Abstract
A strategy for sequential hydrocarbon bioremediation is proposed. The initial O2-requiring transformation is effected by aerobic resting cells, thus avoiding a high oxygen demand. The oxygenated metabolites can then be degraded even under anaerobic conditions when supplemented with a highly water-soluble alternative electron acceptor, such as nitrate. To develop the new strategy, some phenomena were studied by examining Pseudomonas aeruginosa fermentation. The effects of dissolved oxygen (DO) concentration on n-hexadecane biodegradation were investigated first. Under microaerobic conditions, the denitrification rate decreased as the DO concentration decreased, implying that the O2-requiring reactions were rate limiting. The effects of different nitrate and nitrite concentrations were examined next. When cultivated aerobically in tryptic soy broth supplemented with 0 to 0.35 g of NO2−-N per liter, cells grew in all systems, but the lag phase was longer in the presence of higher nitrite concentrations. However, under anaerobic denitrifying conditions, even 0.1 g of NO2−-N per liter totally inhibited cell growth. Growth was also inhibited by high nitrate concentrations (>1 g of NO3−-N per liter). Cells were found to be more sensitive to nitrate or nitrite inhibition under denitrifying conditions than under aerobic conditions. Sequential hexadecane biodegradation by P. aeruginosa was then investigated. The initial fermentation was aerobic for cell growth and hydrocarbon oxidation to oxygenated metabolites, as confirmed by increasing dissolved total organic carbon (TOC) concentrations. The culture was then supplemented with nitrate and purged with nitrogen (N2). Nitrate was consumed rapidly initially. The live cell concentration, however, also decreased. The aqueous-phase TOC level decreased by about 40% during the initial active period but remained high after this period. Additional experiments confirmed that only about one-half of the derived TOC was readily consumable under anaerobic denitrifying conditions.
Petroleum hydrocarbons are among the most common environmental contaminants (8). Much work has been done to understand the aerobic and anaerobic pathways of microbial hydrocarbon degradation (9, 29). Although desirable for active hydrocarbon biodegradation, completely aerobic conditions are hard to implement in the field because of the low solubility of oxygen in water (5, 42). Hydrogen peroxide can provide more oxygen (20). However, hydrogen peroxide may oxidize cellular components by forming singlet oxygen and hydroxyl radicals (29), and using hydrogen peroxide requires special operational design and handling. Moreover, peroxide can form precipitates with soil components, such as metallic compounds, thus decreasing aquifer permeability (11, 30, 40).
It is plausible that uneven distribution of water flow, nutrients, and microbial populations creates a dynamic spectrum of aerobic, microaerobic, and anaerobic conditions. The ability of microorganisms to degrade hydrocarbons under strictly anaerobic conditions is very limited. It is practically absent for aliphatics compounds; there are extremely rare exceptions to this (10, 13, 36). The ability to degrade aromatic compounds under anaerobic conditions is limited to a few strains, and the degradation is typically much slower than aerobic degradation (4, 15, 24). The initial anaerobic transformation of aromatic compounds has been shown to be oxidative hydroxylation, with the oxygen being derived from water (1, 22), which is a much less thermodynamically favorable reaction than oxidation by O2.
The limitation described above indicates the importance of molecular oxygen (O2) as a direct reactant in hydrocarbon catabolism (e.g., in the initial conversion to alcohol) (29). Although other oxidants, such as NO3− or NO2− (in denitrification), Fe(III), Mn(IV), sulfate, and CO2 (in methanogenesis) (17, 29), can be used by many microorganisms as terminal electron acceptors that replace O2 in respiration, they cannot replace O2 as a direct reactant. Consequently, most anaerobic biodegradation is observed with oxygenated derivatives (e.g., alcohols, aldehydes, acids, phenolic compounds, and amino acids) (3, 16).
Therefore, petroleum bioremediation should address (i) the dual roles of O2 in biodegradation (i.e., for respiration and as a direct reactant) and (ii) the interactions of microbial activities in different environments (aerobic, microaerobic, and anaerobic environments). Sequential biodegradation of hydrocarbons probably occurs in nature; the initial O2-requiring oxidation occurs under aerobic or microaerobic conditions, and the subsequent mineralization may proceed even under anaerobic conditions. The same strategy may be used as an advanced bioremediation approach. The initial oxidation (transformation) is effected by aerobic resting cells in a first remediation stage without promoting active growth that may cause undeliverable oxygen demand and plugging of soil pores by overpopulated microbes. The oxidized metabolites are then degraded under the conditions of stimulated microbial activities where the respiration needs are met by adding a highly water-soluble alternative electron acceptor, such as nitrate.
In this work we studied sequential metabolism of n-hexadecane by Pseudomonas aeruginosa, which is among the most commonly isolated microorganisms in petroleum-contaminated soils and groundwater (34). It is known that P. aeruginosa mineralizes aliphatic compounds. P. aeruginosa strains that attack aromatic and polyaromatic hydrocarbons have also been isolated, including a pyrene-degrading strain that was recently isolated in our lab (33). P. aeruginosa strains are typically active denitrifiers and produce biosurfactants (rhamnolipids) when they are grown on hydrophobic substrates (18, 41). Compared to other anaerobic respiration mechanisms, denitrification is more favorable energetically, and benign nitrogen (N2) is the predominant product of this process (17, 29). The rhamnolipids produced are also very beneficial to bioremediation, because they solubilize and mobilize hydrocarbons and other non-aqueous-phase liquids into the aqueous phase, where they can be biodegraded or removed by advective transport (2, 37).
Because they are hydrophobic, hydrocarbons in the environment are often adsorbed or trapped in pores by capillary action; thus, they are not readily accessible to microbes (26). Some microorganisms produce extracellular biosurfactants that solubilize and facilitate the penetration of hydrocarbons through the hydrophilic cell wall; then the hydrocarbons can be degraded by enzymes integrated in the cytoplasmic membrane (39). The most important examples of such organisms are the rhamnolipid-producing Pseudomonas species (28, 44) and the sophorolipid-producing Torulopsis species (21). The biosurfactants of these species are extremely effective and create low surface tensions at much lower concentrations than those required for synthetic surfactants (14).
It should be noted that rhamnolipids are actively synthesized by stationary-phase or resting P. aeruginosa cultures (44). Therefore, they are also produced during the first stage of the proposed sequential bioremediation process and contribute to mobilizing and solubilizing the contaminants during the subsequent mineralization stage. Because P. aeruginosa has these versatile, unique metabolic characteristics, it is the most suitable species for initial studies of sequential hydrocarbon metabolism.
MATERIALS AND METHODS
Microorganism and medium.
A pure culture of P. aeruginosa ATCC 10145 was maintained on tryptic soy agar slants (30 g of tryptic soy broth [TSB] per liter, 1.5% agar) and was subcultured regularly. To prepare an inoculum, a loopful of cells from an agar slant was first transferred into a test tube containing 10 ml of TSB and incubated at 35°C for 24 h. For studies in which TSB or glucose medium (basal medium [see below] containing 2 g of glucose per liter) was used, the cells were transferred and grown in 50 ml of TSB at 30°C for 2 days with magnetic stirring at 300 rpm before they were used as the inoculum. For the experiments in which n-hexadecane was the carbon substrate, the cells in 10 ml of TSB were harvested by centrifugation and added to a 500-ml Erlenmeyer flask containing 100 ml of basal medium supplemented with 5 ml of hexadecane; the pH of this medium was adjusted to 7.0. The culture was incubated for 7 days with magnetic stirring at 30°C and then subcultured again in the hexadecane-containing medium for another 4 days before it was used as the inoculum. The basal medium contained (per liter) 4.0 g of NH4Cl, 2.5 g of K2HPO4, 0.5 g of NaCl, 0.3 g of MgSO4 · 7H2O, 0.03 g of FeCl3 · 6H2O, 0.01 g of CaCl2, and 0.01 g of MnCl2 · 4H2O.
Experimental setup and procedures. (i) Microaerobic denitrification and hexadecane metabolism: effect of oxygen concentration.
To study the full spectrum of aerobic, microaerobic, and anaerobic conditions in soil, we performed experiments to confirm the existence of microaerobic denitrification and the effects of low oxygen concentrations on hexadecane metabolism by P. aeruginosa. Different volumes of an inoculated growth medium containing hexadecane, nitrate, and ammonium were added to nine 50-ml vials. An excess of ammonium (0.65 g of NH4+-N per liter) was present to ensure that the nitrate was consumed only for respiration, because the assimilatory nitrate reductases are repressed by ammonium (27). The vials were stirred at the same speed (100 rpm) by using a multipoint electromagnetic stirrer. The caps on vials 1 through 5 were loosened slightly in order to allow gas phase diffusion. Vials 6 through 9 were tightly capped. Since the gas-liquid interfacial areas were the same, the increasing medium volume from vial 1 to vial 5 resulted in a decreasing trend in dissolved oxygen (DO) concentration. Vials 6 through 9 were the most anaerobic vials; the headspaces in these vials were very small, and there was no gas replenishment. Resazurin, a common redox indicator, was added in order to determine the extent of anaerobiosis; this compound is colorless under anaerobic conditions and pink under aerobic conditions (29). The resultant color confirmed the decreasing DO concentration trend. Samples were taken periodically and used for nitrate analysis.
(ii) Effects of nitrate and nitrite concentrations.
Two sets of experiments were performed to study the effects of nitrate and/or nitrite concentrations on the growth of P. aeruginosa under aerobic conditions and under anaerobic denitrifying conditions. The experiments were conducted at room temperature (22 ± 1°C). TSB containing various initial concentrations of nitrite (0, 0.05, 0.10, 0.15, 0.20, 0.25, 0.30, and 0.35 g of NO2−-N per liter) were used for the aerobic experiments. The denitrifying experiments were performed with glucose media containing either nitrate alone (0.5, 1, 2, 3, or 4 g of NO3−-N per liter) or 0.5 g of NO3−-N per liter plus 0.1, 0.2, or 0.3 g of NO2−-N per liter. Very pure sodium nitrate (>99.9% pure; Sigma) was used to minimize the accompanying residual nitrite content. The aerobic experiments were performed with shallow medium (4 ml of medium) that was vigorously shaken (∼250 rpm) in culture tubes capped with stainless steel closures that allowed good gas exchange. On the other hand, the tubes used for the anaerobic denitrifying experiments were tightly capped, filled with 10 ml of medium, and shaken gently at ∼60 rpm. The broth optical density at 460 nm was measured with a Spectronic 20 colorimeter (Bausch & Lomb) in order to determine cell concentrations.
(iii) Hexadecane metabolism in the sequential aerobic and anaerobic denitrifying process.
Experiments were carried out at 30°C in 2-liter Erlenmeyer flasks containing 800 ml of basal medium supplemented with 0.7% (vol/vol) hexadecane; the medium was magnetically stirred at 400 rpm. Initially, fermentation was carried out with surface aeration; a slow flow of filter-sterilized air through the reactor headspace resulted in cell growth and stationary-phase hydrocarbon oxidation to oxygenated metabolites, including rhamnolipids. The pH was controlled at 6.5 to 6.7 by automatic addition of 2 N NaOH during this period. Surface aeration was used instead of submerged air sparging in order to avoid severe foaming due to biosurfactant production. The culture was then driven to anaerobic denitrifying conditions by purging nitrogen (N2) and adding NaNO3. Ammonium (0.08 g of NH4Cl per liter) was also added to ensure that nitrate was consumed only for respiration and not for assimilation. Before the N2 was introduced into the fermentor, it was passed through an oversaturated NaSO3 solution in order to minimize the residual oxygen content. N2 purging was performed periodically during anaerobic cultivation, especially after each sample was removed. Because denitrification led to an increase in pH, the pH of the anaerobic culture was controlled by automatic addition of 2 N HCl.
Analytical methods.
The cell protein and rhamnolipid concentrations were measured by standard Lowry and anthrone methods, respectively, as described previously (44). A total organic carbon (TOC) analyzer (model TOC-5000A; Shimadzu) was used to measure the TOC content in the aqueous phase after a sample (10 ml) had been extracted with hexane to remove hexadecane. Hexadecane contents were analyzed by high-performance liquid chromatography by using a normal-phase Supelcosil LC-Si column (25 cm by 4.6 mm by 5 μm) and a refractive index detector (model HP 1047A). The mobile phase was n-hexane at a flow rate of 1.0 ml per min.
Ammonium (NH4+) and combined nitrate and nitrite (NOx−) concentrations were determined by using an ammonia electrode (model M-44325; Markson Science) (12, 25). A wide range of the ammonium concentrations (1 to 1,000 mg of NH4+-N per liter) could be measured accurately, while only nitrate and nitrite concentrations ranging from 1 to 20 mg of NOx−-N per liter could be measured. Therefore, each sample was diluted to the proper range prior to the analysis. For the separate nitrite analysis we used the standard method described by Gerhardt (19); 0.1 ml of the sulfanilamide reagent (1% sulfanilamide and 10% HCl; LabChem Inc.) was added to a 5-ml sample in a test tube, and then the preparation was mixed and kept in the dark for 2 to 8 min. Then 0.1 ml of the N-1(naphthyl)-ethylenediamine dihydrochloride reagent (0.1%; LabChem Inc.) was added, and the preparation was mixed and left to stand for at least 10 min. The absorbance at 543 nm was measured with a spectrophotometer.
RESULTS AND DISCUSSION
Microaerobic denitrification and hexadecane metabolism: effect of oxygen concentration.
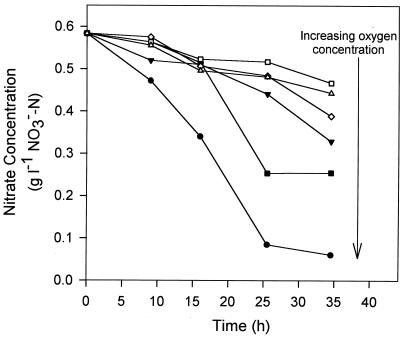
The nitrate consumption profiles obtained with vials containing different DO concentrations are summarized in Fig. 1. Denitrification clearly occurred under the microaerobic conditions studied. This finding is consistent with the previous report that the bacteria in a groundwater microcosm ignored O2 at levels that were less than 8% of air saturation and switched to other electron acceptors (6). Denitrification in the presence of even higher DO levels, termed aerobic denitrification, has also been reported for several species and strains (32, 38, 43).
FIG. 1.
Increase of the denitrification rate with increasing DO concentration during n-hexadecane metabolism by P. aeruginosa under microaerobic conditions. Symbols: ●, vial 1; ■, vial 2; ▾, vial 3; ◊, vial 4; □, vial 5; ▵, vial 6.
More surprisingly, the denitrification rate decreased as the DO concentration decreased. This phenomenon could be attributed to slower hexadecane oxidation as the DO concentration decreased at the very low DO concentrations used. As mentioned above, biodegradation of aliphatic hydrocarbons requires O2 as a direct reactant, as in the initial conversion to alcohol. As the DO concentration decreased, the rate of hydrocarbon oxidation was limited, which in turn slowed the electron donation which drove the electron-accepting denitrification process.
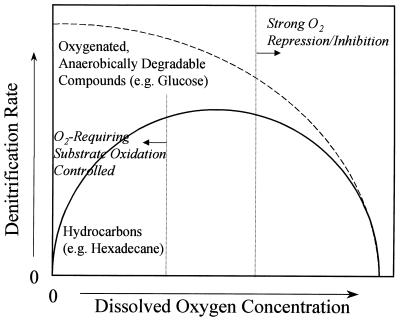
On the other hand, oxygen repression and inhibition of denitrifying enzymes should occur at high DO concentrations (27). Therefore, as shown schematically in Fig. 2, the dependence of nitrate respiration on DO concentration may be controlled by two different mechanisms; it may be hydrocarbon oxidation limited at low DO concentrations and oxygen repression-inhibition limited at high DO concentrations. The denitrification rate for oxygenated, anaerobically degradable substrates (such as glucose and fatty acids) probably always decreases as the DO concentration decreases because of the prevailing oxygen repression-inhibition effect (Fig. 2). More studies are required to confirm and quantitatively evaluate the profiles shown in Fig. 2.
FIG. 2.
Proposed dependence of the denitrification rate of P. aeruginosa on DO concentration for different substrates. In addition to the well-known oxygen repression and inhibition, the denitrification rate is also subject to the limitation of electron donation from O2-requiring hydrocarbon oxidation at very low DO concentrations.
Effects of nitrate and nitrite concentrations.
Denitrification by P. aeruginosa is known to involve sequential reduction of nitrate (NO3−) to nitrite (NO2−), nitric oxide (NO), nitrous oxide (N2O), and nitrogen (N2) (23). Accumulation of intermediate NO2− during the initial denitrifying period has been observed (7), because nitrate reductases are first induced by the nitrate added and then the nitrite formed from nitrate reduction induces expression of nitrite reductases. Before nitrite reductases are fully synthesized, transient nitrite accumulation is likely to occur. Nitrite is, however, highly toxic (31) and may cause cell death or metabolic inhibition when denitrification is used for sequential bioremediation. Therefore, we examined P. aeruginosa growth in the presence of various nitrite and/or nitrate concentrations.
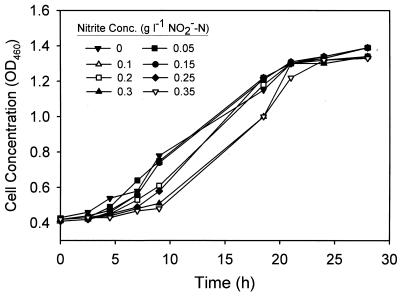
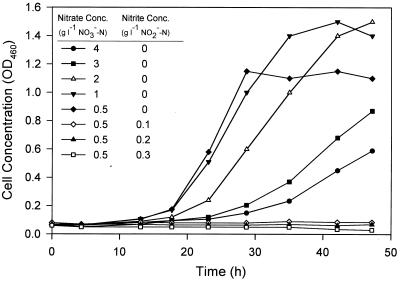
The optical densities at 460 nm of cultures grown aerobically in TSB supplemented with 0 to 0.35 g of NO2−-N per liter are shown in Fig. 3. Cells were able to grow in all systems, but the lag phases were longer at higher nitrite concentrations. The results obtained under anaerobic denitrifying conditions when glucose medium was used are shown in Fig. 4. The presence of nitrite, even at a concentration of 0.1 g of NO2−-N per liter, completely inhibited cell growth. Higher nitrate concentrations also retarded cell growth, probably by inhibiting (or repressing) the nitrite reductases and thus causing nitrite to accumulate to inhibitory levels in the systems (35). P. aeruginosa was found to be more sensitive to nitrate-nitrite inhibition under anaerobic denitrifying conditions than under aerobic conditions.
FIG. 3.
Effect of nitrite concentration on aerobic growth of P. aeruginosa in TSB. OD460, optical density at 460 nm.
FIG. 4.
Effects of nitrite and/or nitrate concentrations on growth of P. aeruginosa in a glucose-based medium under anaerobic denitrifying conditions. OD460, optical density at 460 nm.
Hexadecane metabolism in sequential aerobic and anaerobic denitrifying processes.
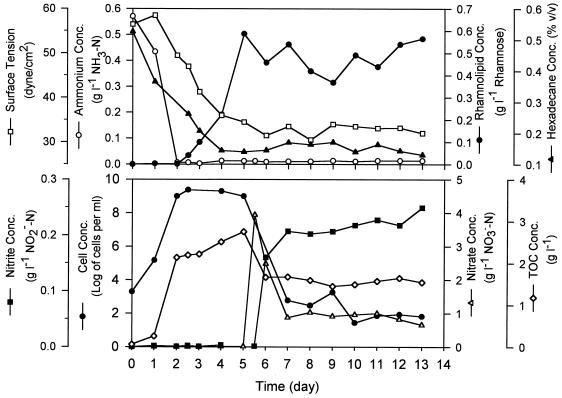
The results obtained in a typical experiment carried out under sequential aerobic and anaerobic denitrifying conditions are shown in Fig. 5. The cell growth profile corresponded very well to the ammonium consumption profile, and the culture reached the stationary phase when the N source was depleted. The rates of hexadecane consumption and aqueous-phase TOC production under aerobic conditions were higher in the exponential growth phase than in the stationary phase. The increase in the TOC level confirmed that hydrocarbon was converted to oxygenated organic compounds by cell metabolism. Rhamnolipids were also produced, and the presence of rhamnolipids was accompanied by reduced medium surface tension (Fig. 5).
FIG. 5.
Results of a typical experiment to examine n-hexadecane metabolism by P. aeruginosa under sequential aerobic and anaerobic denitrifying conditions.
Following the switch to anaerobic denitrifying conditions, nitrate was consumed rapidly initially (during the first day or so). The live cell concentration, however, also decreased. The aqueous-phase TOC level decreased by about 40% during the same active period but was maintained at a high level afterwards. The hexadecane concentration remained constant throughout the anaerobic period, as expected based on the anaerobic recalcitrance of this compound. The rhamnolipid profile fluctuated noticeably at the later stage of the experiment, when an uncharacteristic brown color, in addition to the normal green color, appeared in the anthrone analysis. The brown color might have interfered with the optical measurements and caused the fluctuation. It is not known whether rhamnolipids were significantly degraded. Note that the medium surface tension remained low during this period. The fact that all properties were relatively constant after the initial 2 days of anaerobic denitrification indicated that microbial metabolism was active only during a brief initial period following the switch.
We examined the possible causes of cell death under anaerobic denitrifying conditions. These causes included toxicity of nitrate and nitrite and depletion of readily consumable energy substrates. As shown in Fig. 5, the nitrite concentration increased to about 0.20 g NO2−-N per liter during the rapid initial denitrification stage and remained high (0.20 to 0.25 g NO2−-N per liter) until the end. According to the results described above for the effects of nitrate and nitrite concentrations (Fig. 4), the high nitrite level should definitely have halted cell growth. Nonetheless, we are not certain that it caused cell death.
The possibility that exhaustion of readily consumable TOC caused the sharp decline in cell concentration was examined with two additional experiments in which we used media having different initial TOC concentrations (about 8 and 2 g liter−1). The media used were the cell-free aqueous broth media from two P. aeruginosa fermentations collected by centrifugation after different periods of stationary-phase production of oxygenated metabolites (2 weeks for the high-TOC medium and 2 days for the low-TOC medium). For comparison, the TOC concentration before the switch to denitrification in the previous sequential experiment was 2.8 g liter−1 (Fig. 5), which was within the range covered in the two experiments performed later. The cell (protein) concentrations were, however, very different, about 1.2 g of cell proteins per liter at the switch to denitrifying conditions in the sequential experiment but only 0.01 to 0.04 g liter−1 in the two experiments performed later. (The cell protein concentration in the sequential experiment was not measured but was estimated from the available N source concentration based on our experience with similar systems.) While the high cell concentration led to cell death in the former experiment (Fig. 5), the low inoculum levels in the latter experiments allowed the cells to grow as described in more detail below.
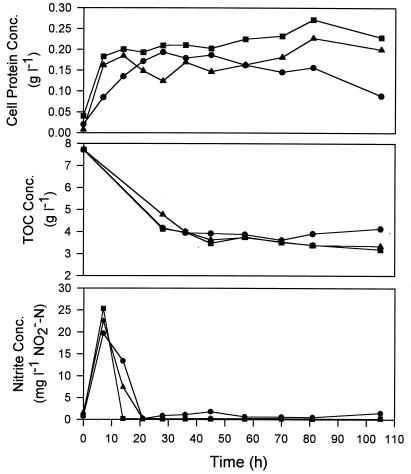
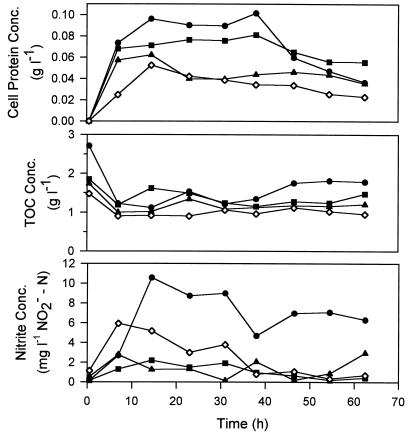
The experiments performed with the media containing high and low TOC concentrations were carried out by using various initial nitrate concentrations (0.35 to 3.30 g of NO3−-N per liter) so that the effects of TOC concentrations on nitrite accumulation could also be investigated. The cell protein, TOC, and nitrite concentration profiles obtained in the high- and low-TOC experiments are shown in Fig. 6 and 7, respectively. Not shown but also measured were the changes in pH and the ammonium and nitrate concentrations in the experiments. The pH increased gradually to 6.2 to 7.2, as expected if denitrification occurred. The excess ammonium added initially (0.1 g of NH4+-N per liter) was consumed during cell growth, but the ammonium was never depleted (the remaining NH4+-N concentration in all systems was more than 0.06 g liter−1). Nitrate was consumed during denitrification but also was never depleted because of the low cell concentrations used in the experiments. The cell protein concentrations were up to 0.25 g liter−1 in the high-TOC systems and 0.1 g liter−1 in the low-TOC systems (Fig. 6 and 7). Thus, pH and ammonium and nitrate concentrations did not limit cell growth.
FIG. 6.
Cell growth, TOC consumption, and transient nitrite accumulation profiles obtained in experiments performed with the high-TOC medium containing different initial nitrate concentrations. Symbols: ●, 2.7 g of NO3−-N per liter; ▴, 0.8 g of NO3−-N per liter; ■, 0.4 g of NO3−-N per liter.
FIG. 7.
Cell growth, TOC consumption, and transient nitrite accumulation profiles obtained in experiments performed with the low-TOC medium containing different initial nitrate concentrations. Symbols: ●, 3.3 g of NO3−-N per liter; ■, 1.3 g of NO3−-N per liter; ▴, 0.7 g of NO3−-N per liter; ◊, 0.35 g of NO3−-N per liter.
Nitrite accumulation appeared to be affected by the available energy substrate and the cell concentration. In the case of a high TOC concentration (8 g liter−1) and a low initial cell concentration (0.01 to 0.04 g of cell proteins per liter), the nitrite concentration increased initially to 20 to 25 mg of NO2−-N per liter but decreased to almost zero within 20 h (Fig. 6). Slow denitrification by the low concentration of cells did not result in a high level of nitrite during the initial period before nitrite reductases were fully expressed. In addition, the availability of readily consumable TOC allowed the cells to recover from the unbalanced nitrate and nitrite reduction conditions. In the case of the lower TOC concentration (2.8 g liter−1) and much higher cell concentration (about 1.2 g of cell proteins per liter), the nitrite concentration increased rapidly to 150 to 200 mg of NO2−-N per liter and remained high throughout the experiment (Fig. 5). The readily consumable TOC was probably depleted by the high cell concentration before nitrite reductases could be completely synthesized. When the lowest TOC concentration (2 g liter−1) and the lowest initial cell concentration (0.01 g of cell proteins per liter) were used, the lowest initial nitrite concentrations were observed (range, 2 to 10 mg of NO2−-N per liter) (Fig. 7). The nitrite concentrations declined during fermentation but at much slower rates than the rates shown in Fig. 6, presumably because of the lower concentrations of consumable TOC that remained after the development of nitrite reductases. Note that during nitrite (and nitrate) reduction cells accept the electrons donated from substrate oxidation. On the other hand, the effects of different nitrate concentrations on nitrite accumulation were not clearly significant. The high nitrate concentration (3.3 g of NO3−-N per liter) appeared to result in more nitrite accumulation in the low-TOC experiment (Fig. 7) but not in the high-TOC experiment (Fig. 6).
The TOC concentration always decreased rapidly during a short initial period and then remained relatively constant afterwards, as shown in Fig. 5 through 7. The decrease was about one-half the initial TOC concentration, regardless of the different initial TOC and cell concentrations employed. The profiles corresponded very well with the cell growth profiles in the two experiments whose results are shown in Fig. 6 and 7. As no other growth-limiting factors could be identified, the results strongly suggest that only about one-half of the TOC (oxygenated metabolites of hexadecane) could be readily consumed by P. aeruginosa under anaerobic denitrifying conditions. The depletion of the readily consumable portions resulted in a cessation of cell growth in the two experiments with low inoculum levels (Fig. 6 and 7) and, plausibly, to cell death in the previous sequential experiment. It is possible that substantially higher fractions of oxygenated metabolites can be consumed if microbial consortia under mixed aerobic, microaerobic, and anaerobic denitrifying conditions are used instead of a single species under strictly anaerobic denitrifying conditions, as in this study. This must be verified by future study.
In conclusion, the O2-requiring reactions of hydrocarbon metabolism were rate limiting under microaerobic conditions. P. aeruginosa was more sensitive to nitrate-nitrite inhibition under anaerobic denitrifying conditions than under aerobic conditions. For the sequential bioremediation strategy, an initial period should be included for induction of nitrate-nitrite reductases in the presence of low nitrate concentrations in order to minimize the transient accumulation of toxic nitrite. Furthermore, in the single-species systems that have been studied, only about one-half of the TOC derived from hexadecane metabolism by aerobic resting or stationary-phase cells are readily consumed under anaerobic denitrifying conditions. In future work, microbial consortia should be employed to mineralize higher fractions of oxygenated metabolites. Our results improved the knowledge of sequential biodegradation of hydrocarbons and laid an important foundation for further development of advanced bioremediation strategies.
ACKNOWLEDGMENTS
This work was supported by the Ohio Board of Regents (Ohio Bioprocessing Research Consortium), by grant BES-9900694 from the National Science Foundation, and by a University of Akron faculty research grant.
REFERENCES
- 1.Altenschmidt U, Fuchs G. Anaerobic degradation of toluene in denitrifying Pseudomonas sp.: indication for toluene methylhydroxylation and benzoyl-CoA as central aromatic intermediate. Arch Microbiol. 1991;156:152–158. doi: 10.1007/BF00290990. [DOI] [PubMed] [Google Scholar]
- 2.Bai G, Brusseau M L, Miller R M. Biosurfactant-enhanced removal of residual hydrocarbon from soil. J Contam Hydrol. 1997;25:157–170. [Google Scholar]
- 3.Bakker G. Anaerobic degration of aromatic compounds in the presence of nitrate. FEMS Lett. 1977;1:103–107. [Google Scholar]
- 4.Ball H A, Reinhard M. Monoaromatic hydrocarbon transformation under anaerobic conditions at Seal Beach, California: laboratory studies. Environ Toxicol Chem. 1996;15:114–122. [Google Scholar]
- 5.Barker J F, Patrick G C, Major D W. Natural attenuation of aromatic hydrocarbons in a shallow sand aquifer. Ground Water Monit Rev. 1987;7:64–71. [Google Scholar]
- 6.Bengtsson G, Annadotter H. Nitrate reduction in a groundwater microcosm determined by 15N gas chromatography-mass spectrometry. Appl Environ Microbiol. 1989;55:2861–2870. doi: 10.1128/aem.55.11.2861-2870.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaszczyk M. Comparison of denitrification by Paracoccus denitrificans, Pseudomonas stutzeri and Pseudomonas aeruginosa. Acta Microbiol Pol. 1992;4:203–210. [PubMed] [Google Scholar]
- 8.Boulding J R. EPA environmental engineering sourcebook. Chelsea, Mich: Ann Arbor Press; 1996. [Google Scholar]
- 9.Bouwer E J, McCarty P L. Transformations of halogenated organic compounds under denitrification conditions. Appl Environ Microbiol. 1983;45:1295–1299. doi: 10.1128/aem.45.4.1295-1299.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bregnard T P A, Hoehener P, Haener A, Zeyer J. Degradation of weathered diesel fuel by microorganisms from a contaminated aquifer in aerobic and anaerobic microcosms. Environ Toxicol Chem. 1996;15:299–307. [Google Scholar]
- 11.Chapelle F H. Ground-water microbiology and geochemistry. New York, N.Y: John Wiley & Sons; 1992. [Google Scholar]
- 12.Clesceri L S, Greenberg A E, Trussel R R. Standard methods for the examination of water and wastewater. 17th ed. Washington, D.C.: American Public Health Association; 1989. [Google Scholar]
- 13.Coates J D, Woodward J, Allen J, Philip P, Lovley D R. Anaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments. Appl Environ Microbiol. 1997;63:3589–3593. doi: 10.1128/aem.63.9.3589-3593.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper D G, Paddock D A. Production of a biosurfactant from Torulopsis bombicola. Appl Environ Microbiol. 1984;47:173–176. doi: 10.1128/aem.47.1.173-176.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coschigano P W, Young L Y. Identification and sequence analysis of two regulatory genes involved in anaerobic toluene metabolism by strain T1. Appl Environ Microbiol. 1997;63:652–660. doi: 10.1128/aem.63.2.652-660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dangel W, Tschech A, Fuchs G. Anaerobic metabolism of cyclohexanol by denitrifying bacteria. Arch Microbiol. 1988;150:358–362. doi: 10.1007/BF00408307. [DOI] [PubMed] [Google Scholar]
- 17.Fenchel T, Finlay B J. Ecology and evolution in anoxic worlds. Oxford, United Kingdom: Oxford University Press; 1995. [Google Scholar]
- 18.Gamble T N, Betlach M R, Tiedje J M. Numerically dominant denitrifying bacteria from world soils. Appl Environ Microbiol. 1977;33:926–939. doi: 10.1128/aem.33.4.926-939.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B. Manual of methods for general bacteriology. Washington, D.C.: American Society for Microbiology; 1981. [Google Scholar]
- 20.Gersberg R M, Dawsey W J, Ridgway H F. Biodegradation of dissolved aromatic hydrocarbons in gasoline-contaminated groundwater using denitrification. In: Calabrese E J, Kostecki P T, editors. Petroleum contaminated soils. Vol. 2. Chelsea, Mich: Lewis Publishers; 1989. pp. 211–217. [Google Scholar]
- 21.Gobbert U, Lang S, Wagner F. Sophorose lipid formation by resting cells of Torulopsis bombicola. Biotechnol Lett. 1984;6:225–230. [Google Scholar]
- 22.Grbic-Galic D. Microbial degradation of homocyclic and heterocyclic aromatic hydrocarbons under anaerobic conditions. Dev Ind Microbiol. 1989;30:237–253. [Google Scholar]
- 23.Hernandez D, Dias F M, Rowe J J. Nitrate transport and its regulation by O2 in Pseudomonas aeruginosa. Arch Biochem Biophys. 1991;286:159–163. doi: 10.1016/0003-9861(91)90022-b. [DOI] [PubMed] [Google Scholar]
- 24.Hess A, Zarda B, Hahn D, Haner A, Stax D, Hohener P, Zeyer J. In situ analysis of denitrifying toluene- and m-xylene-degrading bacteria in a diesel fuel-contaminated laboratory aquifer column. Appl Environ Microbiol. 1997;63:2136–2141. doi: 10.1128/aem.63.6.2136-2141.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju L-K, Trivedi H K. Monitoring of denitrification by Pseudomonas aeruginosa using on-line fluorescence technique. Biotechnol Tech. 1992;6:549–554. [Google Scholar]
- 26.King R B, Long G M, Sheldon J K. Practical environmental bioremediation. Boca Raton, Fla: Lewis Publishers; 1992. [Google Scholar]
- 27.Knowles R. Denitrification. Microbiol Rev. 1982;46:43–70. doi: 10.1128/mr.46.1.43-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosaric N. Biosurfactants: production, properties, and applications. New York, N.Y: Marcel Dekker; 1993. [Google Scholar]
- 29.Madigan M T, Martinko J M, Parker J. Brock biology of microorganisms. 8th ed. Upper Saddle River, N.J: Prentice-Hall; 1997. [Google Scholar]
- 30.Major D W, Mayfield C I, Baker J F. Biotransformation of benzene by denitrification in aquifer sand. Ground Water. 1988;26:8–14. [Google Scholar]
- 31.McCarty M. The health effects of nitrate, nitrite, and N-nitroso compounds. Washington, D.C.: National Academy Press; 1981. [Google Scholar]
- 32.Patureau D, Bernet N, Moletta R. Effect of oxygen on denitrification in continuous chemostat culture with Comamonas sp. SGLY2. J Ind Microbiol. 1996;16:124–128. [Google Scholar]
- 33.Ramirez N. Elucidation of sorption, desorption and biodegradation phenomena of pyrene spiked soils. M.S. thesis. Akron, Ohio: The University of Akron; 1999. [Google Scholar]
- 34.Ridgway H F, Safarik J, Phipps D, Carl P, Clark D. Identification and catabolic activity of well-derived gasoline-degrading bacteria from a contaminated aquifer. Appl Environ Microbiol. 1990;56:3565–3575. doi: 10.1128/aem.56.11.3565-3575.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowe J J, Yarbrough J M, Rake J B, Eagon R G. Nitrite inhibition of aerobic bacteria. Curr Microbiol. 1979;2:51–54. [Google Scholar]
- 36.Rueter P, Rabus R, Wilkes H, Aeckersberg F, Rainey F A, Jannasch H W, Widdel F. Anaerobic oxidation of hydrocarbons in crude oil by new types of surphate-reducing bacteria. Nature. 1994;372:455–458. doi: 10.1038/372455a0. [DOI] [PubMed] [Google Scholar]
- 37.Scheibenbogen K, Zytner R G, Lee H, Trevors J T. Enhanced removal of selected hydrocarbons from soil by Pseudomonas aeruginosa UG2 biosurfactants and some chemical surfactants. J Chem Tech Biotechnol. 1994;59:53–59. [Google Scholar]
- 38.Stouthamer A H, de Boer A P N, van der Oost J, van Spanning R J M. Emerging principles of inorganic nitrogen metabolism in Paracoccus denitrificans and related bacteria. Antonie Leeuwenhoek. 1997;71:33–41. doi: 10.1023/a:1000113824961. [DOI] [PubMed] [Google Scholar]
- 39.Syldatk C, Wagner F. Production of biosurfactants. In: Kosaric N, Cairns W L, Gray N C C, editors. Biosurfactants and biotechnology. New York, N.Y: Marcel Dekker; 1987. pp. 89–120. [Google Scholar]
- 40.Thomas J M, Ward C H. In situ biorestoration of organic contaminants in the subsurface. Environ Sci Technol. 1989;23:760–766. [Google Scholar]
- 41.Tiedje J M. Biology of anaerobic microorganisms. New York, N.Y: John Wiley & Sons; 1988. Ecology of denitrification and dissimilatory nitrate reduction to ammonium; pp. 179–243. [Google Scholar]
- 42.Wilson J T, Leach L E, Henson M, Jones J N. In-situ biorestoration as a groundwater remediation technique. Ground Water Monit Rev. 1986;6:56–64. [Google Scholar]
- 43.Wilson L P, Bouwer E J. Biodegradation of aromatic compounds under mixed oxygen/denitrifying conditions: a review. J Ind Microbiol Biotechnol. 1997;18:116–130. doi: 10.1038/sj.jim.2900288. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Ju L-K. Extracellular particles of polymeric material formed in n-hexadecane fermentation by Pseudomonas aeruginosa. J Bacteriol. 1997;179:193–202. doi: 10.1016/s0168-1656(97)00150-8. [DOI] [PubMed] [Google Scholar]