Abstract
The thermophilic aerobic bacterium Bacillus thermoleovorans Hamburg 2 grows at 60°C on naphthalene as the sole source of carbon and energy. In batch cultures, an effective substrate degradation was observed. The carbon balance, including naphthalene, metabolites, biomass, and CO2, was determined by the application of [1-13C]naphthalene. The incorporation of naphthalene-derived carbon into the bulk biomass as well as into specified biomass fractions such as fatty acids and amino acids was confirmed by coupled gas chromatography-mass spectrometry (GC-MS) and isotope analyses. Metabolites were characterized by GC-MS; the established structures allow tracing the degradation pathway under thermophilic conditions. Apart from typical metabolites of naphthalene degradation known from mesophiles, intermediates such as 2,3-dihydroxynaphthalene, 2-carboxycinnamic acid, and phthalic and benzoic acid were identified for the pathway of this bacterium. These compounds indicate that naphthalene degradation by the thermophilic B. thermoleovorans differs from the known pathways found for mesophilic bacteria.
The naphthalene metabolism of mesophilic microorganisms under aerobic conditions has been intensely investigated, and detailed information has been presented on degradation rates, metabolic pathways, and the involved enzymes (7, 8, 11, 25). In contrast, little is known about the metabolism of naphthalene or other polycyclic aromatic hydrocarbons (PAH) by thermophilic bacteria. Several studies on the growth of thermophilic microorganisms on aromatic compounds such as benzoic acid, cresols, or phenols have been carried out; however, respective degradation pathways are largely unresearched (1, 4, 19, 20). The degradation of xenobiotics by thermophilic microorganisms provides crucial advantages compared to mesophilic organisms, especially when they are applied in biotechnological processes. Limited bioavailability as a result of the low water solubility of hydrophobic contaminants may be overcome due to a higher water solubility at elevated temperatures. The water solubility of naphthalene, for example, rises from 30 mg liter−1 at 20°C to 130 mg liter−1 at 60°C (26). Moreover, diffusion rates increase at higher temperatures with an additional positive impact on bioavailability.
The bacteria applied in this degradation study were isolated from a compost consisting of wooden ties treated with lignite tar. They were able to utilize naphthalene as a sole source of carbon and energy. Stable isotope labeled [1-13C]naphthalene was used as a model contaminant. The fate of naphthalene was traced by means of the technique of 13C isotope analysis, which has been successfully applied to trace metabolic pathways (3, 18, 22). Stable isotope labeling enabled us to trace quantitatively the transformation of the xenobiotic carbon into specific fractions such as CO2, biomass, and metabolites. Moreover, the incorporation of the xenobiotic carbon into the bacterial fatty and amino acid fraction was determined on a molecular level. We describe here structures of naphthalene metabolites produced by the thermophilic Bacillus thermoleovorans Hamburg 2 and discuss possible ways of their formation.
MATERIALS AND METHODS
Bacteria.
Microorganisms were isolated from a contaminated compost consisting of lignite-treated wooden ties mixed with sugar beet mud. Enrichment cultures were obtained after incubation of samples in a mineral salt medium with naphthalene aerobically at 60°C. An isolate was obtained and classified by the Deutsche Sammlung für Mikroorganismen und Zellkulturen (DSMZ) as Bacillus thermoleovorans Hamburg 2. Optimal growth was achieved at 60°C and at pH 6.5 (14).
Degradation studies.
The new isolate was inoculated with naphthalene in a mineral salt medium (NH4Cl [1.00 g], Na2HPO4 · 2H2O [0.42 g], NaH2PO4 · H2O [0.38 g], MgCl2 · 7H2O [0.10 g], CaCl2 · 2H2O [0.10 g], KCl [0.04 g], FeSO4 · 7H2O [1 mg] per 1 liter of demineralized water). The pH was adjusted to 6.6 with 2 M HCl and 1 ml of 10× concentrated trace element, and vitamin solutions were added as described for medium 141 (10). Batch experiments were performed in 100-ml screw-top flasks which were connected to a CO2 trap by a gas tight valve. After bacterial growth was visible the valve was opened and CO2 was trapped in a NaOH solution (10 ml) and sampled at the end of the experiment. The total gas volume of the system was about 120 ml, providing sufficient oxygen for degradation experiment. Naphthalene degradation was performed with 13C-labeled naphthalene and unlabeled naphthalene (38 to 74 μM) in 25 ml of medium. These experiments were used to establish the carbon balance and for structural characterization of biomass and metabolites. Additionally, 3 mM naphthalene in a batch reactor was used for the degradation curve. Inoculation was carried out with 1 ml of a cell suspension which was taken from cultures growing in the logarithmic phase. The cultures were kept shaking at their optimum growth temperature at 60°C for 32 to 96 h, until the liquid became yellowish and turbid. The closed flasks were stored at −20°C until chemical analysis. In control experiments without inoculation, no loss of naphthalene was observed.
Chemicals.
Naphthalene and reference compounds for the identification of metabolites were purchased the highest purity available from Aldrich (Steinheim, Germany); BaCl2 p.a., NaOH p.a., HCl Suprapur, silica gel 60 (70 to 230 mesh), celite, and all other chemicals were obtained from Merck (Darmstadt, Germany). Organic solvents (p.a. quality) were purchased from Merck and distilled before use. Sodium carbonate (Na213CO3) with an isotopic purity of at least 99% was obtained from Isotec. [1-13C]naphthalene was synthesized according to the method of Staab and Haenel (27). The product was purified by column chromatography. The chemical structure and the correct label position were determined by 1H and 13C nuclear magnetic resonance spectroscopy. Gas chromatography-mass spectrometry (GC-MS) analyses revealed that the chemical and isotopic purity of [1-13C]naphthalene was better than 98%.
Chemical analyses. (i) Bacterial growth.
Growth was determined by microscopic cell counting by using a Neubauer counting chamber with a depth of 0.02 mm and a small square area of 0.0025 mm2.
(ii) Naphthalene degradation curve.
Each point of the growth curve was determined by extracting a whole batch culture with 3 ml of hexane. Naphthalene was quantified by GC measurements with an external calibration.
(iii) Naphthalene degradation balance at the end of the experiment.
A 1-ml portion of the medium was extracted with 0.5 ml of an internal standard solution (10 mg of perdeuterated phenanthrene per liter of hexane) to measure the residual naphthalene concentration. For quantification the crude extract was analyzed by GC.
(iv) Metabolites.
A 15-ml portion of the medium was acidified to pH 2 with HCl and then extracted three times with 5 ml of diethyl ether. The solution was concentrated to 1 ml by vacuum evaporation. The residual extract was dried over anhydrous sodium sulfate. Derivatization of carboxylic acids and aromatic hydroxyl groups was performed with ethereal diazomethane. The derivatized extract was exchanged into hexane and fractioned by column chromatography (silica gel, 1 by 10 cm, 70 to 230 mesh). Solvents of increasing polarity (hexane-dichloromethane [70:30, vol/vol], dichloromethane, diethyl ether) were applied. The fractions were analyzed by GC and GC-MS.
(v) Metabolites and fatty acids.
Biomass was hydrolyzed under alkaline conditions. In a glass ampoule, 1.2 g of NaOH was added to 15 ml of medium (2 M NaOH). The ampoule was sealed under an argon stream and heated at 100°C for 3 h. The reaction mixture was acidified to pH 2 (HCl, 6 M) and extracted with diethyl ether. After drying over anhydrous sodium sulfate and derivatization with diazomethane, the sample was fractionated by column chromatography as described above.
(vi) Identification of metabolites.
Transformation products were identified by coupled GC-MS and coinjection of authentic standards.
(vii) Carbonate.
CO2 was trapped in 2 M NaOH (10 ml), and the carbonate formed was precipitated with a saturated BaCl2 solution. The precipitate was washed, dried, and weighed. The isotopic composition was determined by isotope ratio MS (IR-MS).
(viii) Biomass.
The stable carbon isotope composition of the biomass was determined by centrifuging 10 ml of the culture medium on 0.1 g of carbon-free celite. The solid was washed with a phosphate buffer solution (10 mM) and dried. The total carbon content of the homogenized solid was determined with a Leco CHN 100 elementary analyser (Leco Instruments, Kirchheim, Germany). The carbon isotope composition was determined by IR-MS.
(ix) Amino acids.
A 2-ml portion of the liquid culture was hydrolyzed with 6 M HCl (110°C, 22 h). The reaction mixture was derivatized with 2-propanol and trifluoroacetate according to the method of Silfer (24). For identification and isotopic composition GC-MS and IR Monitoring GC-MS (IRMGC-MS) measurements were performed.
(x) GC.
GC analyses were performed with a Carlo Erba Fractovap 4160, equipped with a 30-m capillary column (DB-5, J & W Scientific; internal diameter, 0.32 mm, 0.25-μm film thickness) and a flame ionization detector. Hydrogen was used as the carrier gas; the temperature program was 80°C (5-min isothermal), 80 to 310°C (4°C/min), and 310°C (10-min isothermal). The injection mode was on column. For determining the concentration of naphthalene and the respective metabolites, an internal standard was employed (perdeuterated phenanthrene at 10 mg per liter in hexane).
(xi) GC-MS.
GC-MS measurements were performed with a Carlo Erba 4160 gas chromatograph (DB-5 HT, capillary column; length, 60 m; 0.32 mm; film thickness, 0.25 μm; carrier gas, helium; same temperature program as for GC measurements) coupled with a CH7A mass spectrometer (Finnigan, Bremen, Germany). The following conditions were applied: ionization mode, EI+; ionization energy, 70 eV; emission current, 200 μA; source temperature, 250°C; mass range, m/z 50 to 500.
(xii) IR-MS.
The 13C/12C ratio for the precipitated barium carbonate and for the biomass was determined on a MAT 251 (Finnigan) mass spectrometer connected to an elemental analyzer. The isotopic composition of carbon is expressed in relative differences to the Pee Dee Belemnite standard (PDB) in terms of δ values (δ 13C ‰ = [(13C/12C)sample/(13C/12C)std − 1] × 1,000 [15]). Naphthalene equivalents transformed into biomass or into carbonate were calculated according to the method of Richnow et al. (23) by using the stable carbon isotope distribution and the carbon concentration. Experiments with nonlabeled naphthalene were used to determine the natural isotopic 13C signature, which is necessary for background corrections.
To calibrate the mass spectrometer for the determination of 13C-enriched material reference standards IAEA-309 UL-Glucose A (δ 13C = 93.9‰ [PDB]) and IAEA-309 UL-Glucose B (δ 13C = 535.38‰ [PDB]) were purchased from Analytical Quality Control Service (Vienna, Austria).
(xiii) IRMGC-MS.
The compound-specific composition of the stable carbon isotopes was determined by using a Finnigan MAT 252 IRMGC-MS system. For calibration, external and internal CO2 standards were applied. The reproducibility of compounds highly enriched in 13C (δ values of >4,000‰) was ±200‰. The absolute deviation of the δ value of highly 13C-enriched compounds was checked by measuring 13C-labeled compounds of a known isotopic composition ([1-13C]naphthalene, [9-13C]anthracene, [9-13C]anthraquinone, [3-13C]fluorene, and [1-13C]hexadecane). GC-MS and IRMGC-MS analyses demand the derivatization of amino acids and fatty acids. The addition of carbon-containing groups by derivatization requires a correction of the isotope values. δ 13C values of amino acids and fatty acids were corrected for derivatization (24).
RESULTS AND DISCUSSION
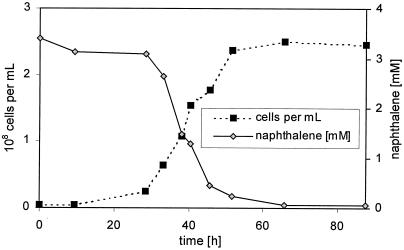
The thermophilic bacterium B. thermoleovorans Hamburg 2 grows with naphthalene as the sole source of carbon and energy. Optimal growth was achieved at 60°C. Naphthalene degradation in batch cultures correlates well with the cell number, indicating that naphthalene has been utilized for cell growth (Fig. 1). During a lag phase of 25 h the naphthalene concentration barely changes and the cell number increases very slowly. Between 25 and 50 h, cell growth correlates well with a decrease of the naphthalene concentration. Upon substrate depletion after 50 h, the cell growth rate decreases, reaching a plateau level at a cell number of 2.5 × 108 cells per ml. B. thermoleovorans was able to degrade naphthalene to a concentration below the detection limit of 0.4 μM.
FIG. 1.
Growth of B. thermoleovorans Hamburg 2 on naphthalene. Growth was performed aerobically in a batch reactor with 3 mM naphthalene in a mineral medium at 60°C.
Naphthalene degradation balance.
The naphthalene carbon balance was calculated for two experiments. Mineralization of 13C-labeled naphthalene was quantified by the production of 13CO2, whereby the major part of the substrate was found to be mineralized. The amount of 13C-labeled carbonate accounted for 77 and 82% of the applied naphthalene, while the remaining naphthalene was ≤0.1% after 96 h of incubation (Table 1). Metabolites were present in negligible concentrations with respect to the naphthalene balance. No enrichment of organic intermediates was observed. As calculated from the carbon content and the isotopic signature of the cell material, 17 and 21% of the applied 13C amounts were found in the cell biomass, which is in the regular range for substrate conversion into cells (2). A carbon balance for naphthalene of 98% proves to be close to the quantitative recovery.
TABLE 1.
[1-13C]naphthalene balance after degradation in a mineral medium for 96 h at 60°C
| Naphthalene type | Expt 1
|
Expt 2
|
||
|---|---|---|---|---|
| μMa | % | μMa | % | |
| Applied naphthalene | 74.42 | 100.0 | 37.98 | 100.0 |
| Remaining naphthalene | 0.09 | 0.1 | 0.02 | 0.1 |
| Mineralized naphthalene | 57.62 | 77.4 | 31.24 | 82.2 |
| Metabolites | <0.01 | <0.1 | <0.01 | <0.1 |
| Naphthalene transformed into biomass | 15.62 | 21.0 | 6.61 | 17.4 |
| Total balanced naphthalene | 73.33 | 98.5 | 37.9 | 99.7 |
Naphthalene equivalents.
Amino acid fraction.
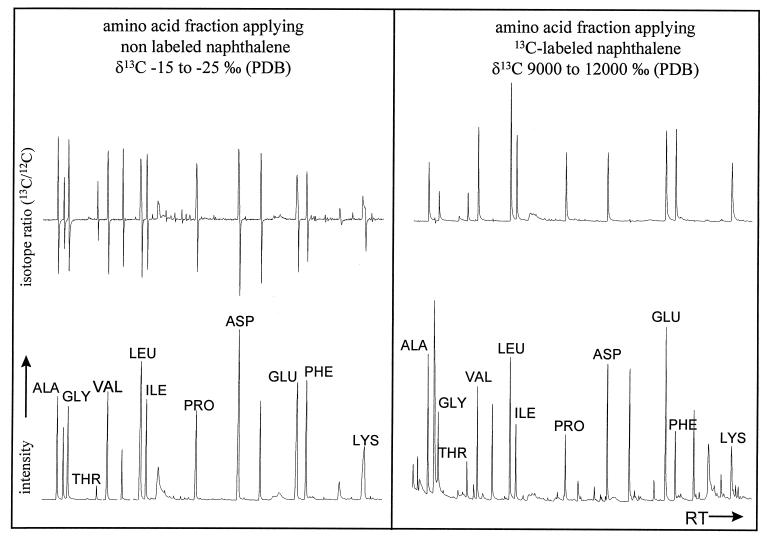
The incorporation of naphthalene-derived carbon into the amino acid fraction was investigated with IRMGC-MS, a sensitive tool to screen for an enrichment in 13C. Figure 2 shows the IRMGC-MS chromatogram of the hydrolyzable amino acid fraction of the 13C study in comparison with the chromatogram of an experiment with unlabeled naphthalene. The bottom traces depict the relative intensities of the individual compounds separated by GC (comparable with a flame ionization detection); the upper traces show the instantaneous ratio of 13CO2 to 12CO2 eluting from the GC column. In these chromatograms a peak above the baseline implies a relative enrichment in 13C, whereas a peak below the baseline indicates a relative enrichment in 12C, with the baseline representing the isotopic composition of the instrumental background. In the experiment with unlabeled naphthalene, bidirectional swings in the ratio indicate that the chromatographic column tends slightly to separate molecules containing 13C from those containing only 12C. The 13C experiment reveals amino acids which are highly enriched in 13C isotopes. Their δ 13C values ranged from 9,000 to 12,000‰ (PDB) after correction for derivatization, indicating a significant incorporation of naphthalene-related carbon. This 13C incorporation clearly demonstrated that B. thermoleovorans used naphthalene-derived carbon for its amino acid synthesis.
FIG. 2.
IRMGC-MS chromatograms of the hydrolyzable amino acid fraction of an experiment with nonlabeled naphthalene compared to a degradation study with 13C-labeled naphthalene. ALA, alanine; GLY, glycine; THR, threonine; VAL, valine; LEU, leucine; ILE, isoleucine; PRO, proline; ASP, aspartic acid; GLU, glutamic acid; PHE, phenylalanine; LYS, lysine.
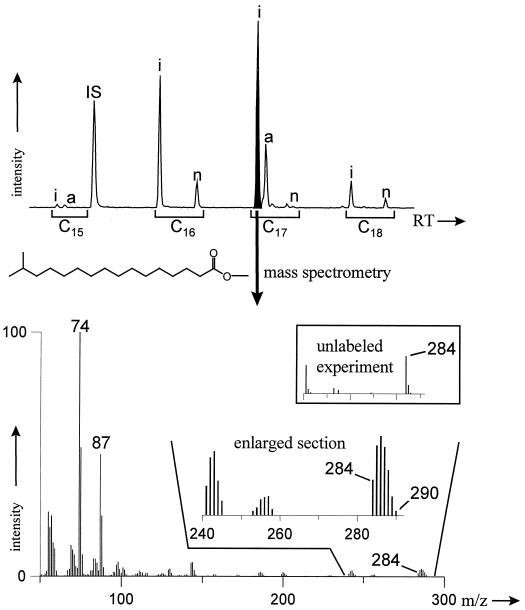
Fatty acid fraction.
The fatty acid pattern of B. thermoleovorans was typical for thermophilic bacteria growing under the applied conditions (21). Iso- and ante-iso-branched chain fatty acids were dominating, with iso-C17:0 as the most abundant compound (Fig. 3). Mass spectra revealed a significant incorporation of the 13C label into the fatty acid fraction. The methyl ester of the iso-C17:0 fatty acid provided a molecular peak of m/z = 284 for the unlabeled compound but revealed molecular masses from 284 to 290 in the degradation experiments with the 13C-labeled substrate (Fig. 3). Considering the natural abundance of the 13C isotope, molecular peaks showed a shift of 0 to 5 atomic mass units, indicating an incorporation of up to 5 13C atoms per molecule. Analysis of the stable isotope distribution by IRMGC-MS revealed δ 13C values in the same order of magnitude as those of the amino acids (10,000 to 12,000‰ [PDB] after correction for derivatization). The results prove the utilization of naphthalene-derived carbon in the fatty acid anabolism of B. thermoleovorans.
FIG. 3.
(Top panel) Partial reconstructed total ion chromatogram of the fatty acid fraction (as methyl esters). C15 to C18 indicate the number of carbon atoms. i, iso (ω2-methyl alkanoic acid); a, ante-iso (ω3-methyl alkanoic acid); n, linear alkanoic acid. (Bottom panel) Mass spectrum of the iso C17:0 fatty acid methyl ester highly enriched in 13C. The frame shows the comparison with an experiment with unlabeled naphthalene.
Metabolites.
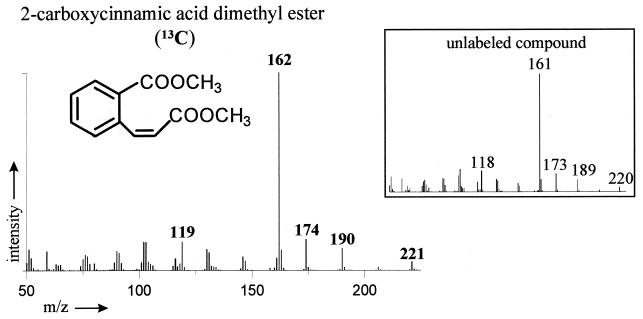
Individual metabolites account for <0.1% of the applied naphthalene after 96 h of incubation. Analysis revealed naphthalene metabolites well known from the mesophilic pathway, as well as yet unknown degradation products. Intermediates could be identified as naphthalene derivatives by means of the 13C label in conjunction with GC-MS analysis. As shown for 2-carboxycinnamic acid, metabolites of [1-13C]naphthalene reveal a mass shift of 1 atomic mass unit compared to the unlabeled reference substance (Fig. 4). The naphthalene metabolites produced by B. thermoleovorans are collected in Table 2 and are opposed to metabolites known from the naphthalene degradation by mesophilic pseudomonads and Bacillus sp.
FIG. 4.
Mass spectrum of 13C-labeled 2-carboxycinnamic acid. Compared to the unlabeled compound, the masses of the molecular peak and the respective fragment peaks are increased by one mass unit. y axis, intensity; x axis, m/z.
TABLE 2.
Naphthalene transformation products of the thermophilic B. thermoleovorans compared with intermediates of mesophilic pseudomonads and Bacillus sp.
| Producta | Presence (+) of:
|
|
|---|---|---|
| Intermediates produced by thermophilic B. thermoleovorans | Intermediates produced by mesophilic organisms (references 6, 7, and 9) | |
| 1-Naphthol | + | + |
| 2-Naphthol | + | + |
| cis-1,2-Dihydro-1,2-dihydroxynaphthalene | + | |
| 1,2-Dihydroxynaphthalene (I) | + | |
| 2,3-Dihydroxynaphthalene (VI) | + | |
| 4-(2-Hydroxyphenyl)-2-oxo-but-3-enoic acid | + | |
| 2-Hydroxycinnamic acid (III) | + | |
| Coumarin (chromen-2-one) (IV) | + | + |
| 3-(2-Hydroxyphenyl)-propanoic acid | + | |
| 2,3-Dihydrocoumarin (chroman-2-one) | + | |
| 2-Hydroxybenzoic acid (salicylic acid) (V) | + | + |
| Benzene-1,2-diol (catechol) | + | |
| 3-(2-Carboxyphenyl)-2-propenoic acid (2-carboxycinnamic acid)b (VII) | + | |
| Phthalic acid (VIII) | + | |
| Benzoic acid (IX) | + | |
Roman numerals in parentheses correspond to the structures in Fig. 5.
cis and trans configuration(s) not determined.
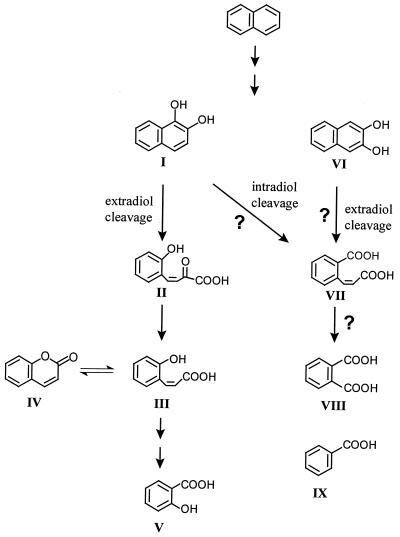
Primary oxidation products of B. thermoleovorans included 1-naphthol, 2-naphthol, and 2,3-dihydroxynaphthalene. 1-Naphthol and 2-naphthol have been described as intermediates during the cometabolization of naphthalene by other Bacillus strains (6). Phenolic metabolites are mostly rearrangement products of arene oxides, which are produced after the oxidation of PAH with molecular oxygen by cytochrome P450-dependent monooxygenases. Somewhat striking is the co-occurrence of dihydroxy-naphthalene derivatives, because they are formed by a basically different enzymatic reaction. The formation of diol structures is catalyzed by dioxygenases, which introduce molecular oxygen into the aromatic ring (7). The co-occurrence of mono- and dioxygenases has been already described in earlier studies with a naphthalene-degrading Mycobacterium sp. (17).
2,3-Dihydroxynaphthalene is an atypical metabolite which we found to be in contrast to its isomer, 1,2-dihydroxynaphthalene, a well-known intermediate after oxygenation of naphthalene by a 1,2-dioxygenase and subsequent rearomatization of the produced dihydrodiol (16). As far as we know, the occurrence of 2,3-dihydroxynaphthalene has not been reported in previous naphthalene degradation studies. However, the ability of mesophilic bacteria such as soil pseudomonads to transform this intermediate may not be surprising because these organisms have been shown to degrade anthracene via the formation of 2,3-dihydroxynaphthalene (7). These findings reveal that soil pseudomonads which can degrade naphthalene and anthracene are able to transform the particular substrate to 1,2-dihydroxynaphthalene and 2,3-dihydroxynaphthalene, respectively.
4-(2-Hydroxyphenyl)-2-oxo-but-3-enoic acid (2-hydroxybenzal pyruvic acid), the ring fission product in the naphthalene degradation pathway of mesophilic pseudomonads, was not detected in our studies with B. thermoleovorans. However, several side chain degradation products of 2-hydroxybenzal pyruvic acid, such as coumarin (the cyclization product of 2-hydroxycinnamic acid), 2-hydroxybenzene-3-propanoic acid, and its cyclization product, 3,4-dihydrocoumarin, as well as salicylic acid, were identified. Metabolites such as salicylic acid and coumarin do fit in the previously published pathways of the bacterial naphthalene metabolism (9, 11). 2-Hydroxyphenyl-3-propanoic acid is likely to result from a hydrogenation reaction of its unsaturated analogue 2-hydroxycinnamic acid. A further cyclization reaction would produce 3,4-dihydrocoumarin.
In addition, other transformation products of B. thermoleovorans were identified that have not been reported to be part of the known naphthalene degradation pathway. 2-Carboxycinnamic acid, phthalic acid, and benzoic acid, as well as the above-mentioned 2,3-dihydroxynaphthalene, were determined. 2-Carboxycinnamic acid was assumed as a ring fission product in early naphthalene degradation studies with soil pseudomonads by Fernley and Evans (12), but no structural assignment was obtained. Sequential induction experiments with synthetic 2-carboxycinnamic acid failed (12). In our study, phthalic acid, a second transformation product with a 2-carboxy functionality was identified. Phthalic acid may arise from further degradation of 2-carboxycinnamic acid. A subsequent decarboxylation reaction would provide benzoic acid.
The co-occurrence of 2,3-dihydroxynaphthalene, 2-carboxycinnamic acid, and phthalic acid beside cinnamic acid and salicylic acid strongly suggests that B. thermoleovorans produces enzymes of different naphthalene degradation pathways (Fig. 5). The formation of 2,3-dihydroxynaphthalene points to an initial oxidation of naphthalene by a 2,3-dioxygenase, whereas the presence of 2-hydroxybenzene derivatives indicates the simultaneous activity of an 1,2-dioxygenase.
FIG. 5.
Metabolites of naphthalene formed by mesophilic and thermophilic microorganisms (hypothetical scheme of precursor product relationship).
The formation of 2-carboxycinnamic acid may be well explained by an ortho-type cleavage (intradiol) of 1,2-dihydroxynaphthalene or a meta-type cleavage (extradiol) of 2,3-dihydroxynaphthalene (Fig. 5). However, the occurrence of 2,3-dihydroxynaphthalene, 2-carboxycinnamic acid, and phthalic acid points to a completely different degradation pathway and not to an unusual meta cleavage of 1,2-dihydroxynaphthalene.
ACKNOWLEDGMENTS
We acknowledge the financial support from the Deutsche Forschungsgemeinschaft (grants SFB 188-D6, -D5, and -B6).
We thank M. Leosson and D. Stüben (Universität Karlsruhe) for the measurement of the isotopic compositions of biomass and carbonates and P. Albrecht and P. Wehrung for providing IRMGC-MS facilities.
REFERENCES
- 1.Adams D, Ribbons D W. The metabolism of aromatic compounds by thermophilic bacilli. Appl Biochem Biotechnol. 1988;17:231–244. [Google Scholar]
- 2.Alexander M. Biodegradation and bioremedation. San Diego, Calif: Academic Press, Inc.; 1994. p. 12. [Google Scholar]
- 3.Beller H R, Reinhard M, Grbić-Galić D. Metabolic by-products of anaerobic toluene degradation by sulfate-reducing enrichment cultures. Appl Environ Microbiol. 1992;58:3192–3195. doi: 10.1128/aem.58.9.3192-3195.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buswell J A, Twomey D G. Utilization of phenol and cresols by Bacillus stearothermophilus, strain PH24. J Gen Microbiol. 1975;87:377–379. doi: 10.1099/00221287-87-2-377. [DOI] [PubMed] [Google Scholar]
- 5.Cerniglia C E, Hebert R L, Szaniszlo P J, Gibson D T. Fungal transformation of naphthalene. Arch Microbiol. 1978;117:135–143. doi: 10.1007/BF00402301. [DOI] [PubMed] [Google Scholar]
- 6.Cerniglia C E, Freeman J P, Evans F E. Evidence for an arene oxide-NIH shift pathway in the transformation of naphthalene to 1-naphthol by Bacillus cereus. Arch Microbiol. 1984;138:283–286. doi: 10.1007/BF00410891. [DOI] [PubMed] [Google Scholar]
- 7.Cerniglia C E, Heitkamp M A. Microbial degradation of polycyclic aromatic hydrocarbons (PAH) in the aquatic environment. In: Varanasi M, editor. Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 48–68. [Google Scholar]
- 8.Cerniglia C E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–368. [Google Scholar]
- 9.Davies J I, Evans W C. Oxidative metabolism of naphthalene by soil pseudomonads. Biochem J. 1964;91:251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deutsche Sammlung für Mikroorganismen und Zellkulturen. Catalogue of strains. Braunschweig, Germany: Deutsche Sammlung für Mikroorganismen und Zellkulturen; 1993. [Google Scholar]
- 11.Gibson D T, Subramanian V. Microbial degradation of aromatic hydrocarbons. In: Gibson D T, editor. Microbial degradation of organic compounds. New York, N.Y: Marcel Dekker; 1984. pp. 182–252. [Google Scholar]
- 12.Fernley H N, Evans W C. Oxidative metabolism of poycyclic hydrocarbons by soil pseudomonads. Nature. 1958;182:373–375. doi: 10.1038/182373a0. [DOI] [PubMed] [Google Scholar]
- 13.Harms H, Bosma T N P. Mass transfer limitation of microbial growth and pollutant degradation. J Ind Microbiol Biotechnol. 1997;18:97–105. [Google Scholar]
- 14.Hebenbrock S, Feitkenhauer H, Märkl H, Antranikian G. Biodeterioration and biodegradation. DECHEMA monographs. Vol. 133. Weinheim, Germany: VCH Verlagsgesellschaft; 1996. Biodegradation of aromatic and aliphatic hydrocarbons by thermophilic microorganisms and their cultivation in bioreactors; pp. 615–622. [Google Scholar]
- 15.Hoefs J. Stable isotope geochemistry. Berlin, Germany: Springer Verlag; 1997. [Google Scholar]
- 16.Jerina D M, Daly H W, Jeffrey A M, Gibson D T. cis-1,2-Dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971;142:394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- 17.Kelley L, Freeman J P, Cerniglia C E. Identification of metabolites from degradation of naphthalene by a Mycobacterium sp. Biodegradation. 1990;1:283–290. doi: 10.1007/BF00119765. [DOI] [PubMed] [Google Scholar]
- 18.Migaud M E, Chee-Sanford J C, Tiedje J M, Frost J W. Benzylfumaric, benzylmaleic, and Z- and E-phenylitaconic acids: characterization and correlation with a metabolite generated by Azoarcus tolulyticus Tol-4 during anaerobic toluene degradation. Appl Environ Microbiol. 1996;62:974–978. doi: 10.1128/aem.62.3.974-978.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller R, Antranikian G, Maloney S, Sharp R. Thermophilic degradation of environmental pollutants. Adv Biochem Eng Biotechnol. 1998;61:155–169. [Google Scholar]
- 20.Mutzel A, Reinscheid U, Antranikian G, Müller R. Isolation and characterization of a thermophilic Bacillus strain, that degrades phenol and cresols as sole carbon source at 70°C. Appl Microbiol Biotechnol. 1996;46:593–596. [Google Scholar]
- 21.Oshima M, Miyagawa A. Comparative studies on the fatty acid composition of moderately and extremely thermophilic bacteria. Lipids. 1974;9:476–480. doi: 10.1007/BF02534274. [DOI] [PubMed] [Google Scholar]
- 22.Richnow H H, Eschenbach A, Seifert R, Wehrung P, Albrecht P, Michaelis W. The use of 13C-labelled polycyclic aromatic hydrocarbons for the analysis of their transformation in soils. Chemosphere. 1998;36:2211–2224. doi: 10.1016/s0045-6535(97)10193-x. [DOI] [PubMed] [Google Scholar]
- 23.Richnow H H, Eschenbach A, Mahro B, Kästner M, Annweiler E, Seifert R, Michaelis W. The formation of nonextractable soil bound residues—a stable isotope approach. Environ Sci Technol. 1999;33:3761–3767. [Google Scholar]
- 24.Silfer J A, Engel M H, Macko S A, Jumeau E J. Stable carbon isotope analysis of amino acid enantionmers by conventional isotope ratio mass spectrometry and combined gas chromatography/isotope ratio mass spectrometry. Anal Chem. 1991;63:370–374. [Google Scholar]
- 25.Smith M R. The biodegradation of aromatic hydrocarbons by bacteria. Biodegradation. 1990;1:191–206. doi: 10.1007/BF00058836. [DOI] [PubMed] [Google Scholar]
- 26.Solubility Data Series. Hydrocarbons C8 to C36. International Union of Pure and Applied Chemistry (IUPAC). 38, part II. New York, N.Y: Pergamon Press; 1989. Hydrocarbons with water and seawater. [Google Scholar]
- 27.Staab H A, Haenel M. [1-13C]-Naphthalin: Synthesis, NMR-Spektrum, ESR-Spektrum des Radikalanions und Automerisierungsversuche. Chem Ber. 1970;103:1095–1100. [Google Scholar]