Abstract
Over the decades, various interventions have been developed and utilized to treat epilepsy. However, the majority of epileptic patients are often first prescribed anti-epileptic drugs (AED), now known as anti-seizure drugs (ASD), as the first line of defense to suppress their seizures and regain their quality of life. ASDs exert their anti-convulsant effects through various mechanisms of action, including regulation of ion channels, blocking glutamate-mediated stimulating neurotransmitter interaction, and enhancing the inhibitory GABA transmission. About one-third of epileptic patients are often resistant to anti-convulsant drugs, while others develop numerous side effects, which may lead to treatment discontinuation and further deterioration of quality of life. Common side effects of ASDs include headache, nausea and dizziness. However, more adverse effects, such as auditory and visual problems, skin problems, liver dysfunction, pancreatitis and kidney disorders may also be witnessed. Some ASDs may even result in life-threatening conditions as well as serious abnormalities, especially in patients with comorbidities and in pregnant women. Nevertheless, some clinicians had observed a reduction in the development of side effects post individualized ASD treatment. This suggests that a careful and well-informed ASD recommendation to patients may be crucial for an effective and side-effect-free control of their seizures. Therefore, this review aimed to elucidate the anticonvulsant effects of ASDs as well as their side effect profile by discussing their mechanism of action and reported adverse effects based on clinical and preclinical studies, thereby providing clinicians with a greater understanding of the safety of current ASDs.
Keywords: AED, ASD, pharmacological treatment, adverse effect, comorbidity, anticonvulsant
1. INTRODUCTION
Epilepsy can affect people of all ages, gender, races, social classes and geographical locations [1]. Persistent, and recurrent seizures tend to have neurobiological, cognitive, psychological and social consequences, which may dampen the quality of life of epileptic patients [2]. Epileptic seizures as well as paroxysmal events (non-seizure symptoms), maybe a reflection and a cause of various underlying neural mechanisms of the disease [3]. For example, increasing levels of glutamate may cause excitotoxic neuronal damage, which may result in epileptogenesis and seizure formation. However, seizures themselves may propagate the neuronal damage further by altering the neuronal and glial expression of glutamate receptors, thereby modifying their function and causing prolonged neuronal hyperexcitability [4]. Similarly, this duality in the pathological relationship may also be extended to various other mechanistic pathways, such as gamma-aminobutyric-acid (GABA) neurotransmission, neuroinflammation and oxidative stress in epilepsy, making it a heterogeneous disease.
There are many types of epilepsy; some are named after their known cause, while others are categorized based on the type of seizures despite having an unknown cause. Approximately half of the world’s epilepsy cases are of unknown causes [5, 6]. Nevertheless, numerous epilepsy models in preclinical studies have been conducted to understand the pathophysiology of different forms of epilepsy, thereby identifying the responsible biomarkers and in turn, developing effective drugs and treatments for them [7].
Approaches such as seizure foci surgery, vagus nerve stimulation (VNS), deep brain stimulation (DBS), and the ketogenic diet have been effectively utilized in the treatment of epilepsy since the 1900s [8]. Surgical seizure foci resection has been accepted as an effective method in suppressing seizures, even in drug-resistant patients, till today [9]. However, like all surgery, complication issues and risks may deter some patients and even neurologists from suggesting seizure foci surgery. Similar risk factors may also exist for VNS and DBS due to surgical invasion. Although the ketogenic diet has proven to be effective in epileptic children who are drug-resistant, the first line of defense against epilepsy has always been anti-epileptic drugs (AEDs), or recently more appropriately branded as anti-seizure drugs (ASDs). AEDs or ASDs have successfully allowed nearly 70% of epileptic patients to control/manage their seizures with ease [10]. However, the success/effective rate of pharmacological treatment approaches with anti-convulsants may vary depending on the etiology of seizure disorder [11]. There are approximately 30 types of ASDs to this date, which possess various molecular targets and mechanisms of action, such as blocking the excitatory mechanism (action potentials) and/or strengthening the inhibitory mechanisms [10-13]. Besides that, ASDs may also modulate the voltage-gated sodium (Na+) channels and/or voltage-gated calcium (Ca2+) channels [14, 15].
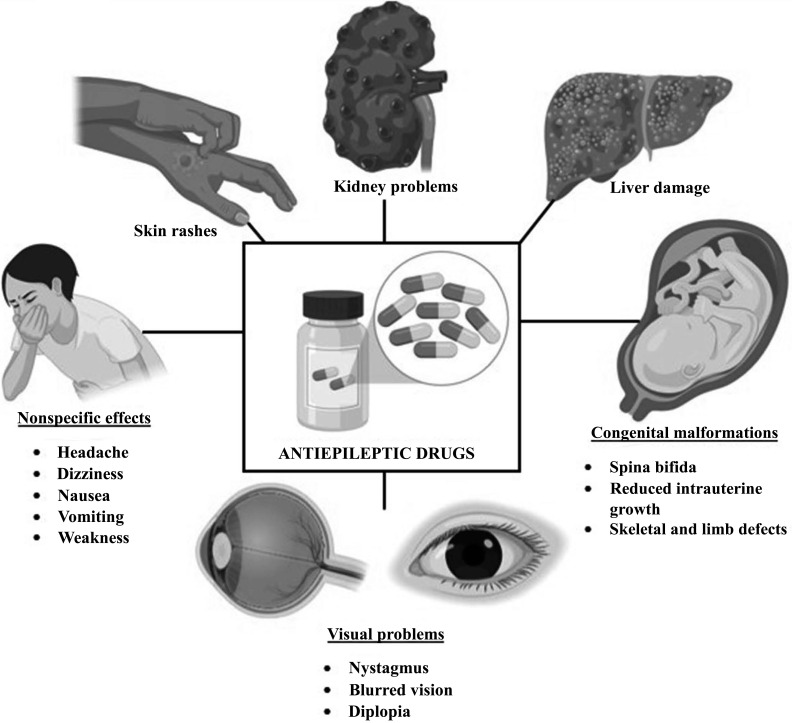
Although effective, most anti-convulsant drugs often have a narrow therapeutic index that separates them from being beneficial or detrimental. Small changes in the pharmacokinetics of ASDs may lead to a decreased therapeutic activity and an increased toxic effect, leading to the development of adverse effects [16]. These adverse effects may be acute and reversible or chronic and permanent [17] (Fig. 1).
Fig. (1).
Side effects of anti-seizure drugs. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
Some ASDs may cause deterioration in the central nervous system, neuronal death, aplastic anemia, liver failure or even death, such as sudden unexpected death in epilepsy (SUDEP). ASDs have also been shown to cause recurrence of seizures as well as temporary paralysis in patients with cerebral lesions or patients with previous symptomatic seizures [18]. Apart from these, non-specific retinal and neurotoxic visual anomalies, such as diplopia, blurred vision and nystagmus, may also occur with an overdose or long-term ASD usage [19]. Most of these previously described adverse effects are rare and dependent on various factors. However, common side effects that may be experienced across most ASD usage include headache, dizziness, drowsiness, hearing disturbances, nausea, and vomiting [20]. Since the type of ASDs, the duration of usage, the pharmacokinetics of the ASD and the pharmacodynamics of the ASD in patients may influence the effectiveness of the ASD as well as the type of side/adverse effects developed, it is therefore, important to understand the individual-based role of these factors among common ASDs before actively prescribing them to epileptic patients. This will ensure that epileptic patients gain the best form of treatment for their debilitating condition in order to have a greater quality of life.
This review elucidates the mechanism of action of common ASDs as well as their side effect profile, based on clinical and preclinical studies. This review hopes to provide guidance to physicians and clinicians in choosing the most appropriate, safe, and individually catered antiepileptic drugs for epileptic patients. This may be especially helpful for epileptic patients who often present complexity with ASDs, such as those who are pregnant, in their old age, or have various other comorbidities together with the seizures.
2. BROAD AND NARROW SPECTRUM ANTI-SEIZURE DRUGS
Anti-seizure drugs are classified according to their mechanism of action as well as the type of epilepsy they are effective against in patients. Currently, ASDs can be divided into two categories; broad spectrum and narrow spectrum ASDs. Broad-spectrum ASDs are effective against a wide variety of seizures (focal, partial and generalized), while narrow-spectrum ASDs are only effective against a certain type of seizure (focal or partial). Thus, broad-spectrum ASDs are usually given as the first-line medication, particularly when the type of seizure is uncertain. In this review, the broad-spectrum ASDs include valproic acid, topiramate, lamotrigine, levetiracetam, felbamate, zonisamide, perampanel, rufinamide and brivarcetam (Table 1). The narrow spectrum ASDs include phenytoin, oxcarbazepine, lacosamide, gabapentin, vigabatrin, carbamazepine, tiagabine, stiripentol, eslicarbazepine acetate and cenobamate (Table 1). Understanding each ASD’s mechanism of action and their specific adverse effects may guide clinicians and physicians in making a well-informed prescription of ASD for their patients, where the main objective is to prevent/halt seizure spread with minimal side effects, thereby ensuring epileptic patients have a good quality of life.
Table 1.
Mechanism of action, side effect profile, and risk factors of anti-seizure drugs based on clinical and preclinical studies.
| Anti-seizure Drug | Mechanism of Action | Study Subjects | Side Effect Profile | Risk Factors | References |
|---|---|---|---|---|---|
| Broad Spectrum ASD | |||||
| Valproate | ▪ Enhance GABA levels ▪ Block sodium (Na+), calcium (Ca2+) and potassium (K+) channels ▪ NMDA receptor antagonist |
Women with epilepsy who were pregnant or who were of childbearing age | Teratogenic effects | * Pregnancy * Child bearing age * Pediatric patients * Genetic mutations (POLG) |
[23] |
| Human placentas | Reduced placental folate concentrations | [24] | |||
| Human placentas | Decreased mRNA expression of genes encoding folate and amino acid and fatty acid transporters | [25] | |||
| Alpers syndrome patients | Increased apoptotic sensitivity | [26] | |||
| Valproate-induced rats | Increased thiobarbituric acid reagent content and NO | [27] | |||
| Epileptic children on a low therapeutic dose of valproate monotherapy | Decreased appetite, abdominal pain, vomiting, diarrhea, enuresis, skin rash and abnormal color vision | [28] | |||
| Topiramate | ▪ Enhance GABA-chloride influx ▪ Block sodium (Na+) channels ▪ NMDA antagonist ▪ Block carbonic anhydrase |
Adults with epilepsy or migraine | Impairment of verbal function, memory, and attention | * High dosage * Infants * Pregnancy * Drug interaction with other ASD |
[29] |
| 1–24 months of age with refractory partial-onset seizure | Hyperammonemia | [30] | |||
| Children with West syndrome | Sleeping state, poor oral intake, and numbness | [31] | |||
| Pregnant women | Increased seizure frequency | [32] | |||
| Lamotrigine | ▪ Block sodium (Na+) and calcium (Ca2+) channels | Patients with eosinophilia and systemic symptoms (DRESS) | Hypersensitivity | * Chronic usage *Age (older) |
[33] |
| Patients with SJS or TEN | SCAR | [34] | |||
| Levetiracetam | ▪ Inhibit sodium (Na+) channels ▪ Increase GABAergic transmission ▪ Bind to SV2A |
Patients with epilepsy | Decreased dopaminergic activity and aggression side effects | * High dosage | [35] |
| Felbamate | ▪ NMDA-glutamate receptor antagonist ▪ Blocks sodium and calcium conduction |
Children with drug-resistant epilepsy | Aplastic anemia, liver failure, decreased appetite, insomnia, fatigue, irritability, leukopenia, rash, hyperactivity, weakness, vomiting, cognitive deterioration, behavioral change | * Drug interaction with other ASD | [36] |
| Children, adolescents, and adults with epilepsy | Nausea, vomiting, and stomach upset | [37] | |||
| Adult rats | Lowest learning tasks | [38] | |||
| Zonisamide | ▪ Blocks sodium (Na+) and T-type calcium channels | Patients with epilepsy | Major depression | * Drug withdrawal * Gender (males) |
[39] |
| Rufinamide | ▪ Inhibits sodium-dependent action potentials | CF1 mice and Sprague–Dawley rats | Decreased motor activity, ataxia, muscle relaxation, and decreased respiration | * Pediatric patients *LGS patients |
[40] |
| Patients with LGS | Increased in height and weight | [41, 42] | |||
| Patients with LGS | Headache, dizziness, drowsiness, vomiting, nausea, fatigue and diplopia | [43] | |||
| Anti-seizure Drug | Mechanism of Action | Study Subjects | Side Effect Profile | Risk Factors | References |
| Brivaracetam | ▪ Binds to SV2A | Children with epilepsy | Systemic side effects | * Pregnancy | [44, 45] |
| Patients with intellectual disability and epilepsy | Behavioral disorder side effects | [191] | |||
| Women with epilepsy | Embryo death | [46] | |||
| Perampanel | ▪ AMPA-glutamate receptor antagonist | WAG/Rij rats | Psychiatric (depressive-like) comorbidity | * High dosage | [47] |
| Patients with epilepsy | Increase in depressive symptoms | [48] | |||
| Patients with drug-resistant partial seizures | Dizziness, drowsiness, and headache | [108] | |||
| Narrow Spectrum ASD | |||||
| Phenytoin | ▪ Reduce action potential amplitude ▪ Block sodium (Na+) channels |
Pediatric patients with convulsive status epilepticus | Hypotension, cardiac arrhythmias and serious extravasation injuries | * Pediatric patients * Drug interaction with other ASD * Status epilepticus patients * Patients with cardiovascular problems * Genetic mutations (HLA-B, CYP2C variant) * Pregnancy * High dosage |
[49] |
| Phenytoin induced-anticonvulsant hypersensitivity syndrome | Cross-reactivity, fever, liver enzyme elevation, and increased skin problems | [50] | |||
| Patients with drug-induced hypersensitivity syndrome | Severe facial edema, erythema, hyperbilirubinemia, and elevated liver transaminases | [51] | |||
| Animal models treated with 100 mg or 200 mg of phenthionine | Increased ALT levels | [52] | |||
| Patients with localization-related epilepsy | Impairment of cognitive functions, such as attention, memory, and problem solving | [53] | |||
| Embryonic rats | Decreased body weight, cleft lip and/or palate, hydrocephalus, hydronephrosis, long bones growth retardation and ectrodactyly | [54] | |||
| Women exposed to antiepileptic drug monotherapy during pregnancy | Increased risk of major congenital malformations | [55] | |||
| Oxcarbazepine | ▪ Block sodium (Na+), calcium (Ca2+) and potassium (K+) channels | Patients with idiopathic trigeminal neuralgia | Tiredness, sleepiness, memory problems, disturbed sleep, difficulty concentrating and unsteadiness | * Monotherapy *Pediatric patients *Pregnancy |
[56] |
| Children with epilepsy | Nausea, vomiting, skin rash, and hyponatremia | [57] | |||
| A 23-year-old pregnant woman with a history of CPS and mild depression | Dizziness and attacks | [58] | |||
| Lacosamide | ▪ Increase the slow inactivation of sodium (Na+) channels ▪ CRMP2 |
Children with epilepsy | Cardiopulmonary events | * Patients with liver problems * Monotherapy * Adjunctant therapy |
[59] |
| Patients with epilepsy | Increased ALT levels | [60] | |||
| Gabapentin | ▪ Bind to alpha-2-delta subunit of calcium (Ca2+) channels | Patients with partial-onset seizures | Anxiety, agitation, and depression | * Monotherapy * Pregnancy * High dosage * Patients with chronic renal failure * Age (older) |
[61] |
| Candidates for elective lower limb orthopedic surgery | Chill, headache, nausea, vomiting, dizziness, and fever | [62] | |||
| Older adults given high doses of gabapentin | Increased risk of being hospitalized with a mental state | [63] | |||
| Patients with chronic kidney disease | Toxicity | [64] | |||
| Anti-seizure Drug | Mechanism of Action | Study Subjects | Side Effect Profile | Risk Factors | References |
| 1. | 2. | 24-week-old male albino Wistar rats | Decreased locomotor activity and increased defecation | - | [65] |
| Pregnant women (including patients with epilepsy) | Less or similar rates of maternal complications, low birth weight, cesarean section, abortion and malformation | [66] | |||
| Vigabatrin | ▪ Inhibits GABA transaminase | Infants with new-onset and previously treated infantile spasm | Getting fat, edema, extreme irritability, high blood pressure, heart failure, blood sugar control irregularities, increased risk of infection and kidney calcification | * Patients on hormone/steroids treatment * Infants * Chronic usage * High dosage |
[67] |
| Patients with epilepsy | Visual field defect | [68] | |||
| Carbamazepine | ▪ Blocks sodium (Na+) channels | Older adults | Hyponatremia | * High dosage * Age (older and children) |
[69] |
| Tiagabine | ▪ Blocks GABA re-uptake | Adult outpatients with epilepsy | Cognitive side effect intolerance | * High dosage * Patients with comorbidities * Drug interaction with comorbid drugs |
[70] |
| Stiripentol | ▪ Increase GABAA receptor transmission ▪ Increase inhibitory post-synaptic currents ▪ Prolong decay time constant |
Adults with DS | Anorexia, weight loss, imbalance, and fatigue | * Intolerance | [71] |
| Patients with a confirmed clinical and genetic diagnosis of DS | Hyperammonemia encephalopathy | [72] | |||
| Eslicarbazepine acetate | ▪ Enhance slow inactivation of sodium (Na+) channels ▪ Blocks T-type calcium channels |
Children with refractory focal-onset seizures | Headache, nasopharyngitis, and drowsiness | * Age (older) * Patients with comorbidities * Drug interaction with other ASD |
[73] |
| Adults with partial-onset or focal seizures | Dizziness, drowsiness, hyponatremia, headache and ataxia | [74] | |||
| Cenobamate | ▪ Blocks INaP action ▪ Positive allosteric modulation of GABAA receptors |
Adults with uncontrollable focal epilepsy | Dizziness, headache, somnolence, diplopia, fatigue, nystagmus and DRESS | * Pregnancy *Drug interaction with other ASD and/or contraceptives *High dosage *Patients with cardiac comorbidity |
[75-78] |
Abbreviations: ASD: anti-seizure drug, ALT: alanine transaminase, POLG: DNA-polymerase gamma, CPS: complex partial seizures, DS: Dravet Syndrome, DRESS: drug eruption with eosinophilia and systemic symptoms, INaP: persistent sodium current, LGS: Lennox-Gastaut syndrome, NO: nitric oxide, SCAR: severe cutaneous adverse reactions, SJS: Stevens–Johnson syndrome, TEN: toxic epidermal necrolysis, SV2A: synaptic vesicle protein 2A, AMPA: amino-3-hydroxy 5-methyl-4-isoxazolepropionic acid, NMDA: N-methyl-D-aspartate, CRMP2: collapsin response mediator protein 2, GABA: gamma-amino-butryic-acid.
2.1. Broad Spectrum Anti-Seizure Drugs
2.1.1. Valproate
Valproate (valproic acid) is often the first line and standard ASD prescribed to epileptic children due to its effectiveness in treating generalized seizures. The primary mechanism of action of valproate is through the enhancement of inhibitory neurotransmitter GABA. Valproate may increase GABA synthesis, decrease GABA turnover, and prevent GABA degradation, all to enhance GABA pro-inhibitory neurotransmission. Valproate may also block sodium (Na+) channels, calcium (Ca2+) channels, and voltage-gated potassium (K+) channels [21, 22] in attempts to reduce neuronal hyperexcitability. Besides that, valproate may also affect other neurotransmitters, such as neuropeptide Y and may act as an N-methyl-D-aspartate (NMDA) receptor antagonist, both of which may block seizure development.
However, one of the common adverse effects reported from the use of valproate was a teratogenic effect seen in women with childbearing potential [23]. In a study involving human placentas, valproate significantly altered the mRNA levels of the major transporters for folic acid, glucose, choline, thyroid hormones and serotonin [24]. Dysregulation in folic acid transport may reduce placental folate concentrations, which could result in adverse fetal outcomes [24]. Similarly, in another study, even low therapeutic levels of valproate were associated with a trend towards a decreased mRNA expression of folate, amino acid, and fatty acid transporters, which may affect placenta function during early pregnancy [25]. In addition, valproate may also cause major organ malformations and cognitive disturbances in the fetus. Epileptic patients who had valproate exposure during pregnancy witnessed the formation of spina bifida, cardiac craniofacial defects and skeletal/limb defects in their fetus [79]. These adverse genetic effects of valproate during pregnancy may be due to its modulating role on histone deacetylase.
Another well-known side effect of valproate is the pronounced liver toxicity associated with genetic epilepsies. An example of a genetic epileptic disorder with which valproate may cause liver toxicity is the Alpers-Hüttenlocher syndrome, which is caused by a mitochondrial DNA-polymerase mutation [80, 81]. In Alpers syndrome patients, p.A467T, the most common DNA-polymerase (POLG) mutation, has been shown to result in lesser POLG expression, greater mtDNA depletion, and a series of mitochondrial abnormalities. These pathologies may lead to more frequent mitochondrial permeability transition pore (mPTP) opening and increased apoptotic sensitivity due to the induction of mitochondrial superoxide flashes [26]. In a study investigating valproic acid-induced hepatotoxicity in rats, a significant increase in serum enzyme activities was observed for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma glutamyl transferase (GGT). The study also showed that valproate administration increased thiobarbituric acid reagent content and nitric oxide (NO) production in the liver tissue, attributing to increased oxidative/nitrosative stress [27]. Interestingly, a 2018 cohort study noticed that POLG1 was not solely associated with an increased risk of valproate-induced liver toxicity but required a combination of other pathogenic mutations as well [82], thereby suggesting that only epileptic patients with the aforementioned pathologies/mutations may be prone to develop liver toxicity post valproate treatment.
Valproate anti-convulsant drug may also cause hyperammonemia as an adverse effect [83-85]. Valproate may increase the body’s ammonia levels by combining with carnitine and forming valproylcarnitine, thus reducing the amount of carnitine in the body. Carnitine is required for metabolism, where it is responsible for transferring fatty acid chains into the mitochondria for energy production. Valproate may also bind to coenzyme A, preventing carnitine regeneration. Decreased carnitine due to these mechanisms may lead to a decreased beta-oxidation of valproate, thus causing an accumulation of toxic metabolites and reduced ammonia eradication [86]. Other adverse effects, such as decreased appetite, abdominal pain, vomiting, diarrhea, enuresis, and abnormal color vision, were also found in some pediatric epilepsy patients [28].
Valproate may be more effective as a monotherapy due to its possible interaction with other ASDs. Although side effects associated with valproate polytherapy have not been clearly concluded previously, valproate has been shown to decrease lamotrigine clearance and increase its concentration in serum [87], which may contribute to lamotrigine-induced DRESS or SCAR.
Taken together, valproate should not be prescribed as the primary therapy for pregnant epilepsy patients to protect the health of the fetus and for epileptic patients with POLG mutations to prevent liver toxicity. Since a valproate dose-dependent relationship may be applicable for other adverse effects, they may still be prescribed to epileptic patients, but at a lower dosage and as a monotherapy. However, careful monitoring of the dose response should be prioritized.
2.1.2. Topiramate
Topiramate is effectively used as a monotherapy for focal onset and primary generalized tonic-clonic seizures in both adults and children. There are several ways in which topiramate exhibits its anti-seizure properties; enhancing GABA-chloride influx, activating potassium conduction, blocking voltage-gated sodium channels, antagonizing NMDA receptors and blocking carbonic anhydrase (increasing the acidity of the brain, which suppresses seizures).
Paresthesia, fatigue, gastrointestinal problems, memory difficulty, and taste deviation were some of the prominent side effects of topiramate treatment [88]. Topiramate treatment has been associated with significant verbal function, memory and attention impairment in patients with epilepsy [29]. A past case study involving a 39-year-old woman treated with carbamazepine for 2 years was initiated on topiramate 50 mg daily for persistent complex partial seizures. While the dose of the carbamazepine was stable, the patient’s topiramate dose was gradually increased, which caused a 5-7 kg body weight loss, fatigue, and fainting spells in the patient [89]. In addition, the patient also developed oliguria, severe encephalopathy, liver and renal failure [89]. These adverse findings were related to the continuous increase in the drug dosage, thus suggesting that the side effects may be related to the topiramate dosage rather than the topiramate use itself. In contrast, in a clinical trial, adverse events of hyperammonemia were reported in infants receiving topiramate as adjunctive therapy to valproate, but no dose-related changes were detected in the serum ammonia or liver function test results [30]. Thus, this indicates that the combination therapy of topiramate with other ASDs may cause severe side effects that may be just as worse as when taken as a monotherapy. In an evaluation study of the safety, tolerability, and efficacy of oral topiramate treatment in children with West syndrome, it was reported that 35% of the children experienced temporary side effects, such as drowsiness and poor oral intake [31]. In contrast, the use of topiramate during the second and third trimesters of pregnancy showed an increase in the frequency of seizures in 47% of the individuals [32]. The evidence suggest that topiramate is safe and tolerable in children even with genetic comorbidities but could result in greater side effects when prescribed with other ASDs in adults, especially when not accounting for the dosage response. Topiramate may also not be beneficial for pregnant epileptic patients, as there may be an underlying hormonal mechanism (pending verification from further investigation), which may counteract the anti-seizure effects of topiramate.
2.1.3. Lamotrigine
Lamotrigine is a phenyl-triazine derivative that is prescribed as an alternative to valproate. Its main mechanism of action is the blocking of voltage-gated sodium and calcium channels on both the pre- and post-synaptic membrane [90]. It is effective on primary and secondary generalized seizures, focal seizures, as well as on atonic and absence seizures indicated in Lennox-Gastaut syndrome of childhood [91].
The use of lamotrigine may cause common ASD side effects, such as headache, dizziness, drowsiness, fatigue, fever and nausea in patients. More specific to lamotrigine, side effects such as moderate ataxia, bipolar triggering, hypersensitivity and multi-organ failure have been reported with chronic usage [92]. Although rare, hepatotoxicity, teratogenicity, pancreatitis and skin rash have also been witnessed in some epileptic patients [92]. Hypersensitivity to lamotrigine or its components may be the primary side effect of lamotrigine administration [90]. In a previous clinical study, 12 out of 44 patients with drug eruption with eosinophilia and systemic symptoms (DRESS) (mostly above 18 years old) reported sensitivity towards lamotrigine [33].
Interestingly, previous studies have shown that in a pediatric age group, the risk of mild skin reactions associated with lamotrigine was higher than in adults, but the risk of lamotrigine-related severe cutaneous adverse reactions (SCAR) was higher in adults compared to the pediatric group [34], suggesting an age-related factor in lamotrigine pharmacodynamics and hypersensitivity. Hypersensitivity reactions are defined as an exaggerated or inappropriate immune response to an antigen or allergen [93], a reaction that is uncommon with other ASDs. In support, lamotrigine treatment has also been shown to significantly increase the delayed-type hypersensitivity response in a preclinical mouse model, where it significantly inhibited IL-2 and TNF-α secretion in splenocytes stimulated with concanavalin A (ConA) [94].
Unfortunately, the side effect profile of lamotrigine has not been investigated in association with pregnancy [95], but hypersensitivity reaction during pregnancy may cause life-threatening conditions in the mother and fetus. Since hypersensitivity is the only major issue of lamotrigine, understanding and eventually counteracting this effect may greatly promote the usage of the otherwise safe and effective ASD lamotrigine.
2.1.4. Levetiracetam
Besides valproic acid and phenytoin, levetiracetam (LEV) is currently one of the most prescribed ASD by clinicians and highly recommended due to its effectiveness as an anti-convulsant against a broad range of epilepsies while having a low side-effect profile and no known drug interactions [96]. LEV may inhibit the voltage-dependent sodium channels, increase GABAergic transmission and bind to the synaptic vesicle protein 2A (SV2A), which causes neuronal inhibition [97, 98].
Behavioral side effects, such as altered mood states including depression, agitation, hostility, irritability, and hyper-arousal, as well as a variety of behavioral problems, including aggression, have been reported frequently in patients using LEV [99]. In one study, decreased dopaminergic activity and aggression were associated with LEV [35]. In addition, the LEV may also cause common side effects, such as headache, dizziness, drowsiness, forgetfulness, abdominal pain, nausea and vomiting, while neurological side effects, such as convulsion, ataxia, anger, anxiety and confusion, have been observed in high doses of LEV [100].
According to a 2015 meta-analysis, the percentage of patients withdrawing from LEV treatment due to adverse effects showed a certain dose-response relationship with LEV tolerability [101]. However, although the tolerability was clearly reduced with a change in drug dose from 1000 to 2000, an additional dose increase (up to 3000 mg/day) was not associated with a worsening of the tolerance [101]. This contrasted with research findings showing a decreased tolerance to the increasing dose rate of other ASDs [102, 103]. These results show that the relationship between dose and tolerance of LEV should be further investigated to mitigate the potential adverse effects of LEV, which may allow clinicians to treat epileptic patients of any age, type and medical history safely and effectively.
Similar to valproate, LEV may be more effective and tolerable as a monotherapy compared to as a polytherapy, especially in pediatric patients with epilepsy. A high seizure reduction rate and a relatively tolerable side-effect profile have been observed with LEV monotherapy [104, 105]. Although a retrospective review in 2016 reported that there may be no significant differences in adverse effects shown between LEV mono- and poly-therapy in pediatric patients [105], LEV monotherapy may be more effective, less costly and may reduce wastage and possible unknown interactions.
2.1.5. Felbamate
Felbamate is mainly used for the treatment of partial seizures in adults and Lennox Gastaut syndrome in children [106]. The main mechanism of action of the felbamate drug is the interaction with NMDA-glutamate receptors, which in turn blocks sodium and calcium conduction [107].
Common side effects of felbamate are loss of appetite, vomiting, insomnia, nausea, dizziness and headache, while serious side effects include aplastic anemia and liver failure [36, 108]. Therefore, monitoring transaminases, coagulation parameters, and blood counts of patients receiving felbamate may be required. In one study, five out of six patients had experienced nausea, vomiting, and stomach upset due to the use of valproate in addition to the drug felbamate [37]. Similarly, gastrointestinal events were also witnessed in another study that had concurrent administration of valproate and felbamate [109], suggesting a drug interaction side effect for felbamate with other ASDs.
As for cognitive side effects, felbamate-treated animals had the least decline in the learning tasks compared to those treated with phenytoin, carbamazepine or valproate [38]. This suggests that the use of felbamate may be more tolerable compared to those of other ASDs, especially when given as a monotherapy.
2.1.6. Zonisamide
Zonisamide is thought to function by blocking the repetitive firing of the voltage-gated sodium channels and the T-type calcium channels [110]. This drug has an effective therapeutic potential in both childhood partial and generalized epilepsies, particularly myoclonic epilepsies [111].
However, the use of zonisamide may trigger the development of anorexia, weight loss, somnolence, nervousness, headache, and sleep disorders in epileptic individuals. In addition, more serious side effects, such as vertigo, reduced sweating, and pancreatitis, have also been reported in patients occasionally [112].
In a preclinical study, the administration of zonisamide in rats did not affect the serum osteocalcin (a bone formation marker), but it increased the serum pyridinoline (bone resorption marker) levels [113]. This means that zonisamide administration may accelerate bone resorption, leading to bone loss as an adverse effect. However, this relationship between zonisamide and bone health has not been established in clinical studies. In fact, one study in humans showed that zonisamide did not adversely affect bone strength and or its metabolism [114].
Zonisamide has shown to possess the highest rate of drug withdrawal due to intolerance to psychiatric and behavioral side effects when compared to other ASDs [115]. In another study in which 433 patients were treated with zonisamide, the incidence of psychiatric side effects leading to drug discontinuation was observed as high as 6.9%, with those developing depression reaching a 2.5% withdrawal rate, aggressive behavior (1.8%), psychosis (1.4%) and irritability (1.2%) [116]. Major depression has been observed as the most common side effect of zonisamide drug discontinuation [39]. Signs of oxidative stress in testicular tissue due to decreased catalase activities and increased malondialdehyde levels have also been observed in the zonisamide treated patients, which suggest the possibility of male reproductive toxicity as a side effect of zonisamide [117]. Taken together, zonisamide may be better recommended for female epileptic patients rather than males, with close monitoring for psychiatric side effects. Although, it may be wise to understand how zonisamide may affect epileptic patients who are pregnant before prescribing them to expectant female patients.
2.1.7. Rufinamide
Rufinamide has been approved to be used for epileptic patients with Lennox-Gastaut syndrome (LGS), a severe form of epilepsy that begins in childhood. The main mode of action of the rufinamide is through the inhibition of the sodium-dependent action potentials [118].
Neurological side effects of very high doses of intraperitoneal rufinamide have been observed in animals, where decreased motor activity, ataxia, muscle relaxation, and decreased respiration were recorded [40]. In pediatric patients, on the other hand, weight loss as a side effect of rufinamide has been contradictorily observed in previous clinical trials [41, 42]. While in epilepsy patients with LGS syndrome, headache, dizziness, drowsiness, vomiting, nausea, fatigue and diplopia have been observed with rufinamide prescription [43], which deters patients from continuing their treatment. There is still much to learn about rufinamide usage in epileptic patients, but given that severe side effects have not been noticed in clinical trials, it may serve as a potentially effective and safe ASD for epileptic patients, especially for those with LGS.
2.1.8. Brivaracetam
Brivaracetam is a chemical analogue of levetiracetam and a high-affinity ligand of the SV2A glycoprotein. Common side effects of brivaracetam, include headache, drowsiness, dizziness, fatigue and nausea [119]. Brivaracetam-related CNS side effects have been shown to peak during the first week, followed by a decrease in the first 6 weeks in prevalence and in the first 3 weeks in incidence, suggesting adaption of the ASD [120]. Another clinical study performed showed a high rate of central and low systemic side effects, such as irritability and drowsiness (17.3% each), which contradicted with the initial post-marketing studies reporting nervousness, insomnia, depression and anxiety in only 2–3% of patients [44, 45]. Despite this contradiction, the adaptive feature (subsiding of side effects) of brivaracetam may champion it as a safe and effective ASD for epileptic patients.
Nevertheless, the potential for brivaracetam teratogenicity in humans may be indicated where embryo death has been observed at doses significantly higher than the equivalent maximum recommended dose for patients (200 mg daily in humans) [121]. There has also been evidence of fetal developmental toxicity in animal studies at plasma exposures higher than clinical exposures of brivaracetam [46]. Therefore, brivaracetam may not be suitable for pregnant, epileptic patients.
2.1.9. Perampanel
Perampanel is a post-synaptic, and noncompetitive α amino-3-hydroxy 5-methyl-4 isoxazolepropionic acid (AMPA)-type glutamate receptor antagonist [122, 123]. Perampanel is used to treat partial and/or generalized tonic-clonic seizures in those above 12 years old. In clinical studies, the most common side effects of perampanel were determined to be signs of aggressive behavior, dizziness and drowsiness. The fluctuation of glutamate levels in the amygdala, hypothalamus, and periaqueductal gray matter may play an important role in the development of aggressive behavior post perampanel [124, 125].
However, since perampanel has been observed to inhibit the limbic epileptogenic process [126], investigations into its potential to concurrently reduce other neuropsychiatric behaviour, particularly depression, in epileptic patients may be warranted. In support, an animal study showed that the drug attenuated the occurrence of psychiatric (depressive-like) comorbidity post perampanel administration [47]. In contrast, the clinical data observed the opposite for the depression outcome instead, where patients experienced an increase in depressive symptoms after taking perampanel [48]. This increase was also deemed predictive of the physically and verbally aggressive behavior that follows after perampanel usage [48]. This suggests that the limbic system in humans and animals may be wired differently and thus, perampanel effects on the limbic system should be further investigated in order to determine the mechanism to attenuate not only the aggression side effect but also as a potential treatment towards depression in epilepsy patients.
Interestingly, some studies suggest that differences in the plasma concentrations of perampanel influence the occurrence and severity of the side effects [127, 128]. Thus, to minimize the potential of perampanel-related adverse events, it may be recommended that patients initiate therapy with a low dose of perampanel and slowly increase the dose until a clinical effect is achieved. Regardless, perampanel appears to be a relatively safe and effective ASD for epileptic patients.
2.2. Narrow Spectrum Anti-Seizure Drugs
2.2.1. Phenytoin
Another common ASD often prescribed by general physicians to epileptic patients is phenytoin. It is one of the oldest ASDs in the market and primarily used for patients with status epilepticus. Phenytoin elicits its anti-seizure effects by reducing the action potential amplitude of neurons, thereby slowing the neural/synaptic conduction, transmission, and excitability [129]. Phenytoin may bind to the active state of the sodium ion channels to prolong the rapid inactivation of the channel [129], which reduces the repetitive firing of action potentials. Blocking the sodium channel may decrease the sensitivity of the neuronal cell to epileptogenic stimuli by increasing the membrane threshold for depolarization [130].
Although widely and effectively used, phenytoin has been reported to cause hypotension, cardiac arrhythmias, and severe extravasation injuries in patients with pediatric convulsive status epilepticus [49]. Similar side effects were also seen in an ASD comparison study, where status epilepticus patients using phenytoin, in contrast to those using sodium valproate, developed signs of hypotension and bradycardia [131]. The study also suggested that sodium valproate may be more tolerable than phenytoin, particularly with regard to early changes in blood pressure [131]. These findings indicate that phenytoin should not be recommended to patients with cardiovascular problems as it could be life-threatening.
The use of phenytoin may also trigger the development of anticonvulsant hypersensitivity syndrome (AHS) due to cross-reactivity with other ASDs, such as carbamazepine, which may lead to multiple organ failures and subsequent death. In an experiment conducted on 6 epileptic patients, phenytoin reportedly caused severe clinical side effects, such as fever, recurrent liver enzyme elevation and increased skin problems due to cross-reactivity reaction with other ASDs [50]. Thus, potential drug interactions and cross-reactivity should be considered when treating epilepsy with phenytoin, especially in those with combination ASD therapy.
Besides that, the use of phenytoin may also trigger the formation of severe edema and erythema on the face, as well as liver abnormalities due to hyperbilirubinemia or greater than ten-fold elevation in liver transaminases [51]. Based on an animal model, mice that were orally administered 100 mg or 200 mg of phenytoin showed increasing levels of alanine aminotransferase (ALT) levels on every usage but without a high mortality rate [52]. The findings were consistent with clinical cases, where previously normal liver enzyme (ALT) levels were abnormally increased following phenytoin therapy [132].
Phenytoin has also been associated with the formation of severe cutaneous adverse reactions (SCARs) due to variants in the HLA-B and CYP2C genes [133]. SCARs include Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), DRESS, and drug hypersensitivity syndrome (DHS) [133]. Thus, epileptic patients with these genetic variants should not be prescribed phenytoin.
Phenytoin may also have negative effects on higher-level cognitive functions, such as attention, memory and problem solving [53]. Morphological and behavioral anomalies have both been observed in animal and human usage of phenytoin, particularly during pregnancy [134]. It has also been found to cause human fetal hydantoin syndrome (FHS). Phenytoin usage during pregnancy may lead to growth-developmental disruption, congenital heart disease, major malformations, and craniofacial defects, including mental retardation in the fetus [134]. Likewise, the most common defects indicated in rodents treated with phenytoin during pregnancy were skeletal anomalies, such as cleft lip and/or palate, hydrocephalus, hydronephrosis, long bones elongation and ectrodactyly [54]. High-dose phenytoin therapy may cause a delay in dendritogenesis. It was observed that short and thick arbors that lead to abnormal shape were formed instead in a hippocampal neuron culture environment [135]. The dendrites of the neurons receiving phenytoin were seen as under-developed, short, and thick compared to the control culture. The observed adverse effects of the phenytoin drug on dendritogenesis may be due to its inhibitory function on Na+ and Ca2+ influx [135]. Therefore, it is thought that phenytoin, which disrupts ion homeostasis, may increase the risk of mental retardation due to a lack of dendritogenesis. In support, a study involving puppies that were prenatally exposed to phenytoin and other ASDs developed major congenital malformations and perinatal deaths, which was attributed to a dose-dependent risk [55]. These findings suggest that phenytoin should not be recommended to pregnant, epileptic patients or at the very least, the dosage and duration of phenytoin should be closely monitored for them.
2.2.2. Oxcarbazepine
Oxcarbazepine (OXC) is a keto analog of carbamazepine, just as effective as the latter but with lesser side effects. It is a potent anticonvulsant when used alone or in combination with other ASD agents in the treatment of partial seizures [136]. OXC elicits its anticonvulsant effect by blocking the voltage-dependent ionic (sodium, potassium and calcium) transmissions, which in turn reduces synaptic impulse propagation (seizures).
Even though deemed safer than carbamazepine, oxcarbazepine may be related to memory and concentration problems, inappropriate antidiuretic hormone secretion syndrome and hyponatremia [56]. Similar to studies in adults, oxcarbazepine monotherapy was not found to have a negative effect on cognitive function in newly diagnosed children and adolescents with partial seizures [137]. Interestingly, another study showed that polytherapy of levetiracetam and oxcarbazepine resulted in the absence of any adverse effects on cognitive function [138]. This suggests that oxcarbazepine should be recommended as a polytherapy rather than a monotherapy.
Besides that, OXC has also shown an effect on neurocyte apoptosis and brain damage. OXC at a concentration of 281.25 mg/kg or more was seen to induce neurocyte apoptosis and brain damage by triggering Bax/Bcl-2 signaling pathway-mediated caspase 3 activation in neonatal rats [139]. An increase in hippocampal apoptosis was also observed in rat pups who were exposed to OXC prenatally [140]. However, no adverse effects related to the use of maternal OXC were found in a case study involving a pregnant individual and two children [58]. These suggest that OXC may not be completely safe during pregnancy, however, since there havenot been any reports of OXC adversity in clinical studies, OXC may be recommended to epileptic patients who are pregnant, pending further clinical investigations.
Other minor side effects of OXC reported include nausea, vomiting, rash, and hyponatremia, which all have been observed in pediatric epilepsy patients [57]. With the current evidence, OXC polytherapy could be deemed as a safer ASD therapeutic option that may be recommended to epileptic patients, regardless of individual or seizure circumstances.
2.2.3. Lacosamide
Lacosamide is one of the newer ASDs in the market. Although the mechanisms of action of lacosamide are not completely clear, current studies suggest that it selectively increases the slow inactivation of voltage-gated sodium channels, thereby reducing neuronal excitability, as well as modulates epileptogenesis through collapsin response mediator protein 2 (CRMP2) [141, 142].
While lacosamide monotherapy and adjunctant therapy have proven to be effective against focal onset seizures, they may cause side effects, such as dizziness, headache, drowsiness, diplopia, cardiovascular abnormalities, skin rash, hematotoxicity, heart damage, psychological symptoms and suicide risk in epileptic patients [59, 136]. In contrast, there have been observations that cardiopulmonary events were not among the side effects associated with lacosamide treatment in clinical studies [143]. Although lacosamide monotherapy is effective and tolerable even at low doses in patients, especially in those over 65 years of age [144], one cohort study suggested that lacosamide polytherapy may also be effective with lesser side effects, particularly when given in combination with non-sodium channel blocker ASDs, such as LEV and zonisamide [145].
In pre-clinical studies, however, it has been reported that the addition of lacosamide to standard anti-convulsant therapy may be associated with an increase in ALT levels, thereby suggesting liver ischemia but only rarely severe liver damage [60]. In this context, it is thought that the use of lacosomide in individuals with various liver problems may adversely affect the prognosis of the disease. Taken together, the scarcity of studies on the side effects of lacosamide treatment may be the hurdle for its recommendation by a physician to epileptic patients, especially when deciding between monotherapy and polytherapy strategies.
2.2.4. Gabapentin
Gabapentin acts as a GABA mimetic agent. Gabapentin is believed to be the safest ASD in the market but unfortunately not as effective. Similar to lacosamide, the mechanism of action of gabapentin is still not well elucidated. Studies suggest that it elicits its anti-convulsant action through the governance of the voltage-gated calcium channels by binding to the alpha-2-delta subunit [146]. Gabapentin may exhibit both anticonvulsant and anxiolytic effects [146].
Although deemed safe, gabapentin-induced hypoglycemia has been observed in a case study, where the binding of gabapentin to the alpha 2 delta subunit of calcium channels in the pancreas may be the root cause for this side effect [147]. In addition, gabapentin may also lead to various psychological and behavioral problems. Patients receiving monotherapy with gabapentin developed anxiety, agitation, and depression over time [61]. Besides that, nonspecific symptoms, such as chills, headache, nausea-vomiting, dizziness, and fever, have also been reported previously [62]. In a study examining the effects of high and low dose usage of gabapentin in the elderly, the high dose patients with a history of chronic renal failure had a higher risk of hospitalization with an altered mental state [63, 64]. This suggests that gabapentin should be given at a lower dosage for elderly patients with chronic kidney disease.
In preclinical studies, the most common psychological and behavioral side effects associated with gabapentin that have been reported were anxiety/agitation and depression [61]. In a study in which the behavioral side effects of ASDs were measured, the locomotor activity of rats receiving gabapentin decreased significantly, and there was an increase in defecation [65]. However, in a mice model, gabapentin administration did not project any negative effects on the behavioral profile of the mice. Instead, in this mice study, gabapentin reversed the seizure sensitivity and psychiatric comorbidities, which was believed to be due to the use of lower than the therapeutic dose of gabapentin [148].
In addition, women exposed to gabapentin during pregnancy have less or similar rates of maternal complications, low birth weight, cesarean section, abortion, and malformations as other ASDs [66]. Therefore, these findings suggest that gabapentin may be safer than other ASDs as only neuropsychiatric effects have been well documented in epileptic patients. Nevertheless, careful considerations should be taken in regards to the age and health history of epileptic patients before prescribing gabapentin, especially when increasing the effective dosage.
2.2.5. Vigabatrin
Vigabatrin is a very effective ASD, especially for refractory focal seizures and children with infantile spasms [136]. Vigabatrin acts by irreversibly inhibiting the GABA metabolizing enzyme GABA transaminase, thereby increasing GABA concentrations and preventing neuronal excitability.
However, vigabatrin also possesses a range of side effects, such as weight gain, edema, extreme irritability, high blood pressure, heart failure, irregularities in the regulation of blood sugar, increased risk of infection, and kidney calcification, which have been attributed to the combined usage of hormone/steroids [67]. Accordingly, psychomotor agitation, axial hypertonia and MRI abnormalities were also observed, in infants but these were relatively benign [149].
The most common adverse effect seen in patients under vigabatrin therapy was visual field defects, which was observed in approximately 28.6% of patients in a clinical trial [68]. Since vigabatrin increases GABA levels overall [150], this GABA increase in the retinal area leads to unnecessary and prolonged retinal cellular membrane depolarization, followed by Cl-, Na+, and water flux, thereby causing homeostatic imbalance and cellular death [151]. In an animal study conducted in 2020, the relationship between vigabatrin and GABA concentrations in the retina and retinal toxicity of vigabatrin was affirmed [152]. The frequency and severity of the visual field loss caused by vigabatrin have ensured its prescription to only be made to epileptic patients if the anti-seizure benefits outweighed the vision risks. Even then, its use is only recommended for a minimum period to prevent permanent vision damage [152, 153].
Taken together, vigabatrin may only be prescribed to epileptic patients for a short time frame, at a low dosage, in those who are not on any other hormone/steroid medications and most importantly, only given to epileptic patients who have specific epilepsies, such as refractory focal seizures and infantile spasms, in order to minimize the occurrence of its adverse effects.
2.2.6. Carbamazepine
Carbamazepine is commonly used as a partial seizure medication and has been associated with tricyclic anti-depressants. The anti-convulsant activity of the drug comes from the blockade of the voltage-gated sodium channels, thereby inhibiting rapid neuronal firing [154].
The use of carbamazepine potentially causes gastrointestinal side effects, such as nausea, vomiting, dyspepsia, abdominal pain, anorexia, diarrhea and sometimes constipation in most patients. However, depending on the dosage and patient characteristics, drowsiness, ataxia, dizziness, double vision, blurred vision and nystagmus may also be observed. In addition, anticholinergic effects, such as dry mouth, mydriasis, impaired near vision and urinary retention, may also be witnessed, but these tend to be rare [155].
It is believed that carbamazepine may affect the function of the autonomic system by causing changes in the acetylcholine release and reuptake. Thus, examining the possible adverse effects on cardiac and respiratory disorders accompanying seizures may be warranted. Severe skin diseases and allergic reactions, such as erythroderma, exfoliative dermatitis, Stevens-Johnson reaction and toxic epidermal necrolysis, have also been observed in some of the patients. Mental and motor outcomes have only been observed in elderly patients, while symptoms, such as psychiatric disorders, tics, dystonia and worsening in epileptic seizures, have been witnessed in epileptic children [156]. A recent population-based study found that carbamazepine users in people older than 65 years showed a significant increase in risk associated with hyponatremia compared to non-users after 30 days post carbamazepine initiation [157]. Thus, clinicians should consider the risk of hyponatremia when prescribing carbamazepine to elderly patients [69]. Studies on carbamazepine side effect profile in pregnancy have yet to be properly investigated, however,, its use is currently deemed generally safe in pregnant patients.
In summary, it is highly recommended to pay attention to the patient’s age when prescribing carbamazepine. However, the rare adverse effects of carbamazepine on cognitive, psychiatric and skin disease may limit the use of this drug clinically unless further investigations were to be done to identify the commonality in the individuals who present these effects, thereby limiting its use only to those who do not share the identified traits.
2.2.7. Tiagabine
Tiagabine (TGB), a lesser-known ASD, works by increasing the inhibitory activity of GABA by blocking the transporters responsible for the re-uptake of GABA into the presynaptic neurons [158]. This allows more GABA to bind to the receptors on the postsynaptic cell membranes and inhibit neuronal excitability [158].
TGB use was commonly associated with mild symptoms of nausea, headache, dizziness, fatigue, weakness, irritability, memory loss, tremor, diarrhea and depression. In addition, serious side effects, such as stupor, hallucination, tachycardia, and high blood pressure, have also been reported with TGB usage [159]. Investigations into the cognitive effects of TGB suggest a negative effect on verbal memory performance [160]. However, in a preclinical study aiming to investigate the effect of tiagabine on memory and learning, TGB did not elicit any negative impact on cognitive function [161]. Among the new ASDs, TGB and zonisamide drugs have been found to have the highest rate of cognitive side effects after topimarate [162]. Interestingly, in another clinical study, cognitive side effects were only observed with the use of high-dose of TGB and in patients with prior comorbidities [70]. Since these cognitive side effects may be more prone in patients with hypertension, heart disease, or psychiatric treatment, it may be wiser to prescribe other/equivalent drugs in addition to tiagabine for the treatment of epilepsy in patients with comorbidities. Possible drug interactions of TGB and chronic disease drugs should be investigated further to prevent the serious side effects associated with TGB. Besides that, the effect of age and pregnancy should also be investigated in conjunction with TGB usage. Otherwise, TGB may be safely prescribed to epileptic patients.
2.2.8. Stiripentol
Stiripentol (STP) has been approved as an orphan drug in the adjunctive treatment of childhood epilepsy syndrome known as severe myoclonic epilepsy in infancy or Dravet syndrome [163]. Studies on CA3 pyramidal neurons in the neonatal rat hippocampus have determined that STP functions by increasing GABAA receptor-mediated transmission [164]. Stiripentol may also increase the frequency of miniature inhibitory post-synaptic currents and prolong the decay-time constant [164]. In a small cohort, STP has also been shown to be effective in adults with Dravet syndrome as well, but with a reasonable tolerance profile, where side effects such as anorexia, weight loss, imbalance, and fatigue were noticed with intolerance [71]. In one study, hyperammonemia encephalopathy was observed in 77% of patients treated with STP, but treatment with carnitine improved the hyperammonemia and allowed the continued use of stiripentol [72]. There is still much to understand about stiripentol as an ASD, however, as of now, stiripentol may be an effective and safe ASD for patients with Dravet syndrome regardless of age.
2.2.9. Eslicarbazepine Acetate
Eslicarbazepine acetate is a dibenzoazepine anticonvulsant that has been approved as adjunctive therapy for partial-onset epileptic seizures [165]. It acts by selectively enhancing the slow inactivation of the voltage-gated sodium channel blockers [166] and by blocking the T-type calcium channels [167].
In a study examining the efficacy and safety of eslicarbazepine acetate as adjunctive therapy for refractory focal-onset seizures in children, the most frequently reported side effects were headache, nasopharyngitis, and drowsiness [73]. There were also clinical studies in which hyponatremia was commonly observed with eslicarbazepine acetate usage [168]. It was believed that hyponatremia may be caused by eslicarbazepine acetate in elderly patients with epilepsy [169]. Therefore, eslicarbazepine acetate should be carefully evaluated for elderly patients before prescriptions are made. Besides that, in a previous study, patients who were 65 years and older had a significantly higher rate of adverse events compared to younger patients after eslicarbazepine acetate usage [74]. Similarly, in another study, side effects were observed to be significantly higher in patients older than 60 years of age [170]. This is thought to be due to the relatively high rate of comorbidity and the risk of drug-drug interactions in elderly patients [171]. Therefore, eslicarbazepine acetate may be safe and effective for younger epileptic patients rather than elderly epileptic patients. Nevertheless, more studies may be warranted to determine its safety in pregnant and/or younger patients with comorbidities as well.
2.2.10. Cenobamate
Cenobamate (CBM) is a newly approved drug for the treatment of focal-onset seizures in adult patients. The seizure-suppressing effect of CMB is associated with two basic molecular mechanisms; inhibiting the permanent sodium (Na+) current by binding to inactive voltage-gated sodium channels (VGSC) with high affinity [172], thereby altering the threshold and frequency of action potentials, and acting as a positive allosteric modulator of GABAA receptors, independent of the benzodiazepine binding site [172], thereby strengthening the GABA-mediated tonic inhibition of seizures.
In general, patients tend to well-tolerated CMB with only minor side effects, such as dizziness, drowsiness, diplopia, headache, and fatigue, developing from usage [75-78]. However, more severe effects, such as hypersensitivity reactions leading to severe DRESS effect, have been observed in patients receiving 200 mg CBM daily [78]. It has been reported that the adverse effects of CBM treatment may increase in a dose-dependent manner [76]. Besides DRESS, CBM has also been shown to shorten the electrocardiogram QT interval, risking cardiac arrhythmias in a dose-dependent manner, which could be a result of the suppression of cardiac sodium channels [173]. This may also be witnessed as a synergistic effect when used with antiseizure drugs, such as rufinamide that shorten the QT interval [174], suggesting potential risk factors with CBM polytherapy strategy. However, in clinical practice, slower titration of CBM and gradual reduction of ineffective VGSC blocker ASDs, may in turn, improve tolerability and effectivity of CBM polytherapy [78]. Thus, CBM may offer a promising polytherapy approach with effective VGSC blockers for the pharmacological treatment of patients with drug-resistant epilepsy.
CONCLUSION
Anti-seizure drugs are widely used as the first line of treatment strategy against epilepsy, where they may prevent or control the occurrence of seizures in patients, thus allowing them to lead a healthy and good life. However, due to the ASD’s mechanism of action, patients often complain about side effects, such as headache, vomiting, nausea, fatigue and mood swings. While these minor and more common side effects are tolerable, some patients may develop more adverse reactions to certain ASDs, which could dampen their quality of life and even be life-threatening. These adverse side effects include liver toxicity, aplastic anemia, cardiorespiratory dysfunction, teratogenicity and bone loss. Research shows that the spectrum of side effects may vary depending on comorbidity, age, pregnancy status, pre-existing diseases, and adverse drug interactions. Thus, it is important to focus on accurately establishing the side effect profile of ASDs and practice individual-based treatment approaches when prescribing ASDs to patients in order to select the most appropriate and effective drug to control their seizures, regardless of mono- or poly-therapy ASD strategies. Further preclinical studies may be needed to better understand the mechanism by which each ASD exerts these side effects, while more clinical studies may be warranted in understanding the effects of ASD in different patient cohorts, especially for newer generation ASDs. Even though ASDs may only control seizures and not cure epilepsy, it may be the only hope for many epileptic patients to have a good quality of life despite their debilitating disease, and thus, it is pivotal to establish the most appropriate, effective and safe ASD for each patient.
AUTHORS’ CONTRIBUTIONS
EA, BK and CO conceived, carried out the literature review and drafted the manuscript. EA, AA and MFS provided critical inputs, revised and edited the final version of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Beghi E., Giussani G., Sander J.W. The natural history and prognosis of epilepsy. Epileptic Disord. 2015;17(3):243–253. doi: 10.1684/epd.2015.0751. [DOI] [PubMed] [Google Scholar]
- 2.Neligan A., Hauser W.A., Sander J.W. The epidemiology of the epilepsies. Handb. Clin. Neurol. 2012;107:113–133. doi: 10.1016/B978-0-444-52898-8.00006-9. [DOI] [PubMed] [Google Scholar]
- 3.Wei F., Yan L.M., Su T., He N., Lin Z.J., Wang J., Shi Y.W., Yi Y.H., Liao W.P. Ion channel genes and epilepsy: functional alteration, pathogenic potential, and mechanism of epilepsy. Neurosci. Bull. 2017;33(4):455–477. doi: 10.1007/s12264-017-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker-Haliski M., White H.S. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb. Perspect. Med. 2015;5(8):a022863. doi: 10.1101/cshperspect.a022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., Hirsch E., Jain S., Mathern G.W., Moshé S.L., Nordli D.R., Perucca E., Tomson T., Wiebe S., Zhang Y.H., Zuberi S.M. ILAE classification of the epilepsies: Position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurman D.J., Begley C.E., Carpio A., Helmers S., Hesdorffer D.C., Mu J., Touré K., Parko K.L., Newton C.R. The primary prevention of epilepsy: A report of the prevention task force of the international league against epilepsy. Epilepsia. 2018;59(5):905–914. doi: 10.1111/epi.14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lidster K., Jefferys J.G., Blümcke I., Crunelli V., Flecknell P., Frenguelli B.G., Gray W.P., Kaminski R., Pitkänen A., Ragan I., Shah M., Simonato M., Trevelyan A., Volk H., Walker M., Yates N., Prescott M.J. Opportunities for improving animal welfare in rodent models of epilepsy and seizures. J. Neurosci. Methods. 2016;260:2–25. doi: 10.1016/j.jneumeth.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Janmohamed M., Brodie M.J., Kwan P. Pharmacoresistance - Epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology. 2020;168:107790. doi: 10.1016/j.neuropharm.2019.107790. [DOI] [PubMed] [Google Scholar]
- 9.Sheng J., Liu S., Qin H., Li B., Zhang X. Drug-resistant epilepsy and surgery. Curr. Neuropharmacol. 2018;16(1):17–28. doi: 10.2174/1570159X15666170504123316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thijs R.D., Surges R., O’Brien T.J., Sander J.W. Epilepsy in adults. Lancet. 2019;393(10172):689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 11.Angalakuditi M., Angalakuditi N. A comprehensive review of the literature on epilepsy in selected countries in emerging markets. Neuropsychiatr. Dis. Treat. 2011;7:585–597. doi: 10.2147/NDT.S24966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanaya R., Arita K. The new antiepileptic drugs: Their neuropharmacology and clinical indications. Neurol. Med. Chir. (Tokyo) 2016;56(5):205–220. doi: 10.2176/nmc.ra.2015-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czapiński P., Blaszczyk B., Czuczwar S.J. Mechanisms of action of antiepileptic drugs. Curr. Top. Med. Chem. 2005;5(1):3–14. doi: 10.2174/1568026053386962. [DOI] [PubMed] [Google Scholar]
- 14.Rogawski M.A., Löscher W. The neurobiology of antiepileptic drugs. Nat. Rev. Neurosci. 2004;5(7):553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 15.Bialer M., White H.S. Key factors in the discovery and development of new antiepileptic drugs. Nat. Rev. Drug Discov. 2010;9(1):68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- 16.Perucca P., Gilliam F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):792–802. doi: 10.1016/S1474-4422(12)70153-9. [DOI] [PubMed] [Google Scholar]
- 17.Siarava E., Hyphantis T., Pelidou S.H., Kyritsis A.P., Markoula S. Factors related to the adverse events of antiepileptic drugs. Epilepsy Behav. 2020;111:107199. doi: 10.1016/j.yebeh.2020.107199. [DOI] [PubMed] [Google Scholar]
- 18.Symonds J.D., Zuberi S.M., Johnson M.R. Advances in epilepsy gene discovery and implications for epilepsy diagnosis and treatment. Curr. Opin. Neurol. 2017;30(2):193–199. doi: 10.1097/WCO.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 19.Löscher W., Potschka H., Sisodiya S.M., Vezzani A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev. 2020;72(3):606–638. doi: 10.1124/pr.120.019539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feyissa A.M., López C.A.S., Britton J.W. Antiepileptic drug therapy in patients with autoimmune epilepsy. Neurol. Neuroimmunol. Neuroinflamm. 2017;4(4):e353. doi: 10.1212/NXI.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chateauvieux S., Morceau F., Dicato M., Diederich M. Molecular and therapeutic potential and toxicity of valproic acid. J. Biomed. Biotechnol. 2010;2010:479364. doi: 10.1155/2010/479364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomson T., Battino D., Perucca E. Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. Lancet Neurol. 2016;15(2):210–218. doi: 10.1016/S1474-4422(15)00314-2. [DOI] [PubMed] [Google Scholar]
- 23.Meador K.J., Penovich P., Baker G.A., Pennell P.B., Bromfield E., Pack A., Liporace J.D., Sam M., Kalayjian L.A., Thurman D.J., Moore E., Loring D.W. Antiepileptic drug use in women of childbearing age. Epilepsy Behav. 2009;15(3):339–343. doi: 10.1016/j.yebeh.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubinchik-Stern M., Shmuel M., Bar J., Kovo M., Eyal S. Adverse placental effects of valproic acid: Studies in perfused human placentas. Epilepsia. 2018;59(5):993–1003. doi: 10.1111/epi.14078. [DOI] [PubMed] [Google Scholar]
- 25.Tetro N., Imbar T., Wohl D., Eisenberg I., Yagel S., Shmuel M., Eyal S. 2019. [DOI] [PubMed]
- 26.Li S., Guo J., Ying Z., Chen S., Yang L., Chen K., Long Q., Qin D., Pei D., Liu X. Valproic acid-induced hepatotoxicity in Alpers syndrome is associated with mitochondrial permeability transition pore opening-dependent apoptotic sensitivity in an induced pluripotent stem cell model. Hepatology. 2015;61(5):1730–1739. doi: 10.1002/hep.27712. [DOI] [PubMed] [Google Scholar]
- 27.Abdelkader N.F., Elyamany M., Gad A.M., Assaf N., Fawzy H.M., Elesawy W.H. Ellagic acid attenuates liver toxicity induced by valproic acid in rats. J. Pharmacol. Sci. 2020;143(1):23–29. doi: 10.1016/j.jphs.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Nasr Esfahani P., Nasiri J., Badihian S., Yaghini O. Short-term side effects of low dose valproate monotherapy in epileptic children: A Prospective Study. Iran. J. Child. Neurol. 2019;13(2):37–46. [PMC free article] [PubMed] [Google Scholar]
- 29.Clark A.M., Kriel R.L., Leppik I.E., White J.R., Henry T.R., Brundage R.C., Cloyd J.C. Intravenous topiramate: safety and pharmacokinetics following a single dose in patients with epilepsy or migraines taking oral topiramate. Epilepsia. 2013;54(6):1106–1111. doi: 10.1111/epi.12165. [DOI] [PubMed] [Google Scholar]
- 30.Manitpisitkul P., Shalayda K., Todd M., Wang S.S., Ness S., Ford L. Pharmacokinetics and safety of adjunctive topiramate in infants (1-24 months) with refractory partial-onset seizures: a randomized, multicenter, open-label phase 1 study. Epilepsia. 2013;54(1):156–164. doi: 10.1111/epi.12019. [DOI] [PubMed] [Google Scholar]
- 31.Nadig P.L., Sahu J.K., Suthar R., Saini A., Sankhyan N. Topiramate as an adjunct in the management of west syndrome. Indian J. Pediatr. 2020;87(1):6–11. doi: 10.1007/s12098-019-03105-0. [DOI] [PubMed] [Google Scholar]
- 32.Westin A.A., Nakken K.O., Johannessen S.I., Reimers A., Lillestølen K.M., Brodtkorb E. Serum concentration/dose ratio of topiramate during pregnancy. Epilepsia. 2009;50(3):480–485. doi: 10.1111/j.1528-1167.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 33.Tashiro Y., Azukizawa H., Asada H., Niihara H., Morita E., Yamauchi T., Mizukawa Y., Kusakabe Y., Numazawa S., Izumi M., Sueki H., Watanabe H. Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms due to lamotrigine differs from that due to other drugs. J. Dermatol. 2019;46(3):226–233. doi: 10.1111/1346-8138.14776. [DOI] [PubMed] [Google Scholar]
- 34.Wang X.Q., Lv B., Wang H.F., Zhang X., Yu S.Y., Huang X.S., Zhang J.T., Tian C.L., Lang S.Y. Lamotrigine-induced severe cutaneous adverse reaction: Update data from 1999-2014. J. Clin. Neurosci. 2015;22(6):1005–1011. doi: 10.1016/j.jocn.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Helmstaedter C., Mihov Y., Toliat M.R., Thiele H., Nuernberg P., Schoch S., Surges R., Elger C.E., Kunz W.S., Hurlemann R. Genetic variation in dopaminergic activity is associated with the risk for psychiatric side effects of levetiracetam. Epilepsia. 2013;54(1):36–44. doi: 10.1111/j.1528-1167.2012.03603.x. [DOI] [PubMed] [Google Scholar]
- 36.Heyman E., Levin N., Lahat E., Epstein O., Gandelman-Marton R. Efficacy and safety of felbamate in children with refractory epilepsy. Eur. J. Paediatr. Neurol. 2014;18(6):658–662. doi: 10.1016/j.ejpn.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Shah Y.D., Singh K., Friedman D., Devinsky O., Kothare S.V. Evaluating the safety and efficacy of felbamate in the context of a black box warning: A single center experience. Epilepsy Behav. 2016;56:50–53. doi: 10.1016/j.yebeh.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Orczyk J.J., Garraghty P.E. The effects of felbamate on appetitive and aversive instrumental learning in adult rats. Epilepsy Behav. 2018;78:14–19. doi: 10.1016/j.yebeh.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 39.Cavanna A.E., Seri S. Psychiatric adverse effects of zonisamide in patients with epilepsy and mental disorder comorbidities. Epilepsy Behav. 2013;29(2):281–284. doi: 10.1016/j.yebeh.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 40.White H.S., Franklin M.R., Kupferberg H.J., Schmutz M., Stables J.P., Wolf H.H. The anticonvulsant profile of rufinamide (CGP 33101) in rodent seizure models. Epilepsia. 2008;49(7):1213–1220. doi: 10.1111/j.1528-1167.2008.01552.x. [DOI] [PubMed] [Google Scholar]
- 41.Nikanorova M., Brandt C., Auvin S., McMurray R. Real-world data on rufinamide treatment in patients with Lennox-Gastaut syndrome: Results from a European noninterventional registry study. Epilepsy Behav. 2017;76:63–70. doi: 10.1016/j.yebeh.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 42.Mourand I., Crespel A., Gelisse P. Dramatic weight loss with rufinamide. Epilepsia. 2013;54(1):e5–e8. doi: 10.1111/j.1528-1167.2012.03579.x. [DOI] [PubMed] [Google Scholar]
- 43.Panebianco M., Prabhakar H., Marson A.G. Rufinamide add-on therapy for refractory epilepsy. Cochrane Database Syst. Rev. 2018;4:CD011772. doi: 10.1002/14651858.CD011772.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Visa-Reñé N., Raspall-Chaure M., Paredes-Carmona F., Coromina J.S., Macaya-Ruiz A. Clinical experience with brivaracetam in a series of 46 children. Epilepsy Behav. 2020;107:107067. doi: 10.1016/j.yebeh.2020.107067. [DOI] [PubMed] [Google Scholar]
- 45.Villanueva V., López-González F.J., Mauri J.A., Rodriguez-Uranga J., Olivé-Gadea M., Montoya J., Ruiz-Giménez J., Zurita J. BRIVA-LIFE-A multicenter retrospective study of the long-term use of brivaracetam in clinical practice. Acta Neurol. Scand. 2019;139(4):360–368. doi: 10.1111/ane.13059. [DOI] [PubMed] [Google Scholar]
- 46.Khaleghi F., Nemec E.C. II Brivaracetam (Briviact): A novel adjunctive therapy for partial-onset seizures. P&T. 2017;42(2):92–96. [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon O.Y., Park S.P. Depression and anxiety in people with epilepsy. J. Clin. Neurol. 2014;10(3):175–188. doi: 10.3988/jcn.2014.10.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S.A., Jeon J.Y., Kim H.W. Effect of perampanel on aggression in patients with refractory focal epilepsy: A 6-month longitudinal study. Epilepsy Behav. 2020;102:106658. doi: 10.1016/j.yebeh.2019.106658. [DOI] [PubMed] [Google Scholar]
- 49.Lyttle M.D., Rainford N.E.A., Gamble C., Messahel S., Humphreys A., Hickey H., Woolfall K., Roper L., Noblet J., Lee E.D., Potter S., Tate P., Iyer A., Evans V., Appleton R.E. Paediatric Emergency Research in the United Kingdom & Ireland (PERUKI) collaborative. Levetiracetam Versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): A multicentre, open-label, randomised trial. Lancet. 2019;393(10186):2125–2134. doi: 10.1016/S0140-6736(19)30724-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumkamthornkul P., Udnaen S., Tansit T., Tuchinda P., Srinoulprasert Y. Evaluation of a lymphocyte transformation test and cytokine detection assay to identify phenytoin and carbamazepine provoked DRESS or SJS/TEN in epilepsy patients. Int. Immunopharmacol. 2018;63:204–210. doi: 10.1016/j.intimp.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Sasidharanpillai S., Riyaz N., Rajan U., Binitha M.P., Khader A., Mariyath O.K., John R., Puravoor J. Drug reaction with eosinophilia and systemic symptoms: Observations from a tertiary care institution. Indian J. Dermatol. Venereol. Leprol. 2014;80(3):221–228. doi: 10.4103/0378-6323.132249. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki E., Matsuo K., Iida A., Tsuneyama K., Fukami T., Nakajima M., Yokoi T. A novel mouse model for phenytoin-induced liver injury: Involvement of immune-related factors and P450-mediated metabolism. Toxicol. Sci. 2013;136(1):250–263. doi: 10.1093/toxsci/kft184. [DOI] [PubMed] [Google Scholar]
- 53.Aldenkamp A.P., Alpherts W.C., Diepman L., van ’t Slot B., Overweg J., Vermeulen J. Cognitive side-effects of phenytoin compared with carbamazepine in patients with localization-related epilepsy. Epilepsy Res. 1994;19(1):37–43. doi: 10.1016/0920-1211(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 54.Mach M., Dubovický M., Navarová J., Kovacovský P., Ujházy E. Vitamin E supplementation in phenytoin induced developmental toxicity in rats: postnatal study. Neuroendocrinol. Lett. 2006;27(Suppl. 2):69–73. [PubMed] [Google Scholar]
- 55.Tomson T., Battino D., Bonizzoni E., Craig J., Lindhout D., Perucca E., Sabers A., Thomas S.V., Vajda F.E.S. Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: A prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530–538. doi: 10.1016/S1474-4422(18)30107-8. [DOI] [PubMed] [Google Scholar]
- 56.Besi E., Boniface D.R., Cregg R., Zakrzewska J.M. Comparison of tolerability and adverse symptoms in oxcarbazepine and carbamazepine in the treatment of trigeminal neuralgia and neuralgiform headaches using the Liverpool Adverse Events Profile (AEP). J. Headache Pain. 2015;16:563. doi: 10.1186/s10194-015-0563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geng H., Wang C. Efficacy and safety of oxcarbazepine in the treatment of children with epilepsy: A meta-analysis of randomized controlled trials. Neuropsychiatr. Dis. Treat. 2017;13:685–695. doi: 10.2147/NDT.S130269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eisenschenk S. Treatment with oxcarbazepine during pregnancy. Neurologist. 2006;12(5):249–254. doi: 10.1097/01.nrl.0000215743.02301.17. [DOI] [PubMed] [Google Scholar]
- 59.Welsh S.S., Lin N., Topjian A.A., Abend N.S. Safety of intravenous lacosamide in critically ill children. Seizure-Eur J Epilep. 2017;52:76–80. doi: 10.1016/j.seizure.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baulac M., Rosenow F., Toledo M., Terada K., Li T., De Backer M., Werhahn K.J., Brock M. Efficacy, safety, and tolerability of lacosamide monotherapy Versus controlled-release carbamazepine in patients with newly diagnosed epilepsy: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol. 2017;16(1):43–54. doi: 10.1016/S1474-4422(16)30292-7. [DOI] [PubMed] [Google Scholar]
- 61.Marson A.G., Al-Kharusi A.M., Alwaidh M., Appleton R., Baker G.A., Chadwick D.W., Cramp C., Cockerell O.C., Cooper P.N., Doughty J., Eaton B., Gamble C., Goulding P.J., Howell S.J., Hughes A., Jackson M., Jacoby A., Kellett M., Lawson G.R., Leach J.P., Nicolaides P., Roberts R., Shackley P., Shen J., Smith D.F., Smith P.E., Smith C.T., Vanoli A., Williamson P.R.S.S. group. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: An unblinded randomised controlled trial. Lancet. 2007;369(9566):1000–1015. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kheirabadi D., Safavi M.R., Taghvaei M., Habibzadeh M.R., Honarmand A. Comparing the prophylactic effects of oral gabapentin, pregabalin, and celecoxib on postoperative pain management in orthopedic surgery of the lower extremity: A double-blind randomized controlled trial. J. Res. Med. Sci. 2020;25:9. doi: 10.4103/jrms.JRMS_140_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fleet J.L., Dixon S.N., Kuwornu P.J., Dev V.K., Montero-Odasso M., Burneo J., Garg A.X. Gabapentin dose and the 30-day risk of altered mental status in older adults: A retrospective population-based study. PLoS One. 2018;13(3):e0193134. doi: 10.1371/journal.pone.0193134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zand L., McKian K.P., Qian Q. Gabapentin toxicity in patients with chronic kidney disease: a preventable cause of morbidity. Am. J. Med. 2010;123(4):367–373. doi: 10.1016/j.amjmed.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 65.Zimcikova E., Simko J., Karesova I., Kremlacek J., Malakova J. Behavioral effects of antiepileptic drugs in rats: Are the effects on mood and behavior detectable in open-field test? Seizure. 2017;52:35–40. doi: 10.1016/j.seizure.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 66.Montouris G. Gabapentin exposure in human pregnancy: Results from the Gabapentin Pregnancy Registry. Epilepsy Behav. 2003;4(3):310–317. doi: 10.1016/S1525-5050(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 67.Kossoff E.H., Hartman A.L., Rubenstein J.E., Vining E.P. High-dose oral prednisolone for infantile spasms: An effective and less expensive alternative to ACTH. Epilepsy Behav. 2009;14(4):674–676. doi: 10.1016/j.yebeh.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 68.Jackson M.C., Jafarpour S., Klehm J., Thome-Souza S., Coughlin F., Kapur K., Loddenkemper T. Effect of vigabatrin on seizure control and safety profile in different subgroups of children with epilepsy. Epilepsia. 2017;58(9):1575–1585. doi: 10.1111/epi.13836. [DOI] [PubMed] [Google Scholar]
- 69.Kim Y.S., Kim D.W., Jung K.H., Lee S.T., Kang B.S., Byun J.I., Yeom J.S., Chu K., Lee S.K. Frequency of and risk factors for oxcarbazepine-induced severe and symptomatic hyponatremia. Seizure. 2014;23(3):208–212. doi: 10.1016/j.seizure.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 70.Sarkis R.A., Goksen Y., Mu Y., Rosner B., Lee J.W. Cognitive and fatigue side effects of anti-epileptic drugs: an analysis of phase III add-on trials. J. Neurol. 2018;265(9):2137–2142. doi: 10.1007/s00415-018-8971-z. [DOI] [PubMed] [Google Scholar]
- 71.Balestrini S., Sisodiya S.M. Audit of use of stiripentol in adults with Dravet syndrome. Acta Neurol. Scand. 2017;135(1):73–79. doi: 10.1111/ane.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zulfiqar Ali Q., Marques P., Selvarajah A., Tabarestani S., Sadoway T., Andrade D.M. Starting stiripentol in adults with Dravet syndrome? Watch for ammonia and carnitine. Epilepsia. 2020;61(11):2435–2441. doi: 10.1111/epi.16684. [DOI] [PubMed] [Google Scholar]
- 73.Kirkham F., Auvin S., Moreira J., Gama H., Falcão A.C., Rocha J.F., Soares-da-Silva P. Efficacy and safety of eslicarbazepine acetate as adjunctive therapy for refractory focal-onset seizures in children: A double-blind, randomized, placebo-controlled, parallel-group, multicenter, phase-III clinical trial. Epilepsy Behav. 2020;105:106962. doi: 10.1016/j.yebeh.2020.106962. [DOI] [PubMed] [Google Scholar]
- 74.Giráldez B.G., Garamendi-Ruiz I., Zurita J., García A., Querol R., Campos D., Cabeza-Alvarez C., Serrano P., López-González F.J., Molins A., Serratosa J.M. Clinical outcomes of eslicarbazepine acetate monotherapy for focal-onset seizures: A multicenter audit. Acta Neurol. Scand. 2019;140(6):422–428. doi: 10.1111/ane.13162. [DOI] [PubMed] [Google Scholar]
- 75.Buckley C.T., Waters O.R., DeMaagd G. Cenobamate: A New adjunctive agent for drug-resistant focal onset epilepsy. Ann. Pharmacother. 2021;55(3):318–329. doi: 10.1177/1060028020941113. [DOI] [PubMed] [Google Scholar]
- 76.Rosenfeld W.E., Nisman A., Ferrari L. Efficacy of adjunctive cenobamate based on number of concomitant antiseizure medications, seizure frequency, and epilepsy duration at baseline: A post-hoc analysis of a randomized clinical study. Epilepsy Res. 2021;172:106592. doi: 10.1016/j.eplepsyres.2021.106592. [DOI] [PubMed] [Google Scholar]
- 77.Chung S.S., French J.A., Kowalski J., Krauss G.L., Lee S.K., Maciejowski M., Rosenfeld W.E., Sperling M.R., Mizne S., Kamin M. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94(22):e2311–e2322. doi: 10.1212/WNL.0000000000009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krauss G.L., Klein P., Brandt C., Lee S.K., Milanov I., Milovanovic M., Steinhoff B.J., Kamin M. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38–48. doi: 10.1016/S1474-4422(19)30399-0. [DOI] [PubMed] [Google Scholar]
- 79.Ornoy A. Valproic acid in pregnancy: how much are we endangering the embryo and fetus? Reprod. Toxicol. 2009;28(1):1–10. doi: 10.1016/j.reprotox.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 80.Stewart J.D., Horvath R., Baruffini E., Ferrero I., Bulst S., Watkins P.B., Fontana R.J., Day C.P., Chinnery P.F. Polymerase γ gene POLG determines the risk of sodium valproate-induced liver toxicity. Hepatology. 2010;52(5):1791–1796. doi: 10.1002/hep.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saneto R.P., Lee I.C., Koenig M.K., Bao X., Weng S.W., Naviaux R.K., Wong L.J.C. POLG DNA testing as an emerging standard of care before instituting valproic acid therapy for pediatric seizure disorders. Seizure-Eur J Epilep. 2010;19(3):140–146. doi: 10.1016/j.seizure.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hynynen J., Pokka T., Komulainen-Ebrahim J., Myllynen P., Kärppä M., Pylvänen L., Kälviäinen R., Sokka A., Jyrkilä A., Lähdetie J., Haataja L., Mäkitalo A., Ylikotila P., Eriksson K., Haapala P., Ansakorpi H., Hinttala R., Vieira P., Majamaa K., Rantala H., Uusimaa J. Variants p.Q1236H and p.E1143G in mitochondrial DNA polymerase gamma POLG1 are not associated with increased risk for valproate-induced hepatotoxicity or pancreatic toxicity: A retrospective cohort study of patients with epilepsy. Epilepsia. 2018;59(11):2125–2136. doi: 10.1111/epi.14568. [DOI] [PubMed] [Google Scholar]
- 83.Farooq F., Sahib Din J., Khan A.M., Naqvi S., Shagufta S., Mohit A. Valproate-induced hyperammonemic encephalopathy. Cureus. 2017;9(8):e1593. doi: 10.7759/cureus.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baddour E., Tewksbury A., Stauner N. Valproic acid-induced hyperammonemia: Incidence, clinical significance, and treatment management. Ment. Health Clin. 2018;8(2):73–77. doi: 10.9740/mhc.2018.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shah S., Wang R., Vieux U. Valproate-induced hyperammonemic encephalopathy: A case report. J. Med. Case Reports. 2020;14(1):19. doi: 10.1186/s13256-020-2343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carr R.B., Shrewsbury K. Hyperammonemia due to valproic acid in the psychiatric setting. Am. J. Psychiatry. 2007;164(7):1020–1027. doi: 10.1176/ajp.2007.164.7.1020. [DOI] [PubMed] [Google Scholar]
- 87.Lalic M., Cvejic J., Popovic J., Bozic K., Golocorbin-Kon S., Al-Salami H., Mikov M. Lamotrigine and valproate pharmacokinetics interactions in epileptic patients. Eur. J. Drug Metab. Pharmacokinet. 2009;34(2):93–99. doi: 10.1007/BF03191157. [DOI] [PubMed] [Google Scholar]
- 88.Minton G.C., Miller A.D., Bookstaver P.B., Love B.L. Topiramate: safety and efficacy of its use in the prevention and treatment of migraine. J. Cent. Nerv. Syst. Dis. 2011;3:155–168. doi: 10.4137/JCNSD.S4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bjøro K., Gjerstad L., Bentdal O., Osnes S., Schrumpf E. Topiramate and fulminant liver failure. Lancet. 1998;352(9134):1119. doi: 10.1016/S0140-6736(05)79759-2. [DOI] [PubMed] [Google Scholar]
- 90.Egunsola O., Choonara I., Sammons H.M. Safety of lamotrigine in paediatrics: A systematic review. BMJ Open. 2015;5(6):e007711. doi: 10.1136/bmjopen-2015-007711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yasam V.R., Jakki S.L., Senthil V., Eswaramoorthy M., Shanmuganathan S., Arjunan K., Nanjan M.J. A pharmacological overview of lamotrigine for the treatment of epilepsy. Expert Rev. Clin. Pharmacol. 2016;9(12):1533–1546. doi: 10.1080/17512433.2016.1254041. [DOI] [PubMed] [Google Scholar]
- 92.Parker G. Risks associated with lamotrigine prescription: A review and personal observations. Australas. Psychiatry. 2018;26(6):640–642. doi: 10.1177/1039856218760733. [DOI] [PubMed] [Google Scholar]
- 93.Justiz Vaillant A.A., Vashisht R., Zito P.M. 2021. Immediate Hypersensitivity Reactions. [PubMed] [Google Scholar]
- 94.Abu-Rish E.Y., Elhayek S.Y., Mohamed Y.S., Hamad I., Bustanji Y. Evaluation of immunomodulatory effects of lamotrigine in BALB/c mice. Acta Pharm. 2017;67(4):543–555. doi: 10.1515/acph-2017-0035. [DOI] [PubMed] [Google Scholar]
- 95.Kong L., Zhou T., Wang B., Gao Z., Wang C. The risks associated with the use of lamotrigine during pregnancy. Int. J. Psychiatry Clin. Pract. 2018;22(1):2–5. doi: 10.1080/13651501.2017.1341986. [DOI] [PubMed] [Google Scholar]
- 96.Ogunsakin O., Tumenta T., Louis-Jean S., Mahbub A., Rabel P., Olupona T., Alam S. Levetiracetam induced behavioral abnormalities in a patient with seizure disorder: a diagnostic challenge. Case Rep. Psychiatry. 2020;2020:8883802. doi: 10.1155/2020/8883802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Surges R., Volynski K.E., Walker M.C. Is levetiracetam different from other antiepileptic drugs? Levetiracetam and its cellular mechanism of action in epilepsy revisited. Ther. Adv. Neurol. Disord. 2008;1(1):13–24. doi: 10.1177/1756285608094212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomas L., Mirza M.M.F., Shaikh N.A., Ahmed N. Rhabdomyolysis: A rare adverse effect of levetiracetam. BMJ Case Rep. 2019;12(8):e230851. doi: 10.1136/bcr-2019-230851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mbizvo G.K., Dixon P., Hutton J.L., Marson A.G. Levetiracetam add-on for drug-resistant focal epilepsy: An updated cochrane review. Cochrane Database Syst. Rev. 2012;2012(9):CD001901. doi: 10.1002/14651858.CD001901.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Halma E., de Louw A.J., Klinkenberg S., Aldenkamp A.P., IJff D.M., Majoie M. Behavioral side-effects of levetiracetam in children with epilepsy: a systematic review. Seizure. 2014;23(9):685–691. doi: 10.1016/j.seizure.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Verrotti A., Prezioso G., Di Sabatino F., Franco V., Chiarelli F., Zaccara G. The adverse event profile of levetiracetam: A meta-analysis on children and adults. Seizure. 2015;31:49–55. doi: 10.1016/j.seizure.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 102.Zaccara G., Perucca P., Loiacono G., Giovannelli F., Verrotti A. The adverse event profile of lacosamide: a systematic review and meta-analysis of randomized controlled trials. Epilepsia. 2013;54(1):66–74. doi: 10.1111/j.1528-1167.2012.03589.x. [DOI] [PubMed] [Google Scholar]
- 103.Zaccara G., Giovannelli F., Cincotta M., Verrotti A., Grillo E. The adverse event profile of perampanel: Meta-analysis of randomized controlled trials. Eur. J. Neurol. 2013;20(8):1204–1211. doi: 10.1111/ene.12170. [DOI] [PubMed] [Google Scholar]
- 104.Yıldırım M., Bektaş Ö., Akıncı Göktaş Ö., Yüksel M.F., Şahin S., Tıraş Teber S. Levetiracetam monotherapy in children with epilepsy: Experience from a tertiary pediatric neurology center. Epilepsy Behav. 2021;116:107745. doi: 10.1016/j.yebeh.2020.107745. [DOI] [PubMed] [Google Scholar]
- 105.Tekgül H., Gencpinar P., Çavuşoğlu D., Dündar N.O. The efficacy, tolerability and safety of levetiracetam therapy in a pediatric population. Seizure. 2016;36:16–21. doi: 10.1016/j.seizure.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 106.Dozières-Puyravel B., Nasser H., Bellavoine V., Ilea A., Delanoe C., Auvin S. Felbamate for infantile spasms syndrome resistant to first-line treatments. Dev. Med. Child Neurol. 2020;62(5):581–586. doi: 10.1111/dmcn.14427. [DOI] [PubMed] [Google Scholar]
- 107.Sills G.J., Rogawski M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology. 2020;168:107966. doi: 10.1016/j.neuropharm.2020.107966. [DOI] [PubMed] [Google Scholar]
- 108.Walsh S., Chen R., Ershad M., Krueger J. The toxicity of newer and lesser-known anticonvulsant drugs. Curr. Emerg. Hosp. Med. Rep. 2020;8 doi: 10.1007/s40138-020-00220-7. [DOI] [Google Scholar]
- 109.Hooper W.D., Franklin M.E., Glue P., Banfield C.R., Radwanski E., McLaughlin D.B., McIntyre M.E., Dickinson R.G., Eadie M.J. Effect of felbamate on valproic acid disposition in healthy volunteers: Inhibition of beta-oxidation. Epilepsia. 1996;37(1):91–97. doi: 10.1111/j.1528-1157.1996.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 110.Leppik I.E. Zonisamide: chemistry, mechanism of action, and pharmacokinetics. Seizure. 2004;13(Suppl. 1):S5–S9. doi: 10.1016/j.seizure.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 111.Chen B., Choi H., Hirsch L.J., Katz A., Legge A., Buchsbaum R., Detyniecki K. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017;76:24–31. doi: 10.1016/j.yebeh.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 112.Antel J., Hebebrand J. Weight-reducing side effects of the antiepileptic agents topiramate and zonisamide. Handb. Exp. Pharmacol. 2012;(209):433–466. doi: 10.1007/978-3-642-24716-3_20. [DOI] [PubMed] [Google Scholar]
- 113.Takahashi A., Onodera K., Kamei J., Sakurada S., Shinoda H., Miyazaki S., Saito T., Mayanagi H. Effects of chronic administration of zonisamide, an antiepileptic drug, on bone mineral density and their prevention with alfacalcidol in growing rats. J. Pharmacol. Sci. 2003;91(4):313–318. doi: 10.1254/jphs.91.313. [DOI] [PubMed] [Google Scholar]
- 114.Koo D.L., Nam H. Effects of zonisamide monotherapy on bone health in drug-naive epileptic patients. Epilepsia. 2020;61(10):2142–2149. doi: 10.1111/epi.16678. [DOI] [PubMed] [Google Scholar]
- 115.Chen B., Detyniecki K., Choi H., Hirsch L., Katz A., Legge A., Wong R., Jiang A., Buchsbaum R., Farooque P. Psychiatric and behavioral side effects of anti-epileptic drugs in adolescents and children with epilepsy. Eur. J. Paediatr. Neurol. 2017;21(3):441–449. doi: 10.1016/j.ejpn.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 116.White J.R., Walczak T.S., Marino S.E., Beniak T.E., Leppik I.E., Birnbaum A.K. Zonisamide discontinuation due to psychiatric and cognitive adverse events: A case-control study. Neurology. 2010;75(6):513–518. doi: 10.1212/WNL.0b013e3181eccfb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar B., Medhi B., Modi M., Saikia B., Attri S.V., Patial A. A mechanistic approach to explore the neuroprotective potential of zonisamide in seizures. Inflammopharmacology. 2018;26(4):1125–1131. doi: 10.1007/s10787-018-0478-9. [DOI] [PubMed] [Google Scholar]
- 118.Hakimian S., Cheng-Hakimian A., Anderson G.D., Miller J.W. Rufinamide: A new anti-epileptic medication. Expert Opin. Pharmacother. 2007;8(12):1931–1940. doi: 10.1517/14656566.8.12.1931. [DOI] [PubMed] [Google Scholar]
- 119.de Biase S., Gigli G.L., Valente M. Brivaracetam for the treatment of focal-onset seizures: pharmacokinetic and pharmacodynamic evaluations. Expert Opin. Drug Metab. Toxicol. 2020;16(10):853–863. doi: 10.1080/17425255.2020.1813277. [DOI] [PubMed] [Google Scholar]
- 120.Meador K.J., Laloyaux C., Elmoufti S., Gasalla T., Fishman J., Martin M.S., Klein P. Time course of drug-related treatment-emergent adverse side effects of brivaracetam. Epilepsy Behav. 2020;111:107212. doi: 10.1016/j.yebeh.2020.107212. [DOI] [PubMed] [Google Scholar]
- 121.Paolini S.L., Pilato M., Rajasekaran V., Waters J.F.R., Bagic A., Urban A. Outcomes in three cases after brivaracetam treatment during pregnancy. Acta Neurol. Scand. 2020;141(5):438–441. doi: 10.1111/ane.13222. [DOI] [PubMed] [Google Scholar]
- 122.Hanada T., Hashizume Y., Tokuhara N., Takenaka O., Kohmura N., Ogasawara A., Hatakeyama S., Ohgoh M., Ueno M., Nishizawa Y. Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia. 2011;52(7):1331–1340. doi: 10.1111/j.1528-1167.2011.03109.x. [DOI] [PubMed] [Google Scholar]
- 123.Rogawski M.A., Hanada T. Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol. Scand. Suppl. 2013;(197):19–24. doi: 10.1111/ane.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coccaro E.F., Lee R., Vezina P. Cerebrospinal fluid glutamate concentration correlates with impulsive aggression in human subjects. J. Psychiatr. Res. 2013;47(9):1247–1253. doi: 10.1016/j.jpsychires.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang F.M., Fan P.C., Weng W.C., Chang C.H., Lee W.T. The efficacy of perampanel in young children with drug-resistant epilepsy. Seizure. 2020;75:82–86. doi: 10.1016/j.seizure.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 126.Citraro R., Leo A., Franco V., Marchiselli R., Perucca E., De Sarro G., Russo E. Perampanel effects in the WAG/Rij rat model of epileptogenesis, absence epilepsy, and comorbid depressive-like behavior. Epilepsia. 2017;58(2):231–238. doi: 10.1111/epi.13629. [DOI] [PubMed] [Google Scholar]
- 127.Gidal B.E., Ferry J., Majid O., Hussein Z. Concentration-effect relationships with perampanel in patients with pharmacoresistant partial-onset seizures. Epilepsia. 2013;54(8):1490–1497. doi: 10.1111/epi.12240. [DOI] [PubMed] [Google Scholar]
- 128.Steinhoff B.J., Patten A., Williams B., Malhotra M. Efficacy and safety of adjunctive perampanel 4 mg/d for the treatment of focal seizures: A pooled post hoc analysis of four randomized, double-blind, phase III studies. Epilepsia. 2020;61(2):278–286. doi: 10.1111/epi.16428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abou-Khalil B.W. Update on antiepileptic drugs 2019. Continuum (Minneap. Minn.) 2019;25(2):508–536. doi: 10.1212/CON.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 130.Shanmugarajah P.D., Hoggard N., Aeschlimann D.P., Aeschlimann P.C., Dennis G.J., Howell S.J., Reuber M., Grünewald R.A., Hadjivassiliou M. Phenytoin-related ataxia in patients with epilepsy: clinical and radiological characteristics. Seizure. 2018;56:26–30. doi: 10.1016/j.seizure.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 131.Amiri-Nikpour M.R., Nazarbaghi S., Eftekhari P., Mohammadi S., Dindarian S., Bagheri M., Mohammadi H. Sodium valproate compared to phenytoin in treatment of status epilepticus. Brain Behav. 2018;8(5):e00951. doi: 10.1002/brb3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yampayon K., Sukasem C., Limwongse C., Chinvarun Y., Tempark T., Rerkpattanapipat T., Kijsanayotin P. Influence of genetic and non-genetic factors on phenytoin-induced severe cutaneous adverse drug reactions. Eur. J. Clin. Pharmacol. 2017;73(7):855–865. doi: 10.1007/s00228-017-2250-2. [DOI] [PubMed] [Google Scholar]
- 133.Curry B., Mican L., Smith T.L. Phenytoin-induced chronic liver enzyme elevation and hepatic fibrosis: A case report. Ment. Health Clin. 2018;8(4):184–187. doi: 10.9740/mhc.2018.07.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tomson T., Battino D. Teratogenic effects of antiepileptic drugs. Seizure. 2008;17(2):166–171. doi: 10.1016/j.seizure.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 135.Marefat A., Sadeghi L. Neurotoxic effects of phenytoin on primary culture of hippocampal neurons: Neural development retardation. Neurol. Psychiatry Brain Res. 2020;36:52–56. doi: 10.1016/j.npbr.2020.03.001. [DOI] [Google Scholar]
- 136.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] [Google Scholar]
- 137.Donati F., Gobbi G., Campistol J., Rapatz G., Daehler M., Sturm Y., Aldenkamp A.P. The cognitive effects of oxcarbazepine Versus carbamazepine or valproate in newly diagnosed children with partial seizures. Seizure-Eur. J. Epilepsy. 2007;16(8):670–679. doi: 10.1016/j.seizure.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 138.Thelengana A., Shukla G., Srivastava A., Singh M.B., Gupta A., Rajan R., Vibha D., Pandit A.K., Prasad K. Cognitive, behavioural and sleep-related adverse effects on introduction of levetiracetam Versus oxcarbazepine for epilepsy. Epilepsy Res. 2019;150:58–65. doi: 10.1016/j.eplepsyres.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 139.Song Y., Zhong M., Cai F.C. Oxcarbazepine causes neurocyte apoptosis and developing brain damage by triggering Bax/Bcl-2 signaling pathway mediated caspase 3 activation in neonatal rats. Eur. Rev. Med. Pharmacol. 2018;22(1):250–261. doi: 10.26355/eurrev_201801_14126. [DOI] [PubMed] [Google Scholar]
- 140.González-Maciel A., Romero-Velázquez R.M., Alfaro-Rodríguez A., Sanchez A.P., Reynoso-Robles R. Prenatal exposure to oxcarbazepine increases hippocampal apoptosis in rat offspring. J. Chem. Neuroanat. 2020;103:101729. doi: 10.1016/j.jchemneu.2019.101729. [DOI] [PubMed] [Google Scholar]
- 141.Rogawski M.A., Tofighy A., White H.S., Matagne A., Wolff C. Current understanding of the mechanism of action of the antiepileptic drug lacosamide. Epilepsy Res. 2015;110:189–205. doi: 10.1016/j.eplepsyres.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 142.Doty P., Rudd G.D., Stoehr T., Thomas D. Lacosamide. Neurotherapeutics. 2007;4(1):145–148. doi: 10.1016/j.nurt.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McGinnis E., Kessler S.K. Lacosamide use in children with epilepsy: Retention rate and effect of concomitant sodium channel blockers in a large cohort. Epilepsia. 2016;57(9):1416–1425. doi: 10.1111/epi.13466. [DOI] [PubMed] [Google Scholar]
- 144.Del Bianco C., Placidi F., Liguori C., Mari L., Ulivi M., Ornello R., Pisani A., Mercuri N.B., Izzi F. Long-term efficacy and safety of lacosamide and levetiracetam monotherapy in elderly patients with focal epilepsy: A retrospective study. Epilepsy Behav. 2019;94:178–182. doi: 10.1016/j.yebeh.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 145.Villanueva V., López-Gomáriz E., López-Trigo J., Palau J., García M., Villarroya T., Bonet M., Santafé C. Rational polytherapy with lacosamide in clinical practice: results of a Spanish cohort analysis RELACOVA. Epilepsy Behav. 2012;23(3):298–304. doi: 10.1016/j.yebeh.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 146.Evoy K.E., Morrison M.D., Saklad S.R. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403–426. doi: 10.1007/s40265-017-0700-x. [DOI] [PubMed] [Google Scholar]
- 147.Hayes W.J., Ferdinand A., Neabore S., Kappes J.A., Hayes K.M., Berendse J. Patient Case Report: Gabapentin-Induced Hypoglycemia. J. Pharm. Pract. 2020;•••:897190020961229. doi: 10.1177/0897190020961229. [DOI] [PubMed] [Google Scholar]
- 148.Amiri S., Haj-Mirzaian A., Amini-Khoei H., Razmi A., Shirzadian A., Rahimi-Balaei M., Olson C.O., Mohsenzadeh A., Rastegar M., Zarrindast M.R., Ghazi-Khansari M. Protective effects of gabapentin against the seizure susceptibility and comorbid behavioral abnormalities in the early socially isolated mice. Eur. J. Pharmacol. 2017;797:106–114. doi: 10.1016/j.ejphar.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 149.Faulkner M.A., Tolman J.A. Safety and efficacy of vigabatrin for the treatment of infantile spasms. J. Cent. Nerv. Syst. Dis. 2011;3:199–207. doi: 10.4137/JCNSD.S6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang J., Naumann M.C., Tsai Y-T., Tosi J., Erol D., Lin C-S., Davis R.J., Tsang S.H. Vigabatrin-induced retinal toxicity is partially mediated by signaling in rod and cone photoreceptors. PLoS One. 2012;7(8):e43889. doi: 10.1371/journal.pone.0043889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y., Qin Z.H. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010;15(11):1382–1402. doi: 10.1007/s10495-010-0481-0. [DOI] [PubMed] [Google Scholar]
- 152.Chan K., Hoon M., Pattnaik B.R., Ver Hoeve J.N., Wahlgren B., Gloe S., Williams J., Wetherbee B., Kiland J.A., Vogel K.R., Jansen E., Salomons G., Walters D., Roullet J.B., Gibson K.M., McLellan G.J. Vigabatrin-induced retinal functional alterations and second-order neuron plasticity in C57BL/6J Mice. Invest. Ophthalmol. Vis. Sci. 2020;61(2):17. doi: 10.1167/iovs.61.2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Plant G.T., Sergott R.C. Understanding and interpreting vision safety issues with vigabatrin therapy. Acta Neurol. Scand. Suppl. 2011;(192):57–71. doi: 10.1111/j.1600-0404.2011.01601.x. [DOI] [PubMed] [Google Scholar]
- 154.Kanase Y., Kitada T., Tabata H., Makino K., Oshitari T., Ohashi H., Yoshinaga T., Natsugari H., Takahashi H. 4-Substituted carbamazepine derivatives: Conformational analysis and sodium channel-blocking properties. Bioorg. Med. Chem. 2018;26(9):2508–2513. doi: 10.1016/j.bmc.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 155.Gierbolini J., Giarratano M., Benbadis S.R. Carbamazepine-related antiepileptic drugs for the treatment of epilepsy - a comparative review. Expert Opin. Pharmacother. 2016;17(7):885–888. doi: 10.1517/14656566.2016.1168399. [DOI] [PubMed] [Google Scholar]
- 156.Kopecek M., Potmesil P. How long does the pharmacokinetic interaction between carbamazepine and quetiapine last after carbamazepine withdrawal? Neuroendocrinol. Lett. 2017;38(7):475–478. [PubMed] [Google Scholar]
- 157.Gandhi S., McArthur E., Mamdani M.M., Hackam D.G., McLachlan R.S., Weir M.A., Burneo J.G., Garg A.X. Antiepileptic drugs and hyponatremia in older adults: Two population-based cohort studies. Epilepsia. 2016;57(12):2067–2079. doi: 10.1111/epi.13593. [DOI] [PubMed] [Google Scholar]
- 158.Pulman J., Marson A.G., Hutton J.L. Tiagabine add-on for drug-resistant partial epilepsy. Cochrane Database Syst. Rev. 2012;(5):CD001908. doi: 10.1002/14651858.CD001908.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chuang S.H., Reddy D.S. Isobolographic analysis of antiseizure activity of the GABA Type A Receptor-modulating synthetic neurosteroids brexanolone and ganaxolone with tiagabine and midazolam. J. Pharmacol. Exp. Ther. 2020;372(3):285–298. doi: 10.1124/jpet.119.261735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fritz N., Glogau S., Hoffmann J., Rademacher M., Elger C.E., Helmstaedter C. Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy Behav. 2005;6(3):373–381. doi: 10.1016/j.yebeh.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 161.Sałat K., Podkowa A., Mogilski S., Zaręba P., Kulig K., Sałat R., Malikowska N., Filipek B. The effect of GABA transporter 1 (GAT1) inhibitor, tiagabine, on scopolamine-induced memory impairments in mice. Pharmacol. Rep. 2015;67(6):1155–1162. doi: 10.1016/j.pharep.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 162.Javed A., Cohen B., Detyniecki K., Hirsch L.J., Legge A., Chen B., Bazil C., Kato K., Buchsbaum R., Choi H. Rates and predictors of patient-reported cognitive side effects of antiepileptic drugs: An extended follow-up. Seizure. 2015;29:34–40. doi: 10.1016/j.seizure.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 163.Chiron C. Current therapeutic procedures in Dravet syndrome. Dev. Med. Child Neurol. 2011;53(Suppl. 2):16–18. doi: 10.1111/j.1469-8749.2011.03967.x. [DOI] [PubMed] [Google Scholar]
- 164.Quilichini P.P., Chiron C., Ben-Ari Y., Gozlan H. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA-A receptor channels. Epilepsia. 2006;47(4):704–716. doi: 10.1111/j.1528-1167.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 165.Leslie T.K., Brückner L., Chawla S., Brackenbury W.J. Inhibitory effect of eslicarbazepine acetate and S-Licarbazepine on Nav1.5 channels. Front. Pharmacol. 2020;11:555047. doi: 10.3389/fphar.2020.555047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hebeisen S., Pires N., Loureiro A.I., Bonifácio M.J., Palma N., Whyment A., Spanswick D., Soares-da-Silva P. Eslicarbazepine and the enhancement of slow inactivation of voltage-gated sodium channels: a comparison with carbamazepine, oxcarbazepine and lacosamide. Neuropharmacology. 2015;89:122–135. doi: 10.1016/j.neuropharm.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 167.Doeser A., Dickhof G., Reitze M., Uebachs M., Schaub C., Pires N.M., Bonifácio M.J., Soares-da-Silva P., Beck H. Targeting pharmacoresistant epilepsy and epileptogenesis with a dual-purpose antiepileptic drug. Brain. 2015;138(Pt 2):371–387. doi: 10.1093/brain/awu339. [DOI] [PubMed] [Google Scholar]
- 168.Weissinger F., Losch F., Winter Y., Brecht S., Lendemans D., Kockelmann E. Effectiveness of eslicarbazepine acetate in dependency of baseline anticonvulsant therapy: Results from a German prospective multicenter clinical practice study. Epilepsy Behav, . 2019;101(Pt A):106574. doi: 10.1016/j.yebeh.2019.106574. [DOI] [PubMed] [Google Scholar]
- 169.Intravooth T., Staack A.M., Juerges K., Stockinger J., Steinhoff B.J. Antiepileptic drugs-induced hyponatremia: Review and analysis of 560 hospitalized patients. Epilepsy Res. 2018;143:7–10. doi: 10.1016/j.eplepsyres.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 170.Lawthom C., Bermejo P., Campos D., McMurray R., Villanueva V. Effectiveness and safety/tolerability of eslicarbazepine acetate in epilepsy patients Aged ≥ 60 Versus < 60 Years: A subanalysis from the euro-esli study. Neurol. Ther. 2019;8(2):491–504. doi: 10.1007/s40120-019-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ferlazzo E., Sueri C., Gasparini S., Aguglia U. Challenges in the pharmacological management of epilepsy and its causes in the elderly. Pharmacol. Res. 2016;106:21–26. doi: 10.1016/j.phrs.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 172.Latimer D.R., Edinoff A.N., Ruff R.D., Rooney K.C., Penny K.M., Patel S.B., Sabbenahalli S., Kaye A.M., Cornett E.M., Viswanath O., Urits I., Kaye A.D. Cenobamate, a sodium channel inhibitor and positive allosteric modulator of GABAA Ion Channels, for partial onset seizures in adults: A comprehensive review and clinical implications. Neurol. Int. 2021;13(2):252–265. doi: 10.3390/neurolint13020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lattanzi S., Trinka E., Zaccara G., Striano P., Del Giovane C., Silvestrini M., Brigo F. Adjunctive cenobamate for focal-onset seizures in adults: A systematic review and meta-analysis. CNS Drugs. 2020;34(11):1105–1120. doi: 10.1007/s40263-020-00759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schimpf R., Veltmann C., Papavassiliu T., Rudic B., Göksu T., Kuschyk J., Wolpert C., Antzelevitch C., Ebner A., Borggrefe M., Brandt C. Drug-induced QT-interval shortening following antiepileptic treatment with oral rufinamide. Heart Rhythm. 2012;9(5):776–781. doi: 10.1016/j.hrthm.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]