Abstract
Natural-abundance 13C-nuclear magnetic resonance was used to probe the intracellular organic solute content of the moderately halophilic bacterium Tetragenococcus halophila. When grown in complex growth media supplemented or not with NaCl, T. halophila accumulates glycine betaine and carnitine. Unlike other moderate halophiles, T. halophila was not able to produce potent osmoprotectants (such as ectoines and glycine betaine) through de novo synthesis when cultured in defined medium under hyperosmotic constraint. Addition of 2 mM carnitine, glycine betaine, or choline to defined medium improved growth parameters, not only at high salinity (up to 2.5 M NaCl) but also in media lacking NaCl. These compounds were taken up when available in the surrounding medium. The transport activity occurred at low and high salinities and seems to be constitutive. Glycine betaine and carnitine were accumulated by T. halophila in an unmodified form, while exogenously provided choline led to an intracellular accumulation of glycine betaine. This is the first evidence of the existence of a choline-glycine betaine pathway in a lactic acid bacterium. An assay showed that the compatible solutes strikingly repressed the accumulation of glutamate and slightly increased the intracellular potassium level only at high salinity. Interestingly, osmoprotectant-treated cells were able to maintain the intracellular sodium concentration at a relatively constant level (200 to 300 nmol/mg [dry weight]), independent of the NaCl concentration of the medium. In contrast, in the absence of osmoprotectant, the intracellular sodium content increased sharply from 200 to 2,060 nmol/mg (dry weight) when the salinity of the medium was raised from 1 to 2 M. Indeed, the imported compatible solutes play an actual role in regulating the intracellular Na+ content and confer a much higher salt tolerance to T. halophila.
The lactic acid bacterium (LAB) Tetragenococcus halophila is a tetrad-forming gram-positive coccus. Formerly known as Pediococcus halophilus, the bacterium was reclassified in the genus Tetragenococcus, based on 16S rRNA studies (5). T. halophila, the type species of this new genus, was shown to be from a distinct line of descent quite separate from those of both aerococci and pediococci (5). Recently, the existence of a second species, Tetragenococcus muriaticus, has been proposed (30). This bacterium, isolated from traditionally fermented Japanese fish sauce, is closely related to the halotolerant species T. halophila and shows a closer phylogenetic relationship to other LAB of the enterococci and lactobacilli (30).
T. halophila contributes to the biopreservation of vegetable products, such as soybeans during the manufacturing of soy sauce, a well-known condiment in southeast Asia, China, and Japan. The bacterium grows during brine fermentation, where the salt concentration ranges from 12 to 26% (26). T. halophila is also associated with other foods processed under reduced water activities, such as cured anchovies, where it becomes the dominant bacterium at the end of the curing process; this organism can grow under both aerobic and anaerobic conditions (36).
The Tetragenococcus genus like other genera of gram-positive moderately halophilic bacteria (Halobacillus, Marinicoccus, Salinicoccus, and Nesterenkonia) includes only species requiring 0.5 to 30% salt, with an NaCl optimum of 10% (35). Raising the salt concentration of the medium presumably increases osmotic stress, which requires bacteria from various ecological niches to cope with the high and often changing salinity of their environment. The moderately halophilic bacteria, similar to all other microorganisms, need to maintain an osmotic equilibrium between inside and outside of the cells. They can achieve osmotic balance by the accumulation of salts and/or organic molecules (35). Among this heterogenous group of bacteria, T. halophila can tolerate high salt concentrations, which suggests that it has a high osmotic adjustment capacity in order to maintain positive cell turgor. Osmoregulation has been studied extensively in nonhalophilic bacteria such as Escherichia coli, Sinorhizobium meliloti, and Bacillus subtilis (1, 6, 7, 17, 32, 33). Unlike the halophiles, they do not have a strict sodium requirement. Both halophilic and nonhalophilic bacteria have evolved mechanisms that enable them to adapt to high salinity. They protect themselves against deleterious hyperosmotic injury by the uptake or synthesis of a limited number of compounds, termed compatible solutes (7, 17). These are not inhibitory to most cellular processes even at near molar concentrations and may even stabilize the native state of proteins and lipids (38). These compatible solutes include sugars and polyols such as trehalose and glycerol, amino acids such as glutamate and proline, and amino acid derivatives such as betaines and ectoines (7, 12, 17). In the LAB family, the identification and the role of compatible solutes have been investigated for Lactobacillus casei subsp. rhamnosus (10), Lactococcus lactis (22), and Lactobacillus plantarum (9, 18, 19). Glycine betaine is reported to serve as the major effective osmoprotectant in these bacteria. It is not synthesized de novo but accumulates from an exogenous supply. In LAB, all transport systems involved in glycine betaine uptake are not induced but appear to be activated by exposure of the cells to high osmotic pressure. Most of the bacteria studied, e.g., Escherichia coli and B. subtilis, can also accumulate glycine betaine through the conversion of its precursor, choline (6, 7, 17). This contrasts with the studied LAB, which do not convert choline to betaine (20, 22, 34). Carnitine has also been identified as a compatible solute in L. plantarum, where it can be accumulated simultaneously with glycine betaine (19).
Like other LAB, T. halophila is characterized by complex nutritional requirements, including a certain number of growth factors such as nicotinic acid, panthothenic acid, and biotin (5). Sakaguchi (28, 29) reported that T. halophila cannot grow in ordinary synthetic media for LAB but rather requires glycine betaine and carnitine as specific growth factors. However, no further investigations have been made to analyze to what extent uptake or synthesis of these osmolytes or both contribute to osmoprotection in T. halophila. To understand this phenomenon, we determined the nutritional requirements of this organism in a chemically defined medium and show that betaines are not required to support growth of T. halophila. In this article, we also report on the role of glycine betaine, carnitine, and choline in the osmoregulation of T. halophila. Data on the accumulation and transformation of each in the cells are presented and provide evidence that T. halophila is the first LAB which is able to convert choline into glycine betaine under aerobic conditions to be identified.
MATERIALS AND METHODS
Bacteria and growth conditions.
T. halophila ATCC 33315 was obtained from the American Type Culture Collection (Manassas, Va.). Cells were grown aerobically without agitation at 30°C. Anaerobic culture conditions were obtained by using the technique described by Bryand (3). Growth was monitored by optical density measurements at 600 nm (OD600) of appropriately diluted cultures.
Complex growth medium MRS (490 mosmol/kg of H2O) (8) was routinely used, as well as a chemically defined medium (DM) based upon that of Kets et al. (19). DM (165 mosmol/kg of H2O) contained (per liter of deionized water), 2 g of (NH4)2SO4, 3 g of sodium acetate, 2 g of K2HPO4, 100 mg of MgSO4, 50 mg of MnSO4, 1.25 g of Tween 80, 300 mg of l-cysteine, 100 mg of l-alanine, l-asparagine, l-aspartic acid, l-glutamic acid, glycine, l-isoleucine, l-leucine, l-lysine, l-methionine, l-proline, l-threonine, l-tryptophan, l-tyrosine, and l-valine, 50 mg of l-arginine, l-histidine, l-phenylalanine, and l-serine, and 10 mg of adenine, guanine, uracil, and xanthine. The medium was supplemented with (per liter) 10 g of glucose, 5 ml of a solution of vitamins described by Kets et al. (18), and 0.5 ml of a solution of trace elements containing (per liter of deionized water) 0.25% (wt/vol) HCl, 1.5 g of FeCl2 · 4H2O, 190 mg of CoCl2 · 6H2O, 100 mg of MnCl2, 70 mg of ZnCl2, 6 mg of H3BO3, 36 mg of Na2MoO4 · 2H2O, and 2 mg of CuCl2 · 2H2O. Glucose was heat sterilized separately. The vitamin and the trace element solutions were sterilized by passage through a 0.22-μm-pore-size sterile filter (Millipore) and added to the other components. The medium was adjusted to pH 7.5 before use. The osmotic strength of the DM was increased by addition of salts from highly concentrated stock solutions. The osmolality of each medium was measured by freezing-point determination. The dry cell weight of cultures was estimated from washed cells; duplicate subsamples of fresh cell slurry were dried in centrifuge tubes at 80°C for at least 24 h until a constant value was attained (1 OD600 unit, corresponding to 0.31 mg [dry weight] per ml).
Extraction of intracellular solutes.
T. halophila was grown in DM or MRS. Cells were harvested during exponential growth by centrifugation and washed with isotonic medium. The cell pellet was extracted at least twice with 80% (vol/vol) ethanol, under vigorous magnetic stirring, at room temperature, for 30 min. After centrifugation, the supernatants (ethanol-soluble fraction [ESF]) were pooled and evaporated to dryness under reduced pressure at 40°C. The dried residue was finally dissolved in a minimal volume of distilled water and stored in the freezer at −20°C until use for further analysis.
Determination of intracellular potassium and sodium contents.
For the determination of potassium content, samples (10 ml) of a mid-log-phase culture were harvested by centrifugation at 10,000 × g for 10 min and washed with 10 ml of potassium-free isotonic DM. Cells were resuspended in 1 ml of perchloric acid (5%) and extracted for 12 h at 4°C. Extracts were diluted 10 times in a 0.01% CsCl solution and centrifuged at 15,000 × g to remove the cellular debris. The K+ concentrations were measured using an atomic absorption spectrophotometer at 766.5 nm. The same method was used for the determination of intracellular concentrations of sodium, except that cells were washed three times with an isotonic solution of either maltose or KCl.
Determination of glutamate content.
The glutamate content of cell extracts was measured routinely with the corresponding Boehringer (Meylan, France) assay kit, according to the specifications of the supplier.
Chromatographic analysis and NMR spectroscopy.
Chromatographic and electrophoretic analyses of the ethanol-soluble cell extracts were performed as described previously (13). The natural-abundance 13C-nuclear magnetic resonance (NMR) spectra were recorded in the pulsed-Fourier transform mode at an operational frequency of 75.4 MHz as described previously (32). The ESF was evaporated to dryness, and the residues were dissolved in 1 ml of D2O.
Transport assays.
Cells grown in DM with or without osmoprotectants were centrifuged (5,000 × g for 10 min), washed twice with an isotonic medium, resuspended to an OD600 of 5, and maintained at room temperature for 30 min. Osmoprotectant uptake assays were performed as described previously (12), using [methyl-14C]glycine betaine (2.07 GBq mM−1), [methyl-14C]choline (2.07 GBq mM−1), dl-[methyl-14C]carnitine (0.22 GBq mM−1), l-[methyl-14C]carnitine (1.86 GBq mM−1), and d-[methyl-14C]carnitine (0.22 GBq mM−1), each at a final concentration of 10 μM in 400 μl of bacterial suspension. [methyl-14C]glycine betaine was prepared from [methyl-14C]choline as described by Ikuta et al. (11). d-[methyl-14C]carnitine was prepared from dl-[methyl-14C]carnitine as described previously (14).
Determination of the intracellular osmoprotectant levels.
Cells were subcultured in DM containing 0 to 2.5 M NaCl, 2 mM [methyl-14C]glycine betaine, [methyl-14C]choline, l-[methyl-14C]carnitine, or d-[methyl-14C]carnitine. After two to five generations, 2 ml of cell culture was harvested by centrifugation and washed twice with carbon-free DM containing the same NaCl concentration as in the growth media. After extraction with 80% ethanol, the radioactive composition of the ESF was analyzed by paper chromatography and/or electrophoresis (13). The radioactive molecules were visualized using the Packard PhosphorImager. The radioactivity of the ethanol-insoluble fraction (EIF) and CO2 was also determined.
Chemicals.
The radiolabeled compounds [methyl-14C]choline and dl-[methyl-14C]carnitine were obtained from NEN-Dupont de Nemours. All other chemicals were of reagent grade and were obtained from Sigma (L'Isle d'Abeau Chesnes, France).
RESULTS
Growth of T. halophila at high salinity.
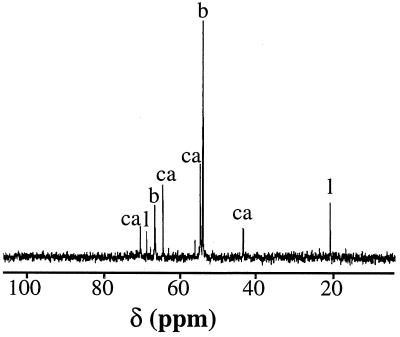
T. halophila was cultured in MRS broth, a complex medium routinely used to cultivate LAB. T. halophila grew in MRS medium containing up to 3.2 M NaCl (Table 1). The growth rate (μ) increased from 0.1 generation h−1 in the absence of sodium chloride to reach a maximal value of 0.16 generation h−1 in medium supplemented with 0.6 M NaCl. Growth was progressively slower as the NaCl concentration increased from 1 to 3.2 M (Table 1). The growth yield was also improved by the addition of NaCl to the culture media, with a maximal OD600 of 3.6 obtained at 1 M NaCl. 13C-NMR analysis of cells grown in MRS medium without or with NaCl (1.5 M) were performed in order to identify the organic solutes accumulated by T. halophila subjected to high salinity. Glycine betaine and carnitine were identified (Fig. 1). These two well-known osmoprotectants were accumulated by T. halophila at high as well as at low salinities (data not shown). Peaks attributed to lactic acid were also detected at low and high salinities.
TABLE 1.
Effect of increasing salinities on the growth of T. halophila in complex mediaa
| NaCl concn (M) | Osmolality (osmol/kg of H2O) | Growth rate (generation h−1) | Maximal OD600 |
|---|---|---|---|
| 0.0 | 0.495 | 0.10 | 2.1 |
| 0.2 | 0.970 | 0.12 | 2.9 |
| 0.6 | 1.869 | 0.16 | 3.5 |
| 1.0 | 2.952 | 0.15 | 3.6 |
| 2.0 | 4.445 | 0.09 | 3.1 |
| 2.4 | 5.180 | 0.05 | 2.9 |
| 3.2 | 7.425 | 0.02 | 1.8 |
Cells were grown aerobically without shaking at 30°C in MRS medium without or with added sodium chloride. Data are the means of at least three independent experiments with less than 10% standard deviation.
FIG. 1.
13C-NMR spectra of ethanolic extracts of T. halophila cells grown in MRS medium supplemented with 1.5 M NaCl. Resonances due to glycine betaine (b), carnitine (ca), and lactate (l) are indicated.
Yeast extract and beef extract, which are components of the MRS medium, contain significant amounts of glycine betaine, choline, and carnitine (10, 19). Hence the above results suggest that T. halophila is able to scavenge some of these solutes from the medium, resulting in a wide salt growth range (Fig. 1; Table 1). Thus the use of the MRS complex medium prevents clear interpretations of the role of these betaines on the moderately halophilic behavior of T. halophila. Consequently, a defined medium lacking betaines was used in order to analyze the precise roles of carnitine, betaine, and choline in the haloadaptative responses of T. halophila.
Effect of exogenous trimethylammonium compounds on the growth of T. halophila subjected to various NaCl concentrations.
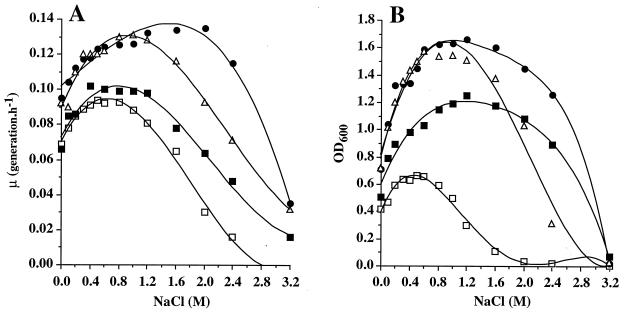
Previous attempts to grow T. halophila in DM failed (29). Here, we observed that T. halophila grew in DM containing the nutrients which commonly compose minimal media used to cultivate LAB (Fig. 2). In the absence of osmoprotectant, T. halophila showed optimal growth at 0.4 to 0.8 M NaCl, with a maximum at 0.5 M NaCl (μ = 0.094 generation h−1 and OD600 = 0.67; Fig. 2).
FIG. 2.
Effect of varied osmolarity on the growth rate (A) and growth yield (B) of T. halophila cells growing in DM in the absence of osmoprotectants (□) or the presence of 2 mM glycine betaine (●), 2 mM dl-carnitine (▵), or 2 mM choline (■).
Growth was slower in DM deprived of NaCl (0.069 generation h−1) as well as in DM with >1 M NaCl. Growth rates decreased to 0.065 and 0.03 generation h−1 in media containing 1.6 and 2 M NaCl, respectively. At 2.4 M NaCl, growth was poor and the cell yield was reduced by 99% compared to that from cells grown in DM supplemented with 0.5 M NaCl (Fig. 2). These experiments showed that T. halophila grows in DM; however both growth rate and growth yield levels are much lower than those obtained with cultures in MRS media. Because important variations of growth between MRS medium and DM were observed, the abilities of exogenously provided glycine betaine (or its precursor choline) and carnitine to function as osmoprotectants in T. halophila were tested. The responses of the bacteria to various salinities in DM supplemented with glycine betaine, choline, or carnitine (2 mM) are described in Fig. 2. After 30 h of incubation, the OD600 values reached in DM supplemented with 1.2 M NaCl in the absence and presence of glycine betaine were 0.3 and 1.7, respectively (Fig. 2B). Similarly enhancement of growth was also observed with carnitine and choline, with maximal OD600 values of 1.65 and 1.25, respectively. Under the test conditions (1.2 M NaCl), growth rates increased from 0.08 generation h−1 in the absence of osmoprotectants to 0.132, 0.128, or 0.1 generation h−1 in the presence of glycine betaine, carnitine, or choline, respectively (Fig. 2A). Growth improvement by the three quaternary ammonium compounds tested was observed over a wide range of salinities, from 0 to 2.5 M NaCl (Fig. 2). This could have resulted from the use of these quaternary ammonium compounds as carbon and/or nitrogen sources by T. halophila. To determine whether T. halophila metabolized the carbon backbone of glycine betaine, carnitine, or choline, each compound was tested as a potential sole carbon source (10 mM) in DM (deprived of glucose). None of these compounds supported the growth of T. halophila. This observation was confirmed with radiolabeled glycine betaine, choline, or carnitine. The radioactivity was never detected in the EIF or in CO2.
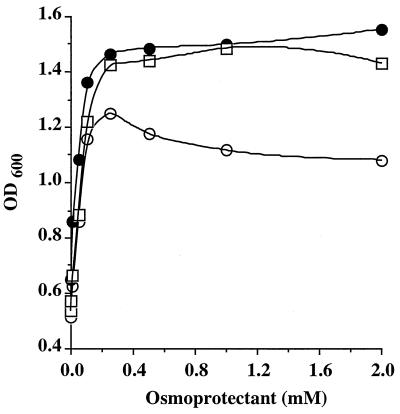
Interestingly, the osmoprotective effects were different depending on which compound was assayed. When cells were grown in DM with no added NaCl, both glycine betaine and carnitine, but not choline, improved the growth yield and the growth rate by 70 and 37%, respectively. Glycine betaine appeared to be the most potent osmoprotectant and allowed the growth of T. halophila in DM containing up to 2.5 M NaCl (Fig. 2), while no growth was observed in the absence of osmoprotectant. Carnitine was as proficient as glycine betaine in improving both the growth rate and the growth yield (Fig. 2). Nevertheless its efficiency decreased when the salinity of the medium exceeded to 2 M NaCl. Choline was less effective than the other trimethylammonium compounds. This osmoprotectant was observed to improve the growth yield over the entire range of salinity, whereas it stimulated both the growth rate and the growth yield at high salinity only (Fig. 2). Hence, each of these compounds resulted in a pronounced osmoprotective effect on stressed cells of T. halophila. Similar stimulation of growth was visible with reduced osmoprotectant concentration. At 10 μM, a slight effect of each osmoprotectant assayed on the growth yield was observed (Fig. 3). A concentration as low as 50 μM betaine increased the growth yield by 1.7- to 2-fold. When betaine was added at 250 μM, the 250 μM concentration was sufficient to protect the cells from the detrimental effects of high salinity and to allow a maximal growth yield (Fig. 3).
FIG. 3.
Glycine betaine, dl-carnitine, and choline improve the growth yield of T. halophila at high osmolarity. T. halophila was grown in DM with 1 M NaCl in the presence of various concentrations of dl-carnitine (●), glycine betaine (□), and choline (○). The cells were grown in 5 ml of medium, without shaking, at 30°C, and the growth yield of each culture was monitored by measurements of OD600 after 56 h of incubation.
Transport characteristics and effect of osmolarity on uptake of compatible solutes.
Transport experiments were performed to determine whether the uptake of glycine betaine, carnitine, and choline (10 μM) was regulated by the NaCl concentration of the medium. Cells were grown in DM lacking NaCl, washed, and resuspended in the same medium without or with added NaCl (from 0.5 to 2 M). The capacities of T. halophila to transport glycine betaine and choline increased slightly from 3.2 and 0.14 nmol/min/mg (dry weight) in DM without NaCl to 4.3 and 0.33 nmol/min/mg (dry weight), respectively, when the cells were shocked with various increased concentrations of NaCl. The results are the means of three independent experiments, and the standard errors did not exceed 5%. These results suggest that rates of glycine betaine and choline uptake are osmotically modulated. On the other hand, the rates of carnitine uptake were similar for all the salinities tested (2 to 2.5 nmol/min/mg [dry weight]).
When the cells were treated with chloramphenicol (100 μg/ml) before the shock, no effect of the protein synthesis inhibitor on the transport of these solutes elicited by osmotic stress (data not shown) was detected. These data indicated that the uptake of these osmoprotectants was not induced by high osmotic pressure.
Cross-competition uptake assays were also performed to determine whether the quaternary ammonium compounds acting as osmoprotectants in T. halophila had similar or different uptake pathways. [14C]glycine betaine or [14C]carnitine was used at a final substrate concentration of 10 μM, and putative unlabeled competitors (choline, glycine, betaine, and carnitine) were added to the transport assay mixtures at a concentration of 1 mM. A 100-fold excess of d-carnitine, l-carnitine, or choline over [14C]glycine betaine had no or very little effect on the glycine betaine uptake activity of T. halophila (Table 2). In marked contrast, the uptake of [14C]glycine betaine by T. halophila was blocked by the addition of a 100-fold excess of unlabeled glycine betaine (Table 2). This lack of competition between carnitine and glycine betaine uptake implies that these two betaines are apparently transported via two different uptake routes in T. halophila.
TABLE 2.
Effect of unlabeled competitors on the uptake of l-[14C]carnitine and [14C]glycine betaine by T. halophilaa
| Osmoprotectant | % Inhibition by:
|
|||
|---|---|---|---|---|
| Glycine betaine | l-Carnitine | d-Carnitine | Choline | |
| l-[14C]carnitine | 70 | 85 | 89 | 59 |
| [14C]glycine betaine | 89 | 2 | 3 | 25 |
Bacteria were grown in DM. Uptake assays were carried out as described in the text with either [14C]glycine betaine or l-[14C]carnitine (10 μM). Unlabeled competitors were added in 100-fold excesses. Results are given as the percent reductions of the uninhibited uptake rates, which were 2.0 and 3.4 nmol/min/mg (dry weight) for l-carnitine and glycine betaine, respectively. The results are the means of three independent experiments. The standard deviations were less than 5%.
The uptake of l-[14C]carnitine by T. halophila was inhibited by the addition of a 100-fold excess of unlabeled carnitine (d or l), glycine betaine, or choline. The inhibitory effect of d- or l-carnitine (85 to 90% inhibition) was slightly greater than that of glycine betaine (70%) or choline (59%) (Table 2). These data are consistent with the existence of two different transport systems in T. halophila: a carnitine transport system taking up carnitine, glycine betaine, and choline and a glycine betaine transport system taking up glycine betaine only.
Identification of the intracellular solutes of osmotically stressed T. halophila.
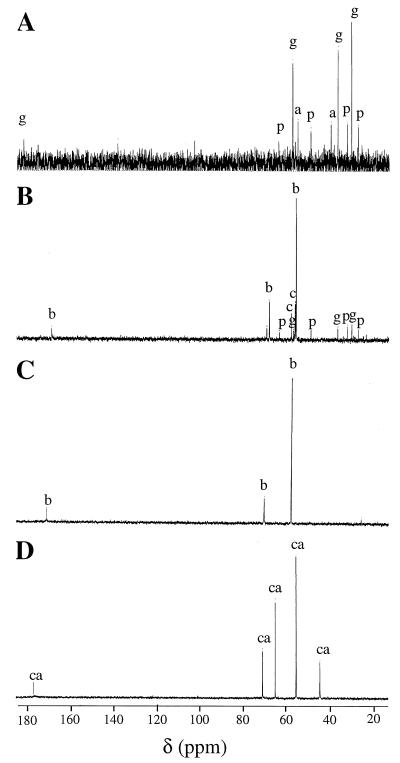
Natural-abundance 13C-NMR analysis was performed to identify the organic osmolytes which might be accumulated in stressed cultures of T. halophila. Cells of T. halophila were grown for 48 h in high-salinity DM in the presence of 2 mM glycine betaine, dl-carnitine, or choline or in the absence of osmoprotectants. Specific signals from glutamate, proline, and aspartate were clearly identified in the spectrum from cells grown in DM with 1 M NaCl (Fig. 4A). Glutamate appeared to be the main organic osmolyte produced in these cells. The 13C-NMR tracings obtained from cells grown in DM with 1 M NaCl and 2 mM choline showed major signals attributed to glycine betaine and minor peaks arising from choline, glutamate, and proline (Fig. 4B). These data indicate that T. halophila oxidized choline into betaine. Hence choline serves as a precursor for the production of glycine betaine.
FIG. 4.
13C-NMR spectra of ethanolic extracts of T. halophila cells grown in DM supplemented with 1 M NaCl in the absence of osmoprotectants (A) or the presence of 2 mM choline (B), 2 mM glycine betaine (C), or 2 mM dl-carnitine (D). The signals were identified from spectra obtained with authentic compounds and represent glutamate (g), aspartate (a), proline (p), choline (c), glycine betaine (b), and carnitine (ca).
The 13C-NMR spectrum from crude ethanolic extracts of cells grown in the presence of 2 mM glycine betaine or carnitine showed only glycine betaine or carnitine signals, respectively (Fig. 4C and D). These two betaines are preferentially accumulated in unmodified forms by T. halophila cultured under hyperosmotic conditions and suppress the accumulation of glutamate and proline.
Osmoregulated accumulation of quaternary ammonium compounds in T. halophila.
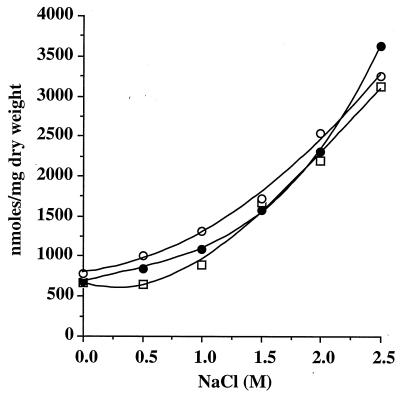
Since 13C-NMR can detect the main representative accumulated compounds only qualitatively, the intracellular amounts of d-carnitine, l-carnitine, and glycine betaine accumulated by T. halophila cells cultivated at various salinities were measured. When T. halophila was grown for two to five generations in the presence of 2 mM d-[14C]carnitine, l-[14C]carnitine, or [14C]glycine betaine in DM with different concentrations of NaCl, the radioactive material which disappeared from the medium was recovered in the ESF. No radioactivity was detected in either the EIF or in the CO2. Chromatographic and electrophoresis analysis of the ESF revealed that the molecule provided accounted for the entire radioactivity of this fraction. Intracellular quantities of glycine betaine, d-carnitine, or l-carnitine increased, but in a nonlinear fashion, when the salt concentration of the medium was raised (Fig. 5). From 0 to 1 M NaCl, the intracellular levels of these betaines increased slightly, by 1.3- to 1.7-fold. Above 1 M NaCl, significant increases in the concentrations of the accumulated solutes were observed. The absolute amounts, however, were quite similar and reached maxima of 3,120, 3,600, and 3,250 nmol/mg (dry weight) for growth in DM with 2.5 M NaCl and glycine betaine, l-carnitine, or d-carnitine respectively. Interestingly, the intracellular concentrations of [14C]glycine betaine, l-[14C]carnitine, and d-[14C]carnitine in stressed cells were 4.7-, 5.3-, and 4.2-fold higher, respectively, than the intracellular concentrations in cells cultivated in DM.
FIG. 5.
Effect of the osmotic strength of growth medium on the accumulation of compatible solutes by T. halophila. Cells were grown for two to five generations in DM containing the indicated NaCl concentrations, in the presence of 2 mM [14C]glycine betaine (□), l-[14C]carnitine (●), or d-[14C]carnitine (○). Cells were harvested, and the ethanol-soluble extracts were subjected to paper chromatography and electrophoresis. Radioactivity was recovered only in carnitine or glycine betaine spots and was measured by scintillation counting.
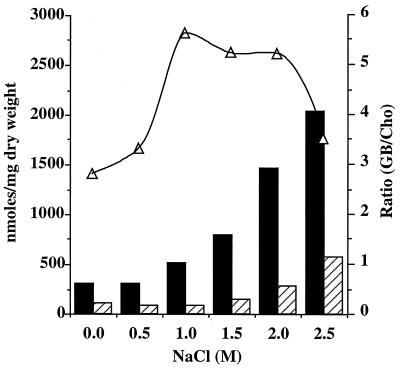
13C-NMR analysis showed that T. halophila was able to oxidize choline into glycine betaine. Therefore, the fate of exogenously provided [14C]choline was analyzed in T. halophila cells grown at various salinities. The cells collected in the late-exponential growth phase converted choline into glycine betaine whatever the medium salinity (Fig. 6). The resulting amount of synthesized glycine betaine remained unchanged from 0 to 1 M NaCl, while it increased in correlation with NaCl concentration from 1 to 2.5 M. A maximal level of 2,037 nmol/mg (dry weight) was reached at 2.5 M NaCl (Fig. 6). Interestingly, the glycine betaine/choline ratio increased from 3 at low salinity (0 to 0.5 M NaCl) to 5 to 6 when the medium salinity was raised to 2 M NaCl. At 2.5 M NaCl the glycine betaine/choline ratio decreased to 3.5.
FIG. 6.
Conversion of choline to betaine in response to increasing salinity. Paper electrophoresis was used for separation, signals corresponding to choline (hatched bar) and glycine betaine (solid bar) were analyzed by phosphorimager, and the radioactivity corresponding to each spot was determined by scintillation counting. ▵, glycine betaine/choline (GB/Cho) ratio.
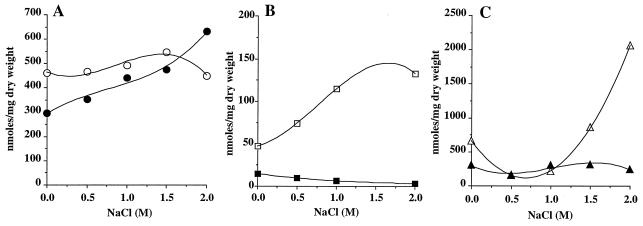
Effect of glycine betaine and osmotic stress on intracellular potassium, sodium, and glutamate. (i) Accumulation of potassium is a primary event in the adaptation of many bacteria to hyperosmotic constraints (7).
To establish whether the response of T. halophila to increased medium salinity involves the accumulation of potassium, the ion content of cytoplasmic extracts of exponentially growing T. halophila cells was analyzed by atomic absorption spectrophotometry. The steady-state potassium concentration of the cytoplasm increased from 460 to 550 nmol/mg (dry weight) when NaCl concentration was raised from 0 to 1.5 M (Fig. 7A). At 2 M NaCl the potassium content decreased slightly to reach 450 nmol/mg (dry weight), which was equivalent to that obtained in DM. When cells were grown in DM containing 0 to 1.5 M NaCl, the addition of 2 mM glycine betaine lowered the steady-state K+ concentrations (300 and 470 nmol/mg [dry weight] at low and high salinity, respectively) (Fig. 7A). In contrast, K+ content was increased to 630 nmol/mg (dry weight) in 2 M NaCl medium in the presence of glycine betaine.
FIG. 7.
Influence of elevated osmolarity, in the absence (open symbols) or presence (solid symbols) of glycine betaine, on the accumulation of potassium (A), glutamate (B), and sodium (C) by T. halophila.
(ii) In many bacteria, the glutamate level increases after osmotic shock to provide counter-ions for a strong increase in the K+ pool.
Glutamate is also required to maintain the steady-state K+ level content (7, 32). In T. halophila, the level of glutamate increased from 47 nmol/mg (dry weight) at low salinity to reach 133 nmol/mg (dry weight) at high salinity (Fig. 7B). When glycine betaine was present in the growth media, the glutamate amount was maintained below 10 nmol/mg (dry weight) regardless of the NaCl concentration of the medium (Fig. 7B).
(iii) All moderately halophilic bacteria require a minimal concentration of Na+ for growth (35).
In T. halophila optimal growth was obtained in DM with salinity ranging from 0.4 to 0.8 M NaCl. Thus the role of Na+ in the moderately halophilic behavior of this organism was studied. In the absence of glycine betaine, the intracellular amount of sodium was relatively high in DM (660 nmol/mg [dry weight]). Then, it decreased three- to fourfold when T. halophila was grown in a salinity range varying from 0.5 to 1 M NaCl. Over 1 M NaCl, the intracellular Na+ content increased linearly with increasing medium salinity, to reach a maximal level of 2,065 nmol/mg (dry weight) when the growth medium was amended with 2 M NaCl (Fig. 7C). In the presence of glycine betaine, the intracellular sodium level fluctuated between 150 and 320 nmol/mg (dry weight) in the medium with salinity ranging from 0 to 2 M NaCl. Carnitine and glycine betaine had the same effect on the intracellular Na+ concentration, which was estimated at 200 to 300 nmol/mg (dry weight) in a range of salinities varying from 0 to 2 M NaCl. Over a wide range of salinity, glycine betaine, like carnitine, maintained the intracellular Na+ concentration close to the level observed in the optimal-growth conditions and in the absence of betaines.
DISCUSSION
We analyzed the physiological osmoregulatory responses of the LAB T. halophila in DM. The effect of osmotic stress on growth was investigated in the presence of the osmoprotectants glycine betaine, carnitine, and choline. The present results show that T. halophila can grow in a chemically defined medium and did not require carnitine or glycine betaine for growth; these solutes have been previously described as specific growth factors for T. halophila cultured in DM (28, 29). In MRS medium and DM, T. halophila tolerates up to 3.2 and 1.6 M NaCl, respectively. The difference in bacterial osmotolerance levels in these two media could be due partly to the presence of a large amount of osmoprotectants in MRS broth and partly to the inability of T. halophila to synthesize efficient osmotically active cellular solutes when cultured in DM with elevated salinity. It was demonstrated by 13C-NMR that T. halophila accumulated only glycine betaine and carnitine as principal organic solutes when cells were grown in MRS without or with NaCl (1.5 M). It has been shown that the presence of glycine betaine and carnitine in MRS broth confers increased osmolarity tolerance to some LAB (10, 19). The addition of glycine betaine, carnitine, or choline to DM containing various NaCl concentrations allowed T. halophila to grow in a wide range of salinities close to that tolerated in MRS media. It is worth noting that glycine betaine and carnitine improved the growth of T. halophila over the whole range of salinities, even in DM lacking NaCl. The growth rate and the growth yield can be increased by adding osmoprotectant to the growth medium and/or by increasing the NaCl concentration up to 1 M NaCl. These observations are paradoxical since it is assumed that increasing the NaCl concentration generates an osmotic stress situation, of which the osmoprotectants alleviate the harmful effect. This supposes that T. halophila requires osmoprotectants for osmoregulatory purposes and osmoprotectants and sodium simultaneously to develop a moderately halophilic behavior. Similar observations have been made for Halomonas elongata, another moderately halophilic bacterium stimulated by glycine betaine and choline at all salinities, including suboptimal growth conditions (4). In contrast, glycine betaine and other osmoprotectants improve the growth of studied LAB only at high osmolarity, while at low osmolarity glycine betaine had an inhibitory effect on the growth of Lactococcus lactis (9, 20, 22).
Natural-abundance 13C-NMR spectroscopy indicated that glutamate was the main organic solute in the cytoplasmic solute pool of T. halophila when cells were grown in DM with 1 M NaCl added. The glutamate level increased two- to threefold when cells were cultured in DM with the NaCl concentration ranging from 0 to 2 M (Fig. 6B). NMR spectra also revealed minor peaks, which were attributed to proline and aspartate. All these amino acids could be accumulated by uptake from the medium and/or by biosynthesis. These solutes have also been found to be the main endogenous osmolytes accumulated by other LAB grown under hyperosmotic conditions (9, 22). Trehalose, which is accumulated by a large number of bacteria under osmotic stress (7, 17), was never detected in T. halophila, either at exponential or at stationary growth phases; this observation has also been reported for other LAB (19, 34). In contrast to most representatives of the moderately halophilic bacteria, for which 13C-NMR analysis led to the identification of ectoine and hydroxyectoine as the main endogenous organic compounds (25, 35, 37), T. halophila did not produce these compounds or any other potent compatible solute.
When glycine betaine or carnitine was present, it was preferentially accumulated and inhibited the accumulation of proline, aspartate, and glutamate. Carnitine or glycine betaine was accumulated in unmodified form by T. halophila at low and high salinities. Their intracellular levels were significantly stimulated (four- to fivefold) only when the salinity of the medium was raised from 1 to 2.5 M NaCl. These results suggest that cells are osmotically stressed at these salinities. On the other hand, the conspicious increase in the accumulated osmoprotectant at salt concentrations >1 M NaCl is probably linked to an activation of the transport of these betaines. Uptake and cross-competition experiments suggested that at least two transport systems operate in T. halophila: the first system transports glycine betaine, and the second transports all the betaines assayed. These uptake systems are not induced and are not activated, or slightly activated, by elevated salinity. The gram-positive model bacterium B. subtilis transports glycine betaine by three separate systems: OpuA, OpuC, and OpuD. Only the OpuC system displays a broad substrate specificity (17). Thus, the T. halophila glycine betaine transport systems are somewhat close to those characterized in B. subtilis but differ from the E. coli ProP and ProU systems, which can recognize all osmoprotectants assayed. Several nonhalophilic bacteria, including Pseudomonas, Agrobacterium, and Rhizobium species (21, 33) and certain halophilic bacteria such as H. elongata (4, 35), have the ability to use l-carnitine and glycine betaine as sole sources of carbon and nitrogen. Brevibacterium linens is able to convert l-carnitine into glycine betaine, which functions as an osmoprotectant in this bacterium, while B. subtilis and E. coli accumulated carnitine under hyperosmotic and aerobic conditions if supplied in the growth medium (14, 16, 21). T. halophila, like other LAB, cannot catabolize any of these compounds under aerobic growth conditions and hence accumulates them only for osmoregulatory purposes (9, 10, 19, 20, 22).
Among the assayed quaternary ammonium compounds, choline appears to be the least efficient for improving both growth yield and growth rate of T. halophila subjected to hyperosmotic constraint. The osmoprotective effect of choline in both gram-positive and gram-negative bacteria has been described (1, 2, 4, 6, 13, 27). In LAB that have been studied, data concerning osmoprotective properties of choline are contradictory. For example, depending on which L. plantarum strain was used, choline has been reported to have osmoprotective effects (9, 20). This solute was accumulated in unmodified form in osmotically stressed L. plantarum cells (9, 20). In Lactococcus lactis, choline did not improve the growth at high osmolarity but was accumulated when provided in the culture medium (34). To our knowledge, T. halophila is the first LAB which can convert choline into glycine betaine under aerobic growth conditions and accumulate glycine betaine during salt stress. Thus, the osmoprotective effect of choline appears to depend on its enzymatic conversion to glycine betaine. The glycine betaine/choline ratio increases from 3 at low salinity (0 to 0.5 M NaCl) to 5 at high salinity (1 to 2 M) which suggests that the choline-glycine betaine pathway is activated and/or induced by elevated salinity. T. halophila thus shares the ability to oxidize choline to glycine betaine for osmoprotective purposes with a number of nonhalophilic and halophilic bacteria (1, 2, 4, 6, 13, 27, 35).
Organic osmolytes are not the only efficient compounds for the adaptation of bacteria to hyperosmotic conditions. Potassium is also known to be a key ion for osmotic adaptation in many bacteria. In T. halophila, the steady-state intracellular concentration of K+ increases from 460 to 550 nmol/mg (dry weight) in DM with added NaCl from 0 to 1.5 M. Under these conditions, glycine betaine inhibits potassium accumulation by 10 to 35%. At >1.5 M NaCl, growth is highly affected and potassium content decreases to reach 450 nmol/mg (dry weight) at 2 M NaCl. Glycine betaine, which greatly improved growth under hyperosmotic conditions (2 M NaCl), allowed the cells to maintain the intracellular K+ concentration at 630 nmol/mg (dry weight). These results suggest that K+ is necessary for growth in media of varied salinities. Nevertheless, it does not seem to be essential in the response to osmotic changes in T. halophila, since (i) the apparent intracellular K+ concentration did not increase in correlation with increasing external NaCl concentration and (ii) the accumulation of glycine betaine does not significantly affect the steady-state intracellular concentration of potassium. The relatively small role of potassium in the achievement of osmotic balance has also been observed in other LAB and most moderately halophilic bacteria (9, 20, 35).
The common denominator for all moderately halophilic bacteria is their requirement for salt and their ability to tolerate high salt concentrations (35). In most cases, a minimum of Na+ is essential for growth. Thus, it was of interest to examine also the change in intracellular sodium concentration in T. halophila subjected to various salinities. In this bacterium, Na+ content was high in DM (660 nmol/mg [dry weight]) and decreased to about 200 nmol/mg (dry weight) in the optimal concentrations of NaCl (0.5 to 1 M). Over these salinities, the Na+ content increased 4- to 10-fold at high osmolarities. At 2 M NaCl, the intracellular concentration of the most prevalent organic anion (glutamate) did not exceed 10% of that of Na+, and 13C-NMR shows that T. halophila does not accumulate other negatively charged osmolytes to balance high levels of cytosolic Na+. In fact, the charge balance in T. halophila cells could be maintained by the accumulation of large amounts of Cl−, as observed in Bacillus haloalkaliphilus and other moderately halophilic bacteria (35). Also, studies based on 23Na-NMR have shown that about 40% of the intracellular Na+ is free and 60% of Na+ is bound to cell components in several moderately halophilic bacteria (35). Thus, T. halophila may not need a large amount of counter-ions equivalent to Na+ if most of these ions are associated with negative charge present on macromolecules. Most microorganisms keep their intracellular Na+ concentration much lower than the external Na+ concentrations (23, 35). However, T. halophila is not the only organism that does not attempt to keep Na+ out of its cytoplasm. For example, Haloanaerobium acetoethylicum, another fermentative halophilic bacterium, also accumulates high levels of Na+ (23). The nonavailability of organic osmoprotectants in the environment may force T. halophila to accumulate high Na+ concentrations. Moreover, the accumulation of salt probably represents an energetically cheaper option than the accumulation of organic osmolytes synthesized de novo. In some anaerobic halophilic bacteria, it was reported that the cells may maintain high intracellular salt (KCl in most cases) concentrations that are at least osmotically equivalent to the external salt (NaCl in most cases) concentrations (23). These bacteria require large amounts of substrates, which supply little energy; thus, the strategy of accumulating salt appears to require much less of the expensive substrate than does the production of organic osmotic solutes (23).
The inability to control the intracellular Na+ level on both sides of the optimal growth salinities may be involved in inhibiting the growth of T. halophila. The presence of betaine or carnitine allows this bacterium to maintain the Na+ concentration at a low level (200 to 300 nmol/mg [dry weight]) at all salinities. This suggests that T. halophila, in the presence of betaines, excludes Na+ to maintain a low concentration, even in an environment containing large amounts of sodium. In many moderately halophilic bacteria, the low intracellular sodium content is achieved by two possible mechanisms of Na+ extrusion: activity of an Na+/H+ antiporter and presence of a primary respiration-driven Na+ pump (35). An effect of betaine on the respiration-driven Na+ pump has previously been described for the moderately halophilic bacterium Halomonas israelensis (24, 31), where glycine betaine induced the stimulation of respiration at high salinities. A respiratory chain is lacking in LAB, but in streptococci and enterococci, two separate Na+ extrusion systems have been described (15), an Na+ ATPase and an Na+/H+ antiporter. These systems function as proton pumps in the membranes of these bacteria, which are unable to generate a large proton potential (15). Although no one doubts the ubiquitous distribution of these Na+ extrusion systems in LAB, if T. halophila possesses these systems, they are probably inhibited under high-salinity conditions, which could explain the high level of Na+ accumulated by the cells under high-salt treatment. In T. halophila, the accumulation of betaines allows physiological conditions that maintain the Na+ concentration inside the cells lower than that outside. The mechanisms by which betaines allow the cells to regulate the intracellular Na+ concentration are still unknown in T. halophila. Finally, as in most bacteria facing adverse hypersaline stress conditions, T. halophila cells have adopted the strategy of accumulating organic osmolytes through a process involving active transport of exogenous osmoprotectants (carnitine and glycine betaine) or through conversion of choline into glycine betaine. In T. halophila, these organic solutes are required not as growth factors but as osmotic and salt stabilizers. Thus, T. halophila has a moderately halophilic behavior provided that osmoprotectants are available in its environment.
ACKNOWLEDGMENTS
The expert technical assistance of Sandrine Papillon is greatly appreciated. We thank Kathryn Mayo for critical reading of the manuscript. We are grateful to J. Hamelin (University of Rennes) for his kind advice on NMR spectroscopy.
Financial support for this study was provided by the Centre National de la Recherche Scientifique, the Direction de la Recherche et des Etudes Doctorales, and the Région Bretagne.
REFERENCES
- 1.Bernard T, Pocard J A, Perroud B, Le Rudulier D. Variations in the response of salt-stressed Rhizobium strains to betaines. Arch Microbiol. 1986;143:359–364. [Google Scholar]
- 2.Boch J, Kempf B, Bremer E. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J Bacteriol. 1994;176:5364–5371. doi: 10.1128/jb.176.17.5364-5371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryand M P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am Clin Nutr. 1972;25:1513–1523. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- 4.Canovas D, Vargas C, Csonka L N, Ventosa A, Nieto J J. Osmoprotectants in Halomonas elongata: high-affinity betaine transport system and choline-betaine pathway. J Bacteriol. 1996;178:7221–7226. doi: 10.1128/jb.178.24.7221-7226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins M D, Williams A M, Wallbanks S. The phylogeny of Aerococcus and Pediococcus as determined by 16s rRNA sequence analysis: description of Tetragenococcus gen. nov. FEMS Microbiol Lett. 1990;70:255–262. doi: 10.1016/s0378-1097(05)80004-7. [DOI] [PubMed] [Google Scholar]
- 6.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 7.Csonka L N, Epstein W. Osmoregulation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 1210–1223. [Google Scholar]
- 8.De Man J C, Rogosa M, Sharpe M E. A medium for cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 9.Glaasker E, Konings W N, Poolman B. Osmotic regulation of intracellular solute pools in Lactobacillus plantarum. J Bacteriol. 1996;178:575–582. doi: 10.1128/jb.178.3.575-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutkins R W, Ellefson W L, Kashket E R. Betaine transport imparts osmotolerance on a strain of Lactobacillus acidophilus. Appl Environ Microbiol. 1987;53:2275–2281. doi: 10.1128/aem.53.10.2275-2281.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikuta S, Matuura K, Imamura S, Misaki H, Horiuti Y. Oxidative pathway of choline to betaine in the soluble fraction prepared from Arthrobacter globiformis. J Biochem. 1977;82:157–163. doi: 10.1093/oxfordjournals.jbchem.a131664. [DOI] [PubMed] [Google Scholar]
- 12.Jebbar M, Talibart R, Gloux K, Bernard T, Blanco C. Osmoprotection of Escherichia coli by ectoine: uptake and accumulation characteristics. J Bacteriol. 1992;174:5027–5035. doi: 10.1128/jb.174.15.5027-5035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jebbar M, Gouesbet G, Himdi-Kabbab S, Blanco C, Bernard T. Osmotic adaptation in Brevibacterium linens: differential effect of proline and glycine betaine on cytoplasmic osmolyte pool. Arch Microbiol. 1995;163:380–386. [Google Scholar]
- 14.Jebbar M, Champion C, Blanco C, Bonnassie S. Carnitine acts as compatible solute in Brevibacterium linens. Res Microbiol. 1998;149:211–219. doi: 10.1016/s0923-2508(98)80081-8. [DOI] [PubMed] [Google Scholar]
- 15.Kakinuma Y. Inorganic cation transport and energy transduction in Enterococcus hirae and other streptococci. Microbiol Mol Biol Rev. 1998;62:1021–1045. doi: 10.1128/mmbr.62.4.1021-1045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kappes R M, Bremer E. Response of Bacillus subtilis to high osmolarity: uptake of carnitine, crotonobetaine and γ-butyrobetaine via the ABC transport system OpuC. Microbiology. 1998;144:83–90. doi: 10.1099/00221287-144-1-83. [DOI] [PubMed] [Google Scholar]
- 17.Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 18.Kets E P W, de Bont J A M. Protective effect of betaine on survival of Lactobacillus plantarum subjected to drying. FEMS Microbiol Lett. 1994;116:251–256. [Google Scholar]
- 19.Kets E P W, Galinski E A, de Bont J A M. Carnitine: a novel compatible solute in Lactobacillus plantarum. Arch Microbiol. 1994;162:243–248. [Google Scholar]
- 20.Kets E P W, Groot M N, Galinski E A, de Bont J A M. Choline and acetylcholine: novel cationic osmolytes in Lactobacillus plantarum. Appl Microbiol Biotechnol. 1997;48:94–98. [Google Scholar]
- 21.Kleber H-P. Bacterial carnitine metabolism. FEMS Microbiol Lett. 1997;147:1–9. doi: 10.1111/j.1574-6968.1997.tb10212.x. [DOI] [PubMed] [Google Scholar]
- 22.Moleenar D, Hagting A, Alkema H, Driessen A J M, Konings W N. Characteristics and osmoregulatory roles of uptake systems for proline and glycine betaine in Lactococcus lactis. J Bacteriol. 1993;175:5438–5444. doi: 10.1128/jb.175.17.5438-5444.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oren A. Bioenergetic aspects of halophilism. Microbiol Mol Biol Rev. 1999;63:334–348. doi: 10.1128/mmbr.63.2.334-348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafaeli-Eshkol D, Avi-Dor Y. Studies on halotolerance in a moderately halophilic bacterium. Effect of betaine on salt resistance of the respiratory system. Biochem J. 1968;109:687–691. doi: 10.1042/bj1090687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regev R, Peri I, Gilboa H, Avi-Dor Y. 13C NMR study of the interrelation between synthesis and uptake of compatible solutes in two moderately halophilic eubacteria. Arch Biochem Biophys. 1990;278:106–112. doi: 10.1016/0003-9861(90)90237-s. [DOI] [PubMed] [Google Scholar]
- 26.Röling W F, Van Verseveld H W. Characterization of Tetragenococcus halophila populations in Indonesian soy mash (Kecap) fermentation. Appl Environ Microbiol. 1996;62:1203–1207. doi: 10.1128/aem.62.4.1203-1207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozwadowski K L, Khachatourians G G, Selvaraj G. Choline oxydase, a catabolic enzyme in Arthrobacter pascens, facilitates adaptation to osmotic stress in Escherichia coli. J Bacteriol. 1991;173:472–478. doi: 10.1128/jb.173.2.472-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi K. Betaine as a growth factor for Pediococcus soyae. VII. Studies on the activities of bacteria in soy sauce brewing. Bull Agric Chem Soc Jpn. 1960;24:489–496. [Google Scholar]
- 29.Sakaguchi K. Carnitine as a growth factor for Pediococcus soyae. X. Studies on the activities of bacteria in soy sauce brewing. Bull Agric Chem Soc Jpn. 1962;26:72–74. [Google Scholar]
- 30.Satomi M, Kimura B, Mizoi M, Sato T, Fujii T. Tetragenococcus muriaticus sp. nov., a new moderately halophilic lactic acid bacterium isolated from fermented squid liver sauce. Int J Syst Bacteriol. 1997;47:832–836. doi: 10.1099/00207713-47-3-832. [DOI] [PubMed] [Google Scholar]
- 31.Shkedy-Vinkler C, Avi-Dor Y. Betaine-induced stimulation of respiration at high osmolarities in a halotolerant bacterium. Biochem J. 1975;150:219–226. doi: 10.1042/bj1500219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talibart R, Jebbar M, Gouesbet G, Himdi-Kabbab S, Wroblewski H, Blanco C, Bernard T. Osmoadaptation in rhizobia: ectoine-induced salt tolerance. J Bacteriol. 1994;176:5210–5217. doi: 10.1128/jb.176.17.5210-5217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talibart R, Jebbar M, Gouffi K, Pichereau V, Gouesbet G, Blanco C, Bernard T, Pocard J A. Transient accumulation of glycine betaine and dynamics of endogenous osmolytes in salt-stressed cultures of Sinorhizobium meliloti. Appl Environ Microbiol. 1997;63:4657–4663. doi: 10.1128/aem.63.12.4657-4663.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uguen P, Blanco C, Hamelin J, Le Pennec J P. Effect of glycine betaine on growth and bacteriocin production by Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1999;65:291–293. doi: 10.1128/aem.65.1.291-293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ventosa A, Nieto J J, Oren A. Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev. 1998;62:504–544. doi: 10.1128/mmbr.62.2.504-544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villar M, De Ruiz Holgado A P, Sanchez J J, Trucco R E, Oliver G. Isolation and characterization of Pediococcus halophila from salted anchovies. Appl Environ Microbiol. 1985;49:664–666. doi: 10.1128/aem.49.3.664-666.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wohlfarth A, Severin J, Galinski E A. The spectrum of compatible solutes in heterotrophic halophilic eubacteria of the family Halomonadaceae. J Gen Microbiol. 1990;136:705–712. [Google Scholar]
- 38.Yancey P H, Clark M E, Hand S C, Bowlus R D, Somero B N. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]