Abstract
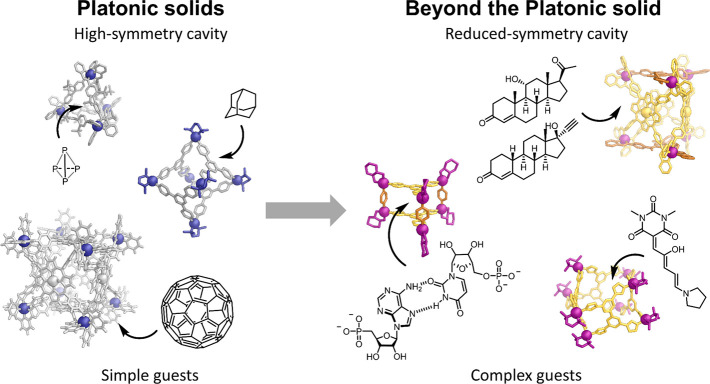
The field of metallosupramolecular chemistry has advanced rapidly in recent years. Much work in this area has focused on the formation of hollow self-assembled metal-organic architectures and exploration of the applications of their confined nanospaces. These discrete, soluble structures incorporate metal ions as ‘glue’ to link organic ligands together into polyhedra.Most of the architectures employed thus far have been highly symmetrical, as these have been the easiest to prepare. Such high-symmetry structures contain pseudospherical cavities, and so typically bind roughly spherical guests. Biomolecules and high-value synthetic compounds are rarely isotropic, highly-symmetrical species. To bind, sense, separate, and transform such substrates, new, lower-symmetry, metal-organic cages are needed. Herein we summarize recent approaches, which taken together form the first draft of a handbook for the design of higher-complexity, lower-symmetry, self-assembled metal-organic architectures.
1. Introduction
1.1. Overview
The field of metallosupramolecular chemistry has advanced rapidly in recent years. Much work in this area has focused on the formation of hollow self-assembled metal–organic architectures and exploration of the applications of their confined nanospaces.1−5 These discrete, soluble structures incorporate metal ions as “glue” to link organic ligands together into polyhedra. Their hollows have found applications in binding and sensing guests,6−8 stabilizing reactive molecules,9−13 and catalyzing reactions as enzymes do.14−19 Most of the architectures employed to date have been highly symmetrical, as these have been the easiest to prepare (Figure 1).20 An understanding of the design principles underpinning the formation of high-symmetry metal–organic cages,1 such as tetrahedra,21−24 cubes,25−28 and octahedra,29−34 has enabled their synthesis and application.35−38 Modification of these structures, either before or after assembly,39−41 can imbue them with new functions.42 Such functions include modulation of the guest-binding properties,43−46 phase transfer (whereby a capsule and its cargo are induced to move between phases),47,48 and enabling the formation of higher-order metal–organic cage-based materials.49−56
Figure 1.
Examples of coordination cages with structures corresponding to Platonic solids, which are well-adapted to pseudospherical guests, contrasted with more complex “beyond Platonic” cages, which are primed for binding of anisotropic guests.25,35,36,59,62−64
Such high-symmetry structures contain pseudospherical cavities and thus bind roughly spherical guests optimally,25,35−38 although asymmetric guests can also be encapsulated.10,12,20,57−59 In some cases more than one smaller guest is bound within a relatively large cavity,57 or the flexibility of a guest enables it to adopt a folded conformation with a complementary size and shape for the cage cavity.18,59,60
Biomolecules and high-value synthetic compounds are rarely isotropic, highly symmetrical species.61 To bind, sense, separate, and transform such substrates, new lower-symmetry metal–organic cages are needed. In response to this need, recent work has focused upon the construction of metal–organic cages with interior cavities of reduced symmetry.
Many early examples of lower-symmetry structures were discovered serendipitously. Only a limited number of structure types beyond the Platonic solids were prepared using established design principles. The great promise of lower-symmetry structures to bind lower-symmetry guests selectively (Figure 1) has motivated efforts to decipher the rules underpinning the formation of complex architectures.62−64 Herein we outline different approaches that taken together form the first draft of a handbook for the design of higher-complexity, lower-symmetry, self-assembled metal–organic architectures.
1.2. Classification of Approaches
The design of metal–organic architectures has been discussed in terms of the following four strategies: the directional-bonding approach,1 the symmetry-interaction approach,65 the molecular-paneling approach,66 and the weak-link approach.67−69 Each of these strategies has been employed to form metallomacrocycles or high-symmetry three-dimensional architectures, often with Platonic geometries. With careful consideration, these design strategies can also be employed to form lower-symmetry structures that deviate from the Platonic solids. However, in this review we have opted for a method of classification that deviates from the strategies noted above because approaches enabling the formation of more complex metal–organic assemblies have recently been established that do not neatly fall within these categories. We focus instead upon the properties of the building blocks along with reaction conditions. This organization lends itself to the aim of this review—to act as a preliminary guide for the further design of complex self-assembled architectures.
Using this building block/reaction condition-based classification, we have identified six broad categories of approach (Figure 2): (1) Heteroleptic Assemblies; (2) Lower-Symmetry Ligands; (3) Ligand Flexibility; (4) Complexity Derived from Solvent, Anions, and Templates; (5) Multimetallics: Heterometallic and Cluster-Containing Architectures; and (6) Geometric Constraints.
Figure 2.
Categorization of approaches to forming complex metal–organic architectures.
Heteroleptic architectures incorporate multiple different ligands (Figure 2a). A particular challenge in this approach is to ensure that the different building blocks integrate into a single product rather than segregating to form simpler structures, each containing only one type of building block. One strategy developed to overcome this challenge involves harnessing the enthalpic and entropic driving forces that govern self-assembly in order to favor a heteroleptic structure.
A similarly intuitive approach involves the use of ligands with greater structural complexity or reduced symmetry, which then translates to the assembly of more complex three-dimensional architectures (Figure 2b).
Flexibility is often incorporated within ligands by the addition of alkyl spacers. Such enhanced flexibility can increase the array of feasible structures in comparison with the use of more rigid ligands, but it can also decrease the predictability of the self-assembly process (Figure 2c).
Complexity based upon solvent, anion, and template effects relies upon altering the self-assembly reaction conditions in order to favor structural complexity (Figure 2d). This method is well-established for producing complex metal–organic architectures. However, as with enhancing ligand flexibility, predicting the outcome of self-assembly using this approach can be challenging.
Multimetallic architectures either contain more than one type of metal center or have vertices that consist of homometallic clusters. Both cases can introduce coordinational flexibility, enabling the formation of architectures with increased structural complexity (Figure 2e).
The sixth approach to generating complex structures in a controlled and predictable manner is the incorporation of geometric constraints into the ligands. These geometric constraints can act to frustrate the formation of simpler structures, thus favoring the construction of architectures with greater complexity (Figure 2f). Examples in which steric control or non-covalent interactions are used to form complex metal–organic structures are also highlighted in this section.
1.3. Scope of the Review
This review focuses on techniques used to prepare metal–organic architectures by self-assembly of organic ligands and metal ions. Some complex structures that form with metal-cluster cores or with metal clusters as vertices70−72 are also included. The term “complex structure” within this review generally refers to structures that deviate from a framework corresponding to one of the high-symmetry Platonic or Archimedean solids. Some examples of structures that outwardly resemble these simple polyhedra, but with reduced symmetry, are included, particularly when the source of the reduced symmetry can be determined.
Although a key motivation for this review is to aid those who might wish to design new lower-symmetry structures for new applications, we focus on construction principles as opposed to the utility and functions of these structures. As the field that we attempt to cover is wide-ranging and fast-moving, omissions in our coverage will be inevitable. We apologize for these in advance.
2. Heteroleptic Assemblies: Incorporation of Multiple Ligands Generates More Complex Architectures
The complexity of metal–organic assemblies can be increased through the use of combinations of multiple ligands, particularly those having different topicities, i.e., with different numbers of metal-binding sites per ligand. In principle, combinations of multiple ligands with different shapes can allow the emergence of unusual architectures with complex geometries. In practice, however, achieving the selective formation of a single structure from a range of possibilities can be challenging. This section explores ways in which this challenge has been overcome, focusing on approaches that may allow general routes to heteroleptic structures.
2.1. Heteroleptic Selectivity by Destabilization of Homoleptic Assemblies
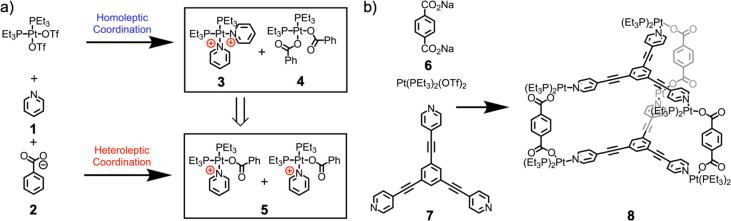
The selective assembly of a single heteroleptic metal–organic architecture is often entropically disfavored. For example, a square-planar metal vertex coordinated by 2 equiv of two different ligands through monodentate donors (i.e., ML12L22) may coexist with other mixed-ligand (i.e., ML11L23, ML13L21) and homoleptic (ML14, ML24) vertices. One way to overcome this tendency is to build in an enthalpic driving force for heteroleptic assembly. Stang et al. found that the principle of charge separation could drive the assembly of less-symmetric structures.73 This approach, shown in Figure 3, depends on the use of platinum(II) centers with two strong-field ligands in a cis configuration and both pyridine (1) and carboxylate (2) donor ligands. After coordination of a pyridine donor to platinum, the pyridine nitrogen atom bears a partial positive charge. When two pyridine donors are adjacent, they repel each other electrostatically (Figure 3, 3). This repulsion is ameliorated when one of the pyridine donors is replaced by a carboxylate (Figure 3, 5). This reduction in repulsion thus leads to the observed preference for heteroleptic coordination.
Figure 3.
(a) Charge separation as a method for driving heteroleptic complex formation, leading to (b) selective formation of mixed-ligand cages.73
The Stang group has employed this concept extensively, for example to form an array of trigonal, tetragonal, and hexagonal prisms and other heteroleptic complexes, by combining cis-PtII(PEt3)2(OTf)2 with 1,4-benzenedicarboxylate (6) (Figure 3) and three-, four-, or sixfold-symmetric pyridine donors.73−75Figure 3 shows the structures of 6, threefold-symmetric donor 7, and the self-assembly product 8. In collaboration with the Huang group, this concept was extended to generate highly emissive platinum(II) metallacages using a fourfold-symmetric pyridine donor component that contains a fluorophore that undergoes aggregation-induced emission.76 The strict spatial separation enforced by the metal–organic architecture preserved the fluorescence in both high- and low-concentration regimes, allowing white-light emission. Similar principles were recently reported in a metallacycle where a high degree of intramolecular twist constrained the incorporated anthracenes, increasing the emission intensity.77 Furthermore, the same group, working with the Sun group, showed that metal–organic capsules can self-assemble into soft superstructures of up to the millimeter scale.78
Combinations of nitrogen-donor and carboxylate ligands have also been used to create molecular rectangles based on palladium.79 The formation of cages containing perylene diimide panels, which can bind polycyclic aromatic hydrocarbons, was recently reported by Zhang et al.80 By combining the orange emission of the cage and blue emission of a captured guest, white-light emission was obtained. Differences in fluorescence quantum yield between the solid-state and solution were also exploited to create hidden messages that were revealed upon exposure of the system to acetonitrile vapor.80
As shown in Figure 4, Severin et al. reported that strained homoleptic assemblies such as 9 rearrange following the addition of an extra ligand.81 Metallomacrocycle 9 is strained, and its strain is alleviated in heteroleptic assembly 11, thus providing a driving force to counter the entropic cost of integrating more building blocks. In homoleptic assembly 9, one carboxylate at each metal center forms a four-membered chelate ring, the strain of which is relieved as these carboxylates become monodentate in flexible trigonal prism 11 following the addition of 2,4,6-tris(pyridin-4-yl)-1,3,5-triazine (10). The resulting monodentate binding endows product 11 with a high degree of flexibility. In the absence of a guest, the trigonal-prismatic framework of 11 collapses in the solid state, forming a compressed structure without an interior cavity. However, when coronene is added, the trigonal prism expands to encapsulate two coronene guests in the solid state. This work shows that flexible coordination cage cavities can be generated not just from flexible organic ligands but also from coordinational flexibility about metal centers.
Figure 4.
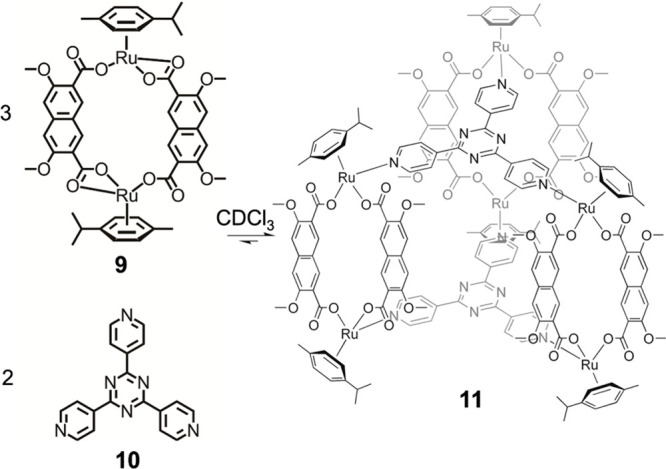
Selective assembly of trigonal prism 11 driven by the removal of a strained four-membered chelate ring in the homoleptic species. Reproduced from ref (81). Copyright 2010 American Chemical Society.
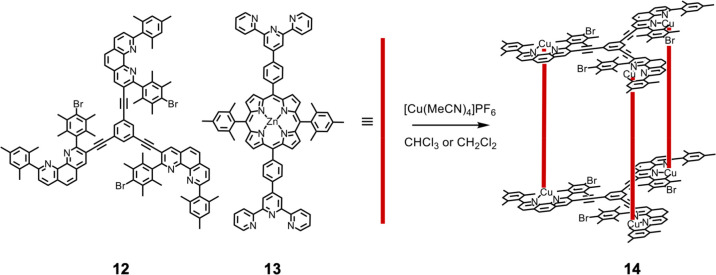
Schmittel and co-workers reported the application of their “heteroleptic terpyridine and phenanthroline metal complexes” (HETTAP) concept to generate myriad of self-assembled structures, including nanoprisms. This approach relies on steric hindrance around the phenanthroline units to prevent homoleptic assembly.82,83 By combining a threefold-symmetric bulky phenanthroline-based ligand (12) with linkers of different lengths (i.e., 13, shown in Figure 5), the authors generated a series of trigonal prisms of differing heights of the general form CuI6L12L23.
Figure 5.
Selective assembly of heteroleptic trigonal prism 14 from precursors 12 and 13 driven by steric restriction involving hindered phenanthrolines (HETTAP).82
The heteroleptic architecture of 14 was further stabilized, eliminating minor byproducts, by the addition of a suitable bridging guest capable of coordinating between the zinc centers in the porphyrins of the ditopic ligands. A planar tridentate pyridine ligand that binds in the central belt of the three porphyrins drives the quantitative formation of the heteroleptic structure. Similar approaches, heteroleptic bis(phenanthroline) complexation (HETPHEN) and heteroleptic pyridine and phenanthroline complexation (HETPYP), have also been shown to selectively yield heteroleptic metal–organic complexes.83
A system may be guided toward heteroleptic assembly through destabilization of alternative homoleptic products that would undergo steric clash. An early seminal example was provided by Yoshizawa and co-workers, who combined sterically hindered and unhindered ligands containing two pyridines to form heteroleptic trigonal prisms.84 Similar approaches have been taken more recently by the Clever group, who used steric bulk to destabilize certain assemblies in order to favor heteroleptic species.85,86 We developed this concept during the selective formation of a copper(I) rhomboidal diporphyrin prism, shown in Figure 6.87 Upon mixing of 8 equiv of the bis(diphenylphosphino)benzene (15) struts, 8 equiv of 2-formylpyridine (16), a guest (17), and 2 equiv of the tetratopic zinc(II)porphyrin (18) with 8 equiv copper(I), rhomboidal prism 19 forms. The offset between the porphyrins within 19 leads to its selective binding of 3,3′-bipyridine (17) between zinc centers.
Figure 6.
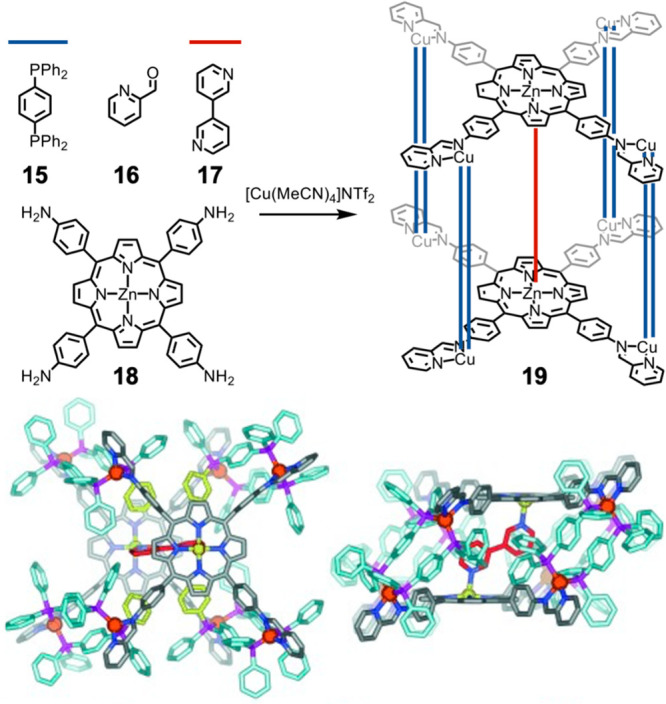
Formation of heteroleptic rhomboidal prism 19 by disfavoring the formation of homoleptic architectures. The offset between the zinc centers in the porphyrins leads to the selective incorporation of 3,3′-bipyridine (17). Adapted with permission from ref (87). Copyright 2015 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
The formation of a homoleptic L2CuI4 porphyrin copper(I) sandwich complex is disfavored by steric clashes between the phenyl groups, and the formation of copper(I) complexes involving the coordination of more than two phosphines is disfavored by the steric bulk of the phenyl groups on phosphorus. The simplest assembly that gives coordinatively saturated copper(I) is thus prism 19. The preference for heteroleptic assembly is likely reinforced by the known preference for copper(I) to selectively form mixed phosphine–pyridine complexes.88
The strategy of using ligands with donors of differing coordinative strength can also drive heteroleptic assembly in concert with the steric effects noted above. As shown in Figure 7, Lehn and co-workers reported a series of cylindrical complexes based on combinations of linear oligo(bipyridine) ligands such as 20 with planar, threefold-symmetric hexaazatriphenylene (HAT) ligand 21 and either silver(I) or copper(I).89,90 The electron-deficient HAT ligands bind less strongly than bipyridines, and their phenyl groups generate steric clashes when two HAT ligands bind around a single metal ion. Assemblies formed from HAT 21 alone would thus be relatively unstable as well as polymeric in nature and thus entropically less favored than the discrete cylindrical assemblies that are observed to form. Lehn et al. used linear ligands containing up to four bipyridine motifs, thus generating cylinders with up to three spatially separated binding pockets. Although the host–guest behavior of this system was not investigated in detail, the principle of using spatially separated binding pockets within the same assembly was further explored by others, such as Clever91,92 and Crowley93 (see Figure 63 in section 7.4).
Figure 7.
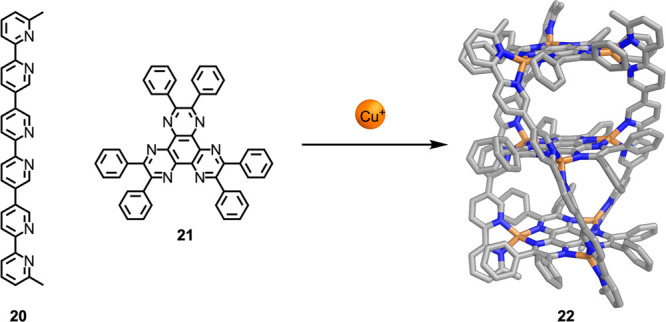
Formation of heteroleptic cylindrical complex 22, with two guest-binding compartments, from tris(bipyridine) 20 and HAT 21.89,90
Figure 63.
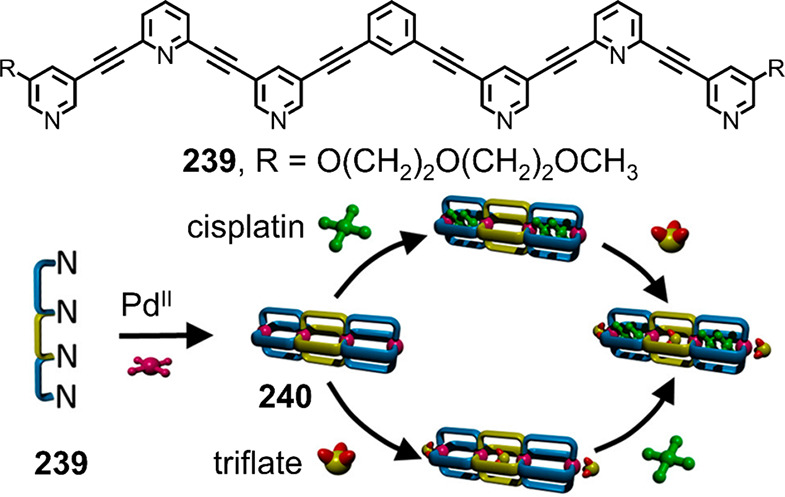
Self-assembly of ligand 239 with PdII into triple-cavity cage 240, which is capable of binding two different types of guest molecules within two distinct types of internal cavities. Reproduced from ref (93). Copyright 2017 American Chemical Society.
2.2. Ligand Shape Complementarity
Clever et al. reported a multitude of different heteroleptic PdII2L4 lantern-type structures, based upon their initial work with analogous homoleptic structures, that contain bidentate ligands incorporating pyridine donors with parallel coordination vectors.94,95 Clever’s approach to forming heteroleptic structures exemplifies the use of shape complementarity.96,97 In the example in Figure 8, bidentate ligand 23 contains isoquinoline donors, and another, 25, contains pyridine donors, each with nonparallel coordination vectors.96 Strain is thus incorporated into homoleptic structures 24 and 26, as the offset coordination vectors cannot close up into a polyhedron by coordinating to square-planar palladium(II) without distortion. When mixed, however, the two ligands come together to form PdII2232252 heteroleptic architecture 27 in which each ligand is cis to its complementary partner, thus forming a tilted lantern architecture. The extension of this concept to a wider variety of ligands subsequently enabled the discovery of an unusual self-penetrating heteroleptic cage architecture.98 Clever and co-workers reported a range of interpenetrated and heteroleptic systems based on similar principles.99−103 Severin and co-workers recently reported the use of similar “banana-shaped” ligands to create heteroleptic cages based on a virtual combinatorial library involving six separate ligands. This led to the formation of a trigonal-antiprismatic [PdII6L6L′6](BF4)12 structure.104
Figure 8.

(a) Formation of homoleptic capsule 24. (b) Formation of homoleptic capsule 26. (c) Formation of heteroleptic lantern complex 27 driven by ligand shape complementarity between 23 and 25. R = hexyl. Reproduced from ref (96). Copyright 2016 American Chemical Society.
The Fujita group reported the assembly of a heteroleptic cantellated tetrahedron from ligands 28 and 29 (Figure 9).105 These ligands have the same angle between coordinating groups but different lengths. Each ligand forms a PdII12L24 cuboctahedral assembly when combined with PdII on its own. However, when combined in a 1:1 ratio, the two diastereomers of product 30 shown in Figure 9 form instead. Rather than narcissistic self-sorting, where each homoleptic assembly forms independently, or random mixing, where a collection of different assemblies form with different ratios of the two ligands incorporated, the system instead produces only PdII1228122912 assemblies. Each cis pair of ligands coordinating the same PdII forms part of a smaller PdII3283 or larger PdII3293 triangular metallomacrocyle, with four of each of these metallomacrocycles covering the cage surface, sharing edges with PdII4282292 rectangles. The PdII1228122912 constitution of 30, as opposed to other ratios of 28 to 29, thus minimizes strain among these triangles and rectangles.
Figure 9.
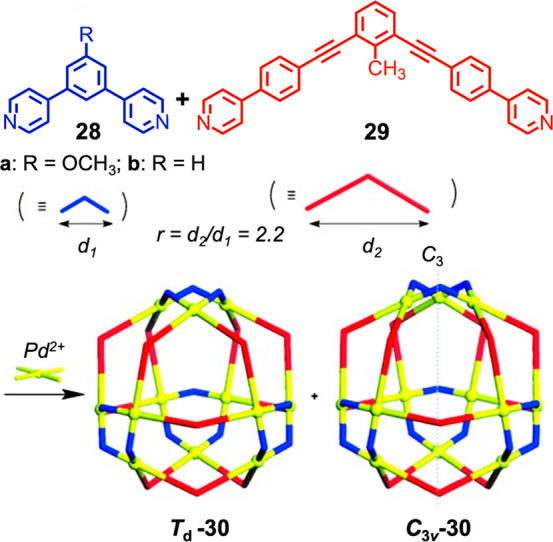
Formation of two diastereomers of heteroleptic cantellated tetrahedron 30 from two ligands, 28 and 29. Adapted with permission from ref (105). Copyright 2014 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Similar principles were used by Benkhäuser and Lützen to create a dinuclear copper(I) molecular kite from subcomponents that did not assemble into discrete, unstrained structures individually.106
2.3. Entropy as a Driving Force for Heteroleptic Assembly
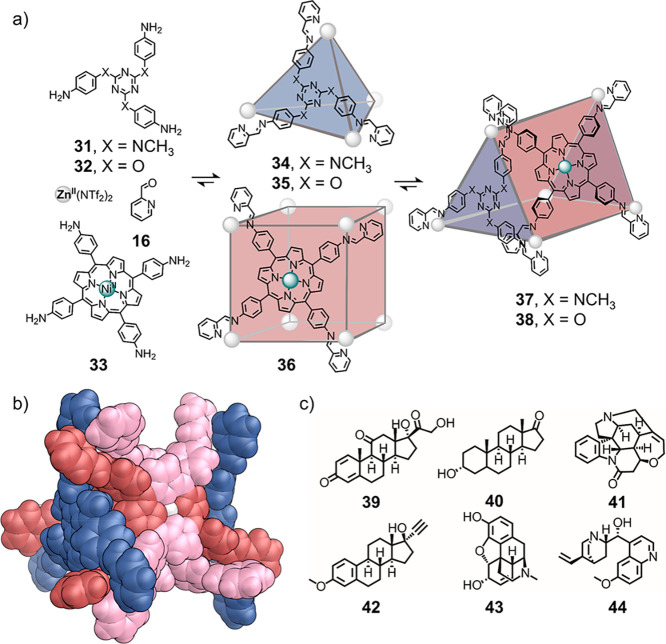
We recently reported a system that undergoes heteroleptic assembly by entropically favoring the mixed architecture (Figure 10).62 Cubes 36 and tetrahedra 34 or 35 are in equilibrium with triangular prisms 37 or 38, respectively. The triangular-prismatic architecture is disfavored enthalpically, but its formation is favored entropically for two reasons. First, the triangular prism has a greater number of conformational microstates: each porphyrin unit adopts a saddled configuration, bowing in or out, in the triangular prism, whereas the porphyrins must lie planar in the cube. Second, the combined cavity volume of triangular prisms 37 or 38 is smaller than the combined volumes of the corresponding cubes and tetrahedra. Thus, fewer solvent molecules are trapped inside the cavities of triangular prisms 37 or 38 relative to the tetrahedra (34 or 35) and cube (36), leading to a more favorable entropy.
Figure 10.
Formation of entropically favored heteroleptic triangular-prismatic complexes that can bind biologically relevant molecules. (a) Assembly of heteroleptic architectures 37 and 38. (b) Crystal structure of 37. (c) Pharmaceutical guests bound by the heteroleptic assemblies. Adapted from ref (62). Copyright 2019 American Chemical Society.
Homoleptic structures, such as 34, 35, and 36, have higher symmetry and more-spherical cavities than the corresponding heteroleptic structures 37 and 38. Such spherical, isotropic cavities are poorly adapted to the binding of more complex, anisotropic molecules of biological interest. A key advantage of the less-symmetric heteroleptic architectures 37 and 38 is the ability to bind higher-value, more complex substrates (e.g., 39–44; Figure 10c) than the more symmetric homoleptic structures.
2.4. Favorable Interactions between Ligands to Drive Heteroleptic Assembly
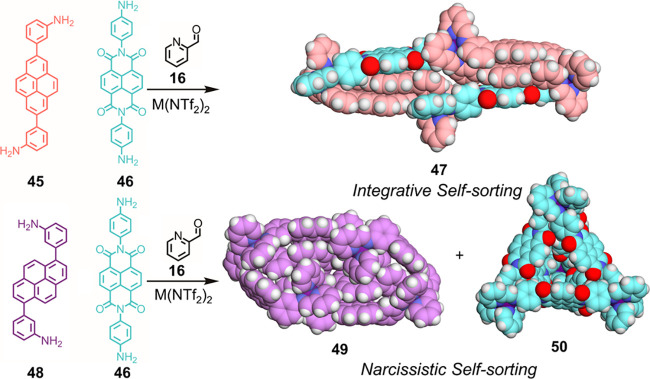
Heteroleptic assembly can be favored by engineering of additional favorable interactions that are not present in the corresponding homoleptic systems. We reported a system of mixed pyrene- and naphthalenediimide-based pyridylimine ligands (Figure 11).107 Alone, each ligand forms a stable homoleptic structure. However, together subcomponents 45 and 46 form FeII4454462 elongated structure 47, which has a different connectivity than either of the homoleptic assemblies. Differentially substituted subcomponent 48, when combined with 46, forms the original homoleptic architectures in an example of narcissistic self-sorting. The selective formation of heteroleptic structure 47 is driven by favorable aromatic stacking interactions between electron-rich and electron-deficient aromatic units that exist only in the mixed architecture. This stacking drives the assembly of the mixed architecture even in the presence of a guest that binds to only one of the possible homoleptic species. This system shows the importance of aromatic stacking interactions in metal–organic architectures.
Figure 11.
Formation of heteroleptic complex 47, favored by aromatic stacking interactions, from the interplay of more electron-rich 45 and more electron-poor 46, and narcissistic self-sorting observed from the combination of 48 and 46 to form homoleptic assemblies 49 and 50.107
Such stacking interactions were also critical in driving the formation of a recently reported twisted trigonal-prismatic architecture.108 Jung and co-workers also reported a catenated architecture based on the stacking of electron-deficient and electron-rich aromatic rings.109 In a similar vein, Yuasa et al. demonstrated that favorable interligand charge-transfer interactions can cause a preference for heteroleptic assemblies over homoleptic alternatives.110
Fujita and co-workers developed a heteroleptic PtII6L2L′3 trigonal prism whose formation is templated by a rigid, flat aromatic guest that binds only in the heteroleptic architecture. Guest binding thus drives selective formation of the heteroleptic trigonal prism. After formation, the guest can be removed by extraction with an apolar solvent, leaving the empty trigonal prism.111 The cavity thus formed can then be used to stabilize the pairing of DNA nucleobases in aqueous solution.63
2.5. Complementary Binding Sites
Stang and co-workers have made extensive use of the square-planar geometric preference of palladium(II) and platinum(II) centers to construct metal–organic assemblies.112 They have obtained heteroleptic assemblies using the concept of complementary binding sites, whereby each component is unable to self-assemble without a complementary partner. As shown in Figure 12, cuboctahedron 53 can be prepared by the assembly of threefold-symmetric, planar metalloligand 51 with bidentate pyridine donor 52. As the metal centers are covalently integrated into one ligand, a second ligand is required for assembly into the nanometer-scale product 53. This work was subsequently extended to form similar chiral adamantanoid cages that incorporate optically active building blocks.113
Figure 12.
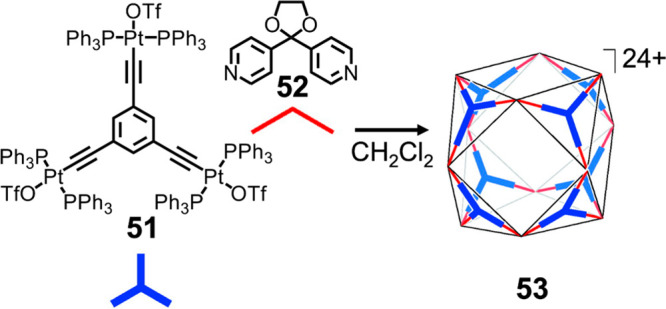
Formation of heteroleptic cuboctahedron 53 driven by the complementarity of binding sites of the different components. Adapted from ref (1). Copyright 2011 American Chemical Society.
Similar principles were previously used by Bosnich and co-workers to selectively generate platinum(II)-based heteroleptic rectangles using terpyridine and monopyridine ligands.114 The Nabeshima115 and Yam116 groups also used this concept to create molecular rectangles, and the area of complementary ligand denticity has recently been reviewed.117 The advantages of combining different donor groups in the same system were further established by Mukherjee and co-workers, who formed open “swings” and “boats” by using pyridine donors in combination with imidazole donors.118 These structures can bind C60 and catalyze Knoevenagel condensations.119
Other groups have further developed the concepts described above to form heteroleptic cages with useful properties. For example, the groups of Ribas, Costas, and Reek reported the formation of a tetragonal-prismatic supramolecular cage from the combination of tetratopic metalloporphyrin tetracarboxylate 55 and macrocycle 54 containing two palladium(II) centers, each coordinated by three nitrogen donors (Figure 13).120 In this system, the coordination preferences of PdII are satisfied by one carboxylate ligand and one macrocyclic ligand, leading to the formation of structure 56 with PdII8544552 composition. This structure encapsulates aminophosphite ligand 57, which coordinates rhodium to form 58. The active supramolecular catalyst thus formed (59) operates with a greater degree of chiral induction due to cage control over the second coordination sphere. Similar capsules have been reported and used for the selective extraction and functionalization of fullerenes.121−123 In collaboration with the von Delius group, the Ribas group recently reported the formation of a “matryoshka” Russian doll-type assembly and its application in the selective formation of a single trans-3 fullerene bisadduct.124
Figure 13.
Formation of heteroleptic tetragonal prism 56 driven by the coordination complementarity of ligands 54 and 55. Cage 56 binds aminophosphite 57, which then binds rhodium (58) to form catalytically active rhodium complex 59. Adapted from ref (120). Copyright 2015 American Chemical Society.
Jin and co-workers reported a system of heteroleptic cages where selective assembly is driven by the interplay between two pairs of distinct chelating sites, a harder O,O′ site and a softer N,N′ site, on a single hydroxamate ligand (60), as shown in Figure 14.125 Half-sandwich iridium and rhodium metal centers assemble with auxiliary pyridine-based ligands, such as 4,4′-bipyridine (61), to form tetragonal and trigonal prisms. The D2-symmetric diastereomer of cage 62 (Figure 14) binds triflate as a guest and template.
Figure 14.
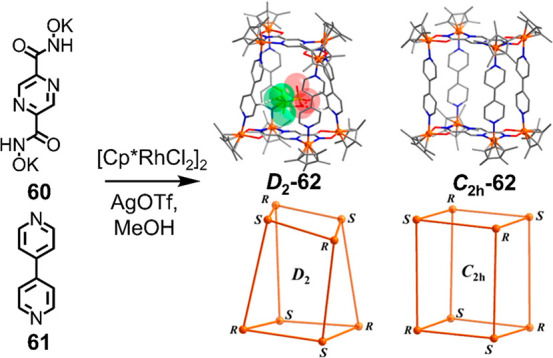
Assembly of molecular prisms with different symmetries based on the hard–soft bis(hydroxamate) donor 60 and 4,4′-bipyridine (61). Adapted from ref (125). Copyright 2015 American Chemical Society.
The hard/soft character of ligand 60 was also used to form heterometallic macrocycles with palladium and iridium centers selectively incorporated into the same framework. Within these heterometallic structures, palladium binds the softer nitrogen donors, whereas iridium binds the harder oxygen donors. One of these macrocycles encapsulated tetrathiafulvalene between parallel hydroxamate ligands.125 The authors recently reported an extension of this system in which symmetric bipyridine 61 is replaced by a bridging unit containing one pyridine and one carboxylate donor site, forming a D2-symmetric heteroleptic species selectively.126
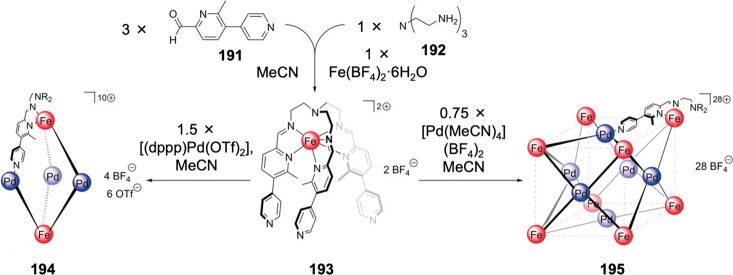
We reported a system of PdII-based macrocycles and cages whose assembly is controlled by the addition of appropriate pyridine-containing templates to the assembled PdII-bound macrocycles. Each PdII is coordinated by three nitrogens from the macrocycle (Figure 15) and one from the bridging ligand.127 The subcomponents 2,6-diformylpyridine (63) and flexible dianiline 64 assemble around palladium(II) templates to generate metal–organic macrocycles containing either three or four PdII centers, depending on the tri- or tetratopic nature of the pyridine template used. When we employed linear, ditopic pyridine template 65, which has a geometry ill-adapted to incorporation within a single macrocycle, three-dimensional capsule 66 was generated. This structure (Figure 15) includes a trimeric macrocycle at each end with bridging ligands 65 between them. Assembly 66 forms cooperatively, with no structures observed containing fewer than three bridging ligands. Structure 66 encloses a small cavity, which was found to bind tetrafluoroborate selectively.
Figure 15.
Formation of complex assembly 66 from subcomponents 63 and 64, PdII(MeCN)4(BF4)2, and N,N′-dipyridylnaphthalenediimide 65. Reproduced from ref (127). Copyright 2019 American Chemical Society.
Similar systems were extended to form truncated tetrahedra and other metal–organic cages by the use of a tritopic aniline ligand. The dynamic pyridylimine bonds formed during self-assembly could be cleanly reduced to form secondary amines, thus disabling the equilibration process and fixing the structures formed.
2.6. Kinetic Traps
Crowley and co-workers reported a novel approach to generating heteroleptic architectures that employs kinetic traps rather than favoring a thermodynamic product (Figure 16).128 PdII2L4 lantern architecture 69, formed from bidentate pyridine-containing ligand 67 with parallel coordination vectors (Figure 16), is combined with another ligand 68 containing 2-aminopyridines. Ligand 68 forms stronger bonds to palladium, so thermodynamics favors its incorporation. When excess ligand 68 is added to PdII2674 lantern 69, PdII2672682 lantern 71 forms selectively in a cis configuration. The selectivity for the cis isomer is attributed to hydrogen bonding between adjacent amino groups. The selective formation of a PdII2672682 lantern, rather than complete substitution to form a homoleptic PdII2684 structure, is attributed to the effects of steric repulsion between the 2-amino groups and incoming pyridine ligands in the proposed associative mechanism. This repulsion increases the energetic barrier to ligand exchange, enabling the selective formation of the heteroleptic species. Calculations suggested that the heteroleptic species is a kinetically trapped metastable species rather than the thermodynamic product, and competition experiments supported this idea. Hydrogen bonding between the pyridine α-CH and adjacent 2-aminopyridine groups is inferred to reinforce this kinetic stability.
Figure 16.
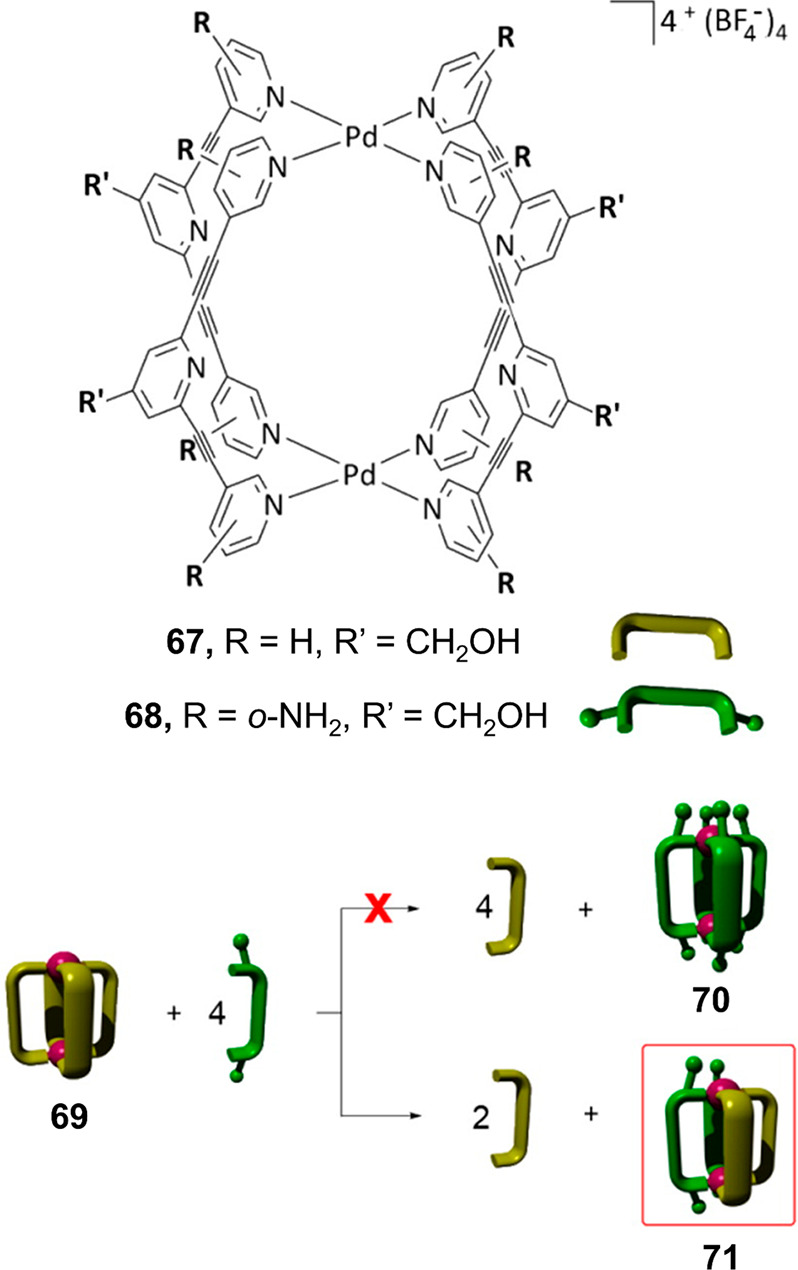
Formation of kinetically trapped heteroleptic molecular lantern complex 71 with selective incorporation of pairs of ligands 67 and 68. Reproduced from ref (128). Copyright 2016 American Chemical Society.
An intriguing use of kinetic control in self-assembly was reported by Lusby, Barran, and co-workers, who used the low lability of cyclometalated platinum corners to create trigonal-prismatic assemblies (Figure 17).129 The identity of the product depends on the sequence of addition rather than the thermodynamic stability of the product. Starting from a platinum complex with one pyridine, one dimethyl sulfoxide, and two phenylato ligands, a bi- or terpyridine ligand is then added. This additional ligand displaces weakly bound dimethyl sulfoxide to form an intermediate complex with either twofold (72) or threefold (74) symmetry. In the case of twofold-symmetric intermediate 72, tritopic pyridine ligand 10 is then added, which forms a new coordination bond trans to a phenylato ligand. This process displaces another phenylato ligand, which is then protonated. The phenyl group thus released is left above the threefold-symmetric face of trigonal prism 73.
Figure 17.
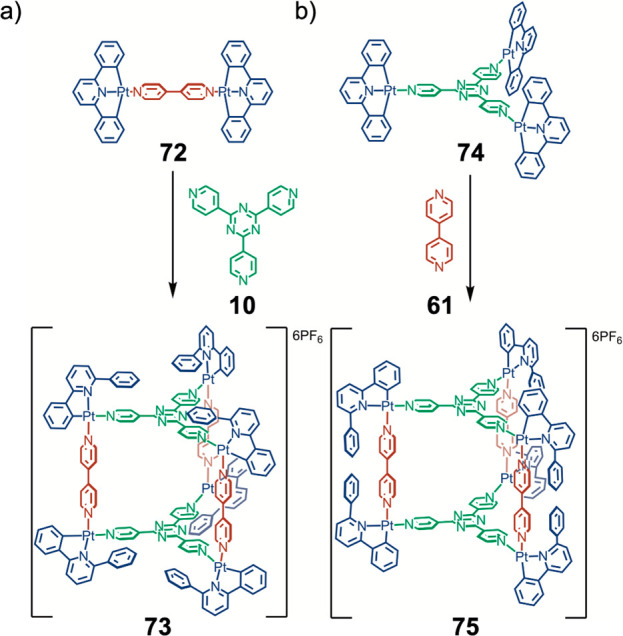
Selective formation of isomeric heteroleptic trigonal prisms 73 and 75 by control over the sequence of addition. (a) Initial addition of ditopic ligand 61. (b) Initial addition of tritopic ligand 10.129
If instead bipyridine 61 is added to threefold-symmetric intermediate 74, the released phenyl groups of product 75 instead stack above the twofold-symmetric ligand. This isomerism is further manifested in the mass spectrometry data collected, where the weaker coordination bonds trans to the phenylato group are observed to rupture preferentially. This approach provides an example of how the sequence of addition can control the outcome of a self-assembly process and thus provides a novel mode of generating structural complexity.
This section has reviewed different approaches for generating heteroleptic structures, which frequently have novel, lower-symmetry architectures. We have explored how control over both the entropy and enthalpy of formation can be used to bias systems toward thermodynamic heteroleptic assembly. More subtly, we have also seen how fine control of the balance of kinetics in a system can enable the formation of kinetically trapped heteroleptic products without preventing the error checking that is vital to the self-assembly of complex architectures.
3. Lower-Symmetry Ligands: Using Reduced-Symmetry Ligands Leads to Reduced-Symmetry Products
The complexity of metal–organic architectures may be increased through the use of components that themselves have more complex structures. This concept has recently been reviewed by Lewis and Crowley.130 Reduced-symmetry ligands can also lead to an increased number of possible structures. Thus, we also evaluate factors that drive the selective formation of one structure from among multiple possibilities.
3.1. Reduced-Symmetry Ligands
M2L4 cages using bis-monodentate ligands and square-planar metal centers have been well-studied and would not be considered “complex” in terms of the scope of this review.131,132 However, several recent publications have reported the formation of M2L4 structures with reduced-symmetry ditopic ligands and a single type of metal ion133−137 or two different types of metal ion,138 leading to greater structural complexity. When M2L4 structures assemble from a reduced-symmetry ditopic ligand, several isomers are possible (Figure 18). Often one or more of these isomers are of lower energy than the others and therefore form preferentially.
Figure 18.
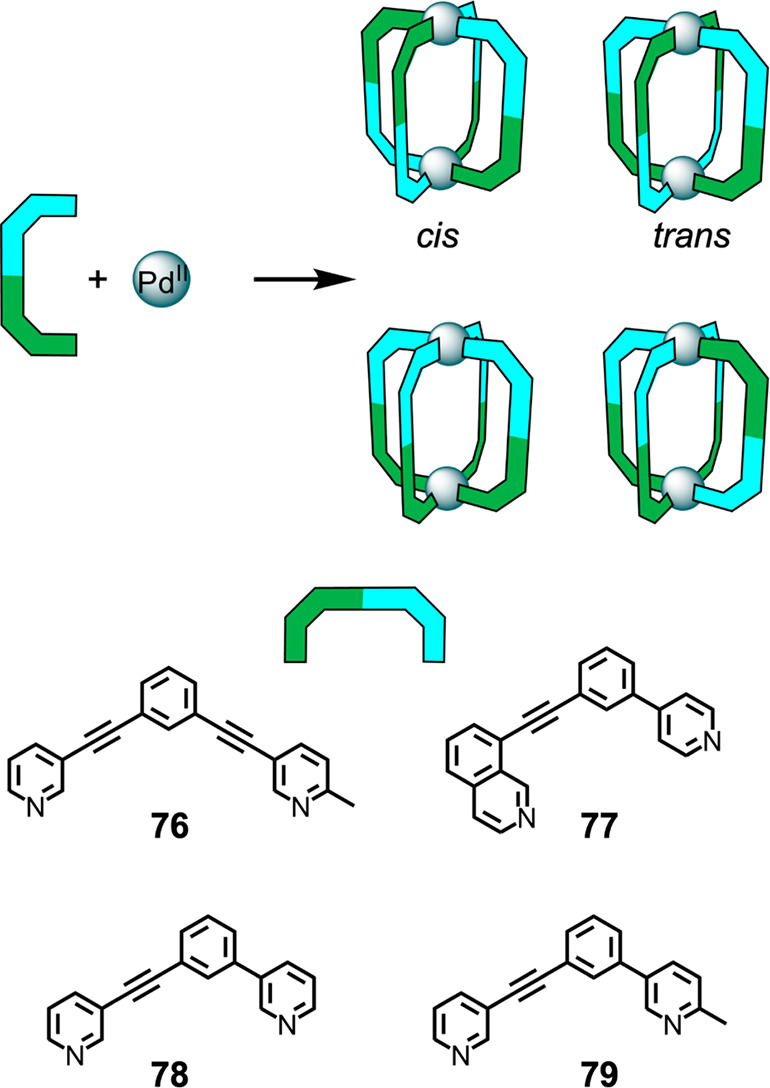
Representations of the four possible isomers of homoleptic PdII2L4 cages that can be formed from one of the reduced-symmetry ditopic ligands 76–79.133
Lewis and co-workers showed that the identity of the preferred isomer of a PdII2L4 cage can be controlled by changing the identity of the ligand (76–79). Hindered ligand 76 produces a C2v-symmetric trans-PdII2L4 isomer in MeCN, minimizing steric clashes, with product identification being supported by DFT calculations.133 Upon an increase in the polarity of the solvent by the use of DMSO, a mixture of the trans-PdII2L4 and cis-PdII2L4 isomers form. This phenomenon is tentatively attributed to selective stabilization of the cis-PdII2L4 isomer by the more polar solvent, which is predicted by DFT to have a larger dipole moment than the trans isomer.
The C2h-symmetric cis-Pd2774 isomer forms selectively in DMSO.133 This selectivity arises from the presence of different binding sites at the two ends of ligand 77, a pyridine and an isoquinoline. Within 77, the planes orthogonal to the coordinate vectors of the nitrogen donor atoms no longer coincide (even when the pyridine and isoquinoline rings are coplanar), thus favoring cis-PdII2774 formation. Subsequent investigations involving ligand 78 indicated that in this case the deviation from coplanarity was not significant enough to yield a single isomer of the PdII2784 complex. However, the greater steric hindrance around the coordination sphere of PdII bound to 79 results in the formation of a single PdII2794 isomer. On the basis of DFT calculations, cis stereochemistry was inferred.
Finally, the addition of steric bulk, in this case via the inclusion of methyl groups in 76 or 79, causes an increase in the helical twist of the structure compared with analogous structures formed by ligands lacking methyl groups. The steric effects of these methyl groups on the conformation of the resulting structure may enable tailoring of the internal cavity space.
The Lewis group has also shown that reduced-symmetry ditopic ligands containing 1,2,3-triazole and isoquinoline binding sites can form a similar PdII2L4 cage as a single cis-PdII2L4 isomer.137 Variation of the substituent on the triazole moiety results in the formation of a series of externally functionalized cages. Because of the uniformity of the main ligand framework among all of the derivatized ligands, dynamic libraries of mixed-ligand cages are obtained when mixtures of the different ligands are used.
Bloch et al. recently demonstrated the use of conformational flexibility in producing reduced-symmetry ligands.139 In their system, a dicarboxylate ligand with a diimine core exists in three different rotational conformations, one of which has lower symmetry. Depending on the crystallization conditions, three distinct cage isomers are isolated from a dynamic library; their structures were determined by single-crystal X-ray crystallography. The three cage isomers each contained either two or four ligands in the reduced-symmetry conformation.
Separate studies reported by Ogata and Yuasa134 and Crowley et al.138 also involved the formation of M2L4 structures with unsymmetrical ditopic ligands. Both utilized the differing labilities of coordination bonds involving different monodentate donors or metal ions to develop mechanisms for guest capture and release. Yuasa et al. altered the stoichiometric ratio of ligand to metal in the reaction mixture to drive the interconversion of a PdII2804 cage, capable of binding anions within its cavity, and a PdII804 complex, which does not bind guests (Figure 19). In this mononuclear complex, the imidazole groups of all four ligands are bound to the PdII center and the four pyridyl donors remain free because imidazole is a stronger donor than pyridine.134
Figure 19.
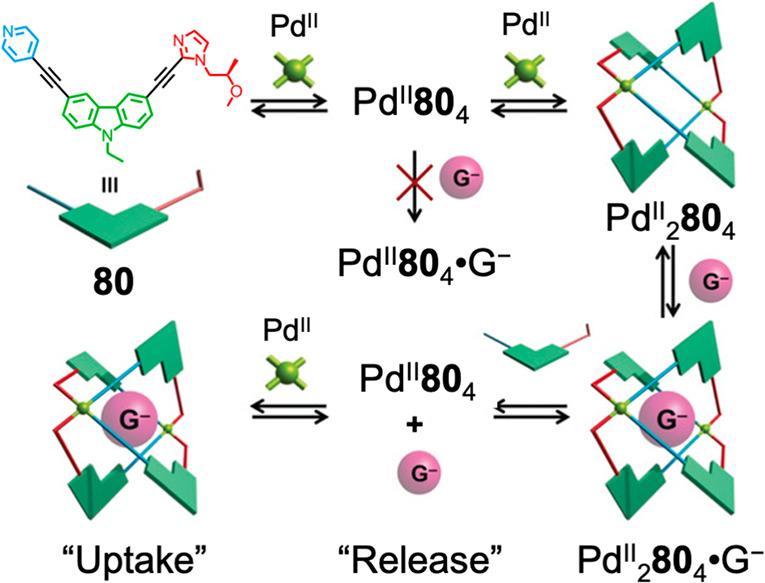
Stepwise self-assembly of a dynamic open PdII2L4 coordination cage using unsymmetrical imidazole–pyridine-based ditopic ligand 80. Stoichiometry-controlled structural transformation of this cage allows anion uptake and release. Adapted with permission from ref (134). Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
An approach introduced by Crowley et al. is based on the design and synthesis of a cage in which the antipodes are PtII, which forms more inert PtII–pyridyl bonds, and PdII, which forms more labile PdII–pyridyl bonds.138 Following its formation (Figure 20), PdIIPtIIL4 cage 83 can open and close reversibly. The addition of 4-dimethylaminopyridine (DMAP) selectively sequesters PdII, forming PdII(DMAP)4 and opening the cage. Subsequent addition of p-toluenesulfonic acid protonates the DMAP ligands and causes their dissociation from the metal centers, releasing PdII and reforming cage 83.
Figure 20.
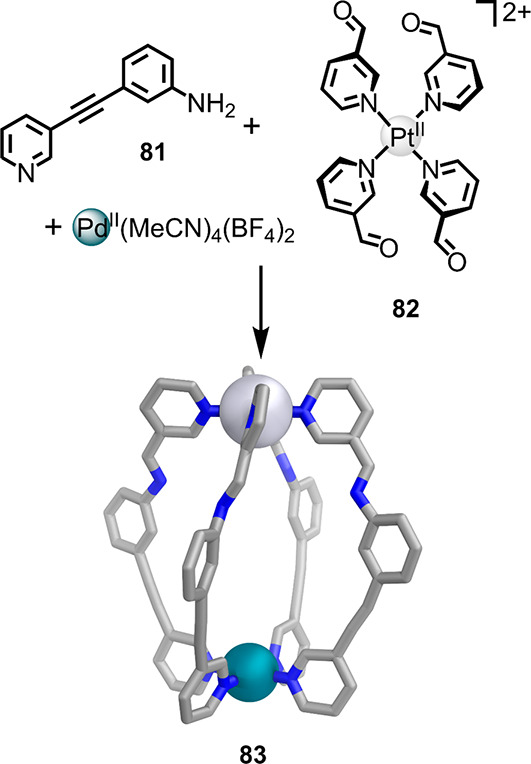
Synthesis of [PdIIPtIIL4](BF4)4 cage 83 via the combination of preformed PtII(3-pyridylcarboxaldehyde)4 complex 82, 3-[2-(3-pyridinyl)ethynyl]aniline (81), and PdII(MeCN)4(BF4)2.138
This stimulus-induced opening and closing of cage 83 also brings about reversible guest uptake and release, illustrating a potential function. Although these structures are relatively simple, they exemplify how functionality can be introduced by the use of reduced-symmetry ligands. These principles may be combined with other rules, detailed elsewhere in this review, that guide the formation of larger and more complex structures to yield architectures of greater complexity and functionality.
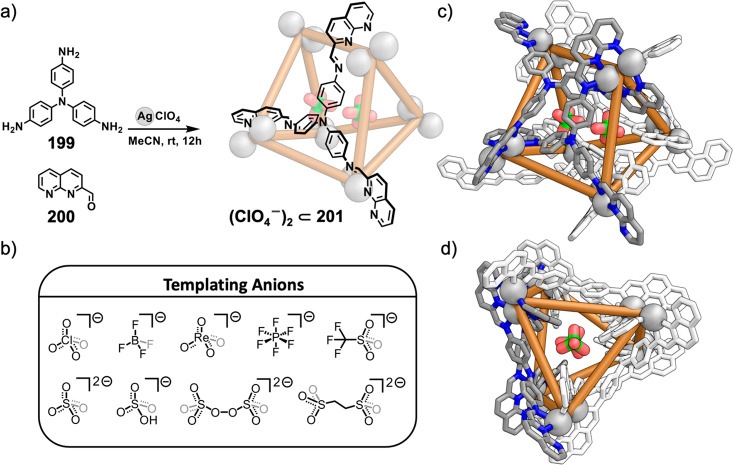
Hooley and co-workers reported the use of a prochiral ligand in the assembly of a desymmetrized FeII4L6 architecture (Figure 21).140 The presence of a prochiral CHOH center in the fluorenone ligand—a motif that they have explored to generate functional capsules141−144—brought about the selective formation of “wizard’s hat” 85, a distorted tetrahedron. The formation of this unusual architecture is favored by a specific pattern of hydrogen bonding involving the −OH groups at the prochiral carbon atoms of the ligands and a templating perchlorate ion at the base of the assembly. An interesting aspect of this assembly is the presence of three mer FeII centers at the base of the structure, which are rare in self-assembled pyridylimine architectures and often drive the assembly of more complex structures, as discussed in subsequent sections.
Figure 21.

Hooley’s “wizard’s hat” assembly 85, stabilized by internal hydrogen bonds and a templating perchlorate ion. The crystal structure shown at the right.140
Along with reduced-symmetry ditopic ligands, tritopic ligands with reduced symmetry can generate complex metal–organic architectures. Su et al. demonstrated the use of such tritopic ligands to form unusual architectures in the preparation of a AgI6L6 tubular structure using an elongated T-shaped ligand.145
Hu et al. used 5-(pyridin-4-yl)isophthalic acid (87) with p-tert-butylthiacalix[4]arene (86) and NiIICl2 to form NiII40 coordination cage 88, with a structure corresponding to the J17 Johnson solid.146 As illustrated in Figure 22, the structure of 88, a gyroelongated square bipyramid, consists of 10 Ni4-p-tert-butylthiacalix[4]arene shuttlecock-like vertices and 16 panels of ligand 87. Four ligands converge at two of the 10 vertices, and five ligands converge at each of the other eight, closing the faces of the structure. In order to form the structure, the ligands coordinate to NiII centers through different donor atoms: through the carboxylate, which can either bridge or chelate NiII, and through the nitrogen donor of pyridine. The phenoxo oxygen and sulfur atoms of the p-tert-butylthiacalix[4]arene units also coordinate to NiII, along with additional 87 units that do not cap the faces of the structure, DMF molecules, chloride ions, and degradation products of DMF in order to satisfy the coordination geometry of NiII.
Figure 22.
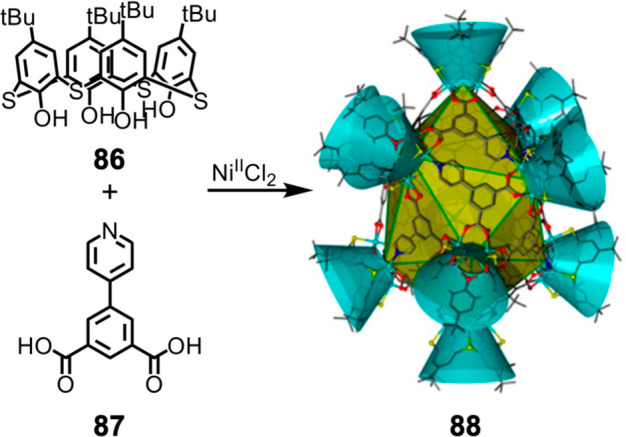
Ni40 coordination cage 88 with a structure corresponding to the J17 Johnson solid, formed from p-tert-butylthiacalix[4]arene (86), 5-(pyridin-4-yl)isophthalic acid (87), and NiIICl2. Reproduced from ref (146). Copyright 2016 American Chemical Society.
Hong et al. employed tritopic ligand 89, which has three binding sites arrayed asymmetrically along its length (Figure 23). The combination of this reduced-symmetry ligand, NiII(ClO4)2, and pyrazole (Pz) in ethanol yields NiII9896Pz6 barrel structure 90.147 In 90, the pyrazole plays two roles, acting as a Lewis base and as an additional donor to satisfy the octahedral coordination sphere of NiII.
Figure 23.
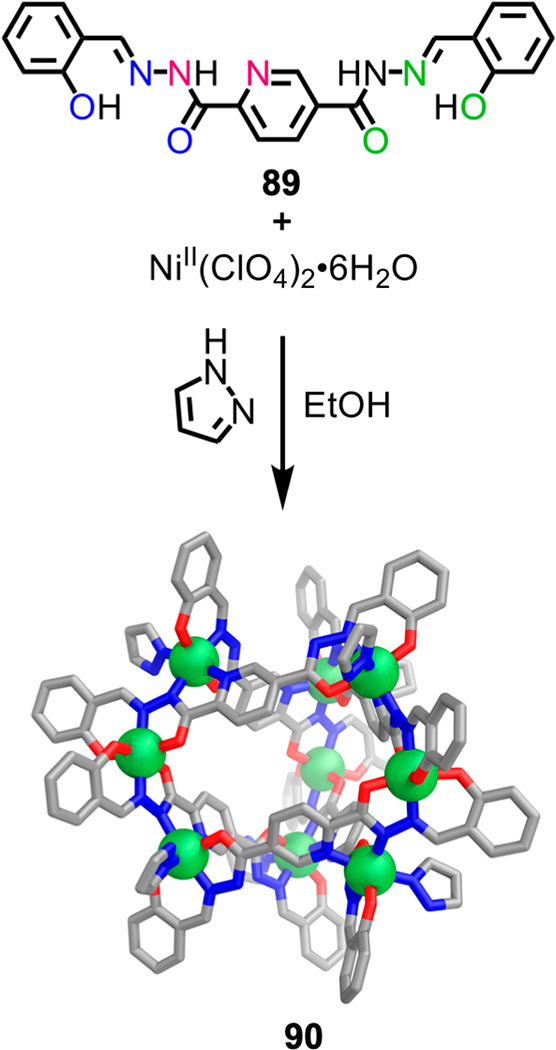
Self-assembly of NiII9896Pz6 barrel structure 90 incorporating asymmetric tritopic ligand 89.147
Li et al. explored the use of desymmetrized tetratopic ligands resembling trapezoids to form metallosupramolecular architectures. Upon combination of these ligands with 180° dipalladium(II) acceptors, ring-in-ring148 or 2D Star-of-David149 structures form.
The reaction of these same ligands with “naked” palladium(II) ions yields three-dimensional structures. One example is PdII249124 sphere-in-sphere architecture 92 (Figure 24), which forms from ligand 91 and PdII.148 The authors drew a contrast between their approach and the one pioneered by Fujita and co-workers.150 The Fujita approach is based on the orthogonal assembly of two ditopic units into “independent” M12L24 spheres connected via flexible linkers to give the M24L24 sphere-in-sphere. In Li’s system (Figure 24) precise preorganization of the entire 3D architecture is enforced by the rigid nature of the ligand. Ligand 91 also reacts with a tritopic platinum(II) unit to form a double-layered pentagonal prism.151
Figure 24.
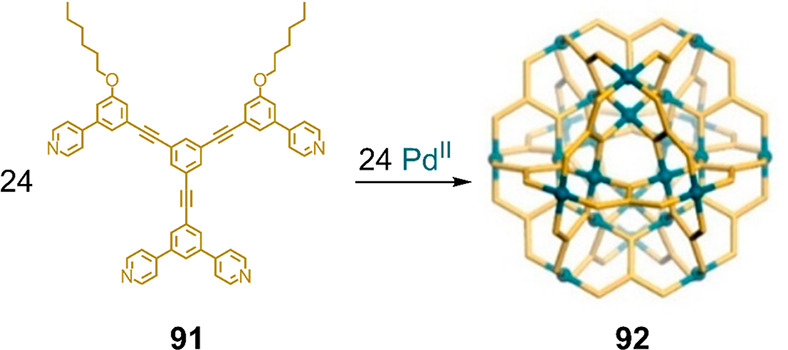
Self-assembly of PdII249124 three-dimensional sphere-in-sphere structure 92. Adapted from ref (148). Copyright 2015 American Chemical Society.
Ligand 91 has donor groups arrayed in two distinct ways; Li et al. also designed ligands with four distinct binding sites that form double-layered macrocyclic structures.152 Their reports exemplify how rational design of new classes of ligands can allow unique metallosupramolecules with high degrees of complexity to be formed.
3.2. Additional Donor Sites
Another approach to designing ligands capable of forming architectures with greater complexity is the modification of ligands that have previously been used to form metal–organic assemblies, for example by appending additional donor sites. This approach was used to design pentatopic ligands 93 and 94 (Figure 25), which form 3D hexagonal-prismatic structures 95 and 96, consisting of two connected 2D double-rimmed “Kandinsky circles”, when combined with octahedrally coordinated cadmium(II) ions.153 Ligands 93 and 94 are based upon a tetratopic donor previously reported by Li et al.,154 with the fifth terpyridine group appended to allow the two circles to be linked.
Figure 25.

Self-assembly of three-dimensional hexagonal-prismatic structures 95 and 96, consisting of two connected 2D double-rimmed “Kandinsky circles”, from CdII and ligands 93 and 94, respectively. Adapted from ref (153). Copyright 2019 American Chemical Society.
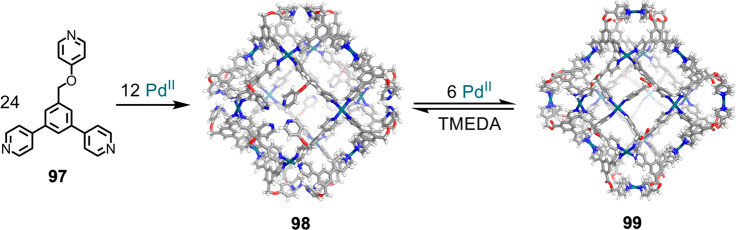
As well as providing a method for the formation of 3D structures from known 2D structures, the ligand-modification approach can be used to increase the complexity of an existing 3D structure. Fujita and co-workers employed this approach to form a PdII189724 stellated cuboctahedron 99 using tripyridyl ligand 97, consisting of a rigid bipyridyl unit with a third pyridyl moiety flexibly tethered to the backbone.155 As shown in Figure 26, the assembly process occurs in a stepwise fashion. The tripyridyl ligand combines with PdII(BF4)2 to yield PdII129724 cuboctahedron 98, analogous to a previously reported complex that incorporates a rigid bipyridine ligand.156
Figure 26.
Stepwise assembly of PdII189724 stellated cuboctahedron 99 from terpyridine 97 and PdII.155
Initial selective complexation with just one type of pyridine donor to form 98 is perhaps surprising at first sight. The authors suggested that the selectivity observed is due to the high kinetic stability of the cuboctahedral framework. Previous work had shown that ligand exchange on a completed cuboctahedron occurs with a half-life of 20 days.157 Kinetic trapping of the cuboctahedron thus drives the selective assembly.
Subsequent addition of more PdII(BF4)2 to intermediate structure 98 resulted in capping of the square faces by the coordination of four “free” pyridyl groups to each new palladium(II) center and consequent stellation of the structure to form 99. Stellation is reversed by the addition of N,N,N′,N′-TMEDA, resulting in the reformation of 98. The authors noted that this reversible opening and closing through stellation may have future applications in guest capture and release.
3.3. Nonplanar Macrocyclic Ligands
As shown in the system in Figure 22, macrocycle-derived subunits can be employed to construct coordination cages.146,158−161 These components often have greater complexity than simpler small-molecule ligands, while still maintaining high symmetry, which increases the complexity of the resulting metal–organic architectures.162,163 Furthermore, the use of macrocycle-derived components also may enable combination of the guest-binding abilities of the macrocycles with those of the higher-order superstructures that the macrocycles form.164−167
Complementing the work of Hu and co-workers, who used p-tert-butylthiacalix[4]arene to form the vertices of a metal–organic polyhedron,146 macrocyclic components have also been employed as the edges and faces of metal–organic cages. Hardie and co-workers reported foundational work in this area using tritopic cyclotriveratrylene (CTV)-related ligands.168−170
The Hardie group’s use of CTV-related ligands to provide an array of new structure types culminated in the report of a “Solomon’s cube”,170 based upon the topology of a Solomon link.171,172 The combination of extended tris(pyridyl)cyclotriguaiacylene (100) with PdII(NO3)2 in DMSO results in PdII41004 structure 101 shown in Figure 27. While resembling a Solomon link,171,172 with alternating under and over crossing points of two rings, 101 has additional connections between the rings, linking them. Consequently, the structure was described as a “Solomon’s cube”, with square faces and eight triply connected vertices.
Figure 27.

Assembly of PdII41004 “Solomon’s cube” 101.170
Structure 101 may thus be considered in terms of its three stereochemically distinct subunits: ligand 100, the Solomon link, and the figure-eight motifs lying on each of four sides of the structure. The crystal structure shows two enantiomers, in which all three of these elements concertedly show opposite handedness.
The driving force for the formation of smaller PdII41004 assembly 101, as opposed to a PdII61008 structure, is likely due to the fact that ligand 100 contains m-pyridine-based arms, as opposed to a linear para ligand regiochemistry, connected to a rigid macrocyclic core. Further stabilization of this topology may come from interligand π-stacking interactions.
Structure 101 in Figure 27 thus demonstrates the ability of nonplanar macrocycle-based ligands to produce more complex structure types than would be observed in analogous cases using planar D3h-symmetric ligands. Interwoven 101 also exemplifies how the use of novel classes of ligands can lead to serendipitous discoveries.
3.4. Metallosupramolecular Chemistry Meets DNA Nanotechnology
Many of the architectures discussed in this review are assembled using small-molecule organic ligands and metal ions. A more exotic example is provided by the metal–nucleic acid cages of Sleiman et al.173 (Figure 28). These structures require stepwise assembly of oligonucleotide strands (102). First, triangles 103 with corners consisting of two 2,9-diphenyl-1,10-phenanthroline ligands (dpp–dpp) are formed through hybridization of three complementary oligonucleotide strands. Second, two triangles are linked with single strands to give 104, and the struts are then rigidified to form trigonal-prismatic structures 105. Finally, site-specific metalation involving the coordination of CuI, AgI, AuI, ZnII, CoII, CdII, or EuII to the dpp–dpp sites, enables the creation of metal–DNA cages 106.173
Figure 28.
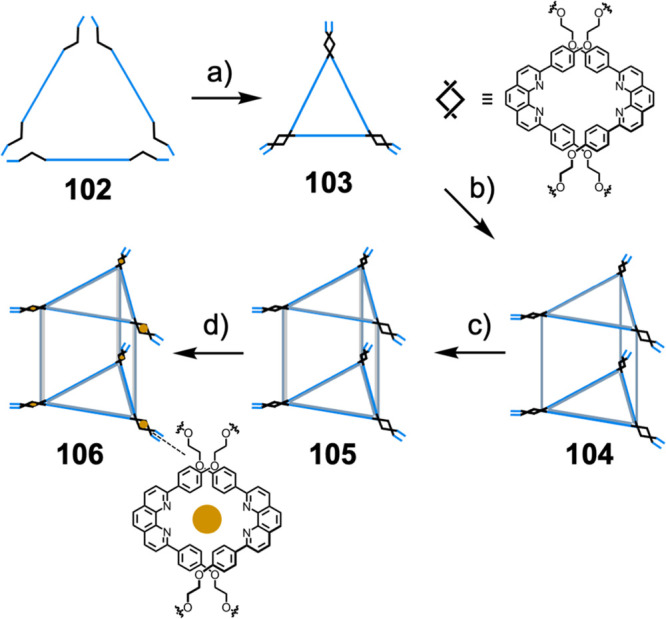
Stepwise assembly of metal–DNA cages from diphenylphenanthroline-containing DNA strands. (a) Hybridization of three complementary oligonucleotide strands. (b) Linking of two triangles with single strands. (c) Rigidification of the linking strands. (d) Site-specific metalation.173
The Sleiman group also demonstrated that the order of the steps could be swapped: premetalation of the triangles followed by single-strand triangle linkage and rigidification results in the same metalated trigonal-prismatic structures. Although the flexibility in the order of construction steps indicates that metal–ligand coordination is not required to template the formation of these trigonal-prismatic structures, metalation of the structures increased their resistance to both chemical and thermal denaturation compared with their demetalated counterparts. Metal coordination was thus demonstrated to enable the formation of robust architectures assembled from strands of DNA, potentially enhancing the range of applications of 3D DNA architectures.174−178
Through highlighting some key examples of complex or reduced-symmetry ligands that have led to novel structures, this section has emphasized the roles of both rational design and serendipity. As a general approach, the use of reduced-symmetry and complex ligands often involves rational design, sometimes with the aid of computational predictions. Postassembly rationalization has in many cases also played a role, enabling the discovery of new assembly rules, which may then be used for future designs.
4. Ligand Flexibility Drives Structural Complexity
Flexible ligands in many cases assemble into high-symmetry architectures.179−185 However, flexibility within a ligand can also extend the scope of structure types beyond those having high symmetries. This section summarizes novel structure types generated via the incorporation of flexibility into the building blocks used to assemble discrete structures. Ligand flexibility often generates serendipitous results, as ligand degrees of freedom are deployed in unforeseen ways.
4.1. Flexible Ditopic Ligands
Ward and co-workers pioneered the construction of metal–organic architectures with flexible ditopic ligands, focusing on ligands containing two bidentate pyrazolylpyridine chelating sites, each attached to a central aromatic group via flexible methylene linkages. These ligands were combined in a 3:2 ratio with octahedral metal centers to yield several distinct structure types. Some of these structures have the geometries of Platonic solids, such as tetrahedra,179−181 and others have lower symmetries and greater complexity.
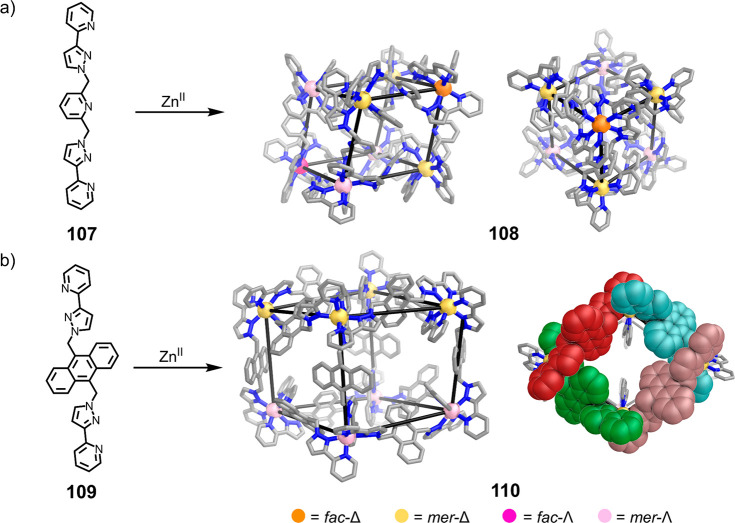
Several of Ward’s M8L12 structures exhibit symmetry reduced from that of a cube.186,187 For example, as shown in Figure 29a, the combination of 107 with ZnII yields ZnII810712 cuboid 108 with S6 symmetry. An antipodal pair of ZnII centers define the S6 axis of the structure. These metal centers have fac stereochemistry but opposite handedness.186 The other six metal centers have mer stereochemistry and are grouped into two sets of three. All of the metal centers within the same set have the same handedness, opposite to that of the other set. Mass spectrometry data showed the formation of an analogous MII8L12 structure, CoII810712, from CoII and 107.
Figure 29.
Two distinct MII8L12 structures formed using ditopic bis(pyrazolylpyridine) ligands and octahedral metal centers.186,187
With anthracene-cored ligand 109, structure 110 was formed, which has the same MII8L12 composition as 108 but significant structural differences. Cuboid 110 consists of two connected ZnII41094 cyclic helical units (Figure 29b).187 Within each tetrameric unit, the four metal centers are trischelated in a mer fashion and have the same absolute configuration. However, as shown in Figure 29b, the handedness of the four metal centers in one tetrameric unit is opposite to that of the metal centers making up the other tetrameric face. The use of CuII(BF4)2 with 109 yields CuII810912, which has a similar structure as 110.
The diversity of structures formed using such ligands was further demonstrated by the formation of unusual NiII4L6 “square” and MII6L9 (MII = ZnII, CoII) “open book” structures using 107 and its modified derivatives.188,189
Ligand 111 reacts with NiII(BF4)2 in a 3:2 ratio in MeOH/CH2Cl2 (Figure 30) to yield a NiII811112 structure, which was initially thought to be cubic.190 However, X-ray crystallography showed that product 112 has an unusual structure, based on C2v-symmetric cuneane formed by the rearrangement of two edges of a cube (Figure 30b). All eight of its metal centers have mer stereochemistry. Interestingly, seven of the metal centers have the same absolute configuration, with the eighth displaying the opposite handedness.
Figure 30.
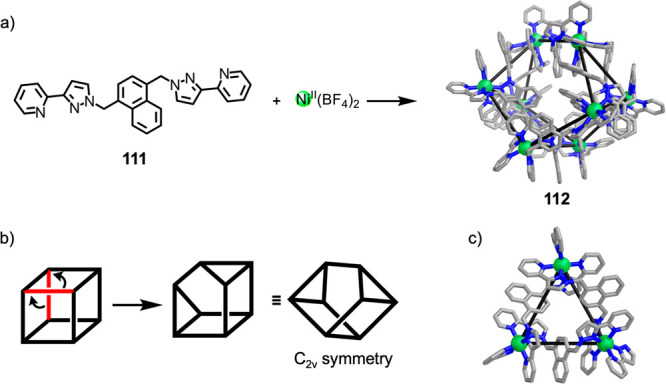
(a) Formation of NiII811112 complex 112 with a structure based on a “cuneane-like” core.190 (b) The “cuneane” structure is obtained by the rearrangement of two edges of a cube. (c) View perpendicular to one of the NiII3L3 cyclic helical units making up the two triangular faces of 112.190
Each of the two triangular faces of 112 is made up of an MII31113 metallomacrocycle (Figure 30c). Such M3L3 units have been observed in structures employing similar ditopic ligands.191−193 The four structure types shown in Figure 31 are built from M3L3 subunits, with their different geometries arising from differences in how these subunits are connected to each other.
Figure 31.

Four different structure types containing M3L3 circular helicate units. (a) Schematic view of MII1611324, (b) CdII1611324,191 (beige and red spheres correspond to mer and fac configurations, respectively), (c) schematic view of MII61139, (d) CuII61139,191 (e) schematic view of MII12113121174, (f) CdII12113121174,192 (g) schematic view of MII1212118, and (h) CdII1212118.193
As shown in Figure 31a, structure 114, a CdII1611324 twisted tetracapped truncated tetrahedron, results from the reaction of 113 and CdII in MeCN; ZnII also forms the analogous structure 115.191 Within 114, four CdII31133 cyclic helical subunits are linked by CdII1133 units, which act as tritopic complex ligands (Figure 31b). The fac-configured CdII centers of the CdII1133 units (red spheres in Figure 31a,b) cap each of the four hexagonal faces of a CdII12 distorted truncated tetrahedral core described by the 12 mer-configured centers (beige spheres in Figure 31a,b) of the four CdII31133 units. When ligand 113 reacts with CuII, the smaller CuII61139 trigonal-prismatic structure 116 forms (Figure 31c). Trigonal prism 116 consists of two CuII31133 circular helical units bridged by three ligands, with some offset between triangular faces leading to distortion toward a trigonal-antiprismatic structure (Figure 31d).
The reaction of NiII(BF4)2 with 113 produces a NiII811312 cubic cage, which does not contain trinuclear helicate units. The observation of different structures with the same ligand but different metal ions was attributed to variations in the ionic radii and stereoelectronic preferences of the metal centers.191 Furthermore, reaction of the same ligand (113) together with flexible tris-bidentate ligand 117 and CdII, CuII, or CoII in a 3:1:3 ratio yields a [MII12117411312] cage with approximately cuboctahedral geometry (Figure 31e).192 Of its eight triangular faces, four are capped by 117, and each of the remaining four consists of an MII31133 circular helical subunit, similar to those found in the other structures.
The fourth structure type, shown in Figure 31g, is an MII1212118 truncated tetrahedral cage framework with idealized T symmetry. This structure results from the reaction of 121, which has a naphthyl central linking group, with CuII, CoII, or CdII.193,194 These structures consist of four M31213 circular helical motifs that are connected directly by six bridging ligands.
A common thread linking the different geometries shown in Figure 31 is the presence of linked MII3L3 circular helicate subunits, where the three metal centers have a mer trischelate geometry. Another important feature of these four structure types is the prevalence of interligand aromatic stacking interactions, often between electron-rich central aromatic moieties on one ligand and electron-deficient pyrazolylpyridine units on another.191−193 This elegant work by the Ward group has thus established the utility of relatively simple, flexible ligands in the construction of assemblies with structures beyond the Platonic solids, whose geometries are controlled by subtle variations in reaction conditions and ligand structure.
1H NMR spectroscopy and mass spectrometry showed that the CdII1611324 structure 114 described above is initially present in solution, but the structure rearranges to give a smaller CdII61139 trigonal prism over weeks in solution.191 Replacing the 1,4-phenyl moiety in 113 with the 1,4-naphthyl in ligand 111 results in a CdII1611124 tetracapped truncated tetrahedron (in contrast to the cuneane structure observed for 111 with NiII, shown in Figure 30a), which does not rearrange in solution. The additional interligand π stacking provided by the naphthyl spacer was inferred to stabilize the tetracapped truncated tetrahedron in solution.195
In contrast, the reaction of 111 with CuII does not selectively yield any species analogous to those shown in Figures 30 and 31. Instead, crystals of an unusual CuII1211115 structure form in low yield, consisting of two CuII31113 units linked by an equatorial belt of six CuII ions, each with a coordination number of 4 or 5.195
Utilizing ligand 111 also allowed Ward et al. to analyze the CdII111121174 analogue of the structures shown in Figure 31e,f in solution. CdII111121174 was shown to exist as three different diastereomers in solution, with T, C3, or S4 symmetry.196 The difference between the diastereomers arises from the different relative helical handednesses of the four CdII3L3 circular helical units in the structure.
Kwong et al. reported the formation of D3-symmetric MII12L18 hexagonal-prismatic architectures following the reaction of 2-formylpyridine 16, m-xylylenediamine 125, and MnII(ClO4)2 or CdII(ClO4)2 in acetonitrile (Figure 32).197 The crystal structure of 126 reveals two M6L6 hexagons having chair conformations, made up of alternating Λ- and Δ-configured metal centers. Bridging ligands connect metal centers with a Λ configuration on one ring with those with a Δ configuration on the other, resulting in mer-Λ and fac-Δ configured metal centers within prism 126. Other metal–organic structures beyond the Platonic solids constructed using similarly flexible ditopic ligands include a HgII4Cl8L4S4-symmetric coordination nanotube198 and a [DyIII8L8(μ2-CH3OH)4]8+ dual triple-stranded helicate.199
Figure 32.
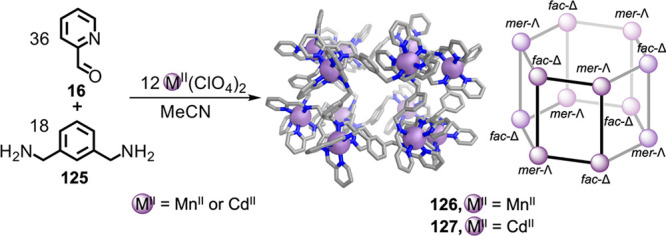
Subcomponent self-assembly of D3-symmetric MII12L18 hexagonal-prismatic structures 126 and 127.197
Mirkin and co-workers developed the “weak-link approach” to forming reduced-symmetry structures with complex functions.200Figure 33 shows a dimeric capsule produced using this approach, incorporating resorcin[4]arene and calix[4]arene subunits linked by platinum(II) centers.201 In the absence of chloride, “weak-link” thioethers coordinate to platinum(II) binding sites. Upon the addition of chloride ions, these thioethers are selectively displaced, causing expansion of the cavity. The addition of silver(I) tetrafluoroborate reverses this expansion by abstracting chloride from the platinum(II) centers and regenerating the closed state of the capsule. In the thioether-coordinated form 131, estradiol 133 is bound selectively. In the chloride-coordinated form 130, two molecules of dextromethorphan·HCl (132) bind instead. Sequential addition of chloride to 131 and silver(I) tetrafluoroborate to 130 brings about reversible binding and release of dextromethorphan, showcasing the ability to reversibly generate cavities with different sizes and shapes and thus control guest binding.
Figure 33.
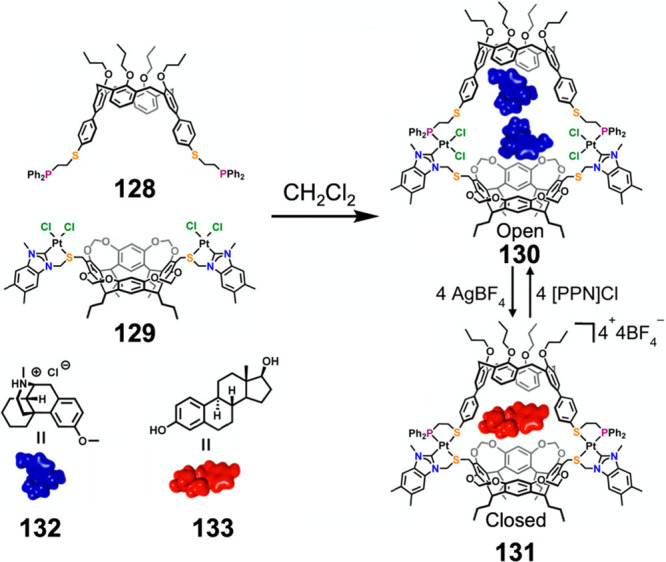
Controlled guest release and uptake by means of the “weak-link” approach using ligands 128 and 129. [PPN]Cl = bis(triphenylphosphine)iminium chloride. Adapted from ref (201). Copyright 2017 American Chemical Society.
4.2. Flexible Tritopic Ligands
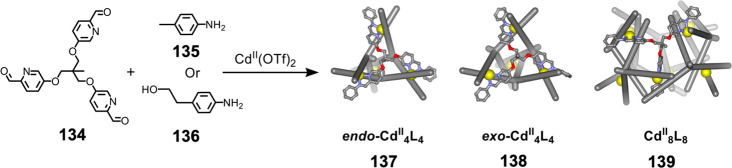
The combination of flexible tris-formylpyridine subcomponent 134 with CdII(OTf)2 and p-toluidine (135) yields a mixture of three products (Figure 34).202 Two of these are T-symmetric CdII4L4 tetrahedra (137 and 138). In 137, the central methyl groups of the ligands point inside the cavity (endo), whereas in 138 these methyl groups point outward (exo). The third, minor, product is CdII8L8 tetragonal antiprism 139 with D4 point symmetry. The eight metal centers defining the vertices of the structure have the same handedness, each with a mer arrangement of ligands.
Figure 34.
Conditions-dependent subcomponent self-assembly of three discrete products: tetrahedra 137 and 138, and CdII8L8 tetragonal antiprism 139 with D4 point symmetry. Adapted from ref (202). Copyright 2016 American Chemical Society.
The relative amount of the CdII8L8 antiprismatic structure 139 grows with increasing concentration because of a reduction in the entropic penalty of forming a larger CdII8L8 species instead of the smaller CdII4L4 complexes. Even more effective at driving the formation of the CdII8L8 structure is the use of 2-(4-aminophenyl)ethanol (136) as a subcomponent in place of 135 and the use of a 1:3 CH2Cl2/MeCN solvent mixture. We hypothesized that these conditions allow the formation of stabilizing hydrogen-bonding interactions between the hydroxy groups of the aniline residues in the CdII8L8 antiprismatic structure. In this example, the analysis of a serendipitous result enabled the rational development of design principles for the optimized preparation of a complex architecture, illustrating the synergy between serendipity and rational design.
Hong et al. used a tris(pyridine) ligand, which had a flexible core similar to that of 134, for the construction of open AgI6L4 cages upon reaction with AgIBF4.203 These cages undergo further assembly to produce higher-order polycatenanes and polycages, depending on the reaction conditions.
4.3. Flexible Tetratopic Ligands
In sections 7.2 and 7.3 we explore how barrel-like and other complex architectures have been constructed using tetratopic ligands that are elongated along one axis or curved. Expanding upon this approach, Duan et al. used tetratopic ligands with flexible linkers separating two bis-tridentate units to prepare structure types that include trigonal-prismatic barrels, cubelike structures, and bicoronal trigonal prisms.204−207 Assembly 141 (Figure 35a) is a Ce81406 cuboidal architecture with pseudo-S4 symmetry formed from CeIII(NO3)3, KOH, and ligand 140.205 The crystal structure of 141 shows that four of its ligands have their long axes aligned, with their central methylene groups bent toward the inside of the cage, and the ligands at the top and bottom of the structure both have their methylene groups bent toward the outside of the cage.
Figure 35.
With CeIII, tetratopic ligands (a) 140, (b) 142, and (c) 144 form cuboid 141 and bicoronal trigonal prisms 143 and 145, respectively.205−207
However, such a pseudocubic structure type does not form when the similar ligands 142 and 144 are used (Figure 35b,c). Instead the Ce8L6 complexes 143 and 145, respectively, are formed. Assembly 143 consists of a Ce61423 trigonal-prismatic framework, with two additional metal centers and three ligands forming a helical pillar within the prism. Two of the tridentate moieties of each ligand in the helical pillar bind to the apical cerium centers, and the other two tridentate sites chelate two of the metal centers making up the prismatic framework (Figure 35b).206 In contrast, 145 has a cagelike structure in which the flexible ligand twists so that the four cerium centers binding to the same ligand are not coplanar.207 Furthermore, stacking interactions between the benzyl groups on neighboring ligands are inferred to stabilize the unusual structure of 145.207
Sun and co-workers also reported the use of flexible tetratopic ligands in the synthesis of unusual “conjoined twin-cages”.208,209 They were further able to control which species formed, either a PdII12L6 cage with three mechanically coupled cavities or two helically isomeric PdII6L3 cages, by the judicious choice of assembly conditions.208
4.4. Flexible Ligands Containing More than One Type of Coordinating Motif
This section considers flexible ligands that bind metal centers using more than one type of donor atom or binding moiety incorporated into the same ligand. Octanuclear helicate 147 (Figure 36), with a cavity large enough to bind amino acids enantioselectively, exemplifies this approach.210 The combination of ZnIICl2 and chiral salen-based ligand 146 produces 147, which consists of two bowl-like ZnII21462 dimers linked by four equatorial zinc centers. Within each dimer, each five-coordinate zinc center is chelated by the N2O2 pocket of one of the ligands, and the two metal centers are linked by two phenalato oxygen atoms. The two pendent pyridyl groups of each ligand remain free to coordinate to additional ZnII ions, whose tetrahedral geometries are satisfied by coordination of two chloride ions, resulting in the formation of the ZnII81464Cl8 structure 147. The use of enantiopure ligand 146 is essential for the formation of cagelike helicate 147. The use of racemic 146 results in the formation of dimeric units containing ligands with opposite handedness, which causes the four peripheral pyridyl groups to point toward different faces of the ZnII2 core, precluding helicate formation.
Figure 36.
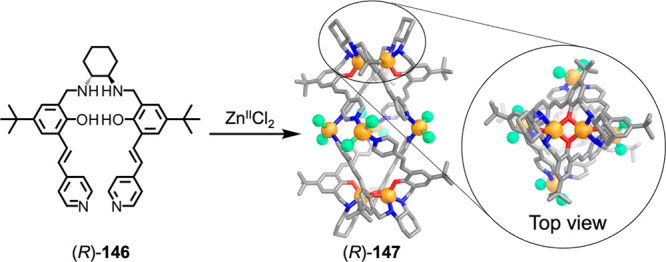
Assembly of octanuclear helicate 147 consisting of two bowl-like ZnII21462 dimers linked by four equatorial ZnIICl2 units.210
Li et al. reported cobalt–imidazolate cage 152, which assembles upon combination of 2-methyl-4-formylimidazole (148), m-xylylenediamine (125), and CoII.211 The 12 ligands form in situ and combine with 12 OH– ions, four water molecules, four octahedral CoIII centers, four tetrahedral CoII ions, and 12 distorted square-pyramidal CoII centers to form a T-symmetric tetartoid structure (Figure 37a). Furthermore, the addition of d- or l-menthol during self-assembly yields enantiopure ΔΔΔΔ-152 or ΛΛΛΛ-152, respectively. The imidazolyl 2-methyl substituent was an effective steric structure-directing feature. This methyl group points inside the pentagonal face of the structure, whereas it could not fit within the smaller window of a cube.
Figure 37.

Subcomponent self-assembly of metal imidazolate (a) tetartoids and (b) cubes. The geometry of the assembled structure is governed by the steric properties of substituent R1. Adapted from ref (211). Copyright 2017 American Chemical Society.
In contrast, when 5-methyl-4-formylimidazole (150) or 4-formylimidazole (151) combines with CoII and m-xylylenediamine, cubic cage 154 or 155 (Figure 37b) forms; in 154, the 5-methyl groups point away from the faces of the structure.212 Thus, the substituent at the 5-position of the imidazolyl ring does not exert steric control over the structure formed, in contrast with the 2-substituent. This work, together with Kwong’s (Figure 32),197 highlights the role that flexible subcomponents play in directing self-assembly. The same simple diamine subcomponent formed complexes with very different structures depending on the steric properties of other subcomponents within the system.
Li recently reported the use of a different flexible bis(imidazole) ligand, 156 (Figure 38), to form bicapped square-antiprismatic structure 157 upon reaction with CuII under solvothermal conditions (Figure 38).213 Single-crystal X-ray diffraction showed the formation of CuII101568 cages that have two types of CuII centers. Eight equatorial CuII ions have a distorted square-pyramidal geometry, with tetradentate chelation of one ligand and monodentate binding of a second. Each of the two axial CuII centers is bound by four imidazolate donors, with additional coordination of anions and water molecules to complete the coordination sphere.
Figure 38.

Self-assembly of adaptable CuII101568 bicapped square antiprism 157.213
Bicapped square-antiprismatic structure 157 can expand or compress vertically to accommodate different anions in its cavity because of its flexible ligands. Among the anions encapsulated (SiF62–, ClO4–, Br–, and Cl–), SiF62– gives the largest cavity volume and Cl– the smallest. Cage compression is triggered through anion exchange, for example, by the addition of KCl to a cage binding ClO4– internally. Li et al. also employed ligand 156 to form a mixed-valence CuII/CuI metallocycle.214 Upon combination of this metallocycle with triethylenediamine in a 2:3 ratio, a trigonal-prismatic structure forms.215 This trigonal prism undergoes a structural transformation to form 157 upon oxidation of CuI to CuII.
4.5. Ligand Flexibility Arising from Substituent Positioning
An alternative way to introduce flexibility into ligands without incorporating alkyl or other flexible linkers is to vary the position of substitution of aryl rings or change the metal-binding moieties so as to provide multiple conformers capable of binding metal ions in different ways.
For example, tri- and tetratopic ligands that employ 3-pyridyl binding sites or imidazoles have been used in place of conformationally locked 4-pyridyl binding sites. When binding to cis-protected square-planar metal centers, such tritopic ligands can form M6L4 open cages (159)216 or bowl-like217 structures. Mukherjee et al. reported PdII61584 open cage 159, which forms from tritopic ligand 158 with imidazole donor groups (Figure 39) and can catalyze Knoevenagel condensations and Diels–Alder reactions within its hydrophobic cavity in water.216 Recently Klajn and co-workers adopted this cage for the investigation of photoswitching in confined environments.218−221
Figure 39.
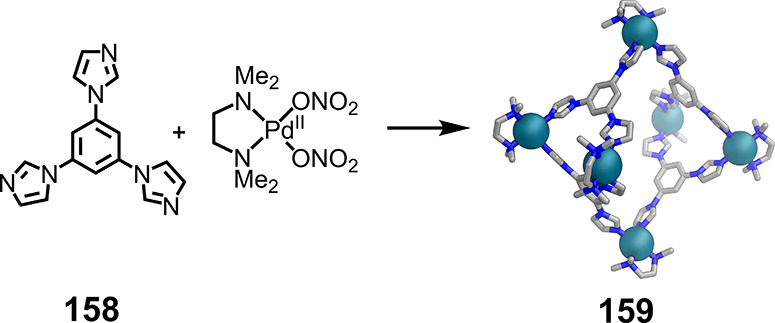
Self-assembly of open PdII61584 cage 159.216
Analogous tetratopic ligands have been shown to form M6L3 trifacial222,223 and M8L4 tetrafacial64,224 barrels that are structurally similar to those formed using the elongated tetratopic ligands discussed in section 7.2. A similar type of ligand flexibility was employed by Schröder and co-workers to form a Cd66 nanosphere with idealized T symmetry. Its dual-shell structure consists of a sphere of 66 CdII centers bridged by μ3-hydroxide, μ3-oxo, and μ5-NO3– anions and enclosed by 12 DMF ligands and 20 tritopic organic capping ligands.225
4.6. Flexible Pseudolinear Polypyridyl Ligands
Fujita and co-workers have demonstrated that in addition to the formation of nanotubes from relatively rigid polypyridyl ligands through guest templation (section 7.4), nanotubular structures are also obtained using more flexible ligands. The combination of PdII(en)(NO3)2 with ligand 160 (Figure 40) and a rodlike guest template results in the formation of PdII6160 end-capped tube 162.226 The flexible nature of the benzenetetracarboxylate-containing core of the ligand allows it to fold and form structure 162 containing only one folded ligand. Selective guest binding within this tube was observed, whereby a biphenylcarboxylate guest bound unidirectionally with the biphenyl group ensconced in the hydrophobic pocket of the tube and the hydrophilic carboxylate exposed to the solvent.
Figure 40.
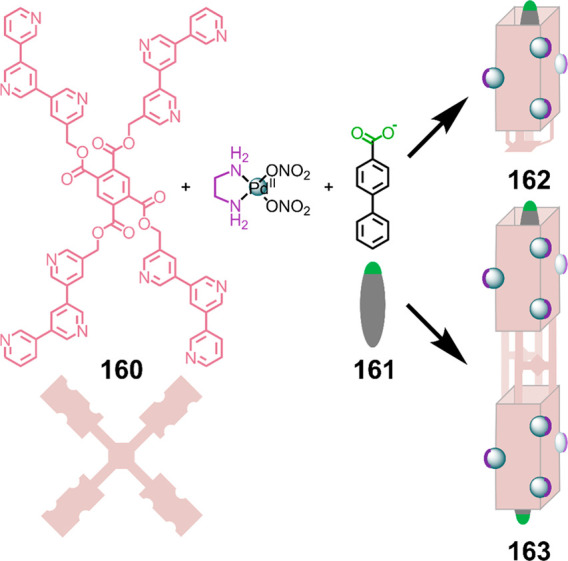
Flexible ligand 160 forms PdII6160 nanotube 162 and PdII121602 nanotube 163.226
At higher concentrations, the longer PdII121602 tube 163 forms as a minor species and was isolated via crystallization. X-ray crystallography revealed a doubly open-ended tube that is 3 nm in length with two template molecules residing inside the cavity.
Similarly, Chand et al. showed that a flexible pseudolinear tripyridine ligand forms a PdII3L4 double-decker cage.227 Upon reduction of the metal:ligand ratio from 3:4 to 1:2, the ligand reconfigures into a U-shaped conformation in which the two terminal pyridines bind to the same PdII center to form a PdIIL2 spiro-type complex and the central pyridine donor of each ligand remains uncoordinated. Interconversion between the two structure types occurred following alteration of the metal:ligand ratio of the reaction mixture.
A consistent theme for this section is that the structures formed from flexible ligands can be difficult to reliably predict, meaning that the results are often serendipitous. However, as elsewhere, rules and hypotheses derived from these initial observations can enable the design of related structures and components to selectively form a desired structure that may have initially been observed to form as one component of a mixture. The adaptability exhibited by some structures formed using flexible ligands is more rarely observed for structures formed with more rigid ligands. The reconfiguration of these more flexible structures can lead to new functions, often related to guest binding.
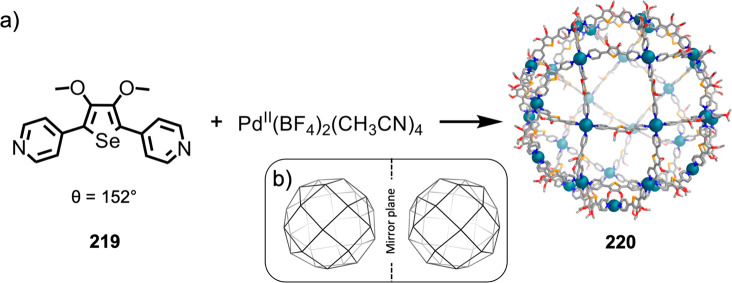
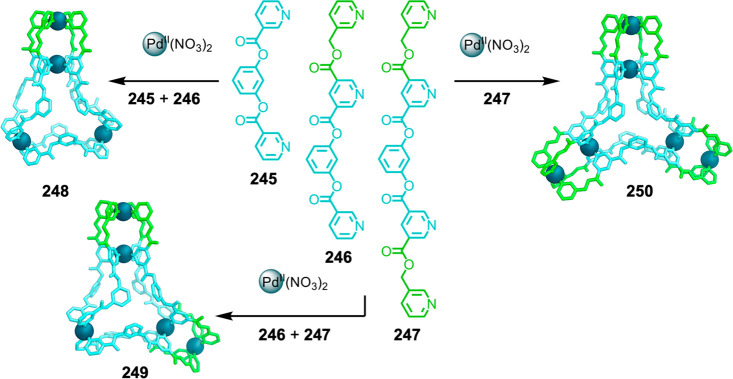
5. Complexity Derived from Solvent, Anions, and Templates
Changes in the environments where metal–organic cages form can alter the structure formed. The course of self-assembly may be reconfigured by changing the solvent, adding a guest, or manipulating external conditions such as temperature and concentration. Although the effects of the environment on the structure may be challenging to predict beforehand, they may be rationalized, and the resulting knowledge can again be used to infer self-assembly rules. In this section we review techniques used to generate complex architectures from simple subcomponents by modulation of the external conditions and by guest addition.
5.1. Solvent- and Concentration-Dependent Complexity
One of the most straightforward methods to direct the assembly of complex architectures is to vary the solvent used. We reported a system where tetrahedral metal–organic cage 165 forms in water but a mixture of methanol and water leads to the selective formation of pentagonal antiprism 166 instead (Figure 41).228 By tuning of the temperature in addition to the solvent, either architecture can be prepared exclusively, with lower temperatures favoring the pentagonal antiprism. This process involves a switch from fac coordination stereochemistry around the metal centers in the tetrahedral cage to all mer metal centers in the pentagonal antiprism, where the lower-symmetry mer coordinative linkages give rise to increased structural complexity.229−232 Antiprism 166 is kinetically stable toward changes in the solvent, requiring heating for a week to convert to the thermodynamically preferred tetrahedron 165 following solvent exchange. However, the pentagonal antiprism is responsive to the addition of a competing aniline: addition of 4-methoxyaniline to a mixture of pentagonal antiprism 166 and tetrahedron 165 brings about the selective disassembly of 166. Severin and co-workers were able to trigger the rearrangement of an octanuclear prismatic cage that forms in chloroform into a tetranuclear complex by exchanging the chloroform solvent for dichloromethane.233 This work illustrates how even quite subtle changes in the solvent can trigger substantial transformations in the assembly structure, especially when the structures have specific binding interactions with that solvent.
Figure 41.

Formation of a self-assembled tetrahedron (165) and pentagonal antiprism (166), controlled by solvent polarity. Adapted with permission from ref (228). Copyright 2013 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Newkome, Wesdemiotis, and co-workers reported an assembly process where at higher concentrations ligand 167 assembles with CdII to produce bisrhombohedral structure 168, whereas at lower concentrations the simpler tetrahedron 169 is favored (Figure 42).234 The use of tris(terpyridine) ligands with CdII provided the delicate balance of lability and stability required for these structures to form. At higher concentrations, the bisrhombohedral architecture forms exclusively, whereas the tetrahedron is the exclusive product at lower concentrations. To confirm the structure of the tetrahedron, which could not be isolated because of rapid equilibration back to the bisrhombohedral architecture, a ruthenium(II)-containing metalloligand, essentially consisting of two units of 167 connected by the coordination of one (blue) terpyridine unit on each 167 to a kinetically inert ruthenium(II) center, was employed. The formation of a similar tetrahedral architecture confirmed the structural assignment of 169. The authors subsequently reported a system wherein the predominant product among three architectures—a cuboctahedron, an octahedron, and a triangular sandwich complex—depended upon the concentration.235 These systems provide a way to generate and switch between complex architectures selectively in solution through manipulation of the concentration.
Figure 42.
(a) Formation and switching between bisrhombohedral complex 168 and tetrahedron 169 in a concentration-dependent process. (b) Schematic view. Adapted from ref (234). Copyright 2014 American Chemical Society.
Shionoya and co-workers reported that tritopic ligand 170 forms bowl-like structure 171 and pseudotetrahedron 172 (Figure 43).236 Conversion between 171 and 172 is governed by different stimuli. Changes in solvent, metal:ligand stoichiometry, guest addition, or pH lead to the formation of one architecture over the other. For example, increasing the proportion of water in the acetonitrile solvent leads to selective formation of capsule 172 from bowl 171, and addition of zinc triflate to a solution of 172 produces 171.
Figure 43.
Formation of bowl 171 or pseudotetrahedron 172 from tris(bipyridyl)porphyrin 170. Reproduced with permission from ref (236). Copyright 2019 American Chemical Society.
Shionoya’s group reported the use of a similar ligand with fourfold symmetry in combination with zinc triflate and a mixed solvent system to generate an unusual D3-symmetric enneahedron.237 We have also made use of solvent effects in a system where a tetrahedron interconverted with dimeric and trimeric stacked structures on the basis of different chemical stimuli.238 Analogous stacked structures had previously been generated from a more rigid, achiral subcomponent, where interconversion between double and triple stacks was controlled by subcomponent substitution.239
5.2. Temperature-Dependent Assembly
Hiraoka and co-workers reported the intriguing system shown in Figure 44, where two similar building blocks sort with a selectivity that varies as a function of temperature.240 Although these capsules are purely organic (Figure 44) and are held together by van der Waals forces, cation−π interactions, and the hydrophobic effect, their novel mechanism of sorting warrants inclusion in this review. The authors used gear-shaped amphiphilic molecules 173 and 174 with hexaphenylbenzene cores that self-assemble to form hexameric cubic architectures. These two hexaphenylbenzenes differ in the presence (173) or absence (174) of methyl groups at three positions (Figure 44). These additional methyl groups have a significant effect on the thermal stability of the formed hexameric architectures, with assembly 175, composed of trimethylated 173, dissociating at 130 °C, whereas assembly 181, formed from non-methylated 174, dissociates at 65 °C. When 173 and 174 were mixed and the mixture was allowed to equilibrate at room temperature in water, a statistical distribution of capsules 175–181 containing both panels was formed as a result of the structural similarities between them. The authors then heated the scrambled system above the disassembly temperature of 181, which led to survival of only 175 at 100 °C. The authors then quenched the system by rapid cooling, trapping it in a metastable state consisting of only the two homogeneous capsules 175 and 181. These capsules then reequilibrated, taking 2 days at 25 °C to reach the equilibrium state of a statistical distribution. This process could be further controlled by binding of guests in the cavities of these self-assembled architectures. This work provides a unique example of control over the statistical distribution of a system of capsules using temperature-quenching techniques analogous to those used in metallurgy.
Figure 44.
Temperature-dependent self-sorting and scrambling behavior driven by quenching equilibria. R = CH3. From ref (240). CC BY 4.0.
5.3. Guest-Templated Assembly
Fujita and co-workers prepared self-assembled heteroleptic trigonal prism 184 (Figure 45), the assembly of which required the presence of certain π-extended guest molecules.241 When no guest was present, oligomeric species and homoleptic octahedral [(ethylenediamine)PdII]6104 predominated. The selective formation of heteroleptic 184 is driven by aromatic stacking interactions between electron-poor cage panels (10) and coronene (183) and also by the steric bulk of the linear bipyridine strut 182, which disfavors the coordination of two linear bipyridine ligands to a single palladium center. When the bipyridine struts are extended, allowing the formation of larger trigonal prisms, three guest molecules stack selectively within the capsule cavity. Both capsules, containing two or three guests, were shown by UV/vis spectroscopy to exhibit charge-transfer character.
Figure 45.

Self-assembly of ditopic 182 and tritopic 10 with (ethylenediamine)PdII(NO3)2 to form guest-templated trigonal prism 184. For clarity, the two bound coronene (183) guests are not shown.241
The Fujita group has also reported systems of capsules formed from PdII with cis-chelating bidentate ligands and pyridine or pyrimidine donor ligands, where guest binding triggers structural transformations. In one example, a trigonal-bipyramidal cage transforms into an octahedral architecture upon guest binding.242 In another, an octahedral PdII20L8 structure transforms to an open PdII8L4 bowl-shaped structure.243 Further to this, Hiraoka and Fujita were able to control the specific constitutional isomer of a Pd3L2 complex that forms from a reduced-symmetry tripyridyl ligand by judicious choice of guest molecules.244 Addition of a flat guest molecule (1,3,5-benzenetricarboxylic acid) favored the formation of a capsule with a relatively flat cavity, whereas the addition of a spherical guest (CBrCl3) resulted in the formation of an isomer with a more spherical cavity, providing an early example of guest control of isomer formation. We reported the transformation of a tetrahedral FeII4L6 cage with porphyrin panel edges upon the addition of C60 or C70. After addition of the fullerene to the system, a FeII3L4 conelike architecture formed. Selective transmetalation of a single iron(II) vertex for copper(I) was favored, resulting in the formation of a heterometallic CuIFeII2L4 structure, exploiting the coordinative unsaturation of iron(II) at a single position.245 Our group also reported the formation of a cuboctahedron that shows cooperative binding of a pair of C60 molecules. The binding of these guests triggers a rearrangement of the original O-symmetric structure to an S6-symmetric analogue that optimizes fullerene binding.246
Müller and Möller reported the formation of a trigonal-bipyramidal capsule that incorporated its template.247 Sodium 5,5-diethylbarbiturate was added to a threefold-symmetric guanidinium-based ligand that chelated three PdII centers, leading to the formation of a trigonal-bipyramidal architecture containing 33 distinct building blocks. While investigating the endohedral functionalization of metal–organic polyhedra via coordination of organophosphonates to polyoxovanadate units at the vertices of the cages, Fang et al. observed the formation of a barrel-like structure instead of a tetrahedral structure that would ordinarily be expected to form.248 The observed preference for the barrel-like structure was attributed to steric effects, as the interior cavity of the tetrahedron would be too small to accommodate the sterically bulky organophosphonate groups. Donnelly, Abrahams, Paterson, and co-workers likewise reported the guest-induced formation of coordination nanotubes that can bind small molecules such as CO2, CS2, and acetonitrile.249
The Yoshizawa group reported a modification of their anthracene-paneled M2L4 lantern architectures wherein guest binding drives the formation of heteroleptic structures (Figure 46).250 They used two pyridine-based bidentate ligands of different lengths, 185 and 186. Each ligand independently assembled with the metal ion to form an M2L4 lantern architecture with spherical internal cavities capable of binding aromatic guests. When the two cages were mixed together, a complex mixture of hetero- and homoleptic architectures formed. However, when C60 was added to this mixture, a single heteroleptic host–guest species was seen, with the composition PdII218521862⊂(C60). Computational models suggested that the cis isomer of this architecture is favored over the trans isomer. The observed preference for the heteroleptic architecture was attributed to the optimization of aromatic stacking interactions between the guest and host.
Figure 46.

Formation of a heteroleptic lantern driven by guest encapsulation. Reproduced with permission from ref (250). Copyright 2015 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
This guest-driven host rearrangement concept might be employed to selectively generate a range of architectures tailored to specific guest-binding tasks. In this approach, the cavity of the host is already filled, thus precluding its use for the binding of guests with lower affinity than the template or necessitating the optimization of template removal. Yoshizawa and co-workers recently reported the use of a guest template to drive a system of equilibrating atropisomeric cages toward a single isomer in a similar system.251
5.4. Anion-Templated Assembly
We reported a system of complex architectures based on anion binding and subcomponent self-assembly (Figure 47).252 Diformylbipyridine subcomponent 187 combines with p-toluidine (135) and cobalt(II) triflimide to generate an initial mixture of architectures. The addition of a triflate template leads to the assembly of a CoII4L6 tetrahedron. However, when lithium perchlorate or potassium hexafluorophosphate is added to either the dynamic library or the tetrahedral architecture, clean conversion to pentagonal prism 188 (Figure 47) is observed upon heating.
Figure 47.

Formation of anion-templated pentagonal prism 188.252
CoII10L15 architecture 188 consists of two parallel CoII5L5 pentagonal rings connected by five bridging ligands, creating a barrel-like structure. This structure is templated by five perchlorate or hexafluorophosphate anions in binding pockets between pairs of bridging ligands. Forming a pentagonal prism in place of the initial tetrahedral structure is favored for reasons that include the maximization of stacking interactions and a better size match of perchlorate or hexafluorophosphate with the smaller cavities found in the pentagonal prism.
The formation of pentagonal prism 188 also generates a central binding pocket surrounded by 10 internally facing pyridine CH groups. This pocket is occupied by a chloride ion, scavenged during synthesis, which is so strongly bound that it cannot be removed by addition of silver(I), in analogous fashion to other self-assembled systems found to bind strongly to halides.253−255 This system thus provides an unusual way to generate a secondary anion binding site by the addition of an initial anionic stimulus. Further work explored different architectures formed by subcomponent self-assembly using this same dialdehyde 187.256−259
Chifotides and Dunbar reported the use of anion−π interactions to control the formation of tetrameric or pentameric helicates, depending on the identity of the anion used.260 The choice of anion during self-assembly likewise dictates the identity of the product formed in the work of Pan, Xu, and co-workers, where the formation of either a PdII2L4 or a PdII3L6 capsule is driven by the addition of nitrate (favoring PdII2L4) or triflate/tetrafluoroborate (favoring PdII3L6).261 This selectivity is driven by differential guest binding within each capsule. Lützen and co-workers also reported a chiral PdII4L8 flexible architecture whose formation is dependent on templation by tetrafluoroborate.262
6. Multimetallics: Heterometallic and Cluster-Containing Architectures
Complexity may be enhanced through the development of heterometallic self-assembled systems, which employ the differing coordination preferences of more than one metal. The development of systems that take advantage of the coordinational flexibility and unusual geometries of metal clusters can also lead to the formation of novel architectures. Either the kinetics or the thermodynamics of self-assembly may be employed to direct the outcome of a multistep process, as described in the examples below.
6.1. Ligand Coordination Preference
An early example of heterometallic supramolecular assemblies was provided by Raymond and Wong, who used ligand 189 that contains both hard and soft donors (Figure 48) to selectively bind two different metal ions.263,264 The catechol group of ligand 189 binds to hard metal centers such as TiIV and SnIV, and the phosphine binds to softer metal centers such as PdII. A stepwise process was initially employed, first installing the catechol-binding metal, as insoluble polymers were observed when phosphine coordination was attempted first. However, under the optimized conditions TiIV- and SnIV-containing mesocates are generated in a single step via selective self-assembly. Similar principles using other ligand designs have been used by the groups of Wang,265 Duan,266,267 Brechin,268 and Youngs269 to generate other trigonal-bipyramidal assemblies.
Figure 48.

Heterobimetallic TiIV2PdII31896 mesocate 190 formed using ligands that contain both hard and soft donors.263,264
Expanding upon the principles developed by Raymond and Wong,263,264 Lützen et al. reported a system incorporating both FeII, which is selectively bound by pyridylimines, and PdII, which binds selectively to monotopic pyridine donors, to form a system capable of complex-to-complex switching with a concomitant spin-state transition (Figure 49).270 Selective binding is driven both by intrinsic ligand preference (avoidance of steric clash at palladium centers ligated by pyridylimines) and by the preassembly of FeII metalloligand 193 using chelating tris(2-aminoethyl)amine (192). This chelating ligand enforces fac hexadentate coordination on the assembly. Subsequent addition of [(dppp)PdII(OTf)2] (dppp = 1,3-bis(diphenylphosphino)propane) causes selective assembly of trigonal bipyramid 194, where two coordination sites on each palladium are occupied by the bidentate phosphine ligand. Employing [PdII(MeCN)4](BF4)2 instead generates cubic architecture 195, which has a structure analogous to one that we prepared using a preassembled platinum complex metalloligand.271 When a less sterically hindered aldehyde subcomponent is added, the more hindered subcomponent 191 is displaced, and the iron(II) transitions from a high-spin state to a low-spin state.
Figure 49.
Self-assembly of subcomponents 191 and 192 with FeII to produce metalloligand 193, which then forms bimetallic trigonal-bipyramidal (194) and cubic (195) architectures. Reproduced with permission from ref (270). Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Crowley and co-workers reported a nonanuclear heterometallic PdII3PtII6 donut-shaped cage, where the use of two different metal-binding sites on each ligand—bidentate triazolylpyridine versus monodentate pyridine—enables heterometallic assembly.272 The architecture catalyzes the light-mediated hetero-Diels–Alder reaction of anthracene with singlet oxygen.
6.2. Combining Kinetically Inert and Labile Metal Ions
The use of a mixture of kinetically inert and labile coordination centers has been explored extensively by the Ward group (Figure 50).273−276 Their stepwise approach involves the initial formation of a coordination complex between three pyrazolylpyridine ligands and kinetically inert RuII or OsII. This complex is formed as a statistical mixture of fac and mer isomers, and the minor fac isomer is then isolated, exploiting the inertness of these metals.277 An example of this fac complex, fac-Ru1133, is represented in red in Figure 50. One coordinating site on each ligand is occupied by the RuII, leaving the other free for coordination to a coordinationally labile metal center. Subsequent addition of labile CoII, CdII, or AgI then results in self-assembly with efficient error checking, as the structures undergo coordinative reconfiguration about the labile metal ion.
Figure 50.
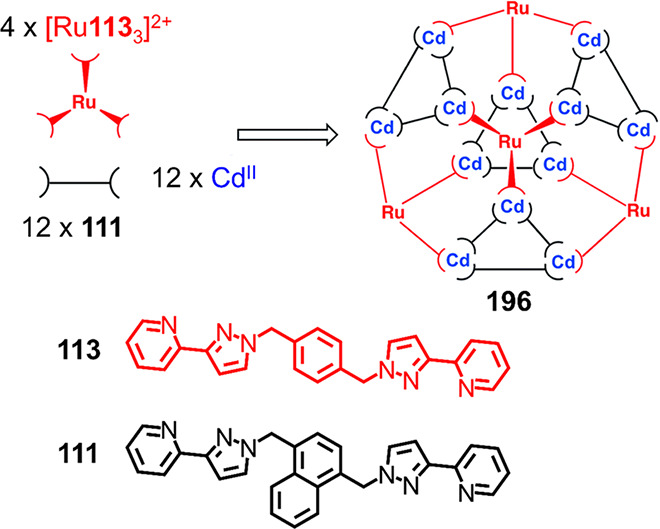
Formation of RuII4CdII121111211312 twisted tetracapped truncated tetrahedral assembly 196 containing both kinetically inert and labile coordination centers and two distinct ligands. From ref (276). CC BY 3.0.
The Ward group further developed this system to install two types of ligands selectively into a heterometallic architecture to form RuII4CdII121111211312 twisted tetracapped truncated tetrahedral array 196 (Figure 50).276 The design of this structure builds upon the homometallic M16L24 structures previously reported by the same group, as depicted in Figure 31a.191
The Jin group also reported a series of heterometallic capsules that combine kinetically inert metals such as rhodium and iridium with kinetically labile metals such as silver and zinc.278
6.3. Multimetallic Vertices
Dicopper “paddlewheel” vertices have been used to generate an array of molecular capsules.279,280 In an elegant example, Schmitt and co-workers generated “capsule-within-a-capsule” 198 using dicopper paddlewheel complexes as nodes (Figure 51).281 Extended tri-m-benzoic acid ligand 197, once deprotonated, reacts with CuII(NO3)2 to form 198. X-ray crystallography revealed a complex octahedron-within-cuboctahedron architecture, with the inner assembly fully linked to the outer one. The architecture, which can be made soluble by postassembly modification with alkylpyridine donors, can absorb 7-amino-4-methylcoumarin from solution.
Figure 51.

(a) Formation of “capsule-within-a-capsule” 198, composed of an octahedron nested within a cuboctahedron. (b, c) Views of 198 from two perspectives. From ref (281). CC BY 4.0.
The potential of using coordinationally flexible bimetallic clusters was recently highlighted by our group in the assembly of a trigonal-prismatic cage that incorporates disilver vertices (Figure 52).282 The nonconverging coordination vectors of 2-formyl-1,8-napthyridine (200) combined with the flexible coordination sphere of silver(I) leads to the formation of disilver vertices. The geometry of tris(4-aminophenyl)amine (199) and an appropriate anionic template (Figure 52b) generate AgI12L6 trigonal prism 201.
Figure 52.
(a) Formation of trigonal prism 201 from silver(I) and subcomponents 199 and 200, which binds (b) pairs of anions, or dianions, driven by the coordinational flexibility of silver(I) and anion templation. (c, d) Two views of the crystal structure of 201. Reproduced from ref (282). Copyright 2019 American Chemical Society.
Crystallographic analysis of 201 showed that two anions are bound within its cavity, held in proximity by the surrounding metal–organic architecture. Two HSO4– anions bind in close proximity, stabilized by additional hydrogen-bonding interactions, as seen in the cyanostars of Flood and colleagues.283 Linear, covalently linked dianions also serve as competent templates for the trigonal prism. Even highly oxidizing species such as peroxodisulfate, which is known to oxidize AgI to AgII,284 can be used, demonstrating the power of self-assembly to alter the properties of structural subunits.2,35,62,285 Silver clusters have also been used to generate a Ag180 nanocage with a diameter of 2.5 nm based on silver “trigons” (three silver ions in a triangular arrangement).286
We further extended this concept to incorporate not just bimetallic corners but also AgI4 and AgI6 clusters as integral structure-directing motifs using the ditopic subcomponent 202.287 As shown in Figure 53b, 202 and 203 initially form mixtures of tetrahedra and three-stranded helicates (204–207). The mixture of 204 and 206 then proceeds to generate six-stranded helicates 208 and 209 in the presence of suitable anionic templates (Figure 53c,d), while analogous structures do not form from subcomponent 203. Key to the formation of these unusual structures is the judicious choice of anion. Whereas the addition of other anions to the equilibrating mixture of 204 and 206 does not effect structural transformation, addition of iodide, bromide, or sulfate triggers rearrangement to a six-stranded helicate that resembles a sheaf of wheat. Its elongated structure was confirmed by X-ray crystallography and solution NMR spectroscopy. This work provides an unusual example of the mutual stabilization between metal clusters and a self-assembled architecture, with anions playing a central role in structuring the metal cluster and therefore the superstructure thus formed.287
Figure 53.
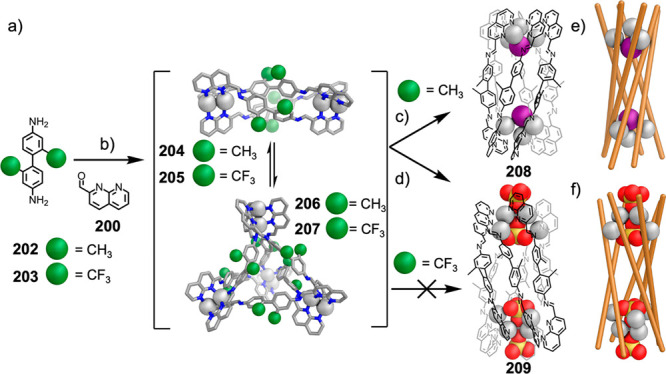
(a–d) Synthesis of six-stranded helicates 208 and 209 capped by Ag4 or Ag6 clusters, formed during self-assembly from dianiline 202 but not from dianiline 203: (a) structures of 202 and 203; (b) formation of 204–207 upon addition of AgNTf2 in MeCN; (c, d) formation of (c) 208 and (d) 209 from 204/206 upon addition of tetrabutylammonium iodide and tetramethylammonium sulfate, respectively. The structures of 204 and 206 are MM3 models, and those of 208 and 209 are based on crystallographic data. (e, f) Simplified representation of six-stranded helicates (e) 208 and (f) 209. Adapted from ref (287). Copyright 2020 American Chemical Society.
Other metal clusters have been used as capsule vertices,288 including polyoxometalate-derived caps,289 which formed a capsule with an unusual “near-miss Johnson” geometry; tungsten–copper synthons, which enabled the formation of distorted octahedral structures;290 tripalladium vertices ligated by tetrazole linkers;291 and trizirconium clusters, which formed the corners of a chiral coordination cage capable of performing sequential asymmetric reactions.292 Manganese has also been employed to generate cages related to truncated tetrahedra.293
6.4. Organometallic Macrocyclic Tubes
Tubular assemblies may be generated by linking macrocyclic ligands with a band of metal centers, as exemplified by the work of Altmann and Pöthig (Figure 54),294 representing an alternate form of structure-directing metal cluster. The reaction of macrocycle 210, containing four imidazolylidene and two pyrazolate rings, with silver(I) and then gold(I) ions leads to the formation of extended tube 211. The cavity of 211 binds the linear guest 1,8-diaminooctane in organic and aqueous solutions. Architecture 211 exemplifies a novel approach to metal-driven self-assembly where the metal ions define a central ring rather than vertices. Such architectures have been used to form mechanically interlocked organometallic [2]rotaxanes.295 In a conceptually related example, Shionoya and co-workers had previously reported a Ag3L2 structure in which two ligand disks were linked by a ring of three silver ions, but solid disk-shaped ligands were used rather than macrocycles.296
Figure 54.
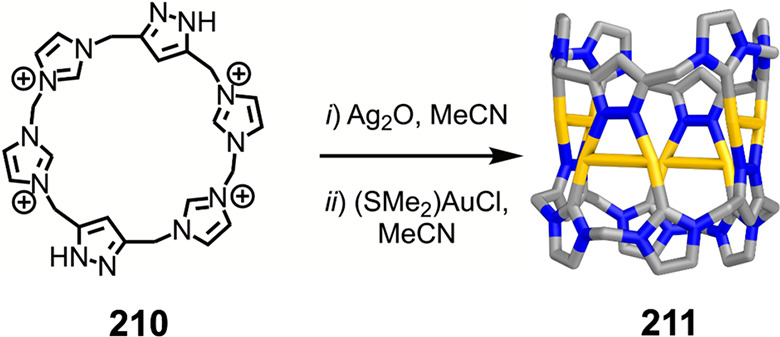
Formation of organometallic macrocyclic tubular structure 211.294
Hetero- and multimetallic self-assembled structures have not been used as extensively as other techniques to generate complex architectures, but there is clearly great potential in this approach. A focus of future work will be utilizing the properties of the multiple metal ions to achieve tasks that cannot be achieved by a single ion. The use of metal clusters with coordinational flexibility as vertices likewise shows promise in the generation of complex architectures from simple ligands.
7. Geometric, Steric, and Subtle Non-covalent Effects
Geometric constraints can shape the self-assembly of high-symmetry structures. Key examples from the Fujita group have shown that even slight alterations of the bend angle (θ, Figure 55), or flexibility, of dipyridyl ligands can result in the formation of polyhedra with dramatically different sizes (Figure 55).297,298 The selective formation of tetrahedra or cubes also depends upon the relative orientations of the coordination vectors in bis-bidentate ligands.28 Similar geometric principles have been shown to drive the formation of more complex, lower-symmetry architectures and to enable discrimination between different structures of high complexity. This section will highlight instructive examples of geometric control along with cases demonstrating the impact of subtle steric effects and non-covalent interactions on the outcome of self-assembly processes.
Figure 55.
Family of spherical, polyhedral structures that Fujita et al. constructed by varying the bend angle, or flexibility, of ditopic “banana-shaped” dipyridine ligands.31,156,297,298
7.1. Bend-Angle Dependence of Ditopic Struts
Key work by Fujita and others has demonstrated that myriad metal–organic structure types with the general formula MIInL2n are formed through the self-assembly of dipyridyl ligands having different bend angles with square-planar PdII and PtII cations. These products are symmetrical polyhedra (Figure 55), including PdII6L12 octahedron 212,31 PdII12L24 cuboctahedron 213,156 PdII24L48 rhombicuboctahedron 214,297,299 and PdII30L60 icosidodecahedron 215.298 Higher-order structures are observed when ligands have a larger bend angle.300,301 However, other dipyridyl ligands have been shown to form architectures that deviate from these Platonic and Archimedean ideals.
Fujita et al. demonstrated that dipyridyl ligand 216 (Figure 56), having a bend angle of 60°, assembles with PdII(NO3)2 in DMSO to give PdII42168 box 217.302 In contrast, carrying out the reaction in CD3CN results in the formation of smaller Pd32166 tube 218. The selective formation of 217 requires the presence of both DMSO and nitrate, with a mixture of 217 and 218 observed when PdII(OTf)2 in DMSO is used. Finally, the ratio of 217 to 218 tracks the DMSO:MeCN ratio of the solvent mixture. Using similar principles, the Yoshizawa group obtained a PdII2L4 polyaromatic capsule that was structurally contracted in comparison with a previously reported capsule using a similar ligand.303
Figure 56.

Solvent-dependent self-assembly of ditopic ligand 216 having a 60° bend angle and PdII(NO3)2 to give PdII4L8 (217) and PdII3L6 (218) box-shaped structures.302
In targeting the next-largest structure in the series of regular PdIInL2n assemblies shown in Figure 55, a PdII60L120 rhombicosidodecahedron, by widening the bend angle of the ditopic ligand, Fujita et al. instead formed an unexpected new architecture. Selenophene-centered ligand 219 (Figure 57) exhibits a bend angle of 152°, a modest increase upon the angle of 149° in the thiophene-centered ligands previously reported to assemble into PdII24L48214 and PdII30L60215 (Figure 55).297−299 The assembly of 219 and PdII in DMSO instead yields structure 220, with a formula of PdII3021960, the same composition as icosidodecahedral structure 215.304
Figure 57.
(a) Formation of metal–organic architecture 220, corresponding to a chiral tetravalent Goldberg polyhedron. (b) Schematic representations of the two enantiomers of 220.304
Refinement of X-ray crystallographic data through the extension of techniques originally developed for protein crystallography revealed that the surface of structure 220 is tiled by eight triangles and 24 squares. Fujita’s team developed a mathematical description of this new class of structures, which they named tetravalent Goldberg polyhedra. According to their nomenclature, 220 is a tet-G(2,1) polyhedron, with the numbers in parentheses describing the relative orientations and spacings of the triangles among its squares. In 220, a given triangle is located two steps horizontally and one step vertically away from its nearest neighbor. Structure 220 is chiral, existing as a pair of enantiomers (Figure 57b). Using graph theory, the authors predicted that PdII48L96tet-G(2,2) structures would be the next largest members of their new series. Meticulous modeling predicted that a ditopic ligand with a bend angle of 152° should favor the formation of such a PdII48L96 architecture, suggesting that the initially observed PdII3021960 structure is a kinetically trapped species.
Through optimization of the conditions of self-assembly and screening of many crystals, the Fujita group was once more able to use their novel crystallographic methods to identify a PdII4821996 structure with the geometry of a larger tet-G(2,2) polyhedron. This remarkable structure demonstrates the power of using the analysis of serendipitous results in the targeting and discovery of new structures. Such structures are among the largest synthetic assemblies known, rivaled only by those incorporating biomolecular building blocks. For example, the Heddle group have used metal-driven assembly of protein subunits to produce large assemblies with unusual structures, such as the snub cube.305
Newkome et al. demonstrated the role that geometric constraints can play in the assembly of heteroleptic structures. They reported that mixing hexatopic ligands 221 and 222 with CdII in a 1:3:12 ratio results in the formation of the CdII48221422212 truncated tetrahedral structure 223 with a molecular weight of approximately 70 kDa (Figure 58).306 Each of the individual bent 222 ligands acts to connect two hexagonal 221 units, four of which make up the faces of the truncated tetrahedron. The rigidity of bent 222 enables the selective formation of the desired structure, which does not form when a more flexible alkyl linker is used as the spacer between the two tris(terpyridine) units. Instead, double-decker hexagons are formed.307
Figure 58.

Self-assembly of heteroleptic truncated tetrahedron 223 (energy-minimized structure from molecular modeling) from ligands 221 and 222 together with CdII. Adapted from ref (306). Copyright 2020 American Chemical Society.
7.2. Stretching Ligands: Elongated Tetratopic Ligands
Metal–organic cages with a high degree of enclosure are often targeted, as well-enclosed cavities tend to exhibit superior guest binding properties. However, other classes of supramolecular host, such as cucurbiturils308 and pillarenes,309 have open-ended structures and have been used in applications where this openness is useful. Control of the degree of enclosure can also affect the guest binding kinetics,310,311 which is a vital parameter in designing functional systems. As a result, metal–organic architectures with open-ended box-, barrel-, and tubelike structures have been investigated.
The combination of elongated tetratopic pyridyl-based ligands and cis-protected square-planar metal centers has been shown to yield a variety of trigonal,312−314 tetragonal,315−318 pentagonal,319 and hexagonal320 barrel-like structures (224–227; Figure 59).321,322 Intriguingly, recent reports have demonstrated that ligands of this class can also form structures with gyrobifastigium,323−325 triangular-orthobicupola,326,327 and square-orthobicupola319 geometries (228–230), in some cases selectively and in others as a minor product. Thus, while the combination of elongated tetrapyridyl ligands with cis-protected square-planar metal centers can yield different structures (Figure 59), control over the thermodynamics of the system is required to ensure that a single product is formed.
Figure 59.

Geometries that can be formed by the combination of elongated tetratopic N-donor ligands and cis-protected square-planar metal centers in a 1:2 ratio. Reproduced with permission from ref (325). Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Recent work by Severin and co-workers sought to uncover the design principles responsible for the assembly of several different structures, particularly taking into account geometric considerations.319 When the authors postulated that the ligands were fully rigid, geometric analysis predicted that larger, often pentagonal, barrels would form. However, tetragonal or trigonal-prismatic barrels are observed in practice. The formation of these entropically favored smaller assemblies is thus attributed to the conformational flexibility of the ligands, which enables them to deviate from planarity, and also to flexibility arising from the potential for a slight misalignment between the coordinate vectors of the ligand and the metal–ligand bonds.
Severin’s group also discovered that ligands containing bulky cores can form structures with gyrobifastigium-like geometries (228; Figure 59).323 This geometry allows a greater distance between bulky ligand cores, thereby reducing steric clashes compared to barrel-like geometries. Further geometric analysis indicated that the gyrobifastigium structure emerges only in a certain window of ligand length-to-width ratios. Further elongation of the ligand precludes gyrobifastigium formation, favoring instead the formation of larger barrel-like assemblies, which also reduce steric clashes between ligand cores.319
Factors beyond simple geometric considerations were also revealed to be important in determining which among the many architectures shown in Figure 59 might predominate. For example, favorable interligand non-covalent interactions can influence the preferred geometry of the structures formed.325 Although analysis of geometric considerations does not yet provide a definitive guide for selectively obtaining each of the seven structure types shown in Figure 59, these principles may be used to guide the targeting of other structure types, using ligands with bulky metal(II) clathrochelate cores.319
Mukherjee et al. further demonstrated the utility of this class of structures using water-soluble tetrafacial barrel 232, selectively formed via the combination of tetrapyridyl ligand 231 and cis-PdII(en)(NO3)2 in a 1:2 ratio (Figure 60). This barrel acts as a carrier for curcumin.315 Complexation within the cavity increases the water solubility of curcumin and also protects it from photodegradation in aqueous solution when it is exposed to either daylight or UV light. The authors attribute the photostabilizing property of the metal–organic barrel to absorption of most of the incident photons by the aromatic panels of the structure, reducing the number absorbed by the curcumin guest. Curcumin has been shown to have pharmacological activity,328 but two factors limiting its potential use in therapeutics are its tendency to undergo photodegradation329 and its low bioavailability arising from poor aqueous solubility,330 both of which may be alleviated by supramolecular carrier 232. The Mukherjee group also reported a urea-functionalized trigonal prism capable of catalyzing Diels–Alder reactions in aqueous media, further demonstrating the potential application of water-soluble open cages.331
Figure 60.
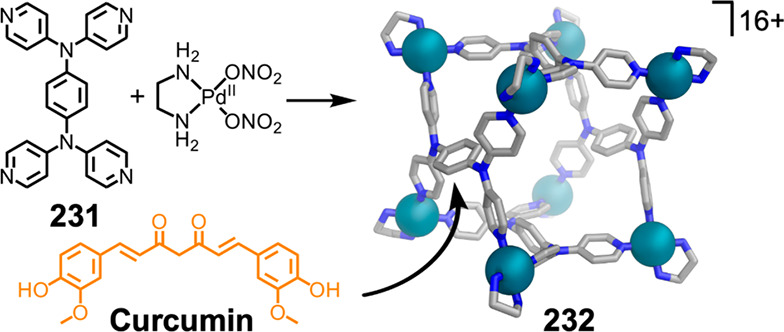
Formation of water-soluble tetrafacial barrel 232 from tetrapyridine 231 and cis-PdII(en)(NO3)2. Barrel 232 increases the water solubility and photostability of curcumin.315
Utilizing the principles discussed above, along with those illustrated in Figure 3, Zhang and co-workers constructed heteroleptic tetragonal barrel-shaped metallacages, which were emissive in both solution and the solid state.332
We reported the formation of a series of tubular MI8L4 structures 235 via subcomponent self-assembly (Figure 61) that have narrower cavities than the other structures discussed above. They are obtained from the reactions of elongated tetraanilines (233a–c), a 2-formylpyridine (16, 234a, or 234b), and AgI or CuI.333,334 The tubelike hosts exist in two possible diastereomeric forms, either D2/D2d-235 or D4-235. The equilibria between diastereomers depend upon the ligand length, the substituents, the identities of the metal ion and the counteranion, and the temperature.334 Furthermore, the linear cavities of the D4-symmetric isomers of these structures bind and stabilize unusual cyanide-based linear guest species such as 236, illustrating an advantage to the formation of elongated cavities.
Figure 61.
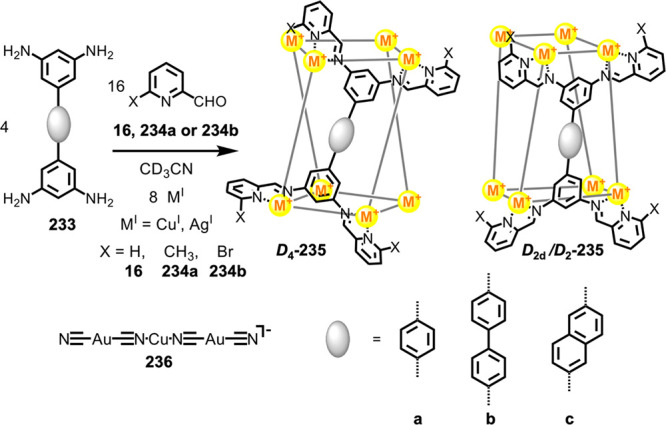
Subcomponent self-assembly of MI8L4 tubelike structures 235 with narrow cavities capable of binding linear cyanoaurate guest complexes such as 236. Adapted from ref (334). Copyright 2014 American Chemical Society.
7.3. Curved versus Planar Ligands
Much of section 7.1 focused upon architectures produced from bent ditopic ligands and their analogues. On the basis of similar reasoning, curvature can be introduced into ligands with higher topicities. When they are planar or nearly planar, such ligands have been employed as panels in the formation of diverse architectures with both high and low symmetries.1,66,335 The deviation of a ligand from planarity can favor the formation of metal–organic complexes with greater complexity and lower symmetry.
Salle et al. used tetrapyridyl ligands based on π-extended tetrathiafulvalenes (exTTF) to construct M4L2336 and M6L3337 ringlike structures as well as a larger M12L6338 species. The combination of curved tetratopic ligand 237 with AgIBF4 in mixed CHCl3/CH3NO2 forms AgI122376 architecture 238 (Figure 62).338 Although an X-ray crystal structure was not obtained for 238, solution studies and molecular modeling enabled its assignment as shown in Figure 62. The modeled structure has a ligand curvature of 87°, close to the value of 86° observed for the free ligand. An analogous structure forms by the assembly of ligand 237 with trans-PdIICl2(MeCN)2 in DMSO.338
Figure 62.
Proposed structure of 238 assembled from ligand 237 and AgIBF4. Reproduced with permission from ref (338). Copyright 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
An additional feature of electron-rich ligand 237 is its ability to undergo two-electron oxidation. The conformational change of the ligand core that accompanies this oxidation was exploited to drive the redox-controlled disassembly and reassembly of a M4L2 coordination cage. This redox-governed process was coupled with guest binding to provide a means of guest release and recapture.339 AgI122376 structure 238 rearranges from the discrete cage into a three-dimensional supramolecular polymer when the ligand units are oxidized to the dicationic state. In contrast, the PdII122376 cage remains intact upon oxidation.338
7.4. Linear Polytopic Ligands
The development of structures that can bind more than one guest is of great interest in the context of new coordination cage applications.57 This goal may be achieved through the design and synthesis of metallosupramolecular structures that contain multiple linked cavities with a high degree of enclosure and are therefore expected to exhibit guest binding. Crowley and co-workers expanded on design principles for the formation of simpler PdII2L4 structures to design pseudolinear polypyridyl ligands that form multicavity structures.93 These architectures contain similar cavities linked end to end.
As shown in Figure 63, combining hexapyridyl ligand 239 and PdII leads to the formation of triple-cavity cage 240. A higher temperature than typically needed for the formation of PdII2L4 complexes is required in order for the error-correcting disassembly of misassembled intermediate structures to occur during the formation of multicavity structures. To illustrate the potential use of such cages, the authors demonstrated the segregated binding of two distinct types of guests within the two different cavity types (terminal and central) within 240. Cisplatin is encapsulated in the terminal cavities, whereas triflate is bound in the central cavity as well as externally at each end of the structure.93
Clever et al. formed double- and triple-cavity cages by expanding upon these principles. A topologically interpenetrated dimeric species forms from the double-cavity cage upon addition of chloride or bromide ions.340 Each of the five segregated interior cavities of the structure can bind chloride or bromide.
Yoshizawa et al. reported the assembly of W-shaped tripyridine ligand 241 with PdII to form PdII32414 double capsule 242.341 When structure 242 is heated with C60, the central PdII is ejected, resulting in the formation of PdII22414·(C60)2 “molecular peanut” 243 (Figure 64), with extensive stabilization from aromatic stacking interactions between the anthracene panels of the host and the fullerene guests outweighing the energetic cost associated with a loss of coordinative saturation of the central pyridine. Capsule 242 also simultaneously binds different guests, diamantane and phenanthrene, with the preference for heterotopic encapsulation of these guests to form 242·(diamantane)(phenanthrene)2 complex 244 attributed to cooperative changes in the volumes of the two cavities that occur upon guest binding. The same group later showed that similar peanut-shaped polyaromatic shells form under much milder conditions when the central pyridine ring is replaced by a phenyl ring, which allows the stepwise encapsulation of two C60 molecules.342
Figure 64.
Molecular double capsule 242, which can form “molecular peanut” 243 upon ejection of the central PdII ion and encapsulation of two C60 molecules or heterotopic (diamantane)(phenanthrene)2 complex 244.341
Chand and co-workers reported that ligands 245–247 form the conjoined cages 248–250 (Figure 65), further developing the concepts introduced above. Instead of different cavities having similar sizes and shapes, ligands were rationally designed to form architectures consisting of laterally or linearly conjoined PdII2L4 and PdII3L6 units (Figure 65).343 Structures 248–250, which were all confirmed by X-ray crystallography, assemble from one or more of the carefully designed ligands 245–247 and PdII in DMSO. In the cases of 248 and 249, integrative self-sorting results in the selective formation of heteroleptic structures. The different types of cavities bind different guests. Small anions, such as NO3– and Cl–, bind within the smaller cavities, and their presence is required to template the formation of structures 248–250. Crystallography also showed that multiple DMSO molecules reside in the larger, trigonal, cavity.
Figure 65.
Conjoined cages 248–250 consisting of laterally or linearly conjoined PdII2L4 and PdII3L6 units; heteroleptic 248 and 249 form selectively via integrative self-sorting of mixtures of the corresponding ligands.343
Fujita et al. reported the use of linear polypyridyl ligands in the formation of a series of nanotubular architectures. The combination of ligand 251 (Figure 66a) with cis-protected PdII and a template such as 4,4′-biphenyldicarboxylate leads to the formation of PdII6L4252; elongated ligands 253 (Figure 66b) and 255 (Figure 66c) lead correspondingly to PdII8L4254 and PdII10L4256. A suitable template is essential to generate well-defined, discrete assemblies.344 The PdII62514 and PdII102554 nanotubes, 252 and 256, respectively, exist as single isomers. However, the PdII82534 structure forms as a mixture of isomers having C2h (254) and D2h symmetries.345
Figure 66.
(a) PdII62514 (252), (b) PdII82534 (254), (c) PdII102554 (256), and (d) PdII122574 (259) nanotubes prepared through assembly of the given ligands with cis-protected PdII centers and a rodlike template.344−346
Later work showed that elongation of these tubelike structures through extension of the ligand with additional pyridine rings was not possible because of poor solubility.346 This problem was circumvented by the design of hexapyridine ligand 257 (Figure 66d) in which two terpyridine units are separated by a spacer. Importantly, the biphenyl spacer, as opposed to an alkyl moiety, ensured that the ligand remained linear instead of folding into a U-shaped conformation. Combination of 257 with cis-protected PdII and the specially designed template molecule 258 allowed the construction of 3.5 nm long nanotube 259.
7.5. Pyrimidine versus Pyridine
The combination of pyridine donors with cis-protected square-planar metal centers has yielded many new coordination cages, some of which are described in this review. Pyrimidine donors are also able to coordinate to MII centers, acting as 120° μ2-bridging ligands.
Triangular hexadentate 1,3,5-tris(3,5-pyrimidyl)benzene (260) is designed to form structures in which the metal centers lie upon the edges of polyhedra as opposed to their vertices.347 The combination of 260 with PdII(en)(NO3)2 in aqueous solution yields enclosed PdII182606 hexahedron 261 with a trigonal-bipyramidal structure consisting of six edge-sharing triangular panels with two metal centers on each edge (Figure 67a).
Figure 67.
Preparation of (a) PdII182606 (261) and (b) PdII152626 (263) hexahedral architectures from ligands 260 and 262, respectively, and PdII(en)(NO3)2. Adapted with permission from ref (348). Copyright 2001 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Subsequent work produced the similar PdII152626 hexahedron 263 (Figure 67b) from modified ligand 262, in which one pyrimidine is replaced with a 3-pyridyl moiety.348 Although 261 and 263 are of similar shape and size, the presence of open clefts at the apical nonbinding sites in 263 is hypothesized to allow easier access of guest molecules into the cavity for binding, in contrast to the more enclosed 261.
Architectures 261 and 263 demonstrate how the geometric constraints of a ligand can result in the formation of novel structures. Subsequent alterations can then be made that allow for a desired structure type to be maintained, but with altered properties. Further replacement of the pyrimidine sites of 262 with 3-pyridyl moieties yields different architectures of both higher and lower symmetries, including a tetrahedron and “open cones”, depending on the conditions used.349
Mukherjee et al. have also used ligands containing pyrimidine moieties to prepare complex architectures. The combination of 1,4-bis(pyrimidin-5-yl)benzene or 4,4′-bis(pyrimidin-5-yl)biphenyl with cis-[(dch)PtII(NO3)2] (dch = 1,2-diaminocyclohexane) in water results in the formation of PtII8L4 nanotubes with lengths of up to 22.0 Å.350 Assembly of hexadentate ligand 264 (Figure 68) with PdII in a 1:1 ratio produces structure 265.351 Crystallography indicates that 265 is a discrete PdII2426424 complex wherein only four of the nitrogen atoms on each ligand bind to PdII, leaving two uncoordinated nitrogen atoms on each ligand (highlighted in purple in Figure 68). The authors described this structure as a “pregnant molecular nanoball”, consisting of a PdII12 “baby ball” within a larger PdII12 “mother ball”. Interestingly, the “mother ball” stabilizes the internal, smaller, structure, as an analogous PdII12L24 cuboctahedral nanosphere does not form from the reaction between pyrimidine and PdII.
Figure 68.
PdII2426424 “pregnant molecular nanoball” 265. N-donor atoms highlighted in purple remain uncoordinated in the observed product structure.351
7.6. Flexible Coordination Geometry of Metal Building Blocks
Many of the examples in previous sections utilize metal ions with rigid coordination spheres. For example, many structures contain PdII or first-row transition metal ions in the +2 oxidation state, adopting exclusively square-planar and pseudo-octahedral coordination geometries, respectively. Ions of the f-block metals can more readily adopt a wider range of coordination numbers and geometries, which can result in the formation of complex metal–organic architectures.
Jeong et al. utilized the coordinative flexibility of lanthanum to assemble the complex [LaIII1826624(CO3)2(H2O)32]2+ structure 267 (Figure 69), which the authors called lanthanitin because of its similarity to the structure of ferritin.352 Crystals of both (S)- and (R)-lanthanitin are obtained from mixtures of LaIIICl3 and the rigid, bent ligand 266 having either (S,S) or (R,R) stereochemistry. Within 267, LaIII ions have different coordination numbers; a mixture of eight- and 10-coordinate LaIII is observed.
Figure 69.

[La1826624(CO3)2(H2O)32]2+, “lanthanitin”.352
Duan and co-workers used a different f-block ion, cerium(IV), to construct a CeIV4L6 basketlike tetragon, using ditopic ligands consisting of two tridentate binding sites connected by a carbazole-based core.353 Four CeIV centers define the four corners of a square and are bridged by four edge-defining ligands. Each of the final two ligands also binds to two of the four cerium ions, defining the bottom of the basket.
Xu and Raymond exploited the variable coordination geometry of lanthanum to form a LaIII8L8 structure with a square-antiprismatic geometry.354 Within the structure, all of the lanthanum centers are nine-coordinate; however, two different coordination geometries are observed: distorted monocapped square-antiprismatic and distorted tricapped trigonal-prismatic. Similarly, Kawai et al. recently reported an octanuclear circular helicate with a D4-symmetric square-antiprismatic geometry that exhibited circularly polarized luminescence (CPL) activity.355
7.7. Non-covalent Interactions and Steric Effects
Previous sections have alluded to the importance of steric control and favorable non-covalent interactions in driving the selective formation of particular structures. In this section we present a few key examples in which these often subtle effects exert a decisive influence on the self-assembly process.
Saalfrank et al. reported an early example wherein steric effects determine self-assembly outcomes.356 The combination of rigid, threefold-symmetric, tris-bidentate pyrazolone-based ligand 268 with gallium(III) acetylacetonate in DMSO resulted in the serendipitous formation of GaIII62686 distorted trigonal-antiprismatic cylinder 269 having D3 symmetry, in which all six gallium centers have the same handedness (Figure 70). Although this structure represents an initially surprising deviation from the expected M4L4 tetrahedral assembly, molecular modeling indicated that the shorter metal–metal distance in a putative M4L4 tetrahedron would cause an increase in unfavorable steric clashes. Further work by the same group utilized molecular modeling to clarify the steric preference for the formation of an FeIII4L4 structure in the case of one ligand versus an FeIII6L6 trigonal antiprism with a different ligand on the basis of favorable aromatic stacking interactions in the trigonal antiprism.357
Figure 70.
GaIII6L6 distorted trigonal antiprism 269 assembles from rigid, threefold-symmetric, tris-bidentate ligand 268 and gallium(III) in DMSO.356
Clever and co-workers described the assembly of PdII22703 bowl 271 (Figure 71) based on a PdII2L4 cage framework lacking a fourth ligand.358 The formation of 271 is driven by the steric demands of its ligands. In the case of ligand 270, steric clash between hydrogen atoms near the coordinating nitrogen of the quinoline is alleviated in a PdII22703 structure, stabilizing 271 with respect to a PdII22704 structure. Upon prolonged heating of a solution of the PdII22703 structure, partial conversion to the PdII22704 structure was observed. However, this conversion could be prevented by the binding of C60 in the open cavity of the bowl.
Figure 71.
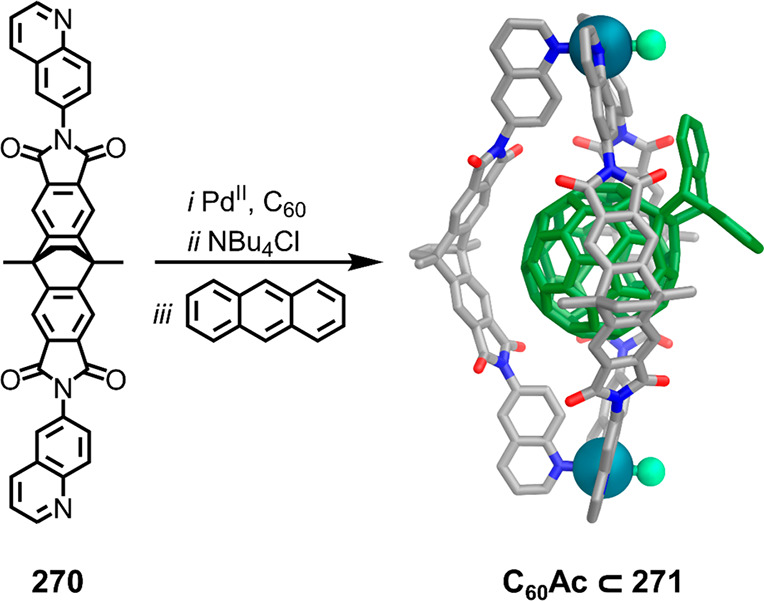
PdII2L3 bowl 271 is formed, rather than a PdII22704 cage, due to the steric demands of quinoline-based ligand 270. Bowl 271 acts as a supramolecular protecting group, enabling the selective formation of a C60–anthracene monoadduct (C60Ac).358
Bowl 271 can also act as a supramolecular protecting group. When a solution of [C60⊂PdII22703Cl2]2+ is treated with 10 equiv of anthracene, the C60–anthracene monoadduct (C60Ac) is selectively formed without undergoing further reaction with anthracene. As shown in Figure 71, the bowl-like cavity of 271 encloses most of the surface of the C60 guest, leaving only a small region available for reaction with anthracene. Finally, a pill-shaped dimer can be formed by bridging the bowls of 2 equiv of 271 with a sterically undemanding terephthalate unit,358 utilizing principles similar to those outlined in section 2.1. The example described above (Figure 71) demonstrates that introducing steric bulk close to the coordinating sites of ligands can increase their propensity to form more complex structures.
Mukherjee and co-workers reported the reaction of (1,1′-bis(diphenylphosphino)ferrocene)platinum(II) with 5,10,15,20-tetrakis(4-pyridyl)porphyrin (272) ligands to form open hexagonal box 273 (Figure 72), as opposed to a more symmetric cubic architecture.359 The bulky ferrocene-derived diphosphine ligand disfavors cube formation and allows formation of hexagonal box 273 with an internal cavity of 43 550 Å3. The formation of 273 depends on the introduction of steric bulk in peripheral coordinating ligands rather than directly affecting ligand–metal binding. This unusual self-assembled architecture senses the presence of zinc(II), with distinct changes in UV/vis absorbance bands observed in methanol solution caused by metalation of the porphyrins.
Figure 72.
Formation of multimetallic porphyrin-based open hexagonal box 273 from tetrapyridylporphyrin 272.359
Liu and co-workers built metal–organic architectures with complex structures using EuIII. The ditopic ligands 274, 276, and 278, each with two pyridine-2,6-dicarboxamide tridentate chelating sites connected by a 1,1′-bi-2-naphthol-derived core, produce architectures 275, 277, and 279, respectively (Figure 73).360 The key difference between the three ligands is the amount of steric bulk in proximity to the tridentate binding sites. All of the EuIII centers within the three structures are nine-coordinate. In 275, each of the six EuIII centers is chelated by three pyridine-2,6-dicarboxamide moieties, resulting in a structure with a twisted triangular-prismatic geometry (Figure 73a).
Figure 73.
Assembly of ligands 274, 276, and 278 with EuIII formed (a) twisted triangular prism 275, (b) “defective” tetrahedron 277 with one missing edge, and (c) “defective” hexahedron 279 with two edges missing. The “missing” edges in the structures are indicated with purple struts.360
Because of the increased steric hindrance near the binding sites in ligands 276 and 278, however, structures 277 and 279 contain EuIII centers that are chelated by only two pyridine-2,6-dicarboxamide units, with the remaining coordination sites on EuIII occupied by water ligands. The result is the formation of “defective” cages 277 and 279 (Figure 73b,c). EuIII42765(H2O)6(OTf)12 architecture 277 has a structure approximating a distorted tetrahedron with one missing edge (Figure 73b). In contrast, EuIII827810(H2O)12(OTf2)24 structure 279 resembles a hexahedral cage in which two edges are missing (Figure 73c).
We also utilized steric effects and non-covalent interactions to drive the formation of barrel-like prismatic structures instead of simple tetrahedra.229 Enforcing mer selectivity at the metal centers on the basis of reduced steric crowding and increased interligand aromatic stacking interactions enabled the formation of M8L12, M10L15, and M12L18 prismatic barrel structures using fluorinated ligands. We hypothesize that the presence of favorable edge-to-face aromatic interactions between the triazine and phenanthroline moieties of neighboring ligands contributed to the stabilization of an unusual M6L4S4-symmetric scalenohedron over a higher-symmetry pseudo-octahedral structure.361 Finally, with Siegel and Baldridge we reported the assembly of an S10-symmetric, fivefold-interlocked [2]catenane from CuI, a corannulene-based pentaaniline, and 2-formyl-6-methylpyridine.362 DFT calculations indicated that interligand aromatic interactions between corannulene units are a key driving force for the interlocking of the two fivefold-symmetric cages.
This section highlights the critical role of geometric and steric factors, as well as the enhancement of non-covalent interactions, in controlling the final structure observed in self-assembly processes. Although a sizable amount of work has already been conducted in this area, these examples also illustrate that much space remains to be explored. Fujita’s contributions, especially those involving the use of bis-monodentate ligands with PdII metal centers, may inspire similar systematic studies focusing on small changes to one geometric feature of other classes of ligand, thereby leading to the discovery of general design principles for complex architectures.
8. Conclusion and Outlook
As we have detailed in this review, recent years have seen the rapid development of many new approaches to the construction of metal–organic structures beyond the Platonic solids. The advent of new applications for supramolecular cages, including in catalysis,363−369 sensing,370−372 molecular separations,373 and biology,374 has provided strong motivation for this development.
As detailed in section 2, incorporating multiple different building blocks into the same structure is an effective way of increasing complexity. However, ensuring that mixed-component structures are formed selectively and that narcissistic sorting is prevented remains challenging. The six methods we outlined for driving heteroleptic architecture formation are variations on the theme of careful ligand selection and pairing. Recent efforts have demonstrated the reliability of heteroleptic approaches for the assembly of coordination cages, producing targeted structures across a range of ligand classes.62,73,82,83,87,89,90,96,107,128,375
Similarly, as noted in section 6, an understanding of ligand coordination preferences can now allow the rational design of new heterometallic architectures. Employing metal ions that form coordination bonds to ligands with differing kinetic lability also provides a useful method of design. However, other methods of producing multimetallic architectures have been less thoroughly investigated, providing scope for future enquiry.
The principles of ligand flexibility, solvent effects, and templation detailed in sections 4 and 5 are well-established, and many early examples of complex architectures depend on these approaches. They can be unpredictable, however, and although serendipitous results are plentiful, targeted design from first principles remains a challenge. The factors that drive the formation of particular structures can be difficult to decipher, even after discovery. Key exceptions include groundbreaking work by Ward et al. involving thorough investigations of the self-assembly processes of flexible di- and tritopic pyrazolylpyridine-based ligands and octahedral metal centers, which led to several new classes of structures.186−196
The reduced-symmetry-ligand and geometry-analysis approaches of sections 3 and 7 have benefited greatly from recent advances. As with heteroleptic approaches, it appears intuitive that reducing the symmetry of a ligand or building block should result in a self-assembled structure of reduced symmetry or increased complexity. However, experience has shown that it can be challenging to obtain single structures as opposed to intractable mixtures. Recent work has nonetheless clarified the circumstances under which a reduction in the symmetry of ligands can result in the formation of a single, complex architecture as opposed to many of them.
Finally, we note that computational methods, including evolutionary algorithms376 and machine learning,377 are playing an increasing role in the discovery of new supramolecular cage structures378−380 and the rationalization of their applications, such as catalytic activity.381 The recent implementation of high-throughput synthetic screening using automation has also vastly increased the capacity for rapid exploration of large chemical spaces.382−384 The advent of artificial intelligence, in particular machine learning, and automated synthetic methods may thus play a key role in the structural prediction and subsequent synthesis of a broad range of low-symmetry metal–organic polyhedral capsules.
Acknowledgments
This work was supported by the European Research Council (695009) and the U.K. Engineering and Physical Sciences Research Council (EPSRC) (EP/P027067/1 and EP/T031603/1). C.T.M. thanks the Leverhulme Trust ECF-2018-684, the Isaac Newton Trust, and Sidney Sussex College, Cambridge, for fellowship support.
Biographies
Charlie T. McTernan studied Chemistry (M.Chem.) at Hertford College, University of Oxford. His Part II project was conducted under the supervision of Prof. Tim Donohoe, investigating the synthesis of isoquinoline motifs using palladium-catalyzed α-arylation. He joined Prof. David Leigh’s group in 2013, funded by a University of Manchester Dean’s Faculty Award. His doctoral research included the synthesis of artificial molecular machines, switchable catalysts, and rotaxanes. In 2017 he joined the Nitschke research group, and then in 2018 he began his Leverhulme Early Career Research Fellowship, jointly funded by the Isaac Newton Trust, and joined Sidney Sussex College as a Research Fellow, where he investigated the synthesis and postassembly modification of diverse metal–organic capsules and ways to tie molecular knots in single molecules. In 2021 he started his independent career at the Francis Crick Institute and King’s College London, exploring how interlocked architectures and metal–organic capsules can be applied in biological settings.
Jack A. Davies received an M.Chem. from the University of Manchester in 2018. During this time, he completed an internship at Nanoco Technologies and a year-long industrial placement in medicinal chemistry at F. Hoffmann-La Roche. His final-year research project was in the field of metallosupramolecular chemistry, conducted under the supervision of Dr. Imogen Riddell. In 2018 he began doctoral research in the group of Prof. Jonathan Nitschke at the University of Cambridge.
Jonathan R. Nitschke was born in Syracuse, New York, USA. He received his Bachelor of Arts in chemistry from Williams College in 1995, remaining confused to this day as to whether chemistry is an art, and his doctorate from the University of California, Berkeley, in 2001, under the supervision of T. Don Tilley. He then undertook postdoctoral studies with Jean-Marie Lehn in Strasbourg, and in 2003 he started his independent research career as a Maître-assistant (fixed-term PI) in the Organic Chemistry Department of the University of Geneva. In 2007 he was appointed University Lecturer at Cambridge, where he has been a full professor since 2014. His research program investigates the self-assembly of complex functional structures from simple molecular precursors and metal ions.
Author Contributions
† C.T.M. and J.A.D. contributed equally.
The authors declare no competing financial interest.
References
- Chakrabarty R.; Mukherjee P. S.; Stang P. J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. 10.1021/cr200077m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashina M.; Tanaka Y.; Lavendomme R.; Ronson T. K.; Pittelkow M.; Nitschke J. R. An Antiaromatic-walled Nanospace. Nature 2019, 574, 511–515. 10.1038/s41586-019-1661-x. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Chen C.; Liu J.; Stang P. J. Recent Developments in the Construction and Applications of Platinum-based Metallacycles and Metallacages via Coordination. Chem. Soc. Rev. 2020, 49, 3889–3919. 10.1039/D0CS00038H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M.; Wu K.; Zhang J.-H.; Su C.-Y. Chiral Metal-Organic Cages/Containers (MOCs): From Structural and Stereochemical Design to Applications. Coord. Chem. Rev. 2019, 378, 333–349. 10.1016/j.ccr.2017.10.031. [DOI] [Google Scholar]
- Yoshizawa M.; Klosterman J. K.; Fujita M. Functional Molecular Flasks: New Properties and Reactions within Discrete, Self-Assembled Hosts. Angew. Chem., Int. Ed. 2009, 48, 3418–3438. 10.1002/anie.200805340. [DOI] [PubMed] [Google Scholar]
- Ahmad N.; Younus H. A.; Chughtai A. H.; Verpoort F. Metal-Organic Molecular Cages: Applications of Biochemical Implications. Chem. Soc. Rev. 2015, 44, 9–25. 10.1039/C4CS00222A. [DOI] [PubMed] [Google Scholar]
- Yamashina M.; Sartin M. M.; Sei Y.; Akita M.; Takeuchi S.; Tahara T.; Yoshizawa M. Preparation of Highly Fluorescent Host-Guest Complexes with Tunable Color upon Encapsulation. J. Am. Chem. Soc. 2015, 137, 9266–9269. 10.1021/jacs.5b06195. [DOI] [PubMed] [Google Scholar]
- Ronson T. K.; Meng W.; Nitschke J. R. Design Principles for the Optimization of Guest Binding in Aromatic-Paneled FeII4L6 Cages. J. Am. Chem. Soc. 2017, 139, 9698–9707. 10.1021/jacs.7b05202. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M.; Kusukawa T.; Fujita M.; Yamaguchi K. Ship-in-a-Bottle Synthesis of Otherwise Labile Cyclic Trimers of Siloxanes in a Self-Assembled Coordination Cage. J. Am. Chem. Soc. 2000, 122, 6311–6312. 10.1021/ja000779c. [DOI] [Google Scholar]
- Takezawa H.; Murase T.; Fujita M. Temporary and Permanent Trapping of the Metastable Twisted Conformer of an Overcrowded Chromic Alkene via Encapsulation. J. Am. Chem. Soc. 2012, 134, 17420–17423. 10.1021/ja308101a. [DOI] [PubMed] [Google Scholar]
- Wang K.; Jordan J. H.; Hu X.-Y.; Wang L. Supramolecular Strategies for Controlling Reactivity within Confined Nanospaces. Angew. Chem., Int. Ed. 2020, 59, 13712–13721. 10.1002/anie.202000045. [DOI] [PubMed] [Google Scholar]
- Dong V. M.; Fiedler D.; Carl B.; Bergman R. G.; Raymond K. N. Molecular Recognition and Stabilization of Iminium Ions in Water. J. Am. Chem. Soc. 2006, 128, 14464–14465. 10.1021/ja0657915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluth M. D.; Bergman R. G.; Raymond K. N. Making Amines Strong Bases: Thermodynamic Stabilization of Protonated Guests in a Highly-Charged Supramolecular Host. J. Am. Chem. Soc. 2007, 129, 11459–11467. 10.1021/ja072654e. [DOI] [PubMed] [Google Scholar]
- Fang Y.; Powell J. A.; Li E.; Wang Q.; Perry Z.; Kirchon A.; Yang X.; Xiao Z.; Zhu C.; Zhang L.; Huang F.; Zhou H.-C. Catalytic Reactions within the Cavity of Coordination Cages. Chem. Soc. Rev. 2019, 48, 4707–4730. 10.1039/C9CS00091G. [DOI] [PubMed] [Google Scholar]
- Brown C. J.; Toste F. D.; Bergman R. G.; Raymond K. N. Supramolecular Catalysis in Metal-Ligand Cluster Hosts. Chem. Rev. 2015, 115, 3012–3035. 10.1021/cr4001226. [DOI] [PubMed] [Google Scholar]
- Pluth M. D.; Bergman R. G.; Raymond K. N. Acid Catalysis in Basic Solution: A Supramolecular Host Promotes Orthoformate Hydrolysis. Science 2007, 316, 85–88. 10.1126/science.1138748. [DOI] [PubMed] [Google Scholar]
- Kaphan D. M.; Levin M. D.; Bergman R. G.; Raymond K. N.; Toste F. D. A Supramolecular Microenvironment Strategy for Transition Metal Catalysis. Science 2015, 350, 1235–1238. 10.1126/science.aad3087. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M.; Tamura M.; Fujita M. Diels-Alder in Aqueous Molecular Hosts: Unusual Regioselectivity and Efficient Catalysis. Science 2006, 312, 251–254. 10.1126/science.1124985. [DOI] [PubMed] [Google Scholar]
- Cullen W.; Misuraca M. C.; Hunter C. A.; Williams N. H.; Ward M. D. Highly Efficient Catalysis of the Kemp Elimination in the Cavity of a Cubic Coordination Cage. Nat. Chem. 2016, 8, 231–236. 10.1038/nchem.2452. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Ronson T. K.; Nitschke J. R. Functional Capsules via Subcomponent Self-Assembly. Acc. Chem. Res. 2018, 51, 2423–2436. 10.1021/acs.accounts.8b00303. [DOI] [PubMed] [Google Scholar]
- Caulder D. L.; Brückner C.; Powers R. E.; König S.; Parac T. N.; Leary J. A.; Raymond K. N. Design, Formation and Properties of Tetrahedral M4L4 and M4L6 Supramolecular Clusters. J. Am. Chem. Soc. 2001, 123, 8923–8938. 10.1021/ja0104507. [DOI] [PubMed] [Google Scholar]
- Bilbeisi R. A.; Clegg J. K.; Elgrishi N.; de Hatten X.; Devillard M.; Breiner B.; Mal P.; Nitschke J. R. Subcomponent Self-Assembly and Guest-Binding Properties of Face-Capped Fe4L48+ Capsules. J. Am. Chem. Soc. 2012, 134, 5110–5119. 10.1021/ja2092272. [DOI] [PubMed] [Google Scholar]
- Yan L.-L.; Tan C.-H.; Zhang G.-L.; Zhou L.-P.; Bünzli J.-C.; Sun Q.-F. Stereocontrolled Self-Assembly and Self-Sorting of Luminescent Europium Tetrahedral Cages. J. Am. Chem. Soc. 2015, 137, 8550–8555. 10.1021/jacs.5b03972. [DOI] [PubMed] [Google Scholar]
- Howlader P.; Zangrando E.; Mukherjee P. S. Self-Assembly of Enantiopure Pd12 Tetrahedral Homochiral Nanocages with Tetrazole Linkers and Chiral Recognition. J. Am. Chem. Soc. 2020, 142, 9070–9078. 10.1021/jacs.0c03551. [DOI] [PubMed] [Google Scholar]
- Meng W.; Breiner B.; Rissanen K.; Thoburn J. D.; Clegg J. K.; Nitschke J. R. A Self-Assembled M8L6 Cubic Cage that Selectively Encapsulates Large Aromatic Guests. Angew. Chem., Int. Ed. 2011, 50, 3479–3483. 10.1002/anie.201100193. [DOI] [PubMed] [Google Scholar]
- Otte M.; Kuijpers P. F.; Troeppner O.; Ivanović-Burmazović I.; Reek J. N. H.; de Bruin B. Encapsulated Cobalt-Porphyrin as a Catalyst for Size-Selective Radical-type Cyclopropanation Reactions. Chem. - Eur. J. 2014, 20, 4880–4884. 10.1002/chem.201400055. [DOI] [PubMed] [Google Scholar]
- Zhou X.-P.; Wu Y.; Li D. Polyhedral Metal-Imidazolate Cages: Control of Self-Assembly and Cage to Cage Transformation. J. Am. Chem. Soc. 2013, 135, 16062–16065. 10.1021/ja4092984. [DOI] [PubMed] [Google Scholar]
- Browne C.; Brenet S.; Clegg J. K.; Nitschke J. R. Solvent-Dependent Host-Guest Chemistry of an Fe8L12 Cubic Capsule. Angew. Chem., Int. Ed. 2013, 52, 1944–1948. 10.1002/anie.201208740. [DOI] [PubMed] [Google Scholar]
- Fujita M.; Oguro D.; Miyazawa M.; Oka H.; Yamaguchi K.; Ogura K. Self-Assembly of Ten Molecules into Nanometre-Sized Organic Host Frameworks. Nature 1995, 378, 469–471. 10.1038/378469a0. [DOI] [Google Scholar]
- Chand D. K.; Biradha K.; Fujita M.; Sakamoto S.; Yamaguchi K. A Molecular Sphere of Octahedral Symmetry. Chem. Commun. 2002, 2486–2487. 10.1039/B206625B. [DOI] [Google Scholar]
- Suzuki K.; Tominaga M.; Kawano M.; Fujita M. Self-Assembly of an M6L12 Coordination Cube. Chem. Commun. 2009, 1638–1640. 10.1039/b822311d. [DOI] [PubMed] [Google Scholar]
- Moon D.; Kang S.; Park J.; Lee K.; John R. P.; Won H.; Seong G. H.; Kim Y. S.; Kim G. H.; Rhee H.; Lah M. S. Face-Driven Corner-Linked Octahedral Nanocages: M6L8 Cages Formed by C3-Symmetric Triangular Facial Ligands Linked via C4-Symmetric Square Tetratopic PdII Ions at Truncated Octahedron Corners. J. Am. Chem. Soc. 2006, 128, 3530–3531. 10.1021/ja060051h. [DOI] [PubMed] [Google Scholar]
- Rizzuto F. J.; Wu W.-Y.; Ronson T. K.; Nitschke J. R. Peripheral Templation Generates an MII6L4 Guest-Binding Capsule. Angew. Chem., Int. Ed. 2016, 55, 7958–7962. 10.1002/anie.201602135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.; Lin Z.; He Z.; Duan C.; Xu C.; Wang Z.; Yan C. Metal-Tunable Nanocages as Artificial Chemosensors. Angew. Chem, Int. Ed. 2008, 47, 877–881. 10.1002/anie.200704206. [DOI] [PubMed] [Google Scholar]
- Mal P.; Breiner B.; Rissanen K.; Nitschke J. R. White Phosphorus is Air-Stable Within a Self-Assembled Tetrahedral Capsule. Science 2009, 324, 1697–1699. 10.1126/science.1175313. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M.; Miyagi S.; Kawano M.; Ishiguro K.; Fujita M. Alkane Oxidation via Photochemical Excitation of a Self-Assembled Molecular Cage. J. Am. Chem. Soc. 2004, 126, 9172–9173. 10.1021/ja047612u. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M.; Kusukawa T.; Kawano M.; Ohhara T.; Tanaka I.; Kurihara K.; Niimura N.; Fujita M. Endohedral Clusterization of Ten Water Molecules into a “Molecular Ice” within the Hydrophobic Pocket of a Self-Assembled Cage. J. Am. Chem. Soc. 2005, 127, 2798–2799. 10.1021/ja043953w. [DOI] [PubMed] [Google Scholar]
- Pearce N.; Champness N. R.. Metal Complexes in Supramolecular Chemistry and Self-Assembly. In Comprehensive Coordination Chemistry III, 3rd ed.; Constable E. C., Parkin G., Que L. Jr., Eds.; Elsevier, 2021; pp 81–98. [Google Scholar]
- Wang M.; Lan W.-J.; Zheng Y.-R.; Cook T. R.; White H. S.; Stang P. J. Post-Self-Assembly Covalent Chemistry of Discrete Multicomponent Metallosupramolecular Hexagonal Prisms. J. Am. Chem. Soc. 2011, 133, 10752–10755. 10.1021/ja204155r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. A.; Pilgrim B. S.; Nitschke J. R. Covalent Post-Assembly Modification in Metallosupramolecular Chemistry. Chem. Soc. Rev. 2018, 47, 626–644. 10.1039/C6CS00907G. [DOI] [PubMed] [Google Scholar]
- Zeng H.; Stewart-Yates L.; Casey L. M.; Bampos N.; Roberts D. A. Covalent Post-Assembly Modification: A Synthetic Multipurpose Tool in Supramolecular Chemistry. ChemPlusChem 2020, 85, 1249–1269. 10.1002/cplu.202000279. [DOI] [PubMed] [Google Scholar]
- McConnell A. J.; Wood C. S.; Neelakandan P. P.; Nitschke J. R. Stimuli-Responsive Metal-Ligand Assemblies. Chem. Rev. 2015, 115, 7729–7793. 10.1021/cr500632f. [DOI] [PubMed] [Google Scholar]
- Han M.; Michel R.; He B.; Chen Y.-S.; Stalke D.; John M.; Clever G. H. Light-Triggered Guest Uptake and Release by a Photochromic Coordination Cage. Angew. Chem., Int. Ed. 2013, 52, 1319–1323. 10.1002/anie.201207373. [DOI] [PubMed] [Google Scholar]
- Akine S.; Miyashita M.; Nabeshima T. A Metallo-Molecular Cage That Can Close the Apertures with Coordination Bonds. J. Am. Chem. Soc. 2017, 139, 4631–4634. 10.1021/jacs.7b00840. [DOI] [PubMed] [Google Scholar]
- Szalóki G.; Croué V.; Carré V.; Aubriet F.; Alévêque O.; Levillain E.; Allain M.; Aragó J.; Ortí E.; Goeb S.; Sallé M. Controlling the Host-Guest Interaction Mode through a Redox Stimulus. Angew. Chem., Int. Ed. 2017, 56, 16272–16276. 10.1002/anie.201709483. [DOI] [PubMed] [Google Scholar]
- McTernan C. T.; Ronson T. K.; Nitschke J. R. Post-assembly Modification of Phosphine Cages Controls Host-Guest Behavior. J. Am. Chem. Soc. 2019, 141, 6837–6842. 10.1021/jacs.9b02604. [DOI] [PubMed] [Google Scholar]
- Grancha T.; Carné-Sánchez A.; Hernández-López L.; Albalad J.; Imaz I.; Juanhuix J.; Maspoch D. Phase Transfer of Rhodium(II)-Based Metal-Organic Polyhedra Bearing Coordinatively Bound Cargo Enables Molecular Separation. J. Am. Chem. Soc. 2019, 141, 18349–18355. 10.1021/jacs.9b10403. [DOI] [PubMed] [Google Scholar]
- Nguyen B.-N. T.; Grommet A. B.; Tron A.; Georges M. C. A.; Nitschke J. R. Heat Engine Drives Transport of an FeII4L4 Cage and Cargo. Adv. Mater. 2020, 32, 1907241. 10.1002/adma.201907241. [DOI] [PubMed] [Google Scholar]
- Andrés M. A.; Carné-Sánchez A.; Sánchez-Laínez J.; Roubeau O.; Coronas J.; Maspoch D.; Gascón I. Ultrathin Films of Porous Metal-Organic Polyhedra for Gas Separation. Chem. - Eur. J. 2020, 26, 143–147. 10.1002/chem.201904141. [DOI] [PubMed] [Google Scholar]
- Ma L.; Haynes C. J. E.; Grommet A. B.; Walczak A.; Parkins C. C.; Doherty C. M.; Longley L.; Tron A.; Stefankiewicz A. R.; Bennett T. D.; Nitschke J. R. Coordination Cages as Permanently Porous Ionic Liquids. Nat. Chem. 2020, 12, 270–275. 10.1038/s41557-020-0419-2. [DOI] [PubMed] [Google Scholar]
- Hosono N.; Kitagawa S. Modular Design of Porous Soft Materials via Self-Organization of Metal-Organic Cages. Acc. Chem. Res. 2018, 51, 2437–2446. 10.1021/acs.accounts.8b00361. [DOI] [PubMed] [Google Scholar]
- Gu Y.; Alt E. A.; Wang H.; Li X.; Willard A. P.; Johnson J. A. Photoswitching Topology in Polymer Networks with Metal-Organic Cages as Crosslinks. Nature 2018, 560, 65–69. 10.1038/s41586-018-0339-0. [DOI] [PubMed] [Google Scholar]
- Zhukhovitskiy A. V.; Zhong M.; Keeler E. G.; Michaelis V. K.; Sun J. E. P.; Hore M. J. A.; Pochan D. J.; Griffin R. G.; Willard A. P.; Johnson J. A. Highly Branched and Loop-Rich Gels via Formation of Metal-Organic Cages Linked by Polymers. Nat. Chem. 2016, 8, 33–41. 10.1038/nchem.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Gu Y.; Keeler E. G.; Park J. V.; Griffin R. G.; Johnson J. A. Star PolyMOCs with Diverse Structures, Dynamics, and Functions by Three-Component Assembly. Angew. Chem., Int. Ed. 2017, 56, 188–192. 10.1002/anie.201609261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutar P.; Suresh V. M.; Jayaramulu K.; Hazra A.; Maji T. K. Binder Driven Self-Assembly of Metal-Organic Cubes Towards Functional Hydrogels. Nat. Commun. 2018, 9, 3587. 10.1038/s41467-018-05818-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga K.; Shionoya M. Nanoarchitectonics for Coordination Asymmetry and Related Chemistry. Bull. Chem. Soc. Jpn. 2021, 94, 839–859. 10.1246/bcsj.20200362. [DOI] [Google Scholar]
- Rizzuto F. J.; von Krbek L. K. S.; Nitschke J. R. Strategies for Binding Multiple Guests in Metal-Organic Cages. Nat. Rev. Chem. 2019, 3, 204–222. 10.1038/s41570-019-0085-3. [DOI] [Google Scholar]
- Takezawa H.; Shitozawa K.; Fujita M. Enhanced Reactivity of Twisted Amides inside a Molecular Cage. Nat. Chem. 2020, 12, 574–578. 10.1038/s41557-020-0455-y. [DOI] [PubMed] [Google Scholar]
- Takezawa H.; Kanda T.; Nanjo H.; Fujita M. Site-Selective Functionalization of Linear Diterpenoids through U-Shaped Folding in a Confined Artificial Cavity. J. Am. Chem. Soc. 2019, 141, 5112–5115. 10.1021/jacs.9b00131. [DOI] [PubMed] [Google Scholar]
- Niki K.; Tsutsui T.; Yamashina M.; Akita M.; Yoshizawa M. Recognition and Stabilization of Unsaturated Fatty Acids by a Polyaromatic Receptor. Angew. Chem., Int. Ed. 2020, 59, 10489–10492. 10.1002/anie.202003253. [DOI] [PubMed] [Google Scholar]
- Patrick G. L.An Introduction to Medicinal Chemistry, 6th ed.; Oxford University Press: Oxford, U.K., 2017. [Google Scholar]
- Rizzuto F. J.; Carpenter J. P.; Nitschke J. R. Multisite Binding of Drugs and Natural Products in an Entropically Favorable, Heteroleptic Receptor. J. Am. Chem. Soc. 2019, 141, 9087–9095. 10.1021/jacs.9b03776. [DOI] [PubMed] [Google Scholar]
- Sawada T.; Yoshizawa M.; Sato S.; Fujita M. Minimal Nucleotide Duplex Formation in Water through Enclathration in Self-Assembled Hosts. Nat. Chem. 2009, 1, 53–56. 10.1038/nchem.100. [DOI] [PubMed] [Google Scholar]
- Saha R.; Devaraj A.; Bhattacharyya S.; Das S.; Zangrando E.; Mukherjee P. S. Unusual Behavior of Donor-Acceptor Stenhouse Adducts in Confined Space of a Water-Soluble PdII8 Molecular Vessel. J. Am. Chem. Soc. 2019, 141, 8638–8645. 10.1021/jacs.9b03924. [DOI] [PubMed] [Google Scholar]
- Caulder D. L.; Raymond K. N. Supramolecules by Design. Acc. Chem. Res. 1999, 32, 975–982. 10.1021/ar970224v. [DOI] [Google Scholar]
- Fujita M.; Umemoto K.; Yoshizawa M.; Fujita N.; Kusukawa T.; Biradha K. Molecular Paneling via Coordination. Chem. Commun. 2001, 509–518. 10.1039/b008684n. [DOI] [Google Scholar]
- Holliday B. J.; Mirkin C. A. Strategies for the Construction of Supramolecular Compounds through Coordination Chemistry. Angew. Chem., Int. Ed. 2001, 40, 2022–2043. . [DOI] [PubMed] [Google Scholar]
- Farrell J. R.; Mirkin C. A.; Liable-Sands L. M.; Rheingold A. L. Strategy for Preparing Molecular Cylinders with Synthetically Programmable Structural Parameters. J. Am. Chem. Soc. 1998, 120, 11834–11835. 10.1021/ja982688+. [DOI] [Google Scholar]
- Holliday B. J.; Farrell J. R.; Mirkin C. A.; Lam K.-C.; Rheingold A. L. Metal-Directed Assembly of Triple-Layered Fluorescent Metallocyclophanes. J. Am. Chem. Soc. 1999, 121, 6316–6317. 10.1021/ja991140f. [DOI] [Google Scholar]
- Mollick S.; Fajal S.; Mukherjee S.; Ghosh S. K. Stabilizing Metal-Organic Polyhedra (MOP): Issues and Strategies. Chem. - Asian J. 2019, 14, 3096–3108. 10.1002/asia.201900800. [DOI] [PubMed] [Google Scholar]
- Gosselin A. J.; Rowland C. A.; Bloch E. D. Permanently Microporous Metal-Organic Polyhedra. Chem. Rev. 2020, 120, 8987–9014. 10.1021/acs.chemrev.9b00803. [DOI] [PubMed] [Google Scholar]
- Lee S.; Jeong H.; Nam D.; Lah M. S.; Choe W. The Rise of Metal-Organic Polyhedra. Chem. Soc. Rev. 2021, 50, 528–555. 10.1039/D0CS00443J. [DOI] [PubMed] [Google Scholar]
- Zheng Y.-R.; Zhao Z.; Wang M.; Ghosh K.; Pollock J. B.; Cook T. R.; Stang P. J. A Facile Approach toward Multicomponent Supramolecular Structures: Selective Self-Assembly via Charge Separation. J. Am. Chem. Soc. 2010, 132, 16873–16882. 10.1021/ja106251f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.-R.; Lan W.-J.; Wang M.; Cook T. R.; Stang P. J. Designed Post-Self-Assembly Structural and Functional Modifications of a Truncated Tetrahedron. J. Am. Chem. Soc. 2011, 133, 17045–17055. 10.1021/ja207217t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.; Zheng Y.-R.; Wang M.; Pollock J. B.; Stang P. J. Construction of Hexagonal Prisms of Variable Size via Coordination-Driven Multicomponent Self-Assembly. Inorg. Chem. 2010, 49, 8653–8655. 10.1021/ic1014219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X.; Cook T. R.; Wang P.; Huang F.; Stang P. J. Highly Emissive Platinum(II) Metallacages. Nat. Chem. 2015, 7, 342–348. 10.1038/nchem.2201. [DOI] [PubMed] [Google Scholar]
- Chen C.; Sun Y.; Zhao Y.; VanderLinden R. T.; Tuo W.; Zhang F.; Zhang S.; Sepehrpour H.; Yan C.; Wang J.; Li D.; Stang P. J. Anthracene-Induced Formation of Highly Twisted Metallacycle and its Crystal Structure and Tunable Assembly Behaviors. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2102602118 10.1073/pnas.2102602118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Zhang F.; Jiang S.; Wang Z.; Ni R.; Wang H.; Zhou W.; Li X.; Stang P. J. Assembly of Metallacages into Soft Suprastructures with Dimensions of up to Micrometers and the Formation of Composite Materials. J. Am. Chem. Soc. 2018, 140, 17297–17307. 10.1021/jacs.8b11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W.; Liu F.; Duan C.; Guo Z.; Zhou S.; Liu Y.; Zhu L. Construction of a Square-Planar Molecular Box: Self-Assembly of Palladium(II) Complexes of 3,6,9,16,19,22-Hexaazatricyclo[22.2.2.211,14]triacon-11,13,24,26(1),27,29-hexaene through Hydrogen-Bonding Interactions. Inorg. Chem. 2001, 40, 7065–7071. 10.1021/ic010101d. [DOI] [PubMed] [Google Scholar]
- Hou Y.; Zhang Z.; Lu S.; Yuan J.; Zhu Q.; Chen W.-P.; Ling S.; Li X.; Zheng Y.-Z.; Zhu K.; Zhang M. Highly Emissive Perylene Diimide-Based Metallacages and their Host-Guest Chemistry for Information Encryption. J. Am. Chem. Soc. 2020, 142, 18763–18768. 10.1021/jacs.0c09904. [DOI] [PubMed] [Google Scholar]
- Mirtschin S.; Slabon-Turski A.; Scopelliti R.; Velders A. H.; Severin K. A Coordination Cage with an Adaptable Cavity Size. J. Am. Chem. Soc. 2010, 132, 14004–14005. 10.1021/ja1063789. [DOI] [PubMed] [Google Scholar]
- Schmittel M.; He B.; Mal P. Supramolecular Multicomponent Self-Assembly of Shape-Adaptive Nanoprisms: Wrapping up C60 with Three Porphyrin Units. Org. Lett. 2008, 10, 2513–2516. 10.1021/ol800796h. [DOI] [PubMed] [Google Scholar]
- Goswami A.; Saha S.; Biswas P. K.; Schmittel M. (Nano)mechanical Motion Triggered by Metal Coordination: from Functional Devices to Networked Multicomponent Catalytic Machinery. Chem. Rev. 2020, 120, 125–199. 10.1021/acs.chemrev.9b00159. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M.; Nagao M.; Kumazawa K.; Fujita M. Side Chain-Directed Complementary cis-Coordination of Two Pyridines on Pd(II); Selective Multicomponent Assembly of Square-, Rectangular-, and Trigonal Prism-Shaped Molecules. J. Organomet. Chem. 2005, 690, 5383–5388. 10.1016/j.jorganchem.2005.06.022. [DOI] [Google Scholar]
- Zhu R.; Bloch W. M.; Holstein J. J.; Mandal S.; Schäfer L. V.; Clever G. H. Donor-Site-Directed Rational Assembly of Heteroleptic cis-[Pd2L2L′2] Coordination Cages from Picolyl Ligands. Chem. - Eur. J. 2018, 24, 12976–12982. 10.1002/chem.201802188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.; Horiuchi S.; Holstein J. J.; Tessarolo J.; Clever G. H. Tunable Fullerene Affinity of Cages, Bowls and Rings Assembled by PdII Coordination Sphere Engineering. Chem. - Eur. J. 2019, 25, 14921–14927. 10.1002/chem.201903317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamurugan G.; Roberts D. A.; Ronson T. K.; Nitschke J. R. Selective Endo and Exo Binding of Mono- and Ditopic Ligands to a Rhomboidal Diporphyrin Prism. Angew. Chem., Int. Ed. 2015, 54, 7539–7543. 10.1002/anie.201501359. [DOI] [PubMed] [Google Scholar]
- Kaeser A.; Mohankumar M.; Mohanraj J.; Monti F.; Holler M.; Cid J.-J.; Moudam O.; Nierengarten I.; Karmazin-Brelot L.; Duhayon C.; Delavaux-Nicot B.; Armaroli N.; Nierengarten J.-F. Heteroleptic Copper(I) Complexes Prepared from Phenanthroline and Bis-Phosphine Ligands. Inorg. Chem. 2013, 52, 12140–12151. 10.1021/ic4020042. [DOI] [PubMed] [Google Scholar]
- Baxter P.; Lehn J.-M.; DeCian A.; Fischer J. Multicomponent Self-Assembly: Spontaneous Formation of a Cylindrical Complex from Five Ligands and Six Metal Ions. Angew. Chem., Int. Ed. 1993, 32, 69–72. 10.1002/anie.199300691. [DOI] [Google Scholar]
- Baxter P. N. W.; Lehn J.-M.; Kneisel B. O.; Baum G.; Fenske D. The Designed Self-Assembly of Multicomponent and Multicompartmental Cylindrical Nanoarchitectures. Chem. - Eur. J. 1999, 5, 113–120. . [DOI] [Google Scholar]
- Han M.; Engelhard D. M.; Clever G. H. Self-assembled Coordination Cages based on Banana-Shaped Ligands. Chem. Soc. Rev. 2014, 43, 1848–1860. 10.1039/C3CS60473J. [DOI] [PubMed] [Google Scholar]
- Löffler S.; Lübben J.; Krause L.; Stalke D.; Dittrich B.; Clever G. H. Triggered Exchange of Anionic for Neutral Guests inside a Cationic Coordination Cage. J. Am. Chem. Soc. 2015, 137, 1060–1063. 10.1021/ja5130379. [DOI] [PubMed] [Google Scholar]
- Preston D.; Lewis J. E. M.; Crowley J. D. Multicavity [PdnL4]2n+ Cages with Controlled Segregated Binding of Different Guests. J. Am. Chem. Soc. 2017, 139, 2379–2386. 10.1021/jacs.6b11982. [DOI] [PubMed] [Google Scholar]
- Freye S.; Hey J.; Torras-Galán A.; Stalke D.; Herbst-Irmer R.; John M.; Clever G. H. Allosteric Binding of Halide Anions by a New Dimeric Interpenetrated Coordination Cage. Angew. Chem., Int. Ed. 2012, 51, 2191–2194. 10.1002/anie.201107184. [DOI] [PubMed] [Google Scholar]
- Regeni I.; Chen B.; Frank M.; Baksi A.; Holstein J. J.; Clever G. H. Coal-Tar Dye-based Coordination Cages and Helicates. Angew. Chem., Int. Ed. 2021, 60, 5673–5678. 10.1002/anie.202015246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch W. M.; Abe Y.; Holstein J. J.; Wandtke C. M.; Dittrich B.; Clever G. H. Geometric Complementarity in Assembly and Guest Recognition of a Bent Heteroleptic cis-[Pd2LA2LB2] Coordination Cage. J. Am. Chem. Soc. 2016, 138, 13750–13755. 10.1021/jacs.6b08694. [DOI] [PubMed] [Google Scholar]
- Pullen S.; Tessarolo J.; Clever G. H. Increasing Structural and Functional Complexity in Self-Assembled Coordination Cages. Chem. Sci. 2021, 12, 7269–7293. 10.1039/D1SC01226F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch W. M.; Holstein J. J.; Hiller W.; Clever G. H. Morphological Control of Heteroleptic cis- and trans-Pd2L2L′2 Cages. Angew. Chem., Int. Ed. 2017, 56, 8285–8289. 10.1002/anie.201702573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K.; Zhang B.; Drechsler C.; Holstein J. J.; Clever G. H. Backbone-Bridging Promotes Diversity in Heteroleptic Cages. Angew. Chem., Int. Ed. 2021, 60, 6403–6407. 10.1002/anie.202012425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.; Johnstone M. D.; Clever G. H. Interpenetrated Cage Structures. Chem. - Eur. J. 2016, 22, 14104–14125. 10.1002/chem.201601752. [DOI] [PubMed] [Google Scholar]
- Schulte T. R.; Holstein J. J.; Schneider L.; Adam A.; Haberhauer G.; Clever G. H. A New Mechanically-Interlocked [Pd2L4] Cage Motif by Dimerization of two Peptide-Based Lemniscates. Angew. Chem.. Int. Ed. 2020, 59, 22489–22493. 10.1002/anie.202010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.-L.; Tessarolo J.; Lee H.; Clever G. H. Multi-Stimuli Control over Assembly and Guest Binding in Metallo-Supramolecular Hosts Based on Dithienylethene Photoswitches. J. Am. Chem. Soc. 2021, 143, 3865–3873. 10.1021/jacs.0c12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarolo J.; Lee H.; Sakuda E.; Umakoshi K.; Clever G. H. Integrative Assembly of Heteroleptic Tetrahedra Controlled by Backbone Steric Bulk. J. Am. Chem. Soc. 2021, 143, 6339–6344. 10.1021/jacs.1c01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudan S.; Li R.-J.; Jansze S. M.; Platzek A.; Rudolf R.; Clever G. H.; Fadaei-Tirani F.; Scopelliti R.; Severin K. Identification of a Heteroleptic Pd6L6L′6 Coordination Cage by Screening of a Virtual Combinatorial Library. J. Am. Chem. Soc. 2021, 143, 1773–1778. 10.1021/jacs.0c12793. [DOI] [PubMed] [Google Scholar]
- Sun Q.-F.; Sato S.; Fujita M. An M12(L1)12(L2)12 Cantellated Tetrahedron: A Case Study on Mixed-Ligand Self-Assembly. Angew. Chem., Int. Ed. 2014, 53, 13510–13513. 10.1002/anie.201408652. [DOI] [PubMed] [Google Scholar]
- Benkhäuser C.; Lützen A. Self-Assembly of Heteroleptic Dinuclear Metallosupramolecular Kites from Multivalent Ligands via Social Self-Sorting. Beilstein J. Org. Chem. 2015, 11, 693–700. 10.3762/bjoc.11.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson T. K.; Roberts D. A.; Black S. P.; Nitschke J. R. Stacking Interactions Drive Selective Self-Assembly and Self-Sorting of Pyrene-Based MII4L6 Architectures. J. Am. Chem. Soc. 2015, 137, 14502–14512. 10.1021/jacs.5b09920. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Ronson T. K.; Xu L.; Nitschke J. R. Transformation Network Culminating in a Heteroleptic Cd6L6L′2 Twisted Trigonal Prism. J. Am. Chem. Soc. 2020, 142, 9152–9157. 10.1021/jacs.0c03798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; Noh T. H.; Jung O.-S. Construction of Hetero-Four-Layered Tripalladium(II) Cyclophanes by Transannular π···π Interactions. Angew. Chem., Int. Ed. 2016, 55, 1005–1009. 10.1002/anie.201509485. [DOI] [PubMed] [Google Scholar]
- Iseki S.; Nonomura K.; Kishida S.; Ogata D.; Yuasa J. Zinc-Ion-Stabilized Charge-Transfer Interactions Drive Self-Complementary or Complementary Molecular Recognition. J. Am. Chem. Soc. 2020, 142, 15842–15851. 10.1021/jacs.0c05940. [DOI] [PubMed] [Google Scholar]
- Kumazawa K.; Biradha K.; Kusukawa T.; Okano T.; Fujita M. Multicomponent Assembly of a Pyrazine-Pillared Coordination Cage That Selectively Binds Planar Guests by Intercalation. Angew. Chem., Int. Ed. 2003, 42, 3909–3913. 10.1002/anie.200351797. [DOI] [PubMed] [Google Scholar]
- Olenyuk B.; Whiteford J. A.; Fechtenkötter A.; Stang P. J. Self-Assembly of Nanoscale Cuboctahedra by Coordination Chemistry. Nature 1999, 398, 796–799. 10.1038/19740. [DOI] [PubMed] [Google Scholar]
- Schweiger M.; Seidel S. R.; Schmitz M.; Stang P. J. Rational Design of Chiral Nanoscale Adamantanoids. Org. Lett. 2000, 2, 1255–1257. 10.1021/ol005781n. [DOI] [PubMed] [Google Scholar]
- Crowley J. D.; Steele I. M.; Bosnich B. Molecular Recognition - Allosterism Generated by Weak Host-Guest Interactions in Molecular Rectangles. Eur. J. Inorg. Chem. 2005, 2005, 3907–3917. 10.1002/ejic.200500393. [DOI] [Google Scholar]
- Yamaki Y.; Nakamura T.; Suzuki S.; Yamamura M.; Minoura M.; Nabeshima T. A Self-Assembled Rectangular Host with Terpyridine-Platinum(II) Moieties that Binds Unsubstituted Pentacene in Solution. Eur. J. Org. Chem. 2016, 2016, 1678–1683. 10.1002/ejoc.201600058. [DOI] [Google Scholar]
- Chan A. K.-W.; Lam W. H.; Tanaka Y.; Wong K. M.-C.; Yam V. W.-W. Multiaddressable Molecular Rectangles with Reversible Host-Guest Interactions: Modulation of pH-Controlled Guest Release and Capture. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 690–695. 10.1073/pnas.1423709112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston D.; Kruger P. E. Using Complementary Ligand Denticity to Direct Metallosupramolecular Structure about Metal Ions with Square-Planar Geometry. ChemPlusChem 2020, 85, 454–465. 10.1002/cplu.202000019. [DOI] [PubMed] [Google Scholar]
- Samanta D.; Shanmugaraju S.; Joshi S. A.; Patil Y. P.; Nethaji M.; Mukherjee P. S. Pillar Height Dependent Formation of Unprecedented Pd8 Molecular Swing and Pd6 Molecular Boat via Multicomponent Self-Assembly. Chem. Commun. 2012, 48, 2298–2300. 10.1039/c2cc16345d. [DOI] [PubMed] [Google Scholar]
- Samanta D.; Mukherjee P. S. Multicomponent Self-Sorting of a Pd7 Molecular Boat and Its Use in Catalytic Knoevenagel Condensation. Chem. Commun. 2013, 49, 4307–4309. 10.1039/c2cc37377g. [DOI] [PubMed] [Google Scholar]
- García-Simón C.; Gramage-Doria R.; Raoufmoghaddam S.; Parella T.; Costas M.; Ribas X.; Reek J. N. H. Enantioselective Hydroformylation by a Rh-Catalyst Entrapped in a Supramolecular Metallocage. J. Am. Chem. Soc. 2015, 137, 2680–2687. 10.1021/ja512637k. [DOI] [PubMed] [Google Scholar]
- García-Simón C.; Garcia-Borràs M.; Gómez L.; Parella T.; Osuna S.; Juanhuix J.; Imaz I.; Maspoch D.; Costas M.; Ribas X. Sponge-Like Molecular Cage for Purification of Fullerenes. Nat. Commun. 2014, 5, 5557. 10.1038/ncomms6557. [DOI] [PubMed] [Google Scholar]
- Fuertes-Espinosa C.; García-Simón C.; Pujals M.; Garcia-Borràs M.; Gómez L.; Parella T.; Juanhuix J.; Imaz I.; Maspoch D.; Costas M.; Ribas X. Supramolecular Fullerene Sponges as Catalytic Masks for Regioselective Functionalisation of C60. Chem. 2020, 6, 169–186. 10.1016/j.chempr.2019.10.010. [DOI] [Google Scholar]
- Fuertes-Espinosa C.; Murillo J.; Soto M. E.; Ceron M. R.; Morales-Martínez R.; Rodríguez-Fortea A.; Poblet J. M.; Echegoyen L.; Ribas X. Highly Selective Encapsulation and Purification of U-Based C78-EMFs within a Supramolecular Nanocapsule. Nanoscale 2019, 11, 23035–23041. 10.1039/C9NR07660C. [DOI] [PubMed] [Google Scholar]
- Ubasart E.; Borodin O.; Fuertes-Espinosa C.; Xu Y.; García-Simón C.; Gómez L.; Juanhuix J.; Gándara F.; Imaz I.; Maspoch D.; von Delius M.; Ribas X. A Three-Shell Supramolecular Complex Enables the Symmetry-Mismatched Chemo- and Regioselective Bis-Functionalization of C60. Nat. Chem. 2021, 13, 420–427. 10.1038/s41557-021-00658-6. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Lin Y.-J.; Li Z.-H.; Jin G.-X. Rational Design of Polynuclear Organometallic Assemblies from a Simple Heteromultifunctional Ligand. J. Am. Chem. Soc. 2015, 137, 13670–13678. 10.1021/jacs.5b08826. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Lin Y.-J.; Li Z.-H.; Stoddart J. F.; Jin G.-X. Coordination-Driven Selective Formation of D2 Symmetric Octanuclear Organometallic Cages. Chem. - Eur. J. 2021, 27, 9524–9528. 10.1002/chem.202101204. [DOI] [PubMed] [Google Scholar]
- Lavendomme R.; Ronson T. K.; Nitschke J. R. Metal and Organic Templates Together Control the Size of Covalent Macrocycles and Cages. J. Am. Chem. Soc. 2019, 141, 12147–12158. 10.1021/jacs.9b06182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston D.; Barnsley J. E.; Gordon K. C.; Crowley J. D. Controlled Formation of Heteroleptic [Pd2(La)2(Lb)2]4+ Cages. J. Am. Chem. Soc. 2016, 138, 10578–10585. 10.1021/jacs.6b05629. [DOI] [PubMed] [Google Scholar]
- Chepelin O.; Ujma J.; Barran P. E.; Lusby P. J. Sequential, Kinetically Controlled Synthesis of Multicomponent Stereoisomeric Assemblies. Angew. Chem., Int. Ed. 2012, 51, 4194–4197. 10.1002/anie.201108994. [DOI] [PubMed] [Google Scholar]
- Lewis J. E. M.; Crowley J. D. Metallo-supramolecular Self-Assembly with Reduced Symmetry Ligands. ChemPlusChem 2020, 85, 815–827. 10.1002/cplu.202000153. [DOI] [PubMed] [Google Scholar]
- McMorran D. A.; Steel P. J. The First Coordinatively Saturated, Quadruply Stranded Helicate and Its Encapsulation of a Hexafluorophosphate Anion. Angew. Chem., Int. Ed. 1998, 37, 3295–3297. . [DOI] [PubMed] [Google Scholar]
- Schmidt A.; Casini A.; Kühn F. E. Self-Assembled M2L4 Coordination Cages: Synthesis and Potential Applications. Coord. Chem. Rev. 2014, 275, 19–36. 10.1016/j.ccr.2014.03.037. [DOI] [Google Scholar]
- Lewis J. E. M.; Tarzia A.; White A. J. P.; Jelfs K. E. Conformational Control of Pd2L4 Assemblies with Unsymmetrical Ligands. Chem. Sci. 2020, 11, 677–683. 10.1039/C9SC05534G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata D.; Yuasa J. Dynamic Open Coordination Cage from Nonsymmetrical Imidazole-Pyridine Ditopic Ligands for Turn-On/Off Anion Binding. Angew. Chem., Int. Ed. 2019, 58, 18424–18428. 10.1002/anie.201911097. [DOI] [PubMed] [Google Scholar]
- Sen S. K.; Natarajan R. Influence of Conformational Change and Interligand Hydrogen Bonding in a Chiral Metal-Organic Cage. Inorg. Chem. 2019, 58, 7180–7188. 10.1021/acs.inorgchem.8b03610. [DOI] [PubMed] [Google Scholar]
- Mishra S. S.; Kompella S. V. K.; Krishnaswamy S.; Balasubramanian S.; Chand D. K. Low-Symmetry Self-Assembled Coordination Complexes with Exclusive Diastereoselectivity: Experimental and Computational Studies. Inorg. Chem. 2020, 59, 12884–12894. 10.1021/acs.inorgchem.0c01964. [DOI] [PubMed] [Google Scholar]
- Lewis J. E. M. Multi-functional, Low Symmetry Pd2L4 Nanocage Libraries. Chem. - Eur. J. 2021, 27, 4454–4460. 10.1002/chem.202005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa L. S.; Findlay J. A.; Wright L. J.; Hartinger C. G.; Crowley J. D. A Reduced-Symmetry Heterobimetallic [PdPtL4]4+ Cage: Assembly, Guest Binding, and Stimulus-Induced Switching. Angew. Chem., Int. Ed. 2020, 59, 11101–11107. 10.1002/anie.202003220. [DOI] [PubMed] [Google Scholar]
- Markwell-Heys A. W.; Schneider M. L.; Madridejos J. M. L.; Metha G. F.; Bloch W. M. Self-Sorting of Porous Cu4L2L′2 Metal-Organic Cages Composed of Isomerisable Ligands. Chem. Commun. 2021, 57, 2915–2918. 10.1039/D0CC08076D. [DOI] [PubMed] [Google Scholar]
- Young M. C.; Holloway L. R.; Johnson A. M.; Hooley R. J. A Supramolecular Sorting Hat: Stereocontrol in Metal-Ligand Self-Assembly by Complementary Hydrogen Bonding. Angew. Chem., Int. Ed. 2014, 53, 9832–9836. 10.1002/anie.201405242. [DOI] [PubMed] [Google Scholar]
- Holloway L. R.; Bogie P. M.; Lyon Y.; Julian R. R.; Hooley R. J. Stereoselective Postassembly CH Oxidation of Self-Assembled Metal-Ligand Cage Complexes. Inorg. Chem. 2017, 56, 11435–11442. 10.1021/acs.inorgchem.7b01958. [DOI] [PubMed] [Google Scholar]
- da Camara B.; Dietz P. C.; Chalek K. R.; Mueller L. J.; Hooley R. J. Selective, Cofactor-Mediated Catalytic Oxidation of Alkanethiols in a Self-Assembled Cage Host. Chem. Commun. 2020, 56, 14263–14266. 10.1039/D0CC05765G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai C.; Bogie P. M.; Holloway L. R.; Dietz P. C.; Mueller L. J.; Hooley R. J. Cofactor-Mediated Nucleophilic Substitution Catalyzed by a Self-Assembled Holoenzyme Mimic. J. Org. Chem. 2019, 84, 12000–12008. 10.1021/acs.joc.9b01880. [DOI] [PubMed] [Google Scholar]
- Holloway L. R.; Bogie P. M.; Lyon Y.; Ngai C.; Miller T. F.; Julian R. R.; Hooley R. J. Tandem Reactivity of a Self-Assembled Cage Catalyst with Endohedral Acid Groups. J. Am. Chem. Soc. 2018, 140, 8078–8081. 10.1021/jacs.8b03984. [DOI] [PubMed] [Google Scholar]
- Wang X.; Huang J.; Xiang S.; Liu Y.; Zhang J.; Eichhöfer A.; Fenske D.; Bai S.; Su C.-Y. Discrete Ag6L6 Coordination Nanotubular Structures Based on a T-Shaped Pyridyl Diphosphine. Chem. Commun. 2011, 47, 3849–3851. 10.1039/c0cc05235c. [DOI] [PubMed] [Google Scholar]
- Hang X.; Liu B.; Zhu X.; Wang S.; Han H.; Liao W.; Liu Y.; Hu C. Discrete {Ni40} Coordination Cage: A Calixarene-Based Johnson-Type (J17) Hexadecahedron. J. Am. Chem. Soc. 2016, 138, 2969–2972. 10.1021/jacs.6b00695. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Jiang F.; Huang Y.; Wu M.; Hong M. Coordination-Driven Face-Directed Self-Assembly of a M9L6 Hexahedral Nanocage from Octahedral Ni(II) Ions and Asymmetric Hydrazone Ligands. Cryst. Growth Des. 2009, 9, 28–31. 10.1021/cg800972x. [DOI] [Google Scholar]
- Sun B.; Wang M.; Lou Z.; Huang M.; Xu C.; Li X.; Chen L.-J.; Yu Y.; Davis G. L.; Xu B.; Yang H.-B.; Li X. From Ring-in-Ring to Sphere-in-Sphere: Self-Assembly of Discrete 2D and 3D Architectures with Increasing Stability. J. Am. Chem. Soc. 2015, 137, 1556–1564. 10.1021/ja511443p. [DOI] [PubMed] [Google Scholar]
- Song B.; Zhang Z.; Wang K.; Hsu C.-H.; Bolarinwa O.; Wang J.; Li Y.; Yin G.-Q.; Rivera E.; Yang H.-B.; Liu C.; Xu B.; Li X. Direct Self-Assembly of a 2D and 3D Star of David. Angew. Chem., Int. Ed. 2017, 56, 5258–5262. 10.1002/anie.201701417. [DOI] [PubMed] [Google Scholar]
- Sun Q.-F.; Murase T.; Sato S.; Fujita M. A Sphere-in-Sphere Complex by Orthogonal Self-Assembly. Angew. Chem.. Int. Ed. 2011, 50, 10318–10321. 10.1002/anie.201104670. [DOI] [PubMed] [Google Scholar]
- Wang H.; Zhou L.-P.; Zheng Y.; Wang K.; Song B.; Yan X.; Wojtas L.; Wang X.-Q.; Jiang X.; Wang M.; Sun Q.-F.; Xu B.; Yang H.-B.; Sue A. C.-H.; Chan Y.-T.; Sessler J. L.; Jiao Y.; Stang P. J.; Li X. Double-Layered Supramolecular Prisms Self-Assembled by Geometrically Non-equivalent Tetratopic Subunits. Angew. Chem.. Int. Ed. 2021, 60, 1298–1305. 10.1002/anie.202010805. [DOI] [PubMed] [Google Scholar]
- Shi J.; Li Y.; Jiang X.; Yu H.; Li J.; Zhang H.; Trainer D. J.; Hla S. W.; Wang H.; Wang M.; Li X. Self-Assembly of Metallo-Supramolecules with Dissymmetrical Ligands and Characterization by Scanning Tunneling Microscopy. J. Am. Chem. Soc. 2021, 143, 1224–1234. 10.1021/jacs.0c12508. [DOI] [PubMed] [Google Scholar]
- Wang H.; Liu C.-H.; Wang K.; Wang M.; Yu H.; Kandapal S.; Brzozowski R.; Xu B.; Wang M.; Lu S.; Hao X.-Q.; Eswara P.; Nieh M.-P.; Cai J.; Li X. Assembling Pentatopic Terpyridine Ligands with Three Types of Coordination Moieties into a Giant Supramolecular Hexagonal Prism: Synthesis, Self-Assembly, Characterization and Antimicrobial Study. J. Am. Chem. Soc. 2019, 141, 16108–16116. 10.1021/jacs.9b08484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Qian X.; Wang K.; Su M.; Haoyang W.-W.; Jiang X.; Brzozowski R.; Wang M.; Gao X.; Li Y.; Xu B.; Eswara P.; Hao X.-Q.; Gong W.; Hou J.-L.; Cai J.; Li X. Supramolecular Kandinsky Circles with High Antibacterial Activity. Nat. Commun. 2018, 9, 1815. 10.1038/s41467-018-04247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q.-F.; Sato S.; Fujita M. An M18L24 Stellated Cuboctahedron through Post-Stellation of an M12L24 Core. Nat. Chem. 2012, 4, 330–333. 10.1038/nchem.1285. [DOI] [PubMed] [Google Scholar]
- Tominaga M.; Suzuki K.; Kawano M.; Kusukawa T.; Ozeki T.; Sakamoto S.; Yamaguchi K.; Fujita M. Finite, Spherical Coordination Networks that Self-Organize from 36 Small Components. Angew. Chem., Int. Ed. 2004, 43, 5621–5625. 10.1002/anie.200461422. [DOI] [PubMed] [Google Scholar]
- Sato S.; Ishido Y.; Fujita M. Remarkable Stabilization of M12L24 Spherical Frameworks through the Cooperation of 48 Pd(II)-Pyridine Interactions. J. Am. Chem. Soc. 2009, 131, 6064–6065. 10.1021/ja900676f. [DOI] [PubMed] [Google Scholar]
- Fox O. D.; Drew M. G. B.; Beer P. D. Resorcarene-Based Nanoarchitectures: Metal-Directed Assembly of a Molecular Loop and Tetrahedron. Angew. Chem., Int. Ed. 2000, 39, 135–140. . [DOI] [PubMed] [Google Scholar]
- Zhong Z.; Ikeda A.; Shinkai S.; Sakamoto S.; Yamaguchi K. Creation of Novel Chiral Cryptophanes by a Self-Assembling Method Utilizing a Pyridyl-Pd(II) Interaction. Org. Lett. 2001, 3, 1085–1087. 10.1021/ol0157205. [DOI] [PubMed] [Google Scholar]
- McKinlay R. M.; Cave G. W. V.; Atwood J. L. Supramolecular Blueprint Approach to Metal-Coordinated Capsules. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 5944–5948. 10.1073/pnas.0408113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgarno S. J.; Power N. P.; Atwood J. L. Metallo-Supramolecular Capsules. Coord. Chem. Rev. 2008, 252, 825–841. 10.1016/j.ccr.2007.10.010. [DOI] [Google Scholar]
- Pei W.-Y; Yang J.; Wu H.; Zhou W.; Yang Y.-W.; Ma J.-F. A Calix[4]resorcinarene-Based Giant Coordination Cage: Controlled Assembly and Iodine Uptake. Chem. Commun. 2020, 56, 2491–2494. 10.1039/D0CC00157K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale S.; Sattin S.; Escudero-Adán E. C.; Martínez-Belmonte M.; de Mendoza J. Giant Regular Polyhedra from Calixarene Carboxylates and Uranyl. Nat. Commun. 2012, 3, 785. 10.1038/ncomms1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers P. F.; Otte M.; Dürr M.; Ivanović-Burmazović I.; Reek J. N. H.; de Bruin B. A Self-Assembled Molecular Cage for Substrate-Selective Epoxidation Reactions in Aqueous Media. ACS Catal. 2016, 6, 3106–3112. 10.1021/acscatal.6b00283. [DOI] [Google Scholar]
- Rizzuto F. J.; Ramsay W. J.; Nitschke J. R. Otherwise Unstable Structures Self-Assemble in the Cavities of Cuboctahedral Coordination Cages. J. Am. Chem. Soc. 2018, 140, 11502–11509. 10.1021/jacs.8b07494. [DOI] [PubMed] [Google Scholar]
- von Krbek L. K. S.; Roberts D. A.; Pilgrim B. S.; Schalley C. A.; Nitschke J. R. Multivalent Crown Ether Receptors Enable Allosteric Regulation of Anion Exchange in an Fe4L6 Tetrahedron. Angew. Chem., Int. Ed. 2018, 57, 14121–14124. 10.1002/anie.201808534. [DOI] [PubMed] [Google Scholar]
- Yang D.; Greenfield J. L.; Ronson T. K.; von Krbek L. K. S.; Yu L.; Nitschke J. R. LaIII and ZnII Cooperatively Template a Metal-Organic Capsule. J. Am. Chem. Soc. 2020, 142, 19856–19861. 10.1021/jacs.0c09991. [DOI] [PubMed] [Google Scholar]
- Sumby C. J.; Hardie M. J. Capsules and Star-Burst Polyhedra: An [Ag2L2] Capsule and a Tetrahedral [Ag4L4] Metallosupramolecular Prism with Cyclotriveratrylene-Type Ligands. Angew. Chem.. Int. Ed. 2005, 44, 6395–6399. 10.1002/anie.200501358. [DOI] [PubMed] [Google Scholar]
- Ronson T. K.; Fisher J.; Harding L. P.; Hardie M. J. Star-Burst Prisms with Cyclotriveratrylene-Type Ligands: A [Pd6L8]12+ Stella Octangular Structure. Angew. Chem.. Int. Ed. 2007, 46, 9086–9088. 10.1002/anie.200703903. [DOI] [PubMed] [Google Scholar]
- Ronson T. K.; Fisher J.; Harding L. P.; Rizkallah P. J.; Warren J. E.; Hardie M. J. Stellated Polyhedral Assembly of a Topologically Complicated Pd4L4 ‘Solomon Cube’. Nat. Chem. 2009, 1, 212–216. 10.1038/nchem.213. [DOI] [PubMed] [Google Scholar]
- Pentecost C. D.; Chichak K. S.; Peters A. J.; Cave G. W. V.; Cantrill S. J.; Stoddart J. F. A Molecular Solomon Link. Angew. Chem., Int. Ed. 2007, 46, 218–222. 10.1002/anie.200603521. [DOI] [PubMed] [Google Scholar]
- Forgan R. S.; Sauvage J.-P.; Stoddart J. F. Chemical Topology: Complex Molecular Knots, Links, and Entanglements. Chem. Rev. 2011, 111, 5434–5464. 10.1021/cr200034u. [DOI] [PubMed] [Google Scholar]
- Yang H.; McLaughlin C. K.; Aldaye F. A.; Hamblin G. D.; Rys A. Z.; Rouiller I.; Sleiman H. F. Metal-Nucleic Acid Cages. Nat. Chem. 2009, 1, 390–396. 10.1038/nchem.290. [DOI] [PubMed] [Google Scholar]
- Aldaye F. A.; Sleiman H. F. Modular Access to Structurally Switchable 3D Discrete DNA Assemblies. J. Am. Chem. Soc. 2007, 129, 13376–13377. 10.1021/ja075966q. [DOI] [PubMed] [Google Scholar]
- Zimmermann J.; Cebulla M. P. J.; Mönninghoff S.; von Kiedrowski G. Self-Assembly of a DNA Dodecahedron from 20 Trisoligonucleotides with C3h Linkers. Angew. Chem., Int. Ed. 2008, 47, 3626–3630. 10.1002/anie.200702682. [DOI] [PubMed] [Google Scholar]
- Bhatia D.; Mehtab S.; Krishnan R.; Indi S. S.; Basu A.; Krishnan Y. Icosahedral DNA Nanocapsules by Modular Assembly. Angew. Chem., Int. Ed. 2009, 48, 4134–4137. 10.1002/anie.200806000. [DOI] [PubMed] [Google Scholar]
- Seeman N. C.; Sleiman H. F. DNA Nanotechnology. Nat. Rev. Mater. 2018, 3, 17068. 10.1038/natrevmats.2017.68. [DOI] [Google Scholar]
- Bujold K. E.; Hsu J. C. C.; Sleiman H. F. Optimized DNA “Nanosuitcases” for Encapsulation and Conditional Release of siRNA. J. Am. Chem. Soc. 2016, 138, 14030–14038. 10.1021/jacs.6b08369. [DOI] [PubMed] [Google Scholar]
- Paul R. L.; Bell Z. R.; Jeffery J. C.; McCleverty J. A.; Ward M. D. Anion-Templated Self-Assembly of Tetrahedral Cage Complexes of Cobalt(II) with Bridging Ligands Containing Two Bidentate Pyrazolyl-Pyridine Binding Sites. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 4883–4888. 10.1073/pnas.052575199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. S.; Mann K. L. V.; Carraz C.-A.; Psillakis E.; Jeffery J. C.; McCleverty J. A.; Ward M. D. Anion-Templated Assembly of a Supramolecular Cage Complex. Angew. Chem.. Int. Ed. 1998, 37, 1279–1281. . [DOI] [PubMed] [Google Scholar]
- Hall B. R.; Manck L. E.; Tidmarsh I. S.; Stephenson A.; Taylor B. F.; Blaikie E. J.; Vander Griend D. A.; Ward M. D. Structures, Host-Guest Chemistry and Mechanism of Stepwise Self-Assembly of M4L6 Tetrahedral Cage Complexes. Dalton Trans. 2011, 40, 12132–12145. 10.1039/c1dt10781j. [DOI] [PubMed] [Google Scholar]
- Bolliger J. L.; Ronson T. K.; Ogawa M.; Nitschke J. R. Solvent Effects upon Guest Binding and Dynamics of a FeII4L4 Cage. J. Am. Chem. Soc. 2014, 136, 14545–14553. 10.1021/ja5077102. [DOI] [PubMed] [Google Scholar]
- Roy B.; Zangrando E.; Mukherjee P. S. Self-Assembly of a Redox Active Water Soluble Pd6L8 ‘Molecular Dice’. Chem. Commun. 2016, 52, 4489–4492. 10.1039/C6CC00042H. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Ronson T. K.; Mosquera J.; Martinez A.; Guy L.; Nitschke J. R. Anion Binding in Water Drives Structural Adaptation in an Azaphosphatrane-Functionalized FeII4L4 Tetrahedron. J. Am. Chem. Soc. 2017, 139, 6574–6577. 10.1021/jacs.7b02950. [DOI] [PubMed] [Google Scholar]
- Mosquera J.; Szyszko B.; Ho S. K. Y.; Nitschke J. R. Sequence-Selective Encapsulation and Protection of Long Peptides by a Self-Assembled FeII8L6 Cubic Cage. Nat. Commun. 2017, 8, 14882. 10.1038/ncomms14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell Z. R.; Harding L. P.; Ward M. D. Self-Assembly of a Molecular M8L12 Cube having S6 Symmetry. Chem. Commun. 2003, 2432–2433. 10.1039/b307172n. [DOI] [PubMed] [Google Scholar]
- Tidmarsh I. S.; Faust T. B.; Adams H.; Harding L. P.; Russo L.; Clegg W.; Ward M. D. Octanuclear Cubic Coordination Cages. J. Am. Chem. Soc. 2008, 130, 15167–15175. 10.1021/ja805605y. [DOI] [PubMed] [Google Scholar]
- Najar A. M.; Tidmarsh I. S.; Adams H.; Ward M. D. Cubes, Squares, and Books: A Simple Transition Metal/Bridging Ligand Combination Can Lead to a Surprising Range of Structural Types with the Same Metal/Ligand Proportions. Inorg. Chem. 2009, 48, 11871–11881. 10.1021/ic901892y. [DOI] [PubMed] [Google Scholar]
- Argent S. P.; Adams H.; Harding L. P.; Ward M. D. A Closed Molecular Cube and an Open Book: Two Different Products from Assembly of the Same Metal Salt and Bridging Ligand. Dalton Trans. 2006, 542–544. 10.1039/B515296H. [DOI] [PubMed] [Google Scholar]
- Stephenson A.; Ward M. D. An Octanuclear Coordination Cage with a ‘Cuneane’ Core - A Topological Isomer of a Cubic Cage. Dalton Trans. 2011, 40, 7824–7826. 10.1039/c0dt01767a. [DOI] [PubMed] [Google Scholar]
- Stephenson A.; Argent S. P.; Riis-Johannessen T.; Tidmarsh I. S.; Ward M. D. Structures and Dynamic Behavior of Large Polyhedral Coordination Cages: An Unusual Cage-to-Cage Interconversion. J. Am. Chem. Soc. 2011, 133, 858–870. 10.1021/ja107403p. [DOI] [PubMed] [Google Scholar]
- Al-Rasbi N. K.; Tidmarsh I. S.; Argent S. P.; Adams H.; Harding L. P.; Ward M. D. Mixed-Ligand Molecular Paneling: Dodecanuclear Cuboctahedral Coordination Cages Based on a Combination of Edge-Bridging and Face-Capping Ligands. J. Am. Chem. Soc. 2008, 130, 11641–11649. 10.1021/ja803847w. [DOI] [PubMed] [Google Scholar]
- Argent S. P.; Adams H.; Riis-Johannessen T.; Jeffery J. C.; Harding L. P.; Mamula O.; Ward M. D. Coordination Chemistry of Tetradentate N-Donor Ligands Containing Two Pyrazolyl-Pyridine Units Separated by a 1,8-Naphthyl Space: Dodecanuclear and Tetranuclear Coordination Cages and Cyclic Helicates. Inorg. Chem. 2006, 45, 3905–3919. 10.1021/ic060157d. [DOI] [PubMed] [Google Scholar]
- Bell Z. R.; Jeffery J. C.; McCleverty J. A.; Ward M. D. Assembly of a Truncated-Tetrahedral Chiral [M12(μ-L)18]24+ Cage. Angew. Chem., Int. Ed. 2002, 41, 2515–2518. . [DOI] [PubMed] [Google Scholar]
- Stephenson A.; Sykes D.; Ward M. D. Cu12 and Cd16 Coordination Cages and their Cu3 and Cd3 Subcomponents, and the Role of Inter-Ligand π-Stacking in Stabilising Cage Complexes. Dalton Trans. 2013, 42, 6756–6767. 10.1039/c3dt50161b. [DOI] [PubMed] [Google Scholar]
- Argent S. P.; Jackson F. C.; Chan H. M.; Meyrick S.; Taylor C. G. P.; Ronson T. K.; Rourke J. P.; Ward M. D. A Family of Diastereomeric Dodecanuclear Coordination Cages Based on Inversion of Chirality of Individual Triangular Cyclic Helicate Faces. Chem. Sci. 2020, 11, 10167–10174. 10.1039/D0SC04347H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham K.-C.; Yiu S.-M.; Kwong H.-L. Dodecanuclear Hexagonal-Prismatic M12L18 Coordination Cages by Subcomponent Self-Assembly. Inorg. Chem. 2013, 52, 5648–5650. 10.1021/ic400665m. [DOI] [PubMed] [Google Scholar]
- Su C.-Y.; Smith M. D.; zur Loye H.-C. Columnar Supramolecular Architecture Self-Assembled from S4-Symmetric Coordination Nanotubes Encapsulating Neutral Guest Molecules. Angew. Chem., Int. Ed. 2003, 42, 4085–4089. 10.1002/anie.200351353. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Ali B.; Wu J.; Guo M.; Yu Y.; Liu Z.; Tang J. Construction of Metallosupramolecular Coordination Complexes: from Lanthanide Helicates to Octahedral Cages Showing Single-Molecule Magnet Behavior. Inorg. Chem. 2019, 58, 3167–3174. 10.1021/acs.inorgchem.8b03249. [DOI] [PubMed] [Google Scholar]
- Gianneschi N. C.; Masar M. S.; Mirkin C. A. Development of a Coordination Chemistry-Based Approach for Functional Supramolecular Structures. Acc. Chem. Res. 2005, 38, 825–837. 10.1021/ar980101q. [DOI] [PubMed] [Google Scholar]
- Mendez-Arroyo J.; d’Aquino A. I.; Chinen A. B.; Manraj Y. D.; Mirkin C. A. Reversible and Selective Encapsulation of Dextromethorphan and β-Estradiol Using an Asymmetric Molecular Capsule Assembled via the Weak-Link Approach. J. Am. Chem. Soc. 2017, 139, 1368–1371. 10.1021/jacs.6b10027. [DOI] [PubMed] [Google Scholar]
- Mosquera J.; Ronson T. K.; Nitschke J. R. Subcomponent Flexibility Enables Conversion between D4-Symmetric CdII8L8 and T-Symmetric CdII4L4 Assemblies. J. Am. Chem. Soc. 2016, 138, 1812–1815. 10.1021/jacs.5b12955. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Jiang F.; Yuan D.; Lyu G.; Chen L.; Hong M. A Controllable and Dynamic Assembly System Based on Discrete Metallocages. Chem. Sci. 2014, 5, 483–488. 10.1039/C3SC52442F. [DOI] [Google Scholar]
- Zhao L.; Wei J.; Zhang J.; He C.; Duan C. Encapsulation of a Quinhydrone Cofactor in the Inner Pocket of Cobalt Triangular Prisms: Combined Light-Driven Reduction of Protons and Hydrogenation of Nitrobenzene. Angew. Chem., Int. Ed. 2017, 56, 15284–15288. 10.1002/anie.201707676. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Lin Z.; He C.; Zhao L.; Duan C. A Symmetry-Controlled and Face-Driven Approach for the Assembly of Cerium-Based Molecular Polyhedra. Dalton Trans. 2010, 39, 11122–11125. 10.1039/c0dt01086c. [DOI] [PubMed] [Google Scholar]
- Jiao Y.; Zhang J.; Zhang L.; Lin Z.; He C.; Duan C. Metal-Organic Polyhedra Containing 36 and 24 Folds of Amide Groups for Selective Luminescent Recognition of Natural Disaccharides. Chem. Commun. 2012, 48, 6022–6024. 10.1039/c2cc30497j. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Qu S.; He C.; Zhang R.; Duan C. Face-driven Octanuclear Cerium(IV) Luminescence Polyhedra: Synthesis and Luminescent Sensing Natural Saccharides. Chem. Commun. 2011, 47, 9387–9389. 10.1039/c1cc12766g. [DOI] [PubMed] [Google Scholar]
- Cai L.-X.; Yan D.-N.; Cheng P.-M.; Xuan J.-J.; Li S.-C.; Zhou L.-P.; Tian C.-B.; Sun Q.-F. Controlled Self-Assembly and Multistimuli-Responsive Interconversions of Three Conjoined Twin-Cages. J. Am. Chem. Soc. 2021, 143, 2016–2024. 10.1021/jacs.0c12064. [DOI] [PubMed] [Google Scholar]
- Cheng P.-M.; Cai L.-X.; Li S.-C.; Hu S.-J.; Yan D.-N.; Zhou L.-P.; Sun Q.-F. Guest-Reaction Driven Cage to Conjoined Twin-Cage Mitosis-Like Host Transformation. Angew. Chem., Int. Ed. 2020, 59, 23569–23573. 10.1002/anie.202011474. [DOI] [PubMed] [Google Scholar]
- Xuan W.; Zhang M.; Liu Y.; Chen Z.; Cui Y. A Chiral Quadruple-Stranded Helicate Cage for Enantioselective Recognition and Separation. J. Am. Chem. Soc. 2012, 134, 6904–6907. 10.1021/ja212132r. [DOI] [PubMed] [Google Scholar]
- Luo D.; Wang X.-Z.; Yang C.; Zhou X.-P.; Li D. Self-Assembly of Chiral Metal-Organic Tetartoid. J. Am. Chem. Soc. 2018, 140, 118–121. 10.1021/jacs.7b11285. [DOI] [PubMed] [Google Scholar]
- Luo D.; Zhou X.-P.; Li D. Beyond Molecules: Mesoporous Supramolecular Frameworks Self-Assembled from Coordination Cages and Inorganic Anions. Angew. Chem., Int. Ed. 2015, 54, 6190–6195. 10.1002/anie.201501081. [DOI] [PubMed] [Google Scholar]
- Wang X.-Z.; Sun M.-Y.; Zheng J.; Luo D.; Qi L.; Zhou X.-P.; Li D. Coordination-Driven Self-Assembly of M10L8 Metal-Organic Bi-Capped Square Antiprisms with Adaptable Cavities. Dalton Trans. 2019, 48, 17713–17717. 10.1039/C9DT04368C. [DOI] [PubMed] [Google Scholar]
- Lai Y.-L.; Wang X.-Z.; Dai R.-R.; Huang Y.-L.; Zhou X.-C.; Zhou X.-P.; Li D. Self-Assembly of Mixed-Valence and Heterometallic Metallocycles: Efficient Catalysts for the Oxidation of Alcohols to Aldehydes in Ambient Air. Dalton Trans. 2020, 49, 7304–7308. 10.1039/D0DT01340D. [DOI] [PubMed] [Google Scholar]
- Lai Y.-L.; Wang X.-Z.; Zhou X.-C.; Dai R.-R.; Zhou X.-P.; Li D. Self-assembly of a Mixed Valence Copper Triangular Prism and Transformation to Cage Triggered by an External Stimulus. Inorg. Chem. 2020, 59, 17374–17378. 10.1021/acs.inorgchem.0c02682. [DOI] [PubMed] [Google Scholar]
- Samanta D.; Mukherjee S.; Patil Y. P.; Mukherjee P. S. Self-Assembled Pd6 Open Cage with Triimidazole Walls and the Use of its Confined Nanospace for Catalytic Knoevenagel- and Diels-Alder Reactions in Aqueous Medium. Chem. - Eur. J. 2012, 18, 12322–12329. 10.1002/chem.201201679. [DOI] [PubMed] [Google Scholar]
- Fujita M.; Yu S.-Y.; Kusukawa T.; Funaki H.; Ogura K.; Yamaguchi K. Self-Assembly of Nanometer-Sized Macrotricyclic Complexes from Ten Small Component Molecules. Angew. Chem., Int. Ed. 1998, 37, 2082–2085. . [DOI] [PubMed] [Google Scholar]
- Canton M.; Grommet A. B.; Pesce L.; Gemen J.; Li S.; Diskin-Posner Y.; Credi A.; Pavan G. M.; Andréasson J.; Klajn R. Improving Fatigue Resistance of Dihydropyrene by Encapsulation within a Coordination Cage. J. Am. Chem. Soc. 2020, 142, 14557–14565. 10.1021/jacs.0c06146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce L.; Perego C.; Grommet A. B.; Klajn R.; Pavan G. M. Molecular Factors Controlling the Isomerization of Azobenzenes in the Cavity of a Flexible Coordination Cage. J. Am. Chem. Soc. 2020, 142, 9792–9802. 10.1021/jacs.0c03444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta D.; Galaktionova D.; Gemen J.; Shimon L. J. W.; Diskin-Posner Y.; Avram L.; Král P.; Klajn R. Reversible Chromism of Spiropyran in the Cavity of a Flexible Coordination Cage. Nat. Commun. 2018, 9, 641. 10.1038/s41467-017-02715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta D.; Gemen J.; Chu Z.; Diskin-Posner Y.; Shimon L. J. W.; Klajn R. Reversible Photoswitching of Encapsulated Azobenzenes in Water. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 9379–9384. 10.1073/pnas.1712787115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N.; Biradha K.; Fujita M.; Sakamoto S.; Yamaguchi K. A Porphyrin Prism: Structural Switching Triggered by Guest Inclusion. Angew. Chem., Int. Ed. 2001, 40, 1718–1721. . [DOI] [PubMed] [Google Scholar]
- Bar A. K.; Mohapatra S.; Zangrando E.; Mukherjee P. S. A Series of Trifacial Pd6 Molecular Barrels with Porphyrin Walls. Chem. - Eur. J. 2012, 18, 9571–9579. 10.1002/chem.201201077. [DOI] [PubMed] [Google Scholar]
- Roy B.; Ghosh A. K.; Srivastava S.; D’Silva P.; Mukherjee P. S. A Pd8 Tetrafacial Molecular Barrel as Carrier for Water Insoluble Fluorophore. J. Am. Chem. Soc. 2015, 137, 11916–11919. 10.1021/jacs.5b08008. [DOI] [PubMed] [Google Scholar]
- Argent S. P.; Greenaway A.; Gimenez-Lopez M. D. C.; Lewis W.; Nowell H.; Khlobystov A. N.; Blake A. J.; Champness N. R.; Schröder M. High-Nuclearity Metal-Organic Nanospheres: A Cd66 Ball. J. Am. Chem. Soc. 2012, 134, 55–58. 10.1021/ja207845u. [DOI] [PubMed] [Google Scholar]
- Tashiro S.; Tominaga M.; Kusukawa T.; Kawano M.; Sakamoto S.; Yamaguchi K.; Fujita M. PdII-Directed Dynamic Assembly of a Dodecapyridine Ligand into End-Capped and Open Tubes: The Importance of Kinetic Control in Self-Assembly. Angew. Chem., Int. Ed. 2003, 42, 3267–3270. 10.1002/anie.200351397. [DOI] [PubMed] [Google Scholar]
- Bandi S.; Pal A. K.; Hanan G. S.; Chand D. K. Stoichiometrically Controlled Revocable Self-Assembled ‘Spiro’ versus Quadruple-Stranded ‘Double-Decker’ Type Coordination Cages. Chem. - Eur. J. 2014, 20, 13122–13126. 10.1002/chem.201403808. [DOI] [PubMed] [Google Scholar]
- Zarra S.; Clegg J. K.; Nitschke J. R. Selective Assembly and Disassembly of a Water-Soluble Fe10L15 Prism. Angew. Chem., Int. Ed. 2013, 52, 4837–4840. 10.1002/anie.201209694. [DOI] [PubMed] [Google Scholar]
- Kieffer M.; Pilgrim B. S.; Ronson T. K.; Roberts D. A.; Aleksanyan M.; Nitschke J. R. Perfluorinated Ligands Induce Meridional Metal Stereochemistry to Generate M8L12, M10L15, and M12L18 Prisms. J. Am. Chem. Soc. 2016, 138, 6813–6821. 10.1021/jacs.6b02445. [DOI] [PubMed] [Google Scholar]
- Metherell A. J.; Ward M. D. Geometric Isomerism in Coordination Cages Based on Tris-chelate Vertices: a Tool to Control both Assembly and Host/Guest Chemistry. Dalton Trans. 2016, 45, 16096–16111. 10.1039/C6DT03041F. [DOI] [PubMed] [Google Scholar]
- Castilla A. M.; Ramsay W. J.; Nitschke J. R. Stereochemistry in Subcomponent Self-Assembly. Acc. Chem. Res. 2014, 47, 2063–2073. 10.1021/ar5000924. [DOI] [PubMed] [Google Scholar]
- Carpenter J. P.; McTernan C. T.; Greenfield J. L.; Lavendomme R.; Ronson T. K.; Nitschke J. R. Controlling the Shape and Chirality of an Eight-Crossing Molecular Knot. Chem. 2021, 7, 1534–1543. 10.1016/j.chempr.2021.03.005. [DOI] [Google Scholar]
- Kilbas B.; Mirtschin S.; Scopelliti R.; Severin K. A Solvent-Responsive Coordination Cage. Chem. Sci. 2012, 3, 701–704. 10.1039/C1SC00779C. [DOI] [Google Scholar]
- Lu X.; Li X.; Guo K.; Xie T.-Z.; Moorefield C. N.; Wesdemiotis C.; Newkome G. R. Probing a Hidden World of Molecular Self-Assembly: Concentration-Dependent, Three-Dimensional Supramolecular Interconversions. J. Am. Chem. Soc. 2014, 136, 18149–18155. 10.1021/ja511341z. [DOI] [PubMed] [Google Scholar]
- Xie T.-Z.; Endres K. J.; Guo Z.; Ludlow J. M. III; Moorefield C. N.; Saunders M. J.; Wesdemiotis C.; Newkome G. R. Controlled Interconversion of Superposed-Bistriangle, Octahedron, and Cuboctahedron Cages Constructed Using a Single, Terpyridinyl-Based Polyligand and Zn2+. J. Am. Chem. Soc. 2016, 138, 12344–12347. 10.1021/jacs.6b07969. [DOI] [PubMed] [Google Scholar]
- Endo K.; Ube H.; Shionoya M. Multi-Stimuli-Responsive Interconversion between Bowl- and Capsule-Shaped Self-Assembled Zinc(II) Complexes. J. Am. Chem. Soc. 2020, 142, 407–416. 10.1021/jacs.9b11099. [DOI] [PubMed] [Google Scholar]
- Nakamura T.; Ube H.; Shiro M.; Shionoya M. A Self-Assembled Multiporphyrin Cage Complex through Three Different Zinc(II) Center Formation under Well-Balanced Aqueous Conditions. Angew. Chem., Int. Ed. 2013, 52, 720–723. 10.1002/anie.201208040. [DOI] [PubMed] [Google Scholar]
- Rizzuto F. J.; Pröhm P.; Plajer A. J.; Greenfield J. L.; Nitschke J. R. Hydrogen-Bond-Assisted Symmetry Breaking in a Network of Chiral Metal-Organic Assemblies. J. Am. Chem. Soc. 2019, 141, 1707–1715. 10.1021/jacs.8b12323. [DOI] [PubMed] [Google Scholar]
- Sørensen A.; Castilla A. M.; Ronson T. K.; Pittelkow M.; Nitschke J. R. Chemical Signals Turn on Guest Binding through Structural Reconfiguration of Triangular Helicates. Angew. Chem., Int. Ed. 2013, 52, 11273–11277. 10.1002/anie.201305245. [DOI] [PubMed] [Google Scholar]
- Zhan Y.-Y.; Kojima T.; Ishii K.; Takahashi S.; Haketa Y.; Maeda H.; Uchiyama S.; Hiraoka S. Temperature-controlled Repeatable Scrambling and Induced-Sorting of Building Blocks between Cubic Assemblies. Nat. Commun. 2019, 10, 1440. 10.1038/s41467-019-09495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M.; Nakagawa J.; Kumazawa K.; Nagao M.; Kawano M.; Ozeki T.; Fujita M. Discrete Stacking of Large Aromatic Molecules within Organic-Pillared Coordination Cages. Angew. Chem., Int. Ed. 2005, 44, 1810–1813. 10.1002/anie.200462171. [DOI] [PubMed] [Google Scholar]
- Wang S.; Sawada T.; Ohara K.; Yamaguchi K.; Fujita M. Capsule-Capsule Conversion by Guest Encapsulation. Angew. Chem., Int. Ed. 2016, 55, 2063–2066. 10.1002/anie.201509278. [DOI] [PubMed] [Google Scholar]
- Wang S.; Sawada T.; Fujita M. Capsule-Bowl Conversion Triggered by a Guest Reaction. Chem. Commun. 2016, 52, 11653–11656. 10.1039/C6CC06551A. [DOI] [PubMed] [Google Scholar]
- Hiraoka S.; Fujita M. Guest-Selected Formation of Pd(II)-Linked Cages from a Prototypical Dynamic Library. J. Am. Chem. Soc. 1999, 121, 10239–10240. 10.1021/ja990733n. [DOI] [Google Scholar]
- Wood D. M.; Meng W.; Ronson T. K.; Stefankiewicz A. R.; Sanders J. K. M.; Nitschke J. R. Guest-Induced Transformation of a Porphyrin-Edged FeII4L6 Capsule into a CuIFeII2L4 Fullerene Receptor. Angew. Chem., Int. Ed. 2015, 54, 3988–3992. 10.1002/anie.201411985. [DOI] [PubMed] [Google Scholar]
- Rizzuto F. J.; Nitschke J. R. Stereochemical Plasticity Modulates Cooperative Binding in a CoII12L6 Cuboctahedron. Nat. Chem. 2017, 9, 903–908. 10.1038/nchem.2758. [DOI] [PubMed] [Google Scholar]
- Müller I. M.; Möller D. Rational Design of a Coordination Cage with a Trigonal-Bipyramidal Shape Constructed from 33 Building Units. Angew. Chem., Int. Ed. 2005, 44, 2969–2973. 10.1002/anie.200463034. [DOI] [PubMed] [Google Scholar]
- Guo J.; Chang Q.; Liu Z.; Wang Y.; Liu C.; Wang M.; Huang D.; Chen G.; Zhao H.; Wang W.; Fang X. How to Not Build a Cage: Endohedral Functionalization of Polyoxometalate-Based Metal-Organic Polyhedra. Chem. Sci. 2021, 12, 7361–7368. 10.1039/D1SC01243F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M.; White K. F.; White J. M.; Abrahams B. F.; Donnelly P. S. Guest-induced Assembly of Bis(thiosemicarbazonato) Zinc(II) Coordination Nanotubes. Angew. Chem.. Int. Ed. 2017, 56, 8370–8374. 10.1002/anie.201701596. [DOI] [PubMed] [Google Scholar]
- Yamashina M.; Yuki T.; Sei Y.; Akita M.; Yoshizawa M. Anisotropic Expansion of an M2L4 Coordination Capsule: Host Capability and Frame Rearrangement. Chem. - Eur. J. 2015, 21, 4200–4204. 10.1002/chem.201406445. [DOI] [PubMed] [Google Scholar]
- Tsutsui T.; Catti L.; Yoza K.; Yoshizawa M. An Atropisomeric M2L4 Cage Mixture Displaying Guest-Induced Convergence and Strong Guest Emission in Water. Chem. Sci. 2020, 11, 8145–8150. 10.1039/D0SC03223A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell I. A.; Smulders M. M. J.; Clegg J. K.; Hristova Y. R.; Breiner B.; Thoburn J. D.; Nitschke J. R. Anion-Induced Reconstitution of a Self-Assembling System to Express a Chloride-Binding Co10L15 Pentagonal Prism. Nat. Chem. 2012, 4, 751–756. 10.1038/nchem.1407. [DOI] [PubMed] [Google Scholar]
- Ayme J.-F.; Beves J. E.; Campbell C. J.; Gil-Ramírez G.; Leigh D. A.; Stephens A. J. Strong and Selective Anion Binding within the Central Cavity of Molecular Knots and Links. J. Am. Chem. Soc. 2015, 137, 9812–9815. 10.1021/jacs.5b06340. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Stephens A. J.; Lemonnier J.-F.; Pirvu L.; Vitorica-Yrezabal I. J.; Robinson C. J.; Leigh D. A. Coordination Chemistry of a Molecular Pentafoil Knot. J. Am. Chem. Soc. 2019, 141, 3952–3958. 10.1021/jacs.8b12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos V.; Stephens A. J.; Jaramillo-Garcia J.; Nussbaumer A. L.; Woltering S. L.; Valero A.; Lemonnier J.-F.; Vitorica-Yrezabal I. J.; Leigh D. A. Allosteric Initiation and Regulation of Catalysis with a Molecular Knot. Science 2016, 352, 1555–1559. 10.1126/science.aaf3673. [DOI] [PubMed] [Google Scholar]
- Riddell I. A.; Hristova Y. R.; Clegg J. K.; Wood C. S.; Breiner B.; Nitschke J. R. Five Discrete Multinuclear Metal-Organic Assemblies from One Ligand: Deciphering the Effects of Different Templates. J. Am. Chem. Soc. 2013, 135, 2723–2733. 10.1021/ja311285b. [DOI] [PubMed] [Google Scholar]
- Riddell I. A.; Ronson T. K.; Clegg J. K.; Wood C. S.; Bilbeisi R. A.; Nitschke J. R. Cation- and Anion-Exchanges Induce Multiple Distinct Rearrangements within Metallosupramolecular Architectures. J. Am. Chem. Soc. 2014, 136, 9491–9498. 10.1021/ja504748g. [DOI] [PubMed] [Google Scholar]
- Riddell I. A.; Ronson T. K.; Nitschke J. R. Mutual Stabilisation between MII4L6 Tetrahedra and MIIX42- Metallate Guests. Chem. Sci. 2015, 6, 3533–3537. 10.1039/C5SC01083G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes C. J. E.; Zhu J.; Chimerel C.; Hernández-Ainsa S.; Riddell I. A.; Ronson T. K.; Keyser U. F.; Nitschke J. R. Blockable Zn10L15 Ion Channels through Subcomponent Self-Assembly. Angew. Chem., Int. Ed. 2017, 56, 15388–15392. 10.1002/anie.201709544. [DOI] [PubMed] [Google Scholar]
- Chifotides H. T.; Giles I. D.; Dunbar K. R. Supramolecular Architectures with π-Acidic 3,6-Bis(2-pyridyl)-1,2,4,5-tetrazine Cavities: Role of Anion-π Interactions in the Remarkable Stability of Fe(II) Metallacycles in Solution. J. Am. Chem. Soc. 2013, 135, 3039–3055. 10.1021/ja3082473. [DOI] [PubMed] [Google Scholar]
- Yu H.-J.; Liu Z.-M.; Pan M.; Wu K.; Wei Z.-W.; Xu Y.-W.; Fan Y.-N.; Wang H.-P.; Su C.-Y. Elucidating Anion-Dependent Formation and Conversion of Pd2L4 and Pd3L6 Metal-Organic Cages by Complementary Techniques. Eur. J. Inorg. Chem. 2018, 2018, 80–85. 10.1002/ejic.201701319. [DOI] [Google Scholar]
- Klein C.; Gütz C.; Bogner M.; Topić F.; Rissanen K.; Lützen A. A New Structural Motif for an Enantiomerically Pure Metallosupramolecular Pd4L8 Aggregate by Anion Templating. Angew. Chem.. Int. Ed. 2014, 53, 3739–3742. 10.1002/anie.201400626. [DOI] [PubMed] [Google Scholar]
- Sun X.; Johnson D. W.; Caulder D. L.; Raymond K. N.; Wong E. H. Rational Design and Assembly of M2M′3L6 Supramolecular Clusters with C3h Symmetry by Exploiting Incommensurate Symmetry Numbers. J. Am. Chem. Soc. 2001, 123, 2752–2763. 10.1021/ja0029376. [DOI] [PubMed] [Google Scholar]
- Sun X.; Johnson D. W.; Caulder D. L.; Powers R. E.; Raymond K. N.; Wong E. H. Exploiting Incommensurate Symmetry Numbers: Rational Design and Assembly of M2M′3L6 Supramolecular Clusters with C3h Symmetry. Angew. Chem., Int. Ed. 1999, 38, 1303–1307. . [DOI] [PubMed] [Google Scholar]
- Wu H.-B.; Wang Q.-M. Construction of Heterometallic Cages with Tripodal Metalloligands. Angew. Chem., Int. Ed. 2009, 48, 7343–7345. 10.1002/anie.200903575. [DOI] [PubMed] [Google Scholar]
- Li X.; Wu J.; He C.; Zhang R.; Duan C. Multicomponent Self-Assembly of a Pentanuclear Ir-Zn Heterometal-Organic Polyhedron for Carbon Dioxide Fixation and Sulfite Sequestration. Chem. Commun. 2016, 52, 5104–5107. 10.1039/C6CC00064A. [DOI] [PubMed] [Google Scholar]
- Li X.; Wu J.; Chen L.; Zhong X.; He C.; Zhang R.; Duan C. Engineering an Iridium-Containing Metal-Organic Molecular Capsule for Induced-Fit Geometrical Conversion and Dual Catalysis. Chem. Commun. 2016, 52, 9628–9631. 10.1039/C6CC04647A. [DOI] [PubMed] [Google Scholar]
- Sanz S.; O’Connor H. M.; Martí-Centelles V.; Comar P.; Pitak M. B.; Coles S. J.; Lorusso G.; Palacios E.; Evangelisti M.; Baldansuren A.; Chilton N. F.; Weihe H.; McInnes E. J. L.; Lusby P. J.; Piligkos S.; Brechin E. K. [MIII2MII3]n+ Trigonal Bipyramidal Cages Based on Diamagnetic and Paramagnetic Metalloligands. Chem. Sci. 2017, 8, 5526–5535. 10.1039/C7SC00487G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison J. C.; Panzner M. J.; Custer P. D.; Reddy D. V.; Rinaldi P. L.; Tessier C. A.; Youngs W. J. Synthesis and Characterization of a Trigonal Bipyramidal Supramolecular Cage Based upon Rhodium and Platinum Metal Centers. Chem. Commun. 2006, 4644–4646. 10.1039/b608991g. [DOI] [PubMed] [Google Scholar]
- Hardy M.; Struch N.; Holstein J. J.; Schnakenburg G.; Wagner N.; Engeser M.; Beck J.; Clever G. H.; Lützen A. Dynamic Complex-to-Complex Transformations of Heterobimetallic Systems Influence the Cage Structure or Spin State of Iron(II) Ions. Angew. Chem., Int. Ed. 2020, 59, 3195–3200. 10.1002/anie.201914629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders M. M. J.; Jiménez A.; Nitschke J. R. Integrative Self-Sorting Synthesis of a Fe8Pt6L24 Cubic Cage. Angew. Chem., Int. Ed. 2012, 51, 6681–6685. 10.1002/anie.201202050. [DOI] [PubMed] [Google Scholar]
- Preston D.; Sutton J. J.; Gordon K. C.; Crowley J. D. A Nona-nuclear Heterometallic Pd3Pt6 “Donut”-Shaped Cage: Molecular Recognition and Photocatalysis. Angew. Chem., Int. Ed. 2018, 57, 8659–8663. 10.1002/anie.201804745. [DOI] [PubMed] [Google Scholar]
- Metherell A. J.; Ward M. D. Stepwise Synthesis of a Ru4Cd4 Coordination Cage Using Inert and Labile Subcomponents: Introduction of Redox Activity at Specific Sites. Chem. Commun. 2014, 50, 6330–6332. 10.1039/C4CC02627F. [DOI] [PubMed] [Google Scholar]
- Metherell A. J.; Ward M. D. Stepwise Assembly of an Adamantoid Ru4Ag6 Cage by Control of Metal Coordination Geometry at Specific Sites. Chem. Commun. 2014, 50, 10979–10982. 10.1039/C4CC05421K. [DOI] [PubMed] [Google Scholar]
- Wragg A. B.; Metherell A. J.; Cullen W.; Ward M. D. Stepwise Assembly of Mixed-Metal Coordination Cages Containing both Kinetically Inert and Kinetically Labile Metal Ions: Introduction of Metal-Centred Redox and Photophysical Activity at Specific Sites. Dalton Trans. 2015, 44, 17939–17949. 10.1039/C5DT02957K. [DOI] [PubMed] [Google Scholar]
- Metherell A. J.; Ward M. D. Imposing Control on Self-Assembly: Rational Design and Synthesis of a Mixed-Metal, Mixed-Ligand Coordination Cage Containing Four Types of Component. Chem. Sci. 2016, 7, 910–915. 10.1039/C5SC03526K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherell A. J.; Cullen W.; Stephenson A.; Hunter C. A.; Ward M. D. Fac and mer Isomers of Ru(II) Tris(pyrazolyl-pyridine) Complexes as Models for the Vertices of Coordination Cages: Structural Characterisation and Hydrogen-bonding Characteristics. Dalton Trans. 2014, 43, 71–84. 10.1039/C3DT52479E. [DOI] [PubMed] [Google Scholar]
- Li H.; Han Y.-F.; Lin Y.-J.; Guo Z.-W.; Jin G.-X. Stepwise Construction of Discrete Heterometallic Coordination Cages Based on Self-Sorting Strategy. J. Am. Chem. Soc. 2014, 136, 2982–2985. 10.1021/ja412667t. [DOI] [PubMed] [Google Scholar]
- Li J.-R.; Zhou H.-C. Bridging-Ligand-Substitution Strategy for the Preparation of Metal-Organic Polyhedra. Nat. Chem. 2010, 2, 893–898. 10.1038/nchem.803. [DOI] [PubMed] [Google Scholar]
- Tranchemontagne D. J.; Ni Z.; O’Keeffe M.; Yaghi O. M. Reticular Chemistry of Metal-Organic Polyhedra. Angew. Chem.. Int. Ed. 2008, 47, 5136–5147. 10.1002/anie.200705008. [DOI] [PubMed] [Google Scholar]
- Byrne K.; Zubair M.; Zhu N.; Zhou X.-P.; Fox D. S.; Zhang H.; Twamley B.; Lennox M. J.; Düren T.; Schmitt W. Ultra-Large Supramolecular Coordination Cages Composed of Endohedral Archimedean and Platonic Bodies. Nat. Commun. 2017, 8, 15268. 10.1038/ncomms15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter J. P.; McTernan C. T.; Ronson T. K.; Nitschke J. R. Anion Pairs Template a Trigonal Prism with Disilver Vertices. J. Am. Chem. Soc. 2019, 141, 11409–11413. 10.1021/jacs.9b05432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatila E. M.; Twum E. B.; Karty J. A.; Flood A. H. Ion Pairing and Co-facial Stacking Drive High-Fidelity Bisulfate Assembly with Cyanostar Macrocyclic Hosts. Chem. - Eur. J. 2017, 23, 10652–10662. 10.1002/chem.201701763. [DOI] [PubMed] [Google Scholar]
- Minisci F.; Citterio A.; Giordano C. Electron-Transfer Processes: Peroxydisulfate, a Useful and Versatile Reagent in Organic Chemistry. Acc. Chem. Res. 1983, 16, 27–32. 10.1021/ar00085a005. [DOI] [Google Scholar]
- Wood C. S.; Ronson T. K.; Belenguer A. M.; Holstein J. J.; Nitschke J. R. Two-Stage Directed Self-Assembly of a Cyclic [3]Catenane. Nat. Chem. 2015, 7, 354–358. 10.1038/nchem.2205. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Su H.-F.; Tan Y.-Z.; Schein S.; Lin S.-C.; Liu W.; Wang S.-A.; Wang W.-G.; Tung C.-H.; Sun D.; Zheng L.-S. Assembly of Silver Trigons into a Buckyball-like Ag180 Nanocage. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 12132–12137. 10.1073/pnas.1711972114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTernan C. T.; Ronson T. K.; Nitschke J. R. Selective Anion Binding Drives the Formation of AgI8L6 and AgI12L6 Six-Stranded Helicates. J. Am. Chem. Soc. 2021, 143, 664–670. 10.1021/jacs.0c11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z.-Z.; Tian C.-B.; Sun Q.-F. Coordination-Assembled Molecular Cages with Metal Cluster Nodes. Chem. Rec. 2021, 21, 498–522. 10.1002/tcr.202000130. [DOI] [PubMed] [Google Scholar]
- Gong Y.; Tao Y.; Xu N.; Sun C.; Wang X.; Su Z. Two Polyoxovanadate-based Metal-Organic Polyhedra with Undiscovered “Near-miss Johnson Solid” Geometry. Chem. Commun. 2019, 55, 10701–10704. 10.1039/C9CC05984A. [DOI] [PubMed] [Google Scholar]
- Bao S.-J.; Xu Z.-M.; Ju Y.; Song Y.-L.; Wang H.; Niu Z.; Li X.; Braunstein P.; Lang J.-P. The Covalent and Coordination Co-driven Assembly of Supramolecular Octahedral Cages with Controllable Degree of Distortion. J. Am. Chem. Soc. 2020, 142, 13356–13361. 10.1021/jacs.0c07014. [DOI] [PubMed] [Google Scholar]
- Howlader P.; Bhandari P.; Chakraborty D.; Clegg J. K.; Mukherjee P. S. Self-Assembly of a Pd8 Macrocycle and Pd12 Homochiral Tetrahedral Cages Using Poly(tetrazolate) Linkers. Inorg. Chem. 2020, 59, 15454–15459. 10.1021/acs.inorgchem.0c02452. [DOI] [PubMed] [Google Scholar]
- Jiao J.; Tan C.; Li Z.; Liu Y.; Han X.; Cui Y. Design and Assembly of Chiral Coordination Cages for Asymmetric Sequential Reactions. J. Am. Chem. Soc. 2018, 140, 2251–2259. 10.1021/jacs.7b11679. [DOI] [PubMed] [Google Scholar]
- Tandon S.; Steuber F. W.; Kathalikkattil A. C.; Venkatesan M.; Watson G. W.; Schmitt W. Modulating Structural and Electronic Properties of Rare Archimedean and Johnson-Type Mn Cages. Inorg. Chem. 2021, 60, 8388–8393. 10.1021/acs.inorgchem.1c00984. [DOI] [PubMed] [Google Scholar]
- Altmann P. J.; Pöthig A. Pillarplexes: A Metal-Organic Class of Supramolecular Hosts. J. Am. Chem. Soc. 2016, 138, 13171–13174. 10.1021/jacs.6b08571. [DOI] [PubMed] [Google Scholar]
- Altmann P. J.; Pöthig A. A pH-Dependent, Mechanically Interlocked Switch: Organometallic [2]Rotaxane vs. Organic [3]Rotaxane. Angew. Chem., Int. Ed. 2017, 56, 15733–15736. 10.1002/anie.201709921. [DOI] [PubMed] [Google Scholar]
- Hiraoka S.; Harano K.; Tanaka T.; Shiro M.; Shionoya M. Quantitative Formation of Sandwich-Shaped Trinuclear Silver(I) Complexes and Dynamic Nature of Their P⇌M Flip Motion in Solution. Angew. Chem.. Int. Ed. 2003, 42, 5182–5185. 10.1002/anie.200351068. [DOI] [PubMed] [Google Scholar]
- Sun Q.-F.; Iwasa J.; Ogawa D.; Ishido Y.; Sato S.; Ozeki T.; Sei Y.; Yamaguchi K.; Fujita M. Self-Assembled M24L48 Polyhedra and Their Sharp Structural Switch upon Subtle Ligand Variation. Science 2010, 328, 1144–1147. 10.1126/science.1188605. [DOI] [PubMed] [Google Scholar]
- Fujita D.; Ueda Y.; Sato S.; Yokoyama H.; Mizuno N.; Kumasaka T.; Fujita M. Self-Assembly of M30L60 Icosidodecahedron. Chem. 2016, 1, 91–101. 10.1016/j.chempr.2016.06.007. [DOI] [Google Scholar]
- Bunzen J.; Iwasa J.; Bonakdarzadeh P.; Numata E.; Rissanen K.; Sato S.; Fujita M. Self-Assembly of M24L48 Polyhedra Based on Empirical Prediction. Angew. Chem., Int. Ed. 2012, 51, 3161–3163. 10.1002/anie.201108731. [DOI] [PubMed] [Google Scholar]
- Harris K.; Fujita D.; Fujita M. Giant Hollow MnL2n Spherical Complexes: Structure, Functionalisation and Applications. Chem. Commun. 2013, 49, 6703–6712. 10.1039/c3cc43191f. [DOI] [PubMed] [Google Scholar]
- Saha S.; Regeni I.; Clever G. H. Structure Relationships between Bis-Monodentate Ligands and Coordination Driven Self-Assemblies. Coord. Chem. Rev. 2018, 374, 1–14. 10.1016/j.ccr.2018.06.010. [DOI] [Google Scholar]
- Suzuki K.; Kawano M.; Fujita M. Solvato-Controlled Assembly of Pd3L6 and Pd4L8 Coordination “Boxes”. Angew. Chem., Int. Ed. 2007, 46, 2819–2822. 10.1002/anie.200605084. [DOI] [PubMed] [Google Scholar]
- Kishida N.; Matsumoto K.; Tanaka Y.; Akita M.; Sakurai H.; Yoshizawa M. Anisotropic Contraction of a Polyaromatic Capsule and its Cavity-Induced Compression Effect. J. Am. Chem. Soc. 2020, 142, 9599–9603. 10.1021/jacs.0c02932. [DOI] [PubMed] [Google Scholar]
- Fujita D.; Ueda Y.; Sato S.; Mizuno N.; Kumasaka T.; Fujita M. Self-Assembly of Tetravalent Goldberg Polyhedra from 144 Small Components. Nature 2016, 540, 563–566. 10.1038/nature20771. [DOI] [PubMed] [Google Scholar]
- Malay A. D.; Miyazaki N.; Biela A.; Chakraborti S.; Majsterkiewicz K.; Stupka I.; Kaplan C. S.; Kowalczyk A.; Piette B. M. A. G.; Hochberg G. K. A.; Wu D.; Wrobel T. P.; Fineberg A.; Kushwah M. S.; Kelemen M.; Vavpetič P.; Pelicon P.; Kukura P.; Benesch J. L. P.; Iwasaki K.; Heddle J. G. An Ultra-stable Gold-coordinated Protein Cage Displaying Reversible Assembly. Nature 2019, 569, 438–442. 10.1038/s41586-019-1185-4. [DOI] [PubMed] [Google Scholar]
- Liu D.; Chen M.; Li K.; Li Z.; Huang J.; Wang J.; Jiang Z.; Zhang Z.; Xie T.; Newkome G. R.; Wang P. Giant Truncated Metallo-Tetrahedron with Unexpected Supramolecular Aggregation Induced Emission Enhancement. J. Am. Chem. Soc. 2020, 142, 7987–7994. 10.1021/jacs.0c02366. [DOI] [PubMed] [Google Scholar]
- Liu D.; Chen M.; Li Y.; Shen Y.; Huang J.; Yang X.; Jiang Z.; Li X.; Newkome G. R.; Wang P. Vertical Assembly of Giant Double- and Triple-Decker Spoked Wheel Supramolecular Structures. Angew. Chem., Int. Ed. 2018, 57, 14116–14120. 10.1002/anie.201809819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow S. J.; Kasera S.; Rowland M. J.; del Barrio J.; Scherman O. A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. 10.1021/acs.chemrev.5b00341. [DOI] [PubMed] [Google Scholar]
- Ogoshi T.; Yamagishi T.-A.; Nakamoto Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 2016, 116, 7937–8002. 10.1021/acs.chemrev.5b00765. [DOI] [PubMed] [Google Scholar]
- Rieth S.; Hermann K.; Wang B.-Y.; Badjić J. D. Controlling the Dynamics of Molecular Encapsulation and Gating. Chem. Soc. Rev. 2011, 40, 1609–1622. 10.1039/C005254J. [DOI] [PubMed] [Google Scholar]
- Sakata Y.; Murata C.; Akine S. Anion-Capped Metallohost Allows Extremely Slow Guest Uptake and On-Demand Acceleration of Guest Exchange. Nat. Commun. 2017, 8, 16005. 10.1038/ncomms16005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivaud S.; Balandier J.-Y.; Chas M.; Allain M.; Goeb S.; Sallé M. A Metal-Directed Self-Assembled Electroactive Cage with Bis(pyrrolo)tetrathiafulvalene (BPTTF) Side Walls. J. Am. Chem. Soc. 2012, 134, 11968–11970. 10.1021/ja305451v. [DOI] [PubMed] [Google Scholar]
- Caskey D. C.; Yamamoto T.; Addicott C.; Shoemaker R. K.; Vacek J.; Hawkridge A. M.; Muddiman D. C.; Kottas G. S.; Michl J.; Stang P. J. Coordination-Driven Face-Directed Self-Assembly of Trigonal Prisms. Face-Based Conformational Chirality. J. Am. Chem. Soc. 2008, 130, 7620–7628. 10.1021/ja710715e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X.; Lin S.; Wang G.; Shang C.; Wang Z.; Liu K.; Fang Y.; Stang P. J. Self-Assembled Perylene Bisimide-Cored Trigonal Prism as an Electron-Deficient Host for C60 and C70 Driven by “Like Dissolves Like. J. Am. Chem. Soc. 2020, 142, 15950–15960. 10.1021/jacs.0c06623. [DOI] [PubMed] [Google Scholar]
- Bhat I. A.; Jain R.; Siddiqui M. M.; Saini D. K.; Mukherjee P. S. Water-Soluble Pd8L4 Self-Assembled Molecular Barrel as an Aqueous Carrier for Hydrophobic Curcumin. Inorg. Chem. 2017, 56, 5352–5360. 10.1021/acs.inorgchem.7b00449. [DOI] [PubMed] [Google Scholar]
- Bhandari P.; Modak R.; Bhattacharyya S.; Zangrando E.; Mukherjee P. S. Self-Assembly of Octanuclear PtII/PdII Coordination Barrels and Uncommon Structural Isomerization of a Photochromic Guest in Molecular Space. JACS Au 2021, 1, 2242–2248. 10.1021/jacsau.1c00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeb S.; Bivaud S.; Croué V.; Vajpayee V.; Allain M.; Sallé M. A Self-Assembled Electro-Active M8L4 Cage Based on Tetrathiafulvalene Ligands. Materials 2014, 7, 611–622. 10.3390/ma7010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S.; Venkateswarulu M.; Sahoo J.; Zangrando E.; De M.; Mukherjee P. S. Self-Assembled PtII8 Metallosupramolecular Tubular Cage as Dual Warhead Antibacterial Agent in Water. Inorg. Chem. 2020, 59, 12690–12699. 10.1021/acs.inorgchem.0c01777. [DOI] [PubMed] [Google Scholar]
- Cecot G.; Marmier M.; Geremia S.; De Zorzi R.; Vologzhanina A. V.; Pattison P.; Solari E.; Fadaei Tirani F.; Scopelliti R.; Severin K. The Intricate Structural Chemistry of MII2nLn-Type Assemblies. J. Am. Chem. Soc. 2017, 139, 8371–8381. 10.1021/jacs.7b04861. [DOI] [PubMed] [Google Scholar]
- Tang J.-H.; Ni R.; He Y.-Q.; Vanderlinden R. T.; Li Y.; Shi B.; Li Z.-Y.; Wang H.; Li X.; Sun Y.; Zhong Y.-W.; Stang P. J. Metal-Organic Pt(II) Hexagonal-Prism Macrocycles and their Photophysical Properties. Inorg. Chem. 2019, 58, 13376–13381. 10.1021/acs.inorgchem.9b02267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Bhadbhade M.; Donald W. A.; Iranmanesh H.; Moore E. G.; Yan H.; Beves J. E. Self-assembled Supramolecular Cages Containing Ruthenium(II) Polypyridyl Complexes. Chem. Commun. 2015, 51, 4465–4468. 10.1039/C4CC10292D. [DOI] [PubMed] [Google Scholar]
- Yamanoi Y.; Sakamoto Y.; Kusukawa T.; Fujita M.; Sakamoto S.; Yamaguchi K. Dynamic Assembly of Coordination Boxes from (en)Pd(II) Unit and a Rectangular Panel-Like Ligand: NMR, CSI-MS, and X-ray Studies. J. Am. Chem. Soc. 2001, 123, 980–981. 10.1021/ja003043o. [DOI] [PubMed] [Google Scholar]
- Cecot G.; Alameddine B.; Prior S.; De Zorzi R.; Geremia S.; Scopelliti R.; Fadaei F. T.; Solari E.; Severin K. Large Heterometallic Coordination Cages with Gyrobifastigium-Like Geometry. Chem. Commun. 2016, 52, 11243–11246. 10.1039/C6CC06066H. [DOI] [PubMed] [Google Scholar]
- Howlader P.; Mondal B.; Purba P. C.; Zangrando E.; Mukherjee P. S. Self-Assembled Pd(II) Barrels as Containers for Transient Merocyanine Form and Reverse Thermochromism of Spiropyran. J. Am. Chem. Soc. 2018, 140, 7952–7960. 10.1021/jacs.8b03946. [DOI] [PubMed] [Google Scholar]
- Cecot G.; Doll M. T.; Planes O. M.; Ramorini A.; Scopelliti R.; Fadaei-Tirani F.; Severin K. Cages vs. Prisms: Controlling the Formation of Metallosupramolecular Architectures with Ligand Side-Chains. Eur. J. Inorg. Chem. 2019, 2019, 2972–2976. 10.1002/ejic.201900483. [DOI] [Google Scholar]
- Bhat I. A.; Devaraj A.; Zangrando E.; Mukherjee P. S. A Discrete Self-Assembled Pd12 Triangular Orthobicupola Cage and its Use for Intramolecular Cycloaddition. Chem. - Eur. J. 2018, 24, 13938–13946. 10.1002/chem.201803039. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S.; Ali S. R.; Venkateswarulu M.; Howlader P.; Zangrando E.; De M.; Mukherjee P. S. Self-Assembled Pd12 Coordination Cage as Photoregulated Oxidase-Like Nanozyme. J. Am. Chem. Soc. 2020, 142, 18981–18989. 10.1021/jacs.0c09567. [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B.; Kumar A.; Bharti A. C. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 2003, 23, 363–398. [PubMed] [Google Scholar]
- Priyadarsini K. I. Photophysics, Photochemistry and Photobiology of Curcumin: Studies from Organic Solutions, Bio-mimetics and Living Cells. J. Photochem. Photobiol., C 2009, 10, 81–95. 10.1016/j.jphotochemrev.2009.05.001. [DOI] [Google Scholar]
- Anand P.; Kunnumakkara A. B.; Newman R. A.; Aggarwal B. B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharmaceutics 2007, 4, 807–818. 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Howlader P.; Das P.; Zangrando E.; Mukherjee P. S. Urea-Functionalized Self-Assembled Molecular Prism for Heterogeneous Catalysis in Water. J. Am. Chem. Soc. 2016, 138, 1668–1676. 10.1021/jacs.5b12237. [DOI] [PubMed] [Google Scholar]
- Mu C.; Zhang Z.; Hou Y.; Liu H.; Ma L.; Li X.; Ling S.; He G.; Zhang M. Tetraphenylethylene-Based Multicomponent Emissive Metallacages as Solid-State Fluorescent Materials. Angew. Chem., Int. Ed. 2021, 60, 12293–12297. 10.1002/anie.202100463. [DOI] [PubMed] [Google Scholar]
- Meng W.; Clegg J. K.; Nitschke J. R. Transformative Binding and Release of Gold Guests from a Self-Assembled Cu8L4 Tube. Angew. Chem., Int. Ed. 2012, 51, 1881–1884. 10.1002/anie.201108450. [DOI] [PubMed] [Google Scholar]
- Meng W.; League A. B.; Ronson T. K.; Clegg J. K.; Isley W. C.; Semrouni D.; Gagliardi L.; Cramer C. J.; Nitschke J. R. Empirical and Theoretical Insights into the Structural Features and Host-Guest Chemistry of M8L4 Tube Architectures. J. Am. Chem. Soc. 2014, 136, 3972–3980. 10.1021/ja412964r. [DOI] [PubMed] [Google Scholar]
- Cook T. R.; Zheng Y.-R.; Stang P. J. Metal-Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal-Organic Materials. Chem. Rev. 2013, 113, 734–777. 10.1021/cr3002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivaud S.; Goeb S.; Croué V.; Dron P. I.; Allain M.; Sallé M. Self-Assembled Containers Based on Extended Tetrathiafulvalene. J. Am. Chem. Soc. 2013, 135, 10018–10021. 10.1021/ja404072w. [DOI] [PubMed] [Google Scholar]
- Bivaud S.; Goeb S.; Croué V.; Allain M.; Pop F.; Sallé M. Tuning the Size of a Redox-Active Tetrathiafulvalene-Based Self-Assembled Ring. Beilstein J. Org. Chem. 2015, 11, 966–971. 10.3762/bjoc.11.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalóki G.; Krykun S.; Croué V.; Allain M.; Morille Y.; Aubriet F.; Carré V.; Voitenko Z.; Goeb S.; Sallé M. Redox-Driven Transformation of a Discrete Molecular Cage into an Infinite 3D Coordination Polymer. Chem. - Eur. J. 2018, 24, 11273–11277. 10.1002/chem.201801653. [DOI] [PubMed] [Google Scholar]
- Croué V.; Goeb S.; Szalóki G.; Allain M.; Sallé M. Reversible Guest Uptake/Release by Redox-Controlled Assembly/Disassembly of a Coordination Cage. Angew. Chem., Int. Ed. 2016, 55, 1746–1750. 10.1002/anie.201509265. [DOI] [PubMed] [Google Scholar]
- Zhu R.; Regeni I.; Holstein J. J.; Dittrich B.; Simon M.; Prévost S.; Gradzielski M.; Clever G. H. Catenation and Aggregation of Multi-Cavity Coordination Cages. Angew. Chem., Int. Ed. 2018, 57, 13652–13656. 10.1002/anie.201806047. [DOI] [PubMed] [Google Scholar]
- Yazaki K.; Akita M.; Prusty S.; Chand D. K.; Kikuchi T.; Sato H.; Yoshizawa M. Polyaromatic Molecular Peanuts. Nat. Commun. 2017, 8, 15914. 10.1038/ncomms15914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K.; Kusaba S.; Tanaka Y.; Sei Y.; Akita M.; Aritani K.; Haga M.-a.; Yoshizawa M. A Peanut-Shaped Polyaromatic Capsule: Solvent-Dependent Transformation and Electronic Properties of a Non-Contacted Fullerene Dimer. Angew. Chem., Int. Ed. 2019, 58, 8463–8467. 10.1002/anie.201903117. [DOI] [PubMed] [Google Scholar]
- Samantray S.; Krishnaswamy S.; Chand D. K. Self-Assembled Conjoined-Cages. Nat. Commun. 2020, 11, 880. 10.1038/s41467-020-14703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi M.; Biradha K.; Fujita M. Quantitative Formation of Coordination Nanotubes Templated by Rodlike Guests. J. Am. Chem. Soc. 1999, 121, 7457–7458. 10.1021/ja991301f. [DOI] [Google Scholar]
- Aoyagi M.; Tashiro S.; Tominaga M.; Biradha K.; Fujita M. Spectroscopic and Crystallographic Studies on the Stability of Self-Assembled Coordination Nanotubes. Chem. Commun. 2002, 2036–2037. 10.1039/B205194J. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T.; Tashiro S.; Tominaga M.; Kawano M.; Ozeki T.; Fujita M. A 3.5-nm Coordination Nanotube. J. Am. Chem. Soc. 2004, 126, 10818–10819. 10.1021/ja047048n. [DOI] [PubMed] [Google Scholar]
- Takeda N.; Umemoto K.; Yamaguchi K.; Fujita M. A Nanometre-sized Hexahedral Coordination Capsule Assembled from 24 Components. Nature 1999, 398, 794–796. 10.1038/19734. [DOI] [Google Scholar]
- Umemoto K.; Tsukui H.; Kusukawa T.; Biradha K.; Fujita M. Molecular Paneling by Coordination: An M15L6 Hexahedral Molecular Capsule having Clefts for Reversible Guest Inclusion. Angew. Chem., Int. Ed. 2001, 40, 2620–2622. . [DOI] [PubMed] [Google Scholar]
- Umemoto K.; Yamaguchi K.; Fujita M. Molecular Paneling via Coordination: Guest-Controlled Assembly of Open Cone and Tetrahedron Structures from Eight Metals and Four Ligands. J. Am. Chem. Soc. 2000, 122, 7150–7151. 10.1021/ja001411i. [DOI] [Google Scholar]
- Bhat I. A.; Zangrando E.; Mukherjee P. S. Coordination-Driven Self-Assembly of Discrete Molecular Nanotubular Architectures. Inorg. Chem. 2019, 58, 11172–11179. 10.1021/acs.inorgchem.9b01763. [DOI] [PubMed] [Google Scholar]
- Bhat I. A.; Samanta D.; Mukherjee P. S. A Pd24 Pregnant Molecular Nanoball: Self-Templated Stellation by Precise Mapping of Coordination Sites. J. Am. Chem. Soc. 2015, 137, 9497–9502. 10.1021/jacs.5b06628. [DOI] [PubMed] [Google Scholar]
- Jeong K. S.; Kim Y. S.; Kim Y. J.; Lee E.; Yoon J. H.; Park W. H.; Park Y. W.; Jeon S.-J.; Kim Z. H.; Kim J.; Jeong N. Lanthanitin: A Chiral Nanoball Encapsulating 18 Lanthanum Ions by Ferritin-Like Assembly. Angew. Chem., Int. Ed. 2006, 45, 8134–8138. 10.1002/anie.200603622. [DOI] [PubMed] [Google Scholar]
- He C.; Wang J.; Zhao L.; Liu T.; Zhang J.; Duan C. A Photoactive Basket-Like Metal-Organic Tetragon Worked as an Enzymatic Molecular Flask for Light Driven H2 Production. Chem. Commun. 2013, 49, 627–629. 10.1039/C2CC37853A. [DOI] [PubMed] [Google Scholar]
- Xu J.; Raymond K. N. Lord of the Rings: An Octameric Lanthanum Pyrazolonate Cluster. Angew. Chem., Int. Ed. 2000, 39, 2745–2747. . [DOI] [PubMed] [Google Scholar]
- Tan Y. B.; Okayasu Y.; Katao S.; Nishikawa Y.; Asanoma F.; Yamada M.; Yuasa J.; Kawai T. Visible Circularly Polarized Luminescence of Octanuclear Circular Eu(III) Helicate. J. Am. Chem. Soc. 2020, 142, 17653–17661. 10.1021/jacs.0c08229. [DOI] [PubMed] [Google Scholar]
- Johnson D. W.; Xu J.; Saalfrank R. W.; Raymond K. N. Self-Assembly of a Three-Dimensional [Ga6(L2)6] Metal-Ligand “Cylinder”. Angew. Chem., Int. Ed. 1999, 38, 2882–2885. . [DOI] [PubMed] [Google Scholar]
- Saalfrank R. W.; Glaser H.; Demleitner B.; Hampel F.; Chowdhry M. M.; Schünemann V.; Trautwein A. X.; Vaughan G. B. M.; Yeh R.; Davis A. V.; Raymond K. N. Self-Assembly of Tetrahedral and Trigonal Antiprismatic Clusters [Fe4(L4)4] and [Fe6(L5)6] on the Basis of Trigonal Tris-Bidentate Chelators. Chem. - Eur. J. 2002, 8, 493–497. . [DOI] [PubMed] [Google Scholar]
- Chen B.; Holstein J. J.; Horiuchi S.; Hiller W. G.; Clever G. H. Pd(II) Coordination Sphere Engineering: Pyridine Cages, Quinoline Bowls, and Heteroleptic Pills Binding One or Two Fullerenes. J. Am. Chem. Soc. 2019, 141, 8907–8913. 10.1021/jacs.9b02207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar A. K.; Chakrabarty R.; Mostafa G.; Mukherjee P. S. Self-assembly of a Nanoscopic Pt12Fe12 Heterometallic Open Molecular Box Containing Six Porphyrin Walls. Angew. Chem., Int. Ed. 2008, 47, 8455–8459. 10.1002/anie.200803543. [DOI] [PubMed] [Google Scholar]
- Tang X.; Chu D.; Gong W.; Cui Y.; Liu Y. Metal-Organic Cages with Missing Linker Defects. Angew. Chem., Int. Ed. 2021, 60, 9099–9105. 10.1002/anie.202017244. [DOI] [PubMed] [Google Scholar]
- Rizzuto F. J.; Kieffer M.; Nitschke J. R. Quantified Structural Speciation in Self-Sorted CoII6L4 Cage Systems. Chem. Sci. 2018, 9, 1925–1930. 10.1039/C7SC04927G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson T. K.; Wang Y.; Baldridge K.; Siegel J. S.; Nitschke J. R. An S10-Symmetric 5-Fold Interlocked [2]Catenane. J. Am. Chem. Soc. 2020, 142, 10267–10272. 10.1021/jacs.0c03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X.; He C.; Zhao L.; Duan C. Photochemical Properties of Host-Guest Supramolecular Systems with Structurally Confined Metal-Organic Capsules. Acc. Chem. Res. 2019, 52, 100–109. 10.1021/acs.accounts.8b00463. [DOI] [PubMed] [Google Scholar]
- Wang Q.-Q.; Gonell S.; Leenders S. H. A. M.; Dürr M.; Ivanović-Burmazović I.; Reek J. N. H. Self-Assembled Nanospheres with Multiple Endohedral Binding Sites Pre-Organize Catalysts and Substrates for Highly Efficient Reactions. Nat. Chem. 2016, 8, 225–230. 10.1038/nchem.2425. [DOI] [PubMed] [Google Scholar]
- Liu W.; Stoddart J. F. Emergent Behaviour in Nanoconfined Molecular Containers. Chem. 2021, 7, 919–947. 10.1016/j.chempr.2021.02.016. [DOI] [Google Scholar]
- Wang Y.; Sun Y.; Shi P.; Sartin M. M.; Lin X.; Zhang P.; Fang H.; Peng P.; Tian Z.; Cao X. Chaperone-Like Chiral Cages for Catalyzing Enantioselective Supramolecular Polymerization. Chem. Sci. 2019, 10, 8076–8082. 10.1039/C9SC02412C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Yang L.; Li X.; Wang J.; Liu X.; He C. Supramolecular Catalysis of Acyl Transfer within Zinc Porphyrin-Based Metal-Organic Cages. Inorg. Chem. 2021, 60, 8802–8810. 10.1021/acs.inorgchem.1c00745. [DOI] [PubMed] [Google Scholar]
- Ikbal S. A.; Colomban C.; Zhang D.; Delecluse M.; Brotin T.; Dufaud V.; Dutasta J.-P.; Sorokin A. B.; Martinez A. Bioinspired Oxidation of Methane in the Confined Spaces of Molecular Cages. Inorg. Chem. 2019, 58, 7220–7228. 10.1021/acs.inorgchem.9b00199. [DOI] [PubMed] [Google Scholar]
- Syntrivanis L.-D.; Némethová I.; Schmid D.; Levi S.; Prescimone A.; Bissegger F.; Major D. T.; Tiefenbacher K. Four-Step Access to the Sesquiterpene Natural Product Presilphiperfolan-1β-ol and Unnatural Derivatives via Supramolecular Catalysis. J. Am. Chem. Soc. 2020, 142, 5894–5900. 10.1021/jacs.0c01464. [DOI] [PubMed] [Google Scholar]
- Wu G.; Chen Y.; Fang S.; Tong L.; Shen L.; Ge C.; Pan Y.; Shi X.; Li H. A Self-Assembled Cage for Wide-Scope Chiral Recognition in Water. Angew. Chem., Int. Ed. 2021, 60, 16594–16599. 10.1002/anie.202104164. [DOI] [PubMed] [Google Scholar]
- Jia C.; Zuo W.; Yang D.; Chen Y.; Cao L.; Custelcean R.; Hostaš J.; Hobza P.; Glaser R.; Wang Y.-Y.; Yang X.-J.; Wu B. Selective Binding of Choline by a Phosphate-Coordination-Based Triple Helicate Featuring an Aromatic Box. Nat. Commun. 2017, 8, 938. 10.1038/s41467-017-00915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F.; Hupatz H.; Yang L.-P.; Schröder H. V.; Li D.-H.; Xin S.; Lentz D.; Witte F.; Xie X.; Paulus B.; Schalley C. A.; Jiang W. Naphthocage: A Flexible yet Extremely Strong Binder for Singly Charged Organic Cations. J. Am. Chem. Soc. 2019, 141, 4468–4473. 10.1021/jacs.9b00445. [DOI] [PubMed] [Google Scholar]
- Chen G.-H.; He Y.-P.; Liang F.-P.; Zhang L.; Zhang J. A Green Separation Process of Ag via a Ti4(embonate)6 Cage. Dalton Trans. 2020, 49, 17194–17199. 10.1039/D0DT03214J. [DOI] [PubMed] [Google Scholar]
- Sepehrpour H.; Fu W.; Sun Y.; Stang P. J. Biomedically Relevant Self-Assembled Metallacycles and Metallacages. J. Am. Chem. Soc. 2019, 141, 14005–14020. 10.1021/jacs.9b06222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch W. M.; Clever G. H. Integrative Self-Sorting of Coordination Cages Based on ‘Naked’ Metal Ions. Chem. Commun. 2017, 53, 8506–8516. 10.1039/C7CC03379F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardo E.; Turcani L.; Miklitz M.; Jelfs K. E. An Evolutionary Algorithm for the Discovery of Porous Organic Cages. Chem. Sci. 2018, 9, 8513–8527. 10.1039/C8SC03560A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcani L.; Greenaway R. L.; Jelfs K. E. Machine Learning for Organic Cage Property Prediction. Chem. Mater. 2019, 31, 714–727. 10.1021/acs.chemmater.8b03572. [DOI] [Google Scholar]
- Berardo E.; Greenaway R. L.; Turcani L.; Alston B. M.; Bennison M. J.; Miklitz M.; Clowes R.; Briggs M. E.; Cooper A. I.; Jelfs K. E. Computationally-Inspired Discovery of an Unsymmetrical Porous Organic Cage. Nanoscale 2018, 10, 22381–22388. 10.1039/C8NR06868B. [DOI] [PubMed] [Google Scholar]
- Abet V.; Szczypiński F. T.; Little M. A.; Santolini V.; Jones C. D.; Evans R.; Wilson C.; Wu X.; Thorne M. F.; Bennison M. J.; Cui P.; Cooper A. I.; Jelfs K. E.; Slater A. G. Inducing Social Self-Sorting in Organic Cages to Tune the Shape of the Internal Cavity. Angew. Chem., Int. Ed. 2020, 59, 16755–16763. 10.1002/anie.202007571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarzia A.; Lewis J. E. M.; Jelfs K. E. High-throughput Computational Evaluation of Low Symmetry Pd2L4 Cages to Aid in System Design. Angew. Chem., Int. Ed. 2021, 60, 20879–20887. 10.1002/anie.202106721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T. A.; Martí-Centelles V.; Wang J.; Lusby P. J.; Duarte F. Rationalizing the Activity of an “Artificial Diels-Alderase”: Establishing Efficient and Accurate Protocols for Calculating Supramolecular Catalysis. J. Am. Chem. Soc. 2020, 142, 1300–1310. 10.1021/jacs.9b10302. [DOI] [PubMed] [Google Scholar]
- Greenaway R. L.; Jelfs K. E. High-Throughput Approaches for the Discovery of Supramolecular Organic Cages. ChemPlusChem 2020, 85, 1813–1823. 10.1002/cplu.202000445. [DOI] [PubMed] [Google Scholar]
- Porwol L.; Kowalski D. J.; Henson A.; Long D.-L.; Bell N. L.; Cronin L. An Autonomous Chemical Robot Discovers the Rules of Inorganic Coordination Chemistry without Prior Knowledge. Angew. Chem., Int. Ed. 2020, 59, 11256–11261. 10.1002/anie.202000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway R. L.; Santolini V.; Bennison M. J.; Alston B. M.; Pugh C. J.; Little M. A.; Miklitz M.; Eden-Rump E. G. B.; Clowes R.; Shakil A.; Cuthbertson H. J.; Armstrong H.; Briggs M. E.; Jelfs K. E.; Cooper A. I. High-Throughput Discovery of Organic Cages and Catenanes Using Computational Screening Fused with Robotic Synthesis. Nat. Commun. 2018, 9, 2849. 10.1038/s41467-018-05271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]