Abstract
Chlorogenic acid (CGA) is a kind of traditional Chinese medicine, abundant in honeysuckle and eucommia, and has a wide range of biological activities, and pharmacological effects. Previous studies have shown that CGA can regulate learning, memory, cognitive ability, coupled with improvement to anxiety, depression, and other post-traumatic stress disorder (PTSD)-like symptoms. This article explores the protective effects of CGA on neurons through its anti-apoptotic effect, inhibition of neuroinflammation and oxidative stress, which may be the mechanisms of its improvement of PTSD-like symptoms. It may provide a new therapeutic strategy for the treatment of PTSD and its comorbidities.
Keywords: Chlorogenic acid, post-traumatic stress disorder, PTSD-like symptoms, learning and memory, apoptosis, oxidative stress
1. INTRODUCTION
Post-traumatic stress disorder (PTSD) is a long-lasting and delayed mental disorder caused by sudden, threatening, or catastrophic life events. The typical main symptoms are intrusive, persistent avoidance symptoms, and persistently increased alertness [1]. Patients with PTSD are often accompanied by comorbidities, such as anxiety and depression [2]. In recent years, with the increase in the incidence of traumatic events such as natural disasters, major accidents, and major diseases, the incidence of PTSD has also increased, which has led to a severe impact on patients' families and society. Unfortunately, so far, the pathogenesis of PTSD is still unclear. The current clinical drugs used to treat PTSD are mainly antidepressants. There are only two drugs, sertraline and paroxetine, approved by the US Food and Drug Administration (FDA) applied to the treatment of PTSD. However, long-term use of antidepressants has noticeable side effects. PTSD is a syndrome with complex pathogenesis, multiple factors, and numerous clinical symptoms. Traditional Chinese medicine has gradually attracted attention because of its high safety, low side effects, and various targets, which may provide better treatment strategies for treating PTSD and its comorbidities.
Chlorogenic acid (CGA) is a kind of carboxyphenolic acid formed by the condensation of quinic acid and caffeic acid in trans-cinnamic acid, mainly derived from eucommia, honeysuckle, chrysanthemum and other plants [3]. A large number of studies have shown that CGA has a wide range of pharmacological effects, such as antioxidant [4], anti-inflammation [5, 6], and anti-tumor [7-9]. CGA has also been proven to have neuroprotective effects and improve learning and memory effects [10-12]. It can improve the survival rate of dopaminergic neurons [13] and inhibit the apoptosis of pyramidal cells in the CA1 region of the hippocampus [14], and reduce oxidative stress and neuroinflammatory responses [15]. Besides, clinical studies have shown that CGA can relieve mental fatigue, headache, and inflammation, and has a specific improvement effect on patients with PTSD-like anxiety [16]. Therefore, CGA may regulate learning, memory and cognitive ability, play anti-anxiety and anti-depression effects and further improve PTSD-like symptoms by protecting neurons, reducing neuroinflammation and oxidative stress. This article mainly investigates the effects of CGA on improving PTSD-like symptoms and its mechanism of action, hoping to provide a reference for its application in the treatment of clinically related diseases in the future.
2. STRUCTURE AND BIOLOGICAL FUNCTION OF CGA
2.1. Structure of CGA
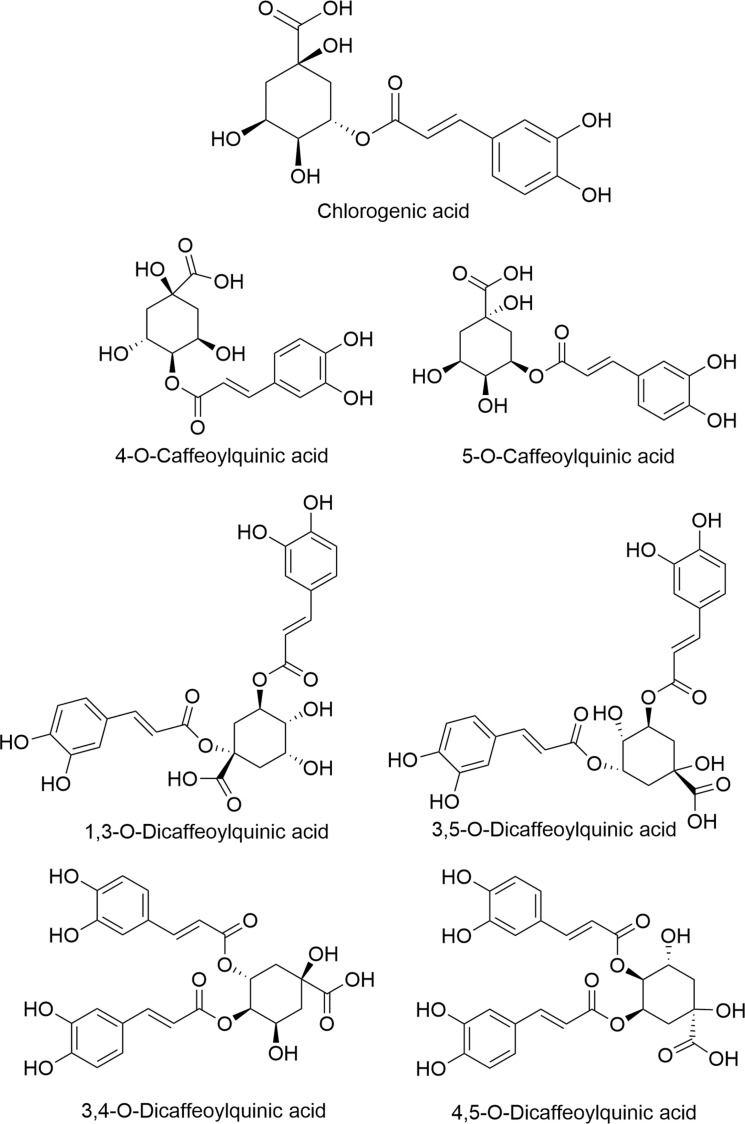
CGA is a phenolic acid compound formed by the condensation of a molecule of caffeic acid and a molecule of quinic acid [3]. Its chemical name is 3-O-caffeoylquinic acid, and its molecular formula is C16H18O9. There are catechol, carboxyl, ester, unsaturated double bond and hydroxyl. Due to the different binding sites and numbers of caffeoyl on quinic acid, chlorogenic acid contains 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 1,3-O-dicaffeoylquinic acid, 3,5-O-caffeoylquinicacid, 3,4-O-caffeoylquinic acid, 4,5-O-dicaffeoylquinic acid, and many other isomers. The structural formula for chlorogenic acid and its isomers are shown in Fig. (1), respectively.
Fig. (1).
Chemical structure of chlorogenic acid and its isomers. The chemical name of CGA is 3-O-caffeoylquinic acid, and its molecular formula is C 16H18O 9 , including catechol, carboxyl, ester, unsaturated double bond and hydroxyl. Due to the different binding sites and numbers of caffeoyl on quinic acid, CGA contains 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 1,3-O-dicaffeoylquinic acid, 3,5-O-caffeoylquinicacid,3,4-O-caffeoylquinic acid, 4,5-O-dicaffeoylquinic acid, and many other isomers.
2.2. Biological Functions of CGA
CGA is widely used in the fields of medicine, chemical industry, and food. It has many biological functions such as anti-inflammatory, anti-oxidation, anti-tumor, anti-virus, anti-bacterial, hypoglycemic, hypolipidemic, and nervous system protection [4, 17, 18]. In the 1980s, CGA was discovered to have anti-cancer effects, which attracted full attention from scientists. Currently, CGA has been found to have inhibitory effects on liver cancer, lung cancer, breast cancer and glioma [7, 19]. A large number of documents have proved that CGA, as a natural compound extracted from various plants, has excellent antibacterial and antiviral activities [20]. Preclinical and clinical studies have shown that CGA plays an essential role in the treatment of obesity and diabetes [21-23]. In addition, chlorogenic acid can improve the imbalance of the enteric microflora and facilitate to enhance the metabolic syndrome in rats elicited by high-carbohydrate and high-fat diet [24, 25]. The phenolic hydroxyl group in the structure of CGA is easy to combine with free radicals, has the ability to scavenge free radicals, and exhibits significant antioxidant activity [26]. Here, CGA can down-regulate the expression of Toll-like receptor 4 (TLR4), inhibit the activation of the downstream nuclear factor-κB (NF-κB) signaling pathway, and ultimately inhibit the release of inflammatory factors, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2), thereby playing an anti-inflammatory effect [27]. CGA additionally encompasses a profound cardioprotective impact by inhibiting the activation of NF-κB and c-Jun N-terminal (JNK) kinase pathways, providing a brand new treatment possibility for the treatment of heart failure [28]. In recent years, scientists have discovered that CGA has neuroprotective effects based on its potent antioxidant and anti-inflammatory effects [29, 30]. An ever-increasing amount of proofs demonstrate that supplementing CGA is a promising strategy for the treatment of neurodegenerative diseases. [31-34]. At the same time, it has been found in a large number of rodent models that CGA can reduce anxiety and depression, improve cognitive and memory dysfunction, and protect neuronal loss [35]. Therefore, CGA may also be used as a potential neuroprotective drug for the treatment of some neurodegenerative diseases and nerve injury-related diseases.
3. CGA IMPROVES PTSD-LIKE SYMPTOMS
3.1. Improvement of Learning, Memory and Cognitive Impairment
The decline of learning and memory and cognitive dysfunction is the features of the primary behaviors in PTSD patients. This is, as of now, a part of proving to affirm the anti-anxiety and anti-depressive impacts of chlorogenic acid [36]. Animal experiments have found that PTSD rats exhibit typical PTSD symptoms, such as anxiety-like behavior, hyperexcitability, cognitive function, and memory decline [37]. Clinical studies have also shown that patients with PTSD have impairments in learning, memory, and cognitive function [38]. Some researchers used Y-maze, Morris water maze, and passive avoidance experiments to detect the effects of CGA on scopolamine-induced memory decline and cognitive dysfunction in mice. They found that CGA can significantly improve the short-term or working memory impairment caused by scopolamine. It can effectively reverse cognitive impairment and reduce the impairment of scopolamine-induced spatial learning in mice [35]. CGA can also reduce the level of acetylcholinesterase activity in the hippocampus and frontal cortex and improve memory and cognitive dysfunction [35]. Besides, CGA can improve the effects of alcohol on the brain damage and cognitive function of newborn rats [39]. Hermawati, Stefanello, and their colleagues used bilateral typical carotid occlusion (BCCO), a single intraperitoneal streptozotocin injection, to establish transient global ischemia (TGI) models and diabetes models, respectively. After the treatment of CGA, the memory dysfunction of the TGI model and the memory decline of rats caused by diabetes were improved effectively [14, 40]. The above studies have confirmed that CGA can improve the learning, memory, and cognitive function of some animal models, but there are few clinically related studies. To detect the effect of CGA intake on cognitive function in the elderly, Kato and colleagues had 8 healthy elderly with subjective memory loss, drink a test beverage (containing 330 mg CGA) before going to bed for six consecutive months, and then evaluate their cognitive status through central nervous system vital signs tests [41]. The results showed that CGA could improve attention, executive, and memory functions of the elderly with subjective memory loss [41]. In addition, some researchers used randomized, double-blind, placebo-controlled trials to detect the effect of CGA on cognitive function. The results are consistent with those of previous studies. CGA can improve the cognitive function of the elderly [42]. The above studies show that CGA can effectively ameliorate learning, memory and cognitive dysfunction, which provides a theoretical basis for improving the symptoms of PTSD and its comorbidities.
3.2. Anti-anxiety and Anti-depression
Patients with PTSD are often associated with comorbidities, such as sleep disturbance, anxiety, and depression [2]. The anti-depression effect of Eucommia ulmoides rich in CGA was investigated with the help of a tail suspension test on mice for seven consecutive days, and the results showed that they could effectively improve depression symptoms in vivo [43]. Subsequently, the same results were found by Song and colleagues. They employed a sucrose preference test, forced swimming test, tail suspension test, as well as an open field test to explore the anti-depression effect of CGA on a rat model of severe depression induced by adrenocorticotropic hormone (ACTH) [44]. The results show that CGA can improve depression-like behavior in rats induced by ACTH. At the same time, CGA also up-regulates the levels of serotonin and dopamine in the serum of depressed rats, showing an antidepressant effect. Furthermore, in the process of investigating the anti-anxiety effect of CGA, the researchers used light/dark test, elevated plus maze and free exploration test to detect the anxiolytic effect of CGA in the mouse anxiety model. In the light/dark test, CGA could increase the number of their transitions from the dark box to the lightbox, the cumulative time spent in the lightbox, and the exercise capacity in the lightbox, producing an anti-anxiety-like effect. In the elevated plus-maze experiment, the cumulative time in the open arm, and the total number of visits to the open arm in the CGA treatment group (20 mg/kg) increased significantly. In the free exploration test, the mice were given CGA (20 mg/kg), and diazepam (1 mg/kg) showed more exercise behavior and exploring behavior in unfamiliar environments than the control group. These results confirm the anti-anxiety effect of CGA [45]. The above studies have shown that CGA has anti-anxiety and anti-depression effects, and such symptoms are often accompanied by PTSD patients.
4. MECHANISM OF CGA TREATMENT OF PTSD-LIKE SYMPTOMS
4.1. Protection of Neurons
It has been demonstrated that neuronal damage can be detected in animal models of PTSD, as well as the loss of neurons and reduction of hippocampal volume in PTSD patients [46], so the protection of neurons may be an essential strategy for the treatment of PTSD. Both in vivo and in vitro experiments have shown that CGA has a protective effect on neurons [44]. In vitro, CGA can promote the release of 5-hydroxytryptamine (5-HT) by up-regulating the expression of synaptic protein I (Syn I), and stimulating the growth of axons and dendrites in raphe neurons from fetus rats. In vivo, CGA can be detected in the cerebrospinal fluid of rats treated with CGA-enriched extract of eucommia, which reaches the level of neuroprotective pharmacological effect, indicating that CGA can cross the blood-cerebrospinal fluid barrier, play its role of protecting neurons and promoting the release of 5-hydroxytryptamine, and up-regulate the expression of Syn I. CGA can also protect neuronal cells by up-regulating the expression of NADPH, also called quinine oxidoreductase 1 (NQO1), mediated by nuclear factor erythroid 2-related factor 2 (Nrf2) [47]. In addition, CGA can also up-regulate the expression of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in the hippocampus [48, 49], and inhibit the loss of hippocampal neurons [50]. Based on the above studies, we speculate that CGA can promote the expression of Syn I, BDNF, NGF, and the release of 5-HT, and directly or indirectly induce the activation of Nrf2 to play a neuroprotective effect on patients with PTSD and related comorbidities.
4.2. Anti-apoptotic Effect
In the adult brain, apoptosis is associated with maintaining the correct number of neurons and eliminating unnecessary or incorrect connections. However, it is interesting to note that one of the pathologic features of PTSD increased apoptosis in the hippocampus, amygdala, and other brain regions [51]. It has been indicated that CGA could protect the apoptosis of PC12 cells induced by hydrogen peroxide [52]. CGA can inhibit nuclear condensation and DNA fragmentation, down-regulate the expression of B cell lymphoma-extra large (Bcl-xL) and cysteinyl aspartate-specific proteinase-3 (Caspase-3), and inhibit the cleavage of poly ADP ribose polymerase. Besides, CGA also inhibits the activation of p38 mitogen-activated protein kinase (p38MAPK) and c-Jun amino-terminal kinase. Furthermore, CGA can also regulate the expression of Bcl-2, Bax, ERK1/2, cyclin D1, and the Shp2/PI3K/Akt pathway to exert anti-apoptotic effects [53-55].
Mitochondria are the central part of the mitochondrial phospholipid membrane that is attacked by reactive oxygen species. The reactive oxygen species can cause the permeability transition pores of the mitochondrial membrane to open, the mitochondrial membrane potential is dissipated, cytochrome C is released, and the downstream apoptosis executive factor Caspase-3 is activated, leading to cell DNA breakage and apoptosis. Previous studies have confirmed that CGA can enhance the intensity of hippocampal neuron mitochondrial membrane potential induced by Aβ25-35 and prevent hippocampal neuronal cell apoptosis [50].
According to the above studies, it is speculated that CGA may inhibit the apoptosis of neurons in hippocampus, amygdala and other brain regions in PTSD patients through apoptosis-related proteins and mitochondria, as well as PI3K/Akt signaling pathway, providing a theoretical basis for the treatment of PTSD and related comorbidities.
4.3. Inhibition of Inflammation and Anti-oxidative Stress
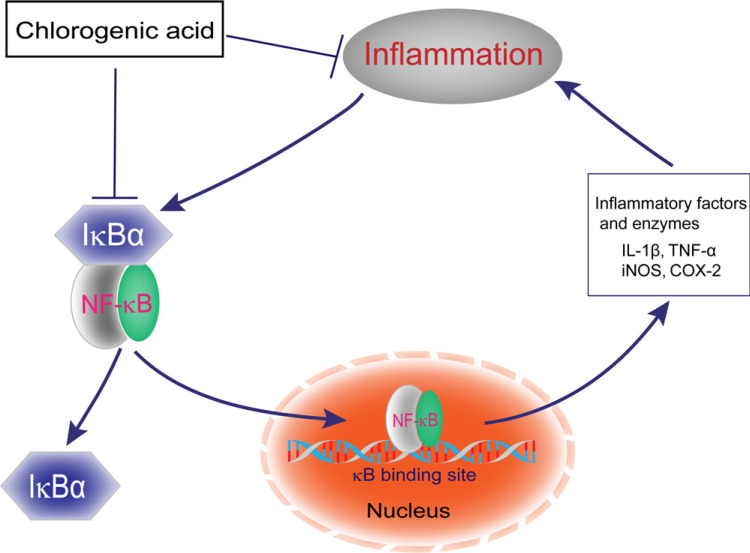
Changes in inflammatory markers are associated with structural and functional changes in brain regions, such as the amygdala, hippocampus, and frontal cortex, which are responsible for regulating stress and emotion. A growing body of research suggests that PTSD patients are associated with changes in some inflammatory markers and various oxidative stress responses [56, 57]. Microglia (MG) is a mononuclear phagocytic cell in the brain, capable of phagocytosis and antigen presentation [58]. In the event of injury or inflammation, activated microglial cells secrete inflammatory and chemokine factors, and overactivated microglia induce neurotoxic effects by overproduction of cytotoxic factors, such as nitric oxide (NO), TNF-α, IL-1β. Shen and colleagues investigated the role of CGA in lipopolysaccharides (LPS) stimulation of microglia and found that CGA can inhibit the production of NO, TNF-α and IL-1β in primary microglia stimulated by LPS [13]. The prevention of the release of neurotoxicity caused by the activation of microglia and ultimately improves the survival rate of neurons. At the same time, another study showed that CGA could not only significantly reduce the release of pro-inflammatory cytokines in macrophages stimulated by LPS, but also attenuate cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), and inhibit the activation of NF-κB signaling pathway from exerting its anti-inflammatory effects [59]. In summary, CGA plays an anti-inflammatory effect by down-regulating the expression of inflammatory factors such as NO, TNF-α, IL-1β, regulating iNOS/COX-2/NF-κB signaling pathways, and further reduces neuroinflammation (Fig. 2).
Fig. (2).
Anti-inflammatory mechanism of chlorogenic acid. In the absence of external stimuli, NF-κB is sequestered and repressed in the cytoplasm by the inhibitory protein I-κBα. When other factors stimulate the body, I-κBα will be degraded and NF-κBis activated. Then the activated NF-κB enters the nucleus and binds to the κB site to increase the release of a variety of cytokines and enzymes, such as TNF-α, IL-1β, iNOS and COX-2, and cause inflammation. However, CGA exerts anti-inflammatory effects by inhibiting the activation of NF-κB. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
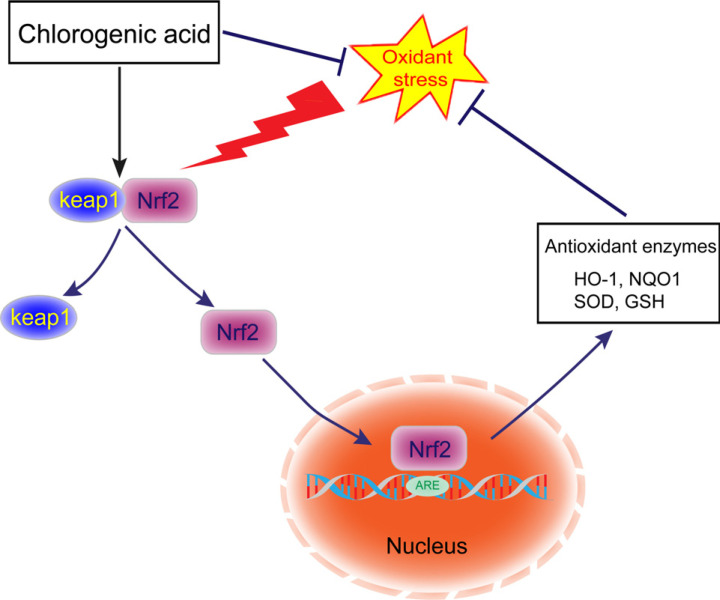
Under normal circumstances, there are a large number of antioxidant enzymes in the brain tissue, such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), which can remove active oxygen and protect neurons from oxidative damage caused by free radicals, thereby avoiding damage to brain cells. Liu et al. [48] found that the protective effect of CGA on cerebral ischemia-reperfusion (CI/R) injury by regulating the oxidative stress-related Nrf2 pathway. Their results showed that the Nrf2 pathway in CI/R rats was inactivation and CGA can reactivate the Nrf2 signaling pathway, which plays a role in reducing oxidative stress. CGA can promote the dissociation of kelch-like ECH-association protein 1 (Keap1) from Nrf2, then the activated Nrf2 is transported into the nucleus, binds to antioxidant response element (ARE), activates the expression of downstream target genes, such as quinone oxidoreductase 1 (NQO1) and heme oxygenase-1 (HO-1), further increase the SOD activity and GSH levels, and reduce the reactive oxygen species (ROS) and lactate dehydrogenase (LDH). HO-1, also known as heat shock protein 32 (Hsp32), is an important member of the vitagene system and has a strong antioxidant effect. Vitagenes is a group of genes involved in maintaining the stability of the intracellular environment under stress, including Hsp32, Hsp70, thioredoxin/thioredoxin reductase system [60]. There is evidence that under oxidative stress, the activation of the vitagene system can play a defensive role in the brain, maintain the redox homeostasis in the cell, and protect the cell itself from oxidative damage [61]. CGA may act as a strong inducer of the vitagene system by activating the Keap1/Nrf2/HO-1 pathway to increase the expression of vitagene. The product reduces the accumulation of malondialdehyde (MDA), inhibits the apoptosis of cerebral cortex cells, and reduces the pathological damage of brain tissue. Other studies have shown that CGA protects neuronal damage by activating antioxidant systems, inhibiting the accumulation of endogenous ROS, restoring mitochondrial membrane potential, and regulating intracellular Ca2+ concentration caused by glutamate over stimulation [62, 63]. Keap1/Nrf2 pathway plays a crucial role in the regulation of excitatory stress response. Other studies have also shown that hormesis may be a common biological effect of natural polyphenols [64]. Hormesis means that mild stress activates the adaptive cellular response of cells to subsequent severe stress, more specifically, it is appropriate over-compensation for the damage of dynamic balance. Strong evidence shows that hormesis is closely related to redox-dependent aging-related neuro-damaging diseases, especially neurodegenerative diseases [65-67]. Therefore, CGA can soar to the protective effect of mitochondria, increase the activity of SOD and GHS, decrease the level of MDA, regulate the Keap1/Nrf2/HO-1 signaling pathway, induce cell quotation transmission, trigger hormesis, and activate the vitagene system to adapt to and protect oxidative stress, and further exert neuroprotective effects, providing a theoretical basis for the treatment of PTSD (Fig. 3).
Fig. (3).
Antioxidant mechanism of chlorogenic acid. Normally, Nrf2 binds to Keap1 and exists in the cytoplasm in an inactive state. When stimulated by reactive oxygen species, Nrf2 dissociates from Keap1, and the activated Nrf2 is transported into the nucleus, binds to ARE, activates the expression of downstream target genes, and plays a role in reducing oxidative stress. However, when ROS accumulates excessively in the body, the antioxidant capacity of the organism will be out of balance, resulting in damage to the cell structure and function. CGA can activate the Nrf2 pathway, promote Nrf2 to enter the nucleus, regulate downstream antioxidant genes, and reduce oxidative stress.
CONCLUSION
In summary, CGA can cross the blood-brain barrier and can regulate learning, memory and cognitive ability by anti-apoptosis, inhibit neuroinflammation and oxidative stress, and play a protective effect on neurons to regulate learning, memory, and cognitive ability, and improve anxiety PTSD-like symptoms such as depression and depression. This provides a theoretical basis for the possible use of CGA as a drug to prevent and treat PTSD and its comorbidities. However, it needs more pre-clinical trials and clinical trials to further verify its clinical application in the future.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by the National Natural Science Foundation of China (81971831 and 81671903), the Personnel Innovation Ability Training Program of Army Medical Center of PLA (2019CXJSC007), Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJZD-K202001102), and the Graduate Student Innovation Program of Chongqing (CYS20345).
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Campbell S.B., Trachik B., Goldberg S., Simpson T.L. Identifying PTSD symptom typologies: A latent class analysis. Psychiatry Res. 2020;285:112779. doi: 10.1016/j.psychres.2020.112779. [DOI] [PubMed] [Google Scholar]
- 2.Gadermann A.M., Alonso J., Vilagut G., Zaslavsky A.M., Kessler R.C. Comorbidity and disease burden in the national comorbidity survey replication (NCS-R). Depress. Anxiety. 2012;29(9):797–806. doi: 10.1002/da.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clifford M.N., Jaganath I.B., Ludwig I.A., Crozier A. Chlorogenic acids and the acyl-quinic acids: discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017;34(12):1391–1421. doi: 10.1039/C7NP00030H. [DOI] [PubMed] [Google Scholar]
- 4.Naveed M., Hejazi V., Abbas M., Kamboh A.A., Khan G.J., Shumzaid M., Ahmad F., Babazadeh D. FangFang, X.; Modarresi-Ghazani, F.; WenHua, L.; XiaoHui, Z. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 5.Arfian N., Wahyudi D.A.P., Zulfatina I.B., Citta A.N., Anggorowati N., Multazam A., Romi M.M., Sari D.C.R. Chlorogenic acid attenuates kidney ischemic/reperfusion injury via reducing inflammation, tubular injury, and myofibroblast formation. BioMed. Res. Int. 2019;2019:5423703. doi: 10.1155/2019/5423703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisht A., Dickens M., Rutherfurd-Markwick K., Thota R., Mutukumira A.N., Singh H. Chlorogenic acid potentiates the anti-inflammatory activity of curcumin in LPS-stimulated THP-1 cells. Nutrients. 2020;12(9):E2706. doi: 10.3390/nu12092706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang S., Wang L.L., Xue N.N., Li C., Guo H.H., Ren T.K., Zhan Y., Li W.B., Zhang J., Chen X.G., Han Y.X., Zhang J.L., Jiang J.D. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics. 2019;9(23):6745–6763. doi: 10.7150/thno.34674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sapio L., Salzillo A., Illiano M., Ragone A., Spina A., Chiosi E., Pacifico S., Catauro M., Naviglio S. Chlorogenic acid activates ERK1/2 and inhibits proliferation of osteosarcoma cells. J. Cell. Physiol. 2020;235(4):3741–3752. doi: 10.1002/jcp.29269. [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Liu J., Xie Z., Rao J., Xu G., Huang K., Li W., Yin Z. Chlorogenic acid inhibits proliferation and induces apoptosis in A498 human kidney cancer cells via inactivating PI3K/Akt/mTOR signalling pathway. J. Pharm. Pharmacol. 2019;71(7):1100–1109. doi: 10.1111/jphp.13095. [DOI] [PubMed] [Google Scholar]
- 10.Tajik N., Tajik M., Mack I., Enck P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur. J. Nutr. 2017;56(7):2215–2244. doi: 10.1007/s00394-017-1379-1. [DOI] [PubMed] [Google Scholar]
- 11.Han J., Miyamae Y., Shigemori H., Isoda H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience. 2010;169(3):1039–1045. doi: 10.1016/j.neuroscience.2010.05.049. [DOI] [PubMed] [Google Scholar]
- 12.Kumar G., Mukherjee S., Paliwal P., Singh S.S., Birla H., Singh S.P., Krishnamurthy S., Patnaik R. Neuroprotective effect of chlorogenic acid in global cerebral ischemia-reperfusion rat model. Naunyn Schmiedebergs Arch. Pharmacol. 2019;392(10):1293–1309. doi: 10.1007/s00210-019-01670-x. [DOI] [PubMed] [Google Scholar]
- 13.Shen W., Qi R., Zhang J., Wang Z., Wang H., Hu C., Zhao Y., Bie M., Wang Y., Fu Y., Chen M., Lu D. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res. Bull. 2012;88(5):487–494. doi: 10.1016/j.brainresbull.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Hermawati E., Arfian N., Mustofa M., Partadiredja G. Chlorogenic acid ameliorates memory loss and hippocampal cell death after transient global ischemia. Eur. J. Neurosci. 2020;51(2):651–669. doi: 10.1111/ejn.14556. [DOI] [PubMed] [Google Scholar]
- 15.Singh S.S., Rai S.N., Birla H., Zahra W., Kumar G., Gedda M.R., Tiwari N., Patnaik R., Singh R.K., Singh S.P. Effect of chlorogenic acid supplementation in MPTP-intoxicated mouse. Front. Pharmacol. 2018;9:757. doi: 10.3389/fphar.2018.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cropley V., Croft R., Silber B., Neale C., Scholey A., Stough C., Schmitt J. Does coffee enriched with chlorogenic acids improve mood and cognition after acute administration in healthy elderly? A pilot study. Psychopharmacology (Berl.) 2012;219(3):737–749. doi: 10.1007/s00213-011-2395-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.K., Park S.U. Chlorogenic acid and its role in biological functions: an up to date. EXCLI J. 2019;18:310–316. doi: 10.17179/excli2019-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao M., Xiang L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020;87:71–88. doi: 10.1016/bs.apha.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Hernandes L.C., Machado A.R.T., Tuttis K., Ribeiro D.L., Aissa A.F., Dévoz P.P., Antunes L.M.G. Caffeic acid and chlorogenic acid cytotoxicity, genotoxicity and impact on global DNA methylation in human leukemic cell lines. Genet. Mol. Biol. 2020;43(3):e20190347. doi: 10.1590/1678-4685-gmb-2019-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santana-Gálvez J., Cisneros-Zevallos L., Jacobo-Velázquez D.A. Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules. 2017;22(3):E358. doi: 10.3390/molecules22030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Lam K.L., Hu J., Ge S., Zhou A., Zheng B., Zeng S., Lin S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019;7(2):579–588. doi: 10.1002/fsn3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagdas D., Etoz B.C., Gul Z., Ziyanok S., Inan S., Turacozen O., Gul N.Y., Topal A., Cinkilic N., Tas S., Ozyigit M.O., Gurun M.S. In vivo systemic chlorogenic acid therapy under diabetic conditions: Wound healing effects and cytotoxicity/genotoxicity profile. Food Chem. Toxicol. 2015;81:54–61. doi: 10.1016/j.fct.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Han X., Zhang Y., Guo J., You Y., Zhan J., Huang W. Chlorogenic acid stimulates the thermogenesis of brown adipocytes by promoting the uptake of glucose and the function of mitochondria. J. Food Sci. 2019;84(12):3815–3824. doi: 10.1111/1750-3841.14838. [DOI] [PubMed] [Google Scholar]
- 24.Bhandarkar N.S., Brown L., Panchal S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet-induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019;62:78–88. doi: 10.1016/j.nutres.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Chen J., Yu B., Chen D., Zheng P., Luo Y., Huang Z., Luo J., Mao X., Yu J., He J. Changes of porcine gut microbiota in response to dietary chlorogenic acid supplementation. Appl. Microbiol. Biotechnol. 2019;103(19):8157–8168. doi: 10.1007/s00253-019-10025-8. [DOI] [PubMed] [Google Scholar]
- 26.Sato Y., Itagaki S., Kurokawa T., Ogura J., Kobayashi M., Hirano T., Sugawara M., Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011;403(1-2):136–138. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 27.Shi H., Dong L., Jiang J., Zhao J., Zhao G., Dang X., Lu X., Jia M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology. 2013;303:107–114. doi: 10.1016/j.tox.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Tian L., Su C.P., Wang Q., Wu F.J., Bai R., Zhang H.M., Liu J.Y., Lu W.J., Wang W., Lan F., Guo S.Z. Chlorogenic acid: A potent molecule that protects cardiomyocytes from TNF-α-induced injury via inhibiting NF-κB and JNK signals. J. Cell. Mol. Med. 2019;23(7):4666–4678. doi: 10.1111/jcmm.14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heitman E., Ingram D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017;20(1):32–39. doi: 10.1179/1476830514Y.0000000146. [DOI] [PubMed] [Google Scholar]
- 30.Nabavi S.F., Tejada S., Setzer W.N., Gortzi O., Sureda A., Braidy N., Daglia M., Manayi A., Nabavi S.M. Chlorogenic acid and mental diseases: from chemistry to medicine. Curr. Neuropharmacol. 2017;15(4):471–479. doi: 10.2174/1570159X14666160325120625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S.S., Rai S.N., Birla H., Zahra W., Rathore A.S., Dilnashin H., Singh R., Singh S.P. Neuroprotective effect of chlorogenic acid on mitochondrial dysfunction-mediated apoptotic death of DA neurons in a parkinsonian mouse model. Oxid. Med. Cell. Longev. 2020;2020:6571484. doi: 10.1155/2020/6571484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao L., Li X., Meng S., Ma T., Wan L., Xu S. Chlorogenic acid alleviates abeta25-35-induced autophagy and cognitive impairment via the mTOR/TFEB signaling pathway. Drug Des. Devel. Ther. 2020;14:1705–1716. doi: 10.2147/DDDT.S235969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombo R., Papetti A. An outlook on the role of decaffeinated coffee in neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2020;60(5):760–779. doi: 10.1080/10408398.2018.1550384. [DOI] [PubMed] [Google Scholar]
- 34.Kumar G., Paliwal P., Mukherjee S., Patnaik N., Krishnamurthy S., Patnaik R. Pharmacokinetics and brain penetration study of chlorogenic acid in rats. Xenobiotica. 2019;49(3):339–345. doi: 10.1080/00498254.2018.1445882. [DOI] [PubMed] [Google Scholar]
- 35.Kwon S.H., Lee H.K., Kim J.A., Hong S.I., Kim H.C., Jo T.H., Park Y.I., Lee C.K., Kim Y.B., Lee S.Y., Jang C.G. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010;649(1-3):210–217. doi: 10.1016/j.ejphar.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Pathak L., Agrawal Y., Dhir A. Natural polyphenols in the management of major depression. Expert Opin. Investig. Drugs. 2013;22(7):863–880. doi: 10.1517/13543784.2013.794783. [DOI] [PubMed] [Google Scholar]
- 37.Alexander K.S., Nalloor R., Bunting K.M., Vazdarjanova A. Investigating individual pre-trauma susceptibility to a PTSD-like phenotype in animals. Front. Syst. Neurosci. 2020;13:85. doi: 10.3389/fnsys.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dossi G., Delvecchio G., Prunas C., Soares J.C., Brambilla P. Neural bases of cognitive impairments in post-traumatic stress disorders: a mini-review of functional magnetic resonance imaging findings. Front. Psychiatry. 2020;11:176. doi: 10.3389/fpsyt.2020.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Z., Li J. Chlorogenic acid prevents alcohol-induced brain damage in neonatal rat. Transl. Neurosci. 2017;8:176–181. doi: 10.1515/tnsci-2017-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefanello N., Schmatz R., Pereira L.B., Rubin M.A., da Rocha J.B., Facco G., Pereira M.E., Mazzanti C.M., Passamonti S., Rodrigues M.V., Carvalho F.B., da Rosa M.M., Gutierres J.M., Cardoso A.M., Morsch V.M., Schetinger M.R. Effects of chlorogenic acid, caffeine, and coffee on behavioral and biochemical parameters of diabetic rats. Mol. Cell. Biochem. 2014;388(1-2):277–286. doi: 10.1007/s11010-013-1919-9. [DOI] [PubMed] [Google Scholar]
- 41.Kato M., Ochiai R., Kozuma K., Sato H., Katsuragi Y. Effect of chlorogenic acid intake on cognitive function in the elderly: A pilot study. Evid. Based Complement. Alternat. Med. 2018;2018:8608497. doi: 10.1155/2018/8608497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saitou K., Ochiai R., Kozuma K., Sato H., Koikeda T., Osaki N., Katsuragi Y. Effect of chlorogenic acids on cognitive function: a randomized, double-blind, placebo-controlled trial. Nutrients. 2018;10(10):1–8. doi: 10.3390/nu10101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J., Chen H., Li H., Tang Y., Yang L., Cao S., Qin D. Antidepressant potential of chlorogenic acid-enriched extract from Eucommia ulmoides Oliver bark with neuron protection and promotion of serotonin release through enhancing synapsin I expression. Molecules. 2016;21(3):260. doi: 10.3390/molecules21030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song J., Zhou N., Ma W., Gu X., Chen B., Zeng Y., Yang L., Zhou M. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food Funct. 2019;10(5):2947–2957. doi: 10.1039/C8FO02599A. [DOI] [PubMed] [Google Scholar]
- 45.Bouayed J., Rammal H., Dicko A., Younos C., Soulimani R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007;262(1-2):77–84. doi: 10.1016/j.jns.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 46.Abdallah C.G., Averill L.A., Akiki T.J., Raza M., Averill C.L., Gomaa H., Adikey A., Krystal J.H. The neurobiology and pharmacotherapy of posttraumatic stress disorder. Annu. Rev. Pharmacol. Toxicol. 2019;59:171–189. doi: 10.1146/annurev-pharmtox-010818-021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J., Lee S., Shim J., Kim H.W., Kim J., Jang Y.J., Yang H., Park J., Choi S.H., Yoon J.H., Lee K.W., Lee H.J. Caffeinated coffee, decaffeinated coffee, and the phenolic phytochemical chlorogenic acid up-regulate NQO1 expression and prevent H2O2-induced apoptosis in primary cortical neurons. Neurochem. Int. 2012;60(5):466–474. doi: 10.1016/j.neuint.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Liu D., Wang H., Zhang Y., Zhang Z. Protective effects of chlorogenic acid on cerebral ischemia/reperfusion injury rats by regulating oxidative stress-related Nrf2 pathway. Drug Des. Devel. Ther. 2020;14:51–60. doi: 10.2147/DDDT.S228751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Ionita R., Postu P.A., Mihasan M., Gorgan D.L., Hancianu M., Cioanca O., Hritcu L. Ameliorative effects of Matricaria chamomilla L. hydroalcoholic extract on scopolamine-induced memory impairment in rats: A behavioral and molecular study. Phytomedicine. 2018;47:113–120. doi: 10.1016/j.phymed.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 50.Shi M., Sun F., Wang Y., Kang J., Zhang S., Li H. CGA restrains the apoptosis of Aβ25-35-induced hippocampal neurons. Int. J. Neurosci. 2020;130(7):700–707. doi: 10.1080/00207454.2019.1702547. [DOI] [PubMed] [Google Scholar]
- 51.Chen X., Jiang Y., Wang J., Liu Y., Xiao M., Song C., Bai Y., Han Y. N.; Han, F. Synapse impairment associated with enhanced apoptosis in post-traumatic stress disorder. Synapse. 2020;74(2):e22134. doi: 10.1002/syn.22134. [DOI] [PubMed] [Google Scholar]
- 52.Cho E.S., Jang Y.J., Hwang M.K., Kang N.J., Lee K.W., Lee H.J. Attenuation of oxidative neuronal cell death by coffee phenolic phytochemicals. Mutat. Res. 2009;661(1-2):18–24. doi: 10.1016/j.mrfmmm.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Zhou R.P., Lin S.J., Wan W.B., Zuo H.L., Yao F.F., Ruan H.B., Xu J., Song W., Zhou Y.C., Wen S.Y., Dai J.H., Zhu M.L., Luo J. Chlorogenic acid prevents osteoporosis by Shp2/PI3K/Akt pathway in ovariectomized rats. PLoS One. 2016;11(12):e0166751. doi: 10.1371/journal.pone.0166751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang S.Q., Wang Y.T., Wei J.X., Shu Y.H., Xiao L., Lu X.M. Beneficial effects of chlorogenic acid on alcohol-induced damage in PC12 cells. Biomed. Pharmacother. 2016;79:254–262. doi: 10.1016/j.biopha.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 55.Chen L., Liu D.N., Wang Y., Liu X.Y., Han S., Zhang K., Li G.Y., Tian X., Wang H.Y., Wang J.H. Treatment with MQA, a derivative of caffeoylquinic acid, provides neuroprotective effects against cerebral ischemia through suppression of the p38 pathway and oxidative stress in rats. J. Mol. Neurosci. 2019;67(4):604–612. doi: 10.1007/s12031-019-01268-1. [DOI] [PubMed] [Google Scholar]
- 56.Wang W., Wang L., Xu H., Cao C., Liu P., Luo S., Duan Q., Ellenbroek B., Zhang X. Characteristics of pro- and anti-inflammatory cytokines alteration in PTSD patients exposed to a deadly earthquake. J. Affect. Disord. 2019;248:52–58. doi: 10.1016/j.jad.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 57.Kim T.D., Lee S., Yoon S. Inflammation in post-traumatic stress disorder (PTSD): a review of potential correlates of PTSD with a neurological perspective. Antioxidants. 2020;9(2):E107. doi: 10.3390/antiox9020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barres B.A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 59.Chen L., Lin X., Xiao J., Tian Y., Zheng B., Teng H. Sonchus oleraceus Linn protects against LPS-induced sepsis and inhibits inflammatory responses in RAW264.7 cells. J. Ethnopharmacol. 2019;236:63–69. doi: 10.1016/j.jep.2019.02.039. [DOI] [PubMed] [Google Scholar]
- 60.Calabrese V.C.C., Mancuso C., Barone E., Calafato S., Bates T., Rizzarelli E., Kostova A.T. Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Front. Biosci. 2009;14:376–397. doi: 10.2741/3250. [DOI] [PubMed] [Google Scholar]
- 61.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rebai O., Belkhir M., Sanchez-Gomez M.V., Matute C., Fattouch S., Amri M. Differential molecular targets for neuroprotective effect of chlorogenic acid and its related compounds against glutamate induced excitotoxicity and oxidative stress in rat cortical neurons. Neurochem. Res. 2017;42(12):3559–3572. doi: 10.1007/s11064-017-2403-9. [DOI] [PubMed] [Google Scholar]
- 63.Mitrea D.R., Malkey R., Florian T.L., Filip A., Clichici S., Bidian C., Moldovan R., Hoteiuc O.A., Toader A.M., Baldea I. Daily oral administration of chlorogenic acid prevents the experimental carrageenan-induced oxidative stress. J. Physiol. Pharmacol. 2020;71(1) doi: 10.26402/jpp.2020.1.04. [DOI] [PubMed] [Google Scholar]
- 64.Brunetti G., Di Rosa G., Scuto M., Leri M., Stefani M., Schmitz-Linneweber C., Calabrese V., Saul N. Healthspan maintenance and prevention of parkinson’s-like phenotypes with hydroxytyrosol and oleuropein aglycone in C. elegans. Int. J. Mol. Sci. 2020;21(7):E2588. doi: 10.3390/ijms21072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fusco R., Scuto M., Cordaro M., D’Amico R., Gugliandolo E., Siracusa R., Peritore A.F., Crupi R., Impellizzeri D., Cuzzocrea S., Di Paola R. N-palmitoylethanolamide-oxazoline protects against middle cerebral artery occlusion injury in diabetic rats by regulating the SIRT1 pathway. Int. J. Mol. Sci. 2019;20(19):E4845. doi: 10.3390/ijms20194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calabrese V., Santoro A., Monti D., Crupi R., Di Paola R., Latteri S., Cuzzocrea S., Zappia M., Giordano J., Calabrese E.J., Franceschi C. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018;115:80–91. doi: 10.1016/j.freeradbiomed.2017.10.379. [DOI] [PubMed] [Google Scholar]
- 67.Di Rosa G., Brunetti G., Scuto M., Trovato Salinaro A., Calabrese E.J., Crea R., Schmitz-Linneweber C., Calabrese V., Saul N. Healthspan enhancement by olive polyphenols in C. elegans wild type and parkinson’s models. Int. J. Mol. Sci. 2020;21(11):E3893. doi: 10.3390/ijms21113893. [DOI] [PMC free article] [PubMed] [Google Scholar]