Abstract
The oxidation of polycyclic aromatic compounds was studied in systems consisting of laccase from Trametes versicolor and so-called mediator compounds. The enzymatic oxidation of acenaphthene, acenaphthylene, anthracene, and fluorene was mediated by various laccase substrates (phenols and aromatic amines) or compounds produced and secreted by white rot fungi. The best natural mediators, such as phenol, aniline, 4-hydroxybenzoic acid, and 4-hydroxybenzyl alcohol were as efficient as the previously described synthetic compounds ABTS [2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)] and 1-hydroxybenzotriazole. The oxidation efficiency increased proportionally with the redox potentials of the phenolic mediators up to a maximum value of 0.9 V and decreased thereafter with redox potentials exceeding this value. Natural compounds such as methionine, cysteine, and reduced glutathione, containing sulfhydryl groups, were also active as mediator compounds.
The concerted action of fungal laccases and oxidizable low-molecular-weight compounds (called mediators in some studies) was found to extend or permit oxidation of nonsubstrate compounds by this enzyme class; an overview of the chronological development of these processes has been recently presented (26). However, this system received widespread attention only when used for bleaching kraft pulp, thus showing promise for biotechnological application (11, 12, 13, 14, 15). Subsequently these laccase mediator systems (LMS), as they are commonly referred to, were also applied to the oxidation of various compounds, and they seems to be useful in preparative synthesis as well (20, 31). Another area of interest with regard to the LMS originates from its application in the degradation of environmental chemicals such as polycyclic aromatic hydrocarbons (PAH) (9, 16, 22, 25).
The choice of the proper mediator substance plays a key role in the general applicability and effectiveness of the system. More than 100 possible mediator compounds have already been described (13), but the most commonly used are still 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 1-hydroxybenzotriazole (HBT). HBT and ABTS are oxidized by laccases to the radical (HBT⋅), the cation radical (ABTS+⋅), and the ABTS dication (ABTS2+); the role of these oxidized species as the essential oxidants for aromatic alcohols has been recently demonstrated (10, 26). In a previous study we reported that the oxidation of a high-molecular-weight PAH model compound is performed by the LMS via ABTS2+ and HBT⋅ by an indirect oxidation without direct contact of the substrate and enzyme (A. Majcherczyk and C. Johannes, submitted for publication).
The most relevant disadvantages of all known effective mediator compounds are either high price or toxicity. Generally, the compounds applied are products of chemical synthesis, and it is still not clear whether the LMS plays a role in natural systems. The ability of white rot fungi secreting only laccases as oxidative enzymes to degrade lignin model compounds (37) or environmentally relevant recalcitrant compounds and the importance of these enzymes in wood degradation (6) could, however, indicate the presence of an LMS with a natural origin. This supposition can also be supported by the lack of a direct correlation between enzyme activity and the biodegradation of aromatic xenobiotic compounds by laccase-producing fungi (33).
The role of free radicals resulting from the oxidation of ABTS or HBT by laccase in the LMS oxidation processes gives rise to the supposition that typical laccase substrates which form radicals can also act as mediator compounds. The present study demonstrates that a number of compounds, either produced by fungi or present during the degradation of lignocellulose substrates, mediate the oxidation of PAH by laccase from Trametes versicolor.
MATERIALS AND METHODS
Chemicals.
Tween 20 was purchased from Sigma (Deisenhofen, Germany). Acenaphthene was obtained from Across Chimica (Neuss, Germany). All other reagents and substrates were provided by Aldrich (Steinheim, Germany) and Fluka (Neu-Ulm, Germany).
Laccase and enzyme assay.
Laccase from T. versicolor was generously donated by Novo Nordisk (Bagsvaerdt, Denmark) and purified as previously described (25). Enzyme activity was determined by oxidation of ABTS (22).
Analysis of PAH.
Samples were analyzed by gas chromatography and mass spectrometry as previously described (25).
Oxidation of PAH by LMS.
All experiments were performed in 0.1 M citric acid–dipotassium hydrogen phosphate buffer (pH 4.5) containing 2.5% acetone (or 1% Tween 20, as indicated), a final concentration of 4 U of enzyme per ml, and a 25 or 5 μM concentration of each PAH as a mixture of 4 or 12 compounds, respectively (see reference 25 for details). All determinations were performed in triplicate. Control samples were prepared in the same manner, but the enzyme was deactivated by 30 min of boiling preceding the addition of the mediator and the substrate.
RESULTS
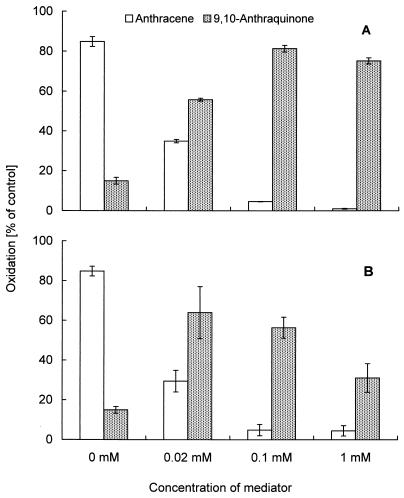
The present study on the oxidation of PAH by LMS and the role of free radicals from natural mediators concentrated mainly on the four compounds that are known to be well oxidized in systems using ABTS or HBT: acenaphthene, acenaphthylene, fluorene, and anthracene (23, 25). The reactions with LMS involving radicals produced from the synthetic mediator compounds by laccase were expected to also take place in the case of other compounds, especially typical laccase substrates such as phenols and aromatic amines. The application of phenol and aniline as natural mediators for the oxidation of anthracene resulted in a very effective removal of PAH and the production of anthraquinone (Fig. 1). Even very low concentrations (0.02 mM) of phenol and aniline increased the oxidation of anthracene by laccase and resulted in a stoichiometric reaction. Higher concentrations of the mediators resulted in an almost complete oxidation of anthracene but (possibly due to the production of coupling products with oxidized mediators) in a nonstoichiometric production of the quinone. No increase in the anthracene oxidation was observed with the application of other laccase substrates (2,6-dimethoxyphenol, pyrogallic acid, ferulic acid, and syringaldazine at 1 mM each); however, 1,4-hydroquinone increased the oxidation from 20% ± 2.9% (oxidation by laccase without mediator) to 33% ± 0.8%.
FIG. 1.
Oxidation of anthracene (84 μM) to 9,10-anthraquinone by laccase (4 U/ml) with different concentrations of phenol (A) and aniline (B). The solution contained 1% Tween 20. Results are means and standard deviations.
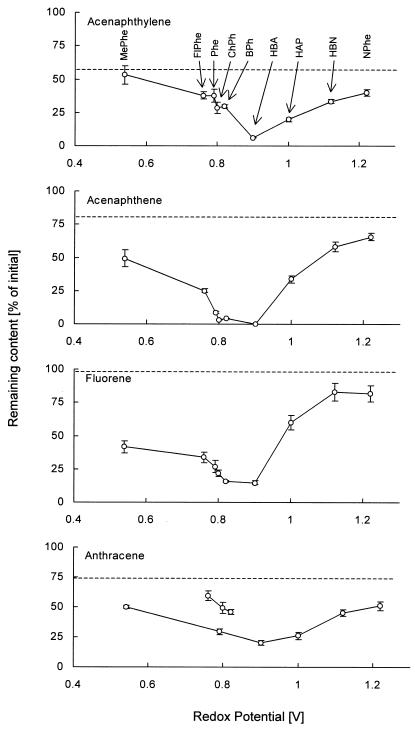
The oxidation of PAH involving direct participation of phenoxy radicals is assumed to depend on the redox potentials (Eoxs) of the individual radical species. Otherwise, the rate of oxidation of the phenolic substrate would decrease after exceeding the redox potential of the enzymes. To confirm this supposition, a number of phenols with increasing Eoxs were tested with regard to their ability to oxidize PAH in LMS. To exclude sterical hindrance, only para-substituted compounds were selected. The highest level of oxidation of all PAH tested was obtained with phenolic mediators with Eoxs of 0.8 to 0.9 V (Fig. 2). Increasing the Eox over 1 V resulted in a rapid decrease of the mediator effect; however, this was still significant even with phenols possessing an Eox of 1.2 V, which far exceeds the redox potentials of common laccases. Only in the cases of anthracene and halogenated phenols did the oxidation not follow the general pattern, but it was still proportional to the Eoxs of the halogenated mediators.
FIG. 2.
Oxidation of four PAH (25 μM each) by laccase (4 U/ml) and the para-substituted phenols (1 mM) 4-methoxyphenol (MePhe) (Eox, 0.54 V versus NHE), 4-fluorophenol (FlPHe) (0.76 V), phenol (Phe) (0.79 V), 4-chlorophenol (ChPh) (0.8 V), 4-bromophenol (BPh) (0.82 V), HBA (0.9 V), 4-hydroxyacetophenone (HAP) (1.0 V), 4-hydroxybenzonitrile (HBN) (1.12 V), and 4-nitrophenol (NPhe) (1.22 V) with respect to their redox potentials (24). The oxidation by laccase without phenols is indicated by dashed lines. Results are means and standard deviations.
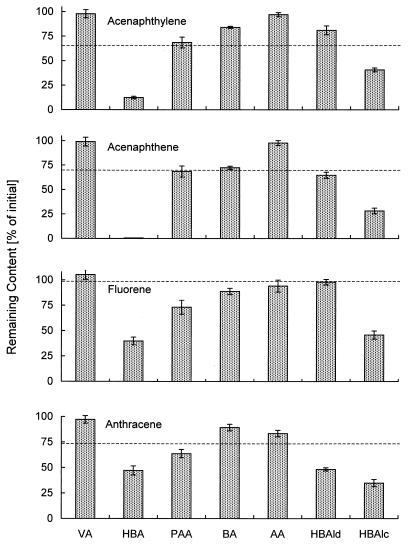
The white rot fungi secrete a large number of low-molecular-weight aromatic compounds, some of which are phenol derivatives and potential laccase substrates (21). The application of such compounds as natural mediators for the oxidation reactions of laccase was demonstrated using the four selected PAH (Fig. 3). The best overall results were obtained with 4-hydroxybenzoic acid (HBA) and 4-hydroxybenzyl alcohol; veratryl and anise alcohols actually inhibited the reaction. In the case of HBA at least 0.1 mM was necessary to significantly increase the oxidation reactions performed by laccase. The system consisting of laccase and HBA also oxidized highly condensed PAH such as benzo[a]pyrene and showed metabolization comparable to that mediated by HBT (Table 1).
FIG. 3.
Oxidation of four PAH (25 μM each) by laccase (4 U/ml) in presence of the natural compounds veratryl alcohol (VA), HBA, phenylacetic acid (PAA), benzaldehyde (BA), anisaldehyde (AA), 4-hydroxybenzaldehyde (HBAld), and 4-hydroxybenzyl alcohol (HBAlc) at 1 mM each. Dashed lines indicate reactions with laccase only. Results are means and standard deviations.
TABLE 1.
Oxidation of 12 PAH by laccase from T. versicolor alone and in the presence of HBA or HBT
| PAH (5 μM) | Oxidation (% of control without enzyme) witha:
|
||
|---|---|---|---|
| Laccase | Laccase + HBA | Laccase + HBT | |
| Naphthalene | 0.7 ± 2.5 | 0.0 ± 1.9 | 0.0 ± 2.1 |
| Acenaphthylene | 7.5 ± 1.4 | 93.7 ± 1.3 | 97.5 ± 0.1 |
| Acenaphthene | 6.9 ± 2.5 | 96.7 ± 1.7 | 100.0 ± 0.0 |
| Fluorene | 1.8 ± 1.7 | 83.9 ± 4.9 | 95.3 ± 0.2 |
| Phenanthrene | 0.0 ± 1.1 | 0.0 ± 1.6 | 7.3 ± 2.7 |
| Anthracene | 73.8 ± 1.2 | 83.9 ± 0.9 | 84.0 ± 0.4 |
| Fluoranthene | 0.0 ± 1.9 | 0.6 ± 2.2 | 6.8 ± 1.2 |
| Pyrene | 0.0 ± 1.6 | 19.6 ± 5.0 | 86.5 ± 1.4 |
| Chrysene | 0.0 ± 2.2 | 0.7 ± 2.8 | 0.0 ± 2.0 |
| Benzo[k]fluoranthene | 0.0 ± 1.9 | 5.4 ± 4.6 | 2.1 ± 5.4 |
| Benzo[a]pyrene | 5.3 ± 1.7 | 52.9 ± 4.9 | 62.0 ± 2.1 |
| Perylene | 0.0 ± 1.4 | 79.9 ± 4.9 | 87.8 ± 2.8 |
Laccase was at 4 U/ml; HBA and HBT were at 1 mM. Results are means and standard deviations.
The selection of the natural mediators was also extended to some amino acids and their derivatives, even though some of them had not been described as being laccase substrates. It was not surprising that the best overall result was obtained using the phenolic amino acid tyrosine (Table 2); however, compounds containing a thiol group (reduced glutathione, cysteine, and methionine) also increased the oxidation of single PAH significantly.
TABLE 2.
Oxidation of PAH by laccase from T. versicolor in the presence of amino acids and derivatives
| Amino acid or derivative (1 mM) | Oxidation (% of control) ofa:
|
|||
|---|---|---|---|---|
| Acenaphthylene | Acenaphthene | Fluorene | Anthracene | |
| None | 42.5 ± 0.1 | 20.1 ± 0.1 | 2.6 ± 0.3 | 25.7 ± 0.6 |
| Methionine | 47.3 ± 3.5 | 8.2 ± 4.2 | 4.9 ± 1.9 | 33.2 ± 3.3 |
| Cysteine | 95.3 ± 0.9 | 5.9 ± 4.1 | 0.1 ± 2.6 | 19.9 ± 4.8 |
| Tyrosine | 97.9 ± 0.3 | 42.5 ± 5.0 | 18.3 ± 6.4 | 44.2 ± 6.2 |
| Tyrosine hydrazide | 97.9 ± 0.1 | 41.0 ± 4.9 | 31.8 ± 2.5 | 63.0 ± 5.9 |
| Reduced glutathione | 99.2 ± 0.1 | 15.0 ± 2.2 | 3.6 ± 2.0 | 34.1 ± 1.9 |
Each PAH was at 25 μM; laccase was at 4 U/ml. Results are means and standard deviations.
DISCUSSION
The oxidation of PAH by the action of LMS proceeds without direct contact between the substrate and the enzyme and involves the action of the low-molecular-weight mediator compound in its oxidized state (Majcherczyk and Johannes, submitted). The primary reactions seem to proceed via abstraction of one electron or hydrogen atom in a single step; however, even the reactions that produce a very good yield involve a large difference between the oxidation potentials of the radicals or cations (ABTS2+, 1.09 V [34]; HBT⋅, 1.0 V [2]) and the single PAH (up to 1.55 V [25]) in a number of cases. The thermodynamically unfavorable reactions resulting from the negative difference in the oxidation potentials of the substrate and oxidant are generally possible if a follow-up process irreversibly removes one of the products from the equilibrium of the first reaction (26, 32, 35). The abstraction of one electron from PAH can be followed by the addition of water (or of the hydroxyl ion) or by further oxidation steps. Similar thermodynamic rules also apply to the oxidation of ABTS and HBT by laccases. The mechanism of these reactions was recently discussed, and the laccase oxidation of ABTS to its dication was demonstrated (26). The reactivities of various PAH possessing similar Eoxs can also be affected by steric hindrance, their electronic structures (25), or the formation of complexes with the oxidant (38).
It can be generally assumed that under the reaction conditions discussed above, all radicals formed by laccase oxidation can potentially act as mediator compounds metabolizing other nonsubstrate compounds, e.g., PAH. This was confirmed by applying phenol, hydroquinone, and aniline as the simplest laccase substrates. The oxidation ability of the LMS using phenols increases with a less negative difference between the oxidation potentials of phenoxy radicals and the desired PAH; however, an increase of the redox potential of the phenol results in a lower rate of oxidation of the phenol by the enzyme (Eoxs of fungal laccases are approximately 0.5 to 0.8 V). The reaction does not terminate with phenols with oxidation potentials higher than the Eox of laccase, because phenols can still be well oxidized below their nominal Eox values (18). These two contradictory processes result in an optimum point, corresponding in our case to phenolic mediators with Eoxs of 0.8 to 0.9 V. It is obvious that conditions which increase the lifetime of the radicals have a positive effect on the oxidation yield. Different results can be expected using mediators in, e.g., water solutions containing detergent or acetone.
One of the best phenolic-type mediators tested was HBA. HBA, together with numerous aromatic compounds, is produced and secreted by white rot fungi (1, 3, 4, 5). Some of these compounds, such as 4-hydroxybenzyl alcohol and 4-hydroxybenzaldehyde, were also found to be active as mediators in the oxidation of PAH. HBA very effectively mediated the oxidation of environmentally relevant highly condensed PAH such as benzo[a]pyrene and perylene and displayed an oxidation pattern comparable to that of the artificial synthetic mediator HBT.
Due to its phenolic character, tyrosine (or its derivatives) also acts as a mediator compound. The positive effect obtained through use of the SH group-containing amino acids and common natural compounds such as reduced glutathione was unexpected. It could indicate that these compounds are also substrates of laccase from T. versicolor, possibly resulting in thiyl radicals as was demonstrated for manganese and horseradish peroxidases (27, 39). Cysteine and glutathione reportedly do not react with laccase from Pycnoporus cinnabarinus and act as a reductant towards semiquinones as well (19).
The degradation of PAH by white rot fungi does not proceed by a single oxidative pathway, and the intracellular degradation of some polycyclic aromatic compounds, e.g., phenanthrene (7), has been reported. The ability of extracellular peroxidases (e.g., ligninase) to metabolize PAH directly or by formation of peroxide radicals was demonstrated in numerous studies. However, the possible physiological role of LMS by the oxidation of these aromatics has not been proved in vivo up to now. 3-Hydroxyanthranilic acid, a compound secreted into the culture medium by P. cinnabarinus and described as a mediator compound for the depolymerization of synthetic lignin (17), was not active in this study.
It seems very probable that the above-described mechanism and natural mediators may play an important role in the degradation of PAH by white rot fungi. The presence of an LMS utilizing natural mediators could explain the ability of laccase-producing fungi to extracellulary metabolize PAH and the lack of a direct correlation between the enzyme activity and degradation.
The mediating phenoxy radicals undergo further oxidation to quinones or polymerize and are thus withdrawn from the in vitro reaction system. Still, the high concentration of mediators used in LMS could be extremely diminished under physiological conditions by the presence of reducing systems that recycle these compounds. The natural mediators described here, especially the two most effective (HBA and 4-hydroxybenzyl alcohol), and their derivatives can be expected to play an important role in the degradation of aromatic compounds by laccase-producing fungi. Both compounds are (i) secreted extracellulary by numerous fungi as mentioned above, (ii) present in situ as common secondary plant metabolites (28, 29, 36), and (iii) released in large amounts during the microbial degradation of lignocellulose (8, 30). Further studies will be done to estimate the physiological importance of the LMS in vivo.
ACKNOWLEDGMENT
This study was supported by the EU Project ERBIC18CT970186.
REFERENCES
- 1.Armand D, Thivend S. The production of phenolic acids by mycelia of hymenomycetes on a glucose medium. C R Acad Sci III. 1965;260:1472–1473. [Google Scholar]
- 2.Aurich H G, Bach G, Hahn K, Küttner G, Weiss W. Aminyloxides (nitroxides). XXV. Reactions of benzotriazolyl oxide radicals with aromatic compounds. J Chem Res. 1977;1977(S):122–123. [Google Scholar]
- 3.Badalyan S M, Doko L, Rapior S, Jacob M, Andary C, Mnatsakanyan B A, Arutyunyan L S, Garibova L V. Chemical and pharmacological study of higher fungi. II. Comparative investigation of caprophores of some Nematoloma species: chemical composition and cultural characteristics. Mikologiya Fitopatologiya. 1996;30:79–86. [Google Scholar]
- 4.Badalyan S M, Mnatsakanyan V A, Arutyunyan L S, Rapior S, Doko L, Jacob M, Serrano J J, Andary C. Chemical and pharmacological study of higher fungi. IV. Comparative investigation of the chemical composition from fruit bodies of five xylotrophic species (Agaricales s.l.) Mikologiya Fitopatologiya. 1997;31:61–66. [Google Scholar]
- 5.Badalyan S M, Rapior S, Doko L, Le Quang J, Jacob M, Serrano J J, Andary C. Chemical and pharmacological study of higher fungi. I. Chemical composition and pharmacological investigation of carpophores of Cortinarius armillatus (Fr.:Fr.) Fr. (Cortinariaceae) Mikologiya Fitopatologiya. 1996;30:37–42. [Google Scholar]
- 6.Bermek H, Li K C, Eriksson K E L. Laccase-less mutants of the white-rot fungus Pycnoporus cinnabarinus cannot delignify kraft pulp. J Biotechnol. 1998;66:117–124. [Google Scholar]
- 7.Bezalel L, Hadar Y, Cerniglia C E. Enzymatic mechanisms involved in phenanthrene degradation by the white rot fungus Pleurotus ostreatus. Appl Environ Microbiol. 1997;63:2495–2501. doi: 10.1128/aem.63.7.2495-2501.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanchette R A, Sutherland J B, Crawford D L. Actinomycetes in discolored wood of living silver maple. Can J Bot. 1981;59:1–7. [Google Scholar]
- 9.Böhmer S, Messner K, Srebotnik E. Proceedings of the 7th International Conference of Biotechnology in the Pulp and Paper Industry. B. Montreal: Canadian Pulp and Paper Association; 1998. Degradation of polycyclic aromatic hydrocarbons by laccase in the presence of mediator compounds; pp. 199–202. [Google Scholar]
- 10.Bourbonnais R, Leech D, Paice M G. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim Biophys Acta. 1998;1379:381–390. doi: 10.1016/s0304-4165(97)00117-7. [DOI] [PubMed] [Google Scholar]
- 11.Bourbonnais R, Paice M G. Demethylation and delignification of kraft pulp by Trametes versicolor laccase in the presence of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonate) Appl Microbiol Biotechnol. 1992;36:823–827. [Google Scholar]
- 12.Bourbonnais R, Paice M G, Reid I D, Lanthier P, Yaguchi M. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl Environ Microbiol. 1995;61:1876–1880. doi: 10.1128/aem.61.5.1876-1880.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Call H-P. Mehrkomponentensystem zum Verändern, Abbau oder Bleichen von Lignin, ligninhaltigen Materialien oder ähnlichen Stoffen sowie Verfahren zu seiner Anwendung. EP 0717143A1[EP 0717143A1] 1996. [Google Scholar]
- 14.Call H-P, Mücke I. History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems (Lignozym-process) J Biotechnol. 1997;53:163–202. [Google Scholar]
- 15.Call H-P, Strittmatter G. Einsatz von lignolytischen Enzymen in der Papier- und Zellstoffindustrie-Neuere Ergebnisse. Papier. 1992;46:32–37. [Google Scholar]
- 16.Collins P J, Kotterman M J, Field J A, Dobson A D. Oxidation of anthracene and benzo[a]pyrene by laccases from Trametes versicolor. Appl Environ Microbiol. 1996;62:4563–4567. doi: 10.1128/aem.62.12.4563-4567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggert C, Temp U, Dean J F, Eriksson K-E L. A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett. 1996;391:144–148. doi: 10.1016/0014-5793(96)00719-3. [DOI] [PubMed] [Google Scholar]
- 18.Fieser L F. An indirect method of studying the oxidation-reduction potentials of unstable systems, including those from the phenols and amines. J Am Chem Soc. 1930;52:5204–5241. [Google Scholar]
- 19.Figueroa-Espinoza M C, Morel M H, Surget A, Asther M, Moukha S, Sigoillot J C, Rouau X. Attempt to cross-link feruloylated arabinoxylans and proteins with a fungal laccase. Food Hydrocolloids. 1999;13:65–71. [Google Scholar]
- 20.Fritz-Langhals E, Kunath B. Synthesis of aromatic aldehydes by laccase-mediator assisted oxidation. Tetrahedron Lett. 1998;39:5955–5956. [Google Scholar]
- 21.Gutierrez A, Caramelo L, Prieto A L, Martinez M J, Martinez A T. Anisaldehyde production and aryl-alcohol oxidase and dehydrogenase activities in ligninolytic fungi of the genus Pleurotus. Appl Environ Microbiol. 1994;60:1783–1788. doi: 10.1128/aem.60.6.1783-1788.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johannes C, Majcherczyk A, Hüttermann A. Degradation of anthracene by laccase of Trametes versicolor in the presence of different mediator compounds. Appl Microbiol Biotechnol. 1996;46:313–317. doi: 10.1007/s002530050823. [DOI] [PubMed] [Google Scholar]
- 23.Johannes C, Majcherczyk A, Hüttermann A. Oxidation of acenaphthene and acenaphthylene by laccase of Trametes versicolor in a laccase-mediator system. J Biotechnol. 1998;61:151–156. [Google Scholar]
- 24.Lind J, Shen X, Eriksen T E, Merenyi G. The one-electron reduction potential of 4-substituted phenoxyl radicals in water. J Am Chem Soc. 1990;112:479–482. [Google Scholar]
- 25.Majcherczyk A, Johannes C, Hüttermann A. Oxidation of polycyclic aromatic hydrocarbons (PAH) by laccase of Trametes versicolor. Enzyme Microb Technol. 1998;22:335–341. [Google Scholar]
- 26.Majcherczyk A, Johannes C, Hüttermann A. Oxidation of aromatic alcohols by laccase from Trametes versicolor mediated by the 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) cation radical and dication. Appl Microbiol Biotechnol. 1999;51:267–276. [Google Scholar]
- 27.McEldoon J P, Dordick J S. Thiol and Mn2+-mediated oxidation of veratryl alcohol by horseradish peroxidase. J Biol Chem. 1991;266:14288–14293. [PubMed] [Google Scholar]
- 28.Mori I, Terashima N. Biosynthesis of p-hydroxybenzoic acid in poplar lignin. J Jpn Wood Res Soc. 1971;17:311–312. [Google Scholar]
- 29.Pearl I A. The nature of aspen lignin. Forest Prod J. 1957;7:88–90. [Google Scholar]
- 30.Pometto A L, Crawford D L. Catabolic fate of Streptomyces viridosporus T7A-produced, acid-precipitable polymeric lignin upon incubation with ligninolytic Streptomyces species and Phanerochaete chrysosporium. Appl Environ Microbiol. 1986;51:171–179. doi: 10.1128/aem.51.1.171-179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potthast A, Rosenau T, Chen C L, Gratzl J S. Selective enzymatic oxidation of aromatic methyl groups to aldehydes. J Org Chem. 1995;60:4320–4321. [Google Scholar]
- 32.Schmidt W, Steckhan E. Milde oxidative Abspaltung der p-Methoxybenzylether-Schutzgruppe durch homogene Elektronenübertragung. Angew Chem. 1978;90:717. [Google Scholar]
- 33.Schützendübel A, Majcherczyk A, Johannes C, Hüttermann A. Degradation of fluorene, anthracene, phenanthrene, fluoranthene, and pyrene lacks connection to the production of extracellular enzymes by Pleurotus ostreatus and Bjerkandera adusta. Int Biodeterior Biodegrad. 1999;43:93–100. [Google Scholar]
- 34.Scott S L, Chen W J, Bakac A, Espenson J H. Spectroscopic parameters electrode potentials, acid ionization constants, and electron exchange rates of the 2,2′-azinobis(3-ethylbenzothiazolone-6-sulfonate) radicals and ions. J Phys Chem. 1993;1993:6710–6714. [Google Scholar]
- 35.Shono T, Matsumura Y, Mizoguchi M, Hayashi J. Oxidation of alcohols by active species generated by electrochemical oxidation of organosulfur compounds. Tetrahedron Lett. 1979;40:3861–3864. [Google Scholar]
- 36.Shunming W, Yinlian Z, Yingpe C. A preliminary study on the phenolic glucosides and phenolic acids from the bark of several species of poplar. Chem Ind Forest Prod. 1985;5:1–8. [Google Scholar]
- 37.Srebotnik E, Jensen K A, Jr, Hammel K E. Fungal degradation of recalcitrant nonphenolic lignin structures without lignin peroxidase. Proc Natl Acad Sci USA. 1994;91:12794–12797. doi: 10.1073/pnas.91.26.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svanholm U, Parker V D. Kinetics and mechanisms of the reactions of organic cation radicals and dications. V. Kinetics of thermodynamically unfavourable electron transfer reactions between cation radicals and aromatic compounds. J Chem Soc Perkin Trans II. 1976;1976:1567–1574. [Google Scholar]
- 39.Wariishi H, Valli K, Renganathan V, Gold M H. Thiol-mediated oxidation of nonphenolic lignin model compounds by manganese peroxidase of Phanerochaete chrysosporium. J Biol Chem. 1989;264:14185–14191. [PubMed] [Google Scholar]