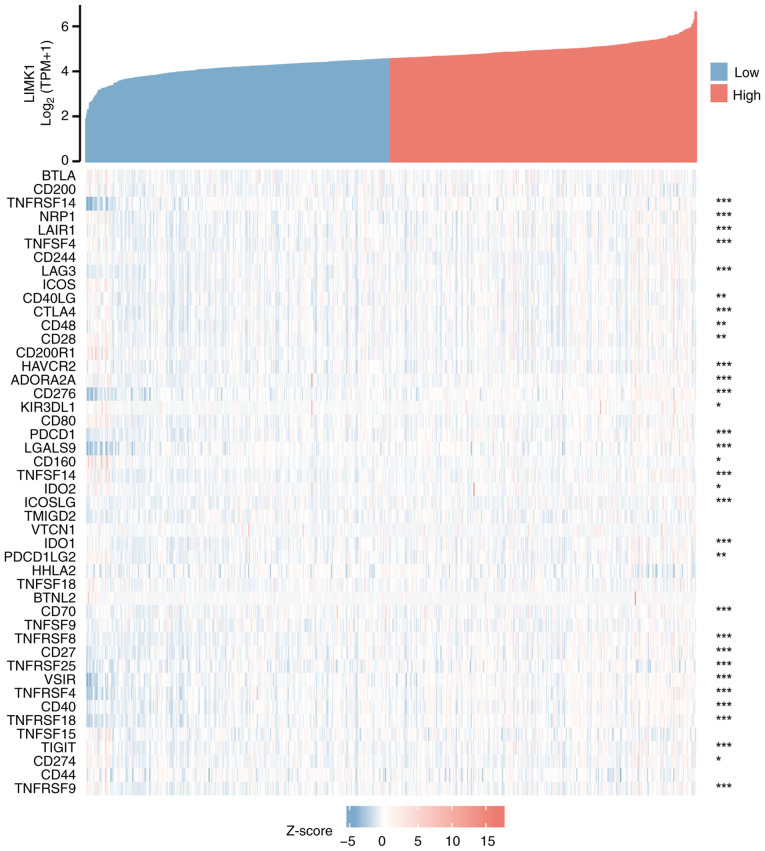
As shown in Fig. 9, the correlations between LIMK1 expression and ~50 immune checkpoint genes in CRC were explored. The results showed that LIMK1 expression was positively correlated with the expression levels of various immune checkpoint genes in CRC. Among them, the most relevant genes were CD276 (r=0.558), TNFRSF4 (r=0.440) and VSIR (r=0.436), suggesting that LIMK1 might serve a significant role in modulating tumor immunity by regulating these immune checkpoint genes.
Figure 9.
Correlation of LIMK1 with 50 immune checkpoint genes in CRC. blank, no significance; *P<0.05, **P<0.01, ***P<0.001. LIMK1, LIM domain kinase 1.
Discussion
CRC has a high morbidity and mortality rate, which urgently requires a robust molecular marker to achieve early diagnosis and treatment. The LIMK protein family includes LIMK1 and LIMK2. It was reported that LIMK1 was highly expressed in a variety of tumors and is related to patient prognosis. Studies have indicated that LIMK1 was upregulated in CRC and causes a poor prognosis (29–31). The related mechanism of LIMK1 regulating CRC progression has been studied (30). However, the association between LIMK1 and tumor immune microenvironment in CRC has not been explored. The present study discussed the association between LIMK1 and CRC from several aspects such as expression levels in tumor samples, clinicopathological features, the correlations with immune cell infiltration and the expression of immune checkpoint genes. First, it confirmed that the expression levels of LIMK1 mRNA and protein were higher in CRC tissues than in normal tissues. Higher LIMK1 expression was correlated with a poor prognosis of CRC including OS, DSS and PFI. In addition, LIMK1 could influence immune cell infiltration and immune checkpoint expression in CRC. In summary, LIMK1 may be a valuable and promising biomarker for the diagnosis of CRC. The findings of the present study laid the foundation that LIMK1 promotes CRC progression from the mechanism of regulating tumor immune microenvironment.
LIMK1, a serine protein kinase, serves a crucial role in the reorganization of actin and microtubule depolymerization (7). Research on LIMK1 has also focused on oncology because of its vital role in promoting tumor cell proliferation, invasion and metastasis (32). It has been reported that LIMK1 expression is upregulated in several types of human cancers, especially in highly malignant neoplasm, such as lung adenocarcinoma, breast cancer and prostate cancer (12,32,33). Furthermore, upregulated LIMK1 is associated with poor patient prognosis. A number of studies have shown that LIMK1 is a significant biomarker that portended a poor prognosis in numerous types of cancer. A study by Huang et al (12) indicates that upregulation of LIMK1 is highly associated with lymph node metastasis and shortened biochemical-free survival in prostate cancer. In ovarian carcinoma, it is reported that high levels of LIMK1 indicate poor tumor differentiation and disease severity (34). In gastric cancer, You et al (35) confirm that with the upregulation of LIMK1, the size of the primary tumor is larger and the number of lymph node metastases greater. It has been confirmed that reducing the expression of LIMK1 can delay tumor growth and peritoneal metastasis in vivo. In CRC research, upregulation of LIMK1 enhances the invasiveness of CRC cells in vitro and in vivo (30). The present study demonstrated that LIMK1 mRNA was highly expressed in a variety of tumor tissues, including CRC. Notably, LIMK1 was downregulated in tumor tissues of LGG by pan-cancer analysis. However, LIMK1 was significantly associated with poor survival in LGG. Few existing studies report the association between LIMK1 and LGG. The expression levels and functions of LIMK1 in LGG needs to be confirmed by further study. LIMK1 was highly expressed in tumor tissues with CRC, whether in paired or unpaired samples. Subsequently, the present study indicated that the protein expression of LIMK1 was upregulated in CRC tumor tissues compared with that in adjacent tissues. It also confirmed that the upregulation of LIMK1 was associated with poor survival. Regarding clinicopathological characteristics, significant positive associations were found between LIMK1 and lymphatic invasion and high TNM stage. Thus, LIMK1 might be more advantageous in the detection of metastatic CRC and prognostic assessment compared with previous screening methods such as CEA, FOBT and CA199, which are more suitable for the diagnosis of early-stage CRC. To prove the accuracy and sensitivity of LIMK1 in the diagnosis of CRC, a ROC curve analysis was performed. The results indicated that the AUC value of LIMK1 was obviously high in the detection of CRC, with 98% sensitivity and 81.9% specificity. Although further studies are needed, LIMK1 may serve as a promising marker for identifying CRC with a poor prognosis.
LIMK1 serves an important role in several signaling pathways, especially those related to tumors (36,37). The present study analyzed the top 10 co-expressed genes that were most related to the expression of LIMK1, of which CFL1, RHOB and RHOC had the highest correlations. In addition, it was found that LIMK1 was involved in a variety of biological processes in the following functional annotations. Zeng et al (38) found that knockdown of Rho GDP dissociation inhibitor 2 could downregulate the malignant biological behavior of gastric cancer cells via the Rac1/Pak1/LIMK1 pathway. In pancreatic cancer, other researchers have indicated that DEP domain-containing protein 1 B could also stimulate cell migration and invasion through this pathway (39). In functional annotations of LIMK1, the present study found that LIMK1 was mainly focused on lamellipodium, ruffle and cell leading edge in CC. The accumulation of LIMK1 in these cellular components probably indicated that it was related to the migration and metastasis of tumor cells. Vainer et al (40) found that VICKZ accumulated at the leading edge of SW480 CRC cells which facilitated the formation of surface morphologies required for cell migration. Rho GTPase-activating protein 5 promotes EMT to accelerate tumor metastasis by regulating RhoA activity in CRC cells (41), which corroborated the results of the present study of LIMK1 enrichment in MF. Axon guidance (42), regulation of actin cytoskeleton (43) and pathogenic E. coli infection (44) pathways are involved in the process of the occurrence of metastasis in CRC. Therefore, combined with the results of the present study, it was hypohesized that the upregulated LIMK1 might directly or indirectly affect the biological functions of tumors by regulating these proteins and pathways.
Another novelty of the present study was that LIMK1 expression was associated with immune cell infiltration in CRC. Several studies have shown that LIMK1 may be involved in the regulation of the immune microenvironment. In T cell immunity, HIV triggers actin polymerization through the LIMK1-cofilin signaling pathway (45). In NK cells, Duvall et al (46) identify LIMK1 as a vital medium to regulate cytoskeletal rearrangement. However, the role of LIMK1 in the immune tumor microenvironment remains to be elucidated. Only some researchers have found that the expression of LIMK1 was enriched in immune (CMS1) subtypes of CRC (29). The present study found that LIMK1 was associated with multiple tumor-infiltrating immune cells in CRC. Among them, LIMK1 was most closely related to CD4+ T cells, macrophages and dendritic cells. In further subgroup analysis, it was found that LIMK1 had stronger correlations with M2 macrophages and Treg cells, according to the analysis of cell surface markers. Among these markers, forkhead box protein 3 (FOXP3) and CD163 showed the highest correlation. FOXP3 is a crucial surface protein in Treg cells, which inhibits cytotoxic T cells from attacking tumor cells (47). Research suggested that macrophages with high expression of CD163 predict poor survival prognosis in a variety of tumors (48). Inspired by immune-related genes, the present study hypothesized that immune checkpoint genes were related to LIMK1 expression levels. MDSCs, as immune suppressive cells, can promote tumor growth, invasion and angiogenesis (49). MDSCs are also been reported to migrate to tumor tissues and exert immunosuppressive functions in CRC (50). A study by Jensen et al (51) found that high LIMK1 expression is associated with poor prognosis of patients with acute myeloid leukemia by influencing MDSCs. Thus, the present study explored the associations between LIMK1 and MDSCs in CRC. According to the results, it was found that the correlation between MDSCs and LIMK1 expression was relatively low in CRC. It was possible that LIMK1 expression could not affect MDSCs recruitment and function in TME. Thus, LIMK1 might be able to regulate the tumor immune microenvironment through affecting immune cell infiltration and immune-related molecules expression. Although more experiments were needed to confirm these speculations, the results suggested that LIMK1 had a significant relationship with immune cell infiltration in CRC.
However, there were several limitations to the present study. First, it only used the online shared database and a small number of clinical samples to analyze the expression of LIMK1 and clinicopathological features of CRC. There are differences in chip consistency in the database. It was important to verify the results using more clinical data. Zhang et al (52) indicated that imbalanced LIMK1 and LIMK2 expression leads to CRC progression and metastasis. Thus, it was appropriate to consider LIMK1 and LIMK2 as common detection and research targets in future studies of the LIMK family. Second, the association between LIMK1 and tumor-infiltrating cells needed to be further confirmed by experiments in vivo or in vitro.
Taken together, the present study showed that LIMK1 was upregulated in CRC and its upregulation trend was closely related to tumor lymph node metastasis and pathological staging in this study. Moreover, it verified the potential association between LIMK1 and tumor-infiltrating lymphocytes in CRC for the first time to the best of the authors' knowledge. This indicated that LIMK1 was probably a powerful indicator for the diagnosis and treatment of CRC.
In brief, LIMK1 was highly expressed in CRC and was closely related to the clinical features and prognosis of patients. In addition, LIMK1 was a promising prognostic indicator that may regulate tumor progression by affecting the tumor immune microenvironment in CRC.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used or analyzed during the current study can be acquired from the corresponding author upon reasonable request.
Authors' contributions
XL and QS analyzed LIMK1 expression data of CRC from the TCGA and GEO databases. DW and YL conducted experimental validation. ZZ and WF designed the experiments and wrote the manuscript. All authors read and approved the final manuscript. ZZ and WF confirm the authenticity of all the raw data.
Ethics approval and consent to participate
This study was approved by the Ethics Review Committee of Tianjin Medical University General Hospital (approval no. 2021-WZ-203) and written informed consent was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical guidelines: Colorectal cancer screening 2021. Am J Gastroenterol. 2021;116:458–479. doi: 10.14309/ajg.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Pan B, Sun L, Chen X, Zeng K, Hu X, Xu T, Xu M, Wang S. Circulating exosomal miR-27a and miR-130a Act as Novel Diagnostic And Prognostic Biomarkers Of Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2018;27:746–754. doi: 10.1158/1055-9965.EPI-18-0067. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Wang C, Song H, Xu Y, Ji G. RNA-Seq profiling of circular RNAs in human colorectal cancer liver metastasis and the potential biomarkers. Mol Cancer. 2019;18:8. doi: 10.1186/s12943-018-0932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nunoue K, Ohashi K, Okano I, Mizuno K. LIMK-1 and LIMK-2, two members of a LIM motif-containing protein kinase family. Oncogene. 1995;11:701–710. [PubMed] [Google Scholar]
- 7.Scott RW, Olson MF. LIM kinases: Function, regulation and association with human disease. J Mol Med (Berl) 2007;85:555–568. doi: 10.1007/s00109-007-0165-6. [DOI] [PubMed] [Google Scholar]
- 8.Kwon J, Seong MJ, Piao X, Jo YJ, Kim NH. LIMK1/2 are required for actin filament and cell junction assembly in porcine embryos developing in vitro. Asian-Australas J Anim Sci. 2020;33:1579–1589. doi: 10.5713/ajas.19.0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bamburg JR, Bernstein BW. Roles of ADF/cofilin in actin polymerization and beyond. F1000 Biol Rep. 2010;2:62. doi: 10.3410/B2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura Y, Yoshioka K, Bernard O, Bereczky B, Itoh K. A role of LIM kinase 1/cofilin pathway in regulating endocytic trafficking of EGF receptor in human breast cancer cells. Histochem Cell Biol. 2006;126:627–638. doi: 10.1007/s00418-006-0198-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Tian J, Wang R, Song M, Zhao R, Chen H, Liu K, Shim J, Zhu F, Dong Z, Lee MH. Dasatinib inhibits lung cancer cell growth and patient derived tumor growth in mice by targeting LIMK1. Front Cell Dev Biol. 2020;8:556532. doi: 10.3389/fcell.2020.556532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang JB, Wu YP, Lin YZ, Cai H, Chen SH, Sun XL, Li XD, Wei Y, Zheng QS, Xu N, Xue XY. Up-regulation of LIMK1 expression in prostate cancer is correlated with poor pathological features, lymph node metastases and biochemical recurrence. J Cell Mol Med. 2020;24:4698–4706. doi: 10.1111/jcmm.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Li D, Fang L. MiR-128-3p suppresses breast cancer cellular progression via targeting LIMK1. Biomed Pharmacother. 2019;115:108947. doi: 10.1016/j.biopha.2019.108947. [DOI] [PubMed] [Google Scholar]
- 14.Kang X, Li W, Liu W, Liang H, Deng J, Wong CC, Zhao S, Kang W, To KF, Chiu PWY, et al. LIMK1 promotes peritoneal metastasis of gastric cancer and is a therapeutic target. Oncogene. 2021;40:3422–3433. doi: 10.1038/s41388-021-01656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu G, Zhou Y, Zhang C, Zhang Y. Upregulation of LIMK1 is correlated with poor prognosis and immune infiltrates in lung adenocarcinoma. Front Genet. 2021;12:671585. doi: 10.3389/fgene.2021.671585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su J, Zhou Y, Pan Z, Shi L, Yang J, Liao A, Liao Q, Su Q. Downregulation of LIMK1-ADF/cofilin by DADS inhibits the migration and invasion of colon cancer. Sci Rep. 2017;7:45624. doi: 10.1038/srep45624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu YH, Lu YX, Zhang ZY, Zhang JM, Zhang WJ, Zheng L, Lin WH, Zhang W, Li XN. SSH3 facilitates colorectal cancer cell invasion and metastasis by affecting signaling cascades involving LIMK1/Rac1. Am J Cancer Res. 2019;9:1061–1073. [PMC free article] [PubMed] [Google Scholar]
- 18.Ito K, Murphy D. Application of ggplot2 to pharmacometric graphics. CPT Pharmacometrics Syst Pharmacol. 2013;2:e79. doi: 10.1038/psp.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509–W514. doi: 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 22.Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49:D605–D612. doi: 10.1093/nar/gkab835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 24.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. doi: 10.1186/gb-2003-4-9-r60. [DOI] [PubMed] [Google Scholar]
- 25.Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L, et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb) 2021;2:100141. doi: 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Liu Y, Zheng L. The diagnostic and prognostic value of LIMK1/2: A pan-cancer analysis. Ann Clin Lab Sci. 2021;51:615–624. [PubMed] [Google Scholar]
- 29.Sousa-Squiavinato ACM, Vasconcelos RI, Gehren AS, Fernandes PV, de Oliveira IM, Boroni M, Morgado-Díaz JA. Cofilin-1, LIMK1 and SSH1 are differentially expressed in locally advanced colorectal cancer and according to consensus molecular subtypes. Cancer Cell Int. 2021;21:69. doi: 10.1186/s12935-021-01770-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Q, Li R, Zhou R, Pan Z, Xu L, Ding Y, Zhao L. LIM kinase 1 interacts with myosin-9 and alpha-actinin-4 and promotes colorectal cancer progression. Br J Cancer. 2017;117:563–571. doi: 10.1038/bjc.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai S, Xu S, Ye Y, Ding K. Identification of an immune-related gene signature to improve prognosis prediction in colorectal cancer patients. Front Genet. 2020;11:607009. doi: 10.3389/fgene.2020.607009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McConnell BV, Koto K, Gutierrez-Hartmann A. Nuclear and cytoplasmic LIMK1 enhances human breast cancer progression. Mol Cancer. 2011;10:75. doi: 10.1186/1476-4598-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q, Jiao D, Hu H, Song J, Yan J, Wu L, Xu LQ. Downregulation of LIMK1 level inhibits migration of lung cancer cells and enhances sensitivity to chemotherapy drugs. Oncol Res. 2013;20:491–498. doi: 10.3727/096504013X13657689382699. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Gan N, Zhou J. Immunohistochemical investigation of the correlation between LIM kinase 1 expression and development and progression of human ovarian carcinoma. J Int Med Res. 2012;40:1067–1073. doi: 10.1177/147323001204000325. [DOI] [PubMed] [Google Scholar]
- 35.You T, Gao W, Wei J, Jin X, Zhao Z, Wang C, Li Y. Overexpression of LIMK1 promotes tumor growth and metastasis in gastric cancer. Biomed Pharmacother. 2015;69:96–101. doi: 10.1016/j.biopha.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Zhao CC, Zhan MN, Liu WT, Jiao Y, Zhang YY, Lei Y, Zhang TT, Zhang CJ, Du YY, Gu KS, Wei W. Combined LIM kinase 1 and p21-activated kinase 4 inhibitor treatment exhibits potent preclinical antitumor efficacy in breast cancer. Cancer Lett. 2020;493:120–127. doi: 10.1016/j.canlet.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Fang J, Chen S, Wang W, Meng S, Liu B. Nonconserved miR-608 suppresses prostate cancer progression through RAC2/PAK4/LIMK1 and BCL2L1/caspase-3 pathways by targeting the 3′-UTRs of RAC2/BCL2L1 and the coding region of PAK4. Cancer Med. 2019;8:5716–5734. doi: 10.1002/cam4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng Y, Ren M, Li Y, Liu Y, Chen C, Su J, Su B, Xia H, Liu F, Jiang H, et al. Knockdown of RhoGDI2 represses human gastric cancer cell proliferation, invasion and drug resistance via the Rac1/Pak1/LIMK1 pathway. Cancer Lett. 2020;492:136–146. doi: 10.1016/j.canlet.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Shi W, Hu W, Ma D, Yan D, Yu K, Zhang G, Cao Y, Wu J, Jiang C, Wang Z. DEP domain-containing protein 1B (DEPDC1B) promotes migration and invasion in pancreatic cancer through the Rac1/PAK1-LIMK1-cofilin1 signaling pathway. Onco Targets Ther. 2020;13:1481–1496. doi: 10.2147/OTT.S229055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vainer G, Vainer-Mosse E, Pikarsky A, Shenoy SM, Oberman F, Yeffet A, Singer RH, Pikarsky E, Yisraeli JK. A role for VICKZ proteins in the progression of colorectal carcinomas: Regulating lamellipodia formation. J Pathol. 2008;215:445–456. doi: 10.1002/path.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian T, Chen ZH, Zheng Z, Liu Y, Zhao Q, Liu Y, Qiu H, Long Q, Chen M, Li L, et al. Investigation of the role and mechanism of ARHGAP5-mediated colorectal cancer metastasis. Theranostics. 2020;10:5998–6010. doi: 10.7150/thno.43427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li VS, Yuen ST, Chan TL, Yan HH, Law WL, Yeung BH, Chan AS, Tsui WY, So S, Chen X, Leung SY. Frequent inactivation of axon guidance molecule RGMA in human colon cancer through genetic and epigenetic mechanisms. Gastroenterology. 2009;137:176–187. doi: 10.1053/j.gastro.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Sousa-Squiavinato ACM, Rocha MR, Barcellos-de-Souza P, de Souza WF, Morgado-Diaz JA. Cofilin-1 signaling mediates epithelial-mesenchymal transition by promoting actin cytoskeleton reorganization and cell-cell adhesion regulation in colorectal cancer cells. Biochim Biophys Acta Mol Cell Res. 2019;1866:418–429. doi: 10.1016/j.bbamcr.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Jobin C. Microbial imbalance and intestinal pathologies: Connections and contributions. Dis Model Mech. 2014;7:1131–1142. doi: 10.1242/dmm.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu X, Guo J, Vorster P, Wu Y. Involvement of LIM kinase 1 in actin polarization in human CD4 T cells. Commun Integr Biol. 2012;5:381–383. doi: 10.4161/cib.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duvall MG, Fuhlbrigge ME, Reilly RB, Walker KH, Kılıç A, Levy BD. Human NK cell cytoskeletal dynamics and cytotoxicity are regulated by LIM kinase. J Immunol. 2020;205:801–810. doi: 10.4049/jimmunol.2000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Facciabene A, Motz GT, Coukos G. T-regulatory cells: Key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, Park YJ. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Transl Med. 2015;49:318–324. doi: 10.4132/jptm.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Xu J, Zhang N, Chen M, Wang H, Zhu D. Targeting the tumour immune microenvironment for cancer therapy in human gastrointestinal malignancies. Cancer Lett. 2019;458:123–135. doi: 10.1016/j.canlet.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 50.Yin K, Xia X, Rui K, Wang T, Wang S. Myeloid-derived suppressor cells: A new and pivotal player in colorectal cancer progression. Front Oncol. 2020;10:610104. doi: 10.3389/fonc.2020.610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen P, Carlet M, Schlenk RF, Weber A, Kress J, Brunner I, Słabicki M, Grill G, Weisemann S, Cheng YY, et al. Requirement for LIM kinases in acute myeloid leukemia. Leukemia. 2020;34:3173–3185. doi: 10.1038/s41375-020-0943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Li A, Shi J, Fang Y, Gu C, Cai J, Lin C, Zhao L, Liu S. Imbalanced LIMK1 and LIMK2 expression leads to human colorectal cancer progression and metastasis via promoting β-catenin nuclear translocation. Cell Death Dis. 2018;9:749. doi: 10.1038/s41419-018-0766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used or analyzed during the current study can be acquired from the corresponding author upon reasonable request.