Key Points
Question
Can daily supplementation with marine ω-3 fatty acids prevent the development of dry eye disease (DED)?
Findings
In this randomized clinical trial of 23 523 US adults who at study entry were free of a previous diagnosis of DED and were not experiencing severe dry eye symptoms, daily supplementation with 1 g of marine ω-3 fatty acids for a median (range) 5.3 (3.8-6.1) years had no significant effect on the incidence of diagnosed DED or reported DED symptoms.
Meaning
The results do not support recommending marine ω-3 fatty acid supplementation to reduce the incidence of DED.
This randomized clinical trial evaluates the impact of daily ω-3 supplementation on the development of incident dry eye disease.
Abstract
Importance
Results of several small randomized clinical trials have suggested that supplements of marine ω-3 fatty acids may be beneficial in treating signs and symptoms of dry eye disease (DED). However, randomized clinical trial data to examine whether ω-3 fatty acid supplements can prevent DED are lacking.
Objective
To evaluate whether long-term daily supplementation with marine ω-3 fatty acids prevents the development of DED.
Design, Setting, and Participants
This was a prespecified ancillary study of the Vitamin D and Omega-3 Trial (VITAL), a nationwide randomized double-blind placebo-controlled 2 × 2 factorial trial of vitamin D and marine ω-3 fatty acids in the primary prevention of cancer and cardiovascular disease. Participants in this ancillary study were 23 523 US adults (men 50 years and older and women 55 years and older) who at study entry were free of a previous diagnosis of DED and were not experiencing severe dry eye symptoms. Participants were enrolled from November 2011 to March 2014, and treatment and follow-up ended on December 31, 2017. Data were analyzed from January 2020 to August 2021.
Interventions
Marine ω-3 fatty acids, 1 g per day.
Main Outcomes and Measures
The primary end point was incident clinically diagnosed DED confirmed by review of the medical records. The secondary end point was a composite of all confirmed incident clinically diagnosed DED cases plus all incident reports of severe DED symptoms.
Results
The mean (SD) age of the 23 523 participants included in the analysis was 67.0 (7.0) years, and 11 349 participants (48.3%) were women. The cohort included 4610 participants (20.0%) who self-identified as Black, 16 481 (71.6%) who self-identified as non-Hispanic White, and 1927 (8.4%) of other racial or ethnic groups or who declined to respond, consolidated owing to small numbers, including American Indian or Alaska Native, Asian, Hispanic or Latino, and Native Hawaiian or Other Pacific Islander. During a median (range) 5.3 (3.8-6.1) years of treatment and follow-up, 472 of 23 523 participants (2.0%) experienced a medical record–confirmed diagnosis of DED. There was no difference in diagnosed DED by randomized ω-3 fatty acid assignment (232 of 11 757 participants [2.0%] with end points in the treated group vs 240 of 11 766 [2.0%] with end points in the placebo group; hazard ratio, 0.97; 95% CI, 0.81-1.16). Similarly, there was no difference between groups for the secondary end point of diagnosed DED plus incident severe DED symptoms (1044 participants [8.9%] with end points in the treated group vs 1074 [9.1%] with end points in the placebo group; hazard ratio, 0.97; 95% CI, 0.89-1.06).
Conclusions and Relevance
In this randomized clinical trial, long-term supplementation with 1 g per day of marine ω-3 fatty acids for a median (range) of 5.3 (3.8-6.1) years did not reduce the incidence of diagnosed DED or a combined end point of diagnosed DED or incident severe DED symptoms. These results do not support recommending marine ω-3 fatty acid supplementation to reduce the incidence of DED.
Trial Registration
ClinicalTrials.gov Identifier: NCT01880463
Introduction
Dry eye disease (DED) is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film causing a variety of symptoms and visual impairment.1,2 In the US, an estimated 16.4 million adults have been diagnosed with DED, and an additional 6 million adults have reported DED symptoms without a formal diagnosis.3 The pathophysiology of DED is incompletely understood, but typically involves a cycle of inflammation, including both innate and adaptive immune responses.4,5,6 Accordingly, management of DED often involves treatment with topical artificial tears plus limited-duration topical steroids; nonglucocorticoid immunomodulatory drugs, such as topical cyclosporine A; or the lymphocyte function–associated antigen (LFA)-1 antagonist lifitegrast.7
ω-3 Fatty acids are also frequently recommended in the management of DED. Basic science studies report beneficial effects with ω-3 fatty acids in several inflammatory processes implicated in the pathogenesis of DED, including production of proinflammatory cytokines and T-lymphocyte proliferation.7 Data from some randomized clinical trials of patients with DED, most of which were of short duration and limited sample size, support a benefit with ω-3 supplements in doses ranging from 420 to 2400 mg per day in reducing 1 or more DED signs and symptoms.8,9,10,11,12
Few studies have examined the efficacy of ω-3 supplements in preventing the onset of DED. Inflammatory processes, acting either directly or in response to changes in tear osmolarity, are believed to play a key role in the earliest stages of DED development,4,5,6 and several cross-sectional studies suggest lower prevalence of DED in persons with higher intake of ω-3 fatty acids.13,14,15 However, to our knowledge, there are no randomized clinical trial data, or even prospective observational data, to examine whether ω-3 fatty acid supplements can reduce the risk of incident DED.
In this report, we present ancillary findings for incident DED from the Vitamin D and Omega-3 Trial (VITAL), a randomized double-blind placebo-controlled 2 × 2 factorial trial designed to test vitamin D and marine ω-3 fatty acid supplements in the primary prevention of cancer and cardiovascular disease among US adults.
Methods
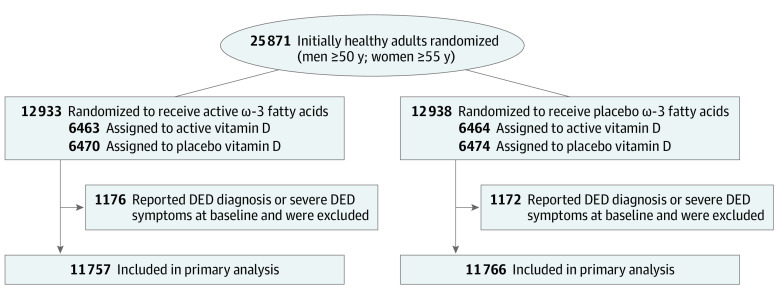
VITAL-Dry Eye is a prespecified ancillary study of VITAL, a nationwide randomized double-blind placebo-controlled 2 × 2 factorial trial of vitamin D3 (cholecalciferol), 2000 IU daily, and marine ω-3 fatty acids (fish oil, eicosapentaenoic acid, and docosahexaenoic acid in a ratio of approximately 1.2:1), 1 g daily, in the primary prevention of cancer and cardiovascular disease among 25 871 adults (men 50 years and older and women 55 years and older). Details of this study are described elsewhere.16,17 Of the 25 871 participants randomized in the parent VITAL trial, VITAL-Dry Eye excluded 2348 participants who at baseline reported a previous diagnosis of DED or dry eye syndrome made by a clinician, or were experiencing severe dry eye symptoms (both dryness and irritation either constantly or often). Compared with those not excluded, excluded participants were older, more likely to be female, and more likely to report a history of diabetes, hypertension, or autoimmune disease. Eligible participants in the parent VITAL trial had no history of cancer (except nonmelanoma skin cancer), myocardial infarction, stroke, transient ischemic attack, or coronary revascularization at study entry. They were required to agree to forgo use of nonstudy fish oil supplements, to limit consumption of supplemental vitamin D and calcium to 800 IU daily and 1200 mg daily, respectively, and to complete a 3-month placebo run-in phase. Safety exclusions included kidney failure or dialysis, severe liver disease, history of hypercalcemia or parathyroid disorders, sarcoidosis or other granulomatous diseases, fish allergy (for the ω-3 intervention), or other serious conditions that would preclude safe participation. Randomization to ω-3 fatty acids, vitamin D, both active agents, or both placebos took place from November 2011 to March 2014 and was computer generated within sex, 5-year age groups, and race (Figure 1). Race and ethnicity data were queried because previous studies have suggested possible racial and ethnic variability in occurrence of eye disease. Participants self-reported race and ethnicity by responding to multiple choice questions and categories were consolidated where appropriate to avoid small numbers. Study pill-taking ended as planned on December 31, 2017, yielding a median (range) treatment period of 5.3 (3.8-6.1) years. The trial was approved by the institutional review board of Brigham and Women’s Hospital, Boston, and was monitored by an external data and safety monitoring board. All participants provided written informed consent before enrollment in the trial. They were not offered any compensation or incentives for participation. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials. Data were analyzed from January 2020 to August 2021. The trial protocol can be found in Supplement 1.
Figure 1. Flow Diagram of Randomization for Incident Clinically Diagnosed Dry Eye Disease (DED).
Baseline questionnaires requested information on clinical and lifestyle risk factors and included a food frequency questionnaire that ascertained participant-reported intake of fish and other foods. Annual questionnaires assessed compliance with randomized treatments, use of nonstudy fish oil or vitamin D supplements, development of major illnesses, and other study end points, including DED, as well as potential adverse effects of the study agents.
Baseline blood samples were collected during the run-in from all willing individuals, including 16 956 randomized participants (65%). Samples were assayed for plasma ω-3 index (eicosapentaenoic acid plus docosahexaenoic acid as a percentage of total fatty acids18) and serum 25-hydroxyvitamin D using liquid chromatography tandem mass spectrometry.
DED Ascertainment
Participants who reported on the baseline questionnaire that they had previously been diagnosed by a clinician with DED or dry eye syndrome or were experiencing severe dry eye symptoms (both dryness and irritation either constantly or often) were excluded from this analysis. On annual follow-up questionnaires, participants were asked 3 validated questions on DED diagnosis and symptoms19: (1) “In the PAST YEAR, have you been diagnosed (by a clinician) with dry eye syndrome or dry eye disease?” (2) “How often are your eyes dry (not wet enough)?” (3) “How often are your eyes irritated?” Response options for the 2 questions about symptoms included “constantly,” “often,” “sometimes,” or “never.”
Participants who reported a new diagnosis of DED postrandomization were asked to provide written consent to review the relevant medical records pertaining to the diagnosis as well as contact information for the treating ophthalmologists or optometrists. Treating clinicians were contacted by mail and asked to complete a standardized questionnaire providing information on the date of initial diagnosis of DED plus additional information related to the diagnosis. Clinicians could also provide the requested information by sending full copies of the patient’s medical record.
The primary end point was incident clinically diagnosed DED confirmed by review of the medical records or by treating clinician questionnaire. The secondary end point was a composite of all confirmed incident clinically diagnosed DED cases plus all incident reports of severe DED symptoms (both dryness and irritation either constantly or often).
Statistical Analysis
The trial was estimated to have 80% or greater power to detect an observed hazard ratio (HR) of 0.85 for the primary end point of incident diagnosed DED in the ω-3 fatty acid group vs placebo. For the secondary composite end point of incident diagnosed DED plus development of severe DED symptoms, estimated power was higher, at 91% or greater to detect an observed HR of 0.85.
Initial analyses compared the distributions of baseline characteristics in the ω-3 and placebo groups using 2-sample t tests, χ2 tests for proportions, and tests for trend for ordinal categories. Kaplan-Meier survival estimates and the log-rank test were used to determine whether there was a difference in time to a DED end point between the ω-3 and placebo groups. Cox proportional hazards models20 were used to estimate the HR of DED among those in the ω-3 group compared with placebo after adjustment for age at baseline, sex, and randomized assignment to vitamin D. Models were also fit separately within 2 baseline age groups: 50 to 64 years and 65 years and older. A potential modifying effect of age on the association between ω-3 treatment and DED was calculated by including a term for the interaction of the study agent and age in the Cox model. The proportionality assumption was tested by including an interaction term of ω-3 treatment with the logarithm of time in Cox models. The proportionality assumption was not violated for the primary end point of incident DED or the secondary combined end point of incident DED plus incident severe symptoms.
Prespecified subgroup analyses were conducted by categories of baseline variables that are possible risk factors for DED (age, sex, race, and major medical comorbidities [diabetes, hypertension, and autoimmune disease]), including baseline plasma ω-3 index and baseline fish intake as well as concurrent randomization to the vitamin D group. Possible effect modification was explored by using interaction terms between subgroup indicators and ω-3 assignment, and tests for trend were conducted when subgroup categories were ordinal. Because it is possible that some DED cases diagnosed during the treatment period may have been prevalent at baseline and to explore possible latent treatment effects, prespecified sensitivity analyses excluded participants with DED diagnosed during the first year and the first 2 years of the trial. Two-sided P values and 95% CIs were calculated, and P <.05 was considered statistically significant. There was no control for multiple hypothesis testing, and no formal adjustment was made to the P values or confidence intervals. Thus, the results regarding secondary end points and subgroups should be interpreted with caution.
Results
The mean (SD) age of the 23 523 participants included in the analysis was 67.0 (7.0) years, and 11 349 participants (48.3%) were women. The cohort included 4610 participants (20.0%) who self-identified as Black, 16 481 (71.6%) who self-identified as non-Hispanic White, and 1927 (8.4%) of other racial or ethnic groups or who declined to respond, consolidated owing to small numbers, including American Indian or Alaska Native, Asian, Hispanic or Latino, and Native Hawaiian or Other Pacific Islander. Baseline characteristics of study participants (Table 1) appeared to be distributed equally between treatment (n = 11 757) and placebo (n = 11 766) groups, although there was a slightly higher proportion of participants with autoimmune disease randomized to ω-3 fatty acids (1878 of 11 757 [16.0%] vs 1762 of 11 766 [15.0%]).
Table 1. Baseline Characteristics Among Participants in the Vitamin D and Omega-3 Trial Who Were Free of Diagnosed Dry Eye Disease or Symptoms at Baselinea.
| Characteristic | Participants, No. (%) | |
|---|---|---|
| Placebo (n = 11 766) | ω-3 Fatty acids (n = 11 757) | |
| Age, mean (SD), y | 67.0 (7.0) | 67.0 (7.0) |
| 50 to <65 | 4575 (38.9) | 4575 (38.9) |
| ≥65 | 7191 (61.1) | 7182 (61.1) |
| Sex | ||
| Female | 5671 (48.2) | 5678 (48.3) |
| Male | 6095 (51.8) | 6079 (51.7) |
| Raceb | ||
| Black | 2299 (20.0) | 2311 (20.1) |
| Non-Hispanic White | 8238 (71.5) | 8243 (71.6) |
| Otherc | 975 (8.5) | 952 (8.3) |
| Diabetes | ||
| No | 10 211 (86.9) | 10 142 (86.4) |
| Yes | 1536 (13.1) | 1593 (13.6) |
| Hypertension history | ||
| No | 5664 (48.4) | 5740 (49.1) |
| Yes | 6034 (51.6) | 5949 (50.9) |
| Hypertension treatment | ||
| No | 5692 (48.7) | 5802 (49.7) |
| Yes | 5987 (51.3) | 5883 (50.3) |
| Autoimmune disease | ||
| No | 10 003 (85.0) | 9878 (84.0) |
| Yes | 1762 (15.0) | 1878 (16.0) |
| Baseline plasma ω-3 level | ||
| Median <2.5% | 3523 (50.2) | 3575 (50.7) |
| Median ≥2.5% | 3499 (49.8) | 3483 (49.3) |
| Fish consumption | ||
| Median <1.5 servings/wk | 6128 (53.0) | 6159 (53.2) |
| Median ≥1.5 servings/wk | 5435 (47.0) | 5411 (46.8) |
| Vitamin D treatment assignment | ||
| Placebo | 5884 (50.0) | 5871 (49.9) |
| Active | 5882 (50.0) | 5886 (50.1) |
Percentages are based on participants with data available.
Race and ethnicity data were queried because previous studies have suggested possible racial and ethnic variability in occurrence of eye disease. Participants self-reported race and ethnicity by responding to multiple choice questions and categories were consolidated where appropriate to avoid small numbers.
Includes American Indian or Alaska Native, Asian, Hispanic or Latino, Native Hawaiian or Other Pacific Islander, and unknown.
The mean (SD) rate of response to questionnaires was 93.2%. The mean (SD) rate of adherence to the trial regimen that was reported by the participants (percentage of participants who took at least two-thirds of the trial capsules) was 82.0% in the ω-3 group and 81.7% in the placebo group. Of the 23 523 participants included in this analysis, 14 080 (59.9%) had provided a blood sample at baseline and a subset (1490 of 14 080 [10.6%]) at 1-year follow-up that could be analyzed for ω-3 index. Among the 1490 participants who had provided a blood sample both at baseline and at 1-year follow-up, the mean (SD) plasma ω-3 index at baseline was 2.6% (1.0%) in the ω-3 group and 2.7% (0.9%) in the placebo group. At 1-year follow-up, the mean ω-3 index rose to 4.1% (an increase of 55.2%) in the ω-3 group and changed by less than 2% in the placebo group.
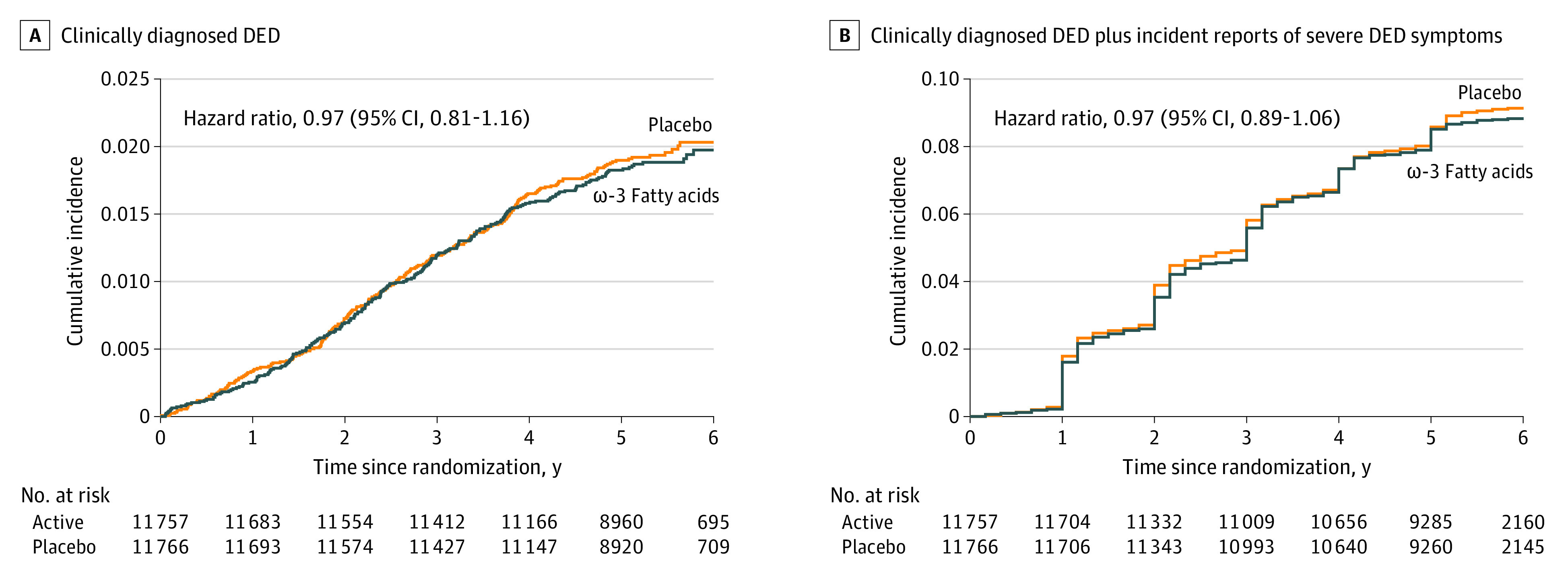
During a median (range) of 5.3 (3.8-6.1) years of treatment and follow-up, 472 of 23 523 participants (2.0%) experienced a medical record–confirmed diagnosis of incident DED, the primary study end point. There was no difference in diagnosed DED by randomized ω-3 fatty acid assignment (232 of 11 757 participants [2.0%] with end points in the treated group vs 240 of 11 766 participants [2.0%] with end points in the placebo group; HR, 0.97; 95% CI, 0.81-1.16) (Table 2). Similarly, there was no difference between groups for the secondary end point of incident DED plus incident severe DED symptoms (1044 participants [8.9%] with end points in the treated group vs 1074 participants [9.1%] with end points in the placebo group; HR, 0.97; 95% CI, 0.89-1.06) (Table 2). The HR for incident severe DED symptoms alone was 0.97 (95% CI, 0.88-1.07).
Table 2. Hazard Ratios (HRs) and 95% CIs for the Primary and Secondary End Points.
| End point | No. with DED end point | HR (95% CI)a | P value | |
|---|---|---|---|---|
| Placebo (n = 11 766) | ω-3 Fatty acids (n = 11 757) | |||
| Primary (incident DED) | 240 | 232 | 0.97 (0.81-1.16) | .72 |
| Secondary (incident DED plus incident severe DED symptoms) | 1074 | 1044 | 0.97 (0.89-1.06) | .49 |
| Excluding year 1 | ||||
| Primary (incident DED) | 198 | 200 | 1.01 (0.83-1.23) | .91 |
| Secondary (incident DED plus incident severe DED symptoms) | 854 | 846 | 0.99 (0.90-1.09) | .81 |
| Excluding years 1-2 | ||||
| Primary (incident DED) | 149 | 145 | 0.97 (0.77-1.22) | .82 |
| Secondary (incident DED plus incident severe DED symptoms) | 597 | 611 | 1.02 (0.91-1.14) | .72 |
Abbreviation: DED, dry eye disease.
Adjusted for age, sex, and vitamin D treatment assignment.
Cumulative incidence rates of incident DED and incident DED plus incident severe DED symptoms are shown in Figure 2. The curves did not differ for the 2 groups at any point during follow-up. HRs were not materially altered when we excluded end points diagnosed during the first year (incident DED HR, 1.01; 95% CI, 0.83-1.23 and incident DED plus incident severe DED symptoms HR, 0.99; 95% CI, 0.90-1.09) and first 2 years (incident DED HR, 0.97; 95% CI, 0.77-1.22 and incident DED plus incident severe DED symptoms HR, 1.02; 95% CI, 0.91-1.14) of follow-up.
Figure 2. Cumulative Incident Rates of Dry Eye Disease (DED) Diagnosis and Symptoms.
Results of prespecified subgroup analyses are presented in Table 3. There was no evidence for modification of the lack of effect of ω-3 fatty acids on incident DED by baseline plasma ω-3 level or fish consumption or by baseline categories of DED risk factors except for a possible lower incidence of diagnosed DED among participants who reported autoimmune disease at study entry and were assigned to the ω-3 group.
Table 3. Hazard Ratios (HRs) and 95% CIs for Incident Dry Eye Disease (DED) Comparing ω-3 Fatty Acids and Placebo Groups.
| Subgroup | Total, No. | No. of incident DED | HR (95% CI)a | |
|---|---|---|---|---|
| Placebo | ω-3 | |||
| Age, y | ||||
| 50 to <65 | 9150 | 61 | 65 | 1.06 (0.75-1.50) |
| ≥65 | 14 373 | 179 | 167 | 0.93 (0.76-1.15) |
| Sex | ||||
| Female | 11 349 | 149 | 154 | 1.03 (0.82-1.29) |
| Male | 12 174 | 91 | 78 | 0.86 (0.64-1.16) |
| Raceb | ||||
| Black | 4610 | 36 | 37 | 1.02 (0.64-1.61) |
| Non-Hispanic White | 16 481 | 183 | 169 | 0.93 (0.75-1.14) |
| Otherc | 1927 | 17 | 19 | 1.18 (0.61-2.28) |
| Diabetes | ||||
| No | 20 353 | 205 | 200 | 0.98 (0.81-1.20) |
| Yes | 3129 | 34 | 31 | 0.87 (0.54-1.42) |
| Hypertension history | ||||
| No | 11 404 | 110 | 94 | 0.85 (0.64-1.12) |
| Yes | 11 983 | 127 | 138 | 1.10 (0.86-1.40) |
| Hypertension treatment | ||||
| No | 11 494 | 110 | 90 | 0.81 (0.61-1.07) |
| Yes | 11 870 | 125 | 141 | 1.15 (0.90-1.46) |
| Autoimmune disease | ||||
| No | 19 881 | 182 | 192 | 1.07 (0.87-1.31) |
| Yes | 3640 | 58 | 40 | 0.65 (0.43-0.97) |
| Baseline plasma ω-3 level | ||||
| Median <2.5% | 7098 | 84 | 63 | 0.75 (0.54-1.04) |
| Median ≥2.5% | 6982 | 92 | 88 | 0.95 (0.71-1.27) |
| Fish consumption | ||||
| Median <1.5 servings/wk | 12 287 | 121 | 128 | 1.07 (0.83-1.37) |
| Median ≥1.5 servings/wk | 10 846 | 115 | 103 | 0.89 (0.68-1.16) |
| Vitamin D treatment assignment | ||||
| Active | 11 768 | 115 | 123 | 1.07 (0.83-1.38) |
| Placebo | 11 755 | 125 | 109 | 0.87 (0.67-1.13) |
Adjusted for age, sex, and vitamin D treatment assignment.
Race and ethnicity data were queried because previous studies have suggested possible racial and ethnic variability in occurrence of eye disease. Participants self-reported race and ethnicity by responding to multiple choice questions and categories were consolidated where appropriate to avoid small numbers.
Includes American Indian or Alaska Native, Asian, Hispanic or Latino, Native Hawaiian or Other Pacific Islander, and unknown.
Discussion
In this randomized clinical trial of 23 523 initially healthy US adults without a reported diagnosis of DED or severe dry eye symptoms at study entry, supplementation with ω-3 fatty acids at a dose of 1 g per day for a median (range) of 5.3 (3.8-6.1) years had no effect on the primary study end point of incident DED. Similarly, there was no difference between treatment groups for the secondary combined end point of incident DED plus incident severe DED symptoms. For each end point, exclusion of end points confirmed early in follow-up had no material impact on effect estimates.
Because it was not feasible to conduct in-person ophthalmic examinations of study participants in this large, geographically dispersed cohort, DED cases were ascertained through participant reports subsequently confirmed by review of relevant medical records. We defined a DED case as a confirmed diagnosis of DED made by a clinician, and we did not distinguish between DED subtypes (aqueous-deficient vs evaporative) or etiologies (eg, contact lens wear, intensive computer use, and meibomian gland dysfunction). Thus, our DED end point had minimal restrictions, was representative of the general DED population seen in real-world clinical practice, and is similar to the disease classification of nonspecific typical DED defined in earlier trials and reviews.8,21
Previous trials of ω-3s and DED were conducted in patients with established DED and examined whether ω-3 supplements, alone or in combination with other eye medications, could reduce DED signs and symptoms. Most of these trials were of short duration (6 months or less) and limited sample size (200 patients or fewer), and many were focused on patients with specific DED etiology.8,9,10,11,12
Of particular relevance to our study, 13 trials were conducted in patients with nonspecific typical DED, a disease classification similar to the DED end point defined in our study, and results were mixed.8 In 12 trials with treatment duration of 6 months or less, ω-3 fatty acids at doses ranging from 420 to 1785 mg per day, when tested alone without other eye medications, appeared to significantly improve DED signs (as measured by tear breakup time, Schirmer test score, and osmolarity) and symptoms (as measured by Ocular Surface Disease Index score). However, the benefit with supplementation, particularly for tear breakup time and Ocular Surface Disease Index scores, weakened with treatment duration and no benefit was observed in trials that combined ω-3 supplementation with other eye medications.8 The 13th trial, the recently completed Dry Eye Assessment and Management (DREAM) study,21 is to our knowledge the longest and largest trial conducted to date among patients with nonspecific typical DED. DREAM randomized 545 patients with moderate to severe DED to 3 g of ω-3 fatty acids daily (n = 349) or placebo (n = 186) for 12 months. Patients were allowed to maintain their current treatments for DED and results were based on mean change in study measures at 6 and 12 months. In final analyses, there was no difference between treatment groups in the primary end point of change in Ocular Surface Disease Index score and no difference in the secondary end points of change in conjunctival staining score, corneal staining score, tear breakup time, and Schirmer test score. Together, these prior randomized clinical trial findings in patients with nonspecific typical DED suggest a possible short-term benefit with ω-3 supplementation in reducing signs and symptoms of DED when tested alone, but not when combined with other eye treatments, even for treatment durations beyond 6 months.
Direct comparison of our findings for incident DED with these earlier findings for DED signs and symptoms is not possible because of differences in disease end points, stage of disease, and ω-3 doses tested. Nonetheless, because the pathophysiologic mechanisms causally linked to DED signs and symptoms are also thought to underlie the earliest stages of DED development,5,6 it seems reasonable and potentially informative to consider the relative efficacy of ω-3 in DED prevention and management. Our finding that long-term supplemental use of ω-3 had no material effect on DED incidence appears broadly consistent with the null findings for DED signs and symptoms observed in the DREAM study. Moreover, we found no benefit with supplementation in newly reported severe DED symptoms, a component of our secondary end point that is more closely related to the end points examined in DREAM. When considered together, neither DREAM, which examined the effect of ω-3 supplementation as an adjuvant in DED management, nor our study, which assessed the individual effect of ω-3 supplementation in DED prevention, provides support for long-term use of ω-3 supplements in reducing risks of DED.
Limitations
Several possible limitations of our study need to be considered, especially in light of the findings of no significant effect. Compliance with study pills remained high (more than 80%) in both treatment groups during follow-up, so poor compliance would be an unlikely explanation. However, the dose of ω-3 fatty acids tested (1 g per day), while greater than the dose associated with short-term symptomatic improvement in several earlier trials, may have been insufficient for primary prevention of DED. An inadequate duration of treatment seems an unlikely explanation because at 5.3 years of treatment and follow-up, VITAL was considerably longer than previous studies. Previous studies support the validity of our questionnaire-based methodology19,22,23 including a good balance of sensitivity and specificity vs commonly used clinical tests for DED.19 Nonetheless, because case identification was based on participant reports, some degree of underascertainment of DED is plausible. Such underascertainment would likely reduce study power, but is not associated with bias in randomized comparisons.24 Random misclassification of diagnosed DED was reduced by the use of medical records to confirm participant reports, and nonrandom or differential misclassification was unlikely because medical records were reviewed by investigators masked to treatment assignment. There was no control for multiple hypothesis testing and no formal adjustment made to the P values or confidence intervals, so results regarding secondary end points and subgroups should be interpreted with caution. The equal distribution of baseline characteristics between treatment groups indicates that confounding by measured factors is unlikely and provides reassurance that other potential confounders, which were either unmeasured (eg, use of contact lenses or artificial tears) or unknown, were also likely to be evenly distributed between the 2 treatment groups.
Conclusions
The findings in this randomized clinical trial of initially healthy US adults show that supplementation with 1 g per day of marine ω-3 fatty acid for 5.3 years showed no effect on incident clinically diagnosed DED or reported severe DED symptoms. These results do not support recommending marine ω-3 fatty acid supplementation to reduce the incidence of DED.
Trial protocol
The members of the VITAL Research Group
Data sharing statement
References
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-283. doi: 10.1016/j.jtos.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 2.Tsubota K, Yokoi N, Shimazaki J, et al. ; Asia Dry Eye Society . New perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15(1):65-76. doi: 10.1016/j.jtos.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 3.Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90-98. doi: 10.1016/j.ajo.2017.06.033 [DOI] [PubMed] [Google Scholar]
- 4.Baudouin C, Irkeç M, Messmer EM, et al. ; ODISSEY European Consensus Group Members . Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018;96(2):111-119. doi: 10.1111/aos.13436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510. doi: 10.1016/j.jtos.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 6.Wei Y, Asbell PA. The core mechanism of dry eye disease is inflammation. Eye Contact Lens. 2014;40(4):248-256. doi: 10.1097/ICL.0000000000000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628. doi: 10.1016/j.jtos.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 8.Chi SC, Tuan HI, Kang YN. Effects of polyunsaturated fatty acids on nonspecific typical dry eye disease: a systematic review and meta-analysis of randomized clinical trials. Nutrients. 2019;11(5):E942. doi: 10.3390/nu11050942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downie LE, Ng SM, Lindsley KB, Akpek EK. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst Rev. 2019;12:CD011016. doi: 10.1002/14651858.CD011016.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannaccare G, Pellegrini M, Sebastiani S, et al. Efficacy of omega-3 fatty acid supplementation for treatment of dry eye disease: a meta-analysis of randomized clinical trials. Cornea. 2019;38(5):565-573. doi: 10.1097/ICO.0000000000001884 [DOI] [PubMed] [Google Scholar]
- 11.Molina-Leyva I, Molina-Leyva A, Bueno-Cavanillas A. Efficacy of nutritional supplementation with omega-3 and omega-6 fatty acids in dry eye syndrome: a systematic review of randomized clinical trials. Acta Ophthalmol. 2017;95(8):e677-e685. doi: 10.1111/aos.13428 [DOI] [PubMed] [Google Scholar]
- 12.Liu A, Ji J. Omega-3 essential fatty acids therapy for dry eye syndrome: a meta-analysis of randomized controlled studies. Med Sci Monit. 2014;20:1583-1589. doi: 10.12659/MSM.891364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318-326. doi: 10.1016/S0002-9394(03)00218-6 [DOI] [PubMed] [Google Scholar]
- 14.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127(6):763-768. doi: 10.1001/archophthalmol.2009.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miljanović B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82(4):887-893. doi: 10.1093/ajcn/82.4.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manson JE, Bassuk SS, Lee IM, et al. The Vitamin D and Omega-3 Trial (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159-171. doi: 10.1016/j.cct.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the Vitamin D and Omega-3 Trial (VITAL). Contemp Clin Trials. 2016;47:235-243. doi: 10.1016/j.cct.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39(1):212-220. doi: 10.1016/j.ypmed.2004.02.030 [DOI] [PubMed] [Google Scholar]
- 19.Gulati A, Sullivan R, Buring JE, Sullivan DA, Dana R, Schaumberg DA. Validation and repeatability of a short questionnaire for dry eye syndrome. Am J Ophthalmol. 2006;142(1):125-131. doi: 10.1016/j.ajo.2006.02.038 [DOI] [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187-220. [Google Scholar]
- 21.Maguire MG, Asbell PA; DREAM Study Research Group . n-3 fatty acid supplementation and dry eye disease. N Engl J Med. 2018;379(7):691. [DOI] [PubMed] [Google Scholar]
- 22.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264-1268. doi: 10.1001/archopht.118.9.1264 [DOI] [PubMed] [Google Scholar]
- 23.Oden NL, Lilienfeld DE, Lemp MA, Nelson JD, Ederer F. Sensitivity and specificity of a screening questionnaire for dry eye. Adv Exp Med Biol. 1998;438:807-820. doi: 10.1007/978-1-4615-5359-5_113 [DOI] [PubMed] [Google Scholar]
- 24.Rothman KJ, Greenland S. Modern Epidemiology. Lippincott-Ravne; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
The members of the VITAL Research Group
Data sharing statement