Abstract
Background
Alzheimer's disease (AD) affects several people worldwide and has devastating impacts on society with a limited number of approaches for its pharmacological treatment. The main causes of AD are not clear yet. However, the formation of senile plaques, neurofibrillary tangles, hyper-phosphorylation of tau protein, and disruption of redox homeostasis may cause AD. These causes have a positive correlation with oxidative stress, producing reactive ions, which are responsible for altering the physiological condition of the body.
Conclusion
Ongoing research recommended the use of phytochemicals as acetylcholinesterase inhibitors to hinder the onset and progression of AD. The natural compound structures, including lignans, flavonoids, tannins, polyphenols, triterpenes, sterols, and alkaloids have anti-inflammatory, antioxidant, and anti-amyloidogenic properties. The purpose of this article is to provide a brief introduction to AD along with the use of natural compounds as new therapeutic approaches for its management.
Keywords: Alzheimer's disease, nutraceuticals, nutrients, phytochemicals, mechanism of AChE in Alzheimer's disease
1. INTRODUCTION
Neurological disorders (ND) are considered as one of the fatal diseases, especially in the urbanized nations. Among those, Alzheimer's disease (AD) is at the highest point on the sequence of neurological disorders [1, 2]. AD was first portrayed and named by a German therapist and pathologist, Alois Alzheimer, in 1906 [3]. AD is the main reason for dementia in aging individuals [4, 5]. Cognitive dysfunction, principally memory misfortunes are the primary side effects related to this illness. Different highlights related to the later phases of AD incorporate language deficiencies, depression, behavioral problems, and psychosis. The etiology of AD is yet not clear. Many pieces of research have been attempted in recent years and concluded with some solid speculations. Some of them are cholinergic theory, amyloid cascade hypothesis, tau hypothesis, oxidative stress hypothesis, zinc dyshomeostasis hypothesis, and calpain cathepsin hypothesis [6-11]. AD is described by neuronal misfortune and progressive cognitive weakness. AD is the main source of dementia worldwide and the rate is expanding quickly, with analysis expected to significantly increase continuously in 2050 [12]. Dementia patients totaled 24.2 million in 2005 and 4.2 million cases emerged every year from 2005 to 2011, with 70% of these cases being a consequence of AD [13]. In developed nations, 1 out of 10 individuals beyond 65 years old is influenced by dementia, with the recurrence of AD nearly doubling within this specific population every 5 years [14]. Around the world, the expense of medicinal consideration for dementia sufferers is approximately 604 billion US$ with the yearly expense of AD per patient ranging between US$42000 to US$56000 in the USA [15, 16]. The average duration of ailment differs somewhere in the range of 4 and 8 years, albeit a few patients may survive up to 20 years after the beginning of the AD [17]. Alzheimer's dementia is assessed to have expanded by 35.4% in 2015, altogether raising the particular expenses of this ailment [18]. Until 1921, it was accepted that the transmission of nerve impulses was 'electrical' in nature. But this hypothesis was not worthy because of two reasons, one being the presence of a gap among neurons and effecter organs and the other a decline in action because of impulses from inhibitory nerves. Otto Loewi demonstrated the 'Chemical' nature of impulse transmission through his analysis of two beating hearts from frogs - one associated with the vagus nerve and accelerator agent nerves; the second one without nerve association. In this investigation, he found the first neurotransmitter acetylcholine (ACh) [19, 20]. Acetylcholinesterase (AChE) is associated with the termination of neurotransmission, while the job of butyrylcholinesterase (BChE) is not comprehended. ACh is a low molecular weight neurotransmitter displayed in both the central and peripheral nervous systems. It is liable for signal transmission from nerves to terminal organs and muscles. Nicotinic (nAChR) and muscarinic acetylcholine receptors (mAChR) are available in the body and these receptors transmit information to many tissues, moreover, they can be found on leukocytes, endothelial cells, nerves, and others [21-24]. AChE is an enzyme converting acetylcholine into choline and acetate. Neurotransmission is halted by the impact of AChE [25, 26]. Acetylcholinesterase inhibitors (AChIs) have been exhibited to improve AD symptoms [27]. Medications for AD are donepezil, rivastigmine [28], and galantamine [29]. These medications were created dependent on the cholinergic hypothesis [30].
The natural sources, particularly plants, give various undiscovered properties of substrates for drug discovery pipeline and offer incredible potential for the improvement of new cholinesterase inhibitors. Past investigations have just introduced the capability of plants as crucial hotspots for cholinesterase inhibitor agents [31, 32]. Therefore, the greater part of the medication depends on the cholinergic theory, which proposes that AD starts as a deficiency in the creation of the synapse acetylcholine.
There are several inhibitors reported for AD management against AChE and BChE [33]. The way that naturally-occurring compounds from plants are viewed as a potential source of new inhibitors has prompted the revelation of a significant number of secondary metabolites and plant extracts with the capacity of inhibiting the AChE, which, as indicated by the cholinergic speculation, expands the levels of the synapse acetylcholine in the brain, hence improving cholinergic capacities in AD patients and alleviating the symptoms of this neurological issue [32]. People with AD and their parental figures are utilizing supplements to halt the movement of the illness [34]. Natural products, for example, curcumin, ginger, and Gingko biloba have been utilized as diets and dietary enhancements to treat human sicknesses, including malignancy, cardiovascular, respiratory, diabetes, metabolic disorders, and neurological issue [35]. It is acknowledged that natural compounds, including vitamins A, C, E, β-carotene, and minerals found in fruits and vegetables are anti-oxidants that offer medical advantages against a few distinctive oxidative stress prompted degenerative sicknesses, including AD [36]. The term vitamin E incorporates diverse fat-soluble compounds, separated into tocopherols and tocotrienols, that have antioxidant activity. α-Tocopherol is the most contemplated, yet a few investigations recommended that tocotrienols may have diverse health-promoting capacities [37]. More information on phytochemicals and their particular targets are fundamental to ensure the safe utilization of these compounds as a possibility for AD treatment [38, 39].
2. MECHANISM OF AChE
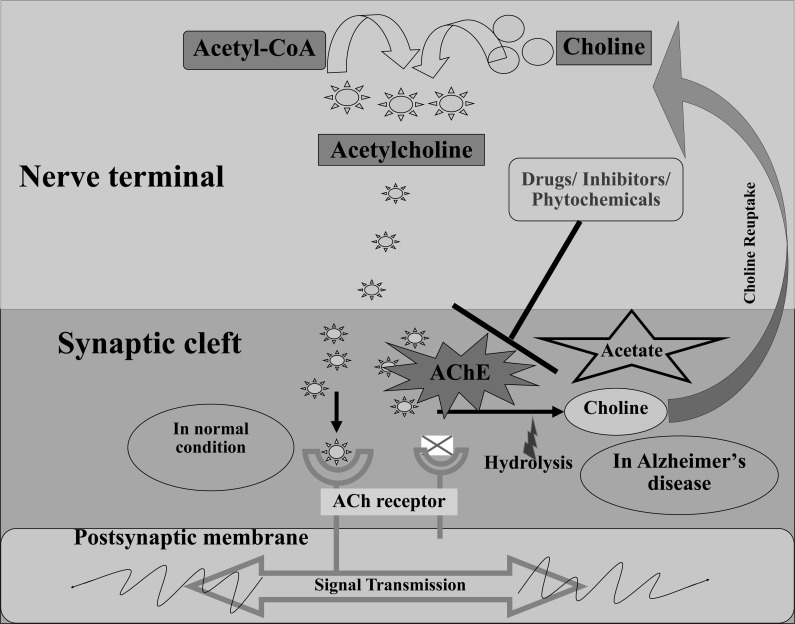
Silman and Sussman (2008) have portrayed a 'structure-function relationship' for AChE (EC 3.1.1.7) and announced that the enzyme was acquired from an enormous group of proteins that, mutually, share a typical α/β fold [40]. Neurotransmitters are called endogenous chemical messengers that empower neurotransmission. They transmit signals over a chemical synapse, for example, neuromuscular connection, from one neuron to another neuron, muscle cell, or gland cell. The essential physiological role of AChE includes the termination of chemical transmission at cholinergic synapses and secretory organs by catalyzing the hydrolysis of the neurotransmitter acetylcholine (ACh), at a high turnover rate (2.5x104 molecules per second) [41, 42]. During neurotransmission, ACh is released from the nerve into the synaptic part and ties to ACh receptors on the post-synaptic layer, transferring the signal from the nerve. AChE, additionally situated on the post-synaptic membrane, ends the signal transmission by hydrolyzing ACh. The liberated choline is taken up again by the pre-synaptic nerve and ACh is incorporated by adding with acetyl-CoA through the choline acetyltransferase [43, 44]. The mechanism of AChE is represented in Fig. (1).
Fig. (1).
Mechanism of AChE in Alzheimer disease. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. ROLE OF NATURAL OR NUTRACEUTICALS COMPOUNDS IN ALZHEIMER’S DISEASE
Dietary supplements have been suggested to cure diseases [45]. Nutraceuticals are the extracts of the compounds from the foods that have health benefits. The term nutraceutical was coined by Stephen Defelice, by combining the words nutrition and pharmaceutical [46]. Nutraceuticals are consumed in the concentrated form like pills, capsules, and beverages having no side effects even at high doses [47]. Even though the causes of AD are not clear however some studies have hypothesized some reasons which include: formation of senile plaques, neurofibrillary tangles, hyper-phosphorylation of tau protein, and disruption of redox homeostasis. These causes have a positive correlation with oxidative stress, producing the reactive ions, which are responsible for altering the physiological condition of the body. To overcome the side effects of known drugs, the researchers are focusing on the identification of the natural bioactive compounds present in foods to treat AD [48]. Consumption of foods consisting of bioactive compounds or appropriate administration of extracted bioactive compounds can have a prophylactic effect against various pathophysiological conditions. Although there are various sources of bioactive compounds used for AD treatment we have only discussed the commonly available. In this section, the effect of various bioactive compounds present in commonly available foods on AD has been summarized and discussed individually.
3.1. Curcumin
Curcumin is a polyphenolic compound derived from the Curcuma Longa. Various studies have shown the curative effect of curcumin on AD. The complete mechanism of the curative effect of curcumin is unknown. However, the three proposed mechanisms are 1) reducing the aggregation of Aβ peptides in the neural tissue due to their anti-inflammatory property [49]. 2) Inhibition of enzymes β-Secretase and AChE responsible for the formation of Aβ fibril [50]. 3) Metal ions present in the synapse regions alleviate the aggregation of Aβ; the phosphorylation of the tau protein bind with metal ions is the main mechanism behind its neuroprotective property [51, 52].
Using protein-ligand docking and ab initio fragment molecular orbital curcumin derivatives have been investigated to inhibit the aggregation of amyloid-β peptides (Aβs) in the brain [53]. Curcumin-loaded nanocapsules have shown an antidepressant-like effect against the Aβ25-35 induced neurotoxicity in mice. Curcumin-loaded nanocapsules were also found more effective than the free curcumin [54]. A study was conducted on streptozotocin-induced rats to find the effect of curcumin on short-term spatial and recognition memory. It has been observed that the doses of 50 and 100 mg kg-1 of curcumin preserved short-term object recognition memory but not short-term spatial memory suggesting a positive effect of curcumin on the former which was not related to hippocampal neurogenesis [55]. An evidence-based in vivo study on albino rats was tested for AD by using the steroid curcumin derivatives. These compounds showed anti-AD activity by enhancing ACh synthesis, GSh level, paraoxonase level, and BCL2 lymphoma level as compared to the untreated group [41]. A model of AD induced by Aβ1-42 peptide in aged female mice has been studied against curcumin lipid-core nanocapsules. It has been reported that after the administration of curcumin lipid-core nanocapsules, displayed significant neuroprotection against Aβ1-42 induced behavioral and neurochemical changes in Alzheimer's model [56]. In a study, selenium nanoparticles encapsulated in Poly-lactide-co-glycolide polymer with curcumin greatly cured the memory and decreased the Aβ load in the brain samples of Alzheimer's mice [57]. The effect of erythropoietin and curcumin on streptozotocin-induced Alzheimer’s dementia rats was studied. It has been reported that the administration of erythropoietin suppressed extrinsic apoptosis while curcumin was effective in combating oxidative stress in streptozotocin-injected rats [58]. Amyloid-binding properties of curcumin analogs viz., demethoxycurcumin, bis-demethoxycurcumin and dimethoxycurcumin in AD have been conducted through in vitro autoradiography and it has been reported that BDMC had the highest affinity for Aβ containing plaques in brain tissues as compared to other curcuminoids. These findings propose that curcumin analogs may serve as a possible radio-ligand for Aβ plaque neuroimaging [59].
3.2. Chitosan
Chitosan is derived by partial deacetylation of chitin which is present in crustacean shells. It is composed of two basic units glucosamine and N-acetylglucosamine. Chitosan shows mucoadhesive properties because of its positive charge. It is insoluble in water and organic solvents with a weak basic nature. The biodegradability, low toxicity, and biocompatibility with other compounds make it suitable for use in biomedical and pharmaceutical formulations. In recent studies, it has been revealed that short-chain chitosan known as chitooligosaccharides have possessed neuroprotective properties because of their ability to inhibit the Aβ and AChE activities. It has also been found to have anti-neuroinflammation and anti-apoptosis effects, which suggest its relation to cure AD [60-63].
In the recent study, chitosan oligosaccharides were chemically modified to prepare peracetylated and N-acetylated chitosan. Their effect against glutamate-induced PC12 cell death was analyzed and they reported that the pretreatment of PC12 cells with the peracetylated chitosan oligosaccharides markedly inhibited glutamate-induced cell death in a concentration-dependent manner. It has also decreased the lactate dehydrogenase release and reactive oxygen species production and loss of mitochondrial membrane potential. Suggesting the peracetylation is essential for the neuroprotective effects of chitosan oligosaccharides [60]. Jia et al. [64] reported that the chitosan oligosaccharides have shown favorable effects on the cognitive impairments in the Aβ1-42 induced rat model of AD. This was due to the inhibition of oxidative stress and neuroinflammatory responses. Other findings confirmed that due to the attenuating oxidative stress by chitooligomers a significant inhibition of dopaminergic neurodegeneration and linked physiological alteration induced by monocrotophos was observed in C. elegans [62]. Chitooligosaccharides with six different molecular weights were studied against the inhibition of AChE and it has been reported that the 90 molecular weight chitooligosaccharide exhibited potent AChE inhibitory activities compared to other molecular weights [65]. Pangestuti & Kim [61] has summarized the neuroprotective properties of chitosan and its derivatives. As per the data chitosan and its derivatives have shown promising properties such as suppression of Aβ formation, AChIs, anti-neuroinflammatory activity, apoptosis inhibitors, etc., which reveal the potential of chitosan and its derivatives as potential therapeutic candidates for ND management. Zhou et al. [63] have reported chitooligosaccharides to protect cultured hippocampal neurons against glutamate-induced neurotoxicity, which suggests that it prevents cultured hippocampal neurons from glutamate-induced cell damage by intrusive with a raise in [Ca2+] and inhibiting caspase-3 activity. In addition to the above studies of chitosan has been used as a vehicle for brain drug delivery because of its good biocompatibility and biodegradability properties [66-71].
3.3. Shilajit
Shilajit is a blackish-brown powder or exudates from high mountain rocks [72]. It takes centuries to produce by the decomposition of plant material from species such as Euphorbia royleana and Trifolium repens [73]. The composition of shilajit may vary from place to place; however the common composition is composed of minerals, mainly selenium, dibenzo-α-pyrones, and humic substances, which include humins, humic acid, and fulvic acid. Other than these molecules, it contains ellagic acid, some fatty acids, resins, latex, gums, albumins, triterpenes, sterols, aromatic carboxylic acids, 3,4-benzocoumarins, amino acids, polyphenols, and phenolic lipids [74, 75].
Effects of shilajit on biogenic free radicals have been estimated by Bhattacharya et al. [76] and they have reported that the processed shilajit 20 and 50 mg kg-1day-1 for 21 days induced a dose-related increase in free radical scavenging enzymes superoxide dismutase, catalase, and glutathione peroxidase activities in frontal cortex and striatum of rats, which suggests the use of shilajit against oxidative stress and geriatric complaints. The improved learning and the memory engram in rats, especially in aged rats, have been reported to increase after the administration of shilajit [77]. Administration of shilajit and Withania somnifer in male wistar rats were studied to find their effect on memory enhancement. It has been reported that the administration of shilajit decrease the AChE straining, restricted to the basal forebrain nuclei, including the medial septum and the vertical limb of the diagonal band [78]. With the help of atomic force techniques, it has been observed that the fulvic acid present in the shilajit slows down the tau protein aggregation thus affecting the fibril length and their morphology. Thus, fulvic acid can be used as a potential nutraceutical for the treatment of AD [72, 79].
3.4. Ginkgo Biloba
Ginkgo biloba tree is widely cultivated in China as a source of food and traditional medicine. Gingko supplements are used to treat AD, dementia, or cognitive impairment. The bioactive components of Ginkgo biloba are flavonoids, terpenoids, and terpene lactones (ginkgolides and bilobalide). The proposed mechanisms of Gingko biloba against AD are the modification of the neurotransmitter system, reducing free radicals, increasing blood supply of the brain, and reducing blood viscosity [80]. Vitolo et al. [81] has found the protective effect of Ginkgolide J against Aβ induced irregular synaptic function and cell death. Oral administration of Ginkgo biloba extract showed the partial recovery of memory deficit and decreased the choline acetyltransferase activity in the hippocampus of rats treated with intraventricular infusion of Aβ for 14 days [82]. Ginkgo biloba a flavonoid-rich antioxidant showed the enhancement of spatial learning and memory of transgenic rats, which was independent of an influence on soluble Aβ or Aβ plaque burden [83]. In a 3 months study, the patient’s attention and memory performance showed significant improvement compared to placebo with the oral administration of Ginkgo biloba extract [84]. Ismail & El-Sonbaty [85] reported the enhanced effect of Ginkgo biloba leaf extract with fermentation against neuroinflammation, stress hormones, apoptosis, and oxidative damage induced by gamma irradiation in the rat brain. The fermentation by Aspergillus niger enhanced the bio-activities of Ginkgo biloba leaf extract as compared to non-fermented extract. Another study has reported the reduced oxidative stress by Ginkgo biloba in the brain tissues of rats induced by electromagnetic waves of mobile phones [86]. The above pieces of evidence suggest the Ginkgo biloba is a potential remedy to treat AD.
3.5. Drumstick
Moringa oleifera is found in Asian and African countries. Its leaves and fruits are consumed by the people [87, 88]. As a food, its extracts are not toxic [87]. The leaves of Moringa oleifera have shown nootropics activity and protection against oxidative stress produced in AD [89]. It was also found that the leaves of Moringa oleifera protect hypobaric hypoxia by altering monoamines in the brain [90]. The effect of Moringa oleifera leaves extract on colchicines infused AD model in rats has been reported to increase superoxide dismutase and catalase activity and decrease lipid peroxidase in the cerebral cortex [91]. Ekong et al. [92] reported the protective effect of
4. ROLE OF ALKALOID COMPOUNDS IN ALZHEIMER’S DISEASE
Alkaloids are a group of natural compounds with organic nitrogen, having low molecular weight primarily found in 25% of the species of higher plants as a secondary metabolite. It is abundant in the Apocynaceae, Fabaceae, Asteraceae, Rubiaceae, Papaveraceae, and Solanaceae families [95, 96]. Since ancient times plant extracts containing alkaloids like Atropa belladonna, Papaver somniferum, Hyoscyamus niger, and Erythroxylum coca have been used for medicinal purposes [97]. These compounds have been used in various pathologies like AD in Table 1.
Table 1.
Role of alkaloid compounds in Alzheimer’s disease.
| S. No. | Alkaloid Compounds | Test | Result | Refs. | |||
|---|---|---|---|---|---|---|---|
| 1 | Aromoline | In vitro | Showed significant human BChE (hBChE) inhibitory activity. | [124] | |||
| 2 | Berberine | Alzheimer’s like disease was induced in rats orally by a mixture of aluminum, cadmium, and fluoride | Improved cognitive behaviors and Docking results showed that berberine inhibited AChE, COX-2, and TACE. | [125] | |||
| 3 | Berberine | Aβ25-35 induced apoptosis in primary neuron cells isolated from the hippocampus of newborn mice | Berberine reversed the effects induced by Aβ25-35. Berberine attenuated the cytotoxic effect of Aβ25-35. Berberine led to a decline in the apoptotic rate. |
[126] | |||
| 4 | Berberine loaded multiwalled carbon nanotubes with polysorbate and phospholipid coating |
In vivo pharmacokinetic studies in rats |
Improvement in the rate and extent of drug absorption in the plasma and brain tissues. Phospholipid-coated and the polysorbate-coated exhibited remarkable recovery in-memory performance from 18th to 20th day. |
[127] | |||
| 5 | Berberine | Ethanol-induced oxidative stress and memory dysfunction in rats | Prevents changes in oxidative stress and cholinesterase activity. | [128] | |||
| 6 | Berberine | Synaptic deficits induced by D-galactose in Male Wistar rats | Synaptic/memory impairments. | [129] | |||
| 7 | Berberine | Twenty-month-old male C57BL/6 mice |
Alleviate postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. | [130] | |||
| 8 | Berberine | Diabetic neuropathy induced by streptozotocin and a high-carbohydrate/high-fat diet in rats. | Beneficial against diabetic neuropathy induced by streptozotocin and a high-carbohydrate/high-fat diet in rats. | [131] | |||
| 9 | Dehydroevodiamine | Rat brain slices | It activates a PP2A Tyr307 site and inhibits phosphorylation of tau in rat brain. |
[132] | |||
| 10 | Galantamine co-administration with adenosine |
Oral tremor induced by galantamine in rats. |
Significantly attenuated the tremulous jaw movements induced by the galantamine. It may be beneficial in reducing parkinsonian motor impairments induced by anticholinesterase treatment. |
[133] | |||
| 11 | Galantamine hydrobromide | Chronic effects of Galantamine hydrobromide on male albino mice. | Galantamine hydrobromide exerted severe perturbations in the cholinergic system in all regions of the brain on chronic exposure, thus eventually leading to behavioral changes. | [134] | |||
| 12 | Galantamine attaching to ceria-containing hydroxyapatite as well ceria-containing Carboxymethyl chitosan-coated hydroxyapatite nanocomposites. |
Ovariectomized AD albino-rats |
Nanoceria-containing uncoated hydroxyapatite-based-galantamine nanocomposite had been found a highly efficient anti-Alzheimer agent, where the nanoceria and hydroxyapatite also showed noteworthy useful effects to galantamine on drug-delivering action, scavenging the hazard reactive oxygen species, repairing the degenerated nerve cells, and discarding the toxic Aβ-amyloid plaques. | [135] | |||
| 13 | Galantamine | AD model mice | Administration of galantamine from the preplaque phase ameliorates the memory decline, improved the unbalanced redox state, and enhanced microglial function. | [136] | |||
| 14 | Huperzine A | Alzheimer Transgenic Mouse Model | It reduces the level of Aβ. | [137] | |||
| 15 | Huperzine C | In vitro | Showed moderate AChE inhibition with an IC50 value of 0.525 ± 0.140 μM |
[138] | |||
| 16 | N-methylasimilobine | In vitro | Exhibited 50% inhibition of AChE at the concentration of 1.5± 0.2 µg mL-1 |
[139] | |||
| 17 | Isorhynchophylline | Amyloid-β Induced Cognitive Impairment in Rats | It restores Aβ -induced a cognitive impairment inhibits neuronal apoptosis and reduces phosphorylation of tau. |
[140] | |||
| S. No. | Alkaloid Compounds | Test | Result | Refs. | |||
| 18 | Galanthamine | In vitro | The inhibitory potential of AChE with IC50 values was 0.35 µmol/L | [141] | |||
| 19 | Palmatine | In vitro, in vivo and ex vivo | Exhibits anti-inflammatory, anti-depressive, anti-pyretic, anti-neurodegenerative properties. | [142] | |||
| 20 | Palmatine and physostigmine | Swiss young male albino mice | Palmatine and physostigmine significantly improved the learning and memory of mice | [143] | |||
| 21 | Palmatine | In vitro | Inhibit PHF6 and full-length tau aggregation and disassemble pre-formed fibrils | [144] | |||
| 22 | Phenserine | Three-month-old male Fischer-344 rats |
Possess the AChE inhibitory activity | [145] | |||
| 23 | Sanguinine | In vitro | Inhibited the activity of AChE | [146] | |||
| 24 | Taspine | In vitro | Inhibited the activity of AChE with an IC50 value of 0.33 ± 0.07 μM | [147] | |||
Moringa oleifera leaf extract on the aluminum-induced temporal cortical degeneration in male albino Wiastar rats. Moringa oleifera leaf extract reduced the serum aluminum concentration and fights against aluminum-induced neurohistopathology in the temporal cortex [92]. Moringa oleifera leaf extract was incubated with the primary culture of embryonic hippocampal neurons and it has been reported that the leaf extract promoted axodendritic maturation and neuroprotection, suggesting its well-being importance for the nervous system [93]. In a recent study, Moringa oleifera was tested against hyperhomocysteinemia induced AD-like pathology in rats, the results showed to prevent the oxidative stress and cognitive impairments induced by homocysteine. It has also decreased the tau hyperphosphorylation and Aβ production in the AD rat model [94]. From the above data, Moringa oleifera is well supported as a good candidate for the treatment of AD.
4.1. Berberine
Berberine is isolated from the Rhizoma coptidis which is a perennial herb and a natural isoquinoline alkaloid. This compound is said to be beneficial for AD by inhibiting Aβ production. It has been reported that the berberine reduced and promoted the hyperphosphorylation of tau and autophagic clearance of tau, respectively, in the mouse model, which strongly supports the berberine as a potential drug candidate for AD [98]. In an experimental model of intracerebroventricular streptozotocin-induced sporadic Alzheimer's-like dementia, administration of berberine has prevented memory loss, anxiogenic behavior, AChE activity, and cell death induced by intracerebroventricular streptozotocin by protecting the progression of neurodegeneration [99]. A neonatal rat model was induced with schizophrenia with the administration of MK-801 and the rodents treated with berberine showed improved motor and cognitive disturbances [100]. The effect of berberine on Aβ-induced impairments in learning and memory of male Wistar rats was reported to prevent memory impairment by restoring the Aβ-induced impairments [101]. Berberine administration was evaluated with western blotting, morris water maze, immunofluorescence staining enzyme-linked immunosorbent assay, and histological analysis, and against AD in mice, it has been reported that berberine significantly enhanced the mice’s spatial learning capacity and memory retention by reducing the Aβ plaque deposition in the hippocampus [102].
4.2. Galantamine
This tertiary alkaloid is isolated from the flowers and bulbs of Galanthus woronowii, Galanthus caucasicus, Narcissus, Leucojum aestivum, and Lycoris radiata [103]. It acts as an AChIs and is approved by the FDA to cure AD, which is available in the market with a generic name as ORAL (ga-LAN-ta-meen)[104]. Galantamine reversibly inhibits cholinesterase, thus preventing the lyses of neurotransmitters [105]. Lyketsos et al. [106] have reported the safety and tolerability of galantamine for up to 18.5 months. The effect of galantamine on cardiac function of newly diagnosed AD patients was evaluated by Isik et al. [107] and they reported that no significant changes in arterial blood pressure occurred at any investigated dosage level. A study was carried to find the metabolite changes in the hippocampal after galantamine treatment for AD. It was reported that the levels of glutamate, glutamate/creatine, and glutamate/N-acetyl aspartate increased after four months of treatment with galantamine [108].
4.3. Palmatine
Palmatine is an isoquinoline alkaloid with various pharmacological effects like AD, age-related disease, cancer, cardiac hypertrophy, osteoporosis, and diabetes [109-111]. It is found in Tinospora cordifolia (Willd.) Miers [112], Corydalis yanhusuo [113], Coptis chinensis Franch. [114], Tinospora sagittata (Oliv.) Gagnep [115], Phellodendron amurense Rupr. [116] and Stephania yunnanensis [117]. Palmatine has shown neuroprotective activity mainly by inhibiting the activity of AChE, BChE, and neuraminidase-1 [118]. It has been reported that palmatine had an antidepressant effect because it regulates brain catalase levels, lipid peroxidation, monoamine oxidase-A activity, plasma nitrite, and corticosterone levels [119]. The synergetic effect of palmatine with berberine has been reported to intensify the inhibition of AChE [120].
4.4. Chelerythrine
Chelerythrine is an alkaloid present in Chelidonium majus L., Sanguinaria canadensis L., Dicranostigma lactucoides, and Zanthoxylum spp. [121]. Chelerythrine inhibits AChE and BChE activity and Aβ aggregation [122]. It has been reported that chelerythrine inhibits the activity of the CYP1A1 enzyme, which can activate some toxic compound to cause cancer [123].
CONCLUSION
Phytochemicals have extraordinary biological action and keep on entering into clinical trials, like alkaloids, nutraceuticals, and nutrients from plants, and it is used for the treatment of various diseases like neurodegenerative issues, cardiovascular ailments, and malignant growth. We emphasized the use of phytochemicals for the effective management of AD in this article. Phytochemicals have the smallest toxicity along with a high level of the repairable property concerning the human body as they are used for AD management. Phytochemicals possess several biological properties like free radical scavenger, anti-inflammatory, anti-aging, and anti-ND properties. For this reason, fruits, vegetables, and spices are recommended for the daily use in the diet because they contain several antioxidant natural compounds. The earth is blessed with diverse foods having nutraceutical properties but due to lack of scientific evidence, they have not been recognized for their curative properties. There is a huge research gap in this field that should be analyzed, evaluated, and summarized in the future.
ACKNOWLEDGMENTS
The authors acknowledged their institutes for providing general support to complete this study.
LIST OF ABBREVIATIONS
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- AChIs
Acetylcholinesterase Inhibitors
- AD
Alzheimer's Disease
- Aβ
Amyloid-beta
- BChE
Butyrylcholinesterase
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ahmad S.S., Akhtar S., Danish R.S.M., Kamal M.A., Sayeed U., Khan M.K.A., Siddiqui M.H., Arif J.M. Screening and elucidation of selected natural compounds for anti- Alzheimer’s potential targeting BACE-1 Enzyme: A case computational study. Curr. Comput. Aided. Drug Des. 2017;13(4):311–318. doi: 10.2174/1573409913666170414123825. [DOI] [PubMed] [Google Scholar]
- 2.Firoz C.K., Jabir N.R., Khan M.S., Mahmoud M., Shakil S., Damanhouri G.A., Zaidi S.K., Tabrez S., Kamal M.A. An overview on the correlation of neurological disorders with cardiovascular disease. Saudi J. Biol. Sci. 2015;•••:19–23. doi: 10.1016/j.sjbs.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berchtold N.C., Cotman C.W. Evolution in the conceptualization of dementia and Alzheimer’s disease: Greco-Roman period to the 1960s. Neurobiol. Aging. 1998;19(3):173–189. doi: 10.1016/S0197-4580(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association 2010 Alzheimer’s disease facts and figures. Alzheimers Dement. 2010;6(2):158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad S.S., Akhtar S., Jamal Q.M., Rizvi S.M., Kamal M.A., Khan M.K., Siddiqui M.H. Multiple targets for the management of Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets. 2016;15(10):1279–1289. doi: 10.2174/1871527315666161003165855. [DOI] [PubMed] [Google Scholar]
- 6.Craig L.A., Hong N.S., McDonald R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011;35(6):1397–1409. doi: 10.1016/j.neubiorev.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Karran E., Mercken M., De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011;10(9):698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 8.Maccioni R.B., Farías G., Morales I., Navarrete L. The revitalized tau hypothesis on Alzheimer’s disease. Arch. Med. Res. 2010;41(3):226–231. doi: 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Markesbery W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997;23(1):134–147. doi: 10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 10.Craddock T.J.A., Tuszynski J.A., Chopra D., Casey N., Goldstein L.E., Hameroff S.R., Tanzi R.E. The zinc dyshomeostasis hypothesis of Alzheimer’s disease. PLoS One. 2012;7(3):e33552. doi: 10.1371/journal.pone.0033552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashima T. Reconsider Alzheimer’s disease by the ‘calpain-cathepsin hypothesis’--a perspective review. Prog. Neurobiol. 2013;105:1–23. doi: 10.1016/j.pneurobio.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Hawking Z.L. Alzheimer’s Disease: The role of mitochondrial dysfunction and potential new therapies. Biosci. Horizons Int. J. Student Res; 2016. p. 9. [Google Scholar]
- 13.Reitz C., Brayne C., Mayeux R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011;7(3):137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu C., Kivipelto M., von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2009;11(2):111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fargo K.N., Aisen P., Albert M., Au R., Corrada M.M., DeKosky S., Drachman D., Fillit H., Gitlin L., Haas M., Herrup K., Kawas C., Khachaturian A.S., Khachaturian Z.S., Klunk W., Knopman D., Kukull W.A., Lamb B., Logsdon R.G., Maruff P., Mesulam M., Mobley W., Mohs R., Morgan D., Nixon R.A., Paul S., Petersen R., Plassman B., Potter W., Reiman E., Reisberg B., Sano M., Schindler R., Schneider L.S., Snyder P.J., Sperling R.A., Yaffe K., Bain L.J., Thies W.H., Carrillo M.C. 2014 Report on the Milestones for the US National Plan to Address Alzheimer’s Disease. Alzheimers Dement. 2014;10(5) Suppl.:S430–S452. doi: 10.1016/j.jalz.2014.08.103. [DOI] [PubMed] [Google Scholar]
- 16.Hurd M.D., Martorell P., Delavande A., Mullen K.J., Langa K.M. Monetary costs of dementia in the United States. N. Engl. J. Med. 2013;368(14):1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J., Brayne C., Matthews F.E. Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ. 2008;336(7638):258–262. doi: 10.1136/bmj.39433.616678.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prince M., Ali G.C., Guerchet M., Prina A.M., Albanese E., Wu Y.T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res. Ther. 2016;8(1):23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto Loewi - Nobel Lecture The chemical transmission of nerve action. https://www.nobelprize.org/prizes/ medicine/1936/loewi/lecture/
- 20.Shaikh S., Verma A., Siddiqui S., Ahmad S.S., Rizvi S.M., Shakil S., Biswas D., Singh D., Siddiqui M.H., Shakil S., Tabrez S., Kamal M.A. Current acetylcholinesterase-inhibitors: a neuroinformatics perspective. CNS Neurol. Disord. Drug Targets. 2014;13(3):391–401. doi: 10.2174/18715273113126660166. [DOI] [PubMed] [Google Scholar]
- 21.Metherate R. Functional connectivity and cholinergic modulation in auditory cortex. Neurosci. Biobehav. Rev. 2011;35(10):2058–2063. doi: 10.1016/j.neubiorev.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessler I., Kirkpatrick C.J. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2008;154(8):1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pohanka M. Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int. J. Mol. Sci. 2012;13(2):2219–2238. doi: 10.3390/ijms13022219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pohanka M., Dobes P. Caffeine inhibits acetylcholinesterase, but not butyrylcholinesterase. Int. J. Mol. Sci. 2013;14(5):9873–9882. doi: 10.3390/ijms14059873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pohanka M. Cholinesterases, a target of pharmacology and toxicology. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2011;155(3):219–229. doi: 10.5507/bp.2011.036. [DOI] [PubMed] [Google Scholar]
- 26.Pohanka M. Acetylcholinesterase inhibitors: A patent review (2008 - present). Expert Opin. Ther. Pat. 2012;22(8):871–886. doi: 10.1517/13543776.2012.701620. [DOI] [PubMed] [Google Scholar]
- 27.Mehta K.M., Yin M., Resendez C., Yaffe K. Ethnic differences in acetylcholinesterase inhibitor use for Alzheimer disease. Neurology. 2005;65(1):159–162. doi: 10.1212/01.wnl.0000167545.38161.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jann M.W. Rivastigmine, a New-Generation Cholinesterase Inhibitor for the Treatment of Alzheimer’s Disease. Pharmacotherapy. Pharmacotherapy Publications Inc.; 2000. pp. 1–12. [DOI] [PubMed] [Google Scholar]
- 29.Zarotsky V., Sramek J.J., Cutler N.R. Galantamine hydrobromide: An agent for Alzheimer’s disease. Am. J. Health Syst. Pharm. 2003;60(5):446–452. doi: 10.1093/ajhp/60.5.446. [DOI] [PubMed] [Google Scholar]
- 30.Chen P.Y., Tsai C.T., Ou C.Y., Hsu W.T., Jhuo M.D., Wu C.H., Shih T.C., Cheng T.H., Chung J.G. Computational analysis of novel drugs designed for use as acetylcholinesterase inhibitors and histamine H3 receptor antagonists for Alzheimer’s disease by docking, scoring and de novo evolution. Mol. Med. Rep. 2012;5(4):1043–1048. doi: 10.3892/mmr.2012.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee P.K., Kumar V., Mal M., Houghton P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14(4):289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Murray A.P., Faraoni M.B., Castro M.J., Alza N.P., Cavallaro V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s Disease therapy. Curr. Neuropharmacol. 2013;11(4):388–413. doi: 10.2174/1570159X11311040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali B., Jamal Q.M., Shams S., Al-Wabel N.A., Siddiqui M.U., Alzohairy M.A., Al Karaawi M.A., Kesari K.K., Mushtaq G., Kamal M.A. In silico analysis of green tea polyphenols as inhibitors of AChE and BChE Enzymes in Alzheimer’s disease treatment. CNS Neurol. Disord. Drug Targets. 2016;15(5):624–628. doi: 10.2174/1871527315666160321110607. [DOI] [PubMed] [Google Scholar]
- 34.Spence J., Chintapenta M., Kwon H.I., Blaszczyk A.T. A Brief review of three common supplements used in Alzheimer’s disease. Consult Pharm. 2017;•••:412–414. doi: 10.4140/TCP.n.2017.412. [DOI] [PubMed] [Google Scholar]
- 35.Reddy P.H., Manczak M., Yin X., Grady M.C., Mitchell A., Tonk S., Kuruva C.S., Bhatti J.S., Kandimalla R., Vijayan M. Protective effects of indian spice curcumin against Amyloid-β in Alzheimer’s disease. J. Alzheimers Dis. 2018;•••:843–866. doi: 10.3233/JAD-170512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thapa A., Carroll N.J. Dietary modulation of oxidative stress in Alzheimer’s Disease. Int. J. Mol. Sci. 2017;18(7):1583. doi: 10.3390/ijms18071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gugliandolo A., Bramanti P., Mazzon E. Role of Vitamin e in the treatment of Alzheimer’s disease: evidence from animal models. Int. J. Mol. Sci. 2017;18(12):2504. doi: 10.3390/ijms18122504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Onofrio G., Sancarlo D., Ruan Q., Yu Z., Panza F., Daniele A., Greco A., Seripa D. Phytochemicals in the treatment of Alzheimer’s disease: A systematic review. Curr. Drug Targets. 2017;18(13):1487–1498. doi: 10.2174/1389450117666161102121553. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad S.S., Waheed T., Rozeen S., Mahmood S., Kamal M.A. Therapeutic study of phytochemicals against cancer and Alzheimer’s disease management. Curr. Drug Metab. 2020;20:1006–1013. doi: 10.2174/1389200221666200103092719. [DOI] [PubMed] [Google Scholar]
- 40.Silman I., Sussman J.L. Acetylcholinesterase: how is structure related to function? Chem. Biol. Interact. 2008;175(1-3):3–10. doi: 10.1016/j.cbi.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 41.Elmegeed G.A., Ahmed H.H., Hashash M.A., Abd-Elhalim M.M., El-kady D.S. Synthesis of novel steroidal curcumin derivatives as anti-Alzheimer’s disease candidates: Evidences-based on in vivo study. Steroids. 2015;101:78–89. doi: 10.1016/j.steroids.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Muñoz-Delgado E., Montenegro M.F., Campoy F.J., Moral-Naranjo M.T., Cabezas-Herrera J., Kovacs G., Vidal C.J. Expression of cholinesterases in human kidney and its variation in renal cell carcinoma types. FEBS J. 2010;277(21):4519–4529. doi: 10.1111/j.1742-4658.2010.07861.x. [DOI] [PubMed] [Google Scholar]
- 43.Bartels C.F., Zelinski T., Lockridge O. Mutation at codon 322 in the human acetylcholinesterase (ACHE) gene accounts for YT blood group polymorphism. Am. J. Hum. Genet. 1993;52(5):928–936. [PMC free article] [PubMed] [Google Scholar]
- 44.Whittaker V.P. The contribution of drugs and toxins to understanding of cholinergic function. Trends Pharmacol. Sci. 1990;11(1):8–13. doi: 10.1016/0165-6147(90)90034-6. [DOI] [PubMed] [Google Scholar]
- 45.Pivi G.A.K., da Silva R.V., Juliano Y., Novo N.F., Okamoto I.H., Brant C.Q., Bertolucci P.H.F. A prospective study of nutrition education and oral nutritional supplementation in patients with Alzheimer’s disease. Nutr. J. 2011;10(1):98. doi: 10.1186/1475-2891-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trottier G., Boström P.J., Lawrentschuk N., Fleshner N.E. Nutraceuticals and prostate cancer prevention: A current review. Nat. Rev. Urol. 2010;7(1):21–30. doi: 10.1038/nrurol.2009.234. [DOI] [PubMed] [Google Scholar]
- 47.Zeisel S.H. Regulation of “nutraceuticals”. Science. 1999;285(5435):1853–1855. doi: 10.1126/science.285.5435.1853. [DOI] [PubMed] [Google Scholar]
- 48.Sadhukhan P., Saha S., Dutta S., Mahalanobish S., Sil P.C. Nutraceuticals: An emerging therapeutic approach against the pathogenesis of Alzheimer’s Disease. Pharmacol. Res. 2018;129:100–114. doi: 10.1016/j.phrs.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 49.He G.L., Luo Z., Yang J., Shen T.T., Chen Y., Yang X.S. Curcumin ameliorates the reduction effect of PGE2 on fibrillar β-Amyloid Peptide (1-42)-induced microglial phagocytosis through the inhibition of EP2-PKA signaling in n9 microglial cells. PLoS One. 2016;11(1):e0147721. doi: 10.1371/journal.pone.0147721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao Z., Zhang A., Lin J., Zheng Z., Shi X., Di W., Qi W., Zhu Y., Zhou G., Fang Y. Telomerase: A target for therapeutic effects of curcumin and a curcumin derivative in Aβ1-42 insult in vitro. PLoS One. 2014;9(7):e101251. doi: 10.1371/journal.pone.0101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghalebani L., Wahlström A., Danielsson J., Wärmländer S.K.T.S., Gräslund A. pH-dependence of the specific binding of Cu(II) and Zn(II) ions to the amyloid-β peptide. Biochem. Biophys. Res. Commun. 2012;421(3):554–560. doi: 10.1016/j.bbrc.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 52.Faller P., Hureau C. A bioinorganic view of Alzheimer’s disease: When misplaced metal ions (re)direct the electrons to the wrong target. Chemistry. 2012;18(50):15910–15920. doi: 10.1002/chem.201202697. [DOI] [PubMed] [Google Scholar]
- 53.Shinzato T., Sato R., Suzuki K., Tomioka S., Sogawa H., Shulga S., Blume Y., Kurita N. Proposal of therapeutic curcumin derivatives for alzheimer’s disease based on ab initio molecular simulations. Chem. Phys. Lett. 2019;•••:136883 [Google Scholar]
- 54.Fidelis E.M., Savall A.S.P., da Luz Abreu E., Carvalho F., Teixeira F.E.G., Haas S.E., Bazanella S.T., Pinton S. Curcumin-loaded nanocapsules reverses the depressant-like behavior and oxidative stress induced by β-Amyloid in mice. Neuroscience. 2019;423:122–130. doi: 10.1016/j.neuroscience.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 55.Bassani T.B., Turnes J.M., Moura E.L.R., Bonato J.M., Cóppola-Segovia V., Zanata S.M., Oliveira R.M.M.W., Vital M.A.B.F. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav. Brain Res. 2017;335:41–54. doi: 10.1016/j.bbr.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Giacomeli R., Izoton J.C., Dos Santos R.B., Boeira S.P., Jesse C.R., Haas S.E. Neuroprotective effects of curcumin lipid-core nanocapsules in a model Alzheimer’s disease induced by β-amyloid 1-42 peptide in aged female mice. Brain Res. 2019;1721:146325. doi: 10.1016/j.brainres.2019.146325. [DOI] [PubMed] [Google Scholar]
- 57.Huo X., Zhang Y., Jin X., Li Y., Zhang L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J. Photochem. Photobiol. B. 2019;190:98–102. doi: 10.1016/j.jphotobiol.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Samy D.M., Ismail C.A., Nassra R.A., Zeitoun T.M., Nomair A.M. Downstream modulation of extrinsic apoptotic pathway in streptozotocin-induced Alzheimer’s dementia in rats: Erythropoietin versus curcumin. Eur. J. Pharmacol. 2016;770:52–60. doi: 10.1016/j.ejphar.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 59.Veldman E.R., Jia Z., Halldin C., Svedberg M.M. Amyloid binding properties of curcumin analogues in Alzheimer’s disease postmortem brain tissue. Neurosci. Lett. 2016;630:183–188. doi: 10.1016/j.neulet.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 60.Hao C., Gao L., Zhang Y., Wang W., Yu G., Guan H., Zhang L., Li C. 2015. [DOI] [PMC free article] [PubMed]
- 61.Pangestuti R., Kim S. Neuroprotective properties of chitosan and its derivatives. 2010. [DOI] [PMC free article] [PubMed]
- 62.Nidheesh T., Salim C., Rajini P.S., Suresh P.V. Antioxidant and neuroprotective potential of chitooligomers in Caenorhabditis elegans exposed to monocrotophos. Carbohydr. Polym. 2016;135:138–144. doi: 10.1016/j.carbpol.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 63.Zhou S., Yang Y., Gu X., Ding F. Neuroscience letters chitooligosaccharides protect cultured hippocampal neurons against glutamate-induced neurotoxicity. 2008. [DOI] [PubMed]
- 64.Jia S., Lu Z., Gao Z., An J., Wu X., Li X., Dai X., Zheng Q., Sun Y. Chitosan oligosaccharides alleviate cognitive deficits in an amyloid-β1-42-induced rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016;83:416–425. doi: 10.1016/j.ijbiomac.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 65.Lee S., Park J., Kim S., Ahn C., Je J. Bioorganic & medicinal chemistry letters chitooligosaccharides suppress the level of protein expression and acetylcholinesterase activity induced by A b 25 – 35 in PC12 Cells. Bioorg. Med. Chem. Lett. 2009;19(3):860–862. doi: 10.1016/j.bmcl.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 66.AnjiReddy K.; Karpagam, S. Chitosan nanofilm and electrospun nanofiber for quick drug release in the treatment of Alzheimer’s disease: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2017;105(Pt 1):131–142. doi: 10.1016/j.ijbiomac.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 67.Elnaggar Y.S.R., Etman S.M., Abdelmonsif D.A., Abdallah O.Y. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: optimization, biological efficacy, and potential toxicity. J. Pharm. Sci. 2015;104(10):3544–3556. doi: 10.1002/jps.24557. [DOI] [PubMed] [Google Scholar]
- 68.Jaruszewski K.M., Ramakrishnan S., Poduslo J.F., Kandimalla K.K. Chitosan enhances the stability and targeting of immuno-nanovehicles to cerebro-vascular deposits of Alzheimer’s disease amyloid protein. Nanomedicine (Lond.) 2012;8(2):250–260. doi: 10.1016/j.nano.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson B., Samanta M.K., Santhi K., Sampath Kumar K.P., Ramasamy M., Suresh B. Significant delivery of tacrine into the brain using magnetic chitosan microparticles for treating Alzheimer’s disease. J. Neurosci. Methods. 2009;177(2):427–433. doi: 10.1016/j.jneumeth.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 70.Wilson B., Samanta M.K., Santhi K., Kumar K.P.S., Ramasamy M., Suresh B. Chitosan nanoparticles as a new delivery system for the anti-Alzheimer drug tacrine. Nanomedicine (Lond.) 2010;6(1):144–152. doi: 10.1016/j.nano.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Yu S., Xu X., Feng J., Liu M., Hu K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019;560:282–293. doi: 10.1016/j.ijpharm.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 72.Cornejo A., Jiménez J.M., Caballero L., Melo F., Maccioni R.B. Fulvic acid inhibits aggregation and promotes disassembly of tau fibrils associated with Alzheimer’s disease. J. Alzheimers Dis. 2011;27(1):143–153. doi: 10.3233/JAD-2011-110623. [DOI] [PubMed] [Google Scholar]
- 73.Agarwal S.P., Khanna R., Karmarkar R., Anwer M.K., Khar R.K. Shilajit: A review. Phytother. Res. 2007;21(5):401–405. doi: 10.1002/ptr.2100. [DOI] [PubMed] [Google Scholar]
- 74.Kong Y.C., But P.P.H., Ng K.H., Cheng K.F., Cambie R.C., Malla S.B. Chemical Studies on a nepalese panacea - Shilajit (I). Pharm. Biol. 1987;25(3):179–182. [Google Scholar]
- 75.Khanna R., Witt M., Khalid Anwer M., Agarwal S.P., Koch B.P. Spectroscopic characterization of fulvic acids extracted from the rock exudate shilajit. Org. Geochem. 2008;39(12):1719–1724. doi: 10.1016/j.orggeochem.2008.08.009. [DOI] [Google Scholar]
- 76.Bhattacharya S.K., Sen A.P., Ghosal S. Effects of shilajit on biogenic free radicals. Phytother. Res. 1995;9(1):56–59. doi: 10.1002/ptr.2650090113. [DOI] [Google Scholar]
- 77.Ghosal S., Lal J., Jaiswal A.K., Bhattacharya S.K. Effects of Shilajit and its active constituents on learning and memory in rats. Phytother. Res. 1993;7(1):29–34. doi: 10.1002/ptr.2650070109. [DOI] [Google Scholar]
- 78.Schliebs R., Liebmann A., Bhattacharya S.K., Kumar A., Ghosal S., Bigl V. Systemic administration of defined extracts from Withania somnifera (Indian Ginseng) and Shilajit differentially affects cholinergic but not glutamatergic and GABAergic markers in rat brain. Neurochem. Int. 1997;30(2):181–190. doi: 10.1016/S0197-0186(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 79.Morales I., Guzmán-Martínez L., Cerda-Troncoso C., Farías G.A., Maccioni R.B. Neuroinflammation in the pathogenesis of Alzheimer’s Disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014;8(1):1–9. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Birks J., Evans J.G. Ginkgo Biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. •••;21(1):CD003120. doi: 10.1002/14651858.CD003120.pub2. [DOI] [PubMed] [Google Scholar]
- 81.Vitolo O., Gong B., Cao Z., Ishii H., Jaracz S., Nakanishi K., Arancio O., Dzyuba S.V., Lefort R., Shelanski M. Protection against β-amyloid induced abnormal synaptic function and cell death by Ginkgolide J. Neurobiol. Aging. 2009;30(2):257–265. doi: 10.1016/j.neurobiolaging.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang F., Nag S., Shiu S.Y.W., Pang S.F. The effects of melatonin and Ginkgo biloba extract on memory loss and choline acetyltransferase activities in the brain of rats infused intracerebroventricularly with β-amyloid 1-40. Life Sci. 2002;71(22):2625–2631. doi: 10.1016/S0024-3205(02)02105-7. [DOI] [PubMed] [Google Scholar]
- 83.Stackman R.W., Eckenstein F., Frei B., Kulhanek D., Nowlin J., Quinn J.F. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp. Neurol. 2003;184(1):510–520. doi: 10.1016/S0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- 84.Maurer K., Ihl R., Dierks T., Frölich L. Clinical efficacy of Ginkgo biloba special extract EGb 761 in dementia of the Alzheimer type. Phytomedicine. 1998;5(6):417–424. doi: 10.1016/S0944-7113(98)80037-8. [DOI] [PubMed] [Google Scholar]
- 85.Ismail A.F.M., El-Sonbaty S.M. Fermentation enhances Ginkgo biloba protective role on gamma-irradiation induced neuroinflammatory gene expression and stress hormones in rat brain. J. Photochem. Photobiol. B. 2016;158:154–163. doi: 10.1016/j.jphotobiol.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 86.Ilhan A., Gurel A., Armutcu F., Kamisli S., Iraz M., Akyol O., Ozen S. Ginkgo biloba prevents mobile phone-induced oxidative stress in rat brain. Clin. Chim. Acta. 2004;340(1-2):153–162. doi: 10.1016/j.cccn.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 87.Mazumder U.K., Gupta M., Chakrabarti S., Pal D. Evaluation of hematological and hepatorenal functions of methanolic extract of Moringa oleifera Lam. root treated mice. Indian J. Exp. Biol. 1999;37(6):612–614. [PubMed] [Google Scholar]
- 88.Maghu T.K., Sharma A., Younis K. 2017. [Google Scholar]
- 89.Mohan M., Kaul N., Punekar A., Girnar R., Junnare P., Patil L. Nootropic Activity of Moringa Oleifera Leaves. J. Nat. Rem. 2005;5(1):59–62. [Google Scholar]
- 90.Ganguly R., Guha D. 2006.
- 91.Ganguly R., Hazra R., Ray K., Guha D. Effect of Moringa Oleifera in experimental model of Alzheimer’s disease : role of antioxidants. Ann. Neurosci. 2005;33-36 doi: 10.5214/ans.0972.7531.2005.120301. [DOI] [Google Scholar]
- 92.Ekong M.B., Ekpo M.M., Akpanyung E.O., Nwaokonko D.U. Neuroprotective effect of Moringa oleifera leaf extract on aluminium-induced temporal cortical degeneration. Metab. Brain Dis. 2017;32(5):1437–1447. doi: 10.1007/s11011-017-0011-7. [DOI] [PubMed] [Google Scholar]
- 93.Hannan M.A., Kang J.Y., Mohibbullah M., Hong Y.K., Lee H., Choi J.S., Choi I.S., Moon I.S. Moringa oleifera with promising neuronal survival and neurite outgrowth promoting potentials. J. Ethnopharmacol. 2014;152(1):142–150. doi: 10.1016/j.jep.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 94.Mahaman Y.A.R., Huang F., Wu M., Wang Y., Wei Z., Bao J., Salissou M.T.M., Ke D., Wang Q., Liu R., Wang J.Z., Zhang B., Chen D., Wang X. Moringa oleifera alleviates homocysteine-induced Alzheimer’s disease-like pathology and cognitive impairments. J. Alzheimers Dis. 2018;63(3):1141–1159. doi: 10.3233/JAD-180091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Funayama S., Cordell G.A. Alkaloids: A Treasury of Poisons and Medicines. Elsevier Inc.; 2014. [Google Scholar]
- 96.Aniszewski T. In: Alkaloids - Secrets of Life : Alkaloid Chemistry, Biological Significance, Applications and Ecological Role. 1st ed. Aniszewski T., editor. Netherlands: Elsevier; 2007. [Google Scholar]
- 97.Brownstein M.J. A brief history of opiates, opioid peptides, and opioid receptors. Proc. Natl. Acad. Sci. USA. 1993;90(12):5391–5393. doi: 10.1073/pnas.90.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen Y., Chen Y., Liang Y., Chen H., Ji X., Huang M. Berberine mitigates cognitive decline in an Alzheimer’s Disease Mouse Model by targeting both tau hyperphosphorylation and autophagic clearance. Biomed. Pharmacother. 2020;121(121):109670. doi: 10.1016/j.biopha.2019.109670. [DOI] [PubMed] [Google Scholar]
- 99.de Oliveira J.S., Abdalla F.H., Dornelles G.L., Adefegha S.A., Palma T.V., Signor C., da Silva Bernardi J., Baldissarelli J., Lenz L.S., Magni L.P., Rubin M.A., Pillat M.M., de Andrade C.M. Berberine protects against memory impairment and anxiogenic-like behavior in rats submitted to sporadic Alzheimer’s-like dementia: Involvement of acetylcholinesterase and cell death. Neurotoxicology. 2016;57:241–250. doi: 10.1016/j.neuro.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 100.Ghotbi Ravandi S., Shabani M., Bashiri H., Saeedi G.M., Khodamoradi M., Nozari M. Ameliorating effects of berberine on MK-801-induced cognitive and motor impairments in a neonatal rat model of schizophrenia. Neurosci. Lett. 2019;706:151–157. doi: 10.1016/j.neulet.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 101.Haghani M., Shabani M., Tondar M. The therapeutic potential of berberine against the altered intrinsic properties of the CA1 neurons induced by Aβ neurotoxicity. Eur. J. Pharmacol. 2015;758:82–88. doi: 10.1016/j.ejphar.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 102.Huang M., Jiang X., Liang Y., Liu Q., Chen S., Guo Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017;91(91):25–33. doi: 10.1016/j.exger.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 103.de Jong C.F., Derks R.J.E., Bruyneel B., Niessen W., Irth H. High-performance liquid chromatography-mass spectrometry-based acetylcholinesterase assay for the screening of inhibitors in natural extracts. J. Chromatogr. A. 2006;1112(1-2):303–310. doi: 10.1016/j.chroma.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 104.Marques L.A., Maada I., de Kanter F.J.J., Lingeman H., Irth H., Niessen W.M.A., Giera M. Stability-indicating study of the anti-Alzheimer’s drug galantamine hydrobromide. J. Pharm. Biomed. Anal. 2011;55(1):85–92. doi: 10.1016/j.jpba.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 105.Maelicke A. Allosteric modulation of nicotinic receptors as a treatment strategy for Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. S. Karger AG; 2000. pp. 11–18. [DOI] [PubMed] [Google Scholar]
- 106.Lyketsos C.G., Reichman W.E., Kershaw P., Zhu Y. Long-term outcomes of galantamine treatment in patients with Alzheimer disease. Am. J. Geriatr. Psychiatry. 2004;12(5):473–482. doi: 10.1097/00019442-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 107.Isik A.T., Bozoglu E., Naharci M.I., Kilic S. Evaluation of the effects of galantamine on cardiac function in elderly patients with Alzheimer’s disease. Am. J. Geriatr. Pharmacother. 2010;8(5):454–459. doi: 10.1016/j.amjopharm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 108.Penner J., Rupsingh R., Smith M., Wells J.L., Borrie M.J., Bartha R. Increased glutamate in the hippocampus after galantamine treatment for Alzheimer disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(1):104–110. doi: 10.1016/j.pnpbp.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 109.Meng F.C., Wu Z.F., Yin Z.Q., Lin L.G., Wang R., Zhang Q.W. Coptidis Rhizoma and its main bioactive components: recent advances in chemical investigation, quality evaluation and pharmacological activity. Chin. Med. 2018;13:13. doi: 10.1186/s13020-018-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ríos J.L., Francini F., Schinella G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Medica. Georg Thieme Verlag; 2015. pp. 975–994. [DOI] [PubMed] [Google Scholar]
- 111.Xu Z., Feng W., Shen Q., Yu N., Yu K., Wang S., Chen Z., Shioda S., Guo Y. Rhizoma Coptidis and Berberine as a natural drug to combat aging and aging-related diseases via anti- oxidation and ampk activation. Aging Dis. 2017;8(6):760–777. doi: 10.14336/AD.2016.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumar P., Srivastava V., Chaturvedi R., Sundar D., Bisaria V.S. Elicitor enhanced production of protoberberine alkaloids from in vitro cell suspension cultures of Tinospora Cordifolia (Willd.) Miers Ex Hook. F. and Thoms. Plant Cell Tissue Organ Cult. 2017;130(2):417–426. doi: 10.1007/s11240-017-1237-0. [DOI] [Google Scholar]
- 113.Zhang Q., Chen C., Wang F.Q., Li C.H., Zhang Q.H., Hu Y.J., Xia Z.N., Yang F.Q. Simultaneous screening and analysis of antiplatelet aggregation active alkaloids from Rhizoma Corydalis. Pharm. Biol. 2016;54(12):3113–3120. doi: 10.1080/13880209.2016.1211714. [DOI] [PubMed] [Google Scholar]
- 114.Yang S.B., Kim E.H., Kim S.H., Kim Y.H., Oh W., Lee J.T., Jang Y.A., Sabina Y., Ji B.C., Yeum J.H. Electrospinning fabrication of poly(vinyl alcohol)/coptis chinensis extract nanofibers for antimicrobial exploits. Nanomaterials (Basel) 2018;8(9):E734. doi: 10.3390/nano8090734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rong Q., Xu M., Dong Q., Zhang Y., Li Y., Ye G., Zhao L. In vitro and in vivo bactericidal activity of Tinospora sagittata (Oliv.) Gagnep. var. craveniana (S.Y.Hu) Lo and its main effective component, palmatine, against porcine Helicobacter pylori. BMC Complement. Altern. Med. 2016;16(1):331. doi: 10.1186/s12906-016-1310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sun M., Xu L., Peng Y., Liu T., Zhang Y., Zhou Z. Multiscale Analysis of the contents of palmatine in the nature populations of Phellodendron Amurense in Northeast China. J. For. Res. 2016;27(2):265–272. doi: 10.1007/s11676-015-0200-3. [DOI] [Google Scholar]
- 117.Xin A., Zhang Y., Zhang Y., Di D., Liu J. Development of an HPLC-DAD method for the determination of five alkaloids in Stephania yunnanensis Lo and in rat plasma after oral dose of Stephania yunnanensis Lo extracts. Biomed. Chromatogr. 2018;32(10):e4292. doi: 10.1002/bmc.4292. [DOI] [PubMed] [Google Scholar]
- 118.Houghton P.J., Ren Y., Howes M.J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006;23(2):181–199. doi: 10.1039/b508966m. [DOI] [PubMed] [Google Scholar]
- 119.Dhingra D., Bhankher A. Behavioral and biochemical evidences for antidepressant-like activity of palmatine in mice subjected to chronic unpredictable mild stress. Pharmacol. Rep. 2014;66(1):1–9. doi: 10.1016/j.pharep.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 120.Xiao H.T., Peng J., Liang Y., Yang J., Bai X., Hao X.Y., Yang F.M., Sun Q.Y. Acetylcholinesterase inhibitors from Corydalis yanhusuo. Nat. Prod. Res. 2011;25(15):1418–1422. doi: 10.1080/14786410802496911. [DOI] [PubMed] [Google Scholar]
- 121.Miao F., Yang X.J., Zhou L., Hu H.J., Zheng F., Ding X.D., Sun D.M., Zhou C.D., Sun W. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat. Prod. Res. 2011;25(9):863–875. doi: 10.1080/14786419.2010.482055. [DOI] [PubMed] [Google Scholar]
- 122.Brunhofer G., Fallarero A., Karlsson D., Batista-Gonzalez A., Shinde P., Gopi Mohan C., Vuorela P. Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: the case of chelerythrine. Bioorg. Med. Chem. 2012;20(22):6669–6679. doi: 10.1016/j.bmc.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 123.Zdarilová A., Vrzal R., Rypka M., Ulrichová J., Dvorák Z. Investigation of sanguinarine and chelerythrine effects on CYP1A1 expression and activity in human hepatoma cells. Food Chem. Toxicol. 2006;44(2):242–249. doi: 10.1016/j.fct.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 124.Hostalkova A., Marikova J., Opletal L., Korabecny J., Hulcova D., Kunes J., Novakova L., Perez D.I., Jun D., Kucera T., Andrisano V., Siatka T., Cahlikova L. Isoquinoline alkaloids from Berberis vulgaris as potential lead compounds for the treatment of Alzheimer’s disease. J. Nat. Prod. 2019;82(2):239–248. doi: 10.1021/acs.jnatprod.8b00592. [DOI] [PubMed] [Google Scholar]
- 125.Hussien H.M., Abd-Elmegied A., Ghareeb D.A., Hafez H.S., Ahmed H.E.A., El-Moneam N.A. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem. Toxicol. 2018;111:432–444. doi: 10.1016/j.fct.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 126.Liang Y., Huang M., Jiang X., Liu Q., Chang X., Guo Y. The neuroprotective effects of Berberine against amyloid β-protein-induced apoptosis in primary cultured hippocampal neurons via mitochondria-related caspase pathway. Neurosci. Lett. 2017;655:46–53. doi: 10.1016/j.neulet.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 127.Lohan S., Raza K., Mehta S.K., Bhatti G.K., Saini S., Singh B. Anti-Alzheimer’s potential of berberine using surface decorated multi-walled carbon nanotubes: A preclinical evidence. Int. J. Pharm. 2017;530(1-2):263–278. doi: 10.1016/j.ijpharm.2017.07.080. [DOI] [PubMed] [Google Scholar]
- 128.Patil S., Tawari S., Mundhada D., Nadeem S. Protective effect of berberine, an isoquinoline alkaloid ameliorates ethanol-induced oxidative stress and memory dysfunction in rats. Pharmacol. Biochem. Behav. 2015;136:13–20. doi: 10.1016/j.pbb.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 129.Zhan P.Y., Peng C.X., Zhang L.H. Berberine rescues D-galactose-induced synaptic/memory impairment by regulating the levels of Arc. Pharmacol. Biochem. Behav. 2014;117:47–51. doi: 10.1016/j.pbb.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 130.Zhang Z., Li X., Li F., An L. Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int. Immunopharmacol. 2016;38:426–433. doi: 10.1016/j.intimp.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 131.Zhou J., Du X., Long M., Zhang Z., Zhou S., Zhou J., Qian G. Neuroprotective effect of berberine is mediated by MAPK signaling pathway in experimental diabetic neuropathy in rats. Eur. J. Pharmacol. 2016;774:87–94. doi: 10.1016/j.ejphar.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 132.Fang J., Liu R., Tian Q., Hong X.P., Wang S.H., Cao F.Y., Pan X.P., Wang J.Z. Dehydro evodiamine attenuates calyculin A-induced tau hyperphosphorylation in rat brain slices. Acta Pharmacol. Sin. 2007;28(11):1717–1723. doi: 10.1111/j.1745-7254.2007.00655.x. [DOI] [PubMed] [Google Scholar]
- 133.Collins L.E., Paul N.E., Abbas S.F., Leser C.E., Podurgiel S.J., Galtieri D.J., Chrobak J.J., Baqi Y., Müller C.E., Salamone J.D. Oral tremor induced by galantamine in rats: a model of the parkinsonian side effects of cholinomimetics used to treat Alzheimer’s disease. Pharmacol. Biochem. Behav. 2011;99(3):414–422. doi: 10.1016/j.pbb.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 134.Kuna Y., Borra N.K. Chronic Effects of Anti-Alzheimer’s Drug, galantamine hydrobromide on cholinergic system of mice brain. J. Pharm. Res. 2013;6(7):714–719. doi: 10.1016/j.jopr.2013.06.010. [DOI] [Google Scholar]
- 135.Wahba S.M.R., Darwish A.S., Kamal S.M. Ceria-containing uncoated and coated hydroxyapatite-based galantamine nanocomposites for formidable treatment of Alzheimer’s disease in ovari ectomized albino-rat model. Mater. Sci. Eng. C. 2016;65:151–163. doi: 10.1016/j.msec.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 136.Saito T., Hisahara S., Iwahara N., Emoto M.C., Yokokawa K., Suzuki H., Manabe T., Matsumura A., Suzuki S., Matsushita T., Kawamata J., Sato-Akaba H., Fujii H.G., Shimohama S. Early administration of galantamine from preplaque phase suppresses oxidative stress and improves cognitive behavior in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Free Radic. Biol. Med. 2019;145:20–32. doi: 10.1016/j.freeradbiomed.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 137.Wang C.Y., Zheng W., Wang T., Xie J.W., Wang S.L., Zhao B.L., Teng W.P., Wang Z.Y. Huperzine A activates Wnt/β-catenin signaling and enhances the nonamyloidogenic pathway in an Alzheimer transgenic mouse model. Neuropsychopharmacology. 2011;36(5):1073–1089. doi: 10.1038/npp.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Feng Z., Chen S., Wang W., Feng L., Dong Y., Zou Y., Ke C., Tang C., Yao S., Zhang H., Gan L., Ye Y., Lin L. Lycodine-type alkaloids from Lycopodiastrum casuarinoides and their acetylcholinesterase inhibitory activity. Fitoterapia. 2019;139(October):104378. doi: 10.1016/j.fitote.2019.104378. [DOI] [PubMed] [Google Scholar]
- 139.Yang Z.D., Zhang X., Du J., Ma Z.J., Guo F., Li S., Yao X.J. An aporphine alkaloid from Nelumbo nucifera as an acetylcholinesterase inhibitor and the primary investigation for structure-activity correlations. Nat. Prod. Res. 2012;26(5):387–392. doi: 10.1080/14786419.2010.487188. [DOI] [PubMed] [Google Scholar]
- 140.Xian Y.F., Mao Q.Q., Wu J.C.Y., Su Z.R., Chen J.N., Lai X.P., Ip S.P., Lin Z.X. Isorhynchophylline treatment improves the amyloid-β-induced cognitive impairment in rats via inhibition of neuronal apoptosis and tau protein hyperphosphorylation. J. Alzheimers Dis. 2014;39(2):331–346. doi: 10.3233/JAD-131457. [DOI] [PubMed] [Google Scholar]
- 141.Lilienfeld S. Galantamine--a novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer’s disease. CNS Drug Rev. 2002;8(2):159–176. doi: 10.1111/j.1527-3458.2002.tb00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kaline S., Chaves M., Feitosa C.M., Da F.P., Santos S., Joilane A., Freire P. Pharmacological activities palmatine alkaloid compound isolated from guatteria friesiana prospects for new drug development. Asian J. Biomed. Pharm. Sci. 2017;6(59):35–39. [Google Scholar]
- 143.Dhingra D., Kumar V. Memory-enhancing activity of palmatine in mice using elevated plus maze and morris water maze. Adv. Pharmacol. Sci. 2012;2012:357368. doi: 10.1155/2012/357368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haj E., Losev Y. Guru KrishnaKumar, V.; Pichinuk, E.; Engel, H.; Raveh, A.; Gazit, E.; Segal, D. Integrating in vitro and in silico approaches to evaluate the “dual functionality” of palmatine chloride in inhibiting and disassembling Tau-derived VQIVYK peptide fibrils. Biochim. Biophys. Acta, Gen. Subj. 2018;1862(7):1565–1575. doi: 10.1016/j.bbagen.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 145.Iijima S., Greig N.H., Garofalo P., Spangler E.L., Heller B., Brossi A., Ingram D.K. Phenserine: a physostigmine derivative that is a long-acting inhibitor of cholinesterase and demonstrates a wide dose range for attenuating a scopolamine-induced learning impairment of rats in a 14-unit T-maze. Psychopharmacology (Berl.) 1993;112(4):415–420. doi: 10.1007/BF02244888. [DOI] [PubMed] [Google Scholar]
- 146.López S., Bastida J., Viladomat F., Codina C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and narcissus extracts. Life Sci. 2002;71(21):2521–2529. doi: 10.1016/S0024-3205(02)02034-9. [DOI] [PubMed] [Google Scholar]
- 147.Rollinger J.M., Schuster D., Baier E., Ellmerer E.P., Langer T., Stuppner H. Taspine: bioactivity-guided isolation and molecular ligand-target insight of a potent acetylcholinesterase inhibitor from Magnolia x soulangiana. J. Nat. Prod. 2006;69(9):1341–1346. doi: 10.1021/np060268p. [DOI] [PMC free article] [PubMed] [Google Scholar]