Abstract
Depression, a well-known mental disorder, has global prevalence, affecting nearly 17% of the population. Due to various limitations of the currently available drugs, people have been adopting traditional herbal medicines to alleviate the symptoms of depression. It is notable to mention that natural products, their derivatives, and their analogs are the main sources for new drug candidates of depression. The mechanisms include interplay with γ-aminobutyric acid (GABA) receptors, serotonergic, dopaminergic noradrenergic systems, and elevation of BDNF levels. The focus of this article is to review the role of signalling molecules in depression and highlight the use of plant-derived natural compounds to counter CNS depression.
Keywords: Antidepressants, plant-derived natural compounds, CNS, neurotransmitters, BDNF, monoamines
1. INTRODUCTION
Depression is a multifactorial mental disorder [1], affecting more than 350 million people globally [2]. It is concomitant with substantial deficits of psychosocial activities and quality of life [3, 4]. Due to the heterogeneous nature of this disease, large disparities in clinical evaluation exist [5], making treatment more challenging. Its aetiology and pathophysiology are still relatively poorly elucidated [6]. Depressive disorders (mainly represented by major depressive disorder) are well-known causes of disability worldwide [7]. According to the Global Burden of Disease study, depression is counted at 4th position in causing disability worldwide, and it is projected to be the 2nd by 2020 [8]. Though there are well-acknowledged chemotherapeutic agents available for mental disorders, a considerable percentage of patients from underdeveloped and developing nations receive no treatment for their disorders due to cost burden, improper counselling, and social stigma regarding depression [9].
Multiple research investigations have revealed imperative insights on various levels of depression, linking it with genetic abnormalities [10], imbalance in levels of neurotransmitters [11], defects in brain anatomy [12], and cognitive deficits [13], so pharmaceutical industries consider high-risk in developing therapeutic agents for depressive disorders. Thus it becomes imperative to look for novel therapies based on natural products [14]. Before considering any novel therapeutic compounds, treatment-resistant depression, slower onset of action, risk of adverse effects, and substance abuse of these compounds must be ascertained, which are major limitations with the use of current pharmacotherapy [15]. Regardless of the fact that drug design and discovery have a high reliance on synthetic chemistry, the contribution of natural products cannot be ignored. Natural products are a potential source of drugs for nervous system-related disorders, cancer, endocrine disorders, etc. [16-25]. WHO list of essential drugs consists of 252 drugs, of which 11% are of plant origin [26]. So there is an absolute chance of finding a natural molecule having desired antidepressant activity. The natural heterocycles and cyclic hydrocarbons (sources include flavonoids, glycosides, alkaloids, terpenes, and saponins from plant sources may instill positive change which researchers are looking for, as they possess unique chemical diversity, even some of these cannot be synthesized by currently known methods. As a result, these natural compounds as novel drug molecules for depression remain untapped. Different scientific reports have focused on validating the supposedly psychoactive phytoconstituents from various medicinal plants, facilitating isolation of bioactive phytoconstituents acting on CNS as an antidepressant. Scientific investigations claiming various phytochemicals as ameliorative agents in depression are limited. However, some key findings have demonstrated that flavonoids, alkaloids, glycosides, terpenes, and saponins isolated from various medicinal plants exhibit antidepressant activity. In this review, we have discussed the potential of various heterocycles and cyclic compounds of plant origin to treat depression. This review will try to understand the research gap in depression, the mechanism of action of selected cyclic and heterocyclic molecules, and in vivo and in vitro activities of these phytoconstituents will also be covered.
2. NEUROTRANSMITTERS AND INTRACELLULAR SIGNALLING MOLECULES OF DEPRESSION
Among the signalling molecules related to depression, significant variation is well established, which can clarify the complexity of the condition. Much evidence has been gained from experiments in animal studies at the cellular and molecular levels regarding depressive conditions.
2.1. Glutamatergic Signaling in Depression
Mounting data indicate that MDD abnormally impacts the glutamatergic system. Among MDD patients, levels of glutamate are elevated in the cerebrospinal fluid, including brain tissue, so antidepressant therapy counteracts this mechanism [27]. In the post-mortem brain, the expression of receptors of glutamate is often atypical [27]. Significantly, NMDA receptor antagonists show strong therapeutic outcomes in patients suffering from depression [28]. Therefore, it can be outlined that in all experimental animals, atypical glutamate receptor expression and downstream signalling are correlated with depression. NMDA also prevents the development of microtubules [29], indicating that the stabilization of microtubule polymers leads to improvements in dendritic morphology and spine development/atrophy. Post-synaptic density protein 95 (PSD95) decreases in PFC in MDD patients, indicating synaptic impairment [30] and a decrease in MAP2 levels [31, 32].
2.2. GSK-3 Signaling and Depression
Various studies confirm that GSK-3b induces neuronal cell death responses [33, 34]. SSRI-dependent antidepressants block GSK-3 action through serine 9 phosphorylation. This occurs via 5-HT1A receptors [35]. Various investigations on different models of depression indicate that pharmacological regulation of GSK-3 expression produces antidepressant effects [36]. B-catenin, a GSK-3 substrate, undergoes proteasomal degradation of B-catenin following phosphorylation [37]. In comparison, cytosolic levels of β-catenin are raised due to inhibition of GSK-3, offering many advantages for cell survival and protection. GSK-3 also regulates neurogenesis in the hippocampal region. Thus it plays a role in depression as it prevents neurogenesis in the hippocampal region [38, 39]. Ironically, DISC-1 binds directly to GSK-3b, limiting its activity, which in turn can lead to modulation of different cellular processes. GSK-3b is blocked by DISC-1 directly. DISC-1 also reverses mice’s depressive actions, as did the GSK-3 pharmacological inhibition [40]. Importantly, GSK-3b SNPs were related to brain structural changes and human depression [41]. GSK-3 inhibition also generates its therapeutic results by deregulating the trafficking of AMPA receptors [42]. This is accomplished by GSK-3b light chain kinesin phosphorylation, which dissociates AMPA receptor cargo (GluR1) [43]. This research also found that a peptide caused antidepressant-like behaviour in mice. Lastly, as per various shreds of evidence, GSK-3 has been found to epigenetically regulate depression [44, 45].
2.3. Wnt Signaling and Depression
Wnts are secreted glycoproteins that act to negatively regulate GSK-3 through the canonical Wnt pathway. Although Wnts play a vital role in growth, they also control neuroplasticity [46-48]. Wnts bind to Fzd receptors, inhibiting GSK-3 by stimulating dishevelled phosphoproteins [49, 50]. Wnt-2 is elevated in many antidepressant procedures, including continuous electroconvulsive treatment in animal models, and in the case of antidepressant treatment, Wnt-2 expression is increased in the hippocampus, and hippocampal induction of Wnt-2 also reduces antidepressant action [51]. Wnt3a expression is also improved in rats after SSRI therapy, in which adult hippocampal neurogenesis is stimulated [52]. Subtypes of Fzd have previously been used in addiction and psychiatric treatments. The viral-mediated hippocampal knock-down of Fzd6 reportedly evokes anhedonic behaviour and heightened fear [53]. Throughout a post-mortem analysis of suicidal cases and animal models of relevance, DLV2 mRNA expressions were substantially reduced in the mouse nucleus accumbens [54]. Such studies strongly link disrupted Wnt signalling with action that is close to depression.
2.4. CREB and Depression
CREB has a role in controlling transcription that activates many signalling events that are important in depression pathology. A review of 26 suicidal victims showed a drop in the expression of CREB in the hippocampal region [55]. Likewise, the CREB function was found to decrease in animal distress experiments [56, 57]. In line with this, CREB rates in the hippocampus are increased after the administration of fluoxetine, which contributes to augmented BDNF production [58-60].In comparison, CREB gets triggered by protein kinase A, which activates in reaction to persistent antidepressant therapy [61], although other kinases activate CREB in a stimulus-reliant manner [61-63]. The antidepressant activity at CREB is therefore hardly unexpected. CREB binds gene targets in the nucleus for controlling neuro-plasticity, cell viability, and cognition [60]. Importantly, BDNF is one of such targets, which might prove beneficial in depressive disorders. Thus disruptions of signalling processes that arise due to depression are distinctly complex. Targeting such signalling proteins provides a vision to develop newer therapies.
2.5. BDNF and Depression
Various neurotrophic factors, particularly researched in association with BDNF, have revealed its extensive connection with depressive disorders. Depressive patients have reduced serum BDNF levels [64, 65]. BDNF mRNA in rodents declines in the hippocampus subjected to stress [66, 67] and PFC [68]. BDNF is also down-regulated after corticosterone therapy [69]. On the other side, BDNF has also been reported to increase after induction of stress [70, 71] and is hypothesized to act as a tool to defend against the consequences of potential stressful incidents. Similarly, the systematic depletion of BDNF in the dentate gyrus decreased the effectiveness of antidepressants [72], and the hippocampal knock-down of BDNF caused anhedonia in rodents [73]. Studies have also confirmed that BDNF also modulates the production of numerous important synaptic proteins [74]. A single variation in the nucleotide sequence of BDNF imparts the vulnerability of humans and rodents towards depressive disorders [75]. Due to compromised dendritic transport [75, 76], this modification results in decreased levels of BDNF. BDNF post-synaptic release triggers presynaptic Rab3a expression [77], which has been found to be downregulated in patients suffering from depression [78]. Impaired BDNF control is inextricably linked to depression. As mentioned in this segment, BDNF activates many main signaling mechanisms that have been shown to be important in animal models for antidepressant responses.
3. UNDERSTANDING RESEARCH GAP TO UNVEIL FUTURE ANTIDEPRESSANTS
The current antidepressant medications are far away from being ideal. The development of newer antidepressant therapeutic compounds has trickled down in the recent past due to the high failure rate of drug development for psychiatric illnesses, so it is important to understand incompetency in antidepressant research. Although it is apparent that antidepressants have significant short- and long-term effects, it is often obvious that complications exist, such as addiction, toxicity, reduced effectiveness over time, relapse, and recurrence concerns. In depression, various neuroscientific abnormalities oscillate from low or high levels of neurotransmitter molecules, impaired brain cells, and cognitive decline. Three theoretical lines of future research investigation may be considered: creating a comprehensive neuroscientific depression model that offers mechanistic linkages between symptoms and the outcomes of antidepressant interventions. The continuation of the search for aetiological and pathophysiological factors involved in depression development. A greater emphasis on translational initiatives to strengthen clinical practice and work utilizing proven fundamental neuroscientific insights. Another relevant aspect is the theoretical problem as to why antidepressants have reduced effectiveness; it is because they work through raising monoamine levels, whereas lower concentrations of these neurotransmitters are not always encountered by individuals with depression. Following the ingestion of antidepressants, monoamine levels improve significantly; however, a therapeutic delay is common. Eventual neurophysiological improvements such as differential activation of monoaminergic receptor rates and downstream intracellular impact on metabotropic enzyme cascades and eventual adjustments to the nuclear transcription of proteins may tend to modulate the therapeutic results.
It is now obvious that other neuromodulators have a role in depression, so there are pretty close chances that current therapeutic targets may be insufficient in producing therapeutic efficacy. So it is thought that an in-depth understanding of molecular targets may culminate the problems like delayed onset, habituation, and adverse drug reactions. Hence depression is considered a multifactorial disease, which requires a multidimensional treatment approach, including psychopharmacological intervention.
Better results can be achieved with faster-acting effective antidepressants. To achieve this reality, different approaches can be applied with the help of advanced tools of genetic and neuroscience. Still, again, it is difficult to predict the outcome of modern health technologies. So the present drug design essentially needs to be faster acting, much efficacious, least complex for physicians to conduct, tolerable by patients, cheap for healthcare providers, otherwise future antidepressants will have a partial impact. All such qualities may be fulfilled by plant-derived drug molecules subjected to experimentation on animal and human subjects.
4. NATURE-INSPIRED COMPOUNDS AS AN EMERGING SOURCE FOR THE TREATMENT OF DEPRESSION
The existence of a wide variety of phytochemicals in medicinal plants is known for their therapeutic potential in the ministration of several diseases, including brain-associated disorders [79-84]. Herbal medicines including saffron, Rhodiola, lavender as well as Echium are used for the treatment of depression. From the last decade, various phytochemical entities with strong anti-depressant activity have been disclosed from various herbal medicines. Constituents that have been well characterized and are under focus on animal models followed by other studies are reviewed here.
4.1. Flavonoids
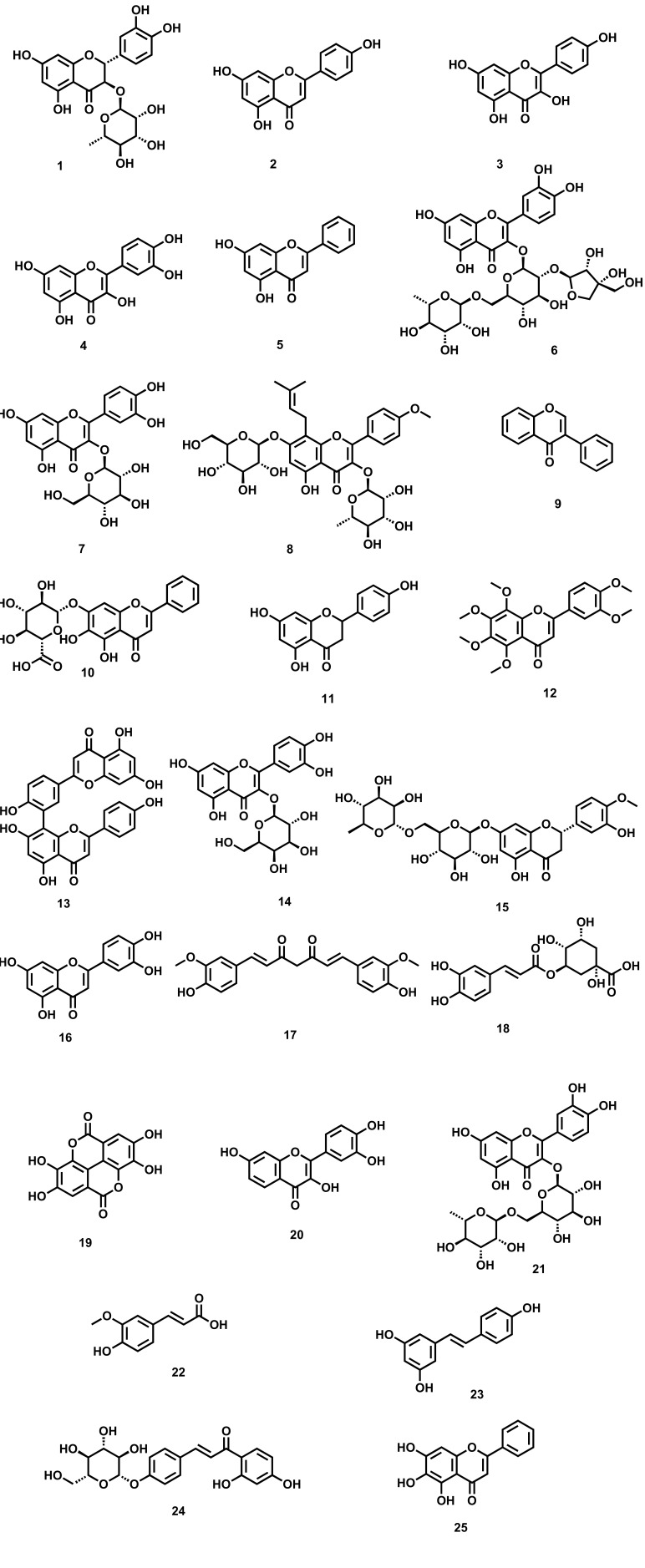
Polyphenols represent a class of compounds that are found ubiquitously in nature [85]. Due to their significant antioxidant potential, they are widely used as alternative medicine in the assistance of various CNS-related disorders [86]. The basic structure of Phenolic compounds consists of an aromatic ring with various hydroxyl groups. Based on the structure, they are categorized into flavonoids, stilbenes, lignans, phenolic acids, and coumarins [87, 88]. Among all, flavonoids represent an important class of phenolic, which is further categorized into isoflavones, anthocyanins, 4-isoflavonoids, and flavan-3-of derivatives [89]. To date, more than 6000 flavonoids have been reported exhibiting diverse pharmacological activities [90-92]. The antidepressant activity of flavonoids isolated from various natural sources has been confirmed in various in-vitro and in-vivo animal studies involving attenuating the levels of various neurotransmitters like 5-HT, NA and DA, and (5-HIAA) besides the balancing of various receptors [93-95]. There are several flavonoids isolated from plants (Fig. 1) which are having outstanding preclinical effects against depression, supporting evaluation of their clinical profile as well [96]. Moreover, various epidemiological evidence support that a diet containing an adequate amount of flavonoids helps in augmenting depression [97-101]. The various plant-derived flavonoids [102], along with their diversified mechanistic cognizance to exhibit antidepressant-like effects, are shown in Table 1.
Fig. (1).
Plant-derived flavonoids against depression (1) Astilbin (2) Apigenin (3) Kaempferol (4) Quercetin (5) Chrysin (6) Quercetin-3-O-apiosy1 (1 → 2)-rhamnosy1 (1 → 6) glucoside (7) Isoquercetrin (8) Icariin (9) Isoflavone (10) Baicalin (11) Naringenin (12) Nobiletin (13) Amentoflavone (14) Hyperoside (15) Hesperidin (16) Luteolin (17) Curcumin (18) Chlorogenic acid (19) Ellagic acid (20) Fisetin (21) Rutin (22) Ferulic acid (23) Resveratrol (24) Isoliquiritin (25) Baicalein.
Table 1.
Plant-derived flavonoids against depression.
| Constituent | Type of Study Cellular/ Animal/ Clinical | Description of Study along with Doses | Mechanism | Refs. |
|---|---|---|---|---|
| Astilbin | CUMS model of depression in mice | Immobility time is significantly reduced at doses 10, 20, and 40 mg/kg (i.p.) without affecting locomotor activity | Activates BDNF signalling pathway | [103] |
| Apigenin | FST in mice | Duration of immobility time is greatly reduced at doses 12.5 and 25 mg/kg (i.p.). | Inhibitory activation on Monoamine oxidase | [104-106] |
| Kaempferol | FST and TST model | Reduces the immobility time at doses 30 mg/kg (o.p) in the FST and TST | Inhibitory activity on Monoamine oxidase | [104, 107] |
| Quercetin | FST model | A decrease in immobility time in comparison to imipramine (15 mg/kg, i.p) | MAO-A inhibition | [108] |
| Chrysin | Brain cells of rats | Suppressing MAO-A relating to an antidepressant-like effect | Inhibitory activity on Monoamine oxidase | [104] |
| Quercetin-3-O-apiosy1 (1 → 2)-rhamnosy1 (1 → 6) glucoside |
PC12 nerve cells | Prevents nerve damage to rat adrenal medulla | Nerve cell protection | [109] |
| Isoquercetrin | FST in rats | Decreases the immobility time at doses 30, 60, and 125 mg/kg similar to imipramine (20 mg/kg) | Inhibition of MAO-B | [110] |
| Icariin | CUMS model of depression in rats | Enhances antioxidant and anti-inflammatory activity at doses (20 or 40 mg/kg) | Acting on (HPA) axis and (HPT) axis | [111] |
| Isoflavone | Postmenopausal women | Reduces depressive symptoms in postmenopausal women | Inhibitory activity on Monoamine oxidase | [112] |
| Baicalin | Murine models | Reduces immobility time in-vivo | MAO-A and B inhibition | [113] |
| Naringenin | Behavioural models of depression |
Reduces immobility time at doses 10, 20, and 50 mg/kg | Elevating NA, 5-HT, and GR levels in the hippocampus region |
[114] |
| Nobiletin | FST, TST | Signifying its potential for the treatment of depression by reducing the immobility time of mice | Acting via interplay with the 5-HT1A receptors | [115] |
| Amentoflavone | FST, TST | Reductions in the duration of immobility time | Interplay with 5-HT2 receptors | [116] |
| Hyperoside | FST | Shortened the immobility time at doses of 30, 60, and 125 mg/kg, in male rats | Elevating the expression of BDNF | [117] |
| Hesperidin | FST | Duration of immobility time gets reduced at doses 0.1, 0.3 and 1 mg/kg (i.p) | Interplay with the 5-HT (1A) receptors | [118, 119] |
| Luteolin | FST, TST | A dose of 50 mg/kg/ reduces immobility time | Enhance GABA A Receptor Cl ion channel complex. | [120] |
| Curcumin | FST, TST, and various other animal models | Curcumin was active in mouse FST and TST at doses 5 and 10 mg/kg | Inhibition of MAO | [121, 122] |
| Chlorogenic acid | TST and FST | When tested in mouse FST and TST, it exhibits antidepressant-like activity | Unclear | [123] |
| Ellagic acid | FST, TST | Ellagic acid reduces immobility period in both FST and TST comparable with fluoxetine at doses 25-100 mg/kg | Acting on adrenergic, serotoninergic, and NO system | [124] |
| Fisetin | FST, TST | Immobility time in FST and TST gets reduced at doses (10 and 20 mg/kg, p.o.) | Inhibition of MAO-A only | [125] |
| Rutin | FST, TST | Immobility time in TST gets reduced at doses (0.3-3.0 mg/kg, p.o.) | Acting via Serotoninergic, noradrenergic pathways | [126] |
| Ferulic acid | FST, TST | (0.01,10 mg/kg, p.o.) | Acting through the Serotoninergic pathway | [127] |
| Resveratrol | FST, TST | Reduces the immobility period in mouse models of FST and TST at doses (20-80 mg/kg, p.o) | Inhibition of MAO-A | [128] |
| Isoliquiritin | FST, TST | Immobility time in FST and TST gets reduced at doses 10, 20, and 40 mg/kg | Raising 5-HT and NE levels | [129] |
| Baicalein | FST, TST | 1, 2 and 4 mg/kg (i.p.) | Increase in BDNF level | [130] |
4.2. Glycosides
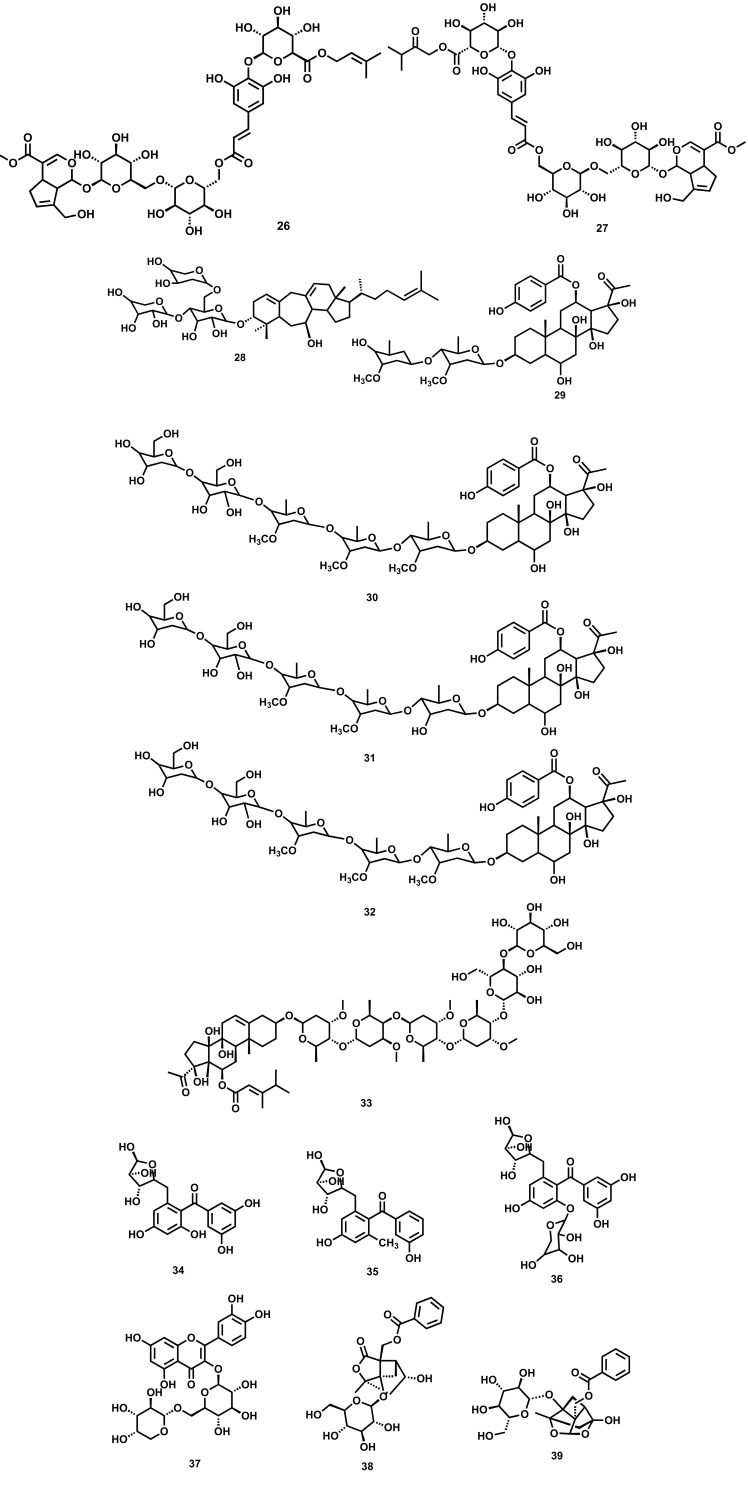
Glycosides are a class of compounds containing a sugar molecule (glycone) attached via a glycosidic linkage to an anomeric carbon of a non-sugar moiety (aglycone). Glycosides as such are various types in nature based on aglycone moiety, including alcoholic, cardiac, steviol, chromone, flavonoid, anthraquinone, coumarin, cyanogenic, iridoid, phenolic, saponins, etc. Glycosides are known for their various pharmacological activities, including antidiabetic, anticancer, antithrombotic, antifungal, analgesic, antiviral, antioxidant, and cardioprotective activity. It has been reported that glycosides isolated from various plant extracts (Fig. 2) exhibit anti-depressant activity mainly by upregulating the level of BDNF in the hippocampus region, which results in promoting synaptic efficacy connectivity of neurons and neuroplasticity. The various plant-derived glycosides along with their diversified mechanistic cognizance to exhibit antidepressant-like effects are shown in Table 2.
Fig. (2).
Plant-derived glycosides against depression (26) Rotunduside G (27) Rotunduside H (28) Cyprotusides B (29) Cynanauriculoside C (30) Cynanauriculoside D (31) Cynanauriculoside E (32) Otophylloside L (33) Cynauricuoside C (34) 3/,4,5/- trihydroxy-6-methoxy-2-O-α-L-arabinosyl benzophenone (35) 3/,4,5/,6-tetrahydroxy-2-O-α-L-arabinosyl benzophenone (36) 3,4-dihydroxy-5-methoxy-2-O-α-L-arabinosyl-6-O-β-D- xylosyl benzophenone (37) Quercetin-3-O-α-L-arabino furanoside (38) Albiflorin (39) paeoniflorin.
Table 2.
Plant-derived glycosides against depression.
| Constituents |
Type of Study Cellular/
Animal/Clinical |
Description of Study along with Doses | Mechanism | Refs. |
|---|---|---|---|---|
| Rotunduside G | FST, TST | At a dose of 50 mg/kg (i.g.), Rotunduside G shows the antidepressant effect | - | [131] |
| Rotunduside H | FST, TST | At the dosage of 50 mg/kg (i.g) | - | [131] |
| Cyprotusides B | In-vivo depression models | Cyprotusides B displayed marked antidepressant activity in the despair mice models | - | [132] |
| Cynanauriculoside C | In-vivo depression models | Exhibits antidepressant activity at the doses of 50 mg/kg (i.g) | - | [133] |
| Cynanauriculoside D | In-vivo depression models | Showed antidepressant activity at doses of 50 mg/kg (i.g) in comparison to fluoxetine (20 mg/kg) | - | [133] |
| Cynanauriculoside E | FST, TST | Exhibits significant antidepressant activity at the doses of 50 mg/kg (i.g) | - | [133] |
| Otophylloside L | FST, TST | At 50 mg/kg doses, It shows its effect in FST and TST | - | [133] |
| Cynauricuoside C | FST, TST | At 50 mg/kg doses, it shows its effect in FST and TST | - | [133] |
| 3/,4,5/-trihydroxy-6-methoxy-2-O-α-L-arabinosyl benzophenone | MOA Inhibition assay | The MAO-A inhibition by these compounds was 310.3 mM, which is comparable with clorgyline 0.5 mM. | Inhibition of MAO-A | [134] |
| 3/,4,5/,6-tetrahydroxy-2-O-α-L-arabinosyl benzophenone | MOA Inhibition assay | The MAO-A inhibition by these compounds was 111.2 mM, which is comparable with clorgyline 0.5 mM. | Inhibition of MAO-A | [134] |
| 3,4-dihydroxy-5-methoxy-2-O-α-L-arabinosyl-6-O-β-D-xylosyl benzophenone | MOA Inhibition assay | The MAO-A inhibition by these compounds was 726.0 mM, which is comparable with clorgyline 0.5 mM. | Inhibition of MAO-A | [134] |
| Quercetin-3-O-α-L-arabino furanoside | MOA Inhibition assay | The MAO-A inhibition by these compounds was 534.1 mM, which is comparable with clorgyline 0.5 mM. | Inhibition of MAO-A | [134] |
| Albiflorin | FST, TST | Reduction in immobility time in FST and TST at doses 3.5, 7.0 and 14.0 mg/kg | Upregulation of hippocampal BDNF expression | [135] |
| Paeoniflorin | CUMS | Decreased ACTH levels in the CUS-treated rats. | Upregulating Noradrenergic receptors | [136] |
| Rotunduside F | Despair mice models | At a dosage of 50 mg/kg, it shows antidepressant activity comparable to fluoxetine (20 mg/kg) | Similar to fluoxetine (20 mg/kg) | [137] |
4.3. Alkaloids
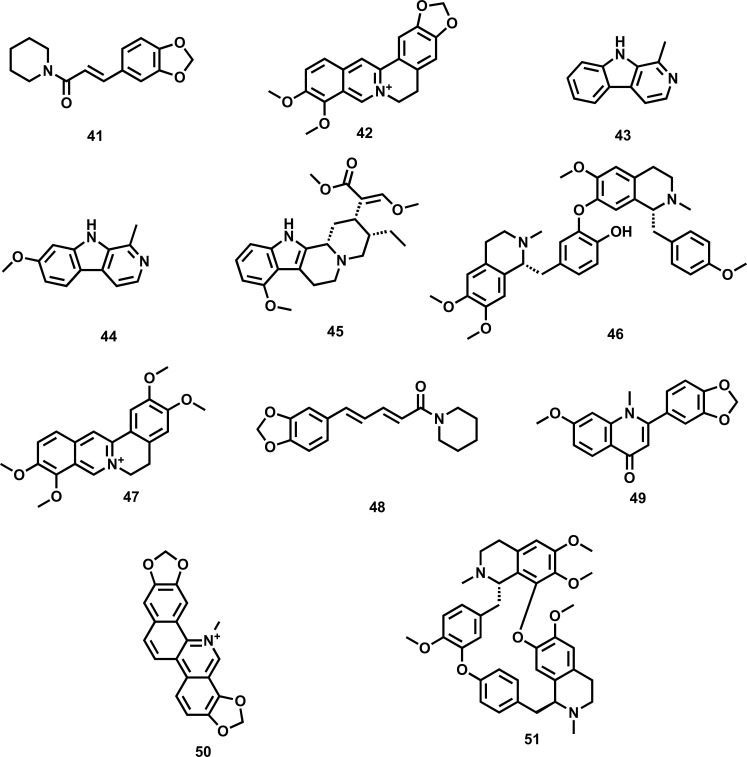
Alkaloids, which are plant secondary metabolites that contain nitrogen in the heterocyclic ring, are known for their wide array of pharmacological activities, including anti-inflammatory, tranquillizer, and antiarthritic. Alkaloids such as harmine, nonharmane, and harmane are reported to exhibit anti-depressant activity (Fig. 3). Besides, harmine also reduces the serum level of ACTH. It has been reported that in animal models of depression, diterpene alkaloids isolated from Aconitum baicalense enhance serotonergic activity. Berberine, a vital plant alkaloid, has been reported to reduce the various symptoms of depression by acting on multiple pathways, including serotonergic, noradrenergic, dopaminergic, L-arginine-NO-cGMP, as well as δ opioid receptor pathway. Piperine isolated from piper longum has been reported to exhibit anti-depressant activity via repression of MAO enzymes, elevating brain 5-HT and BDNF levels, and regulating the HPA axis. The various plant-derived alkaloids along with their diversified mechanistic cognizance to exhibit antidepressant-like effects are shown in Table 3.
Fig. (3).
Plant-derived alkaloids against depression (41) Antiepilepsirine (42) Berberine (43) Harmane (44) Harmine (45) Mitragynine (46) Neferine (47) Palmatine (48) Piperine (49) Punarnavine (50) Sanguinarine (51) Tetrandrine.
Table 3.
Plant-derived alkaloids against depression.
| Constituents |
Type of StudyCellular/
Animal/ Clinical |
Description of Study along with Doses | Mechanism | Refs. |
|---|---|---|---|---|
| Antiepilepsirine | FST, TST | Reduces the immobility time in both FST and TST, at doses 10-20 mg/kg | A decline in DA and 5-HT only |
[138] |
| Berberine | FST, TST | Berberine at a dose of (20 mg/kg, p.o.) elevates 5-HT levels in the frontal cortex and hippocampus | Suppression of MAO-A and B activity | [139] |
| Harmane | FST | Harmane reduces the time of immobility at doses 5-15 mg/kg, i.p. | Acting as Bzd inverse agonist | [140] |
| Harmine | FST UCMS |
Harmine for seven days reverse anhedonia at doses 15 mg/kg | Acting as Bzd inverse agonist | [141] |
| Mitragynine | FST, TST | Significantly reduce the immobility time at doses 10 mg/kg and 30 mg/kg i.p in FST and TST | Acting on HPA axis | [142] |
| Neferine | FST | Compared to imipramine, elicited anti-immobility effects in mice at doses 25 to 100 mg/kg i.p | Acting on HT1a receptor | [143] |
| Palmatine | TST | Compared to fluoxetine (10 mg/kg), it decreases immobility time in unstressed tail suspension test at doses 0.5 and 1 mg/kg | A decrease in MAO-A activity | [144] |
| Piperine | FST, TST SCP |
At doses 2.5, 5, and 10 mg/kg, it reverses plasma corticosterone level and open field activity | Elevating 5-HT but not NE and DA | [145] |
| Punarnavine | FST | At doses 20 and 40 mg/kg, it decreases immobility periods in a forced swim test | A decrease in MAO-A activity | [146] |
| Sanguinarine | FST | SA (2.5, 5, or 10 μg/0.5 μl per side) reduces immobility time in a dose-dependent manner | A decrease in expression of Mkp-1 | [147] |
| Tetrandrine | FST, TST SPT |
Reduces immobility time in both the FST and TST | Increase in 5-HT and NE | [148] |
4.4. Terpenes
Terpenes are a class of phytochemicals that contain an isoprene unit as theirbasic unit and are widely known for their pharmacological activity, including antibacterial, antifungal, antiviral, anticancer, antimalarial, and anti-inflammatory. Some of the terpenes exhibit antidepressant activity involving dopamine D1 and D2 receptors without interacting with noradrenergic receptors. Some terpenes are known to show anti-depressant activity (Fig. 4) by raising the level of 5-HT and norepinephrine levels in the brain. Moreover, the BDNF/TrkB signalling pathway in the cortex region, κ-opioid and endocannabinoid receptors, and elevation of brain monoamine levels are other possible pathways by which terpenes are reported to exhibit anti-depressant activity. The various plant-derived terpenes, along with their diversified mechanistic cognizance to exhibit antidepressant-like effects, are shown in Table 4.
Fig. (4).
Plant-derived terpenes against depression (52) Podoandin (53) Cannabidiol (54) Carvacrol (55) β-Caryophyllene (56) Genipin (57) Hyperforin (58) Salvinorin A (59) Ursolic acid.
Table 4.
Plant-derived terpenes against depression.
| Constituents |
Type of Study Cellular/
Animal/ Clinical |
Description of Study along with Doses | Mechanism | Refs. |
|---|---|---|---|---|
| Podoandin | TST and FST | Reduces the immobility time in FST at doses 10 mg/kg | Acting on HT1a, 5-HT3, dopamine D2 receptors | [149] |
| Cannabidiol | TST and FST | At a dose of 30 mg.kg−1, it induces dose-dependent antidepressant-like effects | Acting on 5-HT1a receptor activation | [150, 151] |
| Carvacrol | FST | Elevate dopamine and serotonin levels at doses 12.5 mg/kg | Action on dopamine D1 and D2 receptors | [152, 153] |
| β-Caryophyllene | TST and FST | Mitigates the stress-related changes in the hippocampus region at doses 25, 50, 100 mg/kg | Action on cannabinoid receptor subtype 2 | [154, 155] |
| Genipin | CUMS model | Elevates the levels of 5-HT, NE in CUMS-induced depressive rats | Elevates 5-HT and NE level | [156] |
| Hyperforin | In-vivo and in-vitro methods were employed | Enhancing BDNF expression in the frontal cortex | Acting on the BDNF/TrkB pathway in the cortex | [157] |
| Salvinorin A | Chronic mild stress (CMS) | Animals treated with Salv A 1 mg /kg reversed anhedonia | Involvement of κ-opioid and endocannabinoid receptors | [158] |
| Ursolic acid | TST and FST in mice | Immobility time gets reduced in FST (10 mg/kg) and in TST (0.01 and 0.1 mg/kg) in comparison to fluoxetine 10 mg/kg | Dopamine D1 and D2 receptors | [159, 160] |
4.5. Saponins
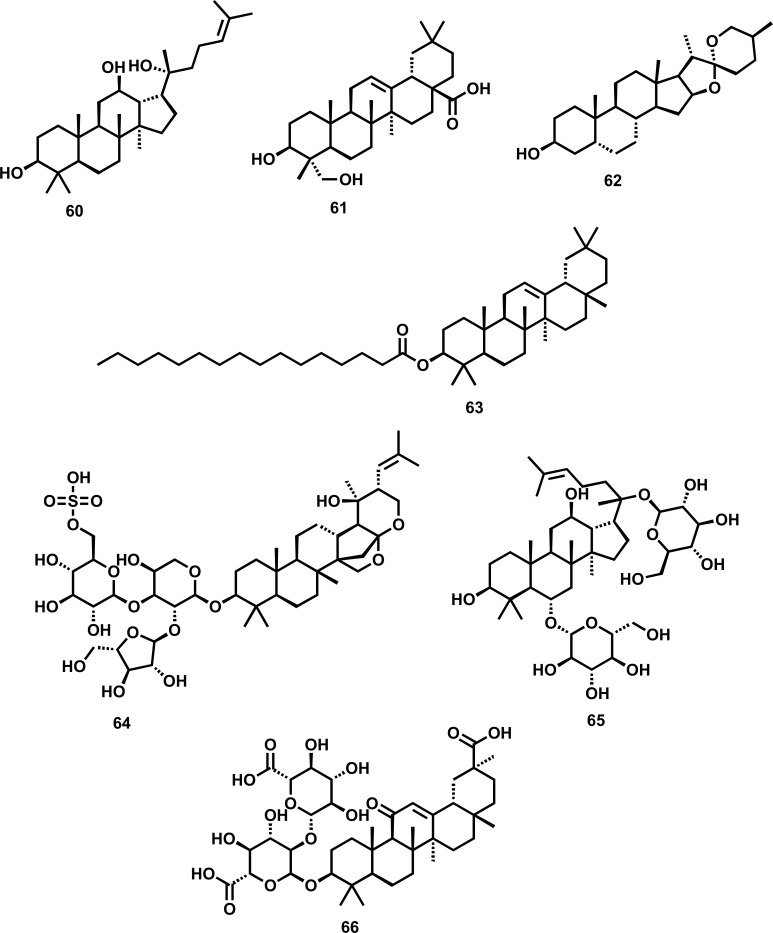
Saponins are a class of compounds known for their foaming ability due to their amphiphilic nature containing a hydrophilic glycone part (sugars/glycosides) and hydrophobic aglycone part (sapogenins). Based on structure, these are classified into steroidal and triterpenoid saponins exhibiting various pharmacological activities, including anti-inflammatory, antimicrobial, and cytotoxic activity. Recently, it has been reported that saponins (Fig. 5) impart a important role in the cure of clinical depression by modulating the various pathways involved in the onset of depression. The various plant-derived glycosides along with their diversified mechanistic cognizance to exhibit antidepressant-like effects are shown in Table 5.
Fig. (5).
Plant-derived saponins against depression (60) Protopanaxadiol (61) Hederagenin (62) Sarsasapogenin (63) β-Amyrin palmitate (64) Bacopaside I (65) Ginsenoside RG I (66) Glycyrrhizin.
Table 5.
Plant-derived saponins against depression.
| Constituents | Type of Study Cellular/Animal/ Clinical | Description of Study along with Doses | Mechanism | Refs. |
|---|---|---|---|---|
| Protopanaxadiol | CSDS-induced depression model | PPD ameliorated behavioural alterations associated with CSDS-induced depression | Elevating 5-HT and NE levels | [161, 162] |
| Hederagenin | By in-vitro method in corticosterone-induced cytotoxicity on PC12 cells In vivo by TST and FST |
Immobility time was reduced by HG (10 mg/kg) in TST after single administration after two consecutive weeks of administration | A decrease in Serum CRF and corticosterone | [163, 164] |
| Sarsasapogenin | Evaluation of cholinergic signalling | Treatment with sarsasapogenin alleviated abnormal cholinergic signalling | Decreases MAO-A and B activity |
[165, 166] |
| β-Amyrin palmitate | FST and TST | Reduction in immobility time of FST and TST model | Mechanisms involving MAO and GABA in the hippocampus. | [167] |
| Constituents | Type of Study Cellular/Animal/ Clinical | Description of Study along with Doses | Mechanism | Refs. |
| Bacopaside I | Shuttle-box escape test, FST, and TST | Measuring the plasma level of corticosterone | MAO-A and MAO-B activity | [168] |
| Ginsenoside RG I | (CUMS)- model | Alleviation of the overexpression of proinflammatory cytokines | Suppression of Glial Activation | [169, 170] |
| Glycyrrhizin | TST and FST | Reduce immobility time of mice in FST and TST model | Acting on α1 adrenoceptor and dopamine D2 receptor | [171] |
CONCLUSION
Depression is a growing psychiatric disorder globally and requires immediate medical attention. Though diverse pharmacotherapeutics are used in treating depression, unfortunately, none of them seems to be felicitous. Strong evidence from different scientific studies support the idea of plant-derived phytochemicals that may offer newer therapeutic tools against depression due to multi-target effects, cost-effectiveness, easy availability, fewer side effects than synthetic prescription drugs. However, to assess the safety and potency of phytochemical with prospective antidepressant activities is also necessary. The current review discusses the available phytochemicals, including curcumin, Berberine, ginsenosides, and naringenin, amid the most studied isolated phytochemicals with antidepressant activity. Furthermore, clinical studies are also essential to confirm the efficacy and safety of these phytochemicals as natural antidepressants. Overall, these data underline the importance to test the tolerability and efficacy of natural products to ameliorate the symptoms or disease progression in depression in the context of controlled clinical trials
ACKNOWLEDGEMENTS
The authors acknowledge the assistance of the University of Kashmir in this research.
LIST OF ABBREVIATIONS
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazole Propionic Acid Receptor
- BDNF
Brain-derived Neurotrophic Factor
- CREB
cAMP Response Element-binding protein
- CSDS
Chronic Social Defeat Stress
- CUMS
Chronic Unpredictable Mild Stress
- DA
Dopamine
- DISC1
Disrupted in Schizophrenia 1
- DLV2
Disheveled-2
- FST
Forced Swim Test
- Fzd
Frizzled Receptors
- GSK
Glycogen Synthase Kinase
- 5-HIAA
5-Hydroxyindoleacetic Acid
- 5HT
5-Hydroxytryptamine
- MDD
Major Depressive Disorder
- mRNA
Messenger RNA
- NA
Noradrenaline
- NMDA
N-methyl-D-aspartate
- PFC
Prefrontal Cortex
- SPT
Sucrose Preference Test
- TST
Tail Suspension Test
- UCMS
Unpredictable Chronic Mild Stress
- Wnt
Wingless-related Integration Site
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Gonda X., Hullam G., Antal P., Eszlari N., Petschner P., Hökfelt T.G., Anderson I.M., Deakin J.F.W., Juhasz G., Bagdy G. Significance of risk polymorphisms for depression depends on stress exposure. Sci. Rep. 2018;8(1):3946. doi: 10.1038/s41598-018-22221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Summergrad P. Investing in global mental health: the time for action is now. Lancet Psychiatry. 2016;3(5):390–391. doi: 10.1016/S2215-0366(16)30031-1. [DOI] [PubMed] [Google Scholar]
- 3.Cho Y., Lee J.K., Kim D.H., Park J.H., Choi M., Kim H.J., Nam M.J., Lee K.U., Han K., Park Y.G. Factors associated with quality of life in patients with depression: A nationwide population-based study. PLoS One. 2019;14(7):e0219455. doi: 10.1371/journal.pone.0219455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greer T.L., Trombello J.M., Rethorst C.D., Carmody T.J., Jha M.K., Liao A., Grannemann B.D., Chambliss H.O., Church T.S., Trivedi M.H. Improvements in psychosocial functioning and health‐related quality of life following exercise augmentation in patients with treatment response but nonremitted major depressive disorder: Results from the Tread Study. Depress. Anxiety. 2016;33(9):870–881. doi: 10.1002/da.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buch A.M., Liston C. Dissecting diagnostic heterogeneity in depression by integrating neuroimaging and genetics. Neuropsychopharmacology. 2020;46(1):156–175. doi: 10.1038/s41386-020-00789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaltenboeck A., Harmer C. The neuroscience of depressive disorders: A brief review of the past and some considerations about the future. Brain Neurosci. Adv. 2018;2:2398212818799269. doi: 10.1177/2398212818799269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrich M.J. Depression is the leading cause of disability around the world. JAMA. 2017;317(15):1517–1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 8.Reddy M.S. Depression: the disorder and the burden. Indian J. Psychol. Med. 2010;32(1):1–2. doi: 10.4103/0253-7176.70510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P.S., Aguilar-Gaxiola S., Alonso J., Angermeyer M.C., Borges G., Bromet E.J., Bruffaerts R., de Girolamo G., de Graaf R., Gureje O., Haro J.M., Karam E.G., Kessler R.C., Kovess V., Lane M.C., Lee S., Levinson D., Ono Y., Petukhova M., Posada-Villa J., Seedat S., Wells J.E. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet. 2007;370(9590):841–850. doi: 10.1016/S0140-6736(07)61414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shadrina M., Bondarenko E.A., Slominsky P.A. Genetics factors in major depression disease. Front. Psychiatry. 2018;9:334. doi: 10.3389/fpsyt.2018.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan J-X., Xia J.J., Deng F.L., Liang W.W., Wu J., Yin B.M., Dong M.X., Chen J.J., Ye F., Wang H.Y., Zheng P., Xie P. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: a targeted metabolomics study. Transl. Psychiatry. 2018;8(1):130. doi: 10.1038/s41398-018-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Ma X., Li M., Liu Y., Zhang J., Huang B., Zhao L., Deng W., Li T., Ma X. Anatomical and functional brain abnormalities in unmedicated major depressive disorder. Neuropsychiatr. Dis. Treat. 2015;11:2415–2423. doi: 10.2147/NDT.S93055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam R.W., Kennedy S.H., Mclntyre R.S., Khullar A. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can. J. Psychiatry. 2014;59(12):649–654. doi: 10.1177/070674371405901206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller G. Is pharma running out of brainy ideas? American Association for the Advancement of Science. Science. 2010;329(5991):502–504. doi: 10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- 15.Cuijpers P., Stringaris A., Wolpert M. Treatment outcomes for depression: challenges and opportunities. Lancet Psychiatry. 2020;7(11):925–927. doi: 10.1016/S2215-0366(20)30036-5. [DOI] [PubMed] [Google Scholar]
- 16.Barkat M.A., Goyal A., Barkat H.A., Salauddin M., Pottoo F.H., Anwer E.T. Herbal medicine: Clinical perspective & regulatory status. Comb. Chem. High Throughput Screen. 2020;24(10):1573–1582. doi: 10.2174/1386207323999201110192942. [DOI] [PubMed] [Google Scholar]
- 17.Mir R.H., Shah A.J., Mohi-Ud-Din R., Pottoo F.H., Dar M.A., Jachak S.M., Masoodi M.H. Natural anti-inflammatory compounds as drug candidates in Alzheimer’s disease. Curr. Med. Chem. 2020;28(23):4799–4825. doi: 10.2174/0929867327666200730213215. [DOI] [PubMed] [Google Scholar]
- 18.Alhmied F., Alammar A., Alsultan B., Alshehri M., Pottoo F.H. Molecular mechanisms of thymoquinone as anticancer agent. Comb. Chem. High Throughput Screen. 2020;24(10):1644–1653. doi: 10.2174/1386207323999201027225305. [DOI] [PubMed] [Google Scholar]
- 19.Mohi-Ud-Din R., Mir R.H., Mir P.A., Farooq S., Raza S.N., Raja W.Y., Masoodi M.H., Singh I.P., Bhat Z.A. Ethnomedicinal uses, phytochemistry and pharmacological aspects of the genus berberis linn: a comprehensive review. Comb. Chem. High Throughput Screen. 2020;••• doi: 10.2174/1386207323999201102141206. [DOI] [PubMed] [Google Scholar]
- 20.Barkat M.A. Evidence-based review on clinical potential of thymoquinone in breast cancer. Nanomedicine for Bioactives. Springer; 2020. pp. 471–486. [Google Scholar]
- 21.Mir R.H., Masoodi M.H. Anti-inflammatory Plant polyphenolics and cellular action mechanisms. Curr. Bioact. Compd. 2020;16(6):809–817. doi: 10.2174/1573407215666190419205317. [DOI] [Google Scholar]
- 22.Amir M., Ahmad N., Sarfaroz M., Ahmad W., Ahmad S., Mujeeb M., Hyder P. F. Tamarindus indica fruit: Pharmacognostical standardization, detection of contaminant, and in vitro antioxidant activity. J. Pharm. Bioallied Sci. 2019;11(4):355–363. doi: 10.4103/jpbs.JPBS_46_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassan R., Masoodi M.H. Saussurea lappa: A comprehensive review on its pharmacological activity and phytochemistry. Curr. Tradit. Med. 2020;6(1):13–23. doi: 10.2174/2215083805666190626144909. [DOI] [Google Scholar]
- 24.Mir R.H., Masoodi M.H. Phytochemical screening and liquid chromatography-mass spectrometry studies of ethyl acetate extract of Origanum vulgare. Int. J. Pharm. Investig. 2020;10(2):132–135. doi: 10.5530/ijpi.2020.2.24. [DOI] [Google Scholar]
- 25.Hassan Mir R., Godavari G., Siddiqui N.A., Ahmad B., Mothana R.A., Ullah R., Almarfadi O.M., Jachak S.M., Masoodi M.H. Design, synthesis, molecular modelling, and biological evaluation of oleanolic acid-arylidene derivatives as potential anti-inflammatory agents. Drug Des. Devel. Ther. 2021;15:385–397. doi: 10.2147/DDDT.S291784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rates S.M.K. Plants as source of drugs. Toxicon. 2001;39(5):603–613. doi: 10.1016/S0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 27.Tokita K., Yamaji T., Hashimoto K. Roles of glutamate signaling in preclinical and/or mechanistic models of depression. Pharmacol. Biochem. Behav. 2012;100(4):688–704. doi: 10.1016/j.pbb.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Zarate C.A., Jr, Singh J.B., Carlson P.J., Brutsche N.E., Ameli R., Luckenbaugh D.A., Charney D.S., Manji H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 29.Kapitein L.C., Hoogenraad C.C. Which way to go? Cytoskeletal organization and polarized transport in neurons. Mol. Cell. Neurosci. 2011;46(1):9–20. doi: 10.1016/j.mcn.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Feyissa A.M. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. 2009. [DOI] [PMC free article] [PubMed]
- 31.Duric V., Banasr M., Licznerski P., Schmidt H.D., Stockmeier C.A., Simen A.A., Newton S.S., Duman R.S. A negative regulator of MAP kinase causes depressive behavior. Nat. Med. 2010;16(11):1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duric V., Banasr M., Stockmeier C.A., Simen A.A., Newton S.S., Overholser J.C., Jurjus G.J., Dieter L., Duman R.S. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int. J. Neuropsychopharmacol. 2013;16(1):69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hongisto V., Vainio J.C., Thompson R., Courtney M.J., Coffey E.T. The Wnt pool of glycogen synthase kinase 3β is critical for trophic-deprivation-induced neuronal death. Mol. Cell. Biol. 2008;28(5):1515–1527. doi: 10.1128/MCB.02227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanis K.Q., Duman R.S. Intracellular signaling pathways pave roads to recovery for mood disorders. Ann. Med. 2007;39(7):531–544. doi: 10.1080/07853890701483270. [DOI] [PubMed] [Google Scholar]
- 35.Li X., Jope R.S. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35(11):2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gould T.D., Einat H., Bhat R., Manji H.K. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int. J. Neuropsychopharmacol. 2004;7(4):387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- 37.Henderson B.R. Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover. Nat. Cell Biol. 2000;2(9):653–660. doi: 10.1038/35023605. [DOI] [PubMed] [Google Scholar]
- 38.Karege F., Perroud N., Burkhardt S., Fernandez R., Ballmann E., La Harpe R., Malafosse A. Protein levels of β-catenin and activation state of glycogen synthase kinase-3β in major depression. A study with postmortem prefrontal cortex. J. Affect. Disord. 2012;136(1-2):185–188. doi: 10.1016/j.jad.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 39.Morales-Garcia J.A., Luna-Medina R., Alonso-Gil S., Sanz-Sancristobal M., Palomo V., Gil C., Santos A., Martinez A., Perez-Castillo A. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem. Neurosci. 2012;3(11):963–971. doi: 10.1021/cn300110c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao Y., Ge X., Frank C.L., Madison J.M., Koehler A.N., Doud M.K., Tassa C., Berry E.M., Soda T., Singh K.K., Biechele T., Petryshen T.L., Moon R.T., Haggarty S.J., Tsai L.H. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-catenin signaling. Cell. 2009;136(6):1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saus E., Soria V., Escaramís G., Crespo J.M., Valero J., Gutiérrez-Zotes A., Martorell L., Vilella E., Menchón J.M., Estivill X., Gratacòs M., Urretavizcaya M. A haplotype of glycogen synthase kinase 3β is associated with early onset of unipolar major depression. Genes Brain Behav. 2010;9(7):799–807. doi: 10.1111/j.1601-183X.2010.00617.x. [DOI] [PubMed] [Google Scholar]
- 42.Wei J., Liu W., Yan Z. Regulation of AMPA receptor trafficking and function by glycogen synthase kinase 3. J. Biol. Chem. 2010;285(34):26369–26376. doi: 10.1074/jbc.M110.121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du J., Wei Y., Liu L., Wang Y., Khairova R., Blumenthal R., Tragon T., Hunsberger J.G., Machado-Vieira R., Drevets W., Wang Y.T., Manji H.K. A kinesin signaling complex mediates the ability of GSK-3β to affect mood-associated behaviors. Proc. Natl. Acad. Sci. USA. 2010;107(25):11573–11578. doi: 10.1073/pnas.0913138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aubry J-M., Schwald M., Ballmann E., Karege F. Early effects of mood stabilizers on the Akt/GSK-3β signaling pathway and on cell survival and proliferation. Psychopharmacology (Berl.) 2009;205(3):419–429. doi: 10.1007/s00213-009-1551-2. [DOI] [PubMed] [Google Scholar]
- 45.Shelton R.C. The molecular neurobiology of depression. Psychiatr. Clin. North Am. 2007;30(1):1–11. doi: 10.1016/j.psc.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciani L., Salinas P.C. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005;6(5):351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 47.Nusse R. Wnt signaling and stem cell control.Cold Spring Harbor symposia on quantitative biology. Cold Spring Harbor Laboratory Press; 2008. [DOI] [PubMed] [Google Scholar]
- 48.Purro S.A., Ciani L., Hoyos-Flight M., Stamatakou E., Siomou E., Salinas P.C. Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. J. Neurosci. 2008;28(34):8644–8654. doi: 10.1523/JNEUROSCI.2320-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kikuchi A., Yamamoto H., Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell. Signal. 2007;19(4):659–671. doi: 10.1016/j.cellsig.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Logan C.Y., Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 51.Okamoto H., Voleti B., Banasr M., Sarhan M., Duric V., Girgenti M.J., Dileone R.J., Newton S.S., Duman R.S. Wnt2 expression and signaling is increased by different classes of antidepressant treatments. Biol. Psychiatry. 2010;68(6):521–527. doi: 10.1016/j.biopsych.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinnock S.B., Blake A.M., Platt N.J., Herbert J. The roles of BDNF, pCREB and Wnt3a in the latent period preceding activation of progenitor cell mitosis in the adult dentate gyrus by fluoxetine. PLoS One. 2010;5(10):e13652. doi: 10.1371/journal.pone.0013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voleti B., Tanis K.Q., Newton S.S., Duman R.S. Analysis of target genes regulated by chronic electroconvulsive therapy reveals role for Fzd6 in depression. Biol. Psychiatry. 2012;71(1):51–58. doi: 10.1016/j.biopsych.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkinson M.B., Dias C., Magida J., Mazei-Robison M., Lobo M., Kennedy P., Dietz D., Covington H., III, Russo S., Neve R., Ghose S., Tamminga C., Nestler E.J. A novel role of the WNT-dishevelled-GSK3β signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J. Neurosci. 2011;31(25):9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dwivedi Y., Rizavi H.S., Conley R.R., Roberts R.C., Tamminga C.A., Pandey G.N. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 56.Qi X., Lin W., Li J., Li H., Wang W., Wang D., Sun M. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol. Dis. 2008;31(2):278–285. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Qi X., Lin W., Li J., Pan Y., Wang W. The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress. Behav. Brain Res. 2006;175(2):233–240. doi: 10.1016/j.bbr.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 58.Nibuya M., Nestler E.J., Duman R.S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 1996;16(7):2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tiraboschi E., Tardito D., Kasahara J., Moraschi S., Pruneri P., Gennarelli M., Racagni G., Popoli M. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. 2004;29(10):1831–1840. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]
- 60.Carlezon W.A., Jr, Duman R.S., Nestler E.J. The many faces of CREB. Trends Neurosci. 2005;28(8):436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Tardito D., Perez J., Tiraboschi E., Musazzi L., Racagni G., Popoli M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacol. Rev. 2006;58(1):115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- 62.Deisseroth K., Tsien R.W. Dynamic multiphosphorylation passwords for activity-dependent gene expression. Neuron. 2002;34(2):179–182. doi: 10.1016/S0896-6273(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 63.Sheng M., Thompson M.A., Greenberg M.E. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252(5011):1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 64.Karege F., Perret G., Bondolfi G., Schwald M., Bertschy G., Aubry J.M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. doi: 10.1016/S0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 65.Shimizu E., Hashimoto K., Okamura N., Koike K., Komatsu N., Kumakiri C., Nakazato M., Watanabe H., Shinoda N., Okada S., Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry. 2003;54(1):70–75. doi: 10.1016/S0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 66.Pizarro J.M., Lumley L.A., Medina W., Robison C.L., Chang W.E., Alagappan A., Bah M.J., Dawood M.Y., Shah J.D., Mark B., Kendall N., Smith M.A., Saviolakis G.A., Meyerhoff J.L. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025(1-2):10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 67.Smith M.A., Makino S., Kvetnansky R., Post R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995;15(3 Pt 1):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 69.Schaaf M.J., de Jong J., de Kloet E.R., Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813(1):112–120. doi: 10.1016/S0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- 70.Fanous S., Hammer R.P., Jr, Nikulina E.M. Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience. 2010;167(3):598–607. doi: 10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marmigère F., Givalois L., Rage F., Arancibia S., Tapia-Arancibia L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13(5):646–655. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- 72.Adachi M., Barrot M., Autry A.E., Theobald D., Monteggia L.M. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol. Psychiatry. 2008;63(7):642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taliaz D., Stall N., Dar D.E., Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol. Psychiatry. 2010;15(1):80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Leary O.F., Wu X., Castren E. Chronic fluoxetine treatment increases expression of synaptic proteins in the hippocampus of the ovariectomized rat: role of BDNF signalling. Psychoneuroendocrinology. 2009;34(3):367–381. doi: 10.1016/j.psyneuen.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 75.Chen Z-Y., Jing D., Bath K.G., Ieraci A., Khan T., Siao C.J., Herrera D.G., Toth M., Yang C., McEwen B.S., Hempstead B.L., Lee F.S. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiaruttini C., Vicario A., Li Z., Baj G., Braiuca P., Wu Y., Lee F.S., Gardossi L., Baraban J.M., Tongiorgi E. Dendritic trafficking of BDNF mRNA is mediated by translin and blocked by the G196A (Val66Met) mutation. Proc. Natl. Acad. Sci. USA. 2009;106(38):16481–16486. doi: 10.1073/pnas.0902833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thakker-Varia S., Alder J., Crozier R.A., Plummer M.R., Black I.B. Rab3A is required for brain-derived neurotrophic factor-induced synaptic plasticity: transcriptional analysis at the population and single-cell levels. J. Neurosci. 2001;21(17):6782–6790. doi: 10.1523/JNEUROSCI.21-17-06782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang H.J., Voleti B., Hajszan T., Rajkowska G., Stockmeier C.A., Licznerski P., Lepack A., Majik M.S., Jeong L.S., Banasr M., Son H., Duman R.S. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 2012;18(9):1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fajemiroye J.O., da Silva D.M., de Oliveira D.R., Costa E.A. Treatment of anxiety and depression: medicinal plants in retrospect. Fundam. Clin. Pharmacol. 2016;30(3):198–215. doi: 10.1111/fcp.12186. [DOI] [PubMed] [Google Scholar]
- 80.Oña G., Bouso J.C. Therapeutic potential of natural psychoactive drugs for central nervous system disorders: A perspective from polypharmacology. Curr. Med. Chem. 2020;••• doi: 10.2174/0929867326666191212103330. [DOI] [PubMed] [Google Scholar]
- 81.Dereli F.T.G., Ilhan M., Akkol E.K. 2019.
- 82.Mir R.H. Natural Anti-inflammatory compounds as drug candidates in Alzheimer’s disease. Curr. Med. Chem. 2021;28(23):4799–4825. doi: 10.2174/0929867327666200730213215. [DOI] [PubMed] [Google Scholar]
- 83.Dar M.A. Extensive phytochemistry, comprehensive traditional uses, and critical pharmacological profile of the great mullein: verbascum thapsus l. Nat. Prod. J. 2019;9(3):158–171. doi: 10.2174/2210315508666180821153531. [DOI] [Google Scholar]
- 84.Bhat M.F., Hassan R., Masoodi M.H. 2018.
- 85.Silva M.L.C. Phenolic compounds, carotenoids and antioxidant activity in plant products. Semin. Cienc. Agrar. 2010;31(3):669–682. doi: 10.5433/1679-0359.2010v31n3p669. [DOI] [Google Scholar]
- 86.Heim K.E., Tagliaferro A.R., Bobilya D.J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002;13(10):572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 87.Herbert B., Harborne J., Moss G. Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants. 1998. [Google Scholar]
- 88.Garcia-Salas P., Morales-Soto A., Segura-Carretero A., Fernández-Gutiérrez A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules. 2010;15(12):8813–8826. doi: 10.3390/molecules15128813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salvamani S. Antiartherosclerotic effects of plant flavonoids. BioMed Res. Int. 2014;2014:480258. doi: 10.1155/2014/480258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ali M., Rauf A., Hadda T.B., Bawazeer S., Abu-Izneid T., Khan H., Raza M., Khan S.A., Shah S.U., Pervez S., Patel S., Orhan I.E. Mechanisms underlying anti-hyperalgesic properties of Kaempferol-3, 7-di-O-α-L-rhamnopyranoside isolated from Dryopteris cycadina. Curr. Top. Med. Chem. 2017;17(4):383–390. doi: 10.2174/1568026616666160824101429. [DOI] [PubMed] [Google Scholar]
- 91.Rauf A., Khan R., Raza M., Khan H., Pervez S., De Feo V., Maione F., Mascolo N. Suppression of inflammatory response by chrysin, a flavone isolated from Potentilla evestita Th. Wolf. In silico predictive study on its mechanistic effect. Fitoterapia. 2015;103:129–135. doi: 10.1016/j.fitote.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 92.Xiao J. Advance on the flavonoid C-glycosides and health benefits. Crit. Rev. Food Sci. Nutr. 2016;56(1):29–45. doi: 10.1080/10408398.2015.1067595. [DOI] [PubMed] [Google Scholar]
- 93.Yan L., Hu Q., Mak M.S., Lou J., Xu S.L., Bi C.W., Zhu Y., Wang H., Dong T.T., Tsim K.W. A Chinese herbal decoction, reformulated from Kai-Xin-San, relieves the depression-like symptoms in stressed rats and induces neurogenesis in cultured neurons. Sci. Rep. 2016;6(1):30014. doi: 10.1038/srep30014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mannucci C., Navarra M., Calzavara E., Caputi A.P., Calapai G. Serotonin involvement in Rhodiola rosea attenuation of nicotine withdrawal signs in rats. Phytomedicine. 2012;19(12):1117–1124. doi: 10.1016/j.phymed.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 95.Lu P., Mamiya T., Lu L., Mouri A., Niwa M., Kim H.C., Zou L.B., Nagai T., Yamada K., Ikejima T., Nabeshima T. Silibinin attenuates cognitive deficits and decreases of dopamine and serotonin induced by repeated methamphetamine treatment. Behav. Brain Res. 2010;207(2):387–393. doi: 10.1016/j.bbr.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 96.Hritcu L. Antidepressant flavonoids and their relationship with oxidative stress. Oxid. Med. Cell. Longev. 2017;2017:5762172. doi: 10.1155/2017/5762172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bouayed J. Polyphenols: a potential new strategy for the prevention and treatment of anxiety and depression. Curr. Nutr. Food Sci. 2010;6(1):13–18. doi: 10.2174/157340110790909608. [DOI] [Google Scholar]
- 98.Chang S-C., Cassidy A., Willett W.C., Rimm E.B., O’Reilly E.J., Okereke O.I. Dietary flavonoid intake and risk of incident depression in midlife and older women. Am. J. Clin. Nutr. 2016;104(3):704–714. doi: 10.3945/ajcn.115.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mihrshahi S., Dobson A.J., Mishra G.D. Fruit and vegetable consumption and prevalence and incidence of depressive symptoms in mid-age women: results from the Australian longitudinal study on women’s health. Eur. J. Clin. Nutr. 2015;69(5):585–591. doi: 10.1038/ejcn.2014.222. [DOI] [PubMed] [Google Scholar]
- 100.Khalid S., Williams C.M., Reynolds S.A. Is there an association between diet and depression in children and adolescents? A systematic review. Br. J. Nutr. 2016;116(12):2097–2108. doi: 10.1017/S0007114516004359. [DOI] [PubMed] [Google Scholar]
- 101.Pase M.P., Scholey A.B., Pipingas A., Kras M., Nolidin K., Gibbs A., Wesnes K., Stough C. Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo-controlled trial. J. Psychopharmacol. 2013;27(5):451–458. doi: 10.1177/0269881112473791. [DOI] [PubMed] [Google Scholar]
- 102.Khan H., Perviz S., Sureda A., Nabavi S.M., Tejada S. Current standing of plant derived flavonoids as an antidepressant. Food Chem. Toxicol. 2018;119:176–188. doi: 10.1016/j.fct.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 103.Lv Q-Q., Wu W.J., Guo X.L., Liu R.L., Yang Y.P., Zhou D.S., Zhang J.X., Liu J.Y. Antidepressant activity of astilbin: involvement of monoaminergic neurotransmitters and BDNF signal pathway. Biol. Pharm. Bull. 2014;37(6):987–995. doi: 10.1248/bpb.b13-00968. [DOI] [PubMed] [Google Scholar]
- 104.Sloley B.D., Urichuk L.J., Morley P., Durkin J., Shan J.J., Pang P.K., Coutts R.T. Identification of kaempferol as a monoamine oxidase inhibitor and potential Neuroprotectant in extracts of Ginkgo biloba leaves. J. Pharm. Pharmacol. 2000;52(4):451–459. doi: 10.1211/0022357001774075. [DOI] [PubMed] [Google Scholar]
- 105.Li R., Zhao D., Qu R., Fu Q., Ma S. The effects of apigenin on lipopolysaccharide-induced depressive-like behavior in mice. Neurosci. Lett. 2015;594:17–22. doi: 10.1016/j.neulet.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 106.Yi L-T., Li J.M., Li Y.C., Pan Y., Xu Q., Kong L.D. Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin. Life Sci. 2008;82(13-14):741–751. doi: 10.1016/j.lfs.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 107.Park S-H., Sim Y.B., Han P.L., Lee J.K., Suh H.W. Antidepressant-like effect of kaempferol and quercitirin, isolated from Opuntia ficus-indica var. saboten. Exp. Neurobiol. 2010;19(1):30–38. doi: 10.5607/en.2010.19.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rinwa P., Kumar A. Quercetin suppress microglial neuroinflammatory response and induce antidepressent-like effect in olfactory bulbectomized rats. Neuroscience. 2013;255:86–98. doi: 10.1016/j.neuroscience.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 109.Yunfeng L. Antidepressant effect of quercetin 3-O-apiosyl (1→ 2)-[rhamnosyl (1→ 6)]-glucoside in mice. Zhongguo Yaolixue Yu Dulixue Zazhi. 2000;14(2):125–127. [Google Scholar]
- 110.Lee M-H., Lin R.D., Shen L.Y., Yang L.L., Yen K.Y., Hou W.C. Monoamine oxidase B and free radical scavenging activities of natural flavonoids in Melastoma candidum D. Don. J. Agric. Food Chem. 2001;49(11):5551–5555. doi: 10.1021/jf010622j. [DOI] [PubMed] [Google Scholar]
- 111.Pan Y., Kong L.D., Li Y.C., Xia X., Kung H.F., Jiang F.X. Icariin from Epimedium brevicornum attenuates chronic mild stress-induced behavioral and neuroendocrinological alterations in male Wistar rats. Pharmacol. Biochem. Behav. 2007;87(1):130–140. doi: 10.1016/j.pbb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 112.Hwang J-S., Lee S.A., Hong S.S., Lee K.S., Lee M.K., Hwang B.Y., Ro J.S. Monoamine oxidase inhibitory components from the roots of Sophora flavescens. Arch. Pharm. Res. 2005;28(2):190–194. doi: 10.1007/BF02977714. [DOI] [PubMed] [Google Scholar]
- 113.Zhu J.T., Choi R.C., Chu G.K., Cheung A.W., Gao Q.T., Li J., Jiang Z.Y., Dong T.T., Tsim K.W. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing β-amyloid-induced cell death. J. Agric. Food Chem. 2007;55(6):2438–2445. doi: 10.1021/jf063299z. [DOI] [PubMed] [Google Scholar]
- 114.Yi L-T., Li C.F., Zhan X., Cui C.C., Xiao F., Zhou L.P., Xie Y. Involvement of monoaminergic system in the antidepressant-like effect of the flavonoid naringenin in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(7):1223–1228. doi: 10.1016/j.pnpbp.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 115.Yi L-T., Xu H.L., Feng J., Zhan X., Zhou L.P., Cui C.C. Involvement of monoaminergic systems in the antidepressant-like effect of nobiletin. Physiol. Behav. 2011;102(1):1–6. doi: 10.1016/j.physbeh.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 116.Ishola I.O., Chatterjee M., Tota S., Tadigopulla N., Adeyemi O.O., Palit G., Shukla R. Antidepressant and anxiolytic effects of amentoflavone isolated from Cnestis ferruginea in mice. Pharmacol. Biochem. Behav. 2012;103(2):322–331. doi: 10.1016/j.pbb.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 117.Zheng M., Liu C., Pan F., Shi D., Zhang Y. Antidepressant-like effect of hyperoside isolated from Apocynum venetum leaves: possible cellular mechanisms. Phytomedicine. 2012;19(2):145–149. doi: 10.1016/j.phymed.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 118.Souza L.C., de Gomes M.G., Goes A.T., Del Fabbro L., Filho C.B., Boeira S.P., Jesse C.R. Evidence for the involvement of the serotonergic 5-HT(1A) receptors in the antidepressant-like effect caused by hesperidin in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;40:103–109. doi: 10.1016/j.pnpbp.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 119.Filho C.B., Del Fabbro L., de Gomes M.G., Goes A.T., Souza L.C., Boeira S.P., Jesse C.R. Kappa-opioid receptors mediate the antidepressant-like activity of hesperidin in the mouse forced swimming test. Eur. J. Pharmacol. 2013;698(1-3):286–291. doi: 10.1016/j.ejphar.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 120.de la Peña J.B.I., Kim C.A., Lee H.L., Yoon S.Y., Kim H.J., Hong E.Y., Kim G.H., Ryu J.H., Lee Y.S., Kim K.M., Cheong J.H. Luteolin mediates the antidepressant-like effects of Cirsium japonicum in mice, possibly through modulation of the GABAA receptor. Arch. Pharm. Res. 2014;37(2):263–269. doi: 10.1007/s12272-013-0229-9. [DOI] [PubMed] [Google Scholar]
- 121.Kulkarni S.K., Bhutani M.K., Bishnoi M. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology (Berl.) 2008;201(3):435–442. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- 122.Kulkarni S., Dhir A., Akula K.K. Potentials of curcumin as an antidepressant. ScientificWorldJournal. 2009;9:1233–1241. doi: 10.1100/tsw.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cropley V., Croft R., Silber B., Neale C., Scholey A., Stough C., Schmitt J. Does coffee enriched with chlorogenic acids improve mood and cognition after acute administration in healthy elderly? A pilot study. Psychopharmacology (Berl.) 2012;219(3):737–749. doi: 10.1007/s00213-011-2395-0. [DOI] [PubMed] [Google Scholar]
- 124.Girish C., Raj V., Arya J., Balakrishnan S. Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur. J. Pharmacol. 2012;682(1-3):118–125. doi: 10.1016/j.ejphar.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 125.Zhen L., Zhu J., Zhao X., Huang W., An Y., Li S., Du X., Lin M., Wang Q., Xu Y., Pan J. The antidepressant-like effect of fisetin involves the serotonergic and noradrenergic system. Behav. Brain Res. 2012;228(2):359–366. doi: 10.1016/j.bbr.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 126.Machado D.G., Bettio L.E., Cunha M.P., Santos A.R., Pizzolatti M.G., Brighente I.M., Rodrigues A.L. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharmacol. 2008;587(1-3):163–168. doi: 10.1016/j.ejphar.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Y.J., Huang X., Wang Y., Xie Y., Qiu X.J., Ren P., Gao L.C., Zhou H.H., Zhang H.Y., Qiao M.Q. Ferulic acid-induced anti-depression and prokinetics similar to Chaihu-Shugan-San via polypharmacology. Brain Res. Bull. 2011;86(3-4):222–228. doi: 10.1016/j.brainresbull.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 128.Xu Y., Wang Z., You W., Zhang X., Li S., Barish P.A., Vernon M.M., Du X., Li G., Pan J., Ogle W.O. Antidepressant-like effect of trans-resveratrol: Involvement of serotonin and noradrenaline system. Eur. Neuropsychopharmacol. 2010;20(6):405–413. doi: 10.1016/j.euroneuro.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 129.Wang W., Hu X., Zhao Z., Liu P., Hu Y., Zhou J., Zhou D., Wang Z., Guo D., Guo H. Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(5):1179–1184. doi: 10.1016/j.pnpbp.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 130.Xiong Z., Jiang B., Wu P.F., Tian J., Shi L.L., Gu J., Hu Z.L., Fu H., Wang F., Chen J.G. Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol. Pharm. Bull. 2011;34(2):253–259. doi: 10.1248/bpb.34.253. [DOI] [PubMed] [Google Scholar]
- 131.Zhou Z.L., Yin W.Q., Yang Y.M., He C.H., Li X.N., Zhou C.P., Guo H. New iridoid glycosides with antidepressant activity isolated from Cyperus rotundus. Chem. Pharm. Bull. (Tokyo) 2016;64(1):73–77. doi: 10.1248/cpb.c15-00686. [DOI] [PubMed] [Google Scholar]
- 132.Zhou Z-L., Lin S-Q., Yin W-Q. New cycloartane glycosides from the rhizomes of Cyperus rotundus and their antidepressant activity. J. Asian Nat. Prod. Res. 2016;18(7):662–668. doi: 10.1080/10286020.2016.1142976. [DOI] [PubMed] [Google Scholar]
- 133.Yang Q-X. Cynanauriculoside C–E, three new antidepressant pregnane glycosides from Cynanchum auriculatum. Phytochem. Lett. 2011;4(2):170–175. doi: 10.1016/j.phytol.2011.02.009. [DOI] [Google Scholar]
- 134.Demirkiran O. Three new benzophenone glycosides with MAO-A inhibitory activity from Hypericum thasium Griseb. Phytochem. Lett. 2012;5(4):700–704. doi: 10.1016/j.phytol.2012.06.018. [DOI] [Google Scholar]
- 135.Wang Y-L., Wang J.X., Hu X.X., Chen L., Qiu Z.K., Zhao N., Yu Z.D., Sun S.Z., Xu Y.Y., Guo Y., Liu C., Zhang Y.Z., Li Y.F., Yu C.X. Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J. Ethnopharmacol. 2016;179:9–15. doi: 10.1016/j.jep.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 136.Qiu F-M., Zhong X.M., Mao Q.Q., Huang Z. Antidepressant-like effects of paeoniflorin on the behavioural, biochemical, and neurochemical patterns of rats exposed to chronic unpredictable stress. Neurosci. Lett. 2013;541:209–213. doi: 10.1016/j.neulet.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 137.Lin S-q. Phenolic glycosides from the rhizomes of Cyperus rotundus and their antidepressant activity. J. Korean Soc. Appl. Biol. Chem. 2015;58(5):685–691. doi: 10.1007/s13765-015-0092-0. [DOI] [Google Scholar]
- 138.Li S., Wang C., Li W., Koike K., Nikaido T., Wang M.W. Antidepressant-like effects of piperine and its derivative, antiepilepsirine. J. Asian Nat. Prod. Res. 2007;9(3-5):421–430. doi: 10.1080/10286020500384302. [DOI] [PubMed] [Google Scholar]
- 139.Peng W-H., Lo K.L., Lee Y.H., Hung T.H., Lin Y.C. Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 2007;81(11):933–938. doi: 10.1016/j.lfs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 140.Farzin D., Mansouri N. Antidepressant-like effect of harmane and other β-carbolines in the mouse forced swim test. Eur. Neuropsychopharmacol. 2006;16(5):324–328. doi: 10.1016/j.euroneuro.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 141.Fortunato J.J., Réus G.Z., Kirsch T.R., Stringari R.B., Fries G.R., Kapczinski F., Hallak J.E., Zuardi A.W., Crippa J.A., Quevedo J. Effects of β-carboline harmine on behavioral and physiological parameters observed in the chronic mild stress model: further evidence of antidepressant properties. Brain Res. Bull. 2010;81(4-5):491–496. doi: 10.1016/j.brainresbull.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 142.Idayu N.F., Hidayat M.T., Moklas M.A., Sharida F., Raudzah A.R., Shamima A.R., Apryani E. Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomedicine. 2011;18(5):402–407. doi: 10.1016/j.phymed.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 143.Sugimoto Y., Furutani S., Nishimura K., Itoh A., Tanahashi T., Nakajima H., Oshiro H., Sun S., Yamada J. Antidepressant-like effects of neferine in the forced swimming test involve the serotonin1A (5-HT1A) receptor in mice. Eur. J. Pharmacol. 2010;634(1-3):62–67. doi: 10.1016/j.ejphar.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 144.Dhingra D., Bhankher A. Behavioral and biochemical evidences for antidepressant-like activity of palmatine in mice subjected to chronic unpredictable mild stress. Pharmacol. Rep. 2014;66(1):1–9. doi: 10.1016/j.pharep.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 145.Li S., Wang C., Wang M., Li W., Matsumoto K., Tang Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80(15):1373–1381. doi: 10.1016/j.lfs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 146.Dhingra D., Valecha R. 2014.
- 147.Chen Y., Wang H., Zhang R., Wang H., Peng Z., Sun R., Tan Q. Microinjection of sanguinarine into the ventrolateral orbital cortex inhibits Mkp-1 and exerts an antidepressant-like effect in rats. Neurosci. Lett. 2012;506(2):327–331. doi: 10.1016/j.neulet.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 148.Gao S., Cui Y.L., Yu C.Q., Wang Q.S., Zhang Y. Tetrandrine exerts antidepressant-like effects in animal models: role of brain-derived neurotrophic factor. Behav. Brain Res. 2013;238:79–85. doi: 10.1016/j.bbr.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 149.Gonçalves A.E., Bürger C., Amoah S.K., Tolardo R., Biavatti M.W., de Souza M.M. The antidepressant-like effect of Hedyosmum brasiliense and its sesquiterpene lactone, podoandin in mice: evidence for the involvement of adrenergic, dopaminergic and serotonergic systems. Eur. J. Pharmacol. 2012;674(2-3):307–314. doi: 10.1016/j.ejphar.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 150.Zanelati T.V., Biojone C., Moreira F.A., Guimarães F.S., Joca S.R. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br. J. Pharmacol. 2010;159(1):122–128. doi: 10.1111/j.1476-5381.2009.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Silote G.P., Sartim A., Sales A., Eskelund A., Guimarães F.S., Wegener G., Joca S. Emerging evidence for the antidepressant effect of cannabidiol and the underlying molecular mechanisms. J. Chem. Neuroanat. 2019;98:104–116. doi: 10.1016/j.jchemneu.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 152.Melo F.H.C., Moura B.A., de Sousa D.P., de Vasconcelos S.M., Macedo D.S., Fonteles M.M., Viana G.S., de Sousa F.C. Antidepressant-like effect of carvacrol (5-Isopropyl-2-methylphenol) in mice: involvement of dopaminergic system. Fundam. Clin. Pharmacol. 2011;25(3):362–367. doi: 10.1111/j.1472-8206.2010.00850.x. [DOI] [PubMed] [Google Scholar]
- 153.Zotti M., Colaianna M., Morgese M.G., Tucci P., Schiavone S., Avato P., Trabace L. Carvacrol: from ancient flavoring to neuromodulatory agent. Molecules. 2013;18(6):6161–6172. doi: 10.3390/molecules18066161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bahi A., Al Mansouri S., Al Memari E., Al Ameri M., Nurulain S.M., Ojha S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol. Behav. 2014;135:119–124. doi: 10.1016/j.physbeh.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 155.Hwang E-S., Kim H.B., Lee S., Kim M.J., Kim K.J., Han G., Han S.Y., Lee E.A., Yoon J.H., Kim D.O., Maeng S., Park J.H. Antidepressant-like effects of β-caryophyllene on restraint plus stress-induced depression. Behav. Brain Res. 2020;380:112439. doi: 10.1016/j.bbr.2019.112439. [DOI] [PubMed] [Google Scholar]
- 156.Wang Q-S., Tian J.S., Cui Y.L., Gao S. Genipin is active via modulating monoaminergic transmission and levels of brain-derived neurotrophic factor (BDNF) in rat model of depression. Neuroscience. 2014;275:365–373. doi: 10.1016/j.neuroscience.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 157.Gibon J., Deloulme J.C., Chevallier T., Ladevèze E., Abrous D.N., Bouron A. The antidepressant hyperforin increases the phosphorylation of CREB and the expression of TrkB in a tissue-specific manner. Int. J. Neuropsychopharmacol. 2013;16(1):189–198. doi: 10.1017/S146114571100188X. [DOI] [PubMed] [Google Scholar]
- 158.Braida D., Capurro V., Zani A., Rubino T., Viganò D., Parolaro D., Sala M. Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br. J. Pharmacol. 2009;157(5):844–853. doi: 10.1111/j.1476-5381.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Colla A.R., Oliveira A., Pazini F.L., Rosa J.M., Manosso L.M., Cunha M.P., Rodrigues A.L. Serotonergic and noradrenergic systems are implicated in the antidepressant-like effect of ursolic acid in mice. Pharmacol. Biochem. Behav. 2014;124:108–116. doi: 10.1016/j.pbb.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 160.Machado D.G., Neis V.B., Balen G.O., Colla A., Cunha M.P., Dalmarco J.B., Pizzolatti M.G., Prediger R.D., Rodrigues A.L. Antidepressant-like effect of ursolic acid isolated from Rosmarinus officinalis L. in mice: evidence for the involvement of the dopaminergic system. Pharmacol. Biochem. Behav. 2012;103(2):204–211. doi: 10.1016/j.pbb.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 161.Xu C., Teng J., Chen W., Ge Q., Yang Z., Yu C., Yang Z., Jia W. 20(S)-protopanaxadiol, an active ginseng metabolite, exhibits strong antidepressant-like effects in animal tests. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(8):1402–1411. doi: 10.1016/j.pnpbp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 162.Jiang N., Lv J.W., Wang H.X., Wang Q., Lu C., Yang Y.J., Huang H., Xia T.J., Lv G.H., Liu X.M. Antidepressant-like effects of 20(S)-protopanaxadiol in a mouse model of chronic social defeat stress and the related mechanisms. Phytother. Res. 2019;33(10):2726–2736. doi: 10.1002/ptr.6446. [DOI] [PubMed] [Google Scholar]
- 163.Zhou D., Jin H., Lin H.B., Yang X.M., Cheng Y.F., Deng F.J., Xu J.P. Antidepressant effect of the extracts from Fructus Akebiae. Pharmacol. Biochem. Behav. 2010;94(3):488–495. doi: 10.1016/j.pbb.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 164.Liang B. Pharmacodynamical evaluation of hederagenin as an antidepressant. Mil. Med. Sci. 2013;37:286–290. [Google Scholar]
- 165.Ren L-X., Luo Y.F., Li X., Zuo D.Y., Wu Y.L. Antidepressant-like effects of sarsasapogenin from Anemarrhena asphodeloides BUNGE (Liliaceae). Biol. Pharm. Bull. 2006;29(11):2304–2306. doi: 10.1248/bpb.29.2304. [DOI] [PubMed] [Google Scholar]
- 166.Feng B., Zhao X.Y., Song Y.Z., Liang W.N., Liu J.L. Sarsasapogenin reverses depressive-like behaviors and nicotinic acetylcholine receptors induced by olfactory bulbectomy. Neurosci. Lett. 2017;639:173–178. doi: 10.1016/j.neulet.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 167.Subarnas A., Tadano T., Nakahata N., Arai Y., Kinemuchi H., Oshima Y., Kisara K., Ohizumi Y. A possible mechanism of antidepressant activity of beta-amyrin palmitate isolated from Lobelia inflata leaves in the forced swimming test. Life Sci. 1993;52(3):289–296. doi: 10.1016/0024-3205(93)90220-W. [DOI] [PubMed] [Google Scholar]
- 168.Liu X., Liu F., Yue R., Li Y., Zhang J., Wang S., Zhang S., Wang R., Shan L., Zhang W. The antidepressant-like effect of bacopaside I: possible involvement of the oxidative stress system and the noradrenergic system. Pharmacol. Biochem. Behav. 2013;110:224–230. doi: 10.1016/j.pbb.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 169.Jiang B., Xiong Z., Yang J., Wang W., Wang Y., Hu Z.L., Wang F., Chen J.G. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br. J. Pharmacol. 2012;166(6):1872–1887. doi: 10.1111/j.1476-5381.2012.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fan C., Song Q., Wang P., Li Y., Yang M., Yu S.Y. Neuroprotective effects of ginsenoside-Rg1 against depression-like behaviors via suppressing glial activation, synaptic deficits, and neuronal apoptosis in rats. Front. Immunol. 2018;9:2889. doi: 10.3389/fimmu.2018.02889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 171.Dhingra D., Sharma A. Evaluation of antidepressant-like activity of glycyrrhizin in mice. Indian J. Pharmacol. 2005;37(6):390. doi: 10.4103/0253-7613.19077. [DOI] [Google Scholar]