Abstract
According to the World Health Organization, Traumatic brain injury (TBI) is the major cause of death and disability and will surpass the other diseases by the year 2020. Patients who suffer TBI face many difficulties which negatively affect their social and personal life. TBI patients suffer from changes in mood, impulsivity, poor social judgment and memory deficits. Both open and closed head injuries have their own consequences. Open head injury associated problems are specific in nature e.g. loss of motor functions whereas closed head injuries are diffused in nature like poor memory, problems in concentration etc. Brain injury may have a detrimental effect on the biochemical processes responsible for the homeostatic and physiological disturbances in the brain. Although significant research has been done in order to decrease the overall TBI-related mortality, many individuals suffer from a life-long disability. In this article, we have discussed the causes of TBI, its consequence and the pathobiology of secondary injury. We have also tried to discuss the evidence-based strategies which are shown to decline the devastating consequences of TBI.
Keywords: Traumatic Brain Injury (TBI), pathology of brain injuries, secondary cascade, excitotoxicity, inflammation, pharmacological therapy
1. INTRODUCTION
Traumatic brain injury (TBI) is one of the major causes of morbidity and mortality, globally. It has a huge socio-economic burden on the healthcare system worldwide. It has been estimated that about 69 million people suffer from TBI each year, worldwide out of which Mild TBI cases are nearly 55.9 million while 5.48 million people suffer from severe TBI based on an estimate [1]. In the US alone, the prevalence of TBI progressed from 0.5 million in the year 2002 to over 2.3-3.17 million individuals in the year 2016-2017[2]. A meta-analysis reported worldwide prevalence of TBI by World Health Organization’s (WHO) region wise classification of the countries showing highest incidence of TBI in regions of America-United States and Canada (1299 cases/ 100,000 people, 95% CI) and European region with 1012 cases/100,000 people (95% CI) while the lowest in African region with 801 cases/100,000 people (95% CI). The extrapolation of this data to regional population showed highest volume of TBI in South-east Asian and Western Pacific regions, annually with 18.3 million and 17.3 million cases, respectively [1]. The incident of TBI in early life has been found to be associated later with the onset of neurodegenerative disorders. TBI results from the penetration of any external physical force into the head causing rapid movement of the brain within the skull. This causes a primary injury that affects neuronal cells, blood vessels, microglia and astrocytes [3]. A severe primary injury initiates a complex cascade of cellular and molecular events including inflammatory, neurochemical and metabolic alterations which contribute to delayed secondary injury [4-12]. Primary injury is a non-reversible and non-curable whereas secondary injury is mostly reversible and can be prevented with suitable surgical interventions and intensive therapeutic measures [13, 14].
The term TBI is more complex than it seems to be and includes coup-contrecoup brain injury, concussion, brain contusion, diffuse axonal injury (DAI), second impact syndrome (SIS), shaken baby syndrome and penetrating injury. A coup-contrecoup brain injury occurs both at the site of impact as well as at the opposite side of the brain. Examples for coup-contrecoup brain injuries include serious car accidents, forceful falls, blows to the head and acts of violence. A concussion is the most common brain injury, which affects brain functioning temporarily. It is a mild injury and usually associated with headaches, nausea, blurry vision, trouble with thinking, disturbances in memory and sleep or mood changes. A contusion is another form of mild injuries that involves bleeding under the skin and often occur in conjunction with the concussion. Axonal damage after TBI may be focal, multifocal or diffuse. Whereas, diffuse axonal injury, is a type of TBI that happens when the brain rapidly shakes inside the skull and the long connecting fibres (axons) in the brain get damaged. DAI may cause injury to many parts of the brain and may even induce coma as a resultant effect. One of the rarest types of brain injury that is mainly found in athletes is SIS. A second brain injury to the patients of SIS may lead to diffuse cerebral swelling, brain herniation and even death within a few minutes. Apart from the individual’s age and gender, various other factors including severity, location and type of injury play an important role in determining the outcome of TBI [2, 7]. The severity of TBI has been categorized based on the Glasgow Coma Scale (GCS). Patients are scored based on the clinical symptoms. It is the overall score that decides whether the injury is mild (score: 13-15), moderate (score: 9-12) or severe (score: <9) [15]. Depending upon the type and severity of TBI, the signs and symptoms may appear immediately after the TBI or may take some days or even weeks. In mild cases, there might be some temporary changes that last only for a few seconds to a few minutes while in severe cases the symptoms persist for several minutes and sometimes even to hours. Patients who survive with TBI often face problems such as anxiety, memory impairments, and behavioural changes. Pharmacological interventions have been promising in dealing with sub-acute as well as chronic phases of TBI. Pharmacological interventions in sub-acute phases aim to prevent the secondary injury whereas in the chronic phase they mainly help the patient to combat the complications of secondary brain injury. Despite the complex nature of TBI, research has been able to explore some of the factors that are responsible for the TBI mediated consequences across the life span of an individual.
1.1. Factors Affecting the Outcome of TBI
1.1.1. Polytrauma
Multi-trauma/polytrauma is defined as the condition, which involves significant injury to more than one body region and is a common consequence of vehicle accidents, war injuries, and slips/falls [16]. Most common type of multi-trauma is traumatic brain injury associated with bone fracture. These co-existing conditions induce a complex post-fracture healing process [17, 18]. TBI patients with skeletal injuries are associated with higher level of serum pyridinolene cross-linked carboxy-terminal telopeptide (1CTP) and insulin-like growth factor binding protein-3 (IGFBP-3) which suggests decreased collagen breakdown in TBI patients [17]. Similarly, extracranial injury may have a potential influence on TBI outcome as disrupted blood-brain barrier (BBB) and immune-privilege brain become vulnerable to secondary damage after TBI [19]. Immune cells located at the site of fracture proliferate and secrete a range of pro-inflammatory cytokines into the blood circulation including interleukin (IL)-1β, tumour necrosis factor (TNF)-α and IL-6 that evokes an extensive inflammatory response [20]. Considering the compromised endothelial integrity of BBB post-TBI, cytokines may have unprecedented access into the injured brain, modulate the neuroinflammatory response and worsens TBI outcomes [21-23]. Few animal model studies reported increased levels of circulating inflammatory cytokines when peripheral bone fracture and TBI were combined. A novel mouse model of polytrauma involving the combined injuries of tibial fracture and closed-skull TBI demonstrated that mice afflicted with both a tibial fracture and TBI had worsened behavioural abnormalities and brain damage compared with mice given only a TBI, due to the presence of aggravated neuroinflammation, edema, and BBB disruption [24].
1.1.2. Infection
Followed by a moderate or severe TBI, most patients require hospitalization especially in an intensive care unit (ICU). As per the Centres for Disease Control and Prevention (CDC) reports, approximately 2.87 million TBI related hospital visits occurred in 2014, out of which 0.288 million TBI related hospitalization. Few findings from experimental models where induction of a peripheral infection or sepsis have been shown to increase the mortality rate and worsen neurobehavioral outcomes in rodents with TBI. During the period of hospital stay, patients are at risk of developing hospital-acquired (nosocomial) infections, especially when the stay exceeds 7 days of ICU hospitalization. Infections acquired after a TBI lead to longer hospitalization and ICU stays, from 6.5 days in non-infected patients with versus 15.2 days in infected patients [25]. Hospital-acquired pneumonia has been reported to worsen post-TBI outcomes and lead to increased intracranial hypertension leading to a longer hospital stay when compared to non-infected TBI patients. Patients with TBI needs regular mechanical ventilation, to avoid airway obstruction and exacerbation of injuries [26]. Ventilator use is the most common cause of in-patient respiratory tract infections, ventilator acquired-pneumonia (VAP) is associated with the nasal entry of pathogens such as Pseudomonas aeruginosa, Staphylococcus aureus, Enterobacteriaceae, Streptococcus pneumonia and Haemophilus influenza [27]. Device and hospitalization-associated exposure of CSF to the environment are independent risk-factor for infections like meningitis and surgical site infection (SSI) [28]. A study reported that insertion of ventricular drain has led to the 3.6-fold and 5.7-fold rise in rates of SSI and meningitis, respectively; while insertion of lumbar drain caused a 30.4-fold and 48.9-fold increase in SSI and meningitis, respectively [29].
2. PHYSIOLOGICAL AND PATHOBIOLOGICAL CONSEQUENCES AFTER TBI
It is very crucial to accentuate that human TBI is a heterogeneous group of disorder with a varying magnitude of pathogenic combinations. The sequela of events resulting from the primary insults to brain and the consequent trickling of secondary cascades, in turn, potentiates the progression of primary pathogenic factors. The primary brain damage and secondary pathological cascade consequent to TBI are summarized in Fig. (1). These consequences are further categorized into physical and biochemical changes.
Fig. (1).
A cascade of the primary and secondary pathological events after traumatic brain injury (TBI). Primary injury includes structural damage that cannot be reverted by therapeutic interventions, and only sensitive to preventive measures. On the other hand, early secondary injury includes necrotic and apoptotic cell death due to the release of excitatory neurotransmitters leading to oxidative stress, mitochondrial dysfunction, damage to the structural proteins and inflammation. Although the short-term inflammatory phase is beneficial because of the activation of microglia which causes phagocytosis of debris and regeneration. But the prolonged inflammation worsens the outcome of TBI. The intermediate phase which includes the release of TNF-α and IL-β within 1 hour of injury and persists for three weeks overlaps with early features of TBI. At last, all these pathogenic events culminate into progressive tissue loss and behavioural changes like motor, sensory, cognitive deficits, post-traumatic epilepsy and electrophysiological changes. AMPA: α-amino-3-hydroxy-5-methyl-4-isooxazolepropionic acid Receptor; Cyt C: Cytochrome c; ER: Endoplasmic reticulum; ICP: Intracranial pressure; NMDA: N-methyl-D-aspartate Receptor; ROS: Reactive oxygen species. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
2.1. Physical Changes
2.1.1. Changes in Cerebral Blood Flow
The brain utilizes approximately 20% of the entire oxygen volume consumed by the body supplied through the cerebral blood flow (CBF) [30, 31]. CBF is auto-regulated through negative feedback mechanism under the control of myogenic, sympathetic mechanism and metabolic processes. Under normal physiological condition where it remains directly proportional to Cerebral Perfusion Pressure (CPP) and inversely proportional to Cerebral Vascular Resistance (CVR) [31-33]. CPP represents the difference between mean arterial pressure (MAP) and intracranial pressure (ICP) [34]. As a consequence of TBI, cerebral blood flow gets impaired due to changes in CPP, marked by low ICP (5mm Hg or less) and altered MAP levels, and an increase in CVR. CPP changes mainly because of two reasons- Firstly, due to reduction in MAP resulting because of hypotension caused by impaired physiological autoregulation mechanisms including prostaglandin dependent vascular regulation [8, 35, 36], as well as reduction in nitric oxide and cholinergic neurotransmission [37, 38]. Secondly, because of edematous fluid accumulation leading to a dramatically increase in ICP. Thus, the changes in MAP/ICP can cause a drastic decrease in CPP. This compromised cerebrovascular autoregulation is unable to maintain the CBF to meet brain metabolic demand [33], and results in brain ischemia which further worsens the neurological outcomes after TBI [39-41]. In the early stages of TBI, hyperaemia may occur which leads to vasoparalysis and a subsequent increase in cerebral blood volume influences the change in ICP. Both cerebral ischemia and hyperaemia show a mismatch between cerebral blood flow and metabolism. For example, low CBF with normal metabolism rate leads to an ischaemic situation and high CBF with normal metabolic rate leads to a hyperemic situation [42-44]. In severe TBI patients, the normobaric and hyperbaric hyperoxia is given to stabilize mitochondrial function, which results in the increased generation of ATP and metabolic rates of oxygen in the brain. In addition, normobaric hyperoxia has no significant impact on the improvement of metabolic rates of oxygen as compared to hyperbaric hyperoxia. Further suggestive of that the demand of oxygen supply is dependent on the magnitude and duration of hyperoxia and might not improve the oxygen demand in areas of low cerebral blood flow [45-47]. Therefore, after TBI, there is an acute hyper-perfusion phase and then ischemic phase that persists for a longer time.
2.1.2. Disruption of the Blood Brain Barrier (BBB)
A continuous layer of brain capillary endothelial cells together with pericytes, a basal lamina and astrocytes form BBB. Tight junctions exist between endothelial cells which form a metabolic and physical barrier restricting the movements of macromolecules between blood and brain and thereby maintain cerebral homeostasis [48]. After TBI, BBB dysfunction may occur as a direct consequence of endothelial cells damage immediately or after several days [22, 35, 49-51]. The processes underlying the disruption of BBB are the production of free radicals and inflammatory mediators (interleukins, TNF-α etc.), up-regulation of matrix metalloproteinases and vascular endothelial growth factor (VEGF) [50]. The increased BBB permeability results in the activation of astrocytes and microglia. The subsequent immune responses involve changes in extracellular homeostasis and neuronal excitability due to alteration in glutamate and potassium buffering or extravasation of inflammatory mediators [52-54]. BBB dysfunction also leads to edema formation and increase in ICP which is responsible for the impairment of cerebral blood flow and ultimately in the development of an ischemic zone and secondary lesion progression.
2.1.3. Edema Formation
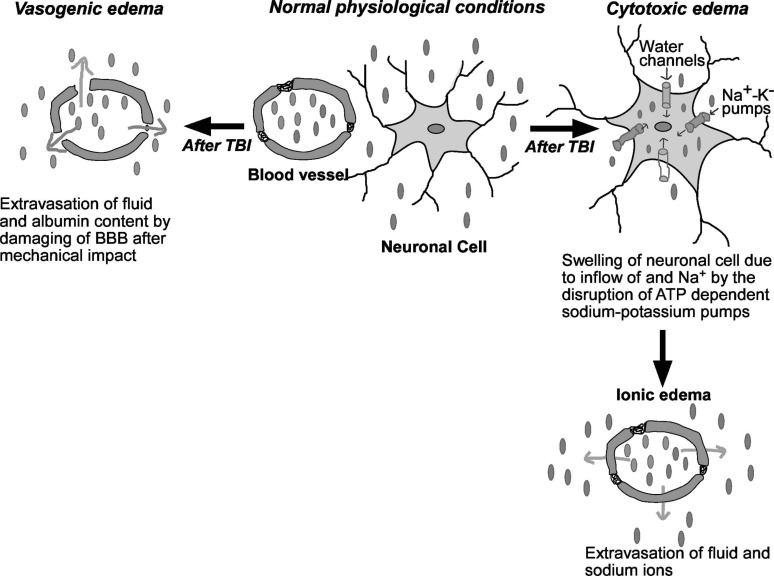
The fluid accumulation inside the brain after TBI is called cerebral edema [55-57]. It is a life-threatening complication and mainly classified into two types: (a) cytotoxic, (b) vasogenic brain edema (Fig. 2).
Fig. (2).
Pathophysiology of vasogenic and cytotoxic edema after traumatic brain injury: Vasogenic edema: After brain insult, disruption of the endothelial tight junctions in the BBB leads to the accumulation of fluid and albumin content in brain parenchyma. Cytotoxic edema: Brain injury induces depletion of ATP production, ultimately responsible for the disturbance in the functioning of ATP-dependent pumps (e.g. Na+-K+ pump). These events cause intra-extracellular ion imbalance. Finally, there is an excessive inflow of Na+ ions and extracellular fluid resulting in cell swelling. Ionic edema: Due to the depletion of extracellular Na+ by the excessive inflow into cells that is a compensatory outflow of Na+ and fluid from blood vessels into cerebral parenchyma. Brown spheres: albumin; Gray spheres: Na+ ions. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
2.1.3.1. Cytotoxic Brain Edema
An increase in intracellular fluid content leading to cellular swelling after TBI is known as cytotoxic brain edema. The failure of the ATP-dependent Na+/K+ pumps due to energy failure is mainly responsible for cytotoxic brain edema. Na+/K+ pump failure leads to an increase in cellular ionic content and an overall increase in cell osmolality which results in the movement of water from the extracellular to the intracellular compartment. Cytotoxic edema is not only responsible for an increase in brain swelling and a rise in ICP but also adversely affects the cellular function by altering intracellular metabolic concentration [58-60]. However, direct quantification of functional outcomes associated with neuronal functions, plasticity and behaviour in diffuse TBI is challenging. As mild to moderate TBI insults does not have any changes in the focal tissue damage and are usually characterised by the axonal swelling and bulbs [61, 62]. After cytotoxic edema, the outpouring of Na+ from blood vessels is hastened as the body tries to compensate for the decrease of extracellular Na+ and fluid [63]. The intravascular Na+ outflow stimulates extravasation of fluid without disruption of BBB and causes fluid accumulation inside the brain parenchyma known as ionic edema. Therefore, cytotoxic edema indirectly causes an increase in brain volume and ICP [64].
2.1.3.2. Vasogenic Brain Edema
It is characterized by mechanical or functional breakdown of BBB [65, 66]. It occurs due to the movement of water from the vasculature to the extracellular space in response to an osmotic gradient created by the leakage of vascular components in the brain parenchyma. It is responsible for an increase in brain water content, brain swelling and ultimately an increase in ICP [59]. Several cell components including membrane channels, auto destructive enzymes, receptors and vascular permeability factors play a role in TBI-induced vasogenic and cytotoxic edema. Some of these are discussed in below section.
2.2. Biochemical Changes
2.2.1. Aquaporins (AQPs)
AQPs are integral components of the cell mammalian membrane and belongs to a family of pore forming proteins [67]. There are 13 AQPs known to exist in mammals, in which AQP1, AQP4 and AQP9 are found to be highly expressed in the brain [68]. AQP1 is highly expressed in the choroid plexus and takes part in the formation of cerebrospinal fluid [69], whereas AQP9 is found in some astrocytic processes at the glia limitans [70]. AQP4 is prominently expressed in the astrocytic foot process surrounding capillaries and involves in maintaining brain water balance by managing water fluxes into and out of the brain parenchyma [71-74]. Increased expression of AQP4 after TBI is responsible for the development of edema [75, 76]. AQP4 plays contrary roles in the pathogenesis of cytotoxic and vasogenic edema. In cytotoxic edema, AQP4 causes the excessive inflow of extracellular fluid into cells whereas in vasogenic edema it helps in the removal of fluid accumulated in the ECF of brain parenchyma by facilitating its reabsorption of fluid. Therefore, AQP4 inhibitors are found to be beneficial in cytotoxic edema by reducing intracellular fluid accumulation, while AQP4 activators are expected to be effective in vasogenic edema acting through clearing the accumulated fluid in the brain parenchyma [64].
2.2.2. Matrix Metalloproteinases (MMPs)
MMPs are a family of zinc-endopeptidases capable of digesting extracellular matrix proteins such as collagen, laminin and fibronectin [77]. Although MMPs support the tissue remodelling by promoting angiogenesis, their over-activation is responsible for the degradation of basal-lamina around brain micro-vessels leading to BBB hyper-permeability [78]. In TBI, the up-regulation of MMPs has been correlated directly to the severity of injury [79-82]. Up-regulation of MMPs especially MMP-9 was observed in astrocytes, microglia, neurons and endothelial cells after experimental brain injury. MMP-9 is also identified as a key-mediator of BBB disruption after brain injury [83-86].
2.2.3. Vasoactive Agents
It is well demonstrated that inflammatory, vasoactive agents can increase the BBB permeability resulting in cerebral edema [87]. Classical as well as neurogenic inflammation, have been found to be involved in producing cerebral edema after brain injuries. In terms of classical inflammation, the bradykinin family of kinins is known for its involvement in the development of edema following acute brain injury [88]. Kallikrein cleaves the kininogen and forms bradykinins with two active peptides (bradykinin and kallidin), producing their actions through two subtypes (B1 and B2 receptors) of bradykinin receptors. Bradykinin peaked within 2 h of TBI whereas bradykinin receptors (B1 and B2) were found to be significantly up-regulated after 24 h of brain insult [89]. Although both B1 and B2 receptors were significantly expressed, only B2 receptors knockout mice had shown a significant reduction in edema and improvement in functional outcomes after TBI [89]. This study suggested that bradykinin B2 receptors play an important role in edema formation after TBI. Treatment with B2 receptor antagonist has also shown a reduction in ICP and contusion volume in a focal contusion model [90]. Tachykinins, the other distinct family of kinins have been suggested to involve in the development of neurogenic inflammation. Neurogenic inflammation is a process that comprises vasodilation, plasma extravasation and neuronal hypersensitivity. It is caused by the variety of neuropeptides released from sensory neurons [91]. Calcitonin gene-related peptide (CGRP) and substance P are the two main neuropeptides implicated in neurogenic inflammation that causes vasodilation and enhances plasma protein extravasation respectively [59].
2.2.4. Sulfonylurea Receptor-1 (SUR-1)
The non-selective (ATP-sensitive) NCCa-ATP channel, was primarily recognized in native reactive astrocytes (NRAs) and later on recognized in neurons and capillary endothelial cells following TBI or stroke. The (SUR-1) receptor regulating NCCa-ATP channel, is a 30-pS channel that allows the conduction of monovalent cations [92, 93]. This channel is not physiologically expressed but gets upregulated in neurons, astrocytes, and capillary endothelial cells after TBI. Even after expression, these channels remain in the inactive state and only get activated after depletion of intracellular energy stores. Activation of these channels leads to cell depolarization, cytotoxic edema and oncotic cell death [94, 95]. SUR-1 receptors present in capillaries also result in the formation of vasogenic edema [92, 96, 97]. Sulfonylurea inhibitors such as glibenclamide are known to exhibit antagonistic action on SUR-1. In TBI, treatment with glibenclamide showed beneficial effects by reducing the edema formation in rats [98-100]. Glibenclamide completed phase 2 clinical trial (ATRAL) and entered phase 3 trial (CHARM) where it seems to be a better alternative compared to the currently available option to treat edema-associated brain swelling i.e. Decompressive Craniectomy [101].
2.2.5. Ionic Homeostasis Imbalance
Immediately after TBI, damage to the neuronal membrane, axonal stretching and opening of voltage-gated K+ channels take place [5]. In addition, non-specific depolarization mediated increase in intracellular Ca2+ ions contribute to the release of the excitatory neurotransmitter including glutamate. The released glutamate acts on N-methyl-D-aspartate (NMDA), D-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and kainate receptors which exacerbate the K+ outflux [102]. Under normal physiological conditions, excessive K+ is taken up by the surrounding glial cells. Extracellular K+ and intracellular Ca2+ increases cortical spreading, metabolic alterations, activation of auto-destructive enzymes, free radical production and decreases ATP production mediating neuronal damage inside the brain [5, 103].
2.2.6. Excitotoxicity
The term excitotoxicity refers to a pathological condition that occurs due to the over-stimulation of the amino acid neurotransmitter receptors especially glutamate, mediating neuronal injury and death [104, 105]. Excitotoxicity is a serious and important neurodegenerative mechanism among all the processes that contribute to both necrotic and apoptotic neuronal cell death following TBI [106-108]. Excitotoxicity attains greater emphasis as it fundamentally suggests the basic mechanism by which the neuronal cells die under pathological conditions. In addition, excitotoxicity represents the massive release of excitatory neurotransmitters in the synaptic and extra-synaptic regions. Glutamate is an important excitatory neurotransmitter, present in small concentration and is highly regulated by mechanisms involving enzymes and transporters in the neuronal and glial cells. However, under pathological conditions like neurodegenerative disorders, TBI, stroke and ischemia these mechanisms are futile in maintaining the amino acid concentration at physiological range in the CNS. As a consequence of oxidative stress, due to rapid Ca2+ influx and excessive ROS generation from the mitochondria. Increased ROS levels result in the breakdown of cell membrane, ultimately influencing the functions of neuronal cells [109-111].
As mentioned earlier, the most devastating feature of excitotoxicity is massive calcium influx and potassium efflux [112]. Neuronal calcium level increases through various mechanisms. TBI is a mechanical injury, calcium enters through the plasma membrane pores formed by tear and shear forces during primary insult [113-115]. The mechanical strain can alter the function of voltage-gated Na+ channels. Increased entry of Na+ ions in the cell and decreased Na+/K+ ATPase pump activity is responsible for the depolarization of the cellular membrane, which then could activate voltage-gated Ca2+ channels. Also, a high intracellular Na+ concentration could cause Na+/Ca2+ exchanger to operate in the reverse direction, which further leads to increased Ca2+ inside the cell [116, 117]. The increased cytoplasmic Ca2+ levels lead to the excessive release of glutamate, which produces neurotoxicity by overstimulating glutamate receptors. On the other end, the dysregulated concentrations of glutamate are also associated with the activation of ion channels (voltage-gated calcium channels) resulting in the rapid influx of Ca2+ ensuing cell death. Moreover, the overstimulation of NMDA receptor is thought to be a major route of Ca2+ entry in the cell [118, 119], and AMPA receptors are primarily more permeable to Na+. However, increased intracellular Na+ levels result in persistent depolarization of the cell membrane, activates NMDA and VGCCs ultimately increases the concentration of cytoplasmic Ca2+. The increased intracellular calcium further results in the release of Ca2+ from intracellular stores like endoplasmic reticulum [108]. This massive rise in intracellular Ca2+ leads to various downstream neurotoxic effects like mitochondrial dysfunction, stimulation of enzymes (caspases, calpains, endonucleases, lipases etc.), production of free radicals, degradation of the integrity of cellular membrane, DNA damage and finally apoptotic and necrotic cell death [108, 120].
2.2.7. Inflammation
Inflammation is the prime or basic immune mechanism, which gets activated in response to invading pathogens or even due to changes in the normal tissue homeostasis. It is usually a beneficial process which protects tissues from harmful substances by restricting their survival and proliferation, ultimately promoting repair and remodelling of tissue matrix [121]. Following tissue injury, M1 stage gets activated which is characterised as the ability of cells to secrete increased pro-inflammatory cytokines and oxidative substances for mediating phagocytosis. The persistent activation of inflammatory responses might result in the potentiation of pro-inflammatory cytokines having deleterious roles in the CNS and periphery [121, 122]. However, extensive and unregulated release of pro-inflammatory cytokines is in turn equally associated with the release of anti-inflammatory cytokines, contributing to the progression of neurodegenerative disorders including TBI and ischemia [66].
Brain injuries both primary mechanical insults and secondary injury result in the impairment of cellular pathways and functions following any trauma or TBI [122]. In addition, TBI stimulates several immune and inflammatory tissue responses. Activation of glial cells and astrocytes, recruitment of leukocytes, neutrophils, macrophages and cytokine release is more prominently reported after TBI as part of immune cell activation, which directly gets scavenged to the targeted site of injury [115, 122, 123]. Similarly, neuroinflammation shows dual opposing roles, as activated astrocytes and glial cells also secrete several neurotoxic substances which have challenging roles in the potentiation of TBI and neuronal cell apoptosis. Therefore, limiting the process of inflammation serves in attenuation of secondary injury and neuronal cell death. For example, minocycline has shown promising therapeutic benefits in several experimental models of TBI, which acts by reducing the activation of microglial cells and cellular inflammatory components following TBI [124-127]. Several other anti-inflammatory agents have also shown significant effects on the recovery of neural functions [128].
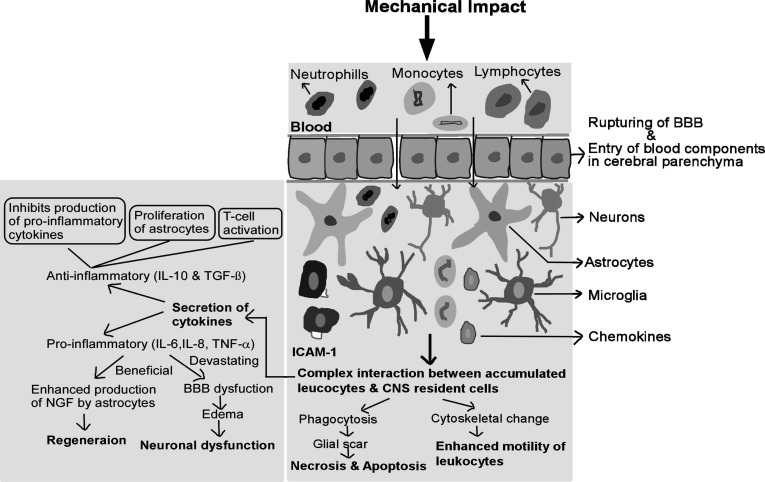
The brain is considered as “immune-privileged” organ as it has a highly selective and tightly regulated BBB that blocks the entry of many foreign pathogens and immune activators [129]. For instance, data from animal studies have suggested that the inflammatory stimulus lipopolysaccharide (LPS), showed delayed response in the brain as compared to other tissues [130]. In TBI, BBB integrity gets compromised exposing the brain cells to peripheral blood products, tissue and cellular debris, prostaglandins and free radicals. These events generate complex immune responses by activating the release of chemokines, adhesion molecules and mobilize glial and other immune cells in the brain [19, 131-133]. These cells then infiltrate the injured tissue along with macrophages and T-cell lymphocytes [134]. Activated polymorphonuclear leukocytes (PMNL) adhere not only to the distorted layer but also to the intact endothelial layer (Fig. 3). The infiltration of these immune cells is facilitated via the up-regulation of endothelial cellular adhesion molecules such as P-selectin, intracellular adhesion molecule (ICAM-1) and vascular adhesion molecule (VCAM-1). Proinflammatory mediators such as TNF-α, interleukin-1β and interleukin-6 become upregulated within few hours after injury. Direct and indirect release of neurotoxic mediators like, nitric oxide, neutrophils, microfilaments and cytokines lead to the progression of tissue damage and tissue scar formation. Further additional release of vasoconstrictors (prostaglandins and leukotrienes) and adhesion of leukocytes and platelets produce microvascular obliteration, BBB lesion, and edema formation. Consequently, it aggravates secondary brain damage and decreases tissue perfusion [6, 129, 132]. However, it is equally important to view the harmful effects of microglial cells, as on one end it results in the clearance of dead cells during acute phases of injury, while on the other side aids in neuronal impairment which later contribute to the pathogenesis of neurodegenerative disorders.
Fig. (3).
Diagrammatic and schematic depiction of inflammatory cascades in TBI. The brain is an immune-privileged organ, switched to a site of several immune and inflammatory reactions after TBI. Compromised BBB increases the infiltration of blood cells, cellular debris and toxin in CNS. Firstly, neutrophils and then monocytes and lymphocytes interact with resident cells i.e. astrocytes, microglia, and neurons to initiate a complex inflammatory response. Macrophages start phagocytosing debris and form a glial scar. Concomitantly, cytoskeletal rearrangement increases the motility of leukocytes. Secretion of pro-inflammatory (IL-6, IL-8, TNF-α tec.) negatively affect BBB integrity and thereby contribute to edema and neuronal dysfunction. On the other hand, secretion of anti-inflammatory cytokines (IL-10, TGF-β) induces the production of NGF by astrocytes leads to regeneration. BBB: Blood-Brain Barrier; IL-6: Interleukin-6; IL-8: Interleukin-8; TNF-α: Tumour necrosis factor-α; NGF: Nerve growth factor; ICAM-1: Intracellular adhesion molecule; IL-10: Interleukin-10; TGF-β: Transforming growth factor-β. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
2.2.8. Activation of Auto-destructive Enzymes
Several enzymes like calpains and caspases (members of a family of cysteine proteases), endonucleases, phospholipases and nitric oxide synthase etc., get activated after TBI. Most of these auto-destructive enzymes are stimulated due to an increase in intracellular [Ca2+]i. The neuron-specific isoform of calpains are activated by µmolar [Ca2+]i range, a level that is achieved by ischaemic and excitotoxic insult following TBI [112, 135]. Cytoskeletal proteins like spectrin, tubulin, tau, microtubule-associated protein and neurofilaments act as substrates for calpains [136, 137]. Several other cell components like cell adhesion molecules, proteases, phosphatases, glutamate receptor and transporter also serve as the substrates for calpains. Therefore, TBI-induced over-activation of calpains can be attributed to abnormal and irreversible changes in the membrane and cytoskeleton of the brain cells [136]. These changes are detrimental to the structural integrity of the cell leading to increased membrane permeability to ions and macromolecules.
Activation of caspases (a hetero-tetramers) has been widely implicated in apoptotic events after CNS injuries, including TBI [136, 138]. Caspases are expressed as inactive pro-forms i.e., zymogens which concomitantly gets activated by both extrinsic and intrinsic pathways [114, 139-141]. In the extrinsic pathway, specific ligands bind to receptors on the cell surface that directly cause caspase activation through adapter proteins. The binding of TNF/lymphotoxin to TNF receptor-1 or fas triggers the activation of caspases [142, 143]. In the intrinsic pathway, caspases get activated intracellularly by the generation of signals from mitochondria. Cytochrome c (Cyt C) released from mitochondria binds to the caspase-activating factor Apaf-1 which oligomerizes with procaspase-9 resulting in induction of pro-caspase-3 [144]. Activated caspases are involved in the breakdown of several groups of the substrate, including enzymes and proteins which have been implicated in the maintenance of structural integrity, signal transduction, transcription and DNA repair [145]. Degradation of these proteins is responsible for the membrane blebbing, DNA fragmentation or condensation and formation of apoptotic bodies and finally cell death [138]. Endonucleases activation after TBI leads to DNA damage and delayed cell death [119, 146]. Phospholipase C (PLC) and Phospholipase A2 (PLA2) are activated due to increase in [Ca2+]i [147, 148]. The activation of PLA2 elicits release and accumulation of free arachidonic acid, changes membrane permeability and increases free radical generation. Activation of PLC results in activation of IP3/DAG second messenger pathway and ultimately increases cytoplasmic Ca2+ influx from intracellular and extracellular stores [148, 149]. Additionally, DAG mediated activation of protein kinase C (PKC) also potentiates the activity of NMDA receptors and VGCCs which further enhance the intracellular Ca2+ levels. Enhanced intracellular Ca2+ binds to enzyme calmodulin and potentiates its activity leading to activation of the nitric oxide synthase (NOS) [150, 151].
Excessive production of nitric oxide (NO) is detrimental to neuronal functioning. Excess NO binds to the superoxide radicals and forms peroxynitrite (ONOO-) which is a highly potent oxidant and has been implicated in oxidizing DNA, proteins and membrane lipids [151]. Superoxide radicals remain under the strict control of endogenous free radical scavenger i.e., Superoxide Dismutase (SOD). However, following traumatic insult, there is excessive production of superoxide radicals, SOD becomes saturated, allowing a high level of peroxynitrite generation and finally leading to neuronal damage [119].
2.2.9. Metabolic Alterations
The cerebral oxygen and glucose consumption (as reflected by cerebral metabolism) are rapidly reduced after TBI along with the reduction of phosphocreatine and ATP levels (as reflected by cerebral energy state). This occurs with considerable temporal and spatial heterogeneity [152-154]. Primary insults result in cell death in a limited region of actual impact but metabolic dysfunction spreads in the remote areas of the brain. In the early phase of the injury, glucose utilization increases for the first 30 min and then continuously decreases for the next 5-10 days [103]. The early hyper glycolysis is due to the disruption of ionic gradients across the neuronal cell membrane which causes potentiation of energy-dependent ionic pumps [9]. The initial phase of hypermetabolism is followed by a long phase of hypo-metabolism where the cells are not able to convert glucose into ATP. This impairment in oxidative metabolism leads to anaerobic metabolism in order to meet energy needs. The anaerobic metabolism leads to the formation of lactic acid. This accumulation of lactic acid in extracellular space may lead to acidosis, membrane damage, disruption of BBB cerebral edema and ultimately neuronal dysfunction [9, 155].
2.2.10. Oxidative Stress
Oxidative stress is centrally involved in the progression of several diseases including TBI and neurological disorders. And is characterized by the imbalanced redox states that are associated with excessive formation of free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) [156]. TBI causes mechanical stress to brain tissue and can initiate sudden biochemical changes at the time of impact that initiates oxidative stress cascades [157]. Consequently, nervous system is highly susceptible to changes in redox potential as it’s an organ with the high demand of oxygen supply and composed of abundant lipids [158, 159]. Imbalanced redox potentials in nervous tissue may damage the brain severely through mechanisms such as an increase in intracellular free Ca2+, release of excitatory amino acids, and neurotoxicity [160]. Aggravated oxidative stress may, in turn, activate several processes inducing cellular damage, mitochondrial dysfunction, impairment of the DNA repair mechanism thereby initiating neurodegenerative cascades involving synaptic disconnection and impaired transport of mitochondria to synapses infuriating the neuronal response to TBI [161-166].
In addition, production of ROS and RNS following TBI is one of the major and most confirmed aspects of secondary injury. Superoxide radical is one of the supreme and primary radicals produced almost immediately following TBI [115, 137, 167, 168]. It is generated by the reduction of one electron of molecular oxygen. Various processes including the increase in the mitochondrial permeability, enzymatic auto degradation of biogenic amine neurotransmitters, oxidation of extravasated haemoglobin, inflammation, conversion of xanthine dehydrogenase to xanthine oxidase, Ca2+ dependent activation of phospholipases and the downstream arachidonic acid events contribute to the generation of free radicals [169]. After TBI the microvascular superoxide radical production is increased and the severity of brain injury can be estimated by measuring biochemical modifications such as ROS-mediated damage. An increase in ROS production has been identified as an important mechanism by which neuronal plasticity is affected during injury [170-172]. Moreover, imbalance in the enzymatic functions may potentiate the generation of superoxide, cellular tissue damages and certainly, if left untreated, leads to cell death and disease. However, superoxide is not an exclusive oxidant but its activity gets enhanced upon interaction with other active oxidant molecules like NO and peroxynitrite [173]. Under physiological conditions, endogenous antioxidant mechanism i.e. superoxide dismutase enzyme converts superoxide radical (O2._) into hydrogen peroxide (H2O2) and reduces the damaging effect of superoxide radical. But, following TBI, superoxide dismutase enzyme activity is reduced resulting in a massive increase in superoxide radicals which may damage the cell membrane, protein and DNA [13, 174].
TBI induces the levels of inducible nitric oxide synthase (iNOS) in the regions of the brain. The induction of iNOS is associated with the release of NO which plays a definite role in the disease pathogenicity [175, 176]. Peroxynitrite (PN, ONOO-) is another free radical donor and one of the most principle reactive species involved in the progression of secondary injury. It is produced by the chemical union of superoxide radical (O2 •-) and nitric oxide (NO•). Nitric oxide and superoxide radicals are not as reactive as peroxynitrite [177]. In addition, peroxynitrite anion can also combine with CO2 and formed nitrosoperoxocarbonate (ONOOCO2) which readily decomposes and give nitro (•NO2) and carbonate radical (•CO3). Collectively, peroxynitrite anion gets decomposed and formed highly reactive •OH, •NO2 and •CO3 [13]. These all free radicals produced following TBI lead to lipid peroxidation, protein nitration and mutation in DNA. Based on the definite role of NO in exacerbating disease pathogenicity in coordination with increased oxidative radicals, it can be suggested that controlling the production of NO might act as a better therapeutic target. In particular, recent evidences have suggested that selective NOS inhibitors were effective in reducing the injury in the immature brains following ischemia [178-181]. In addition, decrease in ascorbate radical formation after TBI by antioxidants have also suggested the definite role of oxidative stress and secondary damage [182]. Ascorbate represents as a cofactor for NO release from S-nitrosothiols and its deficiency result to accumulate the NO reserves. Interestingly, despite the availability of S-nitrosothiols, reduced NO production is largely associated with hypoperfusion after TBI in infants and children [183]. Efficiently, S-nitrosothiols prevents the participation of NO in the formation of peroxynitrite, thus increasing BBB functions. The exciting role of NO either constructive or destructive has been an excellent topic of further consideration, and few elegant reviews can be referred on the role of NO [184-189].
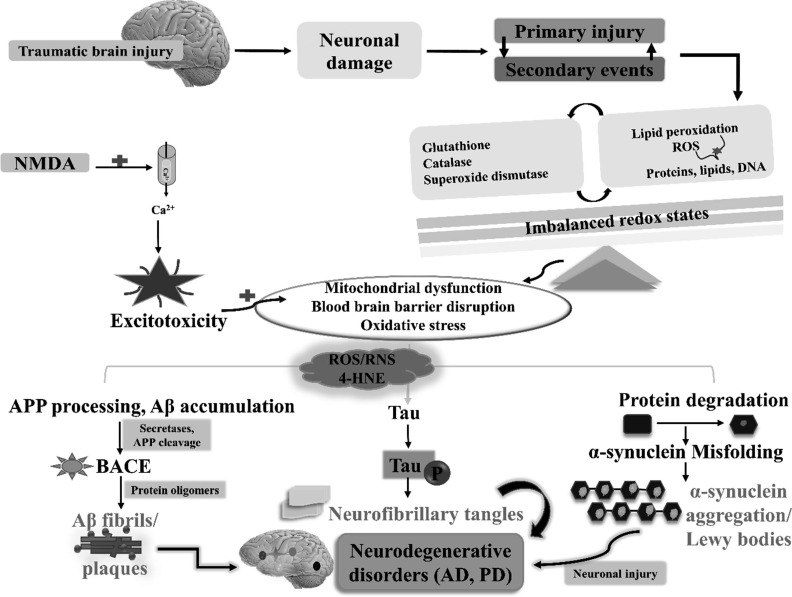
The central nervous system contains a large amount of iron which is responsible for the increase in susceptibility of the brain to oxidative damage especially lipid peroxidation. Under normal physiological conditions, iron is tightly regulated in neuronal tissue. But, following TBI, acidosis and haemorrhages cause disturbances in iron homeostasis [190]. Moreover, extravasated haemoglobin due to the rupturing of cerebral blood vessels and haemorrhage is another source of iron. Haemoglobin itself increases the production of superoxide radicals. The iron leaked following TBI is also responsible for the generation of hydroxyl (OH•) radicals when ferrous ion undergoes oxidation by its reaction with H2O2. The hydroxyl radical is an extremely hostile oxidant that can damage most biological molecules [13]. Several pieces of evidence reported that lipid peroxidation products contribute to Ca2+ toxicity in the cell by the accumulation of 4-HNE that inhibits the activity of glutamate transporters on astrocytes prolonging the NMDA receptor-mediated influx of Ca2+ [191]. Lipid peroxidation can also be responsible for dysregulation of Ca2+ATPase resulting in ionic imbalance [192]. These hostile products cause a defect in the mitochondrial capacity to sequester ions leading to mitochondrial dysfunction [174, 193]. Previous studies suggest that loss of synapse takes place following TBI and are typically associated with sensory, motor, and cognitive impairments in various neurodegenerative conditions, such as major depressive disorder, Alzheimer's disease, Huntington disease, and amyotrophic lateral sclerosis (ALS), as well as aging [194]. Apparently, the dysregulation in the protein processes is associated with the onset of neurodegenerative disorders (Fig. 4). There exists an association between the TBI and AD pathology, and the pathological hallmarks of AD are characterised by the presence of amyloid-β (Aβ) plaques and neurofibrillary tangles of p-Tau. It has been suggested that oxidative imbalance and resultant neuronal damage may play a critical role in the initiation and progression of AD through aggravated production of hyperphosphorylated tau and amyloid plaques, accelerating the vicious pathogenic cycle. Uryu et al., evaluated the levels of 8,12-iso-iPF2α-VI (isoprostane analysis) a marker estimated for lipid peroxidation in urine and brain samples and suggested that repetitive TBI aggravates the oxidative stress and causes accumulation of Aβ impacting cognitive functions in WT and Tg mice [195]. Another important piece of evidence comes from the fact that edaravone (a free radical scavenger) reduced cognitive impairment by attenuating axonal injury and oxidative damage in post-TBI conditions [196]. The defects in antioxidant defence mechanisms resulted in increased Aβ deposition in
Fig. (4).
Selective pathways demonstrating the role of oxidative stress in the progression of neurodegenerative disorders after TBI: TBI induces neuronal loss, accompanied by primary and secondary events. These events are associated with mitochondrial dysfunction, imbalanced redox potential, blood brain disruption and oxidative stress. In addition, increased calcium ion concentration mediated excitotoxicity results in the exacerbation of oxidative stress. Oxidative radicals, in turn, aggravates the tissue damage resulting in the cleavage of APP protein, phosphorylation of Tau and misfolding of α-synuclein protein pathways. Consequently, the alteration in the processes results in cell death leading to the exacerbation of neurodegenerative pathologies. Aβ: Amyloid-β; AD: Alzheimer disease; APP: Amyloid precursor protein, PD: Parkinson disease; RNS: Reactive nitrogen species; ROS: Reactive oxygen species. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
transgenic mice with APP mutation [197]. Plassman et al., in a population based retrospective study, demonstrated that young adults with moderate and severe head injuries are linked with AD and other dementia pathology in the later stages of life [198]. Consequently, the considerable ROS formation increased by the electron transport system within the mitochondria under stressful conditions and aging constitutes a major risk for developing Alzheimer's disease (AD), when no efficient antioxidant system is available [199]. Moreover, several other studies have documented that TBI plays a critical role in the progression and development of AD [200-202]. Autopsy studies have demonstrated that Aβ- plaques were reported in 60% of patients surviving a moderate-to-severe TBI [203]. Moreover, PET neuroimaging has revealed the greater Aβ accumulation in the posterior cingulate region in individuals surviving a moderate-to-severe TBI compared to healthy controls denoting white matter injury [204]. Levels of the proteins involved in Aβ production (BACE1, PS1 and APP) are increased in the injured cortex after TBI, and follow a time course similar to that of Aβ [205-207]. Altogether, it can be anticipated that targeting APP secretases might be an efficient challenge in reducing the Aβ deposition, as it clearly establishes a foundational basis for the over accumulation of Aβ and TBI. Several mechanisms have been expressed stating the link between early development of AD pathology and TBI [208, 209]. Of particular note, emerging evidences from several studies make it unlikely to come to an end line, suggesting more wealth of knowledge on the molecular mechanism needed to be reviewed for the better understanding of the disease pathologically [210, 211].
Similarly, individuals after TBI experience varying amplitude of neurodegenerative manifestations. For example, emerging evidences from human studies have provided a biological basis for the association between TBI and Parkinson's disease. The neuropathologic hallmark of PD is the abnormal accumulation of α-synuclein forming Lewy bodies and Lewy neurites. Furthermore, alpha-synuclein, a protein associated with Parkinson’s disease and Lewy body dementia, has been found elevated on autopsy in 20% of patients with a prior history of TBI [212]. Following severe TBI, α-synuclein may be elevated in the CSF and injured axons of patients died with TBI [213]. Few studies have shown that the expression of alpha-synuclein-positive dopaminergic neurons in the substantia nigra pars compacta was elevated in the ipsilateral hemisphere of a rat TBI model at 60 days post-injury when compared to the contralateral hemisphere of the same injured brain region, and either hemisphere of the sham non-injured rat on the other hand [214]. The level of protein oxidation in the substantia nigra of Parkinson’s patients was double than seen in healthy subjects, while on the other hand, elevated levels of malondialdehyde (thiobarbituric acid reactive substances) and HNE have been reported in the substantia nigra and striatum of Parkinson’s disease patients which suggests the role of oxidative stress in post-TBI associated Parkinson's disease progression [215-217].
2.2.11. Mitochondrial Dysfunction
Mitochondria, which is considered as “power-house” of the cell because it meets around 95% energy demand of the cell, is a membrane-bound organelle, that possesses two membranes i.e. inner and outer membrane. The outer membrane allows movement of certain ions and small molecules whereas the inner membrane-house the components of the electron transport chain (ETC). Neurons are highly dependent on the mitochondria for their energy demand to maintain excitability which is necessary for impulse conduction, neurotransmission, synaptic plasticity [218]. Therefore, mitochondrial function is essential for the survival of neurons. Mitochondrial damage has been implicated following TBI. There are two main contributors to mitochondrial damage. Lipid peroxidation and protein nitration which produces reactive substances and glutamate neurotoxicity [219]. Firstly, a high level of 4-Hydroxynonenal (4-HNE) and 3-Nitrotyrosine (3-NT) are responsible for the impairment of oxidative metabolism and depletion of ATP stores [220, 221]. Secondly, excessive glutamate release causes the overactivation of NMDA receptor, leads to the accumulation of the large amount of cytosolic Ca2+ and overloading of the mitochondria with Ca2+. This cause impairment of mitochondrial respiration, oxidative phosphorylation, ion transport and decrease in mitochondrial Ca2+ buffering capacity (Fig. 5) [13, 219, 222]. Enhanced intracellular Ca2+ also causes the formation of mitochondrial permeability pore (mPTP) in the inner membrane of mitochondria and dumping of Ca2+ pool from the mitochondria matrix to the cytoplasm [222]. This mPTP allows the solutes of molecular mass less than 1500 Dalton to pass freely across the inner mitochondrial membrane. The condition of mitochondrial functions determines the modes of cell death either necrotic or apoptosis [13].
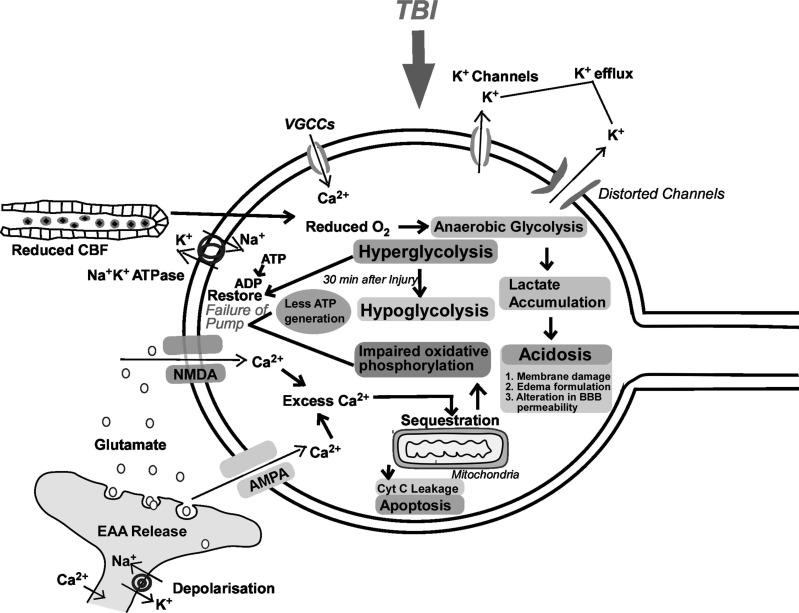
Fig. (5).
Diagrammatic representation of neurometabolic alterations in TBI. Initially, a massive efflux of K+ leads to a strong wave of depolarization. To maintain this ionic dyshomeostasis, neuronal cells carry-out hyper-glycolysis to meets the energy demand and restoring the function of Na+K+ATPase pumps. Simultaneously, distortion of cerebral vasculature due to mechanical impact leads to reduced CBF, ended in the switching of aerobic to anaerobic metabolism and lactic acid accumulation which initiates hypometabolism. This hypometabolic and acidosis state of the cell fails to meet the energy needs and restore the function of ionic pumps. Concurrently, glutamate releases enhance the level of intracellular Ca2+ which sequester inside mitochondria and causes impairment of oxidative phosphorylation. This is the second neurometabolic cascade that fails to restore the function of ionic pumps. AMPA: α-amino-3-hydroxy-5-methyl-4-isooxazolepropionic acid Receptor; CBF: Cerebral Blood Flow; Cyt C: Cytochrome c; EAA: Excitatory amino acids; NMDA: N-methyl-D-aspartate Receptor; VGCCs: Voltage-Gated Calcium Channels. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
2.2.12. Protein Abnormalities
Traumatic brain injury activates multiple secondary injury pathways, increasing the risk for developing multiple neurodegenerative diseases. Although many proteins are found to behave abnormally after TBI, few proteins, which have gained more attention, are discussed below.
Neurofibrillary tangles are abundantly found and widely distributed in approximately one-third of TBI cases. The hyperphosphorylation of tau has been implicated in the pathogenesis of a number of neurodegenerative diseases and has been identified as a component of secondary injury in TBI. Tau is a microtubule-associated phosphoprotein that is essential for polymerization and the stabilization of microtubules, axonal transport, and neuronal health. Physiologic tau phosphorylation is vital to life as a productive response to a variety of stressors produced inside the body. Hyperphosphorylation of tau may result from an imbalance in the activity of tau protein kinases and tau phosphatases. The conversion of physiologic tau to filamentous tau through hyperphosphorylation leads to destabilization of microtubule and is responsible for the formation of neurofibrillary tangles. Some studies have shown distinct pathological patterns of tau protein abnormalities in brain injury, however, the clinical correlation is still elusive [223]. Zanier et al., have shown widespread and progressive tau hyperphosphorylation in a mice model of head trauma. The brain extract of these mice showed potential transmissibility of the tau pathology in naïve mice. Similarly, larger spreading of tau hyper- phosphorylation was observed in patients with single head trauma [224]. In another study, higher expression of different variant of tau phosphorylation for brain dysfunction (cis P-tau) was reported compared to pathological variant of tau (trans P-tau) in severe form of TBI and can be used as a biomarker for secondary damage of TBI [225]. TBI-induced tau oligomers cause cognitive impairment and accelerate the onset of pathology in mice overexpressing human tau at both long and short term after TBI [226]. Asparaginyl endopeptidase, a cysteine proteinase is known to induce tau hyper-phosphorylation in repetitive mild TBI [227]. Similarly, repetitive TBI events exacerbate the accumulation of misfolded amyloid-beta along with tau which spread through a prion-like process and trigger neurodegeneration [228, 229]. Alteration of tau dynamics and protein misfolding induces inflammatory cascade, which may persist for decades after the occurrence of initial TBI event [230].
TDP-43, a DNA/RNA binding protein that controls the expression of multiple genes, is associated with several neurodegenerative diseases including amyotrophic lateral sclerosis, Alzheimer's disease, Huntington's disease etc. Under normal physiological conditions, TDP-43 primarily resides in the nucleus, whereas during pathological stress, it might undergo pathological modifications such as C-terminal truncation, phosphorylation, and aggregation, causing impairment of functions [231]. Several preclinical studies, using fluid percussion injury (FPI) or cortical contusion injury (CCI) or blast model in rodents have shown that TDP-43 was phosphorylated, mis-localized, and cleaved into TDP-25 and TDP-35 following injury. This had accelerated neuronal loss and induction of functional deficits [232-234]. TDP-43A315T transgenic mice had significantly impaired cognition as measured by the Y maze following FPI [233]. In human, single TBI event did not show aggregation of phosphorylated TDP-43 at acute and sub-acute term while increased cytosolic levels of pTDP-43, which indicates a fundamental difference in the aggregation of protein on single or repetitive injury [235].
3. BIOMARKERS FOR TBI
Biomarkers detected from body fluids can aid as potential evaluation tools for TBI patients by acting as intrinsic indicators of cerebral damage thereby providing information about the cellular, biochemical and molecular environments.
Biomarkers in TBI are very vital as they provide insights into injury-induced cellular, biochemical and molecular changes that are difficult to detect using imaging techniques. They have the potential to be used as evaluation and prognostic tools. However, the search for specific TBI biomarkers is very challenging due to the enormously complex processes of numerous pathophysiological events that take place after brain injury. Major hallmarks of primary injury after a TBI event are tissue deformation, axonal shearing, contusion, necrosis and blood barrier function. The main features of secondary injury after TBI events are cerebral edema, increase in inflammatory cytokines, mitochondrial damage, excitotoxicity and ischemia.
The presence of neurofilaments, tau protein, amyloid-β peptides, and spectrin breakdown products following axonal injury were observed on microdialysis in severe TBI patients [236]. The neuroglia ratio, which is measured as the correlation between ubiquitin carboxy-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP), is also considered as a marker of severity of the axonal injury [236, 237]. Neuroinflammatory processes play a crucial role during the secondary cascade of TBI. Increase on IL-18 has been reported in TBI patients with severe disabilities and cognitive impairment. Various MMPs, which have modulatory role on cytokines and cytokine receptors, are also correlated with the TBI and neuroinflammation after TBI. High levels of MMP-8 and MMP-9 after TBI are reported with increased levels of proinflammatory cytokines such as IL-1α, IL-2, and TNF-α [238]. Contrary to that, low levels of MMP-7 have been reported with increased levels of IL-1β, IL-2 and IL-6 in patient with TBI [239]. Decrease serum adiponectin levels are also associated with increased mortality and poor outcomes in TBI patients [240].
Both high intracranial pressure (ICP) and cerebral hypoperfusion (CH) are the outcomes of the inflammatory processes associated with secondary injury. In a study involving patients with increased ICP and CH, elevated serum levels of the cytokines IL-6, IL-8, and TNF-α were detected. The lower serum levels of ceruloplasmin were found in patients with ICH than in patients without ICH [241]. Creatine, a metabolite of L-arginine was another biomarker tested in relation to increased ICP. Serum creatine levels were found to be lower in severe TBI patients with ICH than in those without ICH [242]. While high levels of astroglia-derived S100B in the cerebrospinal fluid (CSF) during both ICP and CH, were observed. Furthermore, high CSF levels of C-tau protein is also correlated with ICH. Higher lactate to pyruvate ratios shows an increased risk of developing ICP in TBI patients [236].
In TBI, a biochemical cascade of secondary injury triggers both necrosis and apoptosis. Caspase-3 and other pro-and anti-apoptotic proteins are increased after severe TBI. Activated caspase-9 and Cyt C in the CSF of patients with severe TBI are detected. In several TBI clinical studies, coagulation abnormalities are observed in approximately half of the total patients [243]. Higher levels of fibrinolytic markers such as prothrombin time (PT), partial thromboplastin time (PTT), fibrin degradation products (FDP), and D-dimer test were reported. PT and FDP were associated with an increased risk of mortality after TBI events. In another study, higher levels of procoagulant microparticles were present in CSF and plasma, resulting in a poor outcome [244].
4. THERAPEUTIC APPROACHES IN THE TREATMENT OF TBI
In TBI sufferers, the primary insult occurs at the time of mechanical impact as discussed and is refractory to any therapeutic strategies. The secondary injury phase encompasses the extremely complex and extended reaction from the gene level to macroscopic one and highly sensitive to therapeutic interventions. Therefore, secondary injury forms the basis for developing and deciding the array of neuroprotective therapies [245]. Different experimental models of TBI have been proved to be very useful in understanding the complex mechanism following trauma and assess the potential of pharmacological interventions. However, in spite of the encouraging results in preclinical studies, most of the compounds failed in subsequent clinical trials. A number of reasons like time of treatment, drug-dosing schedules, and mixed-injury severities have been suggested for these failures [246]. To date, no effective treatment has been identified for TBI because of the heterogeneous nature of the pathology. Another important fact is that most pharmacological strategies have used drugs that hit a single pathophysiological target, although diverse mechanisms are involved in secondary brain injury. The recent discovery of novel modalities of regulated cell death opened an entirely new therapeutic perspective. Cell death following TBI is considered as a major cause for late pathological outcomes and disability in head injury survivors. Various druggable targets such as anti-excitotoxic agents, inhibitors of the inflammatory cascade, cytokines, growth factors, neurorestorative agents, anti-oxidants and inhibitor of apoptosis have been explored to interrupt the cell death in acute brain injury and late secondary damage (described in Table 1).
Table 1.
Pharmacological strategies for treatment and prevention secondary cascade consequent to TBI.
| S. No. | Category | Drugs | Mechanism of Action | Refs. |
|---|---|---|---|---|
| 1. | Anaesthetic agents | Barbiturates, Xenon gas | Reduce the functional activity of the brain and the cerebral metabolic rate of oxygen, thereby lowering the cerebral metabolic demand, the cerebral blood flow and intracranial pressure. | [267] |
| 2. | Anti-excitotoxic agents | NMDA blockers | Selfotel competitively inhibits NMDA receptors, Dexanabinol non competitively inhibits NMDA receptors and Traxoprodil is a non-competitive NMDA antagonist. | [268-270] |
| 3. | Anti-inflammatory drugs and immune modulators | Methylprednisolone, COX-2 inhibitors, interleukin-10, 3,6’-dithiopomalidomide | Anti-inflammatory and immune system modulatory actions. | [245, 271] |
| 5. | Apoptosis inhibitors | Calpain and caspase inhibitors | Epoxy derivatives and some aldehydes inhibit calpains. Z-DEVD-FMK inhibits caspase. | [272, 273] |
| 6. | Ca2+ channel antagonists | Nimodipine | Prevents cerebral vasospasm. | [270] |
| 7. | Cholinergic agents | Citicoline, Rivastigmine | Counteracting excitotoxicity, maintaining cellular adenosine -5’- triphosphate level, stimulating neuronal plasticity. | [274] |
| 8. | Anti-parkinsonian drugs | Amantadine, Bromocriptine, Levodopa with carbidopa | Amantadine enhances dopamine release or inhibits its reuptake presynaptically whereas postsynaptically increases the number and alters the configuration of dopamine receptors. It also competitively antagonizes the NMDA receptor. Bromocriptine has antagonistic action on D2 receptors strongly and to a lesser extent on D1 receptor. A combination of levodopa and carbidopa directly increases the level of dopamine in the brain. |
[275, 276] |
| S. No. | Category | Drugs | Mechanism of Action | Refs. |
| 9. | Hormones | Progesterone, Thyrotropin-releasing hormone and analogues | Reduce edema, modulate glial cell activity, decrease lipid Peroxidation, decreases the expression of pro-inflammatory genes, attenuate mitochondrial dysfunction, decrease proapoptotic and increase anti-apoptotic enzymes. | [277] |
| 10. | Musculotropic vasodilators | Papaverin | Prevents cerebral vasospasm. | [278] |
| 11. | Nootropics | Pyritinol | Facilitates the passing of glucose across the blood brain barrier and increases its metabolism in neuronal tissues. | [279] |
| 12. | Peptide mixtures with neurotrophic actions | Cerebrolysin, Actovegin | Increases aerobic neuronal metabolism, stimulation of protein synthesis, inhibition of reactive oxygen species formation, anti-excitotoxic and anti-apoptotic action. | [280, 281] |
| 13. | Vitamins and nutritional supplements | B vitamins, tioctic acid, selenium, magnesium | Magnesium is a neuroprotective element, it is a non-competitive NMDA antagonist as well as a competitive antagonist at all voltage-gated calcium channels. | [282] |
| 14. | Selective Serotonin reuptake inhibitors | Fluoxetine, Paroxetine, Citalopram, Sertraline | Inhibits the reuptake of serotonin and improves neurobehavioural, neurocognitive, neuropsychiatric deficits especially agitation, depression, psychomotor retardation and recent memory loss. | [283] |
| 15. | Psychostimulants | Methylphenidate | It binds to dopamine transporters and inhibits reuptake and rise extracellular dopamine levels, particularly in the frontal cortex. It also increases the level of norepinephrine and serotonin in the brain. It improves the attention, concentration and motor performance in subjects experiencing TBI. | [284] |
| 16. | Anticonvulsant drugs | Valproic acid | It regulates inhibitory control by affecting the neurotransmitter GABA. It improves neurocognitive symptoms like memory and problem-solving capability as well as neuropsychiatric and neurobehavioural symptoms like depression, mania, destructive and impulsive behaviour, restlessness. | [285, 286] |
| 17. | Neurorestorative agents | Bone marrow stromal cells (MSCs), Erythropoietin | MSCs produce several growth factors like Brain-Derived Growth Factor (BDNF), Vascular Endothelial Growth Factor (VEGF) and basic Fibroblast Growth Factor (bFGF) and these factors enhance angiogenesis and vascular stabilization in lesion boundary. Erythropoietin enhances neurogenesis and improves sensorimotor and spatial learning functions in rat and mouse models. | [287-289] |
4.1. Neuroprotective Approach Through Inhibition of Excitotoxicity and Inflammation
Cellular excitotoxicity is associated with a massive release of neurotransmitters and contributes to the initiation of acute cellular loss (primary injury) and secondary injury cascades. The excitatory amino-acid glutamate causes over stimulation to neurons and astrocytes through ionotropic and metabotropic glutamate receptors (NMDA, AMPA) leading to altered Ca2+, Na+ and K+ fluxes. Increased intracellular Ca2+ further triggers a vicious cycle of detrimental metabolic and uncoupling events. Thus, ligands targeting glutamate receptors (NMDA/AMPA antagonists) have been investigated for the beneficial effect in TBI. Both competitive and non-competitive NMDA antagonists have been tested for the neuroprotective potential in experimental models of TBI. Several NMDA antagonists have demonstrated neuroprotection in preclinical and early clinical studies as mentioned in table 1. However, there were many difficulties associated with the clinical use of NMDA antagonists. It is very difficult to optimize the dose of these agents as the glutamate surge in humans varies from the 10-50 folds after the injury. Secondly, it is hard to achieve the neuroprotection or rescue the neuronal cells once the NMDA receptors get activated or after the glutamate surge which limits the window of use for NMDA antagonists [247]. Third, the competitive antagonists must reach in sufficient concentration to the target region of the brain to overcome the glutamate surge. But the concentration of these drugs may be limited due to compromised flow in the damaged tissue. These antagonists may also cause other CNS and peripheral adverse effects if used in high doses. These difficulties have contributed to the failure of many promising therapeutic agents targeting the NMDA receptors following a traumatic injury including NMDAR antagonists MK-801, Magnesium, while on other hand certain agent like Ketamine, Memantine have proven efficacious in neurological symptoms attenuation. PERK (PKR-like ER Kinase) inhibitor GSK2656157 rescued dendrite and memory loss in primary neurons in mice. AMPA receptor antagonists are also under development which are well absorbed orally (described in Table 1).
Similar to the direct inhibitor of glutamate binding with receptors, ion channel blockers offered neuroprotection against the post-injury glutamate surge. Ca2+ channel blockers (eliprodil, ifenprodil, CP101606 etc.,) also called NMDA non-competitive antagonist, showed significant neuroprotection in initial preclinical studies. CP101606 inhibit selectively NR2B receptor especially in hippocampus and cortex and has a better safety profile at 3-fold of therapeutic concentration in TBI patients, but the randomized control trial for Traxoprodil (CP-101,606) didn’t showed significant improvement in GOS score or mortality although improvement in these parameters were marked. D-Cycloserine also restored the LTP which is impaired in the hippocampus and accelerated the neurobehavioral and cognitive recovery process in mice. The complexity of NMDAR in TBI pathophysiology complicates the translational drug development process [248]. Aptiganel, another similar agent that inhibits the Ca2+ transport through binding on NMDA receptor offered neuroprotection against secondary cascade followed by TBI episode. Blocking another cation Na+ channel has been investigated for excitotoxicity in epilepsy and secondary injury after TBI. Remacemide has been shown to reduce the 70% Ca2+ in cortical neurons in a model of glutamate surge [249]. Similar to remacemide, sipatrigine and BW 1003C87 also reported to block glutamate surge by inhibiting sodium channel, however, the clinical utility of these agents remains to be established [250]. Intracellular free magnesium cation (Mg2+) is also found to significantly decreased persistently till 4 days after TBI episode and it correlates well with the severity of the injury or axonal damage [251, 252]. Mg2+ supplement significantly improved motor recovery however, the learning outcome did not improve in both clinical and preclinical investigations [253]. Here it is pertinent to mention that the schedule of the first administration of Mg2+ supplement is very critical. According to the studies, early administration of Mg2+ (within 24 hours after injury) showed clinical improvement in TBI patients. However, late administration have failed to improve the clinical outcomes [253].
Activation of the inflammatory cascade is another concern after excitotoxicity which needs to be controlled for subsequent secondary outcome followed by TBI. Several studies reported the upregulated levels of inflammatory mediators within 6 hours of injury like TNF-A, IL-6, IL-1B, CCL2, CCL3, CXCL1, CXCL-8, CXCL2, CXCL10, CCR2, CCR5, CXCR4 AND CX3CR1. Thus, anti-inflammatory agents may offer neuroprotection following TBI by attenuating the robust early phase upregulation of inflammatory mediators due to the injury retarding or inhibiting the secondary injury and the chronic neuropathological outcomes [248]. Broad-spectrum anti-inflammatory agents (such as glucocorticoids and progesterone) have been found to be effective in alleviating neuroinflammation in animal studies but failed to improve the behavioural outcome [254, 255]. CRASH, a Randomized placebo-controlled trial, tested the efficacy of methylprednisolone infused to TBI adult patients within 8h of injury resulting to a higher mortality rate [248]. Progesterone, a potent steroidal anti-inflammatory agent, act by inhibiting the TNF-A as well as reduces the inflammation related factors like Complement factor C3 and microglial cell activation inhibition. It also prevents excitotoxicity and decreases apoptosis by inhibiting biochemical alterations like Ca2+ flux, NO production and reduces levels of Caspase-3. Vasogenic edema is reduced due to effect of progesterone on reconstituting BBB and Aquaporin-4 water transporters. The drug went through clinical trial phase 2 randomized controlled trial (PROTECT) and phase 3 trial (SYNAPSE). The efficacy of drug in clinical trial was limited and not as expected from the pre-clinical excellent response. Hence, the study concluded that progesterone showed no clinical advantage compared to the placebo for severe TBI [256].
Similarly, nonsteroidal anti-inflammatory agents (NSAIDs acting via inhibiting COX-1/COX-2) have been shown to reduce the inflammatory mediators in experimental models of TBI, however, their effects are not sufficient to inhibit the tissue damage and improve the neurological functions [257]. Inhibitors of pro-inflammatory cytokine TNF-α (TNF-α antagonist such as etanercept and 3,6-dithiothalidomide) could also be effective in combating neuroinflammation as they showed a decrease in IL-6, IL-1β and TNF-α levels at 3 days post-injury [258, 259]. Etanercept has shown promising effect by attenuating the neuronal and glial apoptosis, microglial activation, cerebral damage and ultimately leading to improved cognition and motor ability. Minocycline, a tetracycline-class antibiotic, has also gained considerable attention due to its anti-inflammatory potential following brain injury [260]. Statins (inhibitors of HMG CoA reductase enzyme) have also been investigated for combating inflammation after brain injury [261]. Similarly, nicotinamide has also offered neuroprotection after brain injury through inhibiting both excitotoxicity and inflammation [262]. Among inflammasome family members, the nucleotide-binding domain leucine-rich repeats family protein 3 (NLRP3) is the most extensively studied following TBI and gained attention as a novel target for the treatment of TBI. Rapamycin, dexmedetomidine and JC124 (small molecule inhibitors for NLRP3) significantly reduces the levels of inflammatory markers IL-1β, TNFα, iNOS and caspase-1 in a post-injury treatment paradigm [263-266].
4.2. Oxidative Stress Pathway Targeting in TBI
Several drugs regulating the oxidative stress cycle are under different phases of drug development process. PEG-SOD was evaluated in clinical trial which failed to show positive outcomes for neurological symptom improvement or patient survival. Superoxide free radical scavengers OPC-14117 was evaluated for HIV-associated cognitive impairment, showing improvement in cognitive scores. This suggests further investigation of drug for TBI may be beneficial. The nitric oxide synthase (NOS) inhibitor like NG-nitro L-arginine methyl ester and 7-nitroindazole showed its efficacy only when administered within an hour of injury in mice, limiting its therapeutic window post-TBI. Ronopterine (4-aminotetrahydrobiopterin, VAS203) has completed phase 2 clinical trial and now entered phase 3, NOSTRA (NOS inhibition in TBI), with no marked alteration in the pressure gradients in brain (ICP, CPP) but has shown an improved Glascow Outcome Score (GOS) with few side effects at higher doses like transitory acute kidney injury. Lipid Peroxidation (LP) inhibitor, tirilazad, has shown potency to neuronal recovery and survival but in the phase 3 clinical study it didn’t showed must efficacy in altering the GOS score but has lowered the mortality in patients. Melatonin and N-acetyl cysteine amide act by Nrf2-ARE signalling, N-acetyl serotonin (melatonin precursor) directly scavenge the oxidants while it indirectly acts through antioxidant enzyme, both shows neuroprotective action but clinical study data is limited for these agents [248, 290].
4.3. Neuroprotective Approach Through Inhibition of Cell Death and Apoptosis
Several drugs targeting apoptosis have been investigated in brain injury which includes caspase inhibitors, neuronal apoptosis inhibitory protein (NAIP) modulators, PARP inhibitors etc. NPS1506 is a modulator of NAIP and NMDA antagonists has been studied in phase-II clinical studies [291]. Rapamycin activated mitophagy combined with MCC950 (NLRP3 inhibitor) attenuates neuroinflammation, mitochondrial damage and cell pressure, and demonstrate better neuroprotective effects post-TBI [264]. Similarly, dexmedetomidine (an inhibitor of apoptosis and NLRP3) has shown a significant reduction in the apoptotic cell death with improvements in behavioural outcomes on post-injury administration alone and in combination with BAY 11-7082 (NF-kB inhibitor) [265]. Another apoptotic inhibitor, tauroursodeoxycholic acid (TUDCA) is also evaluated to show beneficial effects after post-injury treatment via regulation of Akt-related anti-apoptotic signalling pathways [292]. Similarly, post-traumatic administration of the novel p53 inactivator pifithrin-α oxygen analogue offers protection against brain injury-induced neuronal apoptotic loss in hippocampal and improves cognitive deficits in rats [293]. PARP inhibitors (such as PJ34, CV1013, GPI 6150 etc.,) have also shown beneficial effects in TBI mediated cell death and neurological outcome in preclinical as well as clinical studies [294, 295]. Although, several other classes of molecules (such as inhibitors of toll-like receptor pathway/mTOR pathway/p53 etc.,) have been tested for their therapeutic potential in brain injury-induced secondary cell death and apoptosis, details description of each is out of scope within this manuscript [296].
4.4. Neuroprotective Approach by Targeting Proteopathy
One of the major consequences of the severe traumatic brain injury is the accumulation of abnormal protein aggregates, which interrupts the axonal transport or induces inflammation. After a moderate or severe traumatic brain injury, there is a formation of abnormal tau protein aggregates. New tau therapies have shown a promising ability to prevent or reduce tau lesions results from TBI. Some of the strategies, which target the abnormal protein aggregation, are discussed below.
As discussed above, the major function of tau protein is to promote microtubule assembly and maintain microtubule structure. This physiological activity of tau depends upon its extent of phosphorylation. In TBI, tau is abnormally hyperphosphorylated and disrupts the microtubule assembly. Protein phosphatase 2A (PP2A), a heterotrimeric protein, dephosphorylates tau and regulates key signalling pathways in the brain. PP2A contains two subunits: a catalytic C-subunit (PP2Ac) and a regulatory B-subunit (PP2A/PR55) [297]. Regulatory B-subunit represents the major tau phosphatase in the brain and is essential for the dephosphorylation of tau protein. It has been reported that experimental brain insults in rats decreased PP2A/PR55 protein expression and PP2A activity followed by hyperphosphorylation of tau [298, 299]. Similar effects of PP2A/PR55 on tau were found in post-mortem brain samples collected from human TBI patients. Therefore, targeting PP2A/PR55 pharmacologically with sodium selenate may be a new therapeutic approach to reduce the hyperphosphorylation of tau and improve outcome following TBI [300].
Phosphorylated tau exists in two conformations, cis p-tau and trans p-tau, which are regulated by the peptidyl-prolyl isomerase Pin1. Pin1 converts the phosphorylated T231-P motif in tau (p-tau) from the toxic cis form to the physiologic trans isomer. Cis p-tau is an early driver of neurodegenerative disease after brain injury and is responsible for their progression [301, 302]. Mild TBI leads to moderate and transient cis p-tau induction whereas severe TBI or repetitive concussions can lead to robust and persistent cis p-tau induction. In addition, cis p-tau accumulates in the dystrophic neurites of neurons as AD progresses [303]. Tau becomes resistant to dephosphorylation on failure of PP2Ac stabilization ability and prone to aggregation [302]. Another study has reported that tau knockout model has prevented axonopathy and memory deficits in rmCHI [304]. Immunotherapy can be another promising approach against p-tau tangles by targeting the cis P-tau. It has been observed that treating TBI with cis antibody neutralised the noxious cis P-tau thus prevented the widespread tauopathy and neuronal death [302]. Cis mAb enters neurons via FCγ receptors and targets intracellular cis p-tau thereby rendering cis p-tau degradation. In in-vitro stressed neuron models and in in-vivo TBI mouse models, cis p-tau monoclonal antibody (mAb) treatment eliminated cis p-tau induction as well as neurotoxicity induced by it [303]. It also restored many neuropathological and functional outcomes in TBI mouse models. In another study, 2-weeks treatment of TBI mice with cis mAb was able to eliminate cis p-tau effectively and beneficial in reversing of post-TBI structural pathologies [305]. Hence, targeting cis p-tau can be considered as a potential and effective therapy against TBI.
4.5. Pharmacotherapy for Controlling Brain-functional Deficits
The nature of TBI-induced behavioural, cognitive, and neurological deficits is under-diagnosed or poorly understood. The nature of post-injury functional deficits varies on the severity of the injury and post-injury period. There is growing evidence that pharmacological treatment not only improves the functional deficits but also enhances recovery in TBI patients. Pharmacotherapy for TBI that have a direct impact on brain functionality can be classified into the following major categories.
4.5.1. Psychostimulants
These are used to treat post-injury attention deficit hyperactivity disorder (ADHD), motor performance deficiency and deficits in work performance, mood alertness etc. Methylphenidate (inhibitor of dopamine transporter) is being used in the acute phase after TBI (<1 month) and results in better control over the attention, seizures, concentration and motor performance. However, the benefits are limited to work performance and alertness on long term use of methylphenidate (more than 3 months post-TBI). Dexamphetamine and lisdexamfetamine have also shown effective in controlling post-injury agitation and improvement in the speed of information processing, executive function, and working memory in TBI patients on 3 weeks and 12 weeks treatment respectively. However, the evidence for improvement in recovery is still needed to investigate in detail [284, 306-308].
4.5.2. Antidepressants
Depression is another major disability in TBI patient, which is characterized by prolonged and persistent sadness, anhedonia, lack of motivation, disturbance sleep and appetite patterns and/or suicidal thoughts. Selective serotonin reuptake inhibitors (SSRIs such as sertraline, paroxetine and fluoxetine, citalopram) have been found potentially useful for the treatment of agitation, depression, psychomotor retardation, memory loss, and pathological crying in TBI patients in subacute and chronic settings. Unlikely to methylphenidate, SSRIs are not effective in short term treatments (<3 months). Similarly, bupropion (norepinephrine/dopamine-reuptake inhibitor; NDRI) is also equally effective in controlling post-TBI restlessness. These pharmacological interventions are also useful in the treatment of TBI-induced anxiety disorder and other neurological outcomes [283, 309-312].
4.5.3. Anti-convulsants
Post-TBI seizures involve the release of excessive neurotransmitters and disturbed ionic homeostasis, which further complicates the existing damage [286]. Phenytoin, phenobarbital and carbamazepine are considered to be effective in the prevention of provoked seizures after TBI and epileptogenesis in TBI patients. Similarly, valproic acid enhances GABA mediated inhibitory control which not only benefits in post-injury seizure but also helps in the stabilization of CNS and excitotoxicity. The newer anticonvulsants particularly levetiracetam offer advantages due to a better pharmacokinetic profile and fewer drug-drug interactions. Another anticonvulsant, gabapentin (an inhibitor of α2δ-1 submits of the L-type calcium channel) has been shown to have an antiepileptic effect on chronic administration. These anticonvulsants have proven to be beneficial in the first 7 days post-injury [313-315].
4.5.4. Other Agents
Quetiapine and risperidone (antagonists for D2 and 5-HT2 receptors) are being to treat post-TBI mania [316]. Dopaminergic system modulators (amantadine and bromocriptine) are also found to be effective in controlling post-TBI memory, attention and motor function deficits [276]. Modafinil, a GABA modulator, is also found to be beneficial in treating TBI-induced narcolepsy and hypersomnia [317].
The nature of TBI sequelae is complex and heterogeneous. The use of the above described pharmacological interventions is limited to improvement in the behavioural, cognitive and neurological symptoms. Their role in controlling the pathological progression of secondary injury is yet to be established.
4.6. Limitations of Current Clinical Study Design for TBI
The failure reasons predicted based on present and past event of failure of such clinical study includes several factors like insensitivity of available outcome measures, lack of early endpoints and biomarkers to guide clinical trial development of agents with neuroprotective ability. Additionally, the currently available approaches for TBI characterization are unidirectional, i.e. only GCS or GOS score are being evaluated for the outcome measure limiting the therapeutic individualization for TBI management.
CONCLUSION
Increasingly, experimental evidences over the decades have been instrumental in realising the role of diverse mechanisms underlying the disease pathogenicity. The heterogeneity and involvement of multiple mediators contributing to the complexity of secondary neurological damage. Thus, complicates the development of effective strategies for neuroprotection. Because of the complications, diagnostic and therapeutic challenges are being pronouncedly encountered in TBI. Attempts to provide protection by targeting a single cascade may become inadequate. Scientists are diverting the therapeutic strategy from a solo target to a multi-target directed ligand development like in case of Alzheimer’s Disease drug discovery. Such approach might prove beneficial in TBI which is known to have extremely complicated pathophysiological mechanisms and features. In major challenges, it is still persisted to understand the specific mechanism of cellular damage after TBI. Other, specific challenges are identification brain injury regions, meaningful surrogate markers, selection of the most appropriate target signal, timing of administration, methods of administration to achieve CNS effects and dose to elicit the central effect etc. Research efforts are in progress to develop effective clinical strategies such as polypharmacy, combination treatments either in sequence or in a cocktail to provide optimum benefits to the TBI-patients.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by the International Brain Research Organization (IBRO) research fellowship to Ashok Kumar Datusalia. Nidhi Khatri hold a PhD (INSPIRE) fellowship by Department of Science & Technology, Delhi, India. We also thank the Department of Pharmaceuticals, Govt of India for NIPER-Raebareli core grant.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Dewan M.C., Rattani A., Gupta S., Baticulon R.E., Hung Y.C., Punchak M., Agrawal A., Adeleye A.O., Shrime M.G., Rubiano A.M., Rosenfeld J.V., Park K.B. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018:1–18. doi: 10.3171/2017.10.JNS17352. [DOI] [PubMed] [Google Scholar]
- 2.Faul M., Coronado V. Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 2015;127:3–13. doi: 10.1016/B978-0-444-52892-6.00001-5. [DOI] [PubMed] [Google Scholar]
- 3.Saikumar J., Byrns C.N., Hemphill M., Meaney D.F., Bonini N.M. Dynamic neural and glial responses of a head-specific model for traumatic brain injury in Drosophila. Proc. Natl. Acad. Sci. USA. 2020;117(29):17269–17277. doi: 10.1073/pnas.2003909117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis A.E. Mechanisms of traumatic brain injury: Biomechanical, structural and cellular considerations. Crit. Care Nurs. Q. 2000;23(3):1–13. doi: 10.1097/00002727-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Giza C.C., Hovda D.A. The neurometabolic cascade of concussion. J. Athl. Train. 2001;36(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- 6.Werner C., Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 7.McAllister T.W. Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 2011;13(3):287–300. doi: 10.31887/DCNS.2011.13.2/tmcallister. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntosh T.K., Smith D.H., Meaney D.F., Kotapka M.J., Gennarelli T.A., Graham D.I. Neuropathological sequelae of traumatic brain injury: Relationship to neurochemical and biomechanical mechanisms. Lab. Invest. 1996;74(2):315–342. [PubMed] [Google Scholar]
- 9.Prins M., Greco T., Alexander D., Giza C.C. The pathophysiology of traumatic brain injury at a glance. Dis. Model. Mech. 2013;6(6):1307–1315. doi: 10.1242/dmm.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran L.V. Understanding the pathophysiology of traumatic brain injury and the mechanisms of action of neuroprotective interventions. J. Trauma Nurs. 2014;21(1):30–35. doi: 10.1097/JTN.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 11.Freire M.A. Pathophysiology of neurodegeneration following traumatic brain injury. West Indian Med. J. 2012;61(7):751–755. [PubMed] [Google Scholar]
- 12.Kaur P., Sharma S. Recent advances in pathophysiology of traumatic brain injury. Curr. Neuropharmacol. 2018;16(8):1224–1238. doi: 10.2174/1570159X15666170613083606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustafa A.G., Alshboul O.A. Pathophysiology of traumatic brain injury. Neurosciences (Riyadh) 2013;18(3):222–234. [PubMed] [Google Scholar]
- 14.Seule M., Brunner T., Mack A., Hildebrandt G., Fournier J.Y. Neurosurgical and intensive care management of traumatic brain injury. Facial Plast. Surg. 2015;31(4):325–331. doi: 10.1055/s-0035-1562884. [DOI] [PubMed] [Google Scholar]
- 15.Reith F.C.M., Lingsma H.F., Gabbe B.J., Lecky F.E., Roberts I., Maas A.I.R. Differential effects of the Glasgow coma scale score and its components: An analysis of 54,069 patients with traumatic brain injury. Injury. 2017;48(9):1932–1943. doi: 10.1016/j.injury.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 16.Gebhard F., Huber-Lang M. Polytrauma pathophysiology and management principles. Langenbecks Arch. Surg. 2008;393(6):825–831. doi: 10.1007/s00423-008-0334-2. [DOI] [PubMed] [Google Scholar]
- 17.Andermahr J., Elsner A., Brings A.E., Hensler T., Gerbershagen H., Jubel A. Reduced collagen degradation in polytraumas with traumatic brain injury causes enhanced osteogenesis. J. Neurotrauma. 2006;23(5):708–720. doi: 10.1089/neu.2006.23.708. [DOI] [PubMed] [Google Scholar]
- 18.Keel M., Trentz O. Pathophysiology of polytrauma. Injury. 2005;36(6):691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 19.Lucas S.M., Rothwell N.J., Gibson R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006;147(Suppl. 1):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prystaz K., Kaiser K., Kovtun A., Haffner-Luntzer M., Fischer V., Rapp A.E., Liedert A., Strauss G., Waetzig G.H., Rose-John S., Ignatius A. Distinct effects of IL-6 classic and trans-signaling in bone fracture healing. Am. J. Pathol. 2018;188(2):474–490. doi: 10.1016/j.ajpath.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Gentleman S.M., Leclercq P.D., Moyes L., Graham D.I., Smith C., Griffin W.S., Nicoll J.A. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci. Int. 2004;146(2-3):97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Chodobski A., Zink B.J., Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2011;2(4):492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun M., McDonald S.J., Brady R.D., O’Brien T.J., Shultz S.R. The influence of immunological stressors on traumatic brain injury. Brain Behav. Immun. 2018;69:618–628. doi: 10.1016/j.bbi.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Tsitsilonis S., Seemann R., Misch M., Wichlas F., Haas N.P., Schmidt-Bleek K., Kleber C., Schaser K.D. The effect of traumatic brain injury on bone healing: An experimental study in a novel in vivo animal model. Injury. 2015;46(4):661–665. doi: 10.1016/j.injury.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 25.Sharma R., Shultz S.R., Robinson M.J., Belli A., Hibbs M.L., O’Brien T.J., Semple B.D. Infections after a traumatic brain injury: The complex interplay between the immune and neurological systems. Brain Behav. Immun. 2019;79:63–74. doi: 10.1016/j.bbi.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 26.Hui X., Haider A.H., Hashmi Z.G., Rushing A.P., Dhiman N., Scott V.K., Selvarajah S., Haut E.R., Efron D.T., Schneider E.B. Increased risk of pneumonia among ventilated patients with traumatic brain injury: Every day counts! J. Surg. Res. 2013;184(1):438–443. doi: 10.1016/j.jss.2013.05.072. [DOI] [PubMed] [Google Scholar]
- 27.Omar M., Moore L., Lauzier F., Tardif P-A., Dufresne P., Boutin A., Lessard-Bonaventure P., Paquet J., Clément J., Turgeon A.F. Complications following hospital admission for traumatic brain injury: A multicenter cohort study. J. Crit. Care. 2017;41:1–8. doi: 10.1016/j.jcrc.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Kourbeti I.S., Vakis A.F., Papadakis J.A., Karabetsos D.A., Bertsias G., Filippou M., Ioannou A., Neophytou C., Anastasaki M., Samonis G. Infections in traumatic brain injury patients. Clin. Microbiol. Infect. 2012;18(4):359–364. doi: 10.1111/j.1469-0691.2011.03625.x. [DOI] [PubMed] [Google Scholar]
- 29.Kourbeti I.S., Papadakis J.A., Neophytou C., Filippou M., Ioannou A., Karabetsos D.A., Bertsias G., Anastasaki M., Vakis A.F. Infections in patients with traumatic brain injury who undergo neurosurgery. Br. J. Neurosurg. 2011;25(1):9–15. doi: 10.3109/02688697.2010.500411. [DOI] [PubMed] [Google Scholar]
- 30.Heegaard W., Biros M. Traumatic brain injury. Emerg. Med. Clin. North Am. 2007;25(3):655–678. doi: 10.1016/j.emc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Prabhakar H., Sandhu K., Bhagat H., Durga P., Chawla R. Current concepts of optimal cerebral perfusion pressure in traumatic brain injury. J. Anaesthesiol. Clin. Pharmacol. 2014;30(3):318–327. doi: 10.4103/0970-9185.137260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greve M.W., Zink B.J. Pathophysiology of traumatic brain injury. Mt. Sinai J. Med. 2009;76(2):97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- 33.White H., Venkatesh B. Cerebral perfusion pressure in neurotrauma: A review. Anesth. Analg. 2008;107(3):979–988. doi: 10.1213/ane.0b013e31817e7b1a. [DOI] [PubMed] [Google Scholar]
- 34.Len T.K., Neary J.P. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin. Physiol. Funct. Imaging. 2011;31(2):85–93. doi: 10.1111/j.1475-097X.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Baeza A., Reina-de la Torre F., Poca A., Martí M., Garnacho A. Morphological features in human cortical brain microvessels after head injury: A three-dimensional and immunocytochemical study. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003;273(1):583–593. doi: 10.1002/ar.a.10069. [DOI] [PubMed] [Google Scholar]
- 36.Armstead W.M. Differential activation of ERK, p38, and JNK MAPK by nociceptin/orphanin FQ in the potentiation of prostaglandin cerebrovasoconstriction after brain injury. Eur. J. Pharmacol. 2006;529(1-3):129–135. doi: 10.1016/j.ejphar.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 37.Scremin O.U., Li M.G., Jenden D.J. Cholinergic modulation of cerebral cortical blood flow changes induced by trauma. J. Neurotrauma. 1997;14(8):573–586. doi: 10.1089/neu.1997.14.573. [DOI] [PubMed] [Google Scholar]
- 38.DeWitt D.S., Prough D.S. Traumatic cerebral vascular injury: The effects of concussive brain injury on the cerebral vasculature. J. Neurotrauma. 2003;20(9):795–825. doi: 10.1089/089771503322385755. [DOI] [PubMed] [Google Scholar]
- 39.Overgaard J., Tweed W.A. Cerebral circulation after head injury. Part 4: Functional anatomy and boundary-zone flow deprivation in the first week of traumatic coma. J. Neurosurg. 1983;59(3):439–446. doi: 10.3171/jns.1983.59.3.0439. [DOI] [PubMed] [Google Scholar]
- 40.Coles J.P., Fryer T.D., Smielewski P., Chatfield D.A., Steiner L.A., Johnston A.J., Downey S.P., Williams G.B., Aigbirhio F., Hutchinson P.J., Rice K., Carpenter T.A., Clark J.C., Pickard J.D., Menon D.K. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J. Cereb. Blood Flow Metab. 2004;24(2):202–211. doi: 10.1097/01.WCB.0000103022.98348.24. [DOI] [PubMed] [Google Scholar]
- 41.Inoue Y., Shiozaki T., Tasaki O., Hayakata T., Ikegawa H., Yoshiya K., Fujinaka T., Tanaka H., Shimazu T., Sugimoto H. Changes in cerebral blood flow from the acute to the chronic phase of severe head injury. J. Neurotrauma. 2005;22(12):1411–1418. doi: 10.1089/neu.2005.22.1411. [DOI] [PubMed] [Google Scholar]
- 42.Bouma G.J., Muizelaar J.P. Cerebral blood flow, cerebral blood volume, and cerebrovascular reactivity after severe head injury. J. Neurotrauma. 1992;9(Suppl. 1):S333–S348. [PubMed] [Google Scholar]
- 43.Kelly D.F., Kordestani R.K., Martin N.A., Nguyen T., Hovda D.A., Bergsneider M., McArthur D.L., Becker D.P. Hyperemia following traumatic brain injury: Relationship to intracranial hypertension and outcome. J. Neurosurg. 1996;85(5):762–771. doi: 10.3171/jns.1996.85.5.0762. [DOI] [PubMed] [Google Scholar]
- 44.Martin N.A., Patwardhan R.V., Alexander M.J., Africk C.Z., Lee J.H., Shalmon E., Hovda D.A., Becker D.P. Characterization of cerebral hemodynamic phases following severe head trauma: Hypoperfusion, hyperemia, and vasospasm. J. Neurosurg. 1997;87(1):9–19. doi: 10.3171/jns.1997.87.1.0009. [DOI] [PubMed] [Google Scholar]
- 45.Rosenfeld J.V., Maas A.I., Bragge P., Morganti-Kossmann M.C., Manley G.T., Gruen R.L. Early management of severe traumatic brain injury. Lancet. 2012;380(9847):1088–1098. doi: 10.1016/S0140-6736(12)60864-2. [DOI] [PubMed] [Google Scholar]
- 46.Bullock M.R. Hyperoxia. J. Neurosurg. 2008;109(3):421–423. doi: 10.3171/JNS/2008/109/9/0421. [DOI] [PubMed] [Google Scholar]
- 47.De Deyne C.S. Therapeutic hypothermia and traumatic brain injury. Curr. Opin. Anaesthesiol. 2010;23(2):258–262. doi: 10.1097/ACO.0b013e328336ea44. [DOI] [PubMed] [Google Scholar]
- 48.Kago T., Takagi N., Date I., Takenaga Y., Takagi K., Takeo S. Cerebral ischemia enhances tyrosine phosphorylation of occludin in brain capillaries. Biochem. Biophys. Res. Commun. 2006;339(4):1197–1203. doi: 10.1016/j.bbrc.2005.11.133. [DOI] [PubMed] [Google Scholar]
- 49.Lenzlinger P.M., Marx A., Trentz O., Kossmann T., Morganti-Kossmann M.C. Prolonged intrathecal release of soluble Fas following severe traumatic brain injury in humans. J. Neuroimmunol. 2002;122(1-2):167–174. doi: 10.1016/S0165-5728(01)00466-0. [DOI] [PubMed] [Google Scholar]
- 50.Shlosberg D., Benifla M., Kaufer D., Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010;6(7):393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Keeffe E., Kelly E., Liu Y., Giordano C., Wallace E., Hynes M., Tiernan S., Meagher A., Greene C., Hughes S., Burke T., Kealy J., Doyle N., Hay A., Farrell M., Grant G.A., Friedman A., Veksler R., Molloy M.G., Meaney J.F., Pender N., Camarillo D., Doherty C.P., Campbell M. Dynamic blood-brain barrier regulation in mild traumatic brain injury. J. Neurotrauma. 2020;37(2):347–356. doi: 10.1089/neu.2019.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riazi K., Galic M.A., Kuzmiski J.B., Ho W., Sharkey K.A., Pittman Q.J. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc. Natl. Acad. Sci. USA. 2008;105(44):17151–17156. doi: 10.1073/pnas.0806682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vezzani A., Ravizza T., Balosso S., Aronica E. Glia as a source of cytokines: Implications for neuronal excitability and survival. Epilepsia. 2008;49(Suppl. 2):24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 54.David Y., Cacheaux L.P., Ivens S., Lapilover E., Heinemann U., Kaufer D., Friedman A. Astrocytic dysfunction in epileptogenesis: Consequence of altered potassium and glutamate homeostasis? J. Neurosci. 2009;29(34):10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barzó P., Marmarou A., Fatouros P., Hayasaki K., Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J. Neurosurg. 1997;87(6):900–907. doi: 10.3171/jns.1997.87.6.0900. [DOI] [PubMed] [Google Scholar]
- 56.Hudak A.M., Peng L., Marquez de la Plata C., Thottakara J., Moore C., Harper C., McColl R., Babcock E., Diaz-Arrastia R. Cytotoxic and vasogenic cerebral oedema in traumatic brain injury: Assessment with FLAIR and DWI imaging. Brain Inj. 2014;28(12):1602–1609. doi: 10.3109/02699052.2014.936039. [DOI] [PubMed] [Google Scholar]
- 57.Kimbler D.E., Shields J., Yanasak N., Vender J.R., Dhandapani K.M. Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One. 2012;7(7):e41229. doi: 10.1371/journal.pone.0041229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Signoretti S., Marmarou A., Tavazzi B., Lazzarino G., Beaumont A., Vagnozzi R. N-Acetylaspartate reduction as a measure of injury severity and mitochondrial dysfunction following diffuse traumatic brain injury. J. Neurotrauma. 2001;18(10):977–991. doi: 10.1089/08977150152693683. [DOI] [PubMed] [Google Scholar]
- 59.Donkin J.J., Vink R. Mechanisms of cerebral edema in traumatic brain injury: Therapeutic developments. Curr. Opin. Neurol. 2010;23(3):293–299. doi: 10.1097/WCO.0b013e328337f451. [DOI] [PubMed] [Google Scholar]
- 60.Sword J., Masuda T., Croom D., Kirov S.A. Evolution of neuronal and astroglial disruption in the peri-contusional cortex of mice revealed by in vivo two-photon imaging. Brain. 2013;136(Pt 5):1446–1461. doi: 10.1093/brain/awt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan E.B., Johnstone V.P., Alwis D.S., Morganti-Kossmann M-C., Rajan R. Characterising effects of impact velocity on brain and behaviour in a model of diffuse traumatic axonal injury. Neuroscience. 2013;248:17–29. doi: 10.1016/j.neuroscience.2013.05.045. [DOI] [PubMed] [Google Scholar]
- 62.Hellewell S.C., Ziebell J.M., Lifshitz J., Morganti-Kossmann M.C. Injury Models of the Central Nervous System. Springer; 2016. Impact acceleration model of diffuse traumatic brain injury. pp. 253–266. [DOI] [PubMed] [Google Scholar]
- 63.Kahle K.T., Simard J.M., Staley K.J., Nahed B.V., Jones P.S., Sun D. Molecular mechanisms of ischemic cerebral edema: Role of electroneutral ion transport. Physiology (Bethesda) 2009;24:257–265. doi: 10.1152/physiol.00015.2009. [DOI] [PubMed] [Google Scholar]
- 64.Michinaga S., Koyama Y. Pathogenesis of brain edema and investigation into anti-edema drugs. Int. J. Mol. Sci. 2015;16(5):9949–9975. doi: 10.3390/ijms16059949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Unterberg A.W., Stover J., Kress B., Kiening K.L. Edema and brain trauma. Neuroscience. 2004;129(4):1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 66.Len T.K., Neary J.P., Asmundson G.J., Goodman D.G., Bjornson B., Bhambhani Y.N. Cerebrovascular reactivity impairment after sport-induced concussion. Med. Sci. Sports Exerc. 2011;43(12):2241–2248. doi: 10.1249/MSS.0b013e3182249539. [DOI] [PubMed] [Google Scholar]
- 67.Pasantes-Morales H., Cruz-Rangel S. Brain volume regulation: Osmolytes and aquaporin perspectives. Neuroscience. 2010;168(4):871–884. doi: 10.1016/j.neuroscience.2009.11.074. [DOI] [PubMed] [Google Scholar]
- 68.Badaut J., Lasbennes F., Magistretti P.J., Regli L. Aquaporins in brain: Distribution, physiology, and pathophysiology. J. Cereb. Blood Flow Metab. 2002;22(4):367–378. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 69.Oshio K., Watanabe H., Song Y., Verkman A.S., Manley G.T. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005;19(1):76–78. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- 70.Badaut J., Hirt L., Granziera C., Bogousslavsky J., Magistretti P.J., Regli L. Astrocyte-specific expression of aquaporin-9 in mouse brain is increased after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2001;21(5):477–482. doi: 10.1097/00004647-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 71.Agre P., King L.S., Yasui M., Guggino W.B., Ottersen O.P., Fujiyoshi Y., Engel A., Nielsen S. Aquaporin water channels-from atomic structure to clinical medicine. J. Physiol. 2002;542(Pt 1):3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papadopoulos M.C., Verkman A.S. Aquaporin-4 and brain edema. Pediatr. Nephrol. 2007;22(6):778–784. doi: 10.1007/s00467-006-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nag S., Manias J.L., Stewart D.J. Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol. 2009;118(2):197–217. doi: 10.1007/s00401-009-0541-0. [DOI] [PubMed] [Google Scholar]
- 74.Papadopoulos M.C., Verkman A.S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 2013;14(4):265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu H., Yao H.T., Zhang W.P., Zhang L., Ding W., Zhang S.H., Chen Z., Wei E.Q. Increased expression of aquaporin-4 in human traumatic brain injury and brain tumors. J. Zhejiang Univ. Sci. B. 2005;6(1):33–37. doi: 10.1631/jzus.2005.B0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blixt J., Svensson M., Gunnarson E., Wanecek M. Aquaporins and blood-brain barrier permeability in early edema development after traumatic brain injury. Brain Res. 2015;1611:18–28. doi: 10.1016/j.brainres.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Mott J.D., Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004;16(5):558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seo J.H., Guo S., Lok J., Navaratna D., Whalen M.J., Kim K.W., Lo E.H. Neurovascular matrix metalloproteinases and the blood-brain barrier. Curr. Pharm. Des. 2012;18(25):3645–3648. doi: 10.2174/138161212802002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grossetete M., Phelps J., Arko L., Yonas H., Rosenberg G.A. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009;65(4):702–708. doi: 10.1227/01.NEU.0000351768.11363.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirose T., Matsumoto N., Tasaki O., Nakamura H., Akagaki F., Shimazu T. Delayed progression of edema formation around a hematoma expressing high levels of VEGF and mmp-9 in a patient with traumatic brain injury: case report. Neurol. Med. Chir. (Tokyo) 2013;53(9):609–612. doi: 10.2176/nmc.cr2012-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng K., Li C., Shan X., Liu H., Fan W., Wang Z., Zheng P. Matrix metalloproteinases and their tissue inhibitors in serum and cerebrospinal fluid of patients with moderate and severe traumatic brain injury. Neurol. India. 2013;61(6):606–609. doi: 10.4103/0028-3886.125258. [DOI] [PubMed] [Google Scholar]
- 82.Heo J.H., Lucero J., Abumiya T., Koziol J.A., Copeland B.R., del Zoppo G.J. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1999;19(6):624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 83.Rosenberg G.A., Cunningham L.A., Wallace J., Alexander S., Estrada E.Y., Grossetete M., Razhagi A., Miller K., Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: Activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893(1-2):104–112. doi: 10.1016/S0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- 84.Morita-Fujimura Y., Fujimura M., Gasche Y., Copin J.C., Chan P.H. Overexpression of copper and zinc superoxide dismutase in transgenic mice prevents the induction and activation of matrix metalloproteinases after cold injury-induced brain trauma. J. Cereb. Blood Flow Metab. 2000;20(1):130–138. doi: 10.1097/00004647-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 85.Gasche Y., Copin J.C., Sugawara T., Fujimura M., Chan P.H. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2001;21(12):1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Jia F., Pan Y.H., Mao Q., Liang Y.M., Jiang J.Y. Matrix metalloproteinase-9 expression and protein levels after fluid percussion injury in rats: The effect of injury severity and brain temperature. J. Neurotrauma. 2010;27(6):1059–1068. doi: 10.1089/neu.2009.1067. [DOI] [PubMed] [Google Scholar]
- 87.Abbott N.J. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol. 2000;20(2):131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Plesnila N., Schulz J., Stoffel M., Eriskat J., Pruneau D., Baethmann A. Role of bradykinin B2 receptors in the formation of vasogenic brain edema in rats. J. Neurotrauma. 2001;18(10):1049–1058. doi: 10.1089/08977150152693746. [DOI] [PubMed] [Google Scholar]
- 89.Trabold R., Erös C., Zweckberger K., Relton J., Beck H., Nussberger J., Müller-Esterl W., Bader M., Whalley E., Plesnila N. The role of bradykinin B(1) and B(2) receptors for secondary brain damage after traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 2010;30(1):130–139. doi: 10.1038/jcbfm.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zweckberger K., Plesnila N. Anatibant, a selective non-peptide bradykinin B2 receptor antagonist, reduces intracranial hypertension and histopathological damage after experimental traumatic brain injury. Neurosci. Lett. 2009;454(2):115–117. doi: 10.1016/j.neulet.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 91.Geppetti P., Bertrand C., Ricciardolo F.L., Nadel J.A. New aspects on the role of kinins in neurogenic inflammation. Can. J. Physiol. Pharmacol. 1995;73(7):843–847. doi: 10.1139/y95-115. [DOI] [PubMed] [Google Scholar]
- 92.Simard J.M., Chen M., Tarasov K.V., Bhatta S., Ivanova S., Melnitchenko L., Tsymbalyuk N., West G.A., Gerzanich V. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat. Med. 2006;12(4):433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simard J.M., Kent T.A., Chen M., Tarasov K.V., Gerzanich V. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6(3):258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen M., Simard J.M. Cell swelling and a nonselective cation channel regulated by internal Ca2+ and ATP in native reactive astrocytes from adult rat brain. J. Neurosci. 2001;21(17):6512–6521. doi: 10.1523/JNEUROSCI.21-17-06512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen M., Dong Y., Simard J.M. Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J. Neurosci. 2003;23(24):8568–8577. doi: 10.1523/JNEUROSCI.23-24-08568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simard J.M., Geng Z., Woo S.K., Ivanova S., Tosun C., Melnichenko L., Gerzanich V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2009;29(2):317–330. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simard J.M., Kilbourne M., Tsymbalyuk O., Tosun C., Caridi J., Ivanova S., Keledjian K., Bochicchio G., Gerzanich V. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J. Neurotrauma. 2009;26(12):2257–2267. doi: 10.1089/neu.2009.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simard J.M., Sheth K.N., Kimberly W.T., Stern B.J., del Zoppo G.J., Jacobson S., Gerzanich V. Glibenclamide in cerebral ischemia and stroke. Neurocrit. Care. 2014;20(2):319–333. doi: 10.1007/s12028-013-9923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simard J.M., Woo S.K., Tsymbalyuk N., Voloshyn O., Yurovsky V., Ivanova S., Lee R., Gerzanich V. Glibenclamide-10-h treatment window in a clinically relevant model of stroke. Transl. Stroke Res. 2012;3(2):286–295. doi: 10.1007/s12975-012-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zweckberger K., Hackenberg K., Jung C.S., Hertle D.N., Kiening K.L., Unterberg A.W., Sakowitz O.W. Glibenclamide reduces secondary brain damage after experimental traumatic brain injury. Neuroscience. 2014;272:199–206. doi: 10.1016/j.neuroscience.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 101.Pergakis M., Badjatia N., Chaturvedi S., Cronin C.A., Kimberly W.T., Sheth K.N., Simard J.M. BIIB093 (IV glibenclamide): An investigational compound for the prevention and treatment of severe cerebral edema. Expert Opin. Investig. Drugs. 2019;28(12):1031–1040. doi: 10.1080/13543784.2019.1681967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Katayama Y., Becker D.P., Tamura T., Hovda D.A. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 1990;73(6):889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 103.Madikians A., Giza C.C. A clinician’s guide to the pathophysiology of traumatic brain injury. Indian J. Neurotrauma. 2006;3(01):9–17. doi: 10.1016/S0973-0508(06)80004-3. [DOI] [Google Scholar]
- 104.Mark L.P., Prost R.W., Ulmer J.L., Smith M.M., Daniels D.L., Strottmann J.M., Brown W.D., Hacein-Bey L. Pictorial review of glutamate excitotoxicity: Fundamental concepts for neuroimaging. AJNR Am. J. Neuroradiol. 2001;22(10):1813–1824. [PMC free article] [PubMed] [Google Scholar]
- 105.Olney J. 1990.
- 106.Maas A.I., Stocchetti N., Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 107.Mehta A., Prabhakar M., Kumar P., Deshmukh R., Sharma P.L. Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2013;698(1-3):6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 108.Tehse J., Taghibiglou C. The overlooked aspect of excitotoxicity: Glutamate-independent excitotoxicity in traumatic brain injuries. Eur. J. Neurosci. 2019;49(9):1157–1170. doi: 10.1111/ejn.14307. [DOI] [PubMed] [Google Scholar]
- 109.Freire M.A.M., Guimaraes J.S., Santos J.R., Simplício H., Gomes-Leal W. Morphometric analysis of NADPH diaphorase reactive neurons in a rat model of focal excitotoxic striatal injury. Neuropathology. 2016;36(6):527–534. doi: 10.1111/neup.12311. [DOI] [PubMed] [Google Scholar]
- 110.Guimarães J.S., Freire M.A., Lima R.R., Souza-Rodrigues R.D., Costa A.M., dos Santos C.D., Picanço-Diniz C.W., Gomes-Leal W. Mechanisms of secondary degeneration in the central nervous system during acute neural disorders and white matter damage. Rev. Neurol. 2009;48(6):304–310. [PubMed] [Google Scholar]
- 111.Beal M.F. Mechanisms of excitotoxicity in neurologic diseases. FASEB J. 1992;6(15):3338–3344. doi: 10.1096/fasebj.6.15.1464368. [DOI] [PubMed] [Google Scholar]
- 112.Tymianski M., Tator C.H. Normal and abnormal calcium homeostasis in neurons: A basis for the pathophysiology of traumatic and ischemic central nervous system injury. Neurosurgery. 1996;38(6):1176–1195. doi: 10.1097/00006123-199606000-00028. [DOI] [PubMed] [Google Scholar]
- 113.Maxwell W.L., Watt C., Graham D.I., Gennarelli T.A. Ultrastructural evidence of axonal shearing as a result of lateral acceleration of the head in non-human primates. Acta Neuropathol. 1993;86(2):136–144. doi: 10.1007/BF00334880. [DOI] [PubMed] [Google Scholar]
- 114.Büki A., Siman R., Trojanowski J.Q., Povlishock J.T. The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J. Neuropathol. Exp. Neurol. 1999;58(4):365–375. doi: 10.1097/00005072-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 115.Freire M., Falcao D. In: Recent development in neurodegeneration. Howe R.M., editor. New York: Nova Science Publisher; 2021. Inflammatory response, excitotoxicity and oxidative stress following Traumatic Brain Injury. pp. 73–94. [Google Scholar]
- 116.Stys P.K., Lopachin R.M. Mechanisms of calcium and sodium fluxes in anoxic myelinated central nervous system axons. Neuroscience. 1998;82(1):21–32. doi: 10.1016/S0306-4522(97)00230-3. [DOI] [PubMed] [Google Scholar]
- 117.Stys P.K., Waxman S.G., Ransom B.R. Ionic mechanisms of anoxic injury in mammalian CNS white matter: Role of Na+ channels and Na(+)-Ca2+ exchanger. J. Neurosci. 1992;12(2):430–439. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cull-Candy S., Brickley S., Farrant M. NMDA receptor subunits: Diversity, development and disease. Curr. Opin. Neurobiol. 2001;11(3):327–335. doi: 10.1016/S0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 119.Weber J.T. Altered calcium signaling following traumatic brain injury. Front. Pharmacol. 2012;3:60. doi: 10.3389/fphar.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arundine M., Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34(4-5):325–337. doi: 10.1016/S0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 121.Allan S.M., Rothwell N.J. Inflammation in central nervous system injury. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358(1438):1669–1677. doi: 10.1098/rstb.2003.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rothwell N. Interleukin-1 and neuronal injury: Mechanisms, modification, and therapeutic potential. Brain Behav. Immun. 2003;17(3):152–157. doi: 10.1016/S0889-1591(02)00098-3. [DOI] [PubMed] [Google Scholar]
- 123.Streit W.J. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 124.Bye N., Habgood M.D., Callaway J.K., Malakooti N., Potter A., Kossmann T., Morganti-Kossmann M.C. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp. Neurol. 2007;204(1):220–233. doi: 10.1016/j.expneurol.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 125.Guimarães J.S., Freire M.A.M., Lima R.R., Picanço-Diniz C.W., Pereira A., Gomes-Leal W. Minocycline treatment reduces white matter damage after excitotoxic striatal injury. Brain Res. 2010;1329:182–193. doi: 10.1016/j.brainres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 126.Stirling D.P., Khodarahmi K., Liu J., McPhail L.T., McBride C.B., Steeves J.D., Ramer M.S., Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J. Neurosci. 2004;24(9):2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Diguet E., Fernagut P.O., Wei X., Du Y., Rouland R., Gross C., Bezard E., Tison F. Deleterious effects of minocycline in animal models of Parkinson’s disease and Huntington’s disease. Eur. J. Neurosci. 2004;19(12):3266–3276. doi: 10.1111/j.0953-816X.2004.03372.x. [DOI] [PubMed] [Google Scholar]
- 128.Lopes R.S., Cardoso M.M., Sampaio A.O., Barbosa M.S., Jr, Souza C.C., Silva D.A. M.C.; Ferreira, E.M.N.; Freire, M.A.M.; Lima, R.R.; Gomes-Leal, W. Indomethacin treatment reduces microglia activation and increases numbers of neuroblasts in the subventricular zone and ischaemic striatum after focal ischaemia. J. Biosci. 2016;41(3):381–394. doi: 10.1007/s12038-016-9621-1. [DOI] [PubMed] [Google Scholar]
- 129.Fraunberger E., Esser M.J. Neuro-inflammation in pediatric traumatic brain injury-from mechanisms to inflammatory networks. Brain Sci. 2019;9(11):E319. doi: 10.3390/brainsci9110319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Matyszak M.K. Inflammation in the CNS: Balance between immunological privilege and immune responses. Prog. Neurobiol. 1998;56(1):19–35. doi: 10.1016/S0301-0082(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 131.Algattas H., Huang J.H. Traumatic Brain Injury pathophysiology and treatments: Early, intermediate, and late phases post-injury. Int. J. Mol. Sci. 2013;15(1):309–341. doi: 10.3390/ijms15010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Corps K.N., Roth T.L., McGavern D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72(3):355–362. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Potts M.B., Koh S.E., Whetstone W.D., Walker B.A., Yoneyama T., Claus C.P., Manvelyan H.M., Noble-Haeusslein L.J. Traumatic injury to the immature brain: Inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. NeuroRx. 2006;3(2):143–153. doi: 10.1016/j.nurx.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Z., Artelt M., Burnet M., Trautmann K., Schluesener H.J. Early infiltration of CD8+ macrophages/microglia to lesions of rat traumatic brain injury. Neuroscience. 2006;141(2):637–644. doi: 10.1016/j.neuroscience.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 135.Wang K.K., Yuen P.W. Calpain inhibition: An overview of its therapeutic potential. Trends Pharmacol. Sci. 1994;15(11):412–419. doi: 10.1016/0165-6147(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 136.Pineda J.A., Wang K.K., Hayes R.L. Biomarkers of proteolytic damage following traumatic brain injury. Brain Pathol. 2004;14(2):202–209. doi: 10.1111/j.1750-3639.2004.tb00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kermer P., Klöcker N., Bähr M. Neuronal death after brain injury. Models, mechanisms, and therapeutic strategies in vivo. Cell Tissue Res. 1999;298(3):383–395. doi: 10.1007/s004410050061. [DOI] [PubMed] [Google Scholar]
- 138.Knoblach S.M., Nikolaeva M., Huang X., Fan L., Krajewski S., Reed J.C., Faden A.I. Multiple caspases are activated after traumatic brain injury: evidence for involvement in functional outcome. J. Neurotrauma. 2002;19(10):1155–1170. doi: 10.1089/08977150260337967. [DOI] [PubMed] [Google Scholar]
- 139.Liu S., Yin F., Zhang J., Qian Y. The role of calpains in traumatic brain injury. Brain Inj. 2014;28(2):133–137. doi: 10.3109/02699052.2013.860479. [DOI] [PubMed] [Google Scholar]
- 140.Clark R.S., Kochanek P.M., Chen M., Watkins S.C., Marion D.W., Chen J., Hamilton R.L., Loeffert J.E., Graham S.H. Increases in Bcl-2 and cleavage of caspase-1 and caspase-3 in human brain after head injury. FASEB J. 1999;13(8):813–821. doi: 10.1096/fasebj.13.8.813. [DOI] [PubMed] [Google Scholar]
- 141.Clark R.S., Kochanek P.M., Watkins S.C., Chen M., Dixon C.E., Seidberg N.A., Melick J., Loeffert J.E., Nathaniel P.D., Jin K.L., Graham S.H. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J. Neurochem. 2000;74(2):740–753. doi: 10.1046/j.1471-4159.2000.740740.x. [DOI] [PubMed] [Google Scholar]
- 142.Chinnaiyan A.M., O’Rourke K., Tewari M., Dixit V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81(4):505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 143.Varfolomeev E.E., Schuchmann M., Luria V., Chiannilkulchai N., Beckmann J.S., Mett I.L., Rebrikov D., Brodianski V.M., Kemper O.C., Kollet O., Lapidot T., Soffer D., Sobe T., Avraham K.B., Goncharov T., Holtmann H., Lonai P., Wallach D. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9(2):267–276. doi: 10.1016/S1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 144.Reimertz C., Kögel D., Lankiewicz S., Poppe M., Prehn J.H. Ca(2+)-induced inhibition of apoptosis in human SH-SY5Y neuroblastoma cells: degradation of apoptotic protease activating factor-1 (APAF-1). J. Neurochem. 2001;78(6):1256–1266. doi: 10.1046/j.1471-4159.2001.00503.x. [DOI] [PubMed] [Google Scholar]
- 145.Eldadah B.A., Faden A.I. Caspase pathways, neuronal apoptosis, and CNS injury. J. Neurotrauma. 2000;17(10):811–829. doi: 10.1089/neu.2000.17.811. [DOI] [PubMed] [Google Scholar]
- 146.Toescu E.C. Apoptosis and cell death in neuronal cells: Where does Ca2+ fit in? Cell Calcium. 1998;24(5-6):387–403. doi: 10.1016/S0143-4160(98)90062-8. [DOI] [PubMed] [Google Scholar]
- 147.Chao H., Liu Y., Lin C., Xu X., Li Z., Bao Z., Fan L., Tao C., Zhao L., Liu Y., Wang X., You Y., Liu N., Ji J. Activation of bradykinin B2 receptor induced the inflammatory responses of cytosolic phospholipase A2 after the early traumatic brain injury. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864(9 Pt B):2957–2971. doi: 10.1016/j.bbadis.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 148.Dhillon H.S., Carman H.M., Prasad R.M. Regional activities of phospholipase C after experimental brain injury in the rat. Neurochem. Res. 1999;24(6):751–755. doi: 10.1023/A:1020779413122. [DOI] [PubMed] [Google Scholar]
- 149.Dhillon H.S., Carbary T., Dose J., Dempsey R.J., Prasad M.R. Activation of phosphatidylinositol bisphosphate signal transduction pathway after experimental brain injury: A lipid study. Brain Res. 1995;698(1-2):100–106. doi: 10.1016/0006-8993(95)00840-M. [DOI] [PubMed] [Google Scholar]
- 150.Dawson V.L., Dawson T.M. Nitric oxide actions in neurochemistry. Neurochem. Int. 1996;29(2):97–110. doi: 10.1016/0197-0186(95)00149-2. [DOI] [PubMed] [Google Scholar]
- 151.Slemmer J.E., Shacka J.J., Sweeney M.I., Weber J.T. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr. Med. Chem. 2008;15(4):404–414. doi: 10.2174/092986708783497337. [DOI] [PubMed] [Google Scholar]
- 152.Clark R.S., Carcillo J.A., Kochanek P.M., Obrist W.D., Jackson E.K., Mi Z., Wisneiwski S.R., Bell M.J., Marion D.W. Cerebrospinal fluid adenosine concentration and uncoupling of cerebral blood flow and oxidative metabolism after severe head injury in humans. Neurosurgery. 1997;41(6):1284–1292. doi: 10.1097/00006123-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 153.Diringer M.N., Yundt K., Videen T.O., Adams R.E., Zazulia A.R., Deibert E., Aiyagari V., Dacey R.G., Jr, Grubb R.L., Jr, Powers W.J. No reduction in cerebral metabolism as a result of early moderate hyperventilation following severe traumatic brain injury. J. Neurosurg. 2000;92(1):7–13. doi: 10.3171/jns.2000.92.1.0007. [DOI] [PubMed] [Google Scholar]
- 154.Cunningham A.S., Salvador R., Coles J.P., Chatfield D.A., Bradley P.G., Johnston A.J., Steiner L.A., Fryer T.D., Aigbirhio F.I., Smielewski P., Williams G.B., Carpenter T.A., Gillard J.H., Pickard J.D., Menon D.K. Physiological thresholds for irreversible tissue damage in contusional regions following traumatic brain injury. Brain. 2005;128(Pt 8):1931–1942. doi: 10.1093/brain/awh536. [DOI] [PubMed] [Google Scholar]
- 155.Hovda D. Collaborative approach leads to leaps in traumatic brain injury treatment. Mod. Healthc. 2016;45(27):25. [PubMed] [Google Scholar]
- 156.Andreyev A.Y., Kushnareva Y.E., Starkov A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc.) 2005;70(2):200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 157.Cornelius C., Crupi R., Calabrese V., Graziano A., Milone P., Pennisi G., Radak Z., Calabrese E.J., Cuzzocrea S. Traumatic brain injury: Oxidative stress and neuroprotection. Antioxid. Redox Signal. 2013;19(8):836–853. doi: 10.1089/ars.2012.4981. [DOI] [PubMed] [Google Scholar]
- 158.Halliwell B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59(5):1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 159.Packer L., Prilipko L. Free radicals in the brain: Aging, neurological and mental disorders. Springer Science & Business Media; 2012. [Google Scholar]
- 160.Bambrick L., Kristian T., Fiskum G. Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem. Res. 2004;29(3):601–608. doi: 10.1023/B:NERE.0000014830.06376.e6. [DOI] [PubMed] [Google Scholar]
- 161.BERTONI‐FREDDARI C.; Fattoretti, P.; Giorgetti, B.; Solazzi, M.; Balietti, M.; Di Stefano, G.; Casoli, T. Decay of mitochondrial metabolic competence in the aging cerebellum. Ann. N. Y. Acad. Sci. 2004;1019(1):29–32. doi: 10.1196/annals.1297.006. [DOI] [PubMed] [Google Scholar]
- 162.de la Monte S.M., Neely T.R., Cannon J., Wands J.R. Oxidative stress and hypoxia-like injury cause Alzheimer-type molecular abnormalities in central nervous system neurons. Cell. Mol. Life Sci. 2000;57(10):1471–1481. doi: 10.1007/PL00000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Raghavendra Rao V.L., Dhodda V.K., Song G., Bowen K.K., Dempsey R.J. Traumatic brain injury-induced acute gene expression changes in rat cerebral cortex identified by GeneChip analysis. J. Neurosci. Res. 2003;71(2):208–219. doi: 10.1002/jnr.10486. [DOI] [PubMed] [Google Scholar]
- 164.Wu A., Ying Z., Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma. 2004;21(10):1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 165.Wu A., Ying Z., Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004;19(7):1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 166.de la Monte S.M., Luong T., Neely T.R., Robinson D., Wands J.R. Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer’s disease. Lab. Invest. 2000;80(8):1323–1335. doi: 10.1038/labinvest.3780140. [DOI] [PubMed] [Google Scholar]
- 167.Wang W.T., Sun L., Sun C.H. PDIA3-regulted inflammation and oxidative stress contribute to the traumatic brain injury (TBI) in mice. Biochem. Biophys. Res. Commun. 2019;518(4):657–663. doi: 10.1016/j.bbrc.2019.08.100. [DOI] [PubMed] [Google Scholar]
- 168.Kontos H.A., Povlishock J.T. Oxygen radicals in brain injury. Cent. Nerv. Syst. Trauma. 1986;3(4):257–263. doi: 10.1089/cns.1986.3.257. [DOI] [PubMed] [Google Scholar]
- 169.Lewén A., Matz P., Chan P.H. Free radical pathways in CNS injury. J. Neurotrauma. 2000;17(10):871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 170.Beckhauser T.F., Francis-Oliveira J., De Pasquale R. Reactive oxygen species: Physiological and physiopathological effects on synaptic plasticity: Supplementary issue: brain plasticity and repair. J. Exp. Neurosci. 2016;10:S39887. doi: 10.4137/JEN.S39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brewer G.J. Neuronal plasticity and stressor toxicity during aging. Exp. Gerontol. 2000;35(9-10):1165–1183. doi: 10.1016/S0531-5565(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 172.Kamsler A., Segal M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol. Neurobiol. 2004;29(2):167–178. doi: 10.1385/MN:29:2:167. [DOI] [PubMed] [Google Scholar]
- 173.Radi R., Beckman J.S., Bush K.M., Freeman B.A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991;288(2):481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 174.Mustafa A.G., Singh I.N., Wang J., Carrico K.M., Hall E.D. Mitochondrial protection after traumatic brain injury by scavenging lipid peroxyl radicals. J. Neurochem. 2010;114(1):271–280. doi: 10.1111/j.1471-4159.2010.06749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clark R.S., Kochanek P.M., Schwarz M.A., Schiding J.K., Turner D.S., Chen M., Carlos T.M., Watkins S.C. Inducible nitric oxide synthase expression in cerebrovascular smooth muscle and neutrophils after traumatic brain injury in immature rats. Pediatr. Res. 1996;39(5):784–790. doi: 10.1203/00006450-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 176.Rosenberg P.A., Li Y., Ali S., Altiok N., Back S.A., Volpe J.J. Intracellular redox state determines whether nitric oxide is toxic or protective to rat oligodendrocytes in culture. J. Neurochem. 1999;73(2):476–484. doi: 10.1046/j.1471-4159.1999.0730476.x. [DOI] [PubMed] [Google Scholar]
- 177.Kozlov A.V., Bahrami S., Redl H., Szabo C. Alterations in nitric oxide homeostasis during traumatic brain injury. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863(10 Pt B):2627–2632. doi: 10.1016/j.bbadis.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 178.Feng Y., Piletz J.E., Leblanc M.H. Agmatine suppresses nitric oxide production and attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr. Res. 2002;52(4):606–611. doi: 10.1203/00006450-200210000-00023. [DOI] [PubMed] [Google Scholar]
- 179.Nagayama M., Zhang F., Iadecola C. Delayed treatment with aminoguanidine decreases focal cerebral ischemic damage and enhances neurologic recovery in rats. J. Cereb. Blood Flow Metab. 1998;18(10):1107–1113. doi: 10.1097/00004647-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 180.Sinz E.H., Kochanek P.M., Dixon C.E., Clark R.S., Carcillo J.A., Schiding J.K., Chen M., Wisniewski S.R., Carlos T.M., Williams D., DeKosky S.T., Watkins S.C., Marion D.W., Billiar T.R. Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice. J. Clin. Invest. 1999;104(5):647–656. doi: 10.1172/JCI6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gahm C., Holmin S., Wiklund P.N., Brundin L., Mathiesen T. Neuroprotection by selective inhibition of inducible nitric oxide synthase after experimental brain contusion. J. Neurotrauma. 2006;23(9):1343–1354. doi: 10.1089/neu.2006.23.1343. [DOI] [PubMed] [Google Scholar]
- 182.Satchell M.A., Lai Y., Kochanek P.M., Wisniewski S.R., Fink E.L., Siedberg N.A., Berger R.P., DeKosky S.T., Adelson P.D., Clark R.S. Cytochrome c, a biomarker of apoptosis, is increased in cerebrospinal fluid from infants with inflicted brain injury from child abuse. J. Cereb. Blood Flow Metab. 2005;25(7):919–927. doi: 10.1038/sj.jcbfm.9600088. [DOI] [PubMed] [Google Scholar]
- 183.Adelson P.D., Clyde B., Kochanek P.M., Wisniewski S.R., Marion D.W., Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: A preliminary report. Pediatr. Neurosurg. 1997;26(4):200–207. doi: 10.1159/000121192. [DOI] [PubMed] [Google Scholar]
- 184.Dalkara T., Moskowitz M.A. The complex role of nitric oxide in the pathophysiology of focal cerebral ischemia. Brain Pathol. 1994;4(1):49–57. doi: 10.1111/j.1750-3639.1994.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 185.Dalkara T., Moskowitz M.A. Int. Rev. Neurobiol. Vol. 40. Elsevier; 1996. Neurotoxic and neuroprotective roles of nitric oxide in cerebral ischaemia. pp. 319–336. [DOI] [PubMed] [Google Scholar]
- 186.Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9(1):119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Samdani A.F., Dawson T.M., Dawson V.L. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28(6):1283–1288. doi: 10.1161/01.STR.28.6.1283. [DOI] [PubMed] [Google Scholar]
- 188.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Stella A.M.G. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 189.Keynes R.G., Garthwaite J. Nitric oxide and its role in ischaemic brain injury. Curr. Mol. Med. 2004;4(2):179–191. doi: 10.2174/1566524043479176. [DOI] [PubMed] [Google Scholar]
- 190.Hall E.D., Vaishnav R.A., Mustafa A.G. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7(1):51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pedersen W.A., Cashman N.R., Mattson M.P. The lipid peroxidation product 4-hydroxynonenal impairs glutamate and glucose transport and choline acetyltransferase activity in NSC-19 motor neuron cells. Exp. Neurol. 1999;155(1):1–10. doi: 10.1006/exnr.1998.6890. [DOI] [PubMed] [Google Scholar]
- 192.Durmaz R., Kanbak G., Akyüz F., Isiksoy S., Yücel F., Inal M., Tel E. Lazaroid attenuates edema by stabilizing ATPase in the traumatized rat brain. Can. J. Neurol. Sci. 2003;30(2):143–149. doi: 10.1017/S0317167100053415. [DOI] [PubMed] [Google Scholar]
- 193.Singh I.N., Gilmer L.K., Miller D.M., Cebak J.E., Wang J.A., Hall E.D. Phenelzine mitochondrial functional preservation and neuroprotection after traumatic brain injury related to scavenging of the lipid peroxidation-derived aldehyde 4-hydroxy-2-nonenal. J. Cereb. Blood Flow Metab. 2013;33(4):593–599. doi: 10.1038/jcbfm.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Scheff S.W., Price D.A., Hicks R.R., Baldwin S.A., Robinson S., Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J. Neurotrauma. 2005;22(7):719–732. doi: 10.1089/neu.2005.22.719. [DOI] [PubMed] [Google Scholar]
- 195.Uryu K., Laurer H., McIntosh T., Praticò D., Martinez D., Leight S., Lee V.M-Y., Trojanowski J.Q. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 2002;22(2):446–454. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ohta M., Higashi Y., Yawata T., Kitahara M., Nobumoto A., Ishida E., Tsuda M., Fujimoto Y., Shimizu K. Attenuation of axonal injury and oxidative stress by edaravone protects against cognitive impairments after traumatic brain injury. Brain Res. 2013;1490:184–192. doi: 10.1016/j.brainres.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 197.Reddy P.H., Beal M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008;14(2):45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Plassman B.L., Havlik R.J., Steffens D.C., Helms M.J., Newman T.N., Drosdick D., Phillips C., Gau B.A., Welsh-Bohmer K.A., Burke J.R., Guralnik J.M., Breitner J.C. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55(8):1158–1166. doi: 10.1212/WNL.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 199.Clarke J.R., Lyra E. Silva, N.M.; Figueiredo, C.P.; Frozza, R.L.; Ledo, J.H.; Beckman, D.; Katashima, C.K.; Razolli, D.; Carvalho, B.M.; Frazão, R.; Silveira, M.A.; Ribeiro, F.C.; Bomfim, T.R.; Neves, F.S.; Klein, W.L.; Medeiros, R.; LaFerla, F.M.; Carvalheira, J.B.; Saad, M.J.; Munoz, D.P.; Velloso, L.A.; Ferreira, S.T.; De Felice, F.G. Alzheimer-associated Aβ oligomers impact the central nervous system to induce peripheral metabolic deregulation. EMBO Mol. Med. 2015;7(2):190–210. doi: 10.15252/emmm.201404183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Barnes D.E., Kaup A., Kirby K.A., Byers A.L., Diaz-Arrastia R., Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83(4):312–319. doi: 10.1212/WNL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dams-O’Connor K., Gibbons L.E., Bowen J.D., McCurry S.M., Larson E.B., Crane P.K. Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: A population-based study. J. Neurol. Neurosurg. Psychiatry. 2013;84(2):177–182. doi: 10.1136/jnnp-2012-303938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ott A., Stolk R.P., van Harskamp F., Pols H.A., Hofman A., Breteler M.M. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53(9):1937–1942. doi: 10.1212/WNL.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 203.Johnson V.E., Stewart W., Smith D.H. Widespread τ and amyloid-β pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22(2):142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Scott G., Ramlackhansingh A.F., Edison P., Hellyer P., Cole J., Veronese M., Leech R., Greenwood R.J., Turkheimer F.E., Gentleman S.M., Heckemann R.A., Matthews P.M., Brooks D.J., Sharp D.J. Amyloid pathology and axonal injury after brain trauma. Neurology. 2016;86(9):821–828. doi: 10.1212/WNL.0000000000002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Loane D.J., Pocivavsek A., Moussa C.E., Thompson R., Matsuoka Y., Faden A.I., Rebeck G.W., Burns M.P. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat. Med. 2009;15(4):377–379. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Washington P.M., Morffy N., Parsadanian M., Zapple D.N., Burns M.P. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer’s disease mouse model. J. Neurotrauma. 2014;31(1):125–134. doi: 10.1089/neu.2013.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Washington P.M., Villapol S., Burns M.P. Polypathology and dementia after brain trauma: Does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp. Neurol. 2016;275(Pt 3):381–388. doi: 10.1016/j.expneurol.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Abdul-Muneer P.M., Chandra N., Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2015;51(3):966–979. doi: 10.1007/s12035-014-8752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kokiko-Cochran O.N., Godbout J.P. The inflammatory continuum of traumatic brain injury and Alzheimer’s disease. Front. Immunol. 2018;9:672. doi: 10.3389/fimmu.2018.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cantu D., Walker K., Andresen L., Taylor-Weiner A., Hampton D., Tesco G., Dulla C.G. Traumatic brain injury increases cortical glutamate network activity by compromising GABAergic control. Cereb. Cortex. 2015;25(8):2306–2320. doi: 10.1093/cercor/bhu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sugarman M.A., McKee A.C., Stein T.D., Tripodis Y., Besser L.M., Martin B., Palmisano J.N., Steinberg E.G., O’Connor M.K., Au R., McClean M., Killiany R., Mez J., Weiner M.W., Kowall N.W., Stern R.A., Alosco M.L. Failure to detect an association between self-reported traumatic brain injury and Alzheimer’s disease neuropathology and dementia. Alzheimers Dement. 2019;15(5):686–698. doi: 10.1016/j.jalz.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Crane P.K., Gibbons L.E., Dams-O’Connor K., Trittschuh E., Leverenz J.B., Keene C.D., Sonnen J., Montine T.J., Bennett D.A., Leurgans S., Schneider J.A., Larson E.B. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 2016;73(9):1062–1069. doi: 10.1001/jamaneurol.2016.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Spillantini M.G., Crowther R.A., Jakes R., Hasegawa M., Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Acosta S.A., Tajiri N., de la Pena I., Bastawrous M., Sanberg P.R., Kaneko Y., Borlongan C.V. Alpha-synuclein as a pathological link between chronic traumatic brain injury and Parkinson’s disease. J. Cell. Physiol. 2015;230(5):1024–1032. doi: 10.1002/jcp.24830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ihara Y., Chuda M., Kuroda S., Hayabara T. Hydroxyl radical and superoxide dismutase in blood of patients with Parkinson’s disease: Relationship to clinical data. J. Neurol. Sci. 1999;170(2):90–95. doi: 10.1016/S0022-510X(99)00192-6. [DOI] [PubMed] [Google Scholar]
- 216.Yoritaka A., Hattori N., Uchida K., Tanaka M., Stadtman E.R., Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. USA. 1996;93(7):2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Floor E., Wetzel M.G. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 1998;70(1):268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- 218.Wallace D.C. Mitochondria and cancer: Warburg addressed. Cold Spring Harb. Symp. Quant. Biol. 2005;70:363–374. doi: 10.1101/sqb.2005.70.035. [DOI] [PubMed] [Google Scholar]
- 219.Singh I.N., Sullivan P.G., Deng Y., Mbye L.H., Hall E.D. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: Implications for neuroprotective therapy. J. Cereb. Blood Flow Metab. 2006;26(11):1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- 220.Buczek M., Alvarez J., Azhar J., Zhou Y., Lust W.D., Selman W.R., Ratcheson R.A. Delayed changes in regional brain energy metabolism following cerebral concussion in rats. Metab. Brain Dis. 2002;17(3):153–167. doi: 10.1023/A:1019973921217. [DOI] [PubMed] [Google Scholar]
- 221.Mautes A.E., Thome D., Steudel W.I., Nacimiento A.C., Yang Y., Shohami E. Changes in regional energy metabolism after closed head injury in the rat. J. Mol. Neurosci. 2001;16(1):33–39. doi: 10.1385/JMN:16:1:33. [DOI] [PubMed] [Google Scholar]
- 222.Gadelha F.R., Thomson L., Fagian M.M., Costa A.D., Radi R., Vercesi A.E. Ca2+-independent permeabilization of the inner mitochondrial membrane by peroxynitrite is mediated by membrane protein thiol cross-linking and lipid peroxidation. Arch. Biochem. Biophys. 1997;345(2):243–250. doi: 10.1006/abbi.1997.0259. [DOI] [PubMed] [Google Scholar]
- 223.Castellani R.J., Perry G. Tau biology, tauopathy, traumatic brain injury, and diagnostic challenges. J. Alzheimers Dis. 2019;67(2):447–467. doi: 10.3233/JAD-180721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zanier E.R., Bertani I., Sammali E., Pischiutta F., Chiaravalloti M.A., Vegliante G., Masone A., Corbelli A., Smith D.H., Menon D.K., Stocchetti N., Fiordaliso F., De Simoni M.G., Stewart W., Chiesa R. Induction of a transmissible tau pathology by traumatic brain injury. Brain. 2018;141(9):2685–2699. doi: 10.1093/brain/awy193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kondo A., Shahpasand K., Mannix R., Qiu J., Moncaster J., Chen C.H., Yao Y., Lin Y.M., Driver J.A., Sun Y., Wei S., Luo M.L., Albayram O., Huang P., Rotenberg A., Ryo A., Goldstein L.E., Pascual-Leone A., McKee A.C., Meehan W., Zhou X.Z., Lu K.P. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015;523(7561):431–436. doi: 10.1038/nature14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gerson J., Castillo-Carranza D.L., Sengupta U., Bodani R., Prough D.S., DeWitt D.S., Hawkins B.E., Kayed R. Tau oligomers derived from traumatic brain injury cause cognitive impairment and accelerate onset of pathology in Htau mice. J. Neurotrauma. 2016;33(22):2034–2043. doi: 10.1089/neu.2015.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hu W., Tung Y.C., Zhang Y., Liu F., Iqbal K. Involvement of activation of asparaginyl endopeptidase in tau hyperphosphorylation in repetitive mild traumatic brain injury. J. Alzheimers Dis. 2018;64(3):709–722. doi: 10.3233/JAD-180177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Edwards G., III, Moreno-Gonzalez I., Soto C. Amyloid-beta and tau pathology following repetitive mild traumatic brain injury. Biochem. Biophys. Res. Commun. 2017;483(4):1137–1142. doi: 10.1016/j.bbrc.2016.07.123. [DOI] [PubMed] [Google Scholar]
- 229.Edwards G., III, Zhao J., Dash P.K., Soto C., Moreno-Gonzalez I. Traumatic brain injury induces tau aggregation and spreading. J. Neurotrauma. 2020;37(1):80–92. doi: 10.1089/neu.2018.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Collins-Praino L.E., Corrigan F. Does neuroinflammation drive the relationship between tau hyperphosphorylation and dementia development following traumatic brain injury? Brain Behav. Immun. 2017;60:369–382. doi: 10.1016/j.bbi.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 231.Oberstadt M., Claßen J., Arendt T., Holzer M. TDP-43 and cytoskeletal proteins in ALS. Mol. Neurobiol. 2018;55(4):3143–3151. doi: 10.1007/s12035-017-0543-1. [DOI] [PubMed] [Google Scholar]
- 232.Wang H.K., Lee Y.C., Huang C.Y., Liliang P.C., Lu K., Chen H.J., Li Y.C., Tsai K.J. Traumatic brain injury causes frontotemporal dementia and TDP-43 proteolysis. Neuroscience. 2015;300:94–103. doi: 10.1016/j.neuroscience.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 233.Tan X.L., Sun M., Brady R.D., Liu S., Llanos R., Cheung S., Wright D.K., Casillas-Espinosa P.M., Sashindranath M., O’Brien T.J., McDonald S.J., Turner B.J., Shultz S.R. Transactive response DNA-binding protein 43 abnormalities after traumatic brain injury. 2018. [DOI] [PubMed]
- 234.Heyburn L., Abutarboush R., Goodrich S., Urioste R., Batuure A., Statz J., Wilder D., Ahlers S.T., Long J.B., Sajja V.S.S.S. Repeated low-level blast overpressure leads to endovascular disruption and alterations in TDP-43 and piezo2 in a rat model of blast TBI. Front. Neurol. 2019;10:766. doi: 10.3389/fneur.2019.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Johnson V.E., Stewart W., Trojanowski J.Q., Smith D.H. Acute and chronically increased immunoreactivity to phosphorylation-independent but not pathological TDP-43 after a single traumatic brain injury in humans. Acta Neuropathol. 2011;122(6):715–726. doi: 10.1007/s00401-011-0909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mahan M.Y., Thorpe M., Ahmadi A., Abdallah T., Casey H., Sturtevant D., Judge-Yoakam S., Hoover C., Rafter D., Miner J., Richardson C., Samadani U. Glial Fibrillary Acidic Protein (GFAP) Outperforms S100 Calcium-Binding Protein B (S100B) and ubiquitin c-terminal hydrolase L1 (UCH-L1) as predictor for positive computed tomography of the head in trauma subjects. World Neurosurg. 2019;128:e434–e444. doi: 10.1016/j.wneu.2019.04.170. [DOI] [PubMed] [Google Scholar]
- 237.Agoston D.V., Shutes-David A., Peskind E.R. Biofluid biomarkers of traumatic brain injury. Brain Inj. 2017;31(9):1195–1203. doi: 10.1080/02699052.2017.1357836. [DOI] [PubMed] [Google Scholar]
- 238.Roberts D.J., Jenne C.N., Léger C., Kramer A.H., Gallagher C.N., Todd S., Parney I.F., Doig C.J., Yong V.W., Kubes P., Zygun D.A. Association between the cerebral inflammatory and matrix metalloproteinase responses after severe traumatic brain injury in humans. J. Neurotrauma. 2013;30(20):1727–1736. doi: 10.1089/neu.2012.2842. [DOI] [PubMed] [Google Scholar]
- 239.Guilfoyle M.R., Carpenter K.L., Helmy A., Pickard J.D., Menon D.K., Hutchinson P.J. Matrix metalloproteinase expression in contusional traumatic brain injury: A paired microdialysis study. J. Neurotrauma. 2015;32(20):1553–1559. doi: 10.1089/neu.2014.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Takeuchi S., Wada K., Nawashiro H., Uozumi Y., Otani N., Nagatani K., Kobayashi H., Shima K. Decrease in plasma adiponectin level and increase in adiponectin immunoreactivity in cortex and hippocampus after traumatic brain injury in rats. Turk Neurosurg. 2013;23(3):349–354. doi: 10.5137/1019-5149.JTN.7023-12.1. [DOI] [PubMed] [Google Scholar]
- 241.Dash P.K., Redell J.B., Hergenroeder G., Zhao J., Clifton G.L., Moore A. Serum ceruloplasmin and copper are early biomarkers for traumatic brain injury-associated elevated intracranial pressure. J. Neurosci. Res. 2010;88(8):1719–1726. doi: 10.1002/jnr.22336. [DOI] [PubMed] [Google Scholar]
- 242.Gerbatin R.R., Silva L.F.A., Hoffmann M.S., Della-Pace I.D., do Nascimento P.S., Kegler A., de Zorzi V.N., Cunha J.M., Botelho P., Neto J.B.T., Furian A.F., Oliveira M.S., Fighera M.R., Royes L.F.F. Delayed creatine supplementation counteracts reduction of GABAergic function and protects against seizures susceptibility after traumatic brain injury in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;92:328–338. doi: 10.1016/j.pnpbp.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 243.Darwish R.S., Amiridze N.S. Detectable levels of cytochrome C and activated caspase-9 in cerebrospinal fluid after human traumatic brain injury. Neurocrit. Care. 2010;12(3):337–341. doi: 10.1007/s12028-009-9328-3. [DOI] [PubMed] [Google Scholar]
- 244.Samuels J.M., Moore E.E., Silliman C.C., Banerjee A., Cohen M.J., Ghasabyan A., Chandler J., Coleman J.R., Sauaia A. Severe traumatic brain injury is associated with a unique coagulopathy phenotype. J. Trauma Acute Care Surg. 2019;86(4):686–693. doi: 10.1097/TA.0000000000002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Onose G., Daia-Chendreanu C., Haras M., Ciurea A., Anghelescu A. Traumatic brain injury: Current endeavours and trends for neuroprotection and related recovery. Romanian Neurosurgery. 2011;18(1):11–30. [Google Scholar]
- 246.Vink R., Van Den Heuvel C. Recent advances in the development of multifactorial therapies for the treatment of traumatic brain injury. Expert Opin. Investig. Drugs. 2004;13(10):1263–1274. doi: 10.1517/13543784.13.10.1263. [DOI] [PubMed] [Google Scholar]
- 247.Koura S.S., Doppenberg E.M., Marmarou A., Choi S., Young H.F., Bullock R. Relationship between excitatory amino acid release and outcome after severe human head injury. Acta Neurochir. Suppl. (Wien) 1998;71:244–246. doi: 10.1007/978-3-7091-6475-4_70. [DOI] [PubMed] [Google Scholar]
- 248.Jarrahi A., Braun M., Ahluwalia M., Gupta R.V., Wilson M., Munie S., Ahluwalia P., Vender J.R., Vale F.L., Dhandapani K.M., Vaibhav K. Revisiting traumatic brain injury: From molecular mechanisms to therapeutic interventions. Biomedicines. 2020;8(10):E389. doi: 10.3390/biomedicines8100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Smith D.H., Perri B.R., Raghupathi R., Saatman K.E., McIntosh T.K. Remacemide hydrochloride reduces cortical lesion volume following brain trauma in the rat. Neurosci. Lett. 1997;231(3):135–138. doi: 10.1016/S0304-3940(97)00551-X. [DOI] [PubMed] [Google Scholar]
- 250.Sun F.Y., Faden A.I. Neuroprotective effects of 619C89, a use-dependent sodium channel blocker, in rat traumatic brain injury. Brain Res. 1995;673(1):133–140. doi: 10.1016/0006-8993(94)01413-C. [DOI] [PubMed] [Google Scholar]
- 251.Zhao L., Wang W., Zhong J., Li Y., Cheng Y., Su Z., Zheng W., Guan X.D. The effects of magnesium sulfate therapy after severe diffuse axonal injury. Ther. Clin. Risk Manag. 2016;12:1481–1486. doi: 10.2147/TCRM.S109482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chang C.Z., Wu S.C., Kwan A.L., Lin C.L. Magnesium lithospermate B implicates 3′-5′-Cyclic adenosine monophosphate/protein kinase A pathway and N-Methyl-d-aspartate receptors in an experimental traumatic brain injury. World Neurosurg. 2015;84(4):954–963. doi: 10.1016/j.wneu.2015.05.075. [DOI] [PubMed] [Google Scholar]
- 253.Heath D.L., Vink R. Improved motor outcome in response to magnesium therapy received up to 24 hours after traumatic diffuse axonal brain injury in rats. J. Neurosurg. 1999;90(3):504–509. doi: 10.3171/jns.1999.90.3.0504. [DOI] [PubMed] [Google Scholar]
- 254.Brotfain E., Gruenbaum S.E., Boyko M., Kutz R., Zlotnik A., Klein M. Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr. Neuropharmacol. 2016;14(6):641–653. doi: 10.2174/1570159X14666160309123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Flory J.D., Henn-Haase C., Bierer L.M., Lehrner A., Makotkine I., Marmar C.R., Yehuda R. Glucocorticoid functioning in male combat veterans with posttraumatic stress disorder and mild traumatic brain injury. Biol. Psychiatry. 2015;78(3):e5–e6. doi: 10.1016/j.biopsych.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 256.Skolnick B.E., Maas A.I., Narayan R.K., van der Hoop R.G., MacAllister T., Ward J.D., Nelson N.R., Stocchetti N., Investigators S.T. A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 2014;371(26):2467–2476. doi: 10.1056/NEJMoa1411090. [DOI] [PubMed] [Google Scholar]
- 257.Bergold P.J. Treatment of traumatic brain injury with anti-inflammatory drugs. Exp. Neurol. 2016;275(Pt 3):367–380. doi: 10.1016/j.expneurol.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hasturk A.E., Gokce E.C., Yilmaz E.R., Horasanli B., Evirgen O., Hayirli N., Gokturk H., Erguder I., Can B. Therapeutic evaluation of tumor necrosis factor-alpha antagonist etanercept against traumatic brain injury in rats: Ultrastructural, pathological, and biochemical analyses. Asian J. Neurosurg. 2018;13(4):1018–1025. doi: 10.4103/ajns.AJNS_29_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Batsaikhan B., Wang J.Y., Scerba M.T., Tweedie D., Greig N.H., Miller J.P., Hoffer B.J., Lin C.T., Wang J.Y. Post-injury neuroprotective effects of the thalidomide analog 3,6′-dithiothalidomide on traumatic brain injury. Int. J. Mol. Sci. 2019;20(3):E502. doi: 10.3390/ijms20030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sangobowale M., Nikulina E., Bergold P.J. Minocycline plus N-acetylcysteine protect oligodendrocytes when first dosed 12 hours after closed head injury in mice. Neurosci. Lett. 2018;682:16–20. doi: 10.1016/j.neulet.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mountney A., Boutté A.M., Gilsdorf J., Lu X.C., Tortella F.C., Shear D.A. Intravenous administration of simvastatin improves cognitive outcome following severe traumatic brain injury in rats. J. Neurotrauma. 2016;33(16):1492–1500. doi: 10.1089/neu.2015.4139. [DOI] [PubMed] [Google Scholar]
- 262.Alam S.I., Rehman S.U., Kim M.O. Nicotinamide improves functional recovery via regulation of the RAGE/JNK/NF-κB signaling pathway after brain injury. J. Clin. Med. 2019;8(2):E271. doi: 10.3390/jcm8020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kuwar R., Rolfe A., Di L., Xu H., He L., Jiang Y., Zhang S., Sun D. A novel small molecular NLRP3 inflammasome inhibitor alleviates neuroinflammatory response following traumatic brain injury. J. Neuroinflammation. 2019;16(1):81. doi: 10.1186/s12974-019-1471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Chen Y., Meng J., Xu Q., Long T., Bi F., Chang C., Liu W. Rapamycin improves the neuroprotection effect of inhibition of NLRP3 inflammasome activation after TBI. Brain Res. 2019;1710:163–172. doi: 10.1016/j.brainres.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 265.Zheng B., Zhang S., Ying Y., Guo X., Li H., Xu L., Ruan X. Administration of dexmedetomidine inhibited NLRP3 inflammasome and microglial cell activities in hippocampus of traumatic brain injury rats. Biosci. Rep. 2018;38(5):BSR20180892. doi: 10.1042/BSR20180892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Xu X., Yin D., Ren H., Gao W., Li F., Sun D., Wu Y., Zhou S., Lyu L., Yang M., Xiong J., Han L., Jiang R., Zhang J. Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol. Dis. 2018;117:15–27. doi: 10.1016/j.nbd.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 267.Eisenberg H.M., Frankowski R.F., Contant C.F., Marshall L.F., Walker M.D. High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J. Neurosurg. 1988;69(1):15–23. doi: 10.3171/jns.1988.69.1.0015. [DOI] [PubMed] [Google Scholar]
- 268.Palmer G.C. Neuroprotection by NMDA receptor antagonists in a variety of neuropathologies. Curr. Drug Targets. 2001;2(3):241–271. doi: 10.2174/1389450013348335. [DOI] [PubMed] [Google Scholar]
- 269.Maas A.I., Murray G., Henney H., III, Kassem N., Legrand V., Mangelus M., Muizelaar J.P., Stocchetti N., Knoller N., Pharmos T.B.I.i. Efficacy and safety of dexanabinol in severe traumatic brain injury: Results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5(1):38–45. doi: 10.1016/S1474-4422(05)70253-2. [DOI] [PubMed] [Google Scholar]
- 270.Beauchamp K., Mutlak H., Smith W.R., Shohami E., Stahel P.F. Pharmacology of traumatic brain injury: Where is the “golden bullet”? Mol. Med. 2008;14(11-12):731–740. doi: 10.2119/2008-00050.Beauchamp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lin C.T., Lecca D., Yang L.Y., Luo W., Scerba M.T., Tweedie D., Huang P.S., Jung Y.J., Kim D.S., Yang C.H., Hoffer B.J., Wang J.Y., Greig N.H. 3,6′-dithiopomalidomide reduces neural loss, inflammation, behavioral deficits in brain injury and microglial activation. eLife. 2020;9:9. doi: 10.7554/eLife.54726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Berger A.B., Sexton K.B., Bogyo M. Commonly used caspase inhibitors designed based on substrate specificity profiles lack selectivity. Cell Res. 2006;16(12):961–963. doi: 10.1038/sj.cr.7310112. [DOI] [PubMed] [Google Scholar]
- 273.Pietsch M., Chua K.C., Abell A.D. Calpains: Attractive targets for the development of synthetic inhibitors. Curr. Top. Med. Chem. 2010;10(3):270–293. doi: 10.2174/156802610790725489. [DOI] [PubMed] [Google Scholar]
- 274.Hurtado O., Lizasoain I., Moro M.A. Neuroprotection and recovery: Recent data at the bench on citicoline. Stroke. 2011;42(1) Suppl.:S33–S35. doi: 10.1161/STROKEAHA.110.597435. [DOI] [PubMed] [Google Scholar]
- 275.Kraus M.F., Maki P. The combined use of amantadine and l-dopa/carbidopa in the treatment of chronic brain injury. Brain Inj. 1997;11(6):455–460. doi: 10.1080/026990597123430. [DOI] [PubMed] [Google Scholar]
- 276.Frenette A.J., Kanji S., Rees L., Williamson D.R., Perreault M.M., Turgeon A.F., Bernard F., Fergusson D.A. Efficacy and safety of dopamine agonists in traumatic brain injury: A systematic review of randomized controlled trials. J. Neurotrauma. 2012;29(1):1–18. doi: 10.1089/neu.2011.1812. [DOI] [PubMed] [Google Scholar]
- 277.Stein D.G. Brain damage, sex hormones and recovery: A new role for progesterone and estrogen? Trends Neurosci. 2001;24(7):386–391. doi: 10.1016/S0166-2236(00)01821-X. [DOI] [PubMed] [Google Scholar]
- 278.Dalbasti T., Karabiyikoglu M., Ozdamar N., Oktar N., Cagli S. Efficacy of controlled-release papaverine pellets in preventing symptomatic cerebral vasospasm. J. Neurosurg. 2001;95(1):44–50. doi: 10.3171/jns.2001.95.1.0044. [DOI] [PubMed] [Google Scholar]
- 279.Jiménez-Andrade G.Y., Reyes-García G., Sereno G., Ceballos-Reyes G., Vidal-Cantú G.C., Granados-Soto V. Pyritinol reduces nociception and oxidative stress in diabetic rats. Eur. J. Pharmacol. 2008;590(1-3):170–176. doi: 10.1016/j.ejphar.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 280.Gomazkov O.A. Apoptosis in neuronal structures and the role of neurotrophic growth factors. Biochemical mechanisms of brain derived peptide preparations. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova. 2002;(Suppl. 7):17–21. [PubMed] [Google Scholar]
- 281.Reilly P.L. Brain injury: The pathophysiology of the first hours.‘Talk and Die revisited’. J. Clin. Neurosci. 2001;8(5):398–403. doi: 10.1054/jocn.2001.0916. [DOI] [PubMed] [Google Scholar]
- 282.Browne K.D., Leoni M.J., Iwata A., Chen X.H., Smith D.H. Acute treatment with MgSO4 attenuates long-term hippocampal tissue loss after brain trauma in the rat. J. Neurosci. Res. 2004;77(6):878–883. doi: 10.1002/jnr.20215. [DOI] [PubMed] [Google Scholar]
- 283.Silverberg N.D., Panenka W.J. Antidepressants for depression after concussion and traumatic brain injury are still best practice. BMC Psychiatry. 2019;19(1):100. doi: 10.1186/s12888-019-2076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kim J., Whyte J., Patel S., Europa E., Wang J., Coslett H.B., Detre J.A. Methylphenidate modulates sustained attention and cortical activation in survivors of traumatic brain injury: A perfusion fMRI study. Psychopharmacology (Berl.) 2012;222(1):47–57. doi: 10.1007/s00213-011-2622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Dikmen S.S., Machamer J.E., Winn H.R., Anderson G.D., Temkin N.R. Neuropsychological effects of valproate in traumatic brain injury: A randomized trial. Neurology. 2000;54(4):895–902. doi: 10.1212/WNL.54.4.895. [DOI] [PubMed] [Google Scholar]
- 286.Chartrain A.G., Yaeger K., Feng R., Themistocleous M.S., Dangayach N.S., Margetis K., Hickman Z.L. Antiepileptics for post-traumatic seizure prophylaxis after traumatic brain injury. Curr. Pharm. Des. 2017;23(42):6428–6441. doi: 10.2174/1381612823666171031100139. [DOI] [PubMed] [Google Scholar]
- 287.Wang Y., Peng D., Yang X., Huang P., Ye H., Hui Y., Wang X., Sun W., Wu H., Zhang S., Wang L., Sha H., Shang C., Dong H., Hu Q. Study on umbilical cord-matrix stem cells transplantation for treatment of acute traumatic brain injury in rats. Turk Neurosurg. 2019;29(5):750–758. doi: 10.5137/1019-5149.JTN.25463-18.2. [DOI] [PubMed] [Google Scholar]
- 288.Lee J., Cho Y., Choi K.S., Kim W., Jang B.H., Shin H., Ahn C., Lim T.H., Yi H.J. Efficacy and safety of erythropoietin in patients with traumatic brain injury: A systematic review and meta-analysis. Am. J. Emerg. Med. 2019;37(6):1101–1107. doi: 10.1016/j.ajem.2018.08.072. [DOI] [PubMed] [Google Scholar]
- 289.Chrostek M.R., Fellows E.G., Guo W.L., Swanson W.J., Crane A.T., Cheeran M.C., Low W.C., Grande A.W. Efficacy of cell-based therapies for traumatic brain injuries. Brain Sci. 2019;9(10):E270. doi: 10.3390/brainsci9100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Crupi R., Cordaro M., Cuzzocrea S., Impellizzeri D. Management of traumatic brain injury: From present to future. Antioxidants. 2020;9(4):E297. doi: 10.3390/antiox9040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Leoni M.J., Chen X.H., Mueller A.L., Cheney J., McIntosh T.K., Smith D.H. NPS 1506 attenuates cognitive dysfunction and hippocampal neuron death following brain trauma in the rat. Exp. Neurol. 2000;166(2):442–449. doi: 10.1006/exnr.2000.7513. [DOI] [PubMed] [Google Scholar]
- 292.Sun D., Gu G., Wang J., Chai Y., Fan Y., Yang M., Xu X., Gao W., Li F., Yin D., Zhou S., Chen X., Zhang J. Administration of tauroursodeoxycholic acid attenuates early brain injury via Akt pathway activation. Front. Cell. Neurosci. 2017;11:193. doi: 10.3389/fncel.2017.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yang L.Y., Greig N.H., Huang Y.N., Hsieh T.H., Tweedie D., Yu Q.S., Hoffer B.J., Luo Y., Kao Y.C., Wang J.Y. Post-traumatic administration of the p53 inactivator pifithrin-α oxygen analogue reduces hippocampal neuronal loss and improves cognitive deficits after experimental traumatic brain injury. Neurobiol. Dis. 2016;96:216–226. doi: 10.1016/j.nbd.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Besson V.C. Drug targets for traumatic brain injury from poly(ADP-ribose) polymerase pathway modulation. Br. J. Pharmacol. 2009;157(5):695–704. doi: 10.1111/j.1476-5381.2009.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Tao X., Chen X., Mao X., Hou Z., Hao S., Tian R., Zhu Z., Sun M., Liu B. Protective effects of PARP inhibitor, PJ34, is related to down-regulation of calpain and NF-κB in a mouse model of TBI. Brain Inj. 2016;•••:1–11. doi: 10.3109/02699052.2016.1160151. [DOI] [PubMed] [Google Scholar]
- 296.Shi H., Hua X., Kong D., Stein D., Hua F. Role of toll-like receptor mediated signaling in traumatic brain injury. 2019. [DOI] [PubMed]
- 297.Zheng P., Shultz S.R., Hovens C.M., Velakoulis D., Jones N.C., O’Brien T.J. Hyperphosphorylated tau is implicated in acquired epilepsy and neuropsychiatric comorbidities. Mol. Neurobiol. 2014;49(3):1532–1539. doi: 10.1007/s12035-013-8601-9. [DOI] [PubMed] [Google Scholar]
- 298.Bolognin S., Blanchard J., Wang X., Basurto-Islas G., Tung Y.C., Kohlbrenner E., Grundke-Iqbal I., Iqbal K. An experimental rat model of sporadic Alzheimer’s disease and rescue of cognitive impairment with a neurotrophic peptide. Acta Neuropathol. 2012;123(1):133–151. doi: 10.1007/s00401-011-0908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Shultz S.R., Wright D.K., Zheng P., Stuchbery R., Liu S-J., Sashindranath M., Medcalf R.L., Johnston L.A., Hovens C.M., Jones N.C., O’Brien T.J. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain. 2015;138(Pt 5):1297–1313. doi: 10.1093/brain/awv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tan X.L., Wright D.K., Liu S., Hovens C., O’Brien T.J., Shultz S.R. Sodium selenate, a protein phosphatase 2A activator, mitigates hyperphosphorylated tau and improves repeated mild traumatic brain injury outcomes. Neuropharmacology. 2016;108:382–393. doi: 10.1016/j.neuropharm.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 301.Albayram O., Herbert M.K., Kondo A., Tsai C-Y., Baxley S., Lian X., Hansen M., Zhou X.Z., Lu K.P. Function and regulation of tau conformations in the development and treatment of traumatic brain injury and neurodegeneration. Cell Biosci. 2016;6(1):59. doi: 10.1186/s13578-016-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lu K.P., Kondo A., Albayram O., Herbert M.K., Liu H., Zhou X.Z. Potential of the antibody against cis–phosphorylated tau in the early diagnosis, treatment, and prevention of Alzheimer disease and brain injury. JAMA Neurol. 2016;73(11):1356–1362. doi: 10.1001/jamaneurol.2016.2027. [DOI] [PubMed] [Google Scholar]
- 303.Albayram O., Kondo A., Mannix R., Smith C., Tsai C-Y., Li C., Herbert M.K., Qiu J., Monuteaux M., Driver J., Yan S., Gormley W., Puccio A.M., Okonkwo D.O., Lucke-Wold B., Bailes J., Meehan W., Zeidel M., Lu K.P., Zhou X.Z. Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae. Nat. Commun. 2017;8(1):1000. doi: 10.1038/s41467-017-01068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Rubenstein R., Sharma D.R., Chang B., Oumata N., Cam M., Vaucelle L., Lindberg M.F., Chiu A., Wisniewski T., Wang K.K.W., Meijer L. Novel mouse tauopathy model for repetitive mild traumatic brain injury: evaluation of long-term effects on cognition and biomarker levels after therapeutic inhibition of tau phosphorylation. Front. Neurol. 2019;10:124. doi: 10.3389/fneur.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ikonomovic M.D., Abrahamson E.E., Carlson S.W., Graham S.H., Dixon C.E. Novel therapies for combating chronic neuropathological sequelae of TBI. 2019. [DOI] [PMC free article] [PubMed]
- 306.Whyte J., Vaccaro M., Grieb-Neff P., Hart T. Psychostimulant use in the rehabilitation of individuals with traumatic brain injury. J. Head Trauma Rehabil. 2002;17(4):284–299. doi: 10.1097/00001199-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 307.Tenovuo O. Pharmacological enhancement of cognitive and behavioral deficits after traumatic brain injury. Curr. Opin. Neurol. 2006;19(6):528–533. doi: 10.1097/WCO.0b013e328010944f. [DOI] [PubMed] [Google Scholar]
- 308.McDonald B.C., Flashman L.A., Arciniegas D.B., Ferguson R.J., Xing L., Harezlak J., Sprehn G.C., Hammond F.M., Maerlender A.C., Kruck C.L., Gillock K.L., Frey K., Wall R.N., Saykin A.J., McAllister T.W. Methylphenidate and Memory and Attention adaptation training for persistent cognitive symptoms after traumatic brain injury: A Randomized, Placebo-Controlled Trial. Neuropsychopharmacology. 2017;42(9):1766–1775. doi: 10.1038/npp.2016.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhu J., Hamm R.J., Reeves T.M., Povlishock J.T., Phillips L.L. Postinjury administration of L-deprenyl improves cognitive function and enhances neuroplasticity after traumatic brain injury. Exp. Neurol. 2000;166(1):136–152. doi: 10.1006/exnr.2000.7484. [DOI] [PubMed] [Google Scholar]
- 310.Luauté J., Plantier D., Wiart L., Tell L. Care management of the agitation or aggressiveness crisis in patients with TBI. Systematic review of the literature and practice recommendations. Ann. Phys. Rehabil. Med. 2016;59(1):58–67. doi: 10.1016/j.rehab.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 311.Kreitzer N., Ancona R., McCullumsmith C., Kurowski B.G., Foreman B., Ngwenya L.B., Adeoye O. The effect of antidepressants on depression after traumatic brain injury: A meta-analysis. J. Head Trauma Rehabil. 2019;34(3):E47–E54. doi: 10.1097/HTR.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Albrecht J.S., Lydecker A., Peters M., Rao V. Treatment of depression following traumatic brain injury reduces risk of neuropsychiatric outcomes. J. Neurotrauma. 2020;37(23):2542–2548. doi: 10.1089/neu.2019.6957. [DOI] [PubMed] [Google Scholar]
- 313.Saletti P.G., Ali I., Casillas-Espinosa P.M., Semple B.D., Lisgaras C.P., Moshé S.L., Galanopoulou A.S. In search of antiepileptogenic treatments for post-traumatic epilepsy. Neurobiol. Dis. 2019;123:86–99. doi: 10.1016/j.nbd.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Williamson D., Frenette A.J., Burry L.D., Perreault M., Charbonney E., Lamontagne F., Potvin M.J., Giguère J.F., Mehta S., Bernard F. Pharmacological interventions for agitated behaviours in patients with traumatic brain injury: A systematic review. BMJ Open. 2019;9(7):e029604. doi: 10.1136/bmjopen-2019-029604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Massagli T.L. Neurobehavioral effects of phenytoin, carbamazepine, and valproic acid: Implications for use in traumatic brain injury. Arch. Phys. Med. Rehabil. 1991;72(3):219–226. [PubMed] [Google Scholar]
- 316.Morra J.A., Alao A.O. Role of quetiapine in protection of neurodegeneration after traumatic brain injury. Int. J. Psychiatry Med. 2019;55(2):67–73. doi: 10.1177/0091217419838105. [DOI] [PubMed] [Google Scholar]
- 317.Borghol A., Aucoin M., Onor I., Jamero D., Hawawini F. Modafinil for the improvement of patient outcomes following traumatic brain injury. Innov. Clin. Neurosci. 2018;15(3-4):17–23. [PMC free article] [PubMed] [Google Scholar]