Abstract
BACKGROUND
Single-nucleotide polymorphisms (SNPs) of the serotonin type 3 receptor subunit (HTR3) genes have been associated with psychosomatic symptoms, but it is not clear whether these associations exist in irritable bowel syndrome (IBS).
AIM
To assess the association of HTR3 polymorphisms with depressive, anxiety, and somatization symptoms in individuals with IBS.
METHODS
In this retrospective study, 623 participants with IBS were recruited from five specialty centers in Germany, Sweden, the United States, the United Kingdom, and Ireland. Depressive, anxiety, and somatization symptoms and sociodemographic characteristics were collected. Four functional SNPs — HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A — were genotyped and analyzed using the dominant and recessive models. We also performed separate analyses for sex and IBS subtypes. SNP scores were calculated as the number of minor alleles of the SNPs above. The impact of HTR3C c.489C>A was tested by radioligand-binding and calcium influx assays.
RESULTS
Depressive and anxiety symptoms significantly worsened with increasing numbers of minor HTR3C c.489C>A alleles in the dominant model (Fdepressive = 7.475, Pdepressive = 0.006; Fanxiety = 6.535, Panxiety = 0.011). A higher SNP score (range 0-6) was linked to a worsened depressive symptoms score (F = 7.710, P-linear trend = 0.006) in IBS. The potential relevance of the HTR3C SNP was corroborated, showing changes in the expression level of 5-HT3AC variant receptors.
CONCLUSION
We have provided the first evidence that HTR3C c.489C>A is involved in depressive and anxiety symptoms in individuals with IBS. The SNP score indicated that an increasing number of minor alleles is linked to the worsening of depressive symptoms in IBS.
Keywords: Irritable bowel syndrome, 5-HT3 receptor subunit gene polymorphisms, Single-nucleotide polymorphism score, Depression, Anxiety, Somatization
Core Tip: Bringing together high quality data as well as methodological expertise, our results show that: In the dominant model, HTR3C c.489C>A was correlated with depressive and anxiety symptoms in irritable bowel syndrome (IBS); a higher number of minor alleles, which was computed by combining the individual SNP status of HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A, was linked to more severe depressive symptoms in IBS; and the potential relevance of the HTR3C SNP was corroborated in functional assays showing changes in the expression level of 5-HT3AC variant receptors. These results will contribute towards standardization and harmonization of genetic research strategies in IBS.
INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal (GI) disorder characterized by abdominal pain and altered bowel habits[1-4]. The pathophysiology of IBS has not entirely been resolved, but is understood to be biopsychosocial and affected by an impaired function of the central and enteric nervous systems and their crosstalk via the brain-gut axis[5,6]. IBS patients often present with increased comorbid depressive and anxiety symptoms[7-11], highlighting the complex relationship between visceral sensitivity and subjective psychological perceptions[12,13]. Nevertheless, about 50% of IBS patients report GI symptoms but show no comorbid affective symptoms[14].
There is evidence that disturbances of the serotonergic system are important in GI disorders such as IBS and in mental disorders, both of which interact via the brain-gut axis[15,16]. The serotonin type 3 receptors (5-HT3R) modulate key functions in the GI tract[17,18]. In line with such functions, 5-HT3R antagonists are beneficial in the treatment of diarrhea-predominant IBS (IBS-D)[19-22]. 5-HT3Rs are also involved in emotional processing, mood regulation, and visceral perception and have been associated with depressive and anxiety symptoms that represent comorbid phenotypes in IBS[23]. Single-nucleotide polymorphisms (SNPs) in the 5-HT3R subunit genes (HTR3), namely HTR3A c.-42C>T (rs1062613), HTR3B c.386A>C (rs1176744), HTR3C c.489C>A (rs6766410), and HTR3E c.*76G>A (rs56109847), are associated with IBS according to studies investigating the effects of sex or IBS subtypes[12,24-30]. However, whether HTR3 polymorphisms are associated with IBS and comorbid depressive and anxiety symptoms has not been determined because existing studies have missing phenotypic data on comorbidities and small sample sizes. These studies had case-control designs and investigated associations between these polymorphisms in individuals with IBS phenotypes or mental behavioral conditions and controls rather than combining genetic data with specific psychosocial characteristics of IBS patients.
This multicenter observational study focused on a large IBS patient cohort comprising 768 participants from centers in Germany, Sweden, the United States, the United Kingdom, and Ireland with the aim of meeting three objectives: (1) To explore the associations between functional HTR3 polymorphisms and psychosomatic burden (i.e., depressive, anxiety, and somatization symptoms) within an IBS population; (2) To investigate the impact of the HTR3 SNP score (computed as the number of minor alleles) on psychosomatic burden, based on our hypothesis that the observed number of minor alleles was associated with specific mental characteristics in IBS patients; and (3) To perform a functional analysis of variant 5-HT3AC receptors.
MATERIALS AND METHODS
Subjects
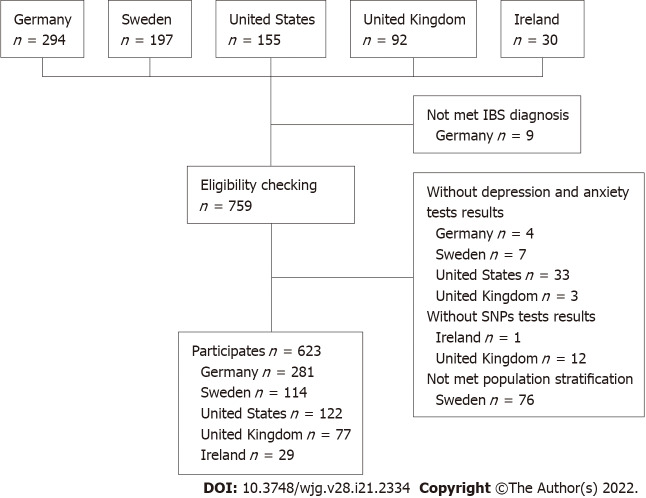
The study population was pooled from five different tertiary care expert centers. German participants were recruited from the Specialty Clinic for Functional GI Disorders at the Department of General Internal Medicine and Psychosomatics of Heidelberg University Hospital[31] and from our clinical partners in the IBS-Net in Hamburg, Krefeld, Berlin, Vilsbiburg, and Munich (www.ibs.uni-hd.de). Swedish participants were recruited at the specialized unit for patients with functional GI disorders at Sahlgrenska University Hospital in Gothenburg. United States participants were recruited at Washington University, Barnes-Jewish Hospital in St. Louis, Missouri. United Kingdom participants were recruited at the Nottingham Digestive Diseases Center and participants from Ireland from a specialty clinic at Cork University Hospital. Participant recruitment is shown in Figure 1.
Figure 1.
Recruitment strategy. IBS: Irritable bowel syndrome.
Written informed consent was obtained from all participants and the experiments were in accordance with the principles of the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report. All studies were approved by the following local Ethics Committees: Heidelberg, Germany: Ethical Committee, Medical Faculty of the Heidelberg University Hospital (S067/2010); Cork, Ireland: Clinical Research Ethics Committee (APC024); Gothenburg, Sweden: Regional Ethical Review Board in Gothenburg (S489-02 and 731-09); Nottingham, United Kingdom: registered at clinical trial clinicaltrials.gov (identifier NCT00745004) and approved by Nottingham Research Ethics Committee 2 (REC reference number 08/H0408/134)[21]; and St-Louis, United States: Washington University St. Louis, Human Research Protection Office (IRB ID #: 201103220).
Inclusion/exclusion criteria
Only patients diagnosed with IBS according to the ROME III criteria were included in the analysis. All participants were of Caucasian ancestry and had comparable population stratification. Patients under 18 years of age or without SNP test results were excluded.
Genotyping
Genomic DNA was isolated from IBS patient blood samples using ethylenediaminetetraacetic acid according to standard protocols[32]. Four polymorphic HTR3 loci, namely HTR3A c.-42C>T (rs1062613), HTR3B c.386A>C (rs1176744), HTR3C c.489C>A (rs6766410), and HTR3E c.*76G>A (rs56109847) were selected as target SNPs for this study. The corresponding primers were designed and synthesized using AssayDesigner 3.1 software. Genotyping was performed at the Department of Human Molecular Genetics at Heidelberg University Hospital using the KASPar® SNP Genotyping System (KBiosciences, Ltd, Hoddesdon, United Kingdom). To analyze HTR3 SNPs, the fluorescence plate reader of the 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, California, United States) was used as recommended. About 10% of the samples were repeat tested to ensure genotyping accuracy.
Data collection
In addition to sociodemographic characteristics and IBS diagnosis, we also collected data on depressive, anxiety, and somatization symptoms[33] and genetic markers of the serotonergic system.
IBS diagnosis: The diagnostic classification of IBS was based on the Scoring Algorithm for Rome III Diagnostic Questionnaire for Adult Functional GI Disorders (SA for Rome III-DQ)[34,35] in all five centers. Percentages of the different IBS subtypes, i.e., constipation-predominant IBS, IBS-D, IBS with mixed bowel habits, and unclassified IBS, were also calculated.
Depressive symptoms: The nine-item depression module from the Patient Health Questionnaire (PHQ-9)[36,37] was used to measure depressive symptoms in the German cohort. The Hospital Anxiety and Depression Scale depression subscale[38] was used to identify depressive symptoms in participants from Sweden, the United Kingdom, and Ireland and the Beck Depression Inventory[39] was used to measure the severity of depressive symptoms in United States participants.
Anxiety symptoms: In the German cohort, symptoms of generalized anxiety were assessed using the brief measurement for generalized anxiety disorder (GAD-7)[40]. In the cohorts from Sweden, the United Kingdom, and Ireland, the HADS anxiety subscale was used to identify anxiety symptoms. The Beck Anxiety Inventory[41] was used to assess anxiety in the United States cohort.
Somatization symptoms: In the cohorts from Germany, Sweden, and the United States, the 15-item somatization module from the PHQ-15[42] was used to identify somatization symptoms.
Genetic markers of the serotonergic system: The four functional SNPs HTR3A c.-42C>T (rs1062613), HTR3B c.386A>C (rs1176744), HTR3C c.489C>A (rs6766410), and HTR3E c.*76G>A (rs56109847) were selected for validation based on previous reports as outlined above[25].
Ligand binding and calcium influx assays
These procedures are described in the Supplementary material.
Statistical analysis
All statistical procedures were carried out using IBM SPSS Statistics 22.0 for Windows. Variables with a skewed distribution were log-transformed prior to further analysis. If different measurements had been collected in the five centers, z values were calculated to enable pooling. The Hardy-Weinberg equilibrium (HWE) of genotype frequency distribution was tested using SHEsis[43]. Genome-wide SNP data were generated by the Bellygenes team of D’Amato M using the Illumina Global Screening array and platform[44,45]. We used the multidimensional scaling approach to correct for population stratification in PLINK[46]. Following the guidance provided at https://github.com/MareesAT/GWA_ tutorial/, our data were anchored by data of the 1000 Genomes project (http://www.1000 genomes.org/). The 10 main components were used as covariates in the association tests to correct for population stratification[46] and exclude outliers. Polymorphisms were analyzed separately using the dominant and the recessive models. Also, stratified analyses based on sex and IBS subtypes were carried out. ANOVA was used to analyze group differences and to check for linear trends in depressive, anxiety, and somatization symptoms. For the independent variable, a SNP score was computed based on the number of minor alleles (i.e., continuous from 0 to 8 for the four SNPs). Based on the first human HTR3 locus-specific variant database (www.htr3.uni-hd.de)[25], scoring criteria were as follows: major allele homozygous variant gene = 0; heterozygous variant gene = 1; and minor allele homozygous variant gene = 2 (for details see Table 1). Statistical comparisons were made between the two groups using the χ2 test or Fisher’s exact test for frequencies and t tests or Mann-Whitney U tests for metric variables. Normal distribution and variance homogeneity were checked as conditions. Statistical tests were two-sided based on an alpha error of 0.05%. All analyses were explorative and not confirmatory. False discovery rates (FDRs) were calculated based on overall P values using the Benjamini-Hochberg method[47]. Significant values that were no longer significant after FDR multiple testing correction were named “nominally significant”.
Table 1.
Strategy for computing the single-nucleotide polymorphism score
|
|
Major allele homozygous genotype
|
Heterozygous variant genotype
|
Minor allele homozygous genotype
|
| HTR3A c.-42C>T (rs1062613) | CC | CT | TT |
| HTR3B c.386A>C (rs1176744) | AA | AC | CC |
| HTR3C c.489C>A (rs6766410) | CC | CA | AA |
| HTR3E c.*76G>A (rs56109847) | GG | GA | AA |
RESULTS
Sociodemographic and symptomatic characteristics
In total, 623 participants from five independent expert centers were included in this study (45.1% from Germany, 18.3% from Sweden, 19.6% from the United States, 12.4% from the United Kingdom, and 4.7% from Ireland). We excluded 76 Swedish participants who did not meet the population stratification criteria (Supplementary Figure 1). Participants from the United Kingdom and Ireland were excluded from the main analysis because the sample size was small. Table 2 presents the sociodemographic characteristics, IBS subtypes, and psychosomatic symptoms of the included participants. Participants had a mean ± SD age of 41.7 ± 16.1 years and 69.5% were female. Overall, IBS patients showed minimal to mild levels of depressive and anxiety symptoms, moderate levels of IBS symptoms, and moderate levels of somatization symptoms.
Table 2.
Sociodemographic and symptomatic characteristics of the study participants
|
|
Total
|
Centers
|
||||
|
Germany
|
Sweden
|
United States
|
United Kingdom
|
Ireland
|
||
| n | 100.0 (623) | 45.1 (281) | 18.3 (114) | 19.6 (122) | 12.4 (77) | 4.7 (29) |
| Age | 41.7 ± 16.1 (18, 91) | 40.7 ± 16.4 (18, 88) | 33.7 ± 11.8 (18, 60) | 54.9 ± 14.6 (25, 91) | 40.8 ± 11.6 (18, 70) | 30.3 ± 8.6 (19, 51) |
| Sex | ||||||
| Female | 69.5 (433) | 63.3 (178) | 69.3 (79) | 77.9 (95) | 72.7 (56) | 86.2 (25) |
| IBS subtypes | ||||||
| IBS-C | 14.1 (87) | 8.9 (25) | 12.3 (14) | 36.7 (44) | 0 | 13.8 (4) |
| IBS-D | 41.7 (258) | 43.4 (122) | 19.6 (22) | 28.3 (34) | 100.0 (77) | 10.3 (3) |
| IBS-M | 42.5 (263) | 45.6 (128) | 67.0 (75) | 31.7 (38) | 0 | 75.9 (22) |
| IBS-U1 | 1.8 (11) | 2.1 (6) | 0.9 (1) | 3.3 (4) | 0 | 0 |
| Depressive symptoms | - | 9.4 ± 5.92 | 5.2 ± 3.54 | 13.2 ± 9.65 | 5.9 ± 4.04 | 4.3 ± 3.24 |
| Anxiety symptoms | - | 7.7 ± 4.83 | 8.3 ± 4.54 | 14.6 ± 9.76 | 9.6 ± 4.64 | 7.8 ± 4.54 |
| Symptom severity | - | 280.3 ± 90.77 | 297.3 ± 100.57 | 43.2 ± 16.28 | - | - |
| Somatization symptoms9 | 14.8±6.3 | 16.2 ± 7.3 | 13.3 ± 4.7 | 13.8 ± 5.0 | - | - |
Sample size, sex, IBS subtypes, and severity of depressive and anxiety symptoms are presented as % (n). Age is presented as mean ± SD (range). Scores of depressive and anxiety symptoms are presented as mean ± SD.
Was not included in the later analysis because of the small sample size.
Evaluated by the nine-item depression module from the Patient Health Questionnaire.
Evaluated by the brief measurement for generalized anxiety disorder.
Evaluated by Hospital Anxiety and Depression Scale.
Evaluated by Beck Depression Inventory.
Evaluated by Beck Anxiety Inventory.
Evaluated by irritable bowel syndrome (IBS) symptom severity scale.
Evaluated by the gastrointestinal symptom rating scale for IBS.
Evaluated by the somatization module for the Patient Health Questionnaire.
IBS: Irritable bowel syndrome; IBS-C: Constipation-predominant IBS; IBS-D: Diarrhea-predominant IBS; IBS-M: IBS with mixed bowel habits; IBS-U: Unclassified IBS.
HTR3 SNP genotypes and allele frequencies
Genotype and allele frequencies of the functional HTR3A c.-42C>T, HTR3B c.386A>, HTR3C c.489C>A, and HTR3E c.*76G>A polymorphisms were calculated. No significant differences in genotype frequency were observed between sexes or IBS subtypes. For HTR3A c.-42C>T, the frequency of the minor T allele was 21.5%; for HTR3B c.386A>C, the frequency of the minor C allele was 29.6%; for HTR3C c.489C>A, the frequency of the minor A allele was 41.6%; and for HTR3E c.*76G>A, the frequency of minor A allele was 6.1%. The genotypic distribution of the four polymorphic loci of rs1062613, rs1176744, rs6766410, and rs56109847 were in accordance with HWE (all P > 0.05). The results are shown in Supplementary Tables 1-3.
HTR3 SNP analysis using the dominant and the recessive model
HTR3 SNPs were separately analyzed using the dominant model and the recessive model stratified for sex and IBS subtypes. Depressive and anxiety symptoms worsened significantly with increasing numbers of minor alleles of HTR3C c.489C>A in the dominant model (Fdepressive = 7.475, Pdepressive = 0.006; Fanxiety = 6.535, Panxiety = 0.011). This seemed to be driven by female sex (Fdepressive = 7.040, Pdepressive = 0.008; Fanxiety = 7.550, Panxiety = 0.006) and IBS-D (Fdepressive = 5.670, Pdepressive = 0.018; Fanxiety = 13.444, Panxiety < 0.001). The same trend was also found for HTR3A c.-42C>T in male participants with depressive symptoms in the dominant model (Fdepressive = 4.149, Pdepressive = 0.043). For the recessive model, depressive and somatization symptoms worsened with increasing numbers of minor alleles of HTR3C c.489C>A (Fdepressive = 6.190, Pdepressive = 0.014) and HTR3B c.386A>C (Fdepressive = 6.482, Pdepressive = 0.011), respectively in IBS-D participants. F values from the ANOVA are shown in Table 3. As mentioned above, the analyses of participants from the United Kingdom and Ireland are presented separately in the Supplementary Table 4.
Table 3.
Differences between single-nucleotide polymorphisms in depressive and anxiety symptoms, separately analyzed with ANOVA using the dominant model and the recessive model
|
Models
|
Mental symptoms
|
SNPs
|
Total
|
Sex
|
IBS subtypes
|
|||
|
Male
|
Female
|
IBS-C
|
IBS-D
|
IBS-M
|
||||
| Dominant model | Depressive symptoms | HTR3A c.-42C>T (rs1062613) | 2.541 | 4.149 ↑ | 0286 | 0.998 | 0.376 | 0.694 |
| HTR3B c.386A>C (rs1176744) | 0.067 | 0.005 | 0.198 | 0.282 | 0.124 | 0.232 | ||
| HTR3C c.489C>A (rs6766410) | 7.475 ↑ | 0.672 | 7.040 ↑ | 0.261 | 5.670 ↑ | 2.328 | ||
| HTR3E c.*76G>A (rs62625044) | 0.054 | 0.013 | 0.180 | 0.208 | 0.461 | 0.010 | ||
| Anxiety symptoms | HTR3A c.-42C>T (rs1062613) | 0.511 | 0.210 | 0.280 | 0.604 | 0.672 | 0.162 | |
| HTR3B c.386A>C (rs1176744) | 0.926 | 0.406 | 0.801 | 0.468 | 1.369 | 0.770 | ||
| HTR3C c.489C>A (rs6766410) | 6.535 ↑ | 0.158 | 7.550 ↑ | 0.186 | 13.444 ↑ | 0.045 | ||
| HTR3E c.*76G>A (rs62625044) | 1.055 | 0.429 | 0.853 | 0.000 | 0.495 | 1.109 | ||
| Somatization symptoms | HTR3A c.-42C>T (rs1062613) | 0.016 | 0.141 | 0.106 | 0.086 | 0.017 | 0.203 | |
| HTR3B c.386A>C (rs1176744) | 0.217 | 0.332 | 0.326 | 0.407 | 0.415 | 2.125 | ||
| HTR3C c.489C>A (rs6766410) | 0.784 | 0.035 | 1.085 | 0.269 | 0.000 | 1.962 | ||
| HTR3E c.*76G>A (rs62625044) | 0.002 | 1.554 | 0.263 | 0.128 | 0.729 | 0.354 | ||
| Recessive model | Depressive symptoms | HTR3A c.-42C>T (rs1062613) | 0.002 | 0.421 | 0.132 | 0.093 | 1.320 | 1.064 |
| HTR3B c.386A>C (rs1176744) | 1.118 | 2.027 | 0.107 | 1.741 | 0.197 | 0.744 | ||
| HTR3C c.489C>A (rs6766410) | 0.047 | 0.821 | 0.401 | 2.306 | 6.190 ↑ | 0.676 | ||
| HTR3E c.*76G>A (rs62625044) | 0.677 | - | 0.574 | 0.541 | - | - | ||
| Anxiety symptoms | HTR3A c.-42C>T (rs1062613) | 0.479 | 0.027 | 0.549 | 0.057 | 0.315 | 0.350 | |
| HTR3B c.386A>C (rs1176744) | 0.872 | 0.081 | 1.691 | 0.476 | 0.361 | 1.923 | ||
| HTR3C c.489C>A (rs6766410) | 0.028 | 2.562 | 0.344 | 0.570 | 3.509 | 2.093 | ||
| HTR3E c.*76G>A (rs62625044) | 0.216 | - | 0.179 | 0.242 | - | - | ||
| Somatization symptoms | HTR3A c.-42C>T (rs1062613) | 0.217 | 0.018 | 0.257 | 0.138 | 0.092 | 0.431 | |
| HTR3B c.386A>C (rs1176744) | 6.482 ↑ | 2.245 | 3.575 | 0.142 | 14.033 ↑ | 0.341 | ||
| HTR3C c.489C>A (rs6766410) | 0.468 | 1.813 | 0.128 | 4.715 ↓ | 0.002 | 0.113 | ||
| HTR3E c.*76G>A (rs62625044) | 0.001 | - | 0.011 | 0.000 | - | - | ||
F values are shown in the table. Arrows represent the direction of the associations. ↑ dependent variable increases from major to minor alleles; ↓ dependent variable decreases from major to minor alleles. The underlined values were significant. Values in bold were “nominally significant” (i.e., lacking significance after false discovery rate multiple testing correction). IBS: Irritable bowel syndrome; IBS-C: Constipation-predominant IBS; IBS-D: Diarrhea-predominant IBS; IBS-M: IBS with mixed bowel habits; SNPs: Single-nucleotide polymorphisms.
Effect of SNP score on psychosomatic symptoms
SNP scores ranged from 0 to 6; 37.1% had one or zero minor alleles of the analyzed HTR3 SNPs and 30.8% had three or more minor alleles of the analyzed HTR3 SNPs. No significant differences in SNP scores were observed between sexes (F = 3.550, P = 0.060) or IBS subtypes (F = 1.485, P = 0.227). ANOVAs were conducted and linear trends were checked to analyze the effect of SNP scores on depressive, anxiety, and somatization symptoms. Overall, an increasing number of minor alleles was linked to worsening depressive symptoms (F = 7.710, P-linear trend = 0.006). However, material trends did not reveal a link between more minor alleles and worsening anxiety or somatization symptoms. By stratifying analyses for sex, an increasing number of minor alleles was linked to worsened depressive symptoms in female participants (F = 5.770, P-linear trend = 0.017). There was no significant association between SNP score and depressive, anxiety, or somatization symptoms when looking at IBS subtypes separately (Table 4). As mentioned above, the analyses of participants from the United Kingdom and Ireland are presented separately in the Supplementary Table 5.
Table 4.
Association of depressive, anxiety, and somatization symptoms with the single-nucleotide polymorphism score of the four tested polymorphisms
|
|
Total
|
Sex
|
IBS subtypes
|
|||
|
Male
|
Female
|
IBS-C
|
IBS-D
|
IBS-M
|
||
| Depressive symptoms | ||||||
| F value | 7.710 | 1.336 | 5.770 | 1.551 | 2.159 | 0.196 |
| P-linear trend value | 0.006 | 0.250 | 0.017 | 0.184 | 0.144 | 0.659 |
| Anxiety symptoms | ||||||
| F value | 2.150 | 0.028 | 2.514 | 1.372 | 2.400 | 1.412 |
| P-linear trend value | 0.143 | 0.866 | 0.114 | 0.245 | 0.123 | 0.236 |
| Somatization symptoms | ||||||
| F value | 1.15 | 0.354 | 1.028 | 0.716 | 2.718 | 0.628 |
| P-linear trend value | 0.283 | 0.553 | 0.311 | 0.614 | 0.102 | 0.429 |
Values in bold were “nominally significant” (i.e., lacking significance after false discovery rate multiple testing correction). IBS: Irritable bowel syndrome; IBS-C: Constipation-predominant IBS; IBS-D: Diarrhea-predominant IBS; IBS-M: IBS with mixed bowel habits.
Functional analysis of variant 5-HT3AC receptors
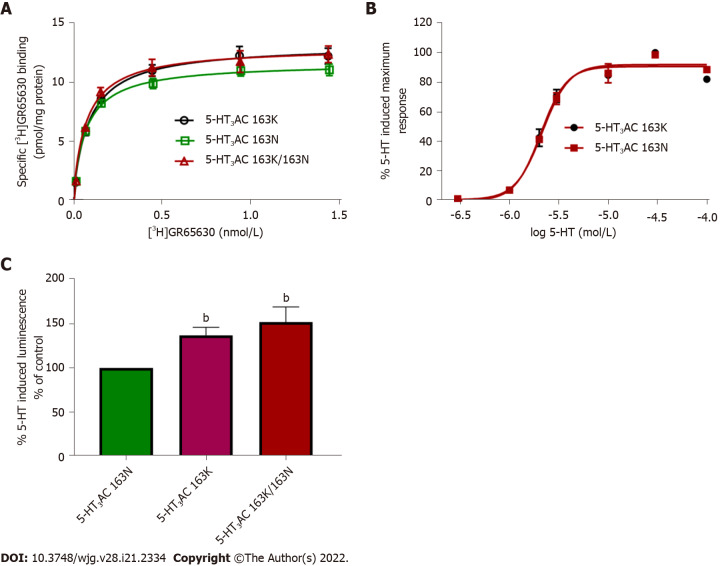
The HTR3C SNP encodes the amino acid exchange p.Asn163Lys (p.N163K), and recombinantly expressed 5-HT3AC receptors harboring variant 5-HT3C subunits that mimic the homozygous minor allele and the heterozygous state presented with increased cell surface expression and enhanced 5-HT maximum response. Radioligand-binding assays revealed higher Bmax values of 117.1% ± 4.38% and 111.9% ± 1.79%. Calcium influx assays showed increased 5-HT-induced maximum effects of 137.2% ± 9.0% and 151.9% ± 17.3% for the minor allele 5-HT3AC 163K or combined 5-HT3AC 163N/5-HT3AC 163K receptors compared with the major allele representing 5-HT3AC 163N receptors, respectively. The affinity of the specific 5-HT3 receptor antagonist [3H]GR65630, as reflected by the Kd values, and the potency of 5-HT, as reflected by the EC50 values, did not differ between the receptor variants (Figure 2).
Figure 2.
Comparative analysis of 5-HT3AC 163N (major allele homozygous), 5-HT3AC 163K (minor allele homozygous), and 5-HT3AC 163K/163N (heterozygous) receptors. A: Radioligand-binding studies Saturation experiments were performed in membranes of HEK293 cells transiently expressing different 5-HT3 subunit combinations in triplicate with six increasing concentrations of [3H]GR65630 (0.02-1.5 nmol/L); Kd values were 0.07 ± 0.01 for 5-HT3AC 163N; 0.08 ± 0.01 for 5-HT3AC 163K, and 0.07 ± 0.01 for 5-HT3AC 163K/163N. n = 6 experiments; B: Concentration response curves were assessed in a calcium influx assay (aequorin assay) in HEK293 cells transiently transfected with different 5-HT3 subunit combinations in quadruplicate with seven increasing concentrations of 5-HT. pEC50 values were as follows: 68 ± 0.03 for 5-HT3AC 163N 5 and 5.66 ± 0.02 for 5-HT3AC 163K; C: Respective maximal calcium influx values (Emax) evoked by 5-HT (10 μmol/L). Data are expressed as percentages of the Emax of the heteromeric 5-HT3AC 163N receptor (% of control), n = 8 experiments. Bars represent mean ± SE. bP < 0.01 from ANOVA followed by Dunnett’s post-test or unpaired Student’s test for comparison of only two groups.
DISCUSSION
Main findings
The 5-HT3 receptors modulate essential functions in the GI tract such as GI motility as well as mood and emotions[19], and HTR3 SNPs have been associated with depression, anxiety, and IBS[25]. In this study, we showed that: (1) In the dominant model, HTR3C c.489C>A was correlated with depressive and anxiety symptoms in IBS; (2) A higher number of minor alleles (i.e., a higher SNP score, which was computed by combining the individual SNP status of HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A) was linked to more severe depressive symptoms in IBS; and (3) The potential relevance of the HTR3C SNP was corroborated in functional assays showing changes in the expression level of 5-HT3AC variant receptors. These findings are discussed in more detail below.
Sample characteristics
Participants with IBS were more frequently female than male, in line with previous findings[48,49] that IBS is more prevalent in young and middle-aged females. Most participants with IBS were only mildly affected by depression and anxiety symptoms. Of note, German participants only visited the Specialty Clinic for Functional GI Disorders at Heidelberg University Hospital after a long history of dealing with IBS[31]; therefore, these participants reported more severe depressive symptoms. However, causal relationships between IBS symptoms, depression, and anxiety are still controversial.
The investigated SNPs rs1062613, rs1176744, rs6766410, and rs56109847 were in accordance with HWE. There was no population stratification, and the sample was representative and excluded genotyping errors. Although participants were recruited from various centers in different countries, there were no obvious selective differences.
Connections between HTR3 SNPs and mental symptoms
HTR3A c.-42C>T (rs1062613): Depressive symptoms were “nominally significantly” more severe with increasing numbers of minor HTR3A c.-42C>T alleles in male participants according to the dominant model. This SNP has been associated with bipolar affective disorder[50], IBS symptom severity, amygdaloid activity[29,51], early life trauma[52], altered emotional networks in the human brain, and the onset of depression[53]. However, these studies only included single sex participants and did not include subgroup analyses.
HTR3B c.386A>C (rs1176744): Somatization symptoms worsened significantly with increasing numbers of minor HTR3B c.386A>C alleles in the dominant model. The HTR3B variant p.Tyr129Ser (rs1176744) has been associated with bipolar affective disorder in males and with major depression in females as well as with pain catastrophizing, a coping style characterized by excessively negative thoughts and emotions related to pain[30,54-56]. This discrepancy may be due to an enhancement or weakening of this association by polymorphic interactions in the serotonin pathways[56].
HTR3C c.489C>A (rs6766410): Depressive and anxiety symptoms worsened significantly with increasing numbers of minor HTR3C c.489C>A alleles in the dominant model. This effect seemed to be driven by female sex and IBS-D. HTR3C c.489C>A was previously associated with IBS-D in female patients[57], but the proportion of male patients was small in this study, which may limit the applicability of these findings. In the recessive model, depressive and anxiety symptoms “nominally significantly” worsened with increasing numbers of minor alleles of HTR3C c.489C>A in Irish participants. However, these results should be interpreted with caution because the Irish sample size was low. As far as we are aware, HTR3C c.489C>A has not been analyzed in individuals with affective disorders before.
HTR3E c.*76G>A (rs56109847): HTR3E is restrictedly and robustly expressed in the GI tract[58,59], suggesting that it plays a special role in 5-HT3 receptor function in the gut. In this study, we did not find a relationship between functional polymorphisms of HTR3E and depressive and anxiety symptoms in IBS patients. This may be attributed to a floor effect because depressive and anxiety symptoms were minimal to mild in our sample[60].
The SNP score and its impact on depressive symptoms
A single gene variant is not sufficient to explain all symptoms shaping the clinical phenotype of a complex disorder like IBS[61]. By computing SNP scores based on the number of minor alleles of rs1062613, rs1176744, rs6766410, and rs56109847, our study revealed that an increasing number of minor alleles is linked to increasing severity of depressive symptoms. However, there was no obvious association between an increasing number of minor alleles and the severity of anxiety or somatization symptoms. Stratification for sex revealed a correlation between increasing numbers of minor alleles and worsening depressive symptoms in female participants.
Functional properties of variant 5-HT3AC receptors
HTR3 genes encode different 5-HT3 subunits to make up heteromeric receptors. The 5-HT3A subunits play a major role in these receptors because they can form functional receptors on their own. The other subunits can only form functional receptors with 5-HT3A and seem to modulate the function and properties of the receptors[62]. How these native receptors might contribute to the pathogenesis of IBS, particularly regarding co-expression patterns of HTR3, has not been established yet. The HTR3A and HTR3E variants reside within untranslated regions and the respective SNPs correlate with increased expression levels, whereas the HTR3B variant changes the channel properties[25]. To gain insight into the pathophysiological relevance of the associated HTR3C variant c.489C>A (rs6766410), we characterized the pharmacological and functional properties of those 5-HT3AC receptors that altered the 5-HT-mediated maximum response and expression of variant 5-HT3AC receptors. However, how structural modifications in these receptors affect their function in vivo and how they modulate the serotonergic system to influence mood, emotional processing, and the manifestation of IBS and comorbid conditions remains to be determined.
Limitations and strengths
Our study has some limitations. First, different instruments were used by different centers to assess phenotypic features. To correct for this, scale scores were converted into z standard scores. However, given that the participants reported no severe psychosomatic symptoms, the discovery chances might be limited. Second, there is no sufficient evidence to show the relationship between risk alleles and respective major/minor alleles as patients and healthy controls were not compared in this study. Similarly, the relative strength of the cumulative effect represented by the SNP score was also affected to a certain extent. Third, our participants were all Caucasian, so the results may not apply to other ethnic groups.
Despite these limitations, our study has some strengths. First, this was a multicenter study so had a large sample size. Large, well-characterized samples like ours are necessary to identify molecular causes of IBS and comorbid conditions[12]. Second, this study investigated the association between polymorphisms in HTR3 genes and comorbid psychosomatic symptoms for the first time. We conducted population stratification tests to ensure that the included populations were comparable. We also performed stratified analyses of sex and IBS subtypes and a more stringent multiple testing correction by FDR. Third, SNP scores have higher power and are better suited to testing multiple instead of single variants. This is useful because the pathogenesis of IBS is complex with multiple factors contributing to the manifestation of various subtypes. Also, individual genes may only play a minor role[12].
Clinical implications and further research
IBS is a complex condition. The continuous improvement of the allelic variation database for HTR3[25] and deep phenotyping combined with gene information (also in other datasets) may help to identify disease subgroups accurately and consistently, thereby facilitating future treatment[33,63]. This will be an important step towards standardization and unification of IBS genetic research strategies.
CONCLUSION
Our results provide the first evidence that the accumulation of HTR3 SNPs (reflected by the SNP score computed by HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A) may play a role in the pathophysiology of depressive and anxiety symptoms in IBS. This study has revealed that depressive and anxiety symptoms significantly worsened from the major to the minor allele of HTR3C c.489C>A in the dominant model and an increasing number of minor alleles are linked to more severe depressive symptoms in IBS.
ARTICLE HIGHLIGHTS
Research background
Over the past decades, genetic evidence on the key players within the serotonergic system including the serotonin type 3 (5-HT3) receptor subunit genes (HTR3) accumulated showing association with irritable bowel syndrome (IBS) as well as mental illnesses. However, it has never been explored whether associations of the single-nucleotide polymorphisms (SNPs) of HTR3 genes to depressive and anxiety symptoms can be replicated within IBS.
Research motivation
In order to address this knowledge gap, This multicenter observational study focused on a large IBS patient cohort comprising 768 participants from centers in Germany, Sweden, the United States, the United Kingdom, and Ireland.
Research objectives
The objectives are: (1) To explore the associations between functional HTR3 polymorphisms and psychosomatic burden within an IBS population; (2) To investigate the impact of the HTR3 SNP score on psychosomatic burden, based on our hypothesis that the observed number of minor alleles was associated with specific mental characteristics in IBS patients; and (3) To perform a functional analysis of variant 5-HT3AC receptors.
Research methods
In this retrospective study, 623 participants with IBS were recruited from five specialty centers in Germany, Sweden, the United States, the United Kingdom, and Ireland. Depressive, anxiety, and somatization symptoms and sociodemographic characteristics were collected. Four functional SNPs — HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A — were genotyped and analyzed using the dominant and recessive models. We also performed separate analyses for sex and IBS subtypes. SNP scores were calculated as the number of minor alleles of the SNPs above. The impact of HTR3C c.489C>A was tested by radioligand-binding and calcium influx assays.
Research results
Bringing together high quality data as well as methodological expertise, our results show that: (1) In the dominant model, HTR3C c.489C>A was correlated with depressive and anxiety symptoms in IBS; (2) A higher number of minor alleles (i.e., the higher the SNP score, which was computed by combining the individual SNP status of HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A) was linked to more severe depressive symptoms in IBS; and (3) The potential relevance of the HTR3C SNP was corroborated in functional assays showing changes in the expression level of 5-HT3AC variant receptors.
Research conclusions
Our results provide the first evidence that the accumulation of HTR3 SNPs (reflected by the SNP score computed by HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A) may play a role in the pathophysiology of depressive and anxiety symptoms in IBS.
Research perspectives
We are confident that these results are of interest to your readership, as they contribute substantially to update current knowledge regarding the role of accumulation of HTR3 SNPs in depressive and anxiety symptoms in IBS patients. In turn, our data will contribute towards standardization and harmonization of genetic research strategies in IBS.
ACKNOWLEDGEMENTS
We would like to thank all patients for their participation in this study and the supporting staff at each site. We acknowledge the kind support of Bartram CR and Hinderhofer K. We would also thank Startt B for proofreading and Bacon C for editing the manuscript. This manuscript results in part from collaboration and network activities promoted under the frame of the international network GENIEUR (Genes in Irritable Bowel Syndrome Research Network Europe), which has been funded by the COST program (BM1106, www.GENIEUR.eu) and is currently supported by the European Society of Neurogastroenterology and Motility (ESNM, www.ESNM.eu).
Footnotes
Institutional review board statement: The study was reviewed and approved by the Ethical Committee, Medical Faculty of the Heidelberg University Hospital (approval No. S067/2010).
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: APC Microbiome Ireland has conducted studies in collaboration with several companies, including GSK, Pfizer, Cremo, Suntory, Wyeth, Mead Johnson, Nutricia, 4D Pharma, and DuPont. Dinan TG has been an invited speaker at meetings organized by Servier, Lundbeck, Janssen, and AstraZeneca and has received research funding from Mead Johnson, Cremo, Suntory Wellness, Nutricia, and 4D Pharma. Clarke G has been an invited speaker at meetings organized by Janssen and is receipt of research funding from Pharmavite. The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: July 19, 2021
First decision: August 9, 2021
Article in press: April 22, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yin ZT, China S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
Contributor Information
Sabrina Berens, Department of General Internal Medicine and Psychosomatics, University Hospital Heidelberg, Heidelberg 69120, Germany.
Yuanjun Dong, Department of Human Molecular Genetics, Institute of Human Genetics, University of Heidelberg, Heidelberg 69120, Germany; Department of General Internal Medicine and Psychosomatics, Internal Medicine II, University Hospital Heidelberg, Heidelberg 69120, Germany.
Nikola Fritz, Department of Human Molecular Genetics, Institute of Human Genetics, University of Heidelberg, Heidelberg 69120, Germany.
Jutta Walstab, Department of Human Molecular Genetics, University of Heidelberg, Heidelberg 69120, Germany.
Mauro D'Amato, Gastrointestinal Genetics Lab, CIC bioGUNE - BRTA, Derio 48160, Spain; IKERBASQUE, Basque Foundation for Science, Bilbao 48001, Spain; Unit of Clinical Epidemiology, Department of Medicine Solna, Karolinska Institutet, Stockholm 17177, Sweden.
Tenghao Zheng, Unit of Clinical Epidemiology, Department of Medicine Solna, Karolinska Institutet, Stockholm 17177, Sweden.
Verena Wahl, Department of Human Molecular Genetics, Institute of Human Genetics, University of Heidelberg, Heidelberg 69120, Germany.
Felix Boekstegers, Institute of Medical Biometry and Informatics, Heidelberg University, Heidelberg 69120, Germany.
Justo Lorenzo Bermejo, Institute of Medical Biometry and Informatics, Heidelberg University, Heidelberg 69120, Germany.
Cristina Martinez, Department of Human Molecular Genetics, Institute of Human Genetics, University of Heidelberg, Heidelberg 69120, Germany; Lleida Institute for Biomedical Research Dr. Pifarré Foundation (IRBLleida), Av. Alcalde Rovira Roure, Lleida 25198, Spain.
Stefanie Schmitteckert, Department of Human Molecular Genetics, Institute of Human Genetics, University of Heidelberg, Heidelberg 69120, Germany.
Egbert Clevers, Department of Clinical and Experimental Medicine, Translational Research Center for Gastrointestinal Disorders, KU Leuven, Leuven 3000, Belgium.
Felicitas Engel, Department of General Internal Medicine and Psychosomatics, Internal Medicine II, University Hospital Heidelberg, Heidelberg 69120, Germany.
Annika Gauss, Department of Gastroenterology, Infectious Diseases and Intoxications, University of Heidelberg, Heidelberg 69120, Germany.
Wolfgang Herzog, Department of General Internal Medicine and Psychosomatics, Heidelberg University, Heidelberg 69120, Germany.
Robin Spiller, Nottingham Digestive Diseases Centre, University of Nottingham, Nottingham NG7 2QL, United Kingdom.
Miriam Goebel-Stengel, Helios Klinikum Rottweil, Rottweil 78628, Germany.
Hubert Mönnikes, Department of Medicine, Institute of Neurogastroenterology (H.M.), Martin-Luther-Hospital, Belin 14193, Germany.
Viola Andresen, Israelitisches Krankenhaus in Hamburg, Hamburg 22297, Germany.
Frieling Thomas, Internal Medicine II, Helios Klinikum Krefeld, Krefeld 47805, Germany.
Jutta Keller, Israelitisches Krankenhaus Hamburg, Hamburg 22297, Ghana.
Christian Pehl, Krankenhaus Vilsbiburg, Vilsbiburg 84137, Germany.
Christoph Stein-Thöringer, Division of Microbiome and Cancer, German Cancer Research Center (DKFZ), Heidelberg 69120, Germany.
Gerard Clarke, Department of Psychiatry and Neurobehavioral Science, University College Cork, Cork T23, Ireland.
Timothy G Dinan, Department of Psychiatry and Neurobehavioral Science, University College Cork, Cork T23, Ireland.
Eamonn M Quigley, Medicine in Digestive Disorders, Department of Medicine, Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist, Houston, TX 77030, United States.
Gregory Sayuk, Division of Gastroenterology, Washington University School of Medicine, Department of Psychiatry, School of Medicine, John Cochran Veteran Affairs Medical Center, St. Louis, MO 63110, United States.
Magnus Simrén, Department of Internal Medicine, Section of Gastroenterology and Hepatology, Sahlgrenska University Hospital, Gothenburg SE-41685, Sweden.
Jonas Tesarz, Department of General Internal Medicine and Psychosomatics, Internal Medicine II, University Hospital Heidelberg, Heidelberg 69120, Germany.
Gudrun Rappold, Department of Human Molecular Genetics, Institute of Human Genetics, University of Heidelberg, Heidelberg 69120, Germany; Interdisciplinary Center for Neurosciences (IZN), University of Heidelberg, Heidelberg 69120, Germany.
Lukas van Oudenhove, Cognitive and Affective Neuroscience Lab, Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH 03748, United States; Laboratory for Brain-Gut Axis Studies, Translational Research Center for Gastrointestinal Disorders, Department of Chronic Diseases, Metabolism, and Ageing, KU Leuven, Leuven 3000, Belgium.
Rainer Schaefert, Department of General Internal Medicine and Psychosomatics, Internal Medicine II, University Hospital Heidelberg, Heidelberg 69120, Germany; Department of Psychosomatic Medicine, Division of Internal Medicine, University Hospital Basel, Basel CH-4031, Switzerland.
Beate Niesler, Interdisciplinary Center for Neurosciences (IZN), University of Heidelberg, Heidelberg 69120, Germany; Department of Human Molecular Genetics, Heidelberg University, Heidelberg 69120, Germany. beate.niesler@med.uni-heidelberg.de.
Data sharing statement
No additional data are available.
References
- 1.Layer P, Andresen V, Pehl C, Allescher H, Bischoff SC, Classen M, Enck P, Frieling T, Haag S, Holtmann G, Karaus M, Kathemann S, Keller J, Kuhlbusch-Zicklam R, Kruis W, Langhorst J, Matthes H, Mönnikes H, Müller-Lissner S, Musial F, Otto B, Rosenberger C, Schemann M, van der Voort I, Dathe K, Preiss JC Deutschen Gesellschaft für Verdauungs- und Stoffwechselkrankheiten; Deutschen Gesellschaft für Neurogastroenterologie und Motilität. Irritable bowel syndrome: German consensus guidelines on definition, pathophysiology and management. Z Gastroenterol. 2011;49:237–293. doi: 10.1055/s-0029-1245976. [DOI] [PubMed] [Google Scholar]
- 2.Dong Y, Baumeister D, Berens S, Eich W, Tesarz J. Stressful Life Events Moderate the Relationship Between Changes in Symptom Severity and Health-related Quality of Life in Patients With Irritable Bowel Syndrome. J Clin Gastroenterol. 2020;54:445–451. doi: 10.1097/MCG.0000000000001261. [DOI] [PubMed] [Google Scholar]
- 3.Palsson OS, Whitehead W, Törnblom H, Sperber AD, Simren M. Prevalence of Rome IV Functional Bowel Disorders Among Adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020;158:1262–1273.e3. doi: 10.1053/j.gastro.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Black CJ, Yiannakou Y, Houghton LA, Ford AC. Epidemiological, Clinical, and Psychological Characteristics of Individuals with Self-reported Irritable Bowel Syndrome Based on the Rome IV vs Rome III Criteria. Clin Gastroenterol Hepatol. 2020;18:392–398.e2. doi: 10.1016/j.cgh.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Stasi C, Caserta A, Nisita C, Cortopassi S, Fani B, Salvadori S, Pancetti A, Bertani L, Gambaccini D, de Bortoli N, Dell'Osso L, Blandizzi C, Marchi S, Bellini M. The complex interplay between gastrointestinal and psychiatric symptoms in irritable bowel syndrome: A longitudinal assessment. J Gastroenterol Hepatol. 2019;34:713–719. doi: 10.1111/jgh.14375. [DOI] [PubMed] [Google Scholar]
- 7.Melchior C, Desprez C, Riachi G, Leroi AM, Déchelotte P, Achamrah N, Ducrotté P, Tavolacci MP, Gourcerol G. Anxiety and Depression Profile Is Associated With Eating Disorders in Patients With Irritable Bowel Syndrome. Front Psychiatry. 2019;10:928. doi: 10.3389/fpsyt.2019.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg DS, Smalley W, Heidelbaugh JJ, Sultan S Amercian Gastroenterological Association. American Gastroenterological Association Institute Guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147:1146–1148. doi: 10.1053/j.gastro.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Simpson CA, Mu A, Haslam N, Schwartz OS, Simmons JG. Feeling down? J Affect Disord. 2020;266:429–446. doi: 10.1016/j.jad.2020.01.124. [DOI] [PubMed] [Google Scholar]
- 10.Gros DF, Antony MM, McCabe RE, Swinson RP. Frequency and severity of the symptoms of irritable bowel syndrome across the anxiety disorders and depression. J Anxiety Disord. 2009;23:290–296. doi: 10.1016/j.janxdis.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Black CJ, Yiannakou Y, Houghton LA, Shuweihdi F, West R, Guthrie E, Ford AC. Anxiety-related factors associated with symptom severity in irritable bowel syndrome. Neurogastroenterol Motil. 2020;32:e13872. doi: 10.1111/nmo.13872. [DOI] [PubMed] [Google Scholar]
- 12.Gazouli M, Wouters MM, Kapur-Pojskić L, Bengtson MB, Friedman E, Nikčević G, Demetriou CA, Mulak A, Santos J, Niesler B. Lessons learned--resolving the enigma of genetic factors in IBS. Nat Rev Gastroenterol Hepatol. 2016;13:77–87. doi: 10.1038/nrgastro.2015.206. [DOI] [PubMed] [Google Scholar]
- 13.Zhang WX, Zhang Y, Qin G, Li KM, Wei W, Li SY, Yao SK. Altered profiles of fecal metabolites correlate with visceral hypersensitivity and may contribute to symptom severity of diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2019;25:6416–6429. doi: 10.3748/wjg.v25.i43.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20:6024–6030. doi: 10.3748/wjg.v20.i20.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56:479–485. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 17.Painsipp E, Shahbazian A, Holzer P. Alosetron, cilansetron and tegaserod modify mesenteric but not colonic blood flow in rats. Br J Pharmacol. 2009;158:1210–1226. doi: 10.1111/j.1476-5381.2009.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trump D. Commentary on: "Ipilimumab vs placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial." Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengeløv L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR, CA184-043 Investigators. Departments of Urology and Immunology and Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN, USA, Electronic address: kwon.eugene@mayo.edu; Johns Hopkins Sidney Kimmel Comprehensive Cancer Center and Brady Urological Institute, Baltimore, MD, USA; Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York, NY, USA; Institut Gustave Roussy, University of Paris-Sud, Villejuif, France; Institut Gustave Roussy, Villejuif, France; VU University Medical Centre, Amsterdam, Netherlands; Vienna General Hospital, Medical University Vienna, Vienna, Austria; Institut Bergonié, Bordeaux, France; CHU Caremeau, Nimes, France; Centro Médico Austral, Buenos Aires, Argentina; Centre Jean Perrin, Clermont-Ferrand, France; St John of God Hospital, Subiaco, WA, Australia; University Hospital of Siena, Istituto Toscano Tumori, Siena, Italy; Hospital de Caridade de Ijuí, Ijuí, Brazil; Nottingham University Hospital, Nottingham, UK; Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA; Netherlands Cancer Institute and Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands; Institute of Oncology Ion Chiricuta and University of Medicine and Pharmacy Iuliu Hatieganu, Cluj-Napoca, Romania; Hospital Británico de Buenos Aires, Buenos Aires, Argentina; Herlev Hospital, Herlev, Denmark; Odense University Hospital, Odense, Denmark; University of Texas MD Anderson Cancer Center, Houston, Urol Oncol. 2016;34:249–250. [Google Scholar]
- 19.Walstab J, Rappold G, Niesler B. 5-HT(3) receptors: role in disease and target of drugs. Pharmacol Ther. 2010;128:146–169. doi: 10.1016/j.pharmthera.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Fukudo S, Ida M, Akiho H, Nakashima Y, Matsueda K. Effect of ramosetron on stool consistency in male patients with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2014;12:953–959.e4. doi: 10.1016/j.cgh.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, Henry A, Hall I, Whorwell P, Spiller R. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617–1625. doi: 10.1136/gutjnl-2013-305989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiller RC. Targeting the 5-HT(3) receptor in the treatment of irritable bowel syndrome. Curr Opin Pharmacol. 2011;11:68–74. doi: 10.1016/j.coph.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Grzesiak M, Beszłej JA, Waszczuk E, Szechiński M, Szewczuk-Bogusławska M, Frydecka D, Dobosz T, Jonkisz A, Lebioda A, Małodobra M, Mulak A. Serotonin-Related Gene Variants in Patients with Irritable Bowel Syndrome and Depressive or Anxiety Disorders. Gastroenterol Res Pract. 2017;2017:4290430. doi: 10.1155/2017/4290430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niesler B, Walstab J, Combrink S, Möller D, Kapeller J, Rietdorf J, Bönisch H, Göthert M, Rappold G, Brüss M. Characterization of the novel human serotonin receptor subunits 5-HT3C,5-HT3D, and 5-HT3E. Mol Pharmacol. 2007;72:8–17. doi: 10.1124/mol.106.032144. [DOI] [PubMed] [Google Scholar]
- 25.Celli J, Rappold G, Niesler B. The Human Serotonin Type 3 Receptor Gene (HTR3A-E) Allelic Variant Database. Hum Mutat. 2017;38:137–147. doi: 10.1002/humu.23136. [DOI] [PubMed] [Google Scholar]
- 26.Guan T, Li T, Cai W, Huang D, Ouyang P, Wang Y, Chen H, Wu K, Ma X. HTR3A and HTR3E gene polymorphisms and diarrhea predominant irritable bowel syndrome risk: evidence from a meta-analysis. Oncotarget. 2017;8:100459–100468. doi: 10.18632/oncotarget.19682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melke J, Westberg L, Nilsson S, Landen M, Soderstrom H, Baghaei F, Rosmond R, Holm G, Björntorp P, Nilsson LG, Adolfsson R, Eriksson E. A polymorphism in the serotonin receptor 3A (HTR3A) gene and its association with harm avoidance in women. Arch Gen Psychiatry. 2003;60:1017–1023. doi: 10.1001/archpsyc.60.10.1017. [DOI] [PubMed] [Google Scholar]
- 28.Niesler B, Weiss B, Fischer C, Nöthen MM, Propping P, Bondy B, Rietschel M, Maier W, Albus M, Franzek E, Rappold GA. Serotonin receptor gene HTR3A variants in schizophrenic and bipolar affective patients. Pharmacogenetics. 2001;11:21–27. doi: 10.1097/00008571-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Kilpatrick LA, Labus JS, Coveleskie K, Hammer C, Rappold G, Tillisch K, Bueller JA, Suyenobu B, Jarcho JM, McRoberts JA, Niesler B, Mayer EA. The HTR3A polymorphism c. -42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology. 2011;140:1943–1951. doi: 10.1053/j.gastro.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada K, Hattori E, Iwayama Y, Ohnishi T, Ohba H, Toyota T, Takao H, Minabe Y, Nakatani N, Higuchi T, Detera-Wadleigh SD, Yoshikawa T. Distinguishable haplotype blocks in the HTR3A and HTR3B region in the Japanese reveal evidence of association of HTR3B with female major depression. Biol Psychiatry. 2006;60:192–201. doi: 10.1016/j.biopsych.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Berens S, Kraus F, Gauss A, Tesarz J, Herzog W, Niesler B, Stroe-Kunold E, Schaefert R. A Specialty Clinic for Functional Gastrointestinal Disorders in Tertiary Care: Concept and Patient Population. Clin Gastroenterol Hepatol. 2017;15:1127–1129. doi: 10.1016/j.cgh.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 32.Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, Gassler N, Fischer C, Whorwell PJ, Atkinson W, Fell C, Büchner KJ, Schmidtmann M, van der Voort I, Wisser AS, Berg T, Rappold G, Niesler B. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–2977. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 33.Boeckxstaens GE, Drug V, Dumitrascu D, Farmer AD, Hammer J, Hausken T, Niesler B, Pohl D, Pojskic L, Polster A, Simren M, Goebel-Stengel M, Van Oudenhove L, Vassallo M, Wensaas KA, Aziz Q, Houghton LA COST Action BM1106 GENIEUR members. Phenotyping of subjects for large scale studies on patients with IBS. Neurogastroenterol Motil. 2016;28:1134–1147. doi: 10.1111/nmo.12886. [DOI] [PubMed] [Google Scholar]
- 34.Dong Y, Baumeister D, Berens S, Eich W, Tesarz J. High Rates of Non-Response Across Treatment Attempts in Chronic Irritable Bowel Syndrome: Results From a Follow-Up Study in Tertiary Care. Front Psychiatry. 2019;10:714. doi: 10.3389/fpsyt.2019.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509–515. [Google Scholar]
- 37.Berens S, Schaefert R, Baumeister D, Gauss A, Eich W, Tesarz J. Does symptom activity explain psychological differences in patients with irritable bowel syndrome and inflammatory bowel disease? J Psychosom Res. 2019;126:109836. doi: 10.1016/j.jpsychores.2019.109836. [DOI] [PubMed] [Google Scholar]
- 38.Montazeri A, Vahdaninia M, Ebrahimi M, Jarvandi S. The Hospital Anxiety and Depression Scale (HADS): translation and validation study of the Iranian version. Health Qual Life Outcomes. 2003;1:14. doi: 10.1186/1477-7525-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong KM, Mak ADP, Yuen SY, Leung ONW, Ma DY, Chan Y, Cheong PK, Lui R, Wong SH, Wu JC. Nature and specificity of altered cognitive functioning in IBS. Neurogastroenterol Motil. 2019;31:e13696. doi: 10.1111/nmo.13696. [DOI] [PubMed] [Google Scholar]
- 40.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 41.Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A) Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S467–S472. doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15:97–98. doi: 10.1038/sj.cr.7290272. [DOI] [PubMed] [Google Scholar]
- 44.Nelson SC, Romm JM, Doheny KF, Pugh EW, Laurie CC. Imputation-based genomic coverage assessments of current genotyping arrays: Illumina HumanCore, OmniExpress, Multi-Ethnic global array and sub-arrays, Global Screening Array, Omni2. 5M, Omni5M, and Affymetrix UK Biobank. 2017 Preprint. Available from: bioRxiv:150219.
- 45.Bonfiglio F, Henström M, Nag A, Hadizadeh F, Zheng T, Cenit MC, Tigchelaar E, Williams F, Reznichenko A, Ek WE, Rivera NV, Homuth G, Aghdassi AA, Kacprowski T, Männikkö M, Karhunen V, Bujanda L, Rafter J, Wijmenga C, Ronkainen J, Hysi P, Zhernakova A, D'Amato M. A GWAS meta-analysis from 5 population-based cohorts implicates ion channel genes in the pathogenesis of irritable bowel syndrome. Neurogastroenterol Motil. 2018;30:e13358. doi: 10.1111/nmo.13358. [DOI] [PubMed] [Google Scholar]
- 46.Marees AT, de Kluiver H, Stringer S, Vorspan F, Curis E, Marie-Claire C, Derks EM. A tutorial on conducting genome-wide association studies: Quality control and statistical analysis. Int J Methods Psychiatr Res. 2018;27:e1608. doi: 10.1002/mpr.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 48.Zhu L, Huang D, Shi L, Liang L, Xu T, Chang M, Chen W, Wu D, Zhang F, Fang X. Intestinal symptoms and psychological factors jointly affect quality of life of patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes. 2015;13:49. doi: 10.1186/s12955-015-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simrén M, Ringström G, Björnsson ES, Abrahamsson H. Treatment with hypnotherapy reduces the sensory and motor component of the gastrocolonic response in irritable bowel syndrome. Psychosom Med. 2004;66:233–238. doi: 10.1097/01.psy.0000116964.76529.6e. [DOI] [PubMed] [Google Scholar]
- 50.Niesler B, Flohr T, Nöthen MM, Fischer C, Rietschel M, Franzek E, Albus M, Propping P, Rappold GA. Association between the 5' UTR variant C178T of the serotonin receptor gene HTR3A and bipolar affective disorder. Pharmacogenetics. 2001;11:471–475. doi: 10.1097/00008571-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Iidaka T, Ozaki N, Matsumoto A, Nogawa J, Kinoshita Y, Suzuki T, Iwata N, Yamamoto Y, Okada T, Sadato N. A variant C178T in the regulatory region of the serotonin receptor gene HTR3A modulates neural activation in the human amygdala. J Neurosci. 2005;25:6460–6466. doi: 10.1523/JNEUROSCI.5261-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gatt JM, Williams LM, Schofield PR, Dobson-Stone C, Paul RH, Grieve SM, Clark CR, Gordon E, Nemeroff CB. Impact of the HTR3A gene with early life trauma on emotional brain networks and depressed mood. Depress Anxiety. 2010;27:752–759. doi: 10.1002/da.20726. [DOI] [PubMed] [Google Scholar]
- 53.Gatt JM, Nemeroff CB, Schofield PR, Paul RH, Clark CR, Gordon E, Williams LM. Early life stress combined with serotonin 3A receptor and brain-derived neurotrophic factor valine 66 to methionine genotypes impacts emotional brain and arousal correlates of risk for depression. Biol Psychiatry. 2010;68:818–824. doi: 10.1016/j.biopsych.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 54.Hammer C, Cichon S, Mühleisen TW, Haenisch B, Degenhardt F, Mattheisen M, Breuer R, Witt SH, Strohmaier J, Oruc L, Rivas F, Babadjanova G, Grigoroiu-Serbanescu M, Hauser J, Röth R, Rappold G, Rietschel M, Nöthen MM, Niesler B. Replication of functional serotonin receptor type 3A and B variants in bipolar affective disorder: a European multicenter study. Transl Psychiatry. 2012;2:e103. doi: 10.1038/tp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frank B, Niesler B, Nöthen MM, Neidt H, Propping P, Bondy B, Rietschel M, Maier W, Albus M, Rappold G. Investigation of the human serotonin receptor gene HTR3B in bipolar affective and schizophrenic patients. Am J Med Genet B Neuropsychiatr Genet. 2004;131B:1–5. doi: 10.1002/ajmg.b.30070. [DOI] [PubMed] [Google Scholar]
- 56.Horjales-Araujo E, Demontis D, Lund EK, Finnerup NB, Børglum AD, Jensen TS, Svensson P, Vase L. Polymorphism in serotonin receptor 3B is associated with pain catastrophizing. PLoS One. 2013;8:e78889. doi: 10.1371/journal.pone.0078889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapeller J, Houghton LA, Walstab J, Boenisch H, Rappold G, Niesler B. 1003 A Coding Variant in the Serotonin Receptor 3C Subunit Is Associated with Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology. 2009;136:A–155. [Google Scholar]
- 58.Niesler B, Frank B, Kapeller J, Rappold GA. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene. 2003;310:101–111. doi: 10.1016/s0378-1119(03)00503-1. [DOI] [PubMed] [Google Scholar]
- 59.Karnovsky AM, Gotow LF, McKinley DD, Piechan JL, Ruble CL, Mills CJ, Schellin KA, Slightom JL, Fitzgerald LR, Benjamin CW, Roberds SL. A cluster of novel serotonin receptor 3-like genes on human chromosome 3. Gene. 2003;319:137–148. doi: 10.1016/s0378-1119(03)00803-5. [DOI] [PubMed] [Google Scholar]
- 60.Maxwell SE, Delaney HD. Measurement and Statistics - an Examination of Construct-Validity. Psychol Bull. 1985;97:85–93. [Google Scholar]
- 61.Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12:592–605. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yaakob NS, Nguyen DT, Exintaris B, Irving HR. The C and E subunits of the serotonin 5-HT3 receptor subtly modulate electrical properties of the receptor. Biomed Pharmacother. 2018;97:1701–1709. doi: 10.1016/j.biopha.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Katsnelson A. Diagnostics: Filling in the missing pieces. Nature. 2016;533:S110–S111. doi: 10.1038/533S110a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.