Abstract
The COVID-19 pandemic has raised considerable concerns that patients with inflammatory bowel disease (IBD), particularly those treated with immunosuppressive therapies, may have an increased risk of SARS-CoV-2 acquisition, develop worse outcomes following COVID-19, and have suboptimal vaccine response compared with the general population. In this review, we summarise data on the risk of COVID-19 and associated outcomes, and latest guidance on SARS-CoV-2 vaccines in patients with IBD. Emerging evidence suggests that commonly used medications for IBD, such as corticosteroids but not biologicals, were associated with adverse outcomes to COVID-19. There has been no increased risk of de novo, or delayed, IBD diagnoses, however, an overall decrease in endoscopy procedures has led to a rise in the number of missed endoscopic-detected cancers during the pandemic. The impact of IBD medication on vaccine response has been a research priority recently. Data suggest that patients with IBD treated with antitumour necrosis factor (TNF) medications had attenuated humoral responses to SARS-CoV-2 vaccines, and more rapid antibody decay, compared with non-anti-TNF-treated patients. Reassuringly, rates of breakthrough infections and hospitalisations in all patients who received vaccines, irrespective of IBD treatment, remained low. International guidelines recommend that all patients with IBD treated with immunosuppressive therapies should receive, at any point during their treatment cycle, three primary doses of SARS-CoV-2 vaccines with a further booster dose as soon as possible. Future research should focus on our understanding of the rate of antibody decay in biological-treated patients, which patients require additional doses of SARS-CoV-2 vaccine, the long-term risks of COVID-19 on IBD disease course and activity, and the potential risk of long COVID-19 in patients with IBD.
Keywords: COVID-19, ulcerative colitis, Crohn's disease, immune response, inflammatory bowel disease, SARS-CoV-2, vaccine, antibody
Key messages.
Patients with inflammatory bowel disease (IBD) did not have an increased risk of COVID-19, and had largely similar outcomes, including hospitalisation, intensive care admission and mortality, compared with the general population.
Risk factors for adverse outcomes to COVID-19 in patients with IBD include advanced age, increasing number of comorbidities, corticosteroid use and increased IBD activity.
Biological-treated patients with IBD who do not have COVID-19 should continue with their pre-pandemic IBD therapies and not be switched electively.
There is no evidence to suggest an increased risk of de novo, or delayed, IBD diagnoses, however, an overall decrease in endoscopy procedures during the pandemic has led to a rise in the number of missed endoscopic-detected cancers.
Patients with IBD should be encouraged to receive a complete course of SARS-CoV-2 vaccine, at any point during their treatment cycle, and be counselled that vaccine response may be attenuated when receiving systemic corticosteroids, antitumour necrosis factor monotherapy or combination therapy, and Janus kinase inhibitors.
More intensive immunisation strategies may be required for patients receiving multiple immunosuppressive medications, however, data reporting vaccine response following multiple SARS-CoV-2 vaccine doses are awaited and will inform future policy recommendations.
Introduction
As of February 2022, globally, there were more than 420 million cases and 5.8 million deaths related to the COVID-19, which is caused by the SARS-CoV-2.1 2 The pandemic remains an ongoing threat to global health due to the emergence of new variants such as Delta (B.1.617.2) and Omicron (B.1.1.529), with considerable escape to antibody neutralisation.3 4 Risk factors for severe infection with COVID-19 include advanced age and underlying medical comorbidities, such as cardiovascular or metabolic diseases.5
Inflammatory bowel diseases (IBD) including Crohn’s disease and ulcerative colitis (UC) are immune-mediated inflammatory diseases (IMIDs) with a rapidly increasing incidence globally.6 There are potential intersections between the pathogenesis of COVID-19 and IBD at the molecular level. Epithelial expression of ACE2 and transmembrane serine protease 2 (TMPRSS2) appear to be essential for viral entry of SARS-CoV-2 into host enterocytes, which result in unopposed renin–angiotensin pathway leading to acute lung injury.7 In patients with IBD, who have inflammation of the gut and are often treated with immunosuppressive medications, the epithelial expression of ACE2 will remain unchanged or even downregulated,8–10 which may impact on the disease spectrum of COVID-19 and its clinical management.
Patients with IBD are at greater risk of developing serious infections and pneumonia,11–13 particularly those treated with biological drugs which are known to be associated with an increased risk of opportunistic infections.14 At the beginning of the pandemic, concerns were raised as to whether patients with IBD may develop worse health outcomes. It also remained uncertain whether patients treated with immunosuppressive drugs have reduced vaccine response, as has been demonstrated previously for other vaccine-preventable infections.15–18 Until recently, patients with IMIDs, including IBD, were excluded from the SARS-CoV-2 vaccine clinical development programmes. Since the roll-out of novel vaccine platforms internationally, many of which have not previously been studied in patients with IBD, many questions regarding the safety and effectiveness of SARS-CoV-2 vaccination in these patients have emerged. In this review, we aim to provide a comprehensive overview on the latest evidence in the management of COVID-19 and IBD, in particular the risk of and outcomes of COVID-19 in patients with IBD, impact of IBD medications on risk of COVID-19 outcomes, whether COVID-19 impacts IBD disease activity, and new data and guidance on SARS-CoV-2 vaccines in patients with IBD, to inform current clinical practice and future research directions.
Impact of IBD on COVID-19
Risk of COVID-19 in patients with IBD
Since the onset of the COVID-19 pandemic, concerns were raised of the possible heightened risk of SARS-CoV-2 infection among patients with IBD and other diseases associated with immune dysregulation. In two small, Italian studies in the early pandemic, patients with IBD had a high seroprevalence of COVID-19,19 and may have had an increased risk for development of SARS-CoV-2 infection compared with the general population.20 21 Conflicting results were, however, reported in a subsequent single-centre Italian study,20 and regional case series from Spain demonstrating a lower adjusted incidence ratio (0.74) of COVID-19 in patients with IBD compared with the general population.22 Two large registry studies across the USA confirmed that the overall incidence rate of COVID-19 among patients with IBD was low (0.23%), and similar to those without IBD.23 Some data from the USA even suggested that patients with IBD had a lower risk of COVID-19 compared with non-IBD cohorts (risk ratio (RR) 0.79, 95% CI 0.72 to 0.86), however, this may be explained by the phenomenon known as ‘shielding’ as some governments advised patients with IBD, who were thought to be at higher risk of severe COVID-19, to stay at home and minimise face-to-face contacts.24 Similar trends were observed across Europe, and in one cohort study in Denmark, the prevalence was lower in patients with IBD than the general population (2.5% vs 3.7%, p<0.01).25 Worldwide, the reported period prevalence of COVID-19 up until early-2022 among patients with IBD ranged from 0% to 5.95%.24–29
In one meta-analysis including 17 studies, the pooled incidence rate per 1000 population was 4.02 in patients with IBD and 6.59 in the general population. There was no significant increase in the pooled relative risk (RR 0.47, 95% CI 0.18 to 1.26) in patients with IBD, and no significant difference by IBD subtype.30 Despite initial concerns, patients with IBD appear to have comparable rates of SARS-CoV-2 infection to that of the general population.
Advanced age has been shown to be an independent risk factor associated with development of COVID-19 in IBD, but no established age cut-off for increased risk has been determined.30 31 Despite initial concerns regarding the effect of immunosuppression on SARS-CoV-2 acquisition, the use of biological drugs has not been associated with increased risk of development of COVID-19.28 30 32
Outcomes of COVID-19 in patients with IBD
Over the course of the pandemic, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) international registry has helped clinicians and patients better understand outcomes of COVID-19 in patients with IBD. Prior to registry closure in January 2022, 7038 cases of COVID-19 from 74 countries had been reported to determine the impact of IBD treatment regimens on outcomes of COVID-19, including hospitalisation and severe COVID-19, defined as a composite of intensive care unit (ICU) admission, mechanical ventilation and death.33–38 Throughout the pandemic, SECURE-IBD has been a hugely informative resource for the IBD community, however, there are significant limitations with how representative the cohort is, in part secondary to the physician-led opt in nature of the registry. Despite being a global registry, cases from the USA account for over one-third of the database, limiting the generalisability of the dataset findings to other populations. Furthermore, patients treated with non-biological therapy were under-represented as almost two-thirds of cases submitted were treated with antitumour necrosis factor (anti-TNF), anti-integrin or IL12/23 inhibitors. Notwithstanding these limitations, SECURE-IBD is the largest cohort to date assessing the impact of IBD treatment on outcomes of COVID-19.
The common presenting symptoms of COVID-19 in patients with IBD are similar to that of the general population. Most presented with fever (67.5%) and cough (59.6%), about one-quarter presented with diarrhoea, anosmia or dyspnoea, and about 10% had gastrointestinal symptoms, including abdominal pain, nausea or vomiting.39 The proportion of gastrointestinal manifestations were higher in patients with IBD but may be confounded by active disease at the time of COVID-19.40
Hospitalisation
Despite minimally higher rates of COVID-19-related hospitalisations in patients with IBD, compared with the general population, the clinical course of hospitalised patients remained similar to those non-hospitalised. One retrospective cohort, conducted early in the pandemic, reported no increased risk of hospitalisation in patients with IBD (RR 1.10, 95% CI 0.74 to 1.40).40 Conversely, two large-scale studies from the Netherlands and USA reported higher rates by 17%–68% in patients with IBD.24 41 In these studies, however, clinical course in hospitalised patients was similar to those not hospitalised, reflecting intercountry variation in threshold for hospital admission. Data for patients with IBD, obtained later in the pandemic, were estimated to range from 21% in European to 66% in Latin American regions.22 42–45 In 1 meta-analysis of 11 studies across the world, the pooled hospitalisation rate due to COVID-19 was 28%.30 Most recently, in one UK-wide population-based study of over 17 million people, including more than 20 000 receiving immunosuppressive medication (OpenSAFELY), there remained an increased risk of hospitalisation in patients with IBD, even after controlling for age, sex and comorbidities.46 However, although these data are extracted at an individual level from 40% of general practices in England, certain confounders were not captured, including shielding status which may have reduced the risk of infection and biased results towards the null. Furthermore, there may have been risk of misclassification of exposure status, given that it would be difficult to determine in this dataset the causal and temporal relationship between active IBD and SARS-CoV-2 infection.
Risk factors associated with COVID-19-related hospitalisation include advanced age, active IBD and the presence of ≥1 non-IBD comorbidity, such as cardiovascular disease.42 43 47 The impact of these risk factors was well demonstrated in SECURE-IBD, where hospitalisation rates for patients with IBD ranged from 5% in children and young people (10–19 years) to 47% in the elderly (>80 years). Rates were higher (60%) in those with more than three comorbidities than those without comorbidity (9%),33 in patients with UC compared with Crohn’s disease (RR 1.55),30 and in Hispanic and black patients compared with white patients (RR 2.5–3.6).48
Critical care, mechanical ventilation and renal replacement therapy
Although initial data suggested that patients with IBD may have had increased critical care admissions, with increased need for mechanical ventilation, over the course of the pandemic, rates have reassuringly been similar to the general population. ICU admission rates for patients with IBD were 3% in SECURE-IBD and 5.3% in an international meta-analysis.30 Compared with the general population, the risk of ICU admission was similar in patients with IBD (RR 0.85, 95% CI 0.69 to 1.06).24 In another population-based study from the Netherlands, ICU admission rates were similar across patients with IBD compared with the general population (12.5% vs 15.7%).41 OpenSAFELY demonstrated a slight increase in risk of admission to ICU and/or death for patients with IBD (HR 1.08, 95% CI 1.01 to 1.16).46 In the SECURE-IBD registry, the overall mechanical ventilation rate was 2%, which increased up to 9% in older patients.33 The relative risk of mechanical ventilation was similar in patients with IBD compared with non-IBD patients (6.3% vs 11.2%, p=1.00),41 as was risk of developing acute renal failure (RR 1.00, 95% CI 0.84 to 1.19) and need for renal replacement therapy (RR 1.00, 95% CI 0.60 to 1.66).24
Mortality
Initial data suggested that the case fatality rate for patients with IBD could be high, ranging from 0% to 33.3%.20 22 41–45 As more nuanced data became available, the rate was considerably much lower; in one meta-analysis, pooled mortality rate was 4.3% in patients with IBD with COVID-19, which was similar to that of the general population.30 In SECURE-IBD, the overall mortality rate was 2%, which was significantly higher in the elderly (20%) than in younger patients (0%).33 Further studies have confirmed that mortality rate from COVID-19 was similar between patients with IBD and those without (RR 0.95, 95% CI 0.71 to 1.26), however, studies that stratified by disease subtype reported that mortality may be higher in patients with UC compared with Crohn’s disease (RR 1.94, 95% CI 1.22 to 3.10).24 30 Multivariable analyses from SECURE-IBD suggest that this association is likely to be confounded by age, sex, smoking status and comorbidity.34 49 50
Risk factors associated with adverse clinical outcomes, including ICU admission, mechanical ventilation and/or death are advanced age, dyspnoea on presentation, active IBD, presence of ≥1 comorbidity and the use of systemic steroids.24 25 34 42–45 47 51 One validated prognostic model used to predict adverse outcomes in patients with IBD with COVID-19, following adjustment for known risk factors including age, male gender, comorbidity, corticosteroid and biological use, demonstrated an excellent discrimination with an area under curve of 0.79 for hospitalisation, 0.88 for ICU admission and 0.94 for death.50
Effect of IBD drugs on COVID-19 outcome
Systemic steroids
Throughout the pandemic, there was extensive evidence demonstrating the detrimental effect of systemic steroids on clinical outcomes of patients with IBD who developed COVID-19. However, most published data are subject to unmeasured confounding between IBD activity, COVID-19 severity and concomitant steroid use. Despite this, data remained convincing and replicable, and helped inform positioning of corticosteroids in the acute management of IBD. Where a decision is taken to stop systemic corticosteroids, careful tapering remains important to avoid the risk of an Addisonian crisis, particularly in the context of intercurrent illness.52
In the SECURE-IBD registry,33 corticosteroid use was independently associated with a 6.9-fold risk of severe COVID-19 and a 11.6-fold risk of death due to COVID-19.33 34 This finding was replicated in a large retrospective cohort covering 4.4 million health plan members in Northern California, some of whom had IBD, whereby the use of oral prednisone prior to SARS-CoV-2 infection was a consistent risk factor for subsequent hospitalisation, ICU admission and death.53 Further studies have also confirmed that corticosteroid use was associated with an increased risk for SARS-CoV-2 infection, hospitalisation and critical care requirement.24 54 55 In a recent meta-analysis, the pooled relative risks of hospitalisation (RR 1.99, 95% CI 1.64 to 2.40), ICU admission (RR 3.41, 95% CI 2.28 to 5.11) and mortality (RR 2.70, 95% CI 1.61 to 4.55) were all significantly higher in patients treated with steroids compared with those who were not.30
5-aminosalicylic acid
Conflicting results have been reported on the effect of 5-aminosalicylic acid (5-ASA) on the disease course of COVID-19 in patients with IBD. Several studies conducted early in the pandemic, including two meta-analyses, demonstrated an increased risk of critical care, ventilator use and mortality in those treated with 5-ASA compared with those not treated with 5-ASA.34 35 56 For instance, pooled relative risks was 1.59 for hospitalisation (95% CI 1.39 to 1.82), 2.38 for ICU admission (95% CI 1.26 to 4.48) and 2.62 for mortality (95% CI 1.67 to 4.11) in patients treated with 5-ASA.30 In a recent, large propensity score matched cohort, 5-ASA was, however, not found to be associated with an increased risk of adverse outcome or mortality.24 As more data from SECURE-IBD emerged, and contrary to the group’s initial report,34 5-ASA was no longer associated with any adverse clinical outcomes, hospitalisation or death in later publications (RR 1.02, 95% CI 0.83 to 1.26) and likely a reflection that the initial association was due to reporting bias, including unreported mild COVID-19 cases in 5-ASA- and sulfasalazine-treated patients and overrepresented mild COVID-19 cases in biological-treated patients, or covariates that were incompletely controlled for.36
Immunomodulators
According to two population-based studies carried out in the USA and France, the use of conventional immunomodulators, namely thiopurines and methotrexate, was not associated with an increased risk of COVID-19 (RR 0.89, 95% CI 0.33 to 2.44), hospitalisation (RR 0.94, 95% CI 0.66 to 1.35), mechanical ventilation or death (RR 0.35, 95% CI 0.09 to 1.43) compared with treatment-naïve patients with IBD.57 58 The pooled relative risks of, ICU admission and mortality were similar between those treated with immunomodulators and those who were not.30 In SECURE-IBD, methotrexate, but not thiopurines, was reported to be associated with a marginally increased risk of hospitalisation and death as a composite outcome (RR 1.26, 95% CI 1.00 to 1.57), but not severe COVID-19 or death.36 Of note, thiopurine monotherapy or combined use with biologicals were also associated with a higher risk of severe COVID-19 when compared with biologicals alone.35
Biological medications
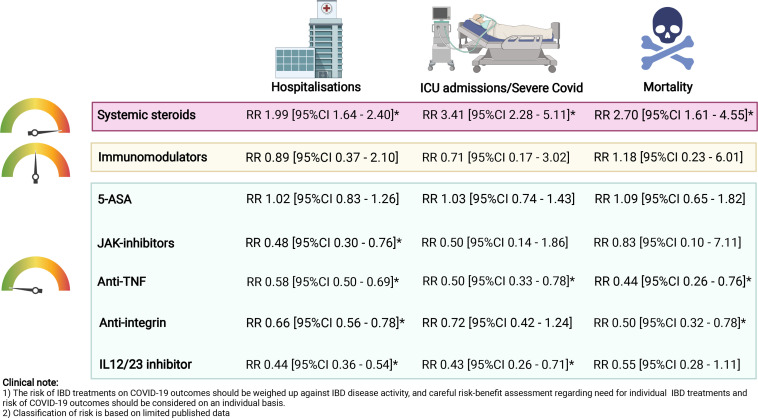
About one in four patients with IBD are treated with biological drugs and, initially, there was uncertainty as to the impact of these therapies on COVID-19. Reassuringly, as the pandemic evolved and new data became available, accumulating evidence has shown that biologicals were safe in patients with IBD(figure 1).
Figure 1.
Impact of IBD treatments on COVID-19 outcomes. Relative risks were calculated using multivariable logistic regression models comparing outcomes of COVID-19 from each medication class to those not treated with that medication. ICU admission encompassed composite outcomes made up by ICU admission, mechanical ventilation and mortality not due to COVID-19. The colours on the indicator represent the collective risk of IBD medications on COVID-19 outcomes: green=low risk, amber=moderate risk, red=high risk. *Indicates significant results where the 95% CI did not cross 1. Figure created with data from refs. 30 36 and using BioRender.com. 5-ASA, 5-aminosalicylic acid; IBD, inflammatory bowel disease; ICU, intensive care unit; JAK-inhibitor, Janus kinase inhibitor; RR, relative risk; TNF, tumour necrosis factor.
Anti-TNF medications
The use of TNF antagonists, the most commonly used biological for patients with IBD, has not been associated with an increased risk of COVID-19.57 Furthermore, anti-TNF-treated patients did not demonstrate an increased risk for ICU admission, mechanical ventilation, or death compared with non-anti-TNF-treated patients34 Similar findings have been replicated in three population-based studies in the USA, France and Denmark.24 25 58
Some studies suggest that the risk of developing severe COVID-19 may be lower in biological-treated patients potentially due to the effect of these drugs in suppressing cytokine inflammatory pathways that underlie COVID-19-associated inflammatory complications.30 56 59 In one meta-analysis, pooled relative risks of hospitalisation (RR 0.34, 95% CI 0.19 to 0.61), ICU admission (RR 0.49, 95% CI 0.33 to 0.72) and mortality (RR 0.22, 95% CI 0.13 to 0.38) were lower in biological-treated patients, most of whom were on anti-TNF, compared with patients treated with other non-biologicals for IBD.30 A meta-analysis also found that patients treated with anti-TNF therapy had decreased risk of hospitalisation and ICU admission compared with corticosteroids or 5-ASA.56
However, patients treated with anti-TNF therapy, in combination with an immunomodulator, showed an increased risk of COVID-19 adverse outcomes. Although a French nationwide study found that in-patient mortality rates were similar between patients treated with anti-TNF monotherapy compared with anti-TNF combination therapy,58 data from SECURE-IBD reported that patients treated with anti-TNF combination therapy had a higher risk of severe COVID-19 than those treated with anti-TNF monotherapy (8.8% vs 2.2%, RR 4.01, 95% CI 1.65 to 9.78).35 In a pooled analysis from three international registries consisting of patients with different IMIDs, anti-TNF monotherapy appeared to have the best safety profile than other commonly prescribed treatment regimens, including anti-TNF combination therapy.60
Anti-integrins
Data of the impact of vedolizumab, an anti-integrin, on COVID-19 outcomes have been conflicting. One report suggested that vedolizumab treatment was associated with an increased risk of developing COVID-19 (RR 1.70, 95% CI 1.16 to 2.48) compared with patients treated with 5-ASA alone.54 Initial data from SECURE-IBD also suggested an increased risk of hospitalisations in vedolizumab- compared with anti-TNF-treated patients (RR 1.39; 95% CI 1.001 to 1.90), but not risk of severe COVID-19.37 54
In more recent data from SECURE-IBD, vedolizumab treatment was found to be associated with a decreased risk of hospitalisation or death (RR 0.66, 95% CI 0.56 to 0.78), and no association with risk of severe COVID-19, compared with patients who were not treated with vedolizumab.36 It is plausible that vedolizumab-treated patients may be at increased risk of COVID-19 compared with patients treated with other IBD medications, in part because the anti-integrin not only binds to effector memory cells in the gut, but also the upper respiratory tract.61 62 It is more likely that initial data were underpowered to detect true differences between patients treated with different IBD medications. As data on patients treated with less commonly prescribed medications, such as vedolizumab, enriched over the course of the pandemic, the increased risk of adverse outcomes to COVID-19 disappeared.
Anti-interleukin 12/23 agents
Although our understanding of risk in patients treated with ustekinumab, an interleukin 12/23 inhibitor, remains limited, in SECURE-IBD, ustekinumab use was associated with a lower risk of hospitalisation or death (RR 0.44, 95% CI 0.36 to 0.54) compared with those not treated with ustekinumab.36 Overall, current evidence highlights that compared with anti-TNF, vedolizumab-treated (RR 0.85, 95% CI 0.56 to 1.28) and ustekinumab-treated (RR 1.25, 95% CI 0.56 to 2.80) patients are not known to be at increased risk of developing severe COVID-19,36 and the relative risk of hospitalisation was similar across patients treated with different biologicals.58
Janus kinase inhibitor
Tofacitinib, a Janus kinase inhibitor (JAKi) approved for the treatment of UC, has been associated with a higher risk of thrombosis and infections, and in particular, herpes zoster.63 Available data on its safety during COVID-19 have been limited. In a subgroup analysis of SECURE-IBD, which included only 37 tofacitinib-treated patients with IBD, tofacitinib use was not associated with an increased risk of hospitalisation, ICU admission, mechanical ventilation, or death when compared with other treatment regimens.38 No thrombotic events were reported. However, in a more recent community-based study from the USA of a larger number of patients with IMIDs including IBD, 31% of patients treated with JAKi had an increased risk of severe COVID-19 (RR 3.35) compared with patients treated with other biologics.53 Reassuringly, recent data showed that tofacitinib was associated with a lower risk of hospitalisation and death (RR 0.48, 95% CI 0.30 to 0.76).36 Tofacitinib has also been shown to be an effective treatment to reduce the risk of death or respiratory failure from COVID-19.64
Impact of COVID-19 on IBD
Impact of COVID-19 on IBD disease activity
Few studies have assessed the impact of COVID-19 on IBD-related disease course, activity and exacerbations, and in particular, impact on patients receiving immunomodulators. The majority of case series or retrospective cohorts have been limited by small sample size and inadequate adjustment for confounders for disease activity. In one cohort from the USA, 118 patients with IBD (62% Crohn’s disease, 55% biological-treated) who developed COVD-19 were followed up to 6 months post-COVID-19.65 Just over one-third of patients reported gastrointestinal symptoms associated with COVID-19, but this may have been confounded by the fact that almost two-thirds of the cohort had symptoms of active IBD around the time of their COVID-19. Reassuringly, there were no significant changes in disease activity, endoscopic evaluation, or laboratory markers pre-COVID-19 and post-COVID-19.
In a retrospective propensity-score matched cohort study of 855 458 patients across 40 healthcare organisations in the USA, risk of developing IBD flare, and risk of de novo IBD, after laboratory-confirmed SARS-CoV-2 infection was assessed.24 Patients with a negative SARS-CoV-2 test were used as controls. Of 4310 patients with IBD with a history of COVID-19, 5.3% and 6.8% had a flare of IBD symptoms within 1 and 3 months, respectively. No significant association was seen with biological therapy. At 3-month follow-up, patients with a history of COVID-19 were 1.3 times (95% CI 1.18 to 1.51) more likely to have an IBD-related disease flare compared with those without a history of COVID-19. These observations may be explained by enteric infection with SARS-CoV-2, and/or upregulation of ACE2 in ileal and colon tissue leading to disease flare.66 67 In this cohort, 774 (0.1%) patients had a new diagnosis of IBD following COVID-19, which was lower compared with a control group without a history of COVID-19 (RR 0.55). The reason for this is unclear although the result remained significant when analysis was restricted to diagnoses at least 6 months following COVID-19.
Risk of long COVID-19 in patients with IBD
Recent evidence has shown that persistent symptoms can remain for many weeks after the acute SARS-CoV-2 infection. This condition is named as long COVID-19 or postacute COVID-19 syndrome, which involves multiple organs including respiratory, cardiovascular, neurological, gastrointestinal and musculoskeletal systems.68 Common symptoms include fatigue, myalgia, dyspnoea, myocardial injury, cognitive impairment and sleep disturbances.69 Studies from different regions have reported variable incidence of long COVID-19, ranging from 33% to 87% at 60 days70 71 and 35% to 76% at 6 months or longer.72 73 One small Italian study reported the clinical characteristics of long COVID-19 in patients with IBD who recovered from acute SARS-CoV-2 infection.74 Prevalence was 39.6%, and two-thirds of patients developed asthenia, one-third neurological manifestations, including anosmia, ageusia, memory loss, dermatological symptoms, myalgia, with a minority developing persistent dyspnoea or depression.
Patients with IBD who developed long COVID-19 were more frequently female, while other demographic characteristics did not differ from those without symptoms of long COVID-19.74 The exact pathogenesis of long COVID-19 remains unknown, however, changes in the gut microbiota post-COVID-19 may be one plausible explanation, as baseline gut microbiome composition has been shown to predict the risk of long COVID-19.75 As more patients are infected with new variants of SAR-CoV-2, the number of patients with IBD who develop long COVID-19 is expected to rise substantially. More research is needed to investigate the pathogenesis of long COVID-19 and its long-term impact on patients with IBD.
Management of IBD during COVID-19 pandemic
International and national guidelines on management of patients with IBD during the COVID-19 pandemic closely aligned with pre-pandemic practice recommendations, in particular with respect to minimisation of systemic corticosteroid use. Although they have largely been based on expert opinion, clinical recommendations have been regularly updated as new data emerged.76–82 As new data accumulated during the multiple COVID-19 waves worldwide, the belief that patients with IBD may be adversely affected by COVID-19, and conversely, that COVID-19 may worsen IBD-related disease activity, has largely abated. A summary of lessons learnt from the conduct of research studies and guideline development during the COVID-19 pandemic is outlined in Box 1.
Box 1. Lessons learnt during the COVID-19 pandemic.
Optimising resources and infrastructure
Use existing research infrastructure, such as disease-specific networks of research-active clinicians and nurses, locally and internationally. During COVID-19, this enabled prioritisation of research questions, standardisation of case record forms across multiple countries, and improved data quality, strengthening data and practice recommendations.
Secure online databases, such as Research Electronic Data Capture (REDCap), allowed rapid setup of studies and surveillance registries, including electronic consent. Consider asking for consent to allow data linkage to national databases of community-based and hospital-based records, and for patients to be recallable.
Patient and public involvement
If face-to-face research study visits are required, aim to minimise clinician and patient contact time to reduce infection risk, and time sample collection during routine hospital infusion appointments. Consider remote sampling, for example, via a postal fingerprick blood testing kit, if local resources allow.
Make use of newsletters, online video and other patient-facing materials to maximise engagement in research. Where possible, communicate individual-level study results back to patients to maintain motivation, particularly for longitudinal studies.
Adaptability and evolving research questions
Regularly review study aims and objectives to address both initial and longer-term outcomes as the pandemic evolves, for example, during the COVID-19 pandemic, vaccine development and risk of long COVID-19.
Data will evolve over time, and publication of data early in the COVID-19 pandemic helped identify immediate gaps in our understanding. Consider publishing study-specific data in real time, via online registries or dashboards, and formal publication of data in the form of living systematic reviews, whereby data are enriched and meta-analysed over the course of the pandemic.
Patients with a known diagnosis of IBD
Most international groups recommended that patients with an established diagnosis of IBD should remain on their pre-pandemic IBD therapy that was successful in inducing and maintaining remission. Patients with a new diagnosis of IBD, or who have an established diagnosis and are experiencing a flare of symptoms, should be managed according to pre-pandemic standard of care. These measures include continuation of immunomodulators, biologicals, JAKi and with a consideration to reduce the use corticosteroid therapy where possible and commencement of newly-initiated subcutaneous therapy to minimise hospital visits.
At the beginning of the pandemic, patients and healthcare providers raised concerns related to risk of nosocomial SARS-CoV-2 infection when attending hospital for maintenance biological therapy and the impact of attendance on healthcare resources.83 Switching between biologicals, for example, from intravenous infliximab to subcutaneous adalimumab was not recommended by most guidelines targeting patients with IBD76 78 unlike those targeting patients with rheumatological conditions.84 85 This is, in part, because recapture of disease remission with a second biological agent, following established remission with a first biological agent, is less durable and more likely to be associated with development of anti-drug antibodies thereby limiting future therapeutic options in IBD.86–88 In some jurisdictions, there have been successful pandemic-related programmes reporting elective switching between alternative delivery routes of the same molecule, specifically from intravenous infliximab to subcutaneous infliximab (CT-P13) in patients with IBD.89 90
Patients with a new presentation of acute severe UC
Managing patients with a new diagnosis of acute severe ulcerative colitis (ASUC) was of particular concern, given the severity of illness, degree of immunosuppression that may be required, and risk of developing COVID-19 in hospitals. Two UK-based RAND appropriateness panels support existing recommendations, particularly the initiation of corticosteroid treatment, escalation to infliximab and timely surgical intervention, irrespective of COVID-19 status, in both children91 and adults92 with IBD.
In the PROTECT-ASUC study, a UK-wide case–control study across 60 acute secondary care hospitals, recruiting 782 patients with ASUC (398 during COVID-19 pandemic and 384 historical controls), more patients received rescue therapy or surgery during the pandemic than in the historical cohort (55% vs 42%, p<0.001), with a shorter time to rescue therapy during the pandemic.93 This difference was driven by a greater use of rescue and primary induction therapies with biologics, ciclosporin or tofacitinib in the pandemic than in the historical cohort; colectomy rates were similar across both groups. Although there was an increased use of outpatient-based pathways for initial administration of intravenous steroids during the pandemic, compared with historical controls, most patients were still managed as an inpatient.94 Although number of cases were small, rates of colectomy were comparable among individuals treated as inpatients and the two cohorts managed as outpatients. Results from these studies suggest that healthcare providers should continue to adapt hospital-based treatment pathways to improve care of patients with newly diagnosed IBD while limiting their risk of SARS-CoV-2 infection.95
Patients with IBD and a diagnosis of COVID-19
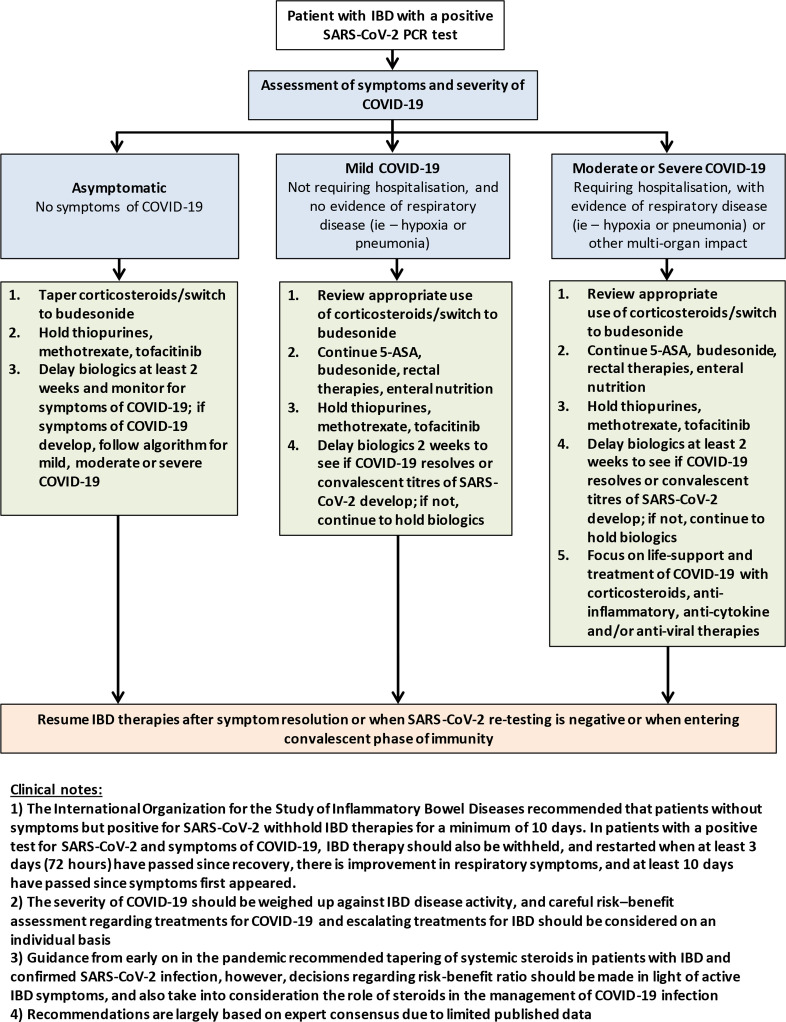
Management of a patient with IBD who tests positive for SARS-CoV-2, with or without symptoms, remains controversial. Consensus from experts recommend modification of IBD therapy in patients who have confirmed COVID-19. (figure 2)76 77 96 General principles of the guidelines include consideration to taper oral corticosteroids or switch to budesonide, with thiopurines, methotrexate and tofacitinib, and delay biological therapy for 2 weeks until recovery. However, most of these recommendations are based on consensus only, and should be considered on an individual basis utilising the most recent data where possible. While steroids have consistently come across as a risk factor for severe COVID-19, the proven benefit of steroids in managing hospitalised patients with COVID-19 suggests that steroids should not always be withdrawn in cases of patients with IBD hospitalised with COVID-19.97
Figure 2.
Treatment considerations for patients with IBD who develop COVID-19 infection. Adapted from the latest European Crohn’s and Colitis Organisation and American Gastroenterological Association guidelines.76 77 5-ASA, 5-aminosalicylic acid; IBD, inflammatory bowel disease.
The International Organisation for the Study of Inflammatory Bowel Diseases recommend that patients without symptoms but positive for SARS-CoV-2 withhold IBD therapies for a minimum of 10 days.96 In patients with a positive test for SARS-CoV-2 and symptoms of COVID-19, IBD therapy should also be withheld, and restarted when at least 3 days (72 hours) have passed since recovery, there is improvement in respiratory symptoms, and at least 10 days have passed since symptoms first appeared.
Vulnerable populations
Children with IBD
Children with IBD receiving immunosuppression, like adults, are considered to be vulnerable and of potentially higher risk of developing adverse outcomes with COVID-19.98 Reassuringly, they have not experienced higher rates of COVID-19, or related morbidity and mortality compared with adults with IBD.99–101 Compared with adults, COVID-19 in children, including those with IBD, followed a milder disease course, and children had less adverse outcomes to COVID-19.99 Like in adults, prepandemic treatment was recommended in children who were newly diagnosed or who experienced disease flare,91 102 as delay in treatment during COVID-19 was associated with disease flares in this age group.103 Longer-term surveillance is important to better understand the relationship between paediatric IBD and development of the newly identified post-COVID-19 viral syndrome, termed multisystem inflammatory syndrome,104 particularly for those on immunosuppressive therapy.105 106
Pregnant women with IBD
Similarly, pregnant women with IBD receiving immunosuppression are a concern for risk of developing adverse outcomes with COVID-19.107 However, throughout the pandemic, this cohort has not yet demonstrated higher rates of COVID-19, or related morbidity and mortality, and adverse pregnancy-related outcomes have been infrequent.100 108 Limited studies have reported pregnancy outcomes in patients with IBD and COVID-19.109 110 In the first case,110 a woman presenting with acute ASUC in her first trimester subsequently tested positive for SARS-CoV-2 and had a spontaneous abortion. The exact cause for this outcome is unclear and could relate to severe exacerbation of UC, impact of COVID-19, genetic abnormalities or a combination of above factors. In the second case,109 a woman with mild IBD symptoms developed COVID-19 at 18 weeks of gestation. She had mild COVID-19 symptoms and pregnancy was not affected following a temporary withdrawal of adalimumab during COVID-19. In a retrospective cohort of 244 pregnant women with IBD (75% on biological therapy), only one case of COVID-19 was reported.108 To date, risk of SARS-CoV-2 infection, or adverse outcomes, in pregnant women with IBD do not appear to be elevated, though larger real-world cohorts will be necessary for more definitive conclusions.
Endoscopy and disease monitoring in patients with IBD
Early in the pandemic, most guidelines have recommended postponing routine or elective endoscopies in patients with IBD,111–115 with an exception of high-risk individuals including those with a new diagnosis (and high index of moderate/severe disease), those admitted with disease flare, bowel obstruction or sclerosing cholangitis.116–120 Careful triage of patients with COVID-19 testing before procedure, appropriate use of personal protective equipment, reprocessing endoscopes and accessories by endoscopic staff are recommended. In addition, less invasive assessment and monitoring approaches using faecal calprotectin and imaging (MRI, CT or ultrasound) were preferred during the peak of the pandemic. One UK-wide analysis of national endoscopy database found that endoscopy procedures during the pandemic were reduced to 80%–95% of normal prepandemic levels, weekly number of endoscopic-detected cancers reduced by 58%, and proportion of missed cancers ranged from 19% (pancreatobiliary) to 72% (colorectal).121 Similar trends were observed in the USA with a reduction of colorectal cancer diagnosis by about 50%.122 Emerging data capturing IBD-related procedures, investigations and hospital attendances continued to show a decrease in volume of these activities during the pandemic compared with prepandemic.123 124
As countries recovered from the most recent wave of COVID-19, national guidelines have prioritised access to endoscopy services postpandemic based on local demand and resources.125–128 The UK has issued updated guidelines which stratified patients based on indication for endoscopy and timing. For example, to perform surveillance colonoscopy within 6 months of the original due date for patients due at an interval of less than 3 years, and for patients due surveillance colonoscopy at an interval of 3 years or more, to perform the endoscopy within 12 months of the original due date.129
Implementation of home biological therapy infusions has been used to reduce hospital attendance during the pandemic. However, in practice, attempts to set up such a service were challenging with lack of resources or suitable monitoring and minimal staff to coordinate care.130 Home-based therapeutic drug monitoring for biological drug and antidrug antibodies whereby patients undergo low-volume intracapillary blood sampling using a fingerprick was found to be equivalent to conventional venepuncture at hospitals or clinics.131 This home-based patient-led innovation132 133 is a potentially useful adjunct to telemedicine and may facilitate safe IBD management while protecting patients from SARS-CoV-2 infection.130 134
SARS-CoV-2 vaccination
The introduction of SARS-CoV-2 vaccines has led to a reduction in SARS-CoV-2 transmission, hospitalisations and deaths. They have been well tolerated, and rates of adverse events post-vaccination have been reported to be similar in patients with IBD compared with the general population, without an increased risk of disease exacerbation.135–137 Previous studies, however, have demonstrated impaired vaccine response following pneumococcal15 18 and influenza vaccination in patients treated with infliximab, but not vedolizumab138 or ustekinumab.139 Therefore, initial concerns were raised that patients on immunosuppressive medications may have attenuated immune responses to SARS-CoV-2 vaccines, with less immune protection from SARS-CoV-2 infection. The majority of published data have focused on vaccine responses to BNT162b2, mRNA-1273 and ChAdOx1 nCoV-19 vaccines, as these were most commonly administered worldwide. The optimal correlate of protection, defined as the immune response needed to protect an individual from a disease,140 for SARS-CoV-2 infection remains to be established. Consequently, surrogates of humoral and cellular immune response have been used to predict vaccine efficacy. These include neutralisation antibody assays,141 antibodies to the spike(S) protein receptor binding domain (anti-S RBD) as a correlate of neutralising antibodies,142 and T cell studies as a measure of cellular immunity.
Humoral response with SARS-CoV-2 vaccination
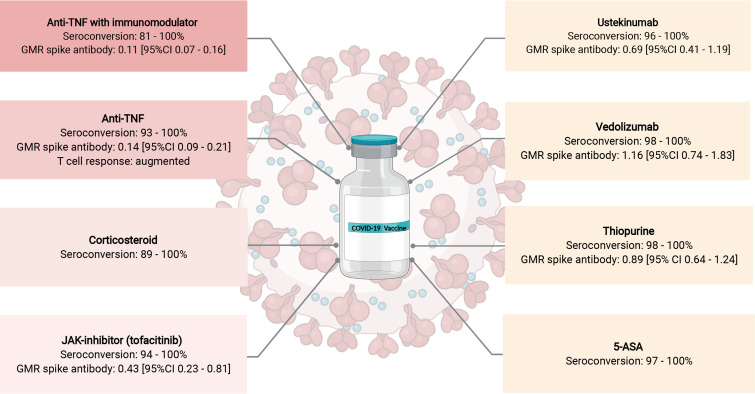
Multiple studies have reported high seroconversion rates following two doses of either the mRNA (BNT162b2, mRNA-1273) or adenoviral vector (ChAdOx1 nCoV-19) vaccines, ranging from 81% to 100% in patients with IBD on immunosuppressive therapy (figure 3).143–146 In one recent meta-analysis of over 9000 patients with IBD, the pooled seroconversion rate was 0.96 (95% CI 0.94 to 0.97).147 Of 31 studies included, seroconversion rates were determined over a range of 28–179 days following a second dose of SARS-CoV-2 vaccine. Variation in vaccine dose and patient sampling intervals, use of different antibody assays, and difficulties with replicating antibody thresholds to define seroconversion, makes interpretation and contextualising of seroconversion rates following vaccination in patients with IBD across different studies challenging.
Figure 3.
Impact of inflammatory bowel disease (IBD) treatments on SARS-CoV-2 vaccine response. GMR spike antibody concentration calculated from multivariable linear regression models comparing the magnitude of spike (S) antibody responses in each medication class to healthy controls. GMR is thought to be equivalent to a fold-change in antibodies. Shaded red boxes represent IBD medications with attenuated vaccine response. Figure created based on data from refs.137 143–146 154 175 using BioRender.com. anti-TNF, anti-tumour necrosis factor; 5-ASA, 5-aminosalicylic acid; GMR, geometric mean ratio; JAK-inhibitor, Janus kinase inhibitor.
Cell-mediated immune responses with SARS-CoV-2 vaccination
Cell-mediated immune responses may also contribute to protective immunity against SARS-CoV-2 infection independent of humoral responses. Studies in both non-immunocompromised148 149 and immunocompromised150 cohorts have demonstrated robust T cell responses correlate with better outcomes to SARS-CoV-2 infection. Uncoupling of humoral and T cell immunity has been reported in non-immunocompromised individuals,151 which may be directly relevant to immunocompromised patients who have attenuated humoral responses if T cell responses are not impaired. Although data in patients with IBD are limited, initial studies are reassuring. CORALE-IBD found a poor correlation between anti-S RBD antibody concentration and T cell clonal response to SARS-CoV-2 vaccines from 303 patients with IBD who were on a range of therapies, although they found that patients with IBD treated with anti-TNF therapy had an augmented T cell clonal depth compared with patients receiving no treatment.152 The CLARITY IBD study, a UK-wide prospective cohort study of patients with IBD investigating the impact of infliximab and vedolizumab, and/or concomitant immunomodulators, on SARS-CoV-2 acquisition, illness and immunity in patients with IBD, reported an uncoupling of anti-S RBD antibodies and anti-spike T cell responses, however, similar T cell responses were observed between infliximab-treated compared with vedolizumab-treated patients after one or two doses of either vaccine.146 Further studies are required to confirm these findings, and to determine the relative contributions of T cell vaccine responses to SARS-CoV-2 immunity in patient with IBD.
IBD medications and vaccine response
Patients treated with anti TNF therapies
Despite high seroconversion rates following vaccination, lower SARS-CoV-2 anti-spike (S) antibody concentrations have been observed in patients receiving anti-TNF treatment, compared with other biological therapies. In CLARITY IBD, following two doses of either the BNT162b2 or ChAdOx1 nCoV-19 vaccines, geometric mean (geometric SD) anti-SARS-CoV-2 S antibody levels were significantly lower in 2279 infliximab-treated compared with 1031 vedolizumab-treated patients (BNT162b2 (infliximab 566.7 U/mL vs vedolizumab 4555.3 U/mL) and ChAdOx1 nCoV-19 (infliximab 184.7 U/mL vs vedolizumab 784.0 U/mL) vaccines).146 Anti-SARS-CoV-2 S antibody concentrations were also lower in patients treated with a concomitant immunomodulator. This association was later confirmed in the RECOVER study, a prospective, controlled multicentre study in Israel.137 Antibody responses following two doses of the BNT162b2 vaccine were compared between 73 healthy controls, 67 anti-TNF treated and 118 non-anti-TNF treated patients with IBD. Four weeks following vaccination with a second dose, anti-TNF treated individuals had significantly lower SARS-CoV-2 anti-S IgG geometric mean concentration compared with healthy controls. Similar observations were seen in this study when assessing neutralising antibodies and when using SARS-CoV-2 spike pseudoparticle neutralisation assays. In RECOVER, neither timing of anti-TNF administration, nor anti-TNF drug levels, were associated with SARS-CoV-2 anti-S concentrations following vaccination, suggesting that SARS-CoV-2 vaccines may be administered at any point during the anti-TNF treatment cycle.
Patients treated with non-anti TNF therapies
Data on whether antibody responses to SARS-CoV-2 vaccines in patients with IBD treated with other biological and non-TNF immunosuppressive therapies are also attenuated continues to emerge. Most studies have shown reassuring results for patients on other biological therapies, but have been limited by small sample sizes within each medication class. In a single-centre cohort study of 602 patients with IBD (292 (48.3%) anti-TNF-treated, 112 (18.6%) vedolizumab-treated, 91 (15.1%) ustekinumab-treated, 51 (8.5%) thiopurine-treated and 36 (6.0%) 5-ASA-treated), antibody responses following a second dose of either the BNT162b2, CX-024414 or ChAdOx1 nCoV-19 vaccine were compared with 168 healthy controls.153 Post-vaccine seropositivity rates were similar among patients with IBD and controls, and median anti-SARS-CoV-2 IgG levels were not significantly different between anti-TNF-treated and non-anti-TNF-treated patients. This may be because of limited number of patients in each group and differences in assay used.
The VIP study from the UK assessed anti-SARS-CoV-2 S protein antibody response in 362 patients with IBD receiving immunosuppressive therapy (78 (12.4%) thiopurine-treated, 72 (19,9%) infliximab/thiopurine-treated, 63 (17.4%) infliximab-treated, 62 (17.1%) vedolizumab-treated, 57 (15.7%) ustekinumab-treated and 30 (8.3%) tofacitinib-treated) and 121 healthy controls, following vaccination with either the BNT162b2 or ChAdOx1 nCoV-19 vaccine.154 Patients treated with infliximab, infliximab/thiopurine and tofacitinib had significantly lower geometric mean anti-SARS-CoV-2 S protein antibody concentrations compared with controls (infliximab: 156.8 U/mL (geometric SD 5.7); p<0.0001, infliximab/thiopurine: 111.1 U/mL (5·7); p<0·0001, tofacitinib: 429·5 U/mL (3·1); p=0·0012, controls: 1578.3 U/mL (3.7)). In contrast, the PREVENT-COVID study compared antibody responses among 317 patients with IBD treated with a range of biological and non-biological therapies and only found diminished antibody responses in patients treated with corticosteroids, but not other medication classes, following two doses of an mRNA vaccine (BNT162b2, mRNA-1273).144 Formal hypothesis testing was unable to be performed in this exploratory analysis as there were only 13 patients being treated with budesonide.
It is unsurprising that reduced antibody responses following mRNA COVID-19 vaccination were also observed in other patient cohorts who have IMIDs, including patients with psoriasis, rheumatoid arthritis and kidney abnormalities.155 The multivariate meta-regression from this systematic review of 5360 patients, some of whom had IBD, demonstrated that treatment with anti-CD20, but not anti-TNF (p=0.058), was associated with lower SARS-CoV-2 antibody concentration following two doses of mRNA vaccination. This finding may, in part, be explained by heterogeneous sample size, disease inclusion, medication use and antibody testing, and further data are awaited to confirm these findings.
Most recently, in a multicentre study of 111 patients with IMIDs, lower seroconversion rates following SARS-CoV-2 vaccination were reported in patients treated with sphingosine 1-posphate (S1P) modulators compared with healthy controls.156 Although these patients were treated with S1P modulators for multiple sclerosis, ozanimod, an S1P inhibitor has received regulatory approval for treatment of UC recently. Whether attenuated seroconversion rates will also be observed in patients with IBD treated with ozanimod remains to be determined.
Patients with prior SARS-CoV-2 infection and different vaccine types
The effect of prior SARS-CoV-2 infection on antibody concentrations and seroconversion, following complete vaccination, has shown a similar direction of effect in healthy patients,157 patients without IBD treated with immunosuppressive therapies,158 and patients with IBD. Anti-SARS-CoV-2 (S) antibody concentrations and seroconversion rates have been reported to be higher in patients with prior SARS-CoV-2 infection before a single dose or both doses of either vaccine, regardless of treatment class.146 153 159 Vaccination with BNT162b2, compared with ChAdOx1 nCoV-19, has been associated with a higher antibody response in patients with IBD, regardless of therapy.146 153 Notably, while thiopurine use is not known to be associated with lower anti-SARS-CoV-2 antibody concentration in individuals who received the ChAdOx1 nCoV-19 vaccine, the higher peak antibody response following mRNA-based SARS-CoV-2 vaccine has led to recommendations for its use in immunosuppressed cohorts.
Durability, vaccine effectiveness and breakthrough infections
Decay of vaccine-induced SARS-CoV-2 antibodies over time, as observed in non-immunosuppressed cohorts,141 160 161 can result in reduced vaccine effectiveness and a subsequent risk of breakthrough infections with SARS-CoV-2.162 Consequently, impaired serological response to SARS-CoV-2 vaccines in patients receiving immunosuppressive therapies may further influence the durability and subsequent vaccine effectiveness in these patients. In this regard, CLARITY IBD demonstrated a shorter antibody half-life in infliximab-treated compared with vedolizumab-treated patients following two doses of BNT162b2 (infliximab: 26.8 days (95% CI 26.2 to 27.5) vs vedolizumab: 47.6 (95% CI 45.5 to 49.8), p<0.0001) and ChAdOx1 nCoV-19 (infliximab: 35.9 days (95% CI 34.9 to 36.8) vs vedolizumab: 58.0 days (95% CI 55.0 to 61.3) p<0.0001) vaccines.146 Similar findings were observed when antibody half-lives of anti-TNF-treated patients were compared with other non-anti-TNF treatments including ustekinumab, 5-ASA and budesonide in addition to vedolizumab (38 days vs 74 days, p=0.045).163 Conversely, in patients with IBD treated with both biological and non-biological therapies, one retrospective cohort study reported vaccine effectiveness of 80.4%, following two doses of an mRNA-based vaccines (BNT162b2, mRNA-1273),164 a rate similar to original vaccine trials.165 166 In this large Veterans Health Administration with IBD cohort, most patients (54.8%, 8048/14,697) were treated with 5-ASA only, and the study was conducted when the major circulating variant was alpha (B1.1.7).
The rates of breakthrough infections in patients with IBD treated with immunosuppressive therapies are less clear. While CLARITY IBD reported more breakthrough infections in infliximab- compared with vedolizumab-treated patients (5.8% (201/3441) vs 3.9% (66/1682), p=0.0039), with a shorter time to a positive SARS-CoV-2 PCR,146 this finding has not yet been observed in other studies.
Two other cohort studies have found no difference in rates of breakthrough infection following two doses of vaccination.167 168 One Israeli case–control study assessed 12 109 patients with IBD who had received two doses of BNT162b2 vaccine.167 The authors reported no difference in rates of breakthrough infections, or time to positive SARS-CoV-2 PCR, in patients treated with anti-TNF or corticosteroids compared with patients with IBD not on these treatments (0.4% vs 0.3%, p=1.0). Similarly, in one large US retrospective cohort, no difference in breakthrough infections between patients with IBD compared with non-IBD controls was found (0.36% vs 0.28%, RR 1.3 (95% CI 0.83 to 2.05)).168 Overall, less than 1% of patients with a breakthrough infection required hospitalisation, regardless of IBD treatment.136 146
Differences in rates of breakthrough infections should be interpreted with caution, as differential findings across studies may be explained by differences in vaccine type, dosing interval, prevalence of SARS-CoV-2 and incidence of dominant variants during the study period.
Third doses and variants of concern
On 26 November 2021, the WHO designated B.1.1.529, or the omicron variant, of SARS-CoV-2 a variant of concern.169 Because of its increased transmissibility and waning population immunity, the omicron variant is driving large numbers of breakthrough and SARS-CoV-2 reinfections, with estimates suggesting serological responses following SARS-CoV-2 vaccination needing to be 40-fold greater than with previous SARS-CoV-2 variants to achieve protective immunity.170 As the majority of published studies predate emergence of omicron, and newer SARS-CoV-2 variants,171 little data have been published on the antibody responses following third, or booster, doses of SARS-CoV-2 vaccines that most countries recommended patients with IBD receive.
Preliminary data from the HERCULES cohort, a multicentre, prospective, non-randomised study assessed serological responses from 85 patients with IBD who received a third dose of SARS-CoV-2 mRNA vaccine.172 They found that all patients were seropositive, and expectedly, median antibody concentrations were higher following a third dose than after the two-dose series. Notably, patients treated with corticosteroids, anti-TNF, mono- or combination therapy, had significantly lower SARS-CoV-2 anti-spike IgG antibody concentrations than patients who were not treated with those therapies (median 38 (IQR 20–120) vs 73 (IQR 58–167), p=0.015). Similar findings were observed in a prospective cohort study of 495 patients with IBD, where corticosteroid use was associated with lower antibodies to SARS-CoV-2 spike protein following a third dose of SARS-CoV-2 vaccine (fold-change: 0.07 (95% CI 0.02 to 0.20)).173
To date, there are no data on outcome and efficacy of fourth dose of vaccine in patients with IBD. According to recommendations from the US Center for Disease Control and Prevention, people aged 12 years and older who are moderately or severely immunocompromised, which includes those on active treatment with high-dose corticosteroids or other drugs that may suppress their immune response, should receive a total of four doses of SARS-CoV-2 vaccine. The four doses are made up of a primary series of three doses of an mRNA SARS-CoV-2 vaccine, plus one booster of an mRNA SARS-CoV-2 vaccine.174 However, the effectiveness of fourth dose vaccine on humoral or cellular immune response and how drugs affect subsequent antibody decay remains unclear.
Conclusion
In summary, accumulating data suggest that there is no increased risk for developing COVID-19 among patients with IBD or risk for de novo IBD after COVID-19 infection. However, older age, increased number of comorbidities and systemic corticosteroid use are risk factors for adverse outcomes following COVID-19 in patients with IBD. Studies suggest no short-term impact of COVID-19 on IBD disease activity, though validation in additional larger cohorts is required, and the risk of long COVID-19 remains unclear. Corticosteroid use appears to be associated with an increased risk of adverse COVID-19 outcomes, but most other medications used to treat IBD, including biologicals, mesalazine and sulfasalazine are not associated with severe COVID-19 outcomes. There is some signal that anti-TNF therapies may exert a protective effect. These results support maintaining patients with IBD on medications that optimally treat their IBD during the COVID-19 pandemic.
The risks of SARS-CoV-2 vaccination are low and SARS-CoV-2 vaccination is strongly recommended in patients with IBD, but the protective immune responses to SARS-CoV-2 vaccination are diminished in some patients with IBD, especially those taking anti-TNF medications. Patients treated with anti-TNF plus immunomodulator combination therapies and JAKi also had poorer antibody responses to SARS-CoV-2 vaccination, which exposes them to a potential increased risk of SARS-CoV-2 infection. These findings support a personalised approach to scheduling vaccine dosing for patients with IBD. Future research should explore factors associated with vaccine hesitancy and its effect in the IBD community, the long-term effect of immunosuppression on vaccine efficacy, and the search for predictive biomarkers of vaccine success, as well as timing of fourth dose of vaccine. Further data on long-term outcomes and the mechanism of decreased serological responses are also warranted.
Footnotes
Twitter: @SimengLin, @LouisHSLau, @nchanchlani1, @DrNickKennedy, @Siew_C_Ng
SL, LHL and NC contributed equally.
Contributors: SL, LL and NC conceived the concepts and drafted the manuscript. NAK and SCN conceived the concepts and critically revised the manuscript. All authors approved the final version of the manuscript.
Funding: SL is suppoted by a Wellcome GW4-CAT fellowship. NC acknowledges support from Crohn's & Colitis UK. SCN was partially funded by InnoHK, The Government of Hong Kong, Special Administrative Region of the People’s Republic of China.
Competing interests: SL reports non-financial support from Pfizer, non-financial support from Ferring, outside the submitted work. LL has no conflict of interest to declare. NC has no conflict of interest to declare. NAK reports grants from F. Hoffmann-La Roche AG, grants from Biogen, grants from Celltrion Healthcare, grants from Galapagos NV, non-financial support from Immundiagnostik, grants and non-financial support from AbbVie, grants and personal fees from Celltrion, personal fees and non-financial support from Janssen, personal fees from Takeda, personal fees and non-financial support from Dr Falk, outside the submitted work. SCN has served as speakers for Janssen, Abbvie, Takeda, Ferring, Tilotts, Menarini, Pfizer and have received research grants from Olympus, Ferring, Janssen and Abbvie, outside the submitted work.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265–9. 10.1038/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature 2022;602:671–5. 10.1038/s41586-021-04389-z [DOI] [PubMed] [Google Scholar]
- 4. Hoffmann M, Krüger N, Schulz S, et al. The omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell 2022;185:447–56. 10.1016/j.cell.2021.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–43. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769–78. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 7. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875–9. 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burgueño JF, Reich A, Hazime H, et al. Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis 2020;26:797–808. 10.1093/ibd/izaa085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suárez-Fariñas M, Tokuyama M, Wei G, et al. Intestinal inflammation modulates the expression of ACE2 and TMPRSS2 and potentially overlaps with the pathogenesis of SARS-CoV-2-related disease. Gastroenterology 2021;160:287–301. 10.1053/j.gastro.2020.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McAllister MJ, Kirkwood K, Chuah SC, et al. Intestinal protein characterisation of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in inflammatory bowel disease (IBD) and fatal COVID-19 infection. Inflammation 2022;45:567–72. 10.1007/s10753-021-01567-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Long MD, Martin C, Sandler RS, et al. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol 2013;108:240–8. 10.1038/ajg.2012.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tinsley A, Navabi S, Williams ED, et al. Increased risk of influenza and Influenza-Related complications among 140,480 patients with inflammatory bowel disease. Inflamm Bowel Dis 2019;25:369–76. 10.1093/ibd/izy243 [DOI] [PubMed] [Google Scholar]
- 13. Kirchgesner J, Lemaitre M, Carrat F, et al. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology 2018;155:337–46. 10.1053/j.gastro.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 14. Bonovas S, Fiorino G, Allocca M, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol 2016;14:1385–97. 10.1016/j.cgh.2016.04.039 [DOI] [PubMed] [Google Scholar]
- 15. Melmed GY, Agarwal N, Frenck RW, et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:148–54. 10.1038/ajg.2009.523 [DOI] [PubMed] [Google Scholar]
- 16. deBruyn JCC, Hilsden R, Fonseca K, et al. Immunogenicity and safety of influenza vaccination in children with inflammatory bowel disease. Inflamm Bowel Dis 2012;18:25–33. 10.1002/ibd.21706 [DOI] [PubMed] [Google Scholar]
- 17. Andrisani G, Frasca D, Romero M, et al. Immune response to influenza A/H1N1 vaccine in inflammatory bowel disease patients treated with anti TNF-α agents: effects of combined therapy with immunosuppressants. J Crohns Colitis 2013;7:301–7. 10.1016/j.crohns.2012.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fiorino G, Peyrin-Biroulet L, Naccarato P, et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis 2012;18:1042–7. 10.1002/ibd.21800 [DOI] [PubMed] [Google Scholar]
- 19. Norsa L, Cosimo P, Indriolo A, et al. Asymptomatic severe acute respiratory syndrome coronavirus 2 infection in patients with inflammatory bowel disease under biologic treatment. Gastroenterology 2020;159:2229–31. 10.1053/j.gastro.2020.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marafini I, Salvatori S, Sena G, et al. Low frequency of COVID-19 in inflammatory bowel diseases. Dig Liver Dis 2020;52:1234–5. 10.1016/j.dld.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rizzello F, Calabrese C, Salice M, et al. COVID-19 in IBD: the experience of a single tertiary IBD center. Dig Liver Dis 2021;53:271–6. 10.1016/j.dld.2020.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taxonera C, Sagastagoitia I, Alba C, et al. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther 2020;52:276–83. 10.1111/apt.15804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khan N, Patel D, Xie D, et al. Are patients with inflammatory bowel disease at an increased risk of developing SARS-CoV-2 than patients without inflammatory bowel disease? results from a nationwide veterans' Affairs cohort study. Am J Gastroenterol 2021;116:808–10. 10.14309/ajg.0000000000001012 [DOI] [PubMed] [Google Scholar]
- 24. Hadi Y, Dulai PS, Kupec J, et al. Incidence, outcomes, and impact of COVID-19 on inflammatory bowel disease: propensity matched research network analysis. Aliment Pharmacol Ther 2022;55:191–200. 10.1111/apt.16730 [DOI] [PubMed] [Google Scholar]
- 25. Attauabi M, Poulsen A, Theede K, et al. Prevalence and outcomes of COVID-19 among patients with inflammatory bowel Disease-A Danish prospective population-based cohort study. J Crohns Colitis 2021;15:540–50. 10.1093/ecco-jcc/jjaa205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mak JWY, Weng M-T, Wei SC, et al. Zero COVID-19 infection in inflammatory bowel disease patients: findings from population-based inflammatory bowel disease registries in Hong Kong and Taiwan. J Gastroenterol Hepatol 2021;36:171–3. 10.1111/jgh.15164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chanchlani N, Lin S, Chee D, et al. Adalimumab and infliximab impair SARS-CoV-2 antibody responses: results from a therapeutic drug monitoring study in 11 422 Biologic-Treated patients. J Crohns Colitis 2022;16:389–97. 10.1093/ecco-jcc/jjab153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kennedy NA, Goodhand JR, Bewshea C, et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut 2021;70:865–75. 10.1136/gutjnl-2021-324388 [DOI] [PubMed] [Google Scholar]
- 29. Sima AR, Saberzadeh-Ardestani B, Vahedi H, et al. Outcomes of COVID-19 in patients with inflammatory bowel disease: comparison with household members and the role of IBD medications. Arch Iran Med 2022;25:17–25. 10.34172/aim.2022.04 [DOI] [PubMed] [Google Scholar]
- 30. Singh AK, Jena A, Kumar-M P, et al. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. United European Gastroenterol J 2021;9:159–76. 10.1177/2050640620972602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gubatan J, Levitte S, Balabanis T, et al. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in northern California. Gastroenterology 2020;159:1141–4. 10.1053/j.gastro.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berte' R, Mazza S, Stefanucci MR, et al. Seroprevalence of SARS-CoV2 in IBD patients treated with biologic therapy. J Crohns Colitis 2021;15:864–8. 10.1093/ecco-jcc/jjaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. COVID-19 and IBD reporting database [Internet]. SECURE-IBD Database, 2020. Available: http://covidibd.org [Accessed 10 Feb 2022].
- 34. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology 2020;159:481–91. 10.1053/j.gastro.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut 2021;70:725–32. 10.1136/gutjnl-2020-322539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ungaro RC, Brenner EJ, Agrawal M, et al. Impact of medications on COVID-19 outcomes in inflammatory bowel disease: analysis of more than 6000 patients from an international registry. Gastroenterology 2022;162:316–9. 10.1053/j.gastro.2021.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agrawal M, Zhang X, Brenner EJ, et al. The impact of Vedolizumab on COVID-19 outcomes among adult IBD patients in the SECURE-IBD registry. J Crohns Colitis 2021;15:1877–84. 10.1093/ecco-jcc/jjab071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Agrawal M, Brenner EJ, Zhang X, et al. Characteristics and outcomes of IBD patients with COVID-19 on tofacitinib therapy in the SECURE-IBD registry. Inflamm Bowel Dis 2021;27:585–9. 10.1093/ibd/izaa303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh AK, Jena A, Kumar-M P, et al. Clinical presentation of COVID-19 in patients with inflammatory bowel disease: a systematic review and meta-analysis. Intest Res 2022;20:134–43. 10.5217/ir.2020.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singh S, Khan A, Chowdhry M, et al. Risk of severe coronavirus disease 2019 in patients with inflammatory bowel disease in the United States: a multicenter research network study. Gastroenterology 2020;159:1575–8. 10.1053/j.gastro.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Creemers RH, Rezazadeh Ardabili A, Jonkers DM, et al. Severe COVID-19 in inflammatory bowel disease patients in a population-based setting. PLoS One 2021;16:e0258271. 10.1371/journal.pone.0258271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut 2020;69:1213–7. 10.1136/gutjnl-2020-321411 [DOI] [PubMed] [Google Scholar]
- 43. Derikx LAAP, Lantinga MA, de Jong DJ, et al. Clinical outcomes of Covid-19 in patients with inflammatory bowel disease: a nationwide cohort study. J Crohns Colitis 2021;15:529–39. 10.1093/ecco-jcc/jjaa215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Queiroz NSF, Martins CdeA, Quaresma AB, et al. COVID-19 outcomes in patients with inflammatory bowel diseases in Latin America: results from SECURE-IBD registry. J Gastroenterol Hepatol 2021;36:3033–40. 10.1111/jgh.15588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bamias G, Kokkotis G, Christidou A, et al. The natural history of COVID-19 in patients with inflammatory bowel disease: a nationwide study by the Hellenic Society for the study of IBD. Eur J Gastroenterol Hepatol 2021;33:e810–7. 10.1097/MEG.0000000000002267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. MacKenna B, Kennedy NA, Mehkar A. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune modifying therapies: a nationwide cohort study in the OpenSAFELY platform [Internet]. bioRxiv 2021. http://medrxiv.org/lookup/doi/10.1101/2021.09.03.21262888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wetwittayakhlang P, Albader F, Golovics PA, et al. Clinical outcomes of COVID-19 and impact on disease course in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol 2021;2021:7591141. 10.1155/2021/7591141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agrawal M, Brenner EJ, Yan Mak JW, et al. COVID-19 outcomes among racial and ethnic minority individuals with inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol 2021;19:2210–3. 10.1016/j.cgh.2021.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roy S, Sheikh SZ, Furey TS. A machine learning approach identifies 5-ASA and ulcerative colitis as being linked with higher COVID-19 mortality in patients with IBD. Sci Rep 2021;11:16522. 10.1038/s41598-021-95919-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sperger J, Shah KS, Lu M, et al. Development and validation of multivariable prediction models for adverse COVID-19 outcomes in patients with IBD. BMJ Open 2021;11:e049740. 10.1136/bmjopen-2021-049740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ricciuto A, Lamb CA, Benchimol EI, et al. Inflammatory bowel disease clinical activity is associated with COVID-19 severity especially in younger patients. J Crohns Colitis 2021. 10.1093/ecco-jcc/jjab172. [Epub ahead of print: 27 Sep 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferraù F, Ceccato F, Cannavò S, et al. What we have to know about corticosteroids use during Sars-Cov-2 infection. J Endocrinol Invest 2021;44:693–701. 10.1007/s40618-020-01384-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Velayos FS, Dusendang JR, Schmittdiel JA. Prior immunosuppressive therapy and severe illness among patients diagnosed with SARS-CoV-2: a community-based study. J Gen Intern Med 2021;36:3794–801. 10.1007/s11606-021-07152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Khan N, Mahmud N, Trivedi C, et al. Risk factors for SARS-CoV-2 infection and course of COVID-19 disease in patients with IBD in the Veterans affair healthcare system. Gut 2021;70:1657–64. 10.1136/gutjnl-2021-324356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ungaro RC, Agrawal M, Park S, et al. Autoimmune and chronic inflammatory disease patients with COVID-19. ACR Open Rheumatol 2021;3:111–5. 10.1002/acr2.11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tripathi K, Godoy Brewer G, Thu Nguyen M, et al. COVID-19 and outcomes in patients with inflammatory bowel disease: systematic review and meta-analysis. Inflamm Bowel Dis 2021. 10.1093/ibd/izab236. [Epub ahead of print: 27 Oct 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Burke KE, Kochar B, Allegretti JR, et al. Immunosuppressive therapy and risk of COVID-19 infection in patients with inflammatory bowel diseases. Inflamm Bowel Dis 2021;27:155–61. 10.1093/ibd/izaa278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meyer A, Semenzato L, Zureik M, et al. Risk of severe COVID-19 in patients treated with IBD medications: a French nationwide study. Aliment Pharmacol Ther 2021;54:160–6. 10.1111/apt.16410 [DOI] [PubMed] [Google Scholar]
- 59. Ardizzone S, Ferretti F, Monico MC, et al. Lower incidence of COVID-19 in patients with inflammatory bowel disease treated with non-gut selective biologic therapy. J Gastroenterol Hepatol 2021;36:3050–5. 10.1111/jgh.15591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Izadi Z, Brenner EJ, Mahil SK, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open 2021;4:e2129639. 10.1001/jamanetworkopen.2021.29639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013;369:711–21. 10.1056/NEJMoa1215739 [DOI] [PubMed] [Google Scholar]
- 62. Feagan BG, Bhayat F, Khalid M, et al. Respiratory tract infections in patients with inflammatory bowel disease: safety analyses from Vedolizumab clinical trials. J Crohns Colitis 2018;12:905–19. 10.1093/ecco-jcc/jjy047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. 10.1056/NEJMoa1606910 [DOI] [PubMed] [Google Scholar]
- 64. Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;385:406–15. 10.1056/NEJMoa2101643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lukin DJ, Funez-dePagnier G, Lima S, et al. No durable impact of COVID-19 on intestinal disease activity in subjects with IBD. Clin Gastroenterol Hepatol 2021;19:2312–4. 10.1016/j.cgh.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Neurath MF. COVID-19 and immunomodulation in IBD. Gut 2020;69:1335–42. 10.1136/gutjnl-2020-321269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mylonaki M, Langmead L, Pantes A, et al. Enteric infection in relapse of inflammatory bowel disease: importance of microbiological examination of stool. Eur J Gastroenterol Hepatol 2004;16:775–8. 10.1097/01.meg.0000131040.38607.09 [DOI] [PubMed] [Google Scholar]
- 68. Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022;22:e102–7. 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Crook H, Raza S, Nowell J, et al. Long covid-mechanisms, risk factors, and management. BMJ 2021;374:n1648. 10.1136/bmj.n1648 [DOI] [PubMed] [Google Scholar]
- 70. Carfì A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020;324:603–5. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chopra V, Flanders SA, O'Malley M, et al. Sixty-Day outcomes among patients hospitalized with COVID-19. Ann Intern Med 2021;174:576–8. 10.7326/M20-5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from Hospital: a cohort study. Lancet 2021;397:220–32. 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021;6:100122. 10.1016/j.lanepe.2021.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Salvatori S, Baldassarre F, Mossa M, et al. Long COVID in inflammatory bowel diseases. J Clin Med 2021;10:5575. 10.3390/jcm10235575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu Q, Mak JWY, Su Q, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022;71:544–52. 10.1136/gutjnl-2021-325989 [DOI] [PubMed] [Google Scholar]
- 76. Magro F, Rahier J-F, Abreu C, et al. Inflammatory bowel disease management during the COVID-19 outbreak: the ten do's and don'ts from the ECCO-COVID Taskforce. J Crohns Colitis 2020;14:S798–806. 10.1093/ecco-jcc/jjaa160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rubin DT, Feuerstein JD, Wang AY, et al. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology 2020;159:350–7. 10.1053/j.gastro.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kennedy NA, Jones G-R, Lamb CA, et al. British Society of gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut 2020;69:984–90. 10.1136/gutjnl-2020-321244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Targownik LE, Bernstein CN, Lakatos PL, et al. Crohn's and colitis Canada's 2021 impact of COVID-19 and inflammatory bowel disease in Canada: risk factors and medications. J Can Assoc Gastroenterol 2021;4:S40–5. 10.1093/jcag/gwab032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nakase H, Matsumoto T, Matsuura M, et al. Expert opinions on the current therapeutic management of inflammatory bowel disease during the COVID-19 pandemic: Japan IBD COVID-19 Taskforce, intractable diseases, the health and labor sciences research. Digestion 2021;102:814–22. 10.1159/000510502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Aysha A-A, Rentsch C, Prentice R, et al. Practical management of inflammatory bowel disease patients during the COVID-19 pandemic: expert commentary from the gastroenterological Society of Australia inflammatory bowel disease faculty. Intern Med J 2020;50:798–804. 10.1111/imj.14889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bernstein CN, Ng SC, Banerjee R, et al. Worldwide management of inflammatory bowel disease during the COVID-19 pandemic: an international survey. Inflamm Bowel Dis 2021;27:836–47. 10.1093/ibd/izaa202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bertani L, Barberio B, Tricò D, et al. Hospitalisation for drug infusion did not increase levels of anxiety and the risk of disease relapse in patients with inflammatory bowel disease during COVID-19 outbreak. J Clin Med 2021;10:3270. 10.3390/jcm10153270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Richez C, Flipo R-M, Berenbaum F, et al. Managing patients with rheumatic diseases during the COVID-19 pandemic: the French Society of rheumatology answers to most frequently asked questions up to may 2020. Joint Bone Spine 2020;87:431–7. 10.1016/j.jbspin.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Akintayo RO, Bahiri R, El Miedany Y, et al. African League against rheumatism (AFLAR) preliminary recommendations on the management of rheumatic diseases during the COVID-19 pandemic. Clin Rheumatol 2021;40:3445–54. 10.1007/s10067-020-05355-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn's disease controlled by maintenance infliximab: prospective randomised switch trial. Gut 2012;61:229–34. 10.1136/gutjnl-2011-300755 [DOI] [PubMed] [Google Scholar]
- 87. Casanova MJ, Chaparro M, Mínguez M, et al. Effectiveness and safety of the sequential use of a second and third anti-TNF agent in patients with inflammatory bowel disease: results from the Eneida registry. Inflamm Bowel Dis 2020;26:606–16. 10.1093/ibd/izz192 [DOI] [PubMed] [Google Scholar]
- 88. Roblin X, Williet N, Boschetti G, et al. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF Trough levels and antidrug antibodies: a prospective randomised trial. Gut 2020;69:1206–12. 10.1136/gutjnl-2019-319758 [DOI] [PubMed] [Google Scholar]
- 89. Argüelles-Arias F, Fernández Álvarez P, Castro Laria L, et al. Switch to infliximab subcutaneous during SARS-CoV-2 pandemic: preliminary results. Rev Esp Enferm Dig 2021. 10.17235/reed.2021.8320/2021 [DOI] [PubMed] [Google Scholar]
- 90. Verma AM, Patel A, Subramanian S, et al. From intravenous to subcutaneous infliximab in patients with inflammatory bowel disease: a pandemic-driven initiative. Lancet Gastroenterol Hepatol 2021;6:88–9. 10.1016/S2468-1253(20)30392-7 [DOI] [PubMed] [Google Scholar]
- 91. Hansen R, Meade S, Beattie RM, et al. Adaptations to the current ECCO/ESPGHAN guidelines on the management of paediatric acute severe colitis in the context of the COVID-19 pandemic: a Rand appropriateness panel. Gut 2021;70:1044–52. 10.1136/gutjnl-2020-322449 [DOI] [PubMed] [Google Scholar]
- 92. Din S, Kent A, Pollok RC, et al. Adaptations to the British Society of gastroenterology guidelines on the management of acute severe UC in the context of the COVID-19 pandemic: a Rand appropriateness panel. Gut 2020;69:1769–77. 10.1136/gutjnl-2020-321927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sebastian S, Walker GJ, Kennedy NA, et al. Assessment, endoscopy, and treatment in patients with acute severe ulcerative colitis during the COVID-19 pandemic (PROTECT-ASUC): a multicentre, observational, case-control study. Lancet Gastroenterol Hepatol 2021;6:271–81. 10.1016/S2468-1253(21)00016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sebastian S, Patel KV, Segal JP, et al. Ambulatory care management of 69 patients with acute severe ulcerative colitis in comparison to 695 inpatients: insights from a multicentre UK cohort study. BMJ Open Gastroenterol 2022;9:e000763. 10.1136/bmjgast-2021-000763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu X-R, Zhang Y-F, Lan N, et al. Practice patterns of colorectal surgery during the COVID-19 pandemic. Dis Colon Rectum 2020;63:1572–4. 10.1097/DCR.0000000000001840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Siegel CA, Christensen B, Kornbluth A, et al. Guidance for restarting inflammatory bowel disease therapy in patients who Withheld immunosuppressant medications during COVID-19. J Crohns Colitis 2020;14:S769–73. 10.1093/ecco-jcc/jjaa135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Turner D, Huang Y, Martín-de-Carpi J, et al. Corona virus disease 2019 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the paediatric IBD Porto group of European Society of paediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr 2020;70:727–33. 10.1097/MPG.0000000000002729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brenner EJ, Pigneur B, Focht G, et al. Benign evolution of SARS-Cov2 infections in children with inflammatory bowel disease: results from two international databases. Clin Gastroenterol Hepatol 2021;19:394–6. 10.1016/j.cgh.2020.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Benchimol EI, Carroll MW, Geist R, et al. Crohn's and colitis Canada's 2021 impact of COVID-19 and inflammatory bowel disease in Canada: children and expectant mothers with inflammatory bowel disease. J Can Assoc Gastroenterol 2021;4:S27–33. 10.1093/jcag/gwab030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ruan W, Ihekweazu F, Walsh S, et al. SARS-COV-2 infection and seroconversion in pediatric inflammatory bowel disease patients. Inflamm Bowel Dis 2021;27:S44–5. 10.1093/ibd/izaa347.108 [DOI] [Google Scholar]
- 102. Arrigo S, Alvisi P, Banzato C, et al. Management of paediatric IBD after the peak of COVID-19 pandemic in Italy: a position paper on behalf of the SIGENP IBD Working group. Dig Liver Dis 2021;53:183–9. 10.1016/j.dld.2020.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Korean Society of Infectious Diseases, Korean Society of Pediatric Infectious Diseases, Korean Society of Epidemiology, et al. Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci 2020;35:e112. 10.3346/jkms.2020.35.e112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607–8. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Meredith J, Khedim C-A, Henderson P, et al. Paediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2 [PIMS-TS] in a Patient Receiving Infliximab Therapy for Inflammatory Bowel Disease. J Crohns Colitis 2021;15:687–91. 10.1093/ecco-jcc/jjaa201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dolinger MT, Person H, Smith R, et al. Pediatric Crohn disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J Pediatr Gastroenterol Nutr 2020;71:153–5. 10.1097/MPG.0000000000002809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. De Lima-Karagiannis A, Juillerat P, Sebastian S, et al. Management of pregnant inflammatory bowel disease patients during the COVID-19 pandemic. J Crohns Colitis 2020;14:S807–14. 10.1093/ecco-jcc/jjaa125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Selinger CP, Fraser A, Collins P, et al. Impact of the coronavirus infectious disease (COVID-19) pandemic on the provision of inflammatory bowel disease (IBD) antenatal care and outcomes of pregnancies in women with IBD. BMJ Open Gastroenterol 2021;8:e000603. 10.1136/bmjgast-2021-000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Prentice RE, Ross AL, Flanagan EK. Management of pregnant women with inflammatory bowel disease and COVID-19-Balancing risks of delayed treatment Recommencement. J Crohns Colitis 2021:jjab214. 10.1093/ecco-jcc/jjab214 [DOI] [PubMed] [Google Scholar]
- 110. Rosen MH, Axelrad J, Hudesman D, et al. Management of acute severe ulcerative colitis in a pregnant woman with COVID-19 infection: a case report and review of the literature. Inflamm Bowel Dis 2020;26:971–3. 10.1093/ibd/izaa109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lui RN, Wong SH, Sánchez-Luna SA, et al. Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol 2020;35:749–59. 10.1111/jgh.15053 [DOI] [PubMed] [Google Scholar]
- 112. Kushnir VM, Berzin TM, Elmunzer BJ, et al. Plans to reactivate gastroenterology practices following the COVID-19 pandemic: a survey of North American centers. Clin Gastroenterol Hepatol 2020;18:2287–94. 10.1016/j.cgh.2020.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Repici A, Pace F, Gabbiadini R, et al. Endoscopy Units and the Coronavirus Disease 2019 Outbreak: A Multicenter Experience From Italy. Gastroenterology 2020;159:363–6. 10.1053/j.gastro.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hennessy B, Vicari J, Bernstein B, et al. Guidance for resuming Gi endoscopy and practice operations after the COVID-19 pandemic. Gastrointest Endosc 2020;92:743–7. 10.1016/j.gie.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chiu PWY, Ng SC, Inoue H, et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for digestive endoscopy (APSDE-COVID statements). Gut 2020;69:991–6. 10.1136/gutjnl-2020-321185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Furfaro F, Vuitton L, Fiorino G, et al. SFED recommendations for IBD endoscopy during COVID-19 pandemic: Italian and French experience. Nat Rev Gastroenterol Hepatol 2020;17:507–16. 10.1038/s41575-020-0319-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chai N, Mei Z, Zhang W, et al. Endoscopy works during the pandemic of coronavirus COVID-19: recommendations by the Chinese Society of digestive endoscopy. United European Gastroenterol J 2020;8:798–803. 10.1177/2050640620930632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Castro Filho EC, Castro R, Fernandes FF, et al. Gastrointestinal endoscopy during the COVID-19 pandemic: an updated review of guidelines and statements from international and national societies. Gastrointest Endosc 2020;92:440–5. 10.1016/j.gie.2020.03.3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ng SC, Mak JWY, Hitz L, et al. COVID-19 pandemic: which IBD patients need to be Scoped-Who gets Scoped now, who can wait, and how to resume to normal. J Crohns Colitis 2020;14:S791–7. 10.1093/ecco-jcc/jjaa128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Iacucci M, Cannatelli R, Labarile N, et al. Endoscopy in inflammatory bowel diseases during the COVID-19 pandemic and post-pandemic period. Lancet Gastroenterol Hepatol 2020;5:598–606. 10.1016/S2468-1253(20)30119-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rutter MD, Brookes M, Lee TJ, et al. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a national endoscopy database analysis. Gut 2021;70:537–43. 10.1136/gutjnl-2020-322179 [DOI] [PubMed] [Google Scholar]
- 122. Kaufman HW, Chen Z, Niles J, et al. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open 2020;3:e2017267. 10.1001/jamanetworkopen.2020.17267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Deputy M, Sahnan K, Worley G, et al. The use of, and outcomes for, inflammatory bowel disease services during the Covid-19 pandemic: a nationwide observational study. Aliment Pharmacol Ther . 2022;55:836-846. 10.1111/apt.16800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lau LHS, Wong SH, Yip TCF, et al. Collateral effect of coronavirus disease 2019 pandemic on hospitalizations and clinical outcomes in gastrointestinal and liver diseases: a Territory-wide observational study in Hong Kong. Gastroenterology 2020;159:1979–81. 10.1053/j.gastro.2020.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Magro F, Nuzzo A, Abreu C, et al. COVID-19 in gastroenterology: where are we now? Current evidence on the impact of COVID-19 in gastroenterology. United European Gastroenterol J 2021;9:750–65. 10.1002/ueg2.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Din S, Gaya D, Kammermeier J, et al. Inflammatory bowel disease clinical service recovery during the COVID-19 pandemic. Frontline Gastroenterol 2022;13:77–81. 10.1136/flgastro-2021-101805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ho KMA, Banerjee A, Lawler M, et al. Predicting endoscopic activity recovery in England after COVID-19: a national analysis. Lancet Gastroenterol Hepatol 2021;6:381–90. 10.1016/S2468-1253(21)00058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Din S, Gaya DR, Arnott IDR. COVID-19: colorectal cancer endoscopic surveillance in IBD. Lancet Gastroenterol Hepatol 2021;6:526–7. 10.1016/S2468-1253(21)00168-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. British Society of Gastroenterology . An update to information and guidance for endoscopy services in the COVID-19 pandemic, 2021. Available: https://www.bsg.org.uk/covid-19-advice/an-update-to-information-and-guidance-for-endoscopy-services-in-the-covid-19-pandemic-2/https://www.bsg.org.uk/covid-19-advice/an-update-to-information-and-guidance-for-endoscopy-services-in-the-covid-19-pandemic-2/ [Accessed 26 Feb 2022].
- 130. Kennedy NA, Hansen R, Younge L, et al. Organisational changes and challenges for inflammatory bowel disease services in the UK during the COVID-19 pandemic. Frontline Gastroenterol 2020;11:343–50. 10.1136/flgastro-2020-101520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chee D, Nice R, Hamilton B, et al. Patient-led remote intracapillary pharmacoKinetic sampling (fingerPRICKS) for therapeutic drug monitoring in patients with inflammatory bowel disease. J Crohns Colitis 2022;16:190–8. 10.1093/ecco-jcc/jjab128 [DOI] [PubMed] [Google Scholar]
- 132. Bossuyt P, Pouillon L, Claeys S, et al. Ultra-proactive therapeutic drug monitoring of infliximab based on point of care testing in inflammatory bowel disease: results of a pragmatic trial. J Crohns Colitis 2022;16:199–206. 10.1093/ecco-jcc/jjab127 [DOI] [PubMed] [Google Scholar]
- 133. Strik AS, Löwenberg M, Mould DR, et al. Efficacy of dashboard driven dosing of infliximab in inflammatory bowel disease patients; a randomized controlled trial. Scand J Gastroenterol 2021;56:145–54. 10.1080/00365521.2020.1856405 [DOI] [PubMed] [Google Scholar]
- 134. Shields S, Dunlop A, Seenan JP, et al. Disease monitoring of biologic treatment in IBD: early impact and future implications of COVID-19 pandemic. Frontline Gastroenterol 2021;12:345–7. 10.1136/flgastro-2020-101563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Botwin GJ, Li D, Figueiredo J, et al. Adverse events after SARS-CoV-2 mRNA vaccination among patients with inflammatory bowel disease. Am J Gastroenterol 2021;116:1746–51. 10.14309/ajg.0000000000001342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Weaver KN, Zhang X, Dai X, et al. Impact of SARS-CoV-2 vaccination on inflammatory bowel disease activity and development of vaccine-related adverse events: results from PREVENT-COVID. Inflamm Bowel Dis 2021:izab302. 10.1093/ibd/izab302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Edelman-Klapper H, Zittan E, Bar-Gil Shitrit A, et al. Lower serologic response to COVID-19 mRNA vaccine in patients with inflammatory bowel diseases treated with Anti-TNFα. Gastroenterology 2022;162:454–67. 10.1053/j.gastro.2021.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Caldera F, Hillman L, Saha S, et al. Immunogenicity of high dose influenza vaccine for patients with inflammatory bowel disease on anti-TNF monotherapy: a randomized clinical trial. Inflamm Bowel Dis 2020;26:593–602. 10.1093/ibd/izz164 [DOI] [PubMed] [Google Scholar]
- 139. Doornekamp L, Goetgebuer RL, Schmitz KS, et al. High Immunogenicity to Influenza Vaccination in Crohn’s Disease Patients Treated with Ustekinumab. Vaccines 2020;8:455. 10.3390/vaccines8030455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021;27:2032–40. 10.1038/s41591-021-01540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–11. 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 142. Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol 2020;5:eabc8413. 10.1126/sciimmunol.abc8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wong S-Y, Dixon R, Martinez Pazos V, et al. Serologic response to messenger RNA coronavirus disease 2019 vaccines in inflammatory bowel disease patients receiving biologic therapies. Gastroenterology 2021;161:715–8. 10.1053/j.gastro.2021.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kappelman MD, Weaver KN, Boccieri M, et al. Humoral immune response to messenger RNA COVID-19 vaccines among patients with inflammatory bowel disease. Gastroenterology 2021;161:1340–3. 10.1053/j.gastro.2021.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Melmed GY, Botwin GJ, Sobhani K, et al. Antibody responses after SARS-CoV-2 mRNA vaccination in adults with inflammatory bowel disease. Ann Intern Med 2021;174:1768–70. 10.7326/M21-2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lin S, Kennedy NA, Saifuddin A, et al. Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat Commun 2022;13:1379. 10.1038/s41467-022-28517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jena A, James D, Singh AK, et al. Effectiveness and durability of COVID-19 vaccination in 9447 patients with IBD: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2022. 10.1016/j.cgh.2022.02.030. [Epub ahead of print: 19 Feb 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020;183:158–68. 10.1016/j.cell.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-Specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020;183:996–1012. 10.1016/j.cell.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med 2021;27:1280–9. 10.1038/s41591-021-01386-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Reynolds CJ, Swadling L, Gibbons JM, et al. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci Immunol 2020;5:eabf3698. 10.1126/sciimmunol.abf3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Li D, Xu A, Mengesha E, et al. The T-cell clonal response to SARS-CoV-2 vaccination in inflammatory bowel disease patients is augmented by anti-TNF therapy and often deficient in antibody-responders. medRxiv 2021. 10.1101/2021.12.08.21267444. [Epub ahead of print: 08 Dec 2021]. [DOI] [Google Scholar]
- 153. Cerna K, Duricova D, Lukas M, et al. Anti-SARS-CoV-2 vaccination and antibody response in patients with inflammatory bowel disease on Immune-modifying therapy: prospective Single-Tertiary study. Inflamm Bowel Dis 2021. 10.1093/ibd/izab301. [Epub ahead of print: 29 Nov 2021]. [DOI] [PubMed] [Google Scholar]
- 154. Alexander JL, Kennedy NA, Ibraheim H, et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol 2022;7:342–52. 10.1016/S2468-1253(22)00005-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sakuraba A, Luna A, Micic D. Serologic response to coronavirus disease 2019 (COVID-19) vaccination in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis. Gastroenterology 2022;162:88–108. 10.1053/j.gastro.2021.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Volkers A, Wieske L, van Dam K, et al. DOP27 Humoral immune response after SARS-CoV-2 vaccination in patients with immune-mediated inflammatory diseases treated with immunosuppressive therapy - a Target to B! study. J Crohns Colitis 2022;16:i079. 10.1093/ecco-jcc/jjab232.066 [DOI] [Google Scholar]
- 157. Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 2021;27:981–4. 10.1038/s41591-021-01325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Prendecki M, Clarke C, Edwards H, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis 2021;80:1322–9. 10.1136/annrheumdis-2021-220626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kennedy NA, Lin S, Goodhand JR, et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021;70:1884–93. 10.1136/gutjnl-2021-324789 [DOI] [PubMed] [Google Scholar]
- 160. Lipsitch M, Krammer F, Regev-Yochay G, et al. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol 2022;22:57–65. 10.1038/s41577-021-00662-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wei J, Pouwels KB, Stoesser N. SARS-CoV-2 anti-spike IgG antibody responses after second dose of ChAdOx1 or BNT162b2 and correlates of protection in the UK general population [Internet]. bioRxiv 2021. http://medrxiv.org/lookup/doi/10.1101/2021.09.13.21263487 [Google Scholar]
- 162. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385:e84. 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Charilaou P, Tricarico C, Battat R, et al. Impact of inflammatory bowel disease therapies on durability of humoral response to SARS-CoV-2 vaccination. Clin Gastroenterol Hepatol 2021. 10.1016/j.cgh.2021.12.007. [Epub ahead of print: 09 Dec 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a Veterans Affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology 2021;161:827–36. 10.1053/j.gastro.2021.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lev-Tzion R, Focht G, Lujan R, et al. COVID-19 vaccine is effective in inflammatory bowel disease patients and is not associated with disease exacerbation. Clin Gastroenterol Hepatol 2021. 10.1016/j.cgh.2021.12.026. [Epub ahead of print: 23 Dec 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hadi YB, Thakkar S, Shah-Khan SM, et al. COVID-19 Vaccination Is Safe and Effective in Patients With Inflammatory Bowel Disease: Analysis of a Large Multi-institutional Research Network in the United States. Gastroenterology 2021;161:1336–9. 10.1053/j.gastro.2021.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. World Health Organization , 2021. Available: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
- 170. Ai J, Zhang H, Zhang Y, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect 2022;11:337–43. 10.1080/22221751.2021.2022440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zhou H, Tada T, Dcosta BM. SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies [Internet]. bioRxiv 2022. https://www.biorxiv.org/content/10.1101/2022.02.15.480166v1 [Google Scholar]
- 172. Schell TL, Knutson KL, Saha S. Humoral immunogenicity of three COVID-19 mRNA vaccine doses in patients with inflammatory bowel disease [Internet]. bioRxiv 2021. http://medrxiv.org/lookup/doi/10.1101/2021.12.22.21268217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Quan J, Ma C, Panaccione R. Serological responses to the first three doses of SARS-CoV-2 vaccination in inflammatory bowel disease: A prospective cohort study [Internet]. bioRxiv 2022. http://medrxiv.org/lookup/doi/10.1101/2022.03.16.22272440 [Google Scholar]
- 174. COVID-19 vaccines for moderately or severely immunocompromised people. Available: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
- 175. Shehab M, Abu-Farha M, Alrashed F, et al. Immunogenicity of BNT162b2 vaccine in patients with inflammatory bowel disease on infliximab combination therapy: a multicenter prospective study. J Clin Med 2021;10:5362. 10.3390/jcm10225362 [DOI] [PMC free article] [PubMed] [Google Scholar]