Abstract
Acetylcholine in the brain promotes arousal and facilitates cognitive functions. Cholinergic neurons in the mesopontine brainstem and basal forebrain are important for activation of the cerebral cortex, which is characterized by the suppression of irregular slow waves, an increase in gamma (30-100 Hz) activity in the electroencephalogram, and the appearance of a hippocampal theta rhythm. During general anesthesia, a decrease in acetylcholine release and cholinergic functions contribute to the desired outcomes of general anesthesia, such as amnesia, loss of awareness and consciousness, and immobility. Animal experiments indicate that inactivation, lesion, or genetic ablation of cholinergic neurons in the basal forebrain potentiated the effects of inhalational and injectable anesthetics, including isoflurane, halothane, propofol, pentobarbital, and in some cases, ketamine. Increased behavioral sensitivity to general anesthesia, faster induction time, and delayed recovery of a loss of righting reflex have been observed in rodents with basal forebrain cholinergic deficits. Cholinergic stimulation in the prefrontal cortex, thalamus, and basal forebrain hastens recovery from general anesthesia. Anticholinesterase accelerates emergence from general anesthesia, but with mixed success, in part depending on the anesthetic used. Cholinergic deficits may contribute to cognitive impairments after anesthesia and operations, which are severe in aged subjects. We propose a cholinergic hypothesis for postoperative cognitive disorder, in line with the cholinergic deficits and cognitive decline in aging and Alzheimer’s disease. The current animal literature suggests that brain cholinergic neurons can regulate the immune and inflammatory response after surgical operation and anesthetic exposure, and anticholinesterase and α7-nicotinic cholinergic agonists can alleviate postoperative inflammatory response and cognitive deficits.
Keywords: Medial septum, nucleus basalis, acetylcholine, general anesthesia, loss of righting reflex, postoperative cognitive disorder, cortical activation, mesopontine brainstem
1. CHOLINERGIC NEURONS AND FUNCTION IN THE MAMMALIAN BRAIN
Acetylcholine (ACh), a low-molecular-weight neurotransmitter in the brain, is known to be involved in arousal, sleep cycle, and cognitive functions. We review the role of ACh in general anesthesia from the following two perspectives: 1) how general anesthetics suppress cholinergic functions, and 2) how cholinergic neurons and cholinergic drugs modulate the effects of general anesthesia. We also review the preclinical literature on the possible role of ACh in postoperative cognitive disorder (POCD). POCD is a long-term disruption of cognitive and behavioral functions after surgical operations, which occurs mostly in the elderly. The decline of cholinergic function in aging and Alzheimer’s disease is well known.
In the periphery, cholinergic neurons are found in the spinal cord and autonomic ganglia. These neurons are essential for movements, glandular secretions, and modulation of smooth muscles. In the brain, cholinergic neurons are concentrated in the mesopontine brainstem, basal forebrain, epithalamus (habenula), and striatum [1-3]. In humans, there are ~20,000 cholinergic neurons in the mesopontine nuclei [4] and ~200,000 in the basal forebrain [5]. These neurons innervate billions of neurons in the brain, notably in the cerebral cortex.
1.1. Mesopontine Brainstem and Basal Forebrain
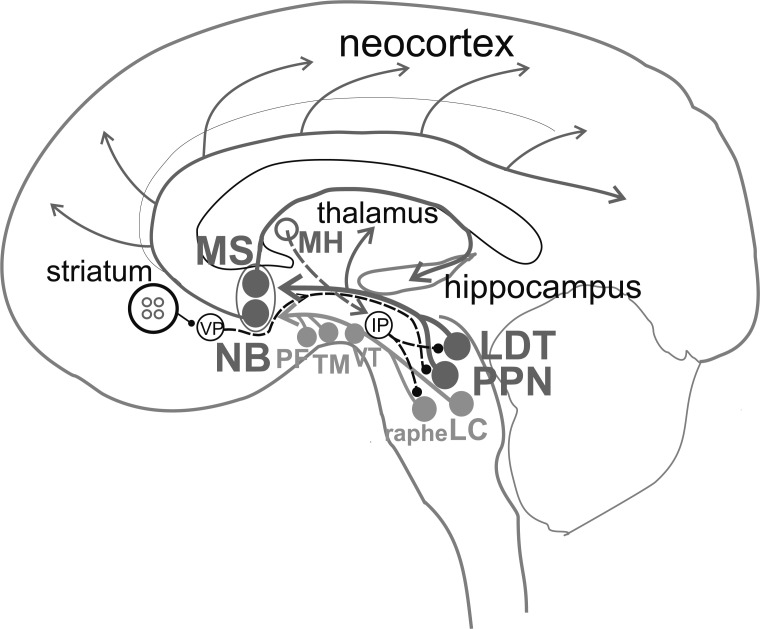
Two nuclei at a midbrain-pons interface, peduculopontine nucleus (PPN) and lateral dorsal tegmentum (LDT), contain cholinergic neurons intermingled with glutamatergic and GABAergic ones [6-8]. The PPN and LDT project ascending axon collaterals to the basal forebrain, hypothalamus, thalamus, and the medial frontal cortex (Fig. 1). The mesopontine cholinergic projections are an integral part of the reticular activating system, promoting arousal and cortical activation. In addition to cholinergic afferents, various subcortical and cortical structures are known to provide inputs to the basal forebrain, including locus coeruleus (norepinephrine), ventral tegmental area (dopamine), dorsal and median raphe (serotonin), perifornical area (orexin), tuberomammillary nucleus (histamine), and glutamatergic inputs from the supramammillary nucleus, amygdala and prefrontal cortex [2, 9-11], and parabrachial nuclei [12].
Fig. (1).
Schematic connections of cholinergic neurons in the brain involved in cortical activation and general anesthesia. Laterodorsal tegmental nucleus (LDT) and pedunculopontine tegmental nucleus (PPN) provide ascending inputs to the thalamus and basal forebrain. In the basal forebrain, cholinergic fibers project from medial septum/diagonal band nuclei (MS) to hippocampus and from nucleus basalis (NB) to neocortex. The basal forebrain also receives ascending inputs from brainstem monoaminergic nuclei, including locus coeruleus (LC) with norepinephrine, median and dorsal raphe with serotonin, and ventral tegmental area (VT) with dopamine. In the hypothalamus, wake-active neurons in the perifornical area (PF) with orexin, and tuberomammillary nucleus (TM) with histamine, project to the basal forebrain. Cholinergic neurons in the medial habenula (MH) activate the interpeduncular nucleus (IP), which in turn inhibits LDT and monoaminergic nuclei. Striatum contains cholinergic interneurons (small red circles), which modulate GABAergic neurons with descending output to ventral pallidum (VP), which projects to PPN/LDT. Cholinergic connections and nuclei are labeled in red; solid line for ascending and dashed line for descending projection; presumed inhibitory connection ends in a filled circle. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
Damage to the mesopontine area results in a coma in humans and animals [13, 14]. In the rat, a comatose state was reported resulting from neuronal damage of the parabrachial nuclei and not from the damage of the LDT and PPN [15, 16]. Other than providing an activation function in normal sleep cycle, cholinergic neurons in PPN/LDT are also responsible for normal gait and posture in humans and animals [17, 18].
The thalamus receives direct cholinergic inputs from PPN and LDT, as well as indirect input from the basal forebrain. Cholinergic release in the thalamus is high during active open field activity in mice [19]. There has been a strong association of cortical arousal with the thalamus. However, neuronal lesions of the thalamus had no or negligible effect on neocortical activation, while lesion or inactivation of the nucleus basalis abolished neocortical activation [12, 20, 21]. In the hypothalamus, several nuclei receive projections from the PPN and LDT, including supramammillary nucleus, tuberomammillary nucleus, and perifornical area; these areas also project to the basal forebrain (Fig. 1).
The cholinergic innervation of the cerebral cortex is mainly derived from the basal forebrain. In the basal forebrain, the nucleus basalis (including magnocellular preoptic area and substantia innominata) projects to the neocortex and amygdala. The medial septum and diagonal band of Broca project to the hippocampus, entorhinal cortex, and olfactory cortex [1-3]. The medial prefrontal cortex appears to be the only cortical area that receives cholinergic afferents from both the basal forebrain and the mesopontine nuclei. GABAergic and glutamatergic neurons in the basal forebrain also project to the cerebral cortex and hippocampus, and they are important in forebrain activation, together with cholinergic neurons [22]. Cholinergic, GABAergic, and glutamatergic neurons are synaptically connected with each other in the medial septum/diagonal band of Broca and nucleus basalis [23-25], such that alteration of cholinergic function may affect GABAergic and glutamatergic outputs to the cerebral cortex. While activation of the hippocampus and neocortex is an important function of the cholinergic neurons of the basal forebrain (below), specific lesion of the cholinergic neurons in the basal forebrain by 192 IgG-saporin does not affect wake-sleep patterns [26] or only increases NREM sleep transiently [27]. However, extensive lesion of basal forebrain neurons in rats induces a “comatose” state without losing the righting reflex [16].
The release of ACh in the neocortex and hippocampus is dependent on behavioral states, with high ACh release during active waking and rapid-eye-movement (REM) sleep and low release during non-rapid-eye-movement (NREM) sleep [28-30]. In freely moving rats, sensory stimulation or wheel-running, compared to non-stimulated immobility, increases ACh release in the hippocampus but not in the sensorimotor cortex [31]. Basal forebrain neurons fire differently to behavioral activation and sleep state [22, 32], and cholinergic basal forebrain neurons fire the highest rate during REM sleep and awake states, as compared to NREM sleep state [33]. ACh level in the pontine medial reticular formation, innervated by cholinergic PPN/LDT neurons, is highest during REM sleep and awake states and lowest during NREM sleep state [8, 34]. Atonia during REM sleep has been associated with ACh release in the medial reticular formation by PPN/LDT neurons in cats [8] or alternatively attributed to glutamatergic neurons in the sublaterodorsal nucleus in rats [15].
ACh is important for the activation of the cerebral cortex. Cortical activation occurs during quiet and active waking and REM sleep, compared to NREM sleep. Activation of the neocortex is characterized by desynchronization of irregular slow waves, including suppressing delta (0-4 Hz) activity and enhancing gamma waves (30-100 Hz) [22, 35], a pattern that is also known as low-voltage fast activity. Activation of the hippocampus is also characterized by suppressing irregular slow waves and enhancing gamma, and in addition, by the appearance of a hippocampal theta rhythm which increases its frequency with activation (4-12 Hz in rodents) [35-38].
A low-frequency (4-6 Hz) hippocampal theta rhythm occurs during sensory stimulation [38-40]. The immobility-theta is abolished by systemic atropine, a muscarinic cholinergic antagonist, and is thus called atropine-sensitive. High-frequency (7-12 Hz) theta rhythm during locomotion is not blocked by atropine (called atropine-resistant), but the theta rhythm is also inferred to contain an atropine-sensitive component [31, 35, 36]. Basal forebrain cholinergic neurons are critical for generating the atropine-sensitive theta rhythm [25, 35-38] and for the suppression of irregular slow waves [35, 41]; they also contribute to the generation of gamma waves in the hippocampus and neocortex [22, 36, 43-45].
1.2. Habenula and Striatum
The habenula at the dorsomedial thalamus provides a bridge between the limbic forebrain and midbrain/hindbrain nuclei [46, 47]. The medial habenula contains cholinergic neurons that project to the interpeduncular nucleus (IPN) through the fascicularis retroflexus. Genetic ablation of the medial habenular neurons in mice results in a large decline in ACh in the IPN, accompanied by hyperactive and compulsive/impulsive behaviors [48]. An intact cholinergic medial habenulo-IPN pathway is apparently required for the normal duration of NREM and REM sleep [49, 50]. Additional evidence suggests the involvement of the non-cholinergic lateral habenula in NREM/ REM sleep durations [51].
Striatal ACh is derived mainly from intrinsic cholinergic interneurons [3], although extrinsic cholinergic afferents come from the brainstem [52]. Cholinergic interneurons constitute 1-2% of all striatal neurons and likely participate in all functions of the striatum through its extensive collaterals [53]. The dorsal striatum is connected with the thalamus and neocortex and participates in sensorimotor functions, including motor learning. The ventral striatum, otherwise known as the nucleus accumbens, receives from the hippocampus, medial prefrontal cortex, and amygdala and links motivation to action, in part through the ventral pallidum [54, 55].
1.3. Acetylcholine Neurochemistry and Receptors
ACh is synthesized by choline-acetyltransferase (ChAT). ACh is packed into synaptic vesicles by a high-affinity vesicular acetylcholine transporter (vAChT). After release at the synaptic cleft, ACh diffuses away and is hydrolyzed by acetylcholinesterase (AChE). Blocking AChE by anticholinesterase, such as physostigmine, is an effective way of increasing ACh concentration, thus enhancing the postsynaptic effect of ACh.
There are two main types of ACh receptors – nicotinic and muscarinic. The nicotinic ACh receptor is ionotropic, and opening nAChRs increases Na+/K+ permeability and depolarizes the postsynaptic cell [56, 57]. A neuronal nAChR is a pentamer consisting of α and β subunits, and various combinations can be made with eight α-subunits and three β-subunits. Some nAChRs are also permeable to Ca2+, which can mediate synaptic plasticity, excitotoxicity, apoptosis, and increase neurotransmitter release from presynaptic terminals [57]. Muscarinic ACh receptors (mAChRs) are G-protein coupled receptors [56, 58] and consist of two main types – M1-like (M1, M3, and M5) or M2-like (M2 and M4). M1-like receptors are coupled to Gq/G11 and inhibit K+ permeability through phospholipase C. M2-like receptors are coupled to Gi/Go, which subsequently activate inward-rectifying K+ channels (postsynaptic inhibition) or inhibit voltage-gated Ca2+ channels (presynaptic inhibition). Presynaptic inhibition decreases the release of ACh (autoreceptor) or other neurotransmitters (heteroreceptors).
General anesthetics act on ligand-gated protein channels [9], and direct action of general anesthetics have been shown on recombinant nAChRs and mAChRs in vitro [59-61]. Volatile anesthetics block nAChRs at subanesthetic doses; however, this was inferred not to contribute to the minimal alveolar concentration (MAC) or LORR in animals [62]. The response of native ACh receptors to general anesthetics in vivo is not known. Response of a central neuron to clinically relevant doses of general anesthesia depends on the action of anesthesia on many types of neuronal receptors/channels, which may include GABAA receptors, N-methyl-D-aspartate (NMDA) receptors, 2-pore K+ channels, Ih channels, and presynaptic Ca2+ channels [9]. The way in which a central neuron responds to a particular general anesthetic cannot yet be determined based on the anesthetic responses of the different channels/receptors of the neuron. Thus, we will focus on the response of brain area to general anesthetics, in relation to how it participates in sleep/wake behavior. We will focus on the loss and recovery of consciousness induced by general anesthesia. Loss of consciousness (LOC) is typically tested by a loss of voluntary response to verbal command in humans. For a large variety of general anesthetics, the anesthetic dose that induces LOC in humans is highly correlated with that inducing a loss of righting reflex in animals [9].
1.4. Acetylcholine, Aging, and Dementia
Acetylcholine is important for cognitive processes such as learning, memory, and attention [63, 64]. Normal aging is associated with a decline in the number of cholinergic neurons in the basal forebrain in rats [65] and humans [66]. Aging is also accompanied by a reduction of cholinergic afferents in the cerebral cortex [67], with the most severe reduction found in the temporal lobe cortical association areas and the entorhinal cortex. A common dementia, Alzheimer's disease (AD), shows early pathology in basal forebrain cholinergic neurons [68-69]. The cholinergic hypothesis for AD, proposed in the 1980s, states that “a serious loss of cholinergic function in the CNS contributed significantly to the cognitive symptoms associated with AD and advance age” [64]. The latter hypothesis has contributed greatly to the development of animal models and cholinergic drugs for the therapeutic treatment of AD [64, 70, 71]. Main medical treatment of AD is AChE inhibitor, which provides modest, and mostly symptomatic improvement of cognitive and behavioral functions in AD patients [64, 70, 71].
2. PARTICIPATION OF CHOLINERGIC NEURONS IN GENERAL ANESTHESIA
2.1. Mesopontine Brainstem and Basal Forebrain
2.1.1. Acetylcholine Release
The level of ACh in many areas of the brain increases with arousal, typically decreases during general anesthesia, and recovers during emergence from anesthesia. A dose-dependent decrease in ACh release in the cerebral cortex was found after administration of barbiturates, propofol, isoflurane, and sevoflurane at 0.5 to 1.5 MAC [30, 72-74]. The decrease of ACh release in the frontal cortex was found to be < 40% of baseline at ≥ 0.5 MAC of isoflurane and sevoflurane, and 25 mg/kg i.p. propofol [74-78]. Sedative doses of midazolam, but not dexmedetomidine, also decreased cortical ACh release [76]. In contrast, 0.3 MAC nitrous oxide increased cortical ACh level [71], and ketamine increased ACh levels in the hippocampus [77] and prefrontal cortex [78].
A theta rhythm in the hippocampus could be induced during surgical anesthesia maintained by several anesthetics [35, 79], notably many inhalational anesthetics (e.g., ether, halothane, sevoflurane, cyclopropane) and some injectable anesthetics (e.g., ketamine and urethane). The theta induced under general anesthesia was blocked by atropine, administered either systematically or locally in the medial septal area [25, 35, 38, 79]. As indicated by the theta rhythm, the activity of septohippocampal cholinergic neurons persists under surgical anesthesia of some anesthetics [35, 79].
Several general anesthetics, halothane [34], isoflurane, enflurane, and ketamine [8], were shown to significantly decrease ACh release in the medial pontine reticular formation. ACh levels in the striatum were also shown to decrease with isoflurane, but the decrease was 2-4 fold smaller than that in the frontal cortex [72].
2.1.2. Cholinergic Drugs
In a study involving human volunteers, physostigmine, an AChE inhibitor that crosses the blood-brain barrier, reversed halothane-induced postoperative somnolence [80]. Physostigmine reversed LOC induced by propofol more consistently (9 of 11 subjects) than LOC induced by sevoflurane (5 of 9 subjects) [81, 82]. Consciousness was indicated by responding to verbal commands, an increase in the amplitude of the auditory steady-state response, and the bispectral index. In a double-blinded, randomized study on women, the bispectral index value while maintaining end-tidal sevoflurane at 0.6% [83], or 3% (1.5 MAC) [84], was not statistically different between physostigmine- and saline-injected groups. Behavioral recovery after discontinuation of sevoflurane, based on ratings of orientation, sedation, and sitting up, was also not different between physostigmine and saline groups [83, 84]. The emergence from desflurane anaesthesia was not altered by physostigmine, except earlier return to spontaneous breathing and shorter extubation time in a group that had > 150 min of anesthesia [85]. In studies on reversal of ketamine anesthesia, one study reported that physostigmine did not reduce recovery time or adverse emergence phenomena associated with ketamine, such as hallucinations and restlessness [86]. In another study, physostigmine was reported to shorten recovery from ketamine anesthesia [87].
Cholinergic agonists have been shown to mitigate or reverse the behavioral effects of a general anesthetic in animals. In rats exposed to ongoing sevoflurane or desflurane concentration that induced LORR, administration of nicotine to the midline thalamus restored righting and mobility [88]. Intrathalamic pretreatment with the nicotinic antagonist, mecamylamine, prevented the nicotine-induced arousal response but did not lower the sevoflurane dose associated with LORR [88]. The lack of effect of a nicotinic antagonist suggests that blocking tonic nicotinic excitation in the midline thalamus does not affect anesthetic response. Intracerebroventricular infusion of AChE inhibitor neostigmine, or muscarinic agonist oxotremorine, in rats, reversed the effects of isoflurane on the electroencephalogram (EEG) and increased spontaneous limb and orofacial exploratory movements [89].
Physostigmine has mixed effects in reversing general anesthesia in animals. After a transient, (< 30 min) increase, halothane MAC in dogs was decreased by i.v. physostigmine [90]. However, isoflurane MAC in rats was significantly increased by physostigmine [91]. Under isoflurane (0.9-1%) anesthesia in rats, physostigmine failed to restore righting but increased EEG activation, as indicated by a theta rhythm and a decreased probability of EEG burst suppression [92]. Paradoxically, during isoflurane anesthesia, a dose of ketamine increased EEG burst suppression but accelerated emergence time [93], which may have resulted from ketamine’s induction of cortical ACh release.
Administration of the mixed cholinergic agonist carbachol into the medial prefrontal cortex in rodents activated the EEG, increased local ACh levels by >500%, and led to behavioral recovery despite continuous administration of 1.9-2.4% sevoflurane [94]. In contrast, administration of carbachol in the parietal cortex also activated the EEG (shown by a low-voltage fast activity) but did not induce behavioral emergence [94]. Microdialysis delivery of caffeine or other adenosine A1 receptor antagonist into the prefrontal cortex of mice increased ACh release locally and in the pontine reticular formation, activated the cortical EEG, and decreased the time of emergence from isoflurane anesthesia [95]. Delivery of an A1 receptor agonist into the prefrontal cortex had the opposite effect of decreasing local ACh levels and arousal [95]. Thus, ACh in the prefrontal cortex may promote arousal and reverse general anesthetic effects, perhaps through prefrontal connections with wake-promoting centers in the brainstem and diencephalon.
2.1.3. Cholinergic Neuronal Inactivation and Stimulation
The specific cholinergic forebrain pathways mediating the effects of general anesthesia in animals have been investigated through inactivation, lesion, or genetic ablation of cholinergic neurons and studied by observing the duration of LORR. Alternatively, stimulation of the basal forebrain by drugs, or optogenetically, tends to decrease the anesthetic-induced behavioral effects.
Early experiments were focused on barbital-induced LORR. Lesion of the medial septum increased the barbital-induced LORR duration [96]. Systemic administration of hemicholinium-3, a choline uptake inhibitor, increased pentobarbital-induced LORR duration and decreased cortical cholinergic activity [97]. In contrast, systemic choline injection [97], septal infusion of bicuculline (GABAA receptor antagonist) or phenylephrine (norepinephrine α1 agonist) shortened the LORR duration induced by pentobarbital, accompanied by an increase in hippocampal cholinergic activity [98, 99]. Systemic methamphetamine also shortened pentobarbital-induced LORR duration by activating dopamine D1 and D2 receptors, but activation of central muscarinic cholinergic receptors was also required [100].
Inactivation of the medial septum by muscimol, a GABAA receptor agonist, decreased the dose of general anesthetic (halothane, pentobarbital, or propofol) required to induce LORR in rats [101]. Electrolytic lesion of the medial septum and vertical limb of the diagonal band increased the duration of LORR induced by both volatile (halothane and isoflurane) and injectable (propofol and pentobarbital) anesthetics [102]. Rats with a selective lesion of medial septal cholinergic neurons by immunotoxin 192 IgG-saporin showed a longer emergence time after isoflurane anesthesia [103] or after cumulative doses of propofol i.p. [104]. Hippocampal 62-100 Hz gamma activity decreased with isoflurane dose, with a decrease that was greater in 192 IgG-saporin lesioned rats than control rats [103]. Similarly, 192 IgG-saporin lesions of the nucleus basalis cholinergic neurons prolonged the duration of LORR induced by propofol, pentobarbital, and halothane [105, 106]. Genetic ablation of the nucleus basalis also prolonged the LORR duration induced by propofol and isoflurane, while stimulation of cholinergic neurons with ‘designer receptor exclusively activated by designer drugs’ or optogenetically hastened emergence [106].
It has been suggested that sensitivity to an anesthesia can be more readily measured under near-equilibrium conditions, rather than using time of LORR onset, recovery, or duration that depends strongly on pharmacokinetics [11]. Anesthetic sensitivity or potency has been estimated in only a few studies on the basal forebrain. Rats with a selective cholinergic lesion of the basal forebrain (by intraventricular 192 IgG-saporin infusion) show enhanced propofol sensitivity compared to control rats, using a composite anesthesia behavioral score [104]. Using a dose that induced LORR in 50% in a population (ED50), electrolytic septal lesioned rats were estimated to have ED50 (LORR) of 112 mg/kg i.p. for propofol and 0.90% for isoflurane, significantly lower than the respective measures of 139 mg/kg i.p. propofol and 1.02% isoflurane in control rats [102]. In mice with no basal forebrain ACh release, following genetic ablation of vAChT in the basal forebrain, ED50 (LORR) was 0.72% for isoflurane and 124 mg/kg i.p. for ketamine, significantly lower than the respective measures of 0.86% isoflurane and 160 mg/kg i.p. ketamine in control wildtype mice [107]. Hippocampal gamma power showed a larger decrease in power in ACh-deficient mice than control mice for pre-LORR doses of isoflurane and ketamine [107].
The duration of loss of tail-pinch or foot-pinch response was measured in a few studies of basal forebrain manipulation. Compared with controls, rats with a selective cholinergic lesion of the medial septum-diagonal band of Broca [103] or nucleus basalis [105], or rats with muscimol inactivation of the medial septum [101] showed increased duration of loss of tail-pinch or foot-pinch response. MAC, or a population measure of the ED50 of immobility to incision pain, to our knowledge, has not been reported in animals with basal forebrain manipulations. MAC typically requires a higher anesthetic dose than ED50 (LORR). The medial septum-diagonal band is recognized as a pro-nociceptive brain area, but more for persistent pain with a long-term cognitive-affective nature [108].
In summary, the above studies suggest that the basal forebrain cholinergic system participates in the induction (sensitivity) and emergence from general anesthesia induced by inhalation anesthetic (halothane and isoflurane), and injectable anesthetics including propofol, pentobarbital, and ketamine.
2.2. Habenula and Striatum
Metabolism (14C-deoxyglucose uptake) in the medial habenula and IPN was found to increase during general anesthesia induced by pentobarbital, ether, and choral hydrate [109]. ACh levels in the IPN were increased by anesthesia during 3% halothane, or 100 mg/kg i.p. ketamine [110]. Infusion of nicotine into the IPN prolonged the duration of recovery from halothane anesthesia, and infusion of a nicotinic antagonist blocked the effect of nicotine but had no effect on anesthesia recovery time on its own [111]. Medial habenula and IPN neurons appear to be anesthesia-active compared to other wake-active neurons; they were also more active during NREM sleep state than the awake state [109]. GABAergic afferents from IPN [112] may be responsible for inhibiting brainstem wake-active neurons, including raphe and LDT neurons. As a result, ACh release in the mesopontine reticular formation would be inhibited [8, 34], and the duration of anesthesia would be prolonged.
Glutamatergic transmission of lateral habenula neurons in mice was shown to be responsible for anesthetic sensitivity to propofol, and interference of glutamate release shifted the ED50 (LORR) of propofol from 3.8 to 10.8 mg/kg i.v. [113]. The lateral habenula projects GABAergic inhibition to wake-related brain areas, including raphe nuclei, ventral tegmental area [114], and LDT/PPN [7].
Inactivation of the ventral striatum (nucleus accumbens) by muscimol increased the potency of several general anesthetics such as pentobarbital and halothane [115]. Ventral striatum output to PPN via the ventral pallidum was suggested to mediate movements and arousal [11, 55]. Thus, ventral striatum inactivation potentiates general anesthesia, but the role of cholinergic striatal neurons is not known. The involvement of the dorsal striatum in general anesthesia is also not known.
3. CHOLINERGIC SYSTEM AND POSTOPERATIVE COGNITIVE DISORDER
Following surgery under general anesthesia, 10-50% of patients over 60 years old may experience postoperative delirium or POCD that lasts for days to years [116-119]. Postoperative delirium is a confused state characterized by impaired attention, abnormal level of consciousness, and thought disorganization. It typically starts within 3 days post-operation and lasts for hours or days [120], or is defined as up to one week in hospital [121]. POCD, a long-lasting state of cognitive impairment, measured by serial performance on a neuropsychological test battery, was reported up to 5 [117] or 7.5 years after coronary artery bypass operation [121]. POCD is known to be related to multiple factors such as age, educational level, neurological status, duration of anesthesia, and the type of surgery. The anesthetic requirement is reduced in the elderly [122], and a poor preoperative cognitive status was associated with a lower propofol requirement [123]. Definitive causes of POCD are not known. Some possible mechanisms of POCD have been suggested, which include inflammation, caspase activation, apoptosis, Alzheimer’s pathology such as amyloid-beta and tau accumulation [119].
POCD mechanisms have been studied in animal models, where predisposing and experimental factors can be better controlled. Here, only the role of ACh in POCD in preclinical studies is reviewed, including some studies in which surgery alone was assumed to induce POCD. The focus on cholinergic involvement is justified because of the important role of ACh in cognitive processing (section 1.4). Furthermore, aging and Alzheimer’s disease are associated with cognitive decline and cholinergic neuronal pathology (section 1.4). A cholinergic hypothesis for POCD may be compared to a cholinergic hypothesis for Alzheimer’s disease, which provides a theoretical framework for understanding cognitive dysfunction and stimulates the development of therapy using cholinergic agents. The cholinergic hypothesis does not exclude other hypotheses and treatment options for POCD. For instance, activation of α5-GABAARs was shown to be responsible for the anesthesia-induced cognitive deficit in mice [124].
Animal studies have shown the role of ACh in POCD. Culley et al. [125] showed that a combination of 2 h exposure to 1.2% isoflurane in 70% nitrous oxide/30% oxygen decreased the performance of both adult (6-months old) and aged (20 months) old Fischer 344 rats on a radial arm maze, tested 2-21 days post-anesthetic exposure. In contrast, propofol anesthesia did not affect maze performance of aged rats [126], suggesting that general anesthesia or suppression of cortical ACh release (found after both isoflurane and propofol anesthesia) is not sufficient to induce post-anesthetic memory deficit. Other studies used the hidden platform in the Morris water maze to test spatial learning and memory. Aged (17-months old) rats, after surgery with 2-h exposure to 1.4-1.7% isoflurane alone, induced a performance deficit in the hidden platform task for 3-7 days after operation; the control group underwent surgery with propofol anesthesia [127]. The study also showed that isoflurane-surgery treated rats with severe impairment in maze performance was associated with a significant reduction in hippocampal ACh levels [127]. Su et al. [128] showed that spatial learning in the water maze was impaired for 2 weeks after isoflurane anesthesia (1.2% for 6 h) in 18-month old mice, accompanied by a significant decrease of ChAT protein levels. Pre-treatment of the aged mice with anticholinesterase donezepil before isoflurane anesthesia attenuated the deficits in learning and in ChAT levels induced by isoflurane anesthesia [128]. In a subsequent study of the same group, the development of POCD in mice was shown to be dependent on the state of central cholinergic neurons [129]. Appendectomy was done with neuroleptic (fentanyl and droperidol) anesthesia, and pretreatment with donepezil prevented the spatial memory impairment and ChAT decrease induced by anesthesia-surgery [129]. In addition, while normal 2-month old adult mice did not show learning impairment after surgery/anesthesia, adult mice with a lesion of the basal forebrain cholinergic neurons by murine-p75-saporin showed deficits of learning and ChAT, similar to aged mice after treatment with anesthesia-surgery [129].
The mechanism by which donezepil mitigates POCD in aged and ACh-deficient rodents is not known. However, an effect of ACh in regulating neuroinflammation and neuropathology is known [130-132], often demonstrated by an α7 nAChR agonist. After exposure to 1.3% isoflurane for 4 h, 20-month old rats showed impairment in learning and remembering a hidden platform in a water maze, and an acute post-anesthesia increase in apoptosis and interleukin-1β (IL-1β), but not necrosis-tumor factor α (TNFα) or IL-10, in the hippocampus [133]. Pretreatment with α7 nAChR agonist GTS-21 in the elderly rats alleviated the isoflurane-induced learning and memory impairment, and the acute apoptosis and IL-1β increase [133]. In a surgical-trauma model on 18-month mice, pretreatment with α7 nAChR agonist, PNU 282987, mitigated the surgery-induced impairment in context-dependent fear conditioning and inflammatory response [134]; surgery was performed to repair an open tibia fracture under chloral hydrate anesthesia, resulting in a rise of pro-inflammatory cytokines IL-10 and TNFα in the blood of elderly mice [134]. In elderly mice that underwent laparotomy under sevoflurane anesthesia, α7 nAChR agonist, varenicline, reduced the surgery-induced DNA damage, tau mislocalization, and cognitive impairment on a novel object recognition test [135]; sevoflurane anesthesia alone did not induce the same effects as surgery with sevoflurane [135]. Electroacupuncture stimulation was shown to reproduce some of the effects of an α7-nAChR agonist. Electroacupuncture stimulation during and following surgery in aged rats alleviated POCD, increased density of α7-nAChR positive neurons, and decreased expression of pro-inflammatory cytokines TNF-α and IL-1β [136]. Aggravation of POCD by amyloid-beta may involve a novel nAChR subtype in the basal forebrain that is highly sensitive to amyloid-beta inhibition [137].
In summary, animal models have shown that aged animals, and those with basal forebrain ACh-deficiency, are susceptible to POCD. Administration of AChE or α7-nAChR agonist mitigates cognitive dysfunctions and reduces inflammatory cytokines after anesthesia and surgery. Questions may be raised regarding whether or not the animal models, or more specifically, the behavioral tests for animal memory in a spatial maze or a fear-conditioning chamber, are appropriate substitutes for the cognitive tests in patients. AChE inhibitor treatment of postoperative delirium did not yield beneficial results in patients [118, 120], but we are not aware of systematic studies of cholinergic drug treatment of POCD patients. Further understanding and definition of the neurocognitive deficits of POCD patients will help the design of more appropriate animal models.
CONCLUSION
An important function of ACh is behavioral and cortical activation, served mainly by cholinergic neurons in the mesopontine brainstem and basal forebrain. Cortical activation modulates the excitability of neurons in the cerebral cortex and establishes network oscillations that facilitate cognitive processing and behavior. Suppression of cholinergic neurons and function by most types of general anesthetics contribute to the desired outcomes of anesthesia, including amnesia, immobility, and loss of awareness. Restoration or enhancement of cholinergic function can facilitate emergence from anesthesia. ACh function is decreased by aging and general anesthesia and it is critical for cognitive processing. Thus, a hypothesis that enhancement of cholinergic function will mitigate long-term postoperative cognitive dysfunction is reasonable. The hypothesis will promote further basic and clinical research on the role of ACh in general anesthesia and POCD.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The study was supported by operating grants from the Canadian Institutes of Health Research (MOP-15685) and Natural Science and Engineering Research Council of Canada (1037-2008) to LSL, and Shenzhen Science and Technology Innovation Committee (Grant No. JCYJ20190809181401666) to TL.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Mesulam M.M., Mufson E.J., Wainer B.H., Levey A.I. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience. 1983;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 2.Semba K. Multiple output pathways of the basal forebrain: organization, chemical heterogeneity, and roles in vigilance. Behav. Brain Res. 2000;115(2):117–141. doi: 10.1016/S0166-4328(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 3.Woolf N.J., Butcher L.L. Cholinergic systems mediate action from movement to higher consciousness. Behav. Brain Res. 2011;221(2):488–498. doi: 10.1016/j.bbr.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 4.Manaye K.F., Zweig R., Wu D., Hersh L.B., De Lacalle S., Saper C.B., German D.C. Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain. Neuroscience. 1999;89(3):759–770. doi: 10.1016/S0306-4522(98)00380-7. [DOI] [PubMed] [Google Scholar]
- 5.Mufson E.J., Ma S.Y., Cochran E.J., Bennett D.A., Beckett L.A., Jaffar S., Saragovi H.U., Kordower J.H. Loss of nucleus basalis neurons containing trkA immunoreactivity in individuals with mild cognitive impairment and early Alzheimer’s disease. J. Comp. Neurol. 2000;427(1):19–30. doi: 10.1002/1096-9861(20001106)427:1<19:AID-CNE2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Semba K., Fibiger H.C. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J. Comp. Neurol. 1992;323(3):387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- 7.Satoh K., Fibiger H.C. Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J. Comp. Neurol. 1986;253(3):277–302. doi: 10.1002/cne.902530302. [DOI] [PubMed] [Google Scholar]
- 8.Lydic R., Baghdoyan H.A. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103(6):1268–1295. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Franks N.P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 2008;9(5):370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 10.Saper C.B., Fuller P.M., Pedersen N.P., Lu J., Scammell T.E. Sleep state switching. Neuron. 2010;68(6):1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung L.S., Luo T., Ma J., Herrick I. Brain areas that influence general anesthesia. Prog. Neurobiol. 2014;122:24–44. doi: 10.1016/j.pneurobio.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Qiu M.H., Chen M.C., Fuller P.M., Lu J. Stimulation of the pontine parabrachial nucleus promotes wakefulness via extra-thalamic forebrain circuit nodes. Curr. Biol. 2016;26(17):2301–2312. doi: 10.1016/j.cub.2016.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posner J.B., Saper C.B., Schiff N., Plum F. Plum and Posner’s Diagnosis of Stupor and Coma. New York, Oxford: 2007. [Google Scholar]
- 14.Parvizi J., Damasio A.R. Neuroanatomical correlates of brainstem coma. Brain. 2003;126(Pt 7):1524–1536. doi: 10.1093/brain/awg166. [DOI] [PubMed] [Google Scholar]
- 15.Lu J., Sherman D., Devor M., Saper C.B. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 16.Fuller P.M., Sherman D., Pedersen N.P., Saper C.B., Lu J. Reassessment of the structural basis of the ascending arousal system. J. Comp. Neurol. 2011;519(5):933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janickova H., Rosborough K., Al-Onaizi M., Kljakic O., Guzman M.S., Gros R., Prado M.A., Prado V.F. Deletion of the vesicular acetylcholine transporter from pedunculopontine/laterodorsal tegmental neurons modifies gait. J. Neurochem. 2017;140(5):787–798. doi: 10.1111/jnc.13910. [DOI] [PubMed] [Google Scholar]
- 18.Karachi C., Grabli D., Bernard F.A., Tandé D., Wattiez N., Belaid H., Bardinet E., Prigent A., Nothacker H.P., Hunot S., Hartmann A., Lehéricy S., Hirsch E.C., François C. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J. Clin. Invest. 2010;120(8):2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein C., Koch K., Hopfeld J., Lobentanzer S., Lau H., Klein J. Impaired hippocampal and thalamic acetylcholine release in P301L tau-transgenic mice. Brain Res. Bull. 2019;152:134–142. doi: 10.1016/j.brainresbull.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Buzsáki G., Bickford R.G., Ponomareff G., Thal L.J., Mandel R., Gage F.H. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J. Neurosci. 1988;8(11):4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dringenberg H.C., Olmstead M.C. Integrated contributions of basal forebrain and thalamus to neocortical activation elicited by pedunculopontine tegmental stimulation in urethane-anesthetized rats. Neuroscience. 2003;119(3):839–853. doi: 10.1016/S0306-4522(03)00197-0. [DOI] [PubMed] [Google Scholar]
- 22.Jones B.E. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol. Sci. 2005;26(11):578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Xu M., Chung S., Zhang S., Zhong P., Ma C., Chang W.C., Weissbourd B., Sakai N., Luo L., Nishino S., Dan Y. Basal forebrain circuit for sleep-wake control. Nat. Neurosci. 2015;18(11):1641–1647. doi: 10.1038/nn.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson J., Manseau F., Ducharme G., Amilhon B., Vigneault E., El Mestikawy S., Williams S. Optogenetic Activation of Septal Glutamatergic Neurons Drive Hippocampal Theta Rhythms. J. Neurosci. 2016;36(10):3016–3023. doi: 10.1523/JNEUROSCI.2141-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dannenberg H., Pabst M., Braganza O., Schoch S., Niediek J., Bayraktar M., Mormann F., Beck H. Synergy of direct and indirect cholinergic septo-hippocampal pathways coordinates firing in hippocampal networks. J. Neurosci. 2015;35(22):8394–8410. doi: 10.1523/JNEUROSCI.4460-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanco-Centurion C., Gerashchenko D., Shiromani P.J. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J. Neurosci. 2007;27(51):14041–14048. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaur S., Junek A., Black M.A., Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J. Neurosci. 2008;28(2):491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kametani H., Kawamura H. Alterations in acetylcholine release in the rat hippocampus during sleep-wakefulness detected by intracerebral dialysis. Life Sci. 1990;47(5):421–426. doi: 10.1016/0024-3205(90)90300-G. [DOI] [PubMed] [Google Scholar]
- 29.Marrosu F., Portas C., Mascia M.S., Casu M.A., Fà M., Giagheddu M., Imperato A., Gessa G.L. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671(2):329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 30.Phillis J.W. Acetylcholine release from the central nervous system: a 50-year retrospective. Crit. Rev. Neurobiol. 2005;17(3-4):161–217. doi: 10.1615/CritRevNeurobiol.v17.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 31.Dudar J.D., Whishaw I.Q., Szerb J.C. Release of acetylcholine from the hippocampus of freely moving rats during sensory stimulation and running. Neuropharmacology. 1979;18(8-9):673–678. doi: 10.1016/0028-3908(79)90034-0. [DOI] [PubMed] [Google Scholar]
- 32.Détári L., Rasmusson D.D., Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Prog. Neurobiol. 1999;58(3):249–277. doi: 10.1016/S0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 33.Lee M.G., Hassani O.K., Alonso A., Jones B.E. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J. Neurosci. 2005;25(17):4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keifer J.C., Baghdoyan H.A., Lydic R. Pontine cholinergic mechanisms modulate the cortical electroencephalographic spindles of halothane anesthesia. Anesthesiology. 1996;84(4):945–954. doi: 10.1097/00000542-199604000-00023. [DOI] [PubMed] [Google Scholar]
- 35.Vanderwolf C.H. Cerebral activity and behavior: control by central cholinergic and serotonergic systems. Int. Rev. Neurobiol. 1988;30:225–340. doi: 10.1016/S0074-7742(08)60050-1. [DOI] [PubMed] [Google Scholar]
- 36.Leung L.S. Generation of theta and gamma rhythms in the hippocampus. Neurosci. Biobehav. Rev. 1998;22(2):275–290. doi: 10.1016/S0149-7634(97)00014-6. [DOI] [PubMed] [Google Scholar]
- 37.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- 38.Bland B.H., Oddie S.D. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav. Brain Res. 2001;127(1-2):119–136. doi: 10.1016/S0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 39.Kramis R., Vanderwolf C.H., Bland B.H. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp. Neurol. 1975;49(1 Pt 1):58–85. doi: 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- 40.Tai S.K., Ma J., Ossenkopp K.P., Leung L.S. Activation of the hippocampus by cholinergic septohippocampal neurons during vestibular stimulation. Hippocampus. 2012;22:914–925. doi: 10.1002/hipo.20955. [DOI] [PubMed] [Google Scholar]
- 41.Vandecasteele M., Varga V., Berényi A., Papp E., Barthó P., Venance L., Freund T.F., Buzsáki G. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc. Natl. Acad. Sci. USA. 2014;111(37):13535–13540. doi: 10.1073/pnas.1411233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee M.G., Chrobak J.J., Sik A., Wiley R.G., Buzsáki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62(4):1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 43.Metherate R., Cox C.L., Ashe J.H. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J. Neurosci. 1992;12(12):4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung L.W.S. Spectral analysis of hippocampal EEG in the freely moving rat: effects of centrally active drugs and relations to evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1985;60(1):65–77. doi: 10.1016/0013-4694(85)90952-6. [DOI] [PubMed] [Google Scholar]
- 45.Berntson G.G., Shafi R., Sarter M. Specific contributions of the basal forebrain corticopetal cholinergic system to electroencephalographic activity and sleep/waking behaviour. Eur. J. Neurosci. 2002;16(12):2453–2461. doi: 10.1046/j.1460-9568.2002.02310.x. [DOI] [PubMed] [Google Scholar]
- 46.Qin C., Luo M. Neurochemical phenotypes of the afferent and efferent projections of the mouse medial habenula. Neuroscience. 2009;161(3):827–837. doi: 10.1016/j.neuroscience.2009.03.085. [DOI] [PubMed] [Google Scholar]
- 47.Viswanath H., Carter A.Q., Baldwin P.R., Molfese D.L., Salas R. The medial habenula: still neglected. Front. Hum. Neurosci. 2014;7:931. doi: 10.3389/fnhum.2013.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi Y., Sano Y., Vannoni E., Goto H., Suzuki H., Oba A., Kawasaki H., Kanba S., Lipp H-P., Murphy N.P., Wolfer D.P., Itohara S. Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Front. Behav. Neurosci. 2013;7:17. doi: 10.3389/fnbeh.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haun F., Eckenrode T.C., Murray M. Habenula and thalamus cell transplants restore normal sleep behaviors disrupted by denervation of the interpeduncular nucleus. J. Neurosci. 1992;12(8):3282–3290. doi: 10.1523/JNEUROSCI.12-08-03282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valjakka A., Vartiainen J., Tuomisto L., Tuomisto J.T., Olkkonen H., Airaksinen M.M. The fasciculus retroflexus controls the integrity of REM sleep by supporting the generation of hippocampal theta rhythm and rapid eye movements in rats. Brain Res. Bull. 1998;47(2):171–184. doi: 10.1016/S0361-9230(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 51.Aizawa H., Yanagihara S., Kobayashi M., Niisato K., Takekawa T., Harukuni R., McHugh T.J., Fukai T., Isomura Y., Okamoto H. The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation. J. Neurosci. 2013;33(20):8909–8921. doi: 10.1523/JNEUROSCI.4369-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dautan D., Huerta-Ocampo I., Witten I.B., Deisseroth K., Bolam J.P., Gerdjikov T., Mena-Segovia J. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J. Neurosci. 2014;34(13):4509–4518. doi: 10.1523/JNEUROSCI.5071-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim S.A.O., Kang U.J., McGehee D.S. Striatal cholinergic interneuron regulation and circuit effects. Front. Synaptic Neurosci. 2014;6:22. doi: 10.3389/fnsyn.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Da Cunha C., Gomez-A A., Blaha C.D. The role of the basal ganglia in motivated behavior. Rev. Neurosci. 2012;23(5-6):747–767. doi: 10.1515/revneuro-2012-0063. [DOI] [PubMed] [Google Scholar]
- 55.Mogenson G.J., Brudzynski S.M., Wu M., Yang C.R., Yim C.Y. In: Limbic Motor Circuits and Neuropsychiatry. Kalivas P.W., Barnes C.D., editors. Boca Raton: CRC Press; 1994. From motivation to action: a review of dopaminergic regulation of limbic–nucleus accumbens–ventral pallidum–pedunculopontine nucleus circuits involved in limbic–motor integration. [Google Scholar]
- 56.Brown D.A. Acetylcholine and cholinergic receptors. Brain Neurosci. Adv. 2019;3:2398212818820506. doi: 10.1177/2398212818820506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yakel J.L. Cholinergic receptors: functional role of nicotinic ACh receptors in brain circuits and disease. Pflugers Arch. 2013;465(4):441–450. doi: 10.1007/s00424-012-1200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thiele A. Muscarinic signaling in the brain. Annu. Rev. Neurosci. 2013;36:271–294. doi: 10.1146/annurev-neuro-062012-170433. [DOI] [PubMed] [Google Scholar]
- 59.Flood P., Ramirez-Latorre J., Role L. α 4 β 2 neuronal nicotinic acetylcholine receptors in the central nervous system are inhibited by isoflurane and propofol, but α 7-type nicotinic acetylcholine receptors are unaffected. Anesthesiology. 1997;86(4):859–865. doi: 10.1097/00000542-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 60.Nietgen G.W., Hönemann C.W., Chan C.K., Kamatchi G.L., Durieux M.E. Volatile anaesthetics have differential effects on recombinant m1 and m3 muscarinic acetylcholine receptor function. Br. J. Anaesth. 1998;81(4):569–577. doi: 10.1093/bja/81.4.569. [DOI] [PubMed] [Google Scholar]
- 61.Hirota K., Hashimoto Y., Lambert D.G. Interaction of intravenous anesthetics with recombinant human M1-M3 muscarinic receptors expressed in chinese hamster ovary cells. Anesth. Analg. 2002;95(6):1607–1610. doi: 10.1097/00000539-200212000-00025. [DOI] [PubMed] [Google Scholar]
- 62.Flood P., Sonner J.M., Gong D., Coates K.M. Heteromeric nicotinic inhibition by isoflurane does not mediate MAC or loss of righting reflex. Anesthesiology. 2002;97(4):902–905. doi: 10.1097/00000542-200210000-00023. [DOI] [PubMed] [Google Scholar]
- 63.Sarter M., Hasselmo M.E., Bruno J.P., Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Brain Res. Rev. 2005;48(1):98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Bartus R.T. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 2000;163(2):495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 65.Smith M.L., Booze R.M. Cholinergic and GABAergic neurons in the nucleus basalis region of young and aged rats. Neuroscience. 1995;67(3):679–688. doi: 10.1016/0306-4522(95)00076-U. [DOI] [PubMed] [Google Scholar]
- 66.de Lacalle S., Iraizoz I., Ma Gonzalo L. Differential changes in cell size and number in topographic subdivisions of human basal nucleus in normal aging. Neuroscience. 1991;43(2-3):445–456. doi: 10.1016/0306-4522(91)90307-A. [DOI] [PubMed] [Google Scholar]
- 67.Geula C., Mesulam M.M. Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer’s disease. Cereb. Cortex. 1996;6(2):165–177. doi: 10.1093/cercor/6.2.165. [DOI] [PubMed] [Google Scholar]
- 68.Whitehouse P.J., Price D.L., Struble R.G., Clark A.W., Coyle J.T., Delon M.R. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215(4537):1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 69.Perry E.K., Johnson M., Kerwin J.M., Piggott M.A., Court J.A., Shaw P.J., Ince P.G., Brown A., Perry R.H. Convergent cholinergic activities in aging and Alzheimer’s disease. Neurobiol. Aging. 1992;13(3):393–400. doi: 10.1016/0197-4580(92)90113-C. [DOI] [PubMed] [Google Scholar]
- 70.Hampel H., Mesulam M.M., Cuello A.C., Farlow M.R., Giacobini E., Grossberg G.T., Khachaturian A.S., Vergallo A., Cavedo E., Snyder P.J., Khachaturian Z.S. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 2018;141(7):1917–1933. doi: 10.1093/brain/awy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Francis P.T., Palmer A.M., Snape M., Wilcock G.K. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J. Neurol. Neurosurg. Psychiatry. 1999;66(2):137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shichino T., Murakawa M., Adachi T., Nakao S., Shinomura T., Kurata J., Mori K. Effects of isoflurane on in vivo release of acetylcholine in the rat cerebral cortex and striatum. Acta Anaesthesiol. Scand. 1997;41(10):1335–1340. doi: 10.1111/j.1399-6576.1997.tb04654.x. [DOI] [PubMed] [Google Scholar]
- 73.Shichino T., Murakawa M., Adachi T., Arai T., Miyazaki Y., Mori K. Effects of inhalation anaesthetics on the release of acetylcholine in the rat cerebral cortex in vivo. Br. J. Anaesth. 1998;80(3):365–370. doi: 10.1093/bja/80.3.365. [DOI] [PubMed] [Google Scholar]
- 74.Kikuchi T., Wang Y., Sato K., Okumura F. In vivo effects of propofol on acetylcholine release from the frontal cortex, hippocampus and striatum studied by intracerebral microdialysis in freely moving rats. Br. J. Anaesth. 1998;80(5):644–648. doi: 10.1093/bja/80.5.644. [DOI] [PubMed] [Google Scholar]
- 75.Dong H.L., Fukuda S., Murata E., Higuchi T. Excitatory and inhibitory actions of isoflurane on the cholinergic ascending arousal system of the rat. Anesthesiology. 2006;104(1):122–133. doi: 10.1097/00000542-200601000-00018. [DOI] [PubMed] [Google Scholar]
- 76.Nemoto C., Murakawa M., Hakozaki T., Imaizumi T., Isosu T., Obara S. Effects of dexmedetomidine, midazolam, and propofol on acetylcholine release in the rat cerebral cortex in vivo. J. Anesth. 2013;27(5):771–774. doi: 10.1007/s00540-013-1589-5. [DOI] [PubMed] [Google Scholar]
- 77.Sato K., Wu J., Kikuchi T., Wang Y., Watanabe I., Okumura F. Differential effects of ketamine and pentobarbitone on acetylcholine release from the rat hippocampus and striatum. Br. J. Anaesth. 1996;77(3):381–384. doi: 10.1093/bja/77.3.381. [DOI] [PubMed] [Google Scholar]
- 78.Pal D., Hambrecht-Wiedbusch V.S., Silverstein B.H., Mashour G.A. Electroencephalographic coherence and cortical acetylcholine during ketamine-induced unconsciousness. Br. J. Anaesth. 2015;114(6):979–989. doi: 10.1093/bja/aev095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stumpf C. Drug action on the electrical activity of the hippocampus. Int. Rev. Neurobiol. 1965;8:77–138. doi: 10.1016/S0074-7742(08)60756-4. [DOI] [PubMed] [Google Scholar]
- 80.Hill G.E., Stanley T.H., Sentker C.R. Physostigmine reversal of postoperative somnolence. Can. Anaesth. Soc. J. 1977;24(6):707–711. doi: 10.1007/BF03006714. [DOI] [PubMed] [Google Scholar]
- 81.Meuret P., Backman S.B., Bonhomme V., Plourde G., Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology. 2000;93(3):708–717. doi: 10.1097/00000542-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 82.Plourde G., Chartrand D., Fiset P., Font S., Backman S.B. Antagonism of sevoflurane anaesthesia by physostigmine: effects on the auditory steady-state response and bispectral index. Br. J. Anaesth. 2003;91(4):583–586. doi: 10.1093/bja/aeg209. [DOI] [PubMed] [Google Scholar]
- 83.Paraskeva A., Papilas K., Fassoulaki A., Melemeni A., Papadopoulos G. Physostigmine does not antagonize sevoflurane anesthesia assessed by bispectral index or enhances recovery. Anesth. Analg. 2002;94(3):569–572. doi: 10.1097/00000539-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 84.Paraskeva A., Staikou C., Diamadis M., Siafaka I., Fassoulaki A. Anesthesia with 1.5 minimum alveolar concentration sevoflurane is not altered by physostigmine as measured by bispectral and clinical indices. J. Clin. Anesth. 2005;17(8):581–585. doi: 10.1016/j.jclinane.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 85.Röhm K.D., Riechmann J., Boldt J., Schöllhorn T., Piper S.N. Retracted: Do patients profit from physostigmine in recovery from desflurane anaesthesia? Acta Anaesthesiol. Scand. 2007;51(3):278–283. doi: 10.1111/j.1399-6576.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 86.Drummond J.C., Brebner J., Galloon S., Young P.S. A randomized evaluation of the reversal of ketamine by physostigmine. Can. Anaesth. Soc. J. 1979;26(4):288–295. doi: 10.1007/BF03006289. [DOI] [PubMed] [Google Scholar]
- 87.Toro-Matos A., Rendon-Platas A.M., Avila-Valdez E., Villarreal-Guzman R.A. Physostigmine antagonizes ketamine. Anesth. Analg. 1980;59(10):764–767. doi: 10.1213/00000539-198010000-00008. [DOI] [PubMed] [Google Scholar]
- 88.Alkire M.T., McReynolds J.R., Hahn E.L., Trivedi A.N. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107(2):264–272. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 89.Hudetz A.G., Wood J.D., Kampine J.P. Cholinergic reversal of isoflurane anesthesia in rats as measured by cross-approximate entropy of the electroencephalogram. Anesthesiology. 2003;99(5):1125–1131. doi: 10.1097/00000542-200311000-00019. [DOI] [PubMed] [Google Scholar]
- 90.Horrigan R.W. Physostigmine and anesthetic requirement for halothane in dogs. Anesth. Analg. 1978;57(2):180–185. doi: 10.1213/00000539-197803000-00006. [DOI] [PubMed] [Google Scholar]
- 91.Zucker J. Central cholinergic depression reduces MAC for isoflurane in rats. Anesth. Analg. 1991;72(6):790–795. doi: 10.1213/00000539-199106000-00013. [DOI] [PubMed] [Google Scholar]
- 92.Kenny J.D., Chemali J.J., Cotten J.F., Van Dort C.J., Kim S.E., Ba D., Taylor N.E., Brown E.N., Solt K. Physostigmine and Methylphenidate Induce Distinct Arousal States During Isoflurane General Anesthesia in Rats. Anesth. Analg. 2016;123(5):1210–1219. doi: 10.1213/ANE.0000000000001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hambrecht-Wiedbusch V.S., Li D., Mashour G.A. Paradoxical Emergence: Administration of Subanesthetic Ketamine during Isoflurane Anesthesia Induces Burst Suppression but Accelerates Recovery. Anesthesiology. 2017;126(3):482–494. doi: 10.1097/ALN.0000000000001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pal D., Dean J.G., Liu T., Li D., Watson C.J., Hudetz A.G., Mashour G.A. Differential Role of Prefrontal and Parietal Cortices in Controlling Level of Consciousness. Curr. Biol. 2018;28(13):2145–2152.e5. doi: 10.1016/j.cub.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Dort C.J., Baghdoyan H.A., Lydic R. Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J. Neurosci. 2009;29(3):871–881. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harvey J.A., Heller A., Moore R.Y., Hunt H.F., Roth L.J. Effect of central nervous system lesions on barbiturate sleeping time in the rat. J. Pharmacol. Exp. Ther. 1964;144:24–36. [PubMed] [Google Scholar]
- 97.Leeuwin R.S., van der Wal J.K., Spanjer W. Interactions of cholinesterase inhibitors and glucocorticoids with ketamine and pentobarbitone-induced general anaesthesia in the rat: possible effects on central cholinergic activity. Br. J. Pharmacol. 1984;82(2):339–347. doi: 10.1111/j.1476-5381.1984.tb10768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalivas P.W., Horita A. Thyrotropin-releasing hormone: neurogenesis of actions in the pentobarbital narcotized rat. J. Pharmacol. Exp. Ther. 1980;212(2):203–210. [PubMed] [Google Scholar]
- 99.Zucker J., Calkins D., Zabawska J., Lai H., Horita A. Effects of intraseptal drug administration on pentobarbital-induced narcosis and hippocampal choline uptake. Pharmacol. Biochem. Behav. 1987;28(4):433–436. doi: 10.1016/0091-3057(87)90501-6. [DOI] [PubMed] [Google Scholar]
- 100.Horita A., Carino M.A., Ukai Y. The analeptic effect of methamphetamine in pentobarbital-narcotized rats is mediated via a dopaminergic-cholinergic mechanism. J. Pharmacol. Exp. Ther. 1994;268(1):311–318. [PubMed] [Google Scholar]
- 101.Ma J., Shen B., Stewart L.S., Herrick I.A., Leung L.S. The septohippocampal system participates in general anesthesia. J. Neurosci. 2002;22(2):RC200. doi: 10.1523/JNEUROSCI.22-02-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leung L.S., Ma J., Shen B., Nachim I., Luo T. Medial septal lesion enhances general anesthesia response. Exp. Neurol. 2013;247:419–428. doi: 10.1016/j.expneurol.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 103.Tai S.K., Ma J., Leung L.S. Medial septal cholinergic neurons modulate isoflurane anesthesia. Anesthesiology. 2014;120(2):392–402. doi: 10.1097/ALN.0b013e3182a7cab6. [DOI] [PubMed] [Google Scholar]
- 104.Laalou F.Z., de Vasconcelos A.P., Oberling P., Jeltsch H., Cassel J.C., Pain L. Involvement of the basal cholinergic forebrain in the mediation of general (propofol) anesthesia. Anesthesiology. 2008;108(5):888–896. doi: 10.1097/ALN.0b013e31816d919b. [DOI] [PubMed] [Google Scholar]
- 105.Leung L.S., Petropoulos S., Shen B., Luo T., Herrick I., Rajakumar N., Ma J. Lesion of cholinergic basal forebrain neurons enhances the response to general anesthetics. Exp. Neurol. 2011;228:259–269. doi: 10.1016/j.expneurol.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 106.Luo T-Y., Cai S., Qin Z-X., Yang S-C., Shu Y., Liu C-X., Zhang Y., Zhang L., Zhou L., Yu T., Yu S-Y. Basal Forebrain Cholinergic Activity Modulates Isoflurane and Propofol Anesthesia. Front. Neurosci. 2020;14:559077. doi: 10.3389/fnins.2020.559077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leung L.S., Chu L., Prado M.A.M., Prado V.F. Forebrain acetylcholine modulates isoflurane and ketamine anesthesia in adult mice. Anesthesiology. 2021;134(4):588–606. doi: 10.1097/ALN.0000000000003713. [DOI] [PubMed] [Google Scholar]
- 108.Ang S.T., Ariffin M.Z., Khanna S. The forebrain medial septal region and nociception. Neurobiol. Learn. Mem. 2017;138:238–251. doi: 10.1016/j.nlm.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 109.Herkenham M. Anesthetics and the habenulo-interpeduncular system: selective sparing of metabolic activity. Brain Res. 1981;210(1-2):461–466. doi: 10.1016/0006-8993(81)90927-6. [DOI] [PubMed] [Google Scholar]
- 110.Hentall I.D., Abate K.L., Wojcik R.S., Andresen M.J. Nicotinic activity in the interpeduncular nucleus of the midbrain prolongs recovery from halothane anesthesia. Neuropharmacology. 1992;31(12):1299–1304. doi: 10.1016/0028-3908(92)90059-X. [DOI] [PubMed] [Google Scholar]
- 111.Taguchi K., Andresen M.J., Hentall I.D. Acetylcholine release from the midbrain interpeduncular nucleus during anesthesia. Neuroreport. 1991;2(12):789–792. doi: 10.1097/00001756-199112000-00015. [DOI] [PubMed] [Google Scholar]
- 112.Wolfman S.L., Gill D.F., Bogdanic F., Long K., Al-Hasani R., McCall J.G., Bruchas M.R., McGehee D.S. Nicotine aversion is mediated by GABAergic interpeduncular nucleus inputs to laterodorsal tegmentum. Nat. Commun. 2018;9(1):2710. doi: 10.1038/s41467-018-04654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gelegen C., Miracca G., Ran M.Z., Harding E.C., Ye Z., Yu X., Tossell K., Houston C.M., Yustos R., Hawkins E.D., Vyssotski A.L., Dong H.L., Wisden W., Franks N.P. Excitatory Pathways from the Lateral Habenula Enable Propofol-Induced Sedation. Curr. Biol. 2018;28(4):580–587.e5. doi: 10.1016/j.cub.2017.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bernard R., Veh R.W. Individual neurons in the rat lateral habenular complex project mostly to the dopaminergic ventral tegmental area or to the serotonergic raphe nuclei. J. Comp. Neurol. 2012;520(11):2545–2558. doi: 10.1002/cne.23080. [DOI] [PubMed] [Google Scholar]
- 115.Ma J., Leung L.S. Limbic system participates in mediating the effects of general anesthetics. Neuropsychopharmacology. 2006;31(6):1177–1192. doi: 10.1038/sj.npp.1300909. [DOI] [PubMed] [Google Scholar]
- 116.Moller J.T., Cluitmans P., Rasmussen L.S., Houx P., Rasmussen H., Canet J., Rabbitt P., Jolles J., Larsen K., Hanning C.D., Langeron O., Johnson T., Lauven P.M., Kristensen P.A., Biedler A., van Beem H., Fraidakis O., Silverstein J.H., Beneken J.E., Gravenstein J.S. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. Lancet. 1998;351(9106):857–861. doi: 10.1016/S0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 117.Newman M.F., Kirchner J.L., Phillips-Bute B., Gaver V., Grocott H., Jones R.H., Mark D.B., Reves J.G., Blumenthal J.A. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N. Engl. J. Med. 2001;344(6):395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 118.Brown E.N., Purdon P.L. The aging brain and anesthesia. Curr. Opin. Anaesthesiol. 2013;26(4):414–419. doi: 10.1097/ACO.0b013e328362d183. [DOI] [PubMed] [Google Scholar]
- 119.Vutskits L., Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. 2016. [DOI] [PubMed]
- 120.Marcantonio E.R. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Evered L., Silbert B., Knopman D.S., Scott D.A., DeKosky S.T., Rasmussen L.S., Oh E.S., Crosby G., Berger M., Eckenhoff R.G. Recommendations for the Nomenclature of Cognitive Change Associated with Anaesthesia and Surgery-2018. Anesthesiology. 2018;129(5):872–879. doi: 10.1097/ALN.0000000000002334. [DOI] [PubMed] [Google Scholar]
- 122.Fodale V., Quattrone D., Trecroci C., Caminiti V., Santamaria L.B. Alzheimer’s disease and anaesthesia: implications for the central cholinergic system. Br. J. Anaesth. 2006;97(4):445–452. doi: 10.1093/bja/ael233. [DOI] [PubMed] [Google Scholar]
- 123.Laalou F.Z., Egard M., Guillot M., Noll E., Taglang G., Pain L. Influence of preoperative cognitive status on propofol requirement to maintain hypnosis in the elderly. Br. J. Anaesth. 2010;105(3):342–346. doi: 10.1093/bja/aeq160. [DOI] [PubMed] [Google Scholar]
- 124.Zurek A.A., Yu J., Wang D.S., Haffey S.C., Bridgwater E.M., Penna A., Lecker I., Lei G., Chang T., Salter E.W., Orser B.A. Sustained increase in α5GABAA receptor function impairs memory after anesthesia. J. Clin. Invest. 2014;124(12):5437–5441. doi: 10.1172/JCI76669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Culley D.J., Baxter M.G., Yukhananov R., Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100(2):309–314. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 126.Lee I.H., Culley D.J., Baxter M.G., Xie Z., Tanzi R.E., Crosby G. Spatial memory is intact in aged rats after propofol anesthesia. Anesth. Analg. 2008;107(4):1211–1215. doi: 10.1213/ane.0b013e31817ee879. [DOI] [PubMed] [Google Scholar]
- 127.Wang H., Xu Z., Feng C., Wang Y., Jia X., Wu A., Yue Y. Changes of learning and memory in aged rats after isoflurane inhalational anaesthesia correlated with hippocampal acetylcholine level. Ann. Fr. Anesth. Reanim. 2012;31(3):e61–e66. doi: 10.1016/j.annfar.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 128.Su D., Zhao Y., Wang B., Xu H., Li W., Chen J., Wang X. Isoflurane-induced spatial memory impairment in mice is prevented by the acetylcholinesterase inhibitor donepezil. PLoS One. 2011;6(11):e27632. doi: 10.1371/journal.pone.0027632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu H., Chen L., Zhang X., Jiang X., Tian W., Yu W., Wang X., Tian J., Su D. Central cholinergic neuronal degeneration promotes the development of postoperative cognitive dysfunction. Lab. Invest. 2019;99(7):1078–1088. doi: 10.1038/s41374-018-0174-9. [DOI] [PubMed] [Google Scholar]
- 130.Tyagi E., Agrawal R., Nath C., Shukla R. Inhibitory role of cholinergic system mediated via alpha7 nicotinic acetylcholine receptor in LPS-induced neuro-inflammation. Innate Immun. 2010;16(1):3–13. doi: 10.1177/1753425909104680. [DOI] [PubMed] [Google Scholar]
- 131.Lehner K.R., Silverman H.A., Addorisio M.E., Roy A., Al-Onaizi M.A., Levine Y., Olofsson P.S., Chavan S.S., Gros R., Nathanson N.M., Al-Abed Y., Metz C.N., Prado V.F., Prado M.A.M., Tracey K.J., Pavlov V.A. Forebrain Cholinergic Signaling Regulates Innate Immune Responses and Inflammation. Front. Immunol. 2019;10:585. doi: 10.3389/fimmu.2019.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cascella M., Bimonte S. The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction. Neural Regen. Res. 2017;12(11):1780–1785. doi: 10.4103/1673-5374.219032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kong F-J., Ma L-L., Zhang H-H., Zhou J-Q. Alpha 7 nicotinic acetylcholine receptor agonist GTS-21 mitigates isoflurane-induced cognitive impairment in aged rats. J. Surg. Res. 2015;194(1):255–261. doi: 10.1016/j.jss.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 134.Gong M., Wang G., Li G., Liu J., Sun P., Xu L., Li J., Guo Y., Zhang M. Dysfunction of inflammation-resolving pathways is associated with postoperative cognitive decline in elderly mice. Behav. Brain Res. 2020;386:112538. doi: 10.1016/j.bbr.2020.112538. [DOI] [PubMed] [Google Scholar]
- 135.Huang C., Chu J.M., Liu Y., Chang R.C., Wong G.T. Varenicline reduces DNA damage, tau mislocalization and post surgical cognitive impairment in aged mice. Neuropharmacology. 2018;143:217–227. doi: 10.1016/j.neuropharm.2018.09.044. [DOI] [PubMed] [Google Scholar]
- 136.Liu P-R., Zhou Y., Zhang Y., Diao S. Electroacupuncture alleviates surgery-induced cognitive dysfunction by increasing α7-nAChR expression and inhibiting inflammatory pathway in aged rats. Neurosci. Lett. 2017;659:1–6. doi: 10.1016/j.neulet.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 137.Liu Q., Huang Y., Xue F., Simard A., DeChon J., Li G., Zhang J., Lucero L., Wang M., Sierks M., Hu G., Chang Y., Lukas R.J., Wu J. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J. Neurosci. 2009;29(4):918–929. doi: 10.1523/JNEUROSCI.3952-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]