Abstract
Effective biomarkers that guide therapeutics with limited adverse effects, have emerged as attractive research topics in cancer diagnosis and treatment. Cancer-derived exosomes, a type of extracellular vesicles representing molecular signatures of cells of origin, could serve as stable reservoirs for potential biomarkers (i.e., proteins, nucleic acids) in non-invasive cancer diagnosis and prognosis. In this review, the physiological and pathological roles of exosomes and their protein components in facilitating tumorigenesis are highlighted. Exosomes carrying proteins can participate in tumor development and progression through multiple signaling pathways, including EMT, invasion and metastasis. Meanwhile, the practical applications of exosomal proteins in detecting and monitoring several solid-tumor cancers (including lung, breast, pancreatic, colorectal and prostate cancers) were also summarized. More clinically relevant, exosomal proteins play pivotal roles in transmitting oncogenic potential or resistance to therapies in recipient cells, which might further support therapeutic strategy determinations.
Keywords: Exosome, tumor-derived exosome, protein biomarker, tumor diagnosis, cancer drug resistance
Introduction
Today, cancer is still a major public health problem worldwide [1]. Despite of significant therapeutic advances in recent decades, the lack of specificity and effectiveness remains major obstacles in clinical treatment. There is an urgent need to identify and validate more effective and less invasive surrogate biomarkers so as to elucidate underlying mechanisms of tumor progression and further provide more potential therapeutic targets for cancer diagnosis and treatment.
Exosomes are extracellular vesicles (EVs) constantly released by most eukaryotic cells. As an intermediate of intercellular communication, exosomes have multiple important biological functions and have been involved in various diseases [2]. In particular, tumor-derived exosomes (TDEs) are implicated in promoting tumor progression, pre-metastasis and immune escape by paracrine subversion of local and distant microenvironments [3]. Emerging evidence supported that exosomes should have a profound impact on the development of cancer therapeutics.
A plenty of key regulators have been identified from tissues and body fluids during tumor progression. However, growing evidence indicated that non-exosomal protein biomarkers have limitations of low accuracy, specificity and reproducibility. Compared with regular tumor biomarkers, exosomes carry cargos reflective of genetic or signaling alterations in cancer cells of origin [4,5], which provides a robust method to monitor cancer progression further guide clinical decisions and treatment strategies.
To date, a wealth of research regarding exosomes in cancer diagnosis and treatment has been reported. Recent reviews have mainly focused on the genetic components of exosomes (i.e., microRNAs) but only a small proportion on exsomal proteins. Considering that detecting key regulatory proteins (e.g., phosphoproteins or other proteins with post-translational modification) can provide more direct information about disease progression, this review highlights the unique features of exosomal proteins in cancer. The application potential and clinical significance to develop exosomal proteins as novel diagnostic and prognostic biomarkers as well as therapeutic targets are summarized in a variety of cancer types.
Biological features of exosomes
Definition, morphology and compositions of exosomes
Exosomes are a class of lipid bilayer-enclosed EVs devoid of intracellular organelles but contain all known molecular constituents within a cell [6,7] (Figure 1). They are produced in late endosomes with size ranging from 30 nm to 150 nm [2,8,9]. The overall composition of exosomes is representative of mixed populations, which includes lipids, nucleic acids and proteins (Figure 1) [10]. The lipid composition mimics plasma membranes [11,12]. Nucleic acids, as key components of exosomes, have multiple functional impacts. For instance, microRNAs (miRNAs) affect gene expression in distant cells through exosomal RNA cargo selection. Exosomal proteome is composited by endosomal, plasma, cytosolic and nuclear proteins, including tetraspanins (CD9, CD81), proteins associated with endosomal sorting complexes required for transport (ESCRT) (Alix, Tsg101), cytoskeletal proteins (actin, tubulin) and cytokines. These different types of proteins are involved in membrane transport and fusion, exosome biogenesis, and can also serve as mediators for cell-cell communication (Figure 1) [6].
Figure 1.
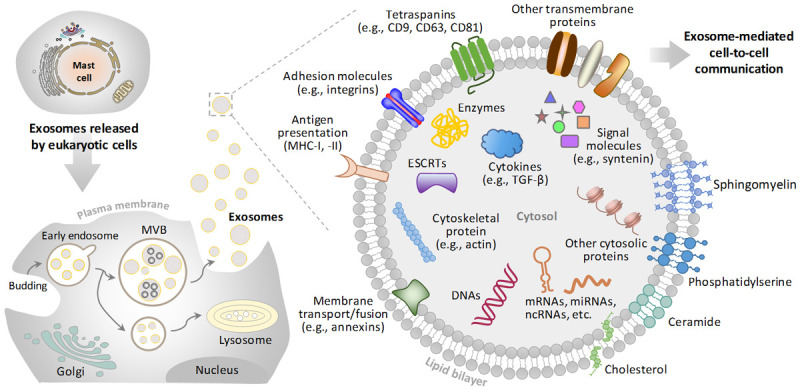
Schematic representation of biogenesis and overall composition of exosomes. Exosomes stem from later endosomes, generated by inward/inner budding from the plasma membrane (PM) or by fusion of internal multivesicular bodies (MVBs) with the PM in most of eukaryotic cells. Exosomes are vesicles with a phospholipid bilayer membrane and are enriched with a range of proteins, RNAs and DNA molecular cargoes. RNAs include mRNA, miRNA, ncRNA, and etc. Exosomes contain endosome-specific tetraspanins (CD9, CD63, CD81), adhesion molecules (e.g., integrins), antigen presentation (MHC-I, -II) and other transmembrane proteins on their membrane surfaces. Exosomes also contain types of cytosolic proteins, including ESCRTs, cytokines and signal molecules. Abbreviations: mRNA, messenger RNA; miRNA, microRNA; ncRNA, non-coding RNA; ESCRT, endosomal sorting complex required for transport; MHC, major histocompatibility complex.
Physical and biological features of exosomes
Exosomes can be secreted by plenty of cell types in vitro, including endothelial cells, epithelial cells, immune cells, tumor cells, and etc. In vivo, exosomes are also broadly observed in numerous body fluids (such as plasma/serum, saliva, urine, reviewed in [9]). Exosomes are formed by inward budding of multivesicular bodies (MVBs) in intracellular endosomes and released by fusing with the plasma membrane (Figure 1). In accordance with this biogenesis and secretion process, exosomes display a heterogeneity by incorporating both plasma membrane and cytosolic components [13,14]. For instance, the highly heterogeneity of TDEs likely reflects the phenotypic state of tumor cells that generate exosomes [15,16]. There is growing evidence showing that cell-derived exosomes act as dynamic mediators of local and systemic cell communication by carrying molecular information [17]. Through transport of essential substances via their cargos, TDEs are capable to modulate tumor microenvironment (TME) during cancer progression [7,18].
Isolation and enrichment of exosomes
Exosomes often coexist in complex biological fluids with many substances (such as lipoprotein or other EVs), thus it is indispensable to obtain non-destructive isolation of exosome [9]. A variant of isolation approaches have been established to purify exosomes for further analysis [19], that have been well summarized in recent review articles [20,21]. Currently, the mainstream isolation and detection methods of exosomes (i.e., purification by ultracentrifugation) could not satisfy the clinical applications. Therefore, efforts to develop new technologies are currently undergoing to obtain high-quality exosomes for theranostics purposes.
Strategies for identification and analysis of exosome proteins
In order to identify and analyze exosome-associated protein biomarkers, an ideal detection is required with characteristics including high-throughput and easy operability, as well as high sensitivity, specificity and stability. The morphology and immunophenotype of exosomes are routinely performed using electron microscopy (EM) [22]. Furthermore, the isolated exosomes can be verified by surface biomarker analysis through ELISA and Western blotting (Figure 2). In these processes, the conservative exosomal proteins will be identified and quantitatively assessed specifically (e.g., tumor-associated proteins). Subsequently, mass spectrometry or extracellular vesicle (EV) arrays (a sandwich ELISA-based method simultaneously studies multiple membrane-associated proteins) for proteome analysis was applied for discovering disease-specific proteins (Figure 2) [13,23,24]. In common, these methods require pre-isolation of exosome and protein extractions, which may be is a cumbersome process.
Figure 2.
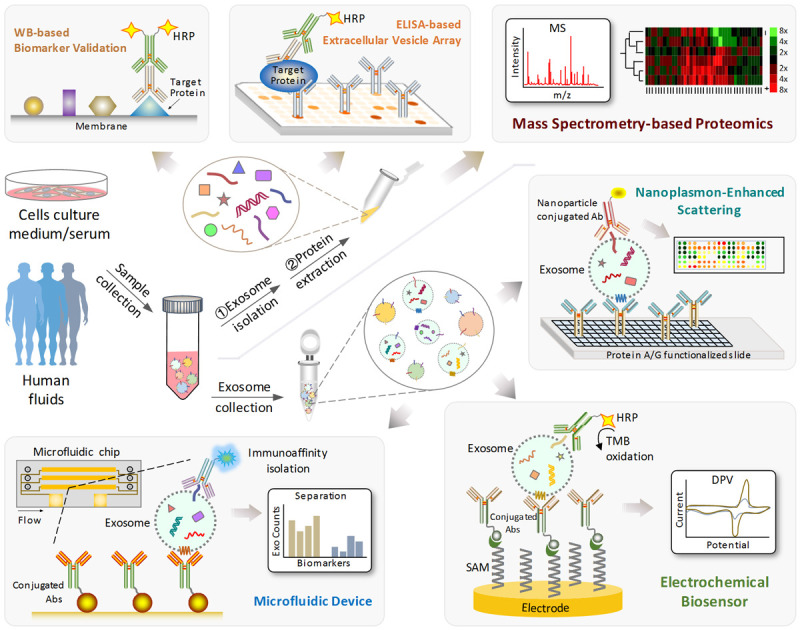
Schematic representation of different exosome isolation and biomarker detection methods. Abbreviations: HRP, horseradish peroxidase; WB, western blotting; ELISA, enzyme-linked immunosorbent assay; MS, Mass Spectrometry; Ab, antibody; SAM, self-assembled monolayers; DPV, differential pulse voltammetry.
Recently, a rapid and high-throughput platform, the microfluidic device was developed to simultaneously isolate and identify exosome surface proteins without pre-purification [25,26]. This microfluidic device is conjugated with multiple functional assays to further investigate the biological mechanisms of exosome surface proteins (Figure 2). In addition, ultrasensitive nanoplasmon enhanced scattering (nPES) assay was also applied to analyze exosomes [27]. The design of nPES is based on a conjugation of exosome-specific antibody and nanoparticles (e.g., gold nanospheres), as well as a sensor chip to produce plasmon effect (Figure 2). In a more efficient way, a combination of microfluidic chip and nPES was developed to achieve better exosome capture [28].
Exosomes with specific surface markers could also be detected using biosensors (e.g., a type of immune-biosensor based on horseradish peroxidase (HRP)-conjugated antibodies, Figure 2) [29]. By keeping the non-disruptive features on exosome integrity, this method provides an ideal platform to study diagnostic biomarkers of disease through a non-invasive test (e.g., blood test). However, it can be only applicable for membrane bound or surface proteins on exosomes but lacks of a broad feasibility for intra-exosomal proteins. Alternatively, proteomic analysis of exosomes by mass spectrometry could identify proteins from the whole proteome secreted by a cell or within biological fluid samples like patient plasma, which features a more effective approach with high potential for diagnostic and therapeutic applications [30,31].
Role of exosomes in cancer
As generally acknowledged, cancer progression is sustained by continuous information exchange between the tumor cells and their stromal microenvironment. These include remodeling tumor microenvironment, promoting angiogenesis, inducing invasion, metastasis and survival, as well as regulating immune escape (Figure 3) [32-34].
Figure 3.
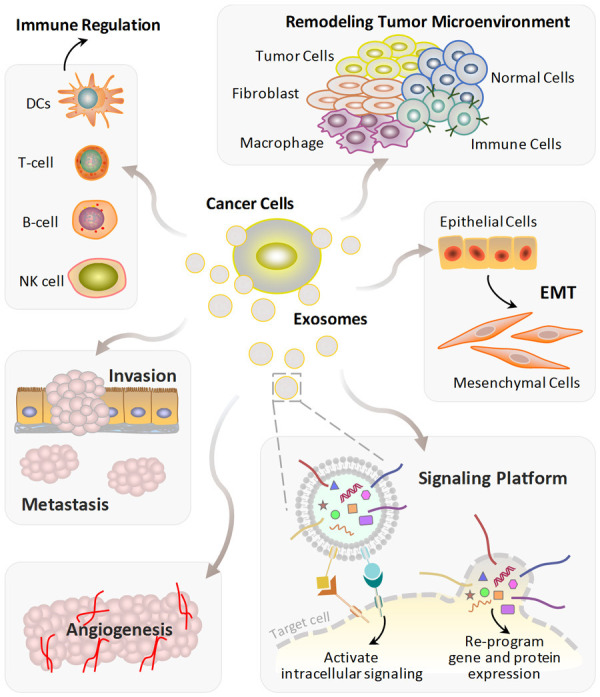
Tumor-derived exosomes (TDEs) elicit various mechanisms to stimulate tumor progression. TDEs can cause the remodeling of tumor microenvironment, promote EMT and angiogenesis, induce tumor invasion and metastasis. Cancer cells remodel B cells, T cells, DCs, and NK cells via exosomes resulting in immune regulation. TDEs also act as signaling platforms to initiate downstream signaling cascades or modulate the gene-expression program through membrane fusion in the target cells. Abbreviations: DCs, dendritic cells; NK cells, nature killer cells; EMT, epithelial-mesenchymal transition.
Exosome in tumor progression
TDEs play important functions in different stages of cancer progression cascade (Figure 3) [35]. For example, TDEs carry several types of main angiogenic stimulatory factors (i.e., vascular endothelial growth factor, VEGF; fibroblast growth factor, FGF; transforming growth factor β, TGF-β; etc.) to induce vascular formation and angiogenesis in cancer [36]. Furthermore, it has been reported that TDEs are also incorporated in inducing epithelial mesenchymal transition (EMT) in recipient cells by activating key regulation signaling pathways, such as TGF-β and WNT/β-catenin signaling pathways [37]. During tumorigenesis, by mediating cellular communication between tumor cells and the surrounding cells, TDEs enhance invasion, migration and establishment of a premetastatic niche. Therefore, TDEs have emerged as a source of information to determine potential regulatory drivers of tumor progression and metastasis [38].
Exosome and tumor microenvironment
Numerous studies have demonstrated that TDEs support the tumor microenvironment through the transfer of their cargos to neighboring or distant cells (including fibroblast, macrophage, immune cells and other normal cells, Figure 3), which is involved in many key processes during cancer progression [39]. For instance, it has been shown that secretion of TDEs increased under hypoxic conditions [40]. The increased TDEs release established a link between hypoxia and tumor aggressiveness. In return, the function of exosomes influenced by hypoxia in various cancer types would further promote hypoxic cell survival in the tumor microenvironment. The complex signaling pathway network between TDEs-mediated cells and the tumor microenvironment is considered to provide a protective environment for their cargo, thereby making them superior targets for cancer screening, monitoring, diagnosis and prognosis evaluation [41,42].
Exosome in cancer immunoregulation
The immunoregulation of TDEs mainly acts through modulating antigen presentation, immune activation or suppression and immune surveillance (Figure 3). Thus, TDEs have a dual function in stimulating immune response. On the one hand, TDEs play a key role in immune system evasion from host immune surveillance. For instance, by transferring antigen components through exosomes to T-cell, immune escape and cell migration are potently stimulated [43]. On the other hand, as an effective cancer immunotherapy, some antigen-positive TDEs have high immunogenicity to improve the antitumor immunity [44]. Understanding exosome biology, especially the molecular mechanisms involved in immune cell targeting, interaction and manipulation, will provide significant insights in immunorecognition and therapeutic intervention of cancer.
Exosome in cancer signaling platform
Secreted by cancer cells, TDEs have been largely considered as a central participant in shuttling specific tumor markers between cells [45]. TDEs contain a variety of membrane proteins (e.g., integrins; major histocompatibility complex, MHC-1, -2; tetraspanins) that can interact with specific ligands on target cells to induce signaling cascades (Figure 3). In addition, the membrane fusion between TDEs and target cells results in the release of cargos (e.g., functional miRNA and proteins) into the cytoplasm, which can in turn re-program the gene-expression profiles in the target cell [2]. Through participating in cellular communications, modulating cell signaling, and contributing to pre-metastatic niche (PMN) formation [46-49], TDEs provide a reservoir of key regulators that have multiple important roles in tumor progression.
Exosome biomarkers in tumor biology
Exosomes present a list of validated and surrogate non-invasive biomarkers with a high accuracy of diagnostic and prognostic information in cancer [50]. Exosome nucleic acids, such as DNA, mRNAs, miRNAs, and ncRNAs (Figure 1) have been shown to be highly associated with the tumor progression of multiple cancer types [51]. As proved in plenty of studies, miRNAs present in TDEs phenocopy those in original tumors [52] and may serve as reliable diagnostic biomarkers to monitor the tumor progression [53]. In addition, the whole-genome sequencing results revealed that DNAs in exosomes may provide detailed information about cancer-specific mutations [54,55], which has a great potential to inform diagnosis and predict cancer therapeutic outcomes. The study of exosome nucleic acids has been well-described in a recent review [56].
Exosomes are also composed of a large variety of proteins (Figure 1) that participate in many biological processes [57]. Proteins in TDEs impact distant cell signaling or promote a niche that sustains tumor microenvironment leading to cancer spreading. It was broadly observed that certain types of proteins were frequently enriched in specific cancer cell-derived exosomes compared with non-tumor cells, providing the potential to apply these proteins in cancer prediction, diagnosis and prognosis [32]. With the development of both proteomic technologies and analytical approaches, research on exosomal proteins is rapidly progressing. In following sections, we will categorize multiple types of exosomal protein biomarkers in different types of cancer.
Exosome proteins as diagnostic and prognostic biomarkers in cancer
As a promising type of novel cancer biomarkers, TDE proteins have several outstanding characteristics [58-60]. First, TDEs have easy accessibility due to their broad existence and strong permeability. Secondly, the specific lipid bilayer membrane structure of exosomes protects proteins from degradation. In addition, certain cancer-associated proteins are enriched in TDEs. Compared to traditional tumor biomarkers, TDE proteins have improved performance and accuracy in determining cancer progression. These quantifiable proteins were shown to be involved in multiple biological functions and metastasis-related pathways in cancer, thus have promise as novel biomarkers for a variety of human cancers [52], including lung, breast, pancreatic, colorectal and prostate cancer (Table 1).
Table 1.
Exosomal proteins as biomarkers in tumor diagnosis and prognosis
| Cancer Types | Protein markers in exosome | Function in tumorigenesis | Ref. |
|---|---|---|---|
| Lung cancer | EGFR | Induce tumor antigen-specific Treg to regulate CD8+ T cells | [61,62] |
| EGFR, GRB2, Src | Regulate recipient cells proliferation in NSCLC | [63] | |
| LRG1 | High expression in urinary exosomes | [64,65] | |
| CD151, TSPAN8 | Modulate extracellular matrix to initiate metastatic | [66,67] | |
| CD171 | Induce EMT to cause metastasis and poor prognosis | [66] | |
| CD91 | A lung adenocarcinoma specific antigen | [68] | |
| CD5L | Block lung epithelial apoptosis to repress immunosurveillance; associated with cancer tissue in clinic | [70] | |
| MUC1 | Selectively enriched in the exosome compartment | [71] | |
| ALDOA, ALDH3A1 | Promote glycolytic activity to enhance motility of recipient cells; related to poor prognosis of lung cancer patients | [74] | |
| BALF | Induce metastasis via vascular endothelial-cadherin way | [75] | |
| Breast cancer | HER2 | Molecular classification of tumor tissues | [77] |
| Fibronectin, Del-1 | Distinguish breast cancer at different status | [78,79] | |
| CD24 | Enriched in pleural effusions and ascites of patients | [80] | |
| CD47 | Prevents cancer cells recognition by innate immune system | [81] | |
| CD82 | Redistribution from tissues to blood due to metastasis | [82] | |
| PKG1, RALGAPA2, NFX1, TJP2 | Phosphoproteins enriched in exosomes of human plasma | [84] | |
| GPC1 | Induce cellular division, differentiation, morphogenesis to identify early stage cancer | [85] | |
| Survivin, survivin-2B | Similar variant pattern in breast cancer tissues; related prominent antiapoptotic pathway | [86] | |
| AnxA2 | Promote angiogenesis; related to TNBC tumor grade and poor survival | [87] | |
| TTLL4 | Mediate microtubule polyglutamylation to alter exosome homeostasis; produce a pre-metastatic niche | [88] | |
| Rab27a, TRAF3IP2 | Inflammatory mediator; involved in metastasis in vivo | [89] | |
| TSP1 | Disrupt intercellular integrity of endothelial cells to induce trans-endothelial migration of cancer cells | [90] | |
| Pancreatic cancer | MIF | Initiate liver pre-metastatic niche formation and metastasis | [95,96] |
| GPC1 | Clinic preoperative and postoperative prognostic index | [97] | |
| CD44v6 | Activate Wnt/β-catenin/PAI-1/TIM-1 to promote the migration and invasion of PCICs | [98,99] | |
| CD44v6/C1QBP | Promote fibrotic liver microenvironment | [100] | |
| Tspan8 | Induce VEGF-independent angiogenesis | [101] | |
| CD151, Tspan8 | Induce EMT, ECM remodeling and pro-inflammatory effect | [102,103] | |
| TJ-Cld7 | Modulate exosomal transporters composition to affect PCICs-derived exosomes and induce PCICs migration | [104] | |
| Myoferlin | Mediate VEGF inclusion to promote tumor growth and angiogenesis | [105] | |
| Integrins | Cause organotropic metastasis | [48] | |
| Integrin β4 | Mediates plectin transfer to induce proliferation, migration and invasion of pancreatic cells | [106] | |
| ZIP4 | Stimulate proliferation, migration and invasion of non-metastatic pancreatic cancer cells | [107] | |
| Survivin | Enhance PDAC cell survival; enriched in PDAC patient serum | [108] | |
| EphA2 | Enriched in recurrent pancreatic cancer | [109] | |
| TNC | Induce local invasion and distant metastasis | [110] | |
| CKAP4 | Related to DKK1 endocytosis and exosome biogenesis | [111] | |
| Colorectal cancer | DKK4 | Related to APC overexpression | [112] |
| Wnt4 | Activate Wnt/β-catenin pathway to induce migration and invasion | [113] | |
| CPNE3 | Highly expressed in tissues and plasma of patients | [114] | |
| Hsp60 | Accumulated in peri-cancerous tissues | [115,116] | |
| GPC1 | Enriched in tumor tissues and plasma of patients | [117] | |
| CD9, CD147 | Abundant in colorectal cancer patient serum | [118] | |
| CEA | Predict metastatic colorectal cancer | [119] | |
| PrP | Promote hypoxic TME of metastasis via increase of endothelial permeability and angiogenic cytokine secretion | [120] | |
| CAPS1 | Promote epithelial cell migration to regulate metastasis | [121] | |
| STX2 | Related to increased expression of Exosome Complex 4 | [122] | |
| Prostate Cancer | HIF-1a | Promotes metastasis via repression of E-cadherin | [124] |
| Integrin ανβ6 | Induce the progression and invasion of cells | [125,126] | |
| Integrin ανβ3 | Increases recipient cells adhesion and migration on vitronectin; activate Src phosphorylation in recipient cells; induce metastatic niche to alter angiogenesis | [48,126] | |
| Integrin α2, α3, β4 | Induce EMT, promoting inflammation, migration and invasion of cancer cells | [127-129] | |
| PKM2 | Induce pre-metastatic niche for bone metastasis | [130] | |
| PLD | Stimulate exosome osteoblast activity for bone metastasis | [131] | |
| Hyal 1 | Stimulate prostate stromal cells mobility for metastatic | [132] | |
| Caveolin-1 | Promote invasion and metastasis via NF-κB signaling | [133] | |
| MMP-9, MMP-14 | Stimulating ERK1/2 phosphorylation | [134,135] | |
| Src, IGF-1R, GRKs, FAK | Induce angiogenesis via VEGF transcription stimulation in TME | [136] | |
| GGT1 | Higher in prostate cancer patients matching tumor tissues | [137] | |
| β-catenin, PCA-3, PSA, PSMA | Enriched in patient’s urinary exosomes | [138] | |
| EpCAM, EGFR, survivin | Detected in exosomes | [139] |
Abbreviations: ALDH3A1, aldehyde dehydrogenase 3-A1; ALDOA, fructose-bisphosphate aldolase; AnxA2, annexin A2; APC, adenomatous polyposis coli; BALF, bronchoalveolar fluid; C1QBP, complement C1q binding protein; CAPS1, calcium-dependent activator protein secretion factor 1; CD44v6, CD44 variant isoform 6; CEA, carcinoembryonic antigen; CKAP4, cytoskeleton-associated protein 4; CPNE3, Copine 3; Del-1, developmental endothelial locus-1; DKK1, dickkopf-related protein 1; DKK4, dickkopf-related protein 4; EGFR, epidermal growth factor receptor; EMT, epithelial mesenchymal transition; EpCAM, epithelial cell adhesion molecule; EphA2, ephrin type-A receptor 2; ERK1/2, extracellular signal-regulated kinases 1/2; FAK, focal adhesion kinase; GGT1, gamma-glutamyl transferase 1; GPC1, glypican 1; GRB2, growth factor receptor-bound protein 2; GRKs, G-protein-coupled receptor kinases; HER2, human epidermal growth factor receptor-2; HIF-1a, hypoxia-inducible factor-1a; Hsp60, heat shock protein-60; Hyal 1, hyaluronidase 1; IGF-1R, insulin-like growth factor 1 receptor; LRG1, leucine-rich alpha-2 glycoprotein 1; MIF, macrophage migration inhibitory factor; MMP, metallopeptidase; MUC1, mucin-1; NFX1, nuclear transcription factor, X-box binding 1; PAI-1, plasminogen activator inhibitor 1; PCA-3, prostate cancer gene-3; PCICs, pancreatic cancer-initiation cells; PDAC, pancreatic ductal adenocarcinoma; PKG1, cGMP-dependent protein kinase 1; PKM2, pyruvate kinase M2; PLD, phospholipase D; PrP, prion protein; PSA, prostate specific antigen; PSMA, prostate specific membrane antigen; Rab27a, Ras-related protein; RALGAPA2, Ral GTPase-activating protein subunit alpha-2; STX2, syntaxin 2; TIM-1, tissue inhibitor of metalloproteases 1; TJ-Cld7, claudin7 in tight junction; TJP2, tight junction protein 2; TME, tumor microenvironment; TNC, tenascin-c; TRAF3IP2, TRAF3 interacting protein 2; Treg, regulatory T cells; TSP1, thrombospondin-1; TSPAN8, tetraspanin 8; TTLL4, tubulin tyrosine ligase like 4; VEGF, vascular endothelial growth factor; ZIP4, zinc transporter.
Lung cancer
Lung cancer is one of the most fatal malignancies and the leading cause of tumor mortality worldwide [1]. The poor survival rates of lung cancer are mainly due to late-stage diagnosis. Thus, it is gaining growing interest to develop new strategies for early detection/diagnosis of lung cancer and novel targeted therapies. In this regard, lung cancer derived-exosomes may provide new insights since they play a pivotal role in regulating physiological functions of surrounding tissue cells and tumor microenvironment (Table 1).
The expression levels of epidermal growth factor receptor (EGFR) in plasma exosome were different between lung cancer patients and healthy individuals. It was also observed that exosomal EGFR in lung cancer induces tumor antigen-specific regulatory T cells (Treg) to inhibit the function of tumor-specific CD8+ T cells, thus accelerating lung cancer progression [61,62]. In particular, proteins associated with signal transduction (i.e., growth factor receptor-bound protein 2 (GRB2), proto-oncogene tyrosine kinase Src (Src) and EGFR), are enriched in plasma exosomes of non-small cell lung cancer (NSCLC). These proteins can actively regulate recipient cells proliferation [63]. In addition, according to liquid biopsy results of urine samples, EGFR or leucine-rich alpha-2 glycoprotein 1 (LRG1) were identified at a remarkably higher expressions in NSCLC patients [64], and could be used as non-invasive diagnosis urinary biomarkers for detecting NSCLC [65].
Exosomes contain enriched amounts of cell-specific markers from endosomal origin, such as tetraspanins CD9, CD63, and CD81. In lung cancer, tetraspanins CD151, CD171 and tetraspanin 8 (TSPAN8) were found in exosomes derived from lung cancer tissues, which were applied as another class of biomarkers to distinguish different pathological types of lung cancer [66]. For example, exosomal CD151 and TSPAN8 were demonstrated in vitro to modulate extracellular matrix and the associated molecules, thus initiate the metastatic process [67]. These proteins are expressed at a significantly higher level in NSCLC patients as compared to healthy individuals [24]. In addition, the serum-released exosomal membrane protein CD91 was also used as a detection index of lung adenocarcinoma [68] and can also act as a reliable biomarker in diagnosing NSCLC [69]. Exosomal CD5L protein expression was detected to be associated with tumor tissues in clinic, suggesting that CD5L may be another potential biomarker for non-invasive diagnosis of NSCLC [70].
Recently, another well-known cancer biomarker, mucin-1 (MUC1), was also found to be sensitive in distinguishing NSCLC patients from healthy counterparts [71]. Additionally, mimecan, cystatin-SA, transforming protein RhoA, thrombospondin-1, protein lifeguard 3, azurocidin and several other exosomal proteins were identified as potential biomarkers for detection of lung cancer (reviewed in [72]). Furthermore, exosome membrane-bound proteins NY-ESO-1, PLAP, Alix and EpCam were verified to be highly correlated to NSCLC overall survival, providing evidence that these proteins may work as prognostic biomarkers for lung cancer [73]. Exosomes from irradiated lung cancer cells regulated the motility of recipient cells by accelerating glycolytic process, where the two metabolic enzymes, exosomal fructose-bisphosphate aldolase (ALDOA) and aldehyde dehydrogenase 3-A1 (ALDH3A1) proteins are elevated and work as important signaling regulators [74]. Moreover, exosomes from lung cancer bronchoalveolar fluid (BALF) promote the migration and invasion of A549 cancer cells by carrying E-cadherin on the surface of exosomes, which provides evidence that E-cadherin may act through a vascular endothelial (VE)-cadherin dependent mechanism to induce lung cancer metastasis [75].
Breast cancer
Recently, female breast cancer has surpassed lung cancer as the most commonly diagnosed cancer worldwide [1]. It is urgent developing therapeutic strategies for early detection and monitoring of breast cancer [76]. As a well-known key regulator in breast cancer, human epidermal growth factor receptor-2 (HER2) was detected in the plasma exosomes and was applied as a non-invasive biomarker in the molecular classification of tumor tissues [77]. In addition, the levels of exosomal fibronectin and developmental endothelial locus-1 (Del-1) were significantly higher in breast cancer patients [78,79]. Strikingly, the plasma levels of both Del-1 and fibronectin almost returned to normal after tumor resection, which suggested that fibronectin and Del-1 may serve as important diagnostic markers to identify patients at different stages and also as prognostic markers for breast cancer treatment (Table 1).
The universal markers CD24 in exosomes has also emerged as a diagnostic indicator of breast cancer [80]. CD24 may have the potential to be used in identification of breast cancer-derived exosomes in pleural effusions and ascites of the patients. Notably, CD47 is another cancer-related surface protein highly expressed in circulating exosomes from breast cancer patients, which facilitates tumor progression by preventing innate immune recognition of cancer cells [81]. Recently, another exosomal tetraspanin CD82 was also detected to be significantly abundant in the serum of breast cancer patients and corresponding cancer tissues [82]. Thus, CD82 may play a key role in malignant breast cancer progression, and can act as an exosome-based biomarker for breast cancer monitoring and diagnosis.
The events of protein phosphorylation usually provide clues about disease status [83]. However, few phosphoproteins in biofluids have been reported as disease markers due to their highly dynamic nature as well as the presence of active phosphatases in biofluids [77]. Several exosome encapsulated phosphoproteins, such as cGMP-dependent protein kinase 1 (PKG1), Ral GTPase-activating protein subunit alpha-2 (RALGAPA2), nuclear transcription factor, X-box-binding protein 1 (NFX1) and tight junction protein 2 (TJP2) are significantly upregulated in breast cancer patients [84], suggesting that they may be employed as a novel type of biomarkers for breast cancer.
Glypican 1 (GPC1) is a lipid raft heparan sulfate proteoglycan located on the cell surface that induces cellular division, differentiation and morphogenesis. GPC1 is specifically enriched on cancer cell-derived exosomes. It was observed that GPC1 levels were elevated on exosomes from breast cancer cells, suggesting a potential use of this exosomal biomarker to identify early breast cancer [85]. As an anti-apoptosis protein, survivin was proved to have diagnostic significance in breast cancer. While survivin-2B, an alternative splice variant of survivin, is a pro-apoptotic protein. Differential expression of both survivin and survivin-2B proteins was found in exosomes from breast cancer patient serum, representing the splice variant pattern in breast cancer tissues [86]. Furthermore, high expression level of exosomal annexin A2 (exo-AnxA2) in triple-negative breast cancer (TNBC) is proved to be closely related to tumor grade, poor overall and disease-free survival, which is attributed to the effect of exo-AnxA2 in promoting angiogenesis. Therefore, exo-AnxA2 represents another potential prognostic biomarker and therapeutic target of TNBC [87].
Recently, a number of specific biomarkers emerged as a new category of potential breast cancer related exosomal protein markers. Tubulin tyrosine ligase like 4 (TTLL4)-mediated microtubule polyglutamylation alters exosome homeostasis by regulating trafficking of MVBs. The TTLL4-derived exosomes produced a pre-metastatic niche for breast cancer cells [88]. Ras-related protein Rab27a, a key player in exosome release, and TRAF3 Interacting Protein 2 (TRAF3IP2), an inflammatory mediator, were both involved in development and metastasis of breast cancer in vivo [89]. While thrombospondin-1 (TSP1) was found to be highly expressed in MDA-MB-231-derived exosomes, which facilitates the trans-endothelial migration of breast cancer cells via disrupting the intercellular integrity of endothelial cells [90].
Pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) is a type of exocrine pancreatic cancer that accounts about 95% of all pancreatic tumors [91]. PDAC remains one of the most devastating gastrointestinal malignancies with poor prognosis and an overall 5-year survival rate of 8%-9% [92]. The lack of accurate diagnostic tests and failure of conventional treatment brings great challenges for developing effective pancreatic cancer therapeutic strategies [92]. Pancreatic cancer-derived exosomes contain various protein molecules (Table 1) that can activate surrounding stromal cells and induce extracellular matrix (ECM) remodeling [93,94]. This further establishes a TME to facilitate metastasis.
Macrophage migration inhibitory factor (MIF) is highly expressed in PDAC-derived exosomes to initiate liver pre-metastatic niche formation and subsequent liver metastasis [95]. These findings suggest that exosomal MIF may be a prognostic marker for the development of hepatic metastasis in pancreatic cancer [96]. Additionally, GPC1 was also isolated from serum exosomes of pancreatic cancer mouse models and pancreatic cancer patients through flow cytometry, exhibiting potentials as both a serological marker and a preoperative and postoperative prognostic index at early and terminal stages of PDAC [97]. This provides high accuracy and sensitivity, thus can be further applied as a detection index for related therapies.
CD44 variant isoform 6 (CD44v6) is a transmembrane protein that was highly expressed in exosomes released by pancreatic cancer-initiation cells (PCICs). PCICs-derived CD44v6-positive exosomes could activate Wnt/β-catenin signaling and up-regulate the expression of plasminogen activator inhibitor 1 (PAI-1) and tissue inhibitor of metalloproteases 1 (TIM-1), thus promoting the migration and invasion of pancreatic cancer cells [98,99]. In another study, exosome-delivered CD44v6/complement C1q binding protein (C1QBP) complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment [100]. Another potential biomarker is tetraspanin 8 (Tspan8), which belongs to tetraspanin protein family. Tspan8-enriched exosomes produced by pancreatic cancer cells can induce VEGF-independent angiogenesis around tumor tissues [101]. In addition, CD151- and Tspan8-postive exosomes were proved to induce EMT, ECM remodeling and pro-inflammatory effect [102] further promote pancreatic tumor progression and metastasis [103].
As for other types of membrane proteins, for instance, by modulating the composition of exosomal transporters and affecting the function of PCICs-derived exosomes, claudin7 in tight junction (TJ-Cld7)-positive exosomes are capable to induce cell migration [104]. While myoferlin can mediate the inclusion of VEGF into exosomes to promote tumor growth and angiogenesis [105]. In addition, integrins-containing exosomes cause pancreatic cancer organotropic metastasis [48]. Integrin β4 mediates the transfer of plectin into exosomes leading to the proliferation, migration, and invasion of pancreatic cells [106]. Zinc transporter ZIP4-positive exosomes, produced by highly metastatic pancreatic cancer cells, can stimulate the proliferation, migration, and invasion of non-metastatic pancreatic cancer cells [107].
Recently, cell survival protein survivin was also found in PDAC cells-derived exosomes to enhance PDAC cell survival and was also highly enriched in exosomes isolated from the serum of PDAC patients [108]. In addition, high expression of exosomal ephrin type-A receptor 2 (Exo-EphA2) in recurrent pancreatic cancer was associated with shorter recurrence-free survival, indicating that high expression of serum Exo-EphA2 represents a novel indication for poor prognosis in patients [109]. Exosomal Tenascin-c (Exo-TNC) was observed to be closely associated with malignant features of pancreatic cancer cells by inducing local invasion and distant metastasis [110]. Moreover, cytoskeleton-associated protein 4 (CKAP4), a novel Dickkopf1 (DKK1) receptor, can also work as a candidate for PDAC diagnosis and therapy prediction. As observed, the secretion of CKAP4-containing exosomes is mediated by DKK1-dependent endocytosis routes [111].
Colorectal cancer
Colorectal cancer is a heterogeneous malignancy with complex carcinogenic mechanisms and aggressive metastasis at later stages, which is the third most common malignancy and the third-leading cause of cancer-related deaths globally [1]. Although great efforts have been made to promote the management of this cancer type, the prognosis of colorectal cancer patients is far from satisfactory. Plenty of experiments demonstrated that colorectal cancer exosomes played a critical role in maintaining cancer cell survival, proliferation and invasion of microenvironment. Therefore, identification of promising diagnostic exosome-related biomarkers (Table 1) would help to explore the underlying mechanisms of colorectal cancer and further promote the development of optimal therapeutic strategies.
Tumor suppressor, adenomatous polyposis coli (APC) is the most commonly mutated protein in colorectal cancer [112], which leads to cancer occurrence and progression. Based on a comparative study of the exosomal proteome between APC overexpression and normal SW480 cells, dickkopf-related protein 4 (DKK4) was identified as a potential exosomal biomarker specifically related to irregulated APC function [112]. While in another study, Wnt4 containing vesicles was delivered to normoxic colorectal cells which activated Wnt/β-catenin pathway to induce cancer cell migration and invasion [113]. Additionally, copine 3 (CPNE3), a membrane-binding protein, is highly expressed in tissues and plasma of patients with colorectal cancer [114]. Moreover, heat shock protein-60 (Hsp60) was observed to be accumulated on the membrane of colorectal cancer cell derived exosomes as well as in peri-cancerous tissues [115], which suggests that Hsp60 positive exosomes may be a novel marker of colorectal cancer [116].
GPC1 is a well-established biomarker in cancer-derived exosomes [97]. Application of GPC1 as a diagnostic marker for colorectal cancer has also been reported. It turned out that GPC1 protein expression in exosomes from plasma of colorectal cancer patients was significantly decreased after surgery [117]. Similarly, CD9 and CD147 positive exosomes were abundant in colorectal cancer patient serum by “ExoScreen” (a tool for detection of exosomes) and the CD147 level dropped after surgery of tumor resection [118]. In another study, serum exosomal carcinoembryonic antigen (CEA) was shown to predict metastatic colorectal cancer with a superior sensitivity and accuracy than serum CEA [119].
Recently, cellular prion protein (PrP)-expressing exosomes were found to promote the microenvironment of metastasis via increase of endothelial permeability and angiogenic cytokine secretion. The hypoxic TME of colorectal cancer increased the PrP-expressing exosome secretion, and the expression of PrP in turn regulated the colorectal tumor progression [120]. Another potential biomarker, calcium-dependent activator protein secretion factor 1 (CAPS1), was detected to be overexpressed in exosomes secreted by colorectal cancer cells that promoted normal epithelial cell migration to regulate metastasis [121]. In addition, syntaxin 2 (STX2), a type of membrane integrated SNARE proteins participating in exocytosis, was found to play a regulatory role on increasing expression of Exosome Complex 4 (EXOSC4), which further drives the proliferation of colorectal cancer [122].
Prostate cancer
Prostate cancer is the most common solid tumor in men and patients with metastatic prostate cancer have relatively high mortality rates [1]. The proteins transferred by exosomes (Table 1) derived from cancer cells to weakly invasive cells have been characterized to play a crucial role in monitoring prostate cancer progression and metastasis increase [123]. For instance, prostate cancer progression was linked to hypoxia and the induction of hypoxia-inducible factor (HIF). The exosomal HIF-1a promotes the occurrence and progression of metastasis via repression of E-cadherin [124].
Integrins on exosomes secreted by prostate cancer cells (integrin α3, β1, ανβ6, ανβ3, etc.) induced the progression and invasion of integrin-negative cells (with no integrin secretion) or epithelial cells [125,126]. For example, exosome-mediated integrin α2 was found to promote the migration and invasion of prostate cancer cells by inducing EMT [127], while integrin α3 could promote inflammation, migration and invasion [128], and similar effect was observed for integrin β4 [129]. In addition, integrin ανβ3 was delivered to TME to activate Src phosphorylation in recipient cells. Integrin ανβ3 present in prostate cancer-derived exosomes may also induce formation of metastatic niche to alter angiogenesis and cell signaling [48].
Exosomal pyruvate kinase M2 (Exo-PKM2) was observed to induce the occurrence of a pre-metastatic niche, thus promoting the bone metastasis of prostate cancer [130]. Similarly, phospholipase D (PLD) in prostate cancer-derived exosomes stimulated the osteoblast activity of exosomes, which may be considered as a potent regulator in bone metastasis establishment [131]. Another prostate cancer-derived exosomal protein, hyaluronidase 1 (Hyal 1) stimulates the mobility of prostate stromal cells thereby enhances the metastatic potential [132]. Exosomal caveolin-1 promotes the invasion and metastasis of prostate cancer cells in an endocrine manner through the NF-κB signaling pathway [133], and exosomal matrix metallopeptidase 9 and 14 (MMP-9 and MMP-14) act by stimulating ERK1/2 phosphorylation [134,135]. Other exosomal proteins, such as Src, insulin-like growth factor 1 receptor (IGF-1R), G-protein-coupled receptor kinases (GRKs) and focal adhesion kinase (FAK), induce prostate cancer angiogenesis via VEGF transcription stimulation in the TME [136].
Recently, serum exosomal gamma-glutamyl transferase 1 (GGT1), a cell surface enzyme, was present with high expression and activity in prostate cancer patients, which may serve as a novel diagnostic marker to screen this cancer type [137]. Investigation of the urinary exosome proteome from prostate cancer patients, identified β-catenin, prostate cancer gene-3 (PCA-3), prostate specific antigen (PSA), and prostate specific membrane antigen (PSMA), which shows the potential for diagnosis and monitoring of prostate cancer [138]. The expression of epithelial cell adhesion molecule (EpCAM), epidermal growth factor receptor (EGFR), survivin, were also observed to be significantly increased in exosomes derived from prostate cancer cells [139].
Exosome protein profiling as therapeutic targets for cancer treatment
The above-mentioned exosomal proteins (Table 1) play an important role in cancer invasion and metastasis through different mechanisms of action. Thus, it is conceivable that these exosomal proteins could serve as promising therapeutic targets. Based on these strategies, numerous agents, diagnostic protocols and clinical assays for anti-tumoral therapy were under development to regulate the exosome functions for therapeutic applications (Table 2) [140,141].
Table 2.
Functions of exosomal proteins in transmitting related drug resistance in cancer
| Drug name | Cancer Type | Exosomal Protein | Related Mechanism | Ref. |
|---|---|---|---|---|
| Cisplatin | Ovarian cancer | AnxA3 | Enhanced secretion of exosomes | [151] |
| Ovarian cancer | CLPTM1L | Ectodomain-dependent way | [152] | |
| NSCLC | PKM2 | Promote glycolysis to neutralize ROS; inhibit apoptosis; reprogram CAFs to affect TME | [153] | |
| 5-Fluorouracil | Colorectal cancer | IDH1 | Mediate NADPH decrease | [154] |
| GDF15, DPP4 | Induce POSTN-Smad signaling | [155,156] | ||
| p-STAT3 | Related to caspase cascade | [157] | ||
| PrP | Hypoxic-exosomal tumor progression | [120] | ||
| Gemcitabine | Breast cancer | EphA2 | Activate ERK1/2 signaling | [158] |
| Pancreatic cancer | EphA2 | Transmit related chemoresistance | [159] | |
| TNBC | AnxA6 | Inhibit EGFR ubiquitination and degradation | [160] | |
| Imatinib | Leukemia | IFITM3, CD146, CD36 | Regulate surface localization | [161] |
| Osimertinib | NSCLC | EGFR | Induce intercellular transfer | [162] |
| ALK-TKIs | ALK-positive NSCLC | Tim-3, Gal-9 | Clinical data of plasma exosome | [163] |
| Taxane | Prostate cancer | Integrin β4, vinculin | Enhance cancer cell migration and invasion | [129] |
| Paclitaxel | PDAC | Survivin | Compromised the effectiveness of paclitaxel with or without ERK inhibitor/chloroquine | [108] |
| Docetaxel | Prostate cancer | MDR-1/P-gp | Transmit related chemoresistance | [164] |
| Celecoxib | Lung cancer | COX-2 | Increase PGE2 and VEGF production to affect TME | [166] |
| Trastuzumab | HER2+ breast cancer | HER2 | HER2 overexpressing exosomes | [167,168] |
| Enzalutamide | Prostate cancer | Syntaxin 6 | Increase CD63 colocalization | [169] |
Abbreviations: ALK, anaplastic lymphoma kinase; ALK-TKIs, ALK-tyrosine-kinase-inhibitors; AnxA3, annexin 3; AnxA6, annexin A6; CAFs, cancer-associated fibroblasts; CLPTM1L, cleft lip and palate transmembrane protein 1-like; COX-2, cyclooxygenase-2; DPP4, dipeptidyl peptidase IV; EphA2, ephrin type-A receptor 2; ERK1/2, extracellular signal-regulated kinases 1/2; Gal-9, galectin-9; GDF15, growth/differentiation factor 15; HER2, human epidermal growth factor receptor 2; IDH1, isocitrate dehydrogenase 1; IFITM3, interferon-induced transmembrane protein 3; MDR-1, multidrug-resistance gene 1; MT, microtubule; PDAC, pancreatic ductal adenocarcinoma; PGE2, prostaglandin E2; P-gp, P-glycoprotein; POSTN, periostin; PrP, prion protein; p-STAT3, phosphorylated signal transducer and activator of transcription 3; ROS, reactive oxygen species; Tim-3, T-cell immunoglobulin- and mucin-domain-containing molecule 3; TKIs, tyrosine kinase inhibitors; TNBC, triple-negative breast cancer; VEGF, vascular endothelial growth factor.
Inhibiting the production of cancer-derived exosomes
The internalization of exosomes by recipient cells often depends on the source and amount of secreted exosomes. A number of compounds have been developed to inhibit TDEs production by targeting different proteins or different stages of exosome biogenesis process, such as RAB27A inhibitors, protein-protein interaction (PPI) inhibitors and calcium channel blocking agents. The mechanisms of these inhibitors are diverse. In addition, further research showed that some clinical therapies for other diseases, like tipifarnib, ketoconazole, cambinol and simvastatin, are also capable to inhibit exosome release in cancer [142]. It is worthy to note that these compounds only affect the exosome release from tumor cells but not from normal cells, which may intrigue a new direction of drug development [143].
Blocking the uptake of cancer-derived exosomes
Besides inhibiting exosome release, another promising strategy for exosome-targeted therapy is blocking the uptake of exosomes by recipient cells via inhibition of membrane fusion, endocytosis, and micropinocytosis [144]. That can efficiently reduce the pro-tumorigenic effect of exosomes. In a cervical cancer model, annexin V treatment prevented phosphatidylserine assisted internalization of exosomes [145]. In addition, targeting protein ligands on exosome surface, such as integrins, tetraspanins, immunoglobulins, lectins, and glycoproteins is another effective strategy to block exosome uptake [146]. For example, TDEs often regulate the formation of pre-metastatic niches through the binding of integrins on TDEs membrane to target cells. Therefore, targeting integrins α6β4 and αvβ5 can decrease exosome uptake and repress lung and liver metastasis [48]. Another type of biomarker, heparan sulfate proteoglycans (HSPGs) serve as internalization receptors for TDEs to induce exosome internalization and other functional activity. The uptake of these internalized HSPGs enriched exosomes could be specifically inhibited by free heparan sulfate (HS) chains. This suggests that targeting key biomarker (i.e., HSPGs) could eventually inhibit TDEs transport further repress the TDEs-related cancer progression [147].
Targeting tumor exosomal proteins to overcome drug resistance
The progression of multidrug resistance is the major obstacle to maintain effective chemotherapy in cancer [148]. Exosome secretion has remarkable influence on numerous signaling networks, which plays a pivotal role in the cancer sustenance. According to the large-scale proteomic analysis of tumor derived exosomes, the important roles of tumor stroma-derived exosomes in inducing both de novo and acquired anti-tumor drug resistance have been uncovered [149]. Thus, targeting specific functions of exosomes provides a proof of concept to prevent and reverse the drug resistance [150].
Exosomes protect cancer cells from the cytotoxic effects of chemotherapy drugs and transfer chemoresistance properties to nearby cells. In particular, exosomal membrane protein or receptors induce drug resistance mainly through regulating specific signal pathway. For example, chemoresistance in castration-resistant prostate cancer was attributed to exosomal caveolin-1 that can elicit NF-κB cascade to affect EMT and cancer stem cell phenotype [133]. The exosomal proteins may act as transmitters or drivers of drug resistance in a variety of cancer types, which provides a promising way to optimize drug response and also encourages implications for the use of new targeted biologics in the treatment of therapy-resistant tumors.
Alkylating agent resistance
In a cisplatin resistant ovarian cancer model, the development of cisplatin resistance was directly correlated with enhanced exocytosis and release of exosomes due to annexin 3 protein expression in exosomes [151]. In the same cell model, the increased levels of exosomal cleft lip and palate transmembrane protein 1-like (CLPTM1L) upon chemotherapy treatment may also confer cisplatin resistance [152]. While in NSCLC, hypoxia-induced exosomes transmit cisplatin resistance to drug-sensitive cells by delivering PKM2, so that exosomal PKM2 may serve as a promising biomarker and therapeutic target for cisplatin resistance [153].
Antimetabolites drug resistance
Exosomes secreted from 5-fluorouracil (5-FU)-resistant colorectal cancer cells transfer a high level of isocitrate dehydrogenase 1 (IDH1, also named NADP+) protein which initiates the resistance of 5-FU-sensitive cells. This effect was attributed to a decreased level of NADPH mediated by IDH1 [154]. In another study, exosomal growth/differentiation factor 15 (GDF15) increased periostin (POSTN) level via Smad signaling to enhance angiogenesis [155]. Subsequent studies found that exosomal dipeptidyl peptidase IV (DPP4) was also a potent inducer of POSTN-Smad signaling pathway. Both GDF15 and DPP4 can be targets for anti-angiogenic therapies [156]. In a similar study, phosphorylated STAT3 (p-STAT3) packaged by exosomes contributed to acquired 5-FU resistance in vitro and in vivo [157]. While in another colorectal cancer murine xenograft model, the expression of Exo-PrP was found to be the key factor related to 5-FU resistance in vivo [120].
The increase of EphA2 in drug-resistant cell-derived exosomes may support an additional mechanism of gemcitabine resistance. As observed in breast cancer cells, the EphA2-Ephrin A1 reversed activated ERK1/2 signaling to promote breast cancer progression [158]. In another model of pancreatic cancer, EphA2 expression could transmit gemcitabine chemoresistance and may serve as a minimally-invasive predictive biomarker for the treatment response [159]. In addition, exosomal annexin A6 (AnxA6) levels in the serum of TNBC patients can be another predictor for gemcitabine resistance with a mechanism regarding to the inhibition of EGFR ubiquitination and degradation [160].
Tyrosine kinase inhibitors (TKIs) resistance
In a study of TKI resistance in chronic myelogenous leukemia (CML), TKI drug (imatinib) resistance was attributed to three surface markers on exosomes released by imatinib-resistant leukemia cells, which were interferon-induced transmembrane protein 3 (IFITM3), CD146 and CD36 [161]. In a NSCLC model, the intercellular transfer of exosomal EGFR represented a novel resistant mechanism of a type of EGFR-TKI, osimertinib [162]. While in anaplastic lymphoma-kinase (ALK)-positive NSCLC patients, a decreased plasma exosome Tim-3 and Galectin-9 levels was shown to be an indication of the resistance response of first generation ALK-TKIs [163].
Microtubule-interfering drug resistance
In taxane-resistant prostate cancer cells, integrin β4 and vinculin were upregulated in exosomes. This provides a basis to develop integrin β4 and vinculin as useful markers for cancer progression with taxane-resistance and further potentiates the establishment of an exosome-based diagnostic system [129]. In KRAS-dependent cancer cells (such as PDAC), survivin enriched exosomes significantly compromised the effectiveness of paclitaxel and the combination of ERK inhibitor with chloroquine (a novel clinical trial for PDAC) [108]. Moreover, transfer of multi-drug resistant proteins to drug-sensitive cells could confer the drug-resistant properties, such as P-glycoprotein (P-gp) [164]. Using a prostate cancer model, resistance to docetaxel was attributed to the enhanced exosome secretion and transporter protein P-glycoprotein (MDR-1/P-gp) exosomal transfer [165]. Extensive and in-depth studies are required to further explain how exosomes mediate and transmit related chemoresistance of microtubule-interfering agents in cancer.
Other drug resistance
The induced expression of COX-2 in lung cancer-derived exosomes by celecoxib treatment was transferred to other cells, resulting in an increased prostaglandin E2 (PGE2) and VEGF production further affecting the tumor microenvironments [166]. In a HER2-positive breast cancer cell model, the resistance to trastuzumab was linked to the secretion of HER2 overexpressing exosomes [167]. Meanwhile, removal of HER2 positive exosomes improved patient responses to trasuzumab [168]. These studies indicated that HER2 could be a useful biomarker for anticipating drug-resistance during treatment. In another enzalutamide-resistant prostate cancer cell model, the upregulation of syntaxin 6 and the increased CD63 colocalization suggested that syntaxin 6 modulated secretion of exosomes to enhance the enzalutamide resistance [169].
Conclusion and future perspectives
As an important tool for intercellular communication and transport, exosomes mediate cell-to-cell information exchange by transmitting their cargos (RNAs, proteins, etc.) to recipient cells and affect several physiological functions of recipient cells. Exosomes provide abundant, stable and specific biological information and are considered as an attractive liquid biopsy specimen with high application values. Exosome-shuttled proteins and nucleic acids have been suggested as novel diagnostic and prognostic indicators for a variety of cancers. Apart from the genetic molecules, exosome-associated proteins have also been broadly examined as potential disease-related biomarkers.
Currently, exploring biomarkers in TDEs has shown great potential but still with obvious limitations. The first and most important limitation is the lack of standardized exosome isolation and characterization techniques to ensure a consistent and reproducible exosome supply. Due to the lack of adequate analysis platforms, the comprehensive assessment of clinically relevant exosomes among miscellaneous populations of cells or body fluid remains challenging. Most of identified functional roles of exosomes are based on in vitro results of isolated exosomes that have limited physiologically relevance under pathological conditions in vivo. Thus, exploring the precise physiological function of exosomes in vivo will be critical to determine their roles in cancer. To establish the diagnostic accuracy of exosomes, the observational properties of identified exosome-derived proteins biomarkers need to be further validated in large, longitudinal studies. More tools are being exploited to uncover the molecular nature of exosomes. Further development of cancer exosomal proteomics, microfluidic techniques and other techniques for exosomal protein isolation and detections will be highly required for the improvement of cancer diagnosis.
Targeting exosomal cargos expresses high diagnostic and prognostic potential in cancer. However, a great deal of research is needed to understand the mechanisms involving in how exosome or exosomal proteins mediate tumor progression. For instance, the majority of these studies analyzed one type of exosome biomarker at a specific stage without tracing across different stages of cancer. Indeed, exosomes can transfer both tumor-promoting molecules (e.g., oncoproteins) and tumor suppressors, indicating their complex roles in cancer biology. Further knowledge is needed to elucidate the signaling pathways and the exact mechanism of involvement of exosomes in tumorigenesis.
Despite many challenges, the non-invasive property features exosomes the next generation of biomarkers in cancer diagnosis. Exosomal proteins play key roles in monitoring exosome-mediated tumor migration, invasion and metastasis and tumor angiogenesis, thus possess a great potential in the transition of more relevant applications in clinic. There is still a long road ahead to revolutionize cancer diagnosis by exciting the potential of exosome biomarkers. By translating the knowledge of experimental and clinical observations into the clinical field, it will open up new therapeutic avenues in personalized diagnosis and precision medicine, and likely bring an optimistic future to cancer patients.
Acknowledgements
This work was supported by Foundation of Nanjing Xiaozhuang University (4172135, J.C.) and Start-Up Funds from China Pharmaceutical University (3150010105, Q.C.).
Disclosure of conflict of interest
None.
Abbreviations
- ALDH3A1
Aldehyde dehydrogenase 3-A1
- ALDOA
Fructose-bisphosphate aldolase
- ALK
Anaplastic lymphoma kinase
- ALK-TKIs
ALK-tyrosine-kinase-inhibitors
- AnxA2
Annexin A2
- AnxA3
Annexin 3
- AnxA6
Annexin A6
- APC
Adenomatous polyposis coli
- BALF
Bronchoalveolar fluid
- C1QBP
Complement C1q binding protein
- CAFs
Cancer-associated fibroblasts
- CAPS1
Calcium-dependent activator protein secretion factor 1
- CD44v6
CD44 variant isoform 6
- CEA
Carcinoembryonic antigen
- CKAP4
Cytoskeleton-associated protein 4
- CLPTM1L
Cleft lip and palate transmembrane protein 1-like
- COX-2
Cyclooxygenase-2
- CPNE3
Copine 3
- Del-1
Developmental endothelial locus-1
- DKK1
Dickkopf-related protein 1
- DKK4
Dickkopf-related protein 4
- DPP4
Dipeptidyl peptidase IV
- EGFR
Epidermal growth factor receptor
- EM
Electron microscopy
- EMT
Epithelial mesenchymal transition
- EpCAM
Epithelial cell adhesion molecule
- EphA2
Ephrin type-A receptor 2
- ERK1/2
Extracellular signal-regulated kinases 1/2
- ESCRT
Endosomal sorting complexes required for transport
- EVs
Extracellular vesicles
- FAK
Focal adhesion kinase
- FGF
Fibroblast growth factor
- Gal-9
Galectin-9
- GDF15
Growth/differentiation factor 15
- GGT1
Gamma-glutamyl transferase 1
- GPC1
Glypican 1
- GRB2
Growth factor receptor-bound protein 2
- GRKs
G-protein-coupled receptor kinases
- HER2
Human epidermal growth factor receptor-2
- HIF-1a
Hypoxia-inducible factor-1a
- Hsp60
Heat shock protein-60
- HRP
Horseradish peroxidase
- Hyal 1
Hyaluronidase 1
- IDH1
Isocitrate dehydrogenase 1
- IFITM3
Interferon-induced transmembrane protein 3
- IGF-1R
Insulin-like growth factor 1 receptor
- LRG1
Leucine-rich alpha-2 glycoprotein 1
- MDR-1
Multidrug-resistance gene 1
- MHC
Major histocompatibility complex
- MIF
Macrophage migration inhibitory factor
- miRNAs
MicroRNAs
- MMP
Metallopeptidase
- mRNAs
Message RNAs
- MT
Microtubule
- MUC1
Mucin-1
- MVBs
Multivesicular bodies
- ncRNAs
Non-coding RNAs
- NFX1
Nuclear transcription factor, X-box binding 1
- nPES
Nanoplasmon enhanced scattering
- NTA
Nanoparticle tracking analysis
- PAI-1
Plasminogen activator inhibitor 1
- PCA-3
Prostate cancer gene-3
- PCICs
pancreatic cancer-initiation cells
- PDAC
Pancreatic ductal adenocarcinoma
- PGE2
Prostaglandin E2
- P-gp
P-glycoprotein
- PKG1
CGMP-dependent protein kinase 1
- PKM2
Pyruvate kinase M2
- PLD
Phospholipase D
- PMN
Pre-metastatic niche
- POSTN
Periostin
- PrP
Prion protein
- PSA
Prostate specific antigen
- PSMA
Prostate specific membrane antigen
- p-STAT3
Phosphorylated signal transducer and activator of transcription 3
- Rab27a
Ras-related protein
- RALGAPA2
Ral GTPase-activating protein subunit alpha-2
- ROS
Reactive oxygen species
- STX2
Syntaxin 2
- TDEs
Tumor-derived exosomes
- TGF-β
Transforming growth factor β
- TIM-1
Tissue inhibitor of metalloproteases 1
- Tim-3
T-cell immunoglobulin- and mucin-domain-containing molecule 3
- TJ-Cld7
Claudin7 in tight junction
- TJP2
Tight junction protein 2
- TKIs
Tyrosine kinase inhibitors
- TME
Tumor microenvironment
- TNBC
Triple-negative breast cancer
- TNC
Tenascin-c
- TRAF3IP2
TRAF3 interacting protein 2
- Treg
regulatory T cells
- TSP1
Thrombospondin-1
- TSPAN8
Tetraspanin 8
- TTLL4
Tubulin tyrosine ligase like 4
- VEGF
Vascular endothelial growth factor
- ZIP4
Zinc transporter
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 3.Hingorani SR. Intercepting cancer communiques: exosomes as heralds of malignancy. Cancer Cell. 2015;28:151–153. doi: 10.1016/j.ccell.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Tang MK, Wong AS. Exosomes: emerging biomarkers and targets for ovarian cancer. Cancer Lett. 2015;367:26–33. doi: 10.1016/j.canlet.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 8.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 11.Ikonen E. Roles of lipid rafts in membrane transport. Curr Opin Cell Biol. 2001;13:470–477. doi: 10.1016/s0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 12.Tan SS, Yin Y, Lee T, Lai RC, Yeo RW, Zhang B, Choo A, Lim SK. Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J Extracell Vesicles. 2013;2:22614. doi: 10.3402/jev.v2i0.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soo CY, Song Y, Zheng Y, Campbell EC, Riches AC, Gunn-Moore F, Powis SJ. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology. 2012;136:192–197. doi: 10.1111/j.1365-2567.2012.03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kucharzewska P, Belting M. Emerging roles of extracellular vesicles in the adaptive response of tumour cells to microenvironmental stress. J Extracell Vesicles. 2013;2:20304. doi: 10.3402/jev.v2i0.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins VR, Dias MS, Hainaut P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr Opin Oncol. 2013;25:66–75. doi: 10.1097/CCO.0b013e32835b7c81. [DOI] [PubMed] [Google Scholar]
- 18.Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SIJ, Wurdinger T, Pegtel DM, van Rheenen J. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, Le Pecq JB. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270:211–226. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 20.Liang Y, Lehrich BM, Zheng S, Lu M. Emerging methods in biomarker identification for extracellular vesicle-based liquid biopsy. J Extracell Vesicles. 2021;10:e12090. doi: 10.1002/jev2.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips W, Willms E, Hill AF. Understanding extracellular vesicle and nanoparticle heterogeneity: novel methods and considerations. Proteomics. 2021;21:e2000118. doi: 10.1002/pmic.202000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arraud N, Linares R, Tan S, Gounou C, Pasquet JM, Mornet S, Brisson AR. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12:614–627. doi: 10.1111/jth.12554. [DOI] [PubMed] [Google Scholar]
- 23.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for extracellular vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorgensen M, Baek R, Pedersen S, Sondergaard EK, Kristensen SR, Varming K. Extracellular vesicle (EV) array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J Extracell Vesicles. 2013;2:20920. doi: 10.3402/jev.v2i0.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F, Liao X, Tian Y, Li G. Exosome separation using microfluidic systems: size-based, immunoaffinity-based and dynamic methodologies. Biotechnol J. 2017;12:1600699. doi: 10.1002/biot.201600699. [DOI] [PubMed] [Google Scholar]
- 26.Ibsen SD, Wright J, Lewis JM, Kim S, Ko SY, Ong J, Manouchehri S, Vyas A, Akers J, Chen CC, Carter BS, Esener SC, Heller MJ. Rapid isolation and detection of exosomes and associated biomarkers from plasma. ACS Nano. 2017;11:6641–6651. doi: 10.1021/acsnano.7b00549. [DOI] [PubMed] [Google Scholar]
- 27.Liang K, Liu F, Fan J, Sun D, Liu C, Lyon CJ, Bernard DW, Li Y, Yokoi K, Katz MH, Koay EJ, Zhao Z, Hu Y. Nanoplasmonic quantification of tumor-derived extracellular vesicles in plasma microsamples for diagnosis and treatment monitoring. Nat Biomed Eng. 2017;1:0021. doi: 10.1038/s41551-016-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang P, Zhou X, He M, Shang Y, Tetlow AL, Godwin AK, Zeng Y. Ultrasensitive detection of circulating exosomes with a 3D-nanopatterned microfluidic chip. Nat Biomed Eng. 2019;3:438–451. doi: 10.1038/s41551-019-0356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moura SL, Martin CG, Marti M, Pividori MI. Electrochemical immunosensing of nanovesicles as biomarkers for breast cancer. Biosens Bioelectron. 2020;150:111882. doi: 10.1016/j.bios.2019.111882. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Gimple RC, Lau WB, Lau B, Fei F, Shen Q, Liao X, Li Y, Wang W, He Y, Feng M, Bu H, Wang W, Zhou S. The present and future of the mass spectrometry-based investigation of the exosome landscape. Mass Spectrom Rev. 2020;39:745–762. doi: 10.1002/mas.21635. [DOI] [PubMed] [Google Scholar]
- 31.Pietrowska M, Funk S, Gawin M, Marczak L, Abramowicz A, Widlak P, Whiteside T. Isolation of exosomes for the purpose of protein cargo analysis with the use of mass spectrometry. Methods Mol Biol. 2017;1654:291–307. doi: 10.1007/978-1-4939-7231-9_22. [DOI] [PubMed] [Google Scholar]
- 32.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 35.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conigliaro A, Cicchini C. Exosome-mediated signaling in epithelial to mesenchymal transition and tumor progression. J Clin Med. 2018;8:26. doi: 10.3390/jcm8010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 39.Han L, Lam EW, Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol Cancer. 2019;18:59. doi: 10.1186/s12943-019-0980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao C, Yang F, Miao S, Liu W, Wang C, Shu Y, Shen H. Role of hypoxia-induced exosomes in tumor biology. Mol Cancer. 2018;17:120. doi: 10.1186/s12943-018-0869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulei D, Petrut B, Tigu AB, Onaciu A, Fischer-Fodor E, Atanasov AG, Ionescu C, Berindan-Neagoe I. Exosomes at a glance - common nominators for cancer hallmarks and novel diagnosis tools. Crit Rev Biochem Mol Biol. 2018;53:564–577. doi: 10.1080/10409238.2018.1508276. [DOI] [PubMed] [Google Scholar]
- 42.Jena BC, Mandal M. The emerging roles of exosomes in anti-cancer drug resistance and tumor progression: an insight towards tumor-microenvironment interaction. Biochim Biophys Acta Rev Cancer. 2021;1875:188488. doi: 10.1016/j.bbcan.2020.188488. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871:455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49:347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Kharaziha P, Ceder S, Li Q, Panaretakis T. Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta. 2012;1826:103–111. doi: 10.1016/j.bbcan.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 50.Wu M, Wang G, Hu W, Yao Y, Yu XF. Emerging roles and therapeutic value of exosomes in cancer metastasis. Mol Cancer. 2019;18:53. doi: 10.1186/s12943-019-0964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding WQ. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA) Mol Cancer. 2015;14:133. doi: 10.1186/s12943-015-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W, Li C, Zhou T, Liu X, Liu X, Li X, Chen D. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16:145. doi: 10.1186/s12943-017-0706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bottani M, Banfi G, Lombardi G. Circulating miRNAs as diagnostic and prognostic biomarkers in common solid tumors: focus on lung, breast, prostate cancers, and osteosarcoma. J Clin Med. 2019;8:1661. doi: 10.3390/jcm8101661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289:3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, Williams C, Rodriguez-Barrueco R, Silva JM, Zhang W, Hearn S, Elemento O, Paknejad N, Manova-Todorova K, Welte K, Bromberg J, Peinado H, Lyden D. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pontecorvi G, Bellenghi M, Puglisi R, Care A, Mattia G. Tumor-derived extracellular vesicles and microRNAs: functional roles, diagnostic, prognostic and therapeutic options. Cytokine Growth Factor Rev. 2020;51:75–83. doi: 10.1016/j.cytogfr.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33:441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 58.Penfornis P, Vallabhaneni KC, Whitt J, Pochampally R. Extracellular vesicles as carriers of microRNA, proteins and lipids in tumor microenvironment. Int J Cancer. 2016;138:14–21. doi: 10.1002/ijc.29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. 2015;9:358–367. doi: 10.1002/prca.201400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He M, Zeng Y. Microfluidic exosome analysis toward liquid biopsy for cancer. J Lab Autom. 2016;21:599–608. doi: 10.1177/2211068216651035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang SH, Li Y, Zhang J, Rong J, Ye S. Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Invest. 2013;31:330–335. doi: 10.3109/07357907.2013.789905. [DOI] [PubMed] [Google Scholar]
- 62.Peng XX, Yu R, Wu X, Wu SY, Pi C, Chen ZH, Zhang XC, Gao CY, Shao YW, Liu L, Wu YL, Zhou Q. Correlation of plasma exosomal microRNAs with the efficacy of immunotherapy in EGFR/ALK wild-type advanced non-small cell lung cancer. J Immunother Cancer. 2020;8:e000376. doi: 10.1136/jitc-2019-000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clark DJ, Fondrie WE, Yang A, Mao L. Triple SILAC quantitative proteomic analysis reveals differential abundance of cell signaling proteins between normal and lung cancer-derived exosomes. J Proteomics. 2016;133:161–169. doi: 10.1016/j.jprot.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Zhang Y, Qiu F, Qiu Z. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis. 2011;32:1976–1983. doi: 10.1002/elps.201000598. [DOI] [PubMed] [Google Scholar]
- 65.Jakobsen KR, Paulsen BS, Baek R, Varming K, Sorensen BS, Jorgensen MM. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles. 2015;4:26659. doi: 10.3402/jev.v4.26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sandfeld-Paulsen B, Jakobsen KR, Baek R, Folkersen BH, Rasmussen TR, Meldgaard P, Varming K, Jorgensen MM, Sorensen BS. Exosomal proteins as diagnostic biomarkers in lung cancer. J Thorac Oncol. 2016;11:1701–1710. doi: 10.1016/j.jtho.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 67.Yue S, Mu W, Zoller M. Tspan8 and CD151 promote metastasis by distinct mechanisms. Eur J Cancer. 2013;49:2934–2948. doi: 10.1016/j.ejca.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 68.Ueda K, Ishikawa N, Tatsuguchi A, Saichi N, Fujii R, Nakagawa H. Antibody-coupled monolithic silica microtips for highthroughput molecular profiling of circulating exosomes. Sci Rep. 2014;4:6232. doi: 10.1038/srep06232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niu L, Song X, Wang N, Xue L, Song X, Xie L. Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci. 2019;110:433–442. doi: 10.1111/cas.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi ES, Faruque HA, Kim JH, Kim KJ, Choi JE, Kim BA, Kim B, Kim YJ, Woo MH, Park JY, Hur K, Lee MY, Kim DS, Lee SY, Kim E. CD5L as an extracellular vesicle-derived biomarker for liquid biopsy of lung cancer. Diagnostics (Basel) 2021;11:620. doi: 10.3390/diagnostics11040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan D, Chen J, Feng C, Wu W, Wang Y, Tong J, Zhou D. Preferential localization of MUC1 glycoprotein in exosomes secreted by non-small cell lung carcinoma cells. Int J Mol Sci. 2019;20:323. doi: 10.3390/ijms20020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun Y, Liu S, Qiao Z, Shang Z, Xia Z, Niu X, Qian L, Zhang Y, Fan L, Cao CX, Xiao H. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Anal Chim Acta. 2017;982:84–95. doi: 10.1016/j.aca.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 73.Lobb RJ, van Amerongen R, Wiegmans A, Ham S, Larsen JE, Moller A. Exosomes derived from mesenchymal non-small cell lung cancer cells promote chemoresistance. Int J Cancer. 2017;141:614–620. doi: 10.1002/ijc.30752. [DOI] [PubMed] [Google Scholar]
- 74.Wang C, Xu J, Yuan D, Bai Y, Pan Y, Zhang J, Shao C. Exosomes carrying ALDOA and ALDH3A1 from irradiated lung cancer cells enhance migration and invasion of recipients by accelerating glycolysis. Mol Cell Biochem. 2020;469:77–87. doi: 10.1007/s11010-020-03729-3. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Liu Z, Li S, Wang M, Dai D, Jing H, Liu L. Upregulation of E-cadherin in bronchoalveolar lavage fluid-derived exosomes in patients with lung cancer. Thorac Cancer. 2020;11:41–47. doi: 10.1111/1759-7714.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Howard J, Wyse C, Argyle D, Quinn C, Kelly P, McCann A. Exosomes as biomarkers of human and feline mammary tumours; a comparative medicine approach to unravelling the aggressiveness of TNBC. Biochim Biophys Acta Rev Cancer. 2020;1874:188431. doi: 10.1016/j.bbcan.2020.188431. [DOI] [PubMed] [Google Scholar]
- 77.Fang S, Tian H, Li X, Jin D, Li X, Kong J, Yang C, Yang X, Lu Y, Luo Y, Lin B, Niu W, Liu T. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLoS One. 2017;12:e0175050. doi: 10.1371/journal.pone.0175050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moon PG, Lee JE, Cho YE, Lee SJ, Jung JH, Chae YS, Bae HI, Kim YB, Kim IS, Park HY, Baek MC. Identification of developmental endothelial locus-1 on circulating extracellular vesicles as a novel biomarker for early breast cancer detection. Clin Cancer Res. 2016;22:1757–1766. doi: 10.1158/1078-0432.CCR-15-0654. [DOI] [PubMed] [Google Scholar]
- 79.Moon PG, Lee JE, Cho YE, Lee SJ, Chae YS, Jung JH, Kim IS, Park HY, Baek MC. Fibronectin on circulating extracellular vesicles as a liquid biopsy to detect breast cancer. Oncotarget. 2016;7:40189–40199. doi: 10.18632/oncotarget.9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rupp AK, Rupp C, Keller S, Brase JC, Ehehalt R, Fogel M, Moldenhauer G, Marme F, Sultmann H, Altevogt P. Loss of EpCAM expression in breast cancer derived serum exosomes: role of proteolytic cleavage. Gynecol Oncol. 2011;122:437–446. doi: 10.1016/j.ygyno.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 81.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, Majeti R, Weissman IL. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Zhong W, Bu J, Li Y, Li R, Nie R, Xiao C, Ma K, Huang X, Li Y. Exosomal protein CD82 as a diagnostic biomarker for precision medicine for breast cancer. Mol Carcinog. 2019;58:674–685. doi: 10.1002/mc.22960. [DOI] [PubMed] [Google Scholar]
- 83.Iliuk AB, Arrington JV, Tao WA. Analytical challenges translating mass spectrometry-based phosphoproteomics from discovery to clinical applications. Electrophoresis. 2014;35:3430–3440. doi: 10.1002/elps.201400153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen IH, Xue L, Hsu CC, Paez JS, Pan L, Andaluz H, Wendt MK, Iliuk AB, Zhu JK, Tao WA. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc Natl Acad Sci U S A. 2017;114:3175–3180. doi: 10.1073/pnas.1618088114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Etayash H, McGee AR, Kaur K, Thundat T. Nanomechanical sandwich assay for multiple cancer biomarkers in breast cancer cell-derived exosomes. Nanoscale. 2016;8:15137–15141. doi: 10.1039/c6nr03478k. [DOI] [PubMed] [Google Scholar]
- 86.Khan S, Bennit HF, Turay D, Perez M, Mirshahidi S, Yuan Y, Wall NR. Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC Cancer. 2014;14:176. doi: 10.1186/1471-2407-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaudhary P, Gibbs LD, Maji S, Lewis CM, Suzuki S, Vishwanatha JK. Serum exosomal-annexin A2 is associated with African-American triple-negative breast cancer and promotes angiogenesis. Breast Cancer Res. 2020;22:11. doi: 10.1186/s13058-020-1251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arnold J, Schattschneider J, Blechner C, Krisp C, Schluter H, Schweizer M, Nalaskowski M, Oliveira-Ferrer L, Windhorst S. Tubulin tyrosine ligase like 4 (TTLL4) overexpression in breast cancer cells is associated with brain metastasis and alters exosome biogenesis. J Exp Clin Cancer Res. 2020;39:205. doi: 10.1186/s13046-020-01712-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alt EU, Worner PM, Pfnur A, Ochoa JE, Schachtele DJ, Barabadi Z, Lang LM, Srivastav S, Burow ME, Chandrasekar B, Izadpanah R. Targeting TRAF3IP2, compared to Rab27, is more effective in suppressing the development and metastasis of breast cancer. Sci Rep. 2020;10:8834. doi: 10.1038/s41598-020-64781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cen J, Feng L, Ke H, Bao L, Li LZ, Tanaka Y, Weng J, Su L. Exosomal thrombospondin-1 disrupts the integrity of endothelial intercellular junctions to facilitate breast cancer cell metastasis. Cancers (Basel) 2019;11:1946. doi: 10.3390/cancers11121946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X, Zeh HJ, Kang R, Kroemer G, Tang D. Cell death in pancreatic cancer: from pathogenesis to therapy. Nat Rev Gastroenterol Hepatol. 2021;18:804–823. doi: 10.1038/s41575-021-00486-6. [DOI] [PubMed] [Google Scholar]
- 92.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 93.Stefanius K, Servage K, de Souza Santos M, Gray HF, Toombs JE, Chimalapati S, Kim MS, Malladi VS, Brekken R, Orth K. Human pancreatic cancer cell exosomes, but not human normal cell exosomes, act as an initiator in cell transformation. Elife. 2019;8:e40226. doi: 10.7554/eLife.40226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sceneay J, Smyth MJ, Moller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 96.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun H, Rana S, Wang Z, Zhao K, Schnolzer M, Provaznik J, Hackert T, Lv Q, Zoller M. The pancreatic cancer-initiating cell marker CD44v6 affects transcription, translation, and signaling: consequences for exosome composition and delivery. J Oncol. 2019;2019:3516973. doi: 10.1155/2019/3516973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Z, von Au A, Schnolzer M, Hackert T, Zoller M. CD44v6-competent tumor exosomes promote motility, invasion and cancer-initiating cell marker expression in pancreatic and colorectal cancer cells. Oncotarget. 2016;7:55409–55436. doi: 10.18632/oncotarget.10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie Z, Gao Y, Ho C, Li L, Jin C, Wang X, Zou C, Mao Y, Wang X, Li Q, Fu D, Zhang YF. Exosome-delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut. 2022;71:568–579. doi: 10.1136/gutjnl-2020-323014. [DOI] [PubMed] [Google Scholar]
- 101.Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner KT, Zoller M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 102.Yue S, Mu W, Erb U, Zoller M. The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget. 2015;6:2366–2384. doi: 10.18632/oncotarget.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao K, Erb U, Hackert T, Zoller M, Yue S. Distorted leukocyte migration, angiogenesis, wound repair and metastasis in Tspan8 and Tspan8/CD151 double knockout mice indicate complementary activities of Tspan8 and CD51. Biochim Biophys Acta Mol Cell Res. 2018;1865:379–391. doi: 10.1016/j.bbamcr.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 104.Kyuno D, Bauer N, Schnolzer M, Provaznik J, Ryschich E, Hackert T, Zoller M. Distinct origin of Claudin7 in early tumor endosomes affects exosome assembly. Int J Biol Sci. 2019;15:2224–2239. doi: 10.7150/ijbs.35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fahmy K, Gonzalez A, Arafa M, Peixoto P, Bellahcene A, Turtoi A, Delvenne P, Thiry M, Castronovo V, Peulen O. Myoferlin plays a key role in VEGFA secretion and impacts tumor-associated angiogenesis in human pancreas cancer. Int J Cancer. 2016;138:652–663. doi: 10.1002/ijc.29820. [DOI] [PubMed] [Google Scholar]
- 106.Shin SJ, Smith JA, Rezniczek GA, Pan S, Chen R, Brentnall TA, Wiche G, Kelly KA. Unexpected gain of function for the scaffolding protein plectin due to mislocalization in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:19414–19419. doi: 10.1073/pnas.1309720110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin H, Liu P, Wu Y, Meng X, Wu M, Han J, Tan X. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018;109:2946–2956. doi: 10.1111/cas.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang WH, Nguyen TT, Hsu CH, Bryant KL, Kim HJ, Ying H, Erickson JW, Der CJ, Cerione RA, Antonyak MA. KRAS-dependent cancer cells promote survival by producing exosomes enriched in survivin. Cancer Lett. 2021;517:66–77. doi: 10.1016/j.canlet.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei Q, Li Z, Feng H, Ren L. Serum exosomal EphA2 is a prognostic biomarker in patients with pancreatic cancer. Cancer Manag Res. 2021;13:3675–3683. doi: 10.2147/CMAR.S304719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qian S, Tan X, Liu X, Liu P, Wu Y. Exosomal Tenascin-c induces proliferation and invasion of pancreatic cancer cells by WNT signaling. Onco Targets Ther. 2019;12:3197–3205. doi: 10.2147/OTT.S192218. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Kimura H, Yamamoto H, Harada T, Fumoto K, Osugi Y, Sada R, Maehara N, Hikita H, Mori S, Eguchi H, Ikawa M, Takehara T, Kikuchi A. CKAP4, a DKK1 receptor, is a biomarker in exosomes derived from pancreatic cancer and a molecular target for therapy. Clin Cancer Res. 2019;25:1936–1947. doi: 10.1158/1078-0432.CCR-18-2124. [DOI] [PubMed] [Google Scholar]
- 112.Lim JW, Mathias RA, Kapp EA, Layton MJ, Faux MC, Burgess AW, Ji H, Simpson RJ. Restoration of full-length APC protein in SW480 colon cancer cells induces exosome-mediated secretion of DKK-4. Electrophoresis. 2012;33:1873–1880. doi: 10.1002/elps.201100687. [DOI] [PubMed] [Google Scholar]
- 113.Huang Z, Yang M, Li Y, Yang F, Feng Y. Exosomes derived from hypoxic colorectal cancer cells transfer Wnt4 to normoxic cells to elicit a prometastatic phenotype. Int J Biol Sci. 2018;14:2094–2102. doi: 10.7150/ijbs.28288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun B, Li Y, Zhou Y, Ng TK, Zhao C, Gan Q, Gu X, Xiang J. Circulating exosomal CPNE3 as a diagnostic and prognostic biomarker for colorectal cancer. J Cell Physiol. 2019;234:1416–1425. doi: 10.1002/jcp.26936. [DOI] [PubMed] [Google Scholar]
- 115.Campanella C, Rappa F, Sciume C, Marino Gammazza A, Barone R, Bucchieri F, David S, Curcuru G, Caruso Bavisotto C, Pitruzzella A, Geraci G, Modica G, Farina F, Zummo G, Fais S, Conway de Macario E, Macario AJ, Cappello F. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer. 2015;121:3230–3239. doi: 10.1002/cncr.29499. [DOI] [PubMed] [Google Scholar]
- 116.Merendino AM, Bucchieri F, Campanella C, Marciano V, Ribbene A, David S, Zummo G, Burgio G, Corona DF, Conway de Macario E, Macario AJ, Cappello F. Hsp60 is actively secreted by human tumor cells. PLoS One. 2010;5:e9247. doi: 10.1371/journal.pone.0009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li J, Chen Y, Guo X, Zhou L, Jia Z, Peng Z, Tang Y, Liu W, Zhu B, Wang L, Ren C. GPC1 exosome and its regulatory miRNAs are specific markers for the detection and target therapy of colorectal cancer. J Cell Mol Med. 2017;21:838–847. doi: 10.1111/jcmm.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, Nonaka R, Yamamoto H, Ishii H, Mori M, Furuta K, Nakajima T, Hayashi H, Sugisaki H, Higashimoto H, Kato T, Takeshita F, Ochiya T. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014;5:3591. doi: 10.1038/ncomms4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yokoyama S, Takeuchi A, Yamaguchi S, Mitani Y, Watanabe T, Matsuda K, Hotta T, Shively JE, Yamaue H. Clinical implications of carcinoembryonic antigen distribution in serum exosomal fraction-measurement by ELISA. PLoS One. 2017;12:e0183337. doi: 10.1371/journal.pone.0183337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yun CW, Lee JH, Go G, Jeon J, Yoon S, Lee SH. Prion protein of extracellular vesicle regulates the progression of colorectal cancer. Cancers (Basel) 2021;13:2144. doi: 10.3390/cancers13092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu B, Sun D, Ma L, Deng Y, Zhang S, Dong L, Chen S. Exosomes isolated from CAPS1-overexpressing colorectal cancer cells promote cell migration. Oncol Rep. 2019;42:2528–2536. doi: 10.3892/or.2019.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang YX, Li YZ, Zhu HF, Zhang ZY, Qian XL, He GY. STX2 drives colorectal cancer proliferation via upregulation of EXOSC4. Life Sci. 2020;263:118597. doi: 10.1016/j.lfs.2020.118597. [DOI] [PubMed] [Google Scholar]
- 123.Junker K, Heinzelmann J, Beckham C, Ochiya T, Jenster G. Extracellular vesicles and their role in urologic malignancies. Eur Urol. 2016;70:323–331. doi: 10.1016/j.eururo.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 124.Hasan D, Gamen E, Abu Tarboush N, Ismail Y, Pak O, Azab B. PKM2 and HIF-1alpha regulation in prostate cancer cell lines. PLoS One. 2018;13:e0203745. doi: 10.1371/journal.pone.0203745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The alphavbeta6 integrin is transferred intercellularly via exosomes. J Biol Chem. 2015;290:4545–4551. doi: 10.1074/jbc.C114.617662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Singh A, Fedele C, Lu H, Nevalainen MT, Keen JH, Languino LR. Exosome-mediated transfer of alphavbeta3 integrin from tumorigenic to nontumorigenic cells promotes a migratory phenotype. Mol Cancer Res. 2016;14:1136–1146. doi: 10.1158/1541-7786.MCR-16-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gaballa R, Ali HEA, Mahmoud MO, Rhim JS, Ali HI, Salem HF, Saleem M, Kandeil MA, Ambs S, Abd Elmageed ZY. Exosomes-mediated transfer of itga2 promotes migration and invasion of prostate cancer cells by inducing epithelial-mesenchymal transition. Cancers (Basel) 2020;12:2300. doi: 10.3390/cancers12082300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bijnsdorp IV, Geldof AA, Lavaei M, Piersma SR, van Moorselaar RJ, Jimenez CR. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J Extracell Vesicles. 2013;2:22097. doi: 10.3402/jev.v2i0.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kawakami K, Fujita Y, Kato T, Mizutani K, Kameyama K, Tsumoto H, Miura Y, Deguchi T, Ito M. Integrin beta4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. Int J Oncol. 2015;47:384–390. doi: 10.3892/ijo.2015.3011. [DOI] [PubMed] [Google Scholar]
- 130.Dai J, Escara-Wilke J, Keller JM, Jung Y, Taichman RS, Pienta KJ, Keller ET. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J Exp Med. 2019;216:2883–2899. doi: 10.1084/jem.20190158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Borel M, Lollo G, Magne D, Buchet R, Brizuela L, Mebarek S. Prostate cancer-derived exosomes promote osteoblast differentiation and activity through phospholipase D2. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165919. doi: 10.1016/j.bbadis.2020.165919. [DOI] [PubMed] [Google Scholar]
- 132.McAtee CO, Booth C, Elowsky C, Zhao L, Payne J, Fangman T, Caplan S, Henry MD, Simpson MA. Prostate tumor cell exosomes containing hyaluronidase Hyal1 stimulate prostate stromal cell motility by engagement of FAK-mediated integrin signaling. Matrix Biol. 2019;78-79:165–179. doi: 10.1016/j.matbio.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin CJ, Yun EJ, Lo UG, Tai YL, Deng S, Hernandez E, Dang A, Chen YA, Saha D, Mu P, Lin H, Li TK, Shen TL, Lai CH, Hsieh JT. The paracrine induction of prostate cancer progression by caveolin-1. Cell Death Dis. 2019;10:834. doi: 10.1038/s41419-019-2066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X, Wilson MJ, Slaton JW, Sinha AA, Ewing SL, Pei D. Increased aggressiveness of human prostate PC-3 tumor cells expressing cell surface localized membrane type-1 matrix metalloproteinase (MT1-MMP) J Androl. 2009;30:259–274. doi: 10.2164/jandrol.108.006494. [DOI] [PubMed] [Google Scholar]
- 135.Vlaeminck-Guillem V. Extracellular vesicles in prostate cancer carcinogenesis, diagnosis, and management. Front Oncol. 2018;8:222. doi: 10.3389/fonc.2018.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.DeRita RM, Zerlanko B, Singh A, Lu H, Iozzo RV, Benovic JL, Languino LR. c-Src, insulin-like growth factor i receptor, g-protein-coupled receptor kinases and focal adhesion kinase are enriched into prostate cancer cell exosomes. J Cell Biochem. 2017;118:66–73. doi: 10.1002/jcb.25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kawakami K, Fujita Y, Matsuda Y, Arai T, Horie K, Kameyama K, Kato T, Masunaga K, Kasuya Y, Tanaka M, Mizutani K, Deguchi T, Ito M. Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer. BMC Cancer. 2017;17:316. doi: 10.1186/s12885-017-3301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, Widmark A. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100:1603–1607. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cho S, Yang HC, Rhee WJ. Simultaneous multiplexed detection of exosomal microRNAs and surface proteins for prostate cancer diagnosis. Biosens Bioelectron. 2019;146:111749. doi: 10.1016/j.bios.2019.111749. [DOI] [PubMed] [Google Scholar]
- 140.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang H, Lu J, Liu J, Zhang G, Lu A. Advances in the discovery of exosome inhibitors in cancer. J Enzyme Inhib Med Chem. 2020;35:1322–1330. doi: 10.1080/14756366.2020.1754814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zocco D, Ferruzzi P, Cappello F, Kuo WP, Fais S. Extracellular vesicles as shuttles of tumor biomarkers and anti-tumor drugs. Front Oncol. 2014;4:267. doi: 10.3389/fonc.2014.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.French KC, Antonyak MA, Cerione RA. Extracellular vesicle docking at the cellular port: extracellular vesicle binding and uptake. Semin Cell Dev Biol. 2017;67:48–55. doi: 10.1016/j.semcdb.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li S, Yi M, Dong B, Jiao Y, Luo S, Wu K. The roles of exosomes in cancer drug resistance and its therapeutic application. Clin Transl Med. 2020;10:e257. doi: 10.1002/ctm2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Milman N, Ginini L, Gil Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug Resist Updat. 2019;45:1–12. doi: 10.1016/j.drup.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 150.Hosseini-Beheshti E, Pham S, Adomat H, Li N, Tomlinson Guns ES. Exosomes as biomarker enriched microvesicles: characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol Cell Proteomics. 2012;11:863–885. doi: 10.1074/mcp.M111.014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yin J, Yan X, Yao X, Zhang Y, Shan Y, Mao N, Yang Y, Pan L. Secretion of annexin A3 from ovarian cancer cells and its association with platinum resistance in ovarian cancer patients. J Cell Mol Med. 2012;16:337–348. doi: 10.1111/j.1582-4934.2011.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parashar D, Geethadevi A, McAllister D, Ebben J, Peterson FC, Jensen DR, Bishop E, Pradeep S, Volkman BF, Dwinell MB, Chaluvally-Raghavan P, James MA. Targeted biologic inhibition of both tumor cell-intrinsic and intercellular CLPTM1L/CRR9-mediated chemotherapeutic drug resistance. NPJ Precis Oncol. 2021;5:16. doi: 10.1038/s41698-021-00152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang D, Zhao C, Xu F, Zhang A, Jin M, Zhang K, Liu L, Hua Q, Zhao J, Liu J, Yang H, Huang G. Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics. 2021;11:2860–2875. doi: 10.7150/thno.51797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang H, Xie S, Liang B, Tang Q, Liu H, Wang D, Huang G. Exosomal IDH1 increases the resistance of colorectal cancer cells to 5-Fluorouracil. J Cancer. 2021;12:4862–4872. doi: 10.7150/jca.58846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng X, Ma N, Wang X, Hu J, Ma X, Wang J, Cao B. Exosomes derived from 5-fluorouracil-resistant colon cancer cells are enriched in GDF15 and can promote angiogenesis. J Cancer. 2020;11:7116–7126. doi: 10.7150/jca.49224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zheng X, Liu J, Li X, Tian R, Shang K, Dong X, Cao B. Angiogenesis is promoted by exosomal DPP4 derived from 5-fluorouracil-resistant colon cancer cells. Cancer Lett. 2021;497:190–201. doi: 10.1016/j.canlet.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 157.Zhang Q, Liu RX, Chan KW, Hu J, Zhang J, Wei L, Tan H, Yang X, Liu H. Exosomal transfer of p-STAT3 promotes acquired 5-FU resistance in colorectal cancer cells. J Exp Clin Cancer Res. 2019;38:320. doi: 10.1186/s13046-019-1314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gao Z, Han X, Zhu Y, Zhang H, Tian R, Wang Z, Cui Y, Wang Z, Niu R, Zhang F. Drug-resistant cancer cell-derived exosomal EphA2 promotes breast cancer metastasis via the EphA2-Ephrin A1 reverse signaling. Cell Death Dis. 2021;12:414. doi: 10.1038/s41419-021-03692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fan J, Wei Q, Koay EJ, Liu Y, Ning B, Bernard PW, Zhang N, Han H, Katz MH, Zhao Z, Hu Y. Chemoresistance transmission via exosome-mediated EphA2 transfer in pancreatic cancer. Theranostics. 2018;8:5986–5994. doi: 10.7150/thno.26650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li T, Tao Z, Zhu Y, Liu X, Wang L, Du Y, Cao J, Wang B, Zhang J, Hu X. Exosomal annexin A6 induces gemcitabine resistance by inhibiting ubiquitination and degradation of EGFR in triple-negative breast cancer. Cell Death Dis. 2021;12:684. doi: 10.1038/s41419-021-03963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hrdinova T, Toman O, Dresler J, Klimentova J, Salovska B, Pajer P, Bartos O, Polivkova V, Linhartova J, Machova Polakova K, Kabickova H, Brodska B, Krijt M, Zivny J, Vyoral D, Petrak J. Exosomes released by imatinibresistant K562 cells contain specific membrane markers, IFITM3, CD146 and CD36 and increase the survival of imatinibsensitive cells in the presence of imatinib. Int J Oncol. 2021;58:238–250. doi: 10.3892/ijo.2020.5163. [DOI] [PubMed] [Google Scholar]
- 162.Wu S, Luo M, To KKW, Zhang J, Su C, Zhang H, An S, Wang F, Chen D, Fu L. Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer. Mol Cancer. 2021;20:17. doi: 10.1186/s12943-021-01307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gao J, Qiu X, Li X, Fan H, Zhang F, Lv T, Song Y. Expression profiles and clinical value of plasma exosomal Tim-3 and Galectin-9 in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;498:409–415. doi: 10.1016/j.bbrc.2018.02.114. [DOI] [PubMed] [Google Scholar]
- 164.Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, Grau GE. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia. 2009;23:1643–1649. doi: 10.1038/leu.2009.76. [DOI] [PubMed] [Google Scholar]
- 165.Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O’Driscoll L. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim J, Hong SW, Kim S, Kim D, Hur DY, Jin DH, Kim B, Kim YS. Cyclooxygenase-2 expression is induced by celecoxib treatment in lung cancer cells and is transferred to neighbor cells via exosomes. Int J Oncol. 2018;52:613–620. doi: 10.3892/ijo.2017.4227. [DOI] [PubMed] [Google Scholar]
- 167.Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della Mina P, Menard S, Filipazzi P, Rivoltini L, Tagliabue E, Pupa SM. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227:658–667. doi: 10.1002/jcp.22773. [DOI] [PubMed] [Google Scholar]
- 168.Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Peak TC, Panigrahi GK, Praharaj PP, Su Y, Shi L, Chyr J, Rivera-Chavez J, Flores-Bocanegra L, Singh R, Vander Griend DJ, Oberlies NH, Kerr BA, Hemal A, Bitting RL, Deep G. Syntaxin 6-mediated exosome secretion regulates enzalutamide resistance in prostate cancer. Mol Carcinog. 2020;59:62–72. doi: 10.1002/mc.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]


