Abstract
As one of the most important elements in our body, zinc plays a part in both the pathophysiology of depression and the antidepressant response. Patients suffering from major depression show significantly reduced zinc levels, which are normalized following successful antidepressant treatment. Recent studies have shown the interaction between zinc, GPR39 and neuropeptides, including galanin and neuropeptide Y (NPY). The zinc-sensing receptor GPR39 forms heterotrimers with 5-HT1A and the galanin receptor GalR1 upon their co-expression in mammalian cells. The oligomerization of these heterotrimers is regulated by the zinc concentration, and this may have an influence on depressive-like behavior. The antidepressant-like effect of zinc is linked to elevated levels of brain-derived neurotrophic factor (BDNF) in brain structures associated with emotion, such as the hippocampus and the amygdala. BDNF regulates neuropeptides, including NPY, cholecystokinin (CCK), and substance P or galanin, which are also implicated in mood disorders. This review focuses for the first time on the interaction between zinc, the GPR39 zinc receptor, BDNF and selected neuropeptides in terms of depression in order to determine its possible role in the neuropharmacology of that illness.
Keywords: GPR39, zinc, neuropeptides, depression, antidepressants, BDNF, GPR39
1. INTRODUCTION
Depression is a common psychiatric disorder, yet its direct pathomechanism is still unknown. The pharmacology of depression requires extensive research to find the appropriate target for a novel, fast-acting and long-lasting antidepressant. The monoaminergic theory of depression, in which inadequate levels of serotonin or noradrenaline in the central nervous system are linked to that illness, has existed for more than half a century. From the findings of studies based on that theory, new antidepressants were synthesized namely, tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRI) and selective noradrenaline inhibitors (SNRI). Unfortunately, all of these require two weeks, sometimes even four, for any antidepressant-like effects to occur. Additionally, more than 30% of depressive patients do not respond to this therapy. A more recent theory of depression is linked to the imbalance between the main excitatory system (mainly glutamatergic) and the main inhibitory system (GABAergic). It is only ketamine that causes antidepressant and long-lasting effects following a single dose, but its dangerous side effects are the reason why ketamine cannot be administered on an outpatient basis. Esketamine in intranasal form, approved by the Food and Drug Administration (FDA), is today highly recommended for treatment-resistant depression (TRD) in adults. However, due to its sedative effect, its potential for abuse, and the dissociation that it can cause, Esketamine should only be administered to patients under direct supervision in healthcare. Ketamine belongs to antagonists of the glutamatergic NMDA receptor. Zinc is also a natural antagonist of NMDA [1], and it shows antidepressant-like properties in both preclinical [2-4] and clinical [5, 6] studies. Deficiency of zinc is linked to clinical major depression [5, 7-9] and depression-like behavior in animal studies [10-12]. Importantly, zinc enhances the expression of BDNF mRNA and protein, and zinc deficiency seems to lower neurotrophic factors. Zinc and the zinc receptor GPR39 are also linked to neuropeptides, and this may be important in understanding the antidepressant-like effects of zinc. This review focuses on the association of zinc and GPR39 with BDNF and selected neuropeptides in depression.
Published literature was searched in PubMed/Medline, Scopus, Web of Science, using appropriate key words. The results were restricted to original peer-reviewed preclinical and clinical studies, as well as reviews. There were no date limits, but the results were limited to English language articles.
2. ZINC, GPR39 AND DEPRESSION
Most patients suffering from major depression have significantly lower levels of serum zinc [8]. Zinc supplementation in the form of imipramine enhances the antidepressant response and improves results in the treatment-resistant form of depression [6]. A double-blind study by Siwek et al. showed a reduction in scores in both the Beck Depression Inventory and the Hamilton Depression Rating Scale following administration of an antidepressant with a diverse mechanism of action, supplemented with zinc [13]. Hence, zinc has the potential both to be a biological marker of major depression and to bring about an antidepressant response.
Zinc deficiency seems to lead to depression-like symptoms. Results from preclinical studies indicate zinc deficiency as a cause of depression-like behavior [10, 12, 14, 15] and related changes in the brain [16, 17].
Zinc can act as a neurotransmitter via the metabotropic GPR39 receptor, which, in the brain, is expressed in the hippocampus, the frontal cortex and the amygdala [18]. Lack of the GPR39 gene results in a depression- and anxiety-like phenotype [19]. Moreover, GPR39 is significantly reduced in both the frontal cortex and the hippocampus of suicide victims [20]. GPR39 seems to play an important role in the antidepressant response. No effect of amine-based drugs was observed in GPR39 knockout mice [21]. Inhibition of the serotonergic or the noradrenergic system resulted in GPR39 down-regulation. Mice lacking GPR39 had lower tryptophan and tyrosine levels [22]. A study by Omar and Tash (2017) showed an increase in mRNA and protein from the GPR39 following the joint administration of zinc and fluoxetine under stress conditions [23]. Our previous study showed a significant increase in cortical GPR39 protein following the administration of a chronic antidepressant with a diverse mechanism of action [24].
2.1. Zinc, GPR39 and Suicide
Major depressive disorder (MDD) is linked to the high risk of suicide [25]. Although low zinc levels are observed in major depressive disorder, there is no data showing zinc levels in suicide [26]. Postmortem studies showed no significant differences in zinc levels in both, the prefrontal cortex and the hippocampus of suicide victims. Despite these results, alterations in zinc interaction with NMDA receptors were observed [27, 28]. Alterations in zinc transport may be associated with the pathophysiology of suicide. In the study by Rafalo-Ulinska, the authors observed increased protein levels of ZnT1, ZnT4-6, but decreased protein level of ZnT3 in the prefrontal cortex of suicide victims [26].
So far, only one study shows the involvement of the GPR39 zinc receptor in the pathophysiology of suicide. We found significantly decreased GPR39 protein levels in both the hippocampus and the prefrontal cortex of suicide victims [20].
3. ZINC, GPR39 AND NEUROPEPTIDES IN DEPRESSION
In recent years, neuropeptides have been linked to major depression due to their modulatory effects on neurotransmission. Neuropeptides may even be involved in the pathophysiology of suicide. Neuropeptide Y (NPY), corticotropin-releasing factor (CRF), substance P, as well as cholecystokinin (CCK) [29] seem to play a part in suicidal behavior. Some of these are linked to zinc, which is known for its antidepressant-like effects in preclinical and clinical studies. The interaction between zinc and selected neuropeptides may play an important role in the neuropharmacology of major depression.
3.1. Neuropeptide Y (NPY)
NPY plays an important role in food intake, in the modulation of cognitive function, and in the control of circadian rhythms. There is strong evidence for its involvement in mood and anxiety disorders [30]. NPY is expressed in the brain, including structures involved in emotions namely, the hippocampus, the amygdala and the olfactory bulb. Significantly reduced levels of NPY have been found in both the prefrontal cortex (-14%) and the caudate nucleus (-27%) of suicide victims [31]. Low NPY levels in cerebrospinal fluid have been found in patients suffering from bipolar disorder who were associated with both previous and prospective suicide attempts [32]. In one study, in which a diet that was low in zinc was administered for 6 weeks, zinc deficiency, which is linked to depression-like behavior, was found to increase hypothalamic NPY [33]. In another study, no significant changes in hypothalamic NPY (only a tendency) were found in rats fed a zinc-deficient diet for 3 weeks [34]. Nevertheless, zinc deficiency is linked to anorexia nervosa, a condition in which mood disturbances are observed. In connection with this finding, zinc supplementation causes weight gain [35]. In our previous study, we observed significantly decreased body weight gain after 2 and 4 weeks of zinc-deficient diet administration, but not after 10 weeks. In the groups receiving a diet low in zinc for 4 and 10 weeks, depression-like behavior was observed [11]. To summarize, it is vital to study the link between zinc deficiency, NPY and depression in order to explain the role of zinc and NPY in the pathomechanism and treatment of depression. The duration of administration of a diet which is low in zinc is also very important. In the above study [34], the length of exposure to such a diet was inconsistent with other studies that presented results relating to depression-like behavior under zinc-deficient conditions. The amount of zinc in zinc-deficient or control diet differed with the diet used in studies showing depression-like behavior under zinc-deficient conditions. In addition, evaluation of NPY concentration in other brain structures such as the hippocampus in zinc-deficient conditions is highly recommended.
3.2. Corticotropin Release Factor (CRF)
In the frontal cortex of suicide victims, Nemeroff et al. found a 23% reduction in the number of CRF binding sites [36]. The role of CRF in stress behavior is widely described. Its hypersecretion is linked to hyperactivity of the hypothalamic-pituitary-adrenal axis (HPA), which is normalized following successful antidepressant treatment [37]. Hyperactivity of the HPA is observed under zinc-deficient conditions conditions that correlate with depression-like behavior [38]. In one study, young rats fed a diet low in zinc were exposed to acute stress. They showed longer immobility times as well as higher corticosterone levels [39]. Abnormal glucocorticoid concentrations in zinc deficiency under conditions of acute stress possibly contribute to the abnormal increase in extracellular glutamate. Zinc is a natural antagonist of NDMA contributes to lowering the concentration of glutamate, which are overexpressed in major depression [1].
3.3. Cholecystokinine (CCK)
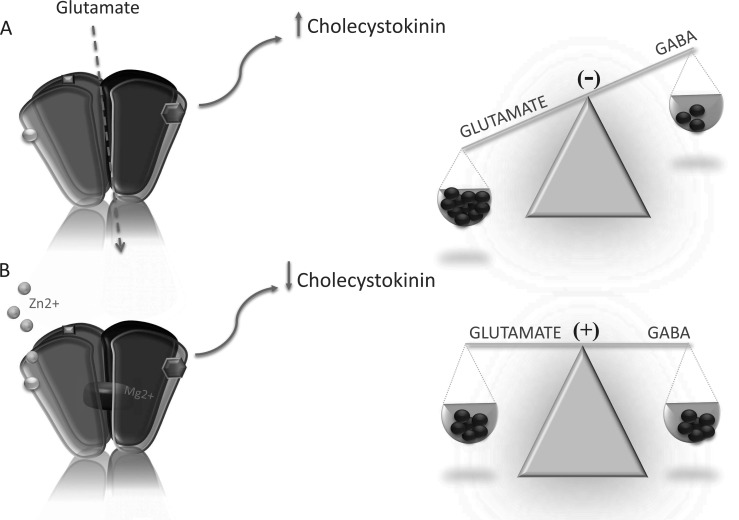
CCK is widely expressed in the mammalian brain. Its physiological functions relate to nociception, thermoregulation, memory processes, feeding and cardio-respiratory control. It also plays a role in mood and anxiety disorders. CCK acts via two metabotropic receptors: CCK1-R and CKK2-R. In one study, the use of the social-defeat paradigm as an animal model of depression was found to cause depression-like symptoms and an increase in cortical CCKergic neurotransmission [40]. The authors of this study found that chronic administration of the CCK2-R antagonist CI-988 prevented all the behavioral and neurobiological changes that were induced by the social-defeat paradigm. Knock-down of CCK in the basolateral amygdala caused antidepressant- and anxiolytic-like effects in mice [41]. In another study, the administration of an antagonist of CCK2-R had an antidepressant effect in the forced swim test in mice [42, 43]. Zinc attenuates the release of CCK in the cerebral cortex [44]. This mechanism is probably linked to NMDA and may be the next explanation for the antidepressant-like effects of zinc (Fig 1). A recent theory of major depression is based on evoked glutamate concentrations in depressed individuals. Zinc as a natural antagonist of the NMDA receptor attenuates excessive glutamatergic neurotransmission. Bandopadhyay and Belleroche showed CCK release to be attenuated through the administration not only of zinc, but also of other NMDA antagonists namely, Mg2+, MK-801, aminophosphonovaleric acid and kynurenic acid showing a direct link between CCK and NMDA [44].
Fig. (1).
The influence of zinc on CCK release via inhibition of NMDA receptor. Glutamate due to NMDA receptor can modulate the release of cholecystokinin (CCK). In the pathophysiology of depression, both the hihg CCK as well as high glutamate levels are observed (A). Zinc lowers glutamate levels by the inhibion of glutamatergic NMDA receptor and therefore may attenuate CCK realease (B). (A higher resolution/colour version of this figure is available in the electronic copy of the article).
3.4. Galanin
In recent years, there has been a fresh insight into the role of zinc in depression. Zinc may act as a neurotransmitter via the GPR39 receptor, which is expressed in the brain, including in the amygdala, the hippocampus and the frontal cortex [46]. GPR39 seems to be involved in both the pathophysiology of depression and in the antidepressant response. Reduced expression of GPR39 is observed not only in animals under restricted zinc conditions but also in suicide victims [20]. The zinc receptor is also necessary for the antidepressant-like effectiveness of monoamine-based drugs [21].
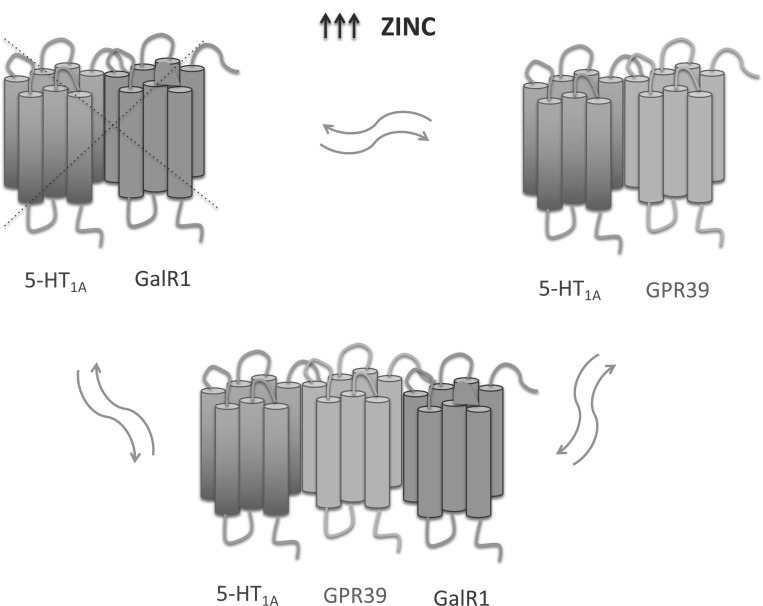
As well as GPR39 and zinc, galanin is linked to depression. Galanin is a peptide, modulating and co-expressed with noradrenalin in the locus coeruleus and serotonin system in the dorsal raphe nucleus [46]. It binds to three receptor subtypes: GAL receptor 1 (GalR1), GalR2, and GalR3. Activation of GalR1 seems to cause depressive-like behavior [46]. Mice with overexpression of galanin show increased immobility time in the forced swim test (FST). Administration of galanin causes depression-like behavior in rats, but this is reversed by administration of the nonselective galanin receptor agonist M35, which shows antidepressant-like properties when administered alone [47]. Galanin (1-15) enhances antidepressant-like effect of 8-OH-DPAT (5-HT1A agonist) [48]. Studies by Tena-Campos et al. have shown a modulatory effect of zinc on the formation of specific GalR1-5-HT1A-GPR39 and 5-HT1A-GPR39 heteroreceptor complexes (Fig. 2) [49]. A previous hypothesis suggested the involvement of the heteromer 5-HT1A-GalR1in depression [49]. However, zinc can disrupt this heteromer through a specific interaction with 5-HT1A [50, 51]. Results from our previous studies have shown a link between GPR39 and serotonergic neurotransmission. Administration for 10 days of p-chlorophenylalanine, which inhibits serotonin synthesis, caused GPR39 down-regulation in the hippocampus of mice. Moreover, rodents lacking GPR39 showed reduced hippocampal tryptophan levels [22]. GPR39 knockout mice do not respond to antidepressants influencing serotonergic transmission, such as imipramine and escitalopram [21]. Tena-Campos et al.’s conclusions suggest that the oligomerization state of GalR1-5-HT1A-GPR39 is regulated by the zinc concentration [49], which, when deficient, may cause depression-like behavior. The authors emphasize that oligomerization between GalR1-5-HT1A-GPR39 may reflect the regulation of depressive-like behavior.
Fig. (2).
A possible role of zinc in the oligomerization states among GPR39, GalR1 and 5-HT1A receptors. At high zinc concentrations 5-HT1A-GPR39 heteromer exists as a dominant form. At low zinc concentration the expression of GPR39 decreases and 5-HT1A-GPR39 heteromer is replaced by 5-HT1A-GalR1 heteromer. The oligomer 5-HT1A-GPR39-GalR1 seems to exsist as an intermediate form between 5-HT1A-GPR39 and 5-HT1A-GalR1 heteromers at adequate zinc concentrations. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
A study by Croll et al. (1994) demonstrated the regulation of neuropeptides by the brain-derived neurotrophic factor (BDNF). The authors found altered expression of hippocampal neuropeptides and neurotrophins following a seizure. They administered BDNF directly to the hippocampus, the cortex and the striatum of rats. BDNF caused an increase in the peptide and mRNA for NPY and CCK in both the hippocampus and the cortex. In the striatum, the peptide and mRNA for NPY, substance P and CCK were significantly elevated. The authors concluded that BDNF may modulate the expression of neuropeptides in a region-specific manner [52].
4. ZINC, GPR39 AND BDNF
4.1. BDNF in Depression
BDNF is a neurotrophic factor that binds to the tropomycin kinase B (TrkB) receptor. BDNF plays a significant role in the development and plasticity of synapses. It is directly involved in the outgrowth of neurites, in neuroplasticity and phenotypic maturation. Studies show that BDNF signaling has an important impact on both behavioral and cognitive functions [53]. Major depression is a common psychiatric disorder, and it may involve suicidal thoughts. Each year about one million people commit suicide worldwide. BDNF and the TrkB receptor seem to play a significant role in the pathophysiology of suicidal behavior. Low levels of BDNF have been found to relate to suicidality rather than major depressive disorder (MDD) specifically [25]. In comparison with control subjects, the messenger RNA and protein levels of both BDNF and TrkB are reduced in the hippocampus and frontal cortex of suicide victims [54, 55]. A study by Kim et al. focused on the plasma BDNF levels in suicidal patients who suffered from MDD. The levels of neurotrophin were significantly reduced in suicidal MDD patients compared with non-suicidal MDD patients and normal controls [56]. On the basis of their results, the authors concluded that levels of BDNF should be considered as a biological marker of suicidal depression. Lee et al.’s study had similar results, showing reduced plasma BDNF levels in untreated MDD patients [57]. Another study confirmed the involvement of BDNF levels, but not only in the suicide group. Most MDD patients showed significantly reduced serum BDNF concentrations [58].
Antidepressant treatment increases the amount of serum BDNF, which is positively correlated with an antidepressant-like effect [59]. Increased BDNF levels were observed following administration of selective norepinephrine inhibitors (SNRI), selective serotonin inhibitors (SSRI) and monoamine oxidase (MAO) inhibitors, and the duration of the elevated levels was consistent with the time course that is necessary for therapeutic improvement [60-62]. In clinical major depression agomelatine has been found to modulate the expression of the BDNF via its interaction with both the melatonergic and the serotonergic receptors [63]. Depressed patients who responded to agomelatine treatment administered for 2 weeks, showed increased serum BDNF levels. An increase in serum BDNF levels is also observed following electroconvulsive therapy (ECT) [64]. Major depressive disorder, mainly treatment-resistant form of depression, may be augmented with atypical antipsychotics as an adjunctive therapy [65]. Some of them increase serum BDNF levels [64].
BDNF produces an antidepressant-like response on its own. A single infusion of BDNF into the dentate gyrus of the hippocampus caused antidepressant-like effects in animal models of depression, including in the learned helplessness test and the forced swim test [66]. Peripheral BDNF administration reduced immobility time in the forced swim test (FST) and attenuated the negative effects caused by the chronic unpredictable stress, as measured in the sucrose preference test [67]. Moreover, the authors found increased neurogenesis following peripheral BDNF administration. In summary, peripheral/serum BDNF may be an important biomarker of major depressive disorder.
4.2. Zinc and BDNF in Depression
As mentioned above, zinc plays an important role both in the pathophysiology of depression and in the antidepressant response. Several studies show a strong link between zinc and BDNF in terms of depression. A zinc-deficient diet, which is linked to depression-like behavior, when administered for 6 weeks, reduced CREB, BDNF and TrkB protein levels in the hippocampus in mice [20]. Rats fed a diet low in zinc showed reduced pCREB and BDNF levels in both the hippocampus [68, 69] and the frontal cortex [69]. In our previous study, we observed reduced CREB, BDNF and TrkB protein levels in the frontal cortex following acute administration of antidepressants with diverse mechanisms of action in zinc-deficient mice. An increased levels of these proteins under zinc-deficient conditions in the frontal cortex following chronic drug administration were observed [70].
Zinc exhibits antidepressant-like properties in commonly used preclinical tests and models of depression. Zinc hydroaspartate administered in a dose of 10 mg/kg not only produced rapid antidepressant effects in conditions of chronic mild stress, but also increased hippocampal BDNF mRNA and protein levels by 17-39% [71]. Chronic unpredictable stress (CUS) caused a significant reduction, by 21%, of BDNF mRNA concentration in the rat hippocampus, with no effect on the frontal cortex [72]. Only joint administration of zinc and imipramine increased BDNF mRNA levels in rats subjected to CUS, but it did not do so in the non-CUS group. Imipramine administered separately did not influence BDNF levels. Manosso et al. investigated signaling pathways that are implicated in the modulation of BDNF [73]. They showed that the antidepressant-like effects of zinc, as measured by the tail suspension test (TST), may be dependent on the activation of PKA, CAMKII, PKC, ERK and P13/GSK-3 signaling pathways.
4.3. GPR39 and BDNF in Depression
Activation of GPR39 triggers biochemical pathways leading to CRE-dependent gene transcription that have an effect on CREB. Enhanced expression of CREB may increase BDNF and TrkB, which is one of the hypotheses concerning the antidepressant effects following GPR39 activation [45]. Our previous study showed significantly decreased CREB and BDNF proteins in the hippocampus, but not in the frontal cortex, of GPR39 knockout mice [74]. Single administration of the GPR39 agonist namely, TC-G 1008 in a dose of 15 mg/kg caused an antidepressant-like response. After twenty-four hours of drug administration, we observed an increase in the BDNF protein level [75]. We hypothesized that the antidepressant-like effects following administration of the GPR39 agonist may result from activation of the hippocampal GPR39/CREB/BDNF/TrkB pathway [45]. Our recent study demonstrated the long-lasting antidepressant-like action of TC-G 1008 [76]. The GPR39 agonist produced an antidepressant response that was observed 0.5 h, 3h, 6h and 24h following a single dose administration, in comparison with imipramine (where activity was observed after 0.5h and 3h), ZnCl2 (0.5h) and MK-801 (0.5h). In this study, we observed only a tendency for decreased BDNF levels in the hippocampus. The difference in findings between this and the previous study was probably due to the different research schemes used. In the earlier study, BDNF was measured 24h after the forced swimming test protocol [75], while in the more recent one, it was measured 30 min after the test took place [76]. BDNF transcription depends on multiple environmental and pharmacological factors. Stress is linked to evoked HPA activity and decreased BDNF levels [77]. Glucocorticoids in high concentrations impair hippocampal neurogenesis and negatively influence synaptic plasticity, probably by modulating BDNF transcription [78].
CONCLUSION
Zinc or GPR39 seems to play an important role in the release of selected neuropeptides, with which they interact. This interaction may be important in terms of therapeutic effect. The levels of BDNF increase following a successful antidepressant treatment. Zinc, as well as zinc receptor, the GPR39, seem to influence BDNF concentrations. However, studies proving direct link between zinc, GPR39 and BDNF are required. Some neuropeptides, such as neuropeptide Y, corticotropin release factor, cholecystokinine, and galanin, play a significant role in both the major depressive disorders and suicide behavior. Zinc produces antidepressant-like effects per se and GPR39 is significantly decreased in suicide victims. Zinc and the zinc receptor GPR39 are linked to neuropeptides. This may be important in understanding the antidepressant-like effects of zinc. Additional studies focusing on zinc and neuropeptides are needed. It is possible that the link between zinc, GPR39, BDNF and neuropeptides may be important in the development of new pharmacological strategies for the antidepressant treatment of major depression.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study was supported by a grant from the National Science Centre (contract UMO-2018/31/B/NZ7/00247).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Mlyniec K. Zinc in the glutamatergic theory of depression. Curr. Neuropharmacol. 2015;13(4):505–513. doi: 10.2174/1570159X13666150115220617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Młyniec K., Davies C.L., de Agüero Sánchez I.G., Pytka K., Budziszewska B., Nowak G. Essential elements in depression and anxiety. Part I. Pharmacol. Rep. 2014;66(4):534–544. doi: 10.1016/j.pharep.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Szewczyk B., Poleszak E., Sowa-Kućma M., Wróbel A., Słotwiński S., Listos J., Wlaź P., Cichy A., Siwek A., Dybała M., Gołembiowska K., Pilc A., Nowak G. The involvement of NMDA and AMPA receptors in the mechanism of antidepressant-like action of zinc in the forced swim test. Amino Acids. 2010;39(1):205–217. doi: 10.1007/s00726-009-0412-y. [DOI] [PubMed] [Google Scholar]
- 4.Cunha M.P., Machado D.G., Bettio L.E.B., Capra J.C., Rodrigues A.L.S. Interaction of zinc with antidepressants in the tail suspension test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(8):1913–1920. doi: 10.1016/j.pnpbp.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Siwek M., Dudek D., Schlegel-Zawadzka M., Morawska A., Piekoszewski W., Opoka W., Zieba A., Pilc A., Popik P., Nowak G. Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J. Affect. Disord. 2010;126(3):447–452. doi: 10.1016/j.jad.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Siwek M., Dudek D., Paul I.A., Sowa-Kućma M., Zieba A., Popik P., Pilc A., Nowak G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: A double blind, placebo-controlled study. J. Affect. Disord. 2009;118(1-3):187–195. doi: 10.1016/j.jad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Swardfager W., Herrmann N., Mazereeuw G., Goldberger K., Harimoto T., Lanctôt K.L. Zinc in depression: A meta-analysis. Biol. Psychiatry. 2013;74(12):872–878. doi: 10.1016/j.biopsych.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Siwek M., Szewczyk B., Dudek D., Styczeń K., Sowa-Kućma M., Młyniec K., Siwek A., Witkowski L., Pochwat B., Nowak G. Zinc as a marker of affective disorders. Pharmacol. Rep. 2013;65(6):1512–1518. doi: 10.1016/S1734-1140(13)71512-3. [DOI] [PubMed] [Google Scholar]
- 9.Maes M., D’Haese P.C., Scharpé S., D’Hondt P., Cosyns P., De Broe M.E. Hypozincemia in depression. J. Affect. Disord. 1994;31(2):135–140. doi: 10.1016/0165-0327(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 10.Młyniec K., Budziszewska B., Reczyński W., Sowa-Kućma M., Nowak G. The role of the GPR39 receptor in zinc deficient-animal model of depression. Behav. Brain Res. 2013;238:30–35. doi: 10.1016/j.bbr.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Młyniec K., Davies C.L., Budziszewska B., Opoka W., Reczyński W., Sowa-Kućma M., Doboszewska U., Pilc A., Nowak G. Time course of zinc deprivation-induced alterations of mice behavior in the forced swim test. Pharmacol. Rep. 2012;64(3):567–575. doi: 10.1016/S1734-1140(12)70852-6. [DOI] [PubMed] [Google Scholar]
- 12.Whittle N., Lubec G., Singewald N. Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids. 2009;36(1):147–158. doi: 10.1007/s00726-008-0195-6. [DOI] [PubMed] [Google Scholar]
- 13.Nowak G., Siwek M., Dudek D., Zięba A., Pilc A. Effect of zinc supplementation on antidepressant therapy in unipolar depression: A preliminary placebo-controlled study. Pol. J. Pharmacol. 2003;55(6):1143–1147. [PubMed] [Google Scholar]
- 14.Tassabehji N.M., Corniola R.S., Alshingiti A., Levenson C.W. Zinc deficiency induces depression-like symptoms in adult rats. Physiol. Behav. 2008;95(3):365–369. doi: 10.1016/j.physbeh.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Młyniec K., Nowak G. Zinc deficiency induces behavioral alterations in the tail suspension test in mice. Effect of antidepressants. Pharmacol. Rep. 2012;64(2):249–255. doi: 10.1016/S1734-1140(12)70762-4. [DOI] [PubMed] [Google Scholar]
- 16.Corniola R.S., Tassabehji N.M., Hare J., Sharma G., Levenson C.W. Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53-mediated mechanisms. Brain Res. 2008;1237:52–61. doi: 10.1016/j.brainres.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Tamano H., Kan F., Kawamura M., Oku N., Takeda A. Behavior in the forced swim test and neurochemical changes in the hippocampus in young rats after 2-week zinc deprivation. Neurochem. Int. 2009;55(7):536–541. doi: 10.1016/j.neuint.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Jackson V.R., Nothacker H-P., Civelli O. GPR39 receptor expression in the mouse brain. Neuroreport. 2006;17(8):813–816. doi: 10.1097/01.wnr.0000215779.76602.93. [DOI] [PubMed] [Google Scholar]
- 19.Siodłak D., Nowak G., Mlyniec K. Interaction between zinc, the GPR39 zinc receptor and the serotonergic system in depression. Brain Res. Bull. 2021;170:146–154. doi: 10.1016/j.brainresbull.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Młyniec K., Doboszewska U., Szewczyk B., Sowa-Kućma M., Misztak P., Piekoszewski W., Trela F., Ostachowicz B., Nowak G. The involvement of the GPR39-Zn(2+)-sensing receptor in the pathophysiology of depression. Studies in rodent models and suicide victims. Neuropharmacology. 2014;79:290–297. doi: 10.1016/j.neuropharm.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Młyniec K., Gaweł M., Nowak G. Study of antidepressant drugs in GPR39 (zinc receptor−/−) knockout mice, showing no effect of conventional antidepressants, but effectiveness of NMDA antagonists. Behav. Brain Res. 2015;287:135–138. doi: 10.1016/j.bbr.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 22.Młyniec K., Gaweł M., Librowski T., Reczyński W., Bystrowska B., Holst B. Investigation of the GPR39 zinc receptor following inhibition of monoaminergic neurotransmission and potentialization of glutamatergic neurotransmission. Brain Res. Bull. 2015;115:23–29. doi: 10.1016/j.brainresbull.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Omar N.N., Tash R.F. Fluoxetine coupled with zinc in a chronic mild stress model of depression: Providing a reservoir for optimum zinc signaling and neuronal remodeling. Pharmacol. Biochem. Behav. 2017;160:30–38. doi: 10.1016/j.pbb.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Młyniec K., Nowak G. GPR39 up-regulation after selective antidepressants. Neurochem. Int. 2013;62(7):936–939. doi: 10.1016/j.neuint.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Orsolini L., Latini R., Pompili M., Serafini G., Volpe U., Vellante F., Fornaro M., Valchera A., Tomasetti C., Fraticelli S., Alessandrini M., La Rovere R., Trotta S., Martinotti G., Di Giannantonio M., De Berardis D. Understanding the complex of suicide in depression: from research to clinics. Psychiatry Investig. 2020;17(3):207–221. doi: 10.30773/pi.2019.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rafalo-Ulinska A., Piotrowska J., Kryczyk A., Opoka W., Sowa-Kucma M., Misztak P., Rajkowska G., Stockmeier C.A., Datka W., Nowak G., Szewczyk B. Zinc transporters protein level in postmortem brain of depressed subjects and suicide victims. J. Psychiatr. Res. 2016;83:220–229. doi: 10.1016/j.jpsychires.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowak G., Szewczyk B., Sadlik K., Piekoszewski W., Trela F., Florek E., Pilc A. Reduced potency of zinc to interact with NMDA receptors in hippocampal tissue of suicide victims. Pol. J. Pharmacol. 2003;55(3):455–459. [PubMed] [Google Scholar]
- 28.Sowa-Kućma M., Szewczyk B., Sadlik K., Piekoszewski W., Trela F., Opoka W., Poleszak E., Pilc A., Nowak G. Zinc, magnesium and NMDA receptor alterations in the hippocampus of suicide victims. J. Affect. Disord. 2013;151(3):924–931. doi: 10.1016/j.jad.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Serafini G., Pompili M., Lindqvist D., Dwivedi Y., Girardi P. The role of neuropeptides in suicidal behavior: A systematic review. BioMed Res. Int. 2013;2013:687575. doi: 10.1155/2013/687575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tural U., Iosifescu D.V. Neuropeptide Y in PTSD, MDD, and chronic stress: A systematic review and meta-analysis. J. Neurosci. Res. 2020;98(5):950–963. doi: 10.1002/jnr.24589. [DOI] [PubMed] [Google Scholar]
- 31.Widdowson P.S., Ordway G.A., Halaris A.E. Reduced neuropeptide Y concentrations in suicide brain. J. Neurochem. 1992;59(1):73–80. doi: 10.1111/j.1471-4159.1992.tb08877.x. [DOI] [PubMed] [Google Scholar]
- 32.Sandberg J.V., Jakobsson J., Pålsson E., Landén M., Mathé A.A. Low neuropeptide Y in cerebrospinal fluid in bipolar patients is associated with previous and prospective suicide attempts. Eur. Neuropsychopharmacol. 2014;24(12):1907–1915. doi: 10.1016/j.euroneuro.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Lee R.G., Rains T.M., Tovar-Palacio C., Beverly J.L., Shay N.F. Zinc deficiency increases hypothalamic neuropeptide Y and neuropeptide Y mRNA levels and does not block neuropeptide Y-induced feeding in rats. J. Nutr. 1998;128(7):1218–1223. doi: 10.1093/jn/128.7.1218. [DOI] [PubMed] [Google Scholar]
- 34.Kwun I.S., Cho Y.E., Lomeda R.A.R., Kwon S.T., Kim Y., Beattie J.H. Marginal zinc deficiency in rats decreases leptin expression independently of food intake and corticotrophin-releasing hormone in relation to food intake. Br. J. Nutr. 2007;98(3):485–489. doi: 10.1017/S0007114507730763. [DOI] [PubMed] [Google Scholar]
- 35.Levenson C.W. Zinc regulation of food intake: New insights on the role of neuropeptide Y. Nutr. Rev. 2003;61(7):247–249. doi: 10.1301/nr.2003.jul.247-249. [DOI] [PubMed] [Google Scholar]
- 36.Nemeroff C.B., Owens M.J., Bissette G., Andorn A.C., Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch. Gen. Psychiatry. 1988;45(6):577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- 37.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J. Psychiatry Neurosci. 2004;29(3):185–193. [PMC free article] [PubMed] [Google Scholar]
- 38.Takeda A., Tamano H., Nishio R., Murakami T. Behavioral abnormality induced by enhanced hypothalamo-pituitary-adrenocortical axis activity under dietary zinc deficiency and its usefulness as a model. Int. J. Mol. Sci. 2016;17(7):1149. doi: 10.3390/ijms17071149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe M., Tamano H., Kikuchi T., Takeda A. Susceptibility to stress in young rats after 2-week zinc deprivation. Neurochem. Int. 2010;56(3):410–416. doi: 10.1016/j.neuint.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Becker C., Zeau B., Rivat C., Blugeot A., Hamon M., Benoliel J.J. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: Involvement of cholecystokinin. Mol. Psychiatry. 2008;13(12):1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- 41.Del Boca C., Lutz P.E., Le Merrer J., Koebel P., Kieffer B.L. Cholecystokinin knock-down in the basolateral amygdala has anxiolytic and antidepressant-like effects in mice. Neuroscience. 2012;218:185–195. doi: 10.1016/j.neuroscience.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernando F., Fuentes J.A., Roques B.P., Ruiz-Gayo M. The CCKB receptor antagonist, L-365,260, elicits antidepressant-type effects in the forced-swim test in mice. Eur. J. Pharmacol. 1994;261(3):257–263. doi: 10.1016/0014-2999(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 43.Hernando F., Fuentes J.A., Fournié-Zaluski M.C., Roques B.P., Ruiz-Gayo M. Antidepressant-like effects of CCK(B) receptor antagonists: Involvement of the opioid system. Eur. J. Pharmacol. 1994;261(3):257–263. doi: 10.1016/0014-2999(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 44.Bandopadhyay R., de Belleroche J. Regulation of CCK release in cerebral cortex by N-methyl-D-aspartate receptors: Sensitivity to APV, MK-801, kynurenate, magnesium and zinc ions. Neuropeptides. 1991;18(3):159–163. doi: 10.1016/0143-4179(91)90108-U. [DOI] [PubMed] [Google Scholar]
- 45.Młyniec K., Singewald N., Holst B., Nowak G. GPR39 Zn(2+)-sensing receptor: A new target in antidepressant development? J. Affect. Disord. 2015;174:89–100. doi: 10.1016/j.jad.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 46.Kuteeva E., Hökfelt T., Wardi T., Ögren S.O. Galanin, galanin receptor subtypes and depression-like behaviour. Cell. Mol. Life Sci. 2008;65(12):1854–1863. doi: 10.1007/s00018-008-8160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogren S.O., Kuteeva E., Hökfelt T., Kehr J. Galanin receptor antagonists: A potential novel pharmacological treatment for mood disorders. CNS Drugs. 2006;20(8):633–654. doi: 10.2165/00023210-200620080-00003. [DOI] [PubMed] [Google Scholar]
- 48.Millón C., Flores-Burgess A., Narváez M., Borroto-Escuela D.O., Santín L., Gago B., Narváez J.A., Fuxe K., Díaz-Cabiale Z. Galanin (1-15) enhances the antidepressant effects of the 5-HT1A receptor agonist 8-OH-DPAT: Involvement of the raphe-hippocampal 5-HT neuron system. Brain Struct. Funct. 2016;221(9):4491–4504. doi: 10.1007/s00429-015-1180-y. [DOI] [PubMed] [Google Scholar]
- 49.Tena-Campos M., Ramon E., Borroto-Escuela D.O., Fuxe K., Garriga P. The zinc binding receptor GPR39 interacts with 5-HT1A and GalR1 to form dynamic heteroreceptor complexes with signaling diversity. Biochim. Biophys. Acta. 2015;1852(12):2585–2592. doi: 10.1016/j.bbadis.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Borroto-Escuela D.O., Narvaez M., Marcellino D., Parrado C., Narvaez J.A., Tarakanov A.O., Agnati L.F., Díaz-Cabiale Z., Fuxe K. Galanin receptor-1 modulates 5-hydroxtryptamine-1A signaling via heterodimerization. Biochem. Biophys. Res. Commun. 2010;393(4):767–772. doi: 10.1016/j.bbrc.2010.02.078. [DOI] [PubMed] [Google Scholar]
- 51.Tena-Campos M., Ramon E., Lupala C.S., Pérez J.J., Koch K.W., Garriga P. Zinc is involved in depression by modulating G protein-coupled receptor heterodimerization. Mol. Neurobiol. 2016;53(3):2003–2015. doi: 10.1007/s12035-015-9153-y. [DOI] [PubMed] [Google Scholar]
- 52.Croll S.D., Wiegand S.J., Anderson K.D., Lindsay R.M., Nawa H. Regulation of neuropeptides in adult rat forebrain by the neurotrophins BDNF and NGF. Eur. J. Neurosci. 1994;6(8):1343–1353. doi: 10.1111/j.1460-9568.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 53.Numakawa T., Richards M., Nakajima S., Adachi N., Furuta M., Odaka H., Kunugi H. The role of brain-derived neurotrophic factor in comorbid depression: Possible linkage with steroid hormones, cytokines, and nutrition. Front. Psychiatry. 2014;5:136. doi: 10.3389/fpsyt.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dwivedi Y., Rizavi H.S., Conley R.R., Roberts R.C., Tamminga C.A., Pandey G.N. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 55.Banerjee R., Ghosh A.K., Ghosh B., Bhattacharyya S., Mondal A.C. Decreased mRNA and protein expression of BDNF, NGF, and their receptors in the hippocampus from suicide: An analysis in human postmortem brain. Clin. Med. Insights Pathol. 2013;6:1–11. doi: 10.4137/CPath.S12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim Y-K., Lee H-P., Won S-D., Park E-Y., Lee H-Y., Lee B-H., Lee S-W., Yoon D., Han C., Kim D-J., Choi S-H. Low plasma BDNF is associated with suicidal behavior in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(1):78–85. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 57.Lee B.H., Kim H., Park S.H., Kim Y.K. Decreased plasma BDNF level in depressive patients. J. Affect. Disord. 2007;101(1-3):239–244. doi: 10.1016/j.jad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Deveci A., Aydemir O., Taskin O., Taneli F., Esen-Danaci A. Serum BDNF levels in suicide attempters related to psychosocial stressors: A comparative study with depression. Neuropsychobiology. 2007;56(2-3):93–97. doi: 10.1159/000111539. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu E., Hashimoto K., Okamura N., Koike K., Komatsu N., Kumakiri C., Nakazato M., Watanabe H., Shinoda N., Okada S., Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry. 2003;54(1):70–75. doi: 10.1016/S0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 60.Nibuya M., Morinobu S., Duman R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nibuya M., Nestler E.J., Duman R.S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 1996;16(7):2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duman R.S. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromol. Med. 2004;5(1):11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 63.Martinotti G., Pettorruso M., De Berardis D., Varasano P.A., Lucidi Pressanti G., De Remigis V., Valchera A., Ricci V., Di Nicola M., Janiri L., Biggio G., Di Giannantonio M. Agomelatine increases BDNF serum levels in depressed patients in correlation with the improvement of depressive symptoms. Int. J. Neuropsychopharmacol. 2016;19(5):pyw003. doi: 10.1093/ijnp/pyw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinotti G., Di Iorio G., Marini S., Ricci V., De Berardis D., Di Giannantonio M. Nerve growth factor and brain-derived neurotrophic factor concentrations in schizophrenia: A review. J. Biol. Regul. Homeost. Agents. 2012;26(3):347–356. [PubMed] [Google Scholar]
- 65.Wright B.M., Eiland E.H., III, Lorenz R. Augmentation with atypical antipsychotics for depression: A review of evidence-based support from the medical literature. Pharmacotherapy. 2013;33(3):344–359. doi: 10.1002/phar.1204. [DOI] [PubMed] [Google Scholar]
- 66.Shirayama Y., Chen A.C-H., Nakagawa S., Russell D.S., Duman R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt H.D., Duman R.S. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35(12):2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doboszewska U., Szewczyk B., Sowa-Kućma M., Młyniec K., Rafało A., Ostachowicz B., Lankosz M., Nowak G. Antidepressant activity of fluoxetine in the zinc deficiency model in rats involves the NMDA receptor complex. Behav. Brain Res. 2015;287:323–330. doi: 10.1016/j.bbr.2015.03.064. [DOI] [PubMed] [Google Scholar]
- 69.Doboszewska U., Szewczyk B., Sowa-Kućma M., Młyniec K., Nowak G. The disruption of CREB/BDNF/TrkB signaling. Dynamic changes induced by zinc deficiency. Pharmacol. Rep. 2013;65(1):119–120. doi: 10.1016/S1734-1140(13)71432-4. [DOI] [Google Scholar]
- 70.Młyniec K., Nowak G. Up-regulation of the GPR39 Zn(2+)-sensing receptor and CREB/BDNF/TrkB pathway after chronic but not acute antidepressant treatment in the frontal cortex of zinc-deficient mice. Pharmacol. Rep. 67(6):1135–1140. doi: 10.1016/j.pharep.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 71.Sowa-Kućma M., Legutko B., Szewczyk B., Novak K., Znojek P., Poleszak E., Papp M., Pilc A., Nowak G. Antidepressant-like activity of zinc: Further behavioral and molecular evidence. J. Neural Transm. (Vienna) 2008;115(12):1621–1628. doi: 10.1007/s00702-008-0115-7. [DOI] [PubMed] [Google Scholar]
- 72.Cieślik K., Sowa-Kućma M., Ossowska G., Legutko B., Wolak M., Opoka W., Nowak G. Chronic unpredictable stress-induced reduction in the hippocampal brain-derived neurotrophic factor (BDNF) gene expression is antagonized by zinc treatment. Pharmacol. Rep. 2011;63(2):537–543. doi: 10.1016/S1734-1140(11)70520-5. [DOI] [PubMed] [Google Scholar]
- 73.Manosso L.M., Moretti M., Ribeiro C.M., Gonçalves F.M., Leal R.B., Rodrigues A.L.S. Antidepressant-like effect of zinc is dependent on signaling pathways implicated in BDNF modulation. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;59:59–67. doi: 10.1016/j.pnpbp.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 74.Młyniec K., Budziszewska B., Holst B., Ostachowicz B., Nowak G. GPR39 (zinc receptor) knockout mice exhibit depression-like behavior and CREB/BDNF down-regulation in the hippocampus. Int. J. Neuropsychopharmacol. 2014;18(3):pyu002. doi: 10.1093/ijnp/pyu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Młyniec K., Starowicz G., Gaweł M., Frąckiewicz E., Nowak G. Potential antidepressant-like properties of the TC G-1008, a GPR39 (zinc receptor) agonist. J. Affect. Disord. 2016;201:179–184. doi: 10.1016/j.jad.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 76.Starowicz G., Jarosz M., Frąckiewicz E., Grzechnik N., Ostachowicz B., Nowak G., Mlyniec K. Long-lasting antidepressant-like activity of the GPR39 zinc receptor agonist TC-G 1008. J. Affect. Disord. 2019;245:325–334. doi: 10.1016/j.jad.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Smith M.A., Makino S., Kvetnansky R., Post R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995;15(3 Pt 1):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suri D., Vaidya V.A. Glucocorticoid regulation of brain-derived neurotrophic factor: Relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]