Abstract
Background
This review aimed to compare the relative effectiveness of different exercise-based cardiac rehabilitation (ExCR) delivery modes (centre-based, home-based, hybrid and technology-enabled ExCR) on key heart failure (HF) outcomes: exercise capacity, health-related quality of life (HRQoL), HF-related hospitalisation and HF-related mortality.
Methods and results
Randomised controlled trials (RCTs) published through 20 June 2021 were identified from six databases, and reference lists of included studies. Risk of bias and certainty of evidence were evaluated using the Cochrane tool and Grading of Recommendations Assessment, Development and Evaluation, respectively. Bayesian network meta-analysis was performed using R. Continuous and binary outcomes are reported as mean differences (MD) and ORs, respectively, with 95% credible intervals (95% CrI). One-hundred and thirty-nine RCTs (n=18 670) were included in the analysis. Network meta-analysis demonstrated improvements in VO2peak following centre-based (MD (95% CrI)=3.10 (2.56 to 3.65) mL/kg/min), home-based (MD=2.69 (1.67 to 3.70) mL/kg/min) and technology-enabled ExCR (MD=1.76 (0.27 to 3.26) mL/kg/min). Similarly, 6 min walk distance was improved following hybrid (MD=84.78 (31.64 to 138.32) m), centre-based (MD=50.35 (30.15 to 70.56) m) and home-based ExCR (MD=36.77 (12.47 to 61.29) m). Incremental shuttle walk distance did not improve following any ExCR delivery modes. Minnesota living with HF questionnaire improved after centre-based (MD=−10.38 (−14.15 to –6.46)) and home-based ExCR (MD=−8.80 (−13.62 to –4.07)). Kansas City Cardiomyopathy Questionnaire was improved following home-based ExCR (MD=20.61 (4.61 to 36.47)), and Short Form Survey 36 mental component after centre-based ExCR (MD=3.64 (0.30 to 6.14)). HF-related hospitalisation and mortality risks reduced only after centre-based ExCR (OR=0.41 (0.17 to 0.76) and OR=0.42 (0.16 to 0.90), respectively). Mean age of study participants was only associated with changes in VO2peak.
Conclusion
ExCR programmes have broader benefits for people with HF and since different delivery modes were comparably effective for improving exercise capacity and HRQoL, the selection of delivery modes should be tailored to individuals’ preferences.
Keywords: cardiac rehabilitation; epidemiology; outcome assessment, health care; heart failure; meta-analysis
What is already known about this subject
Exercise training is an integral component of heart failure (HF) management and can be administered via several delivery modes. However, the relative effectiveness of different delivery modes remains unclear.
What does this study add
This network meta-analysis is the first to demonstrate the relative effectiveness of different exercise-based cardiac rehabilitation (ExCR) delivery modes on functional, patient-reported and clinical outcomes among people with HF.
All delivery modes substantially exceeded the minimal clinically important difference (MCID=1 mL/kg/min) for mean changes in VO2peak (1.76–3.10 mL/kg/min).
All delivery modes except technology-enabled ExCR exceeded the 6 min walk distance MCID (30 m).
All delivery modes exceeded the Minnesota living with HF questionnaire MCID (−5 points), and home-based and technology-enabled modes also exceeded the Kansas City Cardiomyopathy Questionnaire MCID (5.7 points).
Centre-based ExCR reduced HF-related hospitalisations and HF-related mortality by approximately 60% relative to usual care.
How might this impact on clinical practice
ExCR programmes have broader benefits for people with HF and since different delivery modes are comparably beneficial for exercise capacity and quality of life, selection should be tailored for participants’ preferences and goals, clinical history and risk stratification, and priority outcomes.
Background
Heart failure (HF) is a major public health problem associated with high mortality and morbidity,1 as well as significant reductions in exercise capacity and health-related quality of life (HRQoL).2 Clinical guidelines recommend cardiac rehabilitation (CR), a comprehensive intervention comprising exercise and multifactorial education, to achieve and maintain optimal health and prevent further complications for people with HF.3 4
Exercise-based CR (ExCR) is recommended as an integral component of comprehensive HF care.4–6 ExCR is defined as a supervised or unsupervised exercise training provided to people with cardiac disease in or outside clinical settings and can be provided standalone or as a component of comprehensive CR.7 Exercise training improves exercise capacity and quality of life and can reduce hospitalisation and mortality in people with mild-to-moderate chronic HF.8 ExTraMATCH II reported HRQoL and exercise capacity were higher after ExCR than no ExCR control.9 A Cochrane review reported ExCR improved all-cause and HF-specific hospital admissions and HRQoL.10
In pairwise meta-analyses, home-based (HB) ExCR showed significant improvements in exercise capacity and HRQoL over no ExCR control among people with HF.11 12 In a meta-analysis of randomised controlled trials (RCTs), a combination of home-based and centre-based (CB) ExCR showed a 9.72 mL/kg/min increase in VO2peak over no ExCR control but not in HRQoL (9 RCTs, n=306).11 HB ExCR showed greater improvements in VO2peak (2.39 mL/kg/min, 18 RCTs, n=1191) and HRQoL (16 RCTs, n=576; standardised mean difference (MD): 0.38) over no ExCR control.11 There was no statistically significant difference between HB and CB ExCR in improving exercise capacity and HRQoL.11 12
Previous systematic reviews and pairwise meta-analyses reported ExCR has potential health benefits.9–16 Since standard meta-analytical procedures can only consider pairwise comparisons, there is limited understanding of how all delivery modes compare. Network meta-analysis (NMA) overcomes this limitation by enabling simultaneous comparisons between more than two treatments.17
The aim of this systematic review and NMA was to compare the relative effectiveness of centre, home, technology-enabled (TE) and hybrid ExCR interventions on key HF outcomes (exercise capacity, HRQoL, HF-specific hospitalisation and HF-specific mortality) and to discuss the relative pros and cons of different delivery modes.
Methods
We conducted and reported this NMA in accordance with the PRISMA extension statement for reporting of systematic reviews incorporating NMA of healthcare interventions18 and the PRISMA 2020 statement.19
Search strategy
Six electronic databases (MEDLINE, EMBASE, CINAHL, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science and PsycINFO) were searched up to 20 June 2021, for studies that combine two key subject areas: HF and exercise. A search strategy including MeSH and free-text terms was developed for MEDLINE and adapted for other databases. The full study protocol, including a detailed search strategy, was registered with PROSPERO before undertaking study selection (ID: CRD42021264709). The search was limited to English-language reports but not restricted by sample size. Reference lists of included studies and relevant systematic reviews and meta-analyses identified by the database search were manually searched for additional studies. Search results were exported to Covidence for duplicate removal, screening, data extraction and quality assessment.
Eligibility criteria
Eligible studies were RCTs comparing ExCR against usual care (UC) or another ExCR delivery mode among adults (≥18 years) with HF with preserved or reduced ejection fraction. ExCR interventions, either alone or as a component of CR20 lasting a minimum of 4 weeks, were included. A 4-week minimum duration aligns with common 30-day postdischarge mortality and hospitalisation outcomes. For this review, ExCR was grouped based on delivery mode into CB, HB, TE and hybrid. Interventions were classified as CB if >50% of programme delivery occurred in traditional clinical settings (eg, hospitals, rehabilitation centres or comparable community facilities), HB if >50% of programme delivery occurred outside traditional clinical settings (eg, clinician home visits, written resources, self-monitoring diaries) without the use of information communication technologies (ICT), and TE if >50% of programme delivery occurred via ICT (eg, video calls, phone calls or text messages) and outside traditional clinical centres. Interventions were classified as hybrid (HY) if they included ≥2 delivery modes, each contributing 20%–50% to programme delivery. Hybrid programmes could use different delivery modes in parallel or sequentially. Eligible comparators were UC (standard medical care including other components of comprehensive CR but excluding exercise training) or ExCR as defined above.
Outcomes and outcome measures
Studies were included if they reported any of the following ExCR outcomes: exercise capacity, HRQoL, HF-related hospitalisations or HF-related mortality. The primary outcomes were exercise capacity and HRQoL measured on a continuous scale (eg, MD and SD). The secondary outcomes of our analyses were the number of HF-related hospitalisations and HF-related mortality.
Study selection
Two reviewers independently screened all search results (TKT and RAN) and reviewed full-text papers (TKT and KYA) if the title or abstract identified the eligible population and intervention. Discrepancies were resolved by consensus and/or a third reviewer (JCR).
Data extraction
Arm-level data were independently extracted into Covidence by two reviewers (TKT and KYA). For each study, data related to study characteristics (intervention and comparator characteristics (eg, delivery mode), sample size, first author, country, year of publication), study population (eg, mean age, gender) and outcomes of interest (as above) were extracted. For studies that had multiple reports, we extracted data for all relevant outcomes without duplication. If outcome data were reported at multiple time points, exercise capacity and HRQoL were extracted at the postintervention time point while HF-related hospitalisation and HF-related mortality were extracted at the longest follow-up time point. If the assessment period for HF-related hospitalisation or mortality were not explicitly reported, we assumed data represented participants entire trial participation period.
Risk of bias assessment
Two reviewers (TKT and KTK) independently assessed the risk of bias using the Cochrane ‘Risk of Bias 2 (RoB-2)’ tool for RCTs. RoB-2 has five bias domains: bias arising from the randomisation process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome and bias in selection of the reported result.21 Reviewers assigned a judgement of ‘low risk of bias,’ ‘some concerns,’ or ‘high risk of bias’ for each domain item.21 The overall RoB for a study was judged to be at low RoB if all domains were at low RoB, some concerns if at least one domain was at some concerns, and high RoB if at least one domain was at high RoB or judged to have some concerns for multiple domains in a way that substantially lowers confidence in the result.21 Discrepancies were resolved through discussion and involving a third author (KYA) when needed.
Certainty of evidence assessment
The NMA-specific Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool was used to assess the certainty in the evidence22–25 based on the following domains: risk of bias, publication bias, imprecision, inconsistency (heterogeneity), incoherence and indirectness.22 25 Evidence was rated as ‘high’, ‘moderate’, ‘low’ or ‘very low’.22 25 GRADE assessments were performed independently by two reviewers (TKT and KTK). Discrepancies were resolved through discussion.
Statistical analysis
 Bayesian NMA was performed using the gemtc and BUGSnet packages in R. A network graph was generated to provide details of the network geometry. In the network graph, the sizes of the nodes represent the total sample size for each ExCR delivery mode, while line thickness (with a number on it) corresponds to the number of RCTs comparing the ExCR interventions. Model compilation and Markov Chain Monte Carlo simulation were performed to estimate the posterior distributions of model parameters. Continuous and binary outcomes were reported as MDs and OR, respectively, with 95% credible intervals (CrI).
Bayesian NMA was performed using the gemtc and BUGSnet packages in R. A network graph was generated to provide details of the network geometry. In the network graph, the sizes of the nodes represent the total sample size for each ExCR delivery mode, while line thickness (with a number on it) corresponds to the number of RCTs comparing the ExCR interventions. Model compilation and Markov Chain Monte Carlo simulation were performed to estimate the posterior distributions of model parameters. Continuous and binary outcomes were reported as MDs and OR, respectively, with 95% credible intervals (CrI).
Model convergence was evaluated using trace plots and the Gelman-Rubin-Brooks diagnostics. We used the nma.fit function from the BUGSnet package to identify the best fitting model. This function produced a plot of the leverage values along with the corresponding effective number of parameters, total residual deviance and deviance information criterion. Based on this evaluation, we used the random-effects model to estimate direct, indirect and network effect estimates. Incoherence between direct and indirect effect estimates in closed networks was assessed using the nodesplit method in the gemtc package and the nma.fit and nma.compare functions from the BUGSnet package. Forest plots were used to visualise direct, indirect and network effect estimates.
Furthermore, the surface under the cumulative ranking (SUCRA) function from the dmetar package was used to estimate ranking probabilities for all interventions using a SUCRA curve.26 27 The SUCRA score was reported as a percentage, which represents the cumulative probability of a particular intervention being the top-ranking intervention among a set of n interventions. The closer the SUCRA score is to 100%, the higher ranking the intervention in the hierarchy.26 27 Ranking probabilities were visualised in SUCRA plots using the nma.rank function in BUGSnet
The relative effectiveness of ExCR interventions could differ across a variety of factors. We performed network meta-regression to determine if trial-level risk of bias, ExCR treatment duration and participant age influenced the magnitude of effect sizes found in the network.
Results
Study selection and characteristics of included studies
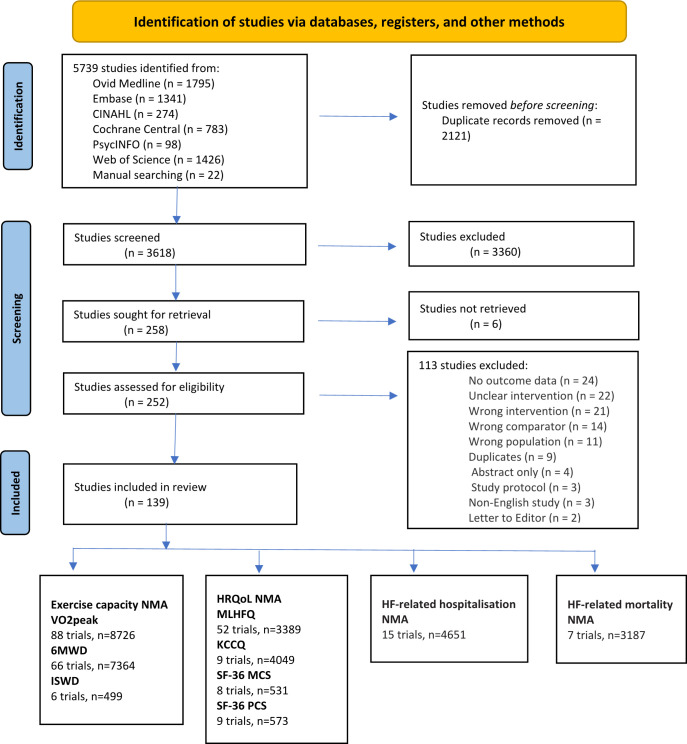
Our systematic search identified 5739 potentially relevant studies, including 22 studies identified from bibliographies of reports of relevant systematic reviews and meta-analysis (figure 1). After full-text screening, we included 139 RCTs, with 18 670 participants conducted between 1996 and 2021. The studies were conducted in 28 countries spread across Europe (eg, UK, Germany, Netherlands, Switzerland), North and South America (eg, USA, Canada, Brazil, Uruguay), Africa (Nigeria), Asia (eg, China, Taiwan) and Australia.
Figure 1.
The PRISMA 2020 flow diagram of study selection.19 6MWD, 6-min walk distance; HF, heart failure; HRQoL, health-related quality of life; ISWD, incremental shuttle walk distance; KCCQ, Kansas City Cardiomyopathy Questionnaire; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NMA, network meta-analysis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SF-36 MCS, Short Form Survey 36 Mental Component Score; SF-36 PCS, SF 36 Physical Component Score; VO2peak, peak oxygen uptake.
All four ExCR delivery modes were represented. Of the 139 trials, 80 were centred-based vs UC, and 35 were HB vs UC, followed by 9 hybrid vs UC, 7 TE vs UC, 4 centre vs HB, 3 CB vs TE and 1 hybrid vs HB. Detailed information about trial treatments is available in online supplemental file 1, and comparisons are summarised in the network plot figure. A small number of studies reported exercise intensity (n=15) and exercise training compliance (n=18). Aerobic (n=84) and aerobic +resistance (n=27) were the most common training modes, followed by flexibility (n=9), resistance (n=8), aerobic +resistance + flexibility (n=7), aerobic +flexibility (n=2) and resistance +flexibility (n=2). Characteristics of included RCTs28–166 are summarised in online supplemental file 1.
openhrt-2021-001949supp001.pdf (416KB, pdf)
The median sample size was 50 participants (range: 10–2331), median participant age was 61.1 years (range: 44–81) and 71.4% of the pooled sample population were male. The median exercise programme duration was 12 weeks (IQR: 12–24 weeks). One study delivered a 10-year programme; however, this comprised three supervised sessions per week for 2 months followed by only two supervised sessions per year.90 The median length of study follow-up was 16 weeks (IQR: 12–26 weeks).
Included studies assessed exercise capacity via peak oxygen uptake (VO2peak, mL/kg/min) or proxy measures including 6 min walk distance (6MWD, m) and incremental shuttle walk distance (ISWD, m). HRQoL was assessed with the Minnesota Living with Heart Failure Questionnaire (MLHFQ), Kansas City Cardiomyopathy Questionnaire (KCCQ) and Short Survey Form 36 (SF-36) mental and physical components (figure 1). HF-related hospitalisations and HF-related mortalities were reported in absolute numbers.
Of the 139 RCTs, 12 trials reported adverse events that occurred during or immediately after exercise training.40 43 53 56 74 90 104 110 132 145 156 158 The reported adverse events were: worsening of HF, hospitalisation due to myocardial infarction, acute coronary syndrome, musculoskeletal injury, shortness of breath, hypoglycaemia, palpitations, angina, arrhythmia, presyncope or syncope, occlusion of peripheral bypass, ectopic heartbeats, hypotension and back pain. No exercise-induced fatal events were reported.
Risk of bias assessment
Sixty-nine (49.6%) of the 139 RCTs had high overall risk of bias (figure 2); 33 (23.7%) studies had high risk of bias due to the randomization process, 25 (18.8%) due to missing outcome data, 27 (19.4%) due to measurement of the outcome, and one due to selection of the reported result. Two studies31 120 had a high risk of bias due to deviations from the intended interventions, where 23 participants crossed over from control to intervention. Of the 139 RCTs, 66 (47.5%) had some concerns about their overall risk of bias: 122 (87.8%) RCTs had some concerns due to the selection of the reported result—studies did not report if they followed a prespecified analysis plan; 72 (51.8%) due to bias in the measurement of the outcome—studies did not report if outcome assessors were blind; and 60 (43.2%) due to the randomization process—studies did not clearly describe allocation concealment. One hundred and thirty-five (97.1%) RCTs had a low risk of bias due to deviations from intended interventions, and 101 (72.7%) due to missing outcome data (figure 2).
Figure 2.
The Cochrane risk of bias graph for the included studies.
NMA outcomes
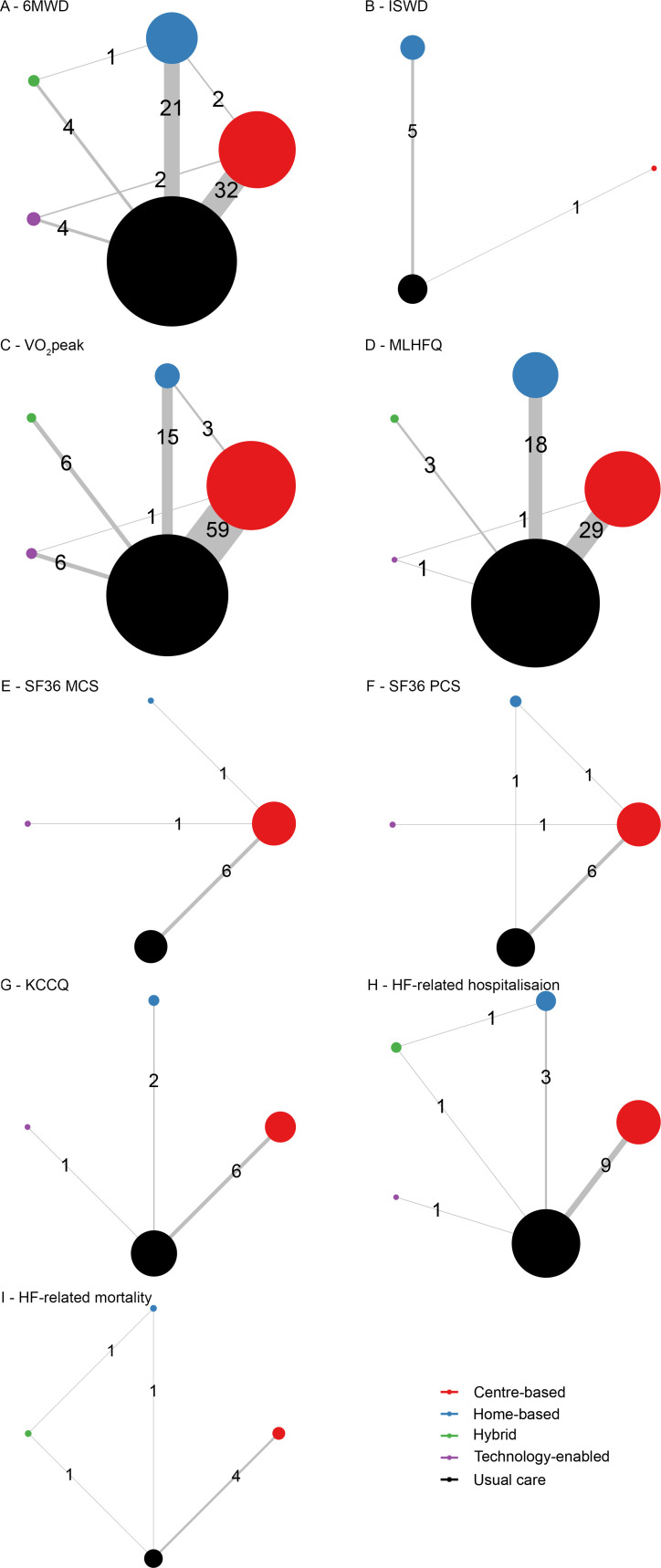
Network plots of eligible comparisons for all outcome measures are shown in figure 3. GRADE assessments of evidence certainty are presented in online supplemental file 2.
Figure 3.
Network geometry for comparisons of treatment effects. 6MWD, 6-min walk distance; HF, heart failure; ISWD, incremental shuttle walk distance; KCCQ, Kansas City Cardiomyopathy Questionnaire; MLHFQ, Minnesota Living with Heart Failure Questionnaire; SF-36 MCS, Short Form Survey 36 Mental Component Score; SF-36 PCS, SF 36 Physical Component Score; VO2peak, peak oxygen uptake.
Exercise capacity
Six-min walk distance
Among 66 comparisons of effects on 6MWD, 32 were between CB ExCR and UC followed by 21 HB ExCR and UC (figure 3). Only hybrid, CB and HB ExCR were associated with increases in 6MWD relative to UC (MD (95% CrI)=84.78 (31.64 to 138.32) m: moderate evidence, MD=50.35 (30.15 to 70.56) m: high evidence and MD=36.77 (12.47 to 61.29) m: moderate evidence, respectively). There were no statistically significant differences between delivery modes (online supplemental files 2 and 3).
SUCRA showed that hybrid ExCR had the highest probability of being ranked first (94.6%), followed by CB ExCR (68.8%) and HB ExCR (46.9%) (online supplemental file 4). There was evidence of network heterogeneity (I2=97.67%) but not incoherence (p>0.1).
Incremental shuttle walk distance
Among six comparisons of effects on ISWD, five were between HB ExCR and UC (figure 3). Neither home or CB programmes improved ISWD compared with UC (HB MD=23.28 (−16.62 to 60.40) m; moderate evidence, and CB MD=9.05 (−70.20 to 88.29) m; low evidence). There was no statistically significant difference between the two ExCR modes (online supplemental files 2 and 3).
Although it did not show statistical significance, SUCRA showed that HB ExCR had the highest probability of being ranked first (76.8%), followed by CB ExCR (48.0%) (online supplemental file 4). There was evidence of network heterogeneity (I2=99.05%).
Peak oxygen uptake
Among 90 comparisons of effects on VO2peak, 59 were between CB ExCR and UC followed by 15 HB ExCR and UC (figure 3). Only CB, HB and TE ExCR were associated with increases in peak oxygen uptake compared with UC (MD=3.10 (2.55 to 3.65) mL/kg/min; high evidence, MD=2.69 (1.67 to 3.70) mL/kg/min; moderate evidence and MD=1.76 (0.26, 3.26) mL/kg/min: low evidence, respectively). There were no statistically significant differences between delivery modes (online supplemental files 2 and 3).
SUCRA showed that CB ExCR had the highest probability of being ranked first (90.5%), followed by HB ExCR (71.8%) and hybrid ExCR (44.1%) (online supplemental file 4). There was evidence of network heterogeneity (I2=94.59%) but not incoherence (p>0.1).
Health-related quality of life
MLHFQ score
Among 52 comparisons of effects on MLHFQ, 29 were between CB ExCR and UC followed by 18 HB ExCR and UC (figure 3). Only centre and HB ExCR showed significant decreases in MLHFQ score compared with UC (MD=−10.38 (−14.15 to –6.46); high evidence, and MD=−8.80 (−13.62 to –4.07); low evidence, respectively). There were no statistically significant differences between delivery modes (online supplemental files 2 and 3).
SUCRA showed that TE ExCR had the highest probability of being ranked first (70.6%), followed by CB ExCR (66.6%) and hybrid ExCR (56.6%) (online supplemental file 4). There was evidence of network heterogeneity (I2=98.05%) but not incoherence (p>0.1).
SF-36 mental component summary score
Among eight comparisons of effects on the SF-36 mental component summary score, six were between CB ExCR and UC (figure 3). Only CB delivery was associated with a statistically significant increase relative to UC (MD (95% CrI)=3.64 (0.30 to 6.14); moderate evidence). There were no statistically significant differences between the CB, HB or TE delivery modes (online supplemental files 2 and 3).
SUCRA showed that CB ExCR had the highest probability of being ranked first (74.7%), followed by TE (70.4%) and HB ExCR (40.2%) (online supplemental file 4). There was evidence of network heterogeneity (I2=83.4%).
SF-36 physical component summary score
Among nine comparisons of effects on SF-36 physical component summary score, six were between CB ExCR and UC (figure 3). No delivery mode improved the SF-36 physical component summary score compared with UC (CB MD=3.24 (−0.37 to 7.35); moderate evidence, HB MD=3.28 (−3.63 to 10.74); high evidence) and TE MD=3.59 (−5.38 to 13.21); moderate evidence). There were no statistically significant differences between the three modes (online supplemental files 2 and 3).
Although it did not show statistical significance, SUCRA showed that CB ExCR had the highest probability of being ranked first (63.8%), followed by TE (62.6%) and HB ExCR (60.4%) (online supplemental file 4). There was evidence of network heterogeneity (I2=98.18%) but not incoherence (p>0.1).
KCCQ score
Among nine comparisons of effects on KCCQ, six were between CB ExCR and UC (figure 3). Only HB ExCR was associated with a significant increase in KCCQ relative to UC (MD=20.61 (4.61 to 36.47); moderate evidence). There were no statistically significant differences between delivery modes (online supplemental files 2 and 3).
SUCRA showed that HB ExCR had the highest probability of being ranked first (95.6%), followed by TE ExCR (56.9%) and CB ExCR (41.5%) (online supplemental file 4). There was evidence of network heterogeneity (I2=98.77%).
HF-related hospitalisation
Among 15 comparisons of effects on HF-related hospitalisation, nine were between CB ExCR and UC, and included relatively short observation periods (4–60 weeks) except for one study with a 520-week treatment period90 (figure 3). CB ExCR was the only delivery mode associated with lower HF-related hospitalisation risk (OR=0.41 (95% CrI 0.17 to 0.76): high evidence), and HF-related hospitalisation risk did not differ between ExCR delivery modes (online supplemental files 2 and 3).
SUCRA showed that hybrid ExCR had the highest probability of being ranked first (75.2%), followed by HB ExCR (71.7%) and CB ExCR (66.2%) (online supplemental file 4). There was evidence of network heterogeneity (I2=87.81%) but not incoherence (p>0.1).
HF-related mortality
Only seven comparisons assessed effects on HF-related mortality; four were between CB ExCR and UC, and included relatively short observation periods (12–60 weeks) except the one study with a 520-week treatment period90 (figure 3). Similar to HF-related hospitalisation, CB ExCR was the only delivery mode associated with lower HF-related mortality risk (OR=0.42 (95% CrI 0.16 to 0.90): moderate evidence), and effects did not differ between ExCR delivery modes (online supplemental files 2 and 3).
SUCRA showed that hybrid ExCR had the highest probability of being ranked first (88.9%), followed by CB ExCR (56.9%) and HB ExCR (45.0%; online supplemental file 4). There was neither network heterogeneity (I2=0) nor incoherence (p>0.1).
Network meta-regression
Mean age of study participants was significantly associated with changes in VO2peak (β (95% CrI)=−1.41 (–2.37 to –0.46)), but not with other outcomes. After controlling for age, only CB, TE and HB ExCR were associated with significant increases in VO2peak relative to UC (MD=3.22 (2.69 to 3.75) mL/kg/min, MD=1.90 (0.46 to 3.33) mL/kg/min and MD=2.52 (1.55 to 3.50) mL/kg/min, respectively). Risk of bias and exercise programme duration were not significantly associated with any outcomes (results not presented).
Discussion
This NMA is the first to demonstrate the relative effectiveness of different ExCR delivery modes on functional, patient-reported and clinical outcomes among people with HF. While the quality of evidence and number of studies included in each comparison varied markedly the overall results across delivery modes are consistent with previous research evaluating the benefits of ExCR among people with HF.167–171
As the mainstay approach in many countries, CB delivery has been studied extensively and was associated with improvements in at least one measure of exercise capacity and HRQoL as well as HF-related hospitalisation and mortality.16 20 172 173 HB delivery was the next most widely studied mode and, consistent with previous pairwise meta-analyses, it was associated with improvements in exercise capacity and HRQoL but not hospitalisation or mortality risks.11 12 Neither centre nor HB delivery modes improved ISWD. Only six studies, with relatively small numbers of participants (ranges from 33 to 65) and a high risk of bias, evaluated the effect of centre and HB ExCR on ISWDe. Effect estimates of comparisons involving few studies with a small number of participants and low to moderate evidence suggest this should be interpreted with caution pending further research. While few published studies have evaluated TE or hybrid11 delivery modes among people with HF, both were associated with improvements in exercise capacity. Neither TE nor hybrid delivery improved HF-related hospitalisation or mortality risk; however, small numbers of studies mean it may be too soon to draw definitive conclusions about the effects of hybrid and TE delivery on clinical outcomes.
While not all delivery modes were effective for all outcome measures, it is important to note we found no evidence of differential effectiveness between delivery modes. Small numbers of comparisons and low to moderate evidence suggest this should be interpreted with caution pending further research, but comparable outcomes between delivery modes are promising given the impact of accessibility barriers on rates of participation in CB programmes (eg, transportation problem and travel costs, distance to rehabilitation centres and rehabilitation costs).174 Effective HB, TE or hybrid delivery modes may help to increase uptake and adherence by enabling people to undertake ExCR in more accessible locations. While these delivery modes did not improve key clinical endpoints compared with UC, mean changes in VO2peak (1.76–3.10 mL/kg/min) substantially exceeded the clinically important difference associated with reduced mortality risk (1 mL/kg/min).169 170 All delivery modes except TE ExCR exceeded minimal clinically important difference (MCID)=30 m of 6MWD,167 168 and HB and TE modes exceeded MCID=5.7 points of KCCQ.171 Similarly, all delivery modes exceeded MCID=−5 points of MLHFQ.175–177
Implementing a range of different ExCR modes in clinical practice—including hybrid options—could be important to maximise uptake rates and adherence by meeting a wide range of participant needs and preferences, and safety concerns.11 Hybrid ExCR could be done in any order/sequence to form a cohesive and comprehensive CR programme. This may be particularly beneficial for people who experience challenges accessing CB programmes, but risk stratification indicates a need for direct supervision by a healthcare professional. For instance, initial CB sessions could be undertaken to manage physical and psychosocial risks, increase participation in group education sessions, and tailor the exercise regimen based on direct observation. When appropriate, subsequent transition into TE178 could aid adherence by reducing accessibility challenges while preserving a level of supervision and monitoring.
In addition to the relative effect estimates, we also reported cumulative ranking probabilities which support to assist decision making by identifying the likelihood of a particular treatment would be best for a specific outcome.27 This may be most useful when the rankings of cumulative probabilities and effect estimates align—as was the case for 6MWD in this review (hybrid ExCR effect estimate=84.78 m, cumulative probability of ranking first=94.6%; CB ExCR effect estimate=50.35 m, cumulative probability of ranking second=68.8%; HB ExCR effect estimate=36.77 m, cumulative probability of ranking third=46.9%). However, use of rankings to inform decision making requires some caution because they do not account for the quality of underpinning evidence, magnitudes of differences between individual treatments, or the possibility differences between treatments may be explained by chance. Moreover, as ranking probabilities relate to a single outcome they do not consider the importance other relevant benefits, harms or pragmatic factors such as cost and complexity.27 Therefore, the selection of ExCR delivery modes should consider a wide range of factors in addition to probability rankings, and the most desirable option(s) may vary between individuals and across healthcare contexts.
This NMA was not without limitations. Few studies with relatively small numbers of participants evaluated TE and hybrid ExCR, therefore, effect estimates of comparisons involving these delivery modes were imprecise. Second, the methods of included studies were not well described, and most studies were judged to have some concerns of risk of bias. Specifically, few studies adequately described the randomisation process (allocation concealment), outcome assessment (outcome assessor blinding) or whether analyses followed a prespecified plan (selection of reported results). Although overall risk of bias was not associated with outcome effects in the network meta-regression, several studies were judged to have high risk of bias. In addition, the results of this NMA could be biased for numerous causes including heterogeneity in study population (eg, gender and age), exercise regimen and intensity of the training, compliance to training. Finally, interpretation of effect estimates on HF-related hospitalisation and mortality are impacted by a very broad range of follow-up periods, and a lack of explicit reporting of the follow-up period in some studies.
Conclusion
ExCR programmes improve functional capacity, quality of life and/or clinical outcomes compared with UC, regardless of whether they are delivered in clinical centres, at home, via digital technologies or a combination of these. ExCR services should consider offering different delivery modes to meet a wider range of participant needs and preferences, and mode selection should consider factors such as individual preferences and goals, clinical history and risk stratification, and priority outcomes.
Footnotes
Twitter: @kteketo, @jrawstorn, @kedirymam331@gmail.com
Contributors: TKT, JCR and RM conceptualised the design of the network meta-analysis and systematic review. TKT and JCR drafted the manuscript. TKT, JCR and RAN contributed to the development of the article search strategy. TKT, RAN, KYA and KTK conducted search screening, study selection, data extraction and quality assessment. TKT performed the data analysis. TKT, JCR, RAN, KYA, KTK and RM have participated in critical revision of the manuscript for important intellectual content. All authors read, provided feedback and approved the final manuscript. TKT is the guarantor who accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: TKT was supported by a Deakin University Dean's Postdoctoral Fellowship (420131).JCR was supported by a National Heart Foundation of Australia Postdoctoral Fellowship (102585).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015;385:812–24. 10.1016/S0140-6736(14)61889-4 [DOI] [PubMed] [Google Scholar]
- 2.Esposito F, Mathieu-Costello O, Shabetai R, et al. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol 2010;55:1945–54. 10.1016/j.jacc.2009.11.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The BACPR standards and core components for cardiovascular disease prevention and rehabilitation, 2017. Available: www.bacpr.com/resources/6A7_BACR
- 4.Dalal HM, Doherty P, Taylor RS. Cardiac rehabilitation. BMJ 2015;351:h5000. 10.1136/bmj.h5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondamudi N, Haykowsky M, Forman DE, et al. Exercise training for prevention and treatment of heart failure. Prog Cardiovasc Dis 2017;60:115–20. 10.1016/j.pcad.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 6.Fletcher GF, Landolfo C, Niebauer J, et al. Promoting physical activity and exercise: JACC health promotion series. J Am Coll Cardiol 2018;72:1622–39. 10.1016/j.jacc.2018.08.2141 [DOI] [PubMed] [Google Scholar]
- 7.Anderson L, Thompson DR, Oldridge N. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008;29:2388–442. 10.1093/eurheartj/ehn309 [DOI] [PubMed] [Google Scholar]
- 9.Taylor RS, Walker S, Smart NA, et al. Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: individual participant meta-analysis. J Am Coll Cardiol 2019;73:1430–43. 10.1016/j.jacc.2018.12.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor RS, Long L, Mordi IR, et al. Exercise-based rehabilitation for heart failure: Cochrane systematic review, meta-analysis, and trial sequential analysis. JACC Heart Fail 2019;7:691–705. 10.1016/j.jchf.2019.04.023 [DOI] [PubMed] [Google Scholar]
- 11.Imran HM, Baig M, Erqou S, et al. Home-based cardiac rehabilitation alone and hybrid with center-based cardiac rehabilitation in heart failure: a systematic review and meta-analysis. J Am Heart Assoc 2019;8:e012779. 10.1161/JAHA.119.012779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zwisler A-D, Norton RJ, Dean SG, et al. Home-based cardiac rehabilitation for people with heart failure: a systematic review and meta-analysis. Int J Cardiol 2016;221:963–9. 10.1016/j.ijcard.2016.06.207 [DOI] [PubMed] [Google Scholar]
- 13.Lewinter C, Doherty P, Gale CP, et al. Exercise-based cardiac rehabilitation in patients with heart failure: a meta-analysis of randomised controlled trials between 1999 and 2013. Eur J Prev Cardiol 2015;22:1504–12. 10.1177/2047487314559853 [DOI] [PubMed] [Google Scholar]
- 14.Haykowsky MJ, Timmons MP, Kruger C, et al. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am J Cardiol 2013;111:1466–9. 10.1016/j.amjcard.2013.01.303 [DOI] [PubMed] [Google Scholar]
- 15.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med 2004;116:693–706. 10.1016/j.amjmed.2003.11.033 [DOI] [PubMed] [Google Scholar]
- 16.Taylor RS, Sagar VA, Davies EJ. Exercise‐based rehabilitation for heart failure. Cochrane Database Syst Rev 2014;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neupane B, Richer D, Bonner AJ, et al. Network meta-analysis using R: a review of currently available automated packages. PLoS One 2014;9:e115065. 10.1371/journal.pone.0115065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2020;2021:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long L, Mordi IR, Bridges C, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019;1:CD003331. 10.1002/14651858.CD003331.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 22.Brignardello-Petersen R, Bonner A, Alexander PE, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 2018;93:36–44. 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Brignardello-Petersen R, Murad MH, Walter SD, et al. GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol 2019;105:60–7. 10.1016/j.jclinepi.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 24.Brignardello-Petersen R, Mustafa RA, Siemieniuk RAC, et al. GRADE approach to rate the certainty from a network meta-analysis: addressing incoherence. J Clin Epidemiol 2019;108:77–85. 10.1016/j.jclinepi.2018.11.025 [DOI] [PubMed] [Google Scholar]
- 25.Puhan MA, Schünemann HJ, Murad MH, et al. A GRADE working group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 26.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 27.Mbuagbaw L, Rochwerg B, Jaeschke R, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev 2017;6:1–5. 10.1186/s13643-017-0473-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brubaker PH, Avis T, Rejeski WJ, et al. Exercise training effects on the relationship of physical function and health-related quality of life among older heart failure patients with preserved ejection fraction. J Cardiopulm Rehabil Prev 2020;40:427–33. 10.1097/HCR.0000000000000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pullen PR, Thompson WR, Benardot D, et al. Benefits of yoga for African American heart failure patients. Med Sci Sports Exerc 2010;42:651–7. 10.1249/MSS.0b013e3181bf24c4 [DOI] [PubMed] [Google Scholar]
- 30.Borland M, Rosenkvist A, Cider A. A group-based exercise program did not improve physical activity in patients with chronic heart failure and comorbidity: a randomized controlled trial. J Rehabil Med 2014;46:461–7. 10.2340/16501977-1794 [DOI] [PubMed] [Google Scholar]
- 31.Mudge AM, Denaro CP, Scott AC, et al. Addition of supervised exercise training to a post-hospital disease management program for patients recently hospitalized with acute heart failure: the EJECTION-HF randomized phase 4 trial. JACC Heart Fail 2018;6:143–52. 10.1016/j.jchf.2017.11.016 [DOI] [PubMed] [Google Scholar]
- 32.Pozehl B, Duncan K, Hertzog M, et al. Heart failure exercise and training cAMP: effects of a multicomponent exercise training intervention in patients with heart failure. Heart Lung 2010;39:S1–13. 10.1016/j.hrtlng.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piotrowicz E, Zieliński T, Bodalski R, et al. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: a randomised controlled study. Eur J Prev Cardiol 2015;22:1368–77. 10.1177/2047487314551537 [DOI] [PubMed] [Google Scholar]
- 34.Maiorana AJ, Naylor LH, Exterkate A, et al. The impact of exercise training on conduit artery wall thickness and remodeling in chronic heart failure patients. Hypertension 2011;57:56–62. 10.1161/HYPERTENSIONAHA.110.163022 [DOI] [PubMed] [Google Scholar]
- 35.Keteyian SJ, Levine AB, Brawner CA, et al. Exercise training in patients with heart failure. A randomized, controlled trial. Ann Intern Med 1996;124:1051–7. 10.7326/0003-4819-124-12-199606150-00004 [DOI] [PubMed] [Google Scholar]
- 36.Guazzi M, Reina G, Tumminello G, et al. Improvement of alveolar-capillary membrane diffusing capacity with exercise training in chronic heart failure. J Appl Physiol 2004;97:1866–73. 10.1152/japplphysiol.00365.2004 [DOI] [PubMed] [Google Scholar]
- 37.Hambrecht R, Schulze PC, Gielen S, et al. Effects of exercise training on insulin-like growth factor-I expression in the skeletal muscle of non-cachectic patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 2005;12:401–6. 10.1097/01.hjr.0000173106.68485.b7 [DOI] [PubMed] [Google Scholar]
- 38.Hwang R, Bruning J, Morris NR, et al. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. J Physiother 2017;63:101–7. 10.1016/j.jphys.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 39.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA 2000;283:3095–101. 10.1001/jama.283.23.3095 [DOI] [PubMed] [Google Scholar]
- 40.Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol 2013;62:584–92. 10.1016/j.jacc.2013.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitzman DW, Brubaker PH, Morgan TM, et al. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail 2010;3:659–67. 10.1161/CIRCHEARTFAILURE.110.958785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitzman DW, Whellan DJ, Duncan P, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med 2021;385:203–16. 10.1056/NEJMoa2026141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016;315:36–46. 10.1001/jama.2015.17346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gary RA, Sueta CA, Dougherty M, et al. Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung 2004;33:210–8. 10.1016/j.hrtlng.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 45.Yeh GY, Wood MJ, Lorell BH, et al. Effects of tai chi mind-body movement therapy on functional status and exercise capacity in patients with chronic heart failure: a randomized controlled trial. Am J Med 2004;117:541–8. 10.1016/j.amjmed.2004.04.016 [DOI] [PubMed] [Google Scholar]
- 46.Smolis-Bąk E, Rymuza H, Kazimierska B, et al. Improvement of exercise tolerance in cardiopulmonary testing with sustained safety after regular training in outpatients with systolic heart failure (NYHA III) and an implantable cardioverter-defibrillator. Prospective 18-month randomized study. Arch Med Sci 2017;13:1094–101. 10.5114/aoms.2016.61938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jónsdóttir S, Andersen KK, Sigurosson AF, et al. The effect of physical training in chronic heart failure. Eur J Heart Fail 2006;8:97–101. 10.1016/j.ejheart.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 48.Servantes DM, Javaheri S, Kravchychyn ACP, et al. Effects of exercise training and CPAP in patients with heart failure and OSA: a preliminary study. Chest 2018;154:808–17. 10.1016/j.chest.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 49.Smart NA, Haluska B, Jeffriess L, et al. Exercise training in heart failure with preserved systolic function: a randomized controlled trial of the effects on cardiac function and functional capacity. Congest Heart Fail 2012;18:295–301. 10.1111/j.1751-7133.2012.00295.x [DOI] [PubMed] [Google Scholar]
- 50.Safiyari-Hafizi H, Taunton J, Ignaszewski A, et al. The health benefits of a 12-week home-based interval training cardiac rehabilitation program in patients with heart failure. Can J Cardiol 2016;32:561–7. 10.1016/j.cjca.2016.01.031 [DOI] [PubMed] [Google Scholar]
- 51.Ricca-Mallada R, Migliaro ER, Silvera G, et al. Functional outcome in chronic heart failure after exercise training: possible predictive value of heart rate variability. Ann Phys Rehabil Med 2017;60:87–94. 10.1016/j.rehab.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 52.Tyni-Lenné R, Gordon A, Sylvén C. Improved quality of life in chronic heart failure patients following local endurance training with leg muscles. J Card Fail 1996;2:111–7. 10.1016/S1071-9164(96)80029-7 [DOI] [PubMed] [Google Scholar]
- 53.Höllriegel R, Winzer EB, Linke A, et al. Long-term exercise training in patients with advanced chronic heart failure: sustained benefits on left ventricular performance and exercise capacity. J Cardiopulm Rehabil Prev 2016;36:117–24. 10.1097/HCR.0000000000000165 [DOI] [PubMed] [Google Scholar]
- 54.Quittan M, Sturm B, Wiesinger GF, et al. Quality of life in patients with chronic heart failure: a randomized controlled trial of changes induced by a regular exercise program. Scand J Rehabil Med 1999;31:223–8. 10.1080/003655099444399 [DOI] [PubMed] [Google Scholar]
- 55.Roveda F, Middlekauff HR, Rondon MUPB, et al. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 2003;42:854–60. 10.1016/s0735-1097(03)00831-3 [DOI] [PubMed] [Google Scholar]
- 56.Mueller S, Winzer EB, Duvinage A, et al. Effect of high-intensity interval training, moderate continuous training, or guideline-based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2021;325:542–51. 10.1001/jama.2020.26812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du H, Newton PJ, Budhathoki C, et al. The home-heart-walk study, a self-administered walk test on perceived physical functioning, and self-care behaviour in people with stable chronic heart failure: a randomized controlled trial. Eur J Cardiovasc Nurs 2018;17:235–45. 10.1177/1474515117729779 [DOI] [PubMed] [Google Scholar]
- 58.Passino C, Del Ry S, Severino S, et al. C-type natriuretic peptide expression in patients with chronic heart failure: effects of aerobic training. Eur J Cardiovasc Prev Rehabil 2008;15:168–72. 10.1097/HJR.0b013e3282f10e9b [DOI] [PubMed] [Google Scholar]
- 59.Pourhabib A, Fotokian Z, Nasiri M. Effects of a group-based aerobic and resistance exercise program on physiological-psychological adaptation in elderly with heart failure. J Clin Gerontol Geriat 2018;9:59–66. [Google Scholar]
- 60.McKelvie RS, Teo KK, Roberts R, et al. Effects of exercise training in patients with heart failure: the exercise rehabilitation trial (EXERT). Am Heart J 2002;144:23–30. 10.1067/mhj.2002.123310 [DOI] [PubMed] [Google Scholar]
- 61.Smolis-Bąk E, Dąbrowski R, Piotrowicz E, et al. Hospital-based and telemonitoring guided home-based training programs: effects on exercise tolerance and quality of life in patients with heart failure (NYHA class III) and cardiac resynchronization therapy. A randomized, prospective observation. Int J Cardiol 2015;199:442–7. 10.1016/j.ijcard.2015.07.041 [DOI] [PubMed] [Google Scholar]
- 62.Erbs S, Höllriegel R, Linke A, et al. Exercise training in patients with advanced chronic heart failure (NYHA IIIB) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ Heart Fail 2010;3:486–94. 10.1161/CIRCHEARTFAILURE.109.868992 [DOI] [PubMed] [Google Scholar]
- 63.de Mello Franco FG, Santos AC, Rondon MUP, et al. Effects of home-based exercise training on neurovascular control in patients with heart failure. Eur J Heart Fail 2006;8:851–5. 10.1016/j.ejheart.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 64.Yeh GY, Wayne PM, Phillips RS. T'ai chi exercise in patients with chronic heart failure. Med 2008;52:195–208. [DOI] [PubMed] [Google Scholar]
- 65.Dziekan G, Myers J, Goebbels U, et al. Effects of exercise training on limb blood flow in patients with reduced ventricular function. Am Heart J 1998;136:22–30. 10.1016/s0002-8703(98)70177-2 [DOI] [PubMed] [Google Scholar]
- 66.Dubach P, Myers J, Dziekan G, et al. Effect of high intensity exercise training on central hemodynamic responses to exercise in men with reduced left ventricular function. J Am Coll Cardiol 1997;29:1591–8. 10.1016/s0735-1097(97)82540-5 [DOI] [PubMed] [Google Scholar]
- 67.Dracup K, Evangelista LS, Hamilton MA, et al. Effects of a home-based exercise program on clinical outcomes in heart failure. Am Heart J 2007;154:877–83. 10.1016/j.ahj.2007.07.019 [DOI] [PubMed] [Google Scholar]
- 68.Gary R, Lee SYS. Physical function and quality of life in older women with diastolic heart failure: effects of a progressive walking program on sleep patterns. Prog Cardiovasc Nurs 2007;22:72–80. 10.1111/j.0889-7204.2007.05375.x [DOI] [PubMed] [Google Scholar]
- 69.Evangelista LS, Doering LV, Lennie T, et al. Usefulness of a home-based exercise program for overweight and obese patients with advanced heart failure. Am J Cardiol 2006;97:886–90. 10.1016/j.amjcard.2005.10.025 [DOI] [PubMed] [Google Scholar]
- 70.Karapolat H, Demir E, Bozkaya YT, et al. Comparison of hospital-based versus home-based exercise training in patients with heart failure: effects on functional capacity, quality of life, psychological symptoms, and hemodynamic parameters. Clin Res Cardiol 2009;98:635–42. 10.1007/s00392-009-0049-6 [DOI] [PubMed] [Google Scholar]
- 71.Evans RA, Singh SJ, Collier R, et al. Generic, symptom based, exercise rehabilitation; integrating patients with COPD and heart failure. Respir Med 2010;104:1473–81. 10.1016/j.rmed.2010.04.024 [DOI] [PubMed] [Google Scholar]
- 72.Wielenga RP, Huisveld IA, Bol E, et al. Safety and effects of physical training in chronic heart failure. Results of the chronic heart failure and graded exercise study (change). Eur Heart J 1999;20:872–9. 10.1053/euhj.1999.1485 [DOI] [PubMed] [Google Scholar]
- 73.Myers J, Gademan M, Brunner K, et al. Effects of high-intensity training on indices of ventilatory efficiency in chronic heart failure. J Cardiopulm Rehabil Prev 2012;32:9–16. 10.1097/HCR.0b013e3182343bdf [DOI] [PubMed] [Google Scholar]
- 74.Edelmann F, Gelbrich G, Düngen H-D, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (exercise training in diastolic heart failure) pilot study. J Am Coll Cardiol 2011;58:1780–91. 10.1016/j.jacc.2011.06.054 [DOI] [PubMed] [Google Scholar]
- 75.Kulcu DG, Kurtais Y, Tur BS, et al. The effect of cardiac rehabilitation on quality of life, anxiety and depression in patients with congestive heart failure. A randomized controlled trial, short-term results. Eura Medicophys 2007;43:489–97. [PubMed] [Google Scholar]
- 76.Myers J, Gianrossi R, Schwitter J, et al. Effect of exercise training on postexercise oxygen uptake kinetics in patients with reduced ventricular function. Chest 2001;120:1206–11. 10.1378/chest.120.4.1206 [DOI] [PubMed] [Google Scholar]
- 77.Vordos Z, Kouidi E, Mavrovouniotis F, et al. Impact of traditional Greek dancing on jumping ability, muscular strength and lower limb endurance in cardiac rehabilitation programmes. Eur J Cardiovasc Nurs 2017;16:150–6. 10.1177/1474515116636980 [DOI] [PubMed] [Google Scholar]
- 78.Nilsson BB, Westheim A, Risberg MA. Effects of group-based high-intensity aerobic interval training in patients with chronic heart failure. Am J Cardiol 2008;102:1361–5. 10.1016/j.amjcard.2008.07.016 [DOI] [PubMed] [Google Scholar]
- 79.Koukouvou G, Kouidi E, Iacovides A, et al. Quality of life, psychological and physiological changes following exercise training in patients with chronic heart failure. J Rehabil Med 2004;36:36–41. 10.1080/11026480310015549 [DOI] [PubMed] [Google Scholar]
- 80.Santa-Clara H, Abreu A, Melo X, et al. High-intensity interval training in cardiac resynchronization therapy: a randomized control trial. Eur J Appl Physiol 2019;119:1757–67. 10.1007/s00421-019-04165-y [DOI] [PubMed] [Google Scholar]
- 81.Kiilavuori K, Näveri H, Leinonen H, et al. The effect of physical training on hormonal status and exertional hormonal response in patients with chronic congestive heart failure. Eur Heart J 1999;20:456–64. 10.1053/euhj.1998.1277 [DOI] [PubMed] [Google Scholar]
- 82.Klecha A, Kawecka-Jaszcz K, Bacior B, et al. Physical training in patients with chronic heart failure of ischemic origin: effect on exercise capacity and left ventricular remodeling. Eur J Cardiovasc Prev Rehabil 2007;14:85–91. 10.1097/HJR.0b013e3280114f12 [DOI] [PubMed] [Google Scholar]
- 83.Tyni-Lenné R, Dencker K, Gordon A, et al. Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. Eur J Heart Fail 2001;3:47–52. 10.1016/s1388-9842(00)00087-8 [DOI] [PubMed] [Google Scholar]
- 84.Peng X, Su Y, Hu Z, et al. Home-based telehealth exercise training program in Chinese patients with heart failure: a randomized controlled trial. Medicine 2018;97:e12069. 10.1097/MD.0000000000012069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klocek M, Kubinyi A, Bacior B, et al. Effect of physical training on quality of life and oxygen consumption in patients with congestive heart failure. Int J Cardiol 2005;103:323–9. 10.1016/j.ijcard.2004.10.021 [DOI] [PubMed] [Google Scholar]
- 86.Shoemaker MJ, Oberholtzer NL, Jongekrijg LE. Exercise- and psychosocial-based interventions to improve daily activity in heart failure: a pilot study. Home Health Care Management & Practice 2017;29:111–20. [Google Scholar]
- 87.Ajiboye OA, Anigbogu CN, Ajuluchukwu JN. Exercise training improves functional walking capacity and activity level of Nigerians with chronic biventricular heart failure. Hong Kong Physiotherapy Journal 2015;33:42–9. [Google Scholar]
- 88.Aksoy S, Findikoglu G, Ardic F, et al. Effect of 10-week supervised moderate-intensity intermittent vs. continuous aerobic exercise programs on vascular adhesion molecules in patients with heart failure. Am J Phys Med Rehabil 2015;94:898–911. 10.1097/PHM.0000000000000306 [DOI] [PubMed] [Google Scholar]
- 89.Belardinelli R, Capestro F, Misiani A, et al. Moderate exercise training improves functional capacity, quality of life, and endothelium-dependent vasodilation in chronic heart failure patients with implantable cardioverter defibrillators and cardiac resynchronization therapy. Eur J Cardiovasc Prev Rehabil 2006;13:818–25. 10.1097/01.hjr.0000230104.93771.7d [DOI] [PubMed] [Google Scholar]
- 90.Belardinelli R, Georgiou D, Cianci G, et al. 10-year exercise training in chronic heart failure: a randomized controlled trial. J Am Coll Cardiol 2012;60:1521–8. 10.1016/j.jacc.2012.06.036 [DOI] [PubMed] [Google Scholar]
- 91.Belardinelli R, Lacalaprice F, Faccenda E, et al. Effects of short-term moderate exercise training on sexual function in male patients with chronic stable heart failure. Int J Cardiol 2005;101:83–90. 10.1016/j.ijcard.2004.05.020 [DOI] [PubMed] [Google Scholar]
- 92.Butterfield JA, Faddy SC, Davidson P, et al. Exercise training in patients with stable chronic heart failure: effects on thoracic impedance cardiography and B-type natriuretic peptide. J Cardiopulm Rehabil Prev 2008;28:33–7. 10.1097/01.HCR.0000311506.49398.6d [DOI] [PubMed] [Google Scholar]
- 93.Chen Y, Funk M, Wen J, et al. Effectiveness of a multidisciplinary disease management program on outcomes in patients with heart failure in China: a randomized controlled single center study. Heart Lung 2018;47:24–31. 10.1016/j.hrtlng.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 94.Chen Y-W, Wang C-Y, Lai Y-H, et al. Home-based cardiac rehabilitation improves quality of life, aerobic capacity, and readmission rates in patients with chronic heart failure. Medicine 2018;97:e9629. 10.1097/MD.0000000000009629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chien C-L, Lee C-M, Wu Y-W, et al. Home-based exercise improves the quality of life and physical function but not the psychological status of people with chronic heart failure: a randomised trial. J Physiother 2011;57:157–63. 10.1016/S1836-9553(11)70036-4 [DOI] [PubMed] [Google Scholar]
- 96.Chou C-H, Fu T-C, Tsai H-H, et al. High-intensity interval training enhances mitochondrial bioenergetics of platelets in patients with heart failure. Int J Cardiol 2019;274:214–20. 10.1016/j.ijcard.2018.07.104 [DOI] [PubMed] [Google Scholar]
- 97.Corvera-Tindel T, Doering LV, Woo MA, et al. Effects of a home walking exercise program on functional status and symptoms in heart failure. Am Heart J 2004;147:339–46. 10.1016/j.ahj.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 98.Dalal HM, Taylor RS, Jolly K, et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: the REACH-HF multicentre randomized controlled trial. Eur J Prev Cardiol 2019;26:262–72. 10.1177/2047487318806358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Daskapan A, Arikan H, Caglar N, et al. Comparison of supervised exercise training and home-based exercise training in chronic heart failure. Saudi Med J 2005;26:842–7. [PubMed] [Google Scholar]
- 100.Ellis RE, Dodd KJ, Holland AE, et al. Effect of eccentric exercise on quality of life and function in people with chronic heart failure: a pilot randomised controlled trial. Disabil Rehabil 2020;10:1-10. 10.1080/09638288.2020.1836679 [DOI] [PubMed] [Google Scholar]
- 101.Fu T-C, Wang C-H, Lin P-S, et al. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol 2013;167:41–50. 10.1016/j.ijcard.2011.11.086 [DOI] [PubMed] [Google Scholar]
- 102.Gielen S, Adams V, Möbius-Winkler S, et al. Anti-Inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 2003;42:861–8. 10.1016/s0735-1097(03)00848-9 [DOI] [PubMed] [Google Scholar]
- 103.Kaltsatou ACH, Kouidi EI, Anifanti MA, et al. Functional and psychosocial effects of either a traditional dancing or a formal exercising training program in patients with chronic heart failure: a comparative randomized controlled study. Clin Rehabil 2014;28:128–38. 10.1177/0269215513492988 [DOI] [PubMed] [Google Scholar]
- 104.Mandic S, Tymchak W, Kim D, et al. Effects of aerobic or aerobic and resistance training on cardiorespiratory and skeletal muscle function in heart failure: a randomized controlled pilot trial. Clin Rehabil 2009;23:207–16. 10.1177/0269215508095362 [DOI] [PubMed] [Google Scholar]
- 105.Mezzani A, Grassi B, Jones AM, et al. Speeding of pulmonary VO2 on-kinetics by light-to-moderate-intensity aerobic exercise training in chronic heart failure: clinical and pathophysiological correlates. Int J Cardiol 2013;167:2189–95. 10.1016/j.ijcard.2012.05.124 [DOI] [PubMed] [Google Scholar]
- 106.Mueller L, Myers J, Kottman W, et al. Exercise capacity, physical activity patterns and outcomes six years after cardiac rehabilitation in patients with heart failure. Clin Rehabil 2007;21:923–31. 10.1177/0269215507079097 [DOI] [PubMed] [Google Scholar]
- 107.Müller L, Myers J, Kottman W, et al. Long-term myocardial adaptations after cardiac rehabilitation in heart failure: a randomized six-year evaluation using magnetic resonance imaging. Clin Rehabil 2009;23:986–94. 10.1177/0269215509339003 [DOI] [PubMed] [Google Scholar]
- 108.Myers J, Hadley D, Oswald U, et al. Effects of exercise training on heart rate recovery in patients with chronic heart failure. Am Heart J 2007;153:1056–63. 10.1016/j.ahj.2007.02.038 [DOI] [PubMed] [Google Scholar]
- 109.Nilsson BB, Westheim A, Risberg MA, et al. No effect of group-based aerobic interval training on N-terminal pro- B-type natriuretic peptide levels in patients with chronic heart failure. Scand Cardiovasc J 2010;44:223–9. 10.3109/14017431.2010.496869 [DOI] [PubMed] [Google Scholar]
- 110.Nolte K, Herrmann-Lingen C, Wachter R, et al. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex-DHF-P trial. Eur J Prev Cardiol 2015;22:582–93. 10.1177/2047487314526071 [DOI] [PubMed] [Google Scholar]
- 111.Oka RK, De Marco T, Haskell WL, et al. Impact of a home-based walking and resistance training program on quality of life in patients with heart failure. Am J Cardiol 2000;85:365–9. 10.1016/s0002-9149(99)00748-1 [DOI] [PubMed] [Google Scholar]
- 112.Passino C, Severino S, Poletti R, et al. Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol 2006;47:1835–9. 10.1016/j.jacc.2005.12.050 [DOI] [PubMed] [Google Scholar]
- 113.Patwala AY, Woods PR, Sharp L, et al. Maximizing patient benefit from cardiac resynchronization therapy with the addition of structured exercise training: a randomized controlled study. J Am Coll Cardiol 2009;53:2332–9. 10.1016/j.jacc.2009.02.063 [DOI] [PubMed] [Google Scholar]
- 114.Pullen PR, Nagamia SH, Mehta PK, et al. Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. J Card Fail 2008;14:407–13. 10.1016/j.cardfail.2007.12.007 [DOI] [PubMed] [Google Scholar]
- 115.Redwine LS, Wilson K, Pung MA, et al. A randomized study examining the effects of mild-to-moderate group exercises on cardiovascular, physical, and psychological well-being in patients with heart failure. J Cardiopulm Rehabil Prev 2019;39:403–8. 10.1097/HCR.0000000000000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sabelis LWE, Senden PJ, Fijnheer R, et al. Endothelial markers in chronic heart failure: training normalizes exercise-induced vWF release. Eur J Clin Invest 2004;34:583–9. 10.1111/j.1365-2362.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 117.Sabelis LWE, Senden PJ, Te Boekhorst BCM, et al. Does physical training increase insulin sensitivity in chronic heart failure patients? Clin Sci 2004;106:459–66. 10.1042/CS20030254 [DOI] [PubMed] [Google Scholar]
- 118.Sadek Z, Salami A, Youness M, et al. A randomized controlled trial of high-intensity interval training and inspiratory muscle training for chronic heart failure patients with inspiratory muscle weakness. Chronic Illn 2022;18:140-154. 10.1177/1742395320920700 [DOI] [PubMed] [Google Scholar]
- 119.Santos JMT, Kowatsch I, Tsutsui JM, et al. Effects of exercise training on myocardial blood flow reserve in patients with heart failure and left ventricular systolic dysfunction. Am J Cardiol 2010;105:243–8. 10.1016/j.amjcard.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 120.Senden PJ, Sabelis LW, Zonderland ML, et al. The effect of physical training on workload, upper leg muscle function and muscle areas in patients with chronic heart failure. Int J Cardiol 2005;100:293–300. 10.1016/j.ijcard.2004.10.039 [DOI] [PubMed] [Google Scholar]
- 121.Servantes DM, Pelcerman A, Salvetti XM, et al. Effects of home-based exercise training for patients with chronic heart failure and sleep apnoea: a randomized comparison of two different programmes. Clin Rehabil 2012;26:45–57. 10.1177/0269215511403941 [DOI] [PubMed] [Google Scholar]
- 122.Spee RF, Niemeijer VM, Schoots T, et al. High intensity interval training after cardiac resynchronization therapy: an explorative randomized controlled trial. Int J Cardiol 2020;299:169–74. 10.1016/j.ijcard.2019.07.023 [DOI] [PubMed] [Google Scholar]
- 123.Willenheimer R, Rydberg E, Cline C, et al. Effects on quality of life, symptoms and daily activity 6 months after termination of an exercise training programme in heart failure patients. Int J Cardiol 2001;77:25–31. 10.1016/s0167-5273(00)00383-1 [DOI] [PubMed] [Google Scholar]
- 124.Williams AD, Carey MF, Selig S, et al. Circuit resistance training in chronic heart failure improves skeletal muscle mitochondrial ATP production rate-a randomized controlled trial. J Card Fail 2007;13:79–85. 10.1016/j.cardfail.2006.10.017 [DOI] [PubMed] [Google Scholar]
- 125.Witham MD, Argo IS, Johnston DW, et al. Long-term follow-up of very old heart failure patients enrolled in a trial of exercise training. Am J Geriatr Cardiol 2007;16:243–8. 10.1111/j.1076-7460.2007.06488.x [DOI] [PubMed] [Google Scholar]
- 126.Witham MD, Gray JM, Argo IS, et al. Effect of a seated exercise program to improve physical function and health status in frail patients > or = 70 years of age with heart failure. Am J Cardiol 2005;95:1120–4. 10.1016/j.amjcard.2005.01.031 [DOI] [PubMed] [Google Scholar]
- 127.Xueyu L, Hao Y, Shunlin X, et al. Effects of low-intensity exercise in older adults with chronic heart failure during the transitional period from hospital to home in China: a randomized controlled trial. Res Gerontol Nurs 2017;10:121–8. 10.3928/19404921-20170411-02 [DOI] [PubMed] [Google Scholar]
- 128.Jaarsma T, Klompstra L, Ben Gal T, et al. Effects of exergaming on exercise capacity in patients with heart failure: results of an international multicentre randomized controlled trial. Eur J Heart Fail 2021;23:114–24. 10.1002/ejhf.1754 [DOI] [PubMed] [Google Scholar]
- 129.Zeitler EP, Piccini JP, Hellkamp AS, et al. Exercise training and pacing status in patients with heart failure: results from HF-ACTION. J Card Fail 2015;21:60–7. 10.1016/j.cardfail.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maldonado-Martín S, Brubaker PH, Eggebeen J, et al. Association between 6-minute walk test distance and objective variables of functional capacity after exercise training in elderly heart failure patients with preserved ejection fraction: a randomized exercise trial. Arch Phys Med Rehabil 2017;98:600–3. 10.1016/j.apmr.2016.08.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barrow DE, Bedford A, Ives G, et al. An evaluation of the effects of tai chi Chuan and chi Kung training in patients with symptomatic heart failure: a randomised controlled pilot study. Postgrad Med J 2007;83:717–21. 10.1136/pgmj.2007.061267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jones LW, Douglas PS, Khouri MG, et al. Safety and efficacy of aerobic training in patients with cancer who have heart failure: an analysis of the HF-ACTION randomized trial. J Clin Oncol 2014;32:2496–502. 10.1200/JCO.2013.53.5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Austin J, Williams R, Ross L, et al. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail 2005;7:411–7. 10.1016/j.ejheart.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 134.Jolly K, Taylor RS, Lip GYH, et al. A randomized trial of the addition of home-based exercise to specialist heart failure nurse care: the Birmingham rehabilitation uptake Maximisation study for patients with congestive heart failure (BRUM-CHF) study. Eur J Heart Fail 2009;11:205–13. 10.1093/eurjhf/hfn029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen D-M, Yu W-C, Hung H-F, et al. The effects of Baduanjin exercise on fatigue and quality of life in patients with heart failure: a randomized controlled trial. Eur J Cardiovasc Nurs 2018;17:456–66. 10.1177/1474515117744770 [DOI] [PubMed] [Google Scholar]
- 136.Gary RA, Dunbar SB, Higgins MK, et al. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res 2010;69:119–31. 10.1016/j.jpsychores.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.de Meirelles LR, Matsuura C, Resende AdeC, et al. Chronic exercise leads to antiaggregant, antioxidant and anti-inflammatory effects in heart failure patients. Eur J Prev Cardiol 2014;21:1225–32. 10.1177/2047487313491662 [DOI] [PubMed] [Google Scholar]
- 138.Cider A, Schaufelberger M, Sunnerhagen KS, et al. Hydrotherapy-a new approach to improve function in the older patient with chronic heart failure. Eur J Heart Fail 2003;5:527–35. 10.1016/s1388-9842(03)00048-5 [DOI] [PubMed] [Google Scholar]
- 139.Piotrowicz E, Piotrowski W, Piotrowicz R. Positive effects of the reversion of depression on the Sympathovagal balance after Telerehabilitation in heart failure patients. Ann Noninvasive Electrocardiol 2016;21:358–68. 10.1111/anec.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Blumenthal JA, Babyak MA, O'Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA 2012;308:465–74. 10.1001/jama.2012.8720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Davidson PM, Cockburn J, Newton PJ, et al. Can a heart failure-specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients? Eur J Cardiovasc Prev Rehabil 2010;17:393–402. 10.1097/HJR.0b013e328334ea56 [DOI] [PubMed] [Google Scholar]
- 142.Brubaker PH, Moore JB, Stewart KP, et al. Endurance exercise training in older patients with heart failure: results from a randomized, controlled, single-blind trial. J Am Geriatr Soc 2009;57:1982–9. 10.1111/j.1532-5415.2009.02499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spee RF, Niemeijer VM, Wijn PF, et al. Effects of high-intensity interval training on central haemodynamics and skeletal muscle oxygenation during exercise in patients with chronic heart failure. Eur J Prev Cardiol 2016;23:1943–52. 10.1177/2047487316661615 [DOI] [PubMed] [Google Scholar]
- 144.Giannuzzi P, Temporelli PL, Corrà U, et al. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: results of the exercise in left ventricular dysfunction and chronic heart failure (ELVD-CHF) trial. Circulation 2003;108:554–9. 10.1161/01.CIR.0000081780.38477.FA [DOI] [PubMed] [Google Scholar]
- 145.Belardinelli R, Georgiou D, Cianci G, et al. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 1999;99:1173–82. 10.1161/01.cir.99.9.1173 [DOI] [PubMed] [Google Scholar]
- 146.Andryukhin A, Frolova E, Vaes B, et al. The impact of a nurse-led care programme on events and physical and psychosocial parameters in patients with heart failure with preserved ejection fraction: a randomized clinical trial in primary care in Russia. Eur J Gen Pract 2010;16:205–14. 10.3109/13814788.2010.527938 [DOI] [PubMed] [Google Scholar]
- 147.Fraga R, Franco FG, Roveda F, et al. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail 2007;9:630–6. 10.1016/j.ejheart.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 148.Sturm B, Quittan M, Wiesinger GF, et al. Moderate-intensity exercise training with elements of step aerobics in patients with severe chronic heart failure. Arch Phys Med Rehabil 1999;80:746–50. 10.1016/s0003-9993(99)90221-6 [DOI] [PubMed] [Google Scholar]
- 149.Yeh GY, McCarthy EP, Wayne PM, et al. Tai chi exercise in patients with chronic heart failure: a randomized clinical trial. Arch Intern Med 2011;171:750–7. 10.1001/archinternmed.2011.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lans C, Cider Åsa, Nylander E, et al. Peripheral muscle training with resistance exercise bands in patients with chronic heart failure. Long-term effects on walking distance and quality of life; a pilot study. ESC Heart Fail 2018;5:241–8. 10.1002/ehf2.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Norman JF, Kupzyk KA, Artinian NT, et al. The influence of the HEART Camp intervention on physical function, health-related quality of life, depression, anxiety and fatigue in patients with heart failure. Eur J Cardiovasc Nurs 2020;19:64–73. 10.1177/1474515119867444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bernocchi P, Vitacca M, La Rovere MT, et al. Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: a randomised controlled trial. Age Ageing 2018;47:82–8. 10.1093/ageing/afx146 [DOI] [PubMed] [Google Scholar]
- 153.Piotrowicz E, Baranowski R, Bilinska M, et al. A new model of home-based telemonitored cardiac rehabilitation in patients with heart failure: effectiveness, quality of life, and adherence. Eur J Heart Fail 2010;12:164–71. 10.1093/eurjhf/hfp181 [DOI] [PubMed] [Google Scholar]
- 154.Główczyńska R, Piotrowicz E, Szalewska D, et al. Effects of hybrid comprehensive telerehabilitation on cardiopulmonary capacity in heart failure patients depending on diabetes mellitus: subanalysis of the TELEREH-HF randomized clinical trial. Cardiovasc Diabetol 2021;20:106. 10.1186/s12933-021-01292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Flynn KE, Piña IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1451–9. 10.1001/jama.2009.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reeves GR, Whellan DJ, O'Connor CM, et al. A novel rehabilitation intervention for older patients with acute decompensated heart failure: the REHAB-HF pilot study. JACC Heart Fail 2017;5:359–66. 10.1016/j.jchf.2016.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gary RA, Cress ME, Higgins MK, et al. A combined aerobic and resistance exercise program improves physical functional performance in patients with heart failure: a pilot study. J Cardiovasc Nurs 2012;27:418–30. 10.1097/JCN.0b013e31822ad3c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lang CC, Smith K, Wingham J, et al. A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH-HFpEF pilot study. BMJ Open 2018;8:e019649. 10.1136/bmjopen-2017-019649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Willenheimer R, Erhardt L, Cline C, et al. Exercise training in heart failure improves quality of life and exercise capacity. Eur Heart J 1998;19:774–81. 10.1053/euhj.1997.0853 [DOI] [PubMed] [Google Scholar]
- 161.Azhar G, Raza S, Pangle A, et al. Potential beneficial effects of dietary protein supplementation and exercise on functional capacity in a pilot study of individuals with heart failure with preserved ejection fraction. Gerontol Geriatr Med 2020;6:2333721420982808:233372142098280. 10.1177/2333721420982808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Beckers PJ, Denollet J, Possemiers NM, et al. Maintaining physical fitness of patients with chronic heart failure: a randomized controlled trial. Eur J Cardiovasc Prev Rehabil 2010;17:660–7. 10.1097/HJR.0b013e328339ccac [DOI] [PubMed] [Google Scholar]
- 163.Piotrowicz E, Pencina MJ, Opolski G, et al. Effects of a 9-week hybrid comprehensive Telerehabilitation program on long-term outcomes in patients with heart failure: the telerehabilitation in heart failure patients (TELEREH-HF) randomized clinical trial. JAMA Cardiol 2020;5:300–8. 10.1001/jamacardio.2019.5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Silva MSVda, Bocchi EA, Guimaraes GV, et al. Benefits of exercise training in the treatment of heart failure: study with a control group. Arq Bras Cardiol 2002;79:351–62. 10.1590/s0066-782x2002001300003 [DOI] [PubMed] [Google Scholar]
- 165.Hasanpour-Dehkordi A, Yadollahi M, Tali SS. Effect of exercise training on dimensions of quality of life and fatigue in people with congestive heart failure class II and III: a randomized controlled trial. Indian J Med Spec 2020;11:15–20. [Google Scholar]
- 166.Piotrowicz E, Stepnowska M, Leszczyńska-Iwanicka K, et al. Quality of life in heart failure patients undergoing home-based telerehabilitation versus outpatient rehabilitation-a randomized controlled study. Eur J Cardiovasc Nurs 2015;14:256–63. 10.1177/1474515114537023 [DOI] [PubMed] [Google Scholar]
- 167.Shoemaker MJ, Curtis AB, Vangsnes E, et al. Triangulating clinically meaningful change in the six-minute walk test in individuals with chronic heart failure: a systematic review. Cardiopulm Phys Ther J 2012;23:5. [PMC free article] [PubMed] [Google Scholar]
- 168.Shoemaker MJ, Curtis AB, Vangsnes E, et al. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm Phys Ther J 2013;24:21. [PMC free article] [PubMed] [Google Scholar]
- 169.Keteyian SJ, Brawner CA, Ehrman JK, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise parameters: implications for clinical trials and clinical practice. Chest 2010;138:950–5. 10.1378/chest.09-2624 [DOI] [PubMed] [Google Scholar]
- 170.Corrà U, Mezzani A, Bosimini E, et al. Prognostic value of time-related changes of cardiopulmonary exercise testing indices in stable chronic heart failure: a pragmatic and operative scheme. Eur J Cardiovasc Prev Rehabil 2006;13:186–92. 10.1097/01.hjr.0000189807.22224.54 [DOI] [PubMed] [Google Scholar]
- 171.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150:707–15. 10.1016/j.ahj.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 172.Taylor RS, Dalal HM, McDonagh ST. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat Rev Cardiol 2021:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Davies EJ, Moxham T, Rees K. Exercise based rehabilitation for heart failure. Cochrane Database Systematic Rev 2010;4. [DOI] [PubMed] [Google Scholar]
- 174.Bakhshayeh S, Sarbaz M, Kimiafar K, et al. Barriers to participation in center-based cardiac rehabilitation programs and patients’ attitude toward home-based cardiac rehabilitation programs. Physiother Theory Pract 2021;37:158–68. 10.1080/09593985.2019.1620388 [DOI] [PubMed] [Google Scholar]
- 175.Sagar VA, Davies EJ, Briscoe S, et al. Exercise-based rehabilitation for heart failure: systematic review and meta-analysis. Open Heart 2015;2:e000163. 10.1136/openhrt-2014-000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota living with heart failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. pimobendan multicenter Research Group. Am Heart J 1992;124:1017–25. 10.1016/0002-8703(92)90986-6 [DOI] [PubMed] [Google Scholar]
- 177.Rector TS, Johnson G, Dunkman WB, et al. Evaluation by patients with heart failure of the effects of enalapril compared with hydralazine plus isosorbide dinitrate on quality of life. V-HeFT II. The V-HeFT Va cooperative studies group. Circulation 1993;87:VI71–7. [PubMed] [Google Scholar]
- 178.Patel S, Park H, Bonato P, et al. A review of wearable sensors and systems with application in rehabilitation. J Neuroeng Rehabil 2012;9:1–17. 10.1186/1743-0003-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2021-001949supp001.pdf (416KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.