Abstract
An inverse correlation between the incidence of cancer and neurodegenerative disease has been observed, with the prevalence of cancer peaking around 60 years of age, then slowly tapering off as neurodegenerative diseases increase in the elderly. Although the diseases rarely occur concurrently, the same genes are differentially expressed between the diseases, with four transcription factors found to be in common for their expression. In the brain, mature astrocytes are the origin of astrocytoma, which make up 58.2% of malignant brain tumors in patients 65 or older, while GFAP+ astrocyte-like neural stem cells from the subventricular zone give rise to glioblastoma and anaplastic astrocytoma, which make up 41.6%. Likewise, in neurodegenerative disease, a decrease in astrocyte density is observed in early disease states, and senescent astrocytes increase. Because astrocytes coordinate synaptic function, astrocyte dysfunction likely contributes to or causes initial synapse loss and cognitive decline seen in neurodegenerative disease. In non-disease states, astrocytes retain their ability to successfully re-enter the cell cycle through adult astrogenesis to maintain the neuroenvironment, and controlled astrocytic proliferation could be an important contributor to neurological function. Disruption to this astrogenic balance could account for the inverse correlation of cell cycle dysregulation resulting in malignant astrocytes and tumorigenesis, and astrocytic senescence and cell death without self-renewal in aging resulting in neurodegenerative disease. The current understanding of the astrocytic roles of the transcription factors that could be the cause of this imbalance will be discussed, as well as possible therapeutic approaches to modulate their expression in the astrocyte.
Keywords: Astrocyte, glioblastoma, astrocytoma, glioma, Alzheimer’s disease, neurodegenerative disease, dementia
1. INTRODUCTION
Astrocytes have a unique keystone role in the brain, from coordinating synaptic function [1-6], to forming a portion of the blood-brain barrier [7, 8], to communicating with microglia to combat injury and disease [9-11]. Additionally, astrocytes regulate extracellular ions, neurotransmitters and proteins [12-15]. Astrocytes are dynamic and participate in neuroinflammation by undergoing reactive astrocytosis/astrogliosis in the event of a localized injury, insult, or disease accompanied by morphological changes and alterations in the expression of hundreds of genes [16-18]. Likewise, in healthy human tissue, unlike neurons, astrocytes retain their ability to successfully re-enter the cell cycle to replenish the cell population and maintain the cellular environment through adult astrogenesis [19-27]. Astrocytes or glial fibrillary acidic protein (GFAP)+ astrocyte-like neural stem cells are the cell type of origin of most brain cancers [28, 29], while an additional analysis of five astrocyte subtypes demonstrated they each had a malignant counterpart in tumor formation [30]. Conversely, astrocyte senescence and atrophy have been observed early in dementia [31, 32]. Similarly, in the aging brain, astrocytes exhibit a senescence-associated secretory phenotype in a chronic inflammatory environment [31], and an inverse correlation between the incidence of cancer and neurodegenerative disease has long been observed [33]. A gene expression profile was conducted and 40 genes were found to be expressed in opposing directions between neurodegenerative disease and cancer, with four transcription factors that typically regulate cellular proliferation, apoptosis and inflammation responsible for their expression: activator protein 1 (AP-1), nuclear factor of activated T cells (NFAT), CCAAT/enhancer-binding protein beta (C/EBPβ) and E2F transcription factor 1 (E2F1) [34]. Because of astrocytes’ unique position in synthesizing many physiological functions of the central nervous system, it is useful for potential therapeutic development of neurological diseases to consider the current knowledge of the role of these transcription factors in astrocytes, in the context of the role of astrocytes in neurodegenerative disease and cancer.
2. THE ROLE OF ASTROCYTES IN DEMENTIAS AND CANCER
2.1. Neurodegenerative Diseases
As it is now clear that astrocytes are integral to synaptic communication and synaptogenesis [2, 5, 35-41] and it is known that synapse loss correlates with cognitive decline and disease progression in dementias such as Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) [42, 43], astrocyte dysfunction could be the cause of neurodegenerative disease [44-46]. Many types of dementias involve aggregation of proteins that can be removed and degraded by astrocytes in healthy tissue, which can also recruit microglia to facilitate degradation [47]. Another common hallmark of the different types of dementia in aged individuals is the narrowing of blood vessels within the brain, leading to damage to the surrounding neurons, of which astrocyte dysfunction is a driving factor [48]. Vascular dementias are more common in the elderly population, and the incidence increases significantly over the age of 85 [47].
In the aged brain, astrocytic senescence alters transcriptional activity that limits synaptic structures and activity. Aging astrocytes upregulate genes associated with inflammation and synapse elimination (C4, SPARC, MHC class I) while downregulating the cholesterol synthesis pathway, essential for presynaptic vesicle formation [49]. A 2 to 3 fold increase in glial fibrillary acidic protein (GFAP) mRNA and protein expression has been found in aging astrocytes, signaling an increase in global astrocyte reactivity rather than a localized response to injury or damage [50, 51]. Aging astrocytes also downregulate Hspa1b, a protective molecular chaperone involved in protein degradation and associated with neurodegenerative processes [49]. Taking these findings together, astrocytes become more reactive with age, causing a change in genomic expression that downregulates synaptic structures and activity and disrupts protein degradation processes in a manner similar to neurodegenerative disease.
Additionally, upon internalization of proteins that accumulate post-mortem in disease, such as α-synuclein (αS) and amyloid-β (aβ), the genetic expression profile of astrocytes changes, with neuroinflammatory genes upregulated. Subsequently, one clear increase is the initial protective response of GFAP as astrogliosis [52, 53]. Astrogliosis has been observed in response to αS in tissue culture, in transgenic mouse models overexpressing αS, and in post mortem tissue of patients diagnosed with neurodegenerative disease [54-59]. Initially believed to coincide with proliferation, this is now not always the case [60]. Although astrogliosis was originally thought to be an all-or-none phenomenon in injury and disease, it is now known that astrocytes respond to neurodegeneration by upregulating growth factors, cytokines, chemokines and anti-oxidant enzymes [9, 17] and that mutant GFAP in Alexander disease dysregulates autophagy [61]. It appears that the role of astrogliosis is likely neuroprotective to initially degenerating nervous tissue, and occurs along a continuum of injury or disease severity [16, 62].
Astrocyte atrophy, signified by a reduction in the number of primary processes, size of the cell body, and an overall decrease in astrocytic-marker positive labeling, has been demonstrated to occur in the hippocampus, prefrontal and entorhinal cortices at the earliest stages of AD onset in mouse models [63]. Likewise, in post-mortem evaluations of Parkinson’s disease (PD), a significant reduction in astrocyte density has been observed in the substantia nigra [46, 64]. Because of their function in the regulation of synapses, the general atrophy of astrocytes could impede synaptic signaling, and contribute to cognitive decline evidenced early in the disease [63, 65]. In addition to astrocyte atrophy, synapse loss has also been observed in early disease states [43]. Astrocytes support synaptic health and synaptogenesis through the neuroligins [66] the glypicans [67] and the release of TNFα [68].
Disruption to the astrocyte is particularly impactful to the human brain, where astrocytes in the cortex are 3 fold greater in diameter and have 10 times as many terminal processes, signalling via internal calcium exchange on an order of magnitude 10 times quicker than the rodent [69, 70] When human astrocytes progenitors were implanted in the mouse brain, they became the predominant astrocyte cell type by 7-10 months at the expense of endogenous murine astrocyte [71], incorporated into synapses and increased learning and long term potentiation compared to murine astrocytes [35].
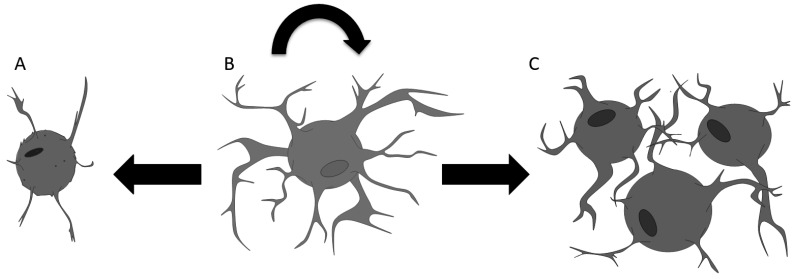
As astrocytes maintain the ability to re-enter the cell cycle in the parenchyma, controlled astrocytic proliferation is likely an important contributor to the neuroenvironment, and there is evidence of increased astrocytic proliferation within rodents undergoing operant learning tasks [72]. Cortical astrocytes are capable of self-renewal throughout the human lifespan [22, 24, 25] and dysregulation of the cell cycle could result in tumorigenesis on one end of the spectrum, while aged atrophied senescent mature astrocytes incapable of re-entering the cell cycle could result in neurodegenerative disease on the other end of the spectrum (Fig. 1).
Fig. (1).
A Model for the Role of Astrocytic Cell Cycle Regulation as the Basis of the Inverse Correlation Between the Incidence of Cancer and Neurodegenerative Disease in Aging Brain. An astrogenic balance maintains the neuroenvironment, as healthy astrocytes (B) can re-enter the cell cycle to replenish cell populations in a regulated controlled manner. In aging, senescent astrocytes (A) are no longer able to reenter the cell cycle to replenish the astrocytic cell population, resulting in astrocytic atrophy and subsequent neurodegeneration. Likewise, the dysregulation of the cell cycle earlier in aging can result in malignant astrocytes (C) and tumorigenesis. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.2. Glioblastoma and Astrocytoma
Longitudinal studies looking at age-related mortality rates revealed that cancer mortality peaks around 60 years and steadily decreases, while the mortality rate of neurodegenerative disease steadily increases after the age of 60 [34]. The incidence of central nervous system (CNS) cancer is steadily increasing, with 83,830 total diagnoses of malignant and non-malignant central nervous system (CNS) cancers expected in 2020 [73]. The 5-year survival rate for glioblastoma from 2012-2016 was just 6.8% [74]. Transcriptomic analysis of various aging tissues may have revealed a possible genetic explanation to the susceptibility or mortality rates of late age-related pathologies seen in humans. In the aging expression of genes involved in inflammation increases, while the expression of cell-cycle genes falls, reflecting genetic expression motifs similar to neurodegenerative diseases and less so to cancer [34]. Notably, researchers found that shared risk alleles between cancer and neurodegenerative disease antagonistically predispose to either type of disease while protecting from the other in an age-dependent manner, supporting previous epidemiological findings [34].
Mature astrocytes have become of interest in the field of neuro-oncology due to their ability to re-enter the cell cycle and proliferate in a physiological setting, suggesting that a dysregulation in this process can lead to the formation of astrocytomas and glioblastoma multiforme (GBM) [75]. In the brain, Surveillance Epidemiology, and End Results Program (SEER) data indicate that mature astrocytes are the root of astrocytoma, which makes up 58.2% of malignant brain tumors in patients 65 or older, and glioblastomas and anaplastic astrocytoma make up 41.6%, originating or GFAP+ astrocyte-like neural stem cells originating from the subventricular zone glioblastoma [29]. However, astrocytes themselves are much more heterogeneous than previously believed, and an analysis of different astrocyte subtypes all expressing Aldh1l1 revealed they all had a malignant analog in brain cancer [30].
Despite their cellular origin, high-grade brain tumors like anaplastic astrocytoma and GBM are genetically and molecularly heterogeneous with respect to the composition of tumor cells, as some tumor types can contain multiple cell types potentially with stem cell-like capacities, thus a significant component of high-grade glioma pathology is the perivascular niche [76, 77]. Apart from astrocytic-origin brain tumors, localized reactive astrocytes, termed tumor-associated astrocytes (TAAs) have been shown to aid the progression and growth of high-grade gliomas and tumor cell lines. At the cellular level, cultured human tumor cells showed that TAAs use this moment of altered genomic expression to communicate with microglia and release anti-inflammatory cytokines like TGFβ and IL-10 via the JAK/STAT pathway, creating a favorable tumor growth environment [78]. In vitro human TAAs are capable of rescuing human glioma cells from cytotoxic effects and apoptosis, suggesting that TAAs play a chemoprotective role for growing tumors [79].
In order to better understand the inverse correlation between cancer and neurodegenerative disease [33], age-related changes in genetic expression were observed in four species human, mice, zebrafish and the short-lived killifish, in samples derived from blood, brain liver and skin [34]. Of the genes antagonistically expressed, four transcription factors were found to be in common that could affect the genes: E2F1, NFAT, C/EBPβ and AP-1 [34]. All four are widely expressed in the body, by astrocytes, and perhaps the most interesting is AP-1, which is part of the c-Jun N-terminal (JNK)/AP1 pathway [80] that is stimulated to induce GFAP expression in astrogliosis that is increased in the aged brain [81]. Although these studies were not completely conducted in human brain tissue in human aging, the induction and repression of genes involved in cell cycle, inflammation and apoptosis in aging have implications for astrocytic physiology and function in neurological disease. Therefore, a consideration of the knowledge of the activity of these four transcription factors to date in astrocytes might shed light on treatments for neurological disease and future research directions.
3. ASTROCYTES AND TRANSCRIPTION FACTORS IMPLICATED IN CANCER AND NEURODEGENE-RATIVE DISEASE
3.1. AP-1
Activator protein 1 (AP-1) is a homodimeric or heterodimeric transcription factor, composed of the Jun and Fos families [82]. It is a basic leucine zipper (bZip) - an alpha helical structure constructed between the conversion of a leucine-zipper domain and the adjacent basic domain [82]. The leucine zipper is needed for homo- and hetero-dimerization, whereas the basic region of the bZIP structure is responsible for DNA binding [83]. Depending on the unique homo- or hetero-dimer arrangements of AP-1, this transcription factor differs in interacting sequence elements [82]. For example, Jun/Jun and Jun/Fos complexes are known to bind to the 12-O-tetradecanoylphorbol-13-acetate (TPA) response element (TRE) sequence with the consensus sequence 5′-TGAG/CTCA-3′; ATF transcription factors recognize the cAMP response elements (CRE) at the consensus site 5′-TGACGTCA-3′; and MAFs interact with MAF recognition elements, consisting of a sequence that is either extension of the TRE sequence -- 5′-TGC-(TRE)-GCA-3′-- or CRE -- 5′-TGC-(CRE)-GCA-3′ [82].
Of the four transcription factors, AP-1 has mostly been studied in astrocytes due to its regulation of the GFAP promoter. Binding of AP-1 to the GFAP promoter increases mRNA expression and protein levels in reactive astrocytes in response to neurological disease, but is not involved in its basal level of expression [84]. In relation to disease, it was found that AP-1 was necessary for increased GFAP production in an Alexander disease model, after kainite treatment to induce seizures, and after injury [84].
Activation of AP-1 in astrocytes can occur through the JNK pathway as a cellular response from stress in a cell environment and is often associated with proliferation, apoptosis, metabolism and DNA repair [85]. The pathway starts with stress to the environment that can range from ionizing radiation, heat, oxidative stress, DNA damage, and inflammatory cytokines. This initiates one of two paths for the JNK pathway along with the Rho family GTPases Cdc42 and Rac receptors [86]. One process for the pathway promotes astrogliosis and/or cellular apoptosis, while the other inhibits signaling for cell survival via STAT and CREB from scaffold proteins [85].
Proteins that accumulate in the brains in patients diagnosed with neurodegenerative disease are degraded by astrocytes through the autophagic process and can also stimulate astrogliosis. aβ aggregates have been known to lead to the apoptosis of astrocytes and astrocytes treated with aβ had an increased expression of the mRNA of AP-1 and increased phosphorylation of JNK and C-JUN [87]. αS, another protein that accumulates in pathological aggregates of neurodegenerative disease stimulates the inflammation response in astrocytes through toll-like receptor 4 (TLR4), which activates the JNK signaling pathway [81, 88].
The AP-1 transcription factor is also necessary for the synthesis of inflammatory secretory cytokines such as IL-2 and IL-6 by astrocytes, via mitogen-activated protein kinase (MAPK) [89]. Once MAPK is activated, phosphorylation of a number of other regulatory proteins such as c-Jun can occur [90]. This allows subsequent steps to follow if the mechanism is proceeding with cell death [89]. If this does not occur when necessary, this could result in the overgrowth of cells, running the potential risk of cancer growth. This is because the AP-1 transcription factor is a mix between the Jun and Fos, allowing it to metabolize and create the following reactions to proceed with the programmed cell death [91]. Notably, AP-1 acts as a mediator in the cell cycle by activating or inhibiting key components. For example, c-Jun and c-Fos increase the rate of cell proliferation, however, another protein in the Jun family, JunB, antagonizes the process [82]. c-Jun is known as an oncogene and tumor promoter due to its ability to hinder the p53 tumor suppressor and by stimulating cyclin D1, promoting the progression from G1 to S phase.
In contrast, JunB inhibits transition to S phase by activating CDKI p16INK4a and repressing cyclin D1. p16 INK4a as well as metalloproteinase 3 have been shown to accumulate in senescent astrocytes in AD [32]. Similarly, stress-induced senescence in astrocytes also results in the accumulation of p16INK4a as well as p21 [92]. Single nucleotide polymorphisms were also discovered to occur in both cancer and neurodegenerative disease in the p16INK4a gene [34].
In gliomas, it has been observed that IL-13Ra2 is overexpressed, but not in normal tissue. Both AP-1 and NFAT were able to induce elevated levels of IL-13Ra2 in glioblastoma multiforme [93]. Subsequently, it was observed that IL-13/IL-13Rα2 used the AP-1 pathway to mediate signal transduction in glial pathologies such as glioblastoma multiforme and astrocytoma [94]. AP-1 was also found to be the transcription factor regulating the expression of γ-synuclein (γS) in astrocytoma cells [95]. γS is upregulated in cancer and also found in inclusions in astrocytes in neurodegenerative disease [96, 97]. RNAi knockdown of γS in astrocytes resulted in a mitotic catastrophe [98], while treatment of astrocytes with γS caused astroprotection and increased proliferation [99].
3.2. E2F1
The E2F family consists of eight total members (E2F1-8) [100]. Several domains compose the configuration of the E2F1 protein. Among them, a nuclear localization signal, a DNA binding domain, a protein binding site for its dimeric partners, a transcriptional activation domain, and a retinoblastoma (Rb) protein-binding domain [101]. In its unphosphorylated form, Rb hinders the ability of E2F1 to bind to its DNA binding site, while phosphorylation of Rb by CDKs frees E2F1 from Rb and the transcription factor can then bind to its dimerization partners (DP), DP1 or DP2 [102]. The formed dimer binds onto the promoter regions of target genes, with a consensus site TTTSSCGCS in which S = G or C [102].
E2F1 undertakes a pivotal role in the cell cycle, apoptosis, and differentiation [100]. Mounting evidence suggests that E2F1 is paramount in the G1 to S phase transition, with its highest expression levels at this transition [102]. E2F1 is a tumor suppressor/oncogene with its increased expression associated with cell cycle activation and proliferation [103, 104]. E2F1 overexpressing mouse astrocytes showed neoplastic transformation with a downregulation of GFAP [105]. Likewise, other studies have exhibited E2F1 as an oncogene in brain tumors [106]. Using glioblastoma and sarcoma cells, it was observed that the ectopic expression of E2F1 prompted a greater level of activity at the promotor for the catalytic subunit of human telomerase in cancer cells [106, 107]. In gliomas, it was found that E2F1 could be repressed if microRNA-10b was inhibited, with effected E2F1 expression through p21 [108].
Like AP-1, the activation of the TLR-4 can affect E2F1. The inflammatory response of astrocytes is, in large, regulated by the activation of toll-like receptors (TLRs), members of the pattern recognition receptor (PRR) family. These transmembrane proteins, expressed both by immune and nonimmune cells, are responsible for the pro-inflammatory reaction to pathogens infecting the CNS [109]. Prolonged activation of TLRs has been linked to both cell death and proliferation, as the receptors can initiate a dual response. Here, the antitumoral effects of TLR activation promote an environment for neural cell degeneration and the protumoral roles support glial cell renewal [109, 110]. This inverse relationship implies that disruption of TLR mediated pathways may accompany neurodegeneration or cancer.
The TLR4 receptor activates the innate immune response of astrocytes [111]. While this receptor generally signals via both the TRIF-dependent pathway and the MyD88-dependent pathway, astrocytic TLR4 has been found to utilize only the latter [112]. Through protein-protein interaction, TLR4 can activate an IL-1R-associated kinase (IRAK) chain reaction, effectively translocating nuclear factor-kappa B (NF-κB) to the nucleus where target genes are bound [113, 114]. NF-κB is another transcription factor that can inhibit E2F1, which subsequently inhibits the release of inflammatory molecules like TNF-α involved in synaptogenesis and IL-6 [112]. The previously mentioned dual effects of TLR activation may be accredited to NF-κB and E2F1 function, as each of the two proteins has been liked to both pro and anti-inflammatory responses in the astrocytic immunity process [113, 115, 116, 117]. However, it is unclear whether the transcriptomic changes are contributors to the differentiation of function in either protein, as human immune and inflammatory aging-regulated genes have been shown to mutually turn the aging signature toward both cancer and neurodegeneration [34].
3.3. NFAT
The nuclear factor of activated T cells (NFAT) is a family of transcription factors consisting of five proteins: NFAT1, NFAT2, NFAT3, NFAT4, and NFAT5 [118]. Besides NFAT5, which responds to osmotic stress, the other members are regulated by calcium and calcineurin CN pathways [119]. The NFAT-homology region (NHR) forms the calcium-regulated region of the proteins and is highly phosphorylated in resting cells, which maintain NFAT in an inactivated state [119]. However, when Ca2+ is released into the cell, it interacts with calmodulin which activates the calcium/calmodulin-dependent serine/threonine phosphatase, CN [118]. Thereafter, CN dephosphorylates serine residues on NFAT, resulting in the transcription factor translocating into the nucleus and coordinating the transcription of specific genes [118, 119]. Moreover, the DNA-binding domain (DBD) of NFATs is highly conserved, which allows all proteins of this family to bind to the DNA core sequence (A/T)GGAAA [119].
In neurodegenerative disease, postmortem studies on AD tissue samples from patients denote increased CN/NFAT levels, with elevated NFAT1 observed in astrocyte nuclei in postmortem brain sections taken from human subjects with mild cognitive impairment [120, 121]. Additionally, there is a bi-directional interaction between CN/NFAT and cytokine factors, suggesting the pathway is preferentially programmed to maintain positive feedback cycles underlying chronic neuroinflammation [122].
Additionally, once activated by CN, NFAT can downregulate expression of glutamate transporter EAAT2/GLT-1 expression leading to glutamate excitotoxicity seen in neurodegenerative disease [123, 124]. On the other side, hindering the activity of NFAT provided protection for the transporter, leading to reduced extracellular glutamate and neuronal survival [125].
An increase in CN also caused a two to three-fold increase in astrocytic complement C3, which stimulated microglia to remove synapses in a mouse model of AD by hindering matricellular factors like SPARC and hevin from partaking in synaptic formation [125]. Indeed, impeding CN/NFAT activity in astrocytes has clearly been demonstrated to lead to synaptoprotection. An increase in amyloid pathology has also been correlated with an increase in CN/NFAT activity, and regions of high amyloid aggregation have been demonstrated to have high CN/NFAT activity. Inhibition of NFAT caused a decrease in amyloid plaque load and soluble Aβ peptide levels [124]. Aβ also affected the CN/NFAT pathway to contribute to the synaptic disturbances as well [126]. In A53T mice, overexpression of αS stimulates CN expression and nuclear translocation of NFAT in midbrain dopaminergic neurons, suggesting a role in neurodegenerative pathology as well as cancer [127].
In cancer, the specific NFAT isoform differs in its proliferative or suppressive effects on cell growth, thus, each phenotype bestows a difference in cancer development [119, 128]. It has also been demonstrated that NFAT1 is overexpressed in glioblastoma multiforme, and appears to be responsible for invasive potential but not proliferation in glioblastoma multiforme cells [129]. In astrocytes, CN/NFAT and AP-1 activities on separate occasions have been shown to drive the expression of MMP3 as an early neuroinflammatory response for cell migration [104, 130]. Coincidently in a tumor setting, NFAT regulated MMP3 expression aids in tumor growth and metastasis, while increased AP-1 expression promotes tumorigenesis [131, 132].
Calcium dysregulation occurs in reactive astrocytes in disease [133]. Calcium release from the endoplasmic reticulum in astrocytes can occur through activation of g-protein coupled transmitter receptors which open IP3 calcium channels on the endoplasmic reticulum membrane [134, 135]. Although L-type calcium channels to allow entry of extracellular calcium has been implicated in disease and CN activation, only small changes are required to stimulate CN/NFAT signaling [136]. Purinergic receptors are among those that can stimulate intracellular calcium release to activate CN/NFAT, and can also spread to proximal astrocytes [137, 138]. It has been shown that P2Y1 receptor activation led to increased proliferation of astrocytic cells [139]. However, at the highest concentrations of ATP, a decrease in proliferation rate was observed, an effect possibly explained by the activation of the P2X7 astrocytic receptor, which is regarded as an inhibitor of proliferation [140]. In astrocytoma cells, the P2Y2 receptor-stimulated the upregulation of genes associated with the prevention of apoptosis while downregulating pro-apoptotic genes and continuation of the cell cycle [141]. Likewise, activation of P2Y6 in astrocytes by UDP caused increased internal calcium and CN/NFAT expression and release of chemokines [142]. Similarly, CN/NFAT is stimulated by injury, which can also be mimicked by P2Y receptor activation in astroctyes, by upregulating NFATc1 but not NFATc2 [143].
3.4. C/EBPβ
The CCAAT/enhancer-binding protein beta (C/EBPβ) belongs to the family of C/EBP transcription factors [144]. This group includes five other members: C/EBPα, C/EBPγ, C/EBPδ, C/EBPϵ and C/EBPζ. Similar to AP-1, the C/EBP proteins are also characterized by a highly conserved bZIP domain [144]. Moreover, the various proteins have the ability to form heterodimers in all intrafamilial combinations due to their conserved bZIP domain [145]. Besides C/EBPζ, the other members can interact with the same DNA sequence site, RTTGCGYAAY, where R is A or G, and Y is C or T [145]. Due to its leucine zipper, C/EBPζ can still dimerize with the other members, however, because it contains two proline residues that disrupt the alpha-helical structure, C/EBPζ/C/EBP heterodimers bind to a different DNA sequence with the consensus site PuPuPuTGCAAT(A/C)CCC, where Pu is a purine [145].
Although much of the research on astrocytes has focused on C/EBPδ [146, 147], C/EBPβ expression in astrocytes also has a role in inflammatory responses. C/EBPβ regulates various human astrocyte inflammatory genes induced by interleukin (IL)-1β [148]. C/EBPβ expression is also incited by synaptogenesis stimulator tumor necrosis factor-α and increased expression of C/EBPβ was observed in AD and PD [148]. Silencing of CEBPβ has shown to diminish glial activation and neurodegenerative effects in PD mouse models, whereas upregulation of CEBPβ expression occurs in reactive astrocytes [149, 150]. C/EBPβ, therefore, has a pro-inflammatory role in astrocytes [151, 152].
Similarly, C/EBPβ mRNA and protein levels correlated with high-grade gliomas and patients with lower expression revealed a longer survival time, while in vitro silencing of C/EBPβ impeded both glioma cell proliferation and invasion [153]. And lastly, one of the transcription factors that enhances brain-derived neurotrophic factor, which is upregulated in cancer and downregulated in neurodegeneration, is C/EBPβ.
4. EFFECTING AGE-RELATED NEUROLOGICAL DISEASE THROUGH ASTROCYTE-SPECIFIC MODULATION OF TRANSCRIPTION FACTORS
4.1. Astrocyte Complexity and Drug Development
The study on the heterogeneity of astrocytes is in its infancy, with one study demonstrating five subtypes alone when only analyzed through the expression of Aldh1l1, all with malignant counterparts [30]. Single-cell RNA sequencing in rodents has described at least seven subtypes of astrocytes [154]. Histological analysis of cortical astrocytes in humans and primates also revealed a unique astrocyte subtype in humans and another astrocyte subtype unique to humans and primates [69, 70]. Similarly, astrocyte activation in injury and disease has traditionally been described as astrogliosis and evidenced by upregulation of GFAP, however, it is now known that there are two different states of reactive astrocytes, A1 and A2 [10]. A1 astrocytes are under the control of microglia, more prevalent in senescence, and toxic to neurons and oligodendrocytes, while A2 reactive astrocytes provide a healthy environment for recovery after injury and disease [10, 155]. Despite this growing complexity, astrocytes associated with the blood-brain barrier (BBB) have an advantage in intravenous central nervous system treatments as treatments can theoretically be more effectively delivered to this cell type [156]. Therefore, as more becomes understood on astrocyte heterogeneity, it should be noted that in developing astrocyte-specific treatments, the diversity of the different subtypes of astrocytes and their variety of function must be considered for directed therapies.
4.2. Modulators of AP-1, NFAT, E2F-1 and C/EBPβ
Pharmacological development of molecules to affect transcription factors has traditionally been difficult, because of the broad implications of affecting the transcription of many different genes [157]. Avenues to indirectly target transcription factors through inhibition and activation can be at the expression level itself, through protein/protein interaction, through binding in an activation/inhibition pocket, or at the protein/DNA binding level [158]. Traditionally, DNA binding drugs were developed to inhibit transcription in cancer through recognition of helical structures and resulted in broad effects [158]. More specific inhibitors were then developed, such as the ability to bind AP-1 recognition element 5’-CATATG-3’ can be blocked by Lambda-1-Rh(MGP)2phi5+ thereby inhibiting AP-1 transcription. Another drug that can inhibit the binding of AP-1 is MLN944, which inhibits c-JUN binding on 5’-aTGAGTCA-3’ sequence [159]. Similarly, attacking signaling pathways that activate transcription factors, such as cyclosporine A and FK506, that inhibit binding of NFAT to CN have been developed in immunosuppression studies and could be delivered specifically to astrocytes [160]. Artificial peptides have also been developed because they recognize similar consensus sequences, such as a novel E2F-1-like peptide shown to regress prostate tumor xenografts or to mimic transcription factor activity [161].
However, more promising current therapeutics employ RNA interference (RNAi) through small interfering or silencing RNA (siRNA) to downregulate specific mRNA after transcription. siRNA-based drugs have been clinically approved in the United States for GIVLAARI for treatment of acute hepatic porphyria and in the United States and Europe for ONPATTRO for treatment of hereditary amyloidogenic transthyretin [162]. siRNAs have also shown promise in early clinical phases in possible treatments of glioblastoma [163-165].
In terms of affecting cancer and neurodegeneration, however, more recently, the focus has turned to microRNAs (miRNAs) as a more effective specific method to attack transcription factors without a broad reaching impact and side effect of traditional DNA-binding drugs, and more nuanced than siRNA [166]. Originally considered as transcription factor inhibitors, it now appears that miRNAs can act as activators or enhancers, or work in a positive feedback loop to activate transcription factor activity by inhibiting an inhibitor [167].
For example, in a screen of 191 miRNAs that could regulate the human CN/NFAT pathway, it was found that 32 were induced by the transcription factor, with 11 providing feedback modulation [168]. Of the 11, 6 were negative feedback loop by blocking activators of NFAT (hsa-miR-21-3p, hsa-let-7b-5p, hsa-miR-17-5p, hsa-miR-19a-3p, hsa-miR-92b-3p, hsa-miR-17-3p), while 4 were positive feedback loop regulators by blocking inhibitors of NFAT (hsa-miR-21-5p, hsa-miR-181c-5p, hsa-let-7c-5p, hsa-let-7b-3p, hsa-miR-155-5p). Likewise, when E2F-1, JUN and CEB/P are activated in late states of tumorigenesis, they promote drug resistance and proliferation [169, 170]. It has been demonstrated that transcription factors transcribe microRNAs at these stages to inhibit apoptosis by chemotherapies in a feed-forward mechanism whereby the transcription factor can then in turn be upregulated by the miRNA [166]. RNA library studies have shown that some miRNA’s can inhibit transcription factor activation, and might provide a fruitful avenue for therapeutic development of transcription enhancers [167]. Additionally, a comprehensive analysis of miRNAs known to affect proteins implicated in neurodegenerative disease demonstrated the active roles of miRNAs in senescence and aging as well [171].
However, as many miRNAs either inhibit or activate a pathway, a cocktail of miRNAs delivered to the astrocyte might be necessary to balance transcription factor activity between disease states. Interestingly, a method to measure miRNA activity in vivo has been developed that could provide a promising avenue for this strategy [172]. Using miRNA-mediated single guide RNA (sgRNA), specific DNA can be targeted based on miRNA levels that it senses through the utilization of the CRISPR-Cas9 system [172].
4.3. Astrocyte Specific Delivery of Gene Therapy
In order to deliver siRNA, miRNA, CRISPR or other gene constructs and combinations specifically to astrocytes, the use of viral transfection or nanoparticles is actively being explored [154]. Viral therapies that have advanced to clinical trials have not traditionally been cell-type specific, except through the promoter delivered, and several astrocyte-specific promoters have been utilized, including GFAP, Aldh1l1 and glutamine synthatase [173-176].
The tropism of adeno-associated viruses (AAVs) has typically been more astrocytic with greater access as they cross the blood-brain barrier. AAVs carry a relatively small load (~4.7 kb for conventional viruses). One type, AAV9 had been shown to effectively cross the BBB and preferentially infect astrocytes in adult mice and primates [177, 178], and designed promoters such as GFaABC1D which are only 681 bp, can be utilized to infect the same cells as GFAP and save space if the gene construct is large [179]. However, in initial studies, GFaABC1D proved weaker at transducing astrocytes, and was loaded with miR124 to silence expression in neurons [180], while additional studies showed greater tropism for astrocytes with AAV5 and GFaABC1D [181]. However, for more specific astrocyte transduction, it would be ideal to only infect astrocytes themselves, regardless of the promoter, and further strategies have engineered AAVs via multiplexed CRE-dependent strategies (CREATE), have engineered AAVs with capsids with a higher tropism for specific cell types in the CNS, including astrocytes [182, 183]. Additionally, vectors such as AAV9P1, with a peptide to more specifically recognize and transfect terminally differentiated astrocytes, have been constructed [184]. Using this vector, HIV-1 genes in astrocytes were successfully edited with CRISPR/Cas9 and demonstrated much greater transfection efficiency in astrocytes compared with neurons.
Another option is lentiviruses, which can carry a greater gene load than AAVs, and when constructed with different envelope glycoproteins, it was shown that vesicular stomatitis virus (VSV) and Mokola Virus (MV) more specifically transduced neurons, while the lymphocytic choriomeningitis virus (LCMV) and the Moloney murine leukemia virus (MuLV) glycoproteins were shown to preferentially infect astrocytes [185, 186]. Although the lentivirus is effective in overexpressing genes, gene knockdown in astrocytes is not as efficient because of small hairpin RNA (shRNA) processing. However, when used in combination with the glutamine synthetase promoter specific to astrocytes, as well as two miRNAs, miR9*T-miR124T, it proved effective in allowing gene silencing in astrocytes [176]. However, it should be noted that although neuronally targeted in the CNS, miRNA124 has also been shown to inhibit NFAT activity in smooth muscle, and may demonstrate off-target effects when utilized in a vector to modulate NFAT in astrocytes [187].
Nanoparticles have been used in the treatment of glioblastoma with RNAi therapy and have been developed to cross the BBB [188]. An advantage of nanoparticles is that outside elements can more easily contain antibodies or proteins that would specifically recognize astrocyte transmembrane proteins to allow for astrocyte-specific recognition. One strategy to deliver siRNA to target astrocytes was a construct engineered with antibodies for the bradykinin B2 on chitosan nanoparticles, that would recognize the transferrin receptor and bradykinin B2 receptor specific to the BBB and astrocytes [189]. Another strategy was to deliver lipid nanoparticles constructed with apolipoprotien E to mediate cellular uptake of an mRNA gene carrier. This caused genes to be expressed in astrocytes and some neurons after intracerebroventricular administration. Further development in the treatment of glioblastoma delivered RNAi by constructing gold nanoparticles with rabies virus glycoproteins conjugated to liposomes with apoplipoprotein E, which inhibited miRNA-92b, an miRNA whose expression increases as cancer progresses [165]. Therefore, regulation of the four transcription factors described by activators, inhibitors or modulators in neurological disease could be achieved as viral and nanoparticle postilions guide them with greater astrocytic tropism.
CONCLUSION
Adult astrogenesis is a common process in the brain through adulthood. Astrocyte cell cycle re-entry is therefore a necessary physiological process to maintain the neuroenvironment. Because of the astrocytic role coordinating central nervous system function, as aging occurs, senescent astrocytic inability to successfully re-enter the cell cycle likely contributes to the cognitive decline seen in age-related dementias. Senescent astrocytes increase the inflammatory response, become atrophic, and are unable to properly protect and regulate the central nervous system. Additionally, astrocytes are the cell type origin for the majority of cancers in the brain, and four transcription factors that are involved in cell cycle regulation and apoptosis, are responsible for the genes that are antagonistically expressed in neurodegenerative disease and cancer. Despite the multifaceted complex nature of transcriptional regulation, as well as the growing understanding of the diversity of astrocytes and their subtypes, it is useful to consider the current knowledge on these particular transcription factors in astrocytes, due to their role in the aging brain, glioma and neurodegenerative disease. Similarly, emerging research has indicated that miRNAs may be an effective way to inhibit or activate transcription factors, and viral or nanoparticle gene delivery is evolving to be more cell-specific, including astrocyte-specific, which will advance the pharmacological development of astrocytic treatments. As future studies into astrocytic function and diversity progress, new avenues that connect these transcription factors, pathways and diseases will likely provide an effective window into pharmacological treatment for age-related neurological diseases.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Irene Luccia Pearl for invaluable help with manuscript preparation, as well as the University of Hartford Neuroscience program for support.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Araque A., Carmignoto G., Haydon P.G., Oliet S.H.R., Robitaille R., Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81(4):728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araque A., Parpura V., Sanzgiri R.P., Haydon P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. doi: 10.1016/S0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 3.Haydon P.G., Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86(3):1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 4.Parpura V., Basarsky T.A., Liu F., Jeftinija K., Jeftinija S., Haydon P.G. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369(6483):744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 5.Verkhratsky A., Nedergaard M. Astroglial cradle in the life of the synapse. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369(1654):20130595. doi: 10.1098/rstb.2013.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panatier A., Vallée J., Haber M., Murai K.K., Lacaille J-C., Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146(5):785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Mathiisen T.M., Lehre K.P., Danbolt N.C., Ottersen O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 8.Kress B.T., Iliff J.J., Xia M., Wang M., Wei H.S., Zeppenfeld D., Xie L., Kang H., Xu Q., Liew J.A., Plog B.A., Ding F., Deane R., Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014;76(6):845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S., Swarnkar S., Goswami P., Nath C. Astrocytes and microglia: Responses to neuropathological conditions. Int. J. Neurosci. 2011;121(11):589–597. doi: 10.3109/00207454.2011.598981. [DOI] [PubMed] [Google Scholar]
- 10.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W-S., Peterson T.C., Wilton D.K., Frouin A., Napier B.A., Panicker N., Kumar M., Buckwalter M.S., Rowitch D.H., Dawson V.L., Dawson T.M., Stevens B., Barres B.A. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jha M.K., Jo M., Kim J-H., Suk K. Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist. 2019;25(3):227–240. doi: 10.1177/1073858418783959. [DOI] [PubMed] [Google Scholar]
- 12.Loria F., Vargas J.Y., Bousset L., Syan S., Salles A., Melki R., Zurzolo C. α-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol. 2017;134(5):789–808. doi: 10.1007/s00401-017-1746-2. [DOI] [PubMed] [Google Scholar]
- 13.Hertz L., Chen Y. Importance of astrocytes for potassium ion (K+) homeostasis in brain and glial effects of K+ and its transporters on learning. Neurosci. Biobehav. Rev. 2016;71:484–505. doi: 10.1016/j.neubiorev.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoud S., Gharagozloo M., Simard C., Gris D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells. 2019;8(2):E184. doi: 10.3390/cells8020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen M.K., Mestre H., Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–1024. doi: 10.1016/S1474-4422(18)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sofroniew M.V. In: Astrogliosis. Glia; Barres Freeman, M.R. Stevens B.B.A., editor. New York, NY: Cold Spring Harbor Laboratory Press; 2015. pp. 107–122. [Google Scholar]
- 17.Sofroniew M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burda J.E., Sofroniew M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habela C.W., Olsen M.L., Sontheimer H. ClC3 is a critical regulator of the cell cycle in normal and malignant glial cells. J. Neurosci. 2008;28(37):9205–9217. doi: 10.1523/JNEUROSCI.1897-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimou L., Götz M. Glial cells as progenitors and stem cells: New roles in the healthy and diseased brain. Physiol. Rev. 2014;94(3):709–737. doi: 10.1152/physrev.00036.2013. [DOI] [PubMed] [Google Scholar]
- 21.Batista C.M., Mariano E.D., Barbosa B.J.A.P., Morgalla M., Marie S.K.N., Teixeira M.J., Lepski G. Adult neurogenesis and glial oncogenesis: When the process fails. BioMed Res. Int. 2014;2014:438639. doi: 10.1155/2014/438639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohn T.C., Koob A.O. Adult astrogenesis and the etiology of cortical neurodegeneration. J. Exp. Neurosci. 2015;9(Suppl. 2):25–34. doi: 10.4137/JEN.S25520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicario A., Kisiswa L., Tann J.Y., Kelly C.E., Ibáñez C.F., Beg A., Baltimore D., Busuttil V., Bottero V., Frelin C. Neuron-type-specific signaling by the p75NTR death receptor is regulated by differential proteolytic cleavage. J. Cell Sci. 2015;128(8):1507–1517. doi: 10.1242/jcs.161745. [DOI] [PubMed] [Google Scholar]
- 24.Bardehle S., Krüger M., Buggenthin F., Schwausch J., Ninkovic J., Clevers H., Snippert H.J., Theis F.J., Meyer-Luehmann M., Bechmann I., Dimou L., Götz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat. Neurosci. 2013;16(5):580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- 25.Ge W-P., Jia J-M. Local production of astrocytes in the cerebral cortex. Neuroscience. 2016;323:3–9. doi: 10.1016/j.neuroscience.2015.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhardwaj R.D., Curtis M.A., Spalding K.L., Buchholz B.A., Fink D., Björk-Eriksson T., Nordborg C., Gage F.H., Druid H., Eriksson P.S., Frisén J. Neocortical neurogenesis in humans is restricted to development. Proc. Natl. Acad. Sci. USA. 2006;103(33):12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villarreal A., Rosciszewski G., Murta V., Cadena V., Usach V., Dodes-Traian M.M., Setton-Avruj P., Barbeito L.H., Ramos A.J. Isolation and characterization of Ischemia-Derived Astrocytes (IDAs) with ability to transactivate quiescent astrocytes. Front. Cell. Neurosci. 2016;10:139. doi: 10.3389/fncel.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner D.A., Adamson D.C. Neuronal-astrocyte metabolic interactions: understanding the transition into abnormal astrocytoma metabolism. J. Neuropathol. Exp. Neurol. 2011;70(3):167–176. doi: 10.1097/NEN.0b013e31820e1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J.H., Lee J.E., Kahng J.Y., Kim S.H., Park J.S., Yoon S.J., Um J-Y., Kim W.K., Lee J-K., Park J., Kim E.H., Lee J.H., Lee J.H., Chung W.S., Ju Y.S., Park S.H., Chang J.H., Kang S.G., Lee J.H. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 2018;560(7717):243–247. doi: 10.1038/s41586-018-0389-3. [DOI] [PubMed] [Google Scholar]
- 30.John Lin C-C., Yu K., Hatcher A., Huang T-W., Lee H.K., Carlson J., Weston M.C., Chen F., Zhang Y., Zhu W., Mohila C.A., Ahmed N., Patel A.J., Arenkiel B.R., Noebels J.L., Creighton C.J., Deneen B. Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci. 2017;20(3):396–405. doi: 10.1038/nn.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salminen A., Ojala J., Kaarniranta K., Haapasalo A., Hiltunen M., Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur. J. Neurosci. 2011;34(1):3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- 32.Bhat R., Crowe E.P., Bitto A., Moh M., Katsetos C.D., Garcia F.U., Johnson F.B., Trojanowski J.Q., Sell C., Torres C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS One. 2012;7(9):e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plun-Favreau H., Lewis P.A., Hardy J., Martins L.M., Wood N.W. Cancer and neurodegeneration: between the devil and the deep blue sea. PLoS Genet. 2010;6(12):e1001257. doi: 10.1371/journal.pgen.1001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aramillo Irizar P., Schäuble S., Esser D., Groth M., Frahm C., Priebe S., Baumgart M., Hartmann N., Marthandan S., Menzel U., Müller J., Schmidt S., Ast V., Caliebe A., König R., Krawczak M., Ristow M., Schuster S., Cellerino A., Diekmann S., Englert C., Hemmerich P., Sühnel J., Guthke R., Witte O.W., Platzer M., Ruppin E., Kaleta C. Transcriptomic alterations during ageing reflect the shift from cancer to degenerative diseases in the elderly. Nat. Commun. 2018;9(1):327. doi: 10.1038/s41467-017-02395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han X., Chen M., Wang F., Windrem M., Wang S., Shanz S., Xu Q., Oberheim N.A., Bekar L., Betstadt S., Silva A.J., Takano T., Goldman S.A., Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12(3):342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haydon P.G. GLIA: listening and talking to the synapse. Nat. Rev. Neurosci. 2001;2(3):185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 37.Christopherson K.S., Ullian E.M., Stokes C.C.A., Mullowney C.E., Hell J.W., Agah A., Lawler J., Mosher D.F., Bornstein P., Barres B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Cabezas R., Avila M., Gonzalez J., El-Bachá R.S., Báez E., García-Segura L.M., Jurado Coronel J.C., Capani F., Cardona-Gomez G.P., Barreto G.E. Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front. Cell. Neurosci. 2014;8:211. doi: 10.3389/fncel.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ullian E. M., Sapperstein S. K., Christopherson K. S., Barres B. A. 2001. [DOI] [PubMed]
- 40.Mauch D. H., Nagler K., Schumacher S., Goritz C., Muller E. C., Otto A., Pfrieger F. W. 2001. [DOI] [PubMed]
- 41.Papouin T., Dunphy J., Tolman M., Foley J.C., Haydon P.G. Astrocytic control of synaptic function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1715;2017(372):372. doi: 10.1098/rstb.2016.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Revuelta G.J., Rosso A., Lippa C.F. Neuritic pathology as a correlate of synaptic loss in dementia with lewy bodies. Am. J. Alzheimers Dis. Other Demen. 2008;23(1):97–102. doi: 10.1177/1533317507310565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., Hansen L.A., Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 44.Chung W-S., Welsh C.A., Barres B.A., Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat. Neurosci. 2015;18(11):1539–1545. doi: 10.1038/nn.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pekny M., Pekna M., Messing A., Steinhäuser C., Lee J-M., Parpura V., Hol E.M., Sofroniew M.V., Verkhratsky A. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131(3):323–345. doi: 10.1007/s00401-015-1513-1. [DOI] [PubMed] [Google Scholar]
- 46.Verkhratsky A., Rodríguez J.J., Parpura V. Astroglia in neurological diseases. Future Neurol. 2013;8(2):149–158. doi: 10.2217/fnl.12.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raz L., Knoefel J., Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016;36(1):172–186. doi: 10.1038/jcbfm.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price B.R., Norris C.M., Sompol P., Wilcock D.M. An emerging role of astrocytes in vascular contributions to cognitive impairment and dementia. J. Neurochem. 2018;144(5):644–650. doi: 10.1111/jnc.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boisvert M.M., Erikson G.A., Shokhirev M.N., Allen N.J. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 2018;22(1):269–285. doi: 10.1016/j.celrep.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nichols N.R., Day J.R., Laping N.J., Johnson S.A., Finch C.E. GFAP mRNA increases with age in rat and human brain. Neurobiol. Aging. 1993;14(5):421–429. doi: 10.1016/0197-4580(93)90100-P. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida T., Goldsmith S.K., Morgan T.E., Stone D.J., Finch C.E. Transcription supports age-related increases of GFAP gene expression in the male rat brain. Neurosci. Lett. 1996;215(2):107–110. doi: 10.1016/0304-3940(96)12966-9. [DOI] [PubMed] [Google Scholar]
- 52.Eng L.F., Ghirnikar R.S. GFAP and astrogliosis. Brain Pathol. 1994;4(3):229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 53.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jellinger K.A. Neuropathological spectrum of synucleinopathies. Mov. Disord. 2003;18(Suppl. 6):S2–S12. doi: 10.1002/mds.10557. [DOI] [PubMed] [Google Scholar]
- 55.Koob A.O., Paulino A.D., Masliah E. GFAP reactivity, apolipoprotein E redistribution and cholesterol reduction in human astrocytes treated with alpha-synuclein. Neurosci. Lett. 2010;469(1):11–14. doi: 10.1016/j.neulet.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braidy N., Gai W-P., Xu Y.H., Sachdev P., Guillemin G.J., Jiang X-M., Ballard J.W.O., Horan M.P., Fang Z.M., Chong B.H., Chan D.K. Uptake and mitochondrial dysfunction of alpha-synuclein in human astrocytes, cortical neurons and fibroblasts. Transl. Neurodegener. 2013;2(1):20. doi: 10.1186/2047-9158-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vieira B.D.M., Radford R.A., Chung R.S., Guillemin G.J., Pountney D.L. Neuroinflammation in multiple system atrophy: response to and cause of α-Synuclein aggregation. Front. Cell. Neurosci. 2015;9:437. doi: 10.3389/fncel.2015.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brück D., Wenning G.K., Stefanova N., Fellner L. Glia and alpha-synuclein in neurodegeneration: A complex interaction. Neurobiol. Dis. 2016;85:262–274. doi: 10.1016/j.nbd.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shults C.W., Rockenstein E., Crews L., Adame A., Mante M., Larrea G., Hashimoto M., Song D., Iwatsubo T., Tsuboi K., Masliah E. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J. Neurosci. 2005;25(46):10689–10699. doi: 10.1523/JNEUROSCI.3527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koob A.O. Astrogenesis versus astrogliosis. Neural Regen. Res. 2017;12(2):203–204. doi: 10.4103/1673-5374.200798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang G., Yue Z., Talloczy Z., Goldman J.E. Adaptive autophagy in alexander disease-affected astrocytes. Autophagy. 2008;4(5):701–703. doi: 10.4161/auto.6028. [DOI] [PubMed] [Google Scholar]
- 62.Anderson M.A., Ao Y., Sofroniew M.V. Heterogeneity of reactive astrocytes. Neurosci. Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verkhratsky A., Parpura V. Neurological and psychiatric disorders as a neuroglial failure. Period. Biol. 2014;116(2):115–124. [PMC free article] [PubMed] [Google Scholar]
- 64.Mena M.A., García de Yébenes J. Glial cells as players in parkinsonism: The “Good,” the “Bad,” and the “Mysterious” glia. Neuroscientist. 2008;14(6):544–560. doi: 10.1177/1073858408322839. [DOI] [PubMed] [Google Scholar]
- 65.Capani F., Quarracino C., Caccuri R., Sica R.E.P. Astrocytes as the main players in primary degenerative disorders of the human central nervous system. Front. Aging Neurosci. 2016;8:45. doi: 10.3389/fnagi.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stogsdill J.A., Ramirez J., Liu D., Kim Y.H., Baldwin K.T., Enustun E., Ejikeme T., Ji R-R., Eroglu C. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature. 2017;551(7679):192–197. doi: 10.1038/nature24638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen N.J., Bennett M.L., Foo L.C., Wang G.X., Chakraborty C., Smith S.J., Barres B.A. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486(7403):410–414. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stellwagen D., Malenka R.C. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 69.Oberheim N.A., Takano T., Han X., He W., Lin J.H., Wang F., Xu Q., Wyatt J.D., Pilcher W., Ojemann J.G., Ransom B.R., Goldman S.A., Nedergaard M. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009;29(10):3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oberheim N.A., Wang X., Goldman S., Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29(10):547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 71.Mariani J.N., Zou L., Goldman S.A. Human glial chimeric mice to define the role of glial pathology in human disease. Methods Mol. Biol. 2019;1936:311–331. doi: 10.1007/978-1-4939-9072-6_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rapanelli M., Frick L.R., Zanutto B.S. Learning an operant conditioning task differentially induces gliogenesis in the medial prefrontal cortex and neurogenesis in the hippocampus. PLoS One. 2011;6(2):e14713. doi: 10.1371/journal.pone.0014713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ostrom Q.T., Patil N., Cioffi G., Waite K., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro-oncol. 2020;22(12) Suppl. 2:iv1–iv96. doi: 10.1093/neuonc/noaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ostrom Q.T., Cioffi G., Gittleman H., Patil N., Waite K., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro-oncol. 2019;21(Suppl. 5):v1–v100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rasheed B.K., Wiltshire R.N., Bigner S.H., Bigner D.D. Molecular pathogenesis of malignant gliomas. Curr. Opin. Oncol. 1999;11(3):162–167. doi: 10.1097/00001622-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Brandao M., Simon T., Critchley G., Giamas G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia. 2019;67(5):779–790. doi: 10.1002/glia.23520. [DOI] [PubMed] [Google Scholar]
- 77.Charles N.A., Holland E.C., Gilbertson R., Glass R., Kettenmann H. The brain tumor microenvironment. Glia. 2011;59(8):1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- 78.Henrik Heiland D., Ravi V.M., Behringer S.P., Frenking J.H., Wurm J., Joseph K., Garrelfs N.W.C., Strähle J., Heynckes S., Grauvogel J., Franco P., Mader I., Schneider M., Potthoff A.L., Delev D., Hofmann U.G., Fung C., Beck J., Sankowski R., Prinz M., Schnell O. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat. Commun. 2019;10(1):2541. doi: 10.1038/s41467-019-10493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen W., Wang D., Du X., He Y., Chen S., Shao Q., Ma C., Huang B., Chen A., Zhao P., Qu X., Li X. Glioma cells escaped from cytotoxicity of temozolomide and vincristine by communicating with human astrocytes. Med. Oncol. 2015;32(3):43. doi: 10.1007/s12032-015-0487-0. [DOI] [PubMed] [Google Scholar]
- 80.Vukic V., Callaghan D., Walker D., Lue L-F., Liu Q.Y., Couraud P-O., Romero I.A., Weksler B., Stanimirovic D.B., Zhang W. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer’s brain is mediated by the JNK-AP1 signaling pathway. Neurobiol. Dis. 2009;34(1):95–106. doi: 10.1016/j.nbd.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rannikko E.H., Weber S.S., Kahle P.J. Exogenous α-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci. 2015;16:57. doi: 10.1186/s12868-015-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garces de Los Fayos Alonso I., Liang H-C., Turner S.D., Lagger S., Merkel O., Kenner L. The role of activator protein-1 (AP-1) family members in CD30-positive lymphomas. Cancers (Basel) 2018;10(4):E93. doi: 10.3390/cancers10040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jindrich K., Degnan B.M. The diversification of the basic leucine zipper family in eukaryotes correlates with the evolution of multicellularity. BMC Evol. Biol. 2016;16:28. doi: 10.1186/s12862-016-0598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brenner M., Messing A., Olsen M.L. AP-1 and the injury response of the GFAP gene. J. Neurosci. Res. 2019;97(2):149–161. doi: 10.1002/jnr.24338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson G.L., Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim. Biophys. Acta. 2007;1773(8):1341–1348. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dhanasekaran D.N., Reddy E.P. JNK-signaling: A multiplexing hub in programmed cell death. Genes Cancer. 2017;8(9-10):682–694. doi: 10.18632/genesandcancer.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li G-Q., Cong D-W., Sun P., Meng X. Aβ1-42 regulates astrocytes through JNK/AP-1 pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22(7):2015–2021. doi: 10.26355/eurrev_201804_14730. [DOI] [PubMed] [Google Scholar]
- 88.Fellner L., Irschick R., Schanda K., Reindl M., Klimaschewski L., Poewe W., Wenning G.K., Stefanova N. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia. 2013;61(3):349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flaherty D. Intracellular signaling and t cell activation. Immunology for Pharmacy. Saint Louis: Elsevier; 2012. pp. 55–62. [Google Scholar]
- 90.Zhang Y., Zhou S., Cai W., Han G., Li J., Chen M., Li H. Hypoxia/reoxygenation activates the JNK pathway and accelerates synovial senescence. Mol. Med. Rep. 2020;22(1):265–276. doi: 10.3892/mmr.2020.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gazon H., Barbeau B., Mesnard J-M., Peloponese J-M.J., Jr Hijacking of the AP-1 signaling pathway during development of ATL. Front. Microbiol. 2018;8:2686. doi: 10.3389/fmicb.2017.02686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bitto A., Sell C., Crowe E., Lorenzini A., Malaguti M., Hrelia S., Torres C. Stress-induced senescence in human and rodent astrocytes. Exp. Cell Res. 2010;316(17):2961–2968. doi: 10.1016/j.yexcr.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 93.Wu A., Ericson K., Chao W., Low W.C. NFAT and AP1 are essential for the expression of a glioblastoma multiforme related IL-13Ra2 transcript. Cell. Oncol. 2010;32(5-6):313–329. doi: 10.3233/CLO-2010-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhardwaj R., Suzuki A., Leland P., Joshi B.H., Puri R.K. Identification of a novel role of IL-13Rα2 in human Glioblastoma multiforme: interleukin-13 mediates signal transduction through AP-1 pathway. J. Transl. Med. 2018;16(1):369. doi: 10.1186/s12967-018-1746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Surgucheva I., Surguchov A. γ-synuclein: cell-type-specific promoter activity and binding to transcription factors. J. Mol. Neurosci. 2008;35(3):267–271. doi: 10.1007/s12031-008-9074-6. [DOI] [PubMed] [Google Scholar]
- 96.Fung K-M., Rorke L.B., Giasson B., Lee V.M-Y., Trojanowski J.Q. Expression of alpha-, beta-, and gamma-synuclein in glial tumors and medulloblastomas. Acta Neuropathol. 2003;106(2):167–175. doi: 10.1007/s00401-003-0718-x. [DOI] [PubMed] [Google Scholar]
- 97.Surguchov A., Palazzo R.E., Surgucheva I. Gamma synuclein: subcellular localization in neuronal and non-neuronal cells and effect on signal transduction. Cell Motil. Cytoskeleton. 2001;49(4):218–228. doi: 10.1002/cm.1035. [DOI] [PubMed] [Google Scholar]
- 98.Le T., Winham C.L., Andromidas F., Silver A.C., Jellison E.R., Levesque A.A., Koob A.O. Chimera RNA interference knockdown of γ-synuclein in human cortical astrocytes results in mitotic catastrophe. Neural Regen. Res. 2020;15(10):1894–1902. doi: 10.4103/1673-5374.280329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Winham C.L., Le T., Jellison E.R., Silver A.C., Levesque A.A., Koob A.O. γ-Synuclein Induces human cortical astrocyte proliferation and subsequent BDNF expression and release. Neuroscience. 2019;410:41–54. doi: 10.1016/j.neuroscience.2019.04.057. [DOI] [PubMed] [Google Scholar]
- 100.Denechaud P-D., Fajas L., Giralt A. E2F1, a novel regulator of metabolism. Front. Endocrinol. (Lausanne) 2017;8:311. doi: 10.3389/fendo.2017.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xie Q., Peng S., Tao L., Ruan H., Yang Y., Li T-M., Adams U., Meng S., Bi X., Dong M-Q., Yuan Z. E2F transcription factor 1 regulates cellular and organismal senescence by inhibiting Forkhead box O transcription factors. J. Biol. Chem. 2014;289(49):34205–34213. doi: 10.1074/jbc.M114.587170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ertosun M.G., Hapil F.Z., Osman Nidai O. E2F1 transcription factor and its impact on growth factor and cytokine signaling. Cytokine Growth Factor Rev. 2016;31:17–25. doi: 10.1016/j.cytogfr.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 103.Wu J., Sabirzhanov B., Stoica B.A., Lipinski M.M., Zhao Z., Zhao S., Ward N., Yang D., Faden A.I. Ablation of the transcription factors E2F1-2 limits neuroinflammation and associated neurological deficits after contusive spinal cord injury. Cell Cycle. 2015;14(23):3698–3712. doi: 10.1080/15384101.2015.1104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang R., Müller C., Huynh V., Fung Y.K., Yee A.S., Koeffler H.P. Functions of cyclin A1 in the cell cycle and its interactions with transcription factor E2F-1 and the Rb family of proteins. Mol. Cell. Biol. 1999;19(3):2400–2407. doi: 10.1128/MCB.19.3.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyajima M., Nornes H.O., Sato K., Neuman T. Overexpression of E2F1 in astrocytes leads to neoplastic transformation and changes in expression of retinoblastoma family members. J. Neurosci. Res. 1996;46(1):108–113. doi: 10.1002/(SICI)1097-4547(19961001)46:1<108:AID-JNR13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 106.Alonso M.M., Fueyo J., Shay J.W., Aldape K.D., Jiang H., Lee O-H., Johnson D.G., Xu J., Kondo Y., Kanzawa T., Kyo S., Bekele B.N., Zhou X., Nigro J., McDonald J.M., Yung W.K., Gomez-Manzano C. Expression of transcription factor E2F1 and telomerase in glioblastomas: mechanistic linkage and prognostic significance. J. Natl. Cancer Inst. 2005;97(21):1589–1600. doi: 10.1093/jnci/dji340. [DOI] [PubMed] [Google Scholar]
- 107.Parr M.J., Manome Y., Tanaka T., Wen P., Kufe D.W., Kaelin W.G.J., Jr, Fine H.A. Tumor-selective transgene expression in vivo mediated by an E2F-responsive adenoviral vector. Nat. Med. 1997;3(10):1145–1149. doi: 10.1038/nm1097-1145. [DOI] [PubMed] [Google Scholar]
- 108.Teplyuk N.M., Uhlmann E.J., Wong A.H-K., Karmali P., Basu M., Gabriely G., Jain A., Wang Y., Chiocca E.A., Stephens R., Marcusson E., Yi M., Krichevsky A.M. MicroRNA-10b inhibition reduces E2F1-mediated transcription and miR-15/16 activity in glioblastoma. Oncotarget. 2015;6(6):3770–3783. doi: 10.18632/oncotarget.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Okun E., Griffioen K.J., Lathia J.D., Tang S-C., Mattson M.P., Arumugam T.V. Toll-like receptors in neurodegeneration. Brain Res. Brain Res. Rev. 2009;59(2):278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abarca-Merlin D.M., Maldonado-Bernal C., Alvarez-Arellano L. Toll-like receptors as therapeutic targets in central nervous system tumors. BioMed Res. Int. 2019;2019:5286358. doi: 10.1155/2019/5286358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu Y-C., Yeh W-C., Ohashi P.S. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 112.Krasowska-Zoladek A., Banaszewska M., Kraszpulski M., Konat G.W. Kinetics of inflammatory response of astrocytes induced by TLR 3 and TLR4 ligation. J. Neurosci. Res. 2007;85(1):205–212. doi: 10.1002/jnr.21088. [DOI] [PubMed] [Google Scholar]
- 113.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deguine J., Barton G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014;6:97. doi: 10.12703/P6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lim C-A., Yao F., Wong J.J-Y., George J., Xu H., Chiu K.P., Sung W-K., Lipovich L., Vega V.B., Chen J., Shahab A., Zhao X.D., Hibberd M., Wei C.L., Lim B., Ng H.H., Ruan Y., Chin K.C. Genome-wide mapping of RELA(p65) binding identifies E2F1 as a transcriptional activator recruited by NF-kappaB upon TLR4 activation. Mol. Cell. 2007;27(4):622–635. doi: 10.1016/j.molcel.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 116.Lattke M., Reichel S.N., Baumann B. NF-κB-mediated astrocyte dysfunction initiates neurodegeneration. Oncotarget. 2017;8(31):50329–50330. doi: 10.18632/oncotarget.18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pützer B.M. Targeting E2F1 death signaling: opposing role in cancer control and neurodegeneration. Discov. Med. 2006;6(33):123–127. [PubMed] [Google Scholar]
- 118.Lee J-U., Kim L-K., Choi J-M. Revisiting the concept of targeting NFAT to control T cell immunity and autoimmune diseases. Front. Immunol. 2018;9:2747. doi: 10.3389/fimmu.2018.02747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mognol G.P., Carneiro F.R.G., Robbs B.K., Faget D.V., Viola J.P.B. Cell cycle and apoptosis regulation by NFAT transcription factors: new roles for an old player. Cell Death Dis. 2016;7(4):e2199. doi: 10.1038/cddis.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abdul H.M., Sama M.A., Furman J.L., Mathis D.M., Beckett T.L., Weidner A.M., Patel E.S., Baig I., Murphy M.P., LeVine H., III, Kraner S.D., Norris C.M. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J. Neurosci. 2009;29(41):12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pleiss M.M., Sompol P., Kraner S.D., Abdul H.M., Furman J.L., Guttmann R.P., Wilcock D.M., Nelson P.T., Norris C.M. Calcineurin proteolysis in astrocytes: Implications for impaired synaptic function. Biochim. Biophys. Acta. 2016;1862(9):1521–1532. doi: 10.1016/j.bbadis.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Griffin W.S., Sheng J.G., Royston M.C., Gentleman S.M., McKenzie J.E., Graham D.I., Roberts G.W., Mrak R.E. Glial-neuronal interactions in Alzheimer’s disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998;8(1):65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Su Z.Z., Leszczyniecka M., Kang D.C., Sarkar D., Chao W., Volsky D.J., Fisher P.B. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2). Proc. Natl. Acad. Sci. USA. 2003;100(4):1955–1960. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sompol P., Furman J.L., Pleiss M.M., Kraner S.D., Artiushin I.A., Batten S.R., Quintero J.E., Simmerman L.A., Beckett T.L., Lovell M.A., Murphy M.P., Gerhardt G.A., Norris C.M. Calcineurin/NFAT signaling in activated astrocytes drives network hyperexcitability in Aβ-Bearing Mice. J. Neurosci. 2017;37(25):6132–6148. doi: 10.1523/JNEUROSCI.0877-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Furman J.L., Sompol P., Kraner S.D., Pleiss M.M., Putman E.J., Dunkerson J., Mohmmad Abdul H., Roberts K.N., Scheff S.W., Norris C.M. Blockade of astrocytic Calcineurin/NFAT signaling helps to normalize hippocampal synaptic function and plasticity in a rat model of traumatic brain injury. J. Neurosci. 2016;36(5):1502–1515. doi: 10.1523/JNEUROSCI.1930-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sompol P., Norris C.M. Ca2+, astrocyte activation and calcineurin/NFAT signaling in age-related Neurodegenerative diseases. Front. Aging Neurosci. 2018;10:199. doi: 10.3389/fnagi.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luo J., Sun L., Lin X., Liu G., Yu J., Parisiadou L., Xie C., Ding J., Cai H. A calcineurin- and NFAT-dependent pathway is involved in α-synuclein-induced degeneration of midbrain dopaminergic neurons. Hum. Mol. Genet. 2014;23(24):6567–6574. doi: 10.1093/hmg/ddu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mancini M., Toker A. NFAT proteins: Emerging roles in cancer progression. Nat. Rev. Cancer. 2009;9(11):810–820. doi: 10.1038/nrc2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tie X., Han S., Meng L., Wang Y., Wu A. NFAT1 is highly expressed in, and regulates the invasion of, glioblastoma multiforme cells. PLoS One. 2013;8(6):e66008. doi: 10.1371/journal.pone.0066008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Neria F., del Carmen Serrano-Perez M., Velasco P., Urso K., Tranque P., Cano E. NFATc3 promotes Ca(2+)-dependent MMP3 expression in astroglial cells. Glia. 2013;61(7):1052–1066. doi: 10.1002/glia.22494. [DOI] [PubMed] [Google Scholar]
- 131.Zanconato F., Forcato M., Battilana G., Azzolin L., Quaranta E., Bodega B., Rosato A., Bicciato S., Cordenonsi M., Piccolo S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 2015;17(9):1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shoshan E., Braeuer R.R., Kamiya T., Mobley A.K., Huang L., Vasquez M.E., Velazquez-Torres G., Chakravarti N., Ivan C., Prieto V., Villares G.J., Bar-Eli M. NFAT1 directly regulates il8 and mmp3 to promote melanoma tumor growth and metastasis. Cancer Res. 2016;76(11):3145–3155. doi: 10.1158/0008-5472.CAN-15-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vardjan N., Verkhratsky A., Zorec R. Astrocytic pathological calcium homeostasis and impaired vesicle trafficking in neurodegeneration. Int. J. Mol. Sci. 2017;18(2):E358. doi: 10.3390/ijms18020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Volterra A., Liaudet N., Savtchouk I. Astrocyte Ca2+ Signalling: An unexpected complexity. Nat. Rev. Neurosci. 2014;•••:327–335. doi: 10.1038/nrn3725. [DOI] [PubMed] [Google Scholar]
- 135.Giaume C., Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24(1):50–64. doi: 10.1002/(SICI)1098-1136(199809)24:1<50:AID-GLIA6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 136.Quintana A.R., Wang D., Forbes J.E., Waxham M.N. Kinetics of calmodulin binding to calcineurin. Biochem. Biophys. Res. Commun. 2005;334(2):674–680. doi: 10.1016/j.bbrc.2005.06.152. [DOI] [PubMed] [Google Scholar]
- 137.Centemeri C., Bolego C., Abbracchio M.P., Cattabeni F., Puglisi L., Burnstock G., Nicosia S. Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br. J. Pharmacol. 1997;121(8):1700–1706. doi: 10.1038/sj.bjp.0701293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abbracchio M.P., Ceruti S. Roles of P2 receptors in glial cells: Focus on astrocytes. Purinergic Signal. 2006;2(4):595–604. doi: 10.1007/s11302-006-9016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Quintas C., Fraga S., Gonçalves J., Queiroz G. P2Y receptors on astrocytes and microglia mediate opposite effects in astroglial proliferation. Purinergic Signal. 2011;7(2):251–263. doi: 10.1007/s11302-011-9235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Neary J.T., Shi Y-F., Kang Y., Tran M.D. Opposing effects of P2X(7) and P2Y purine/pyrimidine-preferring receptors on proliferation of astrocytes induced by fibroblast growth factor-2: Implications for CNS development, injury, and repair. J. Neurosci. Res. 2008;86(14):3096–3105. doi: 10.1002/jnr.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chorna N.E., Santiago-Pérez L.I., Erb L., Seye C.I., Neary J.T., Sun G.Y., Weisman G.A., González F.A. P2Y receptors activate neuroprotective mechanisms in astrocytic cells. J. Neurochem. 2004;91(1):119–132. doi: 10.1111/j.1471-4159.2004.02699.x. [DOI] [PubMed] [Google Scholar]
- 142.Kim B., Jeong H.K., Kim J.H., Lee S.Y., Jou I., Joe E.H. Uridine 5′-diphosphate induces chemokine expression in microglia and astrocytes through activation of the P2Y6 receptor. J. Immunol. 2011;186(6):3701–3709. doi: 10.4049/jimmunol.1000212. [DOI] [PubMed] [Google Scholar]
- 143.Pérez-Ortiz J.M., Serrano-Pérez M.C., Pastor M.D., Martín E.D., Calvo S., Rincón M., Tranque P. Mechanical lesion activates newly identified NFATc1 in primary astrocytes: Implication of ATP and purinergic receptors. Eur. J. Neurosci. 2008;27(9):2453–2465. doi: 10.1111/j.1460-9568.2008.06197.x. [DOI] [PubMed] [Google Scholar]
- 144.Johnson P.F. Molecular stop signs: Regulation of cell-cycle arrest by C/EBP transcription factors. J. Cell Sci. 2005;118(Pt 12):2545–2555. doi: 10.1242/jcs.02459. [DOI] [PubMed] [Google Scholar]
- 145.Ramji D.P., Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem. J. 2002;365(Pt 3):561–575. doi: 10.1042/bj20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang S.M., Lee Y.C., Ko C.Y., Lai M.D., Lin D.Y., Pao P.C., Chi J.Y., Hsiao Y.W., Liu T.L., Wang J.M. Increase of zinc finger protein 179 in response to CCAAT/enhancer binding protein delta conferring an antiapoptotic effect in astrocytes of Alzheimer’s disease. Mol. Neurobiol. 2015;51(1):370–382. doi: 10.1007/s12035-014-8714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang S-M., Lim S-W., Wang Y-H., Lin H-Y., Lai M-D., Ko C-Y., Wang J-M. Astrocytic CCAAT/Enhancer-binding protein delta contributes to reactive oxygen species formation in neuroinflammation. Redox Biol. 2018;16:104–112. doi: 10.1016/j.redox.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fields J., Ghorpade A. C/EBPβ regulates multiple IL-1β-induced human astrocyte inflammatory genes. J. Neuroinflammation. 2012;9:177. doi: 10.1186/1742-2094-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ejarque-Ortiz A., Medina M.G., Tusell J.M., Pérez-González A.P., Serratosa J., Saura J. Upregulation of CCAAT/enhancer binding protein beta in activated astrocytes and microglia. Glia. 2007;55(2):178–188. doi: 10.1002/glia.20446. [DOI] [PubMed] [Google Scholar]
- 150.Morales-Garcia J.A., Gine E., Hernandez-Encinas E., Aguilar-Morante D., Sierra-Magro A., Sanz-SanCristobal M., Alonso-Gil S., Sanchez-Lanzas R., Castaño J.G., Santos A., Perez-Castillo A. CCAAT/Enhancer binding protein β silencing mitigates glial activation and neurodegeneration in a rat model of Parkinson’s disease. Sci. Rep. 2017;7(1):13526. doi: 10.1038/s41598-017-13269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cardinaux J.R., Allaman I., Magistretti P.J. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia. 2000;29(1):91–97. doi: 10.1002/(SICI)1098-1136(20000101)29:1<91:AID-GLIA9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 152.Pulido-Salgado M., Vidal-Taboada J.M., Saura J. C/EBPβ and C/EBPδ transcription factors: Basic biology and roles in the CNS. Prog. Neurobiol. 2015;132:1–33. doi: 10.1016/j.pneurobio.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 153.Homma J., Yamanaka R., Yajima N., Tsuchiya N., Genkai N., Sano M., Tanaka R. Increased expression of CCAAT/enhancer binding protein beta correlates with prognosis in glioma patients. Oncol. Rep. 2006;15(3):595–601. [PubMed] [Google Scholar]
- 154.Valori C.F., Guidotti G., Brambilla L., Rossi D. Astrocytes: Emerging therapeutic targets in neurological disorders. Trends Mol. Med. 2019;25(9):750–759. doi: 10.1016/j.molmed.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 155.Yun S.P., Kam T-I., Panicker N., Kim S., Oh Y., Park J-S., Kwon S-H., Park Y.J., Karuppagounder S.S., Park H., Kim S., Oh N., Kim N.A., Lee S., Brahmachari S., Mao X., Lee J.H., Kumar M., An D., Kang S.U., Lee Y., Lee K.C., Na D.H., Kim D., Lee S.H., Roschke V.V., Liddelow S.A., Mari Z., Barres B.A., Dawson V.L., Lee S., Dawson T.M., Ko H.S. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018;24(7):931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Takano T., Tian G.F., Peng W., Lou N., Libionka W., Han X., Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006;9(2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 157.Yan C., Higgins P.J. Drugging the undruggable: transcription therapy for cancer. Biochim. Biophys. Acta. 2013;1835(1):76–85. doi: 10.1016/j.bbcan.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lambert M., Jambon S., Depauw S., David-Cordonnier M-H. Targeting transcription factors for cancer treatment. Molecules. 2018;23(6):E1479. doi: 10.3390/molecules23061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dai J., Punchihewa C., Mistry P., Ooi A.T., Yang D. Novel DNA bis-intercalation by MLN944, a potent clinical bisphenazine anticancer drug. J. Biol. Chem. 2004;279(44):46096–46103. doi: 10.1074/jbc.M404053200. [DOI] [PubMed] [Google Scholar]
- 160.Lee M., Park J. Regulation of NFAT activation: a potential therapeutic target for immunosuppression. Mol. Cells. 2006;22(1):1–7. [PubMed] [Google Scholar]
- 161.Xie X., Bansal N., Shaik T., Kerrigan J.E., Minko T., Garbuzenko O., Abali E.E., Johnson-Farley N., Banerjee D., Scotto K.W., Bertino J.R. A novel peptide that inhibits E2F transcription and regresses prostate tumor xenografts. Oncotarget. 2014;5(4):901–907. doi: 10.18632/oncotarget.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hu B., Zhong L., Weng Y., Peng L., Huang Y., Zhao Y., Liang X-J. Therapeutic siRNA: state of the art. Signal Transduct. Target. Ther. 2020;5(1):101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Das M., Musetti S., Huang L. RNA interference-based cancer drugs: The roadblocks, and the “Delivery” of the promise. Nucleic Acid Ther. 2019;29(2):61–66. doi: 10.1089/nat.2018.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Singh P., Singh A., Shah S., Vataliya J., Mittal A., Chitkara D. RNA interference nanotherapeutics for treatment of glioblastoma multiforme. Mol. Pharm. 2020;17(11):4040–4066. doi: 10.1021/acs.molpharmaceut.0c00709. [DOI] [PubMed] [Google Scholar]
- 165.Grafals-Ruiz N., Rios-Vicil C.I., Lozada-Delgado E.L., Quiñones-Díaz B.I., Noriega-Rivera R.A., Martínez-Zayas G., Santana-Rivera Y., Santiago-Sánchez G.S., Valiyeva F., Vivas-Mejía P.E. Brain targeted gold liposomes improve rnai delivery for glioblastoma. Int. J. Nanomedicine. 2020;15:2809–2828. doi: 10.2147/IJN.S241055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sadeghi M., Ranjbar B., Ganjalikhany M.R., Khan M. F.; Schmitz, U.; Wolkenhauer, O.; Gupta, S.K. MicroRNA and transcription factor gene regulatory network analysis reveals key regulatory elements associated with prostate cancer progression. PLoS One. 2016;11(12):e0168760. doi: 10.1371/journal.pone.0168760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ramchandran R., Chaluvally-Raghavan P. miRNA-mediated RNA activation in mammalian cells. Adv. Exp. Med. Biol. 2017;983:81–89. doi: 10.1007/978-981-10-4310-9_6. [DOI] [PubMed] [Google Scholar]
- 168.Kannambath S. Micro-RNA feedback loops modulating the calcineurin/NFAT signaling pathway. Noncoding RNA. 2016;2(2):E3. doi: 10.3390/ncrna2020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gao Y. Liu, B.; Feng, L.; Sun, B.; He, S.; Yang, Y.; Wu, G.; e, G.; Liu, C.; Gao, Y.; Zhang, E.; Zhu, B. Targeting JUN, CEBPB, and HDAC3: A novel strategy to overcome drug resistance in hypoxic glioblastoma. Front. Oncol. 2019;9:33. doi: 10.3389/fonc.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lopes-Ramos C.M., Barros B.P., Koyama F.C., Carpinetti P.A., Pezuk J., Doimo N.T.S., Habr-Gama A., Perez R.O., Parmigiani R.B. E2F1 somatic mutation within miRNA target site impairs gene regulation in colorectal cancer. PLoS One. 2017;12(7):e0181153. doi: 10.1371/journal.pone.0181153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sharma S., Lu H-C. microRNAs in Neurodegeneration: Current findings and potential impacts. J. Alzheimers Dis. Parkinsonism. 2018;8(1):420. doi: 10.4172/2161-0460.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang X-W., Hu L-F., Hao J., Liao L-Q., Chiu Y-T., Shi M., Wang Y. A microRNA-inducible CRISPR-Cas9 platform serves as a microRNA sensor and cell-type-specific genome regulation tool. Nat. Cell Biol. 2019;21(4):522–530. doi: 10.1038/s41556-019-0292-7. [DOI] [PubMed] [Google Scholar]
- 173.von Jonquieres G., Mersmann N., Klugmann C.B., Harasta A.E., Lutz B., Teahan O., Housley G.D., Fröhlich D., Krämer-Albers E-M., Klugmann M. Glial promoter selectivity following AAV-delivery to the immature brain. PLoS One. 2013;8(6):e65646. doi: 10.1371/journal.pone.0065646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Koh W., Park Y.M., Lee S.E., Lee C.J. AAV-Mediated astrocyte-specific gene expression under human ALDH1L1 promoter in mouse thalamus. Exp. Neurobiol. 2017;26(6):350–361. doi: 10.5607/en.2017.26.6.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Colin A., Faideau M., Dufour N., Auregan G., Hassig R., Andrieu T., Brouillet E., Hantraye P., Bonvento G., Déglon N. Engineered lentiviral vector targeting astrocytes in vivo. Glia. 2009;57(6):667–679. doi: 10.1002/glia.20795. [DOI] [PubMed] [Google Scholar]
- 176.Merienne N., Delzor A., Viret A., Dufour N., Rey M., Hantraye P., Déglon N. Gene transfer engineering for astrocyte-specific silencing in the CNS. Gene Ther. 2015;22(10):830–839. doi: 10.1038/gt.2015.54. [DOI] [PubMed] [Google Scholar]
- 177.Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27(1):59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Samaranch L., Salegio E.A., San Sebastian W., Kells A.P., Foust K.D., Bringas J.R., Lamarre C., Forsayeth J., Kaspar B.K., Bankiewicz K.S. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum. Gene Ther. 2012;23(4):382–389. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee Y., Messing A., Su M., Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56(5):481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- 180.Taschenberger G., Tereshchenko J., Kügler S. A MicroRNA124 target sequence restores astrocyte specificity of gfaABC1D-Driven transgene expression in AAV-mediated gene transfer. Mol. Ther. Nucleic Acids. 2017;8:13–25. doi: 10.1016/j.omtn.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Griffin J.M., Fackelmeier B., Fong D.M., Mouravlev A., Young D., O’Carroll S.J. Astrocyte-selective AAV gene therapy through the endogenous GFAP promoter results in robust transduction in the rat spinal cord following injury. Gene Ther. 2019;26(5):198–210. doi: 10.1038/s41434-019-0075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ravindra Kumar S., Miles T.F., Chen X., Brown D., Dobreva T., Huang Q., Ding X., Luo Y., Einarsson P.H., Greenbaum A., Jang M.J., Deverman B.E., Gradinaru V. Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nat. Methods. 2020;17(5):541–550. doi: 10.1038/s41592-020-0799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Deverman B.E., Pravdo P.L., Simpson B.P., Kumar S.R., Chan K.Y., Banerjee A., Wu W-L., Yang B., Huber N., Pasca S.P., Gradinaru V. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016;34(2):204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kunze C., Börner K., Kienle E., Orschmann T., Rusha E., Schneider M., Radivojkov-Blagojevic M., Drukker M., Desbordes S., Grimm D., Brack-Werner R. Synthetic AAV/CRISPR vectors for blocking HIV-1 expression in persistently infected astrocytes. Glia. 2018;66(2):413–427. doi: 10.1002/glia.23254. [DOI] [PubMed] [Google Scholar]
- 185.Cannon J.R., Sew T., Montero L., Burton E.A., Greenamyre J.T. Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. Exp. Neurol. 2011;228(1):41–52. doi: 10.1016/j.expneurol.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kang K., Peng X., Zhang X., Wang Y., Zhang L., Gao L., Weng T., Zhang H., Ramchandran R., Raj J.U., Gou D., Liu L. MicroRNA-124 suppresses the transactivation of nuclear factor of activated T cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J. Biol. Chem. 2013;288(35):25414–25427. doi: 10.1074/jbc.M113.460287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jensen S.A., Day E.S., Ko C.H., Hurley L.A., Luciano J.P., Kouri F.M., Merkel T.J., Luthi A.J., Patel P.C., Cutler J.I., Daniel W.L., Scott A.W., Rotz M.W., Meade T.J., Giljohann D.A., Mirkin C.A., Stegh A.H. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 2013;5(209):209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gu J., Al-Bayati K., Ho E.A. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv. Transl. Res. 2017;7(4):497–506. doi: 10.1007/s13346-017-0368-5. [DOI] [PubMed] [Google Scholar]
- 189.Tanaka H., Nakatani T., Furihata T., Tange K., Nakai Y., Yoshioka H., Harashima H., Akita H. In vivo introduction of mRNA encapsulated in lipid nanoparticles to brain neuronal cells and astrocytes via intracerebroventricular administration. Mol. Pharm. 2018;15(5):2060–2067. doi: 10.1021/acs.molpharmaceut.7b01084. [DOI] [PubMed] [Google Scholar]