Abstract
Background
Major Depressive Episodes (MDEs) may characterise many psychiatric disorders. Its pharmacotherapy is laid with unmet needs, rendering the testing of new drugs necessary.
Objective
To compare the effects of vortioxetine with those of other antidepressants (OADs) in a 1-year naturalistic setting.
Methods
We included 126 adult patients with anMDE in the course of major depressive (MDD), bipolar (BD), or schizophrenia spectrum disorders (SSOPDs), with or without substance use disorder (SUD), who received 5-20 mg/day oral vortioxetine, and compared them with 100 patients receiving OADs at baseline and after 1, 3, 8, and 12 months on their scores on the MADRS, the CGI-S, the 24-item BPRS, the YMRS, the Hamilton Anxiety Rating Scale, a Visual Analogue Scale for craving, the Columbia-Suicide Severity Rating Scale, and the WHOQOL-BREF.
Results
Patients on vortioxetine improved similarly to those on OADs on all measures, independently from having or not a comorbid SUD. However, they improved with time better than their OADcounterparts if affected by BD or SSOPDs, but not MDD, on the CGI-S, BPRS depression, anxiety, and manic symptoms. SUD hampered the response of anxiety to treatment. Men improved on depression with time better than women.
Conclusion
MDEs responded to vortioxetine similarly to OADs by improving in depression, general psychopathology, anxiety, suicidal thinking, and quality-of-life, independently from SUD comorbidity. MDEs of patients with BD or SSOPDs on vortioxetine responded better than that of patients on OADs. Clinical Trial Registration No. 17354N.
Keywords: Vortioxetine, Antidepressants, Major Depressive Disorder, Bipolar Disorder, Schizophrenia Spectrum and Other Psychotic Disorders, Major Depressive Episode, 1-year outcome
1. INTRODUCTION
Depression is both a condition and a symptom that is present in a variety of conditions. It is characterised bylow mood and a bleak outlook on life in general, as well as other symptoms, which often appear to be opposite in nature. For example, a person with depression may either overeat or be inappetent, have either hypersomnia or insomnia, have psychomotor retardation or agitation. DSM-IV and DSM-5 criteria need to be considered in diagnosing a major depressive episode (MDE) [1].
According to the WHO, major depressive disorder (MDD) affects over 350 million people worldwide. Morbidity estimates carried out in 2012 anticipated that in 2020, MDD would be the main cause of absenteeism from work and that in 2030, it will be the most prevalent illness worldwide. The MDD population shows increased disability and impairment in psychosocial and occupational functioning, increased absenteeism and reduced work productivity (performance), compared with the general population; this results in public health expenditure [2]. These observations support the need to consider MDD as a major welfare issue.
Despite efforts to identify optimal antidepressant treatments, patient needs are still unmet in terms of improvement of depressive symptoms, remission, and restoration of functioning [3]. Other unsolved issues in drug treatment of depression are the time lag to the onset of drug effect and adverse effects that may occur, such as sexual dysfunction, bodyweight increase, and sleep disorder. Furthermore, typical residual symptoms even after first-line treatment are usually cognitive symptoms, particularly in the areas of executive function, memory, attention, and speed of processing, which are associated withremarkable impairment in patients’ functioning [4, 5]. A 3-year prospective study of 267 patients in primary care with initial depression evaluated the presence of individual residual symptoms during depressive episodes and periods of remission [6]. It has been documented that three individual symptoms (cognitive problems, lack of energy, and sleeping problems) dominated the course of depression and were present for 85-94% of the time during depressive episodes and 39-44% of the time during remissions. Residual symptoms are thought to be a predictor of relapse [7]; in fact, patients with depression who completed a cognitive-behaviour therapy (CBT) cycle and scored on the Hamilton Depression Rating Scale (HDRS) 7 or less for 8 weeks or more (full recovery) relapsed significantly less than those who recovered partially (9% vs. 52%, respectively) [8, 9]. Slower response to CBT, unmarried status, and high residual scores on the Dysfunctional Attitudes Scale were independently and additively related to increased risk of relapse [8, 9].
Since low functioning predicts relapse in MDD [10], and cognitive impairment, QoL, and functioning are interrelated [11], there is a need to reduce cognitive impairment in depression so asto enhance the quality of life [6].
Selective Serotonin (5-HT) Reuptake Inhibitors (SSRIs) and Serotonin and Noradrenaline Reuptake Inhibitors (SNRIs) proved to be effective in treating depression, but also have adverse effects that negatively affect the quality of life (QoL) and adherence to treatment; these include sexual impotence, weight gain, and sleep disorders. Hence, there is a need for new drugs that maintain the same antidepressant effectiveness, but are free from side effects that lead to reduced adherence to treatment and/or QoL, especially among younger people with MDD (<35 years).
Vortioxetine was licensed in 2013 by European Medicines Agency (EMA) for the treatment of MDD in adults in the dose range of 5-20 mg/day (the recommended initial dose is 10 mg/day if the patient is younger than 65 years and 5 mg/day if older) [12].
Vortioxetine (1-[2-(2,4-dimethyl-phenylsulfanyl)-phenyl]-piperazine) belongs to a new chemical class of psychotropics, the bis-aryl-sulfanyl amines. The mechanism of action of vortioxetine is claimed to be related to its multimodal activity, which is a combination of direct modulation of receptor activity and inhibition of the serotonin transporter. It is a 5-HT3, 5-HT7 and 5-HT1D receptor antagonist, a 5-HT1B partial agonist, a 5-HT1A agonist and an inhibitor of the 5-HT transporter [13]. Its inhibitory actions on 5-HT3 serotonin receptors placed on GABA interneurons, ultimately result in the enhancement of glutamatergic and serotonergic transmission in the rat forebrain, an effect reversed by the GABAB agonist baclofen, but not by the GABAA agonist muscimol [14]. Furthermore, it counteracts the effects of tryptophan depletion in rat models of depression on NMDA-related markers, unlike the SSRI paroxetine [15]. However, it may also act through alternative mechanisms, like the PI3K/AKT intracellular pathway [16], and by up-regulating the expression of brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (Trk B) in the hippocampus [17]. It is metabolized by multiple CYP450s and does not inhibit or appreciably induce the CYP system. Hence, its potential for clinically relevant interactions with other drugs is low.
Vortioxetine has shown efficacy in short-term studies, in which it reduced depression and anxiety items of scales like the Montgomery-Åsberg Depression Rating Scale (MADRS) and the Hamilton Depression Rating Scale (HAM-D) in many clinical trials [18]. Long term clinical studies (i.e., about one or more years) are fewer.
A peculiar property of vortioxetine is its efficacy on cognitive symptoms of people with MDD. Many randomized clinical studies have confirmed that vortioxetine 5 mg/day enhances performance in cognitive tests more than placebo and other antidepressants [19, 20]. This pro-cognitive effect of vortioxetine was found to be independent ofits antidepressant action [21].
Vortioxetine proved to be safe and effective in many clinical trials and in meta-analyses, both short- and long-term [22-24]. Some of the typical adverse effects seen with other antidepressants, including sexual dysfunction [3, 18, 25, 26], insomnia, and weight gain [3, 18], are observed less with vortioxetine, even after long-term treatment, hence sparing QoL and adherence to treatment.
MDD is often comorbid with substance use disorder (SUD), such as alcohol and illicit drug use disorders. In the US, about 32% of people with MDD have a comorbid SUD [27].
A 2013 review on the current status of co-occurring mood and SUD [28] showsthat estimates of the lifetime prevalence of mood and substance use disorders and the comorbidity of these disorders in the general population can be derived from two US nationally representative large-scale surveys using DSM-IV diagnostic criteria: the National Epidemiologic Survey on Alcoholism and Related Conditions, which surveyed 43,093 people in 2001 and 2002 [29, 30]; and the National Comorbidity Survey Replication, which surveyed 9,282 people in 2001 and 2003 [31, 32]. As a result, comorbid substance use disorder is high in major depression, with lifetime rates of 40.3% for any alcohol use disorder and 17.2% for any drug use disorder. For major depression and alcohol dependence, the lifetime rate is 21%. This figure refers to the rather obsolete term “dependence” and is lower than the major depressive disorder-alcohol use disorder comorbidity.
Illicit drugs are believed to activate the mesolimbic dopaminergic system, as most of them were shown to increase the release of dopamine in the nucleus accumbens [33]. Direct evidence that 5-HT receptor system influences dopaminergic activity in CNS was first available in the late 1980s, when it was reported that the 5-HT3 agonist 2-methyl serotonin increased dopamine release from hyperactive dopaminergic neurons in striatal slices; this effect was blocked by the 5-HT3 antagonist tropisetron [34].
Rewarding properties of drugs can be behaviourally assessed in rodents through place-preference conditioning tests; in this paradigm, blockade of 5-HT3 receptors attenuates the rewarding properties of drugs like morphine and nicotine, possibly by decreasing dopamine release. The presence of the 5-HT3 receptors in the brain and their localization in areas such as the entorhinal cortex, amygdala, and nucleus accumbens, strongly suggestthat they play a role in controlling multiple behaviours that may be related topsychosis, anxiety, depression, and substance use, as shown by studies with highly potent and selective 5-HT3 receptor antagonists (e.g., ondansetron, granisetron) [35].
Many studies have confirmed the ability of 5-HT3 agonists to increase the release of dopamine in the nucleus accumbens, and the ability of 5-HT3 antagonists to block agonist-induced increases in dopamine levels [36]. Due to its ability to modulate heightened dopamine activity in mesolimbic areas, the 5-HT3 receptor has been suggested to be important in the actions of drugs of abuse.
5-HT1A receptors may be both somatodendritic autoreceptors (in the raphé) and postsynaptic receptors (for example, in the hippocampus). It has been concluded [37] that 5-HT1A auto-receptors mainly facilitate psychostimulant addiction-related behaviours through a reduction in 5-HT release in terminal areas. Post-synaptic 5-HT1A receptors, in contrast, predominantly inhibit the expression of various addiction-related behaviours directly. So, 5-HT1A does not appear to represent the “core” receptor involved in reward and addiction, but rather to contribute to the effects of the entire brain 5-HT systems in moderating addictive phenomena. On the other hand, it has been claimed that long-term drug use and addiction impact the 5-HTergic function, which in turn affects behaviours related to drug-seeking and craving [38].
Considering the role of the 5-HTergic systems in drug addiction, the action of vortioxetine on 5-HT receptors might suggest its usefulness in people affected by MDD in comorbidity with SUD. As a matter of fact, vortioxetine has not been tested to date (September 23, 2019) for its effect on patients with depression and comorbid substance use disorder. Hence, we considered itworthy to try vortioxetine in depression associated with SUD.
2. MATERIALS AND METHODS
2.1. Patients
The study was a naturalistic, parallel, longitudinal follow-up, conducted between January 2018 and January 2020 at Von Siebenthal neuropsychiatric hospital.
We enrolled male and female inpatients diagnosed with the Structured Clinical Interview for DSM-5 (SCID-5-CV) [39] as having a current Major Depressive Episode (MDE) during the course of one of the following: Major Depressive Disorder (MDD), Bipolar Disorder (BD), and Schizophrenia Spectrum and Other Psychotic Disorders (SSOPDs).
Patients were excluded from the study if they had acute psychosis, acute suicidal ideation, and any acute psychiatric condition that might require emergency interventions, organic, neurological, or cardiovascular disease. Other exclusion criteria were pregnancy or breastfeeding or planning a pregnancy during the study period.
Of the 253 patients enrolled in the study, 226 patients wished to be compliant with treatment. Patients were exposed to two different treatment conditions. A group of patients (n=100) were treated with various antidepressants (ADs) except vortioxetine, and the other group (n=126) was treated with vortioxetine without OADs. Both the groups were evaluated for 12 months from baseline (BL). The assignment to groups was based on patient preference after they received full information about the treatment alternatives and their relative mechanisms of action.
After meeting inclusion criteria, patients were explained the study aims and methods and provided free, informed consent. The study received approval from the local ethical committee. It was conducted in accordance with the Principles of Human Rights, as adopted by the World Medical Association at the 18th WMA General Assembly, Helsinki, Finland, June 1964, and subsequently amended by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013.
2.2. Treatments
Vortioxetine was administered orally in the 5-20 mg range; dosing was flexible and could be adjusted according to manufacturers’ indications and patients’ needs. Vortioxetine was administered as 5, 10, or 20 mg tablets or in drops (each drop corresponding to 1 mg) in the morning or at meals, with or without food, according to the patient’s taste. When the patient disliked the oral solution formulation, he/she was shifted to the oral tablet formulation. OADs were trazodone (150-300 mg/day), duloxetine (60-120 mg/day), sertraline (50-200 mg/day), paroxetine (10-40 mg/day), escitalopram (10-20 mg/day), venlafaxine (75-225 mg/day), bupropion (150-300 mg/day), amitriptyline (10 mg/day), clomipramine (75-150 mg/day), mianserine (15 mg), and mirtazapine (15 mg), all administered orally. Benzodiazepines or allosteric benzodiazepine receptor modulators were allowed and as needed, to deal with anxiety bouts or insomnia problems. Gabapentin and pregabalin were also allowed. Patients with SSDOPs or BD could also take antipsychotics (risperidone, olanzapine, clozapine, quetiapine, asenapine, aripiprazole, lurasidone or paliperidone among atypicals, and haloperidol and amisulpride among classical neuropleptics). Additionally, patients with BD could also take lamotrigine, oxcarbazepine, valproate or lithium. No monoamine oxidase inhibitors were allowed throughout the study.
2.3. Study Assessments
Efficacy was assessed using the mean change from BL on Clinical Global Impressions scale-Severity of Illness (CGI-S) [40] and on the Montgomery-Åsberg Depression Rating Scale (MADRS) [41] scores. The 24-item Brief Psychiatric Rating Scale (BPRS) [42], i.e., the expanded version of a previously developed 16- [43] and 18-item version [44], the Young Mania Rating Scale (YMRS) [45], and the Hamilton Anxiety Rating Scale (HAM-A) [46] were administered for the assessment of general psychopathology, and manic and anxious symptoms, respectively. Suicidal tendencies were assessed through the Columbia-Suicide Severity Rating Scale (C-SSRS) [47]. A Visual Analogue Scale (VAScrav) [48] was used to evaluate craving in patients with Alcohol and/or Substance Use Disorders (AUD/SUD, respectively). These assessments were conducted at BL, and then 1, 3, 8, and 12 months after BL. Safety and tolerability were assessed through clinical interview, vital signs and laboratory values. The World Health Organization Quality of Life, Brief version (WHOQOL-BREF) [49] was used to evaluate the quality of life (QoL) at BL and after 1, 3, 8, and 12 months. BL was set at the initiation of drug treatment. All rating scales were completed by specifically-trained treating clinicians. The primary outcomes were the scores on the CGI-S and the MADRS, while all others were secondary.
The effects of gender and age on treatment response in each group and in the whole group were investigated byusing gender as an independent variable and age as a covariate and examining them in the determination of the scores on the MADRS and CGI-S across the study.
We established as response criteria, a final CGI-S score of 1 or 2 or atleast 50% drop from baseline scores on the MADRS with an endpoint score of ≤10. Remission criteria were a final CGI-S score of 1 or a final MADRS score <7. We calculated response and remission rates for each treatment group and according to the SUD/nonSUD split and for the SUD and nonSUD subgroups in the entire sample. The effect size for each treatment and for the SUD and nonSUD populations was calculatedwith Cohen’s d [50], adopting Cohen’s original cutoffs (small about 0.20, medium about 0.50, and large about 0.80 or more) and complementing them with Sawilowsky’s [51] cut-offs, i.e., 0.10 very small, 1.20 very large, and 2.00 or above huge.
Safety measures included frequency and severity of adverse events (AEs), serious AEs (SAEs, as reported, plus completion of the UKU scale [52]); discontinuation due to AEs, abnormal laboratory results, physical examination, vital signs, body mass index (BMI), waist/hip measurement and metabolic syndrome parameters. For SUD patients, urine testing for a range of illicit drugs was arranged bi-weekly.
2.4. Statistical Analysis
We analysed the sample as intention-to-treat (ITT), dealing with missing data due to drop-out cases with the conservative last observation carried forward (LOCF) method [53]. Four-way ANOVA for between-subjects factors SUD diagnosis (two levels), DSM-5 diagnosis (three levels) and Treatment (two levels), and five-level repeated-measures factor Time (BL and 1, 3, 8, 12 months) were performed for each considered measure. Mauchly’s Tests of Sphericity were statistically significant, hence, Huynh-Feldt and Greenhouse-Geisser corrections have been applied. Statistical analyses were carried out with the SPSS 25 software (IBM Corporation, Armonk, NY, USA, 2017). The statistical significance cutoff was set at p < 0.05.
3. RESULTS
3.1. Descriptive Statistics
The final sample consisted of 226 Caucasian inpatients, 104 men (46%) and 122 (54%) women diagnosed with MDD (56.6%), BD (27.4%) and SSOPDs (15.9%). Patients’ age ranged from 20 to 81 years, withmean 48.95, andstandard deviation (SD) 14.07. Patients were assessed for alcohol and/or substance-use disorder; 146 (64.6%) reported no alcohol or illicit/recreational substance use, while 80 (35.4%) received a diagnosis of SUD and/or AUD. Regarding BL socio-demographic variables, women were older than men (t(224)=-2.229; p = 0.27), more men than women reported being single while more women than men reported beingmarried (χ2=22.815; p < 0.001), and SSOPDs diagnosis wasmore frequent in men than in women (χ2=12.462; p = 0.002). Other BL socio-demographic variables did not differ between the two genders. Descriptive statistics are shown in Table 1.
Table 1.
Participants’ characteristics. Data are expressed as percentage or means ± SD, as appropriate.
| - |
Study Sample
(n=226) |
Men
(n=104; 46%) |
Women
(n=122; 54%) |
P |
|---|---|---|---|---|
| Age in years (x̄±SD) [Student’s t-test] | 48.95 ± 14.07 | 46.72 ± 13.69 | 50.86 ± 14.17 | p=0.027 |
| AAO of Psychiatric Disorder (x̄±SD) [Student’s t-test] | 13.81 ± 11.61 | 13.04 ± 11.40 | 14.47 ± 11.80 | n.s. |
| Marital Status, N (%) [χ2 test] | ||||
| Single | 105 (46.9) | 66 (63.5) | 39 (32.5) | p < 0.001 |
| Married | 73 (32.6) | 22 (21.2) | 51 (42.5) | p < 0.001 |
| Separated/Divorced | 36 (6.1) | 14 (13.5) | 22 (18.3) | n.s. |
| Widowed | 10 (4.5) | 2 (1.9) | 8 (6.7) | n.s. |
| Educational level, N (%) [χ2 test] | ||||
| Primary School | 15 (6.6) | 5 (4.8) | 10 (8.2) | n.s. |
| Middle School | 109 (48.2) | 50 (48.1) | 59 (48.4) | n.s. |
| High School | 92 (40.7) | 45 (43.3) | 47 (38.5) | n.s. |
| College/University, Master classes, Specialty, Ph.D. | 10 (4.4) | 4 (3.8) | 6 (4.9) | n.s. |
| Diagnosis, N (%) [χ2 test] | ||||
| MDD | 128 (56.6) | 50 (48.1) | 78 (63.9) | n.s. |
| BD | 62 (27.4) | 28 (26.9) | 34 (27.9) | n.s. |
| SSOPDs | 36 (15.9) | 26 (25) | 10 (8.2) | p = 0.002 |
| Presence of Alcohol or Substance Use Disorder, N (%) [χ2 test] | ||||
| No AUD or SUD | 146 (64.6) | 62 (59.6) | 84 (68.9) | n.s. |
| AUD and/or SUD | 80 (35.4) | 42 (40.4) | 38 (31.1) | n.s. |
3.2. Effects of Vortioxetine and other AD Treatments on General Symptomatology and Suicidality
An ITT analysis with LOCF was used with mixed model ANOVAs involving four independent variables, i.e., SUD (presence/absence), DSM-5 diagnosis (MDD, BD, or SSOPDs) and Treatment (vortioxetine or OADs) as between-subjects variables, and Time (BL and 1, 3, 8, and 12 months) as within-subjects variable, and CGI-S and BPRS scores as dependent variables.
Primary outcomes. CGI-S scores indicated a main effect of time (F(3.192,683.060) = 607.410, p < 0.001; from 4.72 at BL to 1.62 after 12 months; Table 2; Supplementary Fig. (5 (832KB, pdf) ) in all conditions. Three interaction effects were significant, i.e., a Time × Treatment effect (F(3.192,683.060) = 2.741, p = 0.039), with a greater improvement for the vortioxetine group compared to OADs; a Time × DSM-5 diagnosis effect (F(6.384, 683.060) = 5.396, p = 0.014), with a greater improvement of clinical global severity in BD (from 4.76 to 1.46) and SSOPDs (from 4.68 to 1.40) patients than MDD (from 4.73 to 1.99); a Time × DSM-5 diagnosis × Treatment effect (F(6.384, 683.060) = 4.921, p < 0.001), showing that BD and SSOPDs patients exposed to vortioxetine treatment manifested a greater improvement of clinical severity compared to patients treated with OADs. In MDD patients, vortioxetine and the OADs showed similar efficacy. No other significant interactions were found.
Table 2.
Within-group main effects of Time.
| Assessment | F (df) | p |
|---|---|---|
| CGI | 607.410 (3.192,683.060) * | <0.001 |
| BPRS | 301.521 (2.345,497.091) * | <0.001 |
| MADRS | 381.106 (2.749,585.544) * | <0.001 |
| C-SSRS | 23.036 (1.300,278.116) ** | <0.001 |
| YMRS | 47.652 (2.606,555.114) * | <0.001 |
| HAM-A | 203.090 (2.838,604.559) * | <0.001 |
| VAScrav | 50.296 (2.512, 185.868) * | <0.001 |
| WHOQOL-BREF | 46.306 (1.599,342.162) ** | <0.001 |
Mauchly's Tests of Sphericity are statistically significant. *=Greenhouse-Geisser correction; **=Hyun-Feldt correction.
For depressive symptomatology, the main effect of time indicatedMADRS scores to significantly decrease over time (F(2.749,585.544) = 381.106, p < 0.001; from 27.31 at BL to 11.02 after 12 months; Table 2; Supplementary Fig. (1 (832KB, pdf) ) in all conditions. Four interaction effects were significant, i.e., a Time × Treatment effect (F(2.749,585.544) = 19.802, p < 0.001) indicating a greater decrease of depressive symptoms in patients treated with vortioxetine (from 28.15 to 7.99); a Time × SUD effect (F(2.749,585.544) = 5.151, p = 0.002), with a greater decrease of depressive symptomatology in patients without SUD, only at 1-month follow-up (from 29.03 to 16.78); a Time×DSM-5 diagnosis effect (F(5.498,585.544) = 4.082, p = 0.001), with a greater improvement of depressive symptoms in BD (from 26.99 to 10.44) and SSOPDs (from 27.21 to 9.11) patients than in MDD (from 27.73 to 13.49); a Time × DSM-5 diagnosis × Treatment effect (F(5.498,585.544) = 4.261, p = 0.001), showing treatment with vortioxetine to decrease depressive symptoms more than treatment with OADs in both BD and SSOPDs patients, while no significant differences between treatments emerged in MDD patients. No other significant interactions were found.
Secondary outcomes. Suicidal ideation and behaviour significantly decreased over time (F(1.300,278.116) = 23.036, p < 0.001; from 2.59 at BL, to 0.04 after 12 months; Table 2; Supplementary Fig. (6 (832KB, pdf) ), in all conditions. A significant Time × SUD interaction effect (F(1.300,278.116) = 4.243, p = 0.030) was also found, indicating a greater decrease of suicidal ideation and behaviour in SUD patients at 1- and 3-month follow-up (from 1.46 to 0.16).
BPRS scores showed a main effect of time (F(2.345,497.091) = 301.521, p < 0.001; from 51.54 at BL to 31.84 after 12 months; Table 2; Supplementary Fig. (4 (832KB, pdf) ) in all conditions. Three interaction effects were significant, a Time × SUD effect (F(2.345,497.091) = 4.917; p = 0.005), with a greater decrease of psychiatric symptoms in patients without SUD, only at 1 month follow-up (from 54.43 to 38.59); a Time × DSM-5 diagnosis effect (F(4.689, 497.091) = 6.282, p < 0.001), with a greater improvement of psychiatric symptoms in BD (from 53.14 to 31.70) and SSOPDs (from 52.65 to 30.20) patients than in MDD (from 48.82 to 33.62); a Time × DSM-5 diagnosis × Treatment effect (F(4.689, 497.091) = 4.008, p = 0.002), showing that vortioxetine treatment was associated with greater improvement of psychiatric symptoms compared to other AD treatment for both BD and SSOPDs patients. Instead, MDD patients showed no significant differences between the two treatment conditions. No other significant interactions were detected.
3.3. Effects of Vortioxetine and other AD Treatments on Manic and Anxious Symptoms
To analyse manic and anxious symptomatology, ITT analysis with LOCF and mixed-model ANOVAs with the aforementioned independent variables were performed, with MADRS, YMRS, HAM-A scores as dependent variables. All treatment conditions were associated with a significant improvement of anxious, depressive and manic symptoms.
Regarding manic symptoms, the main effect of time showed YMRS scores to decrease significantly over time (F(2.606,555.114) = 47.652, p < 0.001; from 7.85 at BL, to 2.91 after 12 months; Table 2; Supplementary Fig. (2 (832KB, pdf) ) in all conditions. Two interaction effects were significant, a Time × DSM-5 diagnosis effect (F(5.212, 555.114) = 8.446, p < 0.001), with a greater improvement of manic symptomatology in BD (from 8.02 to 2.60) and SSOPDs (from 8.75 to 1.47) patients than in MDD (from 6.77 to 4.58); a Time×DSM-5 diagnosis × Treatment effect (F(2.606, 555.114) = 3.151, p = 0.007), showing that the vortioxetine groups reported more decrease of manic symptoms compared to OADs in both BD and SSOPDs patients, but not in MDD patients, who showed no significant differences between treatment conditions. No other significant interactions were found.
Anxious symptoms analysis revealed the main effect of time, indicating HAM-A scores to significantly decrease over time (F(2.838,604.559) = 203.090, p<0.001; from 24.77 at BL to 9.39 after 12 months; Table 2; Supplementary Fig. (3 (832KB, pdf) ) in all conditions. Four interaction effects were significant, i.e., a Time × Treatment effect (F(2.838,604.559) = 5.976, p = 0.001), indicating more anxious symptom decrease in patients treated with vortioxetine (from 25.05 to 6.94); a Time × SUD effect (F(2.838,604.559) = 4.348, p = 0.006), with a greater improvement of anxious symptoms in patients without SUD only at 1-month follow-up (from 26.34 to 14.35); a Time×DSM-5 diagnosis effect (F(5.677,604.559) = 6.335, p < 0.001), with a greater improvement of anxious symptoms in BD (from 25.87 to 8.65) and SSOPDs (from 23.23 to 7.00) patients than in MDD (from 25.21 to 12.50); and a Time×DSM-5 diagnosis × Treatment effect (F(5.677,604.559) = 5.631, p < 0.001), showing vortioxetine to reduce anxious symptoms in both BD and SSOPDs patients more than OADs, while this did not hold true for MDD patients, who did not show different effects between the two treatment conditions. No other significant interactions were found.
3.4. Effects of Vortioxetine and other AD Treatments on Craving in SUD Patients
To evaluate the presence of craving in SUD patients, ITT analysis with LOCF and mixed-model ANOVAs with the above independent variables was performed, with VAScrav scores as the dependent variable.
A main effect of time was shown on craving, that significantly decreased (F(3.465, 256.430) = 99.210, p < 0.001, η2 = 0.573; from 6.39 at BL to 0.94 after 12 months; Table 2; Supplementary Fig. (7 (832KB, pdf) ). Scores on the VAScrav did not differ between vortioxetine and OADs. No other significant effects were found.
3.5. Effects of Vortioxetine and other AD Treatments on Global Health Perception and Treatment Adherence
For QoL, we performed ITT analysis with LOCF and mixed-model ANOVAs with the above-mentioned independent variables and WHOQOL-BREF scores as the dependent variable.
We observed a steady increase in QoL, as shown by the main effect of WHOQOL-BREF scores (F(1.599,342.162)=46.306, p<0.001; from 52.09 at BL, to 69.42 after 12 months; Table 2; Supplementary Fig. (8 (832KB, pdf) ) without significant differences between conditions. No other significant effects were found.
3.6. Drug Treatment
Vortioxetine doses ranged from 5 to 20 mg (mean 15.46, SD ± 4.03, median 15 mg). OADs were also used in the approved dosage ranges that are considered to be effective. The two groups did not differ for occasional benzodiazepine/zolpidem use, or for additional antipsychotic, lithium, oxcarbazepine, lamotrigine, gabapentin or pregabalin medication.
3.7. Response/Remission Rates and Treatment Impact
Detailed response and remission rates according to the treatment group and patients’ belonging to the SUD or nonSUD subgroups are provided in Supplementary Table 1 (832KB, pdf) . About half of the vortioxetine sample was responsive to treatment (55.56%) and had remitted (46.82%) after one year according to both CGI-S and MADRS criteria; this was much less for the OADs treatment group (36% and 12%, respectively). Generally, response and remission rates were higher for the CGI-S criterion than for the MADRS criterion. We did not observe the expected reduced responsiveness and remission rates in the two SUD subgroups; numerically, there was an advantage for both with respect to their respective nonSUD groups. Treatment with either method, vortioxetine or OADs, had a strong positive clinical impact, as assessed with the CGI-S. Effect sizes for all groups were large according to Cohen [50] and huge according to Sawilowsky [51], all beingmore than 3 (Supplementary Table 2 (832KB, pdf) ). For vortioxetine, effect sizes were generally larger numerically; again, belonging to the SUD subgroups was not associated with a weaker effect size, and the opposite if anything.
3.8. Effects of Gender and Age
To control for age and gender effects, we conducted a mixed-model ANOVAs with Treatment and Gender as between-subjects variables, Time as within-subjects variable, age as a covariate, and MADRS and CGI-S scores as dependent variables. Here, there was the main effect of Gender (F(2,643, 584,087) = 3.719, p < 0.015) on MADRS scores, with women responding less than men. There was also the main effect of age on MADRS scores (F(2,634, 579,378) = 3.041, p = 0.035), but despite this, the main effects and interactions remained significant, and their direction remained unchanged. No significant effects emerged with CGI-S scores as a dependent variable when adjusting for gender or age.
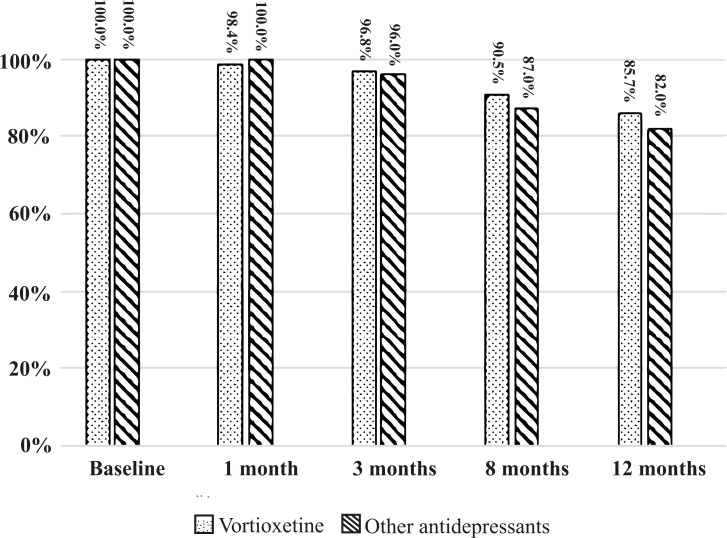
Safety. Regarding treatment adherence/drop-out rates, 18 patients in each group dropped out before the 12-month follow-up. In the vortioxetine group, ten dropped out for nausea, five for safety/inefficacy, and three for personal choice; in the OADs group, ten dropped out for nausea, one for excitement, one for psychomotor agitation, four for inefficacy/poor response, one for tremor, and one for sexual side effects. Treatment adherence at 12 months was 85.7%for patients treated with vortioxetine and 82%for those treated with OADs (Fig. 1).
Fig. (1).
Percentage of patients completing treatment throughout the study. No significant differences between vortioxetine and other antidepressants. Columns indicate the percentage of participants still in the study.
Side effects . During the study period, 68 (53.97%) adverse events occurred in 63 patients on vortioxetine (50%) and 62 (62%) in 55 (55%) patients on OADs. Besides adverse events eventually leading to drop-out, other side effects were tolerated and mild to moderate. During treatment with vortioxetine, 15.08% presented nausea (vs. 12% with OADs), 9.52% gastrointestinal upset (vs. 9% OADs), 3.99% vomiting (vs. 10% OADs), 3.17% diarrhoea (vs. 7% OADs), 2.38% constipation (vs. 4% OADs), 2.38% dizziness (vs. 6% OADs), 2.38% xerostomia (vs. 5% OADs), 0.79% sleep disorders (vs. 2% OADs); no weight gain or sexual side effects occurred with vortioxetine, while with OADs, figures were 6% and 15%, respectively. Adverse events tended to be transient and to improve with time. No late adverse events took place in the vortioxetine group, while there were two cases of late-onset erectile difficulties (2%) and 1 case pf QTc prolongation (1%) in the OADs group.
4. DISCUSSION
In this study, we found comparable effects of both vortioxetine and other antidepressants on primary (CGI-S and MADRS) and secondary outcomes (BPRS, YMRS, HAM-A, C-SSRS, VAScrav, and WHOQOL-BREF). Both the types of treatment were followed by improvements in all scales. However, in patients on vortioxetine, we found a better clinical improvement with time of patients with BD or SSOPDs than with other antidepressants (on both CGI-S and BPRS). This is a previously unreported finding, as most vortioxetine studies do not include patients with BD and even less are conducted on patients with SSOPDs. We further found a greater reduction with time in depression, anxiety, and manic symptoms in patients who were affected by BD and SSOPDs and taking vortioxetine, compared to patients on other antidepressants, while in patients with MDD, depression improved similarly in the two treatment groups. Anxious symptoms of patients with SUD were less likely to respond favourably than those in nonSUD patients. Suicide risk, craving in SUD-comorbid patients, and quality-of-life progressively improved with the two treatment types without intergroup differences.
We followed a BL and 1, 3, 8, and 12 months assessment schedule; this is different from what has been employed by other vortioxetine studies, both naturalistic [54] and randomised [55], only partially overlapping with the latter.
We investigated the effects of vortioxetine vs. OADs in a naturalistic study on MDE symptomatology in a variety of patients with or without SUD comorbidity and MDD, BD or SSOPDs. Although there are no clear indications for the use of vortioxetine in MD and SSOPDs, it may be justified by the presence of MDEs. However, there may be an expansion for its use in other conditions as well, as vortioxetine was found to improve negative symptoms of schizophrenia when added on risperidone [56] and has been advocated in neuropathic pain [57]. In contrast, it did not show significant improvement with respect to placebo in binge-eating disorder [58], and meta-analyses [59, 60] did not confirm an initial positive report of the response of generalised anxiety disorder to vortioxetine [61], but the question of its possible use in other anxiety disorders is left open [62]. Vortioxetine acts through the PI3K/AKT intracellular pathway [16] and could interact with lithium, which also activates this pathway [63], in patients with anMDE and BD, or could strengthen the 5-HT7 antagonism of antipsychotics like risperidone [64] and lurasidone [65] in patients with anSSOPD and MDE receiving such treatment. It is interesting that vortioxetine added on risperidone proved to improve negative symptoms better than the risperidone-placebo combination in one study [66], and the pro-cognitive effects of 5-HT7 antagonism might have played a role in this respect.
There have been no studies focusing on depression-SUD comorbidity so far, except for one study that compared MDD patients with or without comorbid alcohol use disorder [67]. This study found no significant difference in response or remission rates between patients with or without alcohol use disorder, although the latter showed a numerical advantage that was not significant. We found similar response rates for the two treatment groups and for SUD nonSUD subsets, but the absence of SUD in our study wasrelated toa better response of anxious symptomatology, while in Di Nicola et al.’s [67] study, itwas not. Differences in methodology, sample size, and the SUD involved might underlie this discrepancy. We also did not find differences in response/remission rates between patients with or without a comorbid SUD.
In this naturalistic study, we had a relatively low drop-out rate, i.e., 15.93%%, which is markedly lower than the 36.4% rate reported by a similar study, which also had a much shorter duration (12 weeks) than ours [54].
We observed a similar adverse event rate with respect to the figures commonly reported in the literature for both vortioxetine [68-72] and OADs [69], despite the long-term exposure of our patients to their medications. The occurrence was similar to that of side effects in healthy Japanese volunteers [73]. It should be noted that there were three adverse events with a late onset, arising between the sixth and the eighthmonths of treatment in three MDD patients. One was a QTc prolongation, that was promptly managed with short-term mexiletine and disappeared, occurring with 15 mg mirtazapine, the other two were both erectile dysfunctions, and occurred with paroxetine 20 mg and venlafaxine 150 mg. Our data are in line with a relatively safe cardiological profile [74] and a low incidence of sexual side effects with vortioxetine [75].
Limitations. Our study was sufficiently powered for obtaining an alpha of > 0.8; however, by splitting our sample intopatients with and without SUD, we produced undersized subsamples. We performed parametric statistics (data not reported) without testing normality for SUD and nonSUD groups. Furthermore, our study was observational, with no fixed vortioxetine or other antidepressant dosages. The focus ofvortioxetine treatment is currently on cognitive functions, but our study did not assess them. However, we adopted an adequate schedule to assess the effects of medication in patients with an MDE who could have not only MDD but BD or SSOPDs and we analysed data also on the basis of the SUD-nonSUD dichotomy. Our population was diagnostically heterogeneous as for underlying diagnosis, but homogeneous for episode. Our schedule did not foresee a 7- day timepoint from baseline, so we were not able to assess the reported early effects of vortioxetine on depressive symptoms [76,77], although these studies used the intravenous route, which is usually faster in obtaining clinical results. Moreover, although additional medications did not differ between the groups, we cannot exclude they were involved in the final expression of the effects of treatment. The splitting of our sample to various subsamples could have produced smaller groups that were insufficient to allow for possible treatment differences to show-off; furthermore, the results obtained regarding a better efficacy of vortioxetine in the BD and SSOPPD subsamples need further replication, as they are based on relatively small samples.
However, at odds with the bulk of vortioxetine studies [78], we investigated the effects of gender and age and found that women’s depressive episodes respond less than those of men regardless ofwhether they were treated with vortioxetine or OADs and that age influenced MADRS scores without changing the direction of interactions. This partially matches the finding that vortioxetine and sertraline did not differ in efficacy or safety measures in a sample of elderly people with MDD after 6 weeks [79]. Overall, severity was not significantly affected by gender or age. However, the factors affecting the effects of drugs in individuals and populations are multiple and variable [80-82]. Summarising, we are displeased to conclude with the inevitable, dreaded sentence, further studies are needed, but it couldn’t be otherwise.
CONCLUSION
Vortioxetine proved to be as effective in improving a major depressive episode during the course of major depressive disorder, bipolar disorder or schizophrenia spectrum, and other psychotic disorders in patients with or without substance use disorder after a one-year treatment, and showed few and tolerable side effects. It did not differ much from other antidepressants but had generally a larger effect on primary outcomes. Having a bipolar or a schizophrenia spectrum disorder prompted a better clinical response of the major depressive episode in patients taking vortioxetine than in those taking other antidepressants. Men responded better than women. Substance use comorbidity did not affect the response, save for a reduced response of anxiety.
AUTHORS’ CONTRIBUTIONS
GDK, GL, and SDF conceived the study; GL, MM, IC and SDF saw the patients and carried out the treatment; EA, FP, MM, IC and GL assessed the patients; EA, FP, MM, IC, GDK, and GL implemented the database; IC, FP, EA, GL, MM, and GDK performed literature searches; FP and GDK performed statistical analyses; GL, FP, SDF, and GDK wrote the first draft; GL, IC, MM, FP, and GDK wrote Introduction, Methods, and Results; GL, MM, GDK, EA, and SDF wrote the Discussion and Conclusions; GL, GDK and SDF supervised the final form. All authors wrote substantial portions of the paper and viewed and approved the final version.
ACKNOWLEDGEMENTS
The authors wish to thank Ms. Mimma Ariano, Ms. Ales Casciaro, Ms. Teresa Prioreschi, and Ms. Susanna Rospo, Librarians of the Sant’Andrea Hospital, Faculty of Medicine and Psychology, Sapienza University of Rome, for rendering precious bibliographical material accessible.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the local Ethical Committee ASL RM2 Act 3/2018 (Italy).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. The study followed the Principles of Human Rights, as adopted by the World Medical Association at the 18th WMA General Assembly, Helsinki, Finland, June 1964, and subsequently amended by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013.
CONSENT FOR PUBLICATION
All patients provided free, informed consent for participation and treatment received.
STANDARD OF REPORTING
CONSORT guidelines and methodologies were followed.
AVAILABILITY OF DATA AND MATERIALS
Available unrestrictedly upon reasonable demand to the corresponding author.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5 th ed. (DSM-5).; American Psychiatric Association: Arlington, VA; 2013. [Google Scholar]
- 2.Marcus M., Yasamy M.T., van Ommeren M., Chisholm D. Depression. A global public health concern. 2012. Available from: https://www.who.int/mental_health/management/depression/who_paper_depression_wfmh_2012.pdf.
- 3.de Bartolomeis A., Fagiolini A., Maina G. Vortioxetina nel trattamento della depressione maggiore. Riv. Psichiatr. 2016;51(6):215–230. doi: 10.1708/2596.26720. [DOI] [PubMed] [Google Scholar]
- 4.Jaeger J., Berns S., Uzelac S., Davis-Conway S. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 2006;145(1):39–48. doi: 10.1016/j.psychres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Greer T.L., Kurian B.T., Trivedi M.H. Defining and measuring functional recovery from depression. CNS Drugs. 2010;24(4):267–284. doi: 10.2165/11530230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Conradi H.J., Ormel J., de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol. Med. 2011;41(6):1165–1174. doi: 10.1017/S0033291710001911. [DOI] [PubMed] [Google Scholar]
- 7.Yang H., Chuzi S., Sinicropi-Yao L., Johnson D., Chen Y., Clain A., Baer L., McGrath P.J., Stewart J.W., Fava M., Papakostas G.I. Type of residual symptom and risk of relapse during the continuation/maintenance phase treatment of major depressive disorder with the selective serotonin reuptake inhibitor fluoxetine. Eur. Arch. Psychiatry Clin. Neurosci. 2010;260(2):145–150. doi: 10.1007/s00406-009-0031-3. [DOI] [PubMed] [Google Scholar]
- 8.Thase M.E., Simons A.D. Cognitive behavior therapy and relapse of nonbipolar depression: parallels with pharmacotherapy. Psychopharmacol. Bull. 1992;28(2):117–122. [PubMed] [Google Scholar]
- 9.Thase M.E., Simons A.D. The applied use of psychotherapy in the study of the psychobiology of depression. J. Psychother. Pract. Res. 1992;1(1):72–80. [PMC free article] [PubMed] [Google Scholar]
- 10.Ishak W.W., Greenberg J.M., Cohen R.M. Predicting relapse in major depressive disorder using patient-reported outcomes of depressive symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression (IBI-D). J. Affect. Disord. 2013;151(1):59–65. doi: 10.1016/j.jad.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotrena C., Branco L.D., Kochhann R., Shansis F.M., Fonseca R.P. Quality of life, functioning and cognition in bipolar disorder and major depression: A latent profile analysis. Psychiatry Res. 2016;241:289–296. doi: 10.1016/j.psychres.2016.04.102. [DOI] [PubMed] [Google Scholar]
- 12. European Medicines Agency. Brintellix Vortioxetine. 2013. Assessment report for an initial marketing authorisation application. Procedure No. EMEA/H/C/002717. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR _-_Public_assessment_report/human/002717/WC500159447.pdf.
- 13.Bang-Andersen B., Ruhland T., Jørgensen M., Smith G., Frederiksen K., Jensen K.G., Zhong H., Nielsen S.M., Hogg S., Mørk A., Stensbøl T.B. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J. Med. Chem. 2011;54(9):3206–3221. doi: 10.1021/jm101459g. [DOI] [PubMed] [Google Scholar]
- 14.Riga M.S., Sánchez C., Celada P., Artigas F. Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: Focus on glutamatergic and GABAergic neurotransmission. Neuropharmacology. 2016;108:73–81. doi: 10.1016/j.neuropharm.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Hlavacova N., Li Y., Pehrson A., Sanchez C., Bermudez I., Csanova A., Jezova D., Franklin M. Effects of vortioxetine on biomarkers associated with glutamatergic activity in an SSRI insensitive model of depression in female rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;82:332–338. doi: 10.1016/j.pnpbp.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Lv G.B., Wang T.T., Zhu H.L., Wang H.K., Sun W., Zhao L.F. Vortioxetine induces apoptosis and autophagy of gastric cancer AGS cells via the PI3K/AKT pathway. FEBS Open Biol. 2020;10(10):2157–2165. doi: 10.1002/2211-5463.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun B., Lv Y., Xu H., Qi C., Li C., Liu P. Effects of Vortioxetine on depression model rats and expression of BDNF and Trk B in hippocampus. Exp. Ther. Med. 2020;20(3):2895–2902. doi: 10.3892/etm.2020.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldwin D.S., Chrones L., Florea I., Nielsen R., Nomikos G.G., Palo W., Reines E. The safety and tolerability of vortioxetine: Analysis of data from randomized placebo-controlled trials and open-label extension studies. J. Psychopharmacol. 2016;30(3):242–252. doi: 10.1177/0269881116628440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntyre R.S., Lophaven S., Olsen C.K. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int. J. Neuropsychopharmacol. 2014;17(10):1557–1567. doi: 10.1017/S1461145714000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katona C., Hansen T., Olsen C.K. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int. Clin. Psychopharmacol. 2012;27(4):215–223. doi: 10.1097/YIC.0b013e3283542457. [DOI] [PubMed] [Google Scholar]
- 21.McIntyre R.S., Florea I., Tonnoir B., Loft H., Lam R.W., Christensen M.C. Efficacy of vortioxetine on cognitive functioning in working patients with major depressive disorder. J. Clin. Psychiatry. 2017;78(1):115–121. doi: 10.4088/JCP.16m10744. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez E., Perez V., Artigas F. Pharmacology and clinical potential of vortioxetine in the treatment of major depressive disorder. Neuropsychiatr. Dis. Treat. 2014;10:1297–1307. doi: 10.2147/NDT.S41387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Sukhni M., Maruschak N.A., McIntyre R.S. Vortioxetine: a review of efficacy, safety and tolerability with a focus on cognitive symptoms in major depressive disorder. Expert Opin. Drug Saf. 2015;14(8):1291–1304. doi: 10.1517/14740338.2015.1046836. [DOI] [PubMed] [Google Scholar]
- 24.Kelliny M., Croarkin P.E., Moore K.M., Bobo W.V. Profile of vortioxetine in the treatment of major depressive disorder: an overview of the primary and secondary literature. Ther. Clin. Risk Manag. 2015;11:1193–1212. doi: 10.2147/TCRM.S55313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen P.L., Mahableshwarkar A.R., Chen Y., Chrones L., Clayton A.H. Effect of vortioxetine vs. escitalopram on sexual functioning in adults with well-treated major depressive disorder experiencing SSRI-induced sexual dysfunction. J. Sex. Med. 2015;12(10):2036–2048. doi: 10.1111/jsm.12980. [DOI] [PubMed] [Google Scholar]
- 26.Schwasinger-Schmidt T.E., Macaluso M. Other Antidepressants. Handb. Exp. Pharmacol. 2019;250:325–355. doi: 10.1007/164_2018_167. [DOI] [PubMed] [Google Scholar]
- 27.Carey T.L. Use of antidepressants in patients with co-occurring depression and substance use disorders. Handb. Exp. Pharmacol. 2019;250:359–370. doi: 10.1007/164_2018_162. [DOI] [PubMed] [Google Scholar]
- 28.Pettinati H.M., O’Brien C.P., Dundon W.D. Current status of co-occurring mood and substance use disorders: a new therapeutic target. Am. J. Psychiatry. 2013;170(1):23–30. doi: 10.1176/appi.ajp.2012.12010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasin D.S., Hatzenbueler M., Smith S., Grant B.F. Co-occurring DSM-IV drug abuse in DSM-IV drug dependence: results from the national epidemiologic survey on alcohol and related conditions. Drug Alcohol Depend. 2005;80(1):117–123. doi: 10.1016/j.drugalcdep.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Hasin D., Kilcoyne B. Comorbidity of psychiatric and substance use disorders in the United States: current issues and findings from the NESARC. Curr. Opin. Psychiatry. 2012;25(3):165–171. doi: 10.1097/YCO.0b013e3283523dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessler R.C., Chiu W.T., Demler O., Merikangas K.R., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merikangas K.R., Akiskal H.S., Angst J., Greenberg P.E., Hirschfeld R.M., Petukhova M., Kessler R.C. Lifetime and 12- month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch. Gen. Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Chiara G., Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blandina P., Goldfarb J., Craddock-Royal B., Green J.P. Release of endogenous dopamine by stimulation of 5-hydroxytryptamine3 receptors in rat striatum. J. Pharmacol. Exp. Ther. 1989;251(3):803–809. [PubMed] [Google Scholar]
- 35.Tricklebank M.D. Interactions between dopamine and 5-HT3 receptors suggest new treatments for psychosis and drug addiction. Trends Pharmacol. Sci. 1989;10(4):127–129. doi: 10.1016/0165-6147(89)90157-0. [DOI] [PubMed] [Google Scholar]
- 36.Grant K.A. The role of 5-HT3 receptors in drug dependence. Drug Alcohol Depend. 1995;38(2):155–171. doi: 10.1016/0376-8716(95)01120-N. [DOI] [PubMed] [Google Scholar]
- 37.Müller C.P., Carey R.J., Huston J.P., De Souza Silva M.A. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog. Neurobiol. 2007;81(3):133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Kirby L.G., Zeeb F.D., Winstanley C.A. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61(3):421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.First M.B., Williams J.B.W., Karg R.S., Spitzer R.L. Structured Clinical Interview for DSM-5® Disorders—Clinician Version (SCID-5-CV). American Psychiatric Association Publishing, Inc.: Arlington, VA; 2016. [Google Scholar]
- 40.Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare: Rockville, Maryland, Chapter 028 CGI Clinical Global Impressions; 1976. pp. 217–222. [Google Scholar]
- 41.Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134(4):382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 42.Ventura J., Lukoff D., Nuechterlein K.H., Liberman R.P., Green M., Shaner A. Appendix 1: Brief Psychiatric Rating Scale (BPRS) Expanded version (4.0) scales, anchor points and administration manual. Int. J. Methods Psychiatr. Res. 1993;3:227–244. [Google Scholar]
- 43.Overall J.E., Gorham D.R. The Brief Psychiatric Rating Scale. Psychol. Rep. 1962;10(3):799–812. doi: 10.2466/pr0.1962.10.3.799. [DOI] [Google Scholar]
- 44.Overall J.E. Psychological Measurements in Psychopharmacology. Modern Problems in Pharmacopsychiatry. Vol. 7. Pichot, P.; Karger, S., Eds.; Verlag: Basel (CH); 1974. he brief psychiatric rating scale in psychopharmacology research. pp. 67–78. [DOI] [PubMed] [Google Scholar]
- 45.Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133(5):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 47.Posner K., Brown G.K., Stanley B., Brent D.A., Yershova K.V., Oquendo M.A., Currier G.W., Melvin G.A., Greenhill L., Shen S., Mann J.J. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicholson A.N. Visual analogue scales and drug effects in man. Br. J. Clin. Pharmacol. 1978;6(1):3–4. doi: 10.1111/j.1365-2125.1978.tb01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization (WHO) The World Health Organization Quality of Life (WHOQOL)-BREF. World Health Organization: Geneva, Switzerland; 2004. [Google Scholar]
- 50.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge: New York; 1988. [Google Scholar]
- 51.Sawilowsky S. New effect size rules of thumb. J. Mod. Appl. Stat. Methods. 2009;8:467–474. doi: 10.22237/jmasm/1257035100. [DOI] [Google Scholar]
- 52.Lingjaerde O., Ahlfors U.G., Bech P., Dencker S.J., Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr. Scand. Suppl. 1987;334(Suppl. 334):1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 53.Little R., Yau L. Intent-to-treat analysis for longitudinal studies with drop-outs. Biometrics. 1996;52(4):1324–1333. doi: 10.2307/2532847. [DOI] [PubMed] [Google Scholar]
- 54.De Carlo V., Vismara M., Grancini B., Benatti B., Bosi M.F., Colombo A., Viganò C.A., Dell’Osso B. Effectiveness, tolerability, and dropout rates of vortioxetine in comorbid depression: A naturalistic study. Hum. Psychopharmacol. 2020;35(5):e2750. doi: 10.1002/hup.2750. [DOI] [PubMed] [Google Scholar]
- 55.Ostuzzi G., Gastaldon C., Barbato A., D’Avanzo B., Tettamanti M., Monti I., Aguglia A., Aguglia E., Alessi M.C., Amore M., Bartoli F., Biondi M., Bortolaso P., Callegari C., Carrà G., Caruso R., Cavallotti S., Crocamo C., D’Agostino A., De Fazio P., Di Natale C., Giusti L., Grassi L., Martinotti G., Nosé M., Papola D., Purgato M., Rodolico A., Roncone R., Tarsitani L., Turrini G., Zanini E., Amaddeo F., Ruggeri M., Barbui C. Tolerability and efficacy of vortioxetine versus SSRIs in elderly with major depression. Study protocol of the VESPA study: a pragmatic, multicentre, open-label, parallel-group, superiority, randomized trial. Trials. 2020;21(1):695. doi: 10.1186/s13063-020-04460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moazen-Zadeh E., Bayanati S., Ziafat K., Rezaei F., Mesgarpour B., Akhondzadeh S. Vortioxetine as adjunctive therapy to risperidone for treatment of patients with chronic schizophrenia: A randomised, double-blind, placebo-controlled clinical trial. J. Psychopharmacol. 2020;34(5):506–513. doi: 10.1177/0269881120909416. [DOI] [PubMed] [Google Scholar]
- 57.Zuena A.R., Maftei D., Alemà G.S., Dal Moro F., Lattanzi R., Casolini P., Nicoletti F. Multimodal antidepressant vortioxetine causes analgesia in a mouse model of chronic neuropathic pain. Mol. Pain. 2018;14:1744806918808987. doi: 10.1177/1744806918808987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant J.E., Valle S., Cavic E., Redden S.A., Chamberlain S.R. A double-blind, placebo-controlled study of vortioxetine in the treatment of binge-eating disorder. Int. J. Eat. Disord. 2019;52(7):786–794. doi: 10.1002/eat.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pae C-U., Wang S-M., Han C., Lee S-J., Patkar A.A., Masand P.S., Serretti A. Vortioxetine, a multimodal antidepressant for generalized anxiety disorder: a systematic review and meta-analysis. J. Psychiatr. Res. 2015;64:88–98. doi: 10.1016/j.jpsychires.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 60.Qin B., Huang G., Yang Q., Zhao M., Chen H., Gao W., Yang M. Vortioxetine treatment for generalised anxiety disorder: a meta-analysis of anxiety, quality of life and safety outcomes. BMJ Open. 2019;9(11):e033161. doi: 10.1136/bmjopen-2019-033161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bidzan L., Mahableshwarkar A.R., Jacobsen P., Yan M., Sheehan D.V. Vortioxetine (Lu AA21004) in generalized anxiety disorder: results of an 8-week, multinational, randomized, double-blind, placebo-controlled clinical trial. Eur. Neuropsychopharmacol. 2012;22(12):847–857. doi: 10.1016/j.euroneuro.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 62.Yee A., Ng C.G., Seng L.H. Vortioxetine treatment for anxiety disorder: A meta-analysis study. Curr. Drug Targets. 2018;19(12):1412–1423. doi: 10.2174/1389450118666171117131151. [DOI] [PubMed] [Google Scholar]
- 63.Dong H., Zhang X., Dai X., Lu S., Gui B., Jin W., Zhang S., Zhang S., Qian Y. Lithium ameliorates lipopolysaccharide-induced microglial activation via inhibition of toll-like receptor 4 expression by activating the PI3K/Akt/FoxO1 pathway. J. Neuroinflammation. 2014;11:140. doi: 10.1186/s12974-014-0140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith C., Rahman T., Toohey N., Mazurkiewicz J., Herrick- Davis K., Teitler M. Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol. Pharmacol. 2006;70(4):1264–1270. doi: 10.1124/mol.106.024612. [DOI] [PubMed] [Google Scholar]
- 65.Okada M., Fukuyama K., Okubo R., Shiroyama T., Ueda Y. Lurasidone sub-chronically activates serotonergic transmission via desensitization of 5-HT1A and 5-HT7 receptors in dorsal raphe nucleus. Pharmaceuticals (Basel) 2019;12(4):149. doi: 10.3390/ph12040149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waters K.A., Stean T.O., Hammond B., Virley D.J., Upton N., Kew J.N., Hussain I. Effects of the selective 5-HT(7) receptor antagonist SB-269970 in animal models of psychosis and cognition. Behav. Brain Res. 2012;228(1):211–218. doi: 10.1016/j.bbr.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Di Nicola M., Pepe M., Panaccione I., Moccia L., Dattoli L., Molinaro M., Sani G., Janiri L., McIntyre R.S. Effect of vortioxetine in subjects with major depressive and alcohol use disorders: a 6-month retrospective analysis. CNS Spectr. 2020:1–9. doi: 10.1017/S109285292000173X. [DOI] [PubMed] [Google Scholar]
- 68.Nishimura A., Aritomi Y., Sasai K., Kitagawa T., Mahableshwarkar A.R. Randomized, double-blind, placebo-controlled 8-week trial of the efficacy, safety, and tolerability of 5, 10, and 20 mg/day vortioxetine in adults with major depressive disorder. Psychiatry Clin. Neurosci. 2018;72(2):64–72. doi: 10.1111/pcn.12565. [DOI] [PubMed] [Google Scholar]
- 69.Wagner G., Schultes M.T., Titscher V., Teufer B., Klerings I., Gartlehner G. Efficacy and safety of levomilnacipran, vilazodone and vortioxetine compared with other second-generation antidepressants for major depressive disorder in adults: A systematic review and network meta-analysis. J. Affect. Disord. 2018;228:1–12. doi: 10.1016/j.jad.2017.11.056. [DOI] [PubMed] [Google Scholar]
- 70.Zheng J., Wang Z., Li E. The efficacy and safety of 10 mg/day vortioxetine compared to placebo for adult major depressive disorder: a meta-analysis. Afr. Health Sci. 2019;19(1):1716–1726. doi: 10.4314/ahs.v19i1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J., Liu X.F., Feng C., Bao Q., Fu H.R. Efficacy and safety of vortioxetine for the treatment of major depressive disorder: a randomised double-blind placebo-controlled study. Int. J. Psychiatry Clin. Pract. 2019;23(4):245–250. doi: 10.1080/13651501.2017.1397700. [DOI] [PubMed] [Google Scholar]
- 72.Inoue T., Sasai K., Kitagawa T., Nishimura A., Inada I. Randomized, double-blind, placebo-controlled study to assess the efficacy and safety of vortioxetine in Japanese patients with major depressive disorder. Psychiatry Clin. Neurosci. 2020;74(2):140–148. doi: 10.1111/pcn.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsuno K., Nakamura K., Aritomi Y., Nishimura A. Pharmacokinetics, safety, and tolerability of vortioxetine following single- and multiple-dose administration in healthy Japanese adults. Clin. Pharmacol. Drug Dev. 2018;7(3):319–331. doi: 10.1002/cpdd.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bordet C., Rousseau V., Montastruc F., Montastruc J.L. QT prolongation and vortioxetine: a post-marketing study and comparison with other serotonin reuptake inhibitors. Psychopharmacology (Berl.) 2020;237(4):1245–1247. doi: 10.1007/s00213-020-05461-8. [DOI] [PubMed] [Google Scholar]
- 75.Jacobsen P., Zhong W., Nomikos G., Clayton A. Paroxetine, but not vortioxetine, impairs sexual functioning compared with placebo in healthy adults: A randomized, controlled trial. J. Sex. Med. 2019;16(10):1638–1649. doi: 10.1016/j.jsxm.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 76.Vieta E., Florea I., Schmidt S.N., Areberg J., Ettrup A. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int. Clin. Psychopharmacol. 2019;34(4):153–160. doi: 10.1097/YIC.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rancans E., Zambori J., Dalsgaard M., Baayen C., Areberg J., Ettrup A., Florea I. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 7-day randomized, double-blind, placebo-controlled exploratory study. Int. Clin. Psychopharmacol. 2020;35(6):305–312. doi: 10.1097/YIC.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Santos-Casado M., Guisado-Gil A.B., Santos-Ramos B. Systematic review of gender bias in vortioxetine clinical trials. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;110088:110088. doi: 10.1016/j.pnpbp.2020.110088. [DOI] [PubMed] [Google Scholar]
- 79.Borhannejad F., Shariati B., Naderi S., Shalbafan M., Mortezaei A., Sahebolzamani E., Saeb A., Hosein Mortazavi S., Kamalzadeh L., Aqamolaei A., Ali Noorbala A., Namazi-Shabestari A., Akhondzadeh S. Comparison of vortioxetine and sertraline for treatment of major depressive disorder in elderly patients: A double-blind randomized trial. J. Clin. Pharm. Ther. 2020;45(4):804–811. doi: 10.1111/jcpt.13177. [DOI] [PubMed] [Google Scholar]
- 80.Khushboo; Sharma, B. Antidepressants: Mechanism of action, toxicity and possible amelioration. J. Appl. Biotechnol. Bioeng. 2017;3(5):437–448. doi: 10.15406/jabb.2017.03.00082. [DOI] [Google Scholar]
- 81.Gupta V.K., Sharma B. Modulations of mammalian brain functions by antidepressant drugs: role of some phytochemicals as prospective antidepressants. Evid. Based Med. Practice. 2016;2(2):1000103. doi: 10.4172/2471-9919.1000103. [DOI] [Google Scholar]
- 82.Khushboo; Sharma, B. Factors inducing depression as effective tool in therapy. Med. Clin. Arch. 2019;3:1–4. doi: 10.15761/MCA.1000163. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.
Data Availability Statement
Available unrestrictedly upon reasonable demand to the corresponding author.