Abstract
Objective
Recent investigations have revealed that COVID-19 during pregnancy substantially increases the risk of harmful outcomes for mothers and neonates, including preterm death and stillbirth as well as severe maternal morbidity and mortality. Hence, the urgent need to understand the prevalence rate and level of awareness about COVID-19 (SARS-CoV-2 virus infection) and the practice of preventive measures against the disease among pregnant women in Somalia. This study aims to determine the prevalence of COVID-19 among pregnant women seeking antenatal care in the Benadir region (Mogadishu) of Somalia and to assess their knowledge and preventive practices towards COVID-19.
Setting
A hospital-based cross-sectional study involving two major referral maternity hospitals in Mogadishu, Somalia.
Participants
Pregnant women seeking antenatal services were included in our study.
Methods
A total of 477 blood samples were collected from pregnant women attending the two referral hospitals in Mogadishu and screened for COVID-19. The participants were subjected to questionnaire interviews where their detailed history and practice of prevention against COVID-19 were evaluated.
Results
The results showed that 175 (36.7%) were positive while 302 (63.3%) samples were negative for SARS-CoV-2 virus antibodies. Also, out of the 141 pregnant women who had two children or less, 19.4% were positive for IgG/IgM antibodies. Participants who had close contact with patients with COVID-19 were significantly associated for testing positive with a p value 0.0001. Students, teachers, employed people and individuals reported COVID-19 like symptoms were all associated with COVID-19 seropositivity outcomes.
Conclusion
Pregnant women and those with commorbidies should be given special preventive care and health education about COVID-19 transmission.
Keywords: COVID-19, public health, public health
Strengths and limitations of this study.
A cross-sectional study was used to assess the COVID-19 prevalence among pregnant women.
Exclusion of vaccinated pregnant women will prevent false-positive results due to vaccine-induced antibodies.
The selected sites are the two major public hospitals providing free maternal and child care services in Mogadishu and neighbouring states including mothers from internally displaced people camps.
Using questionnaires as a tool for data collection may associate with recall bias.
The reverse transcriptase-PCR is still the gold-standard method in detecting active COVID-19 infection.
Introduction
Since the announcement of the COVID-19 (SARS-CoV-2 virus infection) outbreak as a public health emergency of international concern and its subsequent proclamation as a pandemic in the year 2020, the dramatic loss of human life and the associated public health and socioeconomic challenges that ensued have been devastating. As of 1 December 2021, the number of confirmed COVID-19 cases globally stands at 262 178 403 with 5 215 745 deaths.1 Interestingly, the African continent has the least cases compared with the Americas, Europe or Asia, despite its seeming poor public health system. Similarly, the case-fatality ratio for COVID-19 in Africa is lower than the global case-fatality ratio, which also implies that the outcome of the SARS-CoV-2 virus infection has been less severe among African populations.2 Despite, the low number of confirmed cases in the continent being attributed to the low testing rates, which has continued to undermine the continental response.3 Nonetheless, other factors that have been reported to contribute to the low incidence and mortality rate in Africa are; cross-immunity with malaria, lower population mean age, lower number of individuals with comorbidities like cardiovascular diseases as well as lower pre-COVID-19 era ‘65 year+ mortality rate’.4 5 Notwithstanding these apprehensions, there is no evidence that a large number of COVID-19 deaths have been missed; instead, the low numbers of confirmed cases can be attributed in part to the lessons learnt during the handling of several infectious disease outbreaks that have occurred in the continent including Yellow fever, Ebola, HIV and AIDS.6
Despite having one of Africa’s most fragile healthcare systems, occasioned by the ongoing conflict and destruction of public health infrastructure, the number of cases in Somalia is relatively low. However, cases have in recent months increased tenfold, and there are indications of community transmission beyond the major cities.7 The risk of acquiring COVID-19 is known to be higher among the elderly as well as individuals with underlying comorbidities, including moderate to severe asthma, diabetes, cardiovascular diseases and other respiratory illnesses, including pregnancy which predisposes to severe illness.8–10 The pandemic has disproportionately impacted vulnerable groups such as persons with disabilities and internally displaced people (IDP) living in makeshift camps in Somalia. The above has been driven by the non-adherence to the outlined public health measures and has contributed significantly to the increasing number of cases recorded in the country.
Until recently, many studies have shown that pregnant women do not seem to be at a higher risk of getting COVID-19.11 In other words, being pregnant does not increase the chances of getting COVID-19 more than non-pregnant persons; however, recent studies have shown that COVID-19 during pregnancy is associated with severe outcomes such as high rate of maternal morbidity and mortality and neonatal complications.12–14 There are also emerging evidence that the risk of having stillbirth may be higher among pregnant patients with COVID-19.15 These preneonatal and neonatal period complications are attributed to pregnant women’s reduced respiratory capacity, low immunity and the haemodynamic changes they undergo. The risk of severe maternal outcomes is even higher if they have pulmonary comorbidities, hypertensive disorders and diabetes mellitus.16 Moreover, investigations have shown women to be a vulnerable group during the COVID-19 pandemic. This worry is even more among pregnant women who occasionally experience pregnancy and postpartum mental illnesses (depression, anxiety and postpartum psychosis), resulting in bipolar disorder.17 These situations are a cause for concern in Somalia, whose women of reproductive age represent 38% of the household, with a worrying maternal mortality rate of 692.18 Also, among the 23 102 cases as of 2 December 2021, 26% (amounting >6006 cases) are women.
The main strategy for each country is to vaccine their general public against COVID-19; the WHO and all governments around the globe are doing their best efforts and advocacy for mass vaccination. To date, a total of 7 772 799 316 vaccine doses have been done according to the data released daily by the WHO. The Ministry of Health (MoH) of the federal government of Somalia has been vaccinating the public since 2020 based on vaccines donated by international organisations and some governments. Since the vaccination programme started, the MoH was hesitant to vaccinate pregnant women for lack of evidence; however, on 18 November 2021, they released a newsletter stating that MoH recommends vaccinating pregnant women with a single dose J&J COVID-19 vaccine after the first trimester.
This survey was conducted among pregnant women to study if they were exposed to COVID-19 based on the OnSite COVID-19 IgG/IgM Rapid Test that detects anti-SARS-CoV-2 IgG and IgM antibodies in serum and plasma. The aim was to estimate the prevalence of diagnosed COVID-19 among pregnant women in Somalia’s Benadir region. Also, a questionnaire was administered to consenting participants to determine demographic characteristics and potential risk factors for COVID-19. We also intend to evaluate the presence or otherwise of any association between the participant’s sociodemographic features with their respective COVID-19 status.
Material and methods
Study design
We present a cross-sectional study aiming to estimate the seroprevalence of SARS-CoV-2 antibodies among pregnant women attending referral hospitals in Mogadishu, Somalia, from 31 July 2021 to 31 August 2021 (figure 1). Women who indicated their informed consent and had no history of COVID-19 vaccination were included in the survey. Participants who did not consent to participate in the study were excluded.
Figure 1.
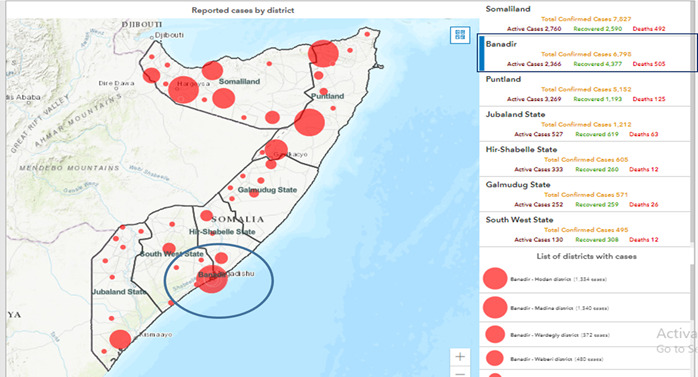
Map of Somalia showing the COVID-19 cases and death (13 November 2021) with the study area (Benadir region) having the second highest number of confirmed cases (6798) and the highest mortality (505). https://covid19som-ochasom.hub.arcgis.com/
Study setting
The selected hospitals are the major two referral hospitals for mother and child in the capital city of Mogadishu. Both hospitals are located on either side of the city and routinely offer free medical services to the mother and child. One hospital in the south provides services to the most vulnerable in the society, mainly the IDP from the regions in the south of Somalia. And the other hospital located in the north of the city covers the city’s north districts, including patients from nearby central Somalia regions. The consenting participants were administered a questionnaire covering demographics, obstetrical history, general health status, COVID-19 status and COVID-19 preventive measures. The questionnaire was prepared using Google form for ease of use, processing and analysing the data generated.
Sample size determination
We assumed a 50% prevalence since no study had previously been done to determine the seroprevalence of COVID-19 antibodies among pregnant women in Somalia.
Therefore, based on the Cochran’s formula for sample size calculation (N = Z2 x P (1-P) ÷ ε2)
where Z is 1.96 (constant), e is the desired level of precision (ie, 5% margin of error at 95% CI), p estimated prevalence (5.7%), and q is 1 – p.
Therefore,
N = Z2 x P (1-P) ÷ ε2
N=3.8416 x 0.5 (1–0.5) ÷ 0.0025
N=384.16 samples.
However, in order to increase our chances of detection, 477 samples were collected.
Serological testing
Participants were tested with the CTK BIOTECH’s OnSite COVID-19 IgG/IgM Rapid Test (California, USA) following the instructions given by the manufacturer. The OnSite COVID-19 IgG/IgM Rapid Test is suitable for detecting individuals with recent or latent infection to SARS-CoV-2 virus infection, indicating recent or prior infection. The OnSite COVID-19 IgG/IgM Rapid Test is one of the most effective tests for detecting previous exposure to SARS-CoV-2 virus infection with a 97.1% sensitivity and 97.8% specificity.
Four trained doctors, assisted by three junior doctors, and a final-year obstetrician-gynaecology postgraduate student participated in the blood sample collection and filling of the Google form questionnaires according to inputs from the participants (pregnant women attending antenatal services). Participants were given a detailed explanation of the study purpose and procedure, while the confidentiality of their data was granted.
The study’s primary outcome was the seroprevalence of IgG and IgM-specific antibodies in a cohort of pregnant women during the study period. In our analysis, we stratified the population according to the results of the serological study (IgG, IgM, and IgG/IgM-positive vs negative group). Those with positive IgG/IgM were further subdivided depending on the presence or absence of COVID-19-related risky behaviours, obstetrical characteristics, history of chronic illnesses and COVID-19 symptoms at any time before the serological study using descriptive and inferential analysis.
Statistical analysis
For the descriptive analysis, the general distribution of all the variables included in this study was assessed by frequency and percentages for categorical variables and means and SD for continuous data using SPSS statistical software V.25. For inferential analysis, we used logistic regression to examine the relationship between predictor variables and the outcome variables.
Patient and public involvement
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting or dissemination plans of our research.
Results
From 31 July 2021 to 31 August 2021, a total of 477 eligible pregnant women were tested for SARS-COV-2 virus infection-specific antibodies. The single-use lateral flow immunoassay test kit is used for detection and differentiation of anti-SARS-CoV-2 virus infection IgG and IgM immunoglobulins where a total of 302 (63.3%) serum samples were found to be negative for SARS-CoV-2 virus infection while 175 (36.7%) turned out to be positive (figure 2A). Similarly, 34% of the circulating antibodies were IgG indicating long-term infection, while IgM circulation was found in only 2% of the women (figure 2B).
Figure 2.
Overall prevalence of SARS-CoV-2 virus antibodies among pregnant women in Mogadishu, Somalia. (A) Bar chart showing antibody negative vs antibody positive. (B) Pie chart showing antibody negative vs IgG, IgM & IgG/IgM.
The COVID-19-related sociodemographic characteristics analysed, including age, occupational status and the number of people in the household, were all found not to be statistically significant (p>0.05). However, other demographics studied like marital status showed that respondents that are married have increased risk of contracting the COVID-19 infection in relation to their counterparts that are not married, and this finding is statistically significant (table 1). Similarly, in terms of the education status, not having formal education was found to be a statistically significant risk factor for contracting COVID-19 with p value of 0.004. Important to note that statistical significance in this case does not equate causation, rather, it implies increased risk.
Table 1.
Sociodemographic characteristics of pregnant women in Mogadishu, Somalia
| Demographics | N | COVID-19 (IgG/IgM) | OR at 95% CI | P value | |
| Positive (175) | Negative (302) | ||||
| Age | |||||
| <25 years | 213 (44.7) | 72 (41.1) | 141 (46.7) | 1.253 (0.860 to 1.826) | 0.241 |
| ≥25 years | 264 (55.3) | 103 (58.9) | 161 (53.3) | Ref.cat | |
| Marital status | |||||
| Married | 341 (71.5) | 109 (62.3) | 232 (76.8) | 2.007 (1.337 to 3.012) | 0.001* |
| Unmarried | 136 (28.2) | 66 (37.7) | 70 (23.2) | Ref.cat | |
| Educational level | |||||
| Informal education | 267 (56.0) | 83 (47.4) | 184 (60.9) | 1.728 (1.187 to 2.518) | 0.004* |
| Formal education | 210 (44.0) | 92 (52.6) | 118 (39.1) | Ref.cat | |
| Occupation | |||||
| Unemployed | 359 (75.3) | 119 (68.0) | 240 (79.5) | 1.822 (1.193 to 2.781) | 0.005* |
| Employed | 118 (24.7) | 56 (32.0) | 62 (20.5) | Ref.cat | |
| Number of household | |||||
| ≤5 | 103 (21.6) | 32 (18.3) | 71 (23.5) | Ref.cat | |
| >5 | 374 (78.4) | 143 (81.7) | 231 (76.5) | 0.728 (0.457 to 1.161) | 0.182 |
*Statistically significant at the 0.05 level.
According to the obstetrical characteristics of the respondents, 34 (19.4%) of women who reported they had given birth to less than two children were found to have circulating levels of SARS-CoV-2 antibodies, and this result was statistically significant at p=0.0001. While the other variables (history of abortion and stage of gestation) were not a potential risk factor to acquiring COVID-19 since none of them was found to be significant (table 2).
Table 2.
Obstetric characteristics of pregnant women in Mogadishu, Somalia
| Characteristic | N | COVID-19 IgG/IgM test | OR 95% CI | P value | |
| Positive (175) | Negative (302) | ||||
| Parity | |||||
| 0–2 | 141 (29.6) | 34 (19.4) | 107 (35.4) | 0.439 (0.289 to 0.684) | 0.0001* |
| >2 | 336 (70.4) | 141 (80.6) | 195 (64.6) | Ref.cat | |
| History of abortion | |||||
| Yes | 105 (22.0) | 35 (20.0) | 70 (23.2) | 0.829 (0.525 to 1.308) | 0.420 |
| No | 372 (78.0) | 56 (22.0) | 50 (16.6) | Ref.cat | |
| Gestational age | |||||
| <13 weeks | 65 (13.6) | 29 (16.6) | 36 (11.9) | Ref.cat | 0.127 |
| 14–26 weeks | 92 (19.3) | 30 (17.1) | 62 (20.5) | 1.665 (0.865 to 3.205) | 0.206 |
*Statistically significant at the 0.05 level.
Some of the unhealthy behaviours reported to increase the risk of other respiratory illnesses were also evaluated in this study. Notable among them is the smoking of cigarettes, Shisha and the local habit of chewing Khat (Catha edulis), a stimulant plant frequently chewed among Somalis. Among these, only the smoking of shisha (4.6% positive) was found to pose a risk for contracting COVID-19 infection due to sharing of pipes.
Furthermore, human-to-human transmission of COVID-19 is precipitated by close contact with family members and is usually high when the number of families in a household is considerable. In order to evaluate the association between the number of people in the household with the risk of contracting the infection, participants were asked questions relating to knowledge about their COVID-19 status, familiarity with the basic clinical signs of COVID-19, the possibility of transmission within family members as well as observance of public health preventive measures (table 3).
Table 3.
COVID-19 status among pregnant women in Mogadishu, Somalia
| Characteristic | N | COVID-19 (IgG/IgM) | OR 95%(CI) | P value | |
| Positive (175) | Negative (302) | ||||
| Were you ever tested for COVID-19? | |||||
| Yes | 139 (29.1) | 67 (38.3) | 72 (23.8) | 1.982 (1.324 to 2.967) | 0.001* |
| No | 338 (70.9) | 108 (61.7) | 230 (76.2) | Ref.cat | |
| What was the result? | |||||
| Positive | 55 (11.6) | 34 (19.5) | 21 (7.0) | 0.283 (0.156 to 0.511) | 0.0001* |
| Negative | 96 (20.2) | 38 (21.8) | 58 (19.2) | 0.698 (0.436 to 1.119) | 0.135 |
| Not applicable | 325 (68.3) | 102 (58.6) | 223 (73.8) | Ref.cat | |
| Have you ever had COVID-19? | |||||
| Yes | 102 (21.4) | 60 (34.3) | 42 (13.9) | 0.639 (0.385 to 1.060) | 0.083 |
| No | 222 (46.5) | 42 (24.0) | 180 (59.6) | 3.911 (2.464 to 6.207) | 0.0001* |
| Not sure | 153 (32.1) | 73 (41.7) | 80 (26.5) | Ref.cat | |
| Did you have COVID-19 symptoms? | |||||
| Yes | 193 (40.5) | 126 (72.0) | 67 (22.2) | 9.019 (5.883 to 13.827) | 0.0001* |
| No | 284 (59.5) | 49 (28.0) | 235 (77.8) | Ref.cat | |
| Did you have fever? | |||||
| Yes | 212 (44.4) | 130 (74.3) | 82 (27.2) | 7.751 (5.076 to 11.836) | 0.0001* |
| No | 265 (55.6) | 45 (25.7) | 220 (72.8) | Ref.cat | |
| Did you have cough? | |||||
| Yes | 216 (45.3) | 138 (78.9) | 78 (25.8) | 10.711 (6.864 to 16.715) | 0.0001* |
| No | 261 (54.7) | 37 (21.1) | 224 (74.2) | Ref.cat | |
| Did you lose your smell? | |||||
| Yes | 180 (37.7) | 127 (72.6) | 53 (17.5) | 12.430 (7.964 to 19.401) | 0.0001* |
| No | 297 (62.3) | 48 (27.4) | 249 (82.5) | Ref.cat | |
| Did you lose your taste? | |||||
| Yes | 166 (34.8) | 118 (67.4) | 48 (15.9) | 10.955 (7.043 to 17.038) | 0.0001* |
| No | 311 (65.2) | 57 (32.6) | 254 (84.1) | Ref.cat | |
| Did you have stomach upset? | |||||
| Yes | 135 (28.3) | 103 (58.9) | 32 (10.6) | 12.070 (7.512 to 19.395) | 0.0001* |
| No | 342 (71.7) | 72 (41.1) | 270 (89.4) | Ref.cat | |
| Did you have shortness of breath? | |||||
| Yes | 170 (35.6) | 122 (69.7) | 48 (15.9) | 12.181 (7.796 to 19.031) | 0.0001* |
| No | 307 (64.4) | 53 (30.3) | 254 (84.1) | Ref.cat | |
| When was the time you had the symptoms | |||||
| <3 months | 87 (18.2) | 48 (27.4) | 39 (12.9) | 0.075 (0.039 to 0.0146) | 0.0001* |
| 3–6 months | 35 (7.3) | 22 (12.6) | 13 (4.3) | 0.055 (0.023 to 0.129) | 0.0001* |
| >6 months | 166 (34.8) | 89 (50.9) | 77 (25.5) | 0.080 (0.044 to 0.145) | 0.0001* |
| Not applicable | 189 (39.6) | 16 (9.1) | 173 (57.3) | Ref.cat | |
| Close contact with someone having COVID-19 | |||||
| Yes | 158 (33.1) | 110 (62.9) | 48 (15.9) | 0.218 (0.117 to 0.407) | 0.0001* |
| No | 256 (53.7) | 44 (25.1) | 212 (70.2) | 2.409 (1.201 to 4.462) | 0.005* |
| Not sure | 63 (13.2) | 21 (12.0) | 42 (13.9) | Ref.cat | |
| Did any household contact colleagues or close friend had COVID-19? | |||||
| Yes | 195 (40.9) | 126 (72.0) | 69 (22.8) | 8.683 (5.674 to 13.288) | 0.0001* |
| No | 282 (59.1) | 49 (28.0) | 233 (77.2) | Ref.cat | |
| Was anyone of your close contacts hospitalised for COVID-19 | |||||
| Yes | 169 (35.4) | 115 (65.7) | 54 (17.9) | 8.802 (5.732 to 13.518) | 0.0001* |
| No | 308 (64.6) | 60 (34.3) | 248 (82.1) | Ref.cat | |
| Did anyone of your close contacts die of COVID-19? | |||||
| Yes | 182 (38.2) | 124 (70.9) | 58 (19.2) | 10.229 (6.628 to 15.785) | 0.0001* |
| No | 295 (61.8) | 51 (29.1) | 244 (80.8) | Ref.cat | |
*Statistically significant at the 0.05 level.
A total of 193 pregnant women reported having had COVID-19 infection, out of which 126 (72%) presented clinical signs indicative of the disease while asymptomatic were 67 (22.2%). The most common symptom presented by the infected women were cough (78.9%), fever (74.3%), shortness of breath (69.7%), loss of smell (72.6%) and stomach upset (58.9%). The timing of symptoms appearing differed significantly between gestations, first trimester (27.4%), second trimester (12.6%) and third trimester (50.9%). Close contact with a family member of someone who had COVID-19 was also a significant finding for testing positive with a p value=0.004. We also observed that the number of close contacts the respondents knew who had A total of 193 pregnant women reported having had COVID-19 infection, out of which 126 (72%) presented clinical signs indicative of the disease while asymptomatic were 67 (22.2%). The most common symptom presented by the infected women were cough (78.9%), fever (74.3%), shortness of breath (69.7%), loss of smell (72.6%) and stomach upset (58.9%). The timing of symptoms appearing differed significantly between gestations, first trimester (27.4%), second trimester (12.6%) and third trimester (50.9%). Close contact with a family member of someone who had COVID-19 was also a significant finding for testing positive with a p value=0.004. We also observed that the number of close contacts the respondents knew whom COVID-19 had was more likely hospitalised (65.7%) or died of the infection (70.9%).
Observing social and physical distances was statistically significant compared with not observing this essential preventive measure (table 4). The result revealed that 41.7% of the respondents admitted to observing distancing among others (168), which is lower than the number of respondents who admitted to not practicing distancing (308), and this was found to be statistically significant (p<0.05). Except for the use of face mask, which showed the number of those using it (268) was higher than those not using (209), the adherence to other preventive measures was poor, particularly frequent hand washing, which showed 53.1% not practicing handwashing compared with 46.9% that claimed they regularly wash their hands. Importantly, this finding was not statistically significant; however, the odds are greater than 1, implying that not adhering to these preventive measures could increase the chances of contracting COVID-19.
Table 4.
Preventive measures observed by the pregnant women in Mogadishu, Somalia
| Characteristic | N | COVID-19 (IgG/IgM) | OR 95% CI | P value | |
| Positive (175) | Negative (302) | ||||
| Do you regularly wear face mask? | |||||
| Yes | 268 (56.2) | 108 (61.7) | 160 (53.0) | Ref.cat | |
| No | 209 (43.8) | 67 (38.3) | 142 (47.0) | 1.431 (0.979 to 2.091) | 0.064 |
| If ‘yes’, what types of mask do you wear? | |||||
| N95 | 43 (9.0) | 18 (10.3) | 25 (8.3) | Ref.cat | |
| Surgical face mask | 79 (16.6) | 23 (13.1) | 56 (18.5) | 1.753 (0.807 to 3.810) | 0.156 |
| Others | 163 (34.2) | 70 (40.0) | 93 (30.8) | 0.957 (0.484 to 1.889) | 0.898 |
| Not applicable | 192 (40.3) | 64 (36.6) | 128 (42.4) | 1.440 (0.732 to 2.831) | 0.290 |
| Do you regularly wash your hands | |||||
| Yes | 205 (43.0) | 82 (46.9) | 123 (40.7) | Ref.cat | |
| No | 272 (57.0) | 93 (53.1) | 179 (59.3) | 1.283 (0.882 to 1.868) | 0.193 |
| Do you keep your distance from others | |||||
| Yes | 168 (35.2) | 73 (41.7) | 95 (31.5) | Ref.cat | |
| No | 309 (64.8) | 102 (58.3) | 207 (68.5) | 1.643 (0.903 to 2.988) | 0.104 |
| Do you avoid handshaking? | |||||
| Yes | 164 (34.4) | 66 (37.7) | 98 (32.5) | Ref.cat | |
| No | 313 (65.6) | 109 (62.3) | 204 (67.5) | 0.858 (0.484 to 1.520) | 0.599 |
Comorbidities of pregnant women with confirmed SARS-CoV-2 virus infection are shown in table 5. There was a significant difference in mean between the COVID-19-positive pregnant women and COVID-19-negative pregnant women across all the comorbidities inquired (diabetes, hypertension, cardiovascular disease and asthma) with a p value of 0.001. The OR was also greater than 1, which means greater odds of association with having any chronic illness and the chances of becoming infected with COVID-19.
Table 5.
Comorbidities of pregnant women with conformed SARS-CoV-2 virus infection
| Characteristic | N | COVID-19 (IgG/IgM) | OR 95% CI | P value | |
| Positive (175) | Negative (302) | ||||
| Do you have diabetes? | |||||
| Yes | 94 (19.7) | 50 (28.6) | 44 (14.6) | 2.345 (1.484 to 3.708) | 0.0001* |
| No | 383 (80.3) | 125 (71.4) | 258 (85.4) | Ref.cat | |
| Do you have hypertension? | |||||
| Yes | 124 (26.0) | 67 (38.3) | 57 (18.9) | 2.667 (1.753 to 4.056) | 0.0001* |
| No | 353 (74.0) | 108 (61.7) | 245 (81.1) | Ref.cat | |
| Do you have cardiac disease? | |||||
| Yes | 85 (17.8) | 46 (26.3) | 39 (12.9) | 2.811 (1.721 to 4.590) | 0.0001* |
| No | 392 (82.2) | 129 (73.7) | 263 (87.1) | Ref.cat | |
| Do you have asthma? | |||||
| Yes | 85 (17.8) | 46 (26.3) | 39 (12.9) | 2.405 (1.494 to 3.870) | 0.0001* |
| No | 392 (82.2) | 129 (73.7) | 263 (87.1) | Ref.cat | |
| Do you have family history of hypertension? | |||||
| Yes | 137 (28.7) | 63 (36.0) | 74 (24.5) | 1.733 (1.156 to 2.598) | 0.008* |
| No | 340 (71.3) | 112 (64.0) | 228 (75.5) | Ref.cat | |
| Do you have family history of diabetes? | |||||
| Yes | 148 (31.0) | 72 (41.1) | 76 (25.2) | 2.079 (1.397 to 3.094) | 0.0001* |
| No | 329 (69.0) | 103 (58.9) | 226 (74.8) | Ref.cat | |
| Do you have family history of cardiac disease? | |||||
| Yes | 155 (32.5) | 76 (43.4) | 79 (26.2) | 2.167 (1.461 to 3.213) | 0.0001* |
| No | 322 (67.5) | 99 (56.6) | 223 (73.8) | Ref.cat | |
| Do you have family history of asthma? | |||||
| Yes | 137 (28.7) | 64 (36.6) | 73 (24.2) | 1.809 (1.206 to 2.712) | 0.004* |
| No | 340 (71.3) | 111 (63.4) | 229 (75.8) | Ref.cat | |
| Do you have family history of obesity? | |||||
| Yes | 193 (40.5) | 89 (50.9) | 104 (34.4) | 1.970 (1.348 to 2.880) | 0.0001* |
| No | 284 (59.5) | 86 (49.1) | 198 (65.6) | Ref.cat | |
| Do you take regular medications? | |||||
| Yes | 137 (28.7) | 66 (37.7) | 71 (23.5) | 0.494 (0.328 to 0.744) | 0.001* |
| Herbal medication | 19 (4.0) | 8 (4.6) | 11 (3.6) | 0.631 (0.246 to 1.617) | 0.338 |
| No | 321 (67.3) | 101 (57.7) | 220 (72.8) | Ref.cat | |
*Statistically significant at <0.05.
Discussion
The cross-sectional approach used was because of the urgent need to understand the status of the disease among pregnant women due to their suppressed immune status, especially given the speed at which the disease was developing. This study investigated the prevalence, knowledge and preventive practices towards COVID-19 among pregnant women seeking antenatal services in two of the major public hospitals in Mogadishu, within the Benadir region of Somalia. The study coincided with the third wave of the COVID-19 outbreak in Somalia around early July 2021. During this period, a total of 477 pregnant women were screened for confirmation of COVID-19 using the CTK BIOTECH’s OnSite COVID-19 IgG/IgM Rapid Test kit, which is capable of identifying SARS-CoV-2 antibodies with 97.1% and 97.8% sensitivity and specificity, respectively.19 To date, the most reliable method of detecting SARS-CoV-2 virus infection is reverse trancriptase-PCR.20 21 Nonetheless, the OnSite COVID-19 IgG/IgM Rapid Test can identify individuals with circulating antibodies against the SARS-CoV-2 virus infection either as a result of recent or prior infection.19 In this study, the predominant immunoglobulin among the COVID-19-positive pregnant women was IgG with 34% circulation followed by IgM with 2%. During the acute phase of COVID-19 infection, IgM blood levels against SARS-CoV-2 virus infection rise rapidly and peak after 2–3 weeks of contracting the virus, followed by SARS-CoV-2 virus infection-specific IgG antibodies appearing and persist in the circulation for months. Because of the inability of the test kit (OnSite COVID-19 IgG/IgM) to distinguish COVID-19 vaccine-induced antibodies and antibodies as a result of SARS-CoV-2 infection, we decide to exclude all pregnant women who have received single or multiple shots of the COVID-19 vaccine. This ensures that only pregnant women with infection (acute or chronic) are diagnosed as positive.22 Based on this, the majority of the COVID-19 positive pregnant women sampled were found to have a chronic long-term infection (predominance of IgG). This outcome is similar to the previous report where titres of IgG targeting N-protein of SARS-CoV2 were recommended as a prognostic factor in understanding the clinical course of COVID-19 and that it should be measured in all patients with SARS-CoV2 infection.23
The overall prevalence of SARS-CoV-2 virus infection among pregnant women was 36.7%, with 3% of the total having an active infection, but none needed critical care. Even though earlier studies have reported a prevalence of 61% among healthcare workers, the 36.7% recorded in this study is distressing, this is because the 61% we referred to was from a study conducted among healthcare workers in Somalia.24 It is not a surprise to see a high number of cases among this category of people because they constitute the frontline workers at the most significant risk of contracting the disease.25 This figure (36.7%) is considerably high when studies were done in Japan and New York, which found a seroprevalence of 0.03% and 16.4%, respectively.16 26
Concerning the adherence to the outlined preventive measures, including avoiding crowd, frequent washing of hands with detergents or disinfectants and the use of face coverings, we observed a worrying trend where the majority of the respondents admitted to not practicing these aforementioned public health guidelines with an increased odd of contracting the disease as a result (OR >1). This undesirable habit of not adhering to the recommended preventive practice may give further credence to the high number of positive cases recorded in this study. We understand that observing these set-out regulations will be challenging and complex because of some Somali culture and traditions of congregating, sharing hugs and shaking hands. Nonetheless, considering the vulnerabilities of pregnant women, there is an urgent need to ensure compliance with these COVID-19 requirements; otherwise, things may only worsen for pregnant women in Somalia. It is important to point out that, based on the results of this study, no difference in terms of increased risk was observed between wearing mask and not wearing. This may not, however, be surprising because public mask wearing is most effective at reducing spread of the virus when the recommended mask is worn properly with high compliance.27 Additionally, because wearing masks has been reported to bring down the overall risk of spreading COVID-19, people have become careless and less likely to abide by standard measures and more willing to take other risks, such as decreasing the physical distance between them and others. Furthermore, despite the results showing no risk of contracting infection regardless of whether a mask is worn or not, in contrast to the popular report on face covering, it is likely that this discrepancy may be because of the careless attitude among people wearing mask for the mere believe that the mask will protect them. Interestingly, only 25.6% of those that claimed they wear mask reported they used the recommended mask (N95%–9% and surgical mask 16.6%). The remaining reported wearing masks made of simple clothes while others considered Islamic face veil (Niqab) to be enough face mask for COVID-19 prevention.
Additional vital findings in this study are the relationship of COVID-19-positive status and comorbidity. Since the first outbreak, we have come to realise that SARS-CoV-2 infects people of all age groups; however, elderly people (above 60 years), as well as individuals with comorbidities such as chronic respiratory disease (asthma patients), diabetes and cardiovascular diseases, are at a greater risk of developing infection with severe outcomes.28 29 Although most of the respondents admitted to not having any of the chronic disease conditions asked, viz hypertension, diabetes, asthma, cardiovascular disease and obesity (74%–82%). Nevertheless, many pregnant women (17.8%–28.7%) indicated they have one or more of these comorbidities, as mentioned earlier, which will increase the risk for them and their unborn children.
Other potential risk factors evaluated in this study, such as parity, history of abortion, stage of gestation, and some unhealthy behaviours like smoking revealed no significant relationship concerning increasing chances of contracting COVID-19 among pregnant women, with an OR of 0.4 to 0.8. However, the risk for contracting the disease for smokers of Shisha was very high with an OR of 3.569, and this finding was statistically significant (p=0.04). Like other studies, none of the different trimesters of pregnancy was associated with a high risk of getting the infection. A study done in Spain found that seroprevalence was similar between women in the first trimester of pregnancy and women in the third trimester, suggesting a similar risk of infection. However, the proportion of women with symptoms and those requiring hospitalisation was higher in the third-trimester group than in the first-trimester group.30 31 On the other hand, available data have already indicated that smoking doubles the risk of having severe COVID-19.32 Lung damage from COVID-19 resembles the damage of smoke from cigarettes and other tobacco products that introduce particulate matter from the environment into the lungs. Notably, the mouthpiece and the hose in the shisha can serve as a means of transmission of the COVID-19 virus, which can also spread through shisha sharing.
Participants who reported COVID-like symptoms were more likely to turn positive when tested for COVID-19. Though some reported no history of COVID-19 infection, this wave arrived simultaneously with the already expected seasonal flue. The order and frequency of symptoms were almost the same with a study done in Mogadishu in which the most typical symptom reported was cough (>75%), followed by fever (>71%) and loss of taste and smell.33 Other studies show that fever and cough were the most typical symptoms reported, followed by stomach upset.34 We also observed that pregnant women who reported having had a previous infection with COVID-19 were more likely to test positive. Though COVID-19-specific immunity may disappear in 3 months, one may believe that since they were infected in previous waves, they still sustain immunity and do not practice preventive measures.35
Despite the findings reported in this study, it is important to emphasise that the method of data collection and assay used are fraught with some limitations as earlier mentioned. One of such limitation is the tendency for the respondents to give a less accurate answer when asked retrospective questions. In order for us to evaluate their current situation with respect to COVID-19-related practices and behaviours, we needed baseline information prior to COVID-19 outbreak. Studies have shown that measurement error can arise when high cognitive questions retrospective inquiries are made as well as the inability of the respondents to remember specific details about their pre-COVID-19 routine hygiene and sanitation practices.36 Also the inability of the rapid test kit to distinguish between cross-reacting antibodies like secretory immunoglobulin A produced against other Corona viruses by the mucosal immunity may result in false-positive detection.37
In conclusion, the COVID-19 high prevalence observed in this study is disturbing considering how vulnerable pregnant women can be, especially in Somalia, where the healthcare services face serious challenges. The poor attitude in observing preventive measures against COVID-19 among pregnant women also warrants serious attention towards raising awareness among midwives and healthcare workers who are in close contact with women delivering babies to reduce infection transmission and ensure prevention and control of the virus.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the support and cooperation of the management of the Benadir and SOS Hospitals. We sincerely appreciate your kind assistance during this study. We also wish to express our gratitude to our medical students for their assistance during data collection.
Footnotes
Twitter: @Jamal Hassan Mohamoud
Contributors: NID and MASN conceived the study and drafted the original protocol. NID, HAD, NAH, MHA and BG contributed to developing the survey questionnaires. HAD, JHM and BG played a major role in the statistical analyses. All the authors participated in, read and approved the final manuscript. NID is acting as guarantor of the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by SIMAD University Ethics Committee. IMRSU/FMHS (FR18) P003. Participants gave informed consent to participate in the study before taking part.
References
- 1.WHO . Somalia: who coronavirus disease (COVID-19) Dashboard with vaccination data. Available: https://covid19.who.int/region/emro/country/so
- 2.Prevent Epidemics . Partnership for evidence-based response to COVID-19 (PERC). Available: https://preventepidemics.org/covid19/perc/ [Accessed 13 Nov 2021].
- 3.Chitungo I, Dzobo M, Hlongwa M, et al. COVID-19: unpacking the low number of cases in Africa. Public Health Pract 2020;1:100038. 10.1016/j.puhip.2020.100038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osei SA, Biney RP, Anning AS, et al. Low incidence of COVID-19 case severity and mortality in Africa; could malaria co-infection provide the missing link? BMC Infect Dis 2022;22:1–11. 10.1186/s12879-022-07064-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawal Y. Africa’s low COVID-19 mortality rate: A paradox? Int J Infect Dis 2021;102:118–22. 10.1016/j.ijid.2020.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nkengasong J. Let Africa into the market for COVID-19 diagnostics. Nature 2020;580:565. 10.1038/d41586-020-01265-0 [DOI] [PubMed] [Google Scholar]
- 7.Crisis Group . COVID-19 in Somalia: a public health emergency in an electoral minefield. Available: https://www.crisisgroup.org/africa/horn-africa/somalia/b155-covid-19-somalia-public-health-emergency-electoral-minefield
- 8.Sanyaolu A, Okorie C, Marinkovic A, et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med 2020;2:1069–76. 10.1007/s42399-020-00363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garba B, Zakaria Z, Salihu MD, et al. Breaking the cycle of the COVID-19 transmission: a challenge for Nigeria. J Glob Health 2020;10. 10.7189/jogh.10.020309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adam MH, Mohamoud JH, Mohamood AS, et al. Seroprevalence of Anti-SARS-CoV-2 antibodies in Benadir region, Somalia. Vaccines 2020;10:220. 10.3390/vaccines10020220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C-L, Liu Y-Y, Wu C-H, et al. Impact of COVID-19 on pregnancy. Int J Med Sci 2021;18:763–7. 10.7150/ijms.49923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ames JL, Ferrara A, Avalos LA, et al. COVID-19 prevalence, symptoms, and sociodemographic disparities in infection among insured pregnant women in northern California. PLoS One 2021;16:e0256891. 10.1371/journal.pone.0256891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr 2021;175:817–26. 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacobucci G. Covid-19: severe infection in pregnancy significantly increases risks, study shows. BMJ 2022;376:o480. 10.1136/bmj.o480 [DOI] [PubMed] [Google Scholar]
- 15.Khalil A, von Dadelszen P, Draycott T, et al. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA 2020;324:705. 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NM M AR, Ld W, SM D WL. Seroprevalence of SARS-CoV-2 during pregnancy and associated outcomes: results from an ongoing prospective cohort study, New York City, 2021. Available: https://europepmc.org/article/PPR/PPR277509 [DOI] [PMC free article] [PubMed]
- 17.Esteban-Gonzalo S, Caballero-Galilea M, González-Pascual JL, et al. Anxiety and worries among pregnant women during the COVID-19 pandemic: a multilevel analysis. Int J Environ Res Public Health 2021;18. 10.3390/ijerph18136875. [Epub ahead of print: 26 Jun 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed MAM, Siewe Fodjo JN, Gele AA, et al. COVID-19 in Somalia: adherence to preventive measures and evolution of the disease burden. Pathogens 2020;9:735. 10.3390/pathogens9090735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagerqvist N, Maleki KT, Verner-Carlsson J, et al. Evaluation of 11 SARS-CoV-2 antibody tests by using samples from patients with defined IgG antibody titers. Sci Rep 2021;11:1–8. 10.1038/s41598-021-87289-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kevadiya BD, Machhi J, Herskovitz J, et al. Diagnostics for SARS-CoV-2 infections. Nat Mater 2021;20:593–605. 10.1038/s41563-020-00906-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R, Han H, Liu F, et al. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta 2020;505:172–5. 10.1016/j.cca.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mouliou DS, Gourgoulianis KI. False-Positive and false-negative COVID-19 cases: respiratory prevention and management strategies, vaccination, and further perspectives. Expert Rev Respir Med 2021;15:993–1002. 10.1080/17476348.2021.1917389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batra M, Tian R, Zhang C, et al. Role of IgG against N-protein of SARS-CoV2 in COVID19 clinical outcomes. Sci Rep 2021;11:1–9. 10.1038/s41598-021-83108-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdi A, Ahmed AY, Abdulmunim M, et al. Preliminary findings of COVID-19 infection in health workers in Somalia: a reason for concern. Int J Infect Dis 2021;104:734–6. 10.1016/j.ijid.2021.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atnafie SA, Anteneh DA, Yimenu DK, et al. Assessment of exposure risks to COVID-19 among frontline health care workers in Amhara region, Ethiopia: a cross-sectional survey. PLoS One 2021;16:e0251000. 10.1371/journal.pone.0251000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arakaki T, Hasegawa J, Sekizawa A, et al. Clinical characteristics of pregnant women with COVID-19 in Japan: a nationwide questionnaire survey. BMC Pregnancy Childbirth 2021;21:1–8. 10.1186/s12884-021-04113-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kai D, Goldstein G-P, Morgunov A. Universal masking is urgent in the COVID-19 pandemic: SEIR and agent based models, empirical validation. Policy Recommendations 2020. http://arxiv.org/abs/2004.13553 [Google Scholar]
- 28.Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health 2020;13:1833–9. 10.1016/j.jiph.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO . Coronavirus disease (COVID-19): situation report, 141, 2020. Available: https://apps.who.int/iris/handle/10665/332390 [Accessed cited 2021 Nov 14].
- 30.Crovetto F, Crispi F, Llurba E. Seroprevalence and clinical spectrum of SARS-CoV-2 infection in the first versus third trimester of pregnancy. medRxiv 2020. [Google Scholar]
- 31.Lopez M, Hawkins-Villarreal A, Goncé A. OP03.02: clinical spectrum of COVID-19 infection throughout gestation. Ultrasound Obstet Gynecol 2021;58:65–6. [Google Scholar]
- 32.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farah Yusuf Mohamud M, Garad Mohamed Y, Mohamed Ali A, et al. Loss of taste and smell are common clinical characteristics of patients with COVID-19 in Somalia: a retrospective double centre study. Infect Drug Resist 2020;13:2631–5. 10.2147/IDR.S263632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen JR, Martin MR, Martin JD, et al. Modeling the onset of symptoms of COVID-19. Front Public Health 2020;8:473. 10.3389/fpubh.2020.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arkhipova-Jenkins I, Helfand M, Armstrong C, et al. Antibody response after SARS-CoV-2 infection and implications for immunity: a rapid living review. Ann Intern Med 2021;174:811–21. 10.7326/M20-7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hipp L, Bünning M, Munnes S. Problems and pitfalls of retrospective survey questions in COVID-19 studies. Surv Res Methods 2020;14:109–45. 10.18148/srm/2020.v14i2.7741 [DOI] [Google Scholar]
- 37.Tsukinoki K, Yamamoto T, Handa K, et al. Detection of cross-reactive immunoglobulin A against the severe acute respiratory syndrome-coronavirus-2 spike 1 subunit in saliva. PLoS One 2021;16:e0249979. 10.1371/journal.pone.0249979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.



