Abstract
BACKGROUND
In the contemporary era of cancer immunotherapy, an abundance of clinical and translational studies have reported radiotherapy (RT) and immunotherapies as a viable option for immunomodulation of many cancer subtypes, with many related clinical trials ongoing. In locally advanced disease, chemotherapy or chemoradiotherapy followed by surgical excision of the tumour remain the principal treatment strategy in oesophageal adenocarcinoma (OAC), however, the use of the host immune system to improve anti-tumour immunity is rapidly garnering increased support in the curative setting.
AIM
To immunophenotype OAC patients’ immune checkpoint (IC) expression with and without radiation and evaluate the effects of checkpoint blockade on cell viability.
METHODS
In the contemporary era of cancer immunotherapy, an abundance of studies have demonstrated that combination RT and IC inhibitors (ICIs) are effective in the immunomodulation of many cancer subtypes, with many related clinical trials ongoing. Although surgical excision and elimination of tumour cells by chemotherapy or chemoradiotherapy remains the gold standard approach in OAC, the propagation of anti-tumour immune responses is rapidly garnering increased support in the curative setting. The aim of this body of work was to immunophenotype OAC patients’ IC expression with and without radiation and to establish the impact of checkpoint blockade on cell viability. This study was a hybrid combination of in vitro and ex vivo models. Quantification of serum immune proteins was performed by enzyme-linked immunosorbent assay. Flow cytometry staining was performed to evaluate IC expression for in vitro OAC cell lines and ex vivo OAC biopsies. Cell viability in the presence of radiation with and without IC blockade was assessed by a cell counting kit-8 assay.
RESULTS
We identified that conventional dosing and hypofractionated approaches resulted in increased IC expression (PD-1, PD-L1, TIM3, TIGIT) in vitro and ex vivo in OAC. There were two distinct subcohorts with one demonstrating significant upregulation of ICs and the contrary in the other cohort. Increasing IC expression post RT was associated with a more aggressive tumour phenotype and adverse features of tumour biology. The use of anti-PD-1 and anti-PD-L1 immunotherapies in combination with radiation resulted in a significant and synergistic reduction in viability of both radiosensitive and radioresistant OAC cells in vitro. Interleukin-21 (IL-21) and IL-31 significantly increased, with a concomitant reduction in IL-23 as a consequence of 4 Gray radiation. Similarly, radiation induced an anti-angiogenic tumour milieu with reduced expression of vascular endothelial growth factor-A, basic fibroblast growth factor, Flt-1 and placental growth factor.
CONCLUSION
The findings of the current study demonstrate synergistic potential for the use of ICIs and ionising radiation to potentiate established anti-tumour responses in the neoadjuvant setting and is of particular interest in those with advanced disease, adverse features of tumour biology and poor treatment responses to conventional therapies.
Keywords: Oesophageal Cancer, Radiotherapy, Immunotherapy, Immunology, Surgery, Oncology
Core Tip: This body of work evaluates the impact of radiotherapy on the immune profile in oesophageal adenocarcinoma with an added caveat of immunotherapy effects on tumour cell killing.
INTRODUCTION
Oesophageal adenocarcinoma (OAC) is rapidly increasing in incidence in the western world, and five year survival rates rarely exceed 40%[1]. Multimodal therapy alongside surgical resection has become standard of care for locally advanced cancer of the oesophagus or the oesophagogastric junction[2]. One option is the CROSS regimen, which includes preoperative administration of carboplatin and paclitaxel with concomitant radiotherapy (RT)[3]. In Europe, radiation is delivered in 23 fractions of 1.8 Gray (Gy), giving a total dose of 41.4 Gy but this varies worldwide, with North American centres delivering up to 50.0-51.4 Gy[4], while Asian regimens can feature cumulative doses of 60 Gy[5].
Hypofractionated RT is where radiation is delivered in fewer fractions of 2.4 Gy to 5.0 Gy, but often the same cumulative dose[6]. This has the potential to reduce costs, increase patient comfort, and could be more effective compared to conventional treatment[7]. Randomised trials in breast and prostate cancer have found that both high- (≥ 5 Gy per fraction) and moderately (2.4-3.4 Gy per fraction) hypo-fractionated RT is non-inferior to traditional regimens[7-10]. As RT is a mainstay of treatment, and oesophageal malignancies are associated with considerable morbidity, there is interest in evaluating whether this paradigm can be applied in the upper gastrointestinal context.
Disappointingly, a pathologic complete response to treatment is observed in less than 30% of patients with oesophageal cancer undergoing chemoradiotherapy[11], and it is this small subgroup that benefits most in terms of survival[12]. More effective strategies are therefore required. One emerging approach is combining chemoradiotherapy with immune checkpoint blockade (ICB). The most widely used ICB involves blocking the interaction of PD-1 expressed on T cells and it’s ligand, PD-L1 expressed on tumour cells[13], and seeks to re-invigorate anti-tumour cytotoxic T cells[14]. Phase III trials of single agent ICB have delivered mixed results in chemorefractory advanced oesophagogastric cancer[15], but some recent encouraging results have been reported in earlier stage disease[16,17].
Radiation can sensitise tumours to immunotherapy through three main mechanisms[18]. First, radiation can increase neoantigen expression and induce immunogenic cell death, whereby release of damage associated molecular patterns (DAMPs) results in more efficient tumour antigen presentation and immune stimulation[19]. Second, radiation induced DNA damage can activate the GMP-AMP stimulator of interferon genes (cGAS-STING) cytosolic DNA sensor, resulting in type I interferon production[20,21]. Finally, RT can result in remodeling of the tumour microenvironment (TME), promoting infiltration of immune cells[22]. The latter effect is particularly affected by radiation dosage and some limited preclinical evidence suggests that hypofractionated RT can have more immunostimulatory effects than conventional fractionation[18]. However, the majority of studies in the literature to date have focused on more inherently immunogenic tumour models like melanoma or non-small cell lung cancer, or common malignancies like breast or colon cancer. There are a number of clinical studies evaluating hypofractionation in the context of squamous cell cancer of the oesophagus, however, data is lacking for OAC. In addition, there are no translational studies characterising immune response in OAC in the context of immunotherapy and thus was the premise for this study.
This study assessed the effects of hypofractionated RT on IC expression in oesophageal cancer cells in vitro and ex vivo and correlated this with clinical outcomes. We also assessed the synergistic effects of ICB and radiation on OAC cell lines. Through this, we aimed to enhance our understanding of the interplay between immunotherapy, radiation and the TME in oesophageal cancer, with the goal of identifying the most effective radiation dosing strategy to combine with immunotherapy.
MATERIALS AND METHODS
Ethics statement
We secured ethical approval for this study from the Tallaght/St James’s Hospital Ethics Committee. All patients provided formal written consent for all sample and variable data collection. During the course of all steps of sample and data collection good clinical practice was maintained and ethical standards upheld. We also pseudonymised patient data to protect privacy.
Specimen collection
We secured tissue from those patients who consented to participate from 2018-2021. Tumour biopsies were obtained from patients with OAC prior to treatment at the National Centre for Oesophageal and Gastric Cancer at St James’s Hospital, Dublin. A total of 17 biopsies were used for analysis with all patient samples being treatment naïve prior to having neoadjuvant therapies to ensure clinical relevance of the study population. A total of 12 men and 5 women with a mean age of 64.23 years (SD11.5) in the study. All patients had locally advanced disease and were T3Nany.
Generation of tumour conditioned media
Tumour treatment naïve tissue samples were added to L-15 (Leibovitz) LonzaTM BioWhittakerTM X-vivo media for in a 12 well plate and subsequently cultured for a period of 24 h at 37 °C, 5% CO2. After the 24 h period expired, the tissue conditioned media was collected for storage at -80 °C.
Quantification of serum immune proteins
Tumour conditioned media (TCM) was collected based on a standard operating procedure designed as per MSD United States instructions (Meso Scale Diagnostics, United States). To assess markers of angiogenesis, vascular injury, pro-inflammatory, cytokines, chemokine as well as soluble checkpoints from TCM, a 54-plex enzyme-linked immunosorbent assay (ELISA) kit was used (Meso Scale Diagnostics, United States). The ELISA was utilized to determine the level of secretions of the following markers: C-reactive protein (CRP), Eotaxin, Eotaxin-3, FGF (basic), Flt-1, GM-CSF, ICAM-1, IFN-γ, interleukin-10 (IL-10), IL-12/IL-23p40, IL-12p70, IL-13, IL-15, IL-16, IL-17A, IL-17A/F, IL-7, IL-8, MCP-1, MCP-4, MDC, MIP-1α, MIP-1β, MIP-3α, PlGF, SAA, TARC, Tie-2, TNF-α, TNF-β, TSLP, VCAM-1, vascular endothelial growth factor (VEGF)-A, VEGF-C and VEGF-D and ICs TIM-3, TIGIT, PD-1, PD-L1, CD276 and CD80 from TCM. These assays were processed according to a standard operating procedure following consultation of the manufacturer’s guidelines. The derived data with respect to all markers were normalised to protein content as determined using a Pierce bicinchoninic acid assay.
Neoplastic tissue sample digestion
In preparation for flow cytometry, the tissue samples were digested to enable phenotyping of the cancer cells. The tissue was resected using a surgical blade and added to collagenase solution (2 mg/mL of collagenase type IV (Sigma) in Hanks Balanced Salt Solution (GE healthcare) supplemented with 4% (v/v) foetal bovine serum) at 37 °C and 1500 rpm on an orbital shaker. The cancer cells were stained with flow cytometry antibodies for subsequent analysis.
Cell culture of OAC cell lines
Human OAC cell lines OE33 were purchased from The European Collection of Authenticated Cell Cultures (ECACC), established from a poorly differentiated stage IIA adenocarcinoma of the lower oesophagus of a 73-year old female patient. An in-house isogenic radioresistant model was generated[23].
Cell viability cholecystokinin octapeptide assay
A cell counting kit-8 (CCK-8) viability assay was used to determine the impact of ionising radiation on the viability of OE33P and passage matched OE33R cells. The impact of anti-PD-1, and anti PD-L1 therapies in isolation, and dual ICB with and without radiation, at both hypofractionation and bolus dosing of clinically relevant doses on the viability of OE33P and R cells was also assessed using a CCK-8 assay. OAC cells (5 × 103) were adhered in a 96 well plate at 37 °C, 5% CO2 overnight. Cells were treated with bolus dosing or three consecutive fractionated doses of radiation with an interval of 24 h using the X-Strahl RS225 irradiator. In addition to this, the cancer cells were treated with and without radiation in the absence or presence of pembrolizumab (10 μg/mL), atezolizumab (10 μg/mL), nivolumab (10 μg/mL) or combination atezolizumab (10 μg/mL) and nivolumab (10 μg/mL), or dual atezolizumab (10 μg/mL) and pembrolizumab (10 μg/mL). All of the data were analysed from three independent experiments (Supplementary material).
Flow cytometry staining for in vitro OAC cell lines and ex vivo OAC biopsies
OE33 cells were trypsinised and stained with zombie aqua viability (Biolegend, United States) dye. Antibodies used for OAC cell lines included: PD-L1-FITC, PD-L2-PE, TIGIT-PE/Cy7, PD-1-APC/Cy7 (Biolegend, United States), OE33P and OE33R cells were fixed with 1% paraformaldehyde solution and acquired using BD FACs CANTO II (BD Biosciences) using Diva software and analysed using FlowJo v10 software (TreeStar Inc.). Tumour tissue biopsies were stained with zombie aqua viability dye (Biolegend, United States) as per manufacturer’s recommendations.
Statistical analysis
GraphPad Prism 9 was utilized to analyze the results. In order to determine statistical differences between treatments in cell lines, a paired parametric statistical t-test was utilized. In order to determine the differences between the OE33P and OE33R cell lines an unpaired parametric t-test was performed. To evaluate any differences between paired treatments of patient samples, Wilcoxon signed rank test was performed. Statistical significance was pre-determined as P ≤ 0.05.
RESULTS
IC expression by an isogenic model of radioresistance following bolus and hypofractionated RT dosing
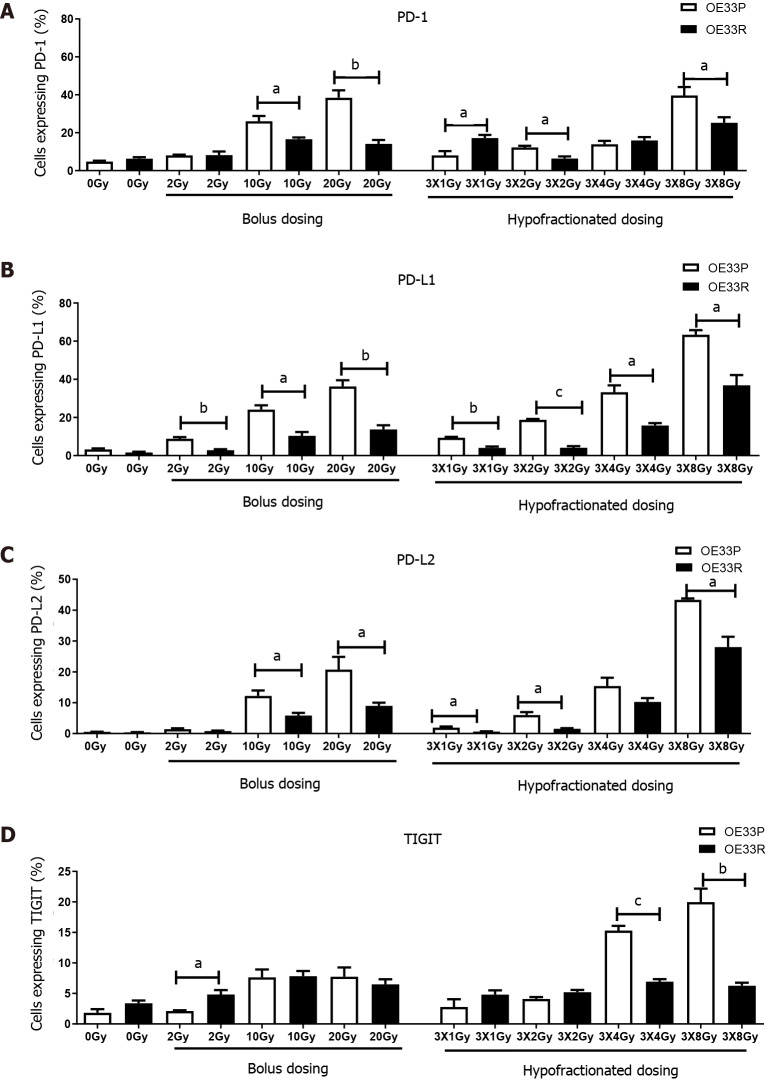
In order to ascertain if different expression levels of IC proteins were detectable on a radiosensitive (OE33P) and a radioresistant (OE33R) OAC cell line at baseline and following variable fractions of radiation, cells were stained with antibodies for a range of IC proteins and assessed by flow cytometry 24 h after the last dose. The administration of fractionated dosing resulted in significantly higher expression of PD-1, PD-L1, PD-L2 and TIGIT (P < 0.05) in both parental and resistant cell lines when compared to bolus dosing (Figure 1). There was a significantly higher expression of checkpoints and their ligands in the parental cell line compared to the passage matched radioresistant cell line. There was also a significantly higher expression of PD-1 and its ligands PD-L1 and PD-L2 with bolus dosing 10 Gy and 20 Gy in OE33P cell lines compared to the radioresistant passage matched cell line (P < 0.05). Globally there was a higher expression of PD-1, PD-L1 and PD-L2 on the parental cell line with fractionated dosing regimens of 3X1 Gy, 3X2 Gy and 3X8 Gy compared to the radioresistant cell line (P < 0.05). In the case of TIGIT, there was a significantly higher upregulation in the OE33P cell line compared to the radioresistant cell line following fractionated dosing of 3X4 Gy and 3X8 Gy (P < 0.05) (Figure 1).
Figure 1.
OE33P and R cell lines were screened for the surface expression of immune checkpoints by flow cytometry. Inhibitory immune checkpoints are expressed at a higher level on parental cell lines than the passage matched radioresistant cell line (n = 3). A: PD-1; B: PD-L1; C: PD-L2; D: TIGIT. Graph shows % expression (± SE). aP < 0.05; bP < 0.01; cP < 0.001 by unpaired parametric t-test.
Cell viability in the context of radiation and IC blockade
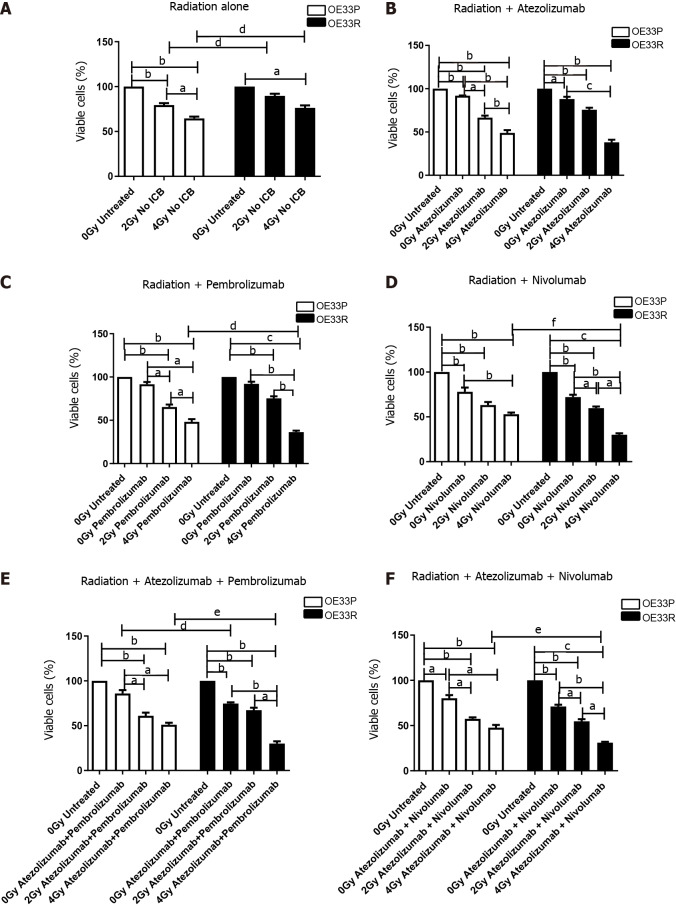
IC blockade alone reduced the viability of both OE33P and OE33R cell lines, for both anti-PD-1 and anti-PD-L1 therapies. Multimodal use of both anti-PD-1 and anti-PD-L1 therapies with ionising radiation resulted in a synergistic reduction in viability in both cell lines (Figure 2).
Figure 2.
Viability (± SE) of OE33P and OE33R cells were assessed using a cell counting kit-8 assay with or without radiation (n = 3). Ionising radiation with immune checkpoint blockade results in a greater reduction in cell viability when compared to either modality alone. Graph shows % expression (± SE). A: Treatment with radiation dosing only; B: Treatment with radiation and single agent immunotherapy Atezolizumab; C: Treatment with radiation and single agent immunotherapy Pembrolizumab; D: Treatment with radiation and single agent immunotherapy Nivolumab; E: Treatment with radiation and dual immunotherapy agents Atezolizumab & Pembrolizumab; F: Treatment with radiation and dual immunotherapy agents Atezolizumab & Nivolumab. aP < 0.05, bP < 0.01, cP < 0.001 paired t-test; dP < 0.05, eP < 0.01, fP < 0.001 unpaired t-test.
In the OE33P cell line, 2 Gy radiation alone reduced viability to 78.49% (± 2.05, P < 0.01) and 4 Gy to 35.48% (± 2.08, P < 0.01) compared with unirradiated cells and there was a significant reduction in viability when comparing 2 Gy to 4 Gy (P < 0.05). In the OE33R cell line, 4 Gy reduced viability to 63.33% (± 2.67, P < 0.05). Both 2 Gy and 4 Gy radiation resulted in a significantly greater reduction in viability in the OE33P cell line compared to the OE33R (P < 0.05) (Figure 2).
Compared with untreated cells, when the OE33P cells were treated with Atezolizumab alone, viability was reduced to 91.3% (± 0.30, P < 0.01) and with the addition of 2 Gy radiation viability reduced to 66.57% (± 2.40, P < 0.01) and to 48.92% (± 5.76, P < 0.01) with 4 Gy radiation. Compared with untreated OE33R cells, viability of OE33R cells treated with Atezolizumab alone was reduced to 88% (± 2.65,P < 0.05), with the addition of 2 Gy radiation viability was reduced to 75.67% (± 2.33, P < 0.01) and 38% (± 3.06, P < 0.01) with 4 Gy radiation (Figure 2B).
In the OE33P cells, Pembrolizumab treatment alone non-significantly reduced viability to 91.54% (± 2.67) compared with the untreated cells, however with the addition of 2 Gy radiation viability reduced to 65.36% (± 2.81, P < 0.01) and 48.18% (± 3.20, P < 0.01) with 4 Gy radiation when compared to untreated OE33P cells. When the OE33R cells were treated with Pembrolizumab, viability was non-significantly reduced to 92% (± 2.52), but the addition of 2 Gy radiation reduced viability to 75.33% (± 2.33, P < 0.01) and 36.33% (± 1.67, P < 0.001) with 4 Gy radiation. Four Gy radiation with Pembrolizumab resulted in a significantly greater reduction in viability in the radioresistant OE33R cell line compared to the radiosensitive OE33P cell line (P < 0.05) (Figure 2C).
In the OE33P cells Nivolumab reduced viability to 77.94% (± 4.79, P < 0.05) and with the addition of 2 Gy radiation viability reduced to 63.21% (± 3.41, P < 0.01) and 52.98% (± 1.82, P < 0.01) with 4 Gy radiation compared with untreated OE33P cells. When the OE33R cells were treated with Nivolumab, viability was reduced to 72% (± 2.62, P < 0.01) and with the addition of 2 Gy radiation viability reduced to 59.67% (± 1.86, P < 0.01) and 30% (± 1.73, P < 0.001) with 4 Gy radiation compared with untreated OE33R cells. Treatment with 4 Gy radiation and Nivolumab resulted in a significantly greater reduction in viability in the OE33R cell line compared to the radiosensitive cell line (P < 0.001) (Figure 2D).
In the OE33P cells, combination Atezolizumab and Pembrolizumab non-significantly reduced viability to 85.94% (± 3.79) but the addition of 2 Gy radiation significantly reduced viability to 61.10% (± 3.44, P < 0.01) and 51.07% (± 2.27, P < 0.01) with 4 Gy radiation compared with untreated OE33P cells. When the OE33R cells were treated with combination Atezolizumab and Pembrolizumab, viability was significantly reduced to 74.67% (± 1.33, P < 0.01), and with the addition of 2 Gy radiation viability was reduced to 67.33% (± 2.73, P < 0.01) and 30% (± 2.52, P < 0.01) with 4 Gy radiation. Four Gy radiation with combination Atezolizumab and Pembrolizumab resulted in a significantly greater reduction in viability in the OE33R cell line compared to the radiosensitive cell line (P < 0.01) (Figure 2E).
In the OE33P cells combination Atezolizumab and Nivolumab reduced viability to 80.18% (± 3.48, P < 0.05) and with the addition of 2 Gy radiation reduced viability further to 57.48% (± 1.64, P < 0.01) and 47.63% (± 3.11, P < 0.01) with 4 Gy radiation, compared with untreated OE33P cells. When the OE33R cells were treated with combination Atezolizumab and Nivolumab, viability was reduced to 71% (± 2.08, P < 0.01) and with the addition of 2 Gy radiation viability reduced to 54.67% (± 2.40, P < 0.01) and 31% (± 1.03, P < 0.001) with 4 Gy radiation compared with untreated OE33R cells. Treatment with 4 Gy radiation and combination Atezolizumab and Nivolumab resulted in a significantly greater reduction in viability in the OE33R cell line compared to the OE33P cell line (P < 0.01) (Figure 2F).
Profiling IC expression in fresh patient tissue samples
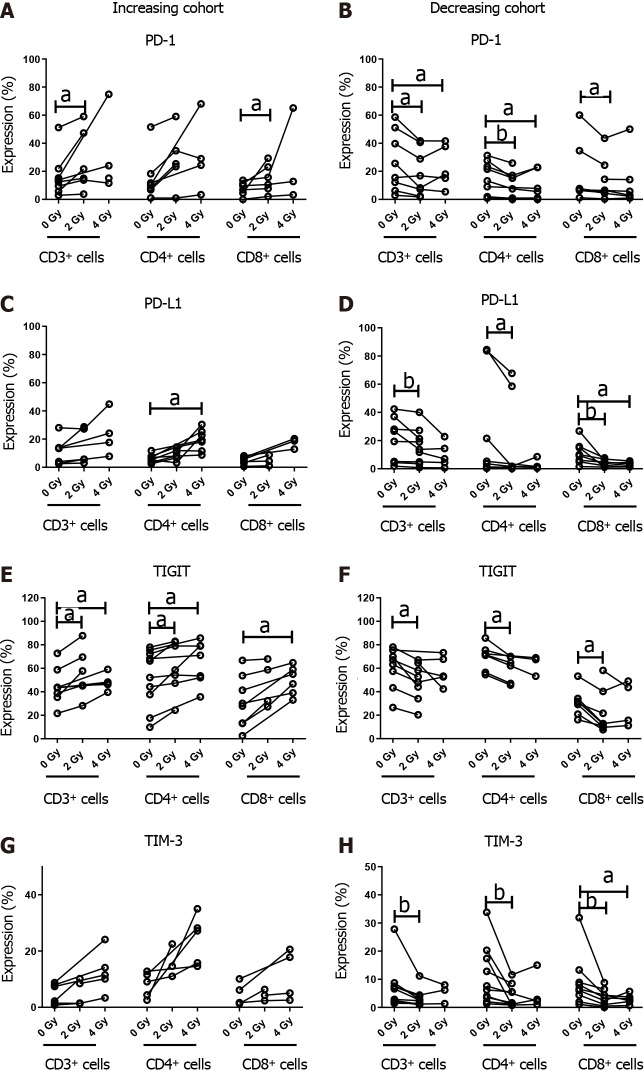
The in vitro data revealed an increase in IC expression on OAC cells post irradiation. To determine if this held true in ex vivo OAC tumour tissue, we profiled IC expression post irradiation with 2 Gy and 4 Gy. Subcohorts of patients demonstrated an upregulation and others a downregulation in checkpoint expression upon exposure to conventional radiation doses and hypofractionation for CD3+, CD3+CD4+, CD3+CD8+ tumour-infiltrating T cells. There was a significant increase in the frequency of CD3+PD-1+ and CD3+CD8+PD-1+ T cells in tumour tissue when irradiated with 2 Gy (26.76 ± 8.80 vs 16.62 ± 5.40 at 0 Gy, and 14.8 ± 4.1 vs 7.70 ± 2.01 at 0 Gy, respectively, P < 0.05). There was a significant increase in the frequency of CD3+CD4+ PD-L1+ cells with 4 Gy radiation (19.4 ± 2.9 vs 6.27 ± 1.02 at 0 Gy, P < 0.05). There was an significant increase in CD3+TIGIT+ and CD3+CD4+TIGIT+ expression with both 2 Gy (55.6 ± 8.6 vs 40.12 ± 5.40, and 61.29 ± 8.20 vs 52.17 ± 7.70, respectively, P < 0.05) and 4 Gy radiation dosing regimens (48.06 ± 3.10 vs 40.12 ± 5.40, and 65.16 ± 6.90 vs 52.17 ± 7.90, respectively, P < 0.05) when compared with unirradiated cells, and an increase in CD3+CD8+ TIGIT+ expression following 4 Gy irradiation (49.55 ± 4.90 vs 31.07 ± 7.70, P < 0.05). Of interest, this population was significantly associated with advanced disease at initial presentation, poorer treatment responses, and adverse features of tumour biology, notably, lymphovascular invasion and perineural invasion.
In the cohort of patients which displayed a reduction in IC protein expression following radiation, there was a significant decrease in expression of PD-1 by CD3+, CD3+CD4+ and CD3+CD8+ cells in tumour tissue when irradiated with 2 Gy vs 0 Gy (18.44 ± 5.90 vs 26.48 ± 7.50, P < 0.05; 10.33 ± 3.40 vs 14.46 ± 3.90, P < 0.01; 12.96 ± 5.10 vs 17.77 ± 8.20, P < 0.05; respectively) and PD-1 expression by CD3+ and CD3+CD4+ when irradiated with 4 Gy vs 0 Gy (21.06 ± 6.90 vs 26.48 ± 7.50, and 10.04 ± 4.20 vs 14.46 ± 3.90, respectively, P < 0.05). There was also a significant decrease CD3+, CD3+CD4+ and CD3+CD8+ cells expressing PD-L1 (15.6 ± 4.2 vs 20.6 ± 5.5, P < 0.01; 18.73 ± 11.50 vs 25.1 ± 13.1, P < 0.05; and 4.13 ± 0.90 vs 11.17 ± 2.80, P < 0.05; respectively) , TIGIT (48.61 ± 5.60 vs 60.13 ± 6.20, 59.88 ± 4.50 vs 69.57 ± 4.10, and 21.67 ± 6.40 vs 30.76 ± 4.50, respectively, P < 0.05) and TIM-3 (3.24 ± 0.90 vs 6.86 ± 2.50, 4.07 ± 1.30 vs 10.91 ± 3.30, and 3.37 ± 119.00 vs 9.13 ± 3.10, respectively, P < 0.01) with 2 Gy radiation. Similar findings were identified with 4 Gy irradiation compared to basal expression by CD3+CD8+ for PD-L1 (3.51 ± 0.60 vs 11.17 ± 2.80, P < 0.05) and TIM-3 (2.93 ± 0.70 vs 9.13 ± 3.10, P < 0.05) (Figure 3).
Figure 3.
Oesophageal adenocarcinoma patients were screened for the surface expression of immune checkpoints ex vivo by flow cytometric analysis. Subcohorts where ionising radiation induced upregulation and downregulation of immune checkpoints (ICs). Inhibitory ICs are expressed at a higher level with conventional and hypofractionated dosing regimens in one cohort (n = 8). Inhibitory ICs are expressed at a lower level with conventional and hypofractionated dosing regimens in a separate cohort (n = 9). A and B: Increasing and decreasing cohort of PD-1; C and D: Increasing and decreasing cohort of PD-L1; E and F: Increasing and decreasing cohort of TIGIT; G and H: Increasing and decreasing cohort of TIM-3. aP < 0.05; bP < 0.01 by Wilcoxon signed rank test.
Clinical correlations
In order to understand potential clinical implications of these cohorts with increased and decreased IC expression post radiation, clinicopathological correlations were made based on patient tumour stage, adverse features of tumour biology, radiation and IC positivity (Table 1). There was a positive correlation basally with PD-1+CD3+ cells and lymphovascular invasion (P = 0.04). In terms of tumour staging, clinically there was a positive association of increasing tumour stage and PD-L1+CD3+ (P = 0.02) basally, PD-L1+CD3+, TIM-3+CD3+ and TIM-3+CD4+ at 2 Gy, and TIM3+CD8+ at 4 Gy (P < 0.05). There was a negative association between PD-1+CD8+ at 2 Gy (P = 0.01). In terms of clinical nodal status, there was a positive association with nodal positivity and PD-L1+CD4+ basally (P < 0.001) and PD-1+CD4+, TIM-3+CD4+, TIM-3+CD8+ at 4 Gy (P < 0.05). Pathologically, advancing tumour stage was negatively associated with TIGIT+CD3+ at 2 and 4 Gy (P < 0.01). Pathological nodal positivity was associated with PD-L1+CD4+ basally at 0 Gy, and TIGIT+CD3+ at 4 Gy (P < 0.05). It was negatively associated with PD-L1+CD8+ cells and TIGIT+CD3+ cells at 2 Gy (P < 0.05).
Table 1.
Clinicopathological characteristics of the study population illustrating the correlation for the percentage of CD3+, CD3+CD4+ and CD3+CD8+ cells expressing immune checkpoints present in oesophageal adenocarcinoma tumour tissue
|
Clinical factor
|
IC expression
|
Radiation dose
|
Spearman r
|
P
value (two-tailed)
|
| Lymphovascular invasion | PD-1 CD3+ | 0 Gy | 0.6396022 | 0.046435 |
| Clinical T stage | PD-L1 CD3+ | 0 Gy | 0.6411189 | 0.024659 |
| PD-1 CD8+ | 2 Gy | -0.7000000 | 0.016471 | |
| PD-L1 CD3+ | 2 Gy | 0.7768986 | 0.004908 | |
| TIM-3 CD3+ | 2 Gy | 0.7171372 | 0.012993 | |
| TIM-3 CD4+ | 2 Gy | 0.7171372 | 0.012993 | |
| TIM-3 CD8+ | 4 Gy | 0.6963106 | 0.025293 | |
| Clinical N stage | PD-L1 CD4+ | 0 Gy | 0.8568931 | 0.000370 |
| PD-1 CD4+ | 4 Gy | 0.7311262 | 0.016282 | |
| TIM-3 CD4+ | 4 Gy | 0.6614951 | 0.037241 | |
| TIM-3 CD8+ | 4 Gy | 0.6614951 | 0.037241 | |
| Pathological T stage | TIGIT CD3+ | 2 Gy | -0.7395740 | 0.014492 |
| TIGIT CD3+ | 4 Gy | -0.8964215 | 0.006267 | |
| Pathological N stage | PD-L1 CD4+ | 0 Gy | 0.6510135 | 0.041473 |
| PD-L1 CD8+ | 2 Gy | -0.6443043 | 0.044345 | |
| TIGIT CD3+ | 2 Gy | -0.7471188 | 0.013014 | |
| TIGIT CD4+ | 4 Gy | 0.8981774 | 0.006011 |
Positive values indicate positive correlation, negative values indicate negative correlation. Spearman correlation. Only significant data shown. Spearman r = 0.40-0.59 moderate, 0.60-0.79 strong and 0.80-1.00 very strong. IC: Immune checkpoint.
Release of angiogenic markers, cytokines, co-stimulatory molecules and soluble checkpoints post irradiation
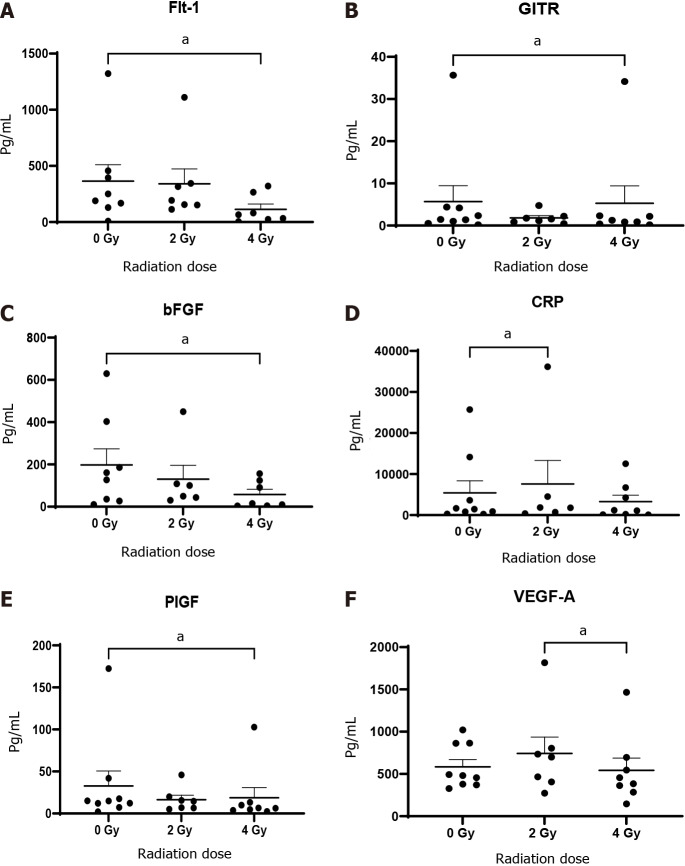
Given the complex interplay in the tumour microenvironment between immunosuppressive factors and anti-tumour immunity, we investigated the expression of cytokines, ICs, co-stimulatory molecules, markers of angiogenesis and vascular injury with and without radiation. The administration of 4 Gy radiation was effective in significantly reducing angiogenic markers over that of untreated 0 Gy tissue; basic fibroblast growth factor (bFGF, 57.7 ± 24.5 vs 197.5 ± 76.2, P < 0.05), Flt-1 (113.5 ± 47.7 vs 364.7 ± 145.8, P < 0.05), placental growth factor (PIGF, 18.7 ± 12.1 vs 32.8 ± 17.8, P < 0.05). Whereas, a significant reduction was observed in VEGF-A following 4 Gy radiation over that of 2 Gy (522.8 ± 144.2 vs 583.7 ± 86.2, P < 0.05) (Figure 4).
Figure 4.
Conditioned media generated using oesophageal adenocarcinoma patient tumour was screened for markers by multiplex immunosorbent assay kit. Angiogenic markers Flt-1, basic fibroblast growth factor (bFGF), placental growth factor (PIGF) and vascular endothelial growth factor (VEGF)-A and vascular injury marker C-reactive protein (CRP) decrease significantly with 4 Gy radiation (n = 9). A: Flt-1; B: GITH; C: bFGF; D: CRP; E: PIGF; F: VEGF-A. aP < 0.05 by Wilcoxon signed rank test.
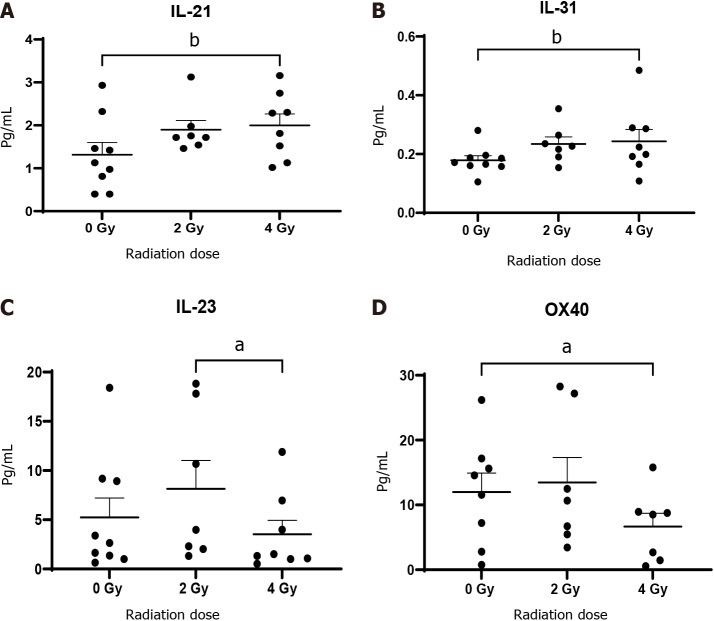
There was a significant increase in the level of IL-21 (1.98 ± 0.30 vs 1.3 ± 0.3, P < 0.01) and IL-31 (0.24 ± 0.04 vs 0.18 ± 0.02, P < 0.01) with the administration of 4 Gy radiation compared to 0 Gy with a significant decrease in IL-23 (3.53 ± 1.40 vs 5.24 ± 1.90, P < 0.05) following 4 Gy radiation compared with 2 Gy. CRP, a marker of vascular injury increased significantly with 2 Gy radiation dosing compared to untreated 0 Gy tissue (7568 ± 5750 vs 5425 ± 2925, P < 0.05) but was reduced with 4 Gy compared to non-irradiated tissue (Figure 5).
Figure 5.
Oesophageal adenocarcinoma patients tumour conditioned media were screened by multiplex immunosorbent assay kit (n = 9). The cytokines interleukin (IL)-21 and IL-31 increase with ionising radiation while IL-23 and OX-40 decrease. A: IL-21; B: IL-31; C: IL-23; D: OX-40. aP < 0.05; bP < 0.01. Wilcoxon signed rank test to compare expression between basal levels and dosing regimens.
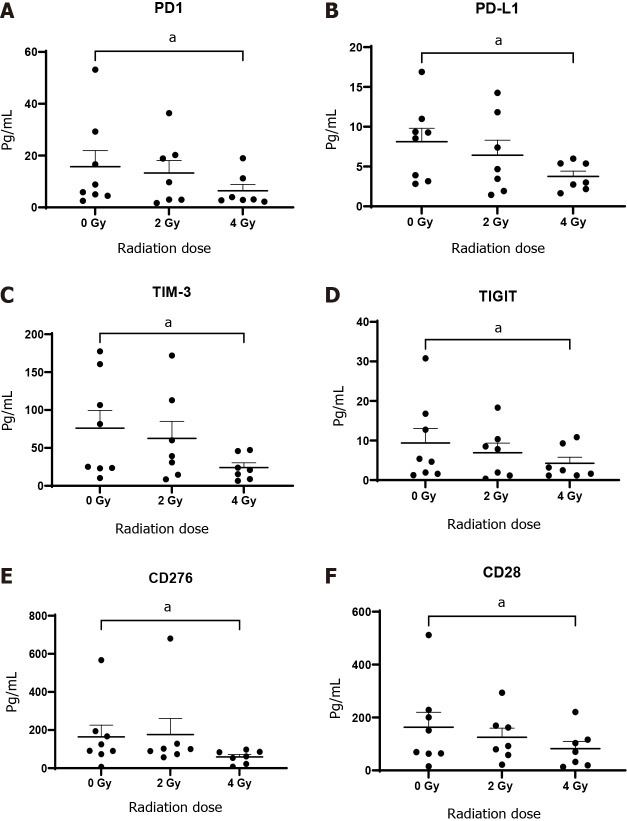
In terms of IC receptor and ligand expression, there was a significant reduction in levels of soluble PD-1 (6.44 ± 2.40 vs 15.72 ± 6.20, P < 0.05), PD-L1 (3.76 ± 0.70 vs 8.12 ± 1.70, P < 0.05), TIM-3 (24.11 ± 6.20 vs 76.02 ± 23.50, P < 0.05), TIGIT (4.26 ± 1.50 vs 39.0 ± 3.6, P < 0.05) and CD276 (58.81 ± 12.80 vs 164.30 ± 61.02, P < 0.05) in the TCM following 4 Gy radiation compared with 0 Gy. In addition, 4 Gy radiation also induced a significant decrease in the release of the soluble co-stimulatory molecules CD28 (82.18 ± 27.70 vs 163.2 ± 56.3, P < 0.05), glucocorticoid-induced TNF receptor (GITR, 5.27 ± 4.10 vs 5.7 ± 3.8, P < 0.05) and OX-40 (6.7 ± 2.1 vs 11.9 ± 2.9, P < 0.05) compared to untreated tissue 0 Gy (Figure 6).
Figure 6.
Oesophageal adenocarcinoma patient’s tumour conditioned media were screened by multiplex immunosorbent assay kit. The inhibitory checkpoints PD-1 and its ligand PD-L1, TIGT, TIM3, immunosuppressive molecule and checkpoint CD276 (B7-H3) and costimulatory molecule CD28 significantly decrease with fractionated radiotherapy (n = 8). A: PD-1; B: PD-L1; C: TIM-3; D: TIGIT; E: CD276; F: CD28. aP < 0.05 by Wilcoxon signed rank test.
DISCUSSION
The visceral appeal of modulating the host immune system is one of simplicity in an effort to harness a profound anti-tumour response and is a principle that has existed since the development of the field of cellular immunology as an entity. Quintessentially, skewing the hosts innate immune system to boost anti-tumour immunity consists of two processes compliant to exploitation: These being the stimulant as well as the reaction. The most logical way to perturb the tumour and its microenvironment is through promoting tumouricidal effects, through systemic chemotherapy or the radiation therapy delivered. Increasing and propagating the anti-tumour responses thereby facilitating immune activation with optimal kinetics may achieve a synergistic anti-tumour response, producing a more profoundly durable effect on the immune system than chemo (radio) therapy alone. In this context, the landmark Checkmate-577 trial has demonstrated significantly improved disease free survival in the adjuvant setting of resectable gastroesophageal cancer[16]. The findings of increased IC expression in vitro and ex vivo through the use of radiation in the current body of work provides promising translational therapeutic rationale for their use in the multimodal paradigm. RT propagates the priming and effector phases of the anti-tumour immune response rendering it an appropriate combination with IC inhibitors (ICIs)[24]. However, inherently radioresistant tumours may pose a particular therapeutic dilemma, as they may not have a similar synergism with ICB as radiosensitive tumours. Ionising radiation is currently under investigation in metastatic oesophageal cancer with pembrolizumab (NCT02642809) and is currently under investigation in the curative setting with neoadjuvant trimodal therapy of Pembrolizumab and chemoradiotherapy in oesophageal squamous cell carcinoma (NCT03792347), with a similar trial investigating durvalumab and chemoradiotherapy in squamous cell carcinoma (SCC) and OAC (NCT02735239). The SKY-SKRAPER-07 trial is currently evaluating anti-PD-L1 Atezolizumab with anti-TIGIT therapy following chemoradiotherapy in advanced oesophageal cancer (NCT04543617). Of note, in a study by Zhao et al[25], they reported that with PD-1 positivity correlated with TIM-3 expression, and CD8+ tumour infiltrating lymphocyte density as a risk factor for recurrence free and overall survival (OS) in oesophageal SCC. The increasing expression in OAC cells and a cohort of patients following RT in this study represents promising therapeutic targets in OAC.
The clinically important observation in this study that half of the patients assessed displayed a reduction in IC expression post RT is an interesting caveat, one which suggests very different susceptibility to ICB in combination with RT and therefore, the stratification of patients into potential responders and non-responders should be addressed. In the same vein, the activation of cGAS-STING signaling, which has been recognized to potentiate systemic anti-tumour immunity and subsequent tumour rejection by dual RT and checkpoint blockade administration is promising even in those with checkpoint downregulation. A study by Vanpouille-Box et al[26], highlighting the importance of the cGAS-STING pathway in response to combination RT and immunotherapy, reported the knockdown of cGAS in murine cancer cells abrogated the priming of CD8+ T cells in tumour-draining lymph nodes and spleen, and prevented the infiltration of abscopal tumours by CD8+ T cells. Importantly, the synergistic and significant reduction in viability of radioresistant OAC cancer cells which we observed in this study following the dual administration of ICIs and ionising radiation is very promising.
With respect to the use of hypofractionation in the curative setting for oesophageal cancer, there is an increasing volume of evidence demonstrating the safety and efficacy of this approach[27]. There are studies demonstrating a survival benefit of this approach particularly in the context of metastatic nodal disease. In one such study, hypofractionated radiotherapy (HFR) administered with taxane based chemotherapy in the management of post-surgery tracheoesophageal groove lymph node (TGLN) metastasis demonstrated improved OS in the HFR group compared with that of the conventional dosing treatment arm [24.1 mo (95%CI, 16.2-32.1 mo) vs 11.9 mo (95%CI, 9.2-14.4 mo), P = 0.024)[28]. Importantly, the study did not find a significant difference in pulmonary complications such as radiation pneumonitis (grades 3-4, 16.0% vs 7.1%; P = 0.314)[28].
Radiation induced lymphopenia is a frequent complication of multimodal cancer therapy and poorer outcomes are directly linked to the severity of lymphopenia[29]. Furthermore, it has been demonstrated that circulating lymphocyte count during neoadjuvant chemoradiation (CRT) in oesophageal cancer patients can predict pathological complete response (pCR) rates and low absolute circulating lymphocytes are associated with poorer outcomes[30]. The widespread adoption of immunotherapy has garnered new support and focus on the preservation of a pool of lymphocytes that are functional in enhancing immune function in the circulation and a study in pancreatic adenocarcinoma demonstrated hypofractionated CRT of 10 Gy in 3 doses over one week resulted in a decrease in the loss of T cells systemically compared to 28 daily doses of 1.8 Gy equating to 50.4 Gy[31,32].
The literature to date is concentrated primarily on evaluation of adverse events and of RT or immunotherapy in isolation. With improvements in targeted radiation delivery modalities, and technological advances, hypofractionated RT is now utilised without evidence of increased toxicities in a number of malignancies[33,34]. The Hypofractionated RT used in this instance was safe, well tolerated and provided robust survival results in those who could not receive chemoradiotherapy[35]. Furthermore, there is data in lung that conventional radiation dosing and immunotherapy is safe and feasible with no increases in adverse events[36-38].
The ATTRACTION 3 trial demonstrated a 50% reduction in serious adverse events in those treated with nivolumab vs conventional chemotherapy in Esophageal Squamous cell carcinoma[39]. In a study evaluating and immunotherapy in renal cell cancer, melanoma and lung, fatigue and pneumonitis were the most common adverse event. They found that toxicity did not correlate with hypofractionation or tumour type. Hypofractionated RT of pulmonary lesions was found to induce a complete response more consistently than in other sites. This study found that combining body Hypofractionated RT with immunotherapy is safe and viable, however, level I evidence is needed[40].
VEGF is a mitogen essential for angiogenesis and Ramucirumab is approved for use in advanced gastroesophageal cancer patients. The use of anti-VEGF agents have shown promise in promoting improved survival when used in combination with chemoradiotherapy in colon cancer, however treatment resistance is a common problem[41]. This can be due compensatory mechanisms resulting in resistance, namely hypoxia- induced increases of other angiogenic promoters such as PIGF[42]. VEGF, bFGF and PIGF are crucial angiogenic promoters linked with tumourigenesis and Flt-1, is involved in tumour growth and metastatic dissemination, most likely via stimulation of macrophage-lineage cells[43]. PIGF/Flt-1 signaling can contribute to colorectal cancer progression through increasing the phosphorylation of p38 mitogen-activated protein kinase (MAPK), thereby upregulating MMP9 expression; resulting in increasing cellular migration/invasion. Therefore inhibition of PIGF/Flt-1 signalling will have therapeutic potential in lower gastrointestinal cancers[44]. In the current study radiation therapy was demonstrated to reduce the expression of these promoters of angiogenesis, which is crucial in the mitigating the risk of metastatic disease for upper gastrointestinal cancers.
The subset of cytokines expressed post radiation and immunotherapy treatment play a key role in determining the subsequent immune response elicited. In this study the OAC tumour tissue released significantly more anti-tumour IL-21 and IL-31 in response to radiation. IL-21 is produced by numerous T helper cells, such as Th1 and Th17 cells, activated Natural Killer T cells[45]. It promotes B cell differentiation into plasma cells, regulates immunoglobulin production, reshaping the tumour microenvironment and influencing the proliferation and/or effector function of both CD4+ and CD8+ T cells, while limiting the differentiation of Tregs[46]. IL-21 has distinct anti-tumour properties as a consequence of its ability to increase the availability of CD8+ T cells through the induction of an early differentiation phenotype and Natural Killer cells[33]. IL-31 has immunoregulatory properties, with a study demonstrating that mice infused with IL-31 had tumour growth disruption and a decreased metastatic burden, supporting the use of IL-31 to offset the risk for metastatic disease development[47]. Similarly, in a breast cancer murine model, the tumouricidal effects of T cells are increased, and myeloid derived suppressor cells and tumour-associated macrophages are reduced in tumours with high expression of IL-31, with an immunophenotype supporting antitumour immunity[48]. While both IL-21 and IL-31 were significantly increased in the TCM, the expression of IL-23, which has been documented to promote tumour metastases was decreased. IL-23 has metastases promoting properties via suppressing the anti-tumour properties of T cells and the anti-metastatic function of NK cells[49]. In addition to this, IL-23 was found to be overexpressed in many human cancers including colorectal and gastric cancer, and was found to be a negative prognostic indicator[50]. Of note CRP, an acute phase protein and marker of vascular injury was found to increase with 2 Gy radiation in our study. There have been epidemiologic studies to suggest that elevated CRP levels in circulation are linked with poorer outcomes in those with solid cancers, whereas elevated levels in apparently healthy subjects, is a potential independent risk factor for future risk of developing cancer of any type including lung, colorectal and gastric cancers due to chronic low inflammatory states, which is of particular relevance in OAC[51]. Therefore, the exact role of CRP in response to RT and immunotherapy requires further study.
Co-stimulatory and IC molecules can have both immunostimulatory and immunosuppressive effects. In this study a range of soluble ICIs and ligands were significantly downregulated following 4 Gy radiation treatment of OAC tumour explants. The role of soluble receptors however, and its effects on immune function remain yet to be elucidated and therefore its potential use as an oncological treatment remain unclear. Through this body of work, we observed a significant down regulation of PD-1, PD-L1, TIM-3 and TIGIT and this was paralleled by a concomitant increase in OAC cell line surface expression and a cohort of OAC tumour explants, which may go some way to explain the decrease in the soluble forms of these IC proteins post irradiation. B7-H3 (also known as CD276) is an IC molecule, with many cancers exhibiting aberrant overexpression and such upregulation is associated with aggressiveness and a poor clinical prognosis[52]. Furthermore, there are studies demonstrating a vital role for B7-H3 in promoting tumourigenesis and metastatic dissemination, proliferation, invasion and migration[52-54]. CD276 promotes tumour proliferation and invasion[55]. In addition, soluble CD276 was found to stimulate the invasion and metastatic dissemination of pancreatic adenocarcinoma cells via the Toll like Receptor 4/nuclear factor kappa-light-chain-enhancer of activated B cells pathway (50). Overall, additional studies are required in gastroesophageal cancers to determine the true function of soluble IC proteins and how they pertain to treatment response and immune regulation.
CD28 which is a co-stimulatory molecule is essential in the augmentation of T cell activation and metabolism, driving tumour-infiltrating T cell glycolysis. It is antagonized by CTLA-4 and PD-1[56]. In the current study, soluble CD28 is reduced with radiation, which may be immunosuppressive. Soluble CD80-Fc has been found to maintain IFN-γ release by PD-1+ specific activated T cells even with PD-L1+ tumour cells[57]. Soluble GITR, which was reduced in this study, represents a potential immunotherapeutic target and is found to be expressed at high levels on Tregs[58,59]. A number of phase 1 trials have identified anti-GITR antibodies to have safe pharmacological profiles, with phase II trials ongoing evaluating its combination with RT and anti-PD-1 therapy (NCT04225039). New promising approaches are focusing on the activation of co-stimulatory pathways to enhance antitumour immune responses. GITR activation can result in the inhibition of T-cell (Treg) function and promote effector T-cell function[58], and may also provide theoretical basis for the clinical application of combinations with monoclonal antibody therapy such as bFGF in molecular targeted therapies[60].
Lastly, OX40 has been demonstrated to have a crucial part to play in maintaining the immune responses in the immediate term and ongoing responses through enhancing T cell expansion, differentiation, and survival[61]. OX40 activation can have a significant impact T cell receptor (TCR) signaling through the PI3-K/PKB pathway, influencing T cell division, survival and cytokine production. This can directly increase calcium influx, and lead to IL-2, IL-4, IL-5, and IFN-γ secretion[62]. OX40 triggering regressed Treg cells, thus this allows Dendritic cells to reach the lymph nodes draining the tumour and in doing so prime the tumour-specific CD8 lymphocytes response [63]. However, in the current study radiation induces a downregulation of OX40 in TCM which may indeed be an immunosuppressive consequence of radiation therapy and one which requires further investigation. Again, more robust studies will be helpful to determine the functions of soluble co-stimulatory molecules as they may have alternate functions compared with their cell membrane bound counterparts in the tumour microenvironment.
CONCLUSION
The introduction of ICIs has resulted in enhanced survival in melanoma and non-small cell lung cancer treatment and has evolved to involve the spectrum of solid gastrointestinal malignancies with positive results of the landmark Checkmate 577 trial in the adjuvant setting most notable to date. However, there remains many issues to be interrogated including an appropriate RT regimen in conjunction with immunotherapies. There is considerable translational, preclinical and clinical data in favour of fractionated RT, and timing of RT delivery and target delineation, there remains disparity and no universal approach applicable to the clinical setting. In the current study, IC blockade in combination with radiation synergistically reduces viability in radioresistant cells and Nivolumab appears most efficacious. There remains a need to delineate the effects of RT on host anti-tumour immunity. Additionally, lymphopenia induced by RT delivery may negate the effects of immunotherapy on offsetting T cell exhaustion, thus protocols that can minimize lymphopenia need careful design for maximal therapeutic potential. Finally, more concentrated and robust studies to determine and validate potential biomarkers to predict those who will be suitable for these treatment modalities are urgently required with profiling next-generation sequencing of tumour mutation burden based profiling, immune signatures, gene profiling signatures and the repertoire of T-cell receptors potential avenues to elucidate this.
ARTICLE HIGHLIGHTS
Research background
Oesophageal cancer is represents a difficult treatment dilemma with poor 5 year overall survival due to presentation at advanced stages due to its indolent nature as well as poor treatment responses to conventional therapies.
Research motivation
The advent of immunotherapy represents a shift in the multimodal treatment paradigm for esophageal cancer and has had mixed results in many solid tumours to date. The Checkmate 577 trial is a landmark study and is sure to revolutionize immune checkpoint blockade as the treatment modality of choice in the adjuvant setting.
Research objectives
To determine the impact of radiotherapy (RT) on immune checkpoint expression, and to determine the prevailing immune milieu in terms of markers of angiogenesis, cytokines and metastatic markers.
Research methods
This hybrid in vitro and ex vivo study is a mixture of flow cytometry, enzyme-linked immunosorbent assay kit work and cell viability by a cell counting kit-8 assay.
Research results
Radiation results in a decrease in angiogenic and metastatic markers with an increase in anti-tumour cytokines. There were two distinct subpopulations, with one cohort of patients demonstrating increased checkpoint expression as a consequence of radiation and a separate cohort demonstrating the opposite effects. The cohort with increased checkpoint expression had poorer treatment responses and were associated with adverse tumour biology.
Research conclusions
Oesophageal cancer represents an immune active tumour and is a viable target in both the neoadjuvant and adjuvant setting and should be combined with RT to exert maximum synergistic effects.
Research perspectives
This seminal study is the first of its kind and is a truly clinical and translational evaluation of the immune landscape of esophageal cancer.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Tallaght/St James’s Hospital Ethics Committee.
Conflict-of-interest statement: There are no conflicts of interest to report.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: International Society for Diseases of the Esophagus, No. ISDE IRE585; European Association for Cancer Research, No. RGEAV21-0745.
Peer-review started: December 3, 2021
First decision: January 27, 2022
Article in press: April 26, 2022
Specialty type: Surgery
Country/Territory of origin: Ireland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casà C, Italy; Kim S, South Korea S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
Contributor Information
Noel E Donlon, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland. donlonn@tcd.ie.
Maria Davern, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Fiona O’Connell, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Andrew Sheppard, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Aisling Heeran, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Anshul Bhardwaj, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Christine Butler, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Ravi Narayanasamy, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Claire Donohoe, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
James J Phelan, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Niamh Lynam-Lennon, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Margaret R Dunne, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Stephen Maher, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Jacintha O’Sullivan, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
John V Reynolds, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Joanne Lysaght, Department of Surgery, Trinity Translational Medicine Institute, St James Hospital, Dublin D08, Ireland.
Data sharing statement
Data generated in this study will be available upon specific request from the corresponding author.
References
- 1.Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, Cunningham D. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. doi: 10.1038/nrdp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383–2396. doi: 10.1016/S0140-6736(17)31462-9. [DOI] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 4.Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 5.Nemoto K, Kawashiro S, Toh Y, Numasaki H, Tachimori Y, Uno T, Jingu K, Matsubara H. Comparison of the effects of radiotherapy doses of 50.4 Gy and 60 Gy on outcomes of chemoradiotherapy for thoracic esophageal cancer: subgroup analysis based on the Comprehensive Registry of Esophageal Cancer in Japan from 2009 to 2011 by the Japan Esophageal Society. Esophagus. 2020;17:122–126. doi: 10.1007/s10388-019-00711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, Yang Y, Guo Q, Ren B, Peng Q, Zou L, Zhu Y, Tian Y. Comparing hypofractionated to conventional fractionated radiotherapy in postmastectomy breast cancer: a meta-analysis and systematic review. Radiat Oncol. 2020;15:17. doi: 10.1186/s13014-020-1463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H, Perera F, Fyles A, Schneider K, Gulavita S, Freeman C. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 8.Murray Brunt A, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield DJ, Chan C, Churn M, Cleator S, Coles CE, Goodman A, Harnett A, Hopwood P, Kirby AM, Kirwan CC, Morris C, Nabi Z, Sawyer E, Somaiah N, Stones L, Syndikus I, Bliss JM, Yarnold JR FAST-Forward Trial Management Group. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, Kindblom J, Ginman C, Johansson B, Björnlinger K, Seke M, Agrup M, Fransson P, Tavelin B, Norman D, Zackrisson B, Anderson H, Kjellén E, Franzén L, Nilsson P. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394:385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 10.Dearnaley D, Syndikus I, Sumo G, Bidmead M, Bloomfield D, Clark C, Gao A, Hassan S, Horwich A, Huddart R, Khoo V, Kirkbride P, Mayles H, Mayles P, Naismith O, Parker C, Patterson H, Russell M, Scrase C, South C, Staffurth J, Hall E. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncol. 2012;13:43–54. doi: 10.1016/S1470-2045(11)70293-5. [DOI] [PubMed] [Google Scholar]
- 11.Donohoe CL, O'Farrell NJ, Grant T, King S, Clarke L, Muldoon C, Reynolds JV. Classification of pathologic response to neoadjuvant therapy in esophageal and junctional cancer: assessment of existing measures and proposal of a novel 3-point standard. Ann Surg. 2013;258:784–792; discussion 792. doi: 10.1097/SLA.0b013e3182a66588. [DOI] [PubMed] [Google Scholar]
- 12.Noble F, Lloyd MA, Turkington R, Griffiths E, O'Donovan M, O'Neill JR, Mercer S, Parsons SL, Fitzgerald RC, Underwood TJ OCCAMS consortium. Multicentre cohort study to define and validate pathological assessment of response to neoadjuvant therapy in oesophagogastric adenocarcinoma. Br J Surg. 2017;104:1816–1828. doi: 10.1002/bjs.10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumour cells in the escape from host immune system and tumour immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 15.Power R, Lowery MA, Reynolds JV, Dunne MR. The Cancer-Immune Set Point in Oesophageal Cancer. Front Oncol. 2020;10:891. doi: 10.3389/fonc.2020.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, Uronis H, Elimova E, Grootscholten C, Geboes K, Zafar S, Snow S, Ko AH, Feeney K, Schenker M, Kocon P, Zhang J, Zhu L, Lei M, Singh P, Kondo K, Cleary JM, Moehler M CheckMate 577 Investigators. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med. 2021;384:1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 17.Smyth EC, Cervantes A. Addition of nivolumab to chemotherapy in patients with advanced gastric cancer: a relevant step ahead, but still many questions to answer. ESMO Open. 2020;5:e001107. doi: 10.1136/esmoopen-2020-001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donlon NE, Power R, Hayes C, Reynolds JV, Lysaght J. Radiotherapy, immunotherapy, and the tumour microenvironment: Turning an immunosuppressive milieu into a therapeutic opportunity. Cancer Lett. 2021;502:84–96. doi: 10.1016/j.canlet.2020.12.045. [DOI] [PubMed] [Google Scholar]
- 19.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumour Immunity in Immunogenic Tumours. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou Y, Liang H, Rao E, Zheng W, Huang X, Deng L, Zhang Y, Yu X, Xu M, Mauceri H, Arina A, Weichselbaum RR, Fu YX. Non-canonical NF-κB Antagonizes STING Sensor-Mediated DNA Sensing in Radiotherapy. Immunity. 2018;49:490–503.e4. doi: 10.1016/j.immuni.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, Harrington KJ. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20:203–217. doi: 10.1038/s41568-020-0246-1. [DOI] [PubMed] [Google Scholar]
- 23.Lynam-Lennon N, Reynolds JV, Pidgeon GP, Lysaght J, Marignol L, Maher SG. Alterations in DNA repair efficiency are involved in the radioresistance of esophageal adenocarcinoma. Radiat Res. 2010;174:703–711. doi: 10.1667/RR2295.1. [DOI] [PubMed] [Google Scholar]
- 24.Gong J, Le TQ, Massarelli E, Hendifar AE, Tuli R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother Cancer. 2018;6:46. doi: 10.1186/s40425-018-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Chen D, Wang W, Zhao T, Wen J, Zhang F, Duan S, Chen C, Sang Y, Zhang Y, Chen Y. Significance of TIM-3 Expression in Resected Esophageal Squamous Cell Carcinoma. Ann Thorac Surg. 2020;109:1551–1557. doi: 10.1016/j.athoracsur.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyu J, Liu T, Li T, Li F, Wang Q, Wang J, Han Y, Zhang J, Peng L, Lang J. Comparison of efficacy, safety, and costs between neoadjuvant hypofractionated radiotherapy and conventionally fractionated radiotherapy for esophageal carcinoma. Cancer Med. 2019;8:3710–3718. doi: 10.1002/cam4.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Yu J, Jiang Y, Pei D, Zhu H, Wang J. Hypofractionated Radiotherapy in Combination With Chemotherapy Improves Outcome of Patients With Esophageal Carcinoma Tracheoesophageal Groove Lymph Node Metastasis. Front Oncol. 2020;10:1540. doi: 10.3389/fonc.2020.01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie X, Lin SH, Welsh JW, Wei X, Jin H, Mohan R, Liao Z, Xu T. Radiation-induced lymphopenia during chemoradiation therapy for non-small cell lung cancer is linked with age, lung V5, and XRCC1 rs25487 genotypes in lymphocytes. Radiother Oncol. 2021;154:187–193. doi: 10.1016/j.radonc.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y, Chen J, Zhao L, Li Q, Zhu J, Yang H, Guo S, Xi M. Prediction of Pathologic Response to Neoadjuvant Chemoradiotherapy in Patients with Esophageal Squamous Cell Carcinoma Incorporating Hematological Biomarkers. Cancer Res Treat. 2021;53:172–183. doi: 10.4143/crt.2020.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Wang P, Zhao Z, Mao Q, Yu J, Li M. A review of radiation-induced lymphopenia in patients with esophageal cancer: an immunological perspective for radiotherapy. Ther Adv Med Oncol. 2020;12:1758835920926822. doi: 10.1177/1758835920926822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crocenzi T, Cottam B, Newell P, Wolf RF, Hansen PD, Hammill C, Solhjem MC, To YY, Greathouse A, Tormoen G, Jutric Z, Young K, Bahjat KS, Gough MJ, Crittenden MR. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer. 2016;4:45. doi: 10.1186/s40425-016-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodin D, Tawk B, Mohamad O, Grover S, Moraes FY, Yap ML, Zubizarreta E, Lievens Y. Hypofractionated radiotherapy in the real-world setting: An international ESTRO-GIRO survey. Radiother Oncol. 2021;157:32–39. doi: 10.1016/j.radonc.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vapiwala N, Wong JK, Handorf E, Paly J, Grewal A, Tendulkar R, Godfrey D, Carpenter D, Mendenhall NP, Henderson RH, Stish BJ, Vargas C, Salama JK, Davis BJ, Horwitz EM. A Pooled Toxicity Analysis of Moderately Hypofractionated Proton Beam Therapy and Intensity Modulated Radiation Therapy in Early-Stage Prostate Cancer Patients. Int J Radiat Oncol Biol Phys. 2021;110:1082–1089. doi: 10.1016/j.ijrobp.2021.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones CM, Spencer K, Hitchen C, Pelly T, Wood B, Hatfield P, Crellin A, Sebag-Montefiore D, Goody R, Crosby T, Radhakrishna G. Hypofractionated Radiotherapy in Oesophageal Cancer for Patients Unfit for Systemic Therapy: A Retrospective Single-Centre Analysis. Clin Oncol (R Coll Radiol) 2019;31:356–364. doi: 10.1016/j.clon.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Spaas M, Lievens Y. Is the Combination of Immunotherapy and Radiotherapy in Non-small Cell Lung Cancer a Feasible and Effective Approach? Front Med (Lausanne) 2019;6:244. doi: 10.3389/fmed.2019.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng L, Xu J, Ye Y, Wang Y, Luo S, Gong X. The Combination of Radiotherapy With Immunotherapy and Potential Predictive Biomarkers for Treatment of Non-Small Cell Lung Cancer Patients. Front Immunol. 2021;12:723609. doi: 10.3389/fimmu.2021.723609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chicas-Sett R, Zafra-Martin J, Morales-Orue I, Castilla-Martinez J, Berenguer-Frances MA, Gonzalez-Rodriguez E, Rodriguez-Abreu D, Couñago F. Immunoradiotherapy as An Effective Therapeutic Strategy in Lung Cancer: From Palliative Care to Curative Intent. Cancers (Basel) 2020;12 doi: 10.3390/cancers12082178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 40.Mohamad O, Diaz de Leon A, Schroeder S, Leiker A, Christie A, Zhang-Velten E, Trivedi L, Khan S, Desai NB, Laine A, Albuquerque K, Iyengar P, Arriaga Y, Courtney K, Gerber DE, Hammers H, Choy H, Timmerman R, Brugarolas J, Hannan R. Safety and efficacy of concurrent immune checkpoint inhibitors and hypofractionated body radiotherapy. Oncoimmunology. 2018;7:e1440168. doi: 10.1080/2162402X.2018.1440168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haibe Y, Kreidieh M, El Hajj H, Khalifeh I, Mukherji D, Temraz S, Shamseddine A. Resistance Mechanisms to Anti-angiogenic Therapies in Cancer. Front Oncol. 2020;10:221. doi: 10.3389/fonc.2020.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macarulla T, Montagut C, Sánchez-Martin FJ, Granja M, Verdaguer H, Sastre J, Tabernero J. The role of PIGF blockade in the treatment of colorectal cancer: overcoming the pitfalls. Expert Opin Biol Ther. 2020;20:15–22. doi: 10.1080/14712598.2020.1677603. [DOI] [PubMed] [Google Scholar]
- 43.Qian BZ, Zhang H, Li J, He T, Yeo EJ, Soong DY, Carragher NO, Munro A, Chang A, Bresnick AR, Lang RA, Pollard JW. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J Exp Med. 2015;212:1433–1448. doi: 10.1084/jem.20141555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei SC, Tsao PN, Weng MT, Cao Z, Wong JM. Flt-1 in colorectal cancer cells is required for the tumour invasive effect of placental growth factor through a p38-MMP9 pathway. J Biomed Sci. 2013;20:39. doi: 10.1186/1423-0127-20-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian Y, Zajac AJ. IL-21 and T Cell Differentiation: Consider the Context. Trends Immunol. 2016;37:557–568. doi: 10.1016/j.it.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng S, Sun Z, Qiao J, Liang Y, Liu L, Dong C, Shen A, Wang Y, Tang H, Fu YX, Peng H. Targeting tumours with IL-21 reshapes the tumour microenvironment by proliferating PD-1intTim-3-CD8+ T cells. JCI Insight. 2020;5 doi: 10.1172/jci.insight.132000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davidi S, Fremder E, Kan T, Raviv Z, Timaner M, Karin N, Hershkovitz D, Arohneim A, Shaked Y. The antiangiogenic role of the pro-inflammatory cytokine interleukin-31. Oncotarget. 2017;8:16430–16444. doi: 10.18632/oncotarget.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kan T, Feldman E, Timaner M, Raviv Z, Shen-Orr S, Aronheim A, Shaked Y. IL-31 induces antitumour immunity in breast carcinoma. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teng MW, Andrews DM, McLaughlin N, von Scheidt B, Ngiow SF, Möller A, Hill GR, Iwakura Y, Oft M, Smyth MJ. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc Natl Acad Sci U S A. 2010;107:8328–8333. doi: 10.1073/pnas.1003251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan J, Smyth MJ, Teng MWL. Interleukin (IL)-12 and IL-23 and Their Conflicting Roles in Cancer. Cold Spring Harb Perspect Biol. 2018;10 doi: 10.1101/cshperspect.a028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48:155–170. doi: 10.3109/10408363.2011.599831. [DOI] [PubMed] [Google Scholar]
- 52.Liu S, Liang J, Liu Z, Zhang C, Wang Y, Watson AH, Zhou C, Zhang F, Wu K, Lu Y, Wang X. The Role of CD276 in Cancers. Front Oncol. 2021;11:654684. doi: 10.3389/fonc.2021.654684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong P, Xiong Y, Yue J, Hanley SJB, Watari H. B7H3 As a Promoter of Metastasis and Promising Therapeutic Target. Front Oncol. 2018;8:264. doi: 10.3389/fonc.2018.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie C, Liu D, Chen Q, Yang C, Wang B, Wu H. Soluble B7-H3 promotes the invasion and metastasis of pancreatic carcinoma cells through the TLR4/NF-κB pathway. Sci Rep. 2016;6:27528. doi: 10.1038/srep27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dai W, Shen G, Qiu J, Zhao X, Gao Q. Aberrant expression of B7-H3 in gastric adenocarcinoma promotes cancer cell metastasis. Oncol Rep. 2014;32:2086–2092. doi: 10.3892/or.2014.3405. [DOI] [PubMed] [Google Scholar]
- 56.Beckermann KE, Hongo R, Ye X, Young K, Carbonell K, Healey DCC, Siska PJ, Barone S, Roe CE, Smith CC, Vincent BG, Mason FM, Irish JM, Rathmell WK, Rathmell JC. CD28 costimulation drives tumour-infiltrating T cell glycolysis to promote inflammation. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haile ST, Dalal SP, Clements V, Tamada K, Ostrand-Rosenberg S. Soluble CD80 restores T cell activation and overcomes tumour cell programmed death ligand 1-mediated immune suppression. J Immunol. 2013;191:2829–2836. doi: 10.4049/jimmunol.1202777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu MMT, Burugu S, Gao D, Yu J, Kos Z, Leung S, Horst BA, Nielsen TO. Evaluation of glucocorticoid-induced TNF receptor (GITR) expression in breast cancer and across multiple tumour types. Mod Pathol. 2020;33:1753–1763. doi: 10.1038/s41379-020-0550-z. [DOI] [PubMed] [Google Scholar]
- 59.van Beek AA, Zhou G, Doukas M, Boor PPC, Noordam L, Mancham S, Campos Carrascosa L, van der Heide-Mulder M, Polak WG, Ijzermans JNM, Pan Q, Heirman C, Mahne A, Bucktrout SL, Bruno MJ, Sprengers D, Kwekkeboom J. GITR ligation enhances functionality of tumour-infiltrating T cells in hepatocellular carcinoma. Int J Cancer. 2019;145:1111–1124. doi: 10.1002/ijc.32181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu M, Xing LQ. Basic fibroblast growth factor as a potential biomarker for diagnosing malignant tumour metastasis in women. Oncol Lett. 2017;14:1561–1567. doi: 10.3892/ol.2017.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Redmond WL, Ruby CE, Weinberg AD. The role of OX40-mediated co-stimulation in T-cell activation and survival. Crit Rev Immunol. 2009;29:187–201. doi: 10.1615/critrevimmunol.v29.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willoughby J, Griffiths J, Tews I, Cragg MS. OX40: Structure and function - What questions remain? Mol Immunol. 2017;83:13–22. doi: 10.1016/j.molimm.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Weixler B, Cremonesi E, Sorge R, Muraro MG, Delko T, Nebiker CA, Däster S, Governa V, Amicarella F, Soysal SD, Kettelhack C, von Holzen UW, Eppenberger-Castori S, Spagnoli GC, Oertli D, Iezzi G, Terracciano L, Tornillo L, Sconocchia G, Droeser RA. OX40 expression enhances the prognostic significance of CD8 positive lymphocyte infiltration in colorectal cancer. Oncotarget. 2015;6:37588–37599. doi: 10.18632/oncotarget.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated in this study will be available upon specific request from the corresponding author.