Abstract
Objective
Slowly expanding lesions (SELs), a subgroup of chronic white matter lesions that gradually expand over time, have been shown to predict disability accumulation in primary progressive multiple sclerosis (MS) disease. However, the relationships between SELs, acute lesion activity (ALA), overall chronic lesion activity (CLA) and disability progression are not well understood. In this study, we examined the ASCEND phase III clinical trial, which compared natalizumab with placebo in secondary progressive MS (SPMS).
Methods
Patients with complete imaging datasets between baseline and week 108 (N=600) were analysed for SEL prevalence (the number and volume of SELs), disability progression, ALA (assessed by gadolinium-enhancing lesions and new T2-hyperintense lesions) and CLA (assessed by T1-hypointense lesion volume increase within baseline T2-non-enhancing lesions identified as SELs and non-SELs).
Results
CLA in both SELs and non-SELs was greater in patients with SPMS with confirmed disability progression than in those with no progression. In the complete absence of ALA at baseline and on study, SEL prevalence was significantly lower, while CLA within non-SELs remained associated with disability progression. Natalizumab decreased SEL prevalence and CLA in SELs and non-SELs compared with placebo.
Conclusions
This study shows that CLA in patients with SPMS is decreased but persists in the absence of ALA and is associated with disability progression, highlighting the need for therapeutics targeting all mechanisms of CLA, including smouldering inflammation and neurodegeneration.
Trial registration number
Keywords: MRI, MULTIPLE SCLEROSIS
WHAT IS ALREADY KNOWN ON THIS TOPIC
It has been demonstrated that it is possible to generate markers of progressive biology in multiple sclerosis (MS) related to chronic lesion activity (CLA) using conventional MRI and that these markers are related to disability and progression. The relationship between acute inflammation and CLA, as well as the contribution to disease progression of CLA, in the absence of ongoing acute inflammation, remains unclear.
WHAT THIS STUDY ADDS
In this study, we demonstrate that ongoing CLA—measured as change over time in T1-hypointense lesion volume—in both slowly expanding lesions (SELs) and non-SELs is an imaging biomarker associated with disability progression in progressive forms of MS even in the absence of acute inflammation. This work also highlights that SEL prevalence and severity are substantially higher in the presence of acute inflammation, which indicates interdependencies between the underpinning pathobiology of acute and chronic lesion activity in MS.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
This study further demonstrates the proof of principle that readily accessible measures of CLA can be used as imaging biomarkers of MS progression. It also supports the need to continue to develop therapies targeting CLA, which may represent the pathological correlate for smouldering inflammation and neurodegenerative pathways of disease progression.
Introduction
Disability progression independent of relapse activity occurs despite highly effective anti-inflammatory disease-modifying therapies (DMTs) in both relapsing1 and progressive forms of multiple sclerosis (MS).2 3 The pathological continuum of MS, from acute and smouldering inflammation to neurodegeneration, includes effects on the brain parenchyma.4 5 Acute demyelination can lead to damage to the denuded axons, resulting in neurodegeneration and axonal loss. In the absence of acute demyelination (as with the use of highly effective anti-inflammatory DMTs), clinical decline continues to occur over the long term,3 6 suggesting that other pathological mechanisms may be responsible for MS disease progression. In this context, there is evidence that brain atrophy may be associated with increasing disability.7 8 Other pathologies such as leptomeningeal inflammation and subpial cortical lesions,9 which are captured by more advanced MRI acquisition methods, may also be associated with increasing disability.10 However, these advanced MRI methods have limitations: their use is generally restricted to specialised centres, and they have been assessed only in small studies thus far.11 There remains a need to identify readily accessible and validated imaging correlates of disability progression that can be used in large, multicentre clinical trials. Understanding the causes and pathophysiology of the chronic neurodegenerative component of the disease process is crucial in this effort.
Extensive histopathologic studies have unravelled the distinct features of white matter lesions in MS and allowed for their categorisation into subtypes: preactive lesions, active lesions, chronic inactive lesions or partly remyelinated plaques, and chronic active (eg, smouldering) or mixed active/inactive lesions.12–14 The extent of chronic active lesion burden correlates with clinical disease severity.13 These chronic active lesions feature a rim of predominantly M1-polarised activated microglia and macrophages, which are associated with continued breakdown of myelin and axonal loss.15 A major limitation of histopathologic studies is their inherently cross-sectional nature; they seldom connect with longitudinal premortem in vivo information on analysed tissue specimens. Recently, MRI methods have been developed to identify subtypes of lesions that might be consistent with the chronic active lesions described by pathologists, including lesions with paramagnetic rims16 and, more recently, slowly expanding lesions (SELs).17 SELs represent a subgroup of chronic lesions in the MS white matter that show gradual expansion over time. It has recently been shown that SELs show only partial correspondence with phase rim lesions (PRLs),18 suggesting that SELs with or without phase rims and PRLs with or without slow expansion represent different aspects or stages of MS pathology within chronic active lesions.
This study assessed chronic lesion activity (CLA) and acute lesion activity (ALA) in the brain white matter of a secondary progressive MS (SPMS) population. CLA as measured by change over time in T1-hypointense lesion volume (T1LV) in SELs and non-SELs was shown to predict disability progression in primary progressive MS.19 CLA was measured in this study by the change in T1LV from baseline to week 108 in SELs, non-SELs and total pre-existing chronic non-enhancing T2 (CNT2) lesions. ALA was defined by having either (1) gadolinium-enhancing (Gd+) T1 lesions at any timepoint in the trial up to week 108, including baseline or (2) any postbaseline new or enlarging T2 lesions. Specifically, we examined the following in an SPMS population treated with placebo: (1) the association between T1-weighted (T1w) MRI features of CLA in SELs and non-SELs and confirmed disability progression, (2) the association between CLA and confirmed disability progression in the absence of ALA and (3) the association between CLA and ALA. We also assessed the effect of natalizumab on SEL prevalence and overall CLA in SELs and non-SELs in patients with SPMS.
Materials and methods
Trial design, patients and MRI
The ASCEND Study was a two-part, multicentre, randomised, double-blind, placebo-controlled phase III study in patients with SPMS to assess the efficacy and safety of natalizumab. Details of the study design and outcomes have previously been described in detail.20 Axial T1w (3D-spoiled gradient echo: TR=28–35 ms; TE=4–11 ms; flip angle=27°–30°; resolution 0.98×0.98×3 mm) and Axial T2w (2D Fast Spin Echo: TR=4000–7400 ms; TE=58–95 ms; resolution=0.98×0.98×3 mm) were acquired at baseline, week 24, week 48, week 72, week 96 and week 108. The SEL analysis population represents the subset of the intention-to-treat population that had available T1w and T2-weighted (T2w) images at all timepoints from baseline to week 108 (including weeks 24, 48, 72 and 96).
Patient and public involvement
No patient was involved in the planning or design of the study.
Clinical measures of disability progression
Expanded Disability Status Scale (EDSS), Timed 25-Foot Walk (T25FW) and 9-Hole Peg Test (9HPT) assessments were performed at baseline and every 12 weeks through week 108. Composite confirmed disability progression was defined as meeting one or more of the following three criteria: an increase of ≥1.0 point from a baseline EDSS Score≤5.5 or an increase of ≥0.5 point from a baseline score≥6.0, an increase of ≥20% from baseline in T25FW time and/or an increase of ≥20% from baseline in 9HPT time (on either hand). Progression was confirmed at a subsequent visit ≥6 months after the possible start of progression and at the end of the trial. To minimise the possibility of capturing disability progression due to clinical relapses, included confirmed disability progression events could not have started or been confirmed ≤74 days after onset of an independent neurology evaluation committee-confirmed clinical relapse. Absence of ALA was defined as no baseline or postbaseline T1 gadolinium-enhancing (Gd+) lesions and no postbaseline new or enlarging T2-hyperintense lesions.
Identification of SELs, ALA and overall CLA
The process of SEL identification has been described in detail elsewhere.19 21 Briefly, SELs are contiguous regions of pre-existing T2 lesions showing constant and concentric local expansion from baseline to week 108. Prior to SEL detection, T2 lesions were identified in baseline scans using a semiautomated method in which a fully automated segmentation of T2 lesions was subsequently manually reviewed and corrected by a single-trained MRI reader.22 In the first stage of SEL detection, SEL candidates are identified as contiguous regions of ≥10 voxels in the baseline T2 lesion mask that (a) are not Gd+ and (b) show a minimum local volumetric expansion, as determined by the Jacobian determinant of the nonlinear deformation between the baseline and week 108 scans. The second stage of SEL detection scores each SEL candidate in turn based on the concentricity and constancy of expansion across time. Considering local expansion between baseline and each intermediate timepoint (weeks 24, 48, 72 and 96) allows for the identification of SEL candidates undergoing constant and gradual expansion across time, while measuring concentricity allows for the identification of SEL candidates exhibiting inside-out radial expansion. Each SEL candidate is assigned an SEL Score, calculated as the sum of the mean normalised measures for constancy and concentricity.
SELs were identified in patients with SPMS from the ASCEND phase III clinical trial. Non-SELs, defined as the portion of the non-enhancing baseline T2 lesion mask not identified as SELs, were also assessed, as were the totality of non-enhancing T2 lesions. The ASCEND SEL analysis population (placebo, n=292; natalizumab, n=308) represents the subset of patients who had available T1w and T2w images at all timepoints from baseline to week 108. Results are presented for SELs with SEL Score≥0. The distribution of SELs and non-SELs by sex, baseline EDSS, age and disease duration are provided in online supplemental tables S1–S3.
bmjno-2021-000240supp001.pdf (89.4KB, pdf)
ALA was defined by having either (1) Gd+ T1 lesions at any timepoint in the trial up to week 108, including baseline or (2) any postbaseline new or enlarging T2 lesions. Gd+ T1 lesions were determined as a consensus of two fully manual identifications by two trained MRI readers, where any discrepancies were adjudicated by a third independent reader. New or enlarging T2 lesions were determined by comparing T2 lesion masks at successive timepoints and automatically identifying focal areas of new T2 lesions, which were not present at the previous timepoint and showed a minimum increase in T2w intensity. These focal areas of new T2 lesions could be entirely in normal-appearing white matter (new) or adjacent to pre-existing T2 lesions (enlarging). All automatically identified new or enlarging T2 lesions were manually reviewed and corrected where necessary. It is important to appreciate that the SEL approach to the detection of lesion expansion is fundamentally different from that used for the detection of so-called new ‘T2 enlarging lesions’, commonly reported in counts of ‘new or enlarging T2 lesions’ in clinical trials (see also Arnold et al23). Methods for the detection of ‘enlarging’ lesions in the context of ‘new or enlarging T2 lesion counts’ (which vary from laboratory to laboratory) have been designed to detect what are essentially new foci of acute white matter lesion activity that are connected by adjacency to areas of pre-existing T2-signal abnormality and therefore may not qualify as ‘de novo’ new lesions (which by definition have to be surrounded by normal-appearing WM).
CLA was measured by the change in T1LV from baseline to week 108 in SELs, non-SELs and total pre-existing CNT2 lesions in patients with SPMS in the placebo arm. T1-hypointense lesions were defined as areas of T2 lesion not showing gadolinium enhancement and with T1w intensity less than or equal to median T1w intensity of grey matter.
Whole brain atrophy was measured via Jacobian integration.24
Statistical analyses
The statistical analysis of SEL data was exploratory and included all patients from ASCEND with no missing or non-evaluable T1w and T2w scans at any timepoint (baseline to week 108; SEL analysis population). No imputation of missing data was performed.
A two-sample proportion test was applied to compare baseline Gd+ T1 lesions between the two treatment groups. CLA was compared between progressors and non-progressors using the Van Elteren test, stratified for progression status, baseline EDSS Score (≤5.5 or ≥6.0) and baseline T2 lesion volume (T2LV) category based on tertiles (≤6908.79 mm3, >6908.79–18 818.49 mm3 and >18 818.49 mm3). Analyses of the association between ALA and SEL prevalence were based on the Van Elteren test, stratified for ALA, baseline EDSS Score (≤5.5 or ≥6.0) and baseline T2LV category based on tertiles (≤6908.79 mm3, >6908.79–18 818.49 mm3 and >18 818.49 mm3). Comparisons of CLA between placebo and natalizumab were based on the Van Elteren test, stratified for treatment, baseline EDSS Score (≤5.5 or≥6.0) and baseline T2LV category based on tertiles (≤6908.79 mm3, >6908.79–18 818.49 mm3 and >18 818.49 mm3). Statistical tests were two-sided and conducted at the 5% significance level without adjustment for multiplicity.
Results
Baseline demographics and brain MRI characteristics of the SPMS analysis population
The baseline demographics and brain MRI characteristics of the analysis population available for SEL detection and the intention-to-treat population from the ASCEND Study dataset are presented in table 1. Age and sex were distributed similarly across the SEL analysis and intention-to-treat populations and between treatment arms. In the SEL analysis population, a greater percentage of natalizumab-treated than placebo-treated individuals had ≥1 Gd+ T1 lesion at baseline (28% vs 19%), but the difference was not statistically significant. In the SEL analysis population, the natalizumab-treated and placebo-treated groups had a similar mean T2LV at baseline (18.1 vs 16.5 cm3). Mean normalised brain volume at baseline was also similar in the two treatment groups.
Table 1.
Baseline characteristics of ASCEND analysis population
| Baseline characteristics | SEL analysis population | ITT population | ||
| Placebo (n=292) |
Natalizumab (n=308) |
Placebo (n=448) |
Natalizumab (n=439) |
|
| Age, mean (SD) | 47.8 (7.6) | 47.4 (7.2) | 47.2 (7.8) | 47.3 (7.4) |
| Female, % | 65 | 62 | 63 | 62 |
| Patients with ≥1 Gd+ T1 lesion, % | 19 | 28 | 22 | 26 |
| Mean (SD) T2LV, cm3 | 16.5 (16.7) | 18.1 (18.5) | 16.2 (16.4) | 17.4 (17.6) |
| Normalised brain volume, mean (SD), cm3 | 1429.8 (81.64) | 1422.0 (81.75) | 1425.8 (83.1) | 1420.9 (82.8) |
ASCEND SEL analysis population represents the subset of the ITT population that had available T1-weighted and T2-weighted images at all timepoints from baseline to week 108 (including weeks 24, 48, 72 and 96).
Gd+, gadolinium enhancing; ITT, intention to treat; SEL, slowly expanding lesion; T1, T1-hypointense; T2LV, T2-hyperintense lesion volume.
Disability progression in SPMS was associated with greater CLA in the placebo arm
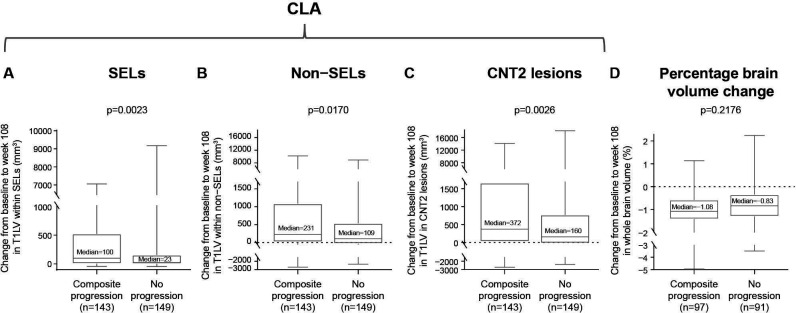
CLA was measured by the change in T1LV from baseline to week 108 in SELs, non-SELs and total pre-existing CNT2 lesions in patients with SPMS treated with placebo. The analysis was restricted to the placebo group to avoid treatment effects, as natalizumab decreases ALA. CLA was compared in patients with composite disability progression (n=143) and patients who remained progression free (n=149). Patients with SPMS with confirmed composite disability progression had significantly more severe CLA than those who were progression free, as measured by T1LV change within SELs, non-SELs and CNT2 lesions (median increase (Q1, Q3) progressors vs non-progressors: 100 (3, 524) vs 23 (0, 155) mm3, p=0.0023; 231 (17, 1090) vs 109 (−29, 538) mm3, p=0.0170; and 372 (26, 1662) vs 160 (−23, 770) mm3, p=0.0026, respectively; figure 1A–C). In contrast, the brain atrophy rate as measured by whole brain volume change from baseline to week 108 did not differ significantly between patients with SPMS with and without composite confirmed disability progression (p=0.2176; figure 1D).
Figure 1.
Association between CLA or whole brain volume loss and composite disability progression in patients with SPMS. Change from baseline to week 108 in T1LV in (A) SELs, (B) non-SELs and (C) CNT2 lesions was significantly associated with composite disability progression in patients with SPMS treated with placebo. No difference in percentage change from baseline to week 108 in (D) whole brain volume was observed in patients with SPMS with composite disability progression compared with those with no progression. Composite progression was confirmed at 24 weeks and end of study on one or more of the EDSS, Timed 25-Foot Walk or 9-Hole Peg Test. In these box-and-whisker representations, the box spans the interquartile range, the median is marked by the horizontal line inside the box and the whiskers are the two lines outside the box that extend to the highest and lowest observations. P values by Van Elteren test; stratified by baseline EDSS Score (≤5.5 or ≥6.0) and baseline T2 lesion volume category based on tertiles (≤6908.79 mm3, >6908.79–18 818.49 mm3 and >18 818.49 mm3). CLA, chronic lesion activity; CNT2, chronic non-enhancing T2; EDSS, Expanded Disability Status Scale; SEL, slowly expanding lesion; SPMS, secondary progressive multiple sclerosis; T1LV, T1-hypointense lesion volume.
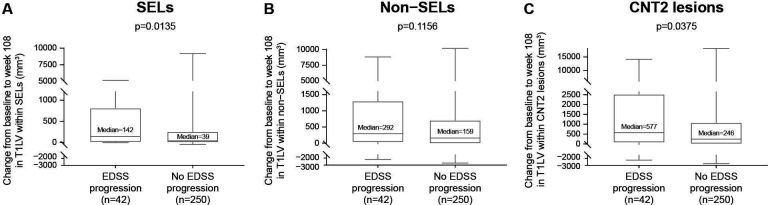
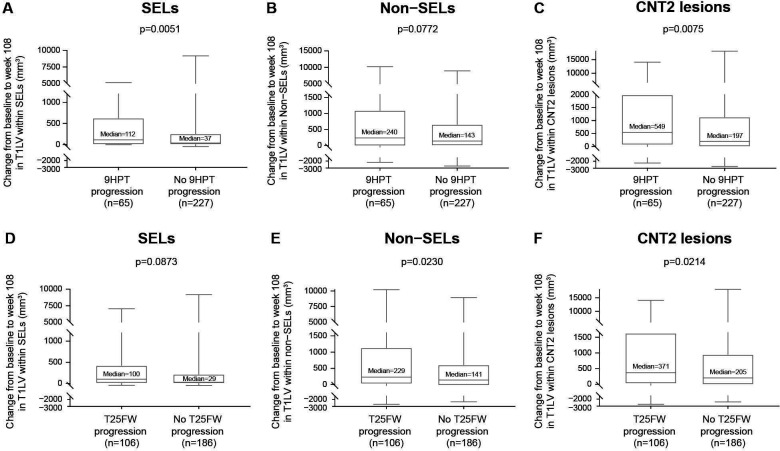
When confirmed disability progression was based only on EDSS Score, CLA as measured by T1LV increase was significantly greater in progressors than non-progressors within SELs (median increase (Q1, Q3) progressors vs non-progressors: 142 (6, 815) vs 39 (0, 258) mm3, p=0.0135; figure 2A) and CNT2 lesions (median increase (Q1, Q3) progressors vs non-progressors: 577 (66, 2529) vs 246 (0, 1090) mm3, p=0.0375; figure 2C), with a consistent trend in the same direction in non-SELs (median increase (Q1, Q3) progressors vs non-progressors: 292 (26, 1302) vs 159 (−9, 710) mm3, p=0.1156; figure 2B). Similarly, when confirmed disability progression was based only on 9HPT Score, CLA as measured by T1LV increase was significantly greater in progressors than non-progressors within SELs (median increase (Q1, Q3) progressors vs non-progressors: 112 (0, 629) vs 37 (0, 258) mm3, p=0.0051; figure 3A) and CNT2 lesions (median increase (Q1, Q3) progressors vs non-progressors: 549 (66, 1995) vs 197 (0, 1139) mm3, p=0.0075; figure 3C) and numerically greater in progressors than non-progressors within non-SELs (median increase (Q1, Q3) progressors vs non-progressors: 240 (−9, 1097) vs 143 (−3, 661) mm3, p=0.0772; figure 3B). Finally, when confirmed disability progression was based only on T25FW, CLA as measured by T1LV increase was significantly greater in progressors than non-progressors within non-SELs (median increase (Q1, Q3) progressors vs non-progressors: 229 (20, 1133) vs 141 (−25, 609) mm3, p=0.0230; figure 3E) and CNT2 lesions (median increase (Q1, Q3) progressors vs non-progressors: 371 (23, 1634) vs 205 (−6, 954) mm3, p=0.0214; figure 3F) and numerically greater in progressors than non-progressors within SELs (median increase (Q1, Q3) progressors vs non-progressors: 100 (3, 426) vs 29 (0, 220) mm3, p=0.0873; figure 3D).
Figure 2.
Association between CLA and EDSS progression. Increase from baseline to week 108 in T1LV within (A) SELs and (C) CNT2 lesions but not (B) non-SELs was associated with EDSS progression in patients with SPMS treated with placebo. (However, a consistent directional trend was observed in non-SELs.) EDSS progression was confirmed at 24 weeks and end of study. In these box-and-whisker representations, the box spans the interquartile range, the median is marked by the horizontal line inside the box and the whiskers are the two lines outside the box that extend to the highest and lowest observations. P values by Van Elteren test; stratified by baseline EDSS Score (≤5.5 or ≥6.0) and by baseline T2 lesion volume category based on tertiles (≤6908.79 mm3, >6908.79–18 818.49 mm3 and >18 818.49 mm3). CLA, chronic lesion activity; CNT2, chronic non-enhancing T2; EDSS, Expanded Disability Status Scale; SEL, slowly expanding lesion; SPMS, secondary progressive multiple sclerosis; T1LV, T1-hypointense lesion volume.
Figure 3.
Association between CLA and 9HPT progression and between CLA and T25FW progression. Change from baseline to week 108 in T1LV within (A) SELs and (C) CNT2 lesions but not (B) non-SELs was associated with 9HPT progression in patients with SPMS treated with placebo. (However, a consistent directional trend was observed in non-SELs.) Change in T1LV from baseline to week 108 within (E) non-SELs and (F) CNT2 lesions but not (D) SELs was associated with T25FW progression in patients with SPMS treated with placebo. (However, a consistent directional trend was observed in SELs.) 9HPT progression and T25FW progression were confirmed at 24 weeks and end of study. In these box-and-whisker representations, the box spans the interquartile range, the median is marked by the horizontal line inside the box and the whiskers are the two lines outside the box that extend to the highest and lowest observations. P values by Van Elteren test; stratified by baseline EDSS Score (≤5.5 or ≥6.0) and by baseline T2 lesion volume category based on tertiles (≤6908.79 mm3, >6908.79–18 818.49 mm3 and >18 818.49 mm3). 9HPT, 9-Hole Peg Test; CLA, chronic lesion activity; CNT2, chronic non-enhancing T2; EDSS, Expanded Disability Status Scale; SEL, slowly expanding lesion; SPMS, secondary progressive multiple sclerosis; T1LV, T1-hypointense lesion volume; T25FW, Timed 25-Foot Walk.
Disability progression in SPMS remained associated with greater CLA in the complete absence of ALA
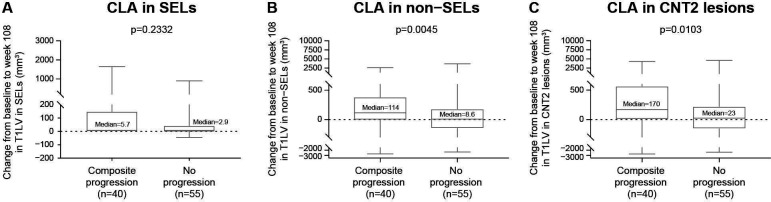
From baseline to week 108, 95 of 292 placebo patients with SPMS had no ALA, defined as no baseline or postbaseline Gd+ T1 lesions and no postbaseline new/enlarging T2 lesions. CLA in patients with SPMS with no ALA was compared in patients with composite confirmed disability progression (n=40) and patients who remained progression free (n=55). Patients exhibiting composite progression had more severe CLA than those who were progression free, as shown by a significant difference in T1LV change within non-SELs (p=0.0045; figure 4B) and CNT2 lesions (p=0.0103; figure 4C) and a trend in the same direction was observed in SELs (p=0.2332; figure 4A). Similar findings were observed in the natalizumab arm (online supplemental figure S1).
Figure 4.
Association between CLA and composite disability progression in the absence of ALA. CLA in (B) non-SELs and (C) CNT2 lesions but not (A) SELs remained associated with composite disability progression in the absence of ALA in patients with SPMS treated with placebo. (However, a consistent directional trend was observed in SELs.) Absence of acute lesion activity was defined as no baseline and postbaseline T1 gadolinium-enhancing and no postbaseline new/enlarging T2 lesions. Composite progression was confirmed at 24 weeks and end of study on one or more of the EDSS, Timed 25-Foot Walk or 9-Hole Peg Test. In these box-and-whisker representations, the box spans the interquartile range, the median is marked by the horizontal line inside the box and the whiskers are the two lines outside the box that extend to the highest and lowest observations. P values by Van Elteren test; stratified by baseline EDSS Score (≤5.5 or ≥6.0) and baseline T2 lesion volume category based on tertiles (≤6908.79 mm3, >6908.79–18 818.49 mm3 and >18 818.49 mm3). ALA, acute lesion activity; CLA, chronic lesion activity; CNT2, chronic non-enhancing T2; EDSS, Expanded Disability Status Scale; SEL, slowly expanding lesion; SPMS, secondary progressive multiple sclerosis; T1LV, T1-hypointense lesion volume.
bmjno-2021-000240supp002.pdf (119.7KB, pdf)
SEL prevalence in patients with SPMS treated with placebo was lower in the absence of ALA
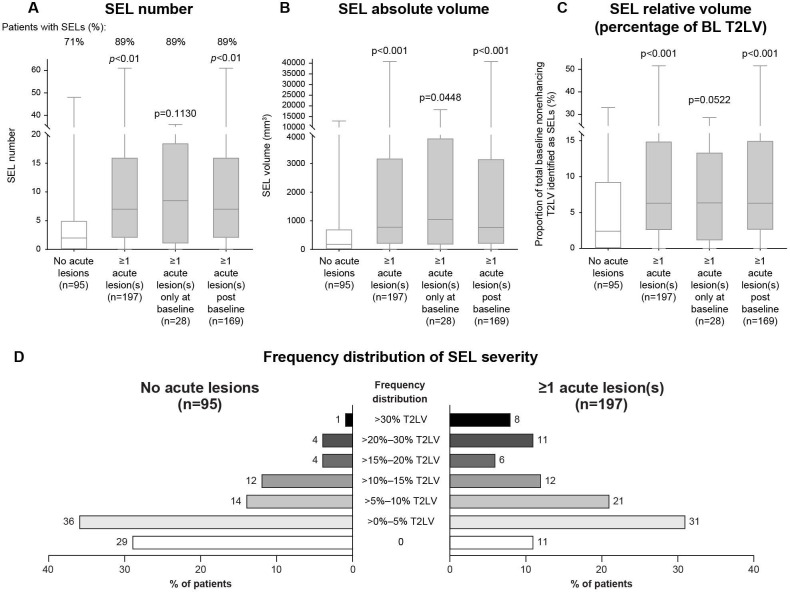
Placebo patients with SPMS with ALA had a higher SEL prevalence as measured by SEL number and volume (based on T2w borders of SELs at baseline) and a higher proportion of baseline T2LV identified as SELs than patients with no ALA (figure 5A–C). The proportion of patients with at least one SEL was also higher in patients with ALA (89%, n=197) in comparison with those with no ALA (71%, n=95). Analysis of the differences in SEL prevalence between patients with SPMS with no ALA (n=95) and those with ALA at baseline only (n=28) or postbaseline (n=169) confirmed that both baseline and postbaseline ALA were associated with a higher SEL prevalence, though the sample size of patients with ALA at baseline only was too small for the associated SEL prevalence to reach significance with respect to SEL number and relative volume (figure 5A, C). An analysis of the frequency distribution of patients by range of SEL prevalence also showed that 19% of patients with ALA had >20% of their total T2 lesion burden identified as SELs, compared with only 5% of patients with no ALA (figure 5D).
Figure 5.
Prevalence of SELs and frequency distribution of SEL severity in the presence versus absence of ALA. SEL (A) number, (B) absolute volume and (C) relative volume (percentage of baseline non-enhancing T2LV) were greater in patients with SPMS treated with placebo who had ALA compared with those with no ALA. (D) The frequency distribution of patients by range of SEL prevalence indicates that patients with ALA had a greater percentage of their total T2 lesion burden identified as SELs compared with patients with no ALA. In these box-and-whisker representations, the box spans the interquartile range, the median is marked by the horizontal line inside the box and the whiskers are the two lines outside the box that extend to the highest and lowest observations. No acute lesion activity was defined as no baseline or postbaseline Gd+ T1 lesions and no postbaseline new/enlarging T2 lesions in weeks 24, 48, 72, 96 and 108. Acute lesions were defined as baseline Gd+ T1 lesions and postbaseline Gd+ T1 lesions and new/enlarging T2 lesions in weeks 24, 48, 72, 96 and 108. P values by Van Elteren test: stratified by baseline EDSS Score (≤5.5 or ≥6.0) and baseline T2LV category based on tertiles (≤6908.79 mm3, >6908.79–18 818.49 mm3 and >18 818.49 mm3). ALA, acute lesion activity; BL, baseline; EDSS, Expanded Disability Status Scale; Gd+, gadolinium enhancing; SEL, slowly expanding lesion; SPMS, secondary progressive multiple sclerosis; T2LV, T2-hyperintense lesion volume.
Natalizumab versus placebo effect on SELs and CLA in patients with SPMS
The placebo-treated and natalizumab-treated arms had similar percentages of patients with ≥1 SEL detected from baseline to week 108 (83% vs 79%).
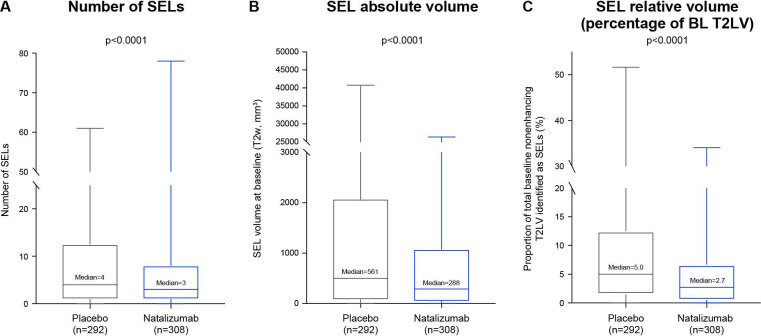
Natalizumab was associated with lower SEL prevalence than placebo, as indicated by a lower number of SELs (median number, 3 vs 4; p<0.0001; figure 6A) and a lower absolute SEL volume (median T2w SEL volume at baseline, 288 vs 561 mm3; p<0.0001; figure 6B). Accordingly, the proportion of total baseline non-enhancing T2LV longitudinally identified as SELs was significantly lower in natalizumab-treated than placebo-treated patients (median proportion, 2.7% vs 5.0%; p<0.0001; figure 6C).
Figure 6.
Effect of natalizumab on SEL prevalence. Natalizumab reduced the (A) number, (B) absolute volume and (C) relative volume (percentage of baseline non-enhancing T2LV) of SELs in patients with SPMS. Box-and-whisker representations: the box spans the interquartile range, the median is marked by the horizontal line inside the box and the whiskers are the two lines outside the box that extend to the highest and lowest observations. P values by Van Elteren test; stratified by baseline Expanded Disability Status Scale Score (≤5.5 or ≥6.0) and baseline T2LV category based on tertiles (≤6908.79 mm3, >6908.79–18 818.49 mm3 and >18 818.49 mm3). BL, baseline; SEL, slowly expanding lesion; SPMS, secondary progressive multiple sclerosis; T2LV, T2-hyperintense lesion volume; T2w, T2 weighted.
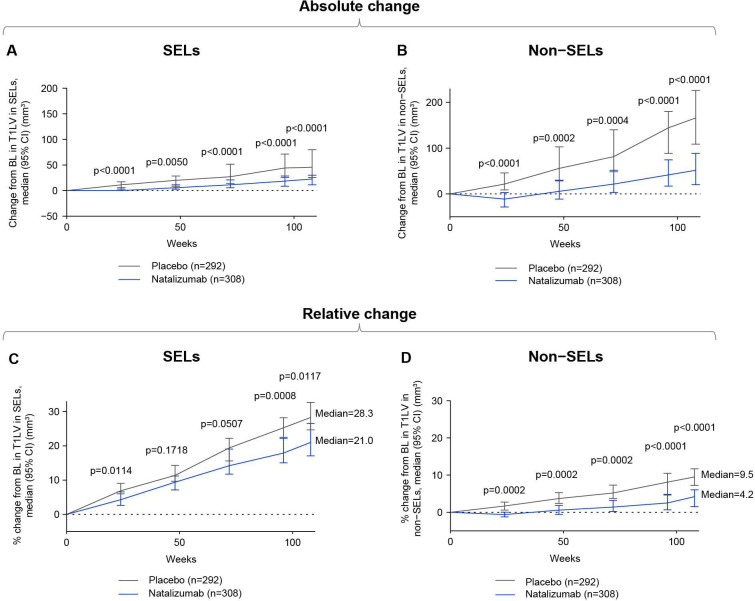
Brain tissue loss associated with CLA as measured by absolute and relative T1LV accumulation was lower in natalizumab-treated than placebo-treated patients within both SELs (figure 7A, C) and non-SELs (figure 7B, D).
Figure 7.
Change in CLA with natalizumab versus placebo. Natalizumab reduced CLA as measured by both (A, B) absolute increase and (C, D) percentage increase in T1LV in SELs and non-SELs compared with placebo in patients with SPMS. Distribution-free quantile confidence limits are displayed. P values by Van Elteren test; stratified by baseline EDSS Score (≤5.5 or ≥6.0) and baseline T2 lesion volume category based on tertiles (≤6908.79 mm3, >6908.79–18 818.49 mm3 and >18 818.49 mm3). BL, baseline; CLA, chronic white matter lesion activity; EDSS, Expanded Disability Status Scale; SPMS, secondary progressive multiple sclerosis; T1LV, T1-hypointense lesion volume.
Discussion
SELs represent a subgroup of MS chronic white matter lesions with continual expansion and tissue destruction and predict clinical progression in progressive-onset MS.19 21 25 In SELs, there is an accumulation over time of T1LV and changes in imaging metrics reflective of gradual microstructural tissue alteration, including a decrease in magnetisation transfer ratio and an increase in diffusion tensor imaging radial diffusivity.25 These findings suggest chronic demyelinating processes and continual axonal/neuronal destruction,25 which have been seen in pathology studies of chronic active lesions.12 13 However, it was recently reported that anti-inflammatory DMTs such as ocrelizumab19 and natalizumab26 may have a modest effect on MRI measures of brain tissue loss in SELs.
In this study, SELs were identified in most patients with SPMS, and confirmed disability progression was associated with more severe CLA as measured by T1LV accumulation in SELs but also in non-SELs. A recent histological case report has confirmed the presence of signs of chronic active lesion pathology in SELs but also in non-SEL tissue.17 These findings are consistent with previously reported findings in relapsing patients with MS in the OPERA I and II dataset and primary progressive patients with MS in the ORATORIO dataset.19 21 Patients with SPMS who progressed in a natural history setting had greater SEL prevalence and more severe CLA as measured by T1LV increase within SELs, non-SELs and total baseline non-enhancing T2 lesions. In the absence of ALA in patients with SPMS, SEL prevalence (as shown by number and volume of SELs) was reduced, though >70% of patients had ≥1 SEL. Importantly, the association of confirmed disability progression with more severe CLA in patients with SPMS remained significant in the absence of ALA. Consistent findings were reported in placebo-treated primary progressive patients with MS in the ORATORIO dataset, in whom ALA, CLA and whole brain atrophy were measured and only CLA in SELs and non-SELs predicted confirmed disability progression over time.19 This indicates that brain tissue loss associated with CLA may be an important driver of disability progression independent of ALA in MS.
Compared with placebo, natalizumab treatment significantly reduced the number and volume of SELs and the proportion of baseline non-enhancing T2 lesions identified as SELs. Natalizumab reduced CLA as measured by T1LV increase in both SELs and non-SELs. The significant association between ALA and SEL prevalence suggests that the effect of natalizumab on CLA in progressive patients with MS could be related to its high efficacy in suppressing acute inflammation. Prior studies demonstrating the effects of natalizumab and the depletion of CD20-expressing cells further support this finding.1 2 27–29 The effects of natalizumab on chronic active lesions have also previously been demonstrated in positron emission tomography studies measuring activated microglia.30 31 In these studies, natalizumab decreased PK11195 uptake, reflective of activated microglia and macrophages, in non-enhancing lesions30 and more specifically at the rim of chronic active lesions.31
It is plausible that the smouldering inflammation that contributes to CLA and disability progression may be influenced by acute inflammation in MS. The recently published long-term brain MRI follow-up of primary progressive patients with MS continuously treated with ocrelizumab over 6.5 years in the ORATORIO Study3 demonstrated an annual increase in T1LV of approximately 3%–6% (reaching a total increase of 37% from baseline at approximately 6 years), which may be attributable to CLA in SELs and non-SELs independent of ALA, as those patients were shown to be devoid of ALA from week 48 onward. Further analytical work will be needed to establish whether the effects of natalizumab and ocrelizumab on CLA (in SELs and non-SELs) and disability progression in progressive forms of MS may be principally explained by their capacity to silence ALA. For example, an extended approach using Bayesian inference for a principal stratum estimand could be used to assess the treatment effect in subgroups characterised by ALA covariate thresholds as a postrandomisation event occurrence.32
Efforts to further refine MS lesion histopathological terminology are ongoing,14 but understanding of the natural history of chronic active lesion phenotypes remains elusive, as their lifespan could begin decades before specimens become available.33 Molecular studies of the lesion rim of chronic active or mixed active/inactive lesions show a predominance of M1-polarised macrophages and activated microglia.15 Innate and adaptive immune system interaction can bidirectionally influence polarisation and perpetuate the disease process.34 Phase rims detected on susceptibility-weighted imaging are thought to represent an imaging biomarker of rims of iron-laden microglia/macrophages and have been linked to disease severity and brain atrophy. SELs35–38 and PRLs seem to both reflect chronic active lesions,26 though recent work has demonstrated only partial concordance between SELs and PRLs, as a substantial proportion of PRLs do not appear to expand over time and some SELs appear to be devoid of phase rims entirely.18 Further work is needed to elucidate the potential complementarity of SEL, non-SEL and PRL measures of CLA and to expand the characterisation of longitudinal tissue alteration properties within distinct MRI phenotypes of chronic active white matter lesions.
Limitations of this study include its extensive focus on the brain; lesions in the spinal cord as well as spinal cord atrophy are also established markers associated with disability.39 40 Similarly, cortical lesions could not be analysed from this dataset. Beyond this, our findings may be limited by the potential misidentification of SELs from acquisition-related artefacts, the measurement noise inherent in MRI studies and the limitations of the SEL detection approach, which ignores chronic active lesions that may not be associated with T1w and T2w expansion. We employed a high detection threshold and stringent criteria for identifying SELs, as a result of which some true SELs may have been classified as non-SELs. In addition, lesion areas of subtle T1 hypointensity may have been missed by this method of SEL detection.
In conclusion, CLA in SELs and non-SELs is an imaging biomarker of chronic active lesions and/or secondary axonal degeneration that reflects ongoing inflammation and is associated with clinical disability progression. We demonstrate that the presence of ALA, whether new Gd+ lesions or new T2-hyperintense lesions, is associated with a higher SEL prevalence in patients with SPMS. This indirect evidence for the influence of ALA on CLA highlights the importance of limiting acute inflammation with highly effective DMTs. However, even in the absence of ALA, the association between CLA and confirmed disability progression persists, although to a more limited extent, with the greater increase in T1w lesion volume in progressors than in non-progressors seen primarily within non-SEL tissue of T2w non-enhancing lesions.
The onset of the effect of natalizumab on CLA was rapid in this study, with a statistically significant reduction in T1w lesion volume increase in SELs and non-SELs seen starting at 24 weeks of treatment. Taken together, these results highlight the need to continue to develop therapies that impact ALA but also more specifically target CLA, which may represent the pathological correlate for smouldering inflammation and neurodegenerative pathways.
Acknowledgments
The authors would like to acknowledge the ASCEND co-investigators for their efforts and contributions.
Footnotes
Twitter: @gavingiovannoni
VB and ICG contributed equally.
Contributors: Study conception and design: VB, ICG, CE, DLA, JK, HC, LZ, CK, AG and SB. Data collection: CE and DLA. Guarantor (accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish): SB. All authors participated in data interpretation and in drafting and revising the manuscript.
Funding: This study was funded by Biogen (grant number not applicable).
Competing interests: VB was an employee of Biogen (Cambridge, Massachusetts, USA) during the completion of the work related to this manuscript and remains a Biogen shareholder. She is now an employee of Agios (Cambridge, Massachusetts, USA), which was not in any way associated with this study. ICG has received fellowship support from Biogen. CE is an employee of NeuroRx Research. DLA has received grants from Biogen, Immunotec and Novartis and consulting fees from Biogen, Celgene, Frequency Therapeutics, Genentech/Roche, Med-Ex Learning, Merck, Novartis, the Population Council, Queens University and Sanofi Aventis and has an ownership interest in NeuroRx. JK, HC, MS, NC, DPB, NF, AG and SB are employees and shareholders of Biogen. LZ and CK were employees of Biogen at the time of these analyses and may hold stock and/or stock options in Biogen. GG has received speaker honoraria and consulting fees from AbbVie, Actelion, Almirall, Atara Bio, Bayer Schering, Biogen, Celgene, FivePrime, GlaxoSmithKline, GW Pharma, Ironwood, Janssen, Merck, Merck KGaA, Novartis, Pfizer, Protein Discovery Laboratories, Sanofi Genzyme, Teva Pharmaceuticals, UCB and Vertex and research support unrelated to this study from Biogen, Ironwood, Merck, Merck KGaA, Novartis, Roche and Takeda.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The datasets generated and analysed during the current study are not publicly available. The authors fully support sharing whenever possible. Requests for deidentified data should be made to Biogen via established company data-sharing policies and processes detailed on the website http://clinicalresearch.biogen.com/.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by an Institutional Review Board at each study site. Participants gave informed consent to participate in the study before taking part.
References
- 1.Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 2020;77:1132–40. 10.1001/jamaneurol.2020.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017;376:209–20. 10.1056/NEJMoa1606468 [DOI] [PubMed] [Google Scholar]
- 3.Wolinsky JS, Arnold DL, Brochet B, et al. Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2020;19:998–1009. 10.1016/S1474-4422(20)30342-2 [DOI] [PubMed] [Google Scholar]
- 4.Filippi M, Preziosa P, Banwell BL, et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain 2019;142:1858–75. 10.1093/brain/awz144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suthiphosuwan S, Sati P, Absinta M, et al. Paramagnetic rim sign in radiologically isolated syndrome. JAMA Neurol 2020;77:653–5. 10.1001/jamaneurol.2020.0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser SL, Kappos L, Arnold DL, et al. Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension. Neurology 2020;95:e1854–67. 10.1212/WNL.0000000000010376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology 2002;59:1412–20. 10.1212/01.WNL.0000036271.49066.06 [DOI] [PubMed] [Google Scholar]
- 8.Rudick RA, Fisher E, Lee JC, et al. Brain atrophy in relapsing multiple sclerosis: relationship to relapses, EDSS, and treatment with interferon beta-1a. Mult Scler 2000;6:365–72. 10.1177/135245850000600601 [DOI] [PubMed] [Google Scholar]
- 9.Correale J, Gaitán MI, Ysrraelit MC, et al. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain 2017;140:527–46. 10.1093/brain/aww258 [DOI] [PubMed] [Google Scholar]
- 10.Filippi M, Preziosa P, Barkhof F. Diagnosis of progressive multiple sclerosis from the imaging perspective: a review. JAMA Neurol 2020;78. 10.1001/jamaneurol.2020.4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews PM. Chronic inflammation in multiple sclerosis - seeing what was always there. Nat Rev Neurol 2019;15:582–93. 10.1038/s41582-019-0240-y [DOI] [PubMed] [Google Scholar]
- 12.Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 2015;78:710–21. 10.1002/ana.24497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luchetti S, Fransen NL, van Eden CG, et al. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol 2018;135:511–28. 10.1007/s00401-018-1818-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhlmann T, Ludwin S, Prat A, et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol 2017;133:13–24. 10.1007/s00401-016-1653-y [DOI] [PubMed] [Google Scholar]
- 15.Jäckle K, Zeis T, Schaeren-Wiemers N, et al. Molecular signature of slowly expanding lesions in progressive multiple sclerosis. Brain 2020;143:2073–88. 10.1093/brain/awaa158 [DOI] [PubMed] [Google Scholar]
- 16.Hametner S, Dal Bianco A, Trattnig S, et al. Iron related changes in MS lesions and their validity to characterize MS lesion types and dynamics with ultra-high field magnetic resonance imaging. Brain Pathol 2018;28:743–9. 10.1111/bpa.12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, Trapp B, Ontaneda D, et al . Histological analysis of slowly expanding lesions in multiple sclerosis: case report [abstract]. Mult Scler 2020;26:P0584. [Google Scholar]
- 18.Elliott C, Belachew S, Fisher E. MRI characterization of chronic active MS lesions by phase rim detection and/or slowly expanding properties [abstract]. Neurology 2021;96:P4101. [Google Scholar]
- 19.Elliott C, Belachew S, Wolinsky JS, et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 2019;142:2787–99. 10.1093/brain/awz212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapoor R, Ho P-R, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol 2018;17:405–15. 10.1016/S1474-4422(18)30069-3 [DOI] [PubMed] [Google Scholar]
- 21.Elliott C, Wolinsky JS, Hauser SL, et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler 2019;25:1915–25. 10.1177/1352458518814117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis SJ. Automatic lesion identification in MRI of multiple sclerosis patients. [Master of Science Thesis]. McGill University, 2004. Available: https://escholarship.mcgill.ca/concern/theses/qz20st101 [Accessed 2 Mar 2022].
- 23.Arnold DL, Belachew S, Gafson AR, et al. Slowly expanding lesions are a marker of progressive MS - No. Mult Scler 2021;27:1681–3. 10.1177/13524585211017020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura K, Guizard N, Fonov VS, et al. Jacobian integration method increases the statistical power to measure gray matter atrophy in multiple sclerosis. Neuroimage Clin 2014;4:10–17. 10.1016/j.nicl.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott C, Arnold DL, Chen H, et al. Patterning chronic active demyelination in slowly expanding/evolving white matter MS lesions. AJNR Am J Neuroradiol 2020;41:1584–91. 10.3174/ajnr.A6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preziosa P, Pagani E, Moiola L, et al. Occurrence and microstructural features of slowly expanding lesions on fingolimod or natalizumab treatment in multiple sclerosis. Mult Scler 2020;1352458520969105. [DOI] [PubMed] [Google Scholar]
- 27.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. 10.1056/NEJMoa044397 [DOI] [PubMed] [Google Scholar]
- 28.Butzkueven H, Kappos L, Pellegrini F, et al. Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results. J Neurol Neurosurg Psychiatry 2014;85:1190–7. 10.1136/jnnp-2013-306936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017;376:221–34. 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 30.Kaunzner UW, Kang Y, Monohan E, et al. Reduction of PK11195 uptake observed in multiple sclerosis lesions after natalizumab initiation. Mult Scler Relat Disord 2017;15:27–33. 10.1016/j.msard.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 31.Sucksdorff M, Tuisku J, Matilainen M, et al. Natalizumab treatment reduces microglial activation in the white matter of the MS brain. Neurol Neuroimmunol Neuroinflamm 2019;6:e574. 10.1212/NXI.0000000000000574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magnusson BP, Schmidli H, Rouyrre N, et al. Bayesian inference for a principal stratum estimand to assess the treatment effect in a subgroup characterized by postrandomization event occurrence. Stat Med 2019;38:4761–71. 10.1002/sim.8333 [DOI] [PubMed] [Google Scholar]
- 33.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Long-term evolution of multiple sclerosis iron rim lesions in 7 T MRI. Brain 2021;144:833–47. 10.1093/brain/awaa436 [DOI] [PubMed] [Google Scholar]
- 34.Strachan-Whaley M, Rivest S, Yong VW. Interactions between microglia and T cells in multiple sclerosis pathobiology. J Interferon Cytokine Res 2014;34:615–22. 10.1089/jir.2014.0019 [DOI] [PubMed] [Google Scholar]
- 35.Kaunzner UW, Kang Y, Zhang S, et al. Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain 2019;142:133–45. 10.1093/brain/awy296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016;126:2597–609. 10.1172/JCI86198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol 2017;133:25–42. 10.1007/s00401-016-1636-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Absinta M, Sati P, Masuzzo F, et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol 2019;76:1474–83. 10.1001/jamaneurol.2019.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Losseff NA, Webb SL, O'Riordan JI, et al. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 1996;119 (Pt 3):701. 10.1093/brain/119.3.701 [DOI] [PubMed] [Google Scholar]
- 40.Dekker I, Sombekke MH, Balk LJ, et al. Infratentorial and spinal cord lesions: cumulative predictors of long-term disability? Mult Scler 2020;26:1381–91. 10.1177/1352458519864933 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjno-2021-000240supp001.pdf (89.4KB, pdf)
bmjno-2021-000240supp002.pdf (119.7KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The datasets generated and analysed during the current study are not publicly available. The authors fully support sharing whenever possible. Requests for deidentified data should be made to Biogen via established company data-sharing policies and processes detailed on the website http://clinicalresearch.biogen.com/.