Abstract
Objectives
To explore the potential use of body mass index (BMI), proteinuria and total lymphocyte count changes in predicting immunological and virological response in individuals with HIV initiated on antiretroviral treatment (ART).
Design
Prospective cohort study.
Setting
Three urban HIV care and treatment centres in Dar es Salaam.
Participants
Individuals with HIV initiating ART.
Outcome measures
HIV viral load ≥1000 copies/mL (viral non-suppression) at 6 months after ART initiation.
Results
Of 215 (out of 220 enrolled) participants who returned for evaluation at 6 months, 147 (66.8%) were women. At 6 months of follow-up, 89.4% (76/85) of participants with sustained weight gain were virally suppressed compared with 31.8% (7/22) with sustained loss, p<0.001. In participants who were lymphopaenic at baseline, an increase to normal total lymphocyte counts at 6 months was associated with an increase in CD4 count compared with participants who remained lymphopaenic, 96.2% (50/52) versus 54.8% (17/31), p<0.001. At baseline, 50.0% (110/220) had proteinuria. In participants without proteinuria from baseline to 6 months, 89.8% (79/88) were virally suppressed compared with participants with proteinuria at baseline and/or 3 months, 85.6% (77/90), those with persistent proteinuria, 30.8% (8/26), and proteinuria at 6 months only, 45.5% (5/11), p<0.001. In modified Poisson regression, the independent predictors other than CD4 cell counts for viral non-suppression at 6 months among individuals with HIV initiating on ART were BMI loss >5% from baseline to 6 months (adjusted RR 2.73, 95% CI (1.36 to 5.47)), lymphopaenia at 6 months (adjusted RR=4.54, 95% CI (2.19 to 9.39)) and proteinuria at 6 months (adjusted RR=2.63, 95% CI (1.25 to 5.54)).
Conclusions
Change in BMI, total lymphocyte count and presence of proteinuria can monitor and predict ART response and may be particularly helpful in settings when CD4 counts and viral load monitoring are unavailable.
Keywords: internal medicine, infectious diseases, HIV & AIDS
Strengths and limitations of this study.
We had complete data on 98% of the originally enrolled participants.
In resource-constrained situations, when viral load and CD4 testing are not easily available, models such as ours with locally determined easily computable prediction cut-offs can be used by clinicians to make clinical decisions.
Our findings require validation in a study with larger sample size.
Local conditions and treatment standards may influence some of the patterns we observed, both in prevalence and in effect.
Introduction
In 2019, about 38 million people were living with HIV globally, with 1.7 million in Tanzania accounting for an estimated prevalence of 4.8% in the Tanzanian population aged 15–49 years.1 Viral load testing is the recommended method for monitoring HIV treatment response.2 However, viral load testing in resource-constrained settings is challenged by limited access, high costs, unavailability at district levels and in areas where available, sometimes a shortage of reagents, compounded by challenges with equipment maintenance,3 as happened during the COVID-19 pandemic.
There is no doubt that viral load testing is effective in monitoring patient treatment adherence and HIV resistance, as per WHO guidelines. However, in resource-constrained areas that may not always be able to perform viral load testing in a timely manner, there is a need for readily available and routinely assessed objective measures that may predict early viral non-suppression or measures that may help with interim evaluation of patients suspected to have treatment failure who will thereafter need additional follow-up with viral load testing. Individuals with HIV are routinely assessed for weight, height, renal function and complete blood counts before initiation of combined antiretroviral treatment (ART) in resource constrained settings including Tanzania. These assessments are repeated at intervals of 3 months, 6 months and biannually after ART initiation. Adverse changes in such parameters from treatment initiation (baseline) or subsequently at follow-up visits provide useful information about treatment responses and may identify a targeted group of patients to be prioritised for viral load testing before a decision to switch the ART regimen.
Routine parameters such as body mass index (BMI), total lymphocyte counts (TLC) and protein in urine are easily available and can be evaluated in combination for HIV treatment monitoring. Weight loss is one of the major manifestations of advanced HIV infection. Factors hypothesised to contribute to weight loss include metabolic alterations, anorexia, malabsorptive disorders, hypogonadism and excessive cytokine production4
Weight gain following ART initiation may reflect slowed resting energy expenditure, resulting from viral suppression and a decrease in HIV enteropathy.5 Weight gain, especially among individuals with low BMI, is associated with improved survival and decreased risk of clinical failure.6 ART responses depend on adherence,7 nutritional status at baseline,8 HIV subtype9 and ART combination regimen.10 In Port Harcourt, Nigeria, among 318 participants with HIV infection aged ≥18 years initiated on ART, almost 70% and 55% of participants gained at least 1 kg weight in the first 6 months and 1 year of treatment, respectively.11 Previous studies in Tanzania have shown that a decrease in nutrition status within the first 3 months of ART initiation was associated with mortality.12
HIV-associated nephropathy is the most common renal biopsy finding in individuals with HIV with a prevalence ranging from 4.7% to 38%.13 Proteinuria and elevated creatinine have been associated with AIDS-defining illness and death.14 Urine assessment for protein by dipstick can detect renal disease and progression, especially as renal biopsy, an invasive procedure, is not readily available in most resource-constrained settings.
HIV is associated with progressive destruction of CD4+ T-helper lymphocytes responsible for the profound immunodeficiency that underlies AIDS.15 As CD4 cells are a subset of lymphocytes, any significant change in CD4 cells will cause a parallel change in TLC.16
This study aimed at assessing the following routinely accessible parameters: BMI, proteinuria and TLC and their patterns of change in monitoring HIV treatment responses at 6 months following ART initiation.
Methods
Study design and population
This cohort study was conducted in three urban care and treatment centres (CTC) in Temeke district, Dar es Salaam: Temeke regional referral hospital, Mbagala Rangi-tatu district hospital and Mbagala Kizuiani dispensary between September 2018 and April 2019. The centres were chosen due to large numbers of ART naïve clients, amounting to 60 or more per clinic per month. The sites have an organised CTC and follow-up plan for clients. Participants were included in the study if they were newly diagnosed with HIV and were ART naïve aged 18 years or older and were able to provide written informed consent. Participants were initiated on ART based on the Tanzanian National guidelines17 with a default regimen of tenofovir, lamivudine and efavirenz unless contraindicated.
Sample size estimation
To determine the minimal detectable relative risks for the study variables, we considered two-sample tests of the expected highest risk category versus the expected lowest risk category. For the dichotomous potential risk factors, we assumed a total number of 215 subjects, split roughly as our actual data are split (with numbers rounded to the nearest five to mimic a prestudy power calculation). For BMI change, we used 80 for the reference group (gain), 125 for stable and 20 for the loss group. The minimum detectable risk ratios were 3.77 for decreased BMI, 2.56 for stable BMI, 2.94 for lymphopenia and for proteinuria, 2.47 for stage greater than 1, 2.47 for age of 40, years and above 2.59 (or <0.11) for female sex, 2.74 for secondary or higher education, 2.44 for unemployment and for never married.
Data collection
We used an interviewer-based structured tool to conduct face-to-face interviews to obtain sociodemographic and baseline characteristics (at treatment initiation) such as age, sex, occupation, the highest level of education attained, marital status and clinically assessed the participant’s WHO HIV clinical stage. A participant’s baseline weight was measured using an Seca weighing scale recording to the nearest 0.5 kg and height using a wall stadiometer recording to the nearest 0.5 cm. BMI was then computed by dividing the weight in kilogram by the height in metres squared, the interpretation of which was adapted from WHO.18
About 10 mL of venous blood was collected aseptically from each participant; 5 mL for CD4 cell counts, analysed using BD FACSCount (Becton Dickenson, USA) and 5 mL for complete blood count to obtain the TLC, analysed by an auto-analyser (Cell DNY1800 from Abbott Diagnostics Division, USA). TLC was categorised as lymphopaenia (<1×109/L), normal lymphocyte (1×109/L to 4×109/L) and lymphocytosis (>4.0×109 /L). We assessed for proteinuria by collecting about 15 mL of a fresh urine sample from each participant into an empty, clean, dry container and tested using CYBOW strips (DFI, Korea). Proteinuria was categorised as negative proteinuria (no proteinuria), trace or 1+proteinuria (equivalent to 30 mg/dL to 100 mg/dL), 2+proteinuria (equivalent to 100 mg/dL to 300 mg/dL), 3+proteinuria (equivalent to 300 mg/dL to 1000 mg/dL) and 4+proteinuria (equivalent to greater than 1000 mg/dL).
At 3 and 6 months after ART initiation, a repeat assessment of participants was done for CD4 cell counts, TLC, proteinuria and weight. At 6 months, an additional 5 mL of blood was collected from each study participant for viral load measurement using the Abbott RealTime HIV-1 assay. Participants were classified as virally suppressed at 6 months after ART initiation if their HIV viral load was <1000 copies/mL, according to Tanzania HIV treatment guidelines. Levels and changes in BMI, lymphocytes and proteinuria were compared between participants who were HIV suppressed and that of HIV not suppressed.
BMI was considered to have changed between one time point and another if it increased or decreased by over 5%. BMI changes from ART initiation to 6 months were categorised into three groups as (1) loss of more than 5%, (2) stable (change between −5% and 5%) or (3) gain of more than 5%. The TLC were categorised as (1) lymphopaenia <1×109 cells/L, (2) normal lymphocyte count 1–4×109 cells/L, (3) lymphocytosis >4×109 cells/L. The TLC pattern change was categorised as (1) lymphopaenic at 6 months, regardless of the status at baseline or 3 months, (2) increased from lymphopaenic to normal or higher and (3) stayed normal or higher (no lymphopaenia seen). Urinary protein patterns were categorised as (1) proteinuria at 6 months regardless of baseline proteinuria status, (2) proteinuria at baseline and/or third month but not 6 months and (3) no proteinuria seen.
Patient and public involvement
Patients or members of the public were not involved in the design, or conduct, or reporting or dissemination plans of the research.
Statistical methods
Data were analysed using IBM SPSS Statistics V.26, R and SAS V.9.4 (Cary, North Carolina). Categorical variables such as age group, sex, marital status, level of education, occupation, categories of BMI, CD4 count, proteinuria, TLC, BMI change, TLC change and proteinuria change were summarised as frequencies and proportions. Continuous variables such as age, BMI and CD4 count were summarised as means and SD. When necessary, small groups were combined for analysis. To determine the association between BMI, TLC or urine protein to CD4 count, we used correlation.
To determine the relationships between individual predictors and viral non-suppression at 6 months, we first used modified Poisson regression for univariable analysis with an assumption that viral non-suppression is a non-rare outcome (more than 10%), to determine which variables to include in the multivariable model.19 20 For multivariable prediction, all predictors in the univariable model with a p value of <0.2 and age, a known confounder, were entered into the modified Poisson regression model. The results of the Poisson regression model were presented as relative risk (RR) and 95% CI (RR; 95% CI). To determine the test characteristics (sensitivity, specificity, positive predictive value, negative predictive value) of a score based on the multivariable model, we used two cut-off levels, based on the first quartile and median of the score among the non-suppressed. The score was the sum of the rounded coefficients for the variables for which the CIs did not include 1 in a model containing only these variables. Since these all rounded to 1, this is equivalent to simply counting the number of these characteristics.
Based on practices in low resourced clinics, communication with the patient and the decision to change the ART regimen depends on the patient’s virological status at 6 months. CD4 cell counts depend on a blood sample collected at the 6-month visit and are, therefore, unavailable for immediate decision-making. We, therefore, excluded all CD4 variables from the model and used parameters available at the time of the 6-month visit to predict viral non-suppression.
Results
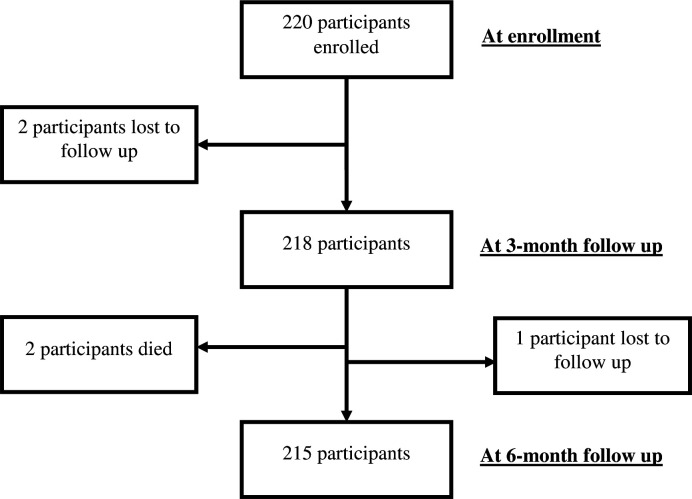
During the recruitment, 220 ART naïve individuals with HIV were initiated on ART and all were enrolled in the study over a month; each participant was followed up for 6 months. Two participants were lost to follow-up at 3 months; two died before 6 months of follow-up and one participant, a long-distance truck driver, was out of the country at the time of the 6-month follow-up. Therefore, our analysis data set includes the remaining 215 participants. Details of enrolment are shown in figure 1.
Figure 1.
Consort diagram.
Baseline characteristics of study participants
Of the 215 participants analysed, the mean age (SD) was 37.1±11.5 years, 146 (68%) were women, 113 (53%) were never married, 45 (21%) had secondary education or higher and 117 (54%) were unemployed (table 1). Most participants, 59.5%, had normal BMI, while 27% were overweight and 13% were underweight. Most participants, 113 (62%), were in WHO HIV clinical stage I, and only eight (4%) were in stage IV, though 93 (43%) had CD4 counts of 350 cells/mL or below; 83 (39%) were lymphopaenic and 111 (52%) had proteinuria at baseline.
Table 1.
Characteristics of 215 study participants at ART initiation, Dar es Salaam, Tanzania, 2019
| Characteristic | n (%) | Mean±SD |
| Age (years) | 37.1±11.5 | |
| Age group (years) | ||
| 18–30 | 69 (32.1) | |
| 31–40 | 72 (33.5) | |
| 41–50 | 45 (20.9) | |
| >51 | 29 (13.5) | |
| Sex | ||
| Female | 146 (67.9) | |
| Male | 69 (32.1) | |
| Level of education | ||
| No education | 10 (4.7) | |
| Primary education | 160 (74.4) | |
| Secondary education | 42 (19.5) | |
| Higher education | 3 (1.4) | |
| Employment status | ||
| Not employed | 117 (54.4) | |
| Employed | 98 (45.6) | |
| Marital status | ||
| Ever married | 102 (47.4) | |
| Never married | 113 (52.6) | |
| Body mass index (kg/m2) | 22.9±4.3 | |
| Underweight | 28 (13.0) | |
| Normal weight | 128 (59.5) | |
| Overweight/obese | 59 (27.4) | |
| WHO HIV clinical stages | ||
| Stage I | 133 (61.9) | |
| Stage II | 30 (14.0) | |
| Stage III | 44 (20.5) | |
| Stage IV | 8 (3.7) | |
| CD4 cell counts (cells/mm3) | 401±253 | |
| <200 | 55 (25.6) | |
| 200–350 | 38 (17.7) | |
| 351–500 | 39 (18.1) | |
| >500 | 83 (38.6) | |
| Lymphocyte counts (x109cells/L) | 1.6±1.2 | |
| <1 | 83 (38.6) | |
| 1–4 | 126 (58.6) | |
| >4 | 6 (2.8) | |
| Proteinuria | ||
| No proteinuria | 104 (48.4) | |
| 1+ (30–100 mg/dL) | 80 (37.2) | |
| 2+ (100–300 mg/dL) | 27 (12.6) | |
| 3+ (300–1000 mg/dL) | 4 (1.9) |
ART, antiretroviral treatment; CD4, Cluster of differentiation 4.
BMI and CD4 count were directly correlated at baseline, 3 and 6 months. TLC and CD4 count were moderately positively correlated, while urine protein and CD4 count were inversely correlated (see online supplemental figures 1, 2 and 3).
bmjopen-2021-059193supp001.pdf (156.3KB, pdf)
Predictors of viral non-suppression at 6 months among individuals with HIV initiated on ART
Only 46 participants (21%) were not virally suppressed at 6 months. The strongest clinical predictors of viral non-suppression in the multivariable analysis were lymphopaenia at 6 months irrespective of baseline lymphocyte status, with 73% of participants with lymphopaenia at 6 months not being suppressed. After adjusting for other factors, lymphopaenia at 6 months was associated with HIV non-suppression (RR=4.54, 95% CI (2.19 to 9.39)). Among participants with a drop in BMI at 6 months of 5% or more from baseline, 80% were non-suppressed (RR=2.73; 95% CI (1.36 to 5.47)). In an alternative analysis, we considered BMI changes of 10%, but only 9 of the non-suppressed participants (20%) had such large decreases. The risk of HIV non-suppression at 6 months was higher among participants with proteinuria at 6 months (RR=2.63; 95% CI (1.25 to 5.54)), table 2. The area under the receiver operating characteristic curve for the multivariable model was 0.895 (figure 2). Baseline HIV stage was not related to HIV non-suppression at 6 months (adjusted RR=1.14, 95% CI (0.63 to 2.08)) for baseline WHO HIV clinical stage II and (adjusted RR=0.82, 95% CI (0.51 to 1.31)) for advanced baseline WHO HIV clinical stages (III and IV)).
Table 2.
Predictors of HIV viral load non-suppression at 6 months among 215 ART naïve participants initiating ART, Dar es Salaam, Tanzania, 2019
| Variable | Total | HIV non-suppression at 6 months n (%) |
RR (95% CI) | Adjusted RR (95% CI) |
| Age (years) | ||||
| <40 | 136 | 26 (19) | 1 | 1 |
| >40 | 79 | 20 (25) | 1.32 (0.79 to 2.21) | 1.43 (0.91 to 2.26) |
| Sex | ||||
| Female | 146 | 35 (24) | 1.50 (0.81 to 2.78) | 1.27 (0.73 to 2.20) |
| Male | 69 | 11 (16) | 1 | 1 |
| Level of education | ||||
| Primary or less | 170 | 37 (22) | 1 | |
| Secondary or higher | 45 | 9 (20) | 0.92 (0.48 to 1.76) | |
| Employment status | ||||
| Not employed | 117 | 24 (21) | 1 | |
| Employed | 98 | 22 (22) | 1.09 (0.66 to 1.83) | |
| Marital status | ||||
| Never married | 113 | 19 (17) | 1 | 1 |
| Ever married | 102 | 27 (26) | 1.57 (0.93 to 2.66) | 1.34 (0.84 to 2.16) |
| Body mass index | ||||
| Change from baseline to 3 months | ||||
| Loss >5% | 28 | 17 (61) | 4.93 (2.41 to 10.09) | |
| Stable | 122 | 21 (17) | 1.40 (0.66 to 2.99) | |
| Gain>5 % | 65 | 8 (12) | 1 | |
| Change from baseline to 6 months | ||||
| Loss >5% | 20 | 16 (80) | 7.11 (3.69 to 13.69) | 2.73 (1.36 to 5.47) |
| Stable | 115 | 21 (18) | 1.62 (0.78 to 3.36) | 1.87 (0.95 to 3.68) |
| Gain>5 % | 80 | 9 (11) | 1 | 1 |
| HIV clinical stage | ||||
| I | 133 | 24 (18) | 1 | 1 |
| II | 30 | 8 (27) | 1.48 (0.74 to 2.97) | 1.14 (0.63 to 2.08) |
| III and IV | 52 | 14 (27) | 1.49 (0.84 to 2.66) | 0.82 (0.51 to 1.31) |
| Total lymphocyte count change from baseline to 6 months | ||||
| Ended lymphopaenic | 37 | 27 (73) | 7.66 (4.32 to 13.60) | 4.54 (2.19 to 9.39) |
| Lymphopaenic to normal | 52 | 7 (13) | 1.41 (0.59 to 3.40) | 1.59 (0.66 to 3.80) |
| Lymphopaenia not seen | 126 | 12 (10) | 1 | 1 |
| Pattern of change in proteinuria | ||||
| Proteinuria at 6 months regardless of baseline proteinuria status | 37 | 24 (65) | 6.73 (3.34 to 13.58) | 2.63 (1.25 to 5.54) |
| Proteinuria at baseline and/or 3 months but not 6 months | 95 | 14 (15) | 1.53 (0.67 to 3.47) | 1.26 (0.62 to 2.57) |
| No proteinuria seen | 83 | 8 (10) | 1 | 1 |
Univariable and multivariable analysis by modified Poisson regression.
ART, antiretroviral therapy; RR, relative risk.
Figure 2.
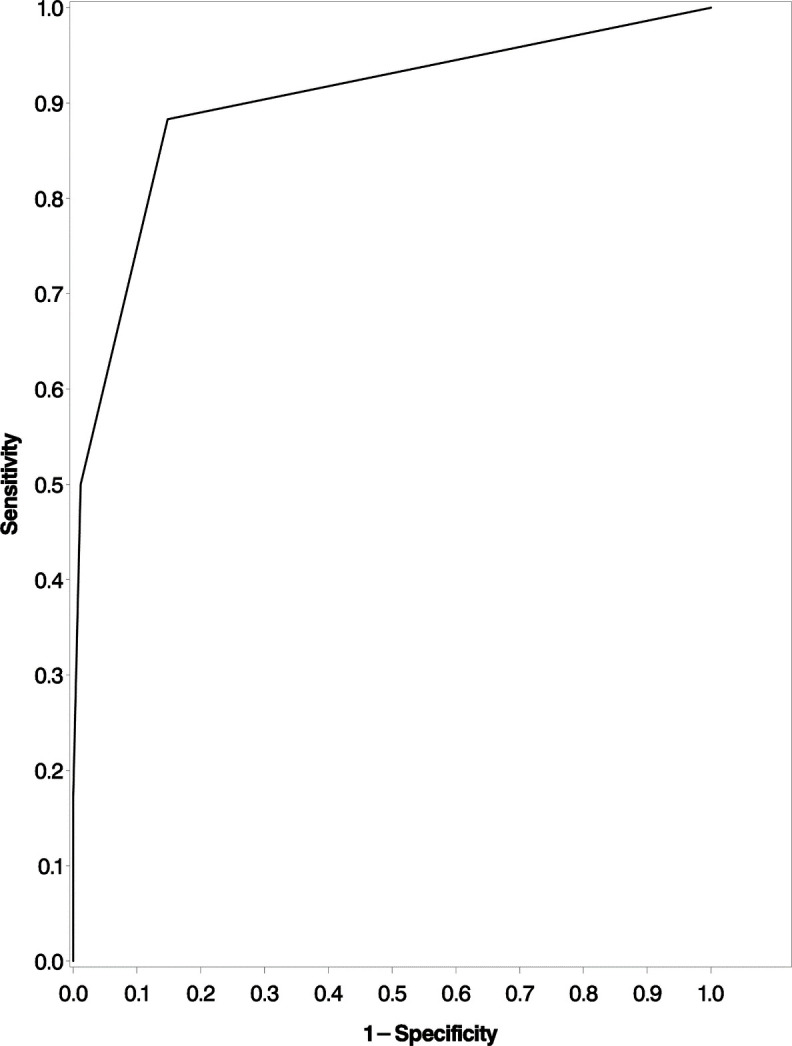
Receiver operating characteristic curve assessing a model using BMI decrease, proteinuria and total lymphocyte counts to predict viral non-suppression among ART naïve individuals with HIV initiated on ART in Dar es Salaam, Tanzania, 2019. BMI, body mass index; ART, antiretroviral treatment.
Using the rounded coefficients of the three variables in a model containing only these variables, which all rounded to 1, we made a ‘prediction score’ with values 0 (n=154, of which 10 were non-suppressed), 1 (n=36, 13 non-suppressed), 2 (n=17, 15 non-suppressed) and 3 (n=8, all non-suppressed). The median value of this score among the non-suppressed was 1.5 and the first quartile value was 1. Thus, having any 2 of the risk factors would be a conservative predictor of non-suppression, and having any one would be less conservative.
Using the median score among the non-suppressed as a cut-off (equivalent to having any two of the components), the sensitivity predicting non-suppression was 0.50, and the specificity was 0.99. Only 12% of the study population met this criterion. When we lowered the cut-off scores to the first quartile value among the non-suppressed (ie, any one of the components), the sensitivity was 0.78 and the specificity was 0.85, with 28% of the study population meeting this criterion.
Discussion
This cohort study recruited ART naïve individuals with HIV from three CTC in Dar es Salaam, Tanzania, with the aim of assessing the utility of TLC, BMI and proteinuria in predicting ART responses at 6 months. The intention of this study is not to replace CD4 cell count testing and HIV viral load monitoring but to assist clinicians when faced with decision-making if these standard monitoring parameters are not easily accessible. Contrary to earlier studies done when the ART medications were not as effective as the current ones,12 patient characteristics at ART initiation did not affect the probability of viral non-suppression at 6 months, whereas patterns of change and the patient’s status at 6 months were highly predictive.
Baseline WHO HIV clinical stage did not help predict HIV non-suppression at 6 months, possibly because under the current ‘Test and Treat’ strategy,21 most individuals initiating ART are in WHO HIV clinical stages I and II (76% of our sample). Current therapy is highly effective except for a few patients whose disease is so advanced that they die before the medication can improve their immune status (two patients in this study). Symptomatic individuals with advanced HIV will likely adhere to treatment to alleviate their symptoms. Perhaps patients with advanced disease fear death and, therefore, are motivated to adhere to ART, with eventual viral suppression. Advanced HIV disease has been shown to be linked with ART adherence.22 Some studies, however, indicate that early HIV stages are linked with high ART adherence and viral suppression.23
Lymphopaenia at 6 months, a decrease in BMI of 5% or more from baseline and proteinuria predicted HIV non-suppression at 6 months. Lymphopaenia at 6 months was the strongest predictor for HIV non-suppression at 6 months. Lymphopaenia at 6 months predicted HIV non-suppression irrespective of a participant’s lymphocyte count (normal or low) before ART initiation. CD4 cells are a subset of lymphocytes and, therefore, lymphopaenia could be indicative of a decline in CD4 cells, as a result of high HIV replication with high viral load signifying viral non-suppression with consequent CD4 cell destruction. Lymphopaenia was significantly associated with CD4 <500 cells/mm3 at all time points. In this study, an increase in total lymphocyte cell count from lymphopaenia to normal lymphocyte counts from baseline to 6 months was significantly associated with an increase in CD4 cell count (additional file 1). TLC is sensitive and specific in predicting CD4 cell counts16 24 though there have been contradictory reports.25 The assessment of TLC among patients on ART, therefore, could serve as an alternative, especially in settings with limited availability of CD4 cell count measurement. A drop in TLC in such a setting can alert a clinician to the likelihood of immunological failure. A drop in lymphocytes could also signal the possibility of immunological non-responders, who will need primary and secondary prophylaxis for opportunistic infection.
Weight loss with consequent BMI drop of 5% or more at 6 months while on ART from weight prior to ART initiation predicts HIV non-suppression, though HIV non-suppression was not associated with being underweight prior to ART initiation, perhaps because of the low prevalence of underweight leading to low power. In this study, sustained weight gain was significantly associated with viral suppression, and sustained weight loss was associated with viral non-suppression at 6 months of ART. An increase in weight and hence BMI may be a sign of immune status improvement signalling a return to health26 27 and improved survival,28 while a decrease in weight and hence BMI may be a sign of HIV progression or decline in CD4 cell counts.5 11 29 Weight loss may be due to HIV itself, opportunistic infections or HIV-associated tumours. Failure to gain weight has been associated with efavirenz toxicity over time as was observed in a study in South Africa.30 Weight loss in both ART naïve and exposed patients has been associated with increased morbidity and mortality.31 32 A study in England observed that each log10 increase in HIV viral load was associated with a 0.92 kg decrease in body weight. However, a decrease in viral load was not significantly associated with weight gain, contrary to our study.33 Since weight changes correlate with the virological response, losing weight should be viewed as an alarming sign of HIV viral non-suppression from any cause. Monitoring of weight and BMI prior to ART initiation and during follow-up is a valuable inexpensive way of identifying individuals with possible viral non-suppression. In an alternative analysis, we considered BMI changes of 10%, but only nine of the non-suppressed participants (20%) had such large decreases, making the 10% decrease not useful as a cut-off in our situation.
Half of the ART-naïve study participants had proteinuria, similar to a report from Zimbabwe.34 The presence of proteinuria was associated with HIV non-suppression. Proteinuria at 6 months was a strong predictor of HIV non-suppression. Proteinuria in individuals with HIV is attributed to a direct effect of HIV on the glomerular and tubular epithelial cells with the progression of HIV disease to AIDS, nephrotoxicity from ART, the progression of chronic kidney disease and death14 35 The higher the viral load, the greater the damage to the kidney.36 We observed a significant proportion of participants with proteinuria of 2+ or above in WHO HIV clinical stage IV. This underscores the fact that, as HIV disease advances, the greater the damage to the kidneys as evidenced by increasing proteinuria. Therefore, routine screening for renal disease may serve not only as a follow-up of renal disease progression but also for HIV treatment response monitoring.
The presence of proteinuria, lymphopaenia and a drop in BMI of 5% are relatively simple parameters to monitor among people living with HIV on ART especially in a setting where viral load monitoring is a challenge. The presence of any of these parameters should alert a clinician on the possibility of viral non-response and review adherence issues including individualised enhanced adherence counselling and subsequent treatment options.
Our findings require validation in a study with a larger sample size. Our small sample may have constrained some predictors of viral non-suppression. Similar studies conducted in different locations are also needed since local conditions and treatment standards may influence some observed patterns, both in prevalence and effect. Furthermore, use of new antiviral drugs and changes in patient characteristics at presentation may change our estimates, and possibly the important predictor variables. We recommend further studies with extended follow-up of patients beyond 6 months to monitor further change in lymphopaenia, proteinuria and drop in BMI of 5% or more especially for individuals maintained on the same regimen after enhanced adherence counselling. We recommend further studies to examine the relationship between virological response and anaemia as well as opportunistic infections and AIDS associated malignancies especially now that ART is initiated early.
One strength of our study is the cohort design with complete follow-up data at 3 and 6 months for 98% of the enrolled participants. Although our scoring system is crude, it is easy to compute and is likely to be valid for a wide variety of situations, whereas a score based on more precise computations would at best work only in our location.
Conclusion
A drop in BMI by more than 5%, persistent lymphopaenia or decrease in TLC to lymphopaenia, and proteinuria at 6 months are associated with HIV non-suppression at 6 months after ART initiation. Scores based on these parameters are easy to use and can serve as alternatives to CD4 cell counts and viral load assessment in facilities with scarcity.
Supplementary Material
Acknowledgments
We are grateful to the participants for their willingness to take part in this study and to the health workers from Temeke regional referral hospital, Mbagala Rangi-tatu district hospital, and Mbagala Kizuiani dispensary for their assistance in participant recruitment and data collection.
Footnotes
Twitter: @DrTumaini
Contributors: Study design: LJ and PM; data collection: LJ and PM; data analysis and interpretation: LJ, PM, BT and EH; manuscript writing: LJ, PM, BT and EH. All the authors gave final approval of the manuscript. PM is the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The dataset analysed during the current study is available upon reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Muhimbili University of Health and Allied Sciences Institutional Review Board with reference number DA287/298/01A/. Participants gave informed consent to participate in the study before taking part. The confidentiality of patient information was ensured. Participants without viral suppression at the 6th month of follow up were managed according to Tanzania National Guidelines for management of HIV and AIDS.
References
- 1.UNAIDS . UNAIDS data, 2020. Available: https://www.unaids.org/en/resources/documents/2020/unaids-data [Accessed 19 Aug 2020].
- 2.World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2016. Available: https://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf [PubMed]
- 3.Roberts T, Bygrave H, Fajardo E, et al. Challenges and opportunities for the implementation of virological testing in resource-limited settings. J Int AIDS Soc 2012;15:17324. 10.7448/IAS.15.2.17324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sepkowitz KA. AIDS--the first 20 years. N Engl J Med 2001;344:1764–72. 10.1056/NEJM200106073442306 [DOI] [PubMed] [Google Scholar]
- 5.Mangili A, Murman DH, Zampini AM, et al. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis 2006;42:836–42. 10.1086/500398 [DOI] [PubMed] [Google Scholar]
- 6.Koethe JR, Lukusa A, Giganti MJ, et al. Association between weight gain and clinical outcomes among malnourished adults initiating antiretroviral therapy in Lusaka, Zambia. J Acquir Immune Defic Syndr 2010;53:507–13. 10.1097/QAI.0b013e3181b32baf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mannheimer S, Friedland G, Matts J, et al. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis 2002;34:1115–21. 10.1086/339074 [DOI] [PubMed] [Google Scholar]
- 8.Paton NI, Sangeetha S, Earnest A, et al. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV Med 2006;7:323–30. 10.1111/j.1468-1293.2006.00383.x [DOI] [PubMed] [Google Scholar]
- 9.Wittkop L, Arsandaux J, Trevino A, et al. CD4 cell count response to first-line combination ART in HIV-2+ patients compared with HIV-1+ patients: a multinational, multicohort European study. J Antimicrob Chemother 2017;72:2869–78. 10.1093/jac/dkx210 [DOI] [PubMed] [Google Scholar]
- 10.Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr 2007;46:187–93. 10.1097/QAI.0b013e31814278c0 [DOI] [PubMed] [Google Scholar]
- 11.Olaleye AO, Owhonda G, Daramola O, et al. Factors associated with weight gain among adult patients initiating antiretroviral therapy in Port Harcourt, Nigeria: a retrospective cohort study. Infect Dis 2017;49:635–8. 10.1080/23744235.2017.1306102 [DOI] [PubMed] [Google Scholar]
- 12.Liu E, Spiegelman D, Semu H, et al. Nutritional status and mortality among HIV-infected patients receiving antiretroviral therapy in Tanzania. J Infect Dis 2011;204:282–90. 10.1093/infdis/jir246 [DOI] [PubMed] [Google Scholar]
- 13.Husain NE, Ahmed MH, Almobarak AO, et al. HIV-Associated nephropathy in Africa: pathology, clinical presentation and strategy for prevention. J Clin Med Res 2018;10:1–8. 10.14740/jocmr3235w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szczech LA, Hoover DR, Feldman JG, et al. Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis 2004;39:1199–206. 10.1086/424013 [DOI] [PubMed] [Google Scholar]
- 15.Imlach S, McBreen S, Shirafuji T, et al. Activated peripheral CD8 lymphocytes express CD4 in vivo and are targets for infection by human immunodeficiency virus type 1. J Virol 2001;75:11555–64. 10.1128/JVI.75.23.11555-11564.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obirikorang C, Quaye L, Acheampong I. Total lymphocyte count as a surrogate marker for CD4 count in resource-limited settings. BMC Infect Dis 2012;12:128. 10.1186/1471-2334-12-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The United Republic of Tanzania, Ministry of Health, Community Development, Gender, Elderly and Children, National AIDS Control Programme . National guidelines for the management of HIV and AIDS. 6th Ed, 2017. Available: https://www.differentiatedservicedelivery.org/Portals/0/adam/Content/NqQGryocrU2RTj58iR37uA/File/Tanzania_NATIONALGUIDELINESFORMANAGEMENTOFHIVANDAIDS6THEDITION2017.pdf
- 18.World Health Organization . Obesity: preventing and managing the global epidemic. WHO technical report series, 2000. Available: https://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ [PubMed]
- 19.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005;162:199–200. 10.1093/aje/kwi188 [DOI] [PubMed] [Google Scholar]
- 20.Dwivedi AK, Mallawaarachchi I, Lee S, et al. Methods for estimating relative risk in studies of common binary outcomes. J Appl Stat 2014;41:484–500. 10.1080/02664763.2013.840772 [DOI] [Google Scholar]
- 21.WHO . The use of antiretroviral drugs for treating and preventing HIV infection guidelines HIV/AIDS programme, 2016. Available: http://apps.who.int/iris/bitstream/10665/85322/1/WHO_HIV_2013.7_eng.pdf [Accessed 19 Jan 2021].
- 22.Biressaw S, Abegaz WE, Abebe M, et al. Adherence to antiretroviral therapy and associated factors among HIV infected children in Ethiopia: unannounced home-based pill count versus caregivers' report. BMC Pediatr 2013;13:132. 10.1186/1471-2431-13-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haberer JE, Bwana BM, Orrell C, et al. ART adherence and viral suppression are high among most non-pregnant individuals with early-stage, asymptomatic HIV infection: an observational study from Uganda and South Africa. J Int AIDS Soc 2019;22:e25232. 10.1002/jia2.25232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwantwi LB, Tunu BK, Boateng D, et al. Body mass index, haemoglobin, and total lymphocyte count as a surrogate for CD4 count in resource limited settings. J Biomark 2017;2017:1–6. 10.1155/2017/7907352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akinola NO, Olasode O, Adediran IA, et al. The search for a predictor of CD4 cell count continues: total lymphocyte count is not a substitute for CD4 cell count in the management of HIV-infected individuals in a resource-limited setting. Clin Infect Dis 2004;39:579–81. 10.1086/422722 [DOI] [PubMed] [Google Scholar]
- 26.Koethe JR, Jenkins CA, Lau B, et al. Higher time-updated body mass index: association with improved CD4+ cell recovery on HIV treatment. J Acquir Immune Defic Syndr 2016;73:197–204. 10.1097/QAI.0000000000001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biadgilign S, Reda AA, Digaffe T. Predictors of mortality among HIV infected patients taking antiretroviral treatment in Ethiopia: a retrospective cohort study. AIDS Res Ther 2012;9:15. 10.1186/1742-6405-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reda AA, Biadgilign S, Deribew A, et al. Predictors of change in CD4 lymphocyte count and weight among HIV infected patients on anti-retroviral treatment in Ethiopia: a retrospective longitudinal study. PLoS One 2013;8:e58595. 10.1371/journal.pone.0058595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naidoo K, Yende-Zuma N, Augustine S. A retrospective cohort study of body mass index and survival in HIV infected patients with and without TB co-infection. Infect Dis Poverty 2018;7:35. 10.1186/s40249-018-0418-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griesel R, Kawuma AN, Wasmann R, et al. Concentration-Response relationships of dolutegravir and efavirenz with weight change after starting antiretroviral therapy. Br J Clin Pharmacol 2022;88:883–93. 10.1111/bcp.15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Sande MAB, Schim van der Loeff MF, Aveika AA, et al. Body mass index at time of HIV diagnosis: a strong and independent predictor of survival. J Acquir Immune Defic Syndr 2004;37:1288–94. 10.1097/01.qai.0000122708.59121.03 [DOI] [PubMed] [Google Scholar]
- 32.Grinspoon S, Mulligan K, Department of Health and Human Services Working Group on the Prevention and Treatment of Wasting and Weight Loss . Weight loss and wasting in patients infected with human immunodeficiency virus. Clin Infect Dis 2003;36:S69–78. 10.1086/367561 [DOI] [PubMed] [Google Scholar]
- 33.Mwamburi DM, Wilson IB, Jacobson DL, et al. Understanding the role of HIV load in determining weight change in the era of highly active antiretroviral therapy. Clin Infect Dis 2005;40:167–73. 10.1086/426591 [DOI] [PubMed] [Google Scholar]
- 34.Fana GT, Ndhlovu CE. Renal dysfunction among anti-retroviral therapy naïve HIV infected patients in Zimbabwe. Cent Afr J Med 2011;57:1–5. [PubMed] [Google Scholar]
- 35.Gupta SK, Smurzynski M, Franceschini N. The effects of HIV-1 viral suppression and non-viral factors on quantitative proteinuria in the HAART era. Antivir Ther 2009;14:543–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Rednor SJ, Ross MJ. Molecular mechanisms of injury in HIV-associated nephropathy. Front Med 2018;5:177. 10.3389/fmed.2018.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059193supp001.pdf (156.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The dataset analysed during the current study is available upon reasonable request to the corresponding author.