Abstract
Almost two decades have passed since the last methamphetamine (METH) abuse epidemic. In recent years, METH abuse in the United States has been rapidly increasing and is currently one of the leading causes of death in our country. Available statistical data indicates re-emergence of METH popularity and suggest an impending third epidemic of METH abuse. Alarmingly, there is no FDA-approved medication for METH use disorder (MUD). This disorder is currently treated with behavioral therapies; however, these therapies have limitations and would benefit from the addition of a MUD pharmacotherapy. Unfortunately, clinical trials have not yet found consistently effective pharmacotherapy for MUD. This review outlines the history of METH use, provides information on current prevalence of METH abuse and MUD, describes medications that have been in clinical trials for MUD, and addresses current as well as potential new treatments for MUD.
Keywords: Methamphetamine use disorder, psychotherapies, pharmacotherapies, addiction, clinical trials
1. INTRODUCTION
1.1. Methamphetamine Use Disorder and Methamphetamine-induced Disorders
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) divides substance-related disorders into two groups: substance use disorders and substance-induced disorders. Substance use disorders (drug addictions) are chronic, relapsing disorders that have been characterized by compulsive seeking and escalated intake of legal or illegal drugs, despite negative consequences. Substance-induced disorders include intoxication, withdrawal, and substance-induced mental disorders such as depression, anxiety or psychosis.
Methamphetamine (METH) is a highly addictive and powerful central nervous system psychostimulant that induces a feeling of intense euphoria and well-being. Due to its strong pleasurable effects, METH is abused worldwide and METH use disorder (MUD) is a worldwide health problem. METH-induced disorders include anxiety, depression, cognitive impairments, insomnia and psychosis. In addition, chronic METH use is a risk factor for developing Parkinson’s disease.
METH-induced depressive-anxious symptoms are usually treated with bupropion or a drive-increasing tricyclic antidepressant such as desipramine [1]. Sleep disturbances and agitation during METH withdrawal are treated with sedating antidepressants or low-potency sedating antipsychotic drugs. MUD patients with psychotic symptoms are medicated with atypical antipsychotic drugs and, if needed and for a short time only, with benzodiazepines. MUD is currently treated using behavioral therapies. There is no FDA-approved medication for MUD. This review provides an overview of current and emerging treatments for this disorder.
1.2. History of Methamphetamine Use
METH was synthesized from ephedrine by Japanese chemist Nagayoshi Nagai in 1893. In 1919, Japanese chemist Akira Ogata was the first to synthesize METH in a crystallized form. METH was used early on as a medical treatment for narcolepsy, asthma, depression, and as a weight-loss drug [2, 3]. People soon discovered its euphoric, energizing side effects. During World War II, METH was used to keep troops awake, enhance endurance, and ward off fatigue on long campaigns [2, 4]. In the United States (US), many pharmaceutical companies manufactured METH to treat extreme obesity in the 50s and 60s. Since the war, METH use increased dramatically, even after it was outlawed by the US in 1970, instigating the first METH epidemic (1940s through the 1960s). The second METH epidemic in the US occurred during the 1990s and early 2000s (https://rockinst.org). Most available METH was made near its users in small batches and small labs during this time. After the sales of ephedrine and pseudoephedrine were strictly limited in 2006 by the Combat Methamphetamine Epidemic Act of 2005 (CMEA), local production was cut substantially, and METH use plateaued for a short while. Mexican Transnational Criminal Organizations (TCOs) have become the primary producer and supplier of low cost, high purity METH in the US, leading to an increase in METH use deaths from METH overdose. The US is on the verge of the third METH epidemic. In Australia and Southeast Asia, the third METH epidemic is already occurring [5]. The world's leading producer of crystal METH is the Golden Triangle (Southeast Asia), specifically Shan State, Myanmar.
METH (N-methylated amphetamine) is currently a schedule-II controlled substance, which has two active optic isoforms, d-enantiomer and l-enantiomer [6]. Desoxyn, which is d-METH, is rarely medically prescribed due to its strong reinforcing properties. It can only be prescribed for attention deficit hyperactivity disorder (ADHD), extreme obesity, or narcolepsy. Therapeutic doses of Desoxyn are 20-25 mg daily, taken every 12 hours, with dose not exceeding 60 mg/day (reference.medscape.com/drugs). l-METH, a vasoconstrictor, is the active constituent of the Vicks Inhaler decongestant, an over-the-counter product containing about 50 mg of the drug [7].
1.3. Prevalence of Methamphetamine Abuse
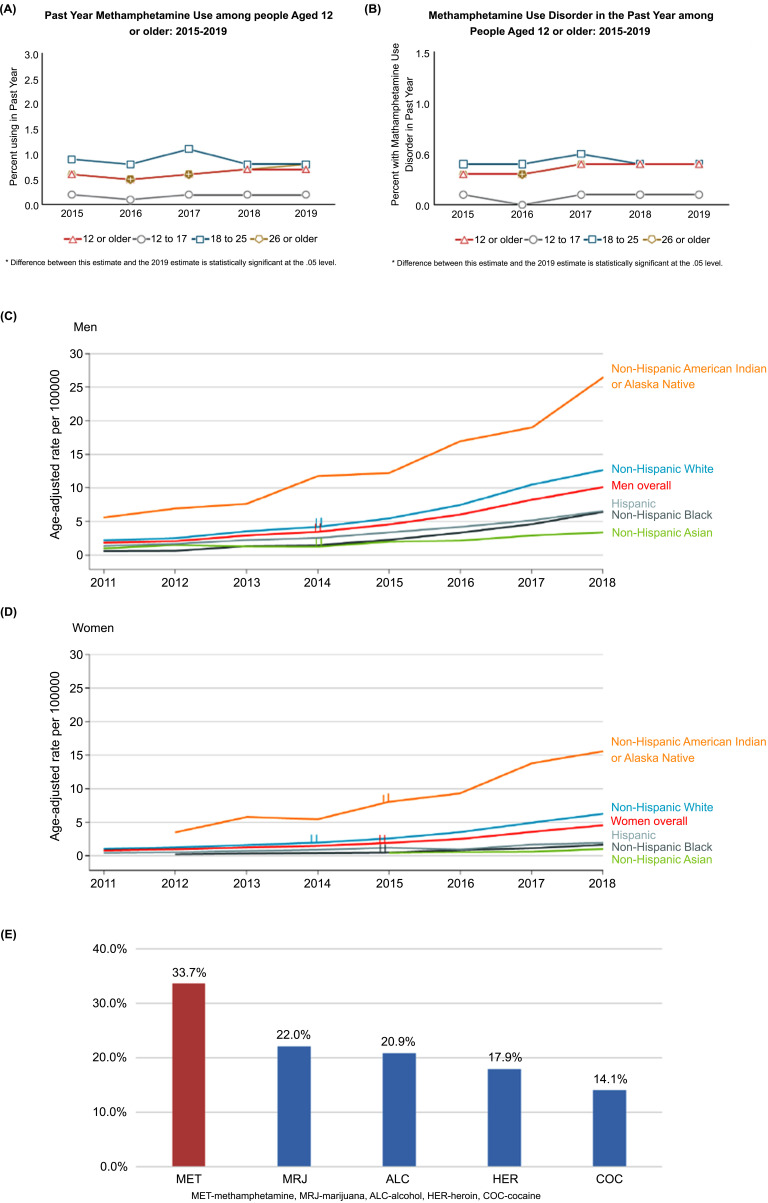
According to the 2020 United Nations World Drug Report, around 27 million people worldwide (corresponding to 0.5% of the adult population) used amphetamines, including METH, amphetamine, and pharmaceutical stimulants, in the past year. Overall, there has been an approximately 40% increase in METH use in the United States between 2016 and 2018 and a further increase between 2018 and 2019 [5]. In the US, 0.7% of the population aged 12 and older, or 1.9 million people, reported the use of METH. METH use declined among young adults (aged 18–25), but increased significantly among adults aged 26 and older from 0.5% (or 1.1 million people) in 2016 to 0.8% (or 1.7 million people) in 2019 (Fig. 1A) [8]. A recent cross-sectional study among a million patients showed a 486.7% increase in METH positive urine from 2013 to 2019 in the US [9]. These data suggest another impending METH epidemic.
Fig. (1).
Prevalence of methamphetamine (METH) use (A) and METH use disorder (B) between 2015 and 2019 in the US [8]. (C, D) Increases in deaths from METH overdose between 2011 and 2018 in the US divided by race and sex [15]. (E) As compared to other abused substances, METH was the most common type of drug involved in substance use-related ED visits in 2020 [18]. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
Not all people who abuse drugs develop substance use disorders. In 2019, about 50% of METH users suffered from MUD, including 19,000 adolescents and 125,000 young adults (Fig. 1A, B) [8]. Among people aged 12 to 25, the percentage with a past-year MUD remained relatively stable between 2015 and 2019. Among adults aged 26 or older, the percentage with a past-year MUD increased from 0.3% (or 539,000 people) in 2016 to 0.4% (or 904,000 people) in 2019 (Fig.1B) [8]. A recent study [10] reported that, since March 2020, there has been a 23% increase in urine samples taken from various healthcare and clinical settings testing positive for METH nationwide, thus indicating an increase in METH abuse during the COVID19 pandemic. Similarly, Centers for Disease Control and Prevention reported a recent increase in METH-related overdose deaths during the pandemic [11].
METH use is linked to a range of serious health risks, including overdose deaths. In the height of the opioid crisis in our country, deaths from METH overdose are rapidly rising [12, 13]. METH-related overdoses started rising markedly in 2009, and they had increased 10-fold by 2019, to over 16,500 [14]. In only 4 years (2013-2017), the rate of METH related overdose deaths more than doubled, and kept rising [12, 13]. Among those aged 26 years and above, deaths involving METH quadrupled among non-Hispanic American Indians and Alaska Natives between 2011 and 2018 (from 4.5 to 20.9 per 100,000 people) overall, with sharp increases for both men (from 5.6 to 26.4 per 100,000) and women (from 3.6 to 15.6 per 100,000) in that group (Fig. 1C, D) [15]. Regarding sex differences, during 2011-2018, age-adjusted rates for METH-involved deaths increased from 1.8 to 10.1 per 100,000 among men (average annual percentage change) and from 0.8 to 4.5 per 100,000 among women. This represents a more than 5-fold increase from 2011 to 2018. Within each sex, non-Hispanic American Indian and Alaska Native individuals had the highest rates, increasing from 5.6 to 26.4 per 100,000 among men during 2011-2018 and from 3.6 to 15.6 per 100,000 among women during 2012-2018. Non-Hispanic White individuals had the second highest rates, increasing from 2.2 to 12.6 per 100,000 among men and from 1.1 to 6.2 per 100,000 among women (Fig. 1C, D) [15].
METH use has increased particularly among people with an existing opioid use disorder (OUD) [16]. People with OUD reported that they sought a synergistic high by combining the drugs or that they wanted to balance the effects of opioids with METH effects. Among treatment-seeking people with OUD, reports of past-month METH use nearly doubled from 18.8%–34.2% between 2011 and 2017 [16]. Synthetic opioids (e.g., illicitly-manufactured fentanyl) have contributed to increases in stimulant-involved deaths [5]. Overall, METH is one of the leading causes of drug overdose deaths in the US. It accounted for 10.6% of deaths in 2016, 49.8% of which involved concomitant use of another drug(s) with heroin (21.8%), fentanyl (11.1%), and cocaine (8.3%) being the top 3 concomitant drugs. It accounted for 15% of deaths in 2017 and 50% of those deaths also involved an opioid [17].
1.4. Health Problems Associated with Methamphetamine Use Disorder
METH overdose is not the only danger to health. METH is highly toxic and its use is associated with pulmonary and cardiovascular pathology, mood and mental problems such as depression, anxiety, insomnia, psychosis, and risk for developing Parkinson’s disease, as well as with cognitive impairments and other health problems [19]. Its abuse frequently co-occurs with other substance use and mental disorders, which adds to the complexity of treating MUD. Furthermore, injecting METH using shared equipment can transmit infectious diseases like HIV or hepatitis B and C. The use of METH by men who have sex with men has been found to be an important factor in the transmission of HIV in that population [20]. Concomitant with the increase in METH use, the number of treatment admissions for amphetamine use disorders increased by 45% between 2012–2016 [21]. According to the Drug Abuse Warning Network (DAWN), METH was the most common type of substance involved in substance use-related emergency room (ER) visits (~34%) in 2020 (Fig. 1E) [18]. METH use-related ER visits varied according to age, sex and community type, being the highest in patients aged 26 to 45, males, and in urban areas, respectively. METH abuse morbidity and mortality risks are well established and mostly associated with cardiovascular and central nervous system toxicity [19, 22].
In general terms, people who abuse METH can be divided into light, moderate and heavy users of the drug. Thus, some people take METH a few times a month while some take the drug every day for extended periods of time, at doses ranging usually from light to moderate [23-25]. A subgroup of individuals abusing METH binge on the drug. Binge use involves a rapid escalation of METH intake, followed by a period of abstinence [25-27]. Humans can binge on METH from 3 to 14 days [27, 28] (over 4 days/week of use on average). The amount of METH consumed is ~1.5 times greater with binge users than with chronic users of the drug.
Heavy METH use has toxic effects on the brain, mainly on the nigrostriatal dopamine pathway, that are mediated by oxidative stress, inflammation and excitotoxicity [19]. People who abuse METH heavily suffer from a variety of neurological consequences of chronic abuse of the drug and have the hardest time quitting METH use [29-31]. Chronic METH users are at higher risk for developing Parkinson’s disease than non-users [32]. Conversely, Parkinson’s disease patients are more prone to addictions [33]. These findings implicate a compromised nigrostriatal pathway in individuals heavily dependent on METH. Animal studies showed that intermittent and long access stimulant self-administration changes the brain in different ways to influence motivated behavior [34]. This data indicates that light, moderate, and heavy METH users represent subpopulations with different changes in the brain and likely need different pharmacotherapies. To date, there have been very few clinical trials involving people who abuse METH heavily and only recent trials have shown promise for these individuals [35]. More clinical trials with people who abuse METH heavily and more studies in animal models of heavy METH use are warranted.
2. ADDICTION CYCLE AND PATHWAYS
Drug addiction is a chronically relapsing disorder characterized by compulsion to seek and take the drug, loss of control in limiting intake, and emergence of a negative emotional state when the drug is not available [36]. The addiction cycle consists of three stages: ‘binge/intoxication', ‘withdrawal/ negative affect', and ‘preoccupation/anticipation' (craving) stage [36]. The preoccupation/anticipation stage of the addiction cycle has long been hypothesized to be a key element of relapse in humans, and remains a focus for identifying the neurobiological mechanisms of relapse and the development of medications for treatment of substance use disorder. Consequently, relapse is the biggest challenge to the efficient treatment of MUD. METH relapse statistics indicate that ~61% of METH users will relapse within one year of completing treatment for MUD and an additional 25% will relapse before 5 years [37].
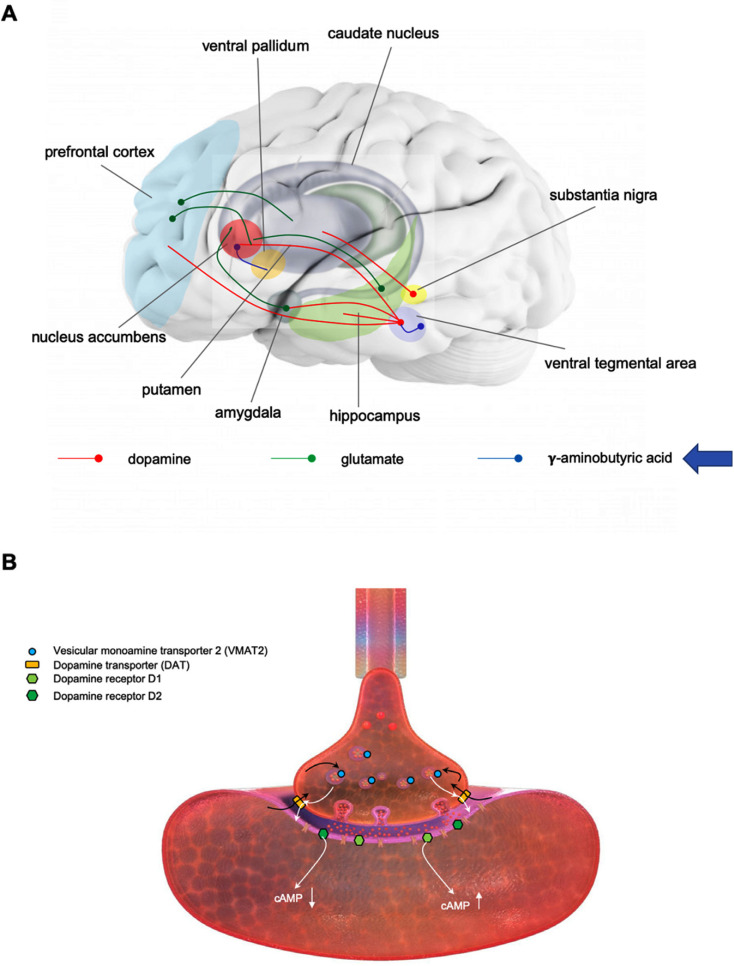
The rewarding/reinforcing effects of drugs are mediated by the nucleus accumbens, whereas the dorsal striatum mediates inhibitory control, spatial learning, cognitive flexibility, stimulus-response learning, and habit [38]. Importantly, there is evidence for a shift in control over drug-seeking behavior from the nucleus accumbens to the dorsal striatum upon development of drug dependence (loss of control over drug taking) [39]. The mesolimbic dopamine pathway (from the ventral tegmental area to the nucleus accumbens) is critical for the acute rewarding effects of METH and other psychostimulant drugs. The extended amygdala plays a central role during the withdrawal/negative affect stage. The preoccupation/anticipation stage mobilizes multiple brain areas [36]. A major neurotransmitter involved in this stage is glutamate. There are three types of instigating factors in relapse: cues, drugs, and stress. Although the neurocircuitries involved in cue-, drug-, and stress-induced reinstatement of drug seeking contain several neurotransmitters and are distinct in a number of aspects, they converge on pathways involving glutamatergic projection from the dorsomedial prefrontal cortex to the core of the nucleus accumbens [40]. In addition, the dopamine projection from the ventral tegmental area to the medial prefrontal cortex and the GABA projection from the nucleus accumbens to the ventral pallidum are involved in cue- and drug-induced relapse whereas stress-induced relapse is also mediated by corticotropin-releasing factor and norepinephrine in amygdala [36]. Chronic drug abuse induces neuroadaptations within the nucleus accumbens and other addiction circuitry areas in experimental animals and humans and these neuroadaptations persist long into withdrawal and contribute to drug cravings and relapse [36, 41]. Fig. (2A) shows key neurotransmitter pathways, and brain areas they connect, involved in addiction and targeted by medications tested for efficacy in MUD.
Fig. (2).
(A) Major neurotransmitter pathways targeted by medications tested for efficacy in methamphetamine use disorder (MUD) and brain areas connected by these pathways. A key brain area mediating drug reward/reinforcement and drug cravings is the nucleus accumbens. This area receives dopaminergic innervations (red) from the ventral tegmental area. This area sends dopaminergic projections also to the prefrontal cortex, hippocampus and amygdala. The nucleus accumbens receives glutamatergic input (green) from the prefrontal cortex, hippocampus and amygdala. GABAergic (blue) interneurons within the ventral tegmental area regulate the activity of dopaminergic neurons projecting to the nucleus accumbens. (B) Methamphetamine (METH) action at the dopaminergic terminal. METH enters the dopaminergic terminal via the dopamine transporter (DAT) where it subsequently enters dopamine storage vesicles via vesicular monoamine transporter 2 (VMAT2) (black arrows). Dopamine is released from the storage vesicles to the cytoplasm and subsequently to the synaptic cleft via METH-induced reversal of the DAT and activates postsynaptic dopamine D1 and D2 receptors. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
3. PHARMACOLOGY OF METHAMPHETAMINE
METH is an indirectly acting sympathomimetic amine that easily crosses the blood brain barrier and distributes throughout the brain [42]. It releases dopamine, serotonin, and noradrenaline from nerve terminals in the central and peripheral nervous system, thus increasing their neurotransmission. METH increases monoaminergic neurotransmission via three primary mechanisms of action: releasing monoamines to the cytosol from the storage vesicles, reversing monoamine transporters, and attenuating monoamine metabolism via inhibition of monoamine oxidases [43-45]. Specifically, due to the chemical structure being similar to monoamines, METH is recognized as a substrate by dopamine, serotonin, and noradrenaline plasma membrane transporters in the brain and is transported into neurons and neuronal terminals [46-49]. Once in the monoaminergic terminals, METH acts on the monoamine storage vesicles and depletes them of neurotransmitters by reversing vesicular monoamine transporter 2 (VMAT2) function and collapsing the pH gradient across the vesicular membrane [50-52]. METH-mediated reversal of the monoamine transporters at the plasma membrane leads to massive release of monoamines into the synaptic cleft [46, 47] (Fig. 2B). After release from terminals, monoamines bind to their receptors and then are metabolized or re-uptaken to neuronal terminals. METH blocks, to some extent, presynaptic re-uptake of monoamines [53]. The net result of this METH action is overstimulation of the monoaminergic pathways in the central and peripheral nervous system that can lead to severe dysfunction or even degeneration of dopaminergic and serotonergic terminals in several brain areas (well-documented in experimental animals), including the striatum, prefrontal cortex, and hippocampus [46, 47]. METH has minimal effect as an agonist at postsynaptic dopamine receptors and it activates them indirectly via released dopamine. In addition, acting via the striato-nigro-thalamo-cortical loop, METH triggers an increase in glutamate in the striatum, which results in excitotoxicity at higher doses of the drug [54].
The positive rewarding effects of METH are mediated mainly by increased dopaminergic neurotransmission, making the dopamine system a favored target for pharmacotherapy in MUD. Serotonin and noradrenaline modulate dopaminergic neurotransmission in the reward circuitry via stimulation of serotonergic and adrenergic receptors in several brain areas [55, 56]. In addition to modulating dopaminergic neurotransmission, certain other properties make the serotonin and noradrenaline system targets for pharmacotherapy for MUD. Serotonin stabilizes mood and regulates impulsivity as well as learning and memory [57] and thus has a role in the development and reinstatement of drug taking [56, 58]. Noradrenaline serves multiple brain functions, including arousal, attention, mood, learning, memory, and the stress response and therefore, it is critically involved in mediating METH effects such as sensitization, drug discrimination, and reinstatement of drug seeking [55, 59].
The rewarding effects of METH decrease in strength over time with chronic use of the drug due to the development of a variety of neuroadaptations [36]. Hypoactivity in the dopaminergic system (decreased levels of dopamine and dopamine D2 receptor) and alterations in hypothalamic-pituitary-adrenal axis functioning develops; irritability and dysphoria emerge in the absence of METH [60]. Regarding dopamine receptors’ roles in MUD, animal studies have shown that activation of dopamine D1 [61-63] and D2 [64-66] receptors mediate METH reward, METH self-administration and METH cravings in withdrawal, with D1 receptor being more strongly involved in these behaviors than D2 receptor. Human studies found an association between D2 receptor deficit and METH seeking (e.g. [67, 68]); however, administration of D2 agonists in clinical trials did not produce the desired effects. One of the reasons for the lack of dopamine-based pharmacotherapies for MUD treatment is a decrease in dopamine D2 receptor levels in the nucleus accumbens and dorsal striatum in chronic METH users - which correlated with low efficacy of direct D2 receptor agonists and dopamine uptake blockers in clinical trials [69-73].
4. CURRENT TREATMENTS FOR METHAMPHETAMINE USE DISORDER
4.1. Non-pharmacological Treatments for Methamphetamine Use Disorder
For now, the best available treatments for MUD are behavioral therapies. They include contingency management (CM), cognitive-behavioral therapy (CBT), the Matrix Model, 12-step facilitation therapy, the mobile medical application reSET®, and other behavioral interventions. Several systematic reviews provide detailed information on the design and the results from human studies on the efficacy of behavioral therapies in MUD [74-77]. The treatments with the most supporting evidence of effectiveness in MUD are presented below.
4.1.1. Contingency Management (CM)
CM uses motivational incentives and tangible rewards to help a person dependent on METH to attain their treatment goals e.g., abstinence from METH. For example, CM participants are provided monetary vouchers in exchange for consecutive urine samples documenting abstinence from METH. A person with METH-positive or missing urine sample is moved down the escalating schedule [78]. The studies that assessed CM efficacy in reducing METH abuse or dependence showed positive outcomes when comparing MUD patients to control group participants or CM to other behavioral therapies [74, 76]. The benefits of CM intervention included reduced drug use, better treatment retention, reduction in psychiatric symptoms, higher utilization of other treatments and medical services, and reductions in risky sexual behavior. Importantly, CM worked not only in research treatment settings but also in community programs for MUD [79, 80]. The studies employed CM varyingly using different reinforcement schedules (continuous reinforcement, intermittent predictable reinforcement, or intermittent, unpredictable reinforcement), different treatment durations (2-4 months), or in specific populations (e.g., men who have sex with men). Despite the differences in experimental design, of the 27 studies on CM effectiveness in MUD, only one found that CM did not effectively reduce METH use [81]. Interestingly, evaluation of CM effectiveness in combination with another treatment (CBT e.g. [82, 83], nurse case management e.g. [84], pharmacotherapy [85], strengths-based case management e.g. [86] or a positive affect intervention e.g. [87]) found no synergistic or additive effects. The sustainability of CM effects post-intervention has not been well-studied. Nevertheless, there is evidence for CM decreasing METH use months post-treatment [88].
Several factors appear to predict CM treatment outcome, including problem severity, race, HIV status, education, and income [76]. For example, CM therapy was the least effective for participants who reported a long history of drug use [89] or more METH use during baseline [86], and it was the most effective in Caucasian participants [88, 89].
4.1.2. Cognitive-behavioral Therapy (CBT)
From the cognitive‐behavioral perspective, substance use is considered the result of coping deficits and maladaptive thinking. CBT for a substance use disorder assumes that drug use is a learned behavior and it emphasizes individual commitment for recovery in order to learn new adaptive behaviors and ways of thinking [90]. Individuals in CBT learn to identify and correct addictive behaviors by applying a range of different skills that can be used to stop METH abuse and to address a range of co-occurring problems. CBT addresses relapse by modifying attitudes and personal core beliefs that might support cravings; it teaches to explore the positive and negative consequences of continued drug use, to recognize cravings early and identify situations in which the patients are vulnerable to using METH, and to develop coping skills to control cravings and avoid these high-risk situations. CBT for substance use disorders can be used in many formats, including individual therapy, group therapy and, more recently, computer‐based therapy that are usually delivered during weekly 60-min sessions lasting 12-20 weeks or as a brief CBT (4-8 sessions) [90, 91].
Application of CBT in lesbian, gay, bisexual, transgender and intersex (LGBTI) communitie consistently showed positive results. Thus, CBT either alone or combined with CM reduced METH use, cravings or relapse during treatment in this population [74, 92]. Similarly, CBT diminished relapse and/or cravings in other individuals with METH use disorder e.g. [91, 93-95]. Of note, although CM and CBT both demonstrated positive outcomes individually, no clear synergism was observed when CM was combined together with CBT [96]; however, when compared, the CM outperformed the CBT, e.g. [82].
4.1.3. The Matrix Model Therapy
The Matrix model therapy is a treatment approach that brings together many different components of addiction treatment, including relapse prevention, family therapy, group therapy, addiction education, and peer support groups, usually administered over the course of a 16-week period [97]. The model incorporates CBT, CM, 12-steps, and motivational interviewing therapy. The program is highly structured and is largely made up of group therapy sessions. The Matrix Model therapy provides a framework for engaging people who abuse METH in treatment and helping them achieve abstinence. Patients learn about issues critical to the addiction, relapse, and recovery process. They receive advice from a trained therapist on how to avoid relapse and how to socialize in a drug-free environment. They are familiarized with self-help programs. As part of the Matrix Model therapy, families are encouraged to actively participate in the recovery of their loved one. The Matrix model therapy has been effective in reducing METH use and craving during treatment and has shown somewhat better retention and abstinence rates than other behavioral interventions [74]. Specifically, when investigated as an independent intervention, the Matrix model therapy reduced METH use and improved craving management and control [98]. When combined with drug court supervision, the therapy improved retention and abstinence rates in individuals with MUD [99]. Two studies that compared Matrix model with treatment-as-usual reported a reduction in METH use, risky behaviors and more days of abstinence for 18 months [100, 101].
4.1.4. Twelve-step Facilitation Therapy
Twelve-step facilitation therapy consists of a structured and brief intervention to facilitate early recovery from alcohol and drug misuse [102]. It is based on cognitive, behavioral and spiritual principles. The 12-step facilitation therapy actively engages people who abuse addictive substances in help groups, so they achieve abstinence from them. Three key ideas are (1) acceptance of one’s addiction, (2) surrender to/acceptance of fellowship and support structure, and (3) active involvement in 12-step meetings and related activities. This therapy has been proven effective in alcohol and cocaine use disorder, but there is surprisingly little literature data on its effectiveness in MUD [103-105], despite the fact that programs treating people with MUD usually either require or recommend participation in 12‐step self‐help meetings [106]. Matrix model therapy incorporates the 12-step program as one component of treatment for MUD and was shown to effectively reduce METH use and improved craving management [98].
4.1.5. reSET Mobile Application
The FDA-approved reSET mobile application, produced by Pear Therapeutics, contains a patient application and clinician dashboard and is designed to deliver CBT to people with MUD and other substance use disorders (with the exception of opioid use disorder). It teaches its users skills that help to achieve abstinence from substance abuse and to increase retention to outpatient programs. This application is aimed to be used in conjunction with outpatient therapy and was shown to be effective in reducing METH use and craving during treatment [74].
4.1.6. Repetitive Transcranial Magnetic Stimulation (rTMS) and Transcranial Direct Current Stimulations (tDCS)
The rTMS is a non-invasive FDA-approved medical procedure for the treatment of depression in adults. This technique relies on the generation of brief magnetic fields using an insulated coil that is placed over the scalp. The tDCS uses a homogenous direct current field delivered at intensities of around 1 mA via two electrodes placed on the scalp. The rTMS acts as a neuro-stimulator and tDCS as a neuro-modulator. The development and maintenance of drug addiction is associated with decreased activity of prefrontal regions, especially the dorsolateral prefrontal cortex (DLPFC) that regulates higher cognitive functions, such as switching attention, working memory, maintaining abstract rules, and inhibiting inappropriate responses (impulse control) [107]. For that reason, the target area for stimulation in rTMS and tDCS studies has been the DLPFC. To date, five randomized clinical studies compared rTMS with sham stimulation or with treatment-as-usual or compared MUD patients with healthy controls and showed a significant reduction in METH craving, executive functions, withdrawal symptoms and/or mood status [108-112]. The rTMS effects could last for 1 month post-intervention [110]. In four of these studies, male or female MUD patients were subjected to 5-20 tDCS sessions of high-frequency (10 Hz) repetitive rTMS over the left DLPFC (except [111] where low-frequency rTMS was used). Zhao and colleagues compared intermittent with continuous burst stimulation (50 Hz) over the left or right DLPFC and reported that decreased craving in male MUD patients was driven by intermittent stimulation of the left DLPFC and continuous stimulation of the right DLPFC, but not by continuous stimulation of the left DLPFC [112].
Similar to rTMS, tDCS significantly reduced METH craving and increased executive functions compared to controls [113-115], lasting throughout the treatment and at least up to 1-month post-treatment [114], despite differences in experimental conditions. The differences included the number of sessions and subjects, sex of the subjects, stimulated side of the DLPFC (right anodal/left cathodal or vice versa), strength of the current, and type of controls (sham stimulation, treatment-as-usual or computerized cognitive addiction therapy).
4.1.7. Emerging Non-pharmacological Treatments for Methamphetamine Use Disorder
Continued efforts to find MUD therapy have produced several interesting findings. Recently, exercise and music therapy were shown to help maintain METH cravings [74, 116].
4.1.8. Summary
The primary interventions with evidence of efficacy in reducing METH use are behavioral therapies. The CM method has been most widely studied in subjects with MUD and overall demonstrated better outcomes than other behavioral therapies. Despite its effectiveness as a therapy for MUD, CM is not widely used, stemming in part from a policy limiting the monetary value of incentives allowable as part of treatment. Utilization of other behavioral treatments is also limited because they require substantial investments in care delivery systems. In addition to not being widely available [117], a limitation of behavioral therapies is the finding that they have moderate and variable efficacy in terms of abstinence and retention and still result in limited long-term recovery and subsequently relapse. This data underscores the need for additional efficacious therapies, such as pharmacotherapies, to help relieve withdrawal symptoms and support motivation for METH-dependent individuals to stay abstinent. Scientific evidence supports CM as the choice of non-pharmacological treatment followed by CBT and then rTMS/tDCS.
4.2. Pharmacological Treatments for Methamphetamine Use Disorder
4.2.1. Ineffective Pharmacotherapies for Methamphetamine Use Disorder
A few recent reviews of medications tested for MUD between 2000 and 2020 have provided exhaustive information on different classes of medications that have been examined and the results of their clinical trials [117-120]. Pharmacotherapies evaluated for MUD aimed to decrease the reinforcing/rewarding effects of METH, decrease cravings and negative effects of withdrawal from METH, or ameliorate comorbid psychiatric conditions and METH abuse-related cognitive impairments. No medication provided sufficient evidence to promote its use in the routine clinical management of MUD. Table 1 provides an alphabetical list of medications tested in randomized placebo-controlled clinical trials for MUD based on the reviews and ClinicalTrials.gov website.
Table 1.
Alphabetical list of medications tested in randomized placebo-controlled clinical trials for methamphetamine-use disorder (reviewed in [117-120] and ClinicalTrials.gov).
| Drug Name | Drug Actions |
|---|---|
| aripiprazole | partial dopamine D2 receptor agonist |
| baclofen | GABAB receptors agonist, muscle relaxant |
| bupropion | dopamine and norepinephrine transporter blocker |
| buspirone | 5-HT1 receptor agonist |
| citicoline | increases norepinephrine and dopamine levels, increases brain metabolism |
| creatine | participates in regenerating ATP |
| dextro-amphetamine | sympathomimetic; increases dopamine, serotonin and norepinephrine levels |
| dextro-methamphetamine | sympathomimetic; increases dopamine, serotonin and norepinephrine levels |
| gabapentin | exerts GABA-like action via Ca2+ channels |
| ibudilast | PDE4 inhibitor and TLR4 antagonist, anti-inflammatory drug |
| methylphenidate | dopamine and norepinephrine transporter blocker |
| mirtazapine | blocker of adrenergic α2 and serotonergic 5-HT2 and 5-HT3 receptors, antidepressant |
| modafinil | weak dopamine reuptake inhibitor and releaser of orexin neuropeptides and histamine, heightening arousal |
| N-acetyl cysteine | increases glutamate levels |
| N-acetyl cysteine/naltrexone | increases glutamate levels, anti-oxidant/opioid receptor blocker |
| naltrexone | blocker of opioid receptors |
| naltrexone + bupropion | opioid receptor blocker/dopamine and noradrenaline transporter blocker |
| ondansetron | 5-HT3 receptor antagonist |
| perindopril | angiotensin receptor blocker |
| pexacerfont | corticotropin-releasing factor 1 antagonist, anti-anxiety drug and antidepressant |
| prazosin | inverse agonist at α1 adrenergic receptor |
| PROMETA | flumazenil/gabapentin/hydroxyzine, targeting histamine, acetylcholine and GABA function |
| sertraline | serotonin reuptake inhibitor |
| risperidone | acts on dopamine, serotonin and noradrenaline receptors |
| risperidone/aripiprazole | act on dopamine, serotonin and noradrenaline receptors |
| risperidone/paliperidone | act on dopamine, serotonin and noradrenaline receptors |
| rivastigmine | cholinesterase inhibitor (increases acetylcholine levels) |
| topiramate | GABAA receptor agonist, AMPA receptor antagonist |
| varenicline | α7 receptor agonist, partial agonist for other nicotinic receptors |
| vigabatrin | GABAA transferase inhibitor (increases GABA levels) |
| vortioxetine (with MBRP) | serotonin transporter blocker, 5-HT3 and 5-HT7 receptor antagonist, 5-HT1B partial agonist, and 5-HT1A agonist |
Abbreviations: GABA, γ-aminobutyric acid; 5-HT, serotonin; MBRP, mindfulness-based relapse prevention; PDE4, phosphodiesterase 4; TLR4, toll-like receptor 4.
The dopaminergic system has been a favored target for MUD pharmacotherapy, and several medications that target dopamine transporter or dopamine D2 receptor have been tested in clinical trials. Agonists for dopamine receptors mimic the action of monoamines to provide modest levels of METH reward/reinforcement. One of the reasons for the lack of efficacy of dopaminergic medications in MUD is a decrease in dopamine D2 receptor levels in the striatal sub-regions in people chronically abusing METH [69-73].
Agonists for receptors for other monoamines (noradrenaline, serotonin) have been tested in clinical trials as potential medications for MUD because they increase dopamine release in the nucleus accumbens and because they decrease negative affective symptoms. Monoamine transporter ligands have been tested for their inhibition of monoamine uptake. Both types of medications, agonists and uptake blockers, increase monoaminergic neurotransmission. Some medications tested in clinical trials had dual actions, e.g., as monoamine stimulators and antidepressants. They included medications targeting opioid, γ-aminobutyric acid (GABA) or the cholinergic system. As aforementioned, the glutamatergic system plays a key role in MUD; consequently, several glutamatergic ligands have been evaluated for their efficacy to treat MUD as well.
To our knowledge, only one clinical trial has addressed the treatment of MUD with comorbid opioid use disorder (OUD) [121].
4.2.2. Emerging Pharmacotherapy for Methamphetamine Use Disorder
A recent study by Trivedi and colleagues found that a combination of oral bupropion and injectable naltrexone was effective in treating adults with moderate or severe MUD in a double-blind, placebo-controlled Phase III clinical trial [35]. The combination was safe and successfully reduced METH use and cravings in a large sample of people with MUD, as compared to placebo. Bupropion is a commonly prescribed medication for depression and nicotine cessation, whereas naltrexone is an opioid antagonist widely prescribed for treating opioid and alcohol use disorders. The findings suggest that this combination therapy may treat METH dependence in people who heavily abuse the drug, particularly if employed in combination with current behavioral treatments, such as CM or CBT. Regarding the mechanism of action in the combination of these two medications, bupropion may alleviate dysphoria associated with METH withdrawal by acting on the dopamine and norepinephrine systems. Alleviating dysphoria may, in turn, reduce cravings and help prevent METH relapse. Naltrexone may reduce the rewarding effects and cravings associated with taking METH via blocking opioid receptors [122, 123]. Interestingly, bupropion or naltrexone administered alone showed limited, inconsistent efficacy combating MUD in previous clinical trials.
4.2.3. Summary
Despite multiple research studies and clinical trials performed, there is no FDA-approved pharmacotherapy for MUD. Clinical trials testing other potential medications for MUD have largely been negative or did not show clear effectiveness (modest at best) or a clear profile of being safe. The medications that have shown the most promise as pharmacotherapies for MUD are bupropion and naltrexone administered together.
4.3. Potential Future Treatments for Methamphetamine Use Disorder
4.3.1. Methamphetamine Immunotherapy
Apart from medications, another novel approach being tested for MUD treatment is the administration of METH antibodies (passive immunotherapy) or compounds that turn the body’s own immune system against METH (active immunotherapy). Passive METH immunotherapy involves vaccination with a pre-produced high affinity monoclonal antibody designed to bind to METH in a bloodstream following METH administration. Active METH immunotherapy involves vaccination with an immunogenic METH-containing conjugate which is able to stimulating specific antibodies capable of sequestering METH in the periphery [124]. Reduction of METH entering the brain diminishes its reinforcing effects, thus reducing METH use and relapse [125].
METH and other drugs of abuse themselves are far too small to be immunogenic; therefore, the first step in active METH immunotherapies is creating a hapten molecule, a chemical derivative of METH, and linking it to immunogenic carrier protein [124]. Subsequently, the conjugate is purified to remove free haptens and mixed with appropriate adjuvants, which help boost the innate immune response. Production of monoclonal METH antibodies involves immunization of mice with immunogenic METH hepten-protein carrier complex, isolation of polyclonal METH antibodies, and complex genetic engineering processes [126]. In both approaches, METH entry into the brain is reduced because immunoglobulins are too large to cross the blood-brain barrier. To date, one monoclonal METH antibody (ch-mAb7F9) capable of effectively holding METH in the bloodstream and disabling its entry into the brain has been produced and tested its safety and tolerability [127, 128]. The antibody is currently tested in Phase 2 trials [129] (ClinicalTrials.gov). No active METH vaccine has reached clinical trials (ClinicalTrials.gov) despite promising early results in preclinical stages [124].
A major challenge in approving active vaccines for medical use is the fact that only a portion of subjects produce high enough titers for the vaccine to be effective. Passive immunotherapy provides better control over antibody dose and, consequently, serum antibody concentration. However, monoclonal antibodies are hampered by a shorter half-life and a higher cost of production than vaccines. Immunotherapy, both passive and active in the forms of monoclonal antibodies and conjugate vaccines, is a promising pharmacologic strategy for combating MUD
4.3.2. Currently Tested Medications for Methamphetamine Use Disorder
Several new medications that bear promise as treatments for MUD are currently in different stages of clinical trials or open. Among those are oxytocin, doxazocin, lobeline, disulfiram, acamprosate, atomoxetine, and entacapone (www.ClinicalTrials.gov).
SUMMARY AND CONCLUSION
Various non-pharmacological approaches have effectively reduced METH use in study participants, with CM producing the strongest effect. For now, psychotherapy is considered the first line of treatment for MUD even though it cannot serve as a standalone treatment of MUD due to poor compliance and high relapse rates once participants are out of a program. Apart from psychotherapy, other non-pharmacological interventions such as rTMS/tDCS or immunotherapies targeting MUD have been studied over recent years and show promise. Active immunotherapy provides specificity, safety, and long-lasting effects whereas passive immunotherapy allows high serum antibody concentration and is suitable for occasions when an immediate effect is required (e.g. METH overdose). Combination of passive and active immunotherapy could produce a better effect than each one alone. MUD immunotherapies would have fewer off-target effects that are prevalent in pharmacotherapies because the blood-brain barrier is not permeable to immunoglobulins. Furthermore, vaccines may increase patient compliance since treatment would consist of a few injections over a long period of time. Combination of psychotherapy with active immunotherapy is likely to decrease METH intake as well as relapse once anti-METH vaccine is developed.
Despite numerous clinical trials conducted to date, there is no consistently effective FDA-approved pharmacotherapy for MUD. Clinical trials testing potential medications for MUD have largely been negative, or did not show clear effectiveness or profile of safety. The medications tested in clinical trials have shown low efficacy in people who use METH moderately and no effect in those who use METH heavily [130-132], with the exception of a very recent study of Trivedi and colleagues who showed the effectiveness of bupropion/naltrexone combination in treating adults with moderate or severe MUD [35]. This is likely because heavy METH use is more damaging to the brain and body than light use of the drug and, therefore, light and heavy METH users respond differently to pharmacotherapies. New therapeutic approaches are needed, particularly for people who use METH heavily, as they suffer the most from METH abuse-related neuropsychological problems [29-31], are less likely to seek treatment than those using the drug moderately [133], and are at high risk of dying from METH overdose.
The reasons for lack of efficacy of clinically tested medications include heterogeneity of METH-abusing population and comorbidity between METH and other psychiatric disorders [19]. Future efforts to combat MUD should include more research on specific sub-populations within people who suffer from MUD, that is, research that would consider gender, race, demographics, existing diseases/disorders, pattern of METH use, and other factors that contribute to the heterogeneity of the MUD-affected population. Furthermore, more clinical research is needed on the co-use of METH and opioids as well as how their combination affects overdose risk. Moreover, to increase the efficacy of pharmaceuticals, particularly dopaminergic, future studies should develop a way to increase the levels of dopamine D2 receptor, which are decreased in individuals chronically abusing METH [69-73]. METH relapse remains the biggest problem in MUD and warrants more pre-clinical and clinical studies.
Early intervention in METH abuse by lowering METH intake is essential not only for preventing METH overdose, but also for subsequent interventions, as greater treatment participation is achieved when METH use is low [133]. Consequently, behavioral therapies should remain the first line of treatment for MUD. In the future, a combination of cognitive therapy(ies) with medication(s), followed by an anti-METH vaccine to maintain low METH intake long-term, will likely work the best against MUD.
ACKNOWLEDGEMENTS
Declared none.
AUTHORS’ CONTRIBUTION
AM conceptualized the idea, searched literature and databases, prepared the manuscript for publication, and affirmed the manuscript in its current form.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Gouzoulis-Mayfrank E., Härtel-Petri R., Hamdorf W., Havemann-Reinecke U., Mühlig S., Wodarz N. Methamphetamine-related disorders. Dtsch. Arztebl. Int. 2017;114(26):455–461. doi: 10.3238/arztebl.2017.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen N. Amphetamine-type stimulants: the early history of their medical and non-medical uses. Int. Rev. Neurobiol. 2015;120:9–25. doi: 10.1016/bs.irn.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen N. Making the first anti-depressant: Amphetamine in American medicine, 1929-1950. J. Hist. Med. Allied Sci. 2006;61(3):288–323. doi: 10.1093/jhmas/jrj039. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen N. Medical science and the military: the Allies’ use of amphetamine during World War II. J. Interdiscip. Hist. 2011;42(2):205–233. doi: 10.1162/JINH_a_00212. [DOI] [PubMed] [Google Scholar]
- 5.UNDOC 2020.
- 6.Hensley D., Cody J.T. Simultaneous determination of amphetamine, methamphetamine, methylenedioxyamphetamine (MDA), methylenedioxymethamphetamine (MDMA), and methylenedioxyethylamphetamine (MDEA) enantiomers by GC-MS. J. Anal. Toxicol. 1999;23(6):518–523. doi: 10.1093/jat/23.6.518. [DOI] [PubMed] [Google Scholar]
- 7.Smith M.L., Nichols D.C., Underwood P., Fuller Z., Moser M.A., Flegel R., Gorelick D.A., Newmeyer M.N., Concheiro M., Huestis M.A. Methamphetamine and amphetamine isomer concentrations in human urine following controlled Vicks VapoInhaler administration. J. Anal. Toxicol. 2014;38(8):524–527. doi: 10.1093/jat/bku077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SAMHSA 2020.
- 9.Twillman R.K., Dawson E., LaRue L., Guevara M.G., Whitley P., Huskey A. Evaluation of trends of near-real-time urine drug test results for methamphetamine, cocaine, heroin, and fentanyl. JAMA Netw. Open. 2020;3(1):e1918514. doi: 10.1001/jamanetworkopen.2019.18514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wainwright J.J., Mikre M., Whitley P., Dawson E., Huskey A., Lukowiak A., Giroir B.P. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 Outbreak. JAMA. 2020;324(16):1674–1677. doi: 10.1001/jama.2020.17694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC Overdose Deaths Accelerating During COVID-19. 2020.
- 12.Jalal H., Buchanich J.M., Roberts M.S., Balmert L.C., Zhang K., Burke D.S. Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science. 2018;361(6408):eaau1184. doi: 10.1126/science.aau1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NIDA Overdose Death Rates. 2018.
- 14.Volkow N. 2020. Rising stimulant deaths show that we face more than just an opioid crisis. [Google Scholar]
- 15.Han B., Cotto J., Etz K., Einstein E.B., Compton W.M., Volkow N.D. Methamphetamine Overdose Deaths in the US by Sex and Race and Ethnicity. JAMA Psychiatry. 2021;78(5):564–567. doi: 10.1001/jamapsychiatry.2020.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis M.S., Kasper Z.A., Cicero T.J. Twin epidemics: The surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend. 2018;193:14–20. doi: 10.1016/j.drugalcdep.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Hedegaard H., Bastian B.A., Trinidad J.P., Spencer M.R., Warner M. Regional Differences in the Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2017. Natl. Vital Stat. Rep. 2019;68(12):1–16. [PubMed] [Google Scholar]
- 18.SAMHSA DAWN and Methamphetamine. 2020.
- 19.Moszczynska A., Callan S.P. Molecular, Behavioral, and Physiological Consequences of Methamphetamine Neurotoxicity: Implications for Treatment. J. Pharmacol. Exp. Ther. 2017;362(3):474–488. doi: 10.1124/jpet.116.238501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vu N.T., Maher L., Zablotska I. Amphetamine-type stimulants and HIV infection among men who have sex with men: implications on HIV research and prevention from a systematic review and meta-analysis. J. Int. AIDS Soc. 2015;18:19273. doi: 10.7448/IAS.18.1.19273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UNDOC 2019.
- 22.Yang X., Wang Y., Li Q., Zhong Y., Chen L., Du Y., He J., Liao L., Xiong K., Yi C.X., Yan J. The main molecular mechanisms underlying methamphetamine- induced neurotoxicity and implications for pharmacological treatment. Front. Mol. Neurosci. 2018;11:186. doi: 10.3389/fnmol.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kesby J.P., Chang A., Markou A., Semenova S. Modeling human methamphetamine use patterns in mice: chronic and binge methamphetamine exposure, reward function and neurochemistry. Addict. Biol. 2018;23(1):206–218. doi: 10.1111/adb.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han E., Yang H., Seol I., Park Y., Lee B., Song J.M. Segmental hair analysis and estimation of methamphetamine use pattern. Int. J. Legal Med. 2013;127(2):405–411. doi: 10.1007/s00414-012-0766-7. [DOI] [PubMed] [Google Scholar]
- 25.Mirecki A., Fitzmaurice P., Ang L., Kalasinsky K.S., Peretti F.J., Aiken S.S., Wickham D.J., Sherwin A., Nobrega J.N., Forman H.J., Kish S.J. Brain antioxidant systems in human methamphetamine users. J. Neurochem. 2004;89(6):1396–1408. doi: 10.1111/j.1471-4159.2004.02434.x. [DOI] [PubMed] [Google Scholar]
- 26.Cho A.K., Melega W.P. Patterns of methamphetamine abuse and their consequences. J. Addict. Dis. 2002;21(1):21–34. doi: 10.1300/J069v21n01_03. [DOI] [PubMed] [Google Scholar]
- 27.Cheng W.S., Garfein R.S., Semple S.J., Strathdee S.A., Zians J.K., Patterson T.L. Binge use and sex and drug use behaviors among HIV(-), heterosexual methamphetamine users in San Diego. Subst. Use Misuse. 2010;45(1-2):116–133. doi: 10.3109/10826080902869620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semple S.J., Patterson T.L., Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. J. Subst. Abuse Treat. 2002;22(3):149–156. doi: 10.1016/S0740-5472(02)00223-4. [DOI] [PubMed] [Google Scholar]
- 29.Logan B.K. Methamphetamine - effects on human performance and behavior. Forensic Sci. Rev. 2002;14(1-2):133–151. [PubMed] [Google Scholar]
- 30.Stock A.K., Rädle M., Beste C. Methamphetamine-associated difficulties in cognitive control allocation may normalize after prolonged abstinence. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;88:41–52. doi: 10.1016/j.pnpbp.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Rusyniak D.E. Neurologic manifestations of chronic methamphetamine abuse. Neurol. Clin. 2011;29(3):641–655. doi: 10.1016/j.ncl.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callaghan R.C., Cunningham J.K., Sykes J., Kish S.J. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012;120(1-3):35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Weintraub D., Koester J., Potenza M.N., Siderowf A.D., Stacy M., Voon V., Whetteckey J., Wunderlich G.R., Lang A.E. Impulse control disorders in Parkinson disease: A cross-sectional study of 3090 patients. Arch. Neurol. 2010;67(5):589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 34.Kawa A.B., Allain F., Robinson T.E., Samaha A.N. The transition to cocaine addiction: the importance of pharmacokinetics for preclinical models. Psychopharmacology (Berl.) 2019;236(4):1145–1157. doi: 10.1007/s00213-019-5164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trivedi M.H., Walker R., Ling W., Dela Cruz A., Sharma G., Carmody T., Ghitza U.E., Wahle A., Kim M., Shores-Wilson K., Sparenborg S., Coffin P., Schmitz J., Wiest K., Bart G., Sonne S.C., Wakhlu S., Rush A.J., Nunes E.V., Shoptaw S. Bupropion and naltrexone in methamphetamine use disorder. N. Engl. J. Med. 2021;384(2):140–153. doi: 10.1056/NEJMoa2020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brecht M.L., Herbeck D. Time to relapse following treatment for methamphetamine use: A long-term perspective on patterns and predictors. Drug Alcohol Depend. 2014;139:18–25. doi: 10.1016/j.drugalcdep.2014.02.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voorn P., Vanderschuren L.J., Groenewegen H.J., Robbins T.W., Pennartz C.M. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27(8):468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Everitt B.J., Robbins T.W. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. 2013;37(9 Pt A):1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Volkow N.D., Koob G.F., McLellan A.T. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scofield M.D., Heinsbroek J.A., Gipson C.D., Kupchik Y.M., Spencer S., Smith A.C., Roberts-Wolfe D., Kalivas P.W. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol. Rev. 2016;68(3):816–871. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalasinsky K.S., Bosy T.Z., Schmunk G.A., Reiber G., Anthony R.M., Furukawa Y., Guttman M., Kish S.J. Regional distribution of methamphetamine in autopsied brain of chronic human methamphetamine users. Forensic Sci. Int. 2001;116(2-3):163–169. doi: 10.1016/S0379-0738(00)00368-6. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki O., Hattori H., Asano M., Oya M., Katsumata Y. Inhibition of monoamine oxidase by d-methamphetamine. Biochem. Pharmacol. 1980;29(14):2071–2073. doi: 10.1016/0006-2952(80)90493-1. [DOI] [PubMed] [Google Scholar]
- 44.Egashira T., Yamanaka Y. Changes in monoamine oxidase activity in mouse brain associated with d-methamphetamine dependence and withdrawal. Biochem. Pharmacol. 1993;46(4):609–614. doi: 10.1016/0006-2952(93)90545-8. [DOI] [PubMed] [Google Scholar]
- 45.Santillo M.F. Inhibition of monoamine oxidase (MAO) by α-ethylphenethylamine and N,α-diethylphenethylamine, two compounds related to dietary supplements. Food Chem. Toxicol. 2014;74:265–269. doi: 10.1016/j.fct.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto B.K., Moszczynska A., Gudelsky G.A. Amphetamine toxicities: classical and emerging mechanisms. Ann. N. Y. Acad. Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volz T.J., Hanson G.R., Fleckenstein A.E. The role of the plasmalemmal dopamine and vesicular monoamine transporters in methamphetamine-induced dopaminergic deficits. J. Neurochem. 2007;101(4):883–888. doi: 10.1111/j.1471-4159.2006.04419.x. [DOI] [PubMed] [Google Scholar]
- 48.Fleckenstein A.E., Metzger R.R., Wilkins D.G., Gibb J.W., Hanson G.R. Rapid and reversible effects of methamphetamine on dopamine transporters. J. Pharmacol. Exp. Ther. 1997;282(2):834–838. [PubMed] [Google Scholar]
- 49.Zaczek R., Culp S., De Souza E.B. Interactions of [3H]amphetamine with rat brain synaptosomes. II. Active transport. J. Pharmacol. Exp. Ther. 1991;257(2):830–835. [PubMed] [Google Scholar]
- 50.Sulzer D., Chen T.K., Lau Y.Y., Kristensen H., Rayport S., Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J. Neurosci. 1995;15(5 Pt 2):4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sulzer D., Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: A mechanism of action. Neuron. 1990;5(6):797–808. doi: 10.1016/0896-6273(90)90339-H. [DOI] [PubMed] [Google Scholar]
- 52.Brown J.M., Hanson G.R., Fleckenstein A.E. Methamphetamine rapidly decreases vesicular dopamine uptake. J. Neurochem. 2000;74(5):2221–2223. doi: 10.1046/j.1471-4159.2000.0742221.x. [DOI] [PubMed] [Google Scholar]
- 53.Fleckenstein A.E., Gibb J.W., Hanson G.R. Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur. J. Pharmacol. 2000;406(1):1–13. doi: 10.1016/S0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- 54.Mark K.A., Soghomonian J.J., Yamamoto B.K. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J. Neurosci. 2004;24(50):11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sofuoglu M., Sewell R.A. Norepinephrine and stimulant addiction. Addict. Biol. 2009;14(2):119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howell L.L., Cunningham K.A. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol. Rev. 2015;67(1):176–197. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lucki I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry. 1998;44(3):151–162. doi: 10.1016/S0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 58.Kirby L.G., Zeeb F.D., Winstanley C.A. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61(3):421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinshenker D., Schroeder J.P. There and back again: A tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32(7):1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- 60.Koob G.F., Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363(1507):3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brennan K.A., Carati C., Lea R.A., Fitzmaurice P.S., Schenk S. Effect of D1-like and D2-like receptor antagonists on methamphetamine and 3,4-methylenedioxymethamphetamine self-administra- tion in rats. Behav. Pharmacol. 2009;20(8):688–694. doi: 10.1097/FBP.0b013e328333a28d. [DOI] [PubMed] [Google Scholar]
- 62.Gu S.M., Cha H.J., Seo S.W., Hong J.T., Yun J. Dopamine D1 receptor antagonist reduces stimulant-induced conditioned place preferences and dopamine receptor supersensitivity. Naunyn Schmiedebergs Arch. Pharmacol. 2020;393(1):131–138. doi: 10.1007/s00210-019-01694-3. [DOI] [PubMed] [Google Scholar]
- 63.Carati C., Schenk S. Role of dopamine D1- and D2-like receptor mechanisms in drug-seeking following methamphetamine self-administration in rats. Pharmacol. Biochem. Behav. 2011;98(3):449–454. doi: 10.1016/j.pbb.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Mizoguchi H., Yamada K., Mizuno M., Mizuno T., Nitta A., Noda Y., Nabeshima T. Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol. Pharmacol. 2004;65(5):1293–1301. doi: 10.1124/mol.65.5.1293. [DOI] [PubMed] [Google Scholar]
- 65.Wee S., Wang Z., Woolverton W.L., Pulvirenti L., Koob G.F. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007;32(10):2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu X., Pan J., Li X., Cui Y., Mao Z., Wu B., Xu H., Zhou W., Liu Y. Inhibition of methamphetamine self-administration and reinstatement by central blockade of angiotensin ii receptor in rats. J. Pharmacol. Exp. Ther. 2019;369(2):244–258. doi: 10.1124/jpet.118.255729. [DOI] [PubMed] [Google Scholar]
- 67.Caprioli D., Venniro M., Zhang M., Bossert J.M., Warren B.L., Hope B.T., Shaham Y. Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J. Neurosci. 2017;37(4):1014–1027. doi: 10.1523/JNEUROSCI.3091-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moeller S.J., Okita K., Robertson C.L., Ballard M.E., Konova A.B., Goldstein R.Z., Mandelkern M.A., London E.D. Low Striatal Dopamine D2-type receptor availability is linked to simulated drug choice in methamphetamine users. Neuropsychopharmacology. 2018;43(4):751–760. doi: 10.1038/npp.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.London E.D. Impulsivity, stimulant abuse, and dopamine receptor signaling. Adv. Pharmacol. 2016;76:67–84. doi: 10.1016/bs.apha.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Martinez D., Slifstein M., Broft A., Mawlawi O., Hwang D.R., Huang Y., Cooper T., Kegeles L., Zarahn E., Abi-Dargham A., Haber S.N., Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: Amphetamine-induced dopamine release in the functional subdivisions of the striatum. J. Cereb. Blood Flow Metab. 2003;23(3):285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 71.Volkow N.D., Chang L., Wang G.J., Fowler J.S., Ding Y.S., Sedler M., Logan J., Franceschi D., Gatley J., Hitzemann R., Gifford A., Wong C., Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. Am. J. Psychiatry. 2001;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 72.Wang G.J., Smith L., Volkow N.D., Telang F., Logan J., Tomasi D., Wong C.T., Hoffman W., Jayne M., Alia-Klein N., Thanos P., Fowler J.S. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol. Psychiatry. 2012;17(9):918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boileau I., Payer D., Houle S., Behzadi A., Rusjan P.M., Tong J., Wilkins D., Selby P., George T.P., Zack M., Furukawa Y., McCluskey T., Wilson A.A., Kish S.J. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: A positron emission tomography study. J. Neurosci. 2012;32(4):1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.AshaRani P.V.; Hombali, A.; Seow, E.; Ong, W.J.; Tan, J.H.; Subramaniam, M. Non-pharmacological interventions for methamphetamine use disorder: A systematic review. Drug Alcohol Depend. 2020;212:108060. doi: 10.1016/j.drugalcdep.2020.108060. [DOI] [PubMed] [Google Scholar]
- 75.Khoramizadeh M., Effatpanah M., Mostaghimi A., Rezaei M., Mahjoub A., Shishehgar S. Treatment of amphetamine abuse/use disorder: A systematic review of a recent health concern. Daru. 2019;27(2):743–753. doi: 10.1007/s40199-019-00282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown H.D., DeFulio A. Contingency management for the treatment of methamphetamine use disorder: A systematic review. Drug Alcohol Depend. 2020;216:108307. doi: 10.1016/j.drugalcdep.2020.108307. [DOI] [PubMed] [Google Scholar]
- 77.Minozzi S., Saulle R., De Crescenzo F., Amato L. Psychosocial interventions for psychostimulant misuse. Cochrane Database Syst. Rev. 2016;9:CD011866. doi: 10.1002/14651858.CD011866.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okafor C.N., Stein D.J., Dannatt L., Ipser J., van Nunen L.J., Lake M.T., Krishnamurti T., London E.D., Shoptaw S. Contingency management treatment for methamphetamine use disorder in South Africa. Drug Alcohol Rev. 2020;39(3):216–222. doi: 10.1111/dar.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shoptaw S., Klausner J.D., Reback C.J., Tierney S., Stansell J., Hare C.B., Gibson S., Siever M., King W.D., Kao U., Dang J. A public health response to the methamphetamine epidemic: the implementation of contingency management to treat methamphetamine dependence. BMC Public Health. 2006;6:214. doi: 10.1186/1471-2458-6-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gόmez W., Olem D., Andrews R., Discepola M.V., Ambrose P., Dilworth S.E., Carrico A.W. Optimizing contingency management with methamphetamine-using men who have sex with men. Cognit. Behav. Pract. 2018;25(2):286–295. doi: 10.1016/j.cbpra.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Menza T.W., Jameson D.R., Hughes J.P., Colfax G.N., Shoptaw S., Golden M.R. Contingency management to reduce methamphetamine use and sexual risk among men who have sex with men: A randomized controlled trial. BMC Public Health. 2010;10:774. doi: 10.1186/1471-2458-10-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rawson R.A., McCann M.J., Flammino F., Shoptaw S., Miotto K., Reiber C., Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 83.Shoptaw S., Reback C.J., Peck J.A., Yang X., Rotheram-Fuller E., Larkins S., Veniegas R.C., Freese T.E., Hucks-Ortiz C. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug Alcohol Depend. 2005;78(2):125–134. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 84.Nyamathi A., Reback C.J., Shoptaw S., Salem B.E., Zhang S., Yadav K. Impact of Tailored interventions to reduce drug use and sexual risk behaviors among homeless gay and bisexual men. Am. J. Men Health. 2017;11(2):208–220. doi: 10.1177/1557988315590837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shoptaw S., Huber A., Peck J., Yang X., Liu J. Jeff Dang; Roll, J.; Shapiro, B.; Rotheram-Fuller, E.; Ling, W. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85(1):12–18. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 86.Corsi K.F., Shoptaw S., Alishahi M., Booth R.E. Interventions to reduce drug use among methamphetamine users at risk for hiv. Curr. HIV/AIDS Rep. 2019;16(1):29–36. doi: 10.1007/s11904-019-00423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carrico A.W., Gόmez W., Jain J., Shoptaw S., Discepola M.V., Olem D., Lagana-Jackson J., Andrews R., Neilands T.B., Dilworth S.E., Evans J.L., Woods W.J., Moskowitz J.T. Randomized controlled trial of a positive affect intervention for methamphetamine users. Drug Alcohol Depend. 2018;192:8–15. doi: 10.1016/j.drugalcdep.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reback C.J., Peck J.A., Dierst-Davies R., Nuno M., Kamien J.B., Amass L. Contingency management among homeless, out-of-treatment men who have sex with men. J. Subst. Abuse Treat. 2010;39(3):255–263. doi: 10.1016/j.jsat.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reback C.J., Peck J.A., Fletcher J.B., Nuno M., Dierst-Davies R. Lifetime substance use and HIV sexual risk behaviors predict treatment response to contingency management among homeless, substance-dependent MSM. J. Psychoactive Drugs. 2012;44(2):166–172. doi: 10.1080/02791072.2012.684633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McHugh R.K., Hearon B.A., Otto M.W. Cognitive behavioral therapy for substance use disorders. Psychiatr. Clin. North Am. 2010;33(3):511–525. doi: 10.1016/j.psc.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yen C.F., Wu H.Y., Yen J.Y., Ko C.H. Effects of brief cognitive-behavioral interventions on confidence to resist the urges to use heroin and methamphetamine in relapse-related situations. J. Nerv. Ment. Dis. 2004;192(11):788–791. doi: 10.1097/01.nmd.0000144699.80765.7f. [DOI] [PubMed] [Google Scholar]
- 92.Harada T., Tsutomi H., Mori R., Wilson D.B. Cognitive-behavioural treatment for amphetamine-type stimulants (ATS)-use disorders. Cochrane Database Syst. Rev. 2018;12:CD011315. doi: 10.1002/14651858.CD011315.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Witkiewitz K., Greenfield B.L., Bowen S. Mindfulness-based relapse prevention with racial and ethnic minority women. Addict. Behav. 2013;38(12):2821–2824. doi: 10.1016/j.addbeh.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suvanchot K.S., Somrongthong R., Phukhao D. Efficacy of group motivational interviewing plus brief cognitive behavior therapy for relapse in amphetamine users with co-occurring psychological problems at Southern Psychiatric Hospital in Thailand. J. Med. Assoc. Thai. 2012;95(8):1075–1080. [PubMed] [Google Scholar]
- 95.Abdoli N., Farnia V., Salemi S., Tatari F., Juibari T.A., Alikhani M., Basanj B. Efficacy of the Marlatt cognitive-behavioral model on decreasing relapse and craving in women with methamphetamine dependence: A clinical trial. J. Subst. Use. 2019;24:229–232. doi: 10.1080/14659891.2018.1549279. [DOI] [Google Scholar]
- 96.Jaffe A., Shoptaw S., Stein J., Reback C.J., Rotheram-Fuller E. Depression ratings, reported sexual risk behaviors, and methamphetamine use: latent growth curve models of positive change among gay and bisexual men in an outpatient treatment program. Exp. Clin. Psychopharmacol. 2007;15(3):301–307. doi: 10.1037/1064-1297.15.3.301. [DOI] [PubMed] [Google Scholar]
- 97.Obert J.L., McCann M.J., Marinelli-Casey P., Weiner A., Minsky S., Brethen P., Rawson R. The matrix model of outpatient stimulant abuse treatment: history and description. J. Psychoactive Drugs. 2000;32(2):157–164. doi: 10.1080/02791072.2000.10400224. [DOI] [PubMed] [Google Scholar]
- 98.Amiri Z., Mirzaee B., Sabet M. Evaluating the efficacy of Regulated 12-Session Matrix Model in reducing susceptibility in methamphetamine-dependent individuals. Int. J. Med. Res. Health Sci. 2016;5:77–85. [Google Scholar]
- 99.Marinelli-Casey P., Gonzales R., Hillhouse M., Ang A., Zweben J., Cohen J., Hora P.F., Rawson R.A. Drug court treatment for methamphetamine dependence: treatment response and posttreatment outcomes. J. Subst. Abuse Treat. 2008;34(2):242–248. doi: 10.1016/j.jsat.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 100.Rawson R.A., Marinelli-Casey P., Anglin M.D., Dickow A., Frazier Y., Gallagher C., Galloway G.P., Herrell J., Huber A., McCann M.J., Obert J., Pennell S., Reiber C., Vandersloot D., Zweben J. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708–717. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 101.Rawson R.A., Gonzales R., Pearce V., Ang A., Marinelli-Casey P., Brummer J. Methamphetamine dependence and human immunodeficiency virus risk behavior. J. Subst. Abuse Treat. 2008;35(3):279–284. doi: 10.1016/j.jsat.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chappel J.N., DuPont R.L. Twelve-step and mutual-help programs for addictive disorders. Psychiatr. Clin. North Am. 1999;22(2):425–446. doi: 10.1016/S0193-953X(05)70085-X. [DOI] [PubMed] [Google Scholar]
- 103.Donovan D.M., Wells E.A. ‘Tweaking 12-Step’: the potential role of 12-Step self-help group involvement in methamphetamine recovery. Addiction. 2007;102(Suppl. 1):121–129. doi: 10.1111/j.1360-0443.2007.01773.x. [DOI] [PubMed] [Google Scholar]
- 104.Hatch-Maillette M., Wells E.A., Doyle S.R., Brigham G.S., Daley D., DiCenzo J., Donovan D., Garrett S., Horigian V.E., Jenkins L., Killeen T., Owens M., Perl H.I. Predictors of 12-Step attendance and participation for individuals with stimulant use disorders. J. Subst. Abuse Treat. 2016;68:74–82. doi: 10.1016/j.jsat.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Winhusen T., Lewis D., Adinoff B., Brigham G., Kropp F., Donovan D.M., Seamans C.L., Hodgkins C.C., Dicenzo J.C., Botero C.L., Jones D.R., Somoza E. Impulsivity is associated with treatment non-completion in cocaine- and methamphetamine-dependent patients but differs in nature as a function of stimulant-dependence diagnosis. J. Subst. Abuse Treat. 2013;44(5):541–547. doi: 10.1016/j.jsat.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galloway G.P., Marinelli-Casey P., Stalcup J., Lord R., Christian D., Cohen J., Reiber C., Vandersloot D. Treatment-as-usual in the methamphetamine treatment project. J. Psychoactive Drugs. 2000;32(2):165–175. doi: 10.1080/02791072.2000.10400225. [DOI] [PubMed] [Google Scholar]
- 107.Bechara A., Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr. Opin. Neurol. 2005;18(6):734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- 108.Su H., Zhong N., Gan H., Wang J., Han H., Chen T., Li X., Ruan X., Zhu Y., Jiang H., Zhao M. High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: A randomised clinical trial. Drug Alcohol Depend. 2017;175:84–91. doi: 10.1016/j.drugalcdep.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 109.Liang Y., Wang L., Yuan T.F. targeting withdrawal symptoms in men addicted to methamphetamine with transcranial magnetic stimulation: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1199–1201. doi: 10.1001/jamapsychiatry.2018.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu T., Li Y., Shen Y., Liu X., Yuan T.F. Gender does not matter: Add-on repetitive transcranial magnetic stimulation treatment for female methamphetamine dependents. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;92:70–75. doi: 10.1016/j.pnpbp.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 111.Yuan J., Liu W., Liang Q., Cao X., Lucas M.V., Yuan T.F. Effect of Low-Frequency repetitive transcranial magnetic stimulation on impulse inhibition in abstinent patients with methamphetamine addiction: a randomized clinical trial. JAMA Netw. Open. 2020;3(3):e200910. doi: 10.1001/jamanetworkopen.2020.0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao D., Li Y., Liu T., Voon V., Yuan T.F. Twice-daily theta burst stimulation of the dorsolateral prefrontal cortex reduces methamphetamine craving: a pilot study. Front. Neurosci. 2020;14:208. doi: 10.3389/fnins.2020.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shahbabaie A., Ebrahimpoor M., Hariri A., Nitsche M.A., Hatami J., Fatemizadeh E., Oghabian M.A., Ekhtiari H. Transcranial DC stimulation modifies functional connectivity of large-scale brain networks in abstinent methamphetamine users. Brain Behav. 2018;8(3):e00922. doi: 10.1002/brb3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alizadehgoradel J., Nejati V., Sadeghi Movahed F., Imani S., Taherifard M., Mosayebi-Samani M., Vicario C.M., Nitsche M.A., Salehinejad M.A. Repeated stimulation of the dorsolateral-prefrontal cortex improves executive dysfunctions and craving in drug addiction: A randomized, double-blind, parallel-group study. Brain Stimul. 2020;13(3):582–593. doi: 10.1016/j.brs.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 115.Xu X., Ding X., Chen L., Chen T., Su H., Li X., Ye Y., Shi W., Ji J., Zhao M., Zhong N., Jiang H. The transcranial direct current stimulation over prefrontal cortex combined with the cognitive training reduced the cue-induced craving in female individuals with methamphetamine use disorder: A randomized controlled trial. J. Psychiatr. Res. 2021;134:102–110. doi: 10.1016/j.jpsychires.2020.12.056. [DOI] [PubMed] [Google Scholar]
- 116.Wu Q., Chen T., Wang Z., Chen S., Zhang J., Bao J., Su H., Tan H., Jiang H., Du J., Zhao M. Effectiveness of music therapy on improving treatment motivation and emotion in female patients with methamphetamine use disorder: A randomized controlled trial. Subst. Abus. 2020;41(4):493–500. doi: 10.1080/08897077.2019.1675117. [DOI] [PubMed] [Google Scholar]
- 117.Ballester J., Valentine G., Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev. Clin. Pharmacol. 2017;10(3):305–314. doi: 10.1080/17512433.2017.1268916. [DOI] [PubMed] [Google Scholar]
- 118.Morley K.C., Cornish J.L., Faingold A., Wood K., Haber P.S. Pharmacotherapeutic agents in the treatment of methamphetamine dependence. Expert Opin. Investig. Drugs. 2017;26(5):563–578. doi: 10.1080/13543784.2017.1313229. [DOI] [PubMed] [Google Scholar]
- 119.Soares E., Pereira F.C. Pharmacotherapeutic strategies for methamphetamine use disorder: mind the subgroups. Expert Opin. Pharmacother. 2019;20(18):2273–2293. doi: 10.1080/14656566.2019.1681970. [DOI] [PubMed] [Google Scholar]
- 120.Paulus M.P., Stewart J.L. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: A Review. JAMA Psychiatry. 2020;77(9):959–966. doi: 10.1001/jamapsychiatry.2020.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tiihonen J., Krupitsky E., Verbitskaya E., Blokhina E., Mamontova O., Föhr J., Tuomola P., Kuoppasalmi K., Kiviniemi V., Zwartau E. Naltrexone implant for the treatment of polydrug dependence: A randomized controlled trial. Am. J. Psychiatry. 2012;169(5):531–536. doi: 10.1176/appi.ajp.2011.11071121. [DOI] [PubMed] [Google Scholar]
- 122.Weber S.C., Beck-Schimmer B., Kajdi M.E., Müller D., Tobler P.N., Quednow B.B. Dopamine D2/3- and μ-opioid receptor antagonists reduce cue-induced responding and reward impulsivity in humans. Transl. Psychiatry. 2016;6(7):e850. doi: 10.1038/tp.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jayaram-Lindström N., Guterstam J., Häggkvist J., Ericson M., Malmlöf T., Schilström B., Halldin C., Cervenka S., Saijo T., Nordström A.L., Franck J. Naltrexone modulates dopamine release following chronic, but not acute amphetamine administration: A translational study. Transl. Psychiatry. 2017;7(4):e1104. doi: 10.1038/tp.2017.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hossain M.K., Hassanzadeganroudsari M., Nurgali K., Apostolopoulos V. Vaccine development against methamphetamine drug addiction. Expert Rev. Vaccines. 2020;19(12):1105–1114. doi: 10.1080/14760584.2020.1857738. [DOI] [PubMed] [Google Scholar]
- 125.de Wit H., Bodker B., Ambre J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology (Berl.) 1992;107(2-3):352–358. doi: 10.1007/BF02245161. [DOI] [PubMed] [Google Scholar]
- 126.Gentry W.B., Rüedi-Bettschen D., Owens S.M. Anti-(+)-methamphetamine monoclonal antibody antagonists designed to prevent the progression of human diseases of addiction. Clin. Pharmacol. Ther. 2010;88(3):390–393. doi: 10.1038/clpt.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stevens M.W., Henry R.L., Owens S.M., Schutz R., Gentry W.B. First human study of a chimeric anti-methamphetamine monoclonal antibody in healthy volunteers. MAbs. 2014;6(6):1649–1656. doi: 10.4161/19420862.2014.976431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stevens M.W., Tawney R.L., West C.M., Kight A.D., Henry R.L., Owens S.M., Gentry W.B. Preclinical characterization of an anti-methamphetamine monoclonal antibody for human use. MAbs. 2014;6(2):547–555. doi: 10.4161/mabs.27620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hiller-Sturmhoefel S. 2020. [Google Scholar]
- 130.Rawson R.A. Current research on the epidemiology, medical and psychiatric effects, and treatment of methamphetamine use. Yao Wu Shi Pin Fen Xi. 2013;21(4):S77–S81. doi: 10.1016/j.jfda.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Courtney K.E., Ray L.A. Methamphetamine: An update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014;143:11–21. doi: 10.1016/j.drugalcdep.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Karila L., Weinstein A., Aubin H.J., Benyamina A., Reynaud M., Batki S.L. Pharmacological approaches to methamphetamine dependence: A focused review. Br. J. Clin. Pharmacol. 2010;69(6):578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brecht M.L., Lovinger K., Herbeck D.M., Urada D. Patterns of treatment utilization and methamphetamine use during first 10 years after methamphetamine initiation. J. Subst. Abuse Treat. 2013;44(5):548–556. doi: 10.1016/j.jsat.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]