Abstract
Physical activity (PA) guidelines for the general population are designed to mitigate the rise of chronic and debilitating diseases brought by inactivity and sedentariness. Although essential, they are insufficient as rates of cardiovascular, pulmonary, renal, metabolic and other devastating and life-long diseases remain on the rise. This systemic failure supports the need for an improved exercise prescription approach that targets the individual. Significant interindividual variability of cardiorespiratory fitness (CRF) responses to exercise are partly explained by biological and methodological factors, and the modulation of exercise volume and intensity seem to be key in improving prescription guidelines. The use of physiological thresholds, such as lactate, ventilation, as well as critical power, have demonstrated excellent results to improve CRF in those struggling to respond to the current homogenous prescription of exercise. However, assessing physiological thresholds requires laboratory resources and expertise and is incompatible for a general population approach. A case must be made that balances the effectiveness of an exercise programme to improve CRF and accessibility of resources. A population-wide approach of exercise prescription guidelines should include free and accessible self-assessed threshold tools, such as rate of perceived exertion, where the homeostatic perturbation induced by exercise reflects physiological thresholds. The present critical review outlines factors for individuals exercise prescription and proposes a new theoretical hierarchal framework to help shape PA guidelines based on accessibility and effectiveness as part of a personalised exercise prescription that targets the individual.
Keywords: Physical fitness, Cardiovascular, Aerobic fitness, Exercise physiology
Key messages.
What is already known
Current physical activity guidelines and homogenous prescription of exercise across health organisations fail to improve cardiorespiratory fitness (CRF) in adults.
Significant interindividual variability of CRF responses to exercise are partly explained by biological and methodological factors.
Using physiological thresholds to determine exercise intensity have demonstrated excellent results to improve CRF in those struggling to respond to exercise, but resources needed such as laboratory facilities and cost associated to evaluate physiological threshold make this method inaccessible for the general population.
What are the new findings
Personalised exercise prescription programmes based on physiological thresholds are more effective at improving CRF in adults.
A population-wide approach of exercise prescription guidelines should include free and accessible self-assessed threshold tools, such as rate of perceived exertion, where the homeostatic perturbation induced by exercise reflects physiological thresholds.
Introduction
The global decline in physical activity (PA) and fitness, and their wide consequences on health, is widely recognised, despite prescribing regular exercise as medicine for decades.1 2 General PA guidelines are provided by many national and international health and exercise governing bodies (eg, WHO, ACSM, CDC, CSEP, Physical Activity Guidelines for Americans, etc), for different age groups, and populations, healthy or suffering from cardiometabolic, renal, pulmonary and other chronic diseases and are designed to ultimately support clinicians and health experts in the prescription of exercise to their patients. These guidelines are evidence-based, simple, clear and constitute fundamental steppingstones for improving cardiorespiratory fitness (CRF) and musculoskeletal fitness across populations (see table 1).
Table 1.
The current physical activity guidelines
| Source | WHO (2020) | ACSM (2017) | Physical Activity Guidelines for Americans (2018) |
| General | Adults aged 18–64 years should do at least 150–300 min of moderate-intensity aerobic physical activity throughout the week or do at least 75–150 min of vigorous-intensity aerobic physical activity throughout the week or an equivalent combination of moderate-intensity and vigorous-intensity activity. | Apparently healthy adults of all ages should do at least 150 min of moderate-intensity aerobic physical activity throughout the week or do at least 75 min of vigorous-intensity aerobic physical activity throughout the week or an equivalent combination of moderate-intensity and vigorous-intensity activity. | Adults should move more and sit less throughout the day. Some physical activity is better than none. For substantial health benefits adults should do at least 150 min to 300 min a week of moderate-intensity or 75 min to 150 min a week of vigorous-intensity aerobic physical activity or an equivalent combination of the two. |
| Aerobic activity | Should be performed in bouts of at least 10 min duration. | Should be performed in one continuous session per day or in multiple sessions of at least 10 min. For very deconditioned individuals exercise bouts of less than 10 min may be beneficial. | Should be performed on at least 3 days a week. All amounts of aerobic activity count towards meeting the key guidelines if they are performed at moderate or vigorous intensity. |
| Muscle strengthening activities | Should be done involving major muscle groups on two or more days a week. | Each major muscle group should be trained 2–3 days per week. | Muscle-strengthening activities of moderate or greater intensity involving all major muscle groups should be done on two or more days a week. |
| For additional health benefits | Increase moderate-intensity aerobic physical activity to more than 300 min per week or more than 150 min of vigorous-intensity aerobic physical activity per week, or an equivalent combination of moderate-intensity and vigorous-intensity activity. | Additional health benefits are gained by engaging in physical activity beyond the equivalent 300 min of moderate-intensity physical activity a week. |
Large individual variation is observed in CRF responses to a standardised training programme with variation ranging from high responders, exhibiting CRF improvements above a predetermined response threshold to a given training stimulus to non-responders where no improvements is observed and even negative responders where a decrease in CRF can be seen.3 A comprehensive evaluation and careful analyses of the distribution of responders to a standardised training programme is essential since numerous factors, including study design and statistical model to determine response thresholds, modulate response variability, and thereby, categorisation of individuals into responders, non-responders and negative responders.4 A recent multicentre trainability study (n=677) suggested that studies conducted using moderate-intensity training, as well as low volume/high-intensity training (HIT) had combined likely non-responders and negative responders at a rate 45% and 52%, respectively, where a change in maximal oxygen consumption (V̇O2max) of approximately −12 to +1 mL/kg/min was observed.5 CRF, classically assessed as V̇O2max, represents a whole-body capability to transport and use oxygen at the tissue level via a multilayered model, initiated from pulmonary ventilation, cardiac output adjustment, O2 transport and diffusion across tissues and mitochondrial metabolism. From this model, multiple sources of variation may influence V̇O2max values and explain the observed large individual variations. This is further exacerbated from the technical challenges of measuring ‘true’ V̇O2max from the many exercise protocols developed over the years that did not include a verification phase.6 7 In their landmark and iconic study, Bouchard et al8 reported significant differences in V̇O2max improvement in response to a 20-week training programme and estimated that 47% of the variation in the response was linked to genetics, a value slightly higher than subsequent reports (26%–34%).9 10 Significant efforts have since been conducted to further explain variation in V̇O2max trainability with factorial assessment that includes age, sex, baseline CRF, sleep, recovery, genetics, as well as modifiable training and statistical factors.4 11–14 Some authors have debated whether non-responders are simply individuals that have received an incorrect exercise dose and how to transform non-responders into responders.13–15 Increasing exercise dosage via volume and intensity supports this notion via greater changes in DNA methylation and gene expression following long-term16 and high-intensity exercise,17 18 resulting in meaningful activation of metabolic pathways necessary to improve V̇O2max. Despite recent efforts attempting to minimise, or even eliminate non-responders and negative responders, there is nonetheless an ongoing need to improve individualised exercise prescription, primarily the volume and intensity components, to meet the goals of the current PA recommendations.
Individuals who self-select exercise overload components such as frequency, duration and intensity through current general exercise guidelines to improve fitness and health may not reach satisfactory levels of PA in order to achieve desirable outcomes or improve CRF, underlining the need to explore alternative strategies, including improving individual exercise prescriptions, and understanding of how to individually programme exercise training. Classical approaches to determine exercise intensity such as %V̇O2max or %heart rate (%HR) retain flawed characteristics when prescribed to the general population as they contextually do not include an individual’s distinct physiological responses to an exercise dose. Meyler et al4 have recently raised good arguments for a more personalised exercise prescription approach that would include physiological thresholds (lactate, gas exchange and ventilatory), where exercise intensity would be better represented by an individual’s response to exercise stimulus. Unfortunately, while sound and reasonable, this approach, commonly used in competitive sports during laboratory assessments, yields little practicality for population-wide PA exercise guidelines, and represents a critical barrier for population’s health. Redefining exercise guidelines for the general population requires a careful assessment of effectiveness, while considering accessibility to resources. To that end, the use of intrinsic self-assessment tools, such as the rate of perceived effort (RPE) scale,19 provide a free and heterogeneous method to manipulate exercise intensity in a reflective way to the individual’s response to homeostatic perturbation. RPE has been shown to be more closely associated with ventilatory thresholds compared with %HR reserve or %V̇O2max20 and has been suggested to be included in public health programmes.21 22 While laboratory-based physiological thresholds are establishing themselves as gold standards to improve exercise prescription guidelines, and the rate of exercise responders, the use of self-assessment effort scales and field-based methods of threshold assessment should receive more considerations as they may better reflect physiological thresholds than the classically used percentage of maximal values (HR, V̇O2), remove accessibility barriers and may be just as effective in improving CRF. The present critical review addresses novel findings regarding CRF modifiable training factors (volume and intensity) to improve exercise prescription guidelines. Relevant literature was also identified to describe the physiological mechanisms linking self-assessed to physiological thresholds to support the proposal of a new hierarchical framework for individualised exercise prescription in healthy adults between the age of 18 and 65 years old.
Factors for individualised exercise prescription
Intrinsic and extrinsic biological factors
Human biology plays a fundamental role in CRF response to training that extends beyond heredity and genes. Intrinsic factors such as sex, age, as well as baseline V̇O2max and HRV each modulate physiological responses from homeostatic perturbations, including changes in V̇O2. The recent review by Meyer et al4 comprehensively addresses biological factors modulating the variability of V̇O2 responses. While sex and age have been suggested to account for less than a combined 10%–16% of the V̇O2 response,23 24 baseline HRV, more precisely high frequency power (high vagal activity), has so far been one of the strongest factors, predicting 27% of the V̇O2 response (n=39), following 8 weeks of aerobic training24 and 34% (n=16) following 12 weeks of interval training.25 Interestingly, baseline V̇O2, as a predictor, has demonstrated mitigating results as Bouchard and Rankinen23 showed that it only explained 1% of the V̇O2 response in their cohort and Hautala et al26 approximately 2%. Subsequent analyses revealed that this phenomenon may be more complex than originally described, and possibly predict more of the V̇O2 response to training, with negative relationships observed, where individuals with lower fitness and initial values would attain greater V̇O2 gains.27 28 This would logically explain diminishing returns from training and a V̇O2 ceiling reached in elite athletes where gaining additional CRF can become nearly impossible and is, in itself, a polarising field of V̇O2 work. Moreover, training studies conducted over weeks, if not months, require the consideration of external factors that further influence biological responses. While many lifestyle, behavioural and environmental factors may come into play, the impact of physical recovery, nutritional status and stress can largely influence phenotyping effects of training.3 The degree of homeostatic perturbation from a predetermined exercise load regulates the timeline associated with musculoskeletal, neural and metabolic recovery necessary to initiate the next training load. Low intensity and volume exercise can require less than 24 hours, whereas high-intensity and volume exercise may need more than 48 hours to adequately recover. Cardiac autonomic recovery, assessed via HRV, offers an excellent assessment of physical recovery despite, its assumed flaws that it does not reflect all physiological systems impacted by exercise.29 Finally, endocrine, immunological and metabolic dysregulation occur from exercise and cannot be easily assessed during recovery but are known to play a critical role in exercise readiness and response to physiological change.
Methodological factors: training volume
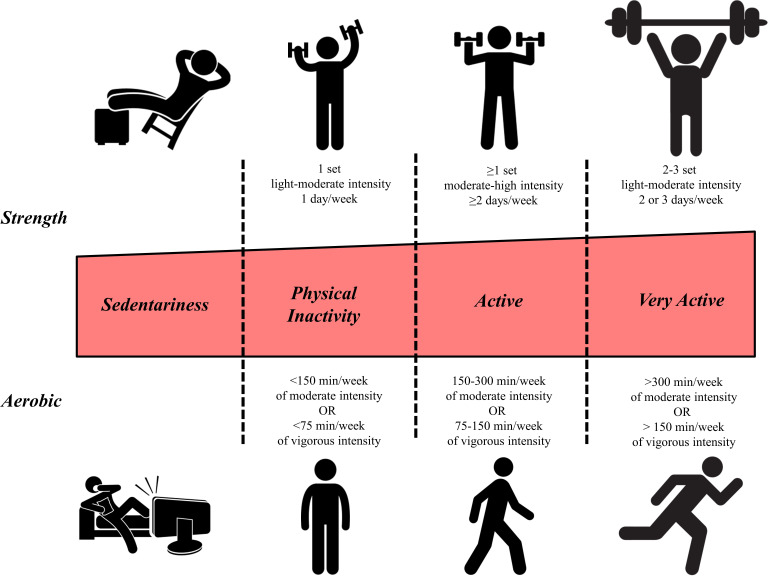
The current ACSM guidelines prescribe a fixed volume of aerobic training consisting of at least ≥150 min per week of moderate intensity or ≥75 min per week of vigorous intensity or a combination of the two (figure 1). Furthermore, for those not meeting the guidelines, it is recommended to gradually work towards meeting the goals commencing with short 5 min activity periods.30 However, individual differences in training status and response exist and therefore appropriate training dose should be considered individually. Sisson et al28 observed a reduced number of V̇O2 non-responders with increasing weekly training volume, via higher energy expenditure relative to body weight, while maintaining training intensity. Montero and Lundby13 studied healthy young males who did not participate in organised sports and observed a decreasing occurrence of non-responders with increasing weekly training volume from 60 (n=16 with 11 non-responders) to 120 (n=15 with six non-responders) and 180 (n=14 with four non-responders) min per week and no non-responders with 240 or 300 min of weekly endurance training. Furthermore, they observed that adding two extra sessions of 60 min per week eliminated non-responders. Importantly, 29% of those exercising 180 min per week, at both moderate and high intensity, did not improve their CRF. Therefore, the current volume guideline of at least 150 min of moderate aerobic exercise might not be sufficient to increase CRF for a significant number of healthy adults. Montero and Lundby13 further suggested a critical duration of >180 ≤240 min per week to increase positive training responses. However, it is crucial to note that a significant proportion of the participants showed a positive training response at training volumes of 60 or 120 min per week highlighting the fact that sufficient training volume to elicit improvement in CRF remains highly individual. Furthermore, a fixed training volume may lead to decreasing gains in aerobic fitness after a training period of 3–6 months, and a subsequent volume increase may be required to pursue further improvements.31
Figure 1.
Physical activity and exercise guidelines across intensity domains for aerobic and resistance training for individuals aged from 18 to 64 years old (adapted from Gibson, Wagner, Hayward 202082).
Methodological factors: training intensity
A key concern remains across aerobic training studies: how intense should endurance training be in order to elicit optimal adaptations. When examining the health and performance effects of training intensity both minimum effective and optimal training intensity should be considered. The minimum training intensity necessary to improve V̇O2max is dependent on its initial value, where individuals with a V̇O2max of >40 mL/kg/min require a minimum intensity of 45% of V̇O2 reserve to increase their V̇O2max, and those with a lower CRF may obtain improvements at 30%–45% of their V̇O2 reserve.32 Exercise at a vigorous intensity (≥6 Metabolic Equivalent (METs), typically ≥60% of V̇O2max) conveys more cardioprotective benefits compared with moderate intensity exercise when total energy expenditure is matched. In their meta-analysis, Scribbans et al33 found no significant difference in improvements in V̇O2 between moderate (60%–70% of V̇O2max), heavy (80%–92.5% of V̇O2max) and severe (100%–250% of V̇O2max) training. However, a meta-analysis conducted by Milanović et al34 that included 723 participants demonstrated that while both endurance and HIT training improved V̇O2max compared with no exercise, HIT training elicited larger improvements (5.5±1.2 mL/kg/min) compared with traditional endurance training (4.9±1.4 mL/kg/min). Moreover, the progression of training intensity also requires considerations. While previously untrained subjects improved their V̇O2max by training at 60% of their heart rate reserve (HRR) 3 days per week for 45 min per session up to 6 months, significant changes in endurance capacity were not observed after the 6-month period.31
When integrating a broader array of indices of cardiometabolic health (body composition, V̇O2max, blood pressure, lipid profile and glucose metabolism) in overweight/obese untrained individuals, high-intensity interval training (HIIT) was more effective in promoting a greater number of positive adaptations compared with moderate intensity continuous training. Recently however, Burton et al35 observed that intense training (80%–90% of V̇O2peak) improved V̇O2peak, but markers of cardiometabolic health (fat metabolism and stress) showed no improvement when daily PA (step count) was reduced. Therefore, a combination of HIT and low/moderate intensity training seems more effective in improving cardiometabolic health compared with HIT alone. Huang et al36 investigated the dose–response relationship of CRF adaptation in sedentary older adults and found the largest V̇O2max adaptations at a mean intensity of 66%–73% of HRR, while a higher training intensity (75%–80% of HRR) resulted in large declines of V̇O2max. Therefore, while in some subject populations the benefits of HIT are undeniable, they might result in negative adaptations in other.
HIIT has received a lot of attention as an effective and time-efficient way of increasing cardiovascular and skeletal muscle health and performance.37 In their meta-analysis, Bacon et al38 observed that HIIT resulted in a greater increase in V̇O2max compared with moderate continuous training. The largest increases were observed when longer intervals of 3–5 min were used. The minimum effective volume of HIT training was also investigated. In inactive and overweight but otherwise healthy men performing one bout of 4 min at 90% HRmax rate three times a week for 10 weeks brought similar improvements to V̇O2max compared with four bouts of 4 min at 90% HRmax.21 However, increasing the number of interval repetitions was more effective in reducing body fat, total cholesterol, low-density lipoproteins (LDL)-cholesterol and ox-LDL cholesterol. The results suggest that already small doses of HIT are an effective way of training for previously inactive subjects.21 Thus, commencing a training programme with a low dose of HIT and gradually increasing the dose as CRF improves might be an effective training strategy.
In elite athletes, a polarised training approach combining moderate and high intensity training has been shown to be superior to both low-volume HIIT and heavy intensity training.39 Laursen40 argued that HIIT and more prolonged training sessions at a lower intensity might cause similar aerobic adaptations in the skeletal muscle via different molecular pathways, while low-intensity training might also promote autonomic balance and recovery. Therefore, their combination in a training programme might result in highest performance improvements. These findings from the training practices of high-level athletes have not yet been fully investigated in the context of general population health. There is limited but emerging evidence that a polarised approach to training might be more effective also in recreational athletes and in young overweight and obese subjects.41 42 Polarised training targets most training time at exercise intensities below VT1 (ventilatory threshold) and above ventilatory threshold 2 (VT2), with limited training between VT1 and VT2, following an undulating non-linear periodisation structure. Studies reporting endurance performance effect sizes comparing polarised (more training time below VT1 and above VT2 – ES 0.85–2.80) versus threshold training (significant training between VT1 and VT2– ES −0.42–2.16) have consistently shown greater improvements following polarised training.39 41 43 In real-life situations, a combination of training intensities might be more practicable and enjoyable, cardiometabolic health benefits more thorough, the physiological training stimulation more varied and a combination of different training intensities could result in better management of training stress.
A hierarchical framework to improve exercise prescription to the individual
Physiological thresholds
Prescribing exercise relative to maximal anchors such as V̇O2max, maximum power or velocity during an incremental test (Wmax, Vmax) or HRmax is a common and traditional approach that fails to account for large individual variation in metabolic response to exercise at a similar percentage of the maximum value.44 The use of physiological thresholds such as VT, lactate threshold (LT) or critical power (CP) and the delta concept representing the difference between thresholds and maximum values have been suggested to lower the instance of non-responders to training.4 45 Threshold-based exercise bouts are, by design, individually modulated to reach the physiological thresholds of each individual rather than a homogenous homeostatic perturbation, inducing heterogeneous responses. Physiological thresholds have a considerable range across individuals where, for example, CP can be as low as ~50% in the untrained, to ~95% V̇O2max in elite athletes.46 The use of VT instead of HRR have previously resulted in improved training responses and fewer non-responders.15 47 Work by Weathermax et al47 demonstrated that those training following an individualised ventilatory thresholds (VT)-based protocol were all considered to respond positively to training (19/19), but 40% of those following an HHR protocol were considered non responders (∆≤4.7% of baseline V̇O2max) (8/20). Training at 95% LT also induced more homogeneous responses among trained and untrained individuals compared with an intensity of 70% V̇O2max.45 While individual thresholds seem to be superior to establishing appropriate and effective training intensities for aerobic training, their application at a population level may be challenging, if not completely impractical. Defining individual ventilatory thresholds, or other physiological limits, requires expensive laboratory equipment and skilled labour and is therefore not an option at a population level.
RPE and self-assessed efforts
While popular among athletes and the general population because of accessible technologies, the efficacy of %HR (or % V̇O2) in guiding exercise intensity has been questioned, and the rating of perceived exertion (RPE) has been shown as a more effective method of targeting exercise intensity corresponding to VT, particularly in less experienced subjects with an RPE of ~13 correlating to VT1.20 The relationship between VT1 and RPE has been investigated in numerous populations, and it is estimated that an RPE of 11–14 will match VT in most individuals, in trained and untrained, independently of body composition.48–50 Two scales have been developed by Borg for RPE. A 6–20 points scale was designed to represent the linear increase of HR with increasing intensity, and another CR10 category ratio scale with a 1–10 system, which was suggested to better represent the non-linear responses of physiological thresholds.51 A review by Hydren and Cohen41 further categorised training zones where intensities at VT1 and below would represent ≤13 and ≤4, intensities between VT1 and VT2 would be 14–16 and 5–6, and ≥VT2 would be ≥17 and ≥7, on the 6–20 and CR10 Borg RPE scales, respectively. These zones and their correspondent RPE score have been suggested to implement polarised training models, which have demonstrated superior outcomes compered with threshold models.41
Beyond athletic training purposes, Tjønna et al21 further suggested the use of RPE for monitoring exercise intensity in public health programmes with a rating of 16 on the Borg scale corresponding to 90% HRmax and would, in most individuals, be above LT and VT1. Importantly, to better prescribe aerobic training to individuals without access to laboratory testing, RPE-based intensity guidelines can be applied. Targeting an RPE of ≤4 (CR10 scale) would be appropriate for moderate, an RPE of 5–6 for vigorous and ≥7 for severe exercise intensity. Supplementing RPE with information on external work (ie, pace in walking/running) and internal physiological response such as heart rate from wearable devices would bring an additional level of sophistication to prescribing and monitoring training.
The application of RPE for exercise and training is easily accessible and effective but nonetheless retains limitations and consideration in its applications, similarly to other methods to assess exercise intensity. Perceived effort (PE) has been defined differently across the literature leading to different ways to how individuals perceive effort per se and to assess the scale validity. Halperin and Emanuel52 have recently addressed this ongoing issue as individuals may understand and perceive the concepts of fatigue, discomfort and heaviness (all key terms used to describe PE yet theoretically independent) differently, directly affecting scale results and selection of exercise intensity. The development of new and population-specific scales, along with variety in each of their versions (aerobic vs resistance exercise, age groups, sports, etc), and how the scale may be defined and presented may also lead to confusion. For adequate implementation of RPE to the general population, the use of the original CR-10 Borg scale would be recommended, along with a simple definition of the scale, and the simple question ‘How effortful is the task?’ should be used.52 The integration of a rating of fatigue scale, which would help remind individuals of the differences in concepts between fatigue and exertion, may help improving RPE validity.53
Another alternative based on intrinsic self-assess effort, termed the ‘isoeffort’ model, has been proposed as an alternative to prescribing intensity instead of physiological or work rate related prescriptions.22 In this model, the total number of intervals, interval duration and rest period duration are prescribed (eg, 4×8 min with 2 min of recovery), and the individual is instructed to ‘solve’ the intensity based on what they can maintain throughout the entire session. Thus, the interval prescription guides towards the desired physiological response.22 The upside of this method is that it requires no physiological testing, but high motivation is needed and is mainly applicable to high intensity training since the aim is to maintain the highest intensity possible.
Finally, the talk test is also an inexpensive, practical and reliable tool to assess exercise intensity, particularly VT1, in healthy and diseased populations.54 55 Described briefly, this test requires exercising individuals to recite a 10–15 s text at the end of an exercise stage of an incremental protocol, following which they are asked the following question: ‘can you talk comfortably?’. They can answer yes, not sure and no, where the answer yes implies the need to increase exercise intensity and not sure or no leads to the end of the test. The reasoning of this test is grounded in the competitive requirements of ventilation for the gas exchange and metabolism of exercise versus speech, as the first inflection point of ventilation that begins to rise exponentially, VT1, represents the point where speech becomes uncomfortable and difficult due to the increased ventilatory demand from exercise.54–57 While simple, this test has shown meaningful validity and reliability when compared with physiological assessed VT1 in elite athletes,56 57 health adults58 59 and even cardiac patients.60 61 Although some considerations are needed when using this test, as most studies have examined the talk test in relation to VT1 only. Its relationship to other thresholds (VT2, lactate), as well as across different exercise testing protocols, also remain unclear.
Mechanisms linking self-assessed to physiological thresholds
The mechanisms describing how perception of effort is so closely associated with VT1, and other physiological variables and thresholds, remain unclear, and whether exertion is primarily modulated via central, peripheral or local pathways requires further investigations. Several concepts have nonetheless been brought forward to explain exertion including metabolically sensitive afferent muscle fibres relaying information to the central nervous system from homeostatic state of muscles under exercising conditions.62 63 The reduction on central motor drive, and subsequently physical performance, seems to also be regulated by exogenous factors,64 psychological preparedness65 and environmental stressors,66 which may further be governed by a sensory tolerance limit, as suggested by Amann and colleagues.63 Although both Borg scales have been heavily used for rate of perceived exertion, the 10-point scale was constructed to reflect the non-linearity of physiological responses during graded exercise tests.19 To that effect, physiologically relevant responses occurring during graded exercise testing, most particularly those occurring at ventilatory, lactate or gas thresholds, would theoretically mirror those of the scale. As such, the oxygen-conforming response, a term coined from the work of Hochachka,67 supports the association between oxygen availability/tissue oxygenation level with local muscle metabolism and contractile functions.68 Interestingly, Drouin et al69 recently demonstrated that perception of effort follows changes in muscle oxygenation during isometric handgrip exercises. It has been established that muscle oxygenation (as determined from changes in O2Hb, HHb, and %O2sat) is closely related to the non-linear ventilatory responses of incremental exercise70 as a result in intracellular fluctuations of mitochondrial oxygen uptake and ATP/Pi ratio. Moreover, changes in muscle oxygenation state seem to be sensitive to both ventilatory thresholds (VT1 and VT2) as well as sexes and training status.70 These insights on the regulatory pathways of RPE and physiological thresholds may not present a complete spectrum of factors; nonetheless, evidence suggests that the oxygen-conformer response and the association between muscle oxygenation and ventilatory thresholds may be, at least in part, responsible.
A new framework for exercise prescription to the general population
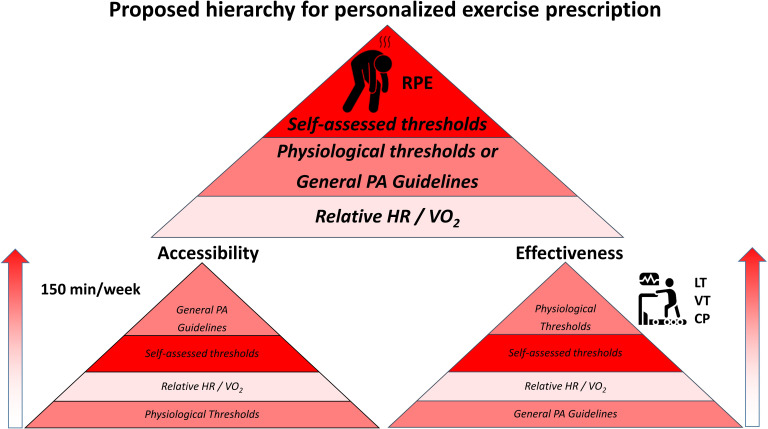
Using RPE to determine exercise intensities for exercise programming have demonstrated a close relationship to VT1 and VT2 and could be just as effective to improve CRF in non-responders and negative responders. This approach is free and widely accessible and should constitute the basis of the exercise prescription guidelines for the general adult population. This method is personalised and reflects the individual’s thresholds, essential to improve CRF. While the foundation of current exercise guidelines includes the recommendation of 150 min/week of moderate PA and is the most accessible way to reach the wider population, this approach remains inheritably flawed and does not result in improved CRF in the general population. Application of more rigorous methods, such as laboratory-based thresholds, for establishing training intensities could be considered based on individual and personalised needs but remains inaccessible for most individuals. To facilitate the understanding of this concept, we developed a hierarchical framework (figure 2) that demonstrates that when both accessibility and effectiveness are considered, prescribing exercise to the general population should favour the use of RPE or other self-assessed thresholds methods, followed by laboratory thresholds or the current PA guidelines, and finally, relative intensities via HR or VO2. This novel framework can be used by clinicians, as well as health and fitness experts, to improve CRF of patients, clients and the general population, in an accessible and effective fashion.
Figure 2.
Hierarchical theoretical framework presenting the level of accessibility and effectiveness of different methods to assess exercise intensity. CP, critical power; LT, lactate threshold; RPE rate of perceived exertion; VT, ventilatory threshold.
Considerations
The present review primarily included studies using V̇O2 changes as a primary variable to evaluate CRF responses to training. While this classical approach has been the bedrock and gold standard to assess CRF for decades, other cardiovascular and pulmonary outcomes can offer valuable information on training adaptations and fitness, including oxygen kinetics, △W/△V̇O2, VE/VCO2 and many others. Nonetheless, the variability in responses of these outcomes have not been extensively investigated, and careful considerations in selecting CRF outcome in training studies should be sought. Additionally, the categorisation of responders in V̇O2max variability studies does not ultimately reflect the exercise benefits obtained from the predetermined training design and should be carefully interpreted. Highly fit and older individuals, for example, may be presented as non-responders following training. Yet, the training design should not be necessarily designated as inadequate or assume that no physiological and health benefits were obtained. While training studies investigating CRF variability are vital in our understanding of the effects of exercise and training on human biology, great care should remain when assessing their primary outcomes.
Moreover, increasing training dose via either intensity or volume have been successful in either eliciting larger improvements in CRF or reducing the number of non-responders.13 28 34 38 However, in some populations, increasing intensity too much might result in negative adaptations.36 It is also apparent that after a while, training at a similar dose fails to elicit further adaptations in CRF.31 Therefore, tailoring training dose to the individual should account for their previous training level and CRF and capacity for recovery. If improvements in CRF are sought, the prescribed training programme should result into a higher training dose compared with the current training regime, but the training dose should not exceed the individual’s capacity for recovery.
There is an abundance of evidence that PA provides key solutions on health benefits both short term and long term. Better CRF is associated with a lower risk of metabolic diseases such as type 2 diabetes mellitus,71 all-cause mortality, cardiorespiratory disease and coronary heart disease related mortality.72 While this review focused primarily on presenting a hierarchal framework based on physiological thresholds and intrinsic self-assessed level of effort, some considerations are needed regarding exercise duration. As little as breaking up long periods of sitting by light activity or even by standing may be enough to acutely induce favourable changes in metabolic parameters.73 Greater health benefits may also be gained with accumulated bouts of 10 min of exercise over days or weeks.74 Oppositely, for young and active individuals, a higher intensity or a larger volume of activity is more effective and may be needed in order to combat the inactivity-induced detriments.75 76
The current review focuses on how to best prescribe exercise with an attempt to improve CRF, but we must acknowledge that health behaviour as well as participating and committing to interventions may be affected by factors reaching beyond biology and methods, including personality characteristics and psychological well-being.77 78 The concept of health-related quality of life refers to a person’s evaluation of physical and mental health over time79 and can be regarded as one the most essential contributors to psychological well-being. Past research has demonstrated that higher levels of health-related quality of life contribute also to increased PA.80 Although the solution to the growing epidemic of physical inactivity and lifestyle-induced diseases sounds simple—exercise more and make healthier food choices—it is difficult for many people to adopt such healthy habits. Targeted, individualised approaches are required as, despite major efforts, physical inactivity has remained a health problem, stemming from many factors.81
Synthesis and conclusions
Chronic disease rate remains on the rise, and current PA guidelines are inadequate to mitigate this worldwide issue. PA guidelines are designed to improve CRF, associated with numerous chronic diseases, cancers and other life-threatening and debilitating conditions. Moreover, large individual variation in training response is observed when standardised and homogenous training programmes and guidelines are prescribed. This might be explained by insufficient control of training intensity resulting in variable homeostatic stress responses interindividually. Since extensive laboratory testing to establish physiological thresholds and individual training zones is not feasible for everyone and might provide an unnecessary barrier to embarking on a training programme, easily available ways of quantifying training intensity should be further examined. The present critical review appraised the value of a novel hierarchal framework that considers accessibility and effectiveness across methods to assess exercise intensity, in order to improve exercise prescription guidelines and lower the instance of non-responders to training to meet the goals of PA guidelines. This framework favours the use of RPE scales, preferably the 10-point scale, at the population level as no equipment is necessary and intensity is intrinsically assessed. RPE requires no devices and has been shown to be an effective method for correlating training intensity to the first ventilatory threshold. However, differences in individual trainability also exist, stemming from biological and methodological sources, such as age, baseline CRF and many other factors, which need to be considered when designing and prescribing exercise.
Footnotes
Contributors: All authors substantially contributed to the conception and design of the work, took part in drafting or extensively revised the manuscript in its current form and approve the submitted version.
Funding: EL, KK and JEP are supported by the Ministry of Education of Finland. DG, KK and JEP are supported by the Amer Cultural Foundation, Finland. KK and JEP are further supported by Business Finland. All authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification or inappropriate data manipulation.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2012;2:1143–211. 10.1002/cphy.c110025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KR, McClave SA, Jampolis MB, et al. The health benefits of exercise and physical activity. Curr Nutr Rep 2016;5:204–12. 10.1007/s13668-016-0175-5 [DOI] [Google Scholar]
- 3.Mann TN, Lamberts RP, Lambert MI. High responders and low responders: factors associated with individual variation in response to standardized training. Sports Med 2014;44:1113–24. 10.1007/s40279-014-0197-3 [DOI] [PubMed] [Google Scholar]
- 4.Meyler S, Bottoms L, Muniz‐Pumares D. Biological and methodological factors affecting response variability to endurance training and the influence of exercise intensity prescription. Exp Physiol 2021;106:1410–24. 10.1113/EP089565 [DOI] [PubMed] [Google Scholar]
- 5.Williams CJ, Gurd BJ, Bonafiglia JT, et al. A Multi-Center Comparison of O2peak Trainability Between Interval Training and Moderate Intensity Continuous Training. Front Physiol 2019;10:19. 10.3389/fphys.2019.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaun GZ. The maximal oxygen uptake verification phase: a light at the end of the tunnel? Sports Med Open 2017;3:44. 10.1186/s40798-017-0112-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poole DC, Jones AM. Measurement of the maximum oxygen uptake V̇o2max: V̇o2peak is no longer acceptable. J Appl Physiol 2017;122:997–1002. 10.1152/japplphysiol.01063.2016 [DOI] [PubMed] [Google Scholar]
- 8.Bouchard C, An P, Rice T, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol 1999;87:1003–8. 10.1152/jappl.1999.87.3.1003 [DOI] [PubMed] [Google Scholar]
- 9.An P, Pérusse L, Rankinen T, et al. Familial aggregation of exercise heart rate and blood pressure in response to 20 weeks of endurance training: the heritage family study. Int J Sports Med 2003;24:57–62. 10.1055/s-2003-37200 [DOI] [PubMed] [Google Scholar]
- 10.Pérusse L, Gagnon J, Province MA, et al. Familial aggregation of submaximal aerobic performance in the heritage family study. Med Sci Sports Exerc 2001;33:597–604. 10.1097/00005768-200104000-00014 [DOI] [PubMed] [Google Scholar]
- 11.Bouchard C, Sarzynski MA, Rice TK, et al. Genomic predictors of the maximal O₂ uptake response to standardized exercise training programs. J Appl Physiol 2011;110:1160–70. 10.1152/japplphysiol.00973.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joyner MJ, Lundby C. Concepts about VO2max and Trainability are context dependent. Exerc Sport Sci Rev 2018;46:138–43. 10.1249/JES.0000000000000150 [DOI] [PubMed] [Google Scholar]
- 13.Montero D, Lundby C. Refuting the myth of non-response to exercise training: 'non-responders' do respond to higher dose of training. J Physiol 2017;595:3377–87. 10.1113/JP273480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson PJ, Atkinson G, Batterham AM. Inter-Individual responses of maximal oxygen uptake to exercise training: a critical review. Sports Med 2017;47:1501–13. 10.1007/s40279-017-0680-8 [DOI] [PubMed] [Google Scholar]
- 15.Wolpern AE, Burgos DJ, Janot JM, et al. Is a threshold-based model a superior method to the relative percent concept for establishing individual exercise intensity? A randomized controlled trial. BMC Sports Sci Med Rehabil 2015;7:16. 10.1186/s13102-015-0011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrès R, Yan J, Egan B, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 2012;15:405–11. 10.1016/j.cmet.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 17.Bryan AD, Magnan RE, Hooper AEC, et al. Physical activity and differential methylation of breast cancer genes assayed from saliva: a preliminary investigation. Ann Behav Med 2013;45:89–98. 10.1007/s12160-012-9411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voisin S, Eynon N, Yan X, et al. Exercise training and DNA methylation in humans. Acta Physiol Oxf Engl 2015;213:39–59. [DOI] [PubMed] [Google Scholar]
- 19.Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics, 1998. [Google Scholar]
- 20.Swaine IL, Emmett J, Murty D, et al. Rating of perceived exertion and heart rate relative to ventilatory threshold in women. Br J Sports Med 1995;29:57–60. 10.1136/bjsm.29.1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tjønna AE, Leinan IM, Bartnes AT, et al. Low- and high-volume of intensive endurance training significantly improves maximal oxygen uptake after 10-weeks of training in healthy men. PLoS One 2013;8:e65382. 10.1371/journal.pone.0065382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seiler S, Sylta Øystein,. How does Interval-Training prescription affect physiological and perceptual responses? Int J Sports Physiol Perform 2017;12:S280–6. 10.1123/ijspp.2016-0464 [DOI] [PubMed] [Google Scholar]
- 23.Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc 2001;33:S446–51. discussion S452-453. 10.1097/00005768-200106001-00013 [DOI] [PubMed] [Google Scholar]
- 24.Hautala AJ, Mäkikallio TH, Kiviniemi A, et al. Cardiovascular autonomic function correlates with the response to aerobic training in healthy sedentary subjects. Am J Physiol Heart Circ Physiol 2003;285:H1747–52. 10.1152/ajpheart.00202.2003 [DOI] [PubMed] [Google Scholar]
- 25.Boutcher SH, Park Y, Dunn SL, et al. The relationship between cardiac autonomic function and maximal oxygen uptake response to high-intensity intermittent-exercise training. J Sports Sci 2013;31:1024–9. 10.1080/02640414.2012.762984 [DOI] [PubMed] [Google Scholar]
- 26.Hautala AJ, Kiviniemi AM, Mäkikallio TH, et al. Individual differences in the responses to endurance and resistance training. Eur J Appl Physiol 2006;96:535–42. 10.1007/s00421-005-0116-2 [DOI] [PubMed] [Google Scholar]
- 27.Maturana FM, Schellhorn P, Erz G, et al. Individual cardiovascular responsiveness to work-matched exercise within the moderate- and severe-intensity domains. Eur J Appl Physiol 2021;121:2039–59. 10.1007/s00421-021-04676-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sisson SB, Katzmarzyk PT, Earnest CP, et al. Volume of exercise and fitness nonresponse in sedentary, postmenopausal women. Med Sci Sports Exerc 2009;41:539–45. 10.1249/MSS.0b013e3181896c4e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanley J, Peake JM, Buchheit M. Cardiac parasympathetic reactivation following exercise: implications for training prescription. Sports Med 2013;43:1259–77. 10.1007/s40279-013-0083-4 [DOI] [PubMed] [Google Scholar]
- 30.Garber CE, Blissmer B, Deschenes MR, et al. American College of sports medicine position stand. quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–59. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- 31.Scharhag-Rosenberger F, Meyer T, Walitzek S, et al. Time course of changes in endurance capacity: a 1-yr training study. Med Sci Sports Exerc 2009;41:1130–7. 10.1249/MSS.0b013e3181935a11 [DOI] [PubMed] [Google Scholar]
- 32.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol 2006;97:141–7. 10.1016/j.amjcard.2005.07.130 [DOI] [PubMed] [Google Scholar]
- 33.Scribbans TD, Vecsey S, Hankinson PB, et al. The Effect of Training Intensity on VO2max in Young Healthy Adults: A Meta-Regression and Meta-Analysis. Int J Exerc Sci 2016;9:230–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milanović Z, Sporiš G, Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med 2015;45:1469–81. 10.1007/s40279-015-0365-0 [DOI] [PubMed] [Google Scholar]
- 35.Burton HM, Wolfe AS, Vardarli E, et al. Background inactivity blunts metabolic adaptations to intense short-term training. Med Sci Sports Exerc 2021;53:1937–44. 10.1249/MSS.0000000000002646 [DOI] [PubMed] [Google Scholar]
- 36.Huang G, Wang R, Chen P, et al. Dose-Response relationship of cardiorespiratory fitness adaptation to controlled endurance training in sedentary older adults. Eur J Prev Cardiol 2016;23:518–29. 10.1177/2047487315582322 [DOI] [PubMed] [Google Scholar]
- 37.Gibala MJ, Little JP, Macdonald MJ, et al. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 2012;590:1077–84. 10.1113/jphysiol.2011.224725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bacon AP, Carter RE, Ogle EA, et al. Vo2Max trainability and high intensity interval training in humans: a meta-analysis. PLoS One 2013;8:e73182. 10.1371/journal.pone.0073182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stöggl T, Sperlich B. Polarized training has greater impact on key endurance variables than threshold, high intensity, or high volume training. Front Physiol 2014;5:33. 10.3389/fphys.2014.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laursen PB. Training for intense exercise performance: high-intensity or high-volume training? Scand J Med Sci Sports 2010;20 Suppl 2:1–10. 10.1111/j.1600-0838.2010.01184.x [DOI] [PubMed] [Google Scholar]
- 41.Hydren JR, Cohen BS. Current scientific evidence for a polarized cardiovascular endurance training model. J Strength Cond Res 2015;29:3523–30. 10.1519/JSC.0000000000001197 [DOI] [PubMed] [Google Scholar]
- 42.Zapata-Lamana R, Henríquez-Olguín C, Burgos C, et al. Effects of polarized training on cardiometabolic risk factors in young overweight and obese women: a Randomized-Controlled trial. Front Physiol 2018;9:1287. 10.3389/fphys.2018.01287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esteve-Lanao J, Foster C, Seiler S, et al. Impact of training intensity distribution on performance in endurance athletes. J Strength Cond Res 2007;21:943–9. 10.1519/R-19725.1 [DOI] [PubMed] [Google Scholar]
- 44.Jamnick NA, Pettitt RW, Granata C, et al. An examination and critique of current methods to determine exercise intensity. Sports Med 2020;50:1729–56. 10.1007/s40279-020-01322-8 [DOI] [PubMed] [Google Scholar]
- 45.Baldwin J, Snow RJ, Febbraio MA. Effect of training status and relative exercise intensity on physiological responses in men. Med Sci Sports Exerc 2000;32:1648–54. 10.1097/00005768-200009000-00020 [DOI] [PubMed] [Google Scholar]
- 46.Lansley KE, Dimenna FJ, Bailey SJ, et al. A 'new' method to normalise exercise intensity. Int J Sports Med 2011;32:535–41. 10.1055/s-0031-1273754 [DOI] [PubMed] [Google Scholar]
- 47.Weatherwax RM, Harris NK, Kilding AE, et al. Incidence of VO2max responders to personalized versus standardized exercise prescription. Med Sci Sports Exerc 2019;51:681–91. 10.1249/MSS.0000000000001842 [DOI] [PubMed] [Google Scholar]
- 48.Hill DW, Cureton KJ, Grisham SC, et al. Effect of training on the rating of perceived exertion at the ventilatory threshold. Eur J Appl Physiol Occup Physiol 1987;56:206–11. 10.1007/BF00640645 [DOI] [PubMed] [Google Scholar]
- 49.Fabre N, Mourot L, Zerbini L, et al. A novel approach for lactate threshold assessment based on rating of perceived exertion. Int J Sports Physiol Perform 2013;8:263–70. 10.1123/ijspp.8.3.263 [DOI] [PubMed] [Google Scholar]
- 50.Elsangedy HM, Krinski K, Costa EC, et al. The rating of perceived exertion is not different at the ventilatory threshold in sedentary women with different body mass indices. J Exerc Sci Fit 2013;11:102–6. 10.1016/j.jesf.2013.11.002 [DOI] [Google Scholar]
- 51.Monnier-Benoit P, Groslambert A, Rouillon J-D. Determination of the ventilatory threshold with affective valence and perceived exertion in trained cyclists: a preliminary study. J Strength Cond Res 2009;23:1752–7. 10.1519/JSC.0b013e3181b74dc1 [DOI] [PubMed] [Google Scholar]
- 52.Halperin I, Emanuel A. Rating of perceived effort: methodological concerns and future directions. Sports Med 2020;50:679–87. 10.1007/s40279-019-01229-z [DOI] [PubMed] [Google Scholar]
- 53.Micklewright D, St Clair Gibson A, Gladwell V, et al. Development and validity of the Rating-of-Fatigue scale. Sports Med 2017;47:2375–93. 10.1007/s40279-017-0711-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed JL, Pipe AL. The talk test: a useful tool for prescribing and monitoring exercise intensity. Curr Opin Cardiol 2014;29:475–80. 10.1097/HCO.0000000000000097 [DOI] [PubMed] [Google Scholar]
- 55.Lyon E, Menke M, Foster C, et al. Translation of incremental talk test responses to steady-state exercise training intensity. J Cardiopulm Rehabil Prev 2014;34:271–5. 10.1097/HCR.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 56.Rodríguez-Marroyo JA, Villa JG, García-López J, et al. Relationship between the talk test and ventilatory thresholds in well-trained cyclists. J Strength Cond Res 2013;27:1942–9. 10.1519/JSC.0b013e3182736af3 [DOI] [PubMed] [Google Scholar]
- 57.Gillespie BD, McCormick JJ, Mermier CM, et al. Talk test as a practical method to estimate exercise intensity in highly trained competitive male cyclists. J Strength Cond Res 2015;29:894–8. 10.1519/JSC.0000000000000711 [DOI] [PubMed] [Google Scholar]
- 58.Jeanes EM, Jeans EA, Foster C, et al. Translation of exercise testing to exercise prescription using the talk test. J Strength Cond Res 2011;25:590–6. 10.1519/JSC.0b013e318207ed53 [DOI] [PubMed] [Google Scholar]
- 59.Persinger R, Foster C, Gibson M, et al. Consistency of the talk test for exercise prescription. Med Sci Sports Exerc 2004;36:1632–6. [PubMed] [Google Scholar]
- 60.Sørensen L, Larsen KSR, Petersen AK. Validity of the talk test as a method to estimate ventilatory threshold and guide exercise intensity in cardiac patients. J Cardiopulm Rehabil Prev 2020;40:330–4. 10.1097/HCR.0000000000000506 [DOI] [PubMed] [Google Scholar]
- 61.Zanettini R, Centeleghe P, Franzelli C, et al. Validity of the talk test for exercise prescription after myocardial revascularization. Eur J Prev Cardiol 2013;20:376–82. 10.1177/2047487312438982 [DOI] [PubMed] [Google Scholar]
- 62.Amann M, Proctor LT, Sebranek JJ, et al. Opioid-Mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 2009;587:271–83. 10.1113/jphysiol.2008.163303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amann M, Venturelli M, Ives SJ, et al. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol 2013;115:355–64. 10.1152/japplphysiol.00049.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol 2017;595:2857–71. 10.1113/JP273185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coquart JBJ, Dufour Y, Groslambert A, et al. Relationships between psychological factors, RPE and time limit estimated by Teleoanticipation. Sport Psychol 2012;26:359–74. 10.1123/tsp.26.3.359 [DOI] [Google Scholar]
- 66.Tyler CJ, Reeve T, Hodges GJ, et al. The effects of heat adaptation on physiology, perception and exercise performance in the heat: a meta-analysis. Sports Medicine 2016;46:1699–724. 10.1007/s40279-016-0538-5 [DOI] [PubMed] [Google Scholar]
- 67.Hochachka PW. Patterns of O2-dependence of metabolism. Adv Exp Med Biol 1988;222:143–51. 10.1007/978-1-4615-9510-6_16 [DOI] [PubMed] [Google Scholar]
- 68.Tschakovsky ME, Pyke KE. Cardiovascular responses to exercise and limitations to human performance. In: Nigel AS, Herbert G, eds. Physiological bases of human performance during work and exercise. New York: Churchill Livingstone, 2008: 5–27. [Google Scholar]
- 69.Drouin PJ, Kohoko ZIN, Mew OK, et al. Fatigue-independent alterations in muscle activation and effort perception during forearm exercise: role of local oxygen delivery. J Appl Physiol 2019;127:111–21. 10.1152/japplphysiol.00122.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Zwaard S, Jaspers RT, Blokland IJ, et al. Oxygenation threshold derived from near-infrared spectroscopy: reliability and its relationship with the first ventilatory threshold. PLoS One 2016;11:e0162914. 10.1371/journal.pone.0162914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jadhav RA, Hazari A, Monterio A, et al. Effect of physical activity intervention in prediabetes: a systematic review with meta-analysis. J Phys Act Health 2017;14:745–55. 10.1123/jpah.2016-0632 [DOI] [PubMed] [Google Scholar]
- 72.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 2009;301:2024–35. 10.1001/jama.2009.681 [DOI] [PubMed] [Google Scholar]
- 73.Gao Y, Silvennoinen M, Pesola AJ, et al. Acute metabolic response, energy expenditure, and EMG activity in sitting and standing. Med Sci Sports Exerc 2017;49:1927–34. 10.1249/MSS.0000000000001305 [DOI] [PubMed] [Google Scholar]
- 74.Chastin SFM, De Craemer M, De Cocker K, et al. How does Light-intensity physical activity associate with adult cardiometabolic health and mortality? systematic review with meta-analysis of experimental and observational studies. Br J Sports Med 2019;53:370–6. 10.1136/bjsports-2017-097563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ekelund U, Brage S, Griffin SJ, et al. Objectively measured moderate- and vigorous-intensity physical activity but not sedentary time predicts insulin resistance in high-risk individuals. Diabetes Care 2009;32:1081–6. 10.2337/dc08-1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pulsford RM, Blackwell J, Hillsdon M, et al. Intermittent walking, but not standing, improves postprandial insulin and glucose relative to sustained sitting: a randomised cross-over study in inactive middle-aged men. J Sci Med Sport 2017;20:278–83. 10.1016/j.jsams.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 77.Raynor DA, Levine H. Associations between the five-factor model of personality and health behaviors among college students. J Am Coll Health 2009;58:73–81. 10.3200/JACH.58.1.73-82 [DOI] [PubMed] [Google Scholar]
- 78.Burgess E, Hassmén P, Pumpa KL. Determinants of adherence to lifestyle intervention in adults with obesity: a systematic review. Clin Obes 2017;7:123–35. 10.1111/cob.12183 [DOI] [PubMed] [Google Scholar]
- 79.Häkkinen A, Rinne M, Vasankari T, et al. Association of physical fitness with health-related quality of life in Finnish young men. Health Qual Life Outcomes 2010;8:15. 10.1186/1477-7525-8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bize R, Johnson JA, Plotnikoff RC. Physical activity level and health-related quality of life in the general adult population: a systematic review. Prev Med 2007;45:401–15. 10.1016/j.ypmed.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 81.Annesi JJ, Unruh JL. Effects of the coach approach intervention on drop-out rates among adults initiating exercise programs at nine YMCAs over three years. Percept Mot Skills 2007;104:459–66. 10.2466/pms.104.2.459-466 [DOI] [PubMed] [Google Scholar]
- 82.Gibson AL, Wagner DR, Heyward VH. Advanced fitness assessment and exercise prescription. In: Human kinetics. Eighth ed, 2019. [Google Scholar]