Abstract
The edible cyanobacterium Spirulina platensis and its chief biliprotein C-Phycocyanin have shown protective activity in animal models of diverse human health diseases, often reflecting antioxidant and anti-inflammatory effects. The beneficial effects of C-Phycocyanin seem likely to be primarily attributable to its covalently attached chromophore Phycocyanobilin (PCB). Within cells, biliverdin is generated from free heme and it is subsequently reduced to bilirubin. Although bilirubin can function as an oxidant scavenger, its potent antioxidant activity reflects its ability to inactivate some isoforms of NADPH oxidase. Free bilirubin can also function as an agonist for the aryl hydrocarbon receptor (AhR); this may explain its ability to promote protective Treg activity in cellular and rodent models of inflammatory disease. AhR agonists also promote transcription of the gene coding for Nrf-2, and hence can up-regulate phase 2 induction of antioxidant enzymes, such as HO-1. Hence, it is proposed that C-Phycocyanin/PCB chiefly exert their protective effects via inhibition of NADPH oxidase activity, as well as by AhR agonism that both induces Treg activity and up-regulates phase 2 induction. This simple model may explain their potent antioxidant/anti-inflammatory effects. Additionally, PCB might mimic biliverdin in activating anti-inflammatory signaling mediated by biliverdin reductase. This essay reviews recent research in which C-Phycocyanin and/or PCB, administered orally, parenterally, or intranasally, have achieved marked protective effects in rodent and cell culture models of Ischemic Stroke and Multiple Sclerosis, and suggests that these agents may likewise be protective for Alzheimer’s disease, Parkinson’s disease, and in COVID-19 and its neurological complications.
Keywords: Phycocyanobilin, C-Phycocyanin, antioxidant, anti-inflammatory, regulatory T cells, Alzheimer’s disease, Multiple Sclerosis, ischemic stroke, Parkinson’s disease, COVID-19
1. INTRODUCTION
Natural complementary therapies intended for oral administration, and generally presumed to be safe, are known as nutraceuticals [1]. Recently, the concept of nutraceuticals has been widening to include whole foods, an extract of foods or herbs, or purified compounds derived from foods or herbs that could be effectively used for preventing and/or treating human health disorders [2]. Indeed, public interest in natural health-promoting foods or their specific components has been rapidly growing. In the US in 2007, 38% of adults had preferred to use such kind of alternative treatments instead of conventional therapies [3]. Certain natural products with prominent antioxidant properties have shown potential as polyvalent therapeutic options for several diseases, with integrated and interconnected target effects [4]. This has led to the development of nutraceutical interventions for several neurodegenerative diseases. Among them, curcumin (grapes), quercetin (apples), resveratrol (grapes), epigallocatechin-3-gallate (green tea), coenzyme Q10, omega-3 polyunsaturated fatty acids, α-lipoic acid, L-sulforaphane (broccoli), thiosulfonate allicin (garlic), rosmarinic or carnosic acids (rosemary), have been found to antagonize pathological mechanisms of neurodegeneration in pre-clinical research [5]. A recent review highlighted the observation that, although most clinical trials using these nutraceuticals have found them to be safe, these studies have recruited small sample sizes and have been of short duration; they typically have proposed that future large-scale well-controlled clinical assays are mandatory to continue developing these and other novel neuroprotective nutraceuticals [6]. Of special interest in this regard are those neurodegenerative conditions that are related to age and inflammation, such as Ischemic Stroke (IS), Multiple Sclerosis (MS), Alzheimer’s (AD) and Parkinson’s diseases (PD), which are major health problems worldwide [7]. An epidemiological estimate has projected a dramatic increase in the global incidence of these disorders, such that by the year 2050, approximately 47 million patients with dementia and other neurodegenerative illnesses [8]. In addition, the novel 2019 coronavirus disease (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), results in a variety of neurological symptoms including headache, confusion, fatigue and cerebrovascular events, with no currently available effective symptomatic or disease-modifying therapies targeting these complications [9]. These challenges will negatively impact on public health systems and global economies, and impose a huge burden on individual quality of life.
A nutraceutical medicine that has gained growing interest in the last two decades is the biliprotein C-Phycocyanin (C-PC) and its prosthetic group Phycocyanobilin (PCB), derived from green-blue algae and cyanobacteria, most notably the cyanobacterial species Spirulina (Arthrospira) platensis (Sp). Sp extracts have served as an important diet supplement during centuries for ancient cultures, such as Mexican Aztecs and Mayans, African communities (used even as the sole nutritional source in famine times), and Chinese culture [10]. Its outstanding nutritional value has been recognized by the US National Aeronautics and Space Administration (NASA), which stated that 1 kg of Sp was in some respects nutritionally equivalent to a fruits and vegetables amount of 1000 kg; hence, it was recommended for supplementing the diet of astronauts during their space missions [11]. Other active ingredients of raw Sp solutions and food-grade C-PC preparations includes minor amounts of different polysaccharides, polyunsaturated fatty acids, carotenoids, essential amino acids, vitamins and minerals [12]. C-PC consists of two protein subunits, α and β, which are linked covalently to the tetrapyrrole chromophore PCB; two molecules of PCB are attached to α subunit, and one to β subunit [13]. Each C-PC polypeptide chain functions to maintain the structural stability of the PCB chromophores, which harvest light energy to fuel the cyanobacteria photosynthesis process [14]. In 1998, a Cuban group was the first to report some of the antioxidant properties of C-PC, demonstrating its potent scavenging activities for hydroxyl radical (•OH), alkoxyl radical (RO•) and superoxide anion (O2•−) [15]; in follow-up studies, C-PC’s ability to scavenge additional oxidant chemical species (e.g. hypochlorite, singlet oxygen, H2O2) was demonstrated, as well as its inhibiting effect on several redox imbalance paradigms [16, 17]. Soon afterwards, the first antioxidant effects of PCB were reported: inhibiting the lipid peroxidation process in artificial liposomes [18], scavenging peroxynitrite species (ONOO–), and inhibiting oxidative damage to DNA [19]. These early results suggested that the prosthetic tetrapyrrolic group might be a critical determinant for the potent antioxidant activities of this biliprotein.
The main objective of this review is to thoroughly expose the pertinent research pointing to the potential utility of PCB as a nutraceutical intervention for major neurodegenerative diseases and COVID-19-related neurological injuries. Our view is supported by data obtained using purified PCB, its human homolog tetrapyrroles (bilirubin and biliverdin), as well as Sp extracts and C-PC preparations, on the reasonable presumption that free PCB or PCB oligopeptides are released from these sources after in vivo administration [20].
2. HOMOLOGY OF PHYCOCYANOBILIN WITH BILIVERDIN AND BILIRUBIN
The heme group (iron-protoporphyrin IX) is an important and ubiquitous cyclic tetrapyrrolic complex critical for life. It binds to different apoproteins with essential physiological functions, including oxygen-carbon dioxide exchange, oxygen storage and transport (hemoglobin, myoglobin), electron transport, redox reactions (catalase, peroxidases), cell signaling, drug metabolism and detoxification (cytochromes) [21]. The cyanobacterial chromophore PCB belongs to a diverse group of natural linear tetrapyrroles occurring also in mammals and plants, which are called “bilins”, a name derived from mammalian “bile pigments”. This includes heme-derived bilins, such as biliverdin (BV) and bilirubin (BR) (Fig. 1), endogenous mammalian tetrapyrrolic compounds with known antioxidant properties participating in the physiological redox cycle catalyzed by heme-oxygenase-1 (HO-1) and biliverdin reductase (BVR) (Fig. 2) [22]. There are many published reports and review papers documenting the therapeutic activities of the BV/BR/HO-1/BVR system, which has shown beneficial effects on diseases of different organs and tissues, such as heart, kidney, liver, intestines, lungs, skin, brain, vasculature and pancreas. Notably, this system has been found to provide benefits in ischemia-reperfusion damage, wounds, endotoxic shock, viral infections, metabolic disorders, proteinopathies and inflammatory conditions [23, 24]. However, excessive levels of free circulating (albumin-unbound) unconjugated BR can exert toxic actions in the body, particularly in the peri-natal brain, whose blood-brain barrier is still not completely developed, producing an encephalopathy known as jaundice of the newborn [25]; other neurotoxicity sequelae can be seen in drug- or genetically-induced hyperbilirubinemia [26]. On the other hand, human health conditions with chronic elevations of unconjugated BR, such as the benign genetic variant Gilbert’s syndrome, are associated with a halving of total mortality, suggesting that a moderate enhancement of BR’s physiological actions may be markedly protective [27].
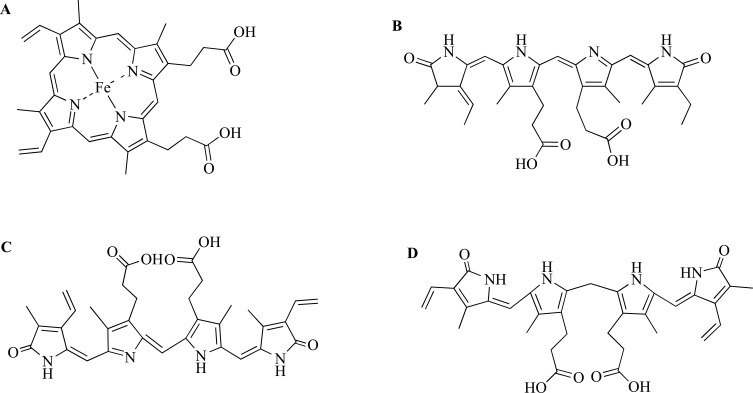
Fig. (1).
Chemical structures of heme (A), Phycocyanobilin (B), Biliverdin (C) and Bilirubin (D). Biliverdin isomers are produced depending on whether the α, β, γ or δ methene bridge of heme is cleaved by HO-1, being the most common cleavage at the α position.
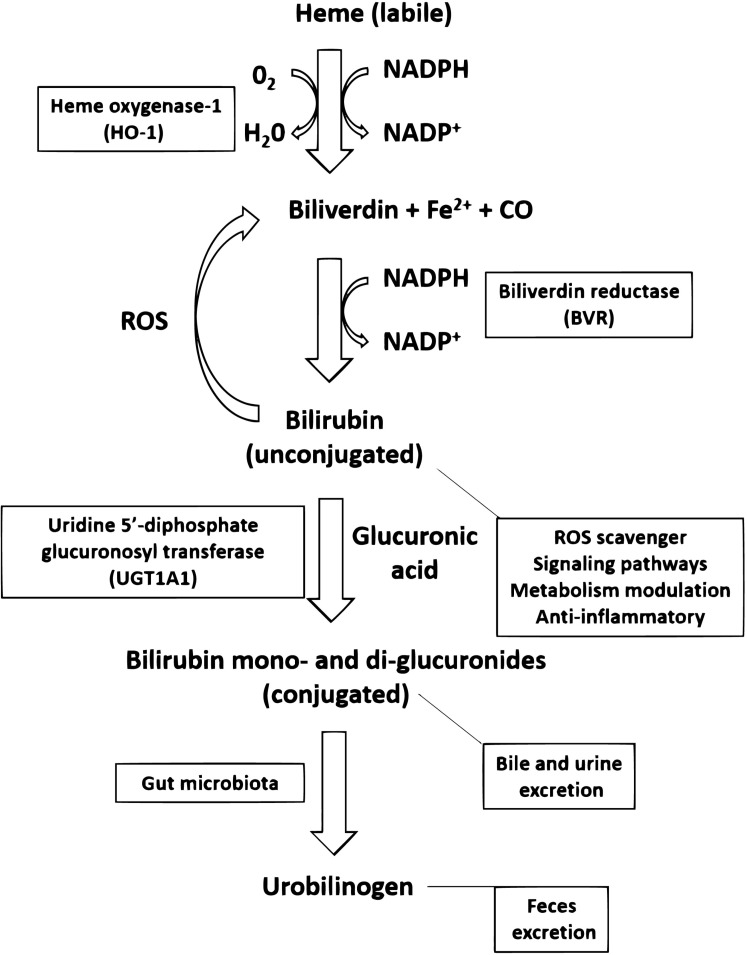
Fig. (2).
Heme catabolism pathway in mammals. Labile (protein free) heme group is catabolized by the inducible enzyme heme oxygenase-1 (HO-1) into Biliverdin, carbon monoxide (CO) and Fe2+. Biliverdin can be converted into Bilirubin by the enzymatic activity of Biliverdin Reductase (BVR, type A in adults, type B in fetus). In the opposite direction, Bilirubin can produce Biliverdin through the reduction of Reactive Oxygen Species (ROS). Bilirubin acts as an endogenous ROS scavenger, mediates signaling pathways, is involved in metabolism modulation and exerts anti-inflammatory actions. In the liver, uridine 5’-diphosphate glucuronosyl transferase (UGT1A1) catalyzes the conjugation of Bilirubin with glucuronic acid producing a hydrophilic derivative for bile and urine excretion. The gut microbiota can transform these Bilirubin mono- and di-glucuronides into urobilinogen, which is excreted in the feces. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Interestingly, mice lacking the BVR gene (resulting in loss of endogenous BR production and the accumulation of BV) showed an increased vulnerability to N-methyl-D-aspartate (NMDA) injection into the brain cortex, resulting in significantly largest lesions [28]. BVR-/- brain slices accumulated around two-fold more O2•– when treated with NMDA than wild-type controls, suggesting that neuronal BR scavenges or prevents the production of NMDAR-induced O2•–, which may be indicative of an endogenous redox regulation in the context of excitotoxicity [28]. BVR is not only responsible for the enzymatic conversion of BV to BR, but also functions as a mediator of cell signaling, leading to cytoprotective and anti-inflammatory effects, and modulating cell growth, tumorigenesis, and apoptosis [29, 30]. It is of intriguing interest that PCB, which is a cyanobacterial metabolite of BV, and structurally homologous to BV, can be converted efficiently by BVR to phycocyanorubin, a compound structurally homologous to BR [31]. Hence, it is reasonable to speculate that the intriguing and versatile antioxidant and anti-inflammatory properties of Sp extracts, C-PC, and PCB demonstrated in pre-clinical studies, including those targeting the central nervous system (CNS), may reflect mimicry of the important physiological protection afforded by BV/BR.
3. PHARMACOLOGICAL ACTIVITIES OF PCB
PCB has a molar mass of 587 Da, whereas that of C-PC is about 36 KDa – approximately 61 times greater. This suggests that it might be possible to obtain this organic pigment from chemical synthesis [32], thereby avoiding pharmaceutical regulations that pertain to products of biological origin. Another advantage regarding the simple PCB chemical structure over polypeptide pharmaceuticals is the absence of any unwanted modifications of amino acids as well as any events of aggregation or enzymatic digestion. The possibility can be envisioned that PCB could be administered covalently linked with bioactive proteins, providing a combination of therapies in a single pharmaceutical product.
Additionally, the binding capacity of PCB with natural polypeptide carriers may pave the way for nutraceutical formulations with improved pharmacokinetic and safety properties. PCB is linked covalently to C-PC cysteine amino acids by thioether bonds (at α84, β84, and β155 positions) [13], a process that could be mediated by the enzymatic catalysis of phycobiliprotein lyases or by spontaneous attachment [33]. Moreover, the reactivity of PCB towards free SH groups present in biological molecules, such as cysteine [34], indicates the possibility of chemically conjugating this tetrapyrrole to appropriate carriers. Indeed, previous reports have demonstrated the stable binding of PCB with several natural proteins. PCB was found to bind to bovine serum albumin (BSA) and this complex showed increased thermal stability and a mutually protective effect against free radical-induced oxidation [35]. Given the versatile and wide use of BSA as a biological carrier of a diverse range of pharmaceutical and nutraceutical compounds, these results point to a possible BSA-PCB stable formulation [36]. Additionally, PCB also binds to human serum albumin (HSA) [37] and to bovine β-lactoglobulin (BLG), the main protein composing the milk's whey of cows; this binding was stable in extreme situations, such as an ample pH range and in experimentally modeled gastrointestinal environment [38]. Interestingly, it was observed that PCB binds to HSA with an affinity similar to BR [39]. This suggests that in conditions of normal BR plasma levels, administered unconjugated PCB may compete with this endogenous human tetrapyrrole for binding to HSA, and use it for its biodistribution throughout the body. On the other hand, PCB might be better absorbed when it is orally administered since it has good stability in acidic environments, in contrast to C-PC that becomes unstable and unfolds at acidic pH [40]. Previous observations have shown that eight PCB oligopeptides bearing 2-13 amino acids are obtained from the pepsin degradation of C-PC in artificial gastric solution (pH 1.2). These chromopeptides evidenced antioxidant, anti-hemolytic and anticancer activities, and notably, those with higher antioxidant effects were similar in this respect to free PCB [41]. It is reasonable to suspect that the physiological effects of orally administered C-PC stem primarily from absorption of these chromopeptides, which might be the primary form in which PCB is absorbed after C-PC administration. PCB appears likely to be the chief mediator of physiological effects of orally administered C-PC.
In order to show the potential health benefits of PCB, we here summarize several reports in which this agent has demonstrated a variety of pharmacological properties in experimental models that might have relevance to the prevention or treatment of human diseases (Table 1).
Table 1.
Pharmacological studies showing potential benefits of PCB for different human pathologies.
| Models of Diseases | Effect of PCB | Refs. |
|---|---|---|
| Pancreatic tumor Cell lines: PA-TU-8902, Mia PaCa-2 and BxPC-3 |
-Prevention of cancer cell proliferation -Blocking of reactive oxygen species generation in mitochondria |
[42] |
| Diabetic nephropathy In vivo model: C57BL/Ks J db/db mice |
-Oral administration in powdered diet supplemented with PCB at 15 mg/kg for 2 weeks -Drop off the redox markers levels in urine and kidney -Down-regulation of Nox2, p22phox, p47phox, TNF-α and HO-1 renal gene expression -Preservation of renal mesangial morphology in diabetic mice |
[43] |
| Liver injury In vivo model: CCl4 injury to liver in C57BL/6 mice |
-Given orally by intragastric gavage at 100 mg/kg daily for 4 days after CCl4 injection -Serum levels restoration of hepatic enzymes and albumin -Rise in hepatic SOD activity -Reduces hepatic cell death -Rise in expression levels of liver regeneration genes (hepatocyte growth factor, HGF and transforming growth factor-alpha, TGF-α) -Higher survival of mice when injected with CCl4 lethal dose |
[44] |
| Ischemic stroke PC12 neuronal cell line In vivo model of stroke penumbra induced by bilateral permanent occlusion of common carotid arteries in Wistar rats |
-Intraperitoneal administration of 47 or 213 μg/kg, in equally divided four subdoses at 30 min, 1, 3 and 6 h post-stroke. -Prevents PC12 death induced by glutamate and H2O2 -Up-regulates the expression of 19 genes (anti-inflammation, neuronal survival) at 1 day post-injury -Modulates the expression of 190 genes that regulate the immune response -Inhibits lipid oxidation and promotes SOD activity in cerebral tissue |
[45] |
| Atherosclerotic injury to endothelial cells In vitro model: cell line EA.hy926 (human umbilical vein endothelial cells) |
-Induces HO-1 gene expression and enzymatic activity -Down-regulates p22Phox expression |
[46] |
| Transplant rejection Allograft response in primary culture of human immune cells |
-Reduces the TNF-α, IFN-γ and IL-17 levels and the proliferation of human peripheral blood mononuclear cells -Inhibits allostimulated CD4+ T cell response -Prevents cytokine production in LPS-stimulated dendritic cells (CD83, CD40, IL-12p70 and IL-23) |
[47] |
| Multiple Sclerosis In vivo experimental autoimmune encephalomyelitis (EAE) model induced in C57BL/6 mice with MOG35–55 peptide |
-Oral administration by intragastric gavage at 0.2, 1 or 5 mg/kg daily for 28 days -EAE disease onset was delayed two or three days for 1 and 5 mg/kg PCB, respectively -EAE clinical severity was ameliorated by 5 mg/kg PCB -Limits the IL-6 and IFN-γ cerebral accumulation in EAE mice |
[48] |
| Type 2 Diabetes Mellitus In vivo model in albino rats induced by streptozotocin |
-Given orally by intragastric gavage 982 μg/kg daily for 30 days -Decreases blood glucose concentration, insulin resistance and lipids levels in diabetic animals -Recovers normal pancreatic cell morphology |
[49] |
Additional evidence suggests that PCB may be the actual mediator of C-PC’s major physiological activities. With respect to prevention of the production of hydroperoxides of linoleic acid, Hirata and colleagues (2000) reported that the effects of C-PC and PCB were comparable in similar concentrations and incubation times. Remarkably, heat denaturation of C-PC did not change its antioxidant activity, suggesting that the specific protein structure of native C-PC was not responsible for its antioxidant effect [18]. Furthermore, the ability of C-PC and PCB to scavenge peroxyl radicals, as quantified by the oxygen radical absorbance capacity (ORAC) assay, was comparable [50], suggesting that PCB was the actual mediator of this effect as well.
In some studies, PCB has shown higher activity than equimolar concentration of C-PC. For example, Scoglio et al. (2016) found that the former was 888 times more potent for inhibiting UDP-α-D-glucose 6-dehydrogenase activity [51]. This enzyme plays a role in the progression of prostate [52], breast [53], ovary [54], and epithelial cancers. Incubation with PCB in nanomolar concentrations has been shown to inhibit the in vitro proliferation of thyroid (FTC-133) and prostate (PC-3) cancer cell lines, inhibiting colony formation by 80-90% [51]. With respect to the inductive impact of PCB (200 µM) and an Sp extract (0.5 g/L) on HO-1 expression in an endothelial cell line (EA.hy926), the mRNA levels rose about 78.0- and 1.6-fold, respectively, whereas HO-1 enzymatic activities rose 261% and 162% of control, respectively [46]. The concentration of PCB in the Sp extract used in this study is estimated to correspond to about 5.6 µM PCB. These findings illustrate that PCB functions as a crucial active component of Sp for inducing HO-1 expression and activity in endothelial cells. It is notable that induction of HO-1 in vascular endothelium exerts anti-atherogenic effects reflecting antioxidant, anti-inflammatory, anti-apoptotic and immunomodulatory actions [55]. Thus, PCB may have potential as a drug or nutraceutical that can be released from whole Sp biomass, C-PC-rich Sp extracts, or purified C-PC preparations, that may find a diverse repertoire of applications.
4. NUTRACEUTICAL INTERVENTION IN NEURO-DEGENERATIVE DISEASES BY C-PC/PCB
4.1. Multiple Sclerosis
MS is a prominent neurological cause of disability, afflicting especially young adults [56]. Its prevalence is higher in the economically developed portions of Europe and North America [57]. MS has two pathogenic phases: acute inflammatory demyelination, followed by progressive axonal degeneration. The first phase of inflammatory demyelination is reversible [58]. However, for unknown reasons, remyelination often fails to normally occur; hence, a therapeutic goal in MS is to improve the remyelination process [59].
Oxidative stress is also a prominent process mediating the pathological mechanisms of MS, as confirmed by studies involving the Experimental Autoimmune Encephalomyelitis (EAE) model of MS, or in biopsies from MS patients. Nitrotyrosine, a marker for ONOO– -mediated protein damage, is prominently present in inflamed CNS tissue both in EAE and MS patient biopsies [60]. Cerebrospinal fluid levels of both nitric oxide (NO) and ONOO– are elevated in MS patients during their acute relapses [61]. Previous studies have shown that PCB can function as a ONOO– scavenger, and can also prevent ONOO– -mediated DNA damage [19]. This suggests that PCB might have the potential to alleviate the pathogenic impact of ONOO– in MS.
PCB can also inhibit lipid peroxidation [18] and scavenge peroxyl radicals [50]. Autopsy studies show that, in spinal cords white matter and cerebral cortex lesions, the products of lipid peroxidation are prominently present in myelin membrane and injured oligodendrocytes [62]. Peroxyl radicals can trigger further lipid peroxidation in a chain reaction of lipid damage, and likely play an important role in the oxidative membrane damage encountered in neurodegenerative diseases such as MS [63]. Based on this, PCB may have the potential to significantly protect CNS tissue by quenching lipid peroxidation chain reactions.
Given the chemical similarity between PCB and the open tetrapyrroles naturally present in humans, such as BV and its enzymatic product BR, it could be anticipated that these might demonstrate similar beneficial actions in experimental models of MS. An early study demonstrated that intraperitoneal administration of BR to diseased rats was able to counteract the clinical deterioration of both acute and chronic EAE, to limit the lipid peroxidation process in spinal cords, as well as to protect primary oligodendrocytes against H2O2 injury [64]. A more detailed assessment of BR’s mechanisms of actions against EAE was reported later. Liu et al. (2008) observed that in vitro BR treatment inhibits the proliferation of CD4+ T cells and PLP-specific CD4+ T cells of SJL/J mice, and decreases the macrophages and dendritic cells expression of co-stimulation mediators and class II MHC [65]. Furthermore, BR significantly ameliorated the clinical and histological course of active and adoptive transfer EAE in SJL/J mice, as well as of acute EAE in Lewis rats, in which a BR-induced rise in the expression of HO-1 (responsible for the conversion of heme to BV, carbon monoxide, and Fe2+) and of BVR in spinal cords was demonstrated [65]. As noted, BVR catalyzes the enzymatic transformation of BV to BR, as well as the conversion of PCB into phycocyanorubin [31]. Thus, it is highly probable that this PCB metabolite may mimic the regulatory activity of BR in different cells. Interestingly, BVR is localized in macrophages plasma membrane, and, when bound by its substrate BV, it can function as a receptor, initiating a signaling cascade upstream of Akt activation that antagonizes the pro-inflammatory effects of lipopolysaccharide, and is in part responsible for the protective effects of BV in models of lethal endotoxic hepatitis [66]. Similarly, it can be speculated that activation of macrophage BVR by BV’s homolog PCB could mediate a positive effect in inflammatory conditions such as MS.
In recent years, we have observed strong protective actions of both PCB and its natural prodrug, the biliprotein C-PC, in rodent EAE models of MS. Treatment with C-PC either before or starting from the day of immunization in Lewis rats with EAE dramatically improved the clinical condition of animals, accompanied by the significant reduction of serum and brain lipids and proteins oxidation, as well as the preservation of myelin structure [67]. Moreover, our study also revealed an up-regulation of regulatory T cells (Treg) markers when MS or healthy people-derived peripheral blood mononuclear cells (PBMC) were treated with C-PC [67]. A later study, employing mice in which EAE has been induced with injections of myelin oligodendrocyte protein (MOG35-55), C-PC suppressed inflammatory infiltrations and demyelination in white matter of the spinal cord, and this effect was associated with improved clinical status [68]. C-PC also promoted axonal preservation, and decreased IL-17 expression in brain tissue and serum. The redox status parameters were also improved in these C-PC-treated mice [68]. Notably, previous reports have demonstrated the relevant involvement of HO-1 on the induction of Treg and its suppressive effect against inflammatory conditions [69, 70]. Protection against EAE was also demonstrated with the treatment of chemical compounds that induce HO-1 expression, corroborated by an enhanced CNS demyelination, paralysis, and mortality in HO-1 knock-out mice as compared with HO-1+/+ animals [71]. Such evidence suggests that these tetrapyrroles (BV, BR and PCB) share the ability of boosting Treg levels and, in this way, control an exacerbated proinflammatory response in different diseases [72].
However, it is still an open question as to what intracellular targets these tetrapyrroles interact with that are capable of initiating the sequence of events leading to Treg specialization. In this regard, a likely candidate is the aryl hydrocarbon receptor (AhR). BR and BV are potent ligand activators of AhR in the cytosol [73], which then promotes the nuclear transcription of specific genes, including HO-1, CYP1A1, CYP2A6, UGT1A1, SLCO1B1, MAPK genes, Nrf2 (a transcription factor for other protective genes), leading to a diverse range of effects [74]. Importantly, it has been shown that agonists for this receptor are potent promoters of Treg differentiation (both Tr1 IL-10 producing cells and foxP3-dependent Treg) [75-77]. Moreover, administration of the natural tryptophan metabolite ITE or the dietary indole derivative di-indolylmethane, that function as physiological AhR agonists, suppresses EAE [78, 79]. This evidence points to the strong possibility that the Treg inducing action of BR/BV/PCB is mediated by their activation of intracellular AhR, which then contributes to their beneficial effect in EAE.
When PCB was tested for its ability to ameliorate EAE, a potent beneficial action was observed in the MOG35-55 mouse model. Its oral administration in a relatively low dose (5 mg/kg of body weight) significantly improved the clinical status of the animals while reducing the cerebral levels of deleterious IL-6, IFN-γ; and a clear tendency to reduction of IL-17 levels was also noted [48]. CNS lesions showing a ubiquitous accumulation of these inflammatory cytokines are widely present in people with MS [80], suggesting that these cytokines are of fundamental importance for pathogenic progression of this condition. In a local microenvironment of abundant TGF-β and IL-6, a transition of Treg into a Th17 cells subset may occur [81], and IL-6 may also limit Treg immunomodulatory functions as well as induce peripheral self-reactive effector T cells [82]. This negative role of IL-6 in MS is also demonstrated in mice lacking this cytokine, which became resistant to EAE induction [83, 84]. Thus, by decreasing these cytokines, PCB counteracts the inflammatory component of MS pathogenesis. Basdeo et al. (2016), employing a cellular model of human allograft responses, found that PCB could diminish dendritic cell function and suppress the pro-inflammatory attack of T cells on allogeneic cells [47]. It is widely recognized that myelin destruction and neuroinflammation in MS are secondary to the imbalance of the effector/regulatory immune components, which is responsible for the tight control of inflammatory self-response [85]. Thus, it is possible to restore the immune balance by: 1) promoting functional Treg; 2) decreasing the effector T cells that escape from regulatory mechanisms [86]. The above-mentioned evidence supports the hypothesis that PCB treatment (given as purified compound or released from its C-PC prodrug) may therefore control the immune disruption in MS by modulating both the regulatory and the effector subsets of the neuroinflammatory response.
In this line of thinking, another interesting observation related to Treg in MS was reported by Dombrowski et al. (2017). These authors have shown that ex vivo, Treg can act to promote the differentiation of oligodendrocyte progenitor cells (OPCs) and aid their ability to induce myelination of brain tissue and re-myelination of damaged brain tissue. Whereas in Treg-deficient mice, oligodendrocytes (ODs) differentiation and remyelination were impaired; this phenomenon was reversed by adoptive transfer of Treg cells [87]. Hence, via induction of Treg, PCB could likely promote remyelination in MS. In Lewis rats with EAE treated with C-PC, transmission electron microscopy analysis of biopsy samples found that myelin remained compact and dense, without signs of axonal damage – a pattern similar to that in healthy animals [67]. Moreover, measurement of the g-ratio (the ratio of axon diameter to myelinated axon diameter, used to quantify axonal myelination) revealed that C-PC treatment kept this ratio in the range observed in healthy mice – whereas this ratio was significantly higher in the EAE mice receiving vehicle [88]. Analogously, in the C-PC-treated EAE mice, areas of spinal cord demyelination were significantly reduced compared to those seen in vehicle-treated EAE mice [68]. Hence, these findings were consistent with those of g-ratio analysis, revealing a protective effect of C-PC on myelin structure [88]. These results demonstrate that C-PC can promote remyelinating activity in various rodent models of EAE, and suggest that administration of PCB might achieve similar results. There is considerable evidence that a failure of remyelination is key to the progression of MS, and that this reflects diminished recruitment of OPCs into inflammatory lesions, and the failure of these cells to differentiate into functionally active ODs capable of myelination [89]. Remyelination may be promoted by measures which recruit OPCs to lesions and aid their differentiation to functional ODs – or that counteract the effect of agents produced in MS lesions that suppress this process. Intriguingly, treatment with C-PC in EAE mice has been found to up-regulate the mRNA expression of a group of genes which play a key role in myelination and re-myelination [68]; these include Mal, Mog, and Mobp, structural components of myelin, and a set of transcription factors (Olig1 – exclusive to ODs, Nkx6-2, and Nkx2-2) that are crucial for OPCs differentiation and maturation [90-92]. Overall, these findings suggest that C-PC/PCB can induce a pattern of gene expression required for OPCs differentiation, maturation to functional ODs, and formation of the axonal sheath, as well as for the development of nodes of Ranvier in newly myelinated axons.
Altogether, these results suggest that PCB has potent anti-inflammatory and immunoregulatory properties, in addition to its antioxidant and remyelinating capacities, supporting its nutraceutical application to MS disease, particularly with respect to its relapsing-remitting phase (RRMS). Although there has been important progress in the development of drugs able to target RRMS, there is an acute need for the development of drugs that can prevent or slow the progression of disability in Primary (PPMS) and Secondary (SPMS) progressive MS [93]. At present, only one drug, Ocrelizumab (Ocrevus®), has been approved for the management of PPMS. It is a humanized monoclonal antibody that targets CD20 and exerts immunosuppressive effects useful in RRMS; however, it has only modest benefit in PPMS [94]. It can be speculated that PCB might be able to oppose myelin destruction and axon degeneration, and to synergize with Ocrelizumab in ameliorating progressive MS. To evaluate this hypothesis, it is desirable to test it in an appropriate animal model for progressive MS. Rodent models of MS have been developed that may be pertinent to the progressive phase of this disorder; these entail demyelination and axonal destruction in the absence of inflammatory T cell involvement [95]. The MOG35-55-induced EAE model, in Biozzi ABH mice, has a chronic phase, following an acute relapsing phase, that may constitute a model for progressive MS; several mechanisms of axon damage may be involved in this syndrome [96]. The cuprizone-induced model of demyelination, which does not involve myelin-reactive T cells, shows neurodegeneration similar to that seen in progressive MS, such as brain atrophy and callosal axon loss [97]. PCB administration should be studied in these animal models mimicking progressive MS, as a complement to previously reported studies evaluating its impact in RRMS models, so that its possible clinical utility in MS could be assessed.
4.2. Ischemic Stroke
One of the major diseases responsible for life-long devastating physical limitations or high number of acute demise among all ages and sex worldwide is stroke [98]. On average, every 40 seconds, someone has a stroke in the US, and someone dies by this cause every 3 minutes 35 seconds, accounting for approximately 1 of every 19 deaths [99]. Etiological classification of stroke includes two categories: ischemic or hemorrhagic, dependent on whether the affected artery is occluded (by an embolus or a thrombus) or ruptured, respectively [100]. Around 85% of stroke cases are ischemic, which present structural and functional brain injury largely correlated with the artery (location) that is occluded, and on the residual (collateral) cerebral blood flow (CBF) present in the different brain regions [101]. Thus, the temporal and spatial evolution of an ischemic event also differs among cerebral regions. In better irrigated areas, the core of the infarction, defined as the region surrounded by the vessel occlusion where cells will eventually die, will not grow as fast as in areas with little or no residual CBF [102]. In contrast, more distal areas from the occluded vessel have a depressed tissue perfusion but still enough to support basal needs for oxygen and glucose, presenting the possibility that cells in these regions might be rescued from death if an appropriate intervention occurs. This area is defined as the ischemic penumbra and denoted as a brain tissue in death risk but with a perfusion level that maintains a basal electrical and biochemical functioning, which allows the possibility to be rescued if an adequate intervention is implemented in a specific time window [103].
Strategies for acute treatment of IS have focused on two research lines: i) the restoration of CBF by thrombolytic drugs or by mechanical reperfusion with thrombectomy devices, ii) the pharmacological interruption of the pathophysiological processes involved in the ischemic cascade by neuroprotectants [104]. Tissue plasminogen activator is a human approved drug for thrombolysis since decades ago, but it has a high risk for cerebral hemorrhage and a narrow therapeutic time window [105]. Mechanical reperfusion has also been used in clinical practice, but it has been reported that 20-30% of successful recanalization is mismatched with clinical positive outcomes [106]. More paradoxically, no neuroprotectant has been able to be effective in the clinics so far, even with a high relative number of candidates showing promise in preclinical settings [107]. However, there is a pressing need to continue developing improved and cost-accessible interventions for IS, owing to its high mortality and the high incidence of disability in stroke survivors, which is a huge burden in terms of patient, family and societal costs [108].
In this sense, natural products may provide an affordable source of neuroprotectants with an adequate safety profile [109]. Thaakur and Sravanthi (2010) showed promising results with spray-dried Spirulina maxima extract in a rat model of cerebral ischemia-reperfusion. This Sp extract was given prophylactically for seven days in food to albino rats subsequently subjected to a two-hour intraluminal filament occlusion of the middle cerebral artery. Sp pretreatment reduced the degeneration of brain neurons and the neurological impairment of ischemic rats, as well as the imbalance in redox parameters, decreasing the level of brain malondialdehyde (MDA) and restoring the activities of brain superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) [110]. Interestingly, the Sp preparation used in this study contained 15% C-PC (denatured by the spray-drying process), suggesting an important role for its digestive metabolites (free PCB and PCB-bound peptides) in the in vivo anti-ischemic actions observed for the cyanobacteria extract. Accordingly, we later tested the idea that Sp-purified C-PC/PCB could be a potential effective modifying treatment for IS. In a model of global transient brain ischemia in gerbils, we observed a strong neuroprotective action of C-PC, given either at 30% purity intragastrically (once daily) during seven days before ischemia, or when given therapeutically at analytical purity in repeated intraperitoneal injections for up to 12 h after the injury [111]. Infarct volume and neurological dysfunction were significantly reduced by C-PC treatment at 24 h following the ischemic injury. Furthermore, C-PC significantly increased neuronal survival in the hippocampus, and promoted the recovery of locomotor activity and survival of ischemic gerbils 7 days after the damage. Biochemical markers of lipid peroxidation were also decreased by C-PC treatment in ischemic brain and serum [111]. These first time-reported results encouraged follow-up studies of the mechanisms and the effects of C-PC/PCB in other experimental settings of IS.
The canonical processes involved in the ischemic cascade mediating cerebral injury, such as energy failure, excitotoxicity, ionic imbalance, oxidative/nitrosative stress, neuroinflammation, blood-brain barrier disruption, and cell death signaling, have been relatively well established [112]. However, taking into account the complex evolution of an ischemic event, it has been postulated that understanding how the different cell types of the brain (neurons, microglia, astrocytes, endothelial cells, pericytes, oligodendrocytes) react to a potential therapy, and how “neurovascular and oligovascular units” are able to recover their normal cell-cell signaling, will foster the development of effective neuroprotectants in humans [113]. Several reports have shed light on the effects of Sp/C-PC/PCB on cells of these brain cells units when they are challenged with ischemia-mimicking injuries. C-PC was able to protect serum and potassium-deprived cerebellar granule cells by interrupting characteristic features of apoptotic death [114]. In the SH-SY5Y human neuronal cell line, Sp extract and C-PC significantly prevented iron-induced cytotoxicity, accompanied by their promotion of the cellular glutathione antioxidant system (GPx: glutathione peroxidase, GR: glutathione reductase, and GSH), as well as limitation of lipid peroxidation [115]. When this neuronal line was subjected to an oxidative insult mediated by tert-butylhydroperoxide and treated with C-PC, we confirmed the protective actions of this biliprotein in vitro in a dose-dependent manner. In a retinal ischemia model in rats, a similar neuroprotective effect of C-PC was reported, in which a single intraocular injection of this drug 15 min before the damage, completely conserved the inner nuclear layer cell density of the retina [116]. Moreover, C-PC prevented Ca2+/phosphate-induced mitochondrial permeability transition in isolated rat brain mitochondria by inhibiting characteristic ischemic intracellular events, such as the production of reactive oxygen species the translocation of cytochrome c (a pro-apoptotic factor) to the extramitochondrial medium, as well as the mitochondrial volume expansion (swelling) and the rupture of its electrochemical membrane potential [116]. Moreover, PCB treatment has also demonstrated protective actions on cultured neurons subjected to ischemic insults, such as the PC12 neuronal cell line subjected to temporary oxygen and glucose deprivation [117], or damaged by the presence of toxic concentrations of L-glutamate or H2O2 [45].
Relevant effects of Sp/C-PC on microglial cells and astrocytes have also been reported. Both Sp extract and C-PC were able to promote the survival of microglial cells in culture subjected to lipopolysaccharide toxicity, and to reduce the mRNA levels of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), TNF-α, and IL-6 in these cells [118]. These genes are involved in the deleterious pro-inflammatory M1 microglial response to brain ischemia; hence, this suggests a positive modulatory action of the biliprotein on ischemic neuroinflammation [119]. Similarly, C-PC demonstrated a strong cytoprotective action on rat primary astrocytes damaged with H2O2, in which the biliprotein reduced the ROS levels, up-regulated the BDNF and NGF gene expression, and down-regulated the expression of proinflammatory factors, such as TNF-α, IL-6 and IL-1β [120]. Other authors corroborated the anti-apoptotic action of C-PC on rat primary astrocytes during trophic factor deprivation, an effect partially explained by its antioxidant activity [121]. Chitosan-coated C-PC liposomes also protected rat primary astrocytes from H2O2 toxicity, while decreasing the expression of TNF-α and inducing the phosphacan and neurocan genes, both glia scar-associated proteoglycans [122]. Previously, it has been observed that these C-PC encapsulated liposomes protected the murine Neuro2A cell line from H2O2 toxicity [123]. Endothelial cells are also crucially involved in ischemic stroke damage, as they regulate brain inflammation [124] and can be damaged by a burst of toxic oxygen and nitrogen species, contributing to blood-brain barrier breakdown, CBF dysregulation, and vascular tone alteration [125]. It has been demonstrated that PCB had a remarkable inducing effect on HO-1 gene expression and enzymatic activity in EA.hy926 endothelial cells in vitro, given that these are derived from the differentiated endothelium of human umbilical cord [46]. PCB also reduced the protein levels of the p22 subunit of NADPH oxidase in these cells [46]. Thus, by activating heme catabolism (releasing BV and carbon monoxide), PCB may sustain an anti-inflammatory and cytoprotective effect on endothelial cells [126]. At the same time, PCB-mediated inhibition of endothelial NADPH oxidase may have a profound effect in controlling the cerebral vascular damage caused by the ischemic event [127].
In order to improve the access of C-PC to the ischemic brain and to increase its neuroprotective time window post-stroke, this biliprotein has also been tested by the intranasal route. C-PC (134 µg/kg) administered intranasally to ischemic rats, showed an effective effect on the infarct volume decrease when applied for up to 9 h post-stroke [120]. Encapsulated C-PC in liposomes with 20% cholesterol also produced a dramatic reduction in the cerebral injury of rats when applied intranasally at 1 mg/mL (around 111.2 µg/kg considering a 300 g rat) 6 h after the ischemic event, decreasing the infarct volume to 23.2% in relation to the vehicle-treated group, even more than the protection seen in injured animals receiving non-encapsulated intranasal C-PC (68.97%) [123]. As chitosan material has been suggested to be a preferred system for delivery to the olfactory bulb (as opposed to the nasopharynx) through nasal mucin adhesion [128], a follow-up study tested the ability of cholesterol C-PC liposomes coated with chitosan for improving the neuroprotective action of the encapsulated biliprotein in IS. When applied intranasally at 0.5 mg/mL (around 13.9 µg/kg considering a 300 g rat) 6 h after stroke, chitosan-coated cholesterol C-PC liposomes significantly reduced the infarct volume (64.9% relative to the vehicle ischemic group) as compared to the non-chitosan C-PC particles (88.6%), despite the fact that the C-PC dose was eight-times smaller due to the presence of chitosan in the nanoformulation [122].
In vivo studies evaluating the administration of PCB as an acute neuroprotective intervention for IS have been reported. In a model of ischemic penumbra in rats, resulting from acute cerebral hypoperfusion induced by the permanent bilateral occlusion of the common carotid arteries, PCB was able to modulate relevant immune and inflammatory genes, as well as to reduce the oxidative imbalance in the brain and serum of ischemic animals [45]. Notably, PCB effectively modulated a range of genes involved in the detrimental pro-inflammatory response in the early phase after an IS, such as IFN-γ, IL-6, CD74, Foxp3, TGF-β, CCL12, IL-4, IL-17A, C/EBPβ, CXCL2, ICAM-1, IL-1β and TNF-α. Furthermore, PCB exerted a positive modulation on the expression levels of several genes acting at the neurovascular unit integrity, including myelination (Mal), energetic metabolism (NADH dehydrogenase), anti-apoptosis (Bcl-2a1), synaptic plasticity (Baiap2) and angiogenesis (VEGF) [45]. More recently, PCB was tested for its ability to ameliorate the brain damage produced by a transient focal cerebral ischemia in rats induced by the vasoconstrictor endothelin-1. In this model, the administration of PCB in cumulative doses up to 6 h after the ischemic event, decreased the infarct volume to 44.1% relative to vehicle ischemic group, and improved the exploratory behavior of the infarcted animals [117]. PCB also prevented cerebral cortex neurodegeneration over a long-term period (28 days after stroke) and preserved the expression levels of myelin basic protein and CNPase enzyme, which are involved in developing myelin sheaths and maintaining myelin-axon integrity, respectively [117]. Accordingly, when the remyelination activity was quantitatively analyzed, PCB showed a significant reduction of the myelin g-ratio (ratio of axon diameter to myelinated axon diameter) in the cerebral cortex of endothelin-1-injured rats as compared to the vehicle-ischemic animals, actually reaching the levels of the sham group [88].
These results open a novel perspective for using PCB either as a purified compound or as C-PC-derived prodrug in nutraceutical or pharmacological formulations for neuroprotection in IS, pointing toward possible combinatory strategies with thrombolytic interventions.
4.3. Alzheimer’s Disease
AD is a devastating neurodegenerative condition leading to dementia and other cognitive impairments, such as memory loss, aphasia, apraxia and dramatic changes in personality behavior; it is the main cause of dementia in the elderly. The histopathological hallmarks of AD and its principal diagnostic criteria include the accumulation of fibrillary beta-amyloid peptide (Aβ40/42) in the core of the extracellular plaques, and the deposition of hyperphosphorylated Tau intracellularly, both of which are critically involved in the synaptic failure, the demise of neurons and brain macroscopic atrophy [129]. Although the majority of AD cases are sporadic and multifactorial, there are three genes whose mutations may cause a familial form of this condition, the amyloid precursor protein (APP), and presenilin 1 (PSEN1) and presenilin 2 (PSEN2) [130]. The current marketed treatments for AD are mainly palliative aiming to ameliorate the patients’ symptoms and improve their day-to-day life. These compounds include three inhibitors of acetylcholinesterase (Donepezil, Galantamine, and Rivastigmine), with the best effect for mild-to-moderate AD and reflecting (temporary) maintenance of acetylcholine neurotransmission. The other approved drug is Memantine (a low affinity NMDA receptor antagonist), for moderate-to-severe AD, which acts through the inhibition of excitotoxicity but preserving the normal functioning of this receptor [131]. However, neither of these compounds are able to halt or slow down the progression of the disease, and novel approaches are needed for achieving this highly desirable goal, as for example novel chemical derivatives [132] or complementary strategies with nutraceuticals [6].
In addition to the amyloid theory of AD pathogenesis, other biological processes are involved in its etiology and progression, which includes an initial transition between normal aging and mild cognitive impairment, which is recognized as an early phase of memory dysfunction previous to AD development [133]. These pathological processes include oxidative stress, dysfunctional glucose metabolism and mitochondrial function [134], neuroinflammation [135], defective insulin signaling [136], metal ion imbalance [137] and alterations in the brain-gut microbiota axis [138]. It is thought that these mechanisms coexist and simultaneously influence each other in AD - as for example, redox dysfunction, which is able to strongly impact the other processes at multiple molecular levels, in individual cells and in cell-cell communication [139].
In this sense, the diverse properties of Sp and its derived C-PC/PCB may support its use as a potentially effective intervention for prevention or management of AD, and a number of previous reports has given insight into this possibility. In PC12 neuronal cells, both Sp extract and C-PC prevented the Aβ1-42 neurotoxicity, suppressed the Aβ1-42-induced increase in poly-ADP ribose polymerase-1 (PARP-1) cleavage and restored the antioxidant defenses (GSH, SOD, GPx, GR) [140]. Moreover, they also lowered the protein levels of APP and BACE1 (β-site APP cleaving enzyme 1), two major factors leading to Aβ production and deposition, and they increased the brain derived neurotrophic factor (BDNF) expression, which plays critical roles in neuronal survival and synaptic function [140]. Sp extract and C-PC have also been proven to be effective in in vivo models of AD-type dementia. In rats with aluminum chloride-induced dementia, oral administration of Sp extract restored the normal levels of antioxidant enzymes (SOD, CAT, GPx), accompanied by the promotion of total antioxidant capacity, GSH and thiol content [141]. The treatment with Sp extract also decreased neuronal death in the cerebral cortex and prevented toxic increases of brain TNF-α and thiobarbituric acid reactive substances, a marker of lipid peroxidation [141]. In the streptozotocin-induced dementia model of AD in rats, the daily intraperitoneal administration of C-PC for 28 days decreased the activity of hippocampal acetylcholinesterase and levels of Bax (pro-apoptotic), TNF-α, and NF-κB (pro-inflammatory factors) [142]. Furthermore, C-PC alleviated the associated dysfunction of the insulin pathway by increasing gene expression of IRS-1, PI3-K and Akt [142]. This result suggests the possibility that C-PC/PCB may have a positive impact on the insulin resistance in AD. Aβ induces oxidative stress and ER stress via NADPH oxidase [143], and this activates JNK, producing an inhibitory phosphorylation of IRS-1, which is responsible for the neuronal insulin resistance of AD. Because of this, the ability of insulin to activate the PI3K-Akt pathway in neurons is compromised. C-PC/PCB may alleviate this pathological situation at the source by preventing NADPH oxidase activation, and thus, the use of PCB to prevent neuronal insulin resistance in AD seems like a promising topic for future research. And since intranasal insulin is showing therapeutic value in AD, a co-administration with PCB may potentially have a synergistic effect [144]. Li et al. (2020) recently reported the effects of C-PC treatment in mice with AD induced by the intracerebroventricular injection of Aβ1-42. In this model, the administration of C-PC significantly prevented cognitive dysfunction (spatial memory) of mice injected with Aβ1-42 [145]. This effect was accompanied by positive modulation of chromatin (HDAC3: histone deacetylase) and post-transcriptional regulation (miRNA-335), and a decrease of IL-6, IL-1β, caspase-3, caspase-9, Bcl-2 and Bax expression levels in the hippocampus [145].
Direct effects of C-PC on the amyloid peptide catabolic pathway have also been reported. In this cascade, APP is sequentially cleaved by β-secretase, acting on an extracellular site and associated to AD, and γ-secretase (functional in normal conditions) within the transmembrane domain, yields the pathogenic Aβ peptides (of 40 or 42 amino acids), which self-interacts leading to aberrant depositions conforming the neuritic plaques [129]. By using an in silico analysis, Singh et al. (2014) showed that the crystallized α/β-dimer of C-PC was able to interact with β-secretase with an interaction energy comparable to the total energy observed between this enzyme and two of its known peptide inhibitors, suggesting that this biliprotein could also act as an enzyme inhibitor [146]. A biological assay with C. elegans transgenic strain CL4176 was performed, in which human Aβ1-42 is expressed in its muscle cells and produces a temperature-dependent paralysis phenotype. Interestingly, the pre-incubation with C-PC produced a significant delay in the onset of C. elegans paralysis, confirming its potential as a blocker of Aβ1-42-induced pathogenesis, which may presumably be through the decreased Aβ1-42 expression, the direct interaction with the amyloid peptide, or by inhibiting its downstream molecular effectors [146]. A subsequent study showed that C-PC directly binds to Aβ40/42 peptide and inhibits its fibril formation, suggesting that the biliprotein could interrupt this crucial mechanism of AD pathogenesis [147].
Given the structural similarity of PCB with BR, it is possible that the positive actions of this human endogenous tetrapyrrole may be mimicked by the cyanobacteria-derived homologs, but avoiding its neurotoxicity issues [26]. In this sense, the heme metabolism system (HO-1/BVR enzymes) has been involved in several aspects of AD. Although reports on the HO-1 expression in AD patients are controversial, some showing either a decrease in their mRNA [148] or enhanced protein levels [149], it has been observed that oxidative damage to this enzyme impairs its function in subjects suffering this condition [150]. A down-regulation of BVR has also been observed in post-mortem brain tissues of AD subjects in comparison to age-matched normal people. This observation was followed by animal studies showing that some compounds such as atorvastatin, which were able to up-regulate both HO-1 and BVR and thus increasing BR production, may ameliorate AD impairment and its associated brain redox imbalance [151]. Moreover, the triple transgenic 3xTg-AD mice exhibit a reduced expression of BVR and a rise in its tyrosine nitration levels, resulting in increased oxidative damage, inflammation and insulin resistance [152]. Notably, intranasal administration of insulin restored BVR activity and reduced disease severity in naïve animals with AD, but not in BVR knock-out AD mice [153]. This finding raises the interesting prospect that intranasal insulin might amplify the putative utility of PCB in AD control by enhancing brain BVR activity and thereby ensuring conversion of PCB to the bilirubin homolog phycocyanorubin. These lines of evidence point toward a possible protective role of the endogenous tetrapyrrolic system against AD deterioration, and support the hypothesis that PCB may also help to prevent this condition.
On the other hand, the brain-gut-microbiota axis has emerged as an important area of research in AD. It has been shown that gut microbiota may affect glutamate metabolism and, therefore, influence the glutamate NMDA receptor and cognitive function in dementia patients [138]. In models of AD, a differential abundance of bacterial strains involved in inflammatory pathways that impact cognitive decline have been reported; moreover, oral interventions with either mixed gut bacteria groups or specific pre-biotic nutrients have shown beneficial impacts on AD symptoms [154]. It, therefore, is notable that C-PC/PCB given orally has shown a favorable impact on gut bacterial strains linked to improvements in AD models. In this regard, the treatment of high-fat diet fed rats with a protease hydrolysate Sp extract resulted in an increased abundance of beneficial bacteria in their gut microbiota, such as Alloprevotella and Ruminococcus [155]. The daily administration of C-PC by oral gavage in pathogen‐free mice for 4 weeks significantly increased the abundance of the Ruminococcaceae, Rikenellaceae and Lactobacillaceae families [156]. Moreover, C-PC-treated animals evidenced higher villus dimensions and goblet cells density in intestinal regions, such as the ileum and the colon, when compared with control mice, suggesting an improvement of intestinal barrier function [156]. Other studies have shown positive modulatory effects of C-PC consumption on the composition of intestinal microbiota in various disease models, such as mice subjected to abdominal x-ray irradiation (rise of Lactobacillus, Bifidobacterium, and Roseburia) [157] or with bleomycin-induced pulmonary fibrosis, in which the biliprotein decreased the levels of bacteria related to inflammation and dramatically increased short-chain fatty acids-producing bacteria [158]. Neyrinck et al. (2017) showed that aged mice (24 months old) fed for 6 weeks with Sp extract (given at 5% with the standard diet), showed a significant increase of Roseburia and Lactobacillus in their intestinal microbiota [159], species which have been associated with attenuated inflammatory conditions [160]. Interestingly, Ruminococcus bacteria is among the main major gut producers of short-chain fatty acids, which are involved in the upregulation of tight junction proteins composing the blood-brain barrier [161], alterations in amyloid metabolism of APPswe/PS1E9 transgenic mice [162], and have been found to be deficient in AD patients [163]. Rikenellaceae family members are hydrogen‐producing bacteria capable of inactivating ROS and therefore protecting against oxidative stress [164]. Formulations with Lactobacillus species, which have been extensively studied for promotion of healthy dietary supplementations, and received the GRAS (generally regarded as safe) certification, have also shown promising beneficial actions in several models of AD, such as 3xTg-AD [165], APP/PS1 [166] and aged SAMP8 mice [167]. In this regard, spray-dried preparations from Sp extract fermented with Lactobacillus significantly ameliorated scopolamine-induced memory deterioration in mice, an effect accompanied by the induction of activated ERK, CREB and BDNF (neuronal survival factors) and inhibition of acetylcholine esterase activities in the mouse hippocampus [168]. In summary, these studies support the potential application of Sp and C-PC-derived PCB for ameliorating the progression of AD.
4.4. Parkinson’s Disease
PD is the second most common neurodegenerative condition after AD, afflicting 1% of aged people above 65 years old [169]. Recent epidemiological data have raised alarms regarding the global impact of PD. In the Global Burden of Disease, Injuries, and Risk Factors Study (GBD) 2015, PD was the neurological disorder fastest growing in prevalence, disability, and deaths; overall patient number more than doubled globally from 1990 to 2015 [170], a fact influenced not only by increasing numbers of older people, but also by environmental and socio-demographic factors [171]. PD is a progressive, debilitating clinical condition associated with bradykinesia, rigidity, resting tremor, and gait impairment; autonomic and cognitive dysfunction may also occur [172]. PD has a major economic impact, estimated to be $23 billion per year in just the U.S. alone [173].
PD remains an incurable condition. None of the available marketed therapies, mainly focused on compensating for the loss of dopaminergic (DA) function, are able to stop the progression of this disease, and even they tend to lose effectiveness over time, and entail side effects, such as motor fluctuations and sleepiness [174]. Dramatically, as PD continues to progress, long-term disability is preceded by a worsening in cognitive and body balance dysfunctions [175]. Thus, intense efforts for investigating and developing effective therapies for PD have been made over the last decades. Novel therapeutic strategies have emerged from advances in the understanding of the pathological mechanisms of PD, offering biological targets for interventions focusing either on disease-modifying treatments through neuroprotection and/or neurorestoration, or symptomatic therapies for motor and non-motor symptoms [176]. Several molecular mechanisms implicated in the pathogenesis of PD mutually interact with perturbed α-synuclein proteostasis; the accumulation of α-synuclein in insoluble cytoplasmic inclusions referred to as Lewy bodies contributes importantly to the death of dopaminergic neurons in the substantia nigra pars compacta (SNc), and also to the cardinal motor symptoms of PD (bradykinesia and rigidity) [177]. Among these mechanisms can be mentioned oxidative stress, mitochondrial damage, neuroinflammation, endoplasmic reticulum stress, and lysosomal dysfunction, which may ultimately provoke the selective death of SNc DA neurons in PD [178, 179].
Based on these features the biological properties described for Sp and its derived C-PC/PCB may effectively counteract the demise of SNc DA neurons in PD. Indeed, several reports have provided supportive evidence for this idea. Pabon et al. (2012) evaluated the effects of Sp extract administration on a PD rat model produced by stereotaxic injection of an adeno-associated virus vector carrying the α-synuclein gene into the SNc [180]. The Sp extract, given as mixed supplementation in normal diet at 0.1% starting 30 days before the surgery, significantly rescued the numbers of dopaminergic tyrosine hydroxylase positive neurons in SNc at 1 and 4 months’ post-injury [180]. This neuroprotective action was accompanied by a reduction of microglial activation and increased expression of CX3CR1, a microglial chemokine receptor that, when stimulated by its ligand (fractalkine or CX3CL1), leads to reduced synthesis of pro-inflammatory cytokines IL-1β and TNF-α [181]. In the 6-hydroxydopamine (6-OHDA) rat model of PD, the chronic oral administration of Sp extract (700 mg/kg/day) was able to reduce oxidative stress, the deficit in locomotor behavior, and DA depletion, while preserving mitochondrial reductase activity in the striatum damaged by this neurotoxin [182]. Lima et al. (2017) showed that the positive actions of Sp supplementation in hemiparkisonean 6-OHDA rats are also related to a decrease in brain inflammation [183]. Moreover, spray-dried Sp extract administration given in combination with amantadine potentiates the action of this PD marketed compound in 6-OHDA-lesioned rats, resulting in improved locomotor activity, increased DA levels, reduced lipid peroxidation, and increased GSH concentrations [184]. This evidence supports the combined use of Sp-derived C-PC/PCB with anti-Parkinson drugs used in the clinical setting, for improving their beneficial effects and reducing their side effects. Moreover, other Sp and C-PC protective actions and molecular mediators were identified in the DJ-1βΔ43 Drosophila melanogaster paraquat-induced neurotoxicity model of PD. The paraquat-treated flies showed an improved locomotor activity and a significant increase in survival when treated with a Sp-enriched diet, showing a remarkable 43 days average lifespan when receiving a 10% Sp diet, as compared to the 19 days average survival of paraquat-treated flies receiving only vehicle; the healthy control group survived 68 days [185]. Furthermore, both the Sp and C-PC-enriched diets reduced the levels of Hsp70 and phosphorylated (activated) JNK in parkinsonian flies, indicating that cellular stress was controlled by these treatments [185].
Interestingly, chemical assays have shown that C-PC inhibits primary fibril nucleation and fibril elongation of α-synuclein (carrying the disease mutation A53T), exhibiting ∼50% inhibition at extremely low molar ratios (200:1 for α-synuclein:C-PC) [147]. This observation suggests that C-PC may directly limit the intracellular aggregation of α-synuclein and, in this way, prevent its toxicity. This idea was tested by Macedo et al. (2017) in a yeast model of PD carrying a galactose-controlled expression plasmid with the human gene of α-synuclein. They reported a significant reduction of α-synuclein intracellular inclusions accompanied by the attenuation in its cytotoxicity when the yeast was treated with C-PC, but no changes in α-synuclein protein levels were observed between the control and the biliprotein-treated cells [186]. Thus, a decreased α-synuclein gene expression didn’t explain the drop in its intracellular inclusions produced by C-PC; rather, direct inhibitory action on α-synuclein fibril assembly could be a reasonable explanation for this effect. Moreover, C-PC also reduced the α-synuclein-induced oxidative stress and proteostasis dysregulation in this yeast model of PD [186].
On the other hand, there is a well-correlated association between oxidative stress and PD [187]. In PD patients, the DA neuronal cells and glia conforming to the SNc evidenced a significant elevation in their ROS levels as a consequence of the dopamine metabolism. This includes the monoamine oxidase- and cyclooxygenase-mediated oxidations of dopamine, generating H2O2 and O2•−, respectively, as well as dopamine auto-oxidation by labile ferric iron (Fe3+); the latter produces diverse ROS (H2O2, O2•−, •OH), pro‐oxidant dopamine‐o‐quinones and acidosis-facilitating aminochromes, all of which contribute to progressive and worsening neuronal loss in PD [188]. Moreover, enhanced iron accumulation in PD patients compared with healthy aged people, most notably in the SNc, may be a factor contributing to the specific spatiotemporal progression of PD neurodegeneration [189]. The potent antioxidant abilities of C-PC/PCB may counteract this oxidative environment in PD, with the potential to abrogate redox-mediated SNc DA loss. By using chemical assays, Bermejo et al. (2008b) demonstrated that both an Sp protein extract and C-PC not only strongly scavenge Fe2+ and Fe3+ ions (most prominently the latter), but also H2O2, O2•− and hypochlorous acid (HClO), while also inhibiting microsomal lipid peroxidation [190]. In a previous work, the same group concluded that the component mainly responsible for the antioxidant activity of Sp extract was its biliprotein C-PC [191]. They have also demonstrated a neuroprotective action of Sp protein extract and of C-PC against iron-induced toxicity in SH-SY5Y neuroblastoma cells [115]. These results are supported by our own observations regarding the inhibitory action of C-PC on an electrochemically generated Fenton reaction, in which H2O2 yields •OH in the presence of Fe2+ [116]. Therefore, by sequestering free iron, C-PC may limit the dopamine auto-oxidation process and its subsequent deleterious effects on neuronal viability. Other important mediators of redox imbalance in PD include microglial NADPH oxidase (producing O2•−) and inducible nitric oxide synthase (iNOS, yielding nitric oxide) [192]; these spontaneously react to yield peroxynitrite, a highly reactive oxidant that readily diffuses across cellular membranes (unlike O2•−) that can rapidly spread oxidative damage to neighboring cells [193]. Several reports have evidenced a neutralizing action of C-PC and PCB on these reactive compounds involved in PD pathogenesis, including peroxynitrite [19], lipid hydroperoxides [18] and NADPH dependent O2•− production [43]. This evidence supports the potential application of C-PC/PCB as complementary therapy for PD [194].
5. C-PC/PCB FOR THE COMPLEMENTARY MANAGEMENT OF COVID-19-INDUCED DAMAGE TO THE NERVOUS SYSTEM
COVID-19 is currently a pandemic affecting most countries of the world, with greater than 123.9 million confirmed cases and over 2.7 million deaths globally as of March 23th, 2021, according to the Johns Hopkins University Coronavirus Resource Center [195]. COVID-19 is caused by the novel SARS-CoV-2, comprised of structural proteins, namely, the spike (S), envelope (E), and membrane (M), which makes up the viral envelope, and a nucleocapsid (N) containing a single-stranded RNA (ssRNA) of 29903 bp that codes for the replicase-transcriptase [196]. SARS-CoV-2 is part of a group of seven known human coronaviruses (all RNA single-stranded), some of which have provoked life-threatening respiratory diseases before, such as SARS-CoV-1 (Hong Kong) [197] and MERS-CoV (Middle East respiratory syndrome-CoV) (Saudi Arabia) [198]. SARS-CoV-2 infection is initiated by the specific interaction between its spike protein (S) and the human receptor angiotensin I-converting enzyme 2 (ACE2), mediated by the S1 receptor binding domain (RBD); followed by the viral and the host cell membranes fusion promoted by the S2 domain [199]. The cleavage at S1/S2 site for releasing each activated domain is mediated by host proteases, including the transmembrane serine protease 2 (TMPRSS2) as well as TMPRSS4 and cathepsins B or L [200, 201]. Another unique characteristic of SARS-CoV-2 is the addition of a furin (serine protease) cleavage sequence containing multi‐basic amino acids (PRRAR) at the S1/S2 site during the biosynthesis of novel virion S protein at the Golgi apparatus inside the host cells [202]. This may expand its transmissibility and tropism, enabling it to infect other ACE2 expressing cells without the presence of TMPRSS2, since virtually all cells normally express furin [203]. Moreover, it has been recently reported that neuropilin-1 (NRP1), known to bind furin-cleaved substrates, significantly potentiates SARS-CoV-2 infectivity in cells, including olfactory neurons facing the nasal cavity expressing NRP1 [204, 205]. Therefore, the distribution of ACE2 receptor, the proteases TMPRSS2 or 4, cathepsin B or L, and furin, as well as NRP1 across different tissues, could be considered critical determinants of the virus’ infectivity and tropism.
Diverse neurological manifestations have been reported in COVID-19 patients. Among these complications, some reflect dysfunction in the CNS such as headache, fatigue, confusion, dizziness, insomnia, epileptic seizures, encephalopathy, acute stroke (ischemic or hemorrhagic), meningitis/encephalitis; others reflect peripheral nervous system (PNS) dysfunction, such as hyposmia/anosmia (reduced/loss of smell), hypogeusia/ageusia (reduced/loss of taste), Guillain-Barré syndrome, neuropathic pain, myalgias, and acute transverse or necrotizing myelitis [206, 207]. In a recent report examining the progression of 509 consecutive COVID-19 patients admitted within a hospital network in Chicago (USA), Liotta et al. (2020) found that neurological manifestations were present at any time during the disease course in 419 patients (82.3%) [208]. This situation becomes more complex given that in aged patients in which immunosenescence coexists with comorbidities like chronic arterial hypertension, diabetes, hyperlipidemia, obesity, among others [209], COVID-19 is likely to be more severe. These are also common risk factors for neurological and neurodegenerative disorders in the elderly [210]. However, their subjacent pathological mechanisms have only begun to be dissected, and knowing how this virus may access and impact the nervous tissue is an urgent need of the hour. These could be the sequelae of either a direct action of SARS-CoV-2 infection on host CNS/PNS cells or an indirect effect due to its “cytokine storm” and hypoxia-induced injury [211].
As COVID-19 infection progresses starting with lung dysfunction manifestations such as hypoxia, inflammation and hypercoagulability, these may impact the nervous system indirectly. The hypoxic environment could result in metabolic acidosis of the brain, accumulation of lactic acid, increased free radicals and oxidative stress, and diminished ATP production in the nervous tissue [212]. Systemic inflammation, reflecting immune efforts to restrict the virus transmission and injury, is also critically involved in COVID-19-induced damage to different organs, including the brain. As a consequence, the individual's immune system generates a burst of inflammatory cells and cytokines mediating a defense response that has been termed as the “cytokine storm”; and controversially, it may also damage the own body [213]. In this sense, a large number of studies have documented the versatile anti-inflammatory and antioxidant activities of Sp extract and its derived C-PC/PCB, supporting its potential use for curtailing the hypoxia-related stress and the cytokine storm progression. Sp extract has strong anti-allergic effects in individuals diagnosed with allergic rhinitis, modulating the levels of pro-inflammatory cytokines [214]. C-PC, a selective cyclooxygenase-2 inhibitor [215], limits nitric oxide production, iNOS expression, TNF-α formation and NF-κB activation in lipopolysaccharide-stimulated RAW 264.7 macrophages, thus limiting some key aspects of deregulated inflammation [216]. Moreover, the administration of either Sp extract, C-PC or PCB has been found to protect the liver under diverse hepatotoxic conditions [217-220]. Given that liver injury is associated with severe disease in COVID-19 patients [221], and also has an impact on the progression of neurological conditions like encephalopathy [222], it can be seen that this liver-safeguarding action of C-PC/PCB may provide indirect neuroprotective activity in these patients. Protection of the brain from COVID-19-induced injury by C-PC/PCB may also be provided by limiting the virus load. It has been recently suggested that compounds with the capacity to inhibit the NOX2 isoform of NADPH oxidase, might be expected to boost the activation of toll-like receptor 7 (TLR7) by single-stranded viral RNA, thereby leading to the induction of type 1 interferon and antiviral antibodies [223]. BR and its homolog PCB have shown inhibiting actions on NOX2-dependent NADPH oxidase activity [43, 224]. Moreover, treatments with either an HO-1 agonist (generating BR) [225] or with Sp extract (releasing PCB in vivo) [226] have shown a significant suppressing activity against influenza virus replication. This activity of PCB may also restrict the thrombotic complications associated with COVID-19 progression, enabling it to protect COVID-19 patients from stroke.
SARS-CoV-2 neuroinvasion may potentially occur through: i) the hematogenous route (by infected leucocytes or direct virus crossing of damaged blood-brain barrier or blood-cerebrospinal fluid barrier), or ii) by retrograde axonal transport (by infected olfactory neurons or peripheral nerves in mucosal epithelium, neuromuscular junctions and enteric terminals) [211]. An increase in blood levels of neurofilament light chain protein (NfL, a marker of axonal and neuronal damage/degeneration) [227] and glial fibrillary acidic protein (GFAP, a marker of astrocytic activation/injury) has been observed in COVID-19 patients [228]. This may indicate the activation of cell death pathways in neurons, as well as activation/damage of astrocytes. ACE2 receptors are found in various brain regions as well as in neuronal and non-neuronal cells (astrocytes, microglia, oligodendrocytes, pericytes, endothelial cells), observed for example in the human middle temporal gyrus and the posterior cingulate cortex [211], suggesting a possibility for SARS-CoV-2 infection of these cells, if it can gain access to the brain parenchyma. Indeed, in a human brain organoid model, Mesci et al. (2020) showed that astrocytes were infected and showed a 4-fold increase in cell death, as well as an impairment of excitatory synaptogenesis [229]. In a human induced pluripotent stem cell (iPSC)-derived BrainSphere model, Bullen et al. (2020) observed that ACE2 is expressed in neuroprogenitor cells and mature BrainSpheres, and short-time incubation with SARS-CoV-2 led to the infection of neurons; virus was detected in neuronal cell bodies, the neurite structures, and in the supernatant over time [230]. Moreover, it was observed that the number of SARS-CoV-2-positive cortical neurons was significantly higher in 60-days than in 15-days-old human brain organoids, suggesting that the virus prefers relatively mature neuronal cell types [231]. Although these authors didn’t detect active replication (viral RNA level increase) of the virus inside neurons, they observed aberrant Tau protein enrichment and phosphorylation in the soma, as well as induction of neuronal death [231].
C-PC/PCB has exerts protective actions against cell death in neurons and astrocytes under several toxic situations that may resemble the brain hypoxia provoked by COVID-19 respiratory distress. C-PC rescued neuronal cells [116] and astrocytes [120] from death induced by oxidative stress, serum deprivation [114] and iron overload [115]. PCB has shown protective actions in cultured neurons from ROS- [45] and oxygen/glucose deprivation-induced [117] demise. Their neuroprotective actions observed in in vivo models of ischemic stroke [111, 117] could also be considered as possibly pertinent to combating cerebrovascular complications of COVID-19. Furthermore, several in silico studies have described docking analysis between PCB and proteins involved in SARS-CoV-2 replication with relevant implications for direct antiviral intervention. PCB was shown to bind to the viral RNA dependent RNA polymerase at its active site [232] and to the main SARS-CoV-2 protease (Mpro), which plays an essential role in processing the polyproteins that are translated from SARS-CoV-2 [233]. Interestingly, docking techniques have showed that the binding energy between PCB and the S spike RBD is close (-7.2 kcal/mol) to the interaction energy between this viral protein domain and its human ACE2 receptor (-12.4 kcal/mol) [234]. Therefore, these computations suggest that PCB may have potential for impeding the infection and replication mechanism of SARS-CoV-2, although further experimental assays are needed to confirm this hypothesis.
6. SAFETY OF C-PC/PCB
C-PC/PCB are natural compounds present in cyanobacteria Sp extracts marketed all over the world either as food supplements or as functional food ingredients [235]. In the US, Sp extract has been approved by the Food and Drug Administration (FDA) for use as color additive in different foods, and as a dietary supplement marketed as capsules, tablets, or powder; it has been certified as GRAS (Generally Recognized As Safe) when it meets certain conditions regarding its microscopic appearance, absence of microcystins, and minimal mold content; this GRAS certification applies to consumption levels from 0.5 to 3 g per serving and up to 6 g per day for a high-end consumer [236, 237]. In other parts of the world, including Canada, Australia, Europe and China, Sp food supplementation has also been accepted for safe consumer use [238]. These facts attest to the absence of any known adverse reports for Sp supplementation in human health [239], in consonance with several preclinical studies on the acute, subchronic and chronic toxicity profile of Sp extracts [240, 241]. Furthermore, reproductive and genotoxic preclinical assays in different animal species have also shown no deleterious effects of Sp treatment [242]. Pregnant mice fed with Sp extract up to 30% (m:m in grams of diet) for 19 days, did not show evidence of embryonal toxicity [243]. Moreover, The Sp pre-treatment (200, 400, 800 mg/kg for 2 weeks) prevented the mutagenic effects of cyclophosphamide in mice from both sexes. This was evidenced by reduced post-implantation losses of fetuses in intoxicated pregnant females when given Sp, as well as an improvement of semen quality (increased sperm concentration and motility) in Sp-treated diseased males [244]. A similar protective and antigenotoxic actions of orally administered Sp aqueous extract were found in mice from both sexes, which were intoxicated with benzo[α]pyrene, a prototypical mutagenic and carcinogenic polycyclic aromatic hydrocarbon present ubiquitously in cigarette smoke, urban air, charbroiled food, vehicle exhaust, asphalt, and coke ovens [245].
This strongly encourages the application of its natural components C-PC/PCB, in the clinical setting. Although the potential harm profile, the pharmacokinetic behavior and the safety for the human use of C-PC/PCB has not been fully assessed, several reports have given some insight into these highly desirable goals. C-PC has shown nontoxic and noncarcinogenic activity in human erythrocytes, and protects them from oxidative damage [246, 247]. The oral administration of C-PC to rats and mice showed no adverse effects even at the highest tested dose (3 g/kg) during an observation period of 14 days, including no alterations in body weight, animal behavior and organ histopathology [248]. More recently, Song et al. (2012) tested the oral chronic ingestion of C-PC at 0.12, 0.4 and 4 g/kg per day in SD rats for 12 weeks, and found that it exerted no toxic effects at any dose on macroscopic, biochemical and physiological parameters, even after a 4-week recovery period [249]. Similar positive results were obtained when albino rats from both sexes received a C-PC diet at 0.25, 0.5, 1, 2 and 5 g/kg for 14 weeks [250]. Interestingly, pilot human assessment of C-PC-enriched Sp extracts has shed light into the safe consumption and nutraceutical benefits of this biliprotein and consequently, its structural component PCB once released after in vivo C-PC metabolism. In one report, 12 subjects experiencing chronic pain received a daily supplementation with a C-PC-concentrated aqueous Sp extract in veggie capsules (with biliprotein levels higher than 30% of Sp dry weight) at 1 g for 4 weeks, or at 0.5 and 0.25 g for 1-week duration, separated by 1-week placebo period. These regimes were associated with a significant reduction for each person’s primary pain area, and an increased ability to perform activities of daily living [251]. The clinical safety of this C-PC-enriched Sp extract was evaluated in 24 subjects of both sexes reporting chronic joint pain, who ingested 2.3 g per day for 2 weeks – amounting to approximately 1 g C-PC daily. No changes were observed for activated partial thromboplastin time, thrombin clotting time, or fibrinogen activity, as well as important parameters of coagulation and platelet function. Moreover, a reduction in the aspartate and the alanine transaminases (AST and ALT) and a rapid and robust relief of chronic pain were induced by this nutraceutical intervention, suggesting an improvement in liver function and the effective resolution of joint associated chronic injury [252]. These results confirmed the previous assessment of this formulation by using in vitro assays, which demonstrated a significant inhibition of ROS formation in polymorphonuclear cells from healthy human donors and no negative effect on a blood clotting assay [253]. In contrast, to the best of our knowledge, toxicological assessment of pure PCB has not been reported so far. Preclinical studies using disease models have shown beneficial actions of PCB given by oral or intraperitoneal routes at different doses and treatment periods (Table 1). As an additional clue, Basdeo et al. (2016) reported that mice treated with either PCB (30 mg/kg) or control (phosphate buffer saline) on days 0, 3, and 5 by oral gavage, did not evidence any differences in their body weight and the macroscopic characteristics of their colons [47]. Although a comprehensive assessment of the toxicological profile of C-PC/PCB is still lacking, the above-mentioned observations suggest that these substances exert no adverse events at their beneficial doses in preclinical models, further supporting the merit of developing it as a nutraceutical supplement for ameliorating neurodegenerative conditions.
CONCLUSION AND PERSPECTIVES
The BV and BR generated intracellularly via HO-1-mediated degradation of heme and the subsequent reductive activity of BVR have important protective regulatory impacts, including inhibition of NADPH oxidase complexes – a key source of oxidative stress in the pathogenesis of a vast array of pathologies – and agonism for AhR. The latter effect plays a key role in the generation of anti-inflammatory Treg cells, and also induces expression of Nrf2, the transcription factor that drives phase 2 induction of a wide range of antioxidant and cytoprotective proteins, while also promoting glutathione synthesis. Additionally, via interaction with BVR, BV may initiate anti-inflammatory cell signaling. These findings help to rationalize the important antioxidant and anti-inflammatory effects of HO-1 activity. Although oral or parenteral administration of BV has potential for replicating these important effects, which likely would be beneficial for prevention and treatment of the many disorders linked to oxidative stress and inflammation, BV is a fine chemical that is currently expensive to synthesize, and it doesn't appear to be any concentrated natural sources of this compound. Furthermore, even if BR were readily available, it is too insoluble for effective oral administration.
However, the BV homologue metabolite PCB constitutes about 0.6% of the dry weight of Spirulina biomass (assuming that C-PC constitutes about 14% of its dry weight). The remarkable antioxidant and anti-inflammatory effects of orally administered Sp extracts or C-PC demonstrated in a wide range of rodent models of human health disorders point to the strong likelihood that PCB, either as the free compound or conjugated to oligopeptides derived from C-PC digestion, is absorbable and mediates protective effects homologous to those achieved by BV/BR. Hence, either whole Sp preparations, Sp extracts enriched in C-PC, or PCB (as the free compound or natural PCB-linked oligopeptides), may have important practical potential for replicating the versatile protective effects which BV/BR mediate physiologically. Given that Sp has long been used as a safe and highly nutritious food in certain human cultures, and is not hard or unduly expensive to produce, its extracts may have outstanding potential for the promotion of human health. The only practical drawback in this regard is that most people find the strong flavor and odor of Sp objectionable, for which reason Sp extracts enriched in PCB and administered in capsule or tablet form may have a bright future.
Oxidant stress and inflammation are almost self-evidently key mediators of a wide range of neurodegenerative disorders, both acute and chronic. In particular, they play a role in the neuronal death and dysfunction associated with acute conditions like IS, and devastating progressive neurodegenerative disorders, most notably MS, Alzheimer’s and Parkinson’s diseases. More recently, SARS-CoV-2 has been found to damage the central and peripheral nervous systems, either directly or indirectly. This essay has emphasized the role of oxidant stress and inflammation in driving the pathogenesis of these disorders, and reviewed the growing evidence that Sp and its key component PCB can intervene positively in their pathogenesis.
The fact that Sp is a food, and that PCB preparations should be susceptible to administration as nutraceuticals, is particularly encouraging, inasmuch as disorders such as MS, AD and PD are best addressed with prevention – and our capacity to identify those who are at risk is currently very limited. The wide availability, safety and affordability of nutraceuticals, at least in comparison to new drugs, make them highly appropriate for widespread use in primary prevention of neurodegeneration. With respect to IS, pre-administration of PCB may lessen the neuronal damage when these events do occur – as suggested by certain rodent studies cited above. However, in acute pathologies such as IS, rapid parenteral administration of purified PCB might prove to be notably protective – as also suggested by rodent studies.
Unfortunately, the pharmacokinetics of orally administered PCB – whether administered with intact C-PC or as a free compound or oligopeptide complex – has so far received little if any study. This needs to be remedied by appropriate research, first in rodents, and then clinically. Moreover, the impact of PCB on NADPH oxidase (and the isoform-specificity of this effect), AhR agonism, and the signaling downstream of BVR needs further evaluation. Ultimately, a wide range of clinical studies will be required, to evaluate its potential utility in a vast range of disorders in which oxidant stress and inflammation play a key pathogenic role. Acute and chronic disorders of the central and peripheral nervous system should be high on the list of those disorders, potentially responsive to PCB, in which this agent receives clinical evaluation.
It should, however be acknowledged that a number of additional nutrients or phytochemicals administrable as nutraceuticals have potential, complementary to that of PCB, for minimizing the pathogenic role of oxidative stress in neurological disorders. In particular, agents that can promote phase 2 induction of antioxidant/cytoprotective enzymes – as PCB can – and that likewise are adequately absorbable and can pass through the blood-brain barrier, merit serious consideration in this regard. Some of these, unlike PCB, can interact directly with the Keap1 protein to induce translocation of the Nrf2 transcription factor to the nucleus, where it can interact with antioxidant response elements of genes coding for phase 2 enzymes to promote their transcription [254]. In particular, lipoic acid appears to have outstanding potential in this regard, as it has shown utility in rodent models of AD, PD, EAE, and, like PCB, can up-regulate BDNF/NGF in certain circumstances [255-260]. Hydroxytyrosol, an antioxidant compound in extra-virgin olive oil, and resveratrol, a component of the skin of grapes and berries, are phase 2 inducers that have shown a similar spectrum of activities in rodents [261-271]. The neurohormone melatonin, like PCB, can aid phase 2 induction in the CNS and provide neuroprotection by promoting increased expression of Nrf2 [272, 273]. Such agents can indirectly suppress NADPH oxidase activity via HO-1 induction [274]. It is reasonable to suspect that concurrent administration of such agents could complement the utility of PCB for combatting neurodegenerative disorders.
Finally, innovations by the nutraceutical or pharmaceutical industry are needed so that it becomes more feasible, convenient, and affordable for people to ingest optimally beneficial amounts of PCB on a daily basis if desired. And the development of a parenterally-administrable form of free PCB may optimize the potential of this agent in the management of oxidative stress emergencies, including IS.
One further comment is warranted. Many medical scientists may be rather blasé about “antioxidants”, based on the fact that clinical trials with certain scavenging antioxidants such as vitamin E, vitamin C, or β-carotene have yielded paltry or even negative results. But PCB is an antioxidant with a difference – it might be characterized as a “source antioxidant”. Although it likewise can scavenge free radicals, it appears to mimic the physiological role of BV/BR as an inhibitor of NADPH oxidase – the most prominent source of excess O2•– in many health disorders. The proinflammatory effects of oxidant stress are mediated largely by H2O2, which modulates the activity of many enzymes by reversible oxidation of sulfhydryl groups. Oxidant scavengers do nothing – directly, at least - to prevent the production or lessen the half-life of H2O2. But BR and PCB prevent its production by choking off a key source of O2•– production – hence their designation as “source antioxidants”. Moreover, via AhR activation and subsequent Nrf2 induction, they can boost the activity of a range of antioxidant enzymes – including those that eliminate H2O2 or function to reverse its oxidizing effects on sulfhydryl groups. Add to that the fact that they may promote Treg activity via AhR, and it can be better understood why PCB has much great anti-inflammatory potential than any mere free radicals scavenger could provide to. We should also bear in mind the marked reduction in global mortality associated with life-long moderate elevation of unconjugated BR in Gilbert’s syndrome subjects. This opens the question if PCB supplementation could mimic this health protection. Clearly, PCB deserves diligent attention from the medical research community since the accumulated evidence strongly supports further development of PCB formulations for testing it in clinical trials.
ACKNOWLEDGEMENTS
Authors would like to thank Alexey Llópiz-Arzuaga for his assistance with CDX (ChemDraw) format, and to Sr. Emilio Fontana from FONTANA INNOVATION SRL for his support.
LIST OF ABBREVIATIONS
- 6-OHDA
6-hydroxydopamine
- AD
Alzheimer's disease
- AhR
Aryl hydrocarbon receptor
- ALT
Alanine transaminase
- APP
Amyloid precursor protein
- AST
Aspartate transaminase
- Aβ
beta-amyloid peptide
- BACE1
β-site APP cleaving enzyme 1
- BDNF
Brain derived neurotrophic factor
- BR
Bilirubin
- BV
Biliverdin
- BVR
Biliverdin reductase
- CAT
Catalase
- CBF
Cerebral blood flow
- CNS
Central nervous system
- COVID-19
Coronavirus disease 2019
- COX-2
Cyclooxygenase-2
- C-PC
C-Phycocyanin
- DA
Dopaminergic
- FDA
Food and Drug Administration
- GFAP
Glial fibrillary acidic protein
- GPx
Glutathione peroxidase
- GR
Glutathione reductase
- GSH
Reduced glutathione
- HO-1
Heme oxigenase-1
- iNOS
Inducible nitric oxide synthase
- iPSC
Induced pluripotent stem cell
- MDA
Malondialdehyde
- MOG
Myelin oligodendrocyte protein
- NfL
Neurofilament light chain protein
- ODs
Oligodendrocytes
- OPCs
Oligodendrocyte progenitor cells
- PARP-1
Poly-ADP ribose polymerase-1
- PBMC
Peripheral blood mononuclear cells
- PCB
Phycocyanobilin
- PNS
Peripheral nervous system
- PSEN1
Presenilin 1
- PSEN2
Presenilin 2
- RBD
Receptor binding domain
- ROS
Reactive oxygen species
- SNc
Substantia nigra pars compacta
- SOD
Superoxide dismutase
- Sp
Spirulin
- Treg
Regulatory T cells
- WHO
World Health Organization
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Giselle Pentón-Rol and Javier Marín-Prida are co-inventors of a patent granted in United States, Canada, European Union and other countries regarding the uses of Phycocyanobilin and its pharmaceutical combinations for the treatment of neurodegenerative and ischemic brain diseases. Mark F. McCarty is co-inventor and co-owner of a United States patent on nutraceutical uses of Phycocyanobilin oligopeptides derived from Spirulina, and of a European Union patent application covering intravenous administration of Phycocyanobilin for treatment of stroke or brain trauma.
REFERENCES
- 1.Venkatakrishnan K., Chiu H-F., Wang C-K. Popular functional foods and herbs for the management of type-2-diabetes mellitus: A comprehensive review with special reference to clinical trials and its proposed mechanism. J. Funct. Foods. 2019;57:425–438. doi: 10.1016/j.jff.2019.04.039. [DOI] [Google Scholar]
- 2.Santini A., Novellino E. Nutraceuticals - shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018;11(6):545–547. doi: 10.1080/17512433.2018.1464911. [DOI] [PubMed] [Google Scholar]
- 3.Nahin R.L., Barnes P.M., Stussman B.J., Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl. Health Stat. Rep. 2009;(18):1–14. [PubMed] [Google Scholar]
- 4.Bernardini S., Tiezzi A., Laghezza Masci V., Ovidi E. Natural products for human health: an historical overview of the drug discovery approaches. Nat. Prod. Res. 2018;32(16):1926–1950. doi: 10.1080/14786419.2017.1356838. [DOI] [PubMed] [Google Scholar]
- 5.Dadhania V.P., Trivedi P.P., Vikram A., Tripathi D.N. Nutraceuticals against neurodegeneration: a mechanistic insight. Curr. Neuropharmacol. 2016;14(6):627–640. doi: 10.2174/1570159X14666160104142223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu H.F., Venkatakrishnan K., Wang C.K. The role of nutraceuticals as a complementary therapy against various neurodegenerative diseases: A mini-review. J. Tradit. Complement. Med. 2020;10(5):434–439. doi: 10.1016/j.jtcme.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dugger B.N., Dickson D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017;9(7):a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu C., De Ronchi D., Fratiglioni L. The epidemiology of the dementias: an update. Curr. Opin. Psychiatry. 2007;20(4):380–385. doi: 10.1097/YCO.0b013e32816ebc7b. [DOI] [PubMed] [Google Scholar]
- 9.Aghagoli G., Gallo M.B., Katchur N.J., Chaves-Sell F., Asaad W.F., Murphy S.A. Neurological involvement in COVID-19 and potential mechanisms: A Review. Neurocrit. Care. 2021;34(3):1062–1071. doi: 10.1007/s12028-020-01049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capelli B., Cysewski G.R. Potential health benefits of spirulina microalgae. Nutrafoods. 2010;9(2):19–26. doi: 10.1007/BF03223332. [DOI] [Google Scholar]
- 11.Mathur M. In: Bioactive Molecules in Food. Mérillon J-M., Ramawat K.G., editors. Cham: Springer International Publishing; 2018. Bioactive Molecules of Spirulina: A Food Supplement. pp. 1–22. [Google Scholar]
- 12.Khan Z., Bhadouria P., Bisen P.S. Nutritional and therapeutic potential of Spirulina. Curr. Pharm. Biotechnol. 2005;6(5):373–379. doi: 10.2174/138920105774370607. [DOI] [PubMed] [Google Scholar]
- 13.Padyana A.K., Bhat V.B., Madyastha K.M., Rajashankar K.R., Ramakumar S. Crystal structure of a light-harvesting protein C-phycocyanin from Spirulina platensis. Biochem. Biophys. Res. Commun. 2001;282(4):893–898. doi: 10.1006/bbrc.2001.4663. [DOI] [PubMed] [Google Scholar]
- 14.Adir N. Elucidation of the molecular structures of components of the phycobilisome: reconstructing a giant. Photosynth. Res. 2005;85(1):15–32. doi: 10.1007/s11120-004-2143-y. [DOI] [PubMed] [Google Scholar]
- 15.Romay C., Armesto J., Remirez D., González R., Ledon N., García I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998;47(1):36–41. doi: 10.1007/s000110050256. [DOI] [PubMed] [Google Scholar]
- 16.Romay Ch., González R., Ledón N., Remirez D., Rimbau V. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003;4(3):207–216. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- 17.Romay C., Gonzalez R., Pizarro M., Lissi E. Kinetics of c-phycocyanin reaction with hypochlorite. J. Protein Chem. 2000;19(2):151–155. doi: 10.1023/A:1007038801482. [DOI] [PubMed] [Google Scholar]
- 18.Hirata T., Tanaka M., Ooike M., Tsunomura T., Sakaguchi M. Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. J. Appl. Phycol. 2000;12(3):435–439. doi: 10.1023/A:1008175217194. [DOI] [Google Scholar]
- 19.Bhat V.B., Madyastha K.M. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: protection against oxidative damage to DNA. Biochem. Biophys. Res. Commun. 2001;285(2):262–266. doi: 10.1006/bbrc.2001.5195. [DOI] [PubMed] [Google Scholar]
- 20.McCarty M.F. Clinical potential of Spirulina as a source of phycocyanobilin. J. Med. Food. 2007;10(4):566–570. doi: 10.1089/jmf.2007.621. [DOI] [PubMed] [Google Scholar]
- 21.Dawson J.H. Probing structure-function relations in heme-containing oxygenases and peroxidases. Science. 1988;240(4851):433–439. doi: 10.1126/science.3358128. [DOI] [PubMed] [Google Scholar]
- 22.Jansen T., Daiber A. Direct Antioxidant properties of bilirubin and biliverdin. Is there a role for biliverdin reductase? Front. Pharmacol. 2012;3:30. doi: 10.3389/fphar.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novák P., Jackson A.O., Zhao G.J., Yin K. Bilirubin in metabolic syndrome and associated inflammatory diseases: New perspectives. Life Sci. 2020;257118032 doi: 10.1016/j.lfs.2020.118032. [DOI] [PubMed] [Google Scholar]
- 24.Jayanti S., Vítek L., Tiribelli C., Gazzin S. The role of bilirubin and the other “yellow players” in neurodegenerative diseases. Antioxidants. 2020;9(9):900. doi: 10.3390/antiox9090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen T.W.R., Wong R.J., Stevenson D.K. Molecular physiology and pathophysiology of bilirubin handling by the blood, liver, intestine, and brain in the newborn. Physiol. Rev. 2020;100(3):1291–1346. doi: 10.1152/physrev.00004.2019. [DOI] [PubMed] [Google Scholar]
- 26.Mancuso C. Bilirubin and brain: A pharmacological approach. Neuropharmacology. 2017;118:113–123. doi: 10.1016/j.neuropharm.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Horsfall L.J., Nazareth I., Pereira S.P., Petersen I. Gilbert’s syndrome and the risk of death: a population-based cohort study. J. Gastroenterol. Hepatol. 2013;28(10):1643–1647. doi: 10.1111/jgh.12279. [DOI] [PubMed] [Google Scholar]
- 28.Vasavda C., Kothari R., Malla A.P., Tokhunts R., Lin A., Ji M., Ricco C., Xu R., Saavedra H.G., Sbodio J.I., Snowman A.M., Albacarys L., Hester L., Sedlak T.W., Paul B.D., Snyder S.H. Bilirubin links heme metabolism to neuroprotection by scavenging superoxide. Cell Chem. Biol. 2019;26(10):1450–1460.e7. doi: 10.1016/j.chembiol.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapitulnik J., Maines M.D. Pleiotropic functions of biliverdin reductase: Cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol. Sci. 2009;30(3):129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien L., Hosick P.A., John K., Stec D.E., Hinds T.D., Jr Biliverdin reductase isozymes in metabolism. Trends Endocrinol. Metab. 2015;26(4):212–220. doi: 10.1016/j.tem.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terry M.J., Maines M.D., Lagarias J.C. Inactivation of phytochrome- and phycobiliprotein-chromophore precursors by rat liver biliverdin reductase. J. Biol. Chem. 1993;268(35):26099–26106. doi: 10.1016/S0021-9258(19)74286-0. [DOI] [PubMed] [Google Scholar]
- 32.Jayasundera K.P., Kinoshita H., Inomata K. Efficient construction of A,B-Rings component for syntheses of phycocyanobilin and its derivative. Chem. Lett. 1998;27(12):1227–1228. doi: 10.1246/cl.1998.1227. [DOI] [Google Scholar]
- 33.Scheer H., Zhao K.H. Biliprotein maturation: the chromophore attachment. Mol. Microbiol. 2008;68(2):263–276. doi: 10.1111/j.1365-2958.2008.06160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bishop J.E., Nagy J.O., O’Connell J.F., Rapoport H. Diastereoselective synthesis of phycocyanobilin-cysteine adducts. J. Am. Chem. Soc. 1991;113(21):8024–8035. doi: 10.1021/ja00021a032. [DOI] [Google Scholar]
- 35.Minic S., Stanic-Vucinic D., Radomirovic M., Radibratovic M., Milcic M., Nikolic M., Cirkovic V.T. Characterization and effects of binding of food-derived bioactive phycocyanobilin to bovine serum albumin. Food Chem. 2018;239:1090–1099. doi: 10.1016/j.foodchem.2017.07.066. [DOI] [PubMed] [Google Scholar]
- 36.Livney Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010;15(1):73–83. doi: 10.1016/j.cocis.2009.11.002. [DOI] [Google Scholar]
- 37.Radibratovic M., Minic S., Stanic-Vucinic D., Nikolic M., Milcic M., Cirkovic Velickovic T. Stabilization of human serum albumin by the binding of phycocyanobilin, a bioactive chromophore of blue-green Alga Spirulina: molecular dynamics and experimental study. PLoS One. 2016;11(12):e0167973. doi: 10.1371/journal.pone.0167973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minic S., Radomirovic M., Savkovic N., Radibratovic M., Mihailovic J., Vasovic T., Nikolic M., Milcic M., Stanic-Vucinic D., Cirkovic V.T. Covalent binding of food-derived blue pigment phycocyanobilin to bovine β-lactoglobulin under physiological conditions. Food Chem. 2018;269:43–52. doi: 10.1016/j.foodchem.2018.06.138. [DOI] [PubMed] [Google Scholar]
- 39.Minic S.L., Milcic M., Stanic-Vucinic D., Radibratovic M., Sotiroudis T.G., Nikolic M.R., Velickovic T.Ć. Phycocyanobilin, a bioactive tetrapyrrolic compound of blue-green alga Spirulina, binds with high affinity and competes with bilirubin for binding on human serum albumin. RSC Advances. 2015;5(76):61787–61798. doi: 10.1039/C5RA05534B. [DOI] [Google Scholar]
- 40.Yan S-G., Zhu L-P., Su H-N., Zhang X-Y., Chen X-L., Zhou B-C., Zhang Y-Z. Single-step chromatography for simultaneous purification of C-phycocyanin and allophycocyanin with high purity and recovery from Spirulina (Arthrospira) platensis. J. Appl. Phycol. 2011;23(1):1–6. doi: 10.1007/s10811-010-9525-7. [DOI] [Google Scholar]
- 41.Minic S.L., Stanic-Vucinic D., Mihailovic J., Krstic M., Nikolic M.R., Cirkovic Velickovic T. Digestion by pepsin releases biologically active chromopeptides from C-phycocyanin, a blue-colored biliprotein of microalga Spirulina. J. Proteomics. 2016;147:132–139. doi: 10.1016/j.jprot.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 42.Koníčková R., Vaňková K., Vaníková J., Váňová K., Muchová L., Subhanová I., Zadinová M., Zelenka J., Dvořák A., Kolář M., Strnad H., Rimpelová S., Ruml T.J., Wong R., Vítek L. Anti-cancer effects of blue-green alga Spirulina platensis, a natural source of bilirubin-like tetrapyrrolic compounds. Ann. Hepatol. 2014;13(2):273–283. doi: 10.1016/S1665-2681(19)30891-9. [DOI] [PubMed] [Google Scholar]
- 43.Zheng J., Inoguchi T., Sasaki S., Maeda Y., McCarty M.F., Fujii M., Ikeda N., Kobayashi K., Sonoda N., Takayanagi R. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304(2):R110–R120. doi: 10.1152/ajpregu.00648.2011. [DOI] [PubMed] [Google Scholar]
- 44.Liu J., Zhang Q.Y., Yu L.M., Liu B., Li M.Y., Zhu R.Z. Phycocyanobilin accelerates liver regeneration and reduces mortality rate in carbon tetrachloride-induced liver injury mice. World J. Gastroenterol. 2015;21(18):5465–5472. doi: 10.3748/wjg.v21.i18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marín-Prida J., Pavón-Fuentes N., Llópiz-Arzuaga A., Fernández-Massó J.R., Delgado-Roche L., Mendoza-Marí Y., Santana S.P., Cruz-Ramírez A., Valenzuela-Silva C., Nazábal-Gálvez M., Cintado-Benítez A., Pardo-Andreu G.L., Polentarutti N., Riva F., Pentón-Arias E., Pentón-Rol G. Phycocyanobilin promotes PC12 cell survival and modulates immune and inflammatory genes and oxidative stress markers in acute cerebral hypoperfusion in rats. Toxicol. Appl. Pharmacol. 2013;272(1):49–60. doi: 10.1016/j.taap.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Strasky Z., Zemankova L., Nemeckova I., Rathouska J., Wong R.J., Muchova L., Subhanova I., Vanikova J., Vanova K., Vitek L., Nachtigal P. Spirulina platensis and phycocyanobilin activate atheroprotective heme oxygenase-1: a possible implication for atherogenesis. Food Funct. 2013;4(11):1586–1594. doi: 10.1039/c3fo60230c. [DOI] [PubMed] [Google Scholar]
- 47.Basdeo S.A., Campbell N.K., Sullivan L.M., Flood B., Creagh E.M., Mantle T.J., Fletcher J.M., Dunne A. Suppression of human alloreactive T cells by linear tetrapyrroles; relevance for transplantation. Transl. Res. 2016;178:81–94.e2. doi: 10.1016/j.trsl.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Cervantes-Llanos M., Lagumersindez-Denis N., Marín-Prida J., Pavón-Fuentes N., Falcon-Cama V., Piniella-Matamoros B., Camacho-Rodríguez H., Fernández-Massó J.R., Valenzuela-Silva C., Raíces-Cruz I., Pentón-Arias E., Teixeira M.M., Pentón-Rol G. Beneficial effects of oral administration of C-Phycocyanin and phycocyanobilin in rodent models of experimental autoimmune encephalomyelitis. Life Sci. 2018;194:130–138. doi: 10.1016/j.lfs.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 49.El-Sayed S.M.H.M. El-Khair BEA, El-Ghobashy RE, El-Assar AM, Hypoglycemic and hypolipidemic effects of Spirulina platensis, Phycocyanin, Phycocyanopeptide and Phycocyanobilin on male diabetic rats. Arab. Univ. J. Agric. Sci. 2018;26:1121–1134. [Google Scholar]
- 50.Benedetti S., Benvenuti F., Scoglio S., Canestrari F. Oxygen radical absorbance capacity of phycocyanin and phycocyanobilin from the food supplement Aphanizomenon flos-aquae. J. Med. Food. 2010;13(1):223–227. doi: 10.1089/jmf.2008.0257. [DOI] [PubMed] [Google Scholar]
- 51.Scoglio S., Lo Curcio V., Catalani S., Palma F., Battistelli S., Benedetti S. Inhibitory effects of Aphanizomenon flos-aquae constituents on human UDP-glucose dehydrogenase activity. J. Enzyme Inhib. Med. Chem. 2016;31(6):1492–1497. doi: 10.3109/14756366.2016.1149478. [DOI] [PubMed] [Google Scholar]
- 52.Aaltomaa S., Lipponen P., Tammi R., Tammi M., Viitanen J., Kankkunen J.P., Kosma V.M. Strong stromal hyaluronan expression is associated with psa recurrence in local prostate cancer. Urol. Int. 2002;69(4):266–272. doi: 10.1159/000066123. [DOI] [PubMed] [Google Scholar]
- 53.Auvinen P., Tammi R., Parkkinen J., Tammi M., Ågren U., Johansson R., Hirvikoski P., Eskelinen M., Kosma V-M. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am. J. Pathol. 2000;156(2):529–536. doi: 10.1016/S0002-9440(10)64757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anttila M.A., Tammi R.H., Tammi M.I., Syrjänen K.J., Saarikoski S.V., Kosma V-M. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000;60(1):150–155. [PubMed] [Google Scholar]
- 55.Araujo J.A., Zhang M., Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012;3:119. doi: 10.3389/fphar.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson L.M., Wallin M.T., Marrie R.A., Culpepper W.J., Langer-Gould A., Campbell J., Buka S., Tremlett H., Cutter G., Kaye W., Wagner L., Larocca N.G. A new way to estimate neurologic disease prevalence in the United States: Illustrated with MS. Neurology. 2019;92(10):469–480. doi: 10.1212/WNL.0000000000007044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Browne P., Chandraratna D., Angood C., Tremlett H., Baker C., Taylor B.V., Thompson A.J. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022–1024. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Losy J. Is MS an inflammatory or primary degenerative disease? J. Neural Transm. (Vienna) 2013;120(10):1459–1462. doi: 10.1007/s00702-013-1079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franklin R.J.M., Ffrench-Constant C. Regenerating CNS myelin - from mechanisms to experimental medicines. Nat. Rev. Neurosci. 2017;18(12):753–769. doi: 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- 60.Cross A.H., Manning P.T., Stern M.K., Misko T.P. Evidence for the production of peroxynitrite in inflammatory CNS demyelination. J. Neuroimmunol. 1997;80(1-2):121–130. doi: 10.1016/S0165-5728(97)00145-8. [DOI] [PubMed] [Google Scholar]
- 61.Cross A.H., Manning P.T., Keeling R.M., Schmidt R.E., Misko T.P. Peroxynitrite formation within the central nervous system in active multiple sclerosis. J. Neuroimmunol. 1998;88(1-2):45–56. doi: 10.1016/S0165-5728(98)00078-2. [DOI] [PubMed] [Google Scholar]
- 62.Haider L., Fischer M.T., Frischer J.M., Bauer J., Höftberger R., Botond G., Esterbauer H., Binder C.J., Witztum J.L., Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134(Pt 7):1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Liu Y., Zhu B., Wang X., Luo L., Li P., Paty D.W., Cynader M.S. Bilirubin as a potent antioxidant suppresses experimental autoimmune encephalomyelitis: implications for the role of oxidative stress in the development of multiple sclerosis. J. Neuroimmunol. 2003;139(1-2):27–35. doi: 10.1016/S0165-5728(03)00132-2. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y., Li P., Lu J., Xiong W., Oger J., Tetzlaff W., Cynader M. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J. Immunol. 2008;181(3):1887–1897. doi: 10.4049/jimmunol.181.3.1887. [DOI] [PubMed] [Google Scholar]
- 66.Wegiel B., Baty C.J., Gallo D., Csizmadia E., Scott J.R., Akhavan A., Chin B.Y., Kaczmarek E., Alam J., Bach F.H., Zuckerbraun B.S., Otterbein L.E. Cell surface biliverdin reductase mediates biliverdin-induced anti-inflammatory effects via phosphatidylinositol 3-kinase and Akt. J. Biol. Chem. 2009;284(32):21369–21378. doi: 10.1074/jbc.M109.027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pentón-Rol G., Martínez-Sánchez G., Cervantes-Llanos M., Lagumersindez-Denis N., Acosta-Medina E.F., Falcón-Cama V., Alonso-Ramírez R., Valenzuela-Silva C., Rodríguez-Jiménez E., Llópiz-Arzuaga A., Marín-Prida J., López-Saura P.A., Guillén-Nieto G.E., Pentón-Arias E. C-Phycocyanin ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Int. Immunopharmacol. 2011;11(1):29–38. doi: 10.1016/j.intimp.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Pentón-Rol G., Lagumersindez-Denis N., Muzio L., Bergami A., Furlan R., Fernández-Massó J.R., Nazabal-Galvez M., Llópiz-Arzuaga A., Herrera-Rolo T., Veliz-Rodriguez T., Polentarutti N., Marín-Prida J., Raíces-Cruz I., Valenzuela-Silva C., Teixeira M.M., Pentón-Arias E. Comparative neuroregenerative effects of C-Phycocyanin and IFN-Beta in a model of multiple sclerosis in mice. J. Neuroimmune Pharmacol. 2016;11(1):153–167. doi: 10.1007/s11481-015-9642-9. [DOI] [PubMed] [Google Scholar]
- 69.Choi B.M., Pae H.O., Jeong Y.R., Kim Y.M., Chung H.T. Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochem. Biophys. Res. Commun. 2005;327(4):1066–1071. doi: 10.1016/j.bbrc.2004.12.106. [DOI] [PubMed] [Google Scholar]
- 70.Xia Z.W., Zhong W.W., Xu L.Q., Sun J.L., Shen Q.X., Wang J.G., Shao J., Li Y.Z., Yu S.C. Heme oxygenase-1-mediated CD4+CD25high regulatory T cells suppress allergic airway inflammation. J. Immunol. 2006;177(9):5936–5945. doi: 10.4049/jimmunol.177.9.5936. [DOI] [PubMed] [Google Scholar]
- 71.Chora Â.A., Fontoura P., Cunha A., Pais T.F., Cardoso S., Ho P.P., Lee L.Y., Sobel R.A., Steinman L., Soares M.P. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J. Clin. Invest. 2007;117(2):438–447. doi: 10.1172/JCI28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCarty M.F. Clinical potential of phycocyanobilin for induction of T regulatory cells in the management of inflammatory disorders. Med. Hypotheses. 2011;77(6):1031–1033. doi: 10.1016/j.mehy.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 73.Phelan D., Winter G.M., Rogers W.J., Lam J.C., Denison M.S. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch. Biochem. Biophys. 1998;357(1):155–163. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- 74.Vítek L. Bilirubin as a signaling molecule. Med. Res. Rev. 2020;40(4):1335–1351. doi: 10.1002/med.21660. [DOI] [PubMed] [Google Scholar]
- 75.Pot C. Aryl hydrocarbon receptor controls regulatory CD4+ T cell function. Swiss Med. Wkly. 2012;142:w13592. doi: 10.4414/smw.2012.13592. [DOI] [PubMed] [Google Scholar]
- 76.Kerkvliet N.I., Steppan L.B., Vorachek W., Oda S., Farrer D., Wong C.P., Pham D., Mourich D.V. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy. 2009;1(4):539–547. doi: 10.2217/imt.09.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., Bradfield C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010;185(6):3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quintana F.J., Murugaiyan G., Farez M.F., Mitsdoerffer M., Tukpah A.M., Burns E.J., Weiner H.L. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2010;107(48):20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rouse M., Singh N.P., Nagarkatti P.S., Nagarkatti M. Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br. J. Pharmacol. 2013;169(6):1305–1321. doi: 10.1111/bph.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lock C., Hermans G., Pedotti R., Brendolan A., Schadt E., Garren H., Langer-Gould A., Strober S., Cannella B., Allard J., Klonowski P., Austin A., Lad N., Kaminski N., Galli S.J., Oksenberg J.R., Raine C.S., Heller R., Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002;8(5):500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 81.Murphy K.M., Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat. Immunol. 2010;11(8):674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishihara K., Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13(4-5):357–368. doi: 10.1016/S1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 83.Eugster H.P., Frei K., Kopf M., Lassmann H., Fontana A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur. J. Immunol. 1998;28(7):2178–2187. doi: 10.1002/(SICI)1521-4141(199807)28:07<2178:AID-IMMU2178>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 84.Okuda Y., Sakoda S., Bernard C.C., Fujimura H., Saeki Y., Kishimoto T., Yanagihara T. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int. Immunol. 1998;10(5):703–708. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- 85.Viglietta V., Baecher-Allan C., Weiner H.L., Hafler D.A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199(7):971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buckner J.H. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 2010;10(12):849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dombrowski Y., O’Hagan T., Dittmer M., Penalva R., Mayoral S.R., Bankhead P., Fleville S., Eleftheriadis G., Zhao C., Naughton M., Hassan R., Moffat J., Falconer J., Boyd A., Hamilton P., Allen I.V., Kissenpfennig A., Moynagh P.N., Evergren E., Perbal B., Williams A.C., Ingram R.J., Chan J.R., Franklin R.J.M., Fitzgerald D.C. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 2017;20(5):674–680. doi: 10.1038/nn.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pentón-Rol G., Marín-Prida J., Falcón-Cama V. C-Phycocyanin and phycocyanobilin as remyelination therapies for enhancing recovery in multiple sclerosis and ischemic stroke: a preclinical perspective. Behav. Sci. (Basel) 2018;8(1):E15. doi: 10.3390/bs8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuhlmann T., Miron V., Cui Q., Wegner C., Antel J., Brück W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131(Pt 7):1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- 90.Ligon K.L., Fancy S.P., Franklin R.J., Rowitch D.H. Olig gene function in CNS development and disease. Glia. 2006;54(1):1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- 91.Fancy S.P., Zhao C., Franklin R.J. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol. Cell. Neurosci. 2004;27(3):247–254. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 92.Southwood C., He C., Garbern J., Kamholz J., Arroyo E., Gow A. CNS myelin paranodes require Nkx6-2 homeoprotein transcriptional activity for normal structure. J. Neurosci. 2004;24(50):11215–11225. doi: 10.1523/JNEUROSCI.3479-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ciotti J.R., Cross A.H. Disease-modifying treatment in progressive Multiple Sclerosis. Curr. Treat. Options Neurol. 2018;20(5):12. doi: 10.1007/s11940-018-0496-3. [DOI] [PubMed] [Google Scholar]
- 94.Montalban X., Hauser S.L., Kappos L., Arnold D.L., Bar-Or A., Comi G., de Seze J., Giovannoni G., Hartung H-P., Hemmer B., Lublin F., Rammohan K.W., Selmaj K., Traboulsee A., Sauter A., Masterman D., Fontoura P., Belachew S., Garren H., Mairon N., Chin P., Wolinsky J.S. Ocrelizumab versus placebo in primary progressive Multiple Sclerosis. N. Engl. J. Med. 2017;376(3):209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 95.Kipp M., Nyamoya S., Hochstrasser T., Amor S. Multiple sclerosis animal models: a clinical and histopathological perspective. Brain Pathol. 2017;27(2):123–137. doi: 10.1111/bpa.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amor S., Smith P.A., Hart B., Baker D. Biozzi mice: of mice and human neurological diseases. J. Neuroimmunol. 2005;165(1-2):1–10. doi: 10.1016/j.jneuroim.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Ohno N., Chiang H., Mahad D.J., Kidd G.J., Liu L., Ransohoff R.M., Sheng Z-H., Komuro H., Trapp B.D. Mitochondrial immobilization mediated by syntaphilin facilitates survival of demyelinated axons. Proc. Natl. Acad. Sci. USA. 2014;111(27):9953–9958. doi: 10.1073/pnas.1401155111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thrift A.G., Cadilhac D.A., Thayabaranathan T., Howard G., Howard V.J., Rothwell P.M., Donnan G.A. Global stroke statistics. Int. J. Stroke. 2014;9(1):6–18. doi: 10.1111/ijs.12245. [DOI] [PubMed] [Google Scholar]
- 99.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Shay C.M., Spartano N.L., Stokes A., Tirschwell D.L., VanWagner L.B., Tsao C.W. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 100.Amarenco P., Bogousslavsky J., Caplan L.R., Donnan G.A., Hennerici M.G. Classification of stroke subtypes. Cerebrovasc. Dis. 2009;27(5):493–501. doi: 10.1159/000210432. [DOI] [PubMed] [Google Scholar]
- 101.Catanese L., Tarsia J., Fisher M. Acute ischemic stroke therapy overview. Circ. Res. 2017;120(3):541–558. doi: 10.1161/CIRCRESAHA.116.309278. [DOI] [PubMed] [Google Scholar]
- 102.Moskowitz M.A., Lo E.H., Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lo E.H. A new penumbra: transitioning from injury into repair after stroke. Nat. Med. 2008;14(5):497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 104.Fisher M., Saver J.L. Future directions of acute ischaemic stroke therapy. Lancet Neurol. 2015;14(7):758–767. doi: 10.1016/S1474-4422(15)00054-X. [DOI] [PubMed] [Google Scholar]
- 105.Campbell B.C., Meretoja A., Donnan G.A., Davis S.M. Twenty-year history of the evolution of stroke thrombolysis with intravenous alteplase to reduce long-term disability. Stroke. 2015;46(8):2341–2346. doi: 10.1161/STROKEAHA.114.007564. [DOI] [PubMed] [Google Scholar]
- 106.Grotta J.C., Hacke W. Stroke neurologist’s perspective on the new endovascular trials. Stroke. 2015;46(6):1447–1452. doi: 10.1161/STROKEAHA.115.008384. [DOI] [PubMed] [Google Scholar]
- 107.O’Collins V.E., Macleod M.R., Donnan G.A., Horky L.L., van der Worp B.H., Howells D.W. 1,026 experimental treatments in acute stroke. Ann. Neurol. 2006;59(3):467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 108.Di Carlo A. Human and economic burden of stroke. Age Ageing. 2009;38(1):4–5. doi: 10.1093/ageing/afn282. [DOI] [PubMed] [Google Scholar]
- 109.Wu P.F., Zhang Z., Wang F., Chen J.G. Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol. Sin. 2010;31(12):1523–1531. doi: 10.1038/aps.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thaakur S., Sravanthi R. Neuroprotective effect of Spirulina in cerebral ischemia-reperfusion injury in rats. . J. Neural Transm., (Vienna), . 2010;117(9):1083–1091. doi: 10.1007/s00702-010-0440-5. [DOI] [PubMed] [Google Scholar]
- 111.Pentón-Rol G., Marín-Prida J., Pardo-Andreu G., Martínez-Sánchez G., Acosta-Medina E.F., Valdivia-Acosta A., Lagumersindez-Denis N., Rodríguez-Jiménez E., Llópiz-Arzuaga A., López-Saura P.A., Guillén-Nieto G., Pentón-Arias E. C-Phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbils. Brain Res. Bull. 2011;86(1-2):42–52. doi: 10.1016/j.brainresbull.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 112.Endres M., Dirnagl U., Moskowitz M.A. The ischemic cascade and mediators of ischemic injury. Handb. Clin. Neurol. 2009;92:31–41. doi: 10.1016/S0072-9752(08)01902-7. [DOI] [PubMed] [Google Scholar]
- 113.Xing C., Hayakawa K., Lok J., Arai K., Lo E.H. Injury and repair in the neurovascular unit. Neurol. Res. 2012;34(4):325–330. doi: 10.1179/1743132812Y.0000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rimbau V., Camins A., Pubill D., Sureda F.X., Romay C., González R., Jiménez A., Escubedo E., Camarasa J., Pallàs M. C-phycocyanin protects cerebellar granule cells from low potassium/serum deprivation-induced apoptosis. Naunyn Schmiedebergs Arch. Pharmacol. 2001;364(2):96–104. doi: 10.1007/s002100100437. [DOI] [PubMed] [Google Scholar]
- 115.Bermejo-Bescós P., Piñero-Estrada E., Villar del Fresno A.M. Neuroprotection by Spirulina platensis protean extract and phycocyanin against iron-induced toxicity in SH-SY5Y neuroblastoma cells. Toxicol. In Vitro. 2008;22(6):1496–1502. doi: 10.1016/j.tiv.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 116.Marín-Prida J., Pentón-Rol G., Rodrigues F.P., Alberici L.C., Stringhetta K., Leopoldino A.M., Naal Z., Polizello A.C., Llópiz-Arzuaga A., Rosa M.N., Liberato J.L., Santos W.F., Uyemura S.A., Pentón-Arias E., Curti C., Pardo-Andreu G.L. C-Phycocyanin protects SH-SY5Y cells from oxidative injury, rat retina from transient ischemia and rat brain mitochondria from Ca2+/phosphate-induced impairment. Brain Res. Bull. 2012;89(5-6):159–167. doi: 10.1016/j.brainresbull.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 117.Pavón-Fuentes N., Marín-Prida J., Llópiz-Arzuaga A., Falcón-Cama V., Campos-Mojena R., Cervantes-Llanos M., Piniella-Matamoros B., Pentón-Arias E., Pentón-Rol G. Phycocyanobilin reduces brain injury after endothelin-1- induced focal cerebral ischaemia. Clin. Exp. Pharmacol. Physiol. 2020;47(3):383–392. doi: 10.1111/1440-1681.13214. [DOI] [PubMed] [Google Scholar]
- 118.Chen J-C., Liu K.S., Yang T-J., Hwang J-H., Chan Y-C., Lee I.T. Spirulina and C-phycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr. Neurosci. 2012;15(6):252–256. doi: 10.1179/1476830512Y.0000000020. [DOI] [PubMed] [Google Scholar]
- 119.Takeuchi H. Neurotoxicity by microglia: Mechanisms and potential therapeutic strategy. Clin. Exp. Neuroimmunol. 2010;1(1):12–21. doi: 10.1111/j.1759-1961.2009.00001.x. [DOI] [Google Scholar]
- 120.Min S.K., Park J.S., Luo L., Kwon Y.S., Lee H.C., Shim H.J., Kim I.D., Lee J.K., Shin H.S. Assessment of C-phycocyanin effect on astrocytes-mediated neuroprotection against oxidative brain injury using 2D and 3D astrocyte tissue model. Sci. Rep. 2015;5:14418. doi: 10.1038/srep14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoon S.J., Choi J.I., Min S.K., Shin H.S. Evaluation of the effect of C-phycocyanin on cultured rat primary hippocampal astrocytes undergoing trophic factor deprivation. Biotechnol. Bioprocess Eng.; BBE. 2019;24(6):870–875. doi: 10.1007/s12257-019-0102-x. [DOI] [Google Scholar]
- 122.Chung G.Y., Shim K.H., Kim H.J., Min S.K., Shin H.S. Chitosan-coated C-phycocyanin liposome for extending the neuroprotective time window against ischemic brain stroke. Curr. Pharm. Des. 2018;24(17):1859–1864. doi: 10.2174/1381612824666180515123543. [DOI] [PubMed] [Google Scholar]
- 123.Min S.K., Kwon Y.S., Cho M.K., Shin H.S. Nasal delivery of antioxidants by cholesterol-incorporated liposomes extends the neuroprotective time window in cerebral ischemia. Curr. Pharm. Des. 2017;23(40):6223–6230. doi: 10.2174/1381612823666170825124515. [DOI] [PubMed] [Google Scholar]
- 124.Anrather J., Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13(4):661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Allen C.L., Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke. 2009;4(6):461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 126.Lee H., Choi Y.K. Regenerative effects of heme oxygenase metabolites on neuroinflammatory diseases. Int. J. Mol. Sci. 2018;20(1):78. doi: 10.3390/ijms20010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ma M.W., Wang J., Zhang Q., Wang R., Dhandapani K.M., Vadlamudi R.K., Brann D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017;12(1):7. doi: 10.1186/s13024-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mistry A., Stolnik S., Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009;379(1):146–157. doi: 10.1016/j.ijpharm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 129.Nelson P.T., Braak H., Markesbery W.R. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009;68(1):1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alonso Vilatela M.E., López-López M., Yescas-Gómez P. Genetics of Alzheimer’s disease. Arch. Med. Res. 2012;43(8):622–631. doi: 10.1016/j.arcmed.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 131.Lane C.A., Hardy J., Schott J.M. Alzheimer’s disease. Eur. J. Neurol. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 132.Maramai S., Benchekroun M., Gabr M.T., Yahiaoui S. Multitarget therapeutic strategies for Alzheimer’s disease: review on emerging target combinations. BioMed Res. Int. 2020;20205120230 doi: 10.1155/2020/5120230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Markesbery W.R. Neuropathologic alterations in mild cognitive impairment: a review. J. Alzheimers Dis. 2010;19(1):221–228. doi: 10.3233/JAD-2010-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019;20(3):148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bolós M., Perea J.R., Avila J. Alzheimer’s disease as an inflammatory disease. Biomol. Concepts. 2017;8(1):37–43. doi: 10.1515/bmc-2016-0029. [DOI] [PubMed] [Google Scholar]
- 136.Akhtar A., Sah S.P. Insulin signaling pathway and related molecules: Role in neurodegeneration and Alzheimer’s disease. Neurochem. Int. 2020;135104707 doi: 10.1016/j.neuint.2020.104707. [DOI] [PubMed] [Google Scholar]
- 137.Wang L., Yin Y.L., Liu X.Z., Shen P., Zheng Y.G., Lan X.R., Lu C.B., Wang J.Z. Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl. Neurodegener. 2020;9:10. doi: 10.1186/s40035-020-00189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chang C.H., Lin C.H., Lane H.Y. d-glutamate and gut microbiota in Alzheimer’s disease. Int. J. Mol. Sci. 2020;21(8):2676. doi: 10.3390/ijms21082676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.von Bernhardi R., Eugenín J. Alzheimer’s disease: redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antioxid. Redox Signal. 2012;16(9):974–1031. doi: 10.1089/ars.2011.4082. [DOI] [PubMed] [Google Scholar]
- 140.Koh E.J., Kim K.J., Choi J., Kang D.H., Lee B.Y. Spirulina maxima extract prevents cell death through BDNF activation against amyloid beta 1-42 (Aβ1-42) induced neurotoxicity in PC12 cells. Neurosci. Lett. 2018;673:33–38. doi: 10.1016/j.neulet.2018.02.057. [DOI] [PubMed] [Google Scholar]
- 141.Yousef M.I., Abdou H.M., Elkader H-T.A.E.A., Hussein H.K., Samra W.E.M.A. Neuroprotective potential of Spirulina platensis against aluminium chloride-induced neural degeneration. Curr. Top. Nutraceutical Res. 2020;18(4):310–318. [Google Scholar]
- 142.Agrawal M., Perumal Y., Bansal S., Arora S., Chopra K. Phycocyanin alleviates ICV-STZ induced cognitive and molecular deficits via PI3-Kinase dependent pathway. Food Chem. Toxicol. 2020;145111684 doi: 10.1016/j.fct.2020.111684. [DOI] [PubMed] [Google Scholar]
- 143.Abramov A.Y., Canevari L., Duchen M.R. β-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J. Neurosci. 2004;24(2):565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ma Q.L., Yang F., Rosario E.R., Ubeda O.J., Beech W., Gant D.J., Chen P.P., Hudspeth B., Chen C., Zhao Y., Vinters H.V., Frautschy S.A., Cole G.M. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J. Neurosci. 2009;29(28):9078–9089. doi: 10.1523/JNEUROSCI.1071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Z., Gan L., Yan S., Yan Y., Huang W. Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease. Transl. Neurosci. 2020;11(1):161–172. doi: 10.1515/tnsci-2020-0101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146.Singh N.K., Hasan S.S., Kumar J., Raj I., Pathan A.A., Parmar A., Shakil S., Gourinath S., Madamwar D. Crystal structure and interaction of phycocyanin with β-secretase: A putative therapy for Alzheimer’s disease. CNS Neurol. Disord. Drug Targets. 2014;13(4):691–698. doi: 10.2174/1871527313666140228114456. [DOI] [PubMed] [Google Scholar]
- 147.Liu Y., Jovcevski B., Pukala T.L. C-Phycocyanin from Spirulina inhibits α-synuclein and amyloid-β fibril formation but not amorphous aggregation. J. Nat. Prod. 2019;82(1):66–73. doi: 10.1021/acs.jnatprod.8b00610. [DOI] [PubMed] [Google Scholar]
- 148.Ishizuka K., Kimura T., Yoshitake J., Akaike T., Shono M., Takamatsu J., Katsuragi S., Kitamura T., Miyakawa T. Possible assessment for antioxidant capacity in Alzheimer’s disease by measuring lymphocyte heme oxygenase-1 expression with real-time RT-PCR. Ann. N. Y. Acad. Sci. 2002;977:173–178. doi: 10.1111/j.1749-6632.2002.tb04814.x. [DOI] [PubMed] [Google Scholar]
- 149.Barone E., Di Domenico F., Mancuso C., Butterfield D.A. The Janus face of the heme oxygenase/biliverdin reductase system in Alzheimer disease: it’s time for reconciliation. Neurobiol. Dis. 2014;62:144–159. doi: 10.1016/j.nbd.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barone E., Di Domenico F., Sultana R., Coccia R., Mancuso C., Perluigi M., Butterfield D.A. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic. Biol. Med. 2012;52(11-12):2292–2301. doi: 10.1016/j.freeradbiomed.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Butterfield D.A., Barone E., Di Domenico F., Cenini G., Sultana R., Murphy M.P., Mancuso C., Head E. Atorvastatin treatment in a dog preclinical model of Alzheimer’s disease leads to up-regulation of haem oxygenase-1 and is associated with reduced oxidative stress in brain. Int. J. Neuropsychopharmacol. 2012;15(7):981–987. doi: 10.1017/S1461145711001118. [DOI] [PubMed] [Google Scholar]
- 152.Barone E., Di Domenico F., Cassano T., Arena A., Tramutola A., Lavecchia M.A., Coccia R., Butterfield D.A., Perluigi M. Impairment of biliverdin reductase-A promotes brain insulin resistance in Alzheimer disease: A new paradigm. Free Radic. Biol. Med. 2016;91:127–142. doi: 10.1016/j.freeradbiomed.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 153.Barone E., Tramutola A., Triani F., Calcagnini S., Di Domenico F., Ripoli C., Gaetani S., Grassi C., Butterfield D. A., Cassano T., Perluigi M. Biliverdin reductase-A mediates the beneficial effects of intranasal insulin in Alzheimer disease. 2019. [DOI] [PubMed]
- 154.Bonfili L., Cecarini V., Gogoi O., Gong C., Cuccioloni M., Angeletti M., Rossi G., Eleuteri A.M. Microbiota modulation as preventative and therapeutic approach in Alzheimer’s disease. FEBS J. 2021;288(9):2836–2855. doi: 10.1111/febs.15571. [DOI] [PubMed] [Google Scholar]
- 155.Hua P., Yu Z., Xiong Y., Liu B., Zhao L. Regulatory efficacy of Spirulina platensis protease hydrolyzate on lipid metabolism and gut microbiota in high-fat diet-fed rats. Int. J. Mol. Sci. 2018;19(12):E4023. doi: 10.3390/ijms19124023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xie Y., Li W., Zhu L., Zhai S., Qin S., Du Z. Effects of phycocyanin in modulating the intestinal microbiota of mice. MicrobiologyOpen. 2019;8(9):e00825. doi: 10.1002/mbo3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lu L., Li W., Sun C., Kang S., Li J., Luo X., Su Q., Liu B., Qin S. Phycocyanin ameliorates radiation-induced acute intestinal toxicity by regulating the effect of the gut microbiota on the TLR4/Myd88/NF-κB pathway. JPEN J. Parenter. Enteral Nutr. 2020;44(7):1308–1317. doi: 10.1002/jpen.1744. [DOI] [PubMed] [Google Scholar]
- 158.Xie Y., Li W., Lu C., Zhu L., Qin S., Du Z. The effects of phycocyanin on bleomycin-induced pulmonary fibrosis and the intestinal microbiota in C57BL/6 mice. Appl. Microbiol. Biotechnol. 2019;103(20):8559–8569. doi: 10.1007/s00253-019-10018-7. [DOI] [PubMed] [Google Scholar]
- 159.Neyrinck A.M., Taminiau B., Walgrave H., Daube G., Cani P.D., Bindels L.B., Delzenne N.M. Spirulina protects against hepatic inflammation in aging: an effect related to the modulation of the gut microbiota? Nutrients. 2017;9(6):633. doi: 10.3390/nu9060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Neyrinck A.M., Schüppel V.L., Lockett T., Haller D., Delzenne N.M. Microbiome and metabolic disorders related to obesity: Which lessons to learn from experimental models? Trends Food Sci. Technol. 2016;57:256–264. doi: 10.1016/j.tifs.2016.08.012. [DOI] [Google Scholar]
- 161.Parker A., Fonseca S., Carding S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11(2):135–157. doi: 10.1080/19490976.2019.1638722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang L., Wang Y., Xiayu X., Shi C., Chen W., Song N., Fu X., Zhou R., Xu Y.F., Huang L., Zhu H., Han Y., Qin C. Altered gut microbiota in a mouse model of Alzheimer’s Disease. J. Alzheimers Dis. 2017;60(4):1241–1257. doi: 10.3233/JAD-170020. [DOI] [PubMed] [Google Scholar]
- 163.Liu P., Wu L., Peng G., Han Y., Tang R., Ge J., Zhang L., Jia L., Yue S., Zhou K., Li L., Luo B., Wang B. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019;80:633–643. doi: 10.1016/j.bbi.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 164.Chen X., Zuo Q., Hai Y., Sun X.J. Lactulose: an indirect antioxidant ameliorating inflammatory bowel disease by increasing hydrogen production. Med. Hypotheses. 2011;76(3):325–327. doi: 10.1016/j.mehy.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 165.Bonfili L., Cecarini V., Berardi S., Scarpona S., Suchodolski J.S., Nasuti C., Fiorini D., Boarelli M.C., Rossi G., Eleuteri A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017;7(1):2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Q.J., Shen Y.E., Wang X., Fu S., Zhang X., Zhang Y.N., Wang R.T. Concomitant memantine and Lactobacillus plantarum treatment attenuates cognitive impairments in APP/PS1 mice. Aging (Albany NY) 2020;12(1):628–649. doi: 10.18632/aging.102645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang X., Yu D., Xue L., Li H., Du J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B. 2020;10(3):475–487. doi: 10.1016/j.apsb.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Choi W.Y., Kang D.H., Lee H.Y. Effect of fermented Spirulina maxima extract on cognitive-enhancing activities in mice with scopolamine-induced dementia. Evid. Based Complement. Alternat. Med. 2018;20187218504 doi: 10.1155/2018/7218504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.de Lau L.M., Breteler M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 170.Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877–897. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dorsey E.R., Elbaz A., Nichols E., Abd-Allah F., Abdelalim A., Adsuar J.C., Ansha M.G., Brayne C., Choi J-Y.J., Collado-Mateo D., Dahodwala N., Do H.P., Edessa D., Endres M., Fereshtehnejad S-M., Foreman K.J., Gankpe F.G., Gupta R., Hankey G.J., Hay S.I., Hegazy M.I., Hibstu D.T., Kasaeian A., Khader Y., Khalil I., Khang Y-H., Kim Y.J., Kokubo Y., Logroscino G., Massano J., Mohamed I.N., Mohammed M.A., Mohammadi A., Moradi-Lakeh M., Naghavi M., Nguyen B.T., Nirayo Y.L., Ogbo F.A., Owolabi M.O., Pereira D.M., Postma M.J., Qorbani M., Rahman M.A., Roba K.T., Safari H., Safiri S., Satpathy M., Sawhney M., Shafieesabet A., Shiferaw M.S., Smith M., Szoeke C.E.I., Tabarés-Seisdedos R., Truong N.T., Ukwaja K.N., Venketasubramanian N., Villafaina S. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Antony P.M.A., Diederich N.J., Krüger R., Balling R. The hallmarks of Parkinson’s disease. FEBS J. 2013;280(23):5981–5993. doi: 10.1111/febs.12335. [DOI] [PubMed] [Google Scholar]
- 173.Huse D.M., Schulman K., Orsini L., Castelli-Haley J., Kennedy S., Lenhart G. Burden of illness in Parkinson’s disease. Mov. Disord. 2005;20(11):1449–1454. doi: 10.1002/mds.20609. [DOI] [PubMed] [Google Scholar]
- 174.Bastide M.F., Meissner W.G., Picconi B., Fasano S., Fernagut P.O., Feyder M., Francardo V., Alcacer C., Ding Y., Brambilla R., Fisone G., Jon Stoessl A., Bourdenx M., Engeln M., Navailles S., De Deurwaerdère P., Ko W.K., Simola N., Morelli M., Groc L., Rodriguez M.C., Gurevich E.V., Quik M., Morari M., Mellone M., Gardoni F., Tronci E., Guehl D., Tison F., Crossman A.R., Kang U.J., Steece-Collier K., Fox S., Carta M., Angela Cenci M., Bézard E. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog. Neurobiol. 2015;132:96–168. doi: 10.1016/j.pneurobio.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 175.Schrag A., Jahanshahi M., Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J. Neurol. Neurosurg. Psychiatry. 2000;69(3):308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Charvin D., Medori R., Hauser R.A., Rascol O. Therapeutic strategies for Parkinson disease: beyond dopaminergic drugs. Nat. Rev. Drug Discov. 2018;17(11):844. doi: 10.1038/nrd.2018.184. [DOI] [PubMed] [Google Scholar]
- 177.Zeng X.S., Geng W.S., Jia J.J., Chen L., Zhang P.P. Cellular and molecular basis of neurodegeneration in Parkinson disease. Front. Aging Neurosci. 2018;10:109. doi: 10.3389/fnagi.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Surmeier D.J. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 2018;285(19):3657–3668. doi: 10.1111/febs.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rocha E.M., De Miranda B., Sanders L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson's disease. Neurobiol. Dis. . 2018;109(Pt B):249–257. doi: 10.1016/j.nbd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 180.Pabon M.M., Jernberg J.N., Morganti J., Contreras J., Hudson C.E., Klein R.L., Bickford P.C. A spirulina-enhanced diet provides neuroprotection in an α-synuclein model of Parkinson’s disease. PLoS One. 2012;7(9):e45256. doi: 10.1371/journal.pone.0045256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cardona A.E., Pioro E.P., Sasse M.E., Kostenko V., Cardona S.M., Dijkstra I.M., Huang D., Kidd G., Dombrowski S., Dutta R., Lee J.C., Cook D.N., Jung S., Lira S.A., Littman D.R., Ransohoff R.M. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 182.Tobón-Velasco J.C., Palafox-Sánchez V., Mendieta L., García E., Santamaría A., Chamorro-Cevallos G., Limón I.D. Antioxidant effect of Spirulina (Arthrospira) maxima in a neurotoxic model caused by 6-OHDA in the rat striatum. J. Neural Transm. (Vienna) 2013;120(8):1179–1189. doi: 10.1007/s00702-013-0976-2. [DOI] [PubMed] [Google Scholar]
- 183.Lima F.A.V., Joventino I.P., Joventino F.P., de Almeida A.C., Neves K.R.T., do Carmo M.R., Leal L.K.A.M., de Andrade G.M., de Barros Viana G.S. Neuroprotective activities of Spirulina platensis in the 6-OHDA model of Parkinson’s disease are related to its anti-inflammatory effects. Neurochem. Res. 2017;42(12):3390–3400. doi: 10.1007/s11064-017-2379-5. [DOI] [PubMed] [Google Scholar]
- 184.Chattopadhyaya I., Gupta S., Mohammed A., Mushtaq N., Chauhan S., Ghosh S. Neuroprotective effect of Spirulina fusiform and amantadine in the 6-OHDA induced Parkinsonism in rats. BMC Complement. Altern. Med. 2015;15:296. doi: 10.1186/s12906-015-0815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kumar A., Christian P.K., Panchal K., Guruprasad B.R., Tiwari A.K. Supplementation of Spirulina (Arthrospira platensis) improves lifespan and locomotor activity in paraquat-sensitive DJ-1β(Δ93) flies, a Parkinson’s disease model in Drosophila melanogaster. J. Diet. Suppl. 2017;14(5):573–588. doi: 10.1080/19390211.2016.1275917. [DOI] [PubMed] [Google Scholar]
- 186.Macedo D., Bertolin T.E., Oro T., Backes L.T.H., Brás I.C., Santos C.N., Tenreiro S., Outeiro T.F. Phycocyanin protects against alpha-synuclein toxicity in yeast. J. Funct. Foods. 2017;38:553–560. doi: 10.1016/j.jff.2017.09.044. [DOI] [Google Scholar]
- 187.Chang K.H., Chen C.M. The role of oxidative stress in Parkinson’s disease. Antioxidants. 2020;9(7):E597. doi: 10.3390/antiox9070597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Trist B.G., Hare D.J., Double K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell. 2019;18(6):e13031. doi: 10.1111/acel.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Genoud S., Roberts B.R., Gunn A.P., Halliday G.M., Lewis S.J.G., Ball H.J., Hare D.J., Double K.L. Subcellular compartmentalisation of copper, iron, manganese, and zinc in the Parkinson’s disease brain. Metallomics. 2017;9(10):1447–1455. doi: 10.1039/C7MT00244K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bermejo P., Piñero E., Villar Á.M. Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulina platensis. Food Chem. 2008;110(2):436–445. doi: 10.1016/j.foodchem.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 191.Piñero E.J.E., Bermejo Bescós P., Villar del Fresno A.M. Antioxidant activity of different fractions of Spirulina platensis protean extract. Farmaco. 2001;56(5-7):497–500. doi: 10.1016/S0014-827X(01)01084-9. [DOI] [PubMed] [Google Scholar]
- 192.Hou L., Bao X., Zang C., Yang H., Sun F., Che Y., Wu X., Li S., Zhang D., Wang Q. Integrin CD11b mediates α-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol. 2018;14:600–608. doi: 10.1016/j.redox.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hemmati-Dinarvand M., Saedi S., Valilo M., Kalantary-Charvadeh A., Alizadeh Sani M., Kargar R., Safari H., Samadi N. Oxidative stress and Parkinson’s disease: conflict of oxidant-antioxidant systems. Neurosci. Lett. 2019;709:134296. doi: 10.1016/j.neulet.2019.134296. [DOI] [PubMed] [Google Scholar]
- 194.McCarty M.F., Lerner A. Nutraceuticals targeting generation and oxidant activity of peroxynitrite may aid prevention and control of Parkinson’s disease. Int. J. Mol. Sci. 2020;21(10):3624. doi: 10.3390/ijms21103624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Johns Hopkins University Coronavirus Resource Center https://coronavirus.jhu.edu
- 196.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 198.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 199.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jaimes J.A., Millet J.K., Whittaker G.R. Proteolytic cleavage of the SARS-CoV-2 spike protein and the role of the novel S1/S2 site. iScience. 2020;23(6):101212. doi: 10.1016/j.isci.2020.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Örd M., Faustova I., Loog M. The sequence at Spike S1/S2 site enables cleavage by furin and phospho-regulation in SARS-CoV2 but not in SARS-CoV1 or MERS-CoV. Sci. Rep. 2020;10(1):16944. doi: 10.1038/s41598-020-74101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cantuti-Castelvetri L., Ojha R. Neuropilin-1 facilitates SARSCoV- 2 cell entry and infectivity. . 2020;370(6518):856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Daly J.L., Simonetti B., Klein K., Chen K.E., Williamson M.K., Antón-Plágaro C., Shoemark D.K., Simón-Gracia L., Bauer M., Hollandi R., Greber U.F., Horvath P., Sessions R.B., Helenius A., Hiscox J.A., Teesalu T., Matthews D.A., Davidson A.D., Collins B.M., Cullen P.J., Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370(6518):861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Padda I., Khehra N., Jaferi U., Parmar M.S. The neurological complexities and prognosis of COVID-19. SN Compr. Clin. Med. 2020:1–12. doi: 10.1007/s42399-020-00527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liotta E.M., Batra A., Clark J.R., Shlobin N.A., Hoffman S.C., Orban Z.S., Koralnik I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann. Clin. Transl. Neurol. 2020;7(11):2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen Y., Klein S.L., Garibaldi B.T., Li H., Wu C., Osevala N.M., Li T., Margolick J.B., Pawelec G., Leng S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021;65101205 doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dumurgier J., Tzourio C. Epidemiology of neurological diseases in older adults. Rev. Neurol. (Paris) 2020;176(9):642–648. doi: 10.1016/j.neurol.2020.01.356. [DOI] [PubMed] [Google Scholar]
- 211.Jakhmola S., Indari O., Chatterjee S., Jha H.C. SARS-CoV-2, an underestimated pathogen of the nervous system. SN Compr. Clin. Med. 2020:1–10. doi: 10.1007/s42399-020-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fan H., Tang X., Song Y., Liu P., Chen Y.L.P., Chen Y. Influence of COVID-19 on cerebrovascular disease and its possible mechanism. Neuropsychiatr. Dis. Treat. 2020;16:1359–1367. doi: 10.2147/NDT.S251173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: A literature review. J. Clin. Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nourollahian M., Rasoulian B., Gafari A., Anoushiravani M., Jabari F., Bakhshaee M. Clinical comparison of the efficacy of spirulina platensis and cetirizine for treatment of allergic rhinitis. Acta Otorhinolaryngol. Ital. 2020;40(3):224–229. doi: 10.14639/0392-100X-N0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Reddy M.C., Subhashini J., Mahipal S.V., Bhat V.B., Srinivas Reddy P., Kiranmai G., Madyastha K.M., Reddanna P. C-Phycocyanin, a selective cyclooxygenase-2 inhibitor, induces apoptosis in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2003;304(2):385–392. doi: 10.1016/S0006-291X(03)00586-2. [DOI] [PubMed] [Google Scholar]
- 216.Cherng S.C., Cheng S.N., Tarn A., Chou T.C. Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2007;81(19-20):1431–1435. doi: 10.1016/j.lfs.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 217.Ma P., Huang R., Jiang J., Ding Y., Li T., Ou Y. Potential use of C-phycocyanin in non-alcoholic fatty liver disease. Biochem. Biophys. Res. Commun. 2020;526(4):906–912. doi: 10.1016/j.bbrc.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 218.Altinok-Yipel F., Tekeli I.O., Ozsoy S.Y., Guvenc M., Sayin S., Yipel M. Investigation of hepatoprotective effect of some algae species on carbon tetrachloride-induced liver injury in rats. Arch. Physiol. Biochem. 2020;126(5):463–467. doi: 10.1080/13813455.2019.1702062. [DOI] [PubMed] [Google Scholar]
- 219.El-Boghdady N.A., Kamel M.A., El-Shamy R.M. Omeprazole and Spirulina platensis ameliorate steatohepatitis in experimental nonalcoholic fatty liver disease. Metab. Syndr. Relat. Disord. 2020;18(9):426–434. doi: 10.1089/met.2019.0129. [DOI] [PubMed] [Google Scholar]
- 220.McCarty M.F., Barroso-Aranda J., Contreras F. Genistein and phycocyanobilin may prevent hepatic fibrosis by suppressing proliferation and activation of hepatic stellate cells. Med. Hypotheses. 2009;72(3):330–332. doi: 10.1016/j.mehy.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 221.Wong Y.J., Tan M., Zheng Q., Li J.W., Kumar R., Fock K.M., Teo E.K., Ang T.L. A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann. Hepatol. 2020;19(6):627–634. doi: 10.1016/j.aohep.2020.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rose C.F., Amodio P., Bajaj J.S., Dhiman R.K., Montagnese S., Taylor-Robinson S.D., Vilstrup H., Jalan R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J. Hepatol. 2020;73(6):1526–1547. doi: 10.1016/j.jhep.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 223.McCarty M.F., DiNicolantonio J.J. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog. Cardiovasc. Dis. 2020;63(3):383–385. doi: 10.1016/j.pcad.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lanone S., Bloc S., Foresti R., Almolki A., Taillé C., Callebert J., Conti M., Goven D., Aubier M., Dureuil B., El-Benna J., Motterlini R., Boczkowski J. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J. 2005;19(13):1890–1892. doi: 10.1096/fj.04-2368fje. [DOI] [PubMed] [Google Scholar]
- 225.Ma L.L., Zhang P., Wang H.Q., Li Y.F., Hu J., Jiang J.D., Li Y.H. heme oxygenase-1 agonist CoPP suppresses influenza virus replication through IRF3-mediated generation of IFN-α/β. Virology. 2019;528:80–88. doi: 10.1016/j.virol.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 226.Chen Y.H., Chang G.K., Kuo S.M., Huang S.Y., Hu I.C., Lo Y.L., Shih S.R. Well-tolerated Spirulina extract inhibits influenza virus replication and reduces virus-induced mortality. Sci. Rep. 2016;6:24253. doi: 10.1038/srep24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mariotto S., Savoldi A., Donadello K., Zanzoni S., Bozzetti S., Carta S., Zivelonghi C., Alberti D., Piraino F., Minuz P., Girelli D., Crisafulli E., Romano S., Marcon D., Marchi G., Gottin L., Polati E., Zanatta P., Monaco S., Tacconelli E., Ferrari S. Nervous system: subclinical target of SARS-CoV-2 infection. J. Neurol. Neurosurg. Psychiatry. 2020;91(9):1010–1012. doi: 10.1136/jnnp-2020-323881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kanberg N., Ashton N.J., Andersson L.M., Yilmaz A., Lindh M., Nilsson S., Price R.W., Blennow K., Zetterberg H., Gisslén M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95(12):e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- 229.Mesci P., Macia A., Saleh A., Martin-Sancho L., Yin X., Snethlage C., Avansini S., Chanda S.K., Muotri A. Sofosbuvir protects human brain organoids against SARS-CoV-2. bioRxiv. 20202020:05.30.125856
- 230.Bullen C.K., Hogberg H.T., Bahadirli-Talbott A., Bishai W.R., Hartung T., Keuthan C., Looney M.M., Pekosz A., Romero J.C., Sillé F.C.M., Um P., Smirnova L. Infectability of human brain sphere neurons suggests neurotropism of SARS-CoV-2. ALTEX. 2020;37(4):665–671. doi: 10.14573/altex.2006111. [DOI] [PubMed] [Google Scholar]
- 231.Ramani A., Müller L., Ostermann P.N., Gabriel E., Abida-Islam P., Müller-Schiffmann A., Mariappan A., Goureau O., Gruell H., Walker A., Andrée M., Hauka S., Houwaart T., Dilthey A., Wohlgemuth K., Omran H., Klein F., Wieczorek D., Adams O., Timm J., Korth C., Schaal H., Gopalakrishnan J. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020;39(20):e106230. doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kiran Raj T.K., Ranjithkumar R., Kanthesh B.M., Gopenath T.S. C-Phycocyanin of Spirulina platensis inhibitis NsP12 required for replication of SARS-CoV-2: a novel finding in-silico. Int. J. Pharm. Sci. Res. 2020;11(9):4271–4278. [Google Scholar]
- 233.Brahmaiah P., Ankit P. In silico screening of food bioactive compounds to predict potential inhibitors of COVID-19 main protease (Mpro) and RNA-dependent RNA polymerase (RdRp). ChemRxiv. 2020
- 234.Petit L., Vernes L., Cadoret J-P. Docking and in silico toxicity assessment of Arthrospira compounds as potential antiviral agents against SARS-CoV-2. J. Appl. Phycol. 2020:1–24. doi: 10.1007/s10811-021-02372-9. [Epub a head of Print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Small E. Spirulina – food for the universe. Biodiversity (Nepean) 2011;12(4):255–265. doi: 10.1080/14888386.2011.642735. [DOI] [Google Scholar]
- 236.2003. http://www.fda.gov/Food/ FoodIngredientsPackaging/GenerallyRecognizedasSafeGRAS/GRASListings/ ucm153944.htm
- 237.Electronic Code of Federal Regulations 2016. http://www.ecfr.gov/cgi-bin/text-idx?mc=true&tpl=true&tpl= ecfrbroowse/Title21/21cfr73_main_02.tpl
- 238.Wu Q., Liu L., Miron A., Klímová B., Wan D., Kuča K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch. Toxicol. 2016;90(8):1817–1840. doi: 10.1007/s00204-016-1744-5. [DOI] [PubMed] [Google Scholar]
- 239.Marles R.J., Barrett M.L., Barnes J., Chavez M.L., Gardiner P., Ko R., Mahady G.B., Low Dog T., Sarma N.D., Giancaspro G.I., Sharaf M., Griffiths J. United States pharmacopeia safety evaluation of spirulina. Crit. Rev. Food Sci. Nutr. 2011;51(7):593–604. doi: 10.1080/10408391003721719. [DOI] [PubMed] [Google Scholar]
- 240.Hutadilok-Towatana N., Reanmongkol W., Satitit S., Panichayupakaranant P., Ritthisunthorn P. A Subchronic toxicity study of Spirulina platensis. Food Sci. Technol. Res. 2008;14(4):351–351. doi: 10.3136/fstr.14.351. [DOI] [Google Scholar]
- 241.Bigagli E., Cinci L., Niccolai A., Tredici M.R., Biondi N., Rodolfi L., Lodovici M., D’Ambrosio M., Mori G., Luceri C. Safety evaluations and lipid-lowering activity of an Arthrospira platensis enriched diet: A 1-month study in rats. Food Res. Int. 2017;102:380–386. doi: 10.1016/j.foodres.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 242.Gutiérrez-Salmeán G., Fabila-Castillo L., Chamorro-Cevallos G. Nutritional and toxicological aspects of Spirulina (Arthrospira). Nutr. Hosp. 2015;32(1):34–40. doi: 10.3305/nh.2015.32.1.9001. [DOI] [PubMed] [Google Scholar]
- 243.Chamorro G., Salazar M. Teratogenic study of Spirulina in mice. Arch. Latinoam. Nutr. 1990;40(1):86–94. [PubMed] [Google Scholar]
- 244.Chamorro-Cevallos G., Garduño-Siciliano L., Barrón B.L., Madrigal-Bujaidar E., Cruz-Vega D.E., Pages N. Chemoprotective effect of Spirulina (Arthrospira) against cyclophosphamide-induced mutagenicity in mice. Food Chem. Toxicol. 2008;46(2):567–574. doi: 10.1016/j.fct.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 245.Chamorro-Cevallos G., Garduño-Siciliano L., Martínez-Galero E., Mojica-Villegas A., Pages N., Gutiérrez-Salmeán G. The protective effect of dietary Arthrospira (Spirulina) maxima against mutagenicity induced by benzo[alpha]pyrene in mice. J. Med. Food. 2014;17(5):527–534. doi: 10.1089/jmf.2013.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pleonsil P., Soogarun S., Suwanwong Y. Anti-oxidant activity of holo- and apo-c-phycocyanin and their protective effects on human erythrocytes. Int. J. Biol. Macromol. 2013;60:393–398. doi: 10.1016/j.ijbiomac.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 247.Romay C., González R. Phycocyanin is an antioxidant protector of human erythrocytes against lysis by peroxyl radicals. J. Pharm. Pharmacol. 2000;52(4):367–368. doi: 10.1211/0022357001774093. [DOI] [PubMed] [Google Scholar]
- 248.Romay C., Ledón N., González R. Further studies on anti-inflammatory activity of phycocyanin in some animal models of inflammation. Inflamm. Res. 1998;47(8):334–338. doi: 10.1007/s000110050338. [DOI] [PubMed] [Google Scholar]
- 249.Song L.F., Liu B., Zhao Y., Tian J.Z., Qin S. Chronic toxicity study of Phycocyanin on Sprague Dawley rats. Chin. Med. Herald. 2012;9:15–21. [Google Scholar]
- 250.Naidu K.A., Sarada R., Manoj G., Khan M.Y., Swamy M.M., Viswanatha S., Murthy K.N., Ravishankar G.A., Srinivas L. Toxicity assessment of Phycocyanin - a blue colorant from blue green alga Spirulina platensis. Food Biotechnol. 1999;13(1):51–66. doi: 10.1080/08905439609549961. [DOI] [Google Scholar]
- 251.Jensen G.S., Attridge V.L., Carter S.G., Guthrie J., Ehmann A., Benson K.F. Consumption of an aqueous cyanophyta extract derived from Arthrospira platensis is associated with reduction of chronic pain: results from two human clinical pilot studies. Nutr. Diet. Suppl. 2016;8:65–70. doi: 10.2147/NDS.S95014. [DOI] [Google Scholar]
- 252.Jensen G.S., Drapeau C., Lenninger M., Benson K.F. Clinical safety of a high dose of Phycocyanin-enriched aqueous extract from Arthrospira (Spirulina) platensis: results from a randomized, double-blind, placebo-controlled study with a focus on anticoagulant activity and platelet activation. J. Med. Food. 2016;19(7):645–653. doi: 10.1089/jmf.2015.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jensen G.S., Attridge V.L., Beaman J.L., Guthrie J., Ehmann A., Benson K.F. Antioxidant and anti-inflammatory properties of an aqueous cyanophyta extract derived from Arthrospira platensis: contribution to bioactivities by the non-phycocyanin aqueous fraction. J. Med. Food. 2015;18(5):535–541. doi: 10.1089/jmf.2014.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jung K.A., Kwak M.K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15(10):7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li B., Tan G.J., Lin H.Q., Zhang J.N., Guo L., Chen L.P. Neuroprotective effects of α-lipoic acid on long-term experimental autoimmune encephalomyelitis. Eur. Rev. Med. Pharmacol. Sci. 2018;22(19):6517–6528. doi: 10.26355/eurrev_201810_16066. [DOI] [PubMed] [Google Scholar]
- 256.Cho J.Y., Um H.S., Kang E.B., Cho I.H., Kim C.H., Cho J.S., Hwang D.Y. The combination of exercise training and alpha-lipoic acid treatment has therapeutic effects on the pathogenic phenotypes of Alzheimer’s disease in NSE/APPsw-transgenic mice. Int. J. Mol. Med. 2010;25(3):337–346. doi: 10.3892/ijmm_00000350. [DOI] [PubMed] [Google Scholar]
- 257.Quinn J.F., Bussiere J.R., Hammond R.S., Montine T.J., Henson E., Jones R.E., Stackman R.W., Jr Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol. Aging. 2007;28(2):213–225. doi: 10.1016/j.neurobiolaging.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 258.Li Y.H., He Q., Yu J.Z., Liu C.Y., Feng L., Chai Z., Wang Q., Zhang H.Z., Zhang G.X., Xiao B.G., Ma C.G. Lipoic acid protects dopaminergic neurons in LPS-induced Parkinson’s disease model. Metab. Brain Dis. 2015;30(5):1217–1226. doi: 10.1007/s11011-015-9698-5. [DOI] [PubMed] [Google Scholar]
- 259.Koriyama Y., Nakayama Y., Matsugo S., Kato S. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2-dependent heme oxygenase-1 induction in the RGC-5 cellline. Brain Res. 2013;1499:145–157. doi: 10.1016/j.brainres.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 260.Karunakaran S., Diwakar L., Saeed U., Agarwal V., Ramakrishnan S., Iyengar S., Ravindranath V. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: protection by alpha-lipoic acid. FASEB J. 2007;21(9):2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- 261.Funakohi-Tago M., Sakata T., Fujiwara S., Sakakura A., Sugai T., Tago K., Tamura H. Hydroxytyrosol butyrate inhibits 6-OHDA-induced apoptosis through activation of the Nrf2/HO-1 axis in SH-SY5Y cells. Eur. J. Pharmacol. 2018;834:246–256. doi: 10.1016/j.ejphar.2018.07.043. [DOI] [PubMed] [Google Scholar]
- 262.Nardiello P., Pantano D., Lapucci A., Stefani M., Casamenti F. Diet supplementation with hydroxytyrosol ameliorates brain pathology and restores cognitive functions in a mouse model of amyloid-β deposition. J. Alzheimers Dis. 2018;63(3):1161–1172. doi: 10.3233/JAD-171124. [DOI] [PubMed] [Google Scholar]
- 263.Peng Y., Hou C., Yang Z., Li C., Jia L., Liu J., Tang Y., Shi L., Li Y., Long J., Liu J. Hydroxytyrosol mildly improve cognitive function independent of APP processing in APP/PS1 mice. Mol. Nutr. Food Res. 2016;60(11):2331–2342. doi: 10.1002/mnfr.201600332. [DOI] [PubMed] [Google Scholar]
- 264.Conde C., Escribano B.M., Luque E., Aguilar-Luque M., Feijóo M., Ochoa J.J., LaTorre M., Giraldo A.I., Lillo R., Agüera E., Santamaría A., Túnez I. The protective effect of extra-virgin olive oil in the experimental model of multiple sclerosis in the rat. Nutr. Neurosci. 2020;23(1):37–48. doi: 10.1080/1028415X.2018.1469281. [DOI] [PubMed] [Google Scholar]
- 265.Farkhondeh T., Folgado S.L., Pourbagher-Shahri A.M., Ashrafizadeh M., Samarghandian S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020;127110234 doi: 10.1016/j.biopha.2020.110234. [DOI] [PubMed] [Google Scholar]
- 266.Chen Y., Shi G.W., Liang Z.M., Sheng S.Y., Shi Y.S., Peng L., Wang Y.P., Wang F., Zhang X.M. Resveratrol improves cognition and decreases amyloid plaque formation in Tg6799 mice. Mol. Med. Rep. 2019;19(5):3783–3790. doi: 10.3892/mmr.2019.10010. [DOI] [PubMed] [Google Scholar]
- 267.Rasheed M.S.U., Tripathi M.K., Patel D.K., Singh M.P. Resveratrol regulates Nrf2-mediated expression of antioxidant and xenobiotic metabolizing enzymes in pesticides-induced parkinsonism. Protein Pept. Lett. 2020;27(10):1038–1045. doi: 10.2174/0929866527666200403110036. [DOI] [PubMed] [Google Scholar]
- 268.Zhang L.F., Yu X.L., Ji M., Liu S.Y., Wu X.L., Wang Y.J., Liu R.T. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food Funct. 2018;9(12):6414–6426. doi: 10.1039/C8FO00964C. [DOI] [PubMed] [Google Scholar]
- 269.Fonseca-Kelly Z., Nassrallah M., Uribe J., Khan R.S., Dine K., Dutt M., Shindler K.S. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front. Neurol. 2012;3:84. doi: 10.3389/fneur.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Shindler K.S., Ventura E., Dutt M., Elliott P., Fitzgerald D.C., Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J. Neuroophthalmol. 2010;30(4):328–339. doi: 10.1097/WNO.0b013e3181f7f833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ge L., Liu L., Liu H., Liu S., Xue H., Wang X., Yuan L., Wang Z., Liu D. Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur. J. Pharmacol. 2015;768:49–57. doi: 10.1016/j.ejphar.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 272.Fang J., Yan Y., Teng X., Wen X., Li N., Peng S., Liu W., Donadeu F.X., Zhao S., Hua J. Melatonin prevents senescence of canine adipose-derived mesenchymal stem cells through activating NRF2 and inhibiting ER stress. Aging (Albany NY) 2018;10(10):2954–2972. doi: 10.18632/aging.101602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chen D., Zhang T., Lee T.H. Cellular mechanisms of melatonin: insight from neurodegenerative diseases. Biomolecules. 2020;10(8):1158. doi: 10.3390/biom10081158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.McCarty M.F., Lerner A. Nutraceutical induction and mimicry of heme oxygenase activity as a strategy for controlling excitotoxicity in brain trauma and ischemic stroke: focus on oxidative stress. Expert Rev. Neurother. 2021;21(2):157–168. doi: 10.1080/14737175.2021.1861940. [DOI] [PubMed] [Google Scholar]