Abstract
A growing body of evidence suggests that astrocytes play a major role in the pathophysiology of Alzheimer’s disease. Given that APOE is primarily expressed in astrocytes, these cells might be an important link between the APOE ε4 allele and the development of Alzheimer’s disease pathology. Here, we investigate this hypothesis in vivo by measuring myo-inositol, a metabolite involved in astrocytic functions, with magnetic resonance spectroscopy. Currently, there is conflicting evidence regarding the relationship between APOE ε4 and myo-inositol concentration. Furthermore, data supporting a relationship between APOE ε4, myo-inositol and Alzheimer’s disease pathology (amyloid-beta and tau proteins) in the preclinical stage of Alzheimer’s disease are limited. A previous study revealed differences in myo-inositol levels between APOE ε4 carriers and non-carriers already in preclinical Alzheimer’s disease participants. However, other reports showed no impact of APOE genotype on the association between myo-inositol and the rate of amyloid-beta accumulation. In the present study, we determined the effect of APOE genotype on the association between myo-inositol and both amyloid-β and tau deposition quantified by PET in 428 cognitively unimpaired elderly and patients with mild cognitive impairment from the Swedish BioFINDER-2 cohort. APOE genotype impacted the associations between myo-inositol and amyloid-β pathology as revealed by an interaction effect between APOE genotype and levels of myo-inositol (P < 0.001) such that higher myo-inositol concentration was related to more amyloid-beta pathology in APOE ε4 carriers only. A similar interaction effect was also found when investigating the effect of APOE on the association between myo-inositol and tau pathology (P < 0.01). Focusing on the APOE ε4 subsample, myo-inositol partially (17%) mediated the association between amyloid-beta and tau pathology (P < 0.05). Furthermore, in a subgroup of participants with available plasma levels of glial fibrillary acidic protein, a marker of astroglial activation and astrocytosis, we found that glial fibrillary acidic protein correlated with myo-inositol only in APOE e4 carriers (APOE ε4 carriers: P < 0.01; APOE ε4 non-carriers: P > 0.8), suggesting that myo-inositol might reflect an aspect of the astrocytic involvement in Alzheimer’s pathology which is specific to the impact of APOE ε4. Therefore, we suggest that myo-inositol is a candidate in vivo marker to study the impact of APOE ε4 on the interplay between astrocytes and the pathophysiology of Alzheimer’s disease.
Keywords: astrocytes, amyloid-β, APOE, myo-inositol, Alzheimer’s disease
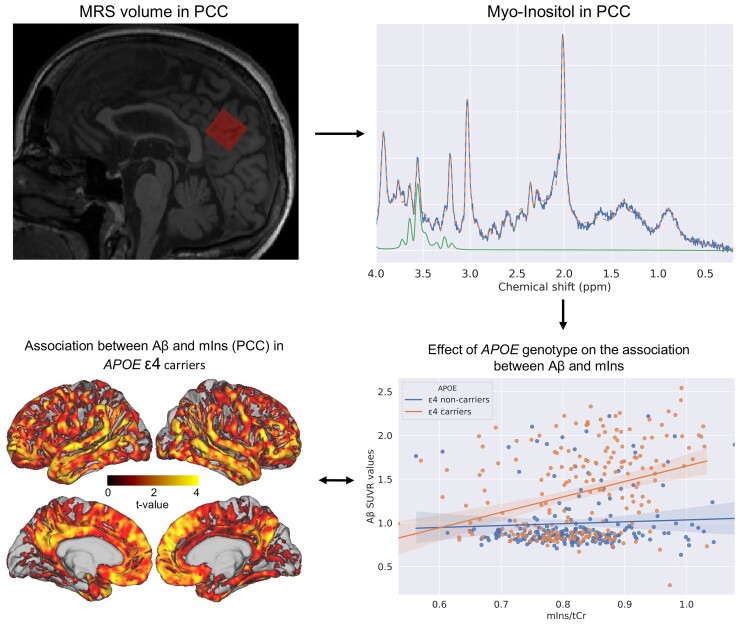
Spotorno et al. measured myo-inositol (mIns), a metabolite involved in astrocytic functions, with magnetic imaging spectroscopy and determined the effect of APOE on the association between mIns and both Aβ- and tau-PET retention. The results showed that higher mIns concentration was related to more Aβ and tau pathology in APOE ε4 carriers only.
Graphical Abstract
Graphical abstract.
Introduction
Although the ε4 allele of APOE gene is the strongest genetic risk factor for late-onset Alzheimer’s disease, the exact mechanism mediating APOE ε4 pathological effect remains elusive. In the central nervous system, APOE is primarily expressed in astrocytes,1 thus the impact of APOE genotype on the pathophysiology of Alzheimer’s disease is likely to involve astrocytic functioning. Studies on astrocytes derived from APOE ε4 human-induced pluripotent stem cells have indeed shown compromised amyloid-β (Aβ) clearance2 as well as impairment in other basic astrocytic functions such as lipid3 and cholesterol2 metabolism, synaptic pruning4 and neurotrophic support5 when compared with APOE ε3 and ε2 carriers.
Mounting evidence from multiple lines of research suggests that astrocytic dysfunction has a critical impact on Alzheimer’s pathology. For example, genetic data have revealed that a significant amount of the risk for sporadic Alzheimer’s disease is associated with genes, such as Clusterin (ApoJ), Sortilin-related receptor 1, Fermitin family member 2 and APOE, which are primarily expressed in astrocytes.1 Moreover, a recent spatial transcriptomics study confirmed that genes associated with inflammatory astrocytes are among the most reactive genes in the proximity of Aβ plaques.6 Reactive astrocytes have also been colocalized with both Aβ plaques7 and neurofibrillary tangles8 in post-mortem studies. Aside from the neuroinflammatory cascade, it has been proposed that both the loss of the normal homeostatic function of astrocytes and the gain of toxic function could impact the clearing of Aβ from the brain via phagocytosis.9 Moreover, astrocytes appear to play an important role also in the tau-related pathological cascade10,11 with some evidence linking these phenomena to APOE genotype.12
Although multiple sources of data point to a critical role of astrocytes in the pathology of Alzheimer’s disease both independently and modulated by APOE, in vivo studies of astrocytic function in humans are still challenging. A possible approach is to use myo-inositol (mIns), a metabolite almost exclusively localized in astrocytes and measurable with magnetic imaging spectroscopy (MRS),13,14 as a proxy of astrocytic function. mIns is the most abundant stereoisomer of inositol15 and serves as a precursor molecule for inositol lipid synthesis and as a physiologically important osmolyte.16 Several MRS studies have previously associated mIns concentration to gliosis and astrocytic disfunctions, notably in ALS and multiple sclerosis.17–20 In addition, reduction in mIns levels has by associated with astrocytic damage and necrosis.21 In the context of the Alzheimer’s disease spectrum, multiple MRS studies have found elevated concentrations of mIns in Alzheimer’s disease patients and even in preclinical Alzheimer’s disease relative to healthy controls22,23 as well as an association between mIns and Aβ accumulation over time.24,25 However, evidence of a link between APOE and mIns are not conclusive, with studies reporting partially contrasting results. For example, a study from our group showed elevated levels of mIns in APOE ε4 carriers without evidence of Aβ accumulation when compared with non-APOE ε4 carriers,22 in-line with previous studies showing the same association in the context of healthy aging.26,27 However, other studies have found no effect of APOE genotype on the levels of mIns in healthy participants, as well as in patients from the Alzheimer’s disease spectrum.24,28,29
In the present study, we explored the interaction between APOE, astrocytes and protein aggregation focusing on non-demented individuals, including Aβ-negative cognitively unimpaired individuals, Aβ-positive cognitively unimpaired individuals (preclinical Alzheimer’s disease) and Aβ-positive patients with mild cognitive impairment (MCI) (prodromal Alzheimer’s disease). Specifically, we investigated the extent to which APOE modulated the association between mIns concentration and both hallmarks of Alzheimer’s disease pathology, Aβ and tau, quantified by 18F-flutemetamol PET and 18F-RO948 PET, respectively. mIns concentration was quantified in a volume located in the precuneus/posterior cingulate cortex (PCC) region, which is an early site of Aβ accumulation and has been recommended as an appropriate region for MRS in studies on Alzheimer’s disease.30 To support inferences about the involvement of astrocytes in the processes under examination, we also quantified plasma glial fibrillary acidic protein (GFAP), a marker of astrocytic activation, which has been shown to correlate with Aβ-PET.31
Materials and methods
Participants
Four hundred and thirty-three participants from the Swedish BioFINDER-2 study were included. Only participants with available MRS, 18F-flutemetamol PET and 18F-RO948 PET acquired within 6 months and age >50 years were included in the study cohort. To capture the entire spectrum of early Alzheimer’s disease development from subthreshold Aβ levels to abnormal Aβ levels and finally cognitive symptoms, cognitively unimpaired participants (CU–Aβ- and CU–Aβ+) and patients with MCI with evidence of Aβ pathology were included (MCI–Aβ+; see Supplementary Materials for inclusion and exclusion criteria). The data were acquired between august 2017 and October 2020 and there is no overlap with the cohort included in previous MRS studies published by our group,22,25 which were based on the first generation of the Swedish BioFINDER study (Swedish BioFINDER-1). Demographic and clinical characteristics are summarized in Table 1. All subjects gave written informed consent according to the Declaration of Helsinki, and the study was approved by the Ethical Review Board of Lund, Sweden.
Table 1.
Demographic summary of the study cohort
| CU—Aβ- | CU—Aβ+ | MCI—Aβ+ | |
|---|---|---|---|
| N (% female) | 259 (56%) | 79 (58%) | 90 (48%) |
| Age | 67 (10) | 73 (9)b | 73 (7)b |
| Years of education | 13 (3) | 12 (4) | 13 (4) |
| MMSE | 29.0 (1.1) | 28.7 (1.3)b | 26.5 (2.3) |
| APOE ε4 (%) | 87 (34%) | 58 (73%)b | 69 (77%)b |
| Tau-positive—accordingly to tau-PET (%)a | 0 | 25 (32%)b | 49 (54%)b |
| Aβ-PET retention in the MRS volume | 0.86 (0.08) | 1.56 (0.33)b | 1.73 (0.39)b |
| Tau-PET retention in the MRS volume | 1.06 (0.10) | 1.12 (0.20)b | 1.23 (0.43)b |
| mIns/tCr in the MRS volume | 0.80 (0.08) | 0.84 (0.09)b | 0.86 (0.11)b |
Values are given as mean (standard deviation).
CU, cognitively unimpaired, MCI, mild cognitive impairment; Aβ+/−, amyloid-β positive/negative according to a previously published cut-off of 0.53 based on Aβ-PET32; MMSE, Mini-Mental State Examination; mIns/tCr, myo-inositol to total creatine ratio.
tau positivity was based on the tau-PET retention in a medial temporal meta-ROI reflecting Braak Stage I–II, using a previously published cut-off of 1.4833
significantly different from the cognitively unimpaired Aβ- group (P < 0.05). See Supplementary Fig. 1 for more details about differences in mIns concentrations across groups. See Supplementary results and Supplementary Fig. 2 for an alternative analysis in which participants were stratified accordingly to Aβ and tau positivity/negativity.
Imaging protocol and analysis
PET protocol
Participants underwent 18F-flutemetamol PET and 18F-RO948 PET on Discovery MI scanners (GE healthcare). 18F-flutemetamol PET images were acquired 90 to 110 min after injection of 185 MBq 18F-flutemetamol, whereas 18F-RO948 PET images were acquired 70 to 90 min after injection of 370 MBq 18F-RO948. Pre-processing and generation of standardized uptake value ratio (SUVRs) maps were carried out as previously described.32,3318F-Flutemetamol scans were normalized using the Pons as reference region, whereas 18F-RO948 images were referenced to the inferior cerebellar grey matter. For all subjects, Aβ status was defined based on the average SUVR values from a cortical composite using a previously published cut-off of 0.53.32
Magnetic imaging spectroscopy–MRI protocol
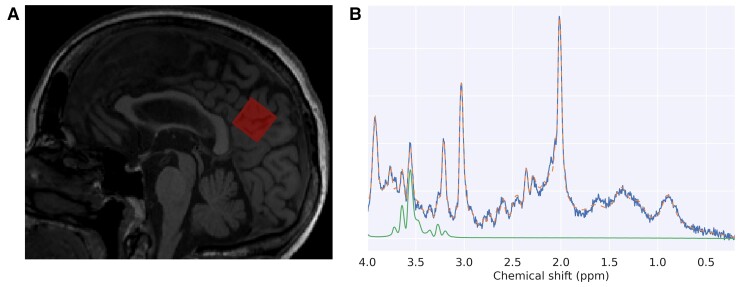
Both MRS and MRI data were acquired on a Siemens Prisma 3T scanner with a 64-channel receiver-coil array (Siemens Healthcare). A 2 × 2 × 2 cm3 MRS voxel was placed on the mid-sagittal plane in the PCC/precuneus area30 (Fig. 1). Single-voxel MRS data were acquired with point-resolved spectroscopy sequence with the following parameters: echo time (TE) = 30 ms; repetition time (TR) = 2000 ms; flip angle = 90 degrees; number of time domain points = 1024, spectral bandwidth = 1200 Hz. Magnetization-prepared rapid gradient-echo anatomical images were acquired with the following acquisition parameters: inversion time = 1100 ms; flip angle = 9 degrees; TE = 2.54 ms; echo spacing = 7.3 ms; TR = 1900 ms; receiver bandwidth = 220 Hz/pixel; and voxel size = 1 × 1 × 1 mm3. Generalized autocalibrating partially parallel acquisitions (Griswold et al., 2002) was applied with acceleration factor of 2 and 24 reference lines.
Figure 1.
Location of the MRS volume. (A) Example of a mid-sagittal slice from one participant depicting the location of the MRS volume in red (posterior cingulate cortex/precuneus). (B) Example of an MRS spectra from the same participant in panel A. The blue line represents the data while the orange dashed line represents the global fit from LCmodel software. The green line depicts the specific fit of myo-inositol from LCmodel.
Magnetic imaging spectroscopy analysis
Metabolite quantification was carried out using LCModel35 and referenced to total creatine [creatine (Cr) + Phosphocreatine (PCr) = tCr] concentration. The ratio to tCr is often used in clinical spectroscopy due to the relative stability of tCr.36 Five spectra were excluded from the analysis due to poor spectral quality (see Supplementary materials for the quality control procedure).
Plasma protocol and analysis
Blood was collected in six EDTA-plasma tubes and centrifuged [2000 g, +4° (C) for 10 min]. After that, plasma was aliquoted into 1.5-ml polypropylene tubes (1 ml plasma in each tube) and stored at −80°C within 30–60 min of collection. GFAP, a marker of astroglial activation and astrocytosis, was analyzed in 288 participants using Discovery kits for HD-X (Quanterix®, Billerica, MA, USA). The analysis was performed by board-certified laboratory technicians who were blinded to clinical diagnoses.
Statistical analysis
The relationships between demographic variables and clinical status (i.e., diagnosis) were evaluated with χ2 ANOVA and student t test (Table 1). To account for the possible confounding effect of the variable amount of grey matter present in the spectroscopic volume, mIns/tCr was modelled in a linear regression framework in the entire cohort against the fraction of grey matter in the volume (see Supplementary materials), and the residuals of this model were used for analyses. In this study, we are targeting the mIns/tCr concentration in the cortex as we measured both Aβ- and tau-PET uptake only in the cortical ribbon. Therefore, although the levels of mIns cannot be computed only from a specific tissue the proposed procedure should help in alleviating the potential confounding effect of the different amount of cortex included in the MRS volume.
The possible association between mIns/tCr ratio and PET retention were investigated using multiple regression analysis. For this purpose, the median values for Aβ-PET and tau-PET retention were derived from the same location of the MRS volume (precuneus/PCC area, see Supplementary materials for the specific procedure). Separate models were run on the full cohort including Aβ-PET or tau-PET retention as outcome (both from the location of the MRS volume), whereas mIns, APOE (coded as a binary value, i.e. ε4 carriers = 1, non-ε4 carriers = 0) and the interaction between the two were modelled as independent variables. The same analyses were repeated using a neocortical meta-ROI for Aβ-PET (prefrontal, lateral temporal, parietal, anterior cingulate, and posterior cingulate/precuneus) and a neocortical meta-ROI for tau-PET encompassing all stage of tau pathology (Braak I-VI).37 Based on previous results showing an association between plasma GFAP and Aβ accumulation,31 we also investigated the association between Aβ-PET retention and plasma GFAP and the potential interaction with APOE genotype running a similar multiple regression model using GFAP instead of mIns/tCr. We also investigated the possible association between GFAP and mIns/tCr in a linear regression framework. The interplay between Aβ, tau, astrocytic disfunction and APOE was further investigated by testing the potential mediation effect of mIns/tCr on the relationship between APOE genotype and Aβ-PET or tau-PET retention. The statistical significance of the mediation effect was estimated with bootstrapping (10 000 samples).38 Supplementary analyses using other commonly assessed metabolites (e.g. total N-acetylaspartate and total choline) were also performed although they were not the focus of the present report.
Sensitivity analyses in APOE ε4 carriers were also performed. First, voxel-wise maps of Aβ-PET and tau-PET retention were regressed against mIns/tCr in the PCC/precuneus. To this aim, the individual PET maps were registered to the MNI space using ANTs routines and restricted to grey matter using the previously generated mask. The voxel-wise analysis was performed with threshold-free, cluster-enhanced permutation statistics using FSL randomize (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise) with 10 000 permutations. Whole-brain statistical significance was set at the stringent family-wise error corrected threshold of P < 0.05. Second, considering that astrocytic dysfunction could have a significant impact on both Aβ and tau pathology, we investigated the potential mediation effect of mIns on the association between Aβ-PET and tau-PET to test whether astrocytic disfunctions contribute to the well-known relation between Aβ and tau accumulation. A further sensitivity analysis including only Aβ positive participants is reported in the Supplementary materials.
All models included age, sex and cognitive status (i.e. cognitive impaired or cognitive un-impaired) as covariates, as well as the tau-PET uptake when investigating Aβ and vice versa. A logarithmic transformation of the age variable was applied to better fit the normal distribution. Analyses were performed in Python 3.7.6 and R v3.6.1.
Data availability
Anonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with EU legislation on the general data protection regulation and decisions by the Swedish Ethical Review Authority and Region Skåne.
Results
Myo-inositol concentration is associated with Aβ-PET retention in APOE ε4 carriers
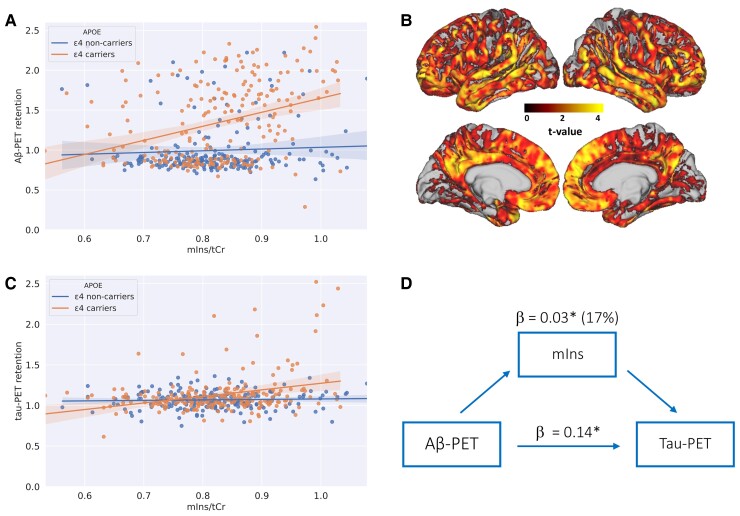
We first focused on the local association between Aβ-PET retention and mIns concentration measured in the same precuneus/PCC region. The results revealed a significant association between these two variables (model R2 = 0.48; mIns/tCr: β=0.36, P < 0.001). When introducing APOE genotype in the model, we found a significant interaction between APOE and mIns/tCr and no main effect of mIns/tCr, showing that the association between mIns and Aβ was present only in APOE ε4 carriers (model R2 = 0.54; mIns/tCr: β = −0.15, P > 0.5; mIns/tCr×APOE: β = 1.21, P < 0.001) (see Fig. 2A and Supplementary Table 1 for the complete statistical results). The analysis using Aβ-PET retention in a neocortical meta-ROI revealed the same results, suggesting that the association between Aβ and mIns/tCr and, most importantly, the effect of interaction with APOE, was not only a local phenomenon (model R2 = 0.54; mIns/tCr: β = −0.06, P > 0.4; mIns/tCr×APOE: β = 0.043, P < 0.001).
Figure 2.
Associations between myo-inositol and both Aβ-PET and tau-PET retention. SUVR, standardized uptake value ratio; mIns/tCr, myo-inositol to total creatine ratio. (A) Co-variation of mIns/tCr and Aβ-PET retention extracted from the same location of the MRS volume. The participants were stratified in APOE ε4 carriers and non-carriers. The translucent area around the regression line represents the 95% confidential interval for the regression estimate. For visualization purposes, the ratio between mIns and tCr was depicted but the statistical analysis was performed on the residualized ratio (see the methods section; Aβ-PET ∼ mIns/tCr: P < 0.001 in APOE ε4 carriers, P > 0.2 in APOE ε4 non-carriers). (B) Results of the voxel-wise analysis in the APOE ε4 carriers: highlighted clusters represent significant (P < 0.05 FWE) positive correlations between Aβ-PET retention and age corrected mIns/tCr from the precuneus region. The colour scale reflects the voxel-wise t-values. Results were projected to surface and overlaid onto MNI (Montreal Neurological Institute) 152 template space using the connectome Workbench (v1.2 https://www.humanconnectome.org/software/connectome-workbench). (C) Co-variation of mIns/tCr and tau-PET retention extracted from the same location of the MRS volume (Fig. 1A). The participants were stratified in APOE ε4 carriers and non-carriers. For visualization purposes, the ratio between mIns and tCr was depicted but the statistical analysis was performed on the residualized ratio (see the methods section; tau-PET ∼ mIns/tCr: P < 0.001 in APOE ε4 carriers, P > 0.3 in APOE ε4 non-carriers). (D) Flow chart representing the mediation analysis in the APOE ε4 carriers. Direct effect = association between Aβ-PET and tau-PET retention extracted from the same region (precuneus/PCC): β = 0.14, P < 0.05; mediation effect of mIns/tCr: β = 0.03, P < 0.05, mIns/tCr explained 17% (βratio) of the effect of Aβ-PET on tau-PET retention.
The voxel-wise analysis of the Aβ-PET data in the APOE ε4 carriers group provided converging evidence showing a positive association between mIns/tCr in the precuneus/PCC and widespread neocortical Aβ-PET retention, which encompassed temporal, parietal and frontal regions (see Fig. 2B). A sensitivity analysis performed in Aβ+ only participants also showed very consistent results (see Supplementary results).
Supplementary analyses of other commonly assessed metabolites (e.g., total N-acetylaspartate, and total choline) revealed no main association with Aβ and no interaction between metabolites levels and APOE (see Supplementary results).
Myo-inositol concentration is associated with tau-PET retention only in APOE ε4 carriers
In the full cohort, we found a significant association between tau-PET retention and mIns/tCr (model R2 = 0.17; mIns/tCr: β = 0.41, P < 0.01). When including APOE genotype, the analysis revealed a significant interaction between mIns/tCr and APOE and no main effect of mIns showing that also the association between tau-PET retention and mIns/tCr was present only in APOE ε4 carriers (model R2 = 0.19; mIns/tCr: β = 0.03, P > 0.8; mIns/tCr×APOE: β = 0.79, P < 0.01) (Fig. 2C). When we replaced tau-PET retention from the MRS volume with tau-PET retention from the neocortical meta-ROI, the interaction between mIns/tCr and APOE was still significant (mIns/tCr×APOE: β = 0.34, P < 0.05). However, when we included Aβ-PET retention in a neocortical meta-ROI in the model, no significant association between tau and mIns/tCr nor an effect of interaction between mIns/tCr and APOE was found (model R2 = 0.20; mIns/tCr: β = 0.09, P > 0.3; mIns/tCr×APOE: β = 0.22, P > 0.1) suggesting that the association of mIns/tCr with tau, was not independent of Aβ. The voxel-wise analyses of the tau-PET maps in the APOE ε4 carriers group revealed no significant associations between tau-PET retention and mIns/tCr.
Myo-inositol concentration mediate the association between Aβ-PET and tau-PET retention in APOE ε4 carriers
Considering the interaction effect of APOE on the association between mIns/tCr and both Aβ-PET and tau-PET retention, and the well-known association between Aβ-PET and tau-PET, we conducted a mediation analysis between these variables in the APOE ε4 carriers. The results revealed that the association between Aβ-PET and tau-PET retention, measured in the same precuneus/PCC region, was partially mediated by mIns concentration (β = 0.029, 95% CI = 0.002–0.07, P < 0.05, mediated effect = 17%) (Fig. 2D and the Supplementary results). In contrast, mIns/tCr does not appear to mediate the relationship between APOE genotype and Aβ-PET or tau-PET retention (both P > 0.9).
The association between plasma glial fibrillary acidic protein levels and Aβ-PET retention is independent of APOE
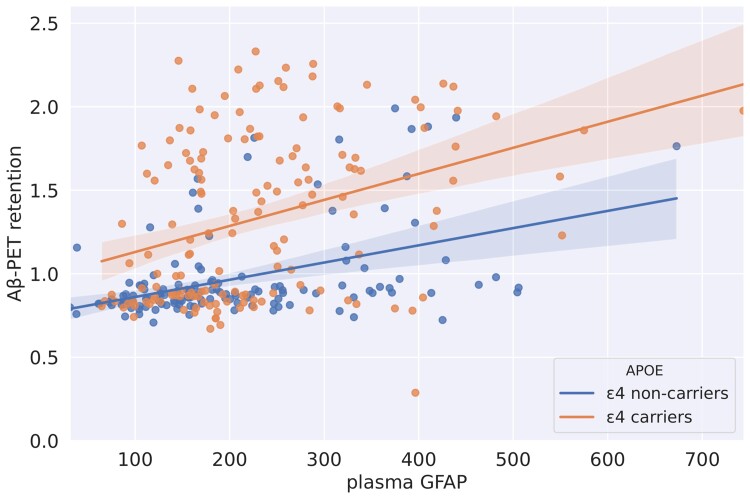
In the subgroup of participants (N = 288: 172 CU–Aβ−; 60 CU–Aβ+; 56 MCI–Aβ+) with available GFAP plasma data a regression model showed an association of Aβ-PET retention with GFAP levels (plasma–GFAP: β = 0.0006, P < 0.05) but no interaction between APOE and GFAP level (Plasma-GFAP×APOE: β = 0.0005, P > 0.1) (Fig. 3 and Supplementary Table 3). In addition, plasma GFAP levels were significantly associated with mIns/tCr but only in APOE ε4 carriers (APOE ε4 carriers: β = 293.73, P < 0.01; APOE ε4 non-carriers: β = 16.76, P > 0.8).
Figure 3.
Association between plasma GFAP levels and Aβ-SUVR values stratified by APOE genotype. SUVR, standardized uptake value ratio; GFAP, glial fibrillary acidic protein. Co-variation of plasma GFAP and Aβ-PET retention extracted from the same location of the MRS volume. The participants were stratified in APOE ε4 carriers and non-carriers (Aβ-PET∼GFAP: P < 0.001 in APOE ε4 carriers, P < 0.001 in APOE ε4 non-carriers). The analysis was run in a subset of 288 participant with available plasma GFAP. The translucent area around the regression line represents the 95% confidential interval for the regression estimate.
Discussion
The study showed that astrocytic function measured using mIns concentration in the precuneus/PCC region was positively associated with Aβ-PET retention in the same region but only in APOE ε4 carriers. Converging evidence from the voxel-wise analysis revealed wide-spread correlations between Aβ-PET retention in neocortical regions and mIns concentrations in the precuneus/PCC suggesting that the association between mIns and Aβ-PET was not limited to the specific location but reflected a broader relationship between mIns and Aβ. Our analyses also suggested that the link between mIns and Aβ is independent from tau accumulation. mIns also appeared to correlate with tau-PET retention only in APOE ε4 carriers and to partially mediate the association between Aβ-PET and tau-PET retention in such group. Interestingly, we also found an association between mIns and plasma level of GFAP but only in APOE ε4 carriers.
In the MRS literature, mIns is widely accepted as a glial marker. mIns has been suggested to be primarily or even exclusively present in glia and, with the highest concentrations, in astrocytes.13,14 mIns concentration has also been previously associated with Aβ accumulation in both cross sectional and longitudinal studies.22,24,25,39 However, the literature on the association between mIns and APOE genotype is mixed40 and evidence in favour or against a link between mIns, Alzheimer’s disease-related protein accumulation and APOE are even scarce.40 The present study provided data supporting of a clear link between APOE ε4, mIns and Aβ, from a large cohort of participants including prodromal and preclinical patients.
The lack of a mediation effect of mIns on the relationship between APOE and Aβ, could suggest that mIns reflected astrocytic response downstream to Aβ misfolding that is exacerbated by the APOE ε4 genotype. The significant mediation effect of mIns on the association between Aβ and tau mIns further suggested that such astrocytic response in APOE ε4 carriers has a deleterious effect on tau accumulation. Several in vitro studies showed that exogenously applied APOE ε4 has more pronounced pro-inflammatory activity compared with APOE ε3 in astrocytes and microglial cells,41,42 which could significantly affect the Aβ-related inflammatory response.43 This specific link is only one of the possible mechanisms that could bring together APOE, mIns and protein accumulation. For example, mIns plays an important role in various cellular processes as the structural basis for secondary messengers, including inositol triphosphates and phosphatidylinositol phosphate lipids44 which are involved in phagocytosis.45 Therefore, different levels of mIns could reflect a deficit in the clearing system of protein aggregates.
A further interesting result of the present study is the differential effect of APOE genotype on mIns concentration and plasma levels of GFAP. Although both mIns and GFAP have been considered to be markers of astrocytic activation, the two markers correlated to one another only in APOE ε4 carriers. Moreover, APOE did not appear to have a significant impact on the association between GFAP and Aβ. This result is in line with neuropathological evidence showing no difference in the quantification of reactive (GFAP-positive) astrocytes between APOE ε4 carriers and non-carriers.46 This observation should be confirmed by further studies, but it is intriguing to speculate that mIns and plasma GFAP might reflect different aspects of the astrocytic involvement in Alzheimer’s pathology.
The results presented here are not completely in line with results from previous studies. For example, Nedelska and colleagues24 found that APOE genotype did not alter the association between baseline mIns and Aβ accumulation over time. Similar results were found by Voevodskaya et al.25 although in this study, the authors did not model an interaction between APOE and mIns. The design of the two studies significantly differed from the design of the present study that was focus on cross-sectional analysis. Moreover, we acquired data using with a different generation of MRI scanner, which might have increased the sensitivity to subtle effects. The proportion of APOE ε4 carriers also differed across studies ranging from the 49% of the present study to the 29% of Nedelska et al24 and 37% of Voevodskaya et al.25. Such difference could also have had an impact on the sensitivity to the effect of APOE on the association between mIns and Alzheimer’s disease pathology.
Some limitation should be considered when interpreting the results of the study. A longitudinal design would be needed to test the temporal dynamic of the interplay between astrocytic response and both Aβ and tau accumulation and the impact of APOE genotype on such dynamic. In addition, the number of APOE ε4 homozygotes was too small to perform a dose-dependent analysis, whereas the number of APOE ε4 copies could have an important impact on the relationships we tried to elucidate. One of the novelties of the study is the comparison between mIns and the plasma level of GFAP as markers of astrocytic activity, but GFAP concentrations were not available for the entire cohort. However, the subgroup analysis including GFAP was still performed in a group of 288 participants. The lack of significant results in the voxel-wise analysis of the association between mIns and tau, also warrants further investigations. This negative result suggests that the association found at the regional level reflected a local phenomenon that did not generalize to other regions. However, this interpretation should be tested by sampling mIns levels in multiple volumes. It is also important to notice that, considering we are focusing on the early stages of the disease process, the amount of tau accumulation is still limited. This intrinsic limitation of the design could have affected our ability to detect associations between tau mIns and APOE genotype.
MRS is a non-invasive and inexpensive technique that can be easily applied in both research and clinical setting. The current results suggest that the quantification of mIns levels could provide a preferential astrocytic marker reflecting some of the impact of APOE ε4 allele on the pathophysiology of Alzheimer’s disease.
Supplementary Material
Abbreviations
- Aβ =
amyloid-beta
- ApoE =
apolipoprotein E
- APOE =
gene encoding the ApoE protein
- GFAP =
glial fibrillary acidic protein
- mIns =
myo-inositol
- PCC =
posterior cingulate cortex
- SUVR =
standardized uptake value ratio
- tCr =
total creatine
Contributor Information
Nicola Spotorno, Clinical Memory Research Unit, Department of Clinical Sciences, Malmö, Lund University, Lund, Sweden.
Chloé Najac, Department of Radiology, Leiden University Medical Center, Leiden, The Netherlands.
Erik Stomrud, Clinical Memory Research Unit, Department of Clinical Sciences, Malmö, Lund University, Lund, Sweden; Memory Clinic, Skåne University Hospital, Malmö, Sweden.
Niklas Mattsson-Carlgren, Clinical Memory Research Unit, Department of Clinical Sciences, Malmö, Lund University, Lund, Sweden; Department of Neurology, Skåne University Hospital, Lund University, Lund, Sweden; Wallenberg Center for Molecular Medicine, Lund University, Lund, Sweden.
Sebastian Palmqvist, Clinical Memory Research Unit, Department of Clinical Sciences, Malmö, Lund University, Lund, Sweden; Memory Clinic, Skåne University Hospital, Malmö, Sweden.
Danielle van Westen, Image and Function, Skane University Hospital, Lund, Sweden; Diagnostic Radiology, Institution for Clinical Sciences, Lund University, Lund, Sweden.
Itamar Ronen, Department of Radiology, Leiden University Medical Center, Leiden, The Netherlands.
Oskar Hansson, Clinical Memory Research Unit, Department of Clinical Sciences, Malmö, Lund University, Lund, Sweden; Memory Clinic, Skåne University Hospital, Malmö, Sweden.
Funding
The work presented was supported by the Swedish Research Council (2016-00906), the Knut and Alice Wallenberg foundation (2017-0383), the Marianne and Marcus Wallenberg foundation (2015.0125), the Swedish Alzheimer Foundation (AF-939932), the Swedish Brain Foundation (FO2021-0293), the Skåne University Hospital Foundation (2020-O000028), Regionalt Forskningsstöd (2020-0314), and the Swedish federal government under the ALF agreement (2018-Projekt0279). The precursor of 18F-flutemetamol was sponsored by GE Healthcare. The precursor of 18F-RO948 was provided by Roche. The funding sources had no role in the design and conduct of the study; in the collection, analysis, interpretation of the data; or in the preparation, review or approval of the manuscript.
Competing interests
O.H. has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from Roche, Genentech, Siemens, Biogen, Alzpath and Cerveau. The other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain Communications online.
References
- 1. Arranz AM, De Strooper B. The role of astroglia in Alzheimer’s disease: Pathophysiology and clinical implications. Lancet Neurol. 2019;18:406–414. [DOI] [PubMed] [Google Scholar]
- 2. Lin YT, Seo J, Gao F, et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98:1141–1154.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sienski G, Narayan P, Bonner JM, et al. APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci Transl Med. 2021;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao J, Davis MD, Martens YA, et al. APOE ɛ4/ɛ4 diminishes neurotrophic function of human iPSC-derived astrocytes. Hum Mol Genet. 2017;26:2690–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung WS, Verghese PB, Chakraborty C, et al. Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc Natl Acad Sci USA. 2016;113:10186–10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen WT, Lu A, Craessaerts K, et al. Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell. 2020;182:976–991.e19. [DOI] [PubMed] [Google Scholar]
- 7. Verkhratsky A, Zorec R, Rodríguez JJ, Parpura V. Astroglia dynamics in ageing and Alzheimer’s disease. Curr Opin Pharmacol. 2016;26:74–79. [DOI] [PubMed] [Google Scholar]
- 8. Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. [DOI] [PubMed] [Google Scholar]
- 9. Rodríguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2016:323:170–182. [DOI] [PubMed] [Google Scholar]
- 10. Chiarini A, Armato U, Gardenal E, Gui L, Dal Prà I. Amyloid β-exposed human astrocytes overproduce phospho-tau and overrelease it within exosomes, effects suppressed by calcilytic NPS 2143-Further implications for Alzheimer’s therapy. Front Neurosci. 2017;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martini-Stoica H, Cole AL, Swartzlander DB, et al. TFEB enhances astroglial uptake of extracellular tau species and reduces tau spreading. J Exp Med. 2018;215:2355–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi Y, Yamada K, Liddelow SA, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017:549:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glanville NT, Byers DM, Cook HW, Spence MW, Palmer FB. Differences in the metabolism of inositol and phosphoinositides by cultured cells of neuronal and glial origin. Biochim Biophys Acta. 1989;1004:169–179. [DOI] [PubMed] [Google Scholar]
- 14. Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. [DOI] [PubMed] [Google Scholar]
- 15. Bouveault L. De l’isomérie optique dans les corps a chaines fermés. Bull Soc Chim Fr. 1894;11:44–147. [Google Scholar]
- 16. Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: Homeostasis, metabolism and functional significance. J Neurochem. 2002;82:736–754. [DOI] [PubMed] [Google Scholar]
- 17. Ratai EM, Alshikho MJ, Zürcher NR, et al. Integrated imaging of [11C]-PBR28 PET. MR diffusion and magnetic resonance spectroscopy:1H. -MRS in amyotrophic lateral sclerosis. NeuroImage Clin. 2018;20:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heckova E, Dal-Bianco A, Strasser B, et al. Extensive brain pathologic alterations detected with 7.0-T MR spectroscopic imaging associated with disability in multiple sclerosis. Radiology. 2022;303:141–150. [DOI] [PubMed] [Google Scholar]
- 19. Bitsch A, Bruhn H, Vougioukas V, et al. Inflammatory CNS demyelination: Histopathologic correlation with in vivo quantitative proton MR spectroscopy. Am J Neuroradiol. 1999;20:1619–1627. [PMC free article] [PubMed] [Google Scholar]
- 20. Fernando KTM, McLean MA, Chard DT, et al. Elevated white matter myo-inositol in clinically isolated syndromes suggestive of multiple sclerosis. Brain. 2004;127:1361–1369. [DOI] [PubMed] [Google Scholar]
- 21. Ciccarelli O, Thomas DL, De Vita E, et al. Low myo-inositol indicating astrocytic damage in a case series of neuromyelitis optica. Ann Neurol. 2013;74:301–305. [DOI] [PubMed] [Google Scholar]
- 22. Voevodskaya O, Sundgren PC, Strandberg O, et al. Myo-inositol changes precede amyloid pathology and relate to APOE genotype in Alzheimer disease. Neurology. 2016;86:1754–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kantarci K, Smith GE, Ivnik RJ, et al. 1H magnetic resonance spectroscopy, cognitive function, and apolipoprotein E genotype in normal aging, mild cognitive impairment and Alzheimer’s disease. J Int Neuropsychol Soc. 2002;8:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nedelska Z, Przybelski SA, Lesnick TG, et al. 1 H-MRS metabolites and rate of β-amyloid accumulation on serial PET in clinically normal adults. Neurology. 2017;89:1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Voevodskaya O, Poulakis K, Sundgren P, et al. Brain myoinositol as a potential marker of amyloid-related pathology: A longitudinal study. Neurology. 2019;92:E395–E405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gomar JJ, Gordon ML, Dickinson D, et al. APOE genotype modulates proton magnetic resonance spectroscopy metabolites in the aging brain. Biol Psychiatry. 2014;75:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Waragai M, Moriya M, Nojo T. Decreased N-acetyl aspartate/myo-inositol ratio in the posterior cingulate cortex shown by magnetic resonance spectroscopy may be one of the risk markers of preclinical Alzheimer’s disease: A 7-year follow-up study. J Alzheimer’s Dis. 2017;60:1411–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suri S, Emir U, Stagg CJ, et al. Effect of age and the APOE gene on metabolite concentrations in the posterior cingulate cortex. Neuroimage. 2017;152:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kantarci K, Jack CR, Xu Y, et al. Regional impairment patterns in mild cogntive impairment and Alzheimer ‘S disease a 1 H MRS study. Neurology. 2000;55:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Öz G, Alger JR, Barker PB, et al. Clinical proton Mr spectroscopy in central nervous system disorders. Radiology. 2014;270:658–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pereira JB, Janelidze S, Smith R, et al. Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer’s disease. Brain. 2021;144:3505–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leuzy A, Smith R, Ossenkoppele R, et al. Diagnostic performance of RO948 F 18 tau positron emission tomography in the differentiation of alzheimer disease from other neurodegenerative disorders. JAMA Neurol. 2020;77:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002;47:1202–1210. [DOI] [PubMed] [Google Scholar]
- 35. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Imaging. 1993;30:672–679. [DOI] [PubMed] [Google Scholar]
- 36. Valenzuela MJ, Sachdev P. Magnetic resonance spectroscopy in AD. Neurology. 2001;56:592–598. [DOI] [PubMed] [Google Scholar]
- 37. Cho H, Choi JY, Hwang MS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016;80:247–258. [DOI] [PubMed] [Google Scholar]
- 38. Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Commun Monogr. 2009;76:408–420. [Google Scholar]
- 39. Kantarci K, Lowe V, Przybelski SA, et al. Magnetic resonance spectroscopy, β-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology. 2011;77:951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Piersson AD, Mohamad M, Rajab F, Suppiah S. Cerebrospinal fluid amyloid beta, tau levels. Apolipoprotein, and:1H. -MRS brain metabolites in Alzheimer’s disease: A systematic review. Acad Radiol. 2021;28:1447–1463. [DOI] [PubMed] [Google Scholar]
- 41. Barger SW, Harmon AD. Microglial activationby Alzheimeramyloid precursor protein and modulationby apolipoprotein E Steven. Nature. 1997;388:878–881. [DOI] [PubMed] [Google Scholar]
- 42. Guo L, Ladu MJ, Van EL. A dual role for apolipoprotein E in neuroinflammation. J Mol Neurosci. 2004;23:205–212. [DOI] [PubMed] [Google Scholar]
- 43. Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. 2013;95:1811–1827. [DOI] [PubMed] [Google Scholar]
- 45. Desale SE, Chinnathambi S. Phosphoinositides signaling modulates microglial actin remodeling and phagocytosis in Alzheimer’s disease. Cell Commun Signal. 2021;19:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Serrano-Pozo A, Betensky RA, Frosch MP, Hyman BT. Plaque-associated local toxicity increases over the clinical course of Alzheimer disease. Am J Pathol. 2016;186:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with EU legislation on the general data protection regulation and decisions by the Swedish Ethical Review Authority and Region Skåne.